Abstract
Tissue stem cells are found in specialized microenvironments (niches) where they are exposed to diverse systemic and local signals that are integrated with cell intrinsic factors to regulate stem cell behavior. In general, systemic signals are utilized to coordinate the response of tissues to acute or long-term changes that affect the whole organism, such as variations in nutrient availability or aging. In contrast, local signaling regulates tissue maintenance by balancing stem cell self-renewal with differentiation under homeostatic conditions and in response to local damage. In this review, we highlight the role of the JAK–STAT pathway in two Drosophila stem cell systems, the testis and intestine, and compare and contrast how activation of this pathway leads to tissue maintenance under both homeostatic conditions and in response to stress or injury.
Introduction
Adult stem cells reside in highly organized and specialized microenvironments, known as niches, within the tissues they sustain. The stem cell niche represents a complex system composed of the stem cells themselves, as well as diverse cellular and acellular components that provide inputs to regulate stem cell behavior [1]. Stem cell maintenance, survival, self-renewal and the initiation of differentiation all depend on the intimate relationship between stem cells and their niche. Therefore, local signaling must be tightly controlled to balance stem cell behavior with the demands upon the tissue.
A number of stem cell niches have been characterized in Drosophila, including the ovary, testis, and intestine, which have served as paradigms for the identification of stem cell niches in more complex mammalian systems (reviewed in [2]). Within these local microenvironments, multiple signal transduction pathways have been shown to regulate stem cell behavior, as well as the size and activity of the niche. Here we review how signaling via the JAK–STAT pathway in Drosophila is utilized in the testis and intestine to regulate stem cell behavior under homeostatic conditions and in response to damage or stress.
The JAK–STAT pathway in Drosophila
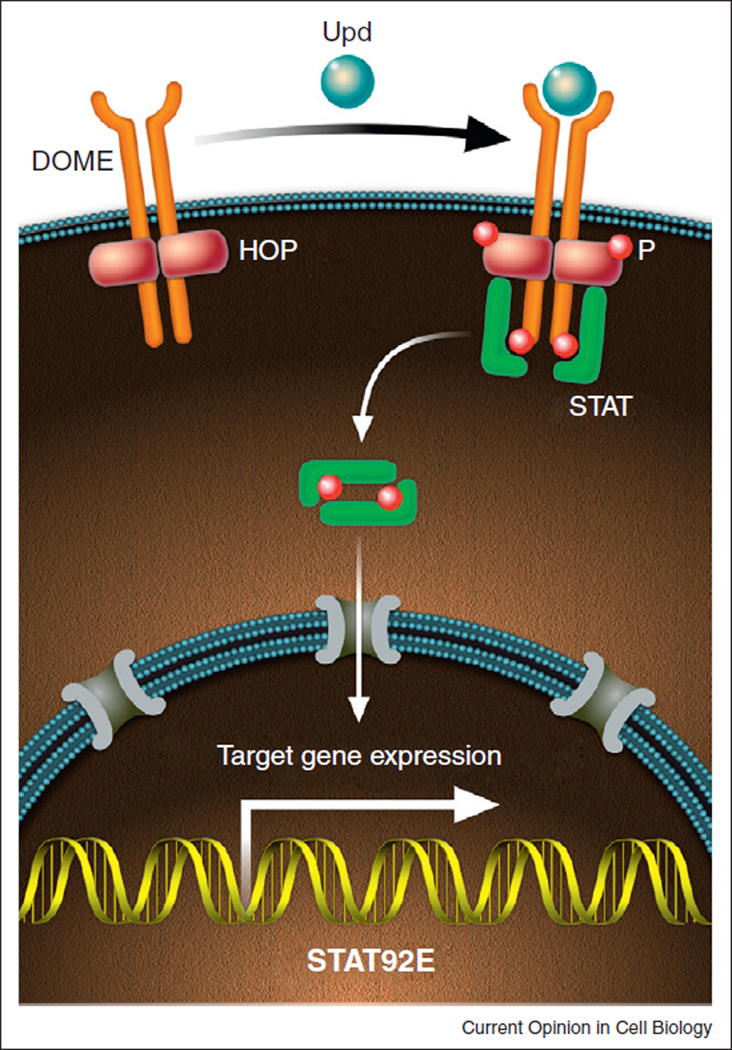
The Janus kinase (JAK)–Signal transducer and activator of transcription (STAT) pathway is an evolutionary conserved signal transduction pathway which is utilized in an array of important biological processes, such as embryonic patterning, cellular proliferation, sex determination, immunity, and regulation of stem cell behavior [3]. The study of the JAK–STAT pathway in vertebrates is complicated by the fact that it functions downstream of numerous cytokine and growth factor receptors and functional redundancy of multiple JAK and Stat homologs. In Drosophila, core components of the pathway include three ligands [Unpaired (Upd, also known as Outstretched/Os), Upd2 and Upd3], a transmembrane receptor, Domeless (Dome); one JAK, Hopscotch (Hop); and one transcription factor, STAT92E (Figure 1; [3]). Molecular and functional data indicate a high level of conservation between the structural components of the insect and mammalian pathways, which is exemplified best by the fact that hyperactivating mutations in mammalian JAKs, which are associated with leukemias and/or myleoproliferative disorders, cause similar blood cell neoplasias in flies [4] and reviewed in [5].
Figure 1.
The canonical JAK–STAT pathway in Drosophila. Binding of the ligand Upd to the transmembrane receptor Dome leads to cross phosphorylation and activation of the receptor-associated Hop kinase. Activation of Hop leads to phosphorylation of the receptor, providing a docking site for cytoplasmatic STAT. Once bound to the Dome/Hop complex, STAT also becomes phosporylated, resulting in the formation of stable dimers that translocate to the nucleus to activate gene expression.
JAK–STAT signaling is initiated in flies when an extracellular ligand, such as Upd, binds to Dome, causing a conformational change that results in phosphorylation and activation of the associated JAKs. JAK phosphorylation creates a docking site for cytoplasmatic STATs. Once bound to the receptor/JAK complex, STATs become phosphorylated, allowing the formation of STAT dimers, which translocate to the nucleus to activate transcription of downstream targets (Figure 1). Conserved negative regulators of the pathway also exist, such as Suppressors of cytokine signaling (SOCS) and Protein inhibitors of activated Stats (PIAS) [3].
The Drosophila testis
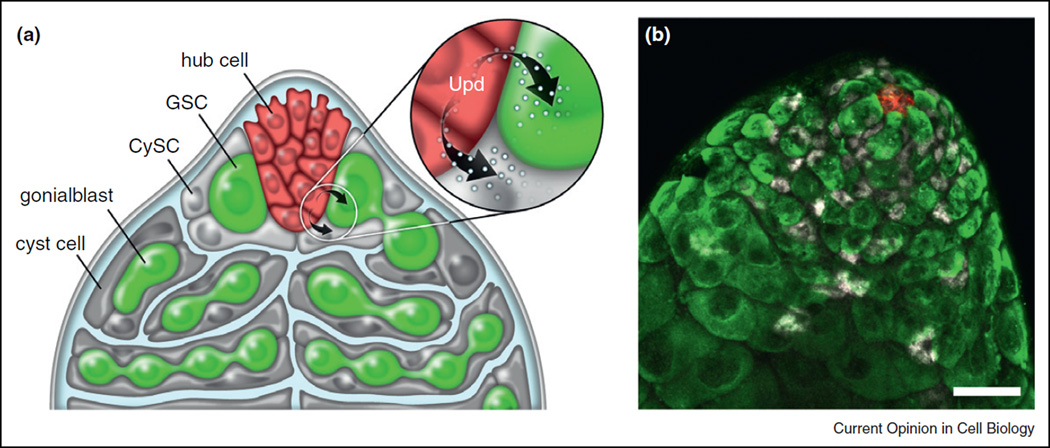
The process of spermatogenesis in Drosophila provides an excellent system to study the role of local signaling in the regulation of stem cell behavior, as two populations of stem cells are located within the same niche at the apical tip of the Drosophila testis, providing a means to compare and contrast how different stem cells respond to the same local signals [6,7]. Germline stem cells (GSCs) arise from primordial germ cells, which form at the posterior end of the developing embryo and follow a programmed migration to coalesce with the somatic component of the gonad [8], while cyst stem cells (CySCs) are derived from a cluster of somatic gonadal precursors present in the embryonic gonad [9]. In the adult, both stem cell populations surround and are in direct contact with a cluster of approximately 10 post-mitotic somatic cells called the hub (Figure 2). Whereas GSCs sustain spermatogenesis, CySCs produce cyst cells that encapsulate the maturing germ cells and ensure differentiation [10–12]. In addition, clonal analysis has demonstrated that CySCs have the potential to generate cells that contribute to the hub, which is a critical component of the stem cell niche in the testis [13–15].
Figure 2.
JAK–STAT signaling in the male germ line. (a) Schematic diagram of the apical tip of the testis. Hub cells (red) Germline stem cells (GSC, green) and Cyst stem cells (CySCs, light gray) are in direct contact with hub cells. Inset highlights the hub as a source of Upd. (b) Immunofluorescence image of the testis apex. Hub cells are marked by expression of the cell surface marker Fasciclin III (FasIII; red). Germ cells are visualized by the germ cell specific marker Vasa (green), and CySCs and cyst cells are apparent based on expression of the transcription factor Traffic-Jam (Tj; white) [54].
JAK–STAT signaling in the testis
Early studies revealed that hub cells specifically produce and secrete Upd, which activates the JAK–STAT pathway in adjacent stem cells to regulate stem cell behavior. Loss of function mutations in hop or clonal analysis with null alleles of STAT92E resulted in loss of both stem cell populations (GSCs and CySCs), whereas ectopic activation of the pathway led to an expanded number of cells that resemble GSCs and CySCs [13,14]. Upd is produced by and secreted from hub cells and can activate JAK–STAT signaling in a non-autonomous manner; however, biochemical studies indicated that the protein is glycosylated and sticks tightly to the extracellular matrix, potentially limiting its diffusion [16,17•]. Interestingly, whereas ectopic expression of upd in germ cells leads to overproliferation of both GSCs and CySCs, forced expression of upd in hub cells does not result in stem cell overproliferation [18], suggesting that hub cells may possess factors that are responsible for modifying Upd in such a way as to limit diffusion. These data also highlight the importance of extracellular matrix as component of stem cell niches.
Therefore, the biochemical properties of the secreted ligand, in combination with restricted expression to a small subset of cells, creates a limited signaling environment localized strategically at the tip of the testis. Accordingly, JAK–STAT activation is apparent only in cells in close proximity to the hub [18,19]. Both CySCs and GSCs possess mechanisms to orient mitotic spindles perpendicular to hub cells to facilitate an asymmetric outcome to stem cell divisions: upon stem cell division, one daughter cell remains adjacent to the hub and close to the source of Upd, while the other daughter cell is displaced away from the hub and initiates differentiation (Figure 2) [20,21].
Elegant studies subsequently demonstrated that activation of the JAK–STAT pathway in CySCs was sufficient to drive proliferation of both CySCs and GSCs, suggesting the presence of another, secreted signal from somatic cells that regulates the behavior of GSCs [22,23••]. Two genes, Zfh-1 and chinmo, were recently identified as important downstream targets of STAT92E in somatic cells [22,24]. Clonal analysis revealed that both genes are required for CySC self-renewal, and overexpression of either in somatic cells resulted in expansion of both soma and germ line [22,24]. These studies indicate that GSC self-renewal is not solely dependent upon the hub and that factors from neighboring CySCs, likely downstream targets of STAT92E, play a critical role in regulating GSC behavior. In addition, the BMP pathway has been proposed to be involved in this cross-talk; however, it is not yet clear whether the signals are derived from the cyst lineage, hub cells or both [23••,25]. Thus, although data indicate that both stem cell populations depend upon the JAK–STAT pathway for maintenance and are competent to respond to Upd, current models suggest that the primary role of the JAK–STAT pathway is to regulate the self-renewal of CySCs directly, which then regulate the proliferation of GSCs in a non-autonomous manner [22,23••]. In addition, activation of JAK–STAT signaling in GSCs directly may regulate adhesion to hub cells [23••]. Modulation of the strength of JAK–STAT signaling throughout the niche, accomplished via negative regulators such as Socs36E, also influences the balance of signaling between soma and germ line [26••].
Future studies should determine how Stat function is regulated differentially in somatic and germ line cells, resulting in the activation of distinct targets unique to each cell type. Such specificity may be achieved by the presence of cell type-specific co-factors or epigenetic modifications that restrict access to promoter regions. Furthermore, it will be interesting to identify factors that are involved in regulating the expression and diffusion of Upd from hub cells, as well as the potential role of other Upd family members, which appear to vary in intensity and duration of JAK–STAT activation [17•]. Lastly, future studies should also reveal the mechanisms by which additional signals, such as the BMP/TGF-β pathway, are integrated with JAK–STAT signaling to regulate tissue homeostasis in the testis [10,23••,27,28].
The Drosophila intestine
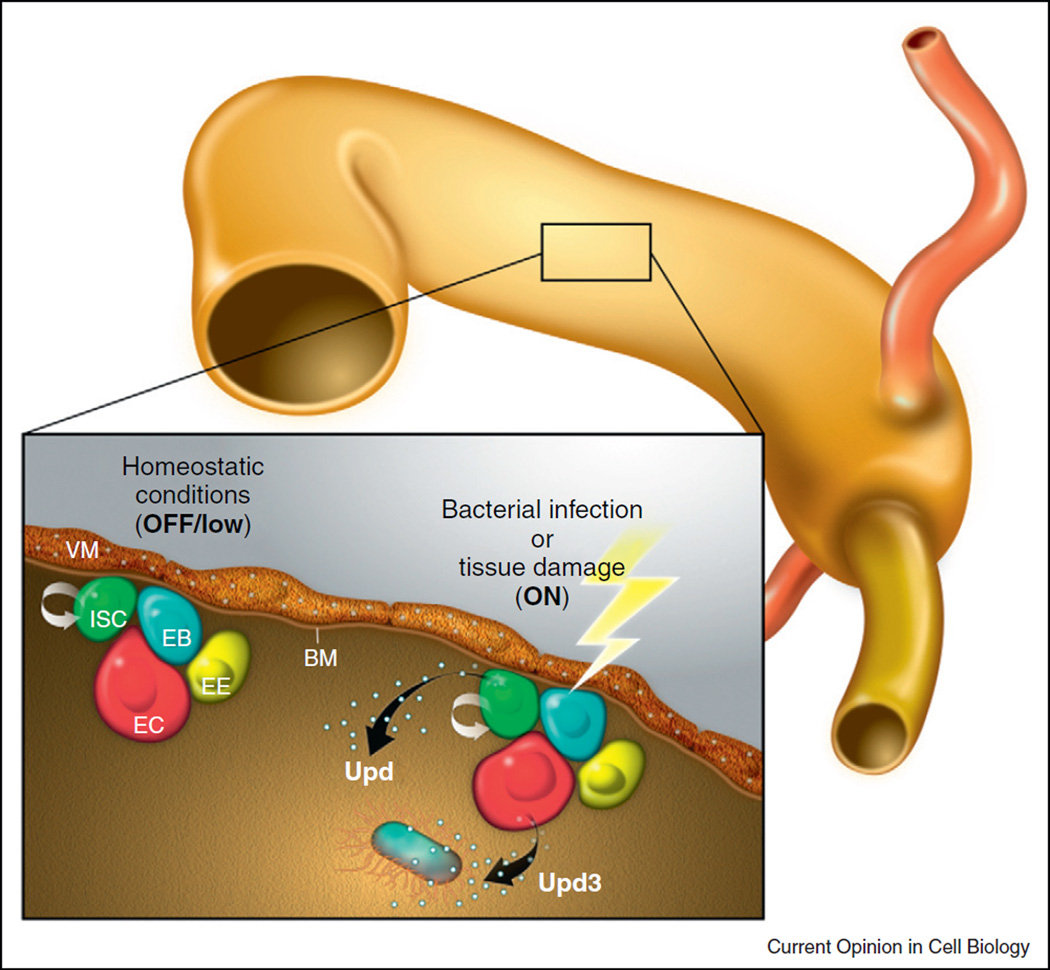
The characterization of intestinal stem cells (ISCs) in the Drosophila posterior midgut has provided an additional, versatile model for the study of mechanisms regulating somatic stem cell behavior [29,30] reviewed in [31]. ISCs are located basally, immediately adjacent to the basement membrane, in close proximity to visceral muscle (Figure 3). In contrast to the testis, ISCs do not reside within a stromal niche adjacent to support cells such as the apical hub [32]. These multipotent ISCs can divide and give rise to a new ISC (self-renewal) and to a population of progenitor cells called enteroblasts (EBs), and recent studies have revealed both symmetric and asymmetric modes of ISC division in response to growth stimuli [33]. Enteroblasts differentiate into either a secretory enteroendocrine cell (EE) that expresses the transcription factor Prospero (Pros) or an absorptive enterocyte (EC), which undergoes endoreplication to become polyploid (Figure 3). In intestines from young flies, the ratio of enteroendocrine cells to enterocytes is roughly 1:9 [30,34].
Figure 3.
JAK–STAT signaling in the posterior midgut. Schematic illustration of the midgut epithelium: Intestinal stem cells (ISCs, green), enteroblasts (EBs, blue), enteroendocrine cells (EEs, yellow), and enterocytes (ECs, red). Under homeostatic conditions, Upd is expressed in the adjacent visceral musculature and activates JAK–STAT signaling in ISCs and EBs. After tissue injury Upd cytokines are upregulated: Upd becomes expressed in progenitor cells (ISCs and EBs), while Upd3 is secreted by enterocytes.
Signaling in the Drosophila midgut
A number of signaling pathways have been implicated in the regulation of ISC behavior and EB differentiation under both homeostatic conditions and in response to aging, bacterial infection, and other environmental changes. For example, ISCs express the Notch ligand Delta (Dl), which activates Notch signaling in the EB daughter to induce differentiation. Accordingly, loss of Notch signaling in the EB causes a hyperplastic phenotype due to ISC-like cells that fail to differentiate and continue proliferating [30,34]. Moreover, it has been shown that the level of N pathway activation in EBs determines their fate (EC vs. EE): ISCs with low Dl expression are typically associated with prospective EE cells that express the Pros+, whereas ISCs with abundant Dl expression are associated with Pros−, presumptive ECs [34,35].
Numerous other factors, in addition to Notch, have been demonstrated to influence the behavior of ISCs and regulate differentiation of progenitor cells. For example, Wingless, which is secreted from the underlying visceral muscle, has been shown to regulate self-renewal of adjacent ISCs [36–38], and signaling via the EGF receptor pathway has been implicated in influencing ISC behavior under homeostatic conditions and in response to stress [38–41]. In addition to local signaling, systemic signals also contribute to the regulation of ISC behavior and gut homeostasis, as signaling via the Drosophila Insulin receptor (dInR) has been shown to regulate ISC proliferation and maintenance [33,42,43].
JAK–STAT signaling in the intestine
In addition to the signaling pathways described above, the JAK–STAT pathway also plays an important role in the regulation of ISC proliferation and tissue homeostasis in the intestine. In young, healthy flies, expression of the Upd ligand has been detected in visceral muscle cells, as revealed by staining with antibodies and promoter reporters (upd-lacZ) [44]. Accordingly, activation of the JAK–STAT pathway is evident in ISCs and EBs, but not EEs and ECs [44,45••,46••,47,48]. These data suggest that Upd diffusion from the muscle could signal to ISCs and, thus, act as a local, secreted signal to influence stem cell behavior, similar to its role in the male germ line.
However, JAK–STAT signaling also plays a prominent role in regulating ISC proliferation in response to damage or bacterial infection in the intestine, in concert with the stress-sensing Jun N-terminal Kinase (JNK) pathway. Jiang et al. elegantly demonstrated that ISC proliferation is stimulated as a consequence of signaling by the damaged ECs that line the lumen of the intestine [45••]. In this way, ISC proliferation is perfectly balanced with the need for replacement of terminally differentiated cell types. Interestingly, reporter lines reveal that Upd expression, which can normally be detected in visceral muscle cells, is now observed in enterocytes, and data indicate that damage leads to increased expression of all three upd family members (upd, upd2, and upd3) in the intestine, as measured by quantitative PCR [45••,46••,49], with upd3 being the most highly upregulated of the three [45••,49]. Notably, the Hippo (Hpo) pathway, an evolutionarily conserved pathway implicated in organ size control and tumorigenesis, has been shown to induce expression of Upd and EGFR ligands in response to damage, both of which would stimulate ISC proliferation [50•,51•,52•]. This transient increase in secreted ligands that stimulate ISC division serves as a remarkably elegant strategy to replenish injured cells in a timely fashion to mediate tissue homeostasis.
In summary, low level signaling, mediated by secretion from the visceral muscle, contributes to homeostatic turnover of the tissue, whereas upregulation of ligand expression, in response to damage or bacterial infection, leads to rapid tissue regeneration and maintenance of integrity of the intestinal epithelium. This dual role of JAK–STAT signaling Drosophila midgut underscores the plasticity and dynamic nature of stem cell niches and provides a paradigm for how normal homeostatic signaling can be modulated to accommodate tissue replacement and repair.
Conclusions and perspectives
Using Drosophila as model system to study local signaling within stem cell niches has provided unique insights into how the JAK–STAT pathway can be used to regulate stem cell behavior in multiple tissues both under homeostatic conditions and in response to injury or stress. Moreover, these studies have provided fundamental insight into how local signaling pathways are used to coordinate stem cell behavior with the demand for replacement of differentiated cells.
In both the testis and intestine, stem cells rely on production of Upd by support cells within the niche (hub cells in the testis and visceral muscle in the posterior midgut) for a basal level of pathway activation and a normal homeostatic turnover of the tissue. However, whereas stem cells within the testis appear to be exposed to a single, localized source of Upd, multiple cells within the intestinal niche can signal to ISCs via Upd and Upd3. It will be interesting to learn whether common targets of STAT92E, in addition to Socs36E and Dome, are expressed in GSCs, CySCs, and ISCs or whether cell type-specific strategies are utilized to elicit different transcriptional outputs. Future studies should also focus on elucidating the roles of other Upd family members in the male germ line and comparing and contrasting posttranslation modification of the Upd proteins that could affect association with the extracellular matrix and ultimately diffusion throughout the niche.
Decreased signaling or pathway inactivation could result in progressive stem cell loss and tissue degeneration, such as what is observed in the testis of aging males [18]. Conversely, an increase in the intensity and/or duration of pathway activation stimulates an increase in stem cell activation in response to damage or infection, to replace damaged enterocytes. Interestingly, in aged flies, precocious activation of ISC proliferation coupled with a block in terminal differentiation of EBs leads to disruption of epithelial integrity (reviewed in [53]), which closely resembles a chronic inflammatory response. Thus, the mechanism by which pathway activation returns to baseline, once damaged cells have been replaced, is of utmost importance. Similar to what occurs in the testis, upregulation of negative regulators of the pathway, such as SOCS36E, likely suppresses signaling to attenuate the response to injury or infection [26••,45••].
As outlined above, the JAK–STAT pathway does not act in isolation to regulate stem cell behavior within the niche. Therefore, one challenge will be to understand how local signals from multiple pathways, sometimes providing antagonistic instructional cues, are perceived by stem cells and integrated with systemic and cell autonomous factors resulting in cell fate decisions. Future studies will continue to elucidate how crosstalk between all of the pathways that regulate stem cell proliferation and maintenance via local signaling ultimately converge to regulate tissue homeostasis and regeneration.
Stem cell niches in invertebrate and vertebrate models share many common features, including architectural organization, cellular and acellular components, and key signaling molecules, such as the JAK–STAT pathway (reviewed in [2]). For example, stem cells in the murine small intestine, the trachea, muscle satellite cells, and long-term hematopoietic stem cells all increase their turnover rate in response to injury. Basic mechanisms that regulate stem cell behavior to accommodate varying tissue demands, such as those discussed above, provide important insights into common strategies also used by mammalian systems (reviewed in [31]). Therefore, knowledge from studies in Drosophila will continue to enhance our understanding of how stem cell deregulation could contribute to disease initiation and progression, and ultimately, result in better therapeutic strategies for regenerative medicine.
Acknowledgements
We would like to thank Jaime Simon for the artwork and an anonymous reviewer for comments on the manuscript, and we apologize to those colleagues whose work could not be referenced directly due to space constraints. D.L.J. is funded by the ACS, NIH and CIRM and the Emerald and G. Harold and Leila Y. Mathers Charitable Foundations. L.P.R. is a GABBA fellow funded by the Portuguese Foundation for Science and Technology (FCT; SFRH/BD/33253/2007).
References and recommended reading
Papers of particular interest, published within the period of review, have been highlighted as:
• of special interest
• • of outstanding interest
- 1.Voog J, Jones DL. Stem cells and the Niche: a dynamic duo. Cell Stem Cell. 2010;6:103–115. doi: 10.1016/j.stem.2010.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Losick VP, Morris LX, Fox DT. Spradling A: Drosophila stem cell niches: a decade of discovery suggests a unified view of stem cell regulation. Dev Cell. 2011;21:159–171. doi: 10.1016/j.devcel.2011.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arbouzova NI, Zeidler MP. JAK/STAT signalling in Drosophila: insights into conserved regulatory and cellular functions. Development. 2006;133:2605–2616. doi: 10.1242/dev.02411. [DOI] [PubMed] [Google Scholar]
- 4.Harrison DA, Binari R, Nahreini TS, Gilman M, Perrimon N. Activation of a Drosophila Janus kinase (JAK) causes hematopoietic neoplasia and developmental defects. EMBO J. 1995;14:2857–2865. doi: 10.1002/j.1460-2075.1995.tb07285.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mathey-Prevot B, Perrimon N. Mammalian and Drosophila blood: JAK of all trades? Cell. 1998;92:697–700. doi: 10.1016/s0092-8674(00)81396-3. [DOI] [PubMed] [Google Scholar]
- 6.Hardy RW, Tokuyasu KT, Lindsley DL, Garavito M. The germinal proliferation center in the testis of Drosophila melanogaster. J Ultrastruct Res. 1979;69:180–190. doi: 10.1016/s0022-5320(79)90108-4. [DOI] [PubMed] [Google Scholar]
- 7.Fuller MT, Spradling AC. Male and female Drosophila germline stem cells: two versions of immortality. Science. 2007;316:402–404. doi: 10.1126/science.1140861. [DOI] [PubMed] [Google Scholar]
- 8.Spradling AC. Developmental genetics of oogenesis. In: Bate M, Martinez-Arias A, editors. The Development of Drosophila melanogaster. I. Cold Spring Harbor Laboratory Press; 1993. pp. 1–70. [Google Scholar]
- 9.Boyle M, DiNardo S. Specification, migration and assembly of the somatic cells of the Drosophila gonad. Development. 1995;121:1815–1825. doi: 10.1242/dev.121.6.1815. [DOI] [PubMed] [Google Scholar]
- 10.Matunis E, Tran J, Gönczy P, Caldwell K, DiNardo S. punt and schnurri regulate a somatically derived signal that restricts proliferation of committed progenitors in the germline. Development. 1997;124:4383–4391. doi: 10.1242/dev.124.21.4383. [DOI] [PubMed] [Google Scholar]
- 11.Kiger AA, White-Cooper H, Fuller MT. Somatic support cells restrict germline stem cell self-renewal and promote differentiation. Nature. 2000;407:750–754. doi: 10.1038/35037606. [DOI] [PubMed] [Google Scholar]
- 12.Tran J, Brenner TJ, DiNardo S. Somatic control over the germline stem cell lineage during Drosophila spermatogenesis. Nature. 2000;407:754–757. doi: 10.1038/35037613. [DOI] [PubMed] [Google Scholar]
- 13.Kiger AA, Jones DL, Schulz C, Rogers MB, Fuller MT. Stem cell self-renewal specified by JAK–STAT activation in response to a support cell cue. Science. 2001;294:2542–2545. doi: 10.1126/science.1066707. [DOI] [PubMed] [Google Scholar]
- 14.Tulina N, Matunis E. Control of stem cell self-renewal in Drosophila spermatogenesis by JAK–STAT signaling. Science. 2001;294:2546–2549. doi: 10.1126/science.1066700. [DOI] [PubMed] [Google Scholar]
- 15.Voog J, D‘Alterio C, Jones DL. Multipotent somatic stem cells contribute to the stem cell niche in the Drosophila testis. Nature. 2008;454:1132–1136. doi: 10.1038/nature07173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Harrison DA, McCoon PE, Binari R, Gilman M, Perrimon N. Drosophila unpaired encodes a secreted protein that activates the JAK signaling pathway. Genes Dev. 1998;12:3252–3263. doi: 10.1101/gad.12.20.3252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Wright VM, Vogt KL, Smythe E, Zeidler MP. Differential activities of the Drosophila JAK/STAT pathway ligands Upd, Upd2 and Upd3. Cell Signal. 2011;23:920–927. doi: 10.1016/j.cellsig.2011.01.020. Authors show that Upd is the most potent of the three ligands (Upd, Upd2 and Upd3) in an ex vivo system. They also show that Upd3 is a secreted molecule and capable of activating JAK/STAT signaling in vivo.
- 18.Boyle M, Wong C, Rocha M, Jones DL. Decline in self-renewal factors contributes to aging of the stem cell niche in the Drosophila testis. Cell Stem Cell. 2007;1:470–478. doi: 10.1016/j.stem.2007.08.002. [DOI] [PubMed] [Google Scholar]
- 19.Wawersik M, Milutinovich A, Casper AL, Matunis E, Williams B, Van Doren M. Somatic control of germline sexual development is mediated by the JAK/STAT pathway. Nature. 2005;436:563–567. doi: 10.1038/nature03849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yamashita Y, Jones DL, Fuller MT. Orientation of asymmetric stem cell division by the APC tumor suppressor and centrosome. Science. 2003;301:1547–1550. doi: 10.1126/science.1087795. [DOI] [PubMed] [Google Scholar]
- 21.Cheng J, Tiyaboonchai A, Yamashita YM, Hunt AJ. Asymmetric division of cyst stem cells in Drosophila testis is ensured by anaphase spindle repositioning. Development. 2011;138:831–837. doi: 10.1242/dev.057901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Leatherman JL, Dinardo S. Zfh-1 controls somatic stem cell self-renewal in the Drosophila testis and nonautonomously influences germline stem cell self-renewal. Cell Stem Cell. 2008;3:44–54. doi: 10.1016/j.stem.2008.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Leatherman JL, Dinardo S. Germline self-renewal requires cyst stem cells and stat regulates niche adhesion in Drosophila testes. Nat Cell Biol. 2010;12:806–811. doi: 10.1038/ncb2086. The authors show that STAT92E is required intrinsically in GSCs for hub attachment but not for self-renewal. A new model is proposed in which GSCs rely on BMP signaling from neighboring CySCs for self-renewal.
- 24.Flaherty MS, Salis P, Evans CJ, Ekas LA, Marouf A, Zavadil J, Banerjee U, Bach EA. chinmo is a functional effector of the JAK/STAT pathway that regulates eye development, tumor formation, and stem cell self-renewal in Drosophila. Dev Cell. 2010;18:556–568. doi: 10.1016/j.devcel.2010.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zheng Q, Wang Y, Vargas E, DiNardo S. magu is required for germline stem cell self-renewal through BMP signaling in the Drosophila testis. Dev Biol. 2011;357:202–210. doi: 10.1016/j.ydbio.2011.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Issigonis M, Tulina N, de Cuevas M, Brawley C, Sandler L, Matunis E. JAK–STAT signal inhibition regulates competition in the Drosophila testis stem cell niche. Science. 2009;326:153–156. doi: 10.1126/science.1176817. The paper demonstrates that inhibition of JAK/STAT signaling via SOCS36E within CySCs prevents them from displacing GSCs from the hub. Therefore, JAK/STAT regulates competition for stem cell occupancy within the testis stem cell niche.
- 27.Shivdasani AA, Ingham PW. Regulation of stem cell maintenance and transit amplifying cell proliferation by tgf-beta signaling in Drosophila spermatogenesis. Curr Biol. 2003;13:2065–2072. doi: 10.1016/j.cub.2003.10.063. [DOI] [PubMed] [Google Scholar]
- 28.Kawase E, Wong MD, Ding BC, Xie T. Gbb/Bmp signaling is essential for maintaining germline stem cells and for repressing bam transcription in the Drosophila testis. Development. 2004;131:1365–1375. doi: 10.1242/dev.01025. [DOI] [PubMed] [Google Scholar]
- 29.Micchelli CA, Perrimon N. Evidence that stem cells reside in the adult Drosophila midgut epithelium. Nature. 2006;439:475–479. doi: 10.1038/nature04371. [DOI] [PubMed] [Google Scholar]
- 30.Ohlstein B, Spradling A. The adult Drosophila posterior midgut is maintained by pluripotent stem cells. Nature. 2006;439:470–474. doi: 10.1038/nature04333. [DOI] [PubMed] [Google Scholar]
- 31.Biteau B, Hochmuth CE, Jasper H. Maintaining tissue homeostasis: dynamic control of somatic stem cell activity. Cell Stem Cell. 2011;9:402–411. doi: 10.1016/j.stem.2011.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Morrison SJ, Spradling AC. Stem cells and niches: mechanisms that promote stem cell maintenance throughout life. Cell. 2008;132:598–611. doi: 10.1016/j.cell.2008.01.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.O‘Brien LE, Soliman SS, Li X, Bilder D. Altered modes of stem cell division drive adaptive intestinal growth. Cell. 2011;147:603–614. doi: 10.1016/j.cell.2011.08.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ohlstein B, Spradling A. Multipotent Drosophila intestinal stem cells specify daughter cell fates by differential notch signaling. Science. 2007;315:988–992. doi: 10.1126/science.1136606. [DOI] [PubMed] [Google Scholar]
- 35.Perdigoto CN, Schweisguth F, Bardin AJ. Distinct levels of Notch activity for commitment and terminal differentiation of stem cells in the adult fly intestine. Development. 2011;138:4585–4595. doi: 10.1242/dev.065292. [DOI] [PubMed] [Google Scholar]
- 36.Lee WC, Beebe K, Sudmeier L, Micchelli CA. Adenomatous polyposis coli regulates Drosophila intestinal stem cell proliferation. Development. 2009;136:2255–2264. doi: 10.1242/dev.035196. [DOI] [PubMed] [Google Scholar]
- 37.Lin G, Xu N, Xi R. Paracrine Wingless signalling controls selfrenewal of Drosophila intestinal stem cells. Nature. 2008;455:1119–1123. doi: 10.1038/nature07329. [DOI] [PubMed] [Google Scholar]
- 38.Xu N, Wang SQ, Tan D, Gao Y, Lin G, Xi R. EGFR, Wingless and JAK/STAT signaling cooperatively maintain Drosophila intestinal stem cells. Dev Biol. 2011;354:31–43. doi: 10.1016/j.ydbio.2011.03.018. [DOI] [PubMed] [Google Scholar]
- 39.Buchon N, Broderick NA, Kuraishi T, Lemaitre B. Drosophila EGFR pathway coordinates stem cell proliferation and gut remodeling following infection. BMC Biol. 2010;8:152. doi: 10.1186/1741-7007-8-152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Biteau B, Jasper H. EGF signaling regulates the proliferation of intestinal stem cells in Drosophila. Development. 2011;138:1045–1055. doi: 10.1242/dev.056671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jiang H, Grenley MO, Bravo MJ, Blumhagen RZ, Edgar BA. EGFR/Ras/MAPK signaling mediates adult midgut epithelial homeostasis and regeneration in Drosophila. Cell Stem Cell. 2011;8:84–95. doi: 10.1016/j.stem.2010.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Biteau B, Karpac J, Supoyo S, DeGennaro M, Lehmann R, Jasper H. Lifespan extension by preserving proliferative homeostasis in Drosophila. PLoS Genetics. 2010;6(10):e1001159. doi: 10.1371/journal.pgen.1001159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Choi NH, Lucchetta E, Ohlstein B. Nonautonomous regulation of Drosophila midgut stem cell proliferation by the insulin-signaling pathway. Proc Natl Acad Sci USA. 2011 doi: 10.1073/pnas.1109348108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lin G, Xu N, Xi R. Paracrine unpaired signaling through the JAK/STAT pathway controls self-renewal and lineage differentiation of drosophila intestinal stem cells. J Mol Cell Biol. 2010;2:37–49. doi: 10.1093/jmcb/mjp028. [DOI] [PubMed] [Google Scholar]
- 45. Jiang H, Patel PH, Kohlmaier A, Grenley MO, McEwen DG, Edgar BA. Cytokine/Jak/Stat signaling mediates regeneration and homeostasis in the Drosophila midgut. Cell. 2009;137:1343–1355. doi: 10.1016/j.cell.2009.05.014. First report that Upd ligands, Upd3 in particular, are upregulated in enterocytes after damage or bacterial infection. In addition, the authors show that JAK/STAT activity promotes progenitor cell differentiation, in part by stimulating Delta/Notch signaling, both under homeostatic and regenerating conditions.
- 46. Buchon N, Broderick NA, Chakrabarti S, Lemaitre B. Invasive and indigenous microbiota impact intestinal stem cell activity through multiple pathways in Drosophila. Genes Dev. 2009;23:2333–2344. doi: 10.1101/gad.1827009. Using a reporter line, the authors show that Upd3 is induced in enterocytes in response to bacteria infection and locally after tissue damage. Together with Ref. [45••] they point to an essential role of JAK/STAT in midgut epithelial turnover.
- 47.Beebe K, Lee WC, Micchelli CA. JAK/STAT signaling coordinates stem cell proliferation and multilineage differentiation in the Drosophila intestinal stem cell lineage. Dev Biol. 2010;338:28–37. doi: 10.1016/j.ydbio.2009.10.045. [DOI] [PubMed] [Google Scholar]
- 48.Liu W, Singh SR, Hou SX. JAK–STAT is restrained by Notch to control cell proliferation of the Drosophila intestinal stem cells. J Cell Biochem. 2010;109:992–999. doi: 10.1002/jcb.22482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Buchon N, Broderick NA, Poidevin M, Pradervand S, Lemaitre B. Drosophila intestinal response to bacterial infection: activation of host defense and stem cell proliferation. Cell Host Microbe. 2009;5:200–211. doi: 10.1016/j.chom.2009.01.003. [DOI] [PubMed] [Google Scholar]
- 50. Staley BK, Irvine KD. Warts and Yorkie mediate intestinal regeneration by influencing stem cell proliferation. Curr Biol. 2010;20:1580–1587. doi: 10.1016/j.cub.2010.07.041. Identify a conserved growth control/tumor suppressor pathway that acts to upregulate Upd and EGFR ligands to regulate ISC proliferation in response to stress or damage.
- 51. Karpowicz P, Perez J, Perrimon N. The Hippo tumor suppressor pathway regulates intestinal stem cell regeneration. Development. 2010;137:4135–4145. doi: 10.1242/dev.060483. See annotation to Ref. [50•].
- 52. Ren F, Wang B, Yue T, Yun EY, Ip YT, Jiang J. Hippo signaling regulates Drosophila intestine stem cell proliferation through multiple pathways. Proc Natl Acad Sci USA. 2010;107:21064–21069. doi: 10.1073/pnas.1012759107. See annotation to Ref. [50•].
- 53.Wang L, Jones DL. The effects of aging on stem cell behavior in Drosophila. Exp Gerontol. 2011;46:340–344. doi: 10.1016/j.exger.2010.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Li MA, Alls JD, Avancini RM, Koo K, Godt D. The large Maf factor Traffic Jam controls gonad morphogenesis in Drosophila. Nat Cell Biol. 2003;5:994–1000. doi: 10.1038/ncb1058. [DOI] [PubMed] [Google Scholar]