Abstract
Cigarette smoke has been associated with a number of pathologies; however, the mechanisms leading to developmental effects are yet to be fully understood. The zebrafish embryo is regarded as a ‘bridge model’; however, not many studies examined its applicability to cigarette smoke toxicity. This study examined the effects of total particulate matter (TPM) from 3R4F reference cigarettes on the early development of zebrafish (Danio rerio). Zebrafish embryos were exposed to two concentrations of TPM (0.4 and 1.4 μg/mL equi-nicotine units) or nicotine at equivalent doses. The exposures began at 2 h post-fertilization (hpf) and lasted until 96 hpf. Several physiological parameters were assessed during or after the exposure. We show that TPM increased mortality, delayed hatching, and increased the incidence of deformities in zebrafish. TPM exposure also increased the incidence of hemorrhage and disrupted the angiogenesis of the major vessels in the brain. Moreover, TPM exposure reduced the larval body length, decreased the heart rate, and reduced the metabolic rate. Biomarkers of xenobiotic metabolism and oxidative stress were also affected. TPM-exposed zebrafish also differed behaviorally: at 24 hpf the embryos had a higher frequency of spontaneous contractions and at 144 hpf the larvae displayed swimming hyperactivity. This study demonstrates that TPM disrupts several aspects of early development in zebrafish. The effects reported for TPM were not attributable to nicotine, since embryos treated with nicotine alone did not differ significantly from the control group. Collectively, our work illustrates the utility of zebrafish as an alternative model to evaluate the toxic effects of cigarette smoke constituents.
Keywords: Cigarette smoke, Total particulate matter, Early development, Toxicity, Behavior, Zebrafish
1. Introduction
It is estimated that cigarette smoking has contributed to premature mortality of >20 million Americans since 1964, and although cigarette smoking among adults in the US has declined from 42% in 1965 to 18% in 2012, it remains the main preventable mortality risk factor with more than 42 million Americans still smoking (US Department of Health and Human Services, 2014). As concluded in the Surgeon General Report (US Department of Health and Human Services, 2004) tobacco smoking impacts almost every organ in the body and the list of tobacco-related diseases continues to increase. Evidence gathered over the past 50 years is sufficient to infer causal relationship between active smoking and lung cancer, hepatocellular carcinoma, and colorectal cancer. Smoking also increases the risk of stroke and tuberculosis, as well as exacerbates asthma, ectopic pregnancy, erectile dysfunction, neovascular and atrophic forms of age-related macular degeneration, rheumatoid arthritis, and diminished overall health (US Department of Health and Human Services, 2014).
Cigarette smoke is a dynamic and complex mixture of >7000 different chemicals, including 69 carcinogens. Cigarette smoke is composed of the i) gas phase, which contains gases and volatile organic compounds, and ii) particulate phase [total particulate matter (TPM)], which, among others, contains nicotine, tobacco-specific nitrosamines, heavy metals, aromatic amines, heterocyclic amines, and polycyclic aromatic hydrocarbons (PAHs) (US Department of Health and Human Services, 2010). It has been shown that many of the components of cigarette smoke, including CO, nicotine, and PAHs (although minor constituents), can permeate across the placenta (Liszewski et al., 2012).
Tobacco smoke exposure in humans during development has been associated with detrimental effects during pre- and postnatal development (Rogers, 2009). It has also been suggested that maternal smoking is associated with 15% of all preterm births, 20–30% cases of low birth weight, and a 150% increase in overall perinatal mortality (Andres and Day, 2000). Maternal smoking was also associated with neonatal malformations, including cardiovascular/heart and musculoskeletal defects (Hackshaw et al., 2011), as well as neurobehavioral effects, including hyperactivity, attention deficit disorder, and learning disabilities (Rogers, 2009).
Although the effects of smoking have been researched extensively since the study of Doll and Hill (1964), the mechanisms through which the various constituents of cigarette smoke cause pathological changes, including developmental defects, remain unknown. The majority of studies investigating the effects of cigarette smoke have utilized mammalian model systems, both in vitro and in vivo. Notably, each model has its own limitations — the in vitro systems allow for a detailed analysis of important cellular pathways and mechanisms, but are unable to provide the whole-organism perspective; conversely, the in vivo systems (such as murine models) permit the analysis of effects on a whole-organism level, but are expensive, labor-intensive, and increasingly criticized ethically (Yang et al., 2009). Although no single model can truly mimic the effects of cigarette smoke on humans, the zebrafish embryo could be regarded as a ‘bridge model’ between in vitro and in vivo mammalian systems, while minimizing the costs associated with in vivo mammalian systems.
Zebrafish (Danio rerio) have been proposed as a model for human disease (Lieschke and Currie, 2007) and have been mostly used in developmental biology and molecular genetics. One of the advantages of the zebrafish model is that the embryos can be exposed as early as 1–4-cell stage for investigation of the effects on very early embryonic development, whereas with murine models the exposure typically begins much later [embryonic day 4, also known as blastocyst stage (at least 32 cells)] (Matta et al., 2007). Zebrafish have also been recognized as a valuable vertebrate model for toxicology and drug discovery (Fako and Furgeson, 2009; Hill et al., 2005; Yang et al., 2009) and a preclinical model for behavioral effects of nicotine (Klee et al., 2011).
More recently (during preparation of this manuscript), the zebrafish model was suggested for the study of developmental toxicity of cigarette smoke condensate (CSC; the authors use the terms ‘CSC’ and ‘TPM’ interchangeably) by Health Canada scientists (Ellis et al., 2014). In that study the embryos were exposed between 6 and 72 hpf to various concentrations of TPM (10–50 μg TPM/mL) that originated from several cigarette brands. The study reported that TPM reduced viability and hatching success, increased incidence of deformities, decreased pigmentation, and resulted in reduced swimming behavior. In addition, the transcript abundance of several genes associated with apoptosis, detoxification, regulation, and pigmentation were affected.
The goal of this study was twofold: (i) to investigate the developmental toxicity of TPM in zebrafish embryos and larvae, and (ii) compare TPM toxicity to that of nicotine. We hypothesize that the effects of TPM on embryo development are driven by the complex nature of this mixture rather than single chemicals, such as nicotine, which is an abundant constituent of TPM. Thus, we have evaluated the development of zebrafish embryos during a four day exposure to TPM or nicotine and assessed multiple developmental, biochemical, and behavioral parameters as summarized in Fig. 1. Several of these parameters were not previously assessed by Ellis et al. (2014).
Fig. 1.
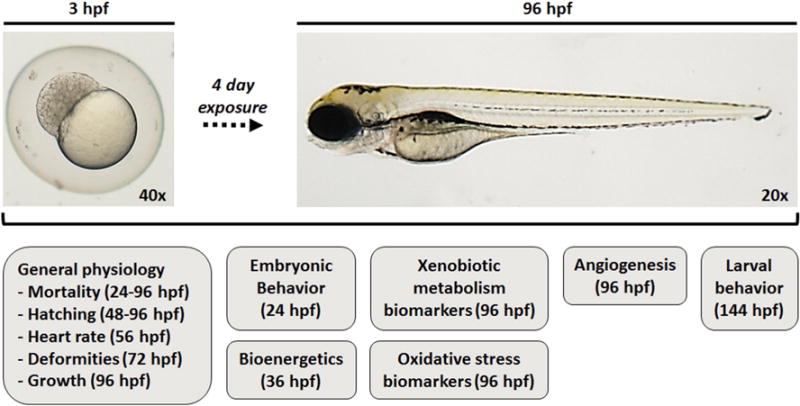
Summary of the parameters that were analyzed at various time points in this study.
2. Materials and methods
2.1. TPM and nicotine
TPM was prepared at Labstat International Inc. (Kitchener, ON, Canada) by mechanical smoking of 3R4F reference cigarettes (University of Kentucky) following the ISO standard 3308:2012, which defines a puff volume of 35 mL, an interval of 60 s between puffs, and a puff duration of 60 s (Johnson et al., 2009). TPM is then dissolved in DMSO at a concentration of 20 mg TPM/mL to generate the stock solution that was used to prepare the working solutions of TPM. The partial analysis of the TPM stock solution was performed at Labstat International and the data are summarized in Table S1. Nicotine hydrogen tartrate salt was purchased from Sigma Aldrich (N5260) and used in nicotine exposures.
2.2. Zebrafish embryo collection
Adult wild type EkkWill zebrafish (EkkWill Waterlife Resources, Ruskin, FL, USA) were maintained in holding tanks on a 14:10 h light–dark cycle at 28 °C in circulating AHAB system (Aquatic Habitats, Apopka, FL, USA) in 60 mg/L salt water (Instant Ocean, Foster & Smith, Rhinelander, WI, USA). Fish were fed brine shrimp in the morning and a mixture of Zeigler’s Adult Zebrafish Complete Diet (Aquatic Habitats) and Cyclop-eeze (Argent Chemical Laboratories, Redmond, WA, USA) in the afternoon. Breeding tanks were set at 5 PM, and embryos were collected the following morning within 1 h of spawning between 9 and 10 AM and kept in 30% Danieau supplemented with 0.00003% methylene blue at 28 °C until separated into experimental groups. All procedures were approved by the Duke University Institutional Animal Care & Use Committee (A279-08-10).
2.3. Experimental set-up
At 2 h post-fertilization (hpf) 15 embryos were randomly assigned into 6 cm diameter glass Petri dishes containing 15 mL 30% Danieau, and separated into treatments. Each treatment had several replicate dishes, such that each dish was used for a specific assay, but the viability, hatching, and deformities were assessed in all replicate dishes. The experiment was repeated using at least 3 zebrafish cohorts obtained during separate breeding events. To make the study less biased towards the inherent variation across replicate dishes and embryo cohorts, the replicate dishes were averaged for the assessment of physiological endpoints (see Table S2 for more details). Thus, all of the reported n-values in this manuscript refer to the number of cohorts, rather than individual embryos or replicate dishes.
The embryos were exposed to two concentrations of TPM, corresponding to 0.4 and 1.4 μg/mL equi-nicotine units [nicotine concentration (μg/mL) in the TPM preparation] as described previously (Arimilli et al., 2012); these concentrations are abbreviated as TPM0.4 and TPM1.4, respectively (see Table S1 for more details on the preparation of TPM0.4 and TPM1.4). These concentrations were chosen based on a preliminary experiment, which showed that TPM0.4 causes several deformities in 10–40% of the embryos. We decided to include a higher dose in order to verify if additional deformities can be observed. Thus, subsequent analyses also included TPM1.4, which is the highest possible concentration using the available TPM stock solution without exceeding 0.1% DMSO in the embryo medium. For an easier comparison with the findings from Ellis et al. (2014), the two aforementioned doses correspond to 6 and 20 μg TPM/mL, respectively. Notably, TPM0.4 is lower than, while TPM1.4 is within the range of concentrations used by Ellis et al. (2014).
Two nicotine concentrations of 0.4 and 1.4 μg/mL were also used; these doses correspond to 2.5 and 8.5 μM of nicotine (see Supplemental material for more details on preparation of nicotine dosing solutions). Final DMSO concentration was 0.1% in all cases. Control embryos received an equivalent volume of DMSO. The exposure lasted 4 days. At the end of the exposure the larvae were euthanized on ice, rinsed with ice-cold distilled water, and flash-frozen in liquid nitrogen unless otherwise specified. The frozen samples were kept at −80 °C until analyzed. Separate trials were conducted to assess bioenergetics and larval behavior (see Supplemental material).
Additional exposures to TPM were performed with embryos starting at 24 hpf (vs. 2 hpf as described above), in order to assess the specificity of TPM effects. Several, but not all endpoints, were assessed in these embryos, including deformities, pericardial area, mortality, and hatching.
2.4. Toxicity assessment
2.4.1. General physiology
During the exposure the embryos/larvae were monitored daily for viability; dead embryos/larvae were removed. Hatching success was assessed starting at 48 hpf. Heart rate was measured at 56 hpf at random using microscopic observations. At 72 hpf larvae were screened for main deformities, including hemorrhage, bent notochord, pericardial edema, string heart, and truncated jaw. Selected larvae were also used to assess pericardial area, which was measured digitally using the bright field images obtained during the CYP1A activity assessment (see details below). Images of selected larvae were also captured at 96 hpf to measure body length and eye diameter. Larval mass was also assessed, using pre-weighed 1.5 mL Eppendorf tubes.
2.4.2. Hemoglobin and angiogenesis staining
During the exposure the embryos to be used for staining were treated with phenylthiourea (0.2 mM) at 24 hpf to prevent pigment formation. At 96 hpf the larvae were fixed in 4% paraformaldehyde for 30 min at room temperature, and transferred to 50% methanol for 5 min and then into 100% methanol; the larvae were stored at −20 °C until stained (see Supplemental material). Hemoglobin analysis was performed using o-Dianisidine staining according to Eisa-Beygi et al. (2013) in preserved embryos to confirm incidences of hemorrhage. Angiogenesis was assessed in preserved larvae, using alkaline phosphatase staining according to Schulte-Merker (2002).
2.4.3. Biomarkers of xenobiotic metabolism
The activity of key enzyme of phase I xenobiotic metabolism – CYP1A (EC 1.14.14.1) – was assessed using the ethoxyresorufin-O-deethylase (EROD) in vivo assay as described in Timme-Laragy et al. (2007). At 72 hpf the larvae were anesthetized and visualized using a rhodamine red-fluorescent filter set under 50× magnification (Zeiss Axioskop, Thornwood, NY). The activity was estimated from the fluorescence intensity of the gastrointestinal tract, which was quantified digitally by IP Lab software (Scanalytics Inc., Fairfax, VA).
The activity of key enzyme of phase II xenobiotic metabolism – glutathione-S-transferase (GST; 2.5.1.18) – was assessed in larval extracts (see Supplemental material) according to Lushchak et al. (2001). Enzyme activity was assessed using a SpectraMax M5 Spectrophotometer (Molecular Devices, Sunnyvale, CA) and SOFTmax Pro software, and normalized to protein concentrations assessed using the bicinchoninic acid assay method (Sigma) and bovine serum albumin (BSA) standards.
2.4.4. Biomarkers of oxidative stress
The activities of antioxidant enzymes and the glutathione concentrations were assessed in preserved larvae. The activities of glutathione peroxidase (GPx; EC 1.11.1.9), glutathione reductase (GR; EC 1.8.1.7), and catalase (CAT; EC 1.11.1.6) were assessed according to Lushchak et al. (2001), and the activity of superoxide dismutase (SOD; EC 1.15.1.1) was assessed using a commercially available kit (Sigma Aldrich) as outlined in Massarsky et al. (2013). The activities were measured in larval extracts (see Supplemental material) and normalized to protein concentrations as above.
The concentrations of total glutathione (TGSH) and oxidized glutathione (GSSG) were measured in larval extracts (see Supplemental material) according to Hermes-Lima and Storey (1996) as adopted for zebrafish embryos (Massarsky et al., 2013). The concentrations of reduced glutathione (GSH) were calculated based on the formula (TGSH = GSH + 2GSSG). The ratio of GSSG:TGSH was also calculated.
2.4.5. Bioenergetics
Bioenergetic analysis was conducted in vivo based on oxygen consumption rate (OCR) measured using the XFe24 Extracellular Flux Analyzer (Seahorse Bioscience, Billerica, MA), according to Stackley et al. (2011) with some modifications. A total of 10 trials were conducted (see Supplemental material). Basal OCR (None) and OCR in the presence of pharmacological agents carbonyl cyanide 4-(trifluoromethoxy) phenylhydrazone (FCCP; a mitochondrial uncoupler), oligomycin (Oligo; an inhibitor of the proton channel of ATP synthase), and sodium azide (NaN3; an inhibitor of cytochrome c oxidase) were measured (see Supplemental material for details).
2.4.6. Behavior
Spontaneous contractions of the trunk are the earliest embryonic motor behavior that is mediated by the spinal circuit independently of the main neurotransmitter systems (Saint-Amant and Drapeau, 2000). The spontaneous contraction frequency was assessed at 24 hpf by counting the number of contractions during a 2 min interval.
Larval behavior was also assessed, using a light:dark behavioral test for larval swimming activity, which also captures larval capacity to adapt to changing environmental stimuli (Ahmad et al., 2012; Padilla et al., 2011). The details are outlined in Supplemental material. Only the TPM0.4-exposed larvae lacking any visual deformities were used for this analysis.
2.5. Statistical analysis
Statistical analyses were conducted using SigmaPlot (SPW 12; Systat Software, Inc., San Jose, CA, USA). One-way Analysis of Variance (ANOVA) with a post-hoc Tukey method was used to assess significant differences in deformities between control/TPM0.4/TPM1.4. Two-way ANOVA with a post-hoc Tukey method was used to compare between control/TPM0.4/TPM1.4 and control/Nic0.4/Nic1.4 when applicable. Two-way ANOVAs were also used to test for significance between treatments (TPM or Nic) in the presence of other experimental factors (e.g. time). The statistical tests are specified in Figure/Table legends. In all cases an n-value of at least 3 was used and p ≤ 0.05 was considered significant.
3. Results
3.1. General physiology
Several effects were observed in TPM-exposed zebrafish. Already at 48 hpf hemorrhage was noted in TPM0.4-exposed embryos, and TPM1.4-exposed embryos displayed hemorrhage and pericardial edema (Fig. 2A). Moreover, TPM1.4 embryos had less pigment at 48 hpf, and displayed the presence of some particles inside the chorion at 24 and 48 hpf (Fig. 2A). In addition, both TPM treatments resulted in pericardial edema, string heart, truncated jaw, and thicker yolk (i.e. slower yolk depletion), but TPM1.4 exposure also resulted in bent notochord (Fig. 2B). The string heart phenotype is shown in Fig. 2C. No developmental differences were observed with nicotine exposure (Fig. 3). The incidence of deformities is summarized in Fig. 4. Furthermore, the extent of pericardial edema was significantly increased with TPM, but not nicotine (Fig. 5A). The aforementioned hemorrhage in TPM-exposed embryos was confirmed using hemoglobin staining, which showed that in control larvae the hemoglobin was localized primarily in pericardium, whereas in TPM-exposed larvae the hemoglobin was also present in other regions (Fig. 5B).
Fig. 2.
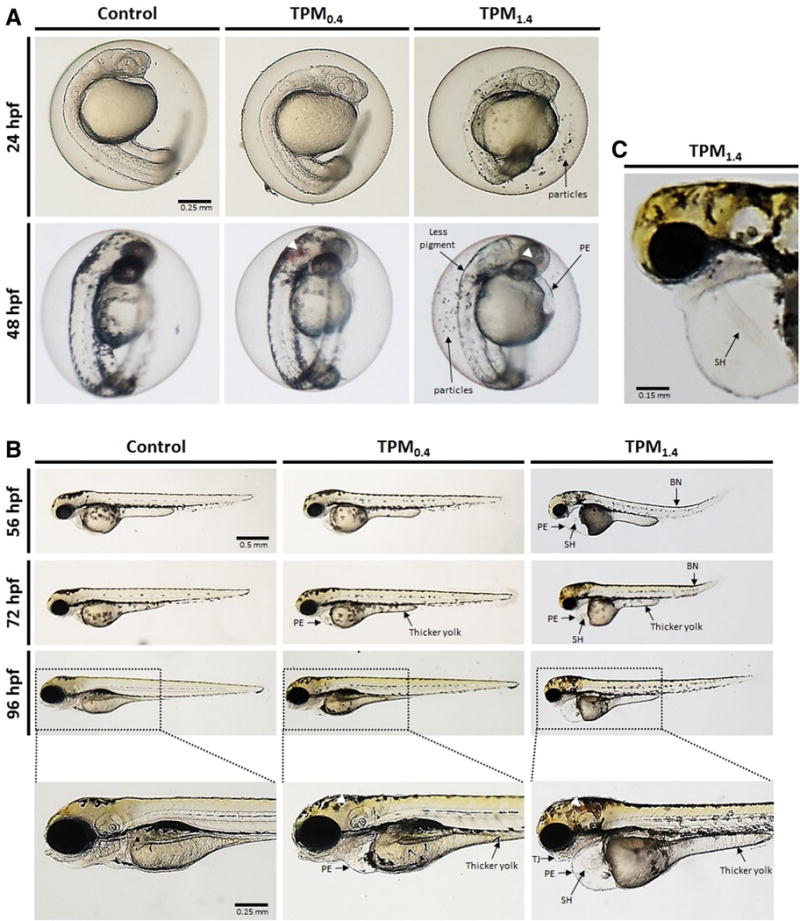
Development of zebrafish embryos throughout the four day exposure to TPM0.4 and TPM1.4. A. Zebrafish embryos at 24 and 48 hpf. B. Zebrafish larvae at 56, 72, and 96 hpf. C. Magnified image of TPM1.4-exposed larvae at 96 hpf displaying the string heart phenotype. The following deformities are abbreviated: pericardial edema (PE), bent notochord (BN), string heart (SH), truncated jaw (TJ). Hemorrhage is indicated by white arrowheads.
Fig. 3.
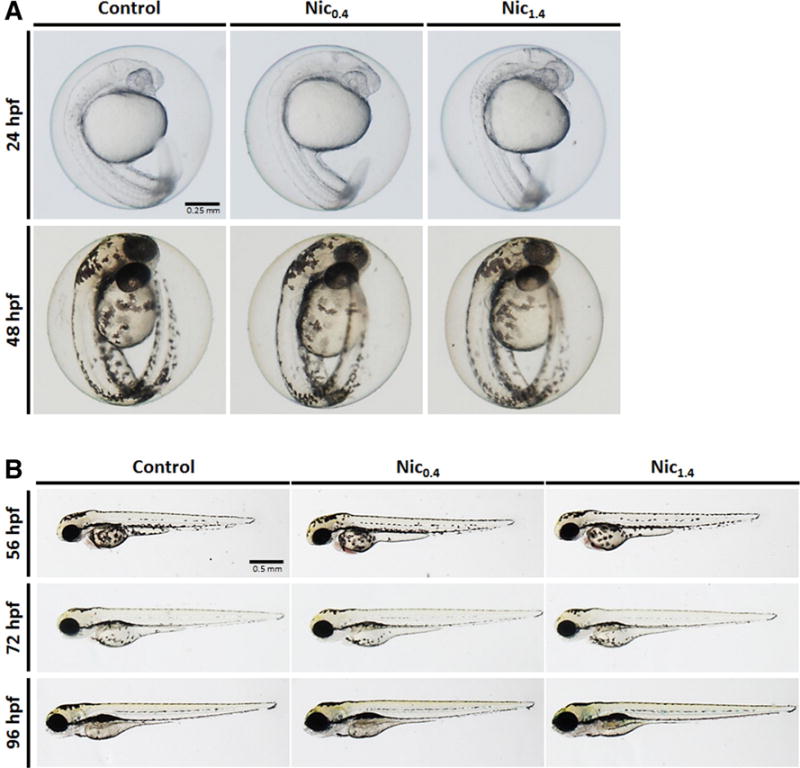
Development of zebrafish embryos throughout the four day exposure to nicotine (Nic) at 0.4 and 1.4 μg/mL. A. Zebrafish embryos at 24 and 48 hpf. B. Zebrafish larvae at 56, 72, and 96 hpf.
Fig. 4.
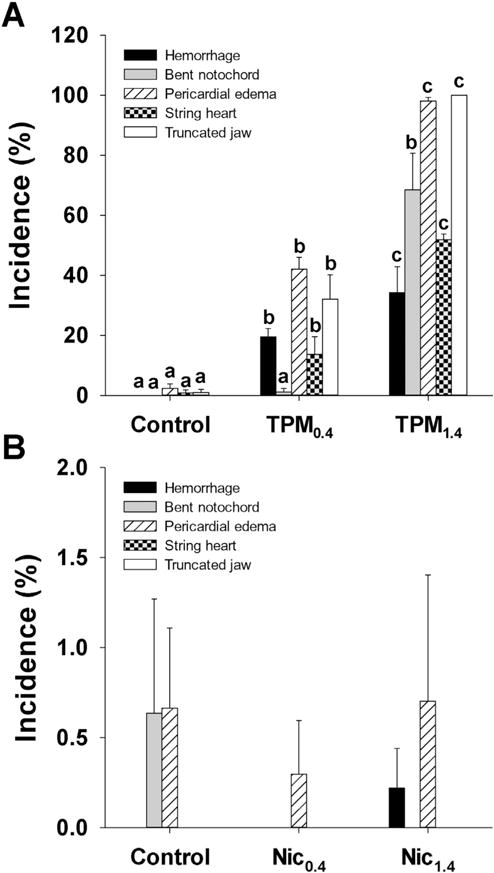
Incidence of deformities in 72 hpf zebrafish larvae exposed to TPM (A) or nicotine (B). Means + SEM (n = 3–6) are displayed. One-way ANOVA with a post hoc Tukey method was used to assess statistical differences. The lower case letters indicate differences between treatments within each type of deformity.
Fig. 5.
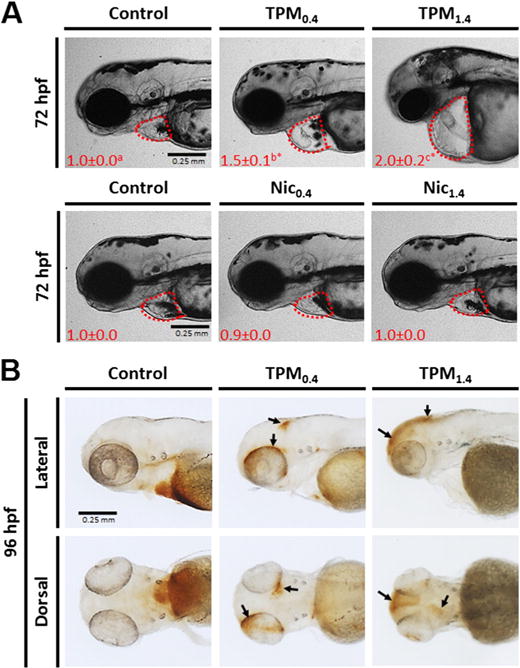
A. Pericardial area in 72 hpf zebrafish larvae exposed to TPM or nicotine. The numbers in red indicate mean ± SEM (n = 3–4) pericardial area as fold change. Two-way ANOVA with a post hoc Tukey method was used to assess statistical differences. The lower case letters indicate differences between concentrations within the same treatment, whereas asterisks (*) indicate significant differences between treatments within a given concentration. B. TPM-exposed zebrafish larvae (96 hpf) were stained for hemoglobin using o-Dianisidine. Areas of hemorrhage are noted with arrows on the lateral and dorsal images.
Moreover, TPM, but not nicotine, increased mortality such that the cumulative mortality rates at 96 hpf were 17% and 38% for TPM0.4 and TPM1.4, respectively, compared to 7% in the control group (Fig. S1). Exposure to TPM, but not nicotine, also affected hatching success, especially with TPM1.4 treatment, where only 75% embryos hatched by 96 hpf (Fig. S2). TPM exposure also affected several other endpoints. Embryos exposed to TPM1.4 had significantly shorter body length at 96 hpf (Fig. S3A) without impact on body mass (Fig. S3B). Both the TPM0.4 and TPM1.4 significantly reduced the diameter of the eye in 96 hpf larvae (Fig. S3C), but these changes were not significant when normalized to the body length (Fig. S3D). The heart rate at 56 hpf was also significantly decreased by TPM1.4 (Fig. S3E). These additional endpoints were not affected by nicotine.
Exposing zebrafish to TPM starting at 24 hpf had similar outcomes. The exposed embryos had the aforementioned deformities (assessed at 72 hpf) in similar proportions as before (Fig. S4A) and had increased pericardial area (assessed at 96 hpf; Fig. S4B). The delay in hatching at 72 hpf was also comparable (Fig. S4C). Among the measured parameters, mortality was the one affected the most by the later exposure start time, such that embryos exposed starting at 24 hpf had lower total mortality (~twice less) at 96 hpf (Fig. S4C) compared with the embryos that were exposed starting at 2 hpf.
3.2. Angiogenesis
The formation of major blood vessels, including dorsal longitudinal anastomotic vessel (DLAV), dorsal aorta (DA), intersegmental vessels (Se), posterior (caudal) cardinal vein (PCV), subintestinal vein (SIV) were not affected by TPM exposure (Fig. 6, Lateral); however, the shape of the heart was affected, with TPM1.4-treated larvae displaying the string heart as noted earlier (Fig. 6, Lateral). The majority of larvae in the control and TPM0.4-treated larvae displayed a typical vascular pattern in the brain, including the mesencephalic vein (MsV), metencephalic artery (MtA), middle cerebral vein (MCeV), posterior (caudal) cerebral vein (PCeV), dorsal longitudinal vein (DLV), and dorsal midline junction (DMJ). This pattern was disrupted in TPM1.4-treated larvae such that some vessels seemed barely visible or even absent (Fig. 6, Dorsal). TPM treatment increased the occurrence of the abnormal vessel pattern in the brain from 10 ± 7% in controls to 27 ± 8% in TPM0.4, and 69 ± 8% in TPM1.4. The increased occurrence of the abnormal vessel pattern in TPM1.4-treated larvae was statistically significant when compared to control and TPM0.4; the difference between TPM0.4 and control groups was not statistically significant. Nicotine did not affect angiogenesis as the vessel patterns in nicotine-exposed and control larvae were similar (data not shown).
Fig. 6.
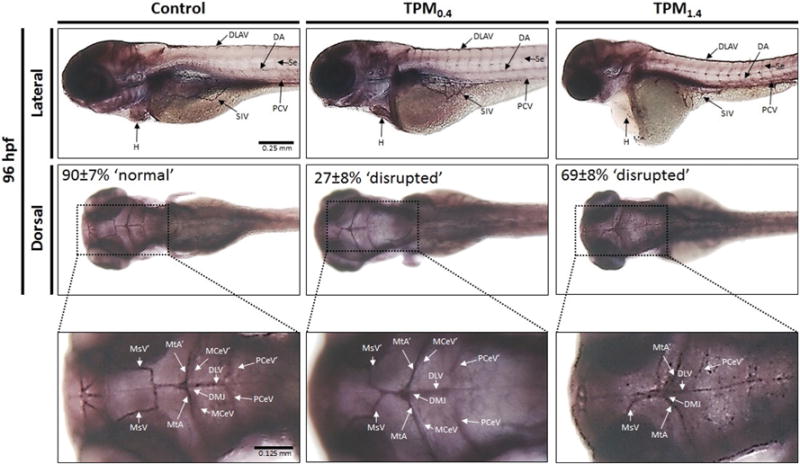
Angiogenesis (alkaline phosphatase staining) in 96 hpf zebrafish larvae exposed to TPM0.4 and TPM1.4. The lateral images show the dorsal longitudinal anastomotic vessel (DLAV), dorsal aorta (DA), intersegmental vessels (Se), posterior (caudal) cardinal vein (PCV), subintestinal vein (SIV), and the heart (H). The dorsal images show the mesencephalic vein (MsV), metencephalic artery (MtA), middle cerebral vein (MCeV), posterior (caudal) cerebral vein (PCeV), dorsal longitudinal vein (DLV), and dorsal midline junction (DMJ). The percentages on the dorsal images correspond to the proportion of larvae displaying the ‘normal’ or ‘disrupted’ phenotype.
3.3. Biomarkers of xenobiotic metabolism
The in vivo activity of CYP1A was significantly reduced by TPM1.4 in 72 hpf larvae (Table 1). In contrast, the activity of GST in extracts of 96 hpf larvae increased in TPM-treated fish, but this increase was only significant at TPM1.4 (Table 1). Nicotine did not significantly affect the activities of either enzyme.
Table 1.
Biomarkers of xenobiotic metabolism (CYP1A and GST activities) and oxidative stress (antioxidant enzymes activities and glutathione concentrations) in zebrafish larvae exposed to TPM or nicotine. Activity of CYP1A was assessed in vivo at 72 hpf. Activities of GST, GR, GPx, GST, SOD, and CAT were determined in whole-body extracts of 96 hpf larvae. Means ± SEM (n = 6–10) are displayed. Two-way ANOVA with a post hoc Tukey method was used to assess statistical differences. The lower case letters indicate differences between concentrations within the same treatment, whereas asterisks (*) indicate significant differences between treatments within a given concentration. Note: the control group is referred to as ‘0 μg/mL’.
| Nicotine content (μg/mL)
|
||||
|---|---|---|---|---|
| 0 | 0.4 | 1.4 | ||
| CYP1A1 | TPM | 1.00 ± 0.06a | 0.93 ± 0.06a | 0.72 ± 0.03b |
| Nic | 1.00 ± 0.11 | 1.00 ± 0.07 | 0.85 ± 0.12 | |
| GST2 | TPM | 102.0 ± 6.9a | 165.4 ± 31.3b* | 284.1 ± 28.2c* |
| Nic | 107.6 ± 5.4 | 112.2 ± 5.2 | 113.5 ± 6.3 | |
| GR2 | TPM | 6.6 ± 0.6a | 11.3 ± 2.2b* | 20.8 ± 1.5c* |
| Nic | 6.2 ± 0.3 | 6.1 ± 0.3 | 6.1 ± 0.3 | |
| GPx2 | TPM | 19.9 ± 3.4 | 15.1 ± 3.1 | 34.4 ± 8.1 |
| Nic | 19.1 ± 2.5 | 29.1 ± 2.8 | 32.9 ± 7.2 | |
| SOD3 | TPM | 12.7 ± 0.6 | 11.3 ± 1.0 | 14.2 ± 3.0 |
| Nic | 12.6 ± 1.5 | 9.9 ± 1.2 | 10.1 ± 1.3 | |
| CAT3 | TPM | 20.1 ± 2.9 | 17.1 ± 3.3 | 15.8 ± 3.0 |
| Nic | 20.0 ± 2.0 | 18.7 ± 1.9 | 19.3 ± 2.3 | |
Fold change.
nmol/min/mg protein.
μmol/min/mg protein.
3.4. Biomarkers of oxidative stress
The activity of GR significantly increased in TPM-exposed larvae. The activities of SOD, GPx, and CAT did not change (Table 1). Furthermore, TGSH concentrations were significantly higher in both TPM0.4- and TPM1.4-treated larvae (Fig. 7). There was also a significant increase in GSSG concentrations and GSSG:TGSH in TPM1.4-treated larvae. GSH concentrations were not significantly affected by TPM. No significant changes in oxidative stress biomarkers were observed in nicotine-treated larvae.
Fig. 7.
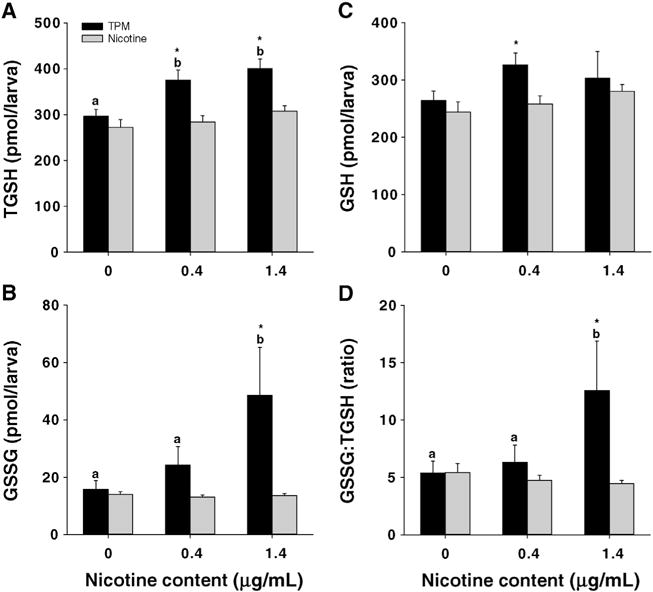
Concentrations of TGSH, GSSG, and GSH, as well as GSSG:TGSH ratios in whole-body extracts of 96 hpf larvae exposed to TPM or nicotine. GSSG:TGSH ratios are reported as percentages. Means ± SEM (n = 6–10) are displayed. Two-way ANOVA with a post hoc Tukey method was used to assess statistical differences. The lower case letters indicate differences between concentrations within the same treatment, whereas asterisks (*) indicate significant differences between treatments within a given concentration. Note: the control group is referred to as ‘0 μg/mL’.
3.5. Bioenergetics
Overall data indicate a significant effect of TPM1.4 but not TPM0.4 on bioenergetics of developing embryos. Basal OCR was significantly decreased in TPM1.4 embryos, resulting in reduced FCCP-induced absolute maximum rate in this group compared to the control and TPM0.4 groups (Fig. 8A). However, total mitochondrial respiration, maximal mitochondrial respiration, spare capacity, and OCR due to ATP synthesis were not significantly affected (Fig. 8B–E). None of the bioenergetic parameters were significantly affected by nicotine.
Fig. 8.
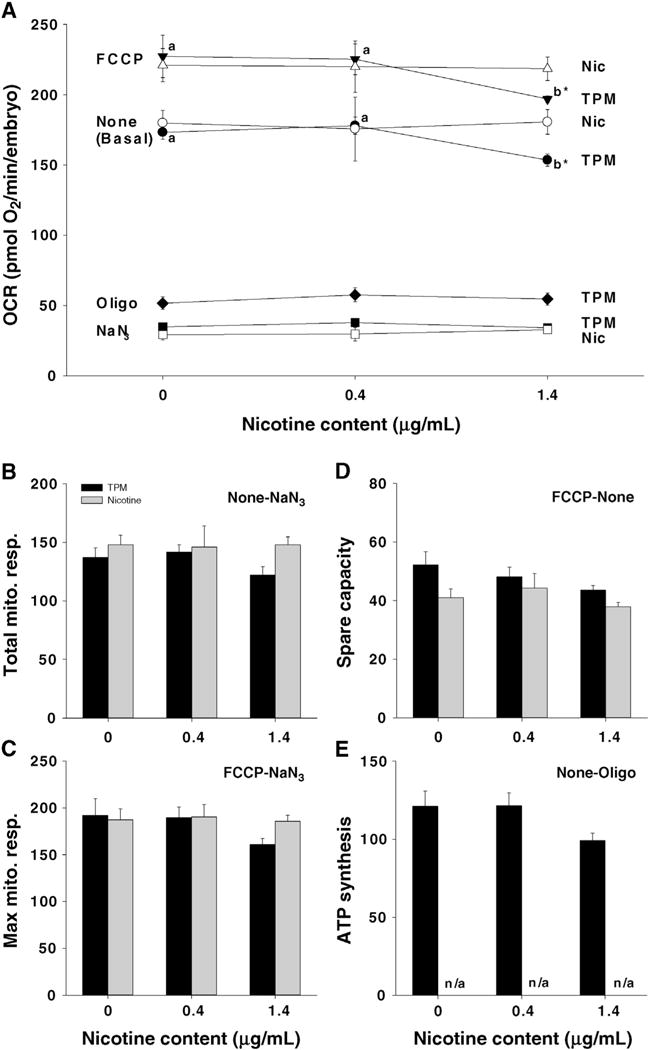
Oxygen consumption rate (OCR) in 36 hpf zebrafish embryos exposed to TPM or nicotine. A. Summary of all measured OCRs under Basal (None), FCCP, Oligo, or NaN3 conditions (Oligo treatments were only performed with TPM). B. Total mitochondrial respiration was calculated as the difference between Basal and NaN3. C. Maximal mitochondrial respiration was calculated as the difference between FCCP and NaN3. D. Spare capacity was the difference between FCCP and Basal. E. OCR due to ATP synthesis was calculated as the difference between Basal and Oligo OCRs. Means ± SEM (n = 3–7) are displayed. Two-way ANOVA with a post-hoc Tukey method was used to assess statistical differences. The lower case letters indicate differences between concentrations within the same treatment, whereas an asterisk (*) indicates differences between treatments within the same concentration. Note: the control group is referred to as ‘0 μg/mL’.
3.6. Behavior
Behavior of exposed embryos and larvae was significantly affected by exposure to TPM, but not nicotine. The spontaneous embryo contractions at 24 hpf were significantly increased in TPM1.4-treated embryos (Fig. 9A). The frequency of spontaneous contractions was not affected in nicotine-alone exposures (Fig. 9A). In larvae, TPM0.4 exposure altered swimming behavior, such that the exposed larvae (only larvae lacking deformities were selected for this assay) showed locomotor hyperactivity compared with controls (Fig. 9B). There was a significant TPM exposure × light/dark interaction. Follow-up tests of TPM0.4 exposure effects during the light and dark conditions showed that there was a significant TPM-induced hyperactivity in the dark condition, whereas there was no significant TPM effect during the light condition. Significant TPM-induced hyperactivity was also seen during the initial dark adaptation period. Swimming behavior was not significantly affected by nicotine (Fig. 9C).
Fig. 9.
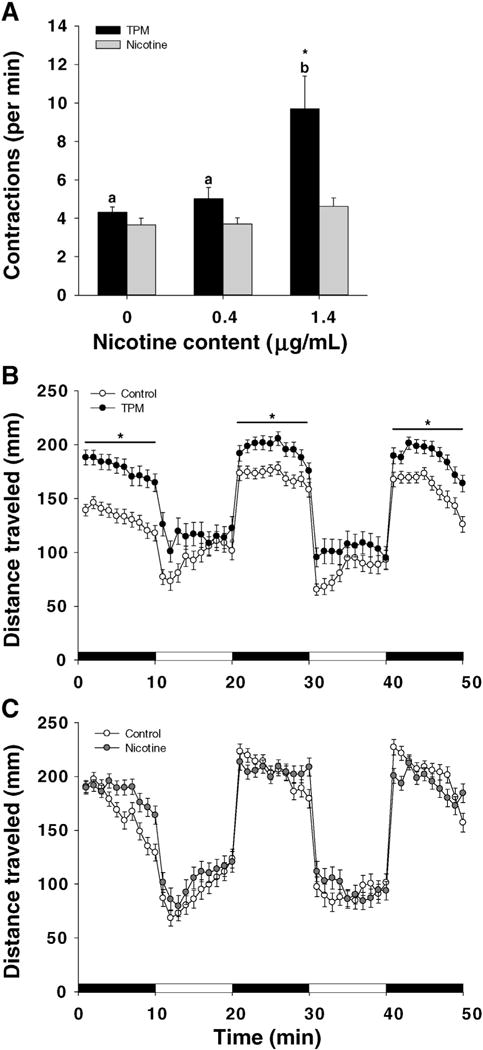
Behavior in zebrafish exposed to TPM or nicotine. A. Spontaneous embryonic contractions were assessed at 24 hpf as the earliest marker of motor-neuron activity. Two-way ANOVA with a post hoc Tukey method was used to assess statistical differences. The lower case letters indicate differences between concentrations within the same treatment, whereas asterisks (*) indicate significant differences between treatments within a given concentration. Means + SEM (n = 4–8) are displayed. Larval behavior in TPM-treated (B) or nicotine-treated (C) larvae. After the exposure the larvae were transferred into fresh Danieau (only larvae without deformities were selected for this assay) and at 144 hpf swimming behavior (50 min in alternating dark and light 10 min periods, starting with a 10 min habituation period in dark) was monitored using Danio Vision. Total distance traveled within each dark/light period is summarized. Means ± SEM (n = 3) are displayed. Two-way ANOVA with a post hoc Tukey method was used to assess statistical differences. The asterisk (*) indicates statistical differences between treatments within the same time period. Note: the control group is referred to as ‘0 μg/mL’.
4. Discussion
This study is one of the first to investigate the applicability of the zebrafish embryo as a ‘bridge model’ for the study of developmental toxicity of TPM. We report that TPM exposure affected developmental, biochemical, and behavioral parameters in zebrafish. Several of the observed adverse effects of TPM, including decreased growth, disrupted vascular development, increased mortality, oxidative stress, and hyperactivity, were consistent with the reported effects of exposure to cigarette smoke in mammalian systems (e.g. Aycicek and Ipek, 2008; Herrmann et al., 2008; Hutchison et al., 1998; Kim et al., 2005; Witschi et al., 1997). Importantly, we also demonstrate that nicotine at equivalent doses was not able to mimic the effects of TPM.
From a developmental perspective, exposure to TPM increased the incidence of deformities, including hemorrhage, bent notochord, pericardial edema, string heart, and truncated jaw. TPM exposure also increased mortality and delayed hatching, as well as decreased larval growth and heart rate. Lastly, TPM disrupted angiogenesis of the major vessels in the brain of zebrafish. While this manuscript was in preparation, Ellis et al. (2014) reported toxicity to zebrafish from exposure to TPM prepared from two reference cigarettes, including 3R4F, and six commercial brands. They also observed phenotypic deformities, delayed hatching, and reduced heart rates at concentrations of 10–50 μg TPM/mL (the concentrations used in our study roughly translate to 6 and 20 μg TPM/mL for TPM0.4 and TPM1.4, respectively). Together, these two reports (Ellis et al. and this manuscript) independently confirm the adverse effects of TPM exposure in the zebrafish model. In addition, we have investigated the biomarkers of xenobiotic metabolism and oxidative stress, mitochondrial function, and behavioral endpoints.
The developmental effects of TPM observed in our study were not attributable to nicotine. The lack of nicotine-specific effects on these endpoints is consistent with previous studies on the effects of nicotine in developing zebrafish embryos (Ali et al., 2012; Parker and Connaughton, 2007; Svoboda et al., 2002) and suggests that other TPM constituents are responsible for these effects. TPM is a complex mixture consisting of several classes of hazardous and potentially hazardous chemicals, including PAHs. While the effects of exposure of many of these chemicals have not been investigated in zebrafish, some of the effects of TPM exposure observed in this study are similar to those observed from PAH exposure. For example, previous studies with PAHs reported pericardial edema, bent notochord, hemorrhaging, and string heart as main morphological changes (Billiard et al., 2006; Carls et al., 2008; Incardona et al., 2004). However, the role of PAHs and others and interactions among the chemicals in TPM-induced toxicity requires further investigation.
In addition to developmental effects, TPM exposure also affected several biochemical parameters. The biomarkers of xenobiotic metabolism were differentially impacted in zebrafish larvae. Specifically, we assessed the activities CYP1A and GST. The role of these enzymes in the toxicity of cigarette smoke has been discussed extensively in the literature, especially with regards to the generation of carcinogenic byproducts resulting from CYP1A activity (e.g. Alexandrov et al., 2002). CYP1A is an important enzyme within phase I xenobiotic metabolism, and is regulated by the aryl hydrocarbon receptor (AhR) (Timme-Laragy et al., 2007). It has long been known that tobacco smoke is a mixture of CYP inducers and inhibitors (Koide et al., 1999), with nicotine and PAHs being considered the main CYP1A inducers (Iba et al., 1998). Among the several hundreds of PAHs identified in cigarette smoke (US Department of Health and Human Services, 2010), we performed the analyses for a selected list of PAHs as part of a panel (Table S1). Among these PAHs several are known AhR agonists – pyrene, benzo [a]anthracene, chrysene, benzo[b]fluoranthene, benzo[k]fluoranthene, benzo[e]pyrene, benzo[a]pyrene, perylene, and indenol[1,2,3-cd]pyrene (Barron et al., 2004) – and one PAH (fluoranthene) that is a CYP1A inhibitor (Timme-Laragy et al., 2007).
We report that the activity of CYP1A was reduced in TPM1.4-exposed larvae. The observed decrease in CYP1A activity in zebrafish larvae contrasts previous studies reporting increased CYP1A activity after exposure to tobacco smoke in mammals (e.g. Czekaj et al., 2005). Although CYP activity was not assessed by Ellis et al. (2014) in zebrafish, the authors did report a TPM-dependent induction in gene expression of AhR2 and several isoforms of CYP1 and CYP2 (other genes associated with AhR signaling and detoxification pathways were also affected). It is not clear why CYP1A activity was decreased in TPM-exposed larvae in our study, but this outcome suggests that the role of AhR pathway in mediating TPM toxicity should be studied in more detail in future studies.
In contrast to CYP1A activity, the activity of GST in whole-larvae homogenates increased. GST is an important enzyme within phase II and conjugates GSH to various electrophilic substrates that are generated in phase I to render these compounds more water soluble (Di Giulio and Meyer, 2008). The increase in GST activity suggests increased xenobiotic metabolism and reflects its protective action against some TPM constituents, as was previously demonstrated for PAHs (Garner and Di Giulio, 2012). Overall, the effects of TPM on CYP1A and GST support the importance of xenobiotic metabolism in mediating its toxicity in zebrafish as in mammals and future work should examine this relationship in more details.
In addition to impacts of xenobiotic metabolism, the toxicity of cigarette smoke has been also discussed in the realm of increased oxidative stress, which in turn, is implicated in the pathogenesis of smoking-related diseases (US Department of Health and Human Services, 2010). Here, we assessed whether TPM exposure induces oxidative stress. Among the several antioxidant enzymes examined, the activity of GR was increased in whole-larvae homogenates. GR regenerates GSH from GSSG; the latter is formed upon the interaction of GSH with ROS (mainly H2O2 and lipid peroxides) (Di Giulio and Meyer, 2008). Elevated GR activity may indicate increased levels of GSSG, which in turn suggests increased generation of ROS. Moreover, the concentrations of TGSH and GSSG were increased in whole-larvae homogenates. The increase in GSSG suggests higher ROS-induced oxidation of GSH. Given that the GSH concentration did not decrease and to explain the increase in TGSH, one may speculate higher GSH synthesis in TPM-exposed larvae to maintain constant GSH levels to neutralize ROS and/or solubilize toxic TPM constituents. Also, GSSG:TGSH increased in TPM-exposed larvae, indicating oxidative stress. Nicotine, did not affect any of the oxidative stress biomarkers, suggesting once again the importance of other TPM constituents. For example, there is abundant evidence on PAH-induced oxidative stress. The mRNA levels of several antioxidant enzymes, including GST, SOD, and GPx, were shown to increase in zebrafish larvae exposed to model PAHs (Timme-Laragy et al., 2009).
In addition to these biochemical effects, other “higher level” effects related to organismal biochemistry and behavior were noted. Bioenergetic analysis showed a decreased OCR in TPM1.4-exposed embryos, suggesting perturbed mitochondrial function or reduced mitochondrial copy number. Consequently, exposure to TPM early in development (3 hpf–36 hpf) may have affected mitochondrial biogenesis by disrupting mitochondrial DNA (mtDNA) replication, leading to reduced levels of mitochondria in TPM1.4-exposed embryos. While it is unknown which chemicals in TPM are responsible for this effect, previous studies have shown that PAH metabolites could induce mtDNA damage and hinder mtDNA replication (Graziewicz et al., 2004; Jung et al., 2009). Interestingly, no significant difference in proton leak (OCR at NaN3) was detected with exposure to TPM, suggesting that mitochondrial membrane integrity was not affected. However, based on the current data, it is difficult to conclude the precise mechanism underlying mitochondrial toxicity of TPM and its potential role in contributing to increased ROS production. Nonetheless, further studies are warranted to better understand such mechanisms.
One of the novel findings in this study is the effect of TPM on behavior. We show that TPM1.4 exposure increased the frequency of spontaneous contractions of the trunk, which is the earliest motor behavior, mediated by the spinal circuit independently of the main neurotransmitter systems (Saint-Amant and Drapeau, 2000). Nicotine did not affect the frequency of spontaneous contractions. This outcome was consistent with Raftery et al. (2014), who exposed zebrafish embryos to nicotine (up to 8 μg/mL) starting at 5 hpf.
Furthermore, larval behavior was also impacted, such that TPM0.4-exposed larvae showed significant hyperactivity at 144 hpf. This was specific to elevated activity during the dark condition, but not the light condition, indicating that the effect was not a generalized hyperkinesis, but rather a specific over-response to the dark condition. Control fish do show higher levels of activity during the dark vs. light condition, but the tobacco exposed fish showed a significantly exaggerated increase in the dark. This low dose effect was seen in larvae that did not show other teratological effects and differed from a dose-dependent decrease in swimming activity as reported by Ellis et al. (2014); however, the latter study used higher TPM concentrations. Interestingly, the nicotine-treated larvae did not differ significantly from the control. This contrasts previous studies that showed nicotine-induced paralysis via alteration of axonal pathfinding in motoneurons and inactivation of acetylcholine receptors in the muscle (Svoboda et al., 2002). The observed hyperactivity with TPM could be mediated by the disruption of cholinergic neurotransmission as suggested for PAHs (Palanikumar et al., 2012). Alternatively, neurotoxicity could also be linked to oxidative stress (Andersen, 2004), and our data do support TPM-induced oxidative stress.
The lack of nicotine-related toxicity in zebrafish contrasts the previously described adverse effects of nicotine on mammalian brain development, which manifest in cognitive and behavioral deficits (e.g. Levin and Slotkin, 1998; Levitt, 1998; Slikker et al., 2005; Slotkin et al., 2006). Several factors, including species-specific differences, metabolism, dose, duration and time of exposure, may explain the lack of developmental toxicity of nicotine in zebrafish model. Also, behavioral and cognitive assessments in zebrafish are much more difficult to execute and interpret. The existing tests are only now starting to catch up in the zebrafish literature, meaning that improved techniques will enable more refined insights into zebrafish behavior. As for the limitations of the zebrafish model, these would include the less precise cognitive/behavioral assessments compared with the mammals and the lack of sensitive equipment to quantify the internal dose of the TPM constituents.
In conclusion, this study evaluated the effects of TPM on early development in zebrafish. Key findings from this study are: 1) TPM exposure resulted in significant physiological and anatomical aberrations in zebrafish; these included increased mortality, delayed hatching, increased incidence of deformities, decreased growth and heart rate, elevated oxidative stress, disrupted angiogenesis, reduced oxygen consumption, and hyperactivity; 2) exposure to nicotine alone was generally non-toxic and led to minimal effects under the experimental conditions. In summary, our work illustrates that zebrafish model is useful to study of cigarette smoke-induced toxicity and bridges the existing in vitro and in vivo models. Further, several biochemical and developmental toxic effects reported herein and by others (Ellis et al., 2014) are consistent with those reported in murine models.
Supplementary Material
Acknowledgments
We thank the following individuals from Duke University: Dr. J.S. Osterberg, A. Taggart, and S.J. Volkoff for zebrafish husbandry; Dr. A.J. Bone, Dr. D. Gooden (SMSF), and M. Chernick for technical assistance. We also thank Dr. S. Eisa-Beygi (University of Toronto) for advice on staining.
Appendix A. Supplementary data
Supplementary data to this article can be found online at http://dx.doi.org/10.1016/j.ntt.2015.09.006.
Footnotes
Conflict of interest
This research was supported by NIH ES010356 and RJR-Leon Golberg Postdoctoral Fellowship. G. L. Prasad is a fulltime employee of R.J. Reynolds Tobacco Co.
Transparency documents
The Transparency documents associated with this article can be found, in online version.
Contributor Information
Nishad Jayasundara, Email: nishad.jayasundara@duke.edu.
Jordan M. Bailey, Email: jordan.bailey@duke.edu.
Anthony N. Oliveri, Email: anthony.olivery@duke.edu.
Edward D. Levin, Email: edlevin@duke.edu.
G.L. Prasad, Email: prasadg@rjrt.com.
Richard T. Di Giulio, Email: richd@duke.edu.
References
- Ahmad F, Noldus L, Tegelenbosch R, Richardson M. Zebrafish embryos and larvae in behavioural assays. Behaviour. 2012;149:1241–1281. [Google Scholar]
- Alexandrov K, Cascorbi I, Rojas M, Bouvier G, Kriek E, Bartsch H. CYP1A1 and GSTM1 genotypes affect benzo[a]pyrene DNA adducts in smokers’ lung: comparison with aromatic/hydrophobic adduct formation. Carcinogenesis. 2002;23:1969–1977. doi: 10.1093/carcin/23.12.1969. [DOI] [PubMed] [Google Scholar]
- Ali S, Champagne DL, Richardson MK. Behavioral profiling of zebrafish embryos exposed to a panel of 60 water-soluble compounds. Behav Brain Res. 2012;228:272–283. doi: 10.1016/j.bbr.2011.11.020. [DOI] [PubMed] [Google Scholar]
- Andersen JK. Oxidative stress in neurodegeneration: cause or consequence? Nat Rev Neurosci. 2004;5:S18–S25. doi: 10.1038/nrn1434. [DOI] [PubMed] [Google Scholar]
- Andres RL, Day MC. Perinatal complications associated with maternal tobacco use. Semin Neonatol. 2000;5:231–241. doi: 10.1053/siny.2000.0025. [DOI] [PubMed] [Google Scholar]
- Arimilli S, Damratoski BE, Bombick B, Borgerding MF, Prasad GL. Evaluation of cytotoxicity of different tobacco product preparations. Regul Toxicol Pharmacol. 2012;64:350–360. doi: 10.1016/j.yrtph.2012.09.004. [DOI] [PubMed] [Google Scholar]
- Aycicek A, Ipek A. Maternal active or passive smoking causes oxidative stress in cord blood. Eur J Pediatr. 2008;167:81–85. doi: 10.1007/s00431-007-0433-z. [DOI] [PubMed] [Google Scholar]
- Barron MG, Heintz R, Rice SD. Relative potency of PAHs and heterocycles as aryl hydrocarbon receptor agonists in fish. Mar Environ Res. 2004;58:95–100. doi: 10.1016/j.marenvres.2004.03.001. [DOI] [PubMed] [Google Scholar]
- Billiard SM, Timme-Laragy AR, Wassenberg DM, Cockman C, Di Giulio RT. The role of the aryl hydrocarbon receptor pathway in mediating synergistic developmental toxicity of polycyclic aromatic hydrocarbons to zebrafish. Toxicol Sci. 2006;92:526–536. doi: 10.1093/toxsci/kfl011. [DOI] [PubMed] [Google Scholar]
- Carls MG, Holland L, Larsen M, Collier TC, Scholz NL, Incardona JP. Fish embryos are damaged by dissolved PAHs, not oil particles. Aquat Toxicol. 2008;88:121–127. doi: 10.1016/j.aquatox.2008.03.014. [DOI] [PubMed] [Google Scholar]
- Czekaj P, Wiaderkiewicz A, Florek E, Wiaderkiewicz R. Tobacco smoke-dependent changes in cytochrome P450 1A1, 1A2, and 2E1 protein expressions in fetuses, newborns, pregnant rats, and human placenta. Arch Toxicol. 2005;79:13–24. doi: 10.1007/s00204-004-0607-7. [DOI] [PubMed] [Google Scholar]
- Di Giulio RT, Meyer JN. Reactive oxygen species and oxidative stress. In: Di Giulio RT, Hinton DE, editors. The Toxicology of Fishes. CRC Press, Taylor & Francis Group; Boca Raton, FL, USA: 2008. pp. 273–324. [Google Scholar]
- Doll R, Hill AB. Mortality in relation to smoking: ten years’ observations of British doctors. Brit Med J. 1964;1:1399–1410. doi: 10.1136/bmj.1.5395.1399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisa-Beygi S, Hatch G, Noble S, Ekker M, Moon TW. The 3-hydroxy-3-methylglutaryl-CoA reductase (HMGCR) pathway regulates developmental cerebral-vascular stability via prenylation-dependent signaling pathway. Dev Biol. 2013;373:258–266. doi: 10.1016/j.ydbio.2012.11.024. [DOI] [PubMed] [Google Scholar]
- Ellis LD, Soo EC, Achenbach JC, Morash MG, Soanes KH. Use of the zebrafish larvae as a model to study cigarette smoke condensate toxicity. PLoS One. 2014;9:e115305. doi: 10.1371/journal.pone.0115305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fako V, Furgeson D. Zebrafish as a correlative and predictive model for assessing biomaterial nanotoxicity. Adv Drug Deliv Rev. 2009;61:478–486. doi: 10.1016/j.addr.2009.03.008. [DOI] [PubMed] [Google Scholar]
- Garner LVT, Di Giulio RT. Glutathione transferase pi class 2 (GSTp2) protects against the cardiac deformities caused by exposure to PAHs but not PCB-126 in zebrafish embryos. Comp Biochem Physiol Part C. 2012;155:573–579. doi: 10.1016/j.cbpc.2012.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graziewicz MA, Sayer JM, Jerina DM, Copeland WC. Nucleotide incorporation by human DNA polymerase gamma opposite benzo[a]pyrene and benzo[c]phenanthrene diol epoxide adducts of deoxyguanosine and deoxyadenosine. Nucleic Acids Res. 2004;32:397–405. doi: 10.1093/nar/gkh213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hackshaw A, Rodeck C, Boniface S. Maternal smoking in pregnancy and birth defects: a systematic review based on 173 687 malformed cases and 11.7 million controls. Hum Reprod Update. 2011;17:589–604. doi: 10.1093/humupd/dmr022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermes-Lima M, Storey KB. Relationship between anoxia exposure and antioxidant status in the frog Rana pipiens. Am J Physiol. 1996;217:918–925. doi: 10.1152/ajpregu.1996.271.4.R918. [DOI] [PubMed] [Google Scholar]
- Herrmann M, King K, Weitzman M. Prenatal tobacco smoke and postnatal secondhand smoke exposure and child neurodevelopment. Curr Opin Pediatr. 2008;20:184–190. doi: 10.1097/MOP.0b013e3282f56165. [DOI] [PubMed] [Google Scholar]
- Hill AJ, Teraoka H, Heidman W, Peterson RE. Zebrafish as a model vertebrate for investigating chemical toxicity. Toxicol Sci. 2005;86:6–19. doi: 10.1093/toxsci/kfi110. [DOI] [PubMed] [Google Scholar]
- Hutchison SJ, Glantz SA, Zhu BQ, Sun YP, Chou TM, Chatterjee K, Deedwania PC, Parmley WW, Sudhir K. In-utero and neonatal exposure to secondhand smoke causes vascular dysfunction in newborn rats. J Am Coll Cardiol. 1998;32:1463–1467. doi: 10.1016/s0735-1097(98)00217-4. [DOI] [PubMed] [Google Scholar]
- Iba MM, Scholl H, Fung J, Thomas PE, Alam J. Induction of pulmonary CYP1A1 by nicotine. Xenobiotica. 1998;28:827–843. doi: 10.1080/004982598239083. [DOI] [PubMed] [Google Scholar]
- Incardona JP, Collier TK, Scholz NL. Defects in cardiac function precede morphological abnormalities in fish embryos exposed to polycyclic aromatic hydrocarbons. Toxicol Appl Pharmacol. 2004;196:191–205. doi: 10.1016/j.taap.2003.11.026. [DOI] [PubMed] [Google Scholar]
- Johnson MD, Schilz J, Djordjevic MV, Rice JR, Shields PG. Evaluation of in vitro assays for assessing the toxicity of cigarette smoke and smokeless tobacco. Cancer Epidemiol Biomark Prev. 2009;18:3263–3304. doi: 10.1158/1055-9965.EPI-09-0965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung D, Cho Y, Collins LB, Swenberg JA, Di Giulio RT. Effects of benzo[a]pyrene on mitochondrial and nuclear DNA damage in Atlantic killifish (Fundulus heteroclitus) from a creosote-contaminated and reference site. Aquat Toxicol. 2009;95:44–51. doi: 10.1016/j.aquatox.2009.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim YJ, Hong YC, Lee KH, Park HJ, Park EA, Moon HS, Ha EH. Oxidative stress in pregnant women and birth weight reduction. Reprod Toxicol. 2005;19:487–492. doi: 10.1016/j.reprotox.2004.10.007. [DOI] [PubMed] [Google Scholar]
- Klee EW, Ebbert JO, Schneider H, Hurt RD, Ekker SC. Zebrafish for the study of the biological effects of nicotine. Nicotine Tob Res. 2011;13:301–312. doi: 10.1093/ntr/ntr010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koide A, Fuwa K, Furukawa F, Hirose M, Nishikawa A, Mori Y. Effect of cigarette smoke on the mutagenic activation of environmental carcinogenesis by rodent liver. Mutat Res-Fund Mol M. 1999;428:165–176. doi: 10.1016/s1383-5742(99)00044-7. [DOI] [PubMed] [Google Scholar]
- Levin ED, Slotkin TA. Developmental neurotoxicity of nicotine. In: Slikker W Jr, Chang LW, editors. Handbook of Developmental Neurotoxicology. Academic Press; San Diego: 1998. pp. 587–615. [Google Scholar]
- Levitt P. Prenatal effects of drugs of abuse on brain development. Drug Alcohol Depend. 1998;51:109–125. doi: 10.1016/s0376-8716(98)00070-2. [DOI] [PubMed] [Google Scholar]
- Lieschke GJ, Currie PD. Animal models of human disease: zebrafish swim into view. Nature. 2007;8:353–367. doi: 10.1038/nrg2091. [DOI] [PubMed] [Google Scholar]
- Liszewski W, Ritner C, Aurigui J, Wong SSY, Hussain N, Krueger W, Oncken C, Bernstein HS. Developmental effects of tobacco smoke exposure during human embryonic stem cell differentiation are mediated through the transforming growth factor-β superfamily member, Nodal. Differentiation. 2012;83:169–178. doi: 10.1016/j.diff.2011.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lushchak VI, Lushchak LP, Mota AA, Hermes-Lima M. Oxidative stress and antioxidant defenses in goldfish Carassius auratus during anoxia and reoxygenation. Am J Physiol Regul Integr Comp Physiol. 2001;280:R100–R107. doi: 10.1152/ajpregu.2001.280.1.R100. [DOI] [PubMed] [Google Scholar]
- Massarsky A, Dupuis L, Taylor J, Eisa-Beygi S, Strek L, Trudeau VL, Moon TW. Assessment of nanosilver toxicity during zebrafish (Danio rerio) development. Chemosphere. 2013;92:59–66. doi: 10.1016/j.chemosphere.2013.02.060. [DOI] [PubMed] [Google Scholar]
- Matta SG, Balfour DJ, Benowitz NL, Boyd RT, Buccafusco JJ, Caggiula AR, Craig CR, Collins AC, Damaj MI, Donny EC, Gardiner PS, Grady SR, Heberlein U, Leonard SS, Levin ED, Lukas RJ, Markou A, Marks MJ, McCallum SE, Parameswaran N, Perkins KA, Picciotto MR, Quik M, Rose JE, Rothenfluh A, Schafer WR, Stolerman IP, Tyndale RF, Wehner JM, Zirger JM. Guidelines on nicotine dose selection for in vivo research. Psychopharmacology. 2007;190:269–319. doi: 10.1007/s00213-006-0441-0. [DOI] [PubMed] [Google Scholar]
- Padilla S, Hunter DL, Padnos B, Frady S, MacPhail RC. Assessing locomotor activity in larval zebrafish: influence of extrinsic and intrinsic variables. Neurotoxicol Teratol. 2011;33:624–630. doi: 10.1016/j.ntt.2011.08.005. [DOI] [PubMed] [Google Scholar]
- Palanikumar L, Kumaraguru AK, Ramakritinan CM, Anand M. Biochemical response of anthracene and benzo [a] pyrene in milkfish Chanos chanos. Ecotoxicol Environ Saf. 2012;75:187–197. doi: 10.1016/j.ecoenv.2011.08.028. [DOI] [PubMed] [Google Scholar]
- Parker B, Connaughton VP. Effects of nicotine on growth and development in larval zebrafish. Zebrafish. 2007;4:59–68. doi: 10.1089/zeb.2006.9994. [DOI] [PubMed] [Google Scholar]
- Raftery TD, Isales GM, Yozzo KL, Volz DC. High-content screening assay for identification of chemicals impacting spontaneous activity in zebrafish embryos. Environ Sci Technol. 2014;48:804–810. doi: 10.1021/es404322p. [DOI] [PubMed] [Google Scholar]
- Rogers JM. Tobacco and pregnancy. Reprod Toxicol. 2009;28:152–160. doi: 10.1016/j.reprotox.2009.03.012. [DOI] [PubMed] [Google Scholar]
- Saint-Amant L, Drapeau P. Motoneuron activity patterns related to the earliest behavior of the zebrafish embryo. J Neurosci. 2000;20:3964–3972. doi: 10.1523/JNEUROSCI.20-11-03964.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulte-Merker S. Looking at embryos. In: Nusslein-Volhard C, Dahm R, editors. Zebrafish — A Practical Approach. Oxford University Press Inc; New York: 2002. pp. 57–58. [Google Scholar]
- Slikker W, Jr, Xu ZA, Levin ED, Slotkin TA. Mode of action: disruption of brain cell replication, second messenger, and neurotransmitter systems during development leading to cognitive dysfunction–developmental neurotoxicity of nicotine. Crit Rev Toxicol. 2005;35:703–711. doi: 10.1080/10408440591007421. [DOI] [PubMed] [Google Scholar]
- Slotkin TA, Pinkerton KE, Seidler FJ. Perinatal environmental tobacco smoke exposure in rhesus monkeys: critical periods and regional selectivity for effects on brain cell development and lipid peroxidation. Environ Health Perspect. 2006;114:34–39. doi: 10.1289/ehp.8286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stackley KD, Beeson CC, Rahn JJ, Chan SSL. Bioenergetic profiling of zebrafish embryonic development. PLoS One. 2011;6:e25652. doi: 10.1371/journal.pone.0025652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svoboda KR, Vijayaraghavan S, Tanguay RL. Nicotinic receptors mediate changes in spinal motoneuron development and axonal pathfinding in embryonic zebrafish exposed to nicotine. J Neurosci. 2002;22:10731–10741. doi: 10.1523/JNEUROSCI.22-24-10731.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timme-Laragy AR, Cockman CJ, Matson CW, Di Giulio RT. Synergistic induction of AHR regulated genes in developmental toxicity from co-exposure to two model PAHs in zebrafish. Aquat Toxicol. 2007;85:241–250. doi: 10.1016/j.aquatox.2007.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timme-Laragy AR, Van Tiem LA, Linney EA, Di Giulio RT. Antioxidant responses and NRF2 in synergistic developmental toxicity of PAHs in zebrafish. Toxicol Sci. 2009;109:217–227. doi: 10.1093/toxsci/kfp038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- US Department of Health and Human Services. The health consequences of smoking: a report of the Surgeon General. Atlanta, GA: US Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health; 2004. [Google Scholar]
- US Department of Health and Human Services. How tobacco smoke causes disease: the biology and behavioral basis for smoking-attributable disease: a report of the Surgeon General. Atlanta, GA: US Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health; 2010. [Google Scholar]
- US Department of Health and Human Services. The health consequences of smoking-50 years of progress: a report of the Surgeon General. Atlanta, GA: US Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health; 2014. [Google Scholar]
- Witschi H, Joad JP, Pinkerton KE. The toxicology of environmental tobacco smoke. Annu Rev Pharmacol Toxicol. 1997;37:29–52. doi: 10.1146/annurev.pharmtox.37.1.29. [DOI] [PubMed] [Google Scholar]
- Yang L, Ho NY, Alshut R, Legradi J, Weiss C, Reischi M, Mikut R, Liebel U, Müller F, Strähle U. Zebrafish embryos as models for embryotoxic and teratological effects of chemicals. Reprod Toxicol. 2009;28:245–253. doi: 10.1016/j.reprotox.2009.04.013. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


