Abstract
Objective
Primary curative treatment of advanced laryngeal cancer may include surgery or chemoradiation, although recommendations vary and both are associated with complications. We evaluated predictors and trends in the use of these modalities and compared rates of complications and overall survival in a population-based cohort of older adults.
Study Design
Retrospective population-based cohort study
Methods
Using SEER cancer registry data linked with Medicare claims, we identified patients over 65 with advanced laryngeal cancer diagnosed 1999-2007 who had total laryngectomy (TL) or chemoradiation (CTRT) within 6 months following diagnosis. We identified complications and estimated the impact of treatment on overall survival, using propensity score methods.
Results
The proportion of patients receiving TL declined from 74% in 1999 to 26% in 2007 (p<0.0001). Almost 20% of CTRT patients had a tracheostomy following treatment and 57% had a feeding tube. TL was associated with an 18% lower risk of death, adjusting for patient and disease characteristics. The benefit of TL was greatest in patients with the highest propensity to receive surgery.
Conclusion
TL remains an important treatment option in well selected older patients. However, treatment selection is complex and, factors such as functional status, patient preference, surgeon expertise and post-treatment support services should play a role in treatment decisions.
Keywords: Treatment, Complications, Survival, Laryngeal Cancer
Introduction
Cancer of the larynx is among the most common cancers of the upper aerodigestive tract, with almost 13,000 new cases diagnosed in the US in 2010.1 Definitive treatment for advanced stage laryngeal cancer (stage III or IV) consists of either total laryngectomy (TL) or chemoradiation (CTRT).2 Radiotherapy (RT) alone has been shown to have poorer efficacy than CTRT and is reserved for the small group of patients for whom substantial comorbidity threatens the safe administration of surgery or CTRT.3, 4
An evolution in the treatment of advanced laryngeal cancer (stage III and IV) has occurred over the past two decades with a fall in the use of primary surgery and a corresponding rise in the use of nonoperative treatment.5-10 This trend originated with the publication of a landmark Veteran Affairs (VA) study in 1991, which showed that induction chemotherapy followed by definitive radiation could preserve the larynx without compromising overall survival.11 In 2003 the Radiation Therapy Oncology Group (RTOG) 91-11 trial concluded that concurrent chemoradiation (CTRT) improved local control compared with induction chemotherapy followed by radiation (RT), and should be considered the standard of care for patients with T3 disease who desire laryngeal preservation.4
While the adoption of primary CTRT as the standard of care has clearly benefited many patients, its increased use has coincided with a rise in functional complications and decreased overall survival, in older patients and those with T4 disease, respectively. 5, 7, 9, 12-14 Furthermore, salvage surgery, as a final curative treatment strategy for patients treated with organ preserving CTRT can be technically complex and have a significant impact on patient morbidity.15, 16 At the population level, little is known about treatment patterns, the proportion of patients receiving various treatment modalities and the factors that influence the type of treatment received. The treatment of older patients is of particular concern with evidence indicating a decreasing benefit of chemotherapy with increasing patient age and a lack of significant benefit when chemotherapy is added to RT in patients over the age of 70.17, 18
The objectives of this study were to evaluate predictors and trends in the use of CTRT and TL and to compare rates of treatment-related complications and overall survival between these modalities in a population-based cohort of older adults.
Materials and Methods
Data
We used Surveillance Epidemiology and End Results (SEER) cancer registry data linked with Medicare claims. SEER is a National Cancer Institute (NCI)-sponsored, consortium of population-based cancer registries covering approximately 28% of Americans in selected states and geographic areas.19 The SEER registries collect information regarding site and extent of disease, clinical and pathologic stage, first course of cancer-directed therapy and sociodemographic characteristics for all newly diagnosed cancer cases, with active follow-up for date and cause of death. Medicare is the primary health insurer for 97% of the US population aged 65 years and older and covers inpatient hospital care (Part A), and outpatient care and physician services (Part B). The SEER-Medicare files were used in accordance with a data-use agreement between the NCI and the Centers for Medicare and Medicaid Services (CMS). This study was approved by the Institutional Review Board at Memorial Sloan-Kettering Cancer Center.
Study Cohort
We identified Medicare beneficiaries aged 66 years or older with a pathologically confirmed primary diagnosis of advanced squamous cell laryngeal cancer (Stage III or IV) between January 1st 1999 and December 31st 2007.20 Patients were categorized into one of two mutually exclusive primary treatment categories, TL or CTRT, based on Medicare claims within 6 months following diagnosis. Primary TL consisted of TL alone (N=103), TL with post-operative RT (N=184) or CTRT (N=41), and included TL with or without a neck dissection. Primary concurrent CTRT included any claim for RT, at least 2 claims for chemotherapy, and no claim for TL within the initial 6-month treatment period. In order to minimize possible misclassification of induction CTRT as concurrent therapy, we excluded patients whose first claim for RT was more than 60 days after their first claim for CT (N=38) (see Appendix Table). From both treatment groups we excluded patients enrolled in a Medicare managed care plan (HMO) and those who did not have continuous Medicare coverage from one year prior to diagnosis through death or end of follow-up. Patients diagnosed only at the time of death, who had a history of another malignancy or who had distant metastases at diagnosis were also excluded.
Outcomes
We evaluated predictors and trends in the use of TL vs. CTRT, differences between these modalities in treatment-related complications and overall survival as well as the rate of salvage surgery in those receiving CTRT. Complications included mucositis, xerostomia, dysphagia, esophageal dilation, esophagitis, pneumonia, sepsis, and venous or pulmonary thromboembolism. These complications were identified by relevant diagnosis and procedure codes between the date of first treatment and death or end of follow-up (see Appendix Table). The number of patients who had an inpatient admission or emergency room (ER) visit for any of the above diagnoses was also identified. Tracheostomy and feeding tube placements were evaluated overall as well as prior to and following treatment initiation. Long-term feeding tube used was based on claims in the Durable Medical Equipment (DME) file for nutritional support or a claim for subsequent feeding tube insertion more than 1 year after initial tube insertion. The rate of salvage surgery in the CTRT group was defined as a claim for TL more than 6 months after diagnosis.
Covariates
Demographic characteristics included patient age, race, geographic location, marital status and residence in a metropolitan versus non-metropolitan county. Median income in the census tract of residence was used as a marker of socioeconomic status, and classified in quartiles. Disease characteristics included clinical tumor stage, site of disease in the larynx, lymph node involvement and year of diagnosis. Comorbidity was estimated using a modification of the Charlson comorbidity index based on inpatient, outpatient and physician claims in the year prior to laryngeal cancer diagnosis.21, 22
Statistical Analysis
Multivariable logistic regression was used to estimate the impact of demographic and clinical characteristics on the likelihood of receiving CTRT relative to TL. The Cochran-Armitage trend test was used to evaluate changes over time in the proportion of patients receiving TL vs. CTRT.
Differences between groups in treatment complications were assessed with chi-square or Fisher's exact tests, as appropriate based on cell counts. We estimated the association between primary treatment modality and the risk of death from any cause using propensity score methods to minimize bias related to the non-random assignment of treatment.23, 24 The propensity to receive surgery was modeled as a function of age, sex, race, census tract median income, marital status, urban-rural residence, geographic region, tumor site, T classification, lymph node involvement, comorbidity score and year of diagnosis using multivariable logistic regression. Death from any cause was estimated in two proportional hazards regression models: one with the propensity score included as a continuous covariate, and the other stratified by propensity score quintiles.25 In sensitivity analyses we examined the impact of excluding patients with T4 disease.26 All analyses were performed in SAS version 9.2 (SAS Institute, Cary, NC).
Results
Cohort Characteristics and Treatment
We identified 759 patients with advanced laryngeal cancer of whom 57% received CTRT and 43% had primary surgery (Table 1). Almost 60% of patients with T4 disease and 34% with T3 disease received TL, respectively. Of those who had T4 disease, 95% were classified as T4a laryngeal cancer. The proportion of patients receiving surgery decreased significantly over the study period (p<0.0001), and this finding persisted even when we excluded cases diagnosed prior to 2000 when several new registries were added to the SEER program (Figure 1). Adjusted for other characteristics, patients with T4 disease had greater odds of receiving TL while those with supraglottic cancers had lower odds of TL (Table 2).
Table 1. Characteristics of cohort by initial treatment modality.
| TL | CTRT | P | |||
|---|---|---|---|---|---|
| No. of patients | % | No. of patients | % | ||
| Total | 328 | 100 | 431 | 100 | |
|
| |||||
| Age at diagnosis | |||||
| 66-69 | 114 | 35 | 151 | 35 | NS |
| 70-74 | 100 | 30 | 139 | 32 | |
| 75-59 | 64 | 20 | 84 | 19 | |
| 80-84 | 34 | 10 | 41 | 10 | |
| 85+ | 16 | 5 | 16 | 4 | |
|
| |||||
| Sex | |||||
| Male | 254 | 77 | 329 | 76 | NS |
| Female | 74 | 23 | 102 | 24 | |
|
| |||||
| Race | |||||
| White | 262 | 80 | 351 | 81 | NS |
| Black | 45 | 14 | 48 | 11 | |
| Other | 21 | 6 | 32 | 7 | |
|
| |||||
| Census tract median income | |||||
| 1st quartile | 95 | 29 | 94 | 2 | NS |
| 2nd quartile | 75 | 23 | 115 | 27 | |
| 3rd quartile | 84 | 26 | 107 | 26 | |
| 4th quartile | 74 | 23 | 115 | 27 | |
|
| |||||
| Urban-rural residence | |||||
| Metropolitan | 264 | 80 | 365 | 85 | NS |
| Non-metropolitan | 64 | 20 | 66 | 15 | |
|
| |||||
| Region | |||||
| Northeast | 71 | 22 | 117 | 27 | NS |
| South | 77 | 23 | 92 | 21 | |
| Midwest | 51 | 16 | 54 | 13 | |
| West | 129 | 39 | 168 | 39 | |
|
| |||||
| Marital Status | |||||
| Married | 161 | 49 | 225 | 52 | NS |
| Not Married | 149 | 45 | 191 | 44 | |
| Unknown | 18 | 5 | 15 | 3 | |
|
| |||||
| Site | |||||
| Glottis | 116 | 35 | 113 | 26 | <0.0001 |
| Supraglottis | 125 | 38 | 267 | 62 | |
| Other | 87 | 27 | 51 | 12 | |
|
| |||||
| Clinical T-stage | |||||
| T1/T2 | 29 | 9 | 125 | 29 | <0.0001 |
| T3 | 112 | 34 | 188 | 44 | |
| T4 | 187 | 57 | 118 | 27 | |
|
| |||||
| Lymph node involvement | |||||
| Negative | 161 | 49 | 177 | 41 | NS |
| Positive | 152 | 46 | 237 | 55 | |
| Unknown | 15 | 5 | 17 | 4 | |
|
| |||||
| Charlson comorbidity score | |||||
| 0 | 184 | 56 | 235 | 55 | NS |
| 1 | 82 | 25 | 123 | 29 | |
| 2+ | 62 | 19 | 73 | 17 | |
|
| |||||
| Year of diagnosis | |||||
| 1999 | 28 | 9 | - | <5 | <0.0001 |
| 2000 | 47 | 14 | - | <10 | |
| 2001 | 51 | 16 | 43 | 10 | |
| 2002 | 38 | 12 | 43 | 10 | |
| 2003 | 36 | 11 | 42 | 10 | |
| 2004 | 36 | 11 | 54 | 13 | |
| 2005 | 27 | 8 | 63 | 15 | |
| 2006 | 40 | 12 | 69 | 16 | |
| 2007 | 25 | 8 | 71 | 16 | |
Abbreviations: TL, total laryngectomy; CTRT, chemoradiation; NS: Not statistically significant at p<0.05
Notes: Other site includes subglottis, overlapping tumors and cancer of the larynx not otherwise specified (NOS). Of those who had T4 disease, 95% had T4a and 5% T4b disease, respectively. Cell counts <11 not shown in accordance with the SEER-Medicare Data Use Agreement.
Figure 1. Trends in total laryngectomy by year of diagnosis.
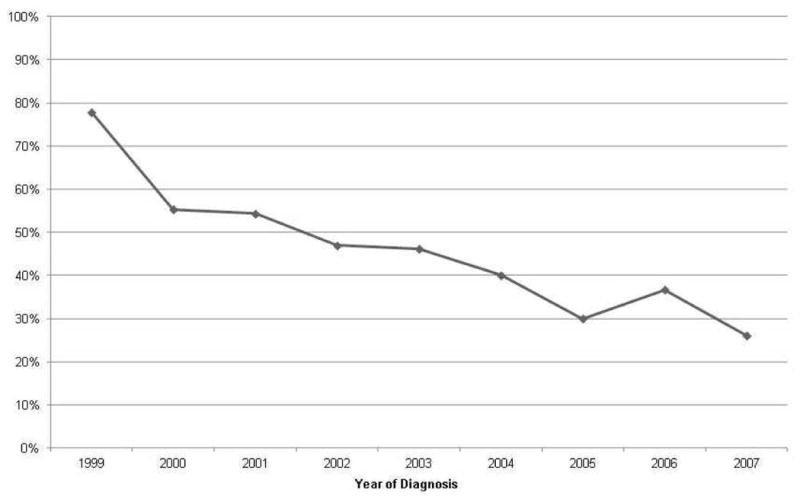
Table 2. Adjusted associations between patient characteristics and receipt of TL (vs. CTRT).
| Characteristic | Adjusted Odds Ratio (95% CI) | ||||
|---|---|---|---|---|---|
|
| |||||
| Age at diagnosis | |||||
| 66-69 | Ref | ||||
| 70-74 | 0.88 (0.59 - 1.31) | ||||
| 75-59 | 1.09 (0.69 - 1.72) | ||||
| 80-84 | 1.06 (0.59 - 1.89) | ||||
| 85+ | 1.14 (0.5 - 2.6) | ||||
|
| |||||
| Sex | |||||
| Male | Ref | ||||
| Female | 0.99 (0.66 - 1.47) | ||||
|
| |||||
| Race | |||||
| White | Ref | ||||
| Black | 1.13 (0.66 - 1.94) | ||||
| Other | 0.67 (0.35 - 1.31) | ||||
|
| |||||
| Census tract median income | |||||
| 1st quartile | Ref | ||||
| 2nd quartile | 0.8 (0.5 - 1.29) | ||||
| 3rd quartile | 0.74 (0.45 - 1.22) | ||||
| 4th quartile | 0.8 (0.46 - 1.38) | ||||
|
| |||||
| Urban-rural residence | |||||
| Metro | Ref | ||||
| Non-metropolitan | 1.25 (0.77 - 2.06) | ||||
|
| |||||
| Region | |||||
| Northeast | Ref | ||||
| South | 1.19 (0.7 - 2.02) | ||||
| Midwest | 1.31 (0.74 - 2.32) | ||||
| West | 1.24 (0.8 - 1.92) | ||||
|
| |||||
| Married | |||||
| Yes | Ref | ||||
| No | 1.01 (0.72 - 1.44) | ||||
| Unknown | 1.48 (0.67 - 3.3) | ||||
|
| |||||
| Site | |||||
| Glottis | Ref | ||||
| Supraglottis | 0.61 (0.41 - 0.91) | ||||
| Other | 1.68 (1.05 - 2.7) | ||||
|
| |||||
| Clinical T-stage | |||||
| T1/T2 | Ref | ||||
| T3 | 2.47 (1.42 - 4.3) | ||||
| T4 | 5.62 (3.26 - 9.67) | ||||
|
| |||||
| Lymph node involvement | |||||
| Negative | Ref | ||||
| Positive | 1.18 (0.81 - 1.73) | ||||
| Unknown | 0.64 (0.28 - 1.46) | ||||
|
| |||||
| Charlson comorbidity score | |||||
| 0 | Ref | ||||
| 1 | 1.04 (0.71 - 1.53) | ||||
| 2+ | 1.37 (0.88 - 2.13) | ||||
|
| |||||
| Year of diagnosis | 0.81 (0.75 - 0.86) | ||||
Abbreviations: TL, total laryngectomy; CTRT, chemoradiation; CI, confidence interval
Notes: Other site includes subglottis, overlapping tumors and cancer of the larynx not otherwise specified.
Complications and Salvage Surgery
While a significantly greater proportion of CTRT patients had claims for mucositis, xerostomia and dysphagia (P<0.0001), the proportion of patients requiring a hospital or ER admission for a treatment-related complication was comparable between the treatment groups (Table 3).
Table 3. Treatment-related complications by initial treatment modality.
| Any claim for | Initial Treatment Modality | P | |||
|---|---|---|---|---|---|
| TL | CTRT | ||||
| No. of patients | % | No. of patients | % | ||
| Mucositis | 15 | 5 | 73 | 17 | <0.0001 |
| Xerostomia | 14 | 4 | 55 | 13 | <0.0001 |
| Dysphagia | 229 | 70 | 359 | 83 | <0.0001 |
| Esophageal stricture requiring dilation | 93 | 28 | 112 | 26 | NS |
| Esophagitis | 64 | 20 | 90 | 21 | NS |
| Pneumonia | 212 | 65 | 257 | 60 | NS |
| Sepsis | 83 | 25 | 127 | 29 | NS |
| Venous/pulmonary TE | 43 | 13 | 71 | 16 | NS |
| Inpatient admission or ED visit for any diagnosis above | 210 | 64 | 290 | 67 | NS |
| Tracheostomy | |||||
| Any time after diagnosis* | 328 | 100 | 174 | 40 | |
| Pre-treatment only | 61 | 19 | 94 | 22 | |
| Post-treatment only | --- | --- | 80 | 19 | |
| Gastrostomy | |||||
| Any time after diagnosis | 128 | 39 | 313 | 73 | |
| Pre-treatment only | 21 | 6 | 68 | 16 | <0.0001 |
| Post-treatment only | 107 | 33 | 245 | 57 | <0.0001 |
| Nutritional support | |||||
| Any time after diagnosis | 64 | 20 | 167 | 39 | <0.0001 |
| ≥1 year after tube placement | 22 | 7 | 60 | 14 | <0.01 |
Abbreviations: TL, total laryngectomy; CTRT, chemoradiation; TE, thromboembolic event
Notes: P-values from unadjusted Fisher's exact test or chi-square test of association between initial treatment modality and complication. Claims for nutritional support identified only patients with a post-treatment claim for gastrostomy tube placement.
All TL patients have a tracheal stoma upon completion of the surgical procedure.
Fifty-seven percent of CTRT patients had a feeding tube inserted following treatment initiation compared with 33% of TL patients (p<0.0001, Table 3). Fourteen percent and 7% of CTRT and TL patients were still tube dependent at one year, respectively. Twenty-two percent of CTRT patients and 19% of TL patients had a tracheostomy prior to treatment initiation and 19% of CTRT patients had a tracheostomy following treatment initiation. All TL patients have a tracheal stoma upon completion of the surgical procedure.
Eleven percent of CTRT patients (N=47) had a claim for TL following the 6-month initial treatment period and the median time to salvage TL was one year.
Survival
Unadjusted for any potential confounders, neither treatment modality was associated with a survival advantage. Controlling for the likelihood of receiving treatment, TL was associated with a 18% lower risk of death from any cause compared with CTRT, stratified by propensity score quintile (adjusted hazard ratio 0.82, p<0.05, Table 4). Results were similar when propensity score was included as a continuous covariate. Within propensity score quintiles, most characteristics were well balanced between patients who received TL and those who received CTRT (data not shown). The survival advantage associated with TL was greatest among patients in the highest propensity score quintile, and this group differed from the other quintiles in several ways. Notably, the share of patients with a T4 tumor in quintile 5 was almost three times greater than in the rest of the cohort (Figure 2). In sensitivity analyses, excluding patients with T4 disease neither treatment modality was associated with a survival advantage.
Table 4. Association between initial treatment modality and risk of death from any cause.
| Model | All Patients HR (95% CI) |
Excluding patients with T4 disease HR (95% CI) |
|---|---|---|
| Unadjusted | 0.97 (0.82 - 1.15) | 1.13 (0.89 - 1.42) |
| Adjusted | ||
| Multivariable model without propensity score | 0.78 (0.64 - 0.95) | 0.94 (0.72 - 1.23) |
| Propensity score as a continuous variable | 0.82 (0.68 - 0.99) | 0.99 (0.77 - 1.28) |
| Stratified by propensity score quintile | 0.82 (0.68 - 0.99) | 1.03 (0.80 - 1.32) |
Abbreviations: HR, hazard ratio; CI, confidence interval
Adjusted HR for impact of TL (compared with CTRT) on risk of death, adjusted for propensity to receive TL. Propensity score estimated as function of age, sex, race, census tract median income, marital status, urban-rural residence, geographic region, tumor site, clinical T stage, lymph node involvement, comorbidity score and year of diagnosis.
Figure 2.
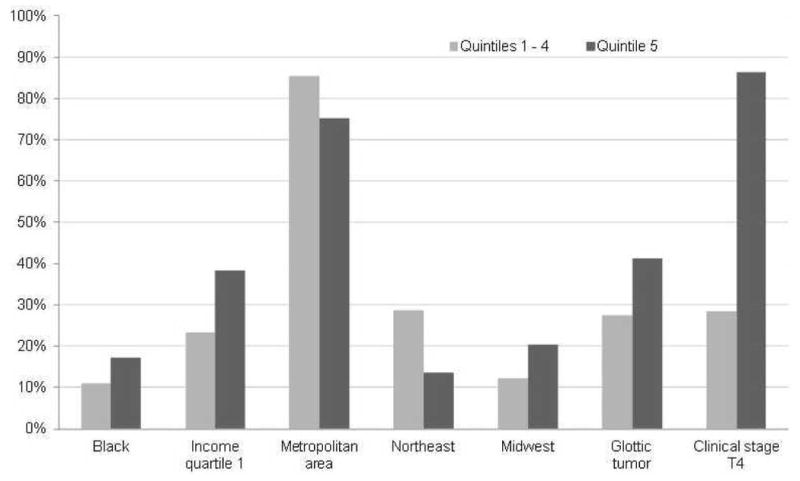
Selected characteristics of patients in propensity score quintile 5 compared with quintiles 1-4*
Legend
*Quintile 5 represents patients with the highest predicted probability of receiving a total laryngectomy, based on age, sex, race, census tract median income, marital status, urban-rural residence, geographic region, tumor site, clinical T stage, lymph node involvement, comorbidity score and year of diagnosis.
Income quartile 1 is lowest quartile of median income in census tract of residence.
Discussion
Following the publication of reports supporting CTRT in advanced laryngeal cancer, specifically in T3 disease, the use of TL has declined significantly over time. 4, 5, 9, 11, 27, 28 The decreased use of surgery we observed during the study period is not surprising. This trend presumably reflects enthusiasm for organ-preserving treatment approaches and clinical trial results suggesting oncologic outcomes comparable to those achieved with surgery.11, 27, 29, 30 While this approach has been greatly effective in many patients, lower rates of organ preservation and higher complication rates in patients with T4 disease have prompted the recommendation of primary TL in this group. 31-34 Our examination of a large, population-based cohort showed that 34% of patients with T3 disease received TL. Of significance, was the finding that a non-negligible fraction of T4 patients received organ preservation therapy (27%), despite NCCN guidelines advising TL.2, 34 These findings suggest wide variations in practice and the influence of other non-clinical factors such as physician specialty and geographic location in treatment choice10, 34-36 In fact, a Canadian population-based study reported variations in TL rates of 6% to 53% among patients with stage III and IV disease and large cause-specific survival differences among cases of potentially comparable prognoses. 36
Inferences about the association between treatment and survival in an observational setting must be made cautiously. Our analysis of survival within propensity score quintiles suggests that the greatest apparent survival advantage is derived by patients with the greatest predicted probability of receiving surgery. This subgroup included a disproportionate share of patients with T4 disease, glottic tumors, black patients and those of lower socioeconomic classes. Indeed when patients with T4 cancers were excluded from the analysis, neither treatment modality was associated with a survival benefit.5, 7 Although it is impossible to infer the cause of death from this analysis, these survival outcomes may be explained by the fact that CTRT is much less efficacious when the cartilaginous skeleton of the larynx is invaded by cancer. In addition, invasion of the laryngeal cartilaginous framework can result in an irretrievably dysfunctional larynx once treated with CTRT, thereby causing severe late toxicity, particularly in the older population. 26, 27 Perhaps more importantly, our findings suggest that patients who receive surgery have been well selected for TL, and therefore represent those most likely to benefit from it.
Acute, often severe treatment-related toxicities leading to interruption or modification of RT delivery may compromise the value of CTRT particularly among older patients and those with coexisting medical conditions or decreased performance status. 37-39,26, 40 While our study demonstrated that a greater proportion of CTRT patients experienced RT-related toxicities including mucositis, xerostomia and dysphagia, the proportion of patients experiencing esophageal stricture requiring dilation, esophagitis, sepsis, pneumonia or thrombosis did not differ significantly between the groups. More importantly, the rate of hospital admissions or ER visits between those receiving CTRT and TL was comparable.
While prolonged disease-free survival and improved cure rates are two of the most important objectives of cancer therapy, the implicit purpose of organ preservation is improved laryngeal function and quality of life.41 Unfortunately, organ preservation does not necessarily result in the preservation of function and does not correlate with the absence of chronic dysphagia, dependence on feeding tubes or a tracheostomy.14, 42, 43 Although the anticipated sequela of total laryngectomy, including a tracheostomy and loss of laryngeal speech, cause considerable physical and psychosocial morbidity to the patient, our results suggest that a number of patients initially treated with organ-preserving therapy will have both poor functional outcomes related to the inability to eat and the inability to breathe through their larynx.44, 45 Fifty seven percent of older patients, initially treated with CTRT, required a feeding tube following treatment and 14% still required nutritional support at one year. Comparable proportions of TL (19%) and CTRT (22%) patients required airway intervention prior to treatment initiation, an indication that these TL patients were appropriately treated but those receiving CTRT should, perhaps, have been more strongly guided toward surgery.
The rate of treatment failure was relatively low compared to what has been reported previously.42, 46, 47 Only 11% of CTRT patients required salvage surgery following the initial 6-month treatment period. It is possible that a portion of patients who received a tracheostomy following CTRT initiation (19%) may have done so as a result of tumor persistence or recurrence, rather than as a result of a complication of therapy.
Several limitations of our analysis should be noted. While we were able to control for important patient and tumor characteristics, there may have been residual confounding by unmeasured factors, such as pre-treatment functional status, other risk factors for complications or salvage surgery in those receiving primary CTRT, and patient and physician preferences. While propensity scores may reduce selection bias, they can only control for observed characteristics. Other, unmeasured factors may be unbalanced, resulting in biased estimates of the association between treatment and outcome.48 Additionally, we may have underestimated rates of treatment complications, as Medicare claims are likely to reflect the most serious diagnosis − those requiring physician evaluation, a hospital stay or a medical procedure.49 Finally, while the complications we identified are likely associated with physical function, social function and quality of life, we were not able to assess these important endpoints directly.
Conclusion
The objective of larynx-preservation therapy is to offer improved function and quality of life in patients with advanced laryngeal cancer, without compromising survival. Our analysis suggests that while this may be the case for many patients, TL remains an important treatment option in certain older patients, particularly patients with extensive disease. Treatment selection is complex and multifactorial, however, and individual patient factors such as functional status and personal preference, in addition to institutional factors including support services and surgical expertise, should play a fundamental role in treatment decisions.
Supplementary Material
Table 1: Site and histology codes
Table 2: Treatment and procedure codes
Table 3: Treatment-related complications codes
Acknowledgments
The authors acknowledge the efforts of the Applied Research Program, NCI; the Office of Research, Development and Information, CMS; Information Management Services (IMS), Inc.; and the Surveillance, Epidemiology, and End Results (SEER) Program tumor registries in the creation of the SEER-Medicare database.
This work was supported by the Health Research Board (Ireland) through the HRB PhD Scholars Program in Health Service Research [Grant: PHD/2007/16] and the National Cancer Institute (1K07CA118189).
Footnotes
Financial disclosures: None
Conflict of Interest: None
References
- 1.Jemal A, Siegel R, Xu J, Ward E. Cancer statistics. CA Cancer J Clin. 2010;60:277–300. doi: 10.3322/caac.20073. 2010. [DOI] [PubMed] [Google Scholar]
- 2.National Comprehensive Cancer Network. Head and Neck Cancers. NCCN Clinical Practice Guidelines in Oncology. 2011;2 [Google Scholar]
- 3.Chen AY, Fedewa S, Pavluck A, et al. Improved survival is associated with treatment at high-volume teaching facilities for patients with advanced stage laryngeal cancer. Cancer. 2010;116:4744–52. doi: 10.1002/cncr.25364. [DOI] [PubMed] [Google Scholar]
- 4.Forastiere AA, Goepfert H, Maor M, et al. Concurrent Chemotherapy and Radiotherapy for Organ Preservation in Advanced Laryngeal Cancer. New England Journal of Medicine. 2003;349:2091–8. doi: 10.1056/NEJMoa031317. [DOI] [PubMed] [Google Scholar]
- 5.Chen AY, Halpern M. Factors Predictive of Survival in Advanced Laryngeal Cancer. Arch Otolaryngol Head Neck Surg. 2007;133:1270–6. doi: 10.1001/archotol.133.12.1270. [DOI] [PubMed] [Google Scholar]
- 6.Chen AY, Schrag N, Hao Y, et al. Changes in treatment of advanced laryngeal cancer 1985-2001. Otolaryngology - Head and Neck Surgery. 2006;135:831–7. doi: 10.1016/j.otohns.2006.07.012. [DOI] [PubMed] [Google Scholar]
- 7.Gourin CG, Conger BT, Sheils WC, et al. The Effect of treatment on survival in patients with advanced laryngeal carcinoma. The Laryngoscope. 2009;119:1312–7. doi: 10.1002/lary.20477. [DOI] [PubMed] [Google Scholar]
- 8.Hoffman HT, Karnell LH, Funk GF, et al. The National Cancer Data Base report on cancer of the head and neck. Arch Otolaryngol Head Neck Surg. 1998;124:951–62. doi: 10.1001/archotol.124.9.951. [DOI] [PubMed] [Google Scholar]
- 9.Hoffman HT, Porter K, Karnell LH, et al. Laryngeal cancer in the United States: changes in demographics, patterns of care, and survival. Laryngoscope. 2006;116:1–13. doi: 10.1097/01.mlg.0000236095.97947.26. [DOI] [PubMed] [Google Scholar]
- 10.Gourin CG, Frick KD. National trends in laryngeal cancer surgery and the effect of surgeon and hospital volume on short-term outcomes and cost of care. The Laryngoscope. 2012;122:88–94. doi: 10.1002/lary.22409. [DOI] [PubMed] [Google Scholar]
- 11.Wolf G, Hong K, Fisher S, et al. Induction chemotherapy plus radiation compared with surgery plus radiation in patients with advanced laryngeal cancer. The Department of Veterans Affairs Laryngeal Cancer Study Group. N Engl J Med. 1991;324:1685–90. doi: 10.1056/NEJM199106133242402. [DOI] [PubMed] [Google Scholar]
- 12.Machtay M, Moughan J, Trotti A, et al. Factors Associated With Severe Late Toxicity After Concurrent Chemoradiation for Locally Advanced Head and Neck Cancer: An RTOG Analysis. Journal of Clinical Oncology. 2008;26:3582–9. doi: 10.1200/JCO.2007.14.8841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Michal SA, Adelstein DJ, Rybicki LA, et al. Multi-agent concurrent chemoradiotherapy for locally advanced head and neck squamous cell cancer in the elderly. Head Neck. 2012;34:1147–52. doi: 10.1002/hed.21891. [DOI] [PubMed] [Google Scholar]
- 14.Francis DO, Weymuller EA, Jr, Parvathaneni U, et al. Dysphagia, stricture, and pneumonia in head and neck cancer patients: does treatment modality matter? Ann Otol Rhinol Laryngol. 2010;119:391–7. doi: 10.1177/000348941011900605. [DOI] [PubMed] [Google Scholar]
- 15.Davis GE, Schwartz SR, Veenstra DL, et al. Cost comparison of surgery vs organ preservation for laryngeal cancer. Archives of otolaryngology--head & neck surgery. 2005;131:21–6. doi: 10.1001/archotol.131.1.21. [DOI] [PubMed] [Google Scholar]
- 16.Ganly I, Patel S, Matsuo J, et al. Postoperative complications of salvage total laryngectomy. Cancer. 2005;103:2073–81. doi: 10.1002/cncr.20974. [DOI] [PubMed] [Google Scholar]
- 17.Bourhis JS, Le Maitre, Pignon J, et al. Impact of age on treatment effect in locally advanced head and neck cancer (HNC): two individual patient data meta-analyses. J Clin Oncol. 2006;24(Suppl 18S):280s. abstract. [Google Scholar]
- 18.Pignon JP, le Maitre A, Maillard E, et al. Meta-analysis of chemotherapy in head and neck cancer (MACH-NC): an update on 93 randomised trials and 17,346 patients. Radiother Oncol. 2009;92:4–14. doi: 10.1016/j.radonc.2009.04.014. [DOI] [PubMed] [Google Scholar]
- 19.Warren JL, Klabunde CN, Schrag D, et al. Overview of the SEER-Medicare data: content, research applications, and generalizability to the United States elderly population. Med Care. 2002;40:IV-3–18. doi: 10.1097/01.MLR.0000020942.47004.03. [DOI] [PubMed] [Google Scholar]
- 20.Greene FL, Page DL, Fleming ID, Fritz A, Bakch CM, Haller DG, Morrow M, editors. AJCC Cancer Staging Manual. 6th. Springer; New York: 2002. [Google Scholar]
- 21.Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol. 1992;45:613–9. doi: 10.1016/0895-4356(92)90133-8. [DOI] [PubMed] [Google Scholar]
- 22.Charlson ME, Pompei P, Ales KL, et al. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373–83. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 23.Rosenbaum PR. Optimal Matching for Observational Studies. Journal of the American Statistical Association. 1989;84:1024–32. [Google Scholar]
- 24.Rubin DB. Estimating causal effects from large data sets using propensity scores. Annals of internal medicine. 1997;127:757–63. doi: 10.7326/0003-4819-127-8_part_2-199710151-00064. [DOI] [PubMed] [Google Scholar]
- 25.D'Agostino RB., Jr Propensity score methods for bias reduction in the comparison of a treatment to a non-randomized control group. Stat Med. 1998;17:2265–81. doi: 10.1002/(sici)1097-0258(19981015)17:19<2265::aid-sim918>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 26.Brizel DM, Esclamado R. Concurrent Chemoradiotherapy for Locally Advanced, Nonmetastatic, Squamous Carcinoma of the Head and Neck: Consensus, Controversy, and Conundrum. Journal of Clinical Oncology. 2006;24:2612–7. doi: 10.1200/JCO.2005.05.2829. [DOI] [PubMed] [Google Scholar]
- 27.Maddox PT, Davies L. Trends in total laryngectomy in the era of organ preservation: a population-based study. Otolaryngol Head Neck Surg. 2012;147:85–90. doi: 10.1177/0194599812438170. [DOI] [PubMed] [Google Scholar]
- 28.Takes RP, Strojan P, Silver CE, et al. Current trends in initial management of hypopharyngeal cancer: the declining use of open surgery. Head Neck. 2012;34:270–81. doi: 10.1002/hed.21613. [DOI] [PubMed] [Google Scholar]
- 29.Forastiere AA, Goepfert H, Maor M, et al. Concurrent chemotherapy and radiotherapy for organ preservation in advanced laryngeal cancer. N Engl J Med. 2003;349:2091–8. doi: 10.1056/NEJMoa031317. [DOI] [PubMed] [Google Scholar]
- 30.Weber RS, Berkey BA, Forastiere A, et al. Outcome of salvage total laryngectomy following organ preservation therapy: the Radiation Therapy Oncology Group trial 91-11. Arch Otolaryngol Head Neck Surg. 2003;129:44–9. doi: 10.1001/archotol.129.1.44. [DOI] [PubMed] [Google Scholar]
- 31.Hamdan J, Feldman L. Survival after organ preserving treatment for T4a laryngeal squamous cell carcinoma. Annals of Oncology. 2010;21:2292–3. doi: 10.1093/annonc/mdq562. [DOI] [PubMed] [Google Scholar]
- 32.Wolf GT. Routine Computed Tomography Scanning for Tumor Staging in Advanced Laryngeal Cancer: Implications for Treatment Selection. Journal of Clinical Oncology. 2010;28:2315–7. doi: 10.1200/JCO.2009.27.3276. [DOI] [PubMed] [Google Scholar]
- 33.National Comprehensive Cancer Network. Head and Neck Cancers. NCCN Clinical Practice Guidelines in Oncology. 2011;2 [Google Scholar]
- 34.Pfister DG, Laurie SA, Weinstein GS, et al. American Society of Clinical Oncology clinical practice guideline for the use of larynx-preservation strategies in the treatment of laryngeal cancer. J Clin Oncol. 2006;24:3693–704. doi: 10.1200/JCO.2006.07.4559. [DOI] [PubMed] [Google Scholar]
- 35.Weeks J, Pfister DG. Outcomes research studies. Oncology (Williston Park) 1996;10:29–34. [PubMed] [Google Scholar]
- 36.Groome PA, O'Sullivan B, Mackillop WJ, et al. Laryngeal cancer treatment and survival differences across regional cancer centres in Ontario, Canada. Clin Oncol (R Coll Radiol) 2011;23:19–28. doi: 10.1016/j.clon.2010.08.027. [DOI] [PubMed] [Google Scholar]
- 37.Trotti A, Bellm LA, Epstein JB, et al. Mucositis incidence, severity and associated outcomes in patients with head and neck cancer receiving radiotherapy with or without chemotherapy: a systematic literature review. Radiother Oncol. 2003;66:253–62. doi: 10.1016/s0167-8140(02)00404-8. [DOI] [PubMed] [Google Scholar]
- 38.Trotti A. Toxicity in head and neck cancer: a review of trends and issues. Int J Radiat Oncol Biol Phys. 2000;47:1–12. doi: 10.1016/s0360-3016(99)00558-1. [DOI] [PubMed] [Google Scholar]
- 39.Givens DJ, Karnell LH, Gupta AK, et al. Adverse events associated with concurrent chemoradiation therapy in patients with head and neck cancer. Archives of otolaryngology--head & neck surgery. 2009;135:1209–17. doi: 10.1001/archoto.2009.174. [DOI] [PubMed] [Google Scholar]
- 40.Brizel DM, Lydiatt W, Colevas AD. Controversies in the locoregional management of head and neck cancer. J Natl Compr Canc Netw. 2011;9:653–62. doi: 10.6004/jnccn.2011.0054. [DOI] [PubMed] [Google Scholar]
- 41.Genden EM, Ferlito A, Rinaldo A, et al. Recent changes in the treatment of patients with advanced laryngeal cancer. Head Neck. 2008;30:103–10. doi: 10.1002/hed.20715. [DOI] [PubMed] [Google Scholar]
- 42.Staton J, Robbins KT, Newman L, et al. Factors Predictive of Poor Functional Outcome after Chemoradiation for Advanced Laryngeal Cancer. Otolaryngology -- Head and Neck Surgery. 2002;127:43–7. doi: 10.1067/mhn.2002.124473. [DOI] [PubMed] [Google Scholar]
- 43.Newman LA, Vieira F, Schwiezer V, et al. Eating and weight changes following chemoradiation therapy for advanced head and neck cancer. Arch Otolaryngol Head Neck Surg. 1998;124:589–92. doi: 10.1001/archotol.124.5.589. [DOI] [PubMed] [Google Scholar]
- 44.DeSanto LW, Olsen KD, Perry WC, et al. Quality of life after surgical treatment of cancer of the larynx. Ann Otol Rhinol Laryngol. 1995;104:763–9. doi: 10.1177/000348949510401003. [DOI] [PubMed] [Google Scholar]
- 45.Williamson JS, Ingrams D, Jones H. Quality of life after treatment of laryngeal carcinoma: a single centre cross-sectional study. Ann R Coll Surg Engl. 2011;93:591–5. doi: 10.1308/147870811X13137608455253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Young VN, Mangus BD, Bumpous JM. Salvage Laryngectomy for Failed Conservative Treatment of Laryngeal Cancer. The Laryngoscope. 2008;118:1561–8. doi: 10.1097/MLG.0b013e31817c1321. [DOI] [PubMed] [Google Scholar]
- 47.Weber RS, Berkey BA, Forastiere A, et al. Outcome of Salvage Total Laryngectomy Following Organ Preservation Therapy: The Radiation Therapy Oncology Group Trial 91-11. Arch Otolaryngol Head Neck Surg. 2003;129:44–9. doi: 10.1001/archotol.129.1.44. [DOI] [PubMed] [Google Scholar]
- 48.Austin PC, Mamdani MM, Stukel TA, et al. The use of the propensity score for estimating treatment effects: administrative versus clinical data. Stat Med. 2005;24:1563–78. doi: 10.1002/sim.2053. [DOI] [PubMed] [Google Scholar]
- 49.Potosky AL, Warren JL, Riedel ER, et al. Measuring complications of cancer treatment using the SEER-Medicare data. Med Care. 2002;40:IV-62–8. doi: 10.1097/00005650-200208001-00009. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table 1: Site and histology codes
Table 2: Treatment and procedure codes
Table 3: Treatment-related complications codes


