Abstract
Childhood poverty is associated with harsh parenting with a risk of transmission to the next generation. This prospective study examined the relations between childhood poverty and non-parent adults’ neural responses to infant cry sounds. While no main effects of poverty were revealed in contrasts of infant cry vs. acoustically matched white noise, a gender by childhood poverty interaction emerged. In females, childhood poverty was associated with increased neural activations in the posterior insula, striatum, calcarine sulcus, hippocampus and fusiform gyrus, while, in males, childhood poverty was associated with reduced levels of neural responses to infant cry in the same regions. Irrespective of gender, neural activation in these regions was associated with higher levels of annoyance with the cry sound and reduced desire to approach the crying infant. The findings suggest gender differences in neural and emotional responses to infant cry sounds among young adults growing up in poverty.
Keywords: poverty, infant cry, caregiving, neuroimaging, gender differences
Introduction
Individuals who grow up in harsh environments including family conflict and insensitive parenting are at risk for a host of adverse developmental outcomes (Repetti, Taylor, & Seeman, 2002) including the replication of insensitive parenting when they become parents (Belsky, 2005; Capaldi & Pears, 2003; Kaufman & Zigler, 1989). Evidence of intergenerational transmission of parenting styles is well documented, particularly in low-income populations (Barnett & The Family Life Project Key, 2008; Hoff, Laursen, & Tardif, 2002; Simons, Whitbeck, Conger, & Wu, 1991). Individuals growing up in poverty are far more likely to experience harsh parenting including physical and emotional maltreatment (Bradley & Corwyn, 2002; Conger & Donnellan, 2007; Grant et al., 2003). Poverty has been associated with an 8–22 fold risk for child abuse (Sedlak & Broadhurst, 1996). Childhood poverty is of particular concern because the experience of harsh parenting may be transmitted to future generations, meaning that when these individuals become parents they may be at a greater risk of repeating harsh parenting behaviors. Thus, parenting quality is an important factor likely contributing to the intergenerational effects of poverty.
However, the underlying neural mechanisms to explain how childhood poverty may result in multigenerational harsh parenting remain largely unexplored. One potential mechanism is impaired negative emotion regulation in responses to infant cry. Individuals who grow up in poverty are more likely to experience emotion dysregulation in adulthood. Studies consistently suggest that childhood poverty is associated with adult depression, substance abuse, and antisocial behaviors, even after controlling for adult levels of socioeconomic status (Cohen, Janicki-Deverts, Chen, & Matthews, 2010; Wadsworth, Evans, Grant, Carter, & Duffy, in press). Childhood poverty and chronic stress are also linked to dysregulated cortisol, a stress hormone (Dozier et al., 2006; Evans, Chen, Miller, & Seeman, 2012; Hostinar, Sullivan, & Gunnar, 2014; Lupien, McEwen, Gunnar, & Heim, 2009; Power, Thomas, Li, & Hertzman, 2012; Seeman, Epel, Gruenewald, Karlamangla, & McEwen, 2010). Elevated cortisol levels may adversely alter the function of brain regions with abundant glucocorticoid receptors and are therefore highly vulnerable to stress hormone exposure, particularly the prefrontal cortex (PFC) and hippocampus (Davidson & McEwen, 2012; Tottenham & Sheridan, 2009). In a study with a non-parent male sample, when cortisol was administered, men who reported exposure to abuse and neglect in childhood exhibited increased hippocampal responses to infant cry sounds (Bos, Montoya, Terburg, & van Honk, 2014). Childhood socioeconomic status (SES) is associated with abnormal structure and function in brain regions important for emotion regulation including the amygdala, striatum, hippocampus, and PFC after controlling for adult socioeconomic status (Gianaros et al., 2011; Hackman, Farah, & Meaney, 2010; Kim et al., 2013; Lupien et al., 2009; Staff et al., 2012). Thus, childhood poverty may be associated with chronic stress exposure and cortisol dysregulation, which may alter neural responses to infant cry sounds and consequently the ability to respond to distressed infants in a sensitive way.
Caregiving behaviors are also linked to increased activation in neural regions related to social and emotional information processing, such as the insula, precuneus, and fusiform gyrus (Barrett & Fleming, 2011; Swain et al., in press; Swain et al., 2014). Previously, first-time human mothers who themselves suffered low quality maternal care in childhood showed smaller brain structure and reduced neural activation in response to infant cry sounds within social information processing areas, such as insula and superior temporal gyrus, as well as emotion regulation regions, such as inferior frontal gyrus and hippocampus (Kim et al., 2010). Therefore, individuals growing up in poverty have elevated risk of harsh parenting. This could impact the maturation of brain regions critical for subsequent caregiving behaviors when they become adults.
In the present longitudinal fMRI study, non-parent men and women at age 24 underwent a neuroimaging session while listening to infant cry sounds. The participants had been followed and their exposure to poverty assessed since age 9. We examined the prospective role of childhood poverty in non-parent adults’ neural responses to infant cry sounds, while controlling for current exposure to poverty. Prior studies of early experience and parental responses to infants have all assessed early experience retrospectively (Ablow, Marks, Feldman, & Huffman, 2013; Kim et al., 2010; Lenzi et al., 2013; Riem, Bakermans-Kranenburg, van Ijzendoorn, Out, & Rombouts, 2012; Strathearn, Fonagy, Amico, & Montague, 2009). Unfortunately, retrospective reports are subject to a variety of recall biases. Moreover, none of these retrospective studies of early experience focused specifically on childhood poverty, but instead used various global indices of adversity. Thus, to our knowledge, this is the only prospective analysis of childhood adversity and parenting function in the adult brain, and the first study of childhood poverty specifically. Moreover, including current exposure to poverty in the analyses allows an examination of the developmental timing (childhood vs. adulthood) of poverty and neural responses to parenting-salient stimuli. Given the well documented significance of childhood SES and harsh parenting (Gershoff, Aber, Raver, & Lennon, 2007; Hoff et al., 2002), we also investigated the potential role of childhood parenting quality received in the associations between childhood poverty exposure and neural responses to infant cry sounds.
Another contribution of this study is inclusion of both men and women. Most studies of early adversity and adult neurological responses to infant signs of distress have relied on parent or non-parent females. Adverse early experience has been considered as a significant risk factor for insensitive parenting in females (Belsky & Jaffee, 2006; Pascoe & Smart Richman, 2009). In animal studies with female rodents, the epigenetic mechanisms of intergenerational transmission of parenting suggest continuity of parenting styles across generations (Champagne, Weaver, Diorio, Sharma, & Meaney, 2003). Adverse childhood experience may also have greater impact on biological stress regulation systems in female than male offspring (Asok, Bernard, Rosen, Dozier, & Roth, 2014; Saavedra-Rodríguez & Feig, 2013). However, in male animal models, biological mechanisms of intergenerational transmission of parenting are poorly understood. In human fathers living in poverty, previous work suggests that while early adversity is associated with insensitive parenting in fathers (Van Ijzendoorn, 1992), adverse early experience can motivate men to become better fathers and be more involved in parenting (Gee, Ro, Shariff-Marco, & Chae, 2009; Kida et al., 2014). This study will examine whether exposure to childhood poverty is associated with neural and behavioral sensitivity to infant cry similarly or differently in females and males.
In the current study, we hypothesized that individuals who grew up in poverty would show altered brain responses to infant cry sounds in both non-parent males and females. Parental care quality received during childhood, a risk factor associated with childhood poverty, may also be associated with neural responses to infant cry sounds. Additionally, we examined whether the associations between childhood poverty and adult neural responses to baby cry stimuli are conditioned by gender.
Methods
Participants
Participants were initially recruited for a larger longitudinal study of poverty and child development at age 9, then followed up at age 13 and 17 (see (Evans, 2003; Evans & Kim, 2012). Among the individuals participating in the three waves of the study, fifty-four individuals were recruited for this neuroimaging study at age 23–24. None had contraindications for fMRI scanning, any current neurological abnormalities, or psychiatric disorders. Among these participants, 10 individuals (6 females, 4 males) were excluded because they were already parents. An additional four persons did not complete the scan due to technical problems or claustrophobia.
Thus, a total of forty young adults without children participated in the current study. The childhood non-poverty group (N=25; male 52%) had never been exposed to poverty from birth until age 17. The childhood poverty group (N=15; male 60%) was exposed to poverty from birth until age 17. Individuals in the childhood poverty group spent most of their childhood in poverty, with a mean percentage of time exposed to poverty from birth until age 17 at 82.49% (SD = 23.27). There were no group differences in gender.
Exposure to poverty was assessed based on family income during childhood (age 9, 13, 17) and the participants’ own income at age 24. The ratio of income-to-needs was computed by dividing total family income (or the participant’s own income) by the poverty threshold at each wave of data collection. This ratio is an annually adjusted, per capita index of income that the U.S. Census Bureau calculates using a standardized formula. Exposure to poverty was defined as an income-to-needs ratio below 1.
An ANOVA to test group-by-gender interaction revealed no significant interaction for income-to-needs ratio in childhood, nor at age 24. The main effect of group status was significant for childhood and current income-to-needs ratios. The childhood poverty group (female 1.38± 0.35; male 0.79± 0.47) had significantly lower income-to-needs ratios than the childhood non-poverty group (female 3.05± 0.83; male 3.41± 1.25) across age 9, 13, and 17, F(1, 36) = 52.66, p < .001. At age 24, the childhood poverty group (female 2.22± 1.38; male 2.35± 1.31) also had significantly lower income-to-needs ratios than the childhood non-poverty group (female 3.73± 2.10; male 5.34± 4.37), F(1, 36) = 5.55, p < .05.
No group-by-gender interaction was found in demographic variables, ethnicity, current age, and relationships status. A main effect of group was found in age, with the non-poverty group (24.13 ± 0.82 years) older than participants in the childhood poverty group (23.12 ± 1.20 years), t(38) = 2.81, p < .01. Thus, age was included as a covariate in analyses. Consistent with the larger SES literature (Evans, 2004), childhood poverty was related to adult relationship status, with persons from middle income backgrounds more likely to be married or with a long term partner, χ2(1, N = 40) = 9.78, p < .05, thus relationship status was also included as a covariate in all analyses. Finally, in order to distinguish the hypothesized influence of childhood poverty from current financial status, concurrent adult poverty exposure was incorporated as a third covariate.
Procedure
After neuroimaging scanning, participants completed questionnaires and behavioral ratings of cry sounds. In previous research waves at age 9, 13, and 17, researchers visited participants’ families at home where participants and participants’ mothers completed questionnaires and interviews on demographic information including family income.
Measures
Parental Bonding Instrument (PBI)
Participants completed the PBI questionnaire (Parker, 1979) at age 24. This instrument is designed to assess childhood parenting style and quality perceived by the child now as an adult. The questionnaire included 12 items on care (sample item: “Mother/Father Spoke to me in a warm and friendly voice,” on a 4 point Likert scale (1 = Very like, 4 = Very unlike)). The participants were asked to answer each item based on reflection on their mother or father in their first 16 years of life. Participants completed the questionnaire twice: once for their mother and the other for their father. Four participants (1 female and 2 males in the childhood poverty group; 1 female in the childhood non-poverty group) did not complete items on paternal care due to father absence in their childhood. The questionnaire has shown good internal consistency and re-test reliability over 20 years (Parker, 1979). Cronbach’s alphas herein were .88 for maternal care and .93 for paternal care.
Behavioral ratings of cry sounds
After scanning, participants listened to the cry sound used in the fMRI paradigm of this study. After hearing the cry sound for 30 seconds, participants rated how much they would want to avoid/approach the crying baby on a five point scale (1=very much want to move away from the baby to 5 = very much want to move closer to the baby). Participants also rated annoyance with the baby cry as well as the white noise control sound stimuli (1=not at all annoyed to 5=very much annoyed).
fMRI paradigm: Infant cry
The Baby Cry paradigm included two auditory stimuli: a standard infant cry, and an acoustically–matched white noise. A standard infant cry was collected during the first two weeks postpartum during the discomfort of a diaper change. The standard infant cry sound has been used in several previous papers with different samples of mothers (Kim et al., 2011; Kim et al., 2010; Swain et al., 2008). This paradigm reliability elicits maternal neural responses (Swain et al., 2014). Using Cool Edit Pro version 2.0 (Syntrillium Software, Phoenix, AZ), the white noise sound enveloped the pattern and volume of the infant cry sound. There were three runs of auditory stimuli, 5 minutes per run. There were four conditions: 1) Passive listening to white noise, 2) Passive listening to infant cry, 3) Listening to cry while imagining it as your baby’s, and 4) Listening to cry as if you’re crying. There were six blocks for each condition, with two blocks per run, and each block was 20s long. The fixation period was 10s long between the conditions. The first and second conditions are used as the standard conditions in the infant cry paradigm [e.g. (De Pisapia et al., 2013; Kim et al., 2010; Laurent & Ablow, 2011; Lorberbaum et al., 2002; Venuti et al., 2012)]. The third and fourth auditory conditions are subject to more variable interpretations by subjects and thus not commonly included for imaging contrasts. The current paper aims to examine non-parent, young adults’ natural and automatic responses to an infant cry, thus only the first and second conditions were included in the analyses in this paper.
For both infant cry and white noise sounds, participants were asked to attend to and experience naturally the emotional state elicited by infant cry sounds. The order of the cry conditions and the white noise condition was pseudo-randomized. The sounds were presented through headphones during the scanning.
fMRI data acquisition and preprocessing
Scanning took place in a 3.0 Tesla Philips magnet scanner in the fMRI laboratory at Veterans Affairs Ann Arbor using a standard eight-channel SENSE head coil. Functional data were acquired (300 T2*-weighted echo-planar-imaging (EPI) volumes; TR = 2,000 ms; TE = 30 ms; flip angle = 90; field of view = 220 mm; matrix size, 64 × 64; 42 axial slices; voxels = 3.44 × 3.44 × 2.80 mm). A high-resolution anatomical T1-weighted image with a 3D gradient recalled echo was also acquired. Functional imaging data were preprocessed and analyzed using Statistical Parametric Mapping 8 (Wellcome Trust Center for Neuroimaging, University College, London; www.fil.ion.ucl.ac.uk/spm). Five images at the beginning of each fMRI run were discarded. Slice timing correction was performed using a middle slice as a reference (slice 21), and then images within each run were realigned to the mean image of the first run to correct for movement. The realigned functional images were spatially normalized to a standard T1 template based on the Montreal Neurological Institute (MNI) reference brain, resampled to 2 × 2 × 2 mm voxels, and then spatially smoothed using an 8mm full width at half maximum (FWHM) Gaussian filter.
fMRI analysis
At the individual level of analysis, fMRI data was regressed onto a general linear model (GLM) containing regressors representing the time periods of each condition, i.e. infant cry and white noise. The six motion correction parameters estimated from the realignment procedure were also entered as covariates of no interest. Regressors were convolved with the canonical haemodynamic response function and low frequency drifts were excluded using a high-pass filter of 0.0078 Hz. At the individual level of analysis, we contrasted images of the blood-oxygen-level–dependent (BOLD) signal changes associated with the infant cry vs. while noise contrast.
At the group level of analysis, contrast images of individual subjects were entered into a random-effect analysis. A multiple regression was performed with childhood poverty (poverty, non-poverty), gender (male, female), and the interaction term of group and gender, as independent variables. The current poverty exposure, current relationship status (single, significant relationship, married), and current age were included in the same model as covariates of no interest. An initial voxel-wise threshold of p < .005 and a minimum cluster size of 275 voxels gave a corrected p < .05. This threshold was determined by Monte-Carlo simulations using the 3dClustSim program of the AFNI toolkit (3dClustSim –mask –both –prefix –fwhmxyz 9.90 11.68 10.45; http://afni.nimh.nih.gov/pub/dist/doc/program_help/3dClustSim.html). The estimates of each suprathreshold region were extracted for each participant using MarsBaR (Marseille boîte à région d’intérêt) (Brett, Anton, Valabregue, & Poline, 2002) and were then entered into Statistical Package for the Social Sciences (SPSS, Inc.) for post-hoc analyses. Additional detail regarding breakdown of the clusters into the proportions of voxels in specific anatomical regions was obtained via the MNI Space utility, as visualized and reported through xjview (http://www.alivelearn.net/).
Using SPSS, a two-way ANCOVA with childhood poverty (poverty, non-poverty) and gender (male, female) as between-subject factors was conducted to test the main and interaction effects on behavioral ratings and childhood parental care quality. Adult current age, current income to needs and current relationship status were included as covariates. Exploratory correlations were also performed to test the associations among childhood poverty exposure, behavioral ratings, childhood parental care quality, and neural activations. The indirect effect of childhood parental care quality in the associations between childhood poverty exposure and neural activations was tested using 95% bias-corrected CIs with bootstrapping procedures (10,000 bootstrap resamples) (Preacher & Hayes, 2008).
Results
fMRI analysis of the associations between childhood poverty and neural activation
The group-level analysis of the infant cry vs. white noise contrast revealed no main effect of childhood poverty, gender, or current poverty exposure. However, significant interaction effects of childhood poverty and gender were identified (Table 1), p < .05, corrected. The clusters were located in the posterior insula and striatum (Figure 1a), calcarine sulcus, hippocampus and precuneus (Figure 1b) and fusiform gyrus and parahippocampus (Figure 1c). Among the covariates, current age was negatively associated with left anterior cingulate cortex activation (t(33)=4.56; x, y, z = −16, −4, 50; ƙ = 334) and being in a relationship was negatively associated with middle frontal gyrus activation (t(33)=4.97; x, y, z = −26, 12, 28; ƙ = 391). When we re-ran all analyses without the covariates, all of the significant gender by poverty interactions reported herein remained significant.
Table 1.
Brain Regions Showing Interactions between Childhood Poverty Exposure and Gender in the Infant Cry vs. While Noise contrasts.
| Area of Activation§ | Brodmann area | Side | # voxels | MNI coordinates
|
t (1, 33) | Post-hoc Analysis | ||
|---|---|---|---|---|---|---|---|---|
| x | y | z | ||||||
| Insula (Putamen/Lentiform nucleus/Precentral Gyrus/Inferior Frontal Gyru) | BA 13/44/47 | L | 392 | −34 | 4 | 4 | 4.38 | Pov: female > male† Non-pov: female < male*** females: Pov > Non-pov** males: Pov < Non-pov** |
| Calcarine sulcus (Precuneus/Caudate/Hippocampus) | – | R | 382 | 26 | −50 | 12 | 3.88 | Pov: female > male** Non-pov: female < male** females: Pov > Non-pov** males: Pov < Non-pov* |
| Fusiform Gyrus (Parahippocampus) | BA 19/37 | R | 449 | 32 | −52 | −10 | 3.70 | Pov: female > male* Non-pov: female < male* females: Pov > Non-pov** males: Pov < Non-pov* |
p < .05, corrected; R = right, L = left; pov = childhood poverty group; non-pov = childhood non-poverty group;
p < .10,
p < .05,
p < .01,
p < .001
The location of the peak coordinate is listed first, then areas included in a cluster are listed in brackets.
Figure 1.
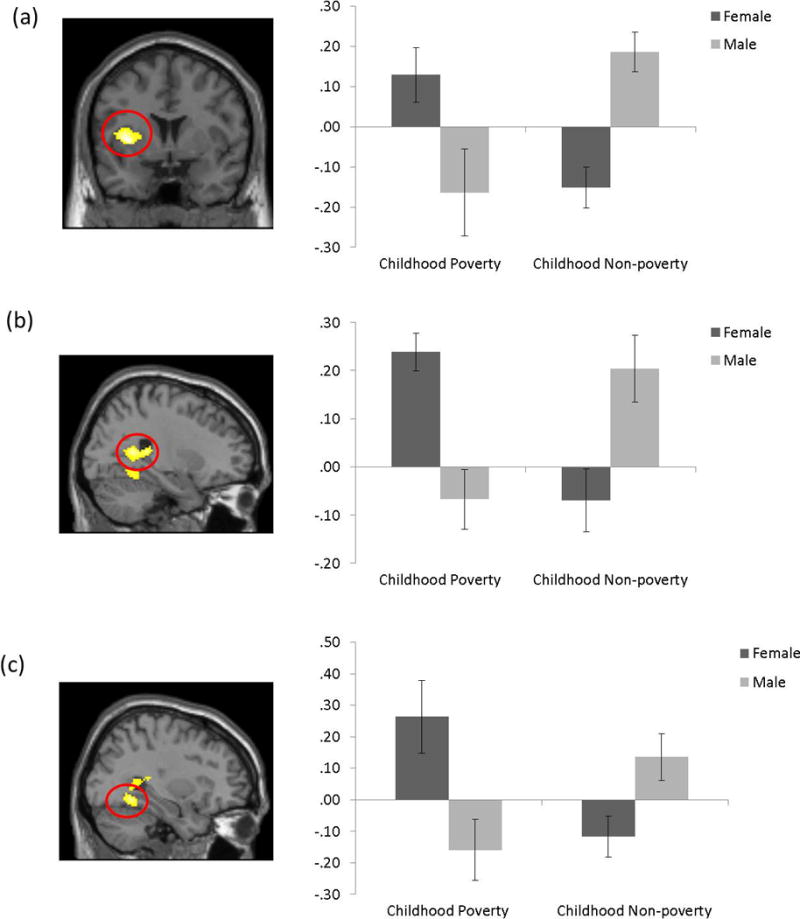
Brain areas in a red circle showing Childhood Poverty-by-Gender on the Infant Cry vs White Noise contrast among males and females, p < .05, corrected. Color bars represent t values of the contrast. Percent signal change estimates for each contrast were averaged across the entire cluster for each participant. A. left posterior insula and striatum (BA13/44; x, y, z = −34, 4, 4); B. right calcarine sulcus, hippocampus, and precuneus (x, y, z = 26, −50, 12); C. right fusiform gyrus and parahippocampus (BA19/37; x, y, z = 32, −52, −10).
A two-way ANCOVA with childhood poverty (poverty, non-poverty) and gender (male, female) as between-subject factors was conducted for the decomposition of the interaction findings of the infant cry vs. white noise contrast. Significant 2-way interactions were further decomposed using t-tests. When the analyses were repeated with covariates (current poverty exposure, current relationship status, and current age), the 2-way interaction remained significant, and no covariate was significant. Thus, the following post-hoc analyses were conducted without covariates. As shown in the post-hoc analysis column in Table 1 and in Figure 1, in adult female participants childhood poverty was associated with increased activation while, in male participants, childhood poverty was associated with reduced activation in the same regions. When gender differences were directly compared in each group, males in the childhood poverty group exhibited reduced activation as compared to females, while in the childhood non-poverty group, females exhibited reduced activation in all regions as compared to males.
To understand the interactions further between two conditions, we used a 3-way ANOVA with condition (infant cry, white noise) as a within-subject factor, and childhood poverty (poverty, non-poverty) and gender (male, female) as between-subject factors. The 3-way interactions were significant in the left insula and right calcarine sulcus, Fs(1, 36) ≥ 16.83, ps < .001. In the cluster of the left insula, the interactions between childhood poverty and gender were driven by the white noise condition, F(1, 36) = 9.56, p < .005. In female participants, individuals who experienced childhood poverty exhibited decreased activations compared to individuals who did not experience childhood poverty during the white noise condition, t(16) = −2.94, p < .05. In contrast, in the cluster of the right calcarine sulcus, the interactions between childhood poverty and gender were driven by the infant cry condition, F(1, 36) = 7.71, p < .01. In female participants, individuals who experienced childhood poverty exhibited increased activations compared to individuals who did not experience childhood poverty during the infant cry condition, t(16) = 2.13, p = .05. No significant effect was detected in males in either region. Neither interaction nor main effect in any region was significant in the cluster of the fusiform gyrus. Thus, the results suggest that the gender by childhood poverty interactions were largely driven by differences in neural responses between two conditions, infant cry and white noise.
Correlations among neural activations, behavioral ratings, and parental care
Two-way ANCOVA was conducted to test group differences in how annoyed participants were by the cry and white noise and how much participants wanted to approach the crying baby. No interaction or main effect was detected for any of the ratings. In exploratory correlation analysis, across males and females, higher ratings of annoyance were correlated with higher activations in the infant cry vs. white noise contrast in all three suprathreshold clusters, rs(40) > .35, ps < .05 (Figure 2a). On the other hand, across males and females, higher ratings of approach were correlated with reduced activations in the infant cry vs. white noise contrast in all three suprathreshold clusters, rs(40) < −.45, ps < .05 (Figure 2b). No significant correlations were detected with the annoyance rating of the white noise sound.
Figure 2.
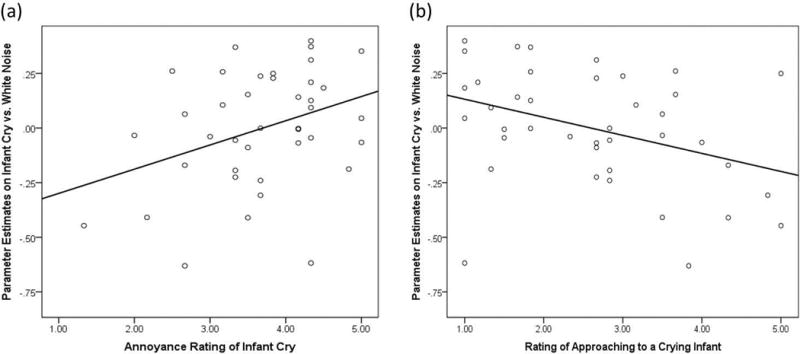
Correlation plots between behavioral ratings and neural activation in left posterior insula and striatum (BA13/44; x, y, z = −34, 4, 4; p < .05, corrected) on the contrast of Infant Cry vs. White Noise condition across males and females. A, Positive correlation using the annoyance ratings of the infant cry sound, r(40) = .35, p < .05; B. Negative correlation using the ratings of approaching a crying baby while listening to the infant cry sound, r(40) = − .37, p < .05.
When the parenting care quality received in childhood was examined, the main effect of childhood poverty status was significant in predicting paternal care quality during childhood, F(1, 35) = 11.65, p < .005. The childhood poverty group (M= 16.08, SD= 9.80) reported lower care provided by a father compared to the childhood non-poverty group (M= 26.50, SD= 4.37). The main effect of gender was also significant, F(1, 35) = 6.06, p < .05. Female participants (M= 25.25, SD= 6.81) reported higher care provided by a father compared to male participants (M= 21.25, SD= 8.97). No interaction or any other main effects were found significant for paternal care. None of the main or interaction effects were significant for maternal care. Given the gender differences, we performed separate exploratory correlation analysis among neural activations and paternal care scores by gender. No significant correlations were detected in females.
However, in males, paternal care scores were positively correlated with neural activation in the posterior insula, r(20) < .76, p < .001 in the infant cry vs. white noise contrast. In a mediation analysis, a significant indirect effect of parental care in the associations between childhood poverty exposure and posterior insula activation was detected among males [indirect effect = 0.10, 95% confidence intervals (CIs) = 0.02–0.21]. The 95% bias-corrected CIs without the inclusion of 0 indicates a statistically significant indirect relationship at p < .05. Thus, low perceived quality of paternal care mediated the associations between childhood poverty exposure and reduced posterior insula activation in males. For females, however, the indirect effect was not significant.
Discussion
The current study examined the role of childhood poverty exposure in neural responses to infant cry in non-parent young adult males and females. Interesting differences in gender emerged in the results. In females, childhood poverty exposure was associated with increased levels of neural responses while listening to an infant cry sound. The regions where childhood poverty was associated with increased levels of activity included the posterior insula, calcarine sulcus, and fusiform gyrus, brain regions involved in sensorimotor and emotional information processing. In contrast, male participants exhibited the opposite patterns in the same brain regions: childhood poverty exposure was associated with reduced levels of neural responses to infant cry. Across gender, increased levels of neural activity in these regions were associated with higher ratings of annoyance with the infant cry and greater tendency to avoid (rather than approach) the crying infant. Therefore, in non-parent females, childhood poverty was linked to more negative emotional responses to infant cry and increased neural activation. On the other hand, in non-parent males, childhood poverty was linked to more positive emotional responses to infant cry and reduced neural activation in the same regions.
Previous studies highlighted the important role of the insula activation in response to infant among mothers and fathers. In response to infant related stimuli, mothers and fathers exhibited increased activation, particularly in the anterior insula, which may be associated with increased empathy toward an infant (Mascaro, Hackett, Gouzoules, Lori, & Rilling, 2014; Riem et al., 2011). However, greater anterior insula responses to infant cry in more intrusive mothers compared to sensitive mothers (Atzil, Hendler, & Feldman, 2011) and very high levels of anterior insula responses to infant images among insensitive fathers (Mascaro et al., 2014) suggest that a high level of anterior insula activation may be associated with increased anxiety that the mothers and fathers experience.
The current study found a poverty-by-gender interaction in the posterior insula, with females exposed to poverty showing increased activation, and males exposed to poverty showing decreased activation, as compared to their non poverty-exposed peers. Studies examining neural responses to physical and social pain suggest different roles of the anterior vs. posterior insula. Posterior insula is involved in detecting one’s own personal experience of pain, while anterior insula is involved in anxiety of personally anticipated pain, as well as empathy toward others in pain (Singer et al., 2004). Thus, perhaps the associations between increased posterior insula activation and increased annoyance/decreased approach ratings may reflect increased experience of negative emotion for participants themselves in response to infant cry sounds.
Furthermore, the associations between increased annoyance/decreased approach ratings and increased activation in other neural regions may suggest upregulation of sensorimotor information processing in response to infant cry sounds. The posterior insula is connected with the somatosensory cortex (Uddin, 2015) as well as the thalamus and striatum (Singer, Critchley, & Preuschoff, 2009), and thus plays an important role in the integration of sensorimotor information. The supplementary motor cortex also emerges in a human maternal brain study of child feedback brain responses that correlated with a measure of empathic concern (Ho et al., 2014) as well as other studies of non-parents using infant pictures (Caria et al., 2012). Other regions identified in the current study, including the striatum and premotor cortex, are involved in sensorimotor information, and the calcarine sulcus, parahippocampus and fusiform gyrus are heavily involved in visual information processing. Increased activation in these visual information processing regions is typically detected in response to auditory stimuli (Sander et al., 2005). Such upregulation of the sensorimotor information processing regions including the striatum, fusiform gyrus, and parahippocampual regions in response to negative emotional information has also been detected in clinical populations with psychopathology compared to healthy controls (Frick, Howner, Fischer, Kristiansson, & Furmark, 2013; Koenigsberg et al., 2009; Surguladze et al., 2005). Therefore, the increased activation in these regions may support experience of negative emotions in response to infant cry sounds.
The finding that childhood exposure to poverty was significantly associated with increased neural responses to infant cry sounds in females is consistent with a previous result from mothers who retrospectively reported low quality maternal care. Their hippocampal responses to infant cry sounds were greater than mothers who retrospectively reported high quality maternal care (Kim et al., 2010). In particular, the hippocampus has a high density of glucocorticoid receptors, thus hippocampal activation has been associated with stress responses to emotional stimuli (Phan, Wager, Taylor, & Liberzon, 2002; Ulrich-Lai & Herman, 2009). Other studies suggest that childhood poverty is associated with reduced hippocampal structure and increased hippocampal responses to an acute stressor (Hanson, Chandra, Wolfe, & Pollak, 2011; Hanson et al., in press; Staff et al., 2012). Therefore, increased neural responses to infant cry in the hippocampus as well as the posterior insula and other sensorimotor processing regions may reflect difficulties in processing and regulating emotional information from the cry. Low-income mothers exhibit a higher rate of postpartum depression (Kim & Bianco, 2014; Segre, O’Hara, Arndt, & Stuart, 2007) and a higher rate of hostility in response to infant cry sounds (Hart & Risley, 1995). More research is needed to understand whether the neural and emotional responses to infant cry sounds can be a risk factor for parental insensitivity toward one’s own infants during the transition to parenthood among first-time, low-income mothers.
On the other hand, in men, childhood exposure to poverty was associated with reduced responses to infant cry sounds in the same neural regions. Furthermore, higher quality of paternal care reported by male participants mediated the links between childhood poverty exposure and reduced neural responses to infant cry. The results may point to a compensatory process in males. Typical non-parent men tend to exhibit more negative and less empathetic responses to infant cry and less physiological responses to infant cry as well (Glocker et al., 2009; Rilling, 2013). However, lower quality paternal care, associated with poverty exposure in childhood, may contribute to increased motivation for non-parent males to compensate for the negative relationships with their own fathers (Kida et al., 2014). This might lead to reduced neural responses that was further associated with a less negative emotional response to infant cry sounds. Although research on males is far scarcer than on females, animal work suggests gender differences in parenting behaviors that may also be informative for understanding the gender differences uncovered herein. Prairie voles are a biparental and socially monogamous species, and thus have been of particular importance in examining neural correlates of paternal and maternal behaviors (McGraw & Young, 2010; Young, Gobrogge, Liu, & Wang, 2011). When exposed to stress, female prairie voles exhibited decreased levels of alloparenting behaviors to unrelated pups; whereas male prairie voles exhibited increased levels of alloparenting behaviors (Scaramella, Neppl, Ontai, & Conger, 2008). These findings, however, should be considered cautiously because they examined the effects of acute stress exposure, whereas the current study examined effects of chronic stress exposure.
It is important to note that although strong interactions between childhood poverty exposure and gender were detected in neural activation, no such interactions were detected in behavioral ratings. Thus, caution is required in making direct links among childhood poverty exposure, neural responses, and behavioral ratings on annoyance and approach/avoidance. It is especially important that the poverty by gender neurological interaction effects are replicated before drawing any definitive conclusions about childhood poverty and neurological responses to infant distress signals. The inconsistent findings may be associated with limited sample size, as well as the fact that the ratings were collected outside of rather than inside the scanner. Observation measures on how individuals interact with infants may help to overcome some of the limitations of behavioral ratings. Thus, future studies should include emotional ratings reported during the neuroimaging scan as well as observation measures.
Finally, we hypothesized childhood poverty would influence adult neural responses to parenting stimuli. One possibility is that the adult neural correlates of poverty shown herein reflect current income status rather than childhood poverty. All of the effects include a covariate for concurrent income levels at age 23–24. When we test the association of concurrent adult income levels, no associations with neural activity are present. Therefore, experiences of poverty in childhood, but not as an adult, appear to be a more significant factor to explain individual differences in neural and emotional responses to infant cry.
The findings from the current study should be considered in light of limitations. First, although in total this neuroimaging study included a relatively large sample of 40 subjects, there was a smaller number of participants in the child poverty group compared to the non-poverty group. There were only six females and nine males in the childhood poverty group. Thus, the findings should be considered as preliminary, and future studies should include more subjects and balanced distributions between poverty and non-poverty groups. Second, the current study involved a hard-to-recruit sample including both males and females with chronic exposure to childhood poverty; however, the sample was limited to non-parents. Previous studies have demonstrated that non-parents show similar neural responses to infant cry compared to parents (Bos et al., 2014; Montoya et al., 2012; Riem et al., 2012; Swain et al., 2014) while others suggested that parents exhibit greater physiological and emotional responses to infant cry across genders (Eisenberger, 2013). Thus, parents may have different neural and behavioral responses to their own infant cry sounds. Also, individuals in poverty are more likely to have children early, even in their teenage years, however our sample was limited to young adults without their own infants. Thus, future studies should examine the neural responses to infant cry sounds among parents from a wider age range.
In sum, the current study sheds light on a question central to many parenting intervention programs, whether poverty and other risk factors impact emotional sensitivity to infants. By recruiting male and female non-parents from low- and middle-income family backgrounds, we uncovered evidence of heterogeneity in poverty background and neural and affective responses to infant cry sounds. In the neural regions potentially involved in negative emotional information processing, non-parent females who grew up in poverty exhibited increased neural responses to infant cry whereas non-parent males who grew up in poverty exhibited reduced neural responses to infant cry. The results suggest that childhood poverty may have more negative effects in women than in men on neural responses to infants. Previous work suggests that harsh parenting styles can be transmitted to the next generation. Relevant to our gender interaction findings, Simons and colleagues (1991) found these multigenerational effects to be greater in women than in men (Simons et al., 1991). Future research with a larger sample is necessary to map out the processes of the intergenerational transmission of both males’ and females’ parenting styles in the context of poverty.
Acknowledgments
The current study was supported by National Institute of Health RC2MD004767, 1R21HD078797-01A1, the W.T. Grant Foundation, the John D. and Catherine T. MacArthur Foundation Network on Socioeconomic Status and Health, the Robert Wood Johnson Foundation and the Centers for Disease Control & Prevention Award # U49/CE002099 via the University of Michigan Injury Center. We thank Erika Blackburn, Sarah Garfinkel, and Robert Varney for assistance with data collection and Christina Congleton for writing assistance.
References
- Ablow JC, Marks AK, Feldman SS, Huffman LC. Associations between first-time expectant women’s representations of attachment and their physiological reactivity to infant cry. Child Development. 2013;84:1373–1391. doi: 10.1111/cdev.12135. [DOI] [PubMed] [Google Scholar]
- Asok A, Bernard K, Rosen JB, Dozier M, Roth TL. Infant-caregiver experiences alter telomere length in the brain. PLOS ONE. 2014;9:e101437. doi: 10.1371/journal.pone.0101437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atzil S, Hendler T, Feldman R. Specifying the neurobiological basis of human attachment: Brain, hormones, and behavior in synchronous and intrusive mothers. Neuropsychopharmacology. 2011;36:2603–2615. doi: 10.1038/npp.2011.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnett MA, The Family Life Project Key, Investigators Mother and grandmother parenting in low-income three-generation rural households. Journal of Marriage and Family. 2008;70:1241–1257. doi: 10.1111/j.1741-3737.2008.00563.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett J, Fleming AS. Annual Research Review: All mothers are not created equal: neural and psychobiological perspectives on mothering and the importance of individual differences. Journal of Child Psychology and Psychiatry. 2011;52:368–397. doi: 10.1111/j.1469-7610.2010.02306.x. [DOI] [PubMed] [Google Scholar]
- Belsky J, Jaffee S. The multiple determinants of parenting. In: Cohen DJ, Cicchetti D, editors. Developmental psychopathology: Risk, disorder, and adaptation. 2nd. Vol. 3. Hoboken, NJ: John Wiley & Sons; 2006. pp. 38–85. [Google Scholar]
- Belsky J, Jaffee SR, Sligo J, Woodward L, Silva PA. Intergenerational transmission of warm-sensitive-stimulating parenting: A prospective study of mothers and fathers of 3-year-olds. Child Development. 2005;76:384–396. doi: 10.1111/j.1467-8624.2005.00852.x. [DOI] [PubMed] [Google Scholar]
- Bos PA, Montoya ER, Terburg D, van Honk J. Cortisol administration increases hippocampal activation to infant crying in males depending on childhood neglect. Human Brain Mapping. 2014;35(10):5116–5126. doi: 10.1002/hbm.22537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley RH, Corwyn RF. Socioeconomic status and child development. Annual Review of Psychology. 2002;53:371–399. doi: 10.1146/annurev.psych.53.100901.135233. [DOI] [PubMed] [Google Scholar]
- Brett M, Anton JL, Valabregue R, Poline JB. Region of interest analysis using the MarsBar toolbox for SPM 99. Neuroimage. 2002;16:S497. [Google Scholar]
- Capaldi DM, Pears KC, Patterson GR, Owen LD. Continuity of parenting practices across generations in an at-risk sample: A prospective comparison of direct and mediated associations. Journal of Abnormal Child Psychology. 2003;31:127–142. doi: 10.1023/a:1022518123387. [DOI] [PubMed] [Google Scholar]
- Caria A, Falco S, Venuti P, Lee S, Esposito G, Rigo P, Birmbauer N, Bornstein MH. Species-specific response to human infant faces in the premotor cortex. Neuroimage. 2012;60:884–893. doi: 10.1016/j.neuroimage.2011.12.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Champagne FA, Weaver IC, Diorio J, Sharma S, Meaney MJ. Natural variations in maternal care are associated with estrogen receptor alpha expression and estrogen sensitivity in the medial preoptic area. Endocrinology. 2003;144:4720–4724. doi: 10.1210/en.2003-0564. [DOI] [PubMed] [Google Scholar]
- Cohen S, Janicki-Deverts D, Chen E, Matthews KA. Childhood socioeconomic status and adult health. Annals of the New York Academy of Sciences. 2010;1186:37–55. doi: 10.1111/j.1749-6632.2009.05334.x. [DOI] [PubMed] [Google Scholar]
- Conger RD, Donnellan MB. An interactionist perspective on the socioeconomic context of human development. Annual Review of Psychology. 2007;58:175–199. doi: 10.1146/annurev.psych.58.110405.085551. [DOI] [PubMed] [Google Scholar]
- Davidson RJ, McEwen BS. Social influences on neuroplasticity: Stress and interventions to promote well-being. Nature Reviews Neuroscience. 2012;15:689–695. doi: 10.1038/nn.3093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Pisapia N, Bornstein MH, Rigo P, Esposito G, De Falco S, Venuti P. Sex differences in directional brain responses to infant hunger cries. Neuroreport. 2013;24:142–146. doi: 10.1097/WNR.0b013e32835df4fa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dozier M, Manni M, Gordon MK, Peloso E, Gunnar MR, Stovall-McClough KC, Levine S. Foster children’s diurnal production of cortisol: An exploratory study. Child Maltreatment. 2006;11:189–197. doi: 10.1177/1077559505285779. [DOI] [PubMed] [Google Scholar]
- Eisenberger NI. An empirical review of the neural underpinnings of receiving and giving social support: Implications for health. Psychosomatic Medicine. 2013;75:545–556. doi: 10.1097/PSY.0b013e31829de2e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans GW. A multimethodological analysis of cumulative risk and allostatic load among rural children. Developmental Psychology. 2003;39:924–933. doi: 10.1037/0012-1649.39.5.924. [DOI] [PubMed] [Google Scholar]
- Evans GW. The environment of childhood poverty. American Psychologist. 2004;59:77–92. doi: 10.1037/0003-066X.59.2.77. [DOI] [PubMed] [Google Scholar]
- Evans GW, Chen E, Miller GE, Seeman TE. How poverty gets under the skin: A lifecourse perspective. In: Maholmes V, King R, editors. The Oxford handbook of poverty and child development. New York: Oxford University Press; 2012. pp. 13–36. [Google Scholar]
- Evans GW, Kim P. Early childhood poverty and adult chronic physiological stress: The mediating role of childhood cumulative risk exposure. Psychological Science. 2012:979–983. doi: 10.1177/0956797612441218. [DOI] [PubMed] [Google Scholar]
- Frick A, Howner K, Fischer H, Kristiansson M, Furmark T. Altered fusiform connectivity during processing of fearful faces in social anxiety disorder. Translational Psychiatry. 2013;3:e312. doi: 10.1038/tp.2013.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gee GC, Ro A, Shariff-Marco S, Chae D. Racial discrimination and health among Asian Americans: Evidence, assessment, and directions for future research. Epidemiologic Reviews. 2009;31:130–151. doi: 10.1093/epirev/mxp009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gershoff ET, Aber JL, Raver CC, Lennon MC. Income is not enough: Incorporating material hardship into models of income associations with parenting and child development. Child Development. 2007;78:70–95. doi: 10.1111/j.1467-8624.2007.00986.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gianaros PJ, Manuck SB, Sheu LK, Kuan DCH, Votruba-Drzal E, Craig AE, Hariri AR. Parental education predicts corticostriatal functionality in adulthood. Cerebral Cortex. 2011;21:896–910. doi: 10.1093/cercor/bhq160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glocker ML, Langleben DD, Ruparel K, Loughead JW, Valdez JN, Griffin MD, Gur RC. Baby schema modulates the brain reward system in nulliparous women. Proceedings of the National Academy of Sciences. 2009;106:9115–9119. doi: 10.1073/pnas.0811620106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant KE, Compas BE, Stuhlmacher AF, Thurm AE, McMahon SD, Halpert JA. Stressors and child and adolescent psychopathology: Moving from markers to mechanisms of risk. Psychological Bulletin. 2003;129:447–466. doi: 10.1037/0033-2909.129.3.447. [DOI] [PubMed] [Google Scholar]
- Hackman DA, Farah MJ, Meaney MJ. Socioeconomic status and the brain: Mechanistic insights from human and animal research. Nature Reviews Neuroscience. 2010;11:651–659. doi: 10.1038/nrn2897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson JL, Chandra A, Wolfe BL, Pollak SD. Association between income and the hippocampus. PLOS ONE. 2011;6:e18712. doi: 10.1371/journal.pone.0018712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson JL, Nacewicz BM, Sutterer MJ, Cayo AA, Schaefer SM, Rudolph KD, Davidson RJ. Behavior problems after early life stress: Contributions of the hippocampus and amygdala. Biological Psychiatry. doi: 10.1016/j.biopsych.2014.04.020. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart B, Risley RR. Meaningful differences. Baltimore: Paul H. Brooks; 1995. [Google Scholar]
- Ho SS, Konrath S, Brown S, Swain JE. Empathy and stress related neural responses in maternal decision making. Frontiers in Neuroscience. 2014;8:152. doi: 10.3389/fnins.2014.00152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoff E, Laursen B, Tardif T. Socioeconomic status and parenting. In: Bornstein MH, editor. Handbook of parenting. 2nd. Mahwah, NJ: Erlbaum; 2002. pp. 231–252. [Google Scholar]
- Hostinar CE, Sullivan RM, Gunnar MR. Psychobiological mechanisms underlying the social buffering of the hypothalamic-pituitary-adrenocortical axis: A review of animal models and human studies across development. Psychological Bulletin. 2014;140:256–282. doi: 10.1037/a0032671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman J, Zigler E. The intergenerational transmission of child abuse. In: Cicchetti D, Carlson V, editors. Child maltreatment: Theory and research on the causes and consequences of child abuse and neglect. Cambridge, UK: Cambridge University Press; 1989. pp. 129–150. [Google Scholar]
- Kida T, Nishitani S, Tanaka M, Takamura T, Sugawara M, Shinohara K. I love my grandkid! An NIRS study of grandmaternal love in Japan. Brain Research. 2014;1542:131–137. [PubMed] [Google Scholar]
- Kim P, Bianco H. How motherhood and poverty change the brain. Zero to Three. 2014;34:29–36. [Google Scholar]
- Kim P, Evans GW, Angstadt M, Ho SS, Sripada CS, Swain JE, Phan KL. Effects of childhood poverty and chronic stress on emotion regulatory brain function in adulthood. The Proceedings of the National Academy of Sciences. 2013;110:18442–18447. doi: 10.1073/pnas.1308240110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim P, Feldman R, Mayes LC, Eicher V, Thompson N, Leckman JF, Swain JE. Breastfeeding, brain activation to own infant cry, and maternal sensitivity. Journal of Child Psychology and Psychiatry. 2011;52:907–915. doi: 10.1111/j.1469-7610.2011.02406.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim P, Leckman JF, Mayes LC, Newman M-A, Feldman R, Swain JE. Perceived quality of maternal care in childhood and structure and function of mothers’ brain. Developmental Science. 2010;13:662–673. doi: 10.1111/j.1467-7687.2009.00923.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koenigsberg HW, Siever LJ, Lee H, Pizzarello S, New AS, Goodman M, Prohovnik I. Neural correlates of emotion processing in borderline personality disorder. Psychiatry Research: Neuroimaging. 2009;172:192–199. doi: 10.1016/j.pscychresns.2008.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laurent HK, Ablow JC. A cry in the dark: Depressed mothers show reduced neural activation to their own infant’s cry. Social Cognitive and Affective Neuroscience. 2012;7(2):125–134. doi: 10.1093/scan/nsq091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenzi D, Trentini C, Pantano P, Macaluso E, Lenzi GL, Ammaniti M. Attachment models affect brain responses in areas related to emotions and empathy in nulliparous women. Human Brain Mapping. 2013;34:1399–1414. doi: 10.1002/hbm.21520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorberbaum JP, Newman JD, Horwitz AR, Dubno JR, Lydiard RB, Hamner MB, George MS. A potential role for thalamocingulate circuitry in human maternal behavior. Biolological Psychiatry. 2002;51:431–445. doi: 10.1016/s0006-3223(01)01284-7. [DOI] [PubMed] [Google Scholar]
- Lupien SJ, McEwen BS, Gunnar MR, Heim C. Effects of stress throughout the lifespan on the brain, behaviour and cognition. Nature Reviews Neuroscience. 2009;10:434–445. doi: 10.1038/nrn2639. [DOI] [PubMed] [Google Scholar]
- Mascaro JS, Hackett PD, Gouzoules H, Lori A, Rilling JK. Behavioral and genetic correlates of the neural response to infant crying among human fathers. Social Cognitive and Affective Neuroscience. 2014;9:1704–1712. doi: 10.1093/scan/nst166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGraw LA, Young LJ. The prairie vole: An emerging model organism for understanding the social brain. Trends in Neurosciences. 2010;33:103–109. doi: 10.1016/j.tins.2009.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montoya JL, Landi N, Kober H, Worhunsky PD, Rutherford HJV, Mencl WE, Potenza MN. Regional brain responses in nulliparous women to emotional infant stimuli. Plos One. 2012;7(5):e36270. doi: 10.1371/journal.pone.0036270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker G. A parental bonding instrument. British Journal of Medical Psychology. 1979;152:1–10. [Google Scholar]
- Pascoe EA, Smart Richman L. Perceived discrimination and health: A meta-analytic review. Psychological Bulletin. 2009;135:531. doi: 10.1037/a0016059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phan KL, Wager T, Taylor SF, Liberzon I. Functional neuroanatomy of emotion: A meta-analysis of emotion activation studies in PET and fMRI. Neuroimage. 2002;16:331–348. doi: 10.1006/nimg.2002.1087. [DOI] [PubMed] [Google Scholar]
- Power C, Thomas C, Li L, Hertzman C. Childhood psychosocial adversity and adult cortisol patterns. British Journal of Psychiatry. 2012;201:199–206. doi: 10.1192/bjp.bp.111.096032. [DOI] [PubMed] [Google Scholar]
- Preacher KJ, Hayes AF. Asymptotic and resampling strategies for assessing and comparing indirect effects in multiple mediator models. Behavior Research Methods. 2008;40:879–891. doi: 10.3758/brm.40.3.879. [DOI] [PubMed] [Google Scholar]
- Repetti RL, Taylor SE, Seeman TE. Risky families: Family social environments and the mental and physical health of offspring. Psychological Bulletin. 2002;128:330–366. [PubMed] [Google Scholar]
- Riem MM, Bakermans-Kranenburg MJ, Pieper S, Tops M, Boksem MA, Vermeiren RR, Rombouts SA. Oxytocin modulates amygdala, insula, and inferior frontal gyrus responses to infant crying: a randomized controlled trial. Biological Psychiatry. 2011;70:291–297. doi: 10.1016/j.biopsych.2011.02.006. [DOI] [PubMed] [Google Scholar]
- Riem MME, Bakermans-Kranenburg MJ, van Ijzendoorn MH, Out D, Rombouts S. Attachment in the brain: adult attachment representations predict amygdala and behavioral responses to infant crying. Attachment & Human Development. 2012;14:533–551. doi: 10.1080/14616734.2012.727252. [DOI] [PubMed] [Google Scholar]
- Rilling JK. The neural and hormonal bases of human parentalcare. Neuropsychologia. 2013;51:731–747. doi: 10.1016/j.neuropsychologia.2012.12.017. [DOI] [PubMed] [Google Scholar]
- Saavedra-Rodríguez L, Feig LA. Chronic social instability induces anxiety and defective social interactions across generations. Biological Psychiatry. 2013;73:44–53. doi: 10.1016/j.biopsych.2012.06.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sander D, Grandjean D, Pourtois G, Schwartz S, Seghier ML, Scherer KR, Vuilleumier P. Emotion and attention interactions in social cognition: Brain regions involved in processing anger prosody. NeuroImage. 2005;28:848–858. doi: 10.1016/j.neuroimage.2005.06.023. [DOI] [PubMed] [Google Scholar]
- Scaramella LV, Neppl TK, Ontai LL, Conger RD. Consequences of socioeconomic disadvantage across three generations: Parenting behavior and child externalizing problems. Journal of Family Psychology. 2008;22:725. doi: 10.1037/a0013190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sedlak AJ, Broadhurst DD. Third National Incidence Study on child abuse and neglect. Washington, D. C.: U. S. Department of Health and Human Services; 1996. [Google Scholar]
- Seeman T, Epel E, Gruenewald T, Karlamangla A, McEwen BS. Socio-economic differentials in peripheral biology: Cumulative allostatic load. Annals of the New York Academy of Sciences. 2010;1186:223–239. doi: 10.1111/j.1749-6632.2009.05341.x. [DOI] [PubMed] [Google Scholar]
- Segre LS, O’Hara MW, Arndt S, Stuart S. The prevalence of postpartum depression: The relative significance of three social status indices. Social Psychiatry and Psychiatric Epidemiology. 2007;42:316–321. doi: 10.1007/s00127-007-0168-1. [DOI] [PubMed] [Google Scholar]
- Simons RL, Whitbeck LB, Conger RD, Wu C-I. Intergenerational transmission of harsh parenting. Developmental Psychology. 1991;27:159. [Google Scholar]
- Singer T, Critchley HD, Preuschoff K. A common role of insula in feelings, empathy and uncertainty. Trends in Cognitive Sciences. 2009;13:334–340. doi: 10.1016/j.tics.2009.05.001. [DOI] [PubMed] [Google Scholar]
- Singer T, Seymour B, O’Doherty J, Kaube H, Dolan RJ, Frith CD. Empathy for pain involves the affective but not sensory components of pain. Science. 2004;303:1157–1162. doi: 10.1126/science.1093535. [DOI] [PubMed] [Google Scholar]
- Staff RT, Murray AD, Ahearn TS, Mustafa N, Fox HC, Whalley LJ. Childhood socioeconomic status and adult brain size: Childhood socioeconomic status influences adult hippocampal size. Annals of Neurology. 2012;71:653–660. doi: 10.1002/ana.22631. [DOI] [PubMed] [Google Scholar]
- Strathearn L, Fonagy P, Amico J, Montague PR. Adult attachment predicts maternal brain and oxytocin response to infant cues. Neuropsychopharmacology. 2009;34:2655–2666. doi: 10.1038/npp.2009.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surguladze S, Brammer MJ, Keedwell P, Giampietro V, Young AW, Travis MJ, Phillips ML. A differential pattern of neural response toward sad versus happy facial expressions in major depressive disorder. Biological Psychiatry. 2005;57:201–209. doi: 10.1016/j.biopsych.2004.10.028. [DOI] [PubMed] [Google Scholar]
- Swain JE, Dayton CJ, Kim P, Ho SS, Tolman RM, Volling BL. Progress on the Paternal Brain: Theory, Animal Models, Human Brain Research and Mental Health Implications. Infant Mental Health Journal. 2014;35:394–408. doi: 10.1002/imhj.21471. [DOI] [PubMed] [Google Scholar]
- Swain JE, Kim P, Spicer J, Ho SS, Dayton CJ, Elmadih A, Abel KM. Approaching the biology of human parental attachment: Brain imaging, oxytocin and coordinated assessments of mothers and fathers. Brain Research. 2014;1580:78–101. doi: 10.1016/j.brainres.2014.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swain JE, Tasgin E, Mayes LC, Feldman R, Constable RT, Leckman JF. Maternal brain response to own baby-cry is affected by cesarean section delivery. Journal of Child Psychology and Psychiatry. 2008;49:1042–1052. doi: 10.1111/j.1469-7610.2008.01963.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tottenham N, Sheridan MA. A review of adversity, the amygdala and the hippocampus: A consideration of developmental timing. Frontiers in Human Neuroscience. 2009;3:68. doi: 10.3389/neuro.09.068.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uddin LQ. Salience processing and insular cortical function and dysfunction. Nature Reviews Neuroscience. 2015;16:55–61. doi: 10.1038/nrn3857. [DOI] [PubMed] [Google Scholar]
- Ulrich-Lai YM, Herman JP. Neural regulation of endocrine and autonomic stress responses. Nature Reviews Neuroscience. 2009;10:397–409. doi: 10.1038/nrn2647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Ijzendoorn MH. Intergenerational transmission of parenting: A review of studies in nonclinical populations. Developmental Review. 1992;12:76–99. [Google Scholar]
- Venuti P, Caria A, Esposito G, De Pisapia N, Bornstein MH, de Falco S. Differential brain responses to cries of infants with autistic disorder and typical development: An fMRI study. Research in Developmental Disabilities. 2012;33:2255–2264. doi: 10.1016/j.ridd.2012.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wadsworth ME, Evans GW, Grant KE, Carter JS, Duffy S. Poverty and the development of psychopathology. In: Chicchetti D, editor. Developmental psychopathology. 3rd. New York: Wiley; in press. [Google Scholar]
- Young KA, Gobrogge KL, Liu Y, Wang Z. The neurobiology of pair bonding: Insights from a socially monogamous rodent. Frontiers in Neuroendocrinology. 2011;32:53–69. doi: 10.1016/j.yfrne.2010.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]


