Abstract
Background/Aims
To quantify aperiodic phonation, nonlinear dynamic methods of acoustic voice analysis, such as correlation dimension, have been shown to be useful. The purpose of this study is to evaluate the validity of nonlinear dynamic analysis as a voice analysis tool for the Parkinson’s disease (PD) treatment effects of deep brain stimulation (DBS) and levodopa.
Methods
In this study, the effects of DBS and levodopa treatment on patients with PD were measured using perturbation, nonlinear dynamic, and perceptual analysis. 19 PD patients that received bilateral (n=9), left, (n=7), or right (n=3) DBS performed sustained vowel phonations, which were recorded before- and after-medication with the stimulator-off and -on. Recordings were also taken of 10 PD patients that did not receive DBS surgery before- and after-medication to provide a baseline.
Results
A mixed two-way ANOVA (surgery, medication) generated significant positive treatment effects of DBS only in mean log-transformed D2, which was supported by mean log-transformed shimmer, vF0, and vAm.
Conclusion
These findings may indicate the validity of nonlinear dynamic analysis as a complement to perceptual analysis in clinical PD voice studies.
Keywords: Parkinson’s disease, voice, perturbation, nonlinear dynamics, perceptual, deep brain stimulation, subthalamic nucleus, levodopa
INTRODUCTION
Parkinson’s disease (PD) is a neurological disease that involves the degeneration of dopamine producing cells in the substantia nigra, causing increased inhibition of the thalamus and the brainstem locomotive center.[1] PD causes both motor and vocal impairment. Impaired Parkinsonian voice has been described as breathy, tremulous, high-pitched, monotone, quiet, and hoarse.[2,3]
The use of levodopa is a standard treatment for PD and has been shown to be effective in combating PD motor symptoms, although its effects on PD voice are inconsistent.[4–9] A new treatment for PD is deep brain stimulation (DBS) of the subthalamic nucleus (STN). DBS treatment involves using a unilateral or bilateral stimulator to target electrical signals to the STN, which is believed to disrupt brain signaling responsible for the motor and vocal impairment symptoms of PD.[1,10,11] DBS has been shown to alleviate motor symptoms, but its effects on voice are less clear.[12–14] Recent studies focusing on the use of levodopa in conjunction with DBS of the STN have argued that DBS of the STN affects neuronal structures involved in voice that are not affected by levodopa, namely non-dopaminergic lesions.[15–17] As a result, the administration of levodopa in conjunction with DBS of the STN is thought to have a greater effect on PD vocal impairment than the use of levodopa alone.[5,16] These studies have applied acoustic perturbation analysis to evaluate the effects of DBS and levodopa.[11,18,19]
Perturbation analysis, however, requires near periodicity in order to reliably extract a pitch from voice samples. Correlation dimension, produced from nonlinear dynamic analysis, can quantify aperiodic voice commonly found in PD patients. Increased correlation dimension may indicate worsening of hoarseness, which perceptually characterizes aperiodic voice.[20–25] In addition, nonlinear vocal fold models are able to capture the pathologic characteristics of PD voice, including reduced vibratory amplitude, incomplete vocal fold closure, increased phonation threshold pressure, vocal tremor, subharmonics, and vocal irregularity.[26] Thus, nonlinear dynamic analysis may be a useful clinical tool for PD voice analysis.
The purpose of this study is to investigate the usefulness of nonlinear dynamic analysis to evaluate the effects of unilateral and bilateral DBS of the STN in conjunction with levodopa on PD voice. PD voices of surgical patients are evaluated in the before-medication stimulator-off (BMSOFF), before-medication stimulator-on (BMSON), after-medication stimulator-off (AMSOFF), and after-medication stimulator-on (AMSON) situations. The results for surgical patients are compared to those for non-surgical patients in the before-medication (BM) and after-medication (AM) situations.
MATERIALS AND METHODS
Participants
The University of Wisconsin IRB and the Committee of Ethics at Shanghai Second Military Medical University Hospital approved the protocol and consent procedure used in this study. An attending neurologist recruited 19 patients diagnosed with idiopathic PD, 11 males and 8 females with an average age of 63.8 years, to undergo DBS-STN surgery (Table 1). The details of the surgery have been discussed elsewhere.[27] Nine patients had bilateral, 7 had left, and 3 had right electrode placement. The decision to undergo DBS surgery was made by the subjects as part of their clinical care independent from the interests of this study. Ten patients diagnosed with idiopathic PD, 6 females and 4 males with a mean age of 66.8 years, that did not undergo surgery were also selected (Table 2). None of the participants suffered from vocal deficits caused by diseases other than PD, symptoms outside of those common to PD as detected by laryngeal endoscopy, cognitive hearing impairment, or depression. The attending neurologist selected patients based on consistency in the criteria listed in Tables 1 and 2.
Table 1.
Gender, age, side of stimulation (STN), Hoehn-Yahr score, UPDRS-III overall motor score and UPDRS-III Item 18 (speech) score, approximate disease duration since diagnosis in years at the time the study was conducted, and medication dosage (M=Madopar, T=Trastal, SCR=Sinernet CR, C=Celance, A=Artane) for patients that received DBS surgery. Hoehn-Yahr and UPDRS-III scores were recorded before the patients underwent surgery and without medication. As a result of lack of regular health care, a few patients have very short disease durations that are inconsistent with the stage of PD because of late diagnosis or diagnosis at the time of the study.
| Patient # | Gender | Age | Side of STN | Hoehn-Yahr | UPDRS-III | UPDRS-III Item 18 (speech) | PD Duration Since Diagnosis (years) | Medication Dosage |
|---|---|---|---|---|---|---|---|---|
| 1 | Female | 68 | Both | III | 51 | 0 | 12 | 250 mg M 100 mg SCR |
| 2 | Female | 62 | Both | III | 60 | 0 | 12 | 375 mg M |
| 3 | Male | 67 | Both | IV | 108 | 2 | 14 | 500 mg SCR |
| 4 | Male | 61 | Left | III | 51 | 0 | 18 | 250 mg M |
| 5 | Male | 50 | Both | III | 78 | 1 | 5 | 625 mg M 400 mg SCR |
| 6 | Male | 56 | Both | III | 58 | 0 | 10 | 250 mg M |
| 7 | Male | 65 | Both | IV | 82 | 1 | 8 | 375 mg M |
| 8 | Female | 65 | Both | III | 79 | 1 | 4 | 687.5 mg M |
| 9 | Female | 57 | Left | V | 100 | 2 | 11 | 375 mg M 200 mg SCR 750 mg C |
| 10 | Male | 72 | Left | III | 67 | 1 | 6 | 750 mg M 500 mg C |
| 11 | Male | 76 | Left | III | 80 | 1 | 13 | 250 mg M |
| 12 | Male | 63 | Left | III | 62 | 1 | 8 | 250 mg M 100 mg SCR |
| 13 | Male | 58 | Left | IV | 84 | 1 | 10 | 250 mg M |
| 14 | Male | 77 | Both | III | 65 | 1 | 7 | 500 mg M 100 mg SCR |
| 15 | Female | 48 | Left | III | 62 | 0 | 10 | 500 mg M |
| 16 | Female | 66 | Right | III | 65 | 0 | 7 | 750 mg M |
| 17 | Female | 65 | Right | III | 65 | 0 | 6 | 562.5 mg M |
| 18 | Female | 68 | Both | IV | 78 | 1 | 10 | 750 mg M |
| 19 | Male | 69 | Right | III | 62 | 1 | 6 | 100 mg T |
| Mean | N/A | 63.8 | N/A | 3.3 (III=3, IV=4, V=5) | 71.4 | 0.7 | 9.3 | N/A |
Table 2.
Gender, age, Hoehn-Yahr score, UPDRS-III overall motor score and UPDRS-III Item 18 (speech) score, approximate disease duration since diagnosis in years at the time the study was conducted, and medication dosage (M=Madopar, T=Trastal, SCR=Sinernet CR, C=Celance, A=Artane) for non-surgical patients. Hoehn-Yahr and UPDRS-III scores were recorded without medication. As a result of lack of regular health care, a few patients have very short disease durations that are inconsistent with the stage of PD because of late diagnosis or diagnosis at the time of the study.
| Patient # | Gender | Age | Hoehn- Yahr | UPDRS-III | UPDRS-III Item 18 (speech) | PD Duration Since Diagnosis (years) | Medication Dosage |
|---|---|---|---|---|---|---|---|
| 1 | Female | 74 | III | 88 | 1 | 2 | 500 mg T 300 mg SCR |
| 2 | Female | 74 | IV | 100 | 2 | 5 | 375 mg M |
| 3 | Female | 73 | III | 76 | 1 | 3 | 375 mg M |
| 4 | Female | 59 | IV | 94 | 1 | 7 | 812.5 mg M 100 mg T |
| 5 | Female | 57 | III | 64 | 0 | 6 | 812.5 mg M 0.1375 mg C 2.5 mg A |
| 6 | Female | 77 | III | 68 | 0 | <1 | 375 mg M |
| 7 | Male | 61 | IV | 96 | 1 | 9 | 750 mg M 100 mg T |
| 8 | Male | 68 | III | 72 | 1 | 4 | 750 mg M 50 mg T |
| 9 | Male | 49 | III | 62 | 0 | 2 | 750 mg M |
| 10 | Male | 76 | IV | 86 | 2 | 4 | 500 mg M 100 mg T |
| Mean | N/A | 66.8 | 3.4 (III=3, IV=4, V=5) | 80.6 | 0.9 | 4.2 | N/A |
Double blindness
The attending neurologist recruited patients. Two research assistants collected the vocal recordings, one controlling the stimulator and administering the medication and another, blind to the stimulator and medication conditions, directing the patient to perform sustained phonations. The patient was blind to the stimulator condition but not to the medication condition. Therefore, double blindness was satisfied with respect to DBS treatment. Blindness of the research assistant was achieved with respect to medication. Another research assistant, unaware of the stimulator or medication conditions, conducted the data analysis, achieving blindness at this stage of data processing with respect to both treatment factors.
Recording procedure
For patients that received DBS, two doctors conducted the recordings in the four situations, BMSOFF, BMSON, AMSOFF, and AMSON. Recordings were taken before-medication (no medication for 12 hours) and after-medication (medication for 1 hour) with the stimulator-off (stimulator deactivated for 30 minutes) and the stimulator-on (stimulator activated for at least 12 hours). For patients that did not undergo surgery, recordings were taken in the BM (no medication for 12 hours) and AM (medication for 1 hour) situations. The smallest amount of medication was administered to achieve the best results and least side effects (Tables 1 and 2). At each time period, sustained /a/ vowel phonations of no less than 5 seconds were recorded in a sound-attenuated room using a head-mounted microphone (AKG Acoustics, Vienna, Austria) positioned at 15 cm from the mouth at a 45 degree angle. Audio files were recorded using Multispeech software (KayPENTAX, Lincoln Park, NJ) at a sampling rate of 25 kHz. The patients were asked to perform the sustained vowel phonations at their habitual loudness and pitch. For each patient, 5 replicate recordings in each of the situations were taken, and 3 replicates were randomly selected for vocal analysis. One-second segments were cut from the middle of these sustained voices, eliminating the offset and onset of phonation. These segments were processed using nonlinear dynamic, perturbation, and perceptual analysis.
Perturbation Analysis
The 3 one-second segments of sustained phonations were analyzed using CSpeech 4.0 software (Paul Milenkovic, Madison, Wisconsin). Percent jitter, percent shimmer, and signal-to-noise ratio (SNR) values were obtained for all vocal segments. The Multi-Dimensional Voice Program (MDVP), model 5105, Version 2.0 (Kay Elemetrics Corporation), was used to obtain the perturbation measures of vF0 (variability in fundamental frequency) and vAm (peak-to-peak amplitude variation).
CSpeech was also used to calculate err, which is a measure of the number of times the algorithm failed to extract a pitch period.[28] An err greater than 10 indicated an unreliable pitch period. The waveforms of samples with err values greater than 10 had type 2 (bifurcations and modulations evident) or type 3 (aperiodic and chaotic) signals. Studies have determined that perturbation analysis is only reliable for nearly periodic voice samples. Thus, percent jitter, percent shimmer, signal-to-noise ratio, vF0, and vAm values for these samples were eliminated.[29]
Nonlinear dynamic analysis
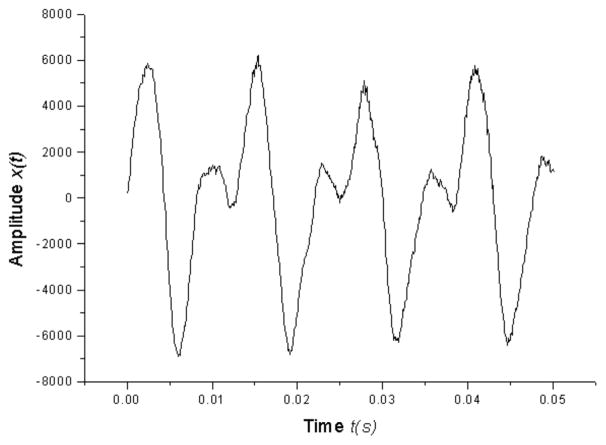
Nonlinearity in human voice production is related to the machinery of the human voice.[22] Correlation dimension, produced from nonlinear dynamic analysis, gives a quantitative estimate of the number of degrees of freedom needed to describe the dynamic system.[30] Higher dimensionality of D2 indicates a more complex system and a higher degree of vocal pathology.[21–25] The theory and usage of nonlinear dynamic methods has been discussed in previous literature.[22,24,30,31–35] Correlation dimension calculations were determined based on past research of excised larynx phonations and live human voices.[21,25,33–35] Figure 1 shows phonatory time series
Figure 1.
Parkinsonian voice acoustic waveform of a patient that received DBS in the BMSON situation.
| (1) |
| (2) |
from a PD patient that received DBS in the BMSON situation. Vocal segments were analyzed over a period of one second (figures were magnified). Figure 2 shows the phase space reconstruction (x(t), x(t +τ)) with τ as the time delay calculated by Fraser and Swinney’s mutual information method (1986). The reconstructed phase space maps the vocal fold vibrations as a function of time, qualitatively showing the dynamic behavior of a signal. Periodic and aperiodic signals produce closed and irregular trajectories respectively. In Grassberger and Procaccia’s correlation dimension (1983),
Figure 2.
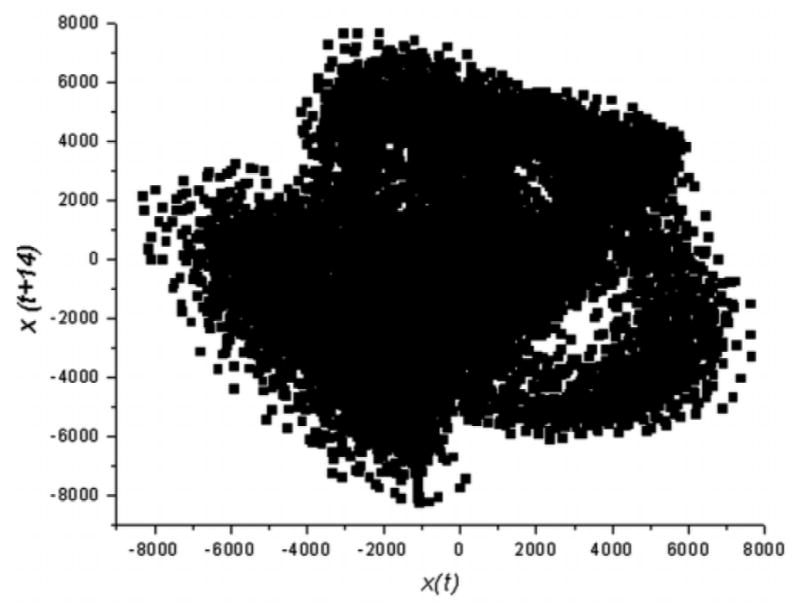
The reconstructed phase space of a Parkinsonian voice in this study.
| (3) |
| (4) |
where r is the radius around Xi and C is calculated using Theiler’s formula (1986). W is the time delay τ and
| (5) |
D2 vs. r is fit with a linear curve in the scaling region. As the embedding dimension m is increased, the slopes of these two curves converge. Figure 3 shows the D2 vs. r curves from the same PD voice. The slopes of the D2 vs. r curves approach 3.038 ± 0.004 in the scaling region, which is the estimated D2 of this voice. Using the steps outlined above, the estimated D2 values of the waveforms were obtained.
Figure 3.
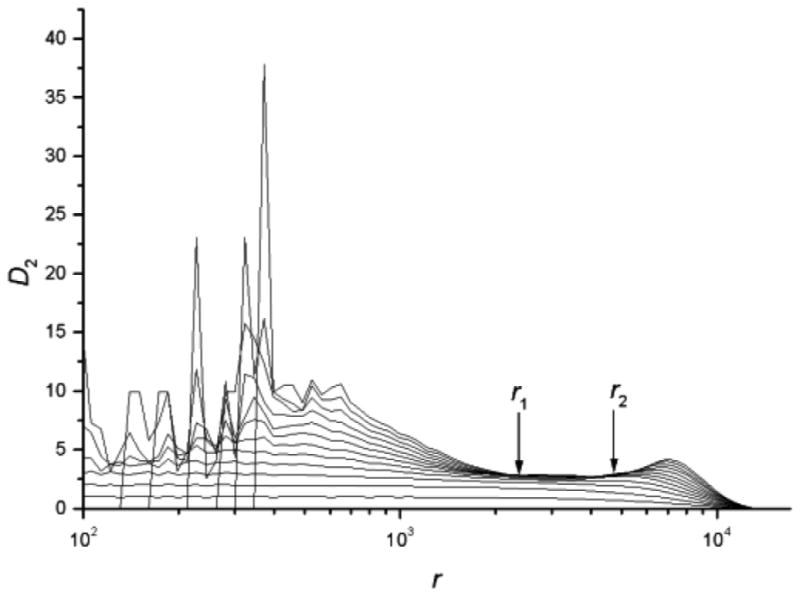
The estimated D2 value versus r. The curves from bottom to top correspond to the embedding dimension m=1, 2, 3, …, 10.
Perceptual analysis
A perceptual rating of general vocal impairment was obtained for all vocal segments to preliminarily investigate the relationship between the D2 and perceptual measures. The one-second segments were rated for general vocal impairment by one graduate student with six years of voice research and perceptual analysis experience. One undergraduate student with two years of voice research experience analyzed 10 percent of the vocal segments to check for inter-rater reliability. These raters spent over 5 hours training for perceptual analysis with Sound Judgment software (Finders University, Adelaide, Australia) and with other clinical experiments involving perceptual analysis, maximizing the accuracy of their perceptual judgments. Neither rater had a history of hearing or communication difficulties. The voice segments were presented in randomized order to each rater through headphones (Targus Inc., Anaheim, CA) in a quiet isolated room. The raters were asked to give scores of general vocal impairment on a scale from 0 to 6 (0=none, 6=severe). In this study, the raters had high inter-rater agreement, with a Spearman’s Rank Order correlation coefficient of 0.972 and p-value of <0.0001. Because of the availability of only two experienced raters, the perceptual ratings were not statistically analyzed.
Statistical analysis
The log-transformed means for each of the voice measures (D2, percent jitter, percent shimmer, SNR, vF0, vAm) were calculated, since the log transformation was found to better meet the assumptions of ANOVA. For each of the six measures, a mixed two-way ANOVA model was fit, with fixed effects for surgery and medication and their interaction, and a random effect for trial nested within patient, the experimental unit. Six tests were run, once with the stimulator-off and once with the stimulator-on conditions for bilateral, left, and right subsets of the surgical group. Each test carried out three comparisons on the fixed effects: a comparison of the no surgery and surgery conditions (BM and AM vs. BMDOFF/BMDON and AMDOFF/AMDON), a comparison of the before- and after-medication conditions (BM and BMDOFF/BMDON vs. AM and AMDOFF/AMDON), and an interaction of the no surgery/surgery and before/after-medication conditions. If the interaction term was statistically significant,, multiple comparisons were carried out using a Fisher’s LSD test. In all, 36 ANOVAS were run (6 measures x 3 subgroups [bilateral, left, right] x 2 conditions [stimulator-off or -on]). Two-sided p-values less than 0.05 were considered significant. Statistical computations were run on SAS 9.1 (SAS Institute, Cary, NC).
Statistical comparisons of the OFF and ON situations were not reported. Previous studies have shown that the stimulator must be turned off for a minimum of 12 hours in order to ensure no effect.[36] In our initial study without the medication treatment factor, there was no significant difference between the OFF and ON situations. As a result, this comparison was eliminated to simplify the statistical analysis.
RESULTS
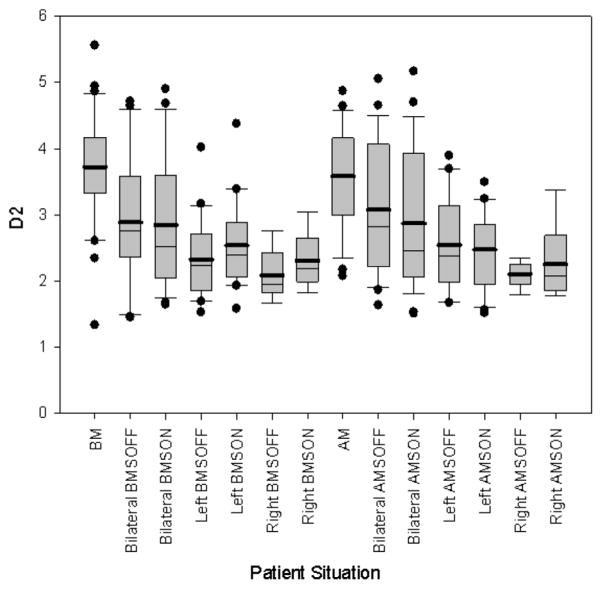
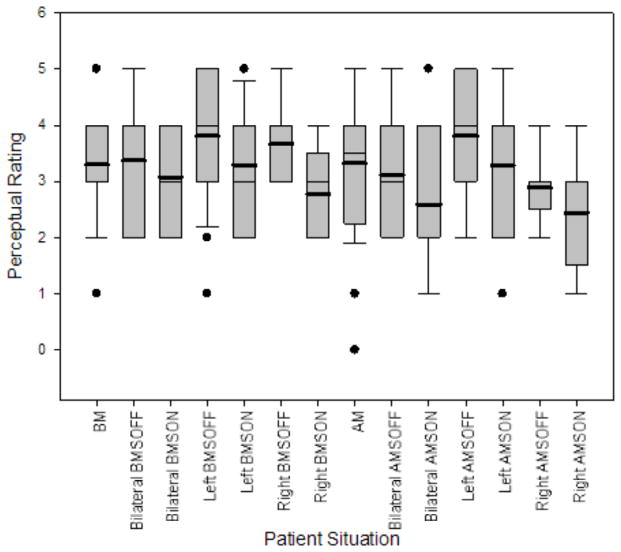
Table 3 shows log-transformed (indicated by “l”) mean D2, percent jitter, percent shimmer, SNR, vF0, and vAm values for control patients in the BM and AM situations and bilateral, left, and right DBS patients in the BMSOFF, BMSON, AMSOFF, and AMSON situations. Table 4A displays the results of the mixed two-way ANOVAs. Table 4B displays the multiple comparisons for significant interaction terms. Figures 4 and 5 display the boxplot for D2 values and perceptual ratings respectively in the various patient situations.
Table 3.
Mean and standard deviation for log-transformed D2, percent jitter, percent shimmer, signal-to-noise ratio (SNR), vF0, and vAm in Parkinson’s disease (PD) subjects in the before and after-medication stimulator-off (BMSOFF, AMSOFF) and before and after-medication stimulator-on (BMSON, AMSON) situations for (A) bilateral (n=9), (B) left (n=7), and (C) right (n=3) patients. Mean and standard deviation for log-transformed D2, percent jitter, percent shimmer, signal-to-noise ratio (SNR), vF0, and vAm for (D) non-surgical patients (n=10) in the before- (BM) and after-medication (AM) situations. Perturbation values for aperiodic samples were eliminated.
| (A) | BMSOFF | BMSON | AMSOFF | AMSON |
|---|---|---|---|---|
| log D2 | 1.011 (±0.325) | 0.988 (±0.346) | 1.074 (±0.323) | 0.994 (±0.354) |
| log % jitter | −0.997 (±0.768) | −1.019 (±0.656) | −1.110 (±0.590) | −1.182 (±0.523) |
| log % shimmer | 1.402 (±0.589) | 1.583 (±0.702) | 1.364 (±0.503) | 1.494 (±0.619) |
| log SNR | 2.783 (±0.278) | 2.699 (±0.368) | 2.816 (±0.231) | 2.786 (±0.323) |
| log vF0 | 0.335 (±0.536) | 0.325 (±0.751) | 0.207 (±0.769) | 0.167 (±0.624) |
| log vAm | 2.150 (±0.388) | 2.215 (±1.061) | 2.101 (±1.049) | 1.576 (±1.635) |
| (B) | BMSOFF | BMSON | AMSOFF | AMSON |
|---|---|---|---|---|
| log D2 | 0.817 (±0.238) | 0.904 (±0.235) | 0.898 (±0.273) | 0.880 (±0.240) |
| log % jitter | −0.772 (±0.637) | −0.704 (±0.730) | −0.640 (±0.334) | −1.111 (±0.530) |
| log % shimmer | 1.582 (±0.366) | 1.792 (±0.635) | 1.839 (±0.213) | 1.514 (±0.527) |
| log SNR | 2.689 (±0.202) | 2.515 (±0.346) | 2.631 (±0.106) | 2.710 (±0.284) |
| log vF0 | 0.223 (±1.031) | 0.247 (±0.855) | 0.411 (±0.488) | 0.372 (±1.151) |
| log vAm | 1.153 (±1.825) | 1.473 (±1.465) | 1.979 (±0.900) | 1.928 (±1.133) |
| (C) | BMSOFF | BMSON | AMSOFF | AMSON |
|---|---|---|---|---|
| log D2 | 0.723 (±0.176) | 0.823 (±0.170) | 0.741 (±0.089) | 0.786 (±0.241) |
| log % jitter | −0.501 (±0.635) | −0.506 (±0.434) | −0.533 (±0.431) | −0.619 (±0.428) |
| log % shimmer | 1.635 (±0.399) | 1.837 (±0.292) | 1.835 (±0.324) | 1.541 (±0.222) |
| log SNR | 2.755 (±0.175) | 2.604 (±0.168) | 2.649 (±0.113) | 2.716 (±0.176) |
| log vF0 | 0.080 (±0.600) | 0.108 (±0.277) | 0.125 (±0.555) | 0.444 (±0.595) |
| log vAm | 1.675 (±1.446) | 2.060 (±0.429) | 1.614 (±1.279) | 1.468 (±1.732) |
| (D) | BM | AM |
|---|---|---|
| log D2 | 1.252 (±0.230) | 1.285 (±0.265) |
| log % jitter | −1.064 (±0.532) | −0.916 (±0.468) |
| log % shimmer | 1.689 (±0.550) | 1.922 (±0.388) |
| log SNR | 2.671 (±0.365) | 2.587 (±0.214) |
| log vF0 | 0.870 (±1.436) | 1.400 (±1.480) |
| log vAm | 2.140 (±1.053) | 2.474 (±0.925) |
Table 4.
(A) shows mixed two-way ANOVA p-values for log-transformed D2, percent jitter, percent shimmer, signal-to-noise ratio (SNR), vF0, and vAm. (B) shows Fisher’s LSD p-values for multiple comparisons of the two significant interaction terms (indicated by **).
| (A) | Stimulator-off | Stimulator-on | |||||
|---|---|---|---|---|---|---|---|
| Effect of surgery | Effect of medication | Interaction | Effect of surgery | Effect of medication | Interaction | ||
| Bilateral | lD2 | 0.033* | 0.226 | 0.690 | 0.019* | 0.609 | 0.709 |
| ljitter | 0.997 | 0.188 | 0.868 | 0.914 | 0.915 | 0.124 | |
| lshimmer | 0.036* | 0.521 | 0.466 | 0.323 | 0.551 | 0.349 | |
| lsnr | 0.097 | 0.891 | 0.655 | 0.446 | 0.948 | 0.559 | |
| lvF0 | 0.006* | 0.394 | 0.172 | 0.008* | 0.494 | 0.131 | |
| lvAm | 0.390 | 0.475 | 0.261 | 0.156 | 0.515 | 0.048** | |
| Left | lD2 | <0.0001* | 0.253 | 0.621 | <0.0001* | 0.916 | 0.515 |
| ljitter | 0.191 | 0.218 | 0.753 | 0.522 | 0.614 | 0.044** | |
| lshimmer | 0.372 | 0.134 | 0.832 | 0.419 | 0.908 | 0.169 | |
| lsnr | 0.451 | 0.750 | 0.927 | 0.963 | 0.555 | 0.340 | |
| lvF0 | 0.023* | 0.186 | 0.513 | 0.043* | 0.256 | 0.439 | |
| lvAm | 0.035* | 0.031* | 0.307 | 0.050 | 0.155 | 0.814 | |
| Right | lD2 | <0.0001* | 0.649 | 0.898 | <0.0001* | 0.972 | 0.559 |
| ljitter | 0.079 | 0.680 | 0.448 | 0.174 | 0.793 | 0.198 | |
| lshimmer | 0.734 | 0.470 | 0.780 | 0.453 | 0.751 | 0.237 | |
| lsnr | 0.430 | 0.695 | 0.794 | 0.645 | 0.802 | 0.688 | |
| lvF0 | 0.037* | 0.423 | 0.485 | 0.078 | 0.265 | 0.806 | |
| lvAm | 0.035* | 0.662 | 0.520 | 0.104 | 0.660 | 0.117 | |
| (B) | BM vs. AM | AM vs. AMSON | AM vs. BMSON | BM vs. AMSON | BM vs. BMSON | BMSON vs. AMSON |
|---|---|---|---|---|---|---|
| ljitter | 0.214 | 0.929 | 0.435 | 0.670 | 0.195 | 0.107 |
| lvAm | 0.281 | 0.026 | 0.345 | 0.131 | 0.848 | 0.235 |
indicates p-values significant at the 0.05 level.
Figure 4.
Boxplot of D2 values for non-surgical patients in the before- (BM) and after-medication (AM) situations and surgical patients divided by bilateral, left, and right in the before- and after-medication stimulator-off (BMSOFF, AMSOFF) and before- and after-medication stimulator-on (BMSON, AMSON) situations.
Figure 5.
Boxplot of perceptual ratings of general vocal impairment for non-surgical patients in the before- (BM) and after-medication (AM) situations and surgical patients divided by bilateral, left, and right in the before- and after-medication stimulator-off (BMSOFF, AMSOFF) and before- and after-medication stimulator-on (BMSON, AMSON) situations.
Effect of DBS (comparison 1)
Mean lD2 was significantly lower in bilateral, left and right DBS patients for the stimulator-off (p=0.033, p<0.0001, p<0.0001) and the stimulator-on conditions (p=0.019, p<0.0001, p<0.0001) than in control patients. Figure 4 shows the significantly lower D2 values in the surgical group than in the control group. Mean lshimmer was significantly lower in bilateral DBS patients for the stimulator-off conditions (p=0.036) than in control patients. Mean lvF0 was significantly lower in bilateral and left DBS patients for the stimulator-off (p=0.006, p=0.023) and the stimulator-on conditions (p=0.008, p=0.043) than in control patients. Mean lvF0 was significantly lower in right DBS patients for the stimulator-off conditions (p=0.037) than in control patients. Mean lvAm was significantly lower in left and right DBS patients for the stimulator-off conditions (p=0.035, p=0.035).
Effect of medication (comparison 2)
Mean lvAm was significantly lower in left DBS patients for the stimulator-off conditions (p=0.031) than in control patients. No other ANOVAs for bilateral, left, or right DBS comparing the before and after-medication conditions generated statistically significant results.
Interaction of DBS and medication (comparison 3)
The interaction term for mean ljitter in left DBS patients for the stimulator-on conditions was statistically significant (p=0.044). The interaction term for mean lvAm in bilateral DBS patients for the stimulator-on conditions was also statistically significant (p=0.048). None of the multiple comparisons were significant for either term.
Perceptual analysis
In comparing the no surgery and surgery conditions (BM and AM vs. BMDOFF/BMDON and AMDOFF/AMDON) in Figure 5, the surgery conditions have similar perceptual ratings. In comparing the before- and after-medication conditions (BM and BMDOFF/BMDON vs. AM and AMDOFF/AMDON) in Figure 5, the medication conditions also have similar perceptual ratings. The mean perceptual ratings for SON situations are lower than that of the respective SOFF situations for bilateral, left, and right DBS patients.
DISCUSSION
The results of this study support the validity of nonlinear dynamic analysis as a clinical tool to evaluate the effects of DBS and levodopa on voice. Mean lD2, produced from nonlinear dynamic analysis, yielded significantly lower values in the bilateral, left, and right subsets of the DBS group than in the control group, indicating an improvement in PD voice. Statistically significant results from perturbation measures show similar trends to correlation dimension, lending credibility to the significant differences between the DBS and control group detected by correlation dimension. Mean lvAm and lvF0 mirrored the results of mean lD2, and mean lshimmer were significantly lower for the bilateral DBS group, supporting an improvement in PD voice. Perturbation results that were not significant showed lower ljitter, lshimmer, lvF0, and lvAm values and higher SNR values for the DBS group, which agree with the results from nonlinear dynamic analysis.
Correlation dimension quantifies vocal irregularity, which may be caused by vocal fold stiffness in PD patients. Decreases in correlation dimension may indicate decreased vocal fold stiffness and more normal contractions of the vocal folds in PD patients, specifically of the antagonistic thyroarytenoid and cricothyroid muscles.[23] The discrepancy between the results from nonlinear dynamic and perturbation analysis may be due to the exclusion of aperiodic vocal samples from perturbation analysis. Previous studies have shown that perturbation analysis is unreliable for aperiodic vocal samples, which are commonly found in PD voice.[20–25,29]
The direct relationship between nonlinear dynamic and perceptual measures of voice is unclear. Correlation dimension quantifies aperiodic vocal patterns and therefore should reflect the perceptual characteristic of hoarseness.[23] In this study, although both perceptual and nonlinear dynamic results indicate improvement in PD voice with DBS, perceptual results show improvement in the stimulator-on condition as compared to the stimulator-off condition whereas nonlinear dynamic results show improvement in the stimulator-on condition as compared to the no surgery condition. Possible reasons for the discrepancy may include the use of a rating of general vocal impairment rather than of hoarseness and the small number of experienced raters. Nonlinear dynamic analysis is more time-effective and cost-effective than perceptual analysis and is the only objective method of analysis that exists for aperiodic voice. Given the unclear relationship between the two types of analysis, however, nonlinear dynamic analysis may only serve as a complement to perceptual analysis in the clinical setting. Further more rigorous studies are needed to elucidate the correlation between nonlinear dynamic and perceptual measures of Parkinsonian voice.
The conclusions of this study on the effectiveness of DBS and levodopa should be regarded with caution, as the purpose of this study was to evaluate the validity of nonlinear dynamic analysis as a clinical tool for detecting improvements in voice from DBS and levodopa treatment. The results indicate that DBS significantly improves voice with or without levodopa treatment and that levodopa treatment may not improve PD voice with or without DBS. Improvements in PD voice associated with levodopa have been shown to decline in the long-run.[10,37,38] This decline in the effectiveness of levodopa may be attributed to an increase in non-dopaminergic lesions outside of the basal ganglia or decreased dopamine transmission within the basal ganglia itself.[15] Patients in this study had Hoehn-Yahr scores of III or IV, indicating a more advanced stage of PD, and more severe vocal impairment as shown by the presence of subharmonics in their vocal waveform. Therefore, effectiveness of levodopa treatment may have already declined, perhaps from the increased prevalence of non-dopaminergic lesions.[4,7,8,39–41] No interaction was found between levodopa and DBS treatment. Although two interaction terms were significant, the multiple comparisons did not generate any significant differences. All other interaction terms were not significant, indicating that low or high levels of levodopa were not correlated with either the stimulator-off or stimulator-on conditions and may show a lack of synergistic effects between the two treatments.
The conclusions of this study have several limitations. The absence of a true stimulator-off condition makes it difficult to pinpoint DBS as the source of the vocal improvement in the PD patients. In addition, the sample size is limited by the inclusion criteria of Parkinson’s disease and qualification for DBS surgery. Furthermore, vocal characteristics, especially in patients with Parkinson’s disease, are highly variable from individual to individual. Although patients within the baseline and surgical groups were selected to be as consistent as possible, differences in the severity of PD as well as vocal machinery may have affected the results. Similar UPDRS-III Item 18 scores, which specifically evaluate speech, however, may indicate that the two groups had similar initial levels of vocal impairment.
This study has applied nonlinear dynamic analysis to the evaluation of aperiodic PD voices with DBS and levodopa treatment. Correlation dimension values indicate improvements in the PD vocal regularity in patients receiving DBS, with neither an apparent added benefit of levodopa treatment nor interaction with levodopa, but these conclusions on treatment effects should be regarded with caution. This study provides support for the validity of nonlinear dynamic analysis as a complementary clinical evaluation tool for PD vocal treatments.
Acknowledgments
This study was supported by NIH Grant No. RO1DC006019 from the National Institute of Deafness and other Communication Disorders (NIDCD) and NSFC (Natural Science Foundation of China) Grant No. 30328029. Alejandro Munoz at the University of Wisconsin-Madison provided statistical analysis expertise.
Contributor Information
Xiao Ping Zhou, Department of Neurosurgery, Shanghai Second Military Medical University.
Victoria S. Lee, Department of Surgery, Otolaryngology, University of Wisconsin-Madison
Emily Q. Wang, Department of Communication Disorders and Sciences, and Department of Otolaryngology and Bronchoesophagology, Rush University Medical Center
Jack J. Jiang, Department of Surgery, Otolaryngology, University of Wisconsin-Madison
References
- 1.Marsden CD. Parkinson’s disease. J Neurol Neurosurg Psychiatry. 1994;57:672–681. doi: 10.1136/jnnp.57.6.672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hanson DG, Gerratt BR, Ward PH. Cinegraphic observations of laryngeal function in Parkinson’s disease. Laryngoscope. 1984;94:348–353. doi: 10.1288/00005537-198403000-00011. [DOI] [PubMed] [Google Scholar]
- 3.Ramig LA, Scherer RC, Titze IR, Ringel SP. Acoustic analysis of voices of patients with neurologic disease: rationale and preliminary data. Ann Otol Rhinol Laryngol. 1988;97:164–172. doi: 10.1177/000348948809700214. [DOI] [PubMed] [Google Scholar]
- 4.Wolfe VI, Garvin JS, Bacon M, Waldrop W. Speech changes in Parkinson’s disease during treatment with L-dopa. J Commun Disord. 1975;8:271–279. doi: 10.1016/0021-9924(75)90019-2. [DOI] [PubMed] [Google Scholar]
- 5.Critchley EM. Speech disorders of Parkinsonism: a review. J Neurol Neurosurg Psychiatry. 1981;44:751–758. doi: 10.1136/jnnp.44.9.751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gentil M, Garcia-Ruiz P, Pollak P, Benabid AL. Effect of stimulation of the subthalamic nucleus on oral control of patients with Parkinsonism. J Neurol Neurosurg Psychiatry. 1999;67:329–333. doi: 10.1136/jnnp.67.3.329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sanabria J, Ruiz PG, Gutierrrez R, Marquez F, Escobar P, Gentil M, Cenjor C. The effect of levodopa on vocal function in Parkinson’s disease. Clin Neuropharmacol. 2001;24:99–102. doi: 10.1097/00002826-200103000-00006. [DOI] [PubMed] [Google Scholar]
- 8.De Letter M, Santens P, Van Borsel JV. The effects of levodopa on word intelligibility in Parkinson’s disease. J Commun Disord. 2005;38:187–196. doi: 10.1016/j.jcomdis.2004.09.001. [DOI] [PubMed] [Google Scholar]
- 9.Goberman AM. Correlation between acoustic speech characteristics and nonspeech motor performance in Parkinson disease. Med Sci Monit. 2005;11:109–116. [PubMed] [Google Scholar]
- 10.Broggi G, Franzini A, Marras C, Romito L, Albanese A. Surgery of Parkinson’s disease: inclusion criteria and follow-up. Neurol Sci. 2003;24(Suppl 1):S38–S40. doi: 10.1007/s100720300037. [DOI] [PubMed] [Google Scholar]
- 11.Hoffman-Ruddy B, Schulz G, Vitek J, Evatt M. A preliminary study of the effects of subthalamic nucleus (STN) deep brain stimulation (DBS) on voice and speech characteristics in Parkinson’s Disease (PD) Clin Linguist Phon. 2001;15:97–101. doi: 10.3109/02699200109167638. [DOI] [PubMed] [Google Scholar]
- 12.Krack P, Batir A, Van Blercom N, Chabardes S, et al. Five-year follow-up of bilateral stimulation of the subthalamic nucleus in advanced Parkinson’s disease. New Engl J Med. 2003;349:1925–1934. doi: 10.1056/NEJMoa035275. [DOI] [PubMed] [Google Scholar]
- 13.Kumar R, Lozano AM, Sime E, Lang AE. Long-term follow-up of thalamic deep brain stimulation for essential and parkinsonian tremor. Neurology. 2003;61:1601–1604. doi: 10.1212/01.wnl.0000096012.07360.1c. [DOI] [PubMed] [Google Scholar]
- 14.Vaillancourt D, Prodoehl J, Metman LV, Bakay RA, Corcos DM. Effects of deep brain stimulation and medication on bradykinesia and muscle activation in Parkinson’s disease. Brain. 2004;127:491–504. doi: 10.1093/brain/awh057. [DOI] [PubMed] [Google Scholar]
- 15.Bejjani BP, Gervais D, Arnulf I, Papadopoulos S, Demeret S, Bonnet AM, Cornu P, Damier P, Agrid Y. Axial parkinsonian symptoms can be improved: the role of levodopa and bilateral subthalamic stimulation. J Neurol Neurosurg Psychiatry. 2000;68:595–600. doi: 10.1136/jnnp.68.5.595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sailer A, Cunic DI, Paradiso GO, Gunraj CA, Wagle-Shukla A, Moro E, Lozano AM, Lang AE, Chen R. Subthalamic nucleus stimulation modulates afferent inhibition in Parkinson disease. Neurology. 2007;68: 356–363. doi: 10.1212/01.wnl.0000252812.95774.aa. [DOI] [PubMed] [Google Scholar]
- 17.Stefani A, Lozano A, Peppe A, Stanzione P, Galati S, Tropepi D, Pierantozzi M, Brusa L, Scarnati E, Mazzone P. Bilateral deep brain stimulation of the pedunculopontine and subthalamic nuclei in severe Parkinson’s disease. Brain. 2007 doi: 10.1093/brain/awl346. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 18.Dromey C, Kumar R, Lang A, Lozano A. An investigation of the effects of subthalamic nucleus stimulation on acoustic measures of voice. Mov Disord. 2000;15:1132–1138. doi: 10.1002/1531-8257(200011)15:6<1132::aid-mds1011>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 19.Gentil M, Chauvin P, Pinto S, Pollak P, Benabid AL. Effect of bilateral stimulation of the subthalamic nucleus on parkinsonian voice. Brain Lang. 2001;78:233–240. doi: 10.1006/brln.2001.2466. [DOI] [PubMed] [Google Scholar]
- 20.Hertrich I, Ackerman H. Gender-specific vocal dysfunctions in Parkinson’s disease: Electroglottographic and acoustic analyses. Ann Otol Rhinol Laryngol. 1995;104:197–202. doi: 10.1177/000348949510400304. [DOI] [PubMed] [Google Scholar]
- 21.Zhang Y, McGilligan C, Zhou L, Vig M, Jiang J. Nonlinear dynamic analysis of voices before and after surgical excision of vocal polyps. J Acoust Soc Am. 2004;115:2270–2277. doi: 10.1121/1.1699392. [DOI] [PubMed] [Google Scholar]
- 22.Titze IR, Baken R, Herzel H. Evidence of chaos in vocal fold vibration. In: Titze IR, editor. Vocal Fold Physiology: Frontiers in Basic Science. San Diego: Singular; 1993. pp. 143–188. [Google Scholar]
- 23.Rahn DA, Chou M, Zhang Y, Jiang J. Phonatory impairment in Parkinson’s disease: Evidence from nonlinear dynamic analysis and perturbation analysis. J Voice. 2007;21:64–71. doi: 10.1016/j.jvoice.2005.08.011. [DOI] [PubMed] [Google Scholar]
- 24.Herzel H, Berry D, Titze IR, Saleh M. Analysis of vocal disorders with methods from nonlinear dynamic analysis. J Speech Lang Hear Res. 1994;37:1008. doi: 10.1044/jshr.3705.1008. [DOI] [PubMed] [Google Scholar]
- 25.Zhang Y, Jiang JJ. Nonlinear dynamic analysis in signal typing of pathological human voices. Electronic Letters. 2003;39:1021–1023. [Google Scholar]
- 26.Yu Z, Jiang J, Rahn DA. Studying vocal vibrations in Parkinson’s disease with a nonlinear model. Chaos. 2005;15:1–9. doi: 10.1063/1.1916186. [DOI] [PubMed] [Google Scholar]
- 27.Benabid AL. Deep brain stimulation for Parkinson’s disease. Curr Opin Neurobiol. 2003;13:696–706. doi: 10.1016/j.conb.2003.11.001. [DOI] [PubMed] [Google Scholar]
- 28.Milenkovic P, Read C. C-Speech Version 4 User’s Manual. Madison, WI: 1992. [Google Scholar]
- 29.Titze IR. Summary statement: workshop on acoustic voice analysis. National Center for Voice and Speech; Denver, CO, USA: 1995. [Google Scholar]
- 30.Grassberger P, Procaccia I. Measuring the strangeness of strange attractors. Physica D. 1983;9:189–208. [Google Scholar]
- 31.Narayanan SS, Alwan AA. A nonlinear dynamical systems analysis of fricative consonants. J Acous Soc Am. 1995;97:2511–2524. doi: 10.1121/1.411971. [DOI] [PubMed] [Google Scholar]
- 32.Kumar A, Mullick SK. Nonlinear dynamical analysis of speech. J Acous Soc Am. 1996;100:615–629. [Google Scholar]
- 33.Jiang JJ, Zhang Y, Ford CN. Nonlinear dynamics of phonations in excised larynx experiments. J Acous Soc Am. 2003;114:2198–2205. doi: 10.1121/1.1610462. [DOI] [PubMed] [Google Scholar]
- 34.Jiang JJ, Zhang Y, McGilligan C. Chaos in voice, from modeling to measurement. J Voice. 2006;20:2–17. doi: 10.1016/j.jvoice.2005.01.001. [DOI] [PubMed] [Google Scholar]
- 35.Zhang Y, Jiang JJ, Biazzo L, Jorgensen M. Perturbation and nonlinear dynamic analyses of voices from patients with unilateral laryngeal paralysis. J Voice. 2005;19:519–528. doi: 10.1016/j.jvoice.2004.11.005. [DOI] [PubMed] [Google Scholar]
- 36.Santens P, De Letter M, Van Borsel J, De Reuck J, Caemaert J. Lateralized effects of subthalamic nucleus stimulation on different aspects of speech in Parkinson’s disease. Brain and Language. 2003;87:253–358. doi: 10.1016/s0093-934x(03)00142-1. [DOI] [PubMed] [Google Scholar]
- 37.The Deep-Brain Stimulation for Parkinson’s Disease Study Group. Deep-brain stimulation of the subthalamic nucleus or the pars interna of the globus pallidus in Parkinson’s disease. New Engl J Med. 2001;345:956–963. doi: 10.1056/NEJMoa000827. [DOI] [PubMed] [Google Scholar]
- 38.Pinto S, Gentil M, Fraix V, Benabid A, Pollak P. Bilateral subthalamic stimulation effects on oral force control in Parkinson’s disease. J Neurol. 2003;250:179–187. doi: 10.1007/s00415-003-0966-7. [DOI] [PubMed] [Google Scholar]
- 39.Goberman AM, Blomgren M. Parkinsonian speech disfluencies: effects of L-dopa-related fluctuations. J Fluency Disord. 2003;28:55–70. doi: 10.1016/s0094-730x(03)00005-6. [DOI] [PubMed] [Google Scholar]
- 40.Poluha PC, Teulings HL, Brookshire RH. Handwriting and speech changes across the levodopa cycle in Parkinson’s disease. Acta Psychol (Amst) 1998;100:71–84. doi: 10.1016/s0001-6918(98)00026-2. [DOI] [PubMed] [Google Scholar]
- 41.Jiang J, Lin E, Wang J, Hanson DG. Glottographic measures before and after levodopa treatment in Parkinson’s disease. Laryngoscope. 1999;109:1287–94. doi: 10.1097/00005537-199908000-00019. [DOI] [PubMed] [Google Scholar]