Abstract
OBJECTIVES
The mechanism of action of methotrexate in rheumatoid arthritis (RA) is complex. It may increase adenosine levels by blocking its conversion to uric acid (UA). This study was done to determine if methotrexate lowers UA in early RA (ERA).
METHODS
Data were obtained from Canadian Early Arthritis Cohort, an incident ERA cohort. All ERA patients with serial UA measurements were included, comparing those with methotrexate use vs. no methotrexate exposure (controls). Analyses were exploratory. Patients with concomitant gout or taking UA-lowering therapies were excluded.
RESULTS
In total, 49 of the 2,524 ERA patients were identified with data available for both pre-methotrexate UA levels and post-methotrexate UA levels (300 µmol/L and 273 µmol/L, respectively; P = 0.035). The control group not taking methotrexate had a mean baseline UA level of 280 µmol/L and a follow-up level of 282 µmol/L (P = 0.448); mean change in UA with methotrexate was −26.8 µmol/L vs. 2.3 µmol/L in the no methotrexate group (P = 0.042). Methotrexate users with a decrease in UA had a disease activity score of 2.37 for 28 joints when compared with the controls (3.26) at 18 months (P = 0.042). Methotrexate users with decreased UA had a lower swollen joint count (SJC) of 0.9 at 18 months, whereas methotrexate users without lowering of UA had an SJC of 4.5 (P = 0.035). Other analyses were not significant.
CONCLUSIONS
Methotrexate response is associated with lowering of serum UA in ERA compared to nonusers. This may be due to changes in adenosine levels. Methotrexate response is associated with lower UA and fewer swollen joints compared to nonresponders.
Keywords: methotrexate, uric acid, early rheumatoid arthritis, Nested case-control
Introduction
Methotrexate is one of the most effective and durable disease-modifying antirheumatic drugs (DMARDs) for the treatment of rheumatoid arthritis (RA).1 It was initially designed in the late 1940s as a stable derivative of aminopterin for the treatment of childhood leukemia.1 Over the last 30 years, methotrexate has been used extensively for RA either as monotherapy or in combination therapy, with high efficacy.1–5
However, our understanding of the mechanism of action of methotrexate continues to evolve. Methotrexate exerts much of its anti-inflammatory effects in RA by increasing local levels of adenosine in inflamed tissues6–11 via inhibition of dihydrofolate reductase and 5-aminoimidazole-4-carboxamide ribonucleotide transformylase.10,11 More specifically, inhibition of these enzymes involved in folate metabolism and purine synthesis ultimately leads to inhibition of adenosine deaminase (ADA), thereby increasing the levels of adenosine.10 The increase in adenosine should follow a reciprocal decrease in xanthine and uric acid (UA; Supplementary Fig. 1).
The clinical response to methotrexate may take several weeks in early RA (ERA) treatment, and early prediction of who will respond to methotrexate is imprecise. In addition, the clinically effective dose of methotrexate and the route of administration are often debated.12–14 Easy methods for early prediction of methotrexate response would aid in personalized medicine. Some studies have suggested measuring intracellular methotrexate polyglutamates as a biomarker, but cost and lack of wide availability have been barriers for routine use.15,16
Changes in serum UA may be useful as a surrogate for the intracellular function of methotrexate. The purpose of this study was to compare changes in serum UA levels in patients with ERA who were treated with methotrexate to those who were not treated with methotrexate and to correlate these changes with clinical outcomes.
Patients and Methods
Study population
Subjects were enrolled from the Canadian Early Arthritis Cohort (CATCH) study, a multicenter, observational, prospective cohort of patients with ERA. CATCH has been recruiting from 19 sites in Canada since July 2007. Data as of September 2014 were used from the database. The inclusion criteria for the CATCH study are patients who are >16 years of age, between six weeks and 12 months of persistent synovitis, and two or more swollen joints or one swollen metacarpophalangeal or proximal inter-phalangeal joint with one or more of the following: positive rheumatoid factor (RF), positive anticyclic citrullinated peptide 2, morning stiffness for >45 minutes, response to nonsteroidal anti-inflammatory drugs, or painful metatarsophalangeal squeeze test. Patients are followed up every three months where case report forms are completed for the first year and twice a year thereafter. During these standard visits, regular blood testing is performed. Serum UA levels are measured in some patients during study visits as part of optional laboratory tests, as per study cohort protocol, at the discretion of their physician. Based on the physician’s discretion, treatment is intensive and mostly aligns with Canadian practice recommendations. All sites had the approval of research ethics boards, and all patients with ERA provided signed consent. Our research complied with the principles of the Declaration of Helsinki. Ethics approval was obtained from the Wstern University Health Research Ethics Board.
Study design, variables, and outcomes
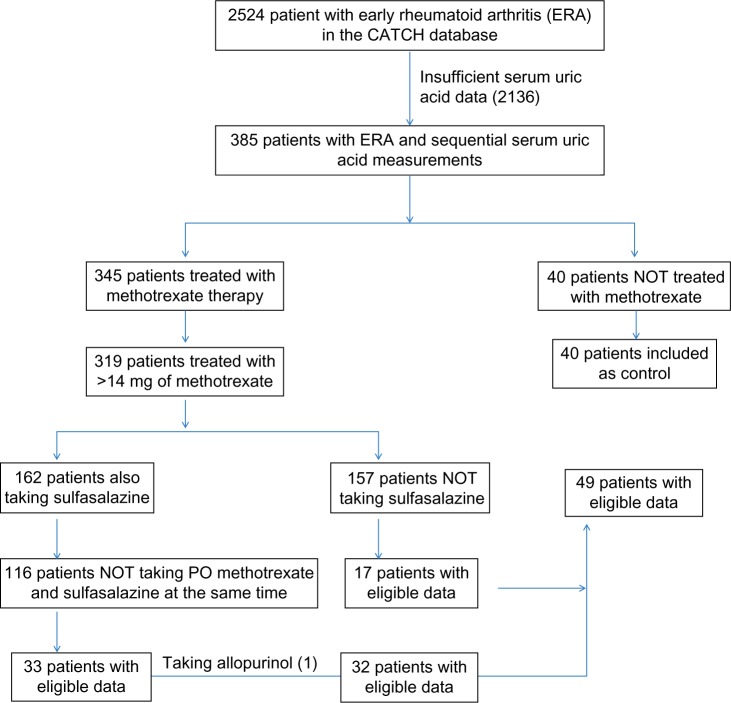
This was a nested case–control study within the CATCH. All patients who met the 2010 ACR/EULAR criteria for RA17 were included if they had serum UA levels performed before and after starting DMARDs and available disease activity score of 28 joints and ESR (DAS(ESR)28) and swollen joint count using 28 joints (SJC28). UA could have been remeasured at any point, while the patient was on a DMARD. Patients were excluded if they were taking oral methotrexate concurrently with oral sulfasalazine, as some data in the literature suggest that a significant drug–drug interaction may lead to poor methotrexate absorption.18 Simultaneous use of subcutaneous methotrexate and oral sulfasalazine was allowed. Data were exploratory, so outcomes included clinical and radiographic outcomes between groups (methotrexate users vs. nonusers and methotrexate users who decreased UA vs. those who did not). Patients were also excluded if they were taking allopurinol or had a history of gout. Patients who were taking an average of ≤14 mg of methotrexate a week were excluded. An average methotrexate dose between 14 mg and 15 mg was allowed to include patients who up-titrated to a stable dose of ≥15 mg per week. Patients with ERA who did not receive any methotrexate were used as controls (Fig. 1), recognizing that the treatment was at the discretion of each enrolling rheumatologist.
Figure 1.
Flow diagram of inclusion and exclusion criteria for patients with ERA.
The following demographic data were obtained: age, sex, the duration of symptoms, the number of DMARDs used, and biological and steroid exposure. Disease activity was measured using the DAS(ESR)28, SJC28, and presence of erosions. Erosions in the CATCH study were determined using the radiographs of the hands and feet performed at baseline, 6 months, and 12 months and then annually, as reported by the local radiologist and/or reviewed by the rheumatologist.
Statistical analysis
All statistical analyses were performed using the SAS software version 9.3 (SAS Institute). Continuous variables were reported as mean values, and the changes in serum UA, DAS(ESR)28, and SJC were compared using the Student’s t-test (P < 0.05 was considered significant). Within groups, UA changes were analyzed by one-tailed t-tests, whereas all other analyses were analyzed by two-tailed t-tests. The analyses for UA changes were one-tailed t-tests because mechanistically we expected the UA levels to change in one direction only (ie, decrease). Comparisons of subsequent demographic and clinical characteristics were performed using one-way analysis of variance (ANOVA) tests and Pearson’s chi-squared analyses. Regression analyses could not be performed due to the small sample size of patients with available data.
Results
There were 2,524 patients in the CATCH database, and 385 subjects with ERA had serial UA measurements performed (Fig. 1). In total, 40 of the 385 patients were not treated with methotrexate (controls). Of the remaining 345 patients, 132 patients were on methotrexate at an average dose of >14 mg per week and were not taking oral methotrexate and sulfasalazine or allopurinol simultaneously. Forty-nine of the methotrexate users had two or more UA results before and during methotrexate treatment and were appropriate for data analyses. The majority of UA levels before methotrexate exposure were taken at baseline, and a large proportion of UA levels after initiation of methotrexate were repeated at 12 months.
Patient demographics are shown in Table 1. Methotrexate users vs. nonusers (controls) were similar with respect to age, proportion of females, baseline, DAS28, SJC, and erosions, whereas the duration of symptoms for methotrexate users was longer than for the control group (197 days vs. 144 days) and the number of DMARD medications also differed (3 vs. 1).
Table 1.
Patient demographics comparing the total CATCH patient population and subgroups based on methotrexate use.
| TOTAL CATCH COHORT | TOTAL METHOTREXATE USERS | INCLUDED METHOTREXATE USERS | INCLUDED METHOTREXATE USERS WITH UA DECREASE | INCLUDED METHOTREXATE USERS WITH UA INCREASE | METHOTREXATE NON-USERS | METHOTREXATE NON-USERS (CONTROLS) | P-VALUE | |
|---|---|---|---|---|---|---|---|---|
| Number of Patients (%) | 2524 (100) | 1885 (75) | 49 (2) | 32 (1.3) | 17 (0.7) | 639 (25) | 40 (1.6) | (N/A) |
| Age Range | 16–92 | 16–92 | 23–78 | 31–68 | 23–78 | 16–92 | 26–83 | (N/A) |
| Age, mean ± SD | 53.08 ± 15.43 | 53.38 ± 15.07 | 52.00 ± 11.43 | 50.78 ± 10.17 | 54.29 ± 13.55 | 52.23 ± 16.44 | 51.00 ± 16.77 | 0.628 |
| Female, no. (%) | 1787 (71) | 1354 (72) | 37 (75) | 22 (69) | 15 (88) | 433 (68) | 31 (77) | 0.824 |
| Meets 2010 ACR/EULAR Classification Criteria for Rheumatoid Arthritis, no. (%) | 1912 (76) | 1541 (82) | 46 (94) | 29 (91) | 17 (100) | 371 (58) | 29 (72) | <0.001 |
| Positive Rheumatoid factor serology at baseline, no. (%) | 1412 (55.9) | 1172 (62) | 34 (69) | 22 (69) | 12 (71) | 240 (38) | 26 (65) | <0.001 |
| Positive Anti-CCP serology at baseline, no. (%) | 1092 (43.3) | 917 (49) | 41 (84) | 27 (84) | 14 (82) | 175 (27) | 21 (53) | <0.001 |
| Symptom duration at baseline, mean ± SD (Days) | 188.37 ± 130.84 | 184.13 ± 106.28 | 197.49 ± 112.94 | 181.44 ± 86.67 | 227.71 ± 149.10 | 201.07 ± 185.45 | 144.25 ± 71.25 | 0.026 |
| Number of DMARDs, mean ± SD | 1.8 ± 1.1 | 2.2 ± 0.9 | 3.0 ± 0.9 | 2.9 ± 0.9 | 3.2 ± 0.9 | 0.6 ± 0.7 | 1.2 ± 0.7 | <0.001 |
| ESR, mean ± SD | ||||||||
| Baseline | 26.5 ± 22.7 | 27.63 ± 22.73 | 24.25 ± 25.61 | 22.03 ± 23.59 | 28.29 ± 29.26 | 22.75 ± 22.12 | 25.03 ± 20.86 | 0.002 |
| 12 months | 15.8 ± 15.7 | 16.00 ± 15.73 | 15.39 ± 17.69 | 13.86 ± 17.68 | 18.33 ± 17.95 | 14.62 ± 15.25 | 14.19 ± 11.91 | 0.881 |
| 18 Months | 15.7 ± 15.6 | 15.95 ± 15.56 | 14.91 ± 16.50 | 10.93 ± 15.51 | 21.63 ± 20.37 | 14.33 ± 15.85 | 14.93 ± 17.95 | 0.391 |
| 24 Months | 16.2 ± 15.6 | 16.18 ± 14.41 | 17.95 ± 19.97 | 13.75 ± 15.60 | 27.00 ± 25.54 | 15.99 ± 16.73 | 19.52 ± 20.23 | 0.203 |
| CRP, mean ± SD | ||||||||
| Baseline | 13.9 ± 17.9 | 14.84 ± 18.65 | 12.74 ± 20.07 | 12.71 ± 17.89 | 12.78 ± 24.15 | 10.82 ± 15.10 | 13.04 ± 15.66 | 0.002 |
| 12 months | 5.6 ± 9.3 | 5.58 ± 9.11 | 5.32 ± 7.90 | 4.38 ± 7.84 | 7.02 ± 7.99 | 5.35 ± 10.11 | 6.84 ± 13.79 | 0.958 |
| 18 Months | 5.4 ± 8.7 | 5.37 ± 8.36 | 4.26 ± 5.57 | 2.67 ± 3.24 | 6.69 ± 7.38 | 5.36 ± 10.47 | 7.83 ± 17.78 | 0.42 |
| 24 Months | 5.5 ± 8.8 | 5.45 ± 8.53 | 6.61 ± 10.88 | 3.55 ± 4.79 | 12.52 ± 16.17 | 6.01 ± 10.52 | 7.64 ± 12.50 | 0.053 |
| Swollen joint count (ACR 28), mean ± SEM | ||||||||
| Baseline | 7.1 ± 6.0 | 7.69 ± 6.20 | 8.18 ± 6.25 | 8.00 ± 6.61 | 8.53 ± 5.69 | 5.10 ± 4.91 | 5.95 ± 4.99 | <0.001 |
| 12 months | 1.9 ± 3.3 | 2.02 ± 3.36 | 3.59 ± 4.38 | 3.45 ± 4.59 | 3.87 ± 4.09 | 1.26 ± 2.42 | 2.03 ± 3.24 | <0.001 |
| 18 Months | 1.6 ± 3.2 | 1.66 ± 3.26 | 2.24 ± 4.47 | 0.89 ± 2.02 | 4.47 ± 6.30 | 1.19 ± 2.35 | 2.30 ± 3.63 | 0.002 |
| 24 Months | 1.4 ± 2.9 | 1.54 ± 3.00 | 1.37 ± 2.13 | 0.82 ± 1.61 | 2.36 ± 2.65 | 0.83 ± 1.65 | 1.18 ± 1.81 | 0.066 |
| DAS28, mean ± SD | ||||||||
| Baseline | 4.9 ± 1.5 | 5.02 ± 1.50 | 4.94 ± 1.41 | 4.93 ± 1.37 | 4.96 ± 1.53 | 4.35 ± 1.38 | 4.61 ± 1.49 | <0.001 |
| 12 months | 2.9 ± 1.4 | 2.93 ± 1.42 | 3.11 ± 1.55 | 2.93 ± 1.52 | 3.43 ±1.60 | 2.61 ± 1.18 | 2.86 ± 1.36 | 0.085 |
| 18 Months | 2.8 ± 1.4 | 2.86 ± 1.43 | 2.67 ± 1.52 | 2.37 ± 1.37 | 3.19 ± 1.66 | 2.73 ± 1.36 | 3.26 ± 1.70 | 0.277 |
| 24 Months | 2.8 ± 1.4 | 2.78 ± 1.38 | 2.70 ± 1.48 | 2.43 ± 1.29 | 3.26 ± 1.75 | 2.67 ± 1.80 | 2.97 ± 1.35 | 0.591 |
Notes: One-way ANOVA and Pearson’s chi-squared analyses were used. DAS28 ≤ 2.6 was defined as remission.
Methotrexate users and controls did not have significantly different baseline UA levels (P = 0.23). Between methotrexate users and controls, the mean follow-up UA levels did not differ significantly owing to high variability within each group. Within each group, the controls had a baseline UA level of 280 µmol/L and follow-up level of 282 µmol/L, providing a net difference of +2 µmol/L (P = 0.448, one-tailed t-test). Methotrexate users had a baseline UA level of 300 µmol/L prior to methotrexate and a follow-up level of 273 µmol/L, with a mean difference of −27 µmol/L (P = 0.035, one-tailed t-test). This statistically significant difference was lost when recalculated with patients taking concurrent sulfasalazine and oral methotrexate. Of the 49 methotrexate users, 32 (65%) had a decrease in UA compared to 18 (45%) of the 40 controls. There was no significant change in mean serum creatinine.
At 18 months, the mean score of DAS(ESR)28 was 2.4 for the 32 methotrexate users who experienced a decrease in UA, while the control group had a higher score of 3.3 (t-test, P = 0.042), as shown in Table 1. In the former group, the SJC at 18 months was 0.9 compared to the 17 patients on methotrexate who did not experience a decrease in UA where the SJC at 18 months was 4.5 (t-test, P = 0.035). The values of DAS(ESR)28 and SJC were not statistically different between the groups at 12 months or 24 months.
Discussion
This study of ERA patients participating in a large multicenter Canadian prospective cohort showed that those treated with methotrexate experienced a decrease in their serum UA levels that correlated with their response to this therapy. These findings support the postulated mechanism of action of methotrexate whereby inhibition of purine synthesis enzymes increases adenosine levels, while it reciprocally decreases UA. Although this mechanism requires further validation, our analysis also revealed that patients were found to have a decrease in their serum UA while receiving methotrexate therapy and were also found to have better clinical outcomes at 18 months by achieving a DAS(ESR)28 defined remission and an SJC28 score of <1.
Our finding that serum UA decreases with methotrexate therapy is in contrast to a previously published work.19 A study published in 2000 by Emery and the Multinational Leflunomide Study Group compared leflunomide versus methotrexate in the treatment of RA. Here, methotrexate was shown to increase UA levels at one year and two years. However, these increases in serum UA levels were also associated with a statistically significant increase in serum creatinine levels. As UA is mainly eliminated via renal excretion, their UA increases may have been subject to confounding. To date, there are no other published reports that examine the effects of methotrexate on serum UA levels.
The mechanism of action of methotrexate is complex. However, major advances in research over the last 30 years indicate that increase in adenosine is a key mechanism in how methotrexate exerts its anti-inflammatory effects. Sub sequently, this has led to further research of adenosine signaling pathways with further therapeutic implications.20 Increased adenosine levels, theoretically, result from decreased xanthine and UA synthesis.9–11 Our outcomes lend further support to this postulated mechanism of action. Furthermore, if the decrease in serum UA levels is from this mechanism of action, it stands to reason that serum UA levels may serve as a surrogate marker for methotrexate therapeutic monitoring. In our cohort, we were able to differentiate patients who respond clinically to methotrexate therapy versus those who do not based on their UA status.
Optimal methotrexate dosing, frequency, and route of administration in RA have been debated.1,12–14 Ideally, for optimal use, all of the abovementioned variables in medication administration would be personalized according to the pharmacodynamic response. In fact, if our hypothesis is that the adenosine–UA pathway is directly linked to the inherent mechanism of action of methotrexate, it is conceivable that serum UA levels may be used to guide the dosing, frequency, and route of administration. A change in UA levels could also be used to explore how well methotrexate is absorbed. Hoekstra et al in 2006 suggested that splitting of methotrexate doses may increase the area under the curve and the intracellular uptake of methotrexate.13 The UA-lowering effect after a single dose of methotrexate lasted only a few days with return to baseline by one week, but steady-state dosing was not done in this study.7 Serial serum UA levels could potentially inform whether splitting of methotrexate dosing could be more effective within an individual, but this has not been studied.
These results are only hypothesis generating. Perhaps early lowering of UA with methotrexate may predict clinical outcomes at 18 months, and a lack of UA reduction could determine a need for methotrexate dose adjustment to obtain optimal pharmacokinetics. It is conceivable that patients tolerating only lower doses of methotrexate have innate enzyme variants that will still increase adenosine levels via this pathway. Further research is needed to test these hypotheses.
There are limitations to this study. This study was a post hoc exploratory analysis of a subset of CATCH patients with a small sample size, and it may have been underpowered, as UA is not routinely performed, especially serially, in the management of ERA. The earliest follow-up UA levels were usually done at 12 months, which was many months following methotrexate initiation. Despite this, we were able to show a significant change in serum UA levels in patients taking methotrexate. In addition, due to limited data, we were not able to perform complex analyses to adjust for potential confounding, such as alcohol consumption, diet, or antihypertensive medications, such as diuretics or angiotensin receptor blockers. Using these prospectively collected data across multiple centers throughout Canada, clinical outcomes were predicted by lowering UA from methotrexate therapy. Baseline characteristics such as antibody status, percentage of patients meeting the 2010 ACR/EULAR classification criteria for RA, the number of DMARDs used, and clinical measures, such as SJC28 and DAS28, did differ between groups. These statistical confounders are likely reflected in certain differences such as that seen between the RF positive group compared to the anti-CCP antibody positive group. Therefore, beyond the primary outcome measure of change in UA in response to methotrexate, these results cannot be generalized. However, within the methotrexate user group, baseline characteristics were very similar between patients who experienced a decrease in UA levels vs. those who did not. Due to small numbers and side effects being uncommon, we did not study UA response and adverse drug reactions to methotrexate.
Conclusions
Methotrexate may be effective in RA via an increase in adenosine levels. In accordance with our hypothesis that this is directly linked to xanthine metabolism, UA levels were shown to be reciprocally decreased in a clinical setting for patients taking methotrexate for ERA. Those who decreased serum UA levels with methotrexate had improved clinical responses compared to controls. More studies are needed to test this hypothesis.
Key Messages
– Methotrexate therapy for the treatment of ERA was associated with a decrease in serum UA levels.
– Monitoring of serum UA levels in response to methotrexate therapy may allow an early prediction of clinical outcomes.
– Further research is needed to elucidate the predictive power of serum UA levels in response to methotrexate therapy in RA.
Acknowledgments
CATCH investigators are Vandana Ahluwalia, Pooneh Akhavan, Hector Arbillaga, Murray Baron, Mary Bell, William Bensen, Gilles Boire, Vivian Bykerk, Alf Cividino, Ines Colmegna, Paul Haraoui, Carol Hitchon, Shahin Jamal, Edward Keystone, Alice Kinkhoff, Majed Kraishi, Maggie Larche, Chris Lyddell, Henri Menard, Dianne Mosher, Bindu Nair, Erin Norris, Chris Penney, Janet Pope, Laurence Rubin, Emily Shaw, Evelyn Sutton, Carter Thorne, and Michel Zummer.
The CATCH study was designed and implemented by the investigators and financially supported initially by Amgen Canada Inc. and Pfizer Canada Inc. via an unrestricted research grant since the inception of CATCH. As of 2011, further support was provided by Hoffmann-La Roche Ltd., United Chemicals of Belgium (UCB) Canada Inc., Bristol-Myers Squibb Canada Co., AbbVie Inc., and Janssen Biotech, Inc. (a wholly owned subsidiary of Johnson & Johnson Inc.). Some funds for analysis were from the Program of Experimental Medicine, Department of Medicine, University of Western Ontario, London, ON, Canada.
Footnotes
ACADEMIC EDITOR: Chuanju Liu, Editor in Chief
PEER REVIEW: Three peer reviewers contributed to the peer review report. Reviewers’ reports totaled 389 words, excluding any confidential comments to the academic editor.
FUNDING: Authors disclose no external funding sources for this nested case-control study within the CATCH study. Funders of the CATCH study are listed in the Acknowledgements section of this paper.
COMPETING INTERESTS: CT discloses grants and personal fees from Abbvie, Amgen, Celgene, Novartis, and Pfizer, and personal fees from Centocor, Genzyme, Hospira, Janssen, Lilly, Merck, Sanofi and Medexus/Medac. VB discloses personal funding from Abbvie, Bristol Myers Squibb, Regeneron and UCB. Other authors disclose no potential conflicts of interest.
Paper subject to independent expert blind peer review. All editorial decisions made by independent academic editor. Upon submission manuscript was subject to anti-plagiarism scanning. Prior to publication all authors have given signed confirmation of agreement to article publication and compliance with all applicable ethical and legal requirements, including the accuracy of author and contributor information, disclosure of competing interests and funding sources, compliance with ethical requirements relating to human and animal study participants, and compliance with any copyright requirements of third parties. This journal is a member of the Committee on Publication Ethics (COPE).
Author Contributions
Conceived and designed the experiments: JJL, GKD, JEP. Analyzed the data: JJL, JEP. Wrote the first draft of the manuscript: JJL. Contributed to the writing of the manuscript: JJL, VPB, GB, BH, CH, CT, DT, SJ, ECK, JEP. Agree with manuscript results and conclusions: JJL, VPB, CH, CT, DT, ECK, JEP. Jointly developed the structure and arguments for the paper: JJL, VPB, GB, BH, CH, CT, DT, SJ, ECK, JEP. Made critical revisions and approved final version: JJL, VPB, GB, BH, CH, CT, DT, SJ, ECK, JEP. All authors reviewed and approved of the final manuscript.
Supplementary Material
Supplementary Figure 1. Purine synthesis pathway. Methotrexate is thought to work by indirectly inhibiting ADA and increasing adenosine levels. ADA is involved in purine synthesis, including UA.
REFERENCES
- 1.Weinblatt ME. Methotrexate in rheumatoid arthritis: a quarter century of development. Trans Am Clin Climatol Assoc. 2013;124:16–25. [PMC free article] [PubMed] [Google Scholar]
- 2.Verschueren P, De Cock D, Corluy L, et al. Methotrexate in combination with other DMARDs is not superior to methotrexate alone for remission induction with moderate-to-high-dose glucocorticoid bridging in early rheumatoid arthritis after 16 weeks of treatment: the CareRA trial. Ann Rheum Dis. 2015;74(1):27–34. doi: 10.1136/annrheumdis-2014-205489. [DOI] [PubMed] [Google Scholar]
- 3.Weinblatt ME, Kaplan H, Germain BF, et al. Methotrexate in rheumatoid arthritis. A five-year prospective multicenter study. Arthritis Rheum. 1994;37(10):1492–8. doi: 10.1002/art.1780371013. [DOI] [PubMed] [Google Scholar]
- 4.Weinblatt ME, Kaplan H, Germain BF, et al. Low-dose methotrexate compared with auranofin in adult rheumatoid arthritis. A thirty-six-week, double-blind trial. Arthritis Rheum. 1990;33(3):330–8. doi: 10.1002/art.1780330305. [DOI] [PubMed] [Google Scholar]
- 5.Williams HJ, Willkens RF, Samuelson CO, Jr, et al. Comparison of low-dose oral pulse methotrexate and placebo in the treatment of rheumatoid arthritis. A controlled clinical trial. Arthritis Rheum. 1985;28(7):721–30. doi: 10.1002/art.1780280702. [DOI] [PubMed] [Google Scholar]
- 6.Katerelos M, Mudge SJ, Stapleton D, et al. 5-aminoimidazole-4-carboxamide ribonucleoside and AMP-activated protein kinase inhibit signalling through NF-kappaB. Immunol Cell Biol. 2010;88(7):754–60. doi: 10.1038/icb.2010.44. [DOI] [PubMed] [Google Scholar]
- 7.Smolenska Z, Kaznowska Z, Zarowny D, Simmonds HA, Smolenski RT. Effect of methotrexate on blood purine and pyrimidine levels in patients with rheumatoid arthritis. Rheumatology (Oxford) 1999;38(10):997–1002. doi: 10.1093/rheumatology/38.10.997. [DOI] [PubMed] [Google Scholar]
- 8.Morabito L, Montesinos MC, Schreibman DM, et al. Methotrexate and sulfasalazine promote adenosine release by a mechanism that requires ecto-5′-nucleotidase-mediated conversion of adenine nucleotides. J Clin Invest. 1998;101(2):295–300. doi: 10.1172/JCI1554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cronstein BN, Eberle MA, Gruber HE, Levin RI. Methotrexate inhibits neutrophil function by stimulating adenosine release from connective tissue cells. Proc Natl Acad Sci U S A. 1991;88(6):2441–5. doi: 10.1073/pnas.88.6.2441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Baggott JE, Vaughn WH, Hudson BB. Inhibition of 5-aminoimidazole-4-carboxamide ribotide transformylase, adenosine deaminase and 5′-adenylate deaminase by polyglutamates of methotrexate and oxidized folates and by 5-aminoimidazole-4-carboxamide riboside and ribotide. Biochem J. 1986;236(1):193–200. doi: 10.1042/bj2360193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Allegra CJ, Drake JC, Jolivet J, Chabner BA. Inhibition of phosphoribosylami-noimidazolecarboxamide transformylase by methotrexate and dihydrofolic acid polyglutamates. Proc Natl Acad Sci U S A. 1985;82(15):4881–5. doi: 10.1073/pnas.82.15.4881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schiff MH, Jaffe JS, Freundlich B. Head-to-head, randomised, crossover study of oral versus subcutaneous methotrexate in patients with rheumatoid arthritis: drug-exposure limitations of oral methotrexate at doses ≥15 mg may be overcome with subcutaneous administration. Ann Rheum Dis. 2014;73(8):1549–51. doi: 10.1136/annrheumdis-2014-205228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hoekstra M, Haagsma C, Neef C, Proost J, Knuif A, van de Laar M. Splitting highzdose oral methotrexate improves bioavailability: a pharmacokinetic study in patients with rheumatoid arthritis. J Rheumatol. 2006;33(3):481–5. [PubMed] [Google Scholar]
- 14.Hoekstra M, Haagsma C, Neef C, Proost J, Knuif A, van de Laar M. Bioavailability of higher dose methotrexate comparing oral and subcutaneous administration in patients with rheumatoid arthritis. J Rheumatol. 2004;31(4):645–8. [PubMed] [Google Scholar]
- 15.den Boer E, de Rotte MC, Pluijm SM, Heil SG, Hazes JM, de Jonge R. Determinants of erythrocyte methotrexate polyglutamate levels in rheumatoid arthritis. J Rheumatol. 2014;41(11):2167–78. doi: 10.3899/jrheum.131290. [DOI] [PubMed] [Google Scholar]
- 16.Dervieux T, Zablocki R, Kremer J. Red blood cell methotrexate polyglutamates emerge as a function of dosage intensity and route of administration during pulse methotrexate therapy in rheumatoid arthritis. Rheumatology (Oxford) 2010;49(12):2337–45. doi: 10.1093/rheumatology/keq216. [DOI] [PubMed] [Google Scholar]
- 17.Aletaha D, Neogi T, Silman AJ, et al. 2010 Rheumatoid arthritis classification criteria: an American College of Rheumatology/European League Against Rheumatism Collaborative Initiative. Arthritis Rheum. 2010;62(9):2569–81. doi: 10.1002/art.27584. [DOI] [PubMed] [Google Scholar]
- 18.Urquhart BL, Gregor JC, Chande N, Knauer MJ, Tirona RG, Kim RB. The human proton-coupled folate transporter (hPCFT): modulation of intestinal expression and function by drugs. Am J Physiol Gastrointest Liver Physiol. 2010;298(2):G248–54. doi: 10.1152/ajpgi.00224.2009. [DOI] [PubMed] [Google Scholar]
- 19.Emery P, Breedveld FC, Lemmel EM, et al. A comparison of the efficacy and safety of leflunomide and methotrexate for the treatment of rheumatoid arthritis. Rheumatology (Oxford) 2000;39(6):655–65. doi: 10.1093/rheumatology/39.6.655. [DOI] [PubMed] [Google Scholar]
- 20.Hasko G, Linden J, Cronstein B, Pacher P. Adenosine receptors: therapeutic aspects for inflammatory and immune diseases. Nat Rev Drug Discov. 2008;7(9):759–70. doi: 10.1038/nrd2638. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1. Purine synthesis pathway. Methotrexate is thought to work by indirectly inhibiting ADA and increasing adenosine levels. ADA is involved in purine synthesis, including UA.