Abstract
Congenital unilateral absence of the hand (amelia) completely deprives individuals of sensorimotor experiences with their absent effector. The consequences of such deprivation on motor planning abilities are poorly understood. Fourteen patients and matched controls performed two grip selection tasks: 1) overt grip selection (OGS), in which they used their intact hand to grasp a three-dimensional object that appeared in different orientations using the most natural (under-or over-hand) precision grip, and 2) prospective grip selection (PGS), in which they selected the most natural grip for either the intact or absent hand without moving. For the intact hand, we evaluated planning accuracy by comparing concordance between grip preferences expressed in PGS vs. OGS. For the absent hand, we compared PGS responses with OGS responses for the intact hand that had been phase shifted by 180°, thereby accounting for mirror symmetrical biomechanical constraints of the two limbs. Like controls, amelic individuals displayed a consistent preference for less awkward grips in both OGS and PGS. Unexpectedly, however, they were slower and less accurate for PGS based on either the intact or the absent hand. We conclude that direct sensorimotor experience with both hands may be important for the typical development or refinement of effector-specific internal representations of either limb.
Keywords: Amputation, Amelia, Prospective, Development, Bilateral, Representation
1. Introduction
The functional organization of primary sensory and motor maps is activity-dependent throughout the lifespan, changing in response to increases or decreases in stimulation. Traumatic loss of an established limb therefore induces reorganization of primary sensory and motor maps (e.g. Merzenich et al., 1983; Donoghue and Sanes, 1988; Sanes et al., 1988; Kaas, 2000). The impact of these reorganizational changes on pre-movement action planning and selection is much less certain. For instance, amputees who experienced traumatic loss of a limb, typically in adulthood, retain the ability to choose responses that would minimize biomechanical awkwardness if using the absent hand (Philip and Frey, 2011). This ability may arise from effector-specific internal representations that are robust to the effects of chronic inactivity (Johnson et al., 2002b), or that are maintained through “cross-activation” during use of the intact limb (Parlow and Kinsbourne, 1989; Lee et al., 2010). The role of experience in the establishment of internal representations that guide motor planning is unclear. Individuals born without a hand (i.e., unilateral upper extremity amelics; “amelic individuals” for brevity) provide a unique opportunity to address this important issue.
Whether amelic individuals possess internal sensorimotor representations of the absent limb remains controversial. Some 7–20% of amelic individuals report phantom sensations for their absent limb(s) (Vetter and Weinstein, 1967; Melzack et al., 1997; Wilkins et al., 1998), suggesting that they may possess internal representations of body parts that have never developed (Brugger et al., 2000). Consistent with this view, transcranial magnetic stimulation (TMS) of motor cortex contralateral to the absent limb can sometimes evoke phantom sensations in amelic individuals (Brugger et al., 2000). However, this is not always the case (Cohen et al., 1991; Pascual-Leone et al., 1996; Mercier et al., 2006; Reilly and Sirigu, 2011). Results from the hand laterality task, considered an implicit test of motor imagery, have likewise been inconsistent. One investigation found that while dominant hand amputees experienced difficulties, three individuals with unilateral amelia were able to identify whether rotated pictures were of left or right hands as accurately as controls (Nico et al., 2004). Their slower response times, however, were taken as evidence for use of non-motor strategies. Conversely, a larger investigation of 14 amelic individuals reported impaired motor imagery for the congenitally absent hand (Funk and Brugger, 2008).
One possibility is that at least gross representations of the limbs develop through maturational processes, possibly including prenatal growth prior to prenatal amputation (e.g. as occurs in amniotic band syndrome), while postnatal movement and associated sensory feedback are necessary only to refine limb representations. Or, congenital phantoms (and perhaps also performances on hand laterality tasks) may depend on representations acquired through observational learning entirely (Price, 2006), or in combination with maturational factors (Brugger et al., 2000).
Exclusive reliance on the hand laterality task to evaluate motor representations in amelic individuals is limiting, because it can elicit various strategies other than motor planning or motor imagery (Daprati et al., 2010; Vannuscorps et al., 2012; Habacha et al., 2014). We instead used a prospective grip selection task (PGS) to investigate the ability of amelic individuals to engage in motor planning and prediction with the absent or intact hands (e.g. Johnson, 2000a; Johnson et al., 2002b; Daprati et al., 2010; Philip and Frey, 2011). In this task, participants decided whether they would prefer an under- or over-hand grasp to engage a stimulus object appearing in numerous orientations. Notably, solving the PGS task appears to involve rapid, implicit, analog motor simulation to evaluate the costs (awkwardness/comfort) of potential response alternatives (Johnson, 2000a), rather than requiring use of explicit motor imagery (Daprati et al., 2010). These internal simulations might play a major role in predicting sensory consequences in advance during ongoing movements (Wolpert et al., 1995), and also in forecasting long-range movement outcomes (Frey, 2010). The PGS task shows consistent engagement of posterior parietal, premotor and cerebellar mechanisms implicated in movement planning and control, but not primary sensory or motor cortices (Johnson et al., 2002a; Jacobs et al., 2010; Marangon et al., 2011; Martin et al., 2011).
If direct experience with a limb is necessary to develop effector-specific internal representations, then – in contrast to traumatic amputees who lost their hands in adulthood (Philip and Frey, 2011) – we anticipated that individuals with unilateral amelia would exhibit impaired grip selection accuracy and speed based on their absent hand, relative to their intact hand and to matched controls. Alternatively, unilateral amelic individuals might exhibit bilateral grip performances comparable to control grip performances (similar to traumatic amputees). This could occur if effector-specific internal representations develop via purely maturational processes, or if “cross activation” allows experience with the intact hand to establish and maintain effector-specific representations for both hands.
2. Materials and methods
2.1. Participants
Fourteen individuals with unilateral upper limb amelia (5 female, ages 10–68 yrs; including 10 adults ages 20–68 yrs, and 4 children ages 10–13 yrs) gave informed to consent to participate in this study, as well as 14 healthy controls matched for age, gender, and handedness.1 See Table 1 for individual details on the amelic participants. When referring to controls, the terms “absent” and “intact” denote the hands that respectively match the absent and intact sides of their yoked amelic participant. Because we assumed that the dominant hand of all amelic participants was their intact hand, control participants also used their dominant hand as the “intact” (and thus the non-dominant hand as “absent”).
Table 1.
Demographic data on amelic participants. Mech=mechanical; myoelec=myoelectric; NA=not available. Strategies: T=imagined a hand with thumb on opposite side, V=visual, M=motor, C=cognitive.
| Amelic participant characteristics | |||||
|---|---|---|---|---|---|
| Age | Sex | Above/Below Elbow | Aff. side | Prosthesis | Strategy |
| 21 | M | BE | L | None | T |
| 54 | M | AE | R | None | T,V |
| 45 | F | BE | R | None | T |
| 39 | M | BE | L | None | T |
| 20 | M | BE | L | None | NA |
| 13 | M | BE | L | None | NA |
| 13 | F | BE | L | None | NA |
| 13 | F | BE | L | Myoelec | NA |
| 10 | F | BE | L | Mech | NA |
| 35 | M | BE | L | None | M,V |
| 40 | F | BE | R | None | M |
| 40 | M | BE | L | None | M |
| 29 | M | BE | L | Mech | T |
| 68 | M | BE | R | Myoelec | C |
All participants had normal or corrected-to-normal visual acuity. No amelic individuals reported pain or other phantom sensations.
Adult participants (> 18 yrs of age) were tested in at the University of Oregon (Eugene, Oregon; N=5 amelics) and University of Missouri (Columbia, MO; N=5 amelics), by overlapping experimenters. Child participants were tested at Shriners Hospital (Portland, Oregon; N=4 amelics) by a separate group of trained experimenters.
All participants performed both tasks, and gave informed consent. Adult participants performed the tasks in counterbalanced order, while child participants always performed PGS (Task 2) first. Procedures were approved by the local IRB and in accordance with the Declaration of Helsinki.
Quantitative data on cognitive impairments and developmental delays were unavailable; however, all participants showed no overt sign of impairment or delay, child participants showed no motor disability (see Section 2.6), and all members of the University of Missouri group were employed at the university.
2.2. Grip selection task
Participants performed the grip selection task as detailed in Philip and Frey (2011). We used an apparatus employed in several previous studies from our lab (Jacobs et al., 2010; Philip and Frey, 2011). In brief, participants sat at a table and reached out to grasp a graphically rendered “widget” (68 mm diameter, ≈1.6° visual angle) on a computer monitor. The same 2D stimulus objects were used in both the OGS and PGS tasks for consistency. Stimuli consisted of a graphically rendered spherical widget with photo-realistic shading to provide the illusion of three-dimensionality. The widget was half-pink and half-tan, with indentations on each side for finger placement, allowing for only two precision grip orientations. To create 3D objects for grasping, these widget stimuli were back-projected through a transparent plastic overlay fitted over the surface of a flat computer monitor. The center of the overlay was a 3D transparent plastic disk that extended 25 mm from the surface of the computer screen. This disk was equivalent in size (32 mm radius) and in position to the projected stimulus object, creating the appearance of a graspable object extending from the surface of the screen. A touch sensor wire (E112 Capacitive Touch Sensor, Quantum Research Group, Pittsburgh PA) was wrapped around the disk’s circumference. See Fig. 1 for example stimuli.
Fig. 1.
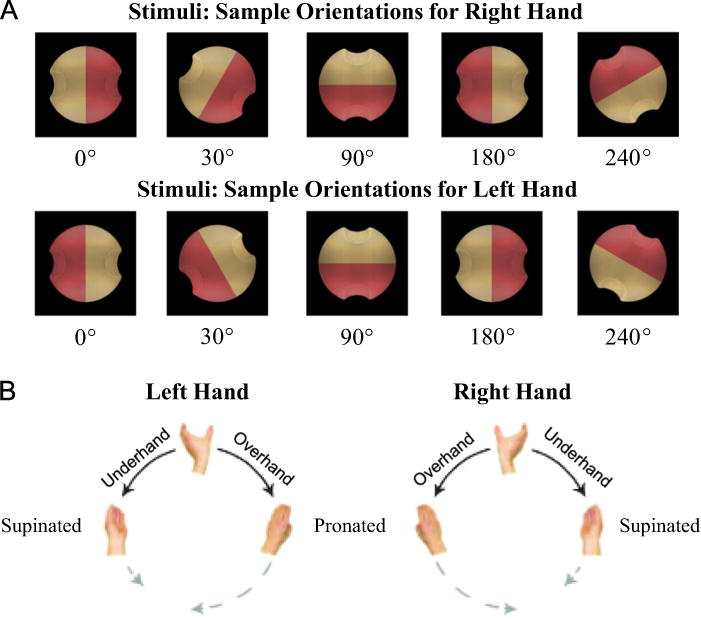
Experimental components. (A) Stimulus object at 4 sample orientations for each hand. (B) Possible hand postures. Adapted from Jacobs et al. (2010).
The widget appeared against a black background in 12 possible orientations: 30° increments rotated around the line-of-sight axis. Stimulus orientations were defined in terms of relative hand orientation (henceforth referred to as orientation, for brevity). Rotation values are defined in terms of external rotation distance (i.e. of a hand). For example, a 0° stimulus for the right-hand would be the same image as a 180° stimulus for the left-hand (as shown in Fig. 1A); in either case, the thumb would be on the tan indentation at a neutral (overhand) posture. This enables direct comparison of grip preferences between left and right hands (Johnson, 2000b).
For each orientation, there were two possible grips, under- or over-hand. These were determined by the color of the stimulus’ indentation on which the participant chose to place their thumb. “Thumb placement” represents this choice in terms of the final hand position. For example, a 0° stimulus orientation allows thumb placements of 0° (on the tan indentation) or 180° (on the pink indentation).
“Rest keys” (as described below) remained on the table throughout, to identify when participants initiated a movement.
2.3. Task 1 procedure: overt grip selection (OGS)
During OGS, participants were required to reach and grasp a stimulus object presented in various orientations in the most comfortable (precision grip) manner by placing the pads of the thumb and forefinger on the small indentations (see Fig. 1A). Participants could use either an over- or under-hand grip (Fig. 1B), and were instructed to perform as quickly and accurately as possible, without making corrections mid-reach.
The OGS task comprised a single session of 96 trials, divided into 4 blocks of 24 trials each (child participants: 4 blocks of 12 trials each). Adult participants used the intact hand throughout. Child participants also had 4 blocks with the absent hand (i.e. stump); OGS from the absent hand is not included in trial counts or analysis, because stump movements were only collected for 4 participants and are difficult to compare with intact hand movements. Thus, Task 1 entailed 8 trials (4 for children) of each combination of 12 stimulus orientations.
Each session began with a single 10-trial practice block to familiarize participants with the OGS task. Each trial comprised the following epochs, as shown in Fig. 2A: (1) A variable delay of 0, 500, 1000 or 1500 ms. (2) A stimulus object, lasting 4000 ms or until response. (3) A key-return period, lasting until the participant returned their hand to a rest key on the table top. (4) An inter-trial interval (ITI), 1500 ms duration. Trial epochs 1–3 included a blue dot (7 mm diameter, 0.13° visual angle) on the same side of the screen as their hand, to warn the participant of an upcoming stimulus and remind them which hand to use.
Fig. 2.
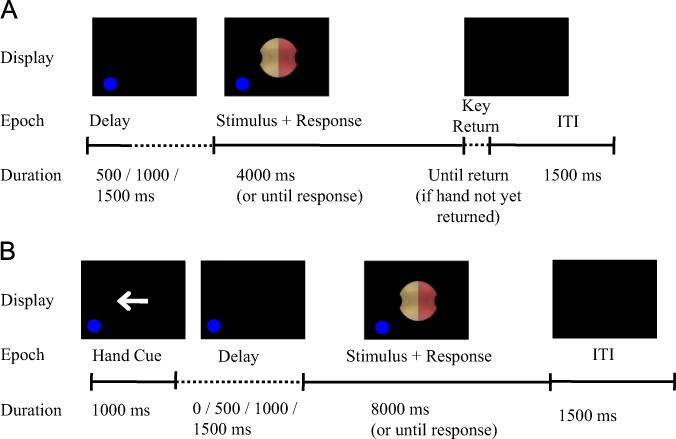
Trial time course. (A) Overt grip selection (OGS) task. (B) Prospective grip selection (PGS) task. Adapted from Philip and Frey (2011).
Child participants also saw a hand cue (left or right arrow) before the variable delay period, because child participants alternated between blocks of OGS with intact hand, and blocks of OGS with absent hand (i.e., stump), as described above.
When the stimulus object appeared, participants were instructed to immediately reach to and grasp it, using the specified hand, to grasp the widget using the most natural (i.e., under- or over-hand) precision grip. The experimenter used a keyboard to record on which color the participant placed his or her thumb. Trials were aborted if the participant moved either hand off the rest key during any trial stage other than the stimulus presentation stage, or if the participant moved the wrong hand off the rest key during the stimulus presentation phase. Onset time (OT) was defined as the time from start from stimulus appearance to release of the rest key, and movement time (MT) was defined as the time between release of the rest key and contact with the on the 3D plastic overlay, as detected by the touch sensor detailed above. MT was not recorded for the child participants.
2.4. Task 2 procedure: prospective grip selection (PGS) task
During PGS, participants were presented with the same stimuli as described above, and instructed to remain still while reporting which side (“pink” or “tan”) of the stimulus their thumb would contact if they were to grasp the object using the specified hand and the most natural precision grip. Participants reported this response by speaking the appropriate color name into a microphone, and the experimenter recorded the chosen color. Microphone input was used only to determine the onset of the vocal response. Response time (RT) was defined as the time from stimulus appearance to start of vocal response. Participants were instructed to respond as quickly and accurately as possible; instructions did not include any mention of imagery or imagination. All participants performed PGS separately with the absent and intact hands.
For adult participants who performed Task 2 (PGS) first, they were presented with 5 OGS trials to familiarize themselves with the precision grasping movements and with the stimuli. During this familiarization session, the stimulus appeared at orientations not used during the main task. Child participants received no familiarization session. In addition, all participants performed 10 practice PGS trials before beginning data collection.
Similar to Task 1, the PGS task comprised a single session of 192 trials, divided into 4 blocks of 48 trials each (Child participants: 96 trials, 8 blocks of 12 trials each). Hand varied between trials, counterbalanced within each block. Thus, Condition 2 entailed 8 trials of each combination of 12 stimulus orientations * 2 hands.
Each PGS trial followed the structure of OGS trials, with three exceptions (Fig. 2B). First, each trial began with a hand choice cue of 1000 ms duration, in the form of an arrow pointing either left or right to indicate the hand used in the current trial. Second, there was no key-return period. Third, the stimulus window lasted up to 8000 ms.
Trials were aborted if the participant moved either hand off the rest key at any time.
2.5. Data collection and analysis
Trials with OT, MT, or RT more than two standard deviations from the within-participant means were eliminated from the analyses; this eliminated 6.0% of OGS trials and 4.9% of PGS trials. Outlier rate did not differ between groups in either task (t-test p > 0.3). Repeated-measures analyses of variance (ANOVA) were performed on participant means as described later in the text, with all post-hoc comparisons carried out via Tukey’s HSD test. Correlations were calculated using Pearson’s r when the assumptions were met. The nonparametric measure of Kendall’s τ was also used because of its robustness in the presence of small sample sizes and outliers. Differences between correlations were tested for statistical significance by using Fisher’s z-transformation, followed by a t-test on the z-transformed values (Howell, 1997).
Inter-rater reliability of response recording was evaluated by having two experimenters code responses (colors) in a subset of participants (N=4 from the University of Missouri group), during both tasks. No differences were found between the two experimenters.
For a simple measure of grip preferences, we computed “choice likelihood” (CL) for each thumb placement. The CL was the probability of choosing to place the thumb in one indentation vs. the opposite indentation. For example, participants could choose to grasp a 0° orientation stimulus with thumb placements of 0° (pink) or 180° (tan). Thus, CL for a 0° thumb placement equaled the count of grasps with 0° thumb placement, divided by the total count of 0° and 180° placements. CL was computed independently for each hand and task.
“Consistency” was calculated for each CL value as the absolute difference between the CL and chance (0.5), to quantify how reliably each participant made the same choice at the same stimulus orientation.
PGS accuracy was determined by comparing the similarity between grip preferences during OGS execution, and grip preferences revealed during PGS selection, following the procedure detailed in Johnson (2000b) and Philip and Frey (2011). Briefly, we estimate an amelic individual’s OGS preferences with their absent hand as the inverse of their preferences for the intact hand. This takes advantage of the fact that left and right upper extremities obey joint constraints that are 180° out of phase (MacKenzie and Iberall, 1994), and prior evidence showing that in healthy adults overt grip preferences for one hand are virtually identical to the inverse of those from the other hand (r=0.99, Johnson, 2000a, Experiment 3). PGS preferences can then be compared to estimated (for the absent hand) and actual (for the intact hand) OGS choices for amelic individuals. Thus, for judgments with hand k and a stimulus in a particular orientation θi,
| (1) |
Accuracy scores were computed separately for each participant, hand, and orientation.
2.6. Bruininks–Oseretsky Test of Motor Proficiency
Child participants underwent further quantitative motor testing via the Bruininks–Oseretsky Test of Motor Proficiency (BOTMP; Bruininks and Oseretsky, 1978). Three hand subtests were used: Fine motor precision (FMP: filling in shapes, drawing lines through paths, connecting dots, folding paper), fine motor integration (FMI: copying shapes), and manual dexterity (MP: placing dots, transferring pennies, sorting cards, placing pegs, stringing blocks). The FMP and MP tests included bimanual tasks; only the MP test involved a time constraint. The BOMT is normed for participants of 4–21 yrs. Because of the small sample size (N=4 in each group), between-groups differences in BOTMP scores were measured using the Wilcoxon Signed Rank test, a nonparametric equivalent of a paired-sample t-test.
3. Results
3.1. Task performance
On average, control participants successfully performed the OGS task on 94 ± 6% of trials, as measured by the frequency of trials on which they moved directly to grasp the stimulus in either a supinated or pronated precision grip aligned at the indentations on the stimulus within 5 s of stimulus presentation. Controls performed the PGS task successfully on 97 ± 3% of trials, as measured by the frequency of trials on which the participant gave a single clear verbal response within 4 s. For amelic participants, the mean success rate was 95 ± 5% for OGS, and 96 ± 3% for PGS. For either task, success rate did not differ significantly between groups (t-test: OGS p=0.690, PGS p=0.454). Given the near-ceiling performance on both tasks, we did not analyze errors in further detail.
3.2. Grip preferences and stimulus orientation
As expected, both controls and amelic individuals exhibited sensitivity to biomechanical constraints when performing the OGS task. Fig. 3 shows the likelihood of control participants (gray lines) and amelic participants (dotted lines) selecting each grip (i.e., the choice likelihood) at each orientation, compared to subjective awkwardness ratings provided by a separate control sample (Philip and Frey, 2011). Both groups strongly preferred less awkward grips in OGS (when sensory feedback is available). The correlation between OGS choice likelihood and awkwardness for controls was −0.914 for the absent hand and −0.855 for the intact hand; for amelic participants, these correlations reached −0.974 and −0.933 respectively (p < 0.001 in all cases). Note that this strong inverse relationship between grip preferences and awkwardness arose even though we calculated the two measures from different populations (choice likelihoods from amelic participants and matched controls, awkwardness ratings from naïve healthy young adult participants). Furthermore, OGS grip preferences with the intact hand were nearly identical across groups (r=0.975, p < 0.0001).
Fig. 3.
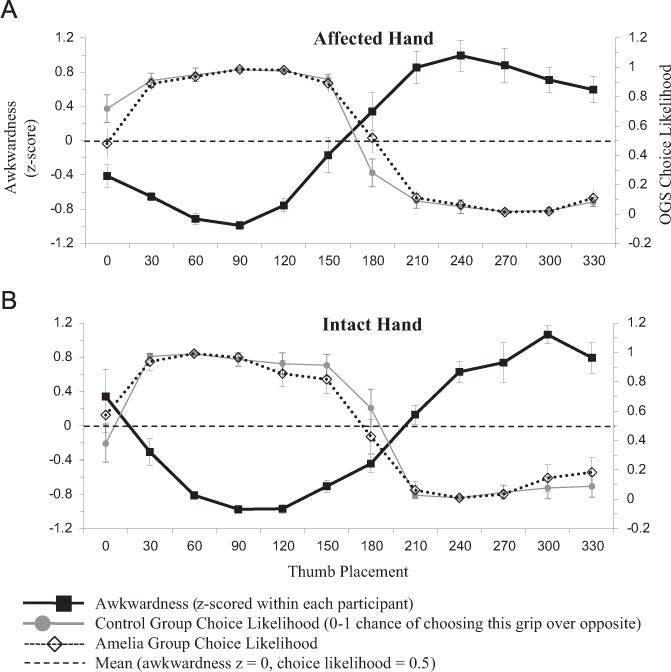
OGS choice likelihood, for each thumb placement. All OGS data from intact hand, mirror-reversed to produce absent hand data; see Section 2 for details. Congruence between high choice likelihood and low awkwardness demonstrates selection of non-awkward grips. All values are group mean±SEM across participants. (A) Affected hand. (B) Intact hand.
Despite the absence of movements (and associated feedback) in PGS, both groups’ grip preferences still showed a strong negative correlation with rated awkwardness, consistent with previous work in healthy adults (Johnson, 2000a; Jacobs et al., 2010; Martin et al., 2011), densely hemiplegic stroke patients (Johnson, 2000b; Johnson et al., 2002b), and traumatic amputees (Philip and Frey, 2011). The correlation between PGS choice likelihood and awkwardness for controls was −0.879 for the absent hand (p < 0.0001) and −0.814 for the intact hand (p < 0.01); the difference between hands was not statistically significant (z= −0.55, p=0.582). For amelic participants, these correlations reached −0.631 (p < 0.05) for the absent hand and −0.854 (p < 0.0001) for the intact hand; the difference between hands was not statistically significant (z=1.24, p=0.215). This difference between hands would have reached significance at α=.05 with 31 participants in each group. Fig. 4 illustrates the relationship between awkwardness, control choice likelihoods, and amelic choice likelihoods, for both hands in the PGS task. Note the match between grip preferences in the amelic and control groups. Mean choice likelihoods were similar between the two groups (absent hand r=0.947, p < 0.001; intact hand r=0.881, p < 0.0001). These high correlations demonstrate that stimulus orientation had similar effects on PGS performances in both amelic participants and controls.
Fig. 4.
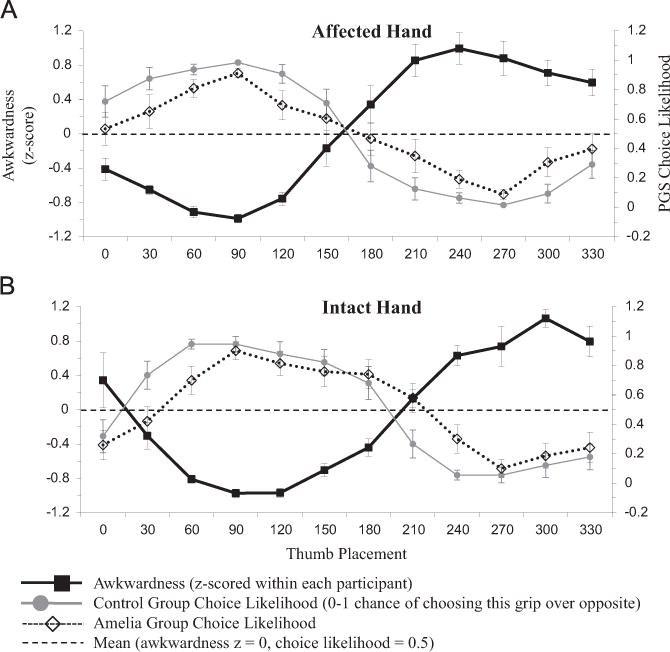
PGS choice likelihood. See Fig. 3 for format details. Note similar grip preference pattern between groups.
As in past studies, we found that preferences in PGS were less consistent than in OGS, which may reflect the more approximate nature of decisions based exclusively on internal representations without feedback (Rodriguez et al., 2008; Macuga et al., 2012). Furthermore, the amelic group showed less consistent grip preferences than controls on both tasks, regardless of the hand involved. We quantified consistency (i.e. choice likelihoods farther from chance) via a 2 (Group: amelia, control)*2 (Hand: absent, intact)*2 (Task: OGS, PGS) ANOVA. We found main effects of Task (F(1,89)=14.37, p < 0.001) and Group (F(1,89)=9.81, p < 0.01), and a non-significant trend toward a Task*Group interaction (F(1,89 (=2.44, p=0.12), but no significant effects of Hand, Hand*Task, or Hand*Group (F < 1.0). Post-hoc tests revealed that the main effect of Task arose from significantly lower consistency during PGS compared to OGS (p < 0.0005), and that the main effect of Group arose from significantly lower consistency for amelic participants than for controls (p < 0.005).
3.3. Effects of unilateral amelia on grip selection accuracy
Of greatest interest was the extent to which grip preferences in PGS, when no actual movements were executed, matched those exhibited in OGS, where participants experienced sensory feedback. To this end, we performed a 12 (Orientation)*2 (Hand: absent, intact)*2 (Group: amelia, control) ANOVA on PGS selection accuracy. PGS accuracy was unaffected by Hand (F < 1.0), but exhibited significant main effects of Orientation (F(11,297)=6.41, p < 0.0001) and, most importantly, of Group (F(1,297)=23.15, p < 0.001). The main effect of group arose from lower selection accuracy for amelic participants (72±33%) compared to controls (89±21%; difference p < 0.0001). Critically, the main effect of Group alongside the non-significant Group*Hand interaction (F < 1.0, p=0.508) reflects the fact that amelic participants had reduced PGS accuracy for both hands, compared to control participants, as shown in Fig. 5A.
Fig. 5.
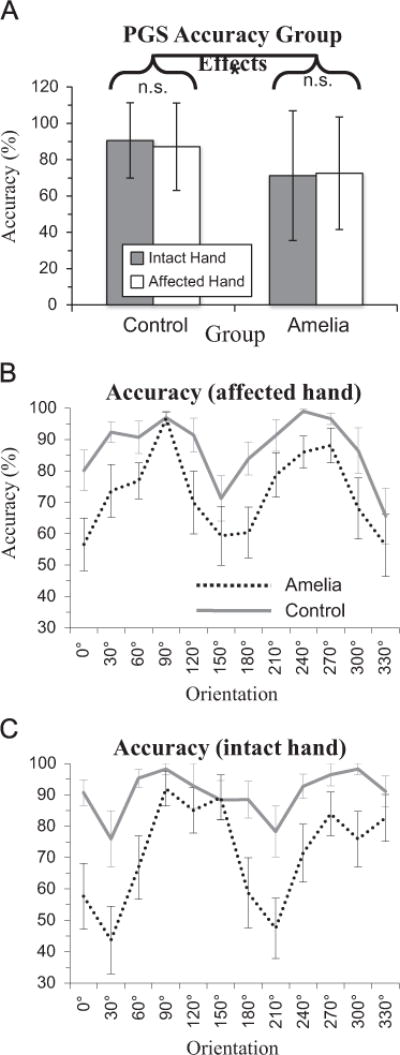
PGS selection accuracy effects. Group mean±SEM. (A) Group effects. Main effect of group (p < 0.001), no effect of hand (p=0.731) or interaction (p=0.508). (B) Effect of orientation on accuracy with affected hand. (C) Effect of orientation on accuracy with intact hand.
For interaction effects, only the Hand*Orientation interaction effect achieved significance (F(11,297)=5.48, p < 0.0001; (p > 0.08 in all other cases). Fig. 5B and C illustrates the orientation effects on selection accuracy.
Table 2 summarizes the effects of unilateral amelia on grip selection accuracy, as well as its effects on grip selection speed (Section 3.4).
Table 2.
Results of ANOVAs for grip selection accuracy (“Acc”) and speed (reaction time, onset time, and movement time) across participants. Significant effects shown in bold for clarity. Post-hoc tests indicate that Group effects arose from lower accuracy and higher RT for amelics. Note that PGS accuracy and RT show Group effects without Group*Hand interaction.
| Grip selection accuracy and speed | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Group
|
Hand
|
Orientation
|
Grp.*Hand
|
Grp.*Orient.
|
Hand*Orient.
|
||||||||
| F | p | F | p | F | p | F | p | F | p | F | p | ||
| PGS | Acc | 23.15 | <0.001 | 0.12 | 0.731 | 5.67 | <0.001 | 0.45 | 0.508 | 1.2 | 0.283 | 2.45 | <0.01 |
| RT | 25.39 | <0.005 | 248.55 | 0.04 | 0.09 | 1 | 0 | 0.965 | 4.31 | <0.0001 | 2.36 | <0.01 | |
| OGS | OT | 0.19 | 0.666 | – | – | 0.53 | 0.878 | – | – | 0.87 | 0.573 | – | – |
| MT | 0.61 | 0.456 | – | – | 5.05 | <.0001 | – | – | 1.26 | 0.261 | – | – | |
3.4. Effects of unilateral amelia on grip selection speed
We also evaluated the effect of group on OGS onset time (OT), OGS movement time (MT), and PGS response time (RT). We quantified OGS effects via 12 (Orientation)*2 (Group: amelia, control) ANOVAs. Because we collected OGS data for the intact hand only, while we collected PGS data for both hands, ANOVAs on PGS data also included an additional 2-level factor (Hand: absent, intact).
Overall, during OGS, the two groups performed indistinguishably. We found no significant main or interaction effects of any factor on OT (p > 0.3). For MT, we found a significant main effect of Orientation (F(11,99)=5.05, p < 0.0001; Fig. 6A), but no other main or interaction effects (p > 0.25). This likely reflects increased time for positions that require greater rotations of the hand.
Fig. 6.
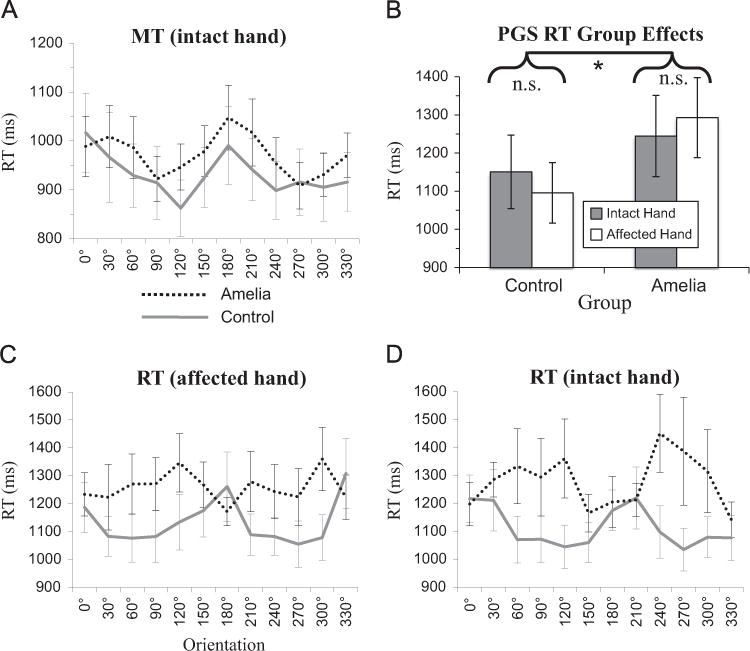
Task speed effects, group mean±SEM. OT not shown due to lack of significant effects. (A) Effect of orientation on OGS MT with intact hand. (B) Group effects on PGS RT. Main effects of group (p < 0.01), but not hand (p=0.98) or interaction (p=0.78). (C) Effect of orientation on RT with affected hand. (D) Effect of orientation on RT with intact hand.
For PGS RT, we found a significant main effect of Group (F (1,297)=17. 0 7, p < 0.01) but no main effects of Hand or Orientation (F < 1.0). Importantly, this arises because amelic participants (1269±396 ms) were slower than control participants (1124±331 ms; difference p < 0.0001) when selecting grips prospectively for either the intact or absent hands, as shown in Fig. 6B. We found significant interaction effects of Hand*Orientation (F(11,297)=2.04, p < 0.05) and Group*Orientation (F(11,27)=4.31, p < 0.001), but not Hand*Group (F < 1.0). The orientation-based interaction effects are shown in Fig. 6C and D, illustrating that the amelic group’s PGS RTs showed a different pattern of orientation-sensitivity for either hand, neither of which was like the orientation profile of controls (which was consistent across hands).
3.5. Task strategies: self-reported
While most participants solve the grip selection task using motor strategies (Daprati et al., 2010), exceptions may be possible among amelic individuals. We asked nine participants to describe how they accomplished the PGS task. As shown in Table 1, 5/9 amelic participants imagined a hand with a thumb on the opposite side compared to the position of their thumb on their intact hand, 3/9 considered movements of their absent hand, 2/9 used the visual properties of the object, and 1/9 used a cognitive strategy of predicting with the intact hand and then flipping. These numbers include two participants who reported using multiple strategies (visual+motor, visual+thumb position). Participants generally had difficulty forming answers to this question, so these results are difficult to interpret. In particular, “imagined a hand with the thumb on the opposite side” may or may not reflect a motor strategy. However, only one amelic participant definitively reported completing the task without using a motor strategy.
3.6. Absence of motor deficits: BOTMP
The Bruininks–Oseretsky Test of Motor Proficiency (BOTMP) revealed no motor impairments in a subset of amelic participants (N=4). Table 3 summarizes our results. The two groups showed equivalent performance at the fine motor precision (amelics=39.3 ± 1.7, controls 40.8 ± 0.5; signrank=1.5, p=0.375) and fine motor integration (amelics=38.8 ± 0.5, controls=38.8 ± 1.5; signrank=3, p = 1.0) tests. Amelics showed a non-significant trend toward lower manual dexterity (amelics=29.0 ± 5.4, controls 36.0 ± 1.4; signrank=1.0, p=0.250), possibly because only the manual dexterity test involved bimanual tasks under time constraint. Overall, we found no evidence that amelic individuals had impairments in grasping or fine motor control.
Table 3.
Results of Bruininks Oseretsky Test of Motor Proficiency (BOTMP) for child participants. BOTMP not normed for adults age 21+. Subtests: FMP=fine motor precision; FMI=fine motor integration; MD=manual dexterity. No group effect on score for any subtest (signed-rank test p > 0.25); see Section 3.6 for details.
| Precision motor skills of child participants | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Age at testing | Gender | Hand pref | FMP score | FMP age equivalent | FMI score | FMI age equivalent | MD score | MD age equivalent | |
| Amelia 1 | 13y+4m | M | R | 41 | 15 | 39 | 13 | 25 | 8 |
| Amelia 2 | 13y+11 m | F | R | 40 | 15 | 39 | 13 | 37 | 19+ |
| Amelia 3 | 13y+1m | F | R | 37 | 8.5 | 39 | 13 | 27 | 8.5 |
| Amelia 4 | 10y+1m | F | R | 39 | 10 | 38 | 10 | 27 | 8.5 |
| Control 1 | 13y+2m | M | R | 40 | 12 | 40 | 15 | 38 | 19+ |
| Control 2 | 14y+2m | F | R | 41 | 19+ | 40 | 10 | 35 | 13 |
| Control 3 | 13y+10m | F | R | 41 | 19+ | 37 | 8.5 | 35 | 15.5 |
| Control 4 | 10y+0m | F | R | 41 | 19+ | 38 | 10 | 36 | 15.5 |
3.7. Confound and outlier tests
We did not predict a bilateral effect of amelia on PGS accuracy, so we performed additional tests to ensure that this unexpected finding did not arise from possible outliers or confounds. For instance, task order (i.e., PGS before OGS) could potentially have a stronger effect on amelic participants than control participants. Furthermore, our data were collected in three sites, by two different groups of experimenters, from two widely divergent age groups. Fig. 7 shows PGS accuracy for each individual participant, including influences of group, hand, task order, age group, and testing site.
Fig. 7.
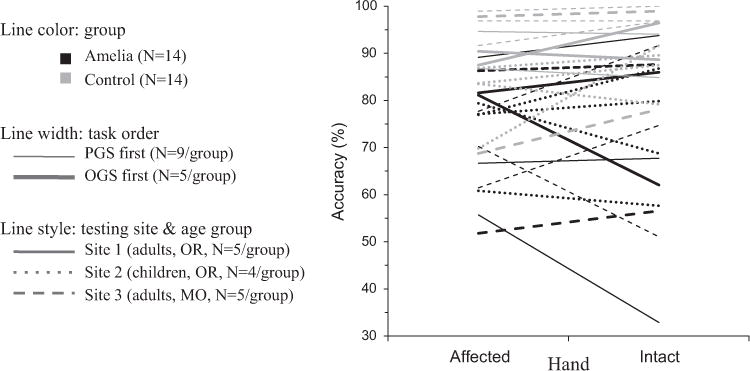
PGS selection accuracy for individual participants. Task orders are not balanced because all child participants did PGS first, while adult participants had task order counterbalanced. Between-group difference not consistently driven by task order, age, or testing site.
These factors involved too few participants to make strong statistical claims, but we performed nonparametric tests (valid for small sample sizes) on possible confounds of task order and testing site (OR adults, OR children, MO adults) on mean PGS accuracy. These two tests could not be combined in a single analysis because all child participants performed PGS first, while adult participants performed the tasks in counterbalanced order, thereby creating a dependency between site and order. A Mann–Whitney U test found no significant effect of task order (z=0.3596, p=0.719), and a Kruskal–Wallis test revealed no significant effect of testing site (χ2=1.2, p=0.5487). Most importantly, Fig. 7 shows that the between-group differences were not consistently driven by other factors.
4. Discussion
Amelic individuals, like controls, show clear preferences for less awkward grip options whether basing PGS decisions on the intact or the absent hand. This evidence is consistent with the hypothesis that planning involves internal representations that capture the biomechanical constraints that would be encountered if using the absent limb, despite lack of experience grasping. However, amelic participants’ PGS performances were slower and less accurate than controls; i.e., amelic participants exhibited a greater disparity between preferences in the PGS vs. OGS task. Unexpectedly, this effect was present in PGS judgments based on either the absent or intact hands. Our finding of bilaterally impaired grip selection for individuals with unilateral upper limb amelia conflicts with both of our a priori hypotheses, which predicted that grip selection accuracy among amelic individuals would be either fully intact compared to controls, or unilaterally impaired.
We offer two possible explanations for this pattern of results. It may be that regardless of the hand involved, amelic individuals employ some alternative mechanism that is slower and less accurate than the effector-specific internal representations that controls presumably use for prospective grip selection. Alternatively, our results may be interpreted as evidence that maturation alone is sufficient for the development of relatively coarse, effector-specific limb representations, but that sensorimotor experience with both hands is necessary for their refinement. We now discuss our results and these possible interpretations.
4.1. Grip selection planning in unilateral amelia
Similar to controls, amelic individuals display a preference for less awkward grip options across stimulus orientations, and both groups exhibit comparable response onset and movement times. Amelic individuals are less consistent in their grip preferences than controls on both the OGS and PGS tasks. This could be attributable to their experience using a single hand to perform all grasping actions, even those for which controls might switch to the opposite hand. However, our BOTMP data (Table 3) suggests that this does not reflect a more general motor planning deficit, because amelic individuals were able to perform as well as controls at a broad variety of fine motor control tasks.
In the PGS task, where grip preferences must be chosen in the absence of movements (and therefore feedback), amelic individuals exhibit response preferences that are sensitive to constraints on pronation/supination of their intact hand, and also to factors that would have constrained movements of the absent limbs if they had developed typically. Like controls, they prefer less awkward grip options, as reflected in the negative correlation between independently obtained awkwardness ratings and grip preferences (Fig. 4). Nevertheless, amelic individuals’ PGS performances based on both hands are slower (Fig. 6B) and less accurate than controls (Fig. 5A). Amelic individuals showed only minor between-hands differences in PGS, reflected in their pattern of reaction times (Fig. 5C vs. D). This bilateral deficit contrasts with traumatic amputees who show no PGS deficits even decades postamputation (Philip and Frey, 2011). Indeed, based on the limited data available, traumatic amputation in early childhood does not induce PGS deficits; in a prior study of traumatic unilateral upper limb amputees, those who underwent amputation at age < 5 (n=2) showed no differences in grip selection accuracy compared those who lost a limb in adulthood (Philip and Frey, 2011, Fig. 8; low-accuracy outliers were ages 22 and 65 at amputation). Therefore, the grip selection deficits detected in the current study seem to depend on experience during a critical period with the first few years of life, possibly including the prenatal period.
4.2. Differences from previous amelia studies
Some previous studies have found intact bilateral motor representations in amelic individuals with phantom sensations (Brugger et al., 2000; Reilly and Sirigu, 2011). However, phantom sensations occur in only 8–12% of individuals with congenital amelia (Melzack et al., 1997; Wilkins et al., 1998), and none of our amelic participants had phantom sensations. Therefore, any phantom-dependent effects would not be reflected here, nor among most amelic individuals.
Previous work with “non-phantom” amelic individuals reported unilateral deficits in motor imagery, in the form of decreased accuracy (Funk and Brugger, 2008) or increased strategy use (Nico et al., 2004), rather than the bilateral deficits we detected here. These studies used a hand laterality task instead of a grip selection task, which could lead to different results for two non-exclusive reasons. First, hand laterality tasks can be solved by multiple strategies (Daprati et al., 2010; Vannuscorps et al., 2012; Habacha et al., 2014), whereas the grip selection task requires movement planning and prediction (Johnson, 2000a; Daprati et al., 2010). Second, motor imagery involves similar neural networks to motor execution; while evidence remains inconclusive, the shared network may include the involvement of contralateral primary sensorimotor cortex in motor imagery (Sharma et al., 2008; Guillot et al., 2012; Macuga and Frey, 2012). Conversely, grip selection tasks do not involve primary sensorimotor cortex, but instead engage higher-level representations in premotor and parietal cortices, and the cerebellum (Jacobs et al., 2010). Therefore, since primary sensorimotor cortex may be involved in motor imagery but not motor prediction (i.e. grip selection), and sensorimotor cortex may reorganize after amputation (Cohen et al., 1991; Lotze et al., 2001; but see Gagné et al. (2011)), we might expect motor limb absence to elicit greater changes in motor imagery than in motor prediction.
4.3. Why does unilateral amelia lead to bilateral deficits?
Here we present four possible explanations for why the amelic individuals’ deficit in prospective grip selection is bilateral rather than unilateral.
First, amelic individuals could show a bilateral deficit because prospective action selection may involve activating two competing effector-specific models, one for each limb. According to the affordance competition hypothesis (Cisek, 2007; Cisek and Kalaska, 2010), the human motor control system simultaneously activates multiple plans for alternative possible movements, and then later selects between these parallel plans. If amelic individuals have a less-refined effector-specific representation for their absent hand (e.g. a representation of a stump’s role in supporting dexterous activity with the intact hand), this could lead to errors at the plan-activation stage, and those errors and/or delays could affect action selection regardless of which effector-specific representation the participant eventually chose. Importantly, this account still assumes that amelic individuals possess effector-specific representations, even of the absent hand.
Second, amelic individuals could use behavioral strategies that are not fully integrated into their prospective internal representation of their hands. If amelic individuals develop personalized strategies for successful one-handed interaction with the world, these strategies could be external to their internal representations of their intact hand. In this case, these strategies would not affect PGS performance, leading to lower accuracy as PGS selections (based on intact hand representation) differ from OGS selections (based on intact hand representation and strategy). However, the amelic individuals’ successful OGS performance makes this explanation unlikely. Our data suggest that amelic individuals perform a grip selection task normally when they can execute movements with their intact hand.
Third, amelic individuals could complete the PGS task using strategies other than movement planning and prediction. Healthy adults and stroke patients generally solve grip selection tasks using motor strategies, compared to mental object rotation tasks, which can be solved through visual or cognitive strategies (Tomasino and Rumiati, 2004; Daprati et al., 2010). Only one of our amelic participants clearly reported using only a non-motor strategy. However, these participant self-reports may or may not accurately reflect their actual implicit processing. If individuals with congenital amelia were unable to successfully perform the PGS task with their absent hand, they may have switched to nonmotor strategies; after the switch, they could potentially have persisted with the non-motor strategy for both hands. The use of a non-motor strategy would lead to reduced influence of biomechanical constraints on responses (Parsons, 1994; Tomasino and Rumiati, 2004), which would appear here as atypical effects of stimulus orientation on performance. Thus, the use of non-motor strategies could explain the abnormal pattern of reaction times in amelic individuals (Fig. 6C and D), but is unlikely to explain reduced PGS accuracy, because amelic individuals’ grip selections depended on stimulus orientation according to the pattern expected from controls (Fig. 4) and from each amelic individual’s OGS selections (Fig. 5B and C). Given the coherent pattern of results across stimulus orientations, another difficulty with this account is explaining how members of the amelic group all managed to adopt functionally-identical alternative strategies.
Finally, these results may suggest that our internal representations capture the approximate biomechanical properties of the typical human body, independent of direct sensorimotor experience. These putative innate representations would require sensory experience for refinement. Unilateral hand absence could have bilateral effects on functional development of limb representations, if refinement of effector-specific representations requires cross-activation in both directions (i.e. from both hands to bilateral cortex), as has been argued for intermanual skill transfer (Parlow and Kinsbourne, 1989; Lee et al., 2010). This would explain why amelic individuals appear capable of selecting actions in a manner that is constrained by the typical properties of the body. Likewise, the absence of experiences with one side could account for why they are both slower and less accurate than controls.
4.4. Limitations
In this study, we did not collect data on cognitive impairments or developmental delays in most patient groups. As a result, we cannot exclude the possibility that the results reflect a cognitive or developmental difference between groups, though this seems unlikely given the equivalent motor performance of the child amelic individuals, and the absence of any overt impairments or deficits in any amelic participants. We also did not collect structural or functional neuroimaging data, which prevents us from identifying hemispheric asymmetries that might underlie any between-group differences.
Among amelic patients, it is difficult to confirm whether their intact hand would have been dominant without amelia. However, this uncertainty about hand dominance does not affect our conclusions, because hand choice (dominant vs. non-dominant) has no effect on grip selection performance (Johnson, 2000a).
4.5. Conclusions
Individuals with congenital unilateral upper limb amelia show a bilateral deficit in predicting the outcome of planned grasping movements. The reason for this is unknown, but four possible causes are suggested here. This bilateral deficit differs strikingly from traumatic unilateral upper limb amputees, who show no deficit with either hand, even decades after amputation (Philip and Frey, 2011). Therefore, bilateral action may play a central role in developing the cerebral mechanisms for bilateral movement planning and control.
Acknowledgments
Funding
This work was funded by Grant R01-NS083377-01 from the National Institute of Neurological Disorders and Stroke (NINDS) to SHF, and Grant 79160 to MA.
Footnotes
One additional pair of child participants (1 amelic individual, 1 control) was removed due to outlying age (8 yrs) and behavior. Extremely long movement onset times for both individuals suggested poor task understanding at that age. PGS selection accuracy was not outlying for the removed amelic individual or control, so the pair’s removal does not influence our main findings.
References
- Brugger P, Kollias SS, Müri RM, Crelier G, Hepp-Reymond MC, Regard M. Beyond re-membering: phantom sensations of congenitally absent limbs. Proc Natl Acad Sci USA. 2000;97:6167–6172. doi: 10.1073/pnas.100510697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruininks RH, Oseretsky N. Bruininks–Oseretsky Test of Motor Proficiency. American Guidance Service; 1978. [Google Scholar]
- Cisek P. Cortical mechanisms of action selection: the affordance competition hypothesis. Philos Trans R Soc B: Biol Sci. 2007;362:1585–1599. doi: 10.1098/rstb.2007.2054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cisek P, Kalaska JF. Neural mechanisms for interacting with a world full of action choices. Annu Rev Neurosci. 2010;33:269–298. doi: 10.1146/annurev.neuro.051508.135409. [DOI] [PubMed] [Google Scholar]
- Cohen LG, Bandinelli S, Findley TW, Hallett M. Motor reorganization after upper limb amputation in man. A study with focal magnetic stimulation. Brain. 1991;114(Pt 1B):615–627. doi: 10.1093/brain/114.1.615. [DOI] [PubMed] [Google Scholar]
- Daprati E, Nico D, Duval S, Lacquaniti F. Different motor imagery modes following brain damage. Cortex. 2010;46:1016–1030. doi: 10.1016/j.cortex.2009.08.002. [DOI] [PubMed] [Google Scholar]
- Donoghue JP, Sanes JN. Organization of adult motor cortex representation patterns following neonatal forelimb nerve injury in rats. J Neurosci. 1988;8:3221–3232. doi: 10.1523/JNEUROSCI.08-09-03221.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frey SH. In: Forecasting the Long-Range Consequences of Manual and Tool Use Actions: Neurophysiological, Behavioral and Computational Considerations. Danion F, Latash M, editors. New York: 2010. pp. 295–313. [Google Scholar]
- Funk M, Brugger P. Mental rotation of congenitally absent hands. J Int Neuropsychol Soc. 2008;14:81–89. doi: 10.1017/S1355617708080041. [DOI] [PubMed] [Google Scholar]
- Gagné M, Hétu S, Reilly KT, Mercier C. The map is not the territory: motor system reorganization in upper limb amputees. Hum Brain Mapp. 2011;32:509–519. doi: 10.1002/hbm.21038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guillot A, Di Rienzo F, MacIntyre T, Moran A, Collet C. Imagining is not doing but involves specific motor commands: a review of experimental data related to motor inhibition. Front Human Neurosci. 2012;6:247. doi: 10.3389/fnhum.2012.00247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habacha H, Molinaro C, Tabben M, Lejeune-Poutrain L. Implementation of specific motor expertise during a mental rotation task of hands. Exp Brain Res. 2014;232:3465–3473. doi: 10.1007/s00221-014-4029-3. [DOI] [PubMed] [Google Scholar]
- Howell D. Statistical Methods for Psychology. Wadsworth; Belmont, CA: 1997. [Google Scholar]
- Jacobs S, Danielmeier C, Frey SH. Human anterior intraparietal and ventral premotor cortices support representations of grasping with the hand or a novel tool. J Cogn Neurosci. 2010;22:2594–2608. doi: 10.1162/jocn.2009.21372. [DOI] [PubMed] [Google Scholar]
- Johnson SH. Thinking ahead: the case for motor imagery in prospective judgements of prehension. Cognition. 2000a;74:33–70. doi: 10.1016/s0010-0277(99)00063-3. [DOI] [PubMed] [Google Scholar]
- Johnson SH. Imagining the impossible: intact motor representations in hemiplegics. NeuroReport. 2000b;11:729–732. doi: 10.1097/00001756-200003200-00015. [DOI] [PubMed] [Google Scholar]
- Johnson SH, Rotte M, Grafton ST, Hinrichs H, Gazzaniga MS, Heinze HJ. Selective activation of a parietofrontal circuit during implicitly imagined prehension. NeuroImage. 2002a;17:1693–1704. doi: 10.1006/nimg.2002.1265. [DOI] [PubMed] [Google Scholar]
- Johnson SH, Sprehn G, Saykin AJ. Intact motor imagery in chronic upper limb hemiplegics: evidence for activity-independent action representations. J Cogn Neurosci. 2002b;14:841–852. doi: 10.1162/089892902760191072. [DOI] [PubMed] [Google Scholar]
- Kaas JH. The reorganization of somatosensory and motor cortex after peripheral nerve or spinal cord injury in primates. Prog Brain Res. 2000;128:173–179. doi: 10.1016/S0079-6123(00)28015-1. [DOI] [PubMed] [Google Scholar]
- Lee M, Hinder MR, Gandevia SC, Carroll TJ. The ipsilateral motor cortex contributes to cross-limb transfer of performance gains after ballistic motor practice. J Physiol. 2010;588:201–212. doi: 10.1113/jphysiol.2009.183855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lotze M, Flor H, Grodd W, Larbig W, Birbaumer N. Phantom movements and pain. An fMRI study in upper limb amputees. Brain. 2001;124:2268–2277. doi: 10.1093/brain/124.11.2268. [DOI] [PubMed] [Google Scholar]
- MacKenzie CL, Iberall T. The Grasping Hand. Elsevier Science B.V; Amsterdam, Netherlands: 1994. p. 482. [Google Scholar]
- Macuga KL, Frey SH. Neural representations involved in observed, imagined, and imitated actions are dissociable and hierarchically organized. NeuroImage. 2012;59:2798–2807. doi: 10.1016/j.neuroimage.2011.09.083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macuga KL, Papailiou AP, Frey SH. Motor imagery of tool use: relationship to actual use and adherence to Fitts’ law across tasks. Exp Brain Res. 2012;218:169–179. doi: 10.1007/s00221-012-3004-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marangon M, Jacobs S, Frey SH. Evidence for context sensitivity of grasp representations in human parietal and premotor cortices. J Neurophysiol. 2011;105:2536–2546. doi: 10.1152/jn.00796.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin K, Jacobs S, Frey SH. Handedness-dependent and -independent cerebral asymmetries in the anterior intraparietal sulcus and ventral premotor cortex during grasp planning. NeuroImage. 2011;57:502–512. doi: 10.1016/j.neuroimage.2011.04.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melzack R, Israel R, Lacroix R, Schultz G. Phantom limbs in people with congenital limb deficiency or amputation in early childhood. Brain. 1997;120:1603–1620. doi: 10.1093/brain/120.9.1603. [DOI] [PubMed] [Google Scholar]
- Mercier C, Reilly KT, Vargas CD, Aballea A, Sirigu A. Mapping phantom movement representations in the motor cortex of amputees. Brain. 2006;129:2202–2210. doi: 10.1093/brain/awl180. [DOI] [PubMed] [Google Scholar]
- Merzenich MM, Kaas JH, Wall J, Nelson RJ, Sur M, Felleman D. Topographic reorganization of somatosensory cortical areas 3b and 1 in adult monkeys following restricted deafferentation. Neuroscience. 1983;8:33–55. doi: 10.1016/0306-4522(83)90024-6. [DOI] [PubMed] [Google Scholar]
- Nico D, Daprati E, Rigal F, Parsons L, Sirigu A. Left and right hand recognition in upper limb amputees. Brain. 2004;127:120–132. doi: 10.1093/brain/awh006. [DOI] [PubMed] [Google Scholar]
- Parlow SE, Kinsbourne M. Asymmetrical transfer of training between hands: implications for interhemispheric communication in normal brain. Brain Cogn. 1989;11:98–113. doi: 10.1016/0278-2626(89)90008-0. [DOI] [PubMed] [Google Scholar]
- Parsons LM. Temporal and kinematic properties of motor behavior reflected in mentally simulated action. J Exp Psychol Hum Percept Perform. 1994;20:709–730. doi: 10.1037//0096-1523.20.4.709. [DOI] [PubMed] [Google Scholar]
- Pascual-Leone A, Peris M, Tormos JM, Pascual AP, Catalá MD. Reorganization of human cortical motor output maps following traumatic forearm amputation. NeuroReport. 1996;7:2068–2070. doi: 10.1097/00001756-199609020-00002. [DOI] [PubMed] [Google Scholar]
- Philip BA, Frey SH. Preserved grip selection planning in chronic unilateral upper extremity amputees. Exp Brain Res. 2011;214:437–452. doi: 10.1007/s00221-011-2842-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price EH. A critical review of congenital phantom limb cases and a developmental theory for the basis of body image. Conscious Cogn. 2006;15:310–322. doi: 10.1016/j.concog.2005.07.003. [DOI] [PubMed] [Google Scholar]
- Reilly KT, Sirigu A. Motor cortex representation of the upper-limb in individuals born without a hand. PLoS One. 2011;6:e18100. doi: 10.1371/journal.pone.0018100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez M, Llanos C, Gonzalez S, Sabate M. How similar are motor imagery and movement? Behav Neurosci. 2008;122:910–916. doi: 10.1037/0735-7044.122.4.910. Epub 2008/08/30. [DOI] [PubMed] [Google Scholar]
- Sanes JN, Suner S, Lando JF, Donoghue JP. Rapid reorganization of adult rat motor cortex somatic representation patterns after motor nerve injury. Proc Natl Acad Sci USA. 1988;85:2003–2007. doi: 10.1073/pnas.85.6.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma N, Jones PS, Carpenter TA, Baron JC. Mapping the involvement of BA 4a and 4p during motor imagery. NeuroImage. 2008;41:92–99. doi: 10.1016/j.neuroimage.2008.02.009. [DOI] [PubMed] [Google Scholar]
- Tomasino B, Rumiati RI. Effects of strategies on mental rotation and hemispheric lateralization: neuropsychological evidence. J Cogn Neurosci. 2004;16:878–888. doi: 10.1162/089892904970753. [DOI] [PubMed] [Google Scholar]
- Vannuscorps G, Pillon A, Andres M. Effect of biomechanical constraints in the hand laterality judgment task: where does it come from? Front Hum Neurosci. 2012;6:299. doi: 10.3389/fnhum.2012.00299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vetter RJ, Weinstein S. The history of the phantom in congenitally absent limbs. Neuropsychologia. 1967;5:335–338. [Google Scholar]
- Wilkins KL, McGrath PJ, Finley GA, Katz J. Phantom limb sensations and phantom limb pain in child and adolescent amputees. Pain. 1998;78:7–12. doi: 10.1016/S0304-3959(98)00109-2. [DOI] [PubMed] [Google Scholar]
- Wolpert DM, Ghahramani Z, Jordan MI. An internal model for sensorimotor integration. Science. 1995;269:1880–1882. doi: 10.1126/science.7569931. [DOI] [PubMed] [Google Scholar]


