Abstract
The protozoan Toxoplasma gondii (T. gondii) manipulates the behavior of its rodent intermediate host to facilitate its passage to its feline definitive host. This is accomplished by a reduction of the aversive response that rodents show towards cat odors, which likely increases the predation risk. Females on average show similar changes as males. However, behaviors that relate to aversion and attraction are usually strongly influenced by the estrus cycle. In this study, we replicated behavioral effects of T. gondii in female rats, as well as expanded it to two novel behavioral paradigms. We also characterized the role of the estrus cycle in the behavioral effects of T. gondii on female rats. Uninfected females preferred to spend more time in proximity to rabbit rather than bobcat urine, and in a dark chamber rather than a lit chamber. Infected females lost both of these preferences, and also spent more time investigating social novelty (foreign bedding in their environment). Taken together, these data suggest that infection makes females less risk averse and more exploratory. Furthermore, this effect was influenced by the estrus cycle. Uninfected rats preferred rabbit urine to bobcat urine throughout the cycle except at estrus and metestrus. In contrast, infected rats lost this preference at every stage of the cycle except estrus. Commensurate with the possibility that this was a hormone-dependent effect, infected rats had elevated levels of circulating progesterone, a known anxiolytic.
Keywords: Toxoplasma gondii, Rat, Female, Progesterone, Behavior, Aversion, Predator aversion, Light aversion, Estrus
1. Introduction
Some parasites, in the process of trying to aid their own transmission, can offer surprising insight to the neurobiology of behavior. The protozoan parasite Toxoplasma gondii (T. gondii) is such an organism. It undergoes sexual reproduction solely in its definitive host, the cat. The oocysts formed are excreted in feces, and consumed by a variety of secondory host species including, of the most evolutionary relevance, rodents. T. gondii infection predominantly occurs in the central nervous system, muscle, and gonads. The cycle is completed when a cat eats an infected rodent. Facilitating that final step, infection by T. gondii reduces the innate fear of cat odor in rodents, at times even turning it into attraction [4]. Moreover, infection also lessens rodent aversion to novelty [9,25]. These behavioral modifications appear to be remarkably specific in rats, as mobility and social behaviors [3], responses to other predators besides cats [12], and learned fear responses are typically unaffected [24].
T. gondii forms diffusely distributed cysts throughout the brain, with a possible amygdalar preference [5,24]. According to some studies, the location of these cysts appears to correlate with the strength of behavioral changes [5,8]. In male rats, infection increases dopamine levels [20], causes dendritic atrophy [18], reduces activation of predator fear circuitry while at the same time increasing activation of sexual arousal circuitry (centered on the posterodorsal medial amygdala) in response to cat odor [10]. T. gondii affects human personality and behavior in some broadly similar ways as well, likely through shared functional architecture of these behaviors with rodents (reviewed in [2]).
There is evidence of gender specificity of T. gondii’s behavioral effects. Female mice show reduced aversion to cat odor while male mice do not [27], and T. gondii-correlated shifts in personality test results often show different, sometimes diametrically opposed directions in men and women [15]. An understanding of T. gondii effects in female rodents is hampered by the fact that prior studies have not controlled for estrus stages.
In this study, we sought to further examine whether the behavior of female rats is affected by T. gondii infection and, specifically, whether any such effects vary as a function of female estrus status.
2. Materials & methods
Studies were conducted according to the guidelines of and were approved by Stanford Administrative Panel on Laboratory Animal Care (APLAC). For behavioral studies, data was recorded using VideoTrack software by ViewPoint Behavior Technology, and analyzed blind to the treatment group.
2.1. T. gondii preparation and infecting the rats
A Prugniaud type II strain of T. gondii (from Boothroyd Laboratory, Stanford University) was maintained as tachyzoites by passage through human foreskin fibroblast monolayers. Infected fibroblasts were scraped and washed with phosphate buffered saline (PBS), resuspended in PBS and syringe-lysed (25 and then 27-gauge needle) to release tachyzoites. Long–Evans strain rats (from Charles River Laboratories) were injected with 10 million tachyzoites (intraperitoneally; i.p.) in sterile PBS at a minimum of 6 weeks prior to behavioral testing. By this time point, sickness effects resulting from acute infection dissipate, and the infection progresses to the chronic phase [24]. Health status was monitored throughout the experiment. Control animals were injected with vehicle (PBS).
2.2. Determination of estrus cycle stage
Estrus cycle status was determined with standard techniques [13,17], using a smear of the vaginal cell lining, collected by gently inserting a dry cotton-tipped applicator into the animal’s vagina and rotating it two or three times. Cells were then smeared on a glass microscope slide, and DiffQuick stain was used to improve visibility of the sample cells. Each cycle stage has a characteristic distribution of cell types in vaginal lining samples [13,17].
2.3. Bobcat urine aversion paradigm
A bobcat urine aversion paradigm was modified from an existing protocol utilizing a cat collar as the odor source [24]. Testing took place in a large, rectangular 76.2 × 30.5 × 33 cm Tupperware box with transparent sides and opaque lid. The short sides of the box had rectangular openings cut into them. The narrow confines of the test arena were intended to sharpen the behavioral preference. The test stimulus was bobcat urine, and the control stimulus rabbit urine (both purchased from PredatorPee.com).
One milliliter of each stimulus was pipetted on a piece of surgical gauze and placed outside of the box, in front of the openings; plastic grating prevented rats from reaching. (It should be noted that while equal quantities of urine were used from bobcat and rabbit, such samples may differ qualitatively, in terms of concentration of pertinent odorants; insight into this could be acquired with a detailed dose–response study. However, the focus of the present paper [and of others examining the behavioral effects of T. gondii, using this volume of urine stimulus] is on the effects of infection on aversion, not on the nature of aversion itself.) Before behavioral testing, rats were habituated to the arena for two 10-minute sessions without stimuli, one day apart. Actual test sessions were 15 minute long, as previously shown to be sufficient to demonstrate these behavioral effects of the parasite [24]. All habituation and test trials took place at the same time of the day. Sample sizes:
Diestrus, 10 control/18 T. gondii; proestrus, 8 control/6 T. gondii; estrus, 5 control/6 T. gondii; metestrus, 10 control/8 T. gondii.
2.4. Light aversion paradigm
The experimental arena (a transparent, plastic 30 × 30 × 120 cm box) was divided into three equal-sized chambers along its length by transparent partitions with openings that allowed passage by rats. One of the opposite-end chambers was designated as the ‘dark’ chamber; it was covered with a black fabric sheet and an opaque cardboard box on all sides, except the side through which the rats could pass to the middle chamber. The opposing ‘light’ chamber was lit brightly from above and a slight angle. Light level at the center of the ‘light’ and ‘dark’ chambers was >1200 lux, and >20 lux, respectively. For testing, rats were placed in the middle chamber and allowed to roam freely for 15 min. All test trials took place at the same time of the day. No habituation was performed as the tested rats did not show strong light aversion in a familiar environment. Sample size: 6 control/6 T. gondii.
2.5. Foreign material aversion paradigm
For this experiment, the arena used for the bobcat urine study was utilized. The stimulus was either 50 ml. of bedding material from the rat’s home cage, or from the cage of an unfamiliar female. Habituation was as described above, and the testing period was 10 min. All habituation and test trials took place at the same time of the day. Sample size: 6 control/6 T. gondii.
2.6. Blood sampling
Blood was collected from an in-dwelling silicone catheter in the right jugular vein. For implantation of the catheter, animals were anesthetized in an isofluorene chamber, and kept under through the surgery using a muzzle that circulated isofluorene. The catheter was inserted through an incision on the animal’s back, with the catheter access port placed through a smaller incision between the shoulder blades [23]. The tube was inserted through an incision in the jugular vein, and fastened by two sutures [23]. Catheters were implanted one week before sampling, and flushed daily with heparinized saline. Samples of 0.2–0.3 ml volumes were collected at 2 pm, and stored at −20 °C in EDTA-coated tubes.
2.7. Progesterone determinations
Progesterone concentrations were determined in whole blood using a quantitative ELISA kit (Enzo Lifesciences Progesterone ELISA Kit, catalog #ADI-900-011), according to the manufacturer’s instructions. Absorbances were read at 405 nm. Sample size: 3 control/4 T. gondii.
2.8. Data analysis/statistics
Preference for a specific odor, dark preference, and preference for foreign conspecific bedding were determined using a 95% confidence interval test. ‘Preference’ in this study was reported as time spent near one stimulus that is in excess of the opposing stimulus. It is determined by subtracting total time, in seconds, spent near stimulus A from time spent near stimulus B. Progesterone levels were compared by two-way ANOVA using the day of testing and T. gondii infection status as variables. The initial day of blood sampling was a function of patency of catheters; thus, animals were not all at the same point in their cycles on the day of first sampling. Rather than analyze samples by day (which would involve grouping samples from different points in the cycle from different animals), the sequence of samples from different animals was standardized by considering each animal’s day of peak progesterone levels to correspond to the beginning of the estrus stage in the cycle; thus, samples from different animals were grouped and analyzed by point in cycle. Data are presented as means +/− SEMs. Analysis was performed using SPSS version 20.0.
3. Results
3.1. Behavioral effects of T. gondii
3.1.1. T. gondii infection blocks the aversion towards bobcat urine
Animals at diestrus stage of the estrus cycle were tested for their preference of rabbit urine over bobcat urine. Uninfected animals showed a clear preference for the rabbit half of the arena (95% confidence interval, range 37.16 to 309.92 s), while T. gondii (toxo) animals (red color) showed no preference for either stimulus (95% confidence interval, range −45.1 to 157.49 s) (Fig. 1).
Fig. 1.
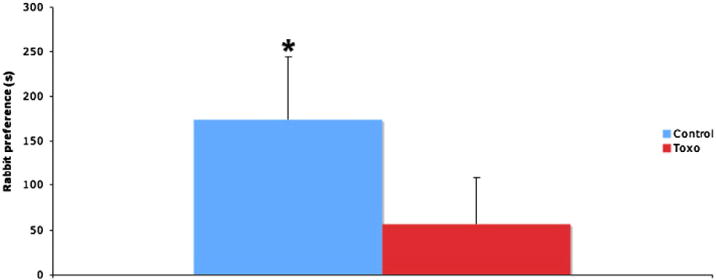
Animals at diestrus stage of the estrus cycle were tested for their preference of rabbit urine over bobcat urine. The columns represent time spent at the half of the test arena close to rabbit urine that is in excess of the time spent at the half of the test arena close to the bobcat urine. Control animals (blue color) showed a clear preference for the rabbit half of the arena (95% confidence interval, range 37.16 to 309.92 s), while T. gondii (toxo) animals (red color) showed no preference for either stimulus (95% confidence interval, range −45.1 to 157.49 s). n = 10 control/18 T. gondii.
3.1.2. Infected female rats show no light aversion
Rats are photophobic [14], so light was used as a second aversive stimulus. It is arguably to the advantage of T. gondii to affect this aversion, since being in open, well-lit spaces potentially increases predation risk for rats. Animals at diestrus stage of the estrus cycle were tested for their preference for a dark chamber over a brightly lit chamber. Control animals showed a clear preference for the dark chamber (95% confidence interval, range 130.3 to 722.0 s) while T. gondii animals showed no preference (95% confidence interval, range −546.9 to 450.9 s) (Fig. 2).
Fig. 2.
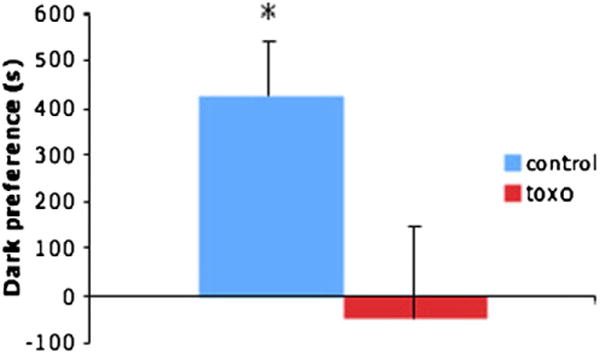
Animals at diestrus stage of the estrus cycle were tested for their preference for a dark chamber over a brightly lit chamber. The columns represent time spent at the chamber of the test arena that was dark that is in excess of dark chamber (dark blue) or dark (light blue). Control animals (blue color) showed a clear preference for the dark chamber (95% confidence interval, range 130.3 to 722.0 s) while T. gondii (toxo) animals (red color) showed no preference (95% confidence interval, range −546.9 to 450.9 s). n = 6 control/6 T. gondii.
3.1.3. Infected female rats are attracted to social novelty
Animals were tested for their preference of a foreign urine over their own urine. Control animals showed no preference for either side (95% confidence interval, −213.6 to 98.2 s), while T. gondii animals showed a significant preference for the unfamiliar bedding (95% confidence interval, 39.8 to 255.1 s) (Fig. 3).
Fig. 3.
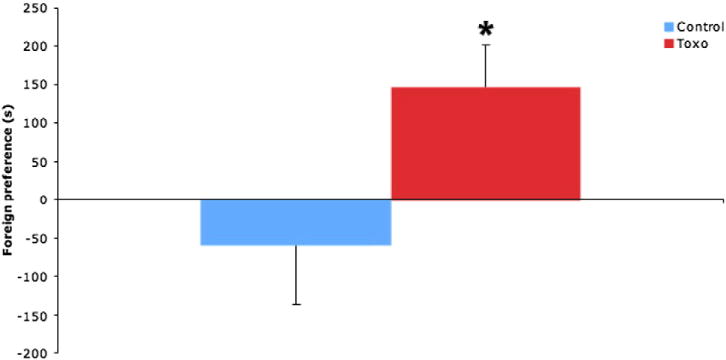
Animals were tested for their preference of a foreign urine over their own urine. The columns represent time spent at the half of the test arena close to bedding from the cage of an unfamiliar conspecific that is in excess of the time spent at the half of the test arena close to bedding from their opwn cage. Control animals (blue color) showed no preference for either side (95% confidence interval, −213.6 to 98.2), while T. gondii (toxo) animals (red color) showed a significant preference for the unfamiliar bedding (95% confidence interval, 39.8 to 255.1). n = 6 control/6 T. gondii.
3.1.4. T. gondii’s influence on the avoidance of bobcat odor is dependent on the stage of the estrus cycle
The aversive behaviors of female rats undergo significant changes depending on the stage of estrus cycle [7]. Animals were tested for their preference of rabbit urine over bobcat urine, across each different stage of the estrus cycle. Control animals showed no preference for either side during metestrus (95% confidence interval, range −104.08 to 276.62 s) or estrus (95% confidence interval, range 42.67 to 496.27 s), but they significantly preferred the rabbit half at every other stage of the cycle. T. gondii animals showed no preference for either side at any stage except estrus, where they significantly preferred the rabbit half of the arena (95% confidence interval, range 157.57 to 387.76 s) (Fig. 4).
Fig. 4.
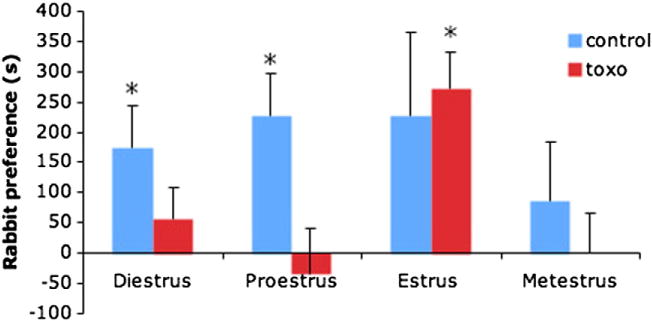
Animals were tested for their preference of rabbit urine over bobcat urine, across each different stage of the estrus cycle. The columns represent time spent at the half of the test arena close to rabbit urine that is in excess of the time spent at the half of the test arena close to the bobcat urine. Control animals (blue color showed no preference for either side during metestrus (95% confidence interval, range −104.08 to 276.62 s) or estrus (95% confidence interval, range 42.67 to 496.27 s), but they significantly preferred the rabbit half at every other stage of the cycle. T. gondii animals (right) showed no preference for either side at any stage except estrus, where they significantly preferred the rabbit half of the arena (95% confidence interval, range 157.57 to 387.76 s).
3.2. Physiological effects of T. gondii
3.2.1. T. gondii infection does not affect the structure of the estrus cycle
Estrus stages of the animals were tracked once a day at acute stage of infection for 16 days before and after infection with T. gondii and chronic stage of infection for 12 days for infected animals and uninfected controls. The frequency of each individual stage is compared for the infected and uninfected conditions. In both acute and chronic phases, there was no statistically significant difference (t-test) at any of the stages between the two groups (Fig. 5).
Fig. 5.
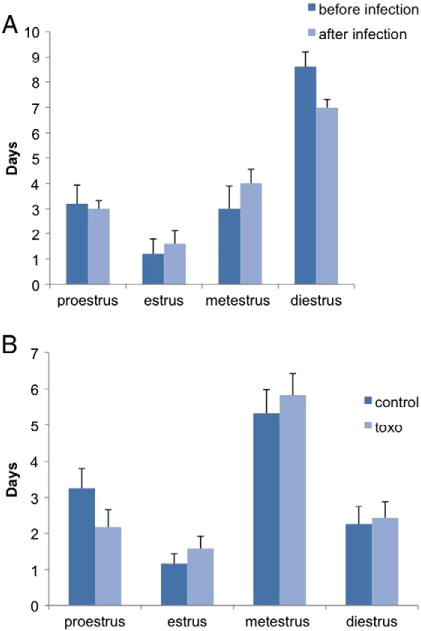
Estrus stages of the animals were tracked once a day at A) acute stage of infection for 16 days before and after infection with T. gondii (n = 5) and B) chronic stage of infection for 12 days for infected animals and uninfected controls (n = 12 for each group). Each column represents the frequency of the specific stage within these time periods. The frequency of each individual stage is compared for the infected and uninfected conditions. In both acute and chronic phases, there was no statistically significant difference (t-test) at any of the stages between the two groups.
3.2.2. T. gondii-infected female rats have higher levels of circulating progesterone
Changing stages of estrus cycle are accompanied by cycling gonadal hormone levels. One such hormone is progesterone. This steroid is a good candidate for being a T. gondii target, as it decreases anxiety. Circulating progesterone levels were measured in blood taken from the jugular vein of rats daily for five days. Commensurate with this anxiolytic role, T. gondii-infected animals had higher circulating progesterone concentrations than control animals (Univariate analysis of variance, main effect of T. gondii (F[1,32] = 14.670, p = 0.001)) (Fig. 6).
Fig. 6.
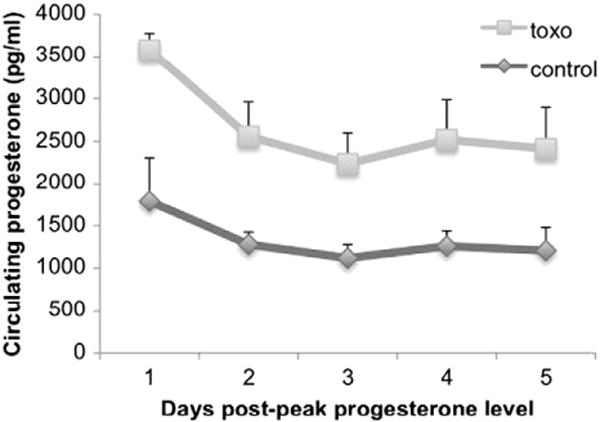
Circulating progesterone levels were measured in blood taken from the jugular vein of rats daily for five days. Each line represents progesterone concentrations across these days, with highest levels set to the first day for each animal. Levels from T. gondii (gray squares) and control (black diamonds) animals are compared. T. gondii animals show and elevated level of circulating progesterone across all five days (univariate analysis of variance, main effect of T. gondii (F[1,32] = 14.670, p = 0.001)). n = 3 control/4 T. gondii.
4. Discussion
The basic behavioral modification associated with T. gondii infection is a reduction of cat aversion. While this effect was documented in both male and female rats [3,4,25], studies that involve both genders were done in mixed populations. This may cause subtle differences to be lost through population average effects. For example, in mice, females show more consistent reduction of cat aversion than males [27]. Furthermore, the effects of the estrus cycle on this behavioral modification may be masked in groups of females that occupy different cycle stages at the time of testing. Here, we confirm that infected female rats show a reduced avoidance of bobcat urine, at levels similar to that in previous reports concerning males, in a manner that is influenced by the estrus cycle.
Our data cast light on the specificity of the parasite’s effects. Past studies primarily focus on T. gondii’s effect on cat avoidance, as this effect is seen as most evolutionarily relevant for progressing T. gondii’s life cycle. However, T. gondii has also been reported to reduce novelty aversion, another type of risk avoidance behavior [9,25]. Here, we corroborate that finding, in that infected females also lost their natural avoidance of bright lights, and increased their investigation of the potential presence of foreign rats. Taken together, these data imply that rather than targeting cat aversion specifically, T. gondii may instead influence the response to risk. Previous reports show that conditioned fear behavior is not affected by T. gondii infection [7], suggesting that circuitry controlling innate versus learned fear is differentially affected by the parasite. It should, however, be noted that past studies show avoidance of other predator odors as unaffected at a similar stimulus intensity [11].
It should also be noted that, while we postulate that the increased preference for the aversive stimuli in this study correspond to a reduction of this aversion, other possibilities, such as a reduction of attraction to non-aversive stimulus, are also present. Further studies that look into measures of attraction and aversion beyond approach can clarify this distinction.
We also report that the manifestation of the behavioral modifications of T. gondii-infected females depends on the stage of the estrus cycle. Cycle status affects anxiety [1], fear [6], and aversion in rodents [7]. Rats in receptive phases of their cycle are less anxious, spending more time in open arms of elevated plus maze and in brightly lit areas, showing decreased latency in leaving dark chambers, decreased freezing after a foot shock, and increased interaction with conspecifics, as compared to animals in diestrus. This effect can last into metestrus, with such rats showing more exploration under high light intensity compared to those in other stages [19]. Our results indicate that uninfected controls avoided bobcat urine at all points during the cycle except for estrus and metestrus. While there are many studies on the disruptive effect of predator exposure on cycling (such as 11), to our knowledge, this is the first report on the effect of the cycle stage on predator aversion behavior. The impact of the cycle on the behavior is, however, quite different in infected animals, as they show no avoidance at any point in the cycle, except at estrus.
The dependency of the observed behavior on the estrus cycle suggests a potential role of sex hormones in T. gondii-derived behavioral modification. While several gonadal hormones are known to influence behavior, progesterone in specific is known to have powerful effects on reducing anxiety. Progesterone lessens various behavioral indices of anxiety [19]; moreover, fluctuations in many of these endpoints over the course of the cycle are progesterone dependent. These anxiolytic effects are likely mediated by metabolites of progesterone binding to the GABAA receptor, which makes the receptor more sensitive to GABA [16,26]. Thus, were infection with T. gondii to increase progesterone concentrations, the hormone’s anxiolytic effects might help explain the parasite’s behavioral effects. Indeed, we observed an increased concentration of circulating progesterone in the physiological range [1,21] in infected animals compared to uninfected controls. The loss of this effect in T. gondii animals during estrus is puzzling, and may be related to the marked spiking of progesterone levels during this stage.
In conclusion, female rats showed behavioral modifications as a result of T. gondii infection similar to those previously reported in male rats. It remains to be determined whether the underlying neurobiology is the same. We previously reported that in T. gondii-infected male rats, cat odor causes activation of the posterodorsal medial amygdala, a response typically elicited by a sexual stimulus, and increases activation of sexual arousal circuitry (centered on the posterodorsal medial amygdala) in response to cat odor. The brain regions that comprise the sexual circuitry in males, however, often have different functions in females, such as regulating maternal behaviors [22]. Because of this, we would expect the behavioral effects to differ in the two sexes if the same circuitry were affected. As the effects appear to be the same, it is more likely that different circuitry will be involved in females; this is a focus of current study.
HIGHLIGHTS.
Toxo-infected females show reduced risk aversion.
Behavioral modifications are dependent on the stage of the estrus cycle.
The infected have elevated progesterone, a possible means for the behavior change.
Acknowledgments
We thank Drs. Andrew K. Evans, Claude M. Nagamine, and Melissa Works for the training they provided. This work was funded by The Stanley Medical Research Institute (06R-1463). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Auger CJ, Forbes-Lorman RM. Progestin receptor-mediated reduction of anxiety-like behavior in male rats. PLoS One. 2008;3(11):e3606. doi: 10.1371/journal.pone.0003606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bech-Nielsen S. Toxoplasma gondii associated behavioural changes in mice, rats and humans: evidence from current research. Prev Vet Med. 2012 Jan;103(1):78–9. doi: 10.1016/j.prevetmed.2011.10.011. [DOI] [PubMed] [Google Scholar]
- 3.Berdoy M, Webster JP, Macdonald DW. Parasite-altered behaviour: is the effect of Toxoplasma gondii on Rattus norvegicus specific? Parasitology. 1995 Nov;111(Pt 4):403–9. doi: 10.1017/s0031182000065902. [DOI] [PubMed] [Google Scholar]
- 4.Berdoy M, Webster JP, Macdonald DW. Fatal attraction in rats infected with Toxoplasma gondii. Proc Biol Sci. 2000 Aug;267(1452):1591–4. doi: 10.1098/rspb.2000.1182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Berenreiterová M, Flegr J, Kuběna AA, Němec P. The distribution of Toxoplasma gondii cysts in the brain of a mouse with latent toxoplasmosis: implications for the behavioral manipulation hypothesis. PLoS One. 2011;6(12):e28925. doi: 10.1371/journal.pone.0028925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen W, Shields J, Huang W, King JA. Female fear: influence of estrus cycle on behavioral response and neuronal activation. Behav Brain Res. 2009 Jul;201(1):8–13. doi: 10.1016/j.bbr.2009.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Díaz-Véliz G, Butrón S, Benavides MS, Dussaubat N, Mora S. Gender, estrous cycle, ovariectomy, and ovarian hormones influence the effects of diazepam on avoidance conditioning in rats. Pharmacol Biochem Behav. 2000 Aug;66(4):887–92. doi: 10.1016/s0091-3057(00)00283-5. [DOI] [PubMed] [Google Scholar]
- 8.Evans AK, Strassmann PS, Lee IP, Sapolsky RM. Patterns of Toxoplasma gondii cyst distribution in the forebrain associate with individual variation in predator odor avoidance and anxiety-related behavior in male Long–Evans rats. Brain Behav Immun. 2014 Mar;37:122–33. doi: 10.1016/j.bbi.2013.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hay J, Hutchison WM, Aitken PP. Congenital Toxoplasma infection and response to novelty in mice. Ann Trop Med Parasitol. 1983 Aug;77(4):437–9. doi: 10.1080/00034983.1983.11811733. [DOI] [PubMed] [Google Scholar]
- 10.House PK, Vyas A, Sapolsky R. Predator cat odors activate sexual arousal pathways in brains of Toxoplasma gondii infected rats. PLoS One. 2011;6(8):e23277. doi: 10.1371/journal.pone.0023277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Koskela E, Horne TJ, Mappes T, Ylonen H. Does risk of small mustelid predation affect the oestrous cycle in the bank vole, Clethrionomys glareolus? Anim Behav. 1996;51:159–1163. [Google Scholar]
- 12.Lamberton PH, Donnelly CA, Webster JP. Specificity of the Toxoplasma gondii-altered behaviour to definitive versus non-definitive host predation risk. Parasitology. 2008 Sep;135(10):1143–50. doi: 10.1017/S0031182008004666. [DOI] [PubMed] [Google Scholar]
- 13.Larsen B, Markovetz AJ, Galask RP. Quantitative alterations in the genital microflora of female rats in relation to the estrous cycle. J Infect Dis. 1976 Nov;134(5):486–9. doi: 10.1093/infdis/134.5.486. [DOI] [PubMed] [Google Scholar]
- 14.Lockard RB. Some effects of light upon the behavior of rodents. Psychol Bull. 1963 Nov;60:509–29. doi: 10.1037/h0046113. [DOI] [PubMed] [Google Scholar]
- 15.Lindová J, Novotná M, Havlícek J, Jozífková E, Skallová A, Kolbeková P, et al. Gender differences in behavioural changes induced by latent toxoplasmosis. Int J Parasitol. 2006 Dec;36(14):1485–92. doi: 10.1016/j.ijpara.2006.07.008. [DOI] [PubMed] [Google Scholar]
- 16.Lovick TA. GABA in the female brain—oestrous cycle-related changes in GABAergic function in the periaqueductal grey matter. Pharmacol Biochem Behav. 2008 Jul;90(1):43–50. doi: 10.1016/j.pbb.2007.12.014. [DOI] [PubMed] [Google Scholar]
- 17.Marcondes FK, Bianchi FJ, Tanno AP. Determination of the estrous cycle phases of rats: some helpful considerations. Braz J Biol. 2002 Nov;62(4A):609–14. doi: 10.1590/s1519-69842002000400008. [DOI] [PubMed] [Google Scholar]
- 18.Mitra R, Sapolsky RM, Vyas A. Toxoplasma gondii infection induces dendritic retraction in basolateral amygdala accompanied by reduced corticosterone secretion. Dis Model Mech. 2013 Mar;6(2):516–20. doi: 10.1242/dmm.009928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mora S, Dussaubat N, Díaz-Véliz G. Effects of the estrous cycle and ovarian hormones on behavioral indices of anxiety in female rats. Psychoneuroendocrinology. 1996 Oct;21(7):609–20. doi: 10.1016/s0306-4530(96)00015-7. [DOI] [PubMed] [Google Scholar]
- 20.Prandovszky E, Gaskell E, Martin H, Dubey JP, Webster JP, McConkey GA. The neurotropic parasite Toxoplasma gondii increases dopamine metabolism. PLoS One. 2011;6(9):e23866. doi: 10.1371/journal.pone.0023866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rodriguez-Sierra JF, Howard JL, Pollard GT, Hendricks SE. Effect of ovarian hormones on conflict behavior. Psychoneuroendocrinology. 1984;9(3):293–300. doi: 10.1016/0306-4530(84)90008-8. [DOI] [PubMed] [Google Scholar]
- 22.Sheehan T, Paul M, Amaral E, Numan MJ, Numan M. Evidence that the medial amygdala projects to the anterior/ventromedial hypothalamic nuclei to inhibit maternal behavior in rats. Neuroscience. 2001;106(2):341–56. doi: 10.1016/s0306-4522(01)00286-x. [DOI] [PubMed] [Google Scholar]
- 23.Thomsen M, Caine SB. Chronic intravenous drug self-administration in rats and mice. Curr Protoc Neurosci. 2005 Aug; doi: 10.1002/0471142301.ns0920s32. [Chapter 9:Unit 9.20] [DOI] [PubMed] [Google Scholar]
- 24.Vyas A, Kim SK, Giacomini N, Boothroyd JC, Sapolsky RM. Behavioral changes induced by Toxoplasma infection of rodents are highly specific to aversion of cat odors. Proc Natl Acad Sci U S A. 2007 Apr;104(15):6442–7. doi: 10.1073/pnas.0608310104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Webster JP, Brunton CF, MacDonald DW. Effect of Toxoplasma gondii upon neophobic behaviour in wild brown rats, Rattus norvegicus. Parasitology. 1994 Jul;109(Pt 1):37–43. doi: 10.1017/s003118200007774x. [DOI] [PubMed] [Google Scholar]
- 26.Wilson MA. Influences of gender, gonadectomy, and estrous cycle on GABA/BZ receptors and benzodiazepine responses in rats. Brain Res Bull. 1992 Aug;29(2):165–72. doi: 10.1016/0361-9230(92)90022-p. [DOI] [PubMed] [Google Scholar]
- 27.Xiao J, Kannan G, Jones-Brando L, Brannock C, Krasnova IN, Cadet JL, et al. Sex-specific changes in gene expression and behavior induced by chronic Toxoplasma infection in mice. Neuroscience. 2012 Mar 29;206:39–48. doi: 10.1016/j.neuroscience.2011.12.051. [DOI] [PubMed] [Google Scholar]


