Abstract
Radiotherapy continues to be a primary modality in the treatment of cancer. DNA damage induced by radiation can promote apoptosis as well as both autophagy and senescence, where autophagy and senescence can theoretically function to prolong tumor survival. A primary aim of this work was to investigate the hypothesis that autophagy and/or senescence could be permissive for DNA repair, thereby facilitating tumor cell recovery from radiation-induced growth arrest and/or cell death. In addition, studies were designed to elucidate the involvement of autophagy and senescence in radiation sensitization by PARP inhibitors and the re-emergence of a proliferating tumor cell population. In the context of this work, the relationship between radiation-induced autophagy and senescence was also determined. Studies were performed using DNA repair proficient HCT116 colon carcinoma cells and a repair deficient Ligase IV (−/−) isogenic cell line. Irradiation promoted a parallel induction of autophagy and senescence that was strongly correlated with the extent of persistent H2AX phosphorylation in both cell lines; however inhibition of autophagy failed to suppress senescence, indicating that the two responses were dissociable. Irradiation resulted in a transient arrest in the HCT116 cells while arrest was prolonged in the Ligase IV (−/−) cells; however, both cell lines ultimately recovered proliferative function, which may reflect maintenance of DNA repair capacity. The PARP inhibitors (Olaparib) and (Niraparib) increased the extent of persistent DNA damage induced by radiation as well as the extent of both autophagy and senescence; neither cell line underwent significant apoptosis by radiation alone or in the presence of the PARP inhibitors. Inhibition of autophagy failed to attenuate radiation sensitization, indicating that autophagy was not involved in the action of the PARP inhibitors. As with radiation alone, despite sensitization by PARP inhibition, proliferative recovery was evident within a period of 10–20 days. While inhibition of DNA repair via PARP inhibition may initially sensitize tumor cells to radiation via the promotion of senescence, this strategy does not appear to interfere with proliferative recovery, which could ultimately contribute to disease recurrence.
1. Introduction
Radiotherapy is used along with other modalities such as surgery, chemotherapy, and immunotherapy to either shrink tumors before surgery or eliminate surviving tumor cells post surgery. While ionizing radiation is ultimately cytotoxic by virtue of inducing DNA damage, specifically double-strand breaks [1–3], radiation also elicits a complex ensemble of responses that can moderate its toxic effects. Among these responses, autophagy and senescence are particularly intriguing because they can contribute to tumor control through autophagic cell death [4] or persistent growth arrest [5], respectively, but can also antagonize apoptosis and thereby shelter a population of dormant cells that may later reinitiate tumor regrowth [6–9].
There is extensive evidence that radiation can promote autophagy [10]. Autophagy can function as a pro-survival mechanism or as pro-death mechanism, depending on the agents used and the experimental systems. The relationship between autophagy and the DNA repair system is unclear, but several studies have shown that autophagy might play a role during exposure to DNA damaging agents [11–15].
It is also well established that various forms of stress, particularly exposure to DNA-damaging agents such as radiation, can promote senescence [5, 16–17]. While senescence has often been considered to be an irreversible form of growth arrest, it is long established that telomerase can be reactivated in cells undergoing replicative senescence, ultimately leading to an immortalized replicating cell population [18]. Furthermore, there is clear experimental evidence for reversibility of senescence under select experimental conditions [19].
With regard to DNA damage and senescence it has been established that ionizing radiation induces DNA damage foci, the majority of which are transient and disappear within hours post-treatment [20–21]. While some foci may persist for months, the repair of double-strand DNA breaks in senescent cells may result in recovery and regrowth. In fact, there is evidence that senescent cells can repopulate after exposure to chemotherapeutic agents and radiation [16, 22–24].
From a clinical perspective, the possibility of sensitization to radiation (and chemotherapy) through the administration of PARP inhibitors to interfere with DNA repair continues to be an area of active inquiry [25–28]. Interestingly, sensitization to radiation has been shown to lead primarily to an increase in senescence with minimal apoptosis [29–30]. Furthermore, the potential involvement of autophagy in radiation sensitization via PARP inhibition has not been investigated; this is relevant as autophagy and senescence have been shown to be closely associated responses in some studies [31–33].
The primary aim of the current work was to understand the involvement of autophagy and senescence in the response to radiation-induced DNA damage, and the interplay between these responses and DNA repair. Our findings revealed that the extent of both autophagy and senescence correlates with the intensity of persistent unrepaired DNA damage. Furthermore, interference with DNA repair via PARP inhibition using Olaparib (AZD 2281) or Niraparib (MK 4827) may initially sensitize cells via increased autophagy and senescence, but not apoptosis. However, this strategy does not appear to interfere with proliferative recovery, which could, in theory, contribute to disease recurrence [34–37].
2. Materials and methods
2.1 Cell lines
HCT116 colon cancer cells were purchased from ATCC and HCT116 Ligase IV-deficient were generated as previously described [38]. HCT116 Ligase IV-deficient and Ligase IV proficient cells lines were maintained as subconfluent cultures in RPMI 1640 medium with 5% fetal bovine serum, 5% bovine calf serum, 2 mM L-glutamine, and penicillin/ (GIBCO Life Technologies, Gaithersburg, MD) and incubated at 37°C, 5% CO2, in a humidified environment. In every experiment, cells were cultured under identical conditions and incubated overnight to allow for adherence prior to irradiation.
ATG5 and ATG7 silencing shCon, shATG5 and shATG7 plasmid constructs were isolated (Qiagen-plasmid midi kit) using bacterial stocks (Sigma-Aldrich). Plasmid constructs were packaged into lentiviral particles using HEK 239T cells and a packaging mixture composed of Lipofectamine (Invitrogen, 11668–019), psPAX2 and pMD2.G packaging constructs (Addgene, 12260, 12259). Growth medium containing the viral particles was collected and used to infect HCT116 cells. Infected cells were then maintained with the selection marker, puromycin (2 µg/mL) throughout the course of the study.
2.2 Time course of radiation-induced effects on cell viability
Cells were plated in 6-well plates (generally 200,000 cells/well) and allowed to adhere overnight. The next day, cells were treated with radiation and the number of viable cells was counted at indicated time points for 5 days. In case of co-administering a drug (PARP inhibitors or autophagy inhibitors) with radiation, cells were pretreated with the drug 3 hours before radiation and drug was washed away 24 hr post radiation. In the case of the apoptosis inhibitor, Z-VAD, cells were pretreated for 3 hours and maintained in the drug throughout the course of the study. At each time point, medium was removed and cells were washed one time with 1X PBS. 500 µL of 0.25% trypsin was added to each well for harvesting and incubated for 5 minutes, then deactivated by 500 µL of fresh medium, to make up 1 mL of cell suspension. Cells were collected in 1 mL conical tubes (Eppendorf) and 10 µL of cell suspension was added to 10 µL of trypan blue (0.4%), placed onto chamber slides of a hemacytometer (Hausser Scientific) and counted under a microscope.
2.3 Clonogenic survival assay
200 cells were plated in 6-well plates and allowed to adhere overnight. After 24 h, cells were pre-incubated with the indicated drug for 3 h and then exposed to the indicated dose of radiation. The following day, drug-containing medium was removed, cells were washed and supplemented with fresh medium that was replaced every other day for two weeks. On the day of staining, cells were fixed with 90% methanol for 10 min, and then stained with 1% crystal violet for another 10 minutes. Colonies were then washed with PBS three times to eliminate excessive crystal violet staining and counted manually.
2.4 Assessment of autophagy by acridine orange staining
50,000 cells were seeded in 6-well plates, permitted to adhere overnight and exposed to radiation the following day. At the various time points, medium was removed and cells washed once with 1X PBS. The acridine orange solution was made up in 1X PBS to a final concentration of 100ng/ml in the dark and protected from light until ready for use. For flow cytometry, 10 µL of acridine orange solution was added to each sample and allowed to incubate for 15 min. Dye-containing medium then was aspired, plates were washed with 1X PBS and fresh medium was added. Photographs were taken with an Olympus 1× 70 microscope and an Olympus SC 35 camera.
The cell population positively stained with acridine orange was quantified by flow cytometry. Treated cells were trypsinized, collected, and centrifuged at 1500 rpm for 5 min. Supernatant was removed and pellets were resuspended in 990 µL of 1X PBS. The cell suspension was filtered through a standard flow cytometry 40 micron filter (BD Falcon). The acridine orange solution was made up in 1X PBS to a final concentration of 100ng/ml in the dark and protected from light until ready for use. For flow cytometry, 10 µL of acridine orange solution was added to each sample and allowed to mix for 15 min. Acridine orange is excited at a wavelength of 525 nM for green flourescence and 620 nM for red fluorescence.
2.5 Transfection of HCT116 cells with RFP-LC3
The RFP-LC3 construct was generated by the Tolkovosky laboratory [39]. 1×106 HCT-116 cells were collected in a pellet, centrifuged, and resuspended with the construct in 100 µL of the Amaxa Nucleofector Kit V. A microgram of the RFP-LC3 vector was added to the suspension. The cell suspension was collected in a cuvette, and then placed in nucleofector device to run program D-032. 500 µL of medium was added to the transfected cells and to transfer them to a Petri dish where cells were maintained under Gentamycin (8 ng/mL) to maintain the stable transfection.
2.6 Cell cycle analysis
At the indicated time points, cells were trypsinized, collected, and centrifuged at 1,500 rpm. The supernatant was aspired, pellets washed in PBS and recentrifuged at 1,500 rpm. The supernatant was removed, 0.2 mL of PBS was added and pellets were gently mixed to form a single cell suspension. 1.8 mL of cold 70% ethanol was added gradually into the cell suspension; cells were vortexed, centrifuged, ethanol was aspired, and cells were washed with PBS prior to addition of a staining solution (0.1% (v/v) Triton-X-100 in 10 mL PBS, 2 mg of DNase free RNase A, and 0.2 mL of the propidium iodide stock (1 mg/ml)) 2 hours prior to flow cytometry.
2.7 Evaluation of senescence by β-galactosidase staining
β-Galactosidase staining was utilized as a marker of senescence. Cells were washed once with 1X PBS and fixed with 2% formaldehyde/ 0.2% glutaraldehyde for 5 min, again washed with PBS and finally incubated overnight in a staining solution composed of 1 mg/mL 5-bromo-4-chloro-3-inolyl-β-galactosidase in dimethylformamide (20 mg/mL stock), 5 mM potassium ferricyanide, 150 mM NaCl, 40 mM citric acid/sodium phosphate, 2 mM MgCl2, at pH 6.0 in CO2 at 37°C. The following day, cells were washed twice with PBS and pictures were taken.
For β-Galactosidase detection by flow cytometry, cells were washed and incubated for 1 h in complete medium containing 100 nM of bafilomycin A1 to induce lysosomal alkalinization. After incubation, C12FDG working solution was added to each well in amount to make the final concentration 33 µM and incubation was continued for another 1 h. Medium was then aspired and cells were washed twice with PBS. Cells were harvested, collected by centrifugation at 1500 rpm, resuspended in PBS and analyzed by flow cytometry as above but with excitation at 490 nm and a 514 nm emission filter. C12FDG is hydrolyzed by upregulated β-galactosidase enzyme and becomes fluorescent at wavelength of 500–510 nM.
2.8 Determination of γH2AX intensity as a marker of DNA damage
5,000 cells were seeded in 4-chamber coverglass slides (Lab-Tek II) and allowed to adhere overnight. On the following day, cells were irradiated and fixed with 4% formaldehyde for 5 min at indicated time points. Cells were washed twice with 1X PBS, incubated at room temperature in 0.05% triton-X for 15 minutes, washed, and incubated with 1X PBS containing 1% of BSA for 30 min to prevent non-specific binding of the antibody. Finally, cells were incubated in a 1:10 dilution of γH2AX antibody (BD Pharmingen) in 1% BSA for 1 h. Images were taken using an LSM 700 confocal microscope (Zeiss).
Alternatively, for flow cytometry, cells were at harvested at the indicated time points, fixed with 90% ethanol and maintained at −20 °C until the day of experiment. Cells then were centrifuged at 3,000 rpm for 5 min and resuspended in 1% BSA for 30 min. γH2AX antibody (BD Pharmingen) was added to the cells in a dilution of (1:200) and incubated at room temperature for 1 hr. Cells were then analyzed by flow cytometry at an excitation wavelength of 488. Raw data were normalized according to the intensity of control samples (normalized mean intensity = intensity of the sample / the intensity of the corresponding control sample within the same experiment)
2.9 Evaluation of DNA damage extent by the comet assay
200,000 cells were plated in 6 cm2 dishes and treated as indicated. After 72 hours, cells were gently scraped from the plates and 100,000 cells were mixed with molten LMAgarose (at 37 °C) at a ratio of 1:10 (v/v). The mixture of cells and LMAgarose was then pipetted onto Comet Slides (Trevigen) and incubated for 30 min at 37 °C. The slides were kept at 4 °C for 10 min prior to being immersed in Lysis Solution (Trevigen) overnight. On the following day, the slides were immersed in 1X Neutral Electrophoresis Buffer for 30 minutes at 4 °C, set onto an electrophoresis tray for 45 minutes and electrophoresed at 1 volt per cm. Slides were then immersed in DNA precipitation solution (1M ammonium acetate in 70% ethanol) for 30 minutes at room temperature, followed by 70% ethanol for another 30 min. Samples were dried at 37°C for 30 min and stained with the working dilution of SYBR Green (Trevigen).
2.10 Evaluation of apoptosis
Following the indicated treatments, cells were harvested at the indicated time points and collected on a cytospin slide, fixed with formaldehyde (4%) for 5 min and washed with 1X PBS twice. Slides were fixed with acetic acid/ethanol (1:2) for 5 min and washed twice with 1X PBS. For the terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) assay, cells were blocked with BSA (1 mg/ml for 30 min) at room temperature, washed twice in PBS, and incubated with enzyme mixture (terminal transferase, 25 mM CoCl2, fluorescein-12dUTP) for 1 h at 37° to allow the enzymatic reaction. After washing with PBS, cells were stained with DAPI and images were taken using a fluorescence microscope.
For the PI/Annexin assay, adherent and non-adherent cells were harvested in Eppendorf tubes, and pellets resuspended in 100 µL of binding buffer (BD Biosciences). 5 µL of Annexin-FITC (BD Biosciences) and 5 µL of PI at 10 µg/mL (BD Biosciences) were added to the cell suspension and incubated for 15 min in the dark at room temperature. 400 µL of Annexin V binding buffer 1X (BD Pharmingen) was added to each sample, and samples were analyzed by flow cytometry at 530 nM.
2.11 Western blotting
At the indicated time points, cells were harvested and mixed with lysis buffer (1 M Tris-HCl, pH 6.8, 10% SDS) containing protease and phosphatase inhibitors (Sigma-Aldrich). Proteins were separated on 12% gels using SDS-PAGE and transferred onto nitrocellulose membranes. The membranes were blocked using nonfat dry milk and PBS for 30 min at room temperature, then incubated with the primary antibody overnight at 4°C. Primary antibodies used were anti-p62 (SQSTM1–Santa Cruz sc-28359), anti-β actin (Santa Cruz sc-47778), anti-ATG5 (Cell Signaling – 12994S), and anti-ATG7 (Cell Signaling – 8558S). All primary antibodies were used at a 1:1,000 dilution. The following day, membranes were incubated with correspondent secondary antibodies for 1h. Secondary antibodies used were goat anti-mouse IgG (Amersham, GE Healthcare) and monkey anti-rabbit IgG (Amersham, GE Healthcare). Membranes were then washed three times and bands were detected using enhanced chemiluminescence detection reagents (Pierce, Rockford, IL).
2.12 Statistical analysis
Statistics were conducted using Statview statistical software (SAS Institute, Cary, NC). The data were expressed as means ± S.E. Comparisons were made using two-way analysis of variance followed by the Bonferroni post hoc test. p values <0.05 were considered as statistically significant.
3. Results
3.1 Response of DNA repair-competent and DNA repair-deficient cell lines to radiation
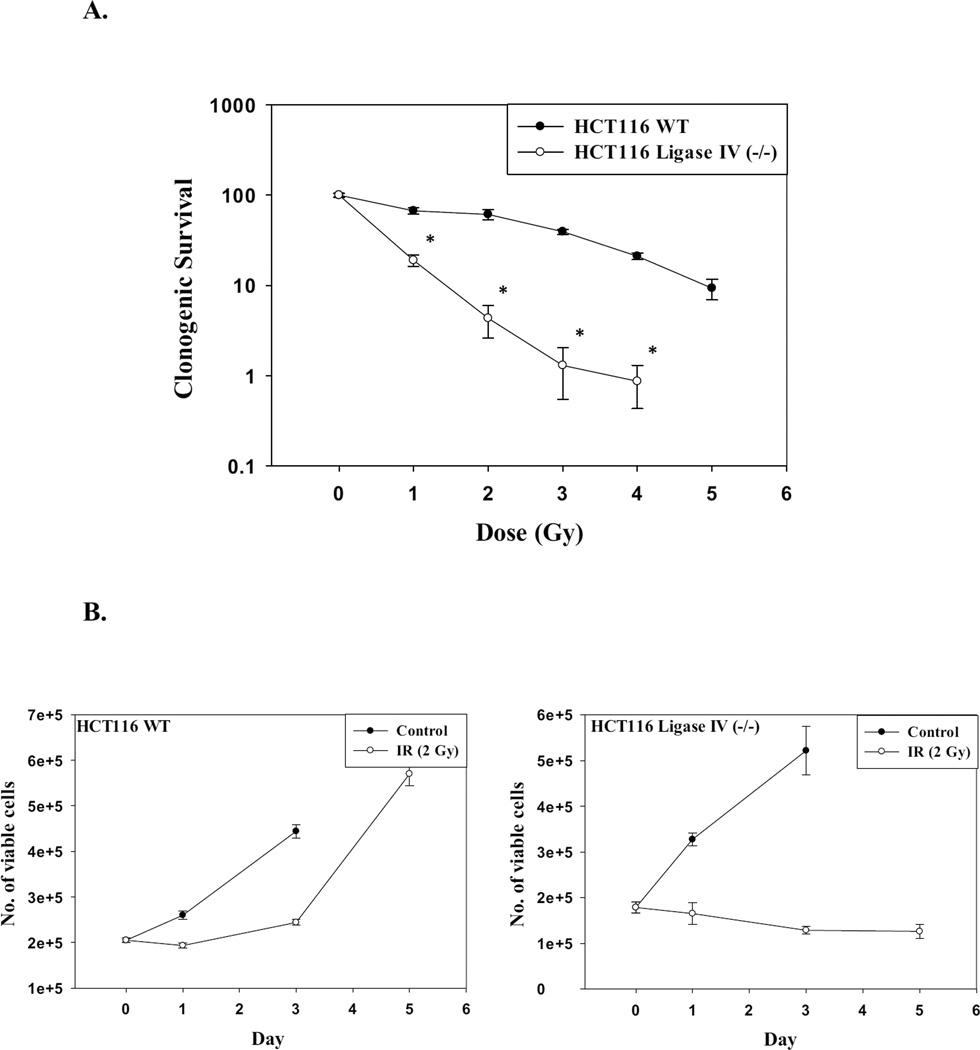
Radiation sensitivity in the HCT116 and the HCT116 Ligase-IV-deficient cell lines was determined by clonogenic survival. Figure 1A shows that HCT116 cells lacking Ligase IV were significantly more sensitive to radiation than the Ligase IV wild-type cells as the clonogenic survival was significantly decreased at lower doses of radiation compared to wild type cells. This finding is consistent with previous reports in the literature indicating that DNA repair-deficient cell lines are more sensitive to radiation than DNA repair-proficient cells [40–42].
Figure 1. Radiation responses of DNA-repair-proficient and Ligase IV deficient HCT116 cell lines.
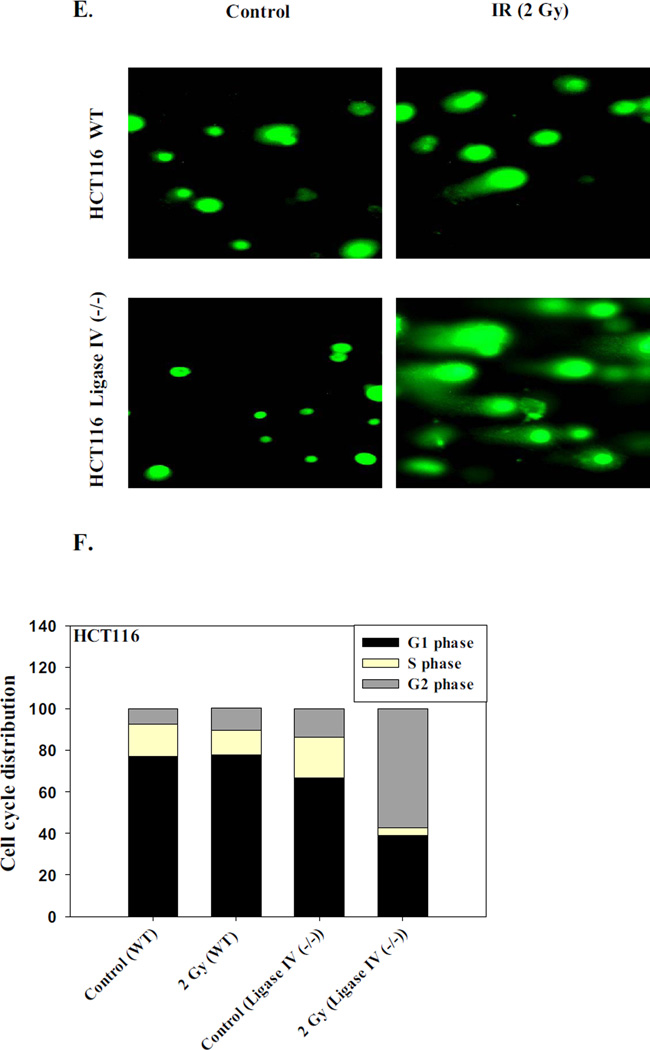
A. Radiosensitivity of HCT116 wt and HCT116 Ligase IV-deficient cells as determined by clonogenic survival (n=3). B. Impact of radiation on cell growth. Cells were exposed to 2 Gy of radiation and the number of viable cells was determined on days 0, 1, 3, and 5 (n=3). Graphs represent pooled data from three replicate experiments. C. Confocal microscope imaging of γH2AX foci formation at 2 Gy irradiation at 72 h post-treatment. D. Mean intensity of γH2AX as determined by flow cytometry 96 hr post treatment (n=3). E. Comet assay. Fluorescent microscope imaging of both cell lines 72 hours post treatment with 2 Gy. F. Cell cycle analysis after exposure of HCT116 Ligase IV (−/−) colon cancer cells to 2 Gy of irradiation at 72 hr post-treatment. Error bars represent standard error. * in A and D indicates p<0.05 compared to the corresponding effect at a similar dose of radiation in the HCT116 wt cells) (n=3).
Radiation sensitivity was further compared by performing temporal response studies in which the HCT116 and the HCT116 Ligase-IV deficient cell lines were exposed to a radiation dose of 2 Gy and viable cell number was monitored over time. Figure 1B shows that growth of the HCT116 cells was inhibited only transiently followed by relatively rapid recovery of proliferative capacity whereas radiation produced a sustained growth inhibition (with a slight decline in viable cell number between days 3 and 5) in the HCT116 Ligase IV-deficient cells.
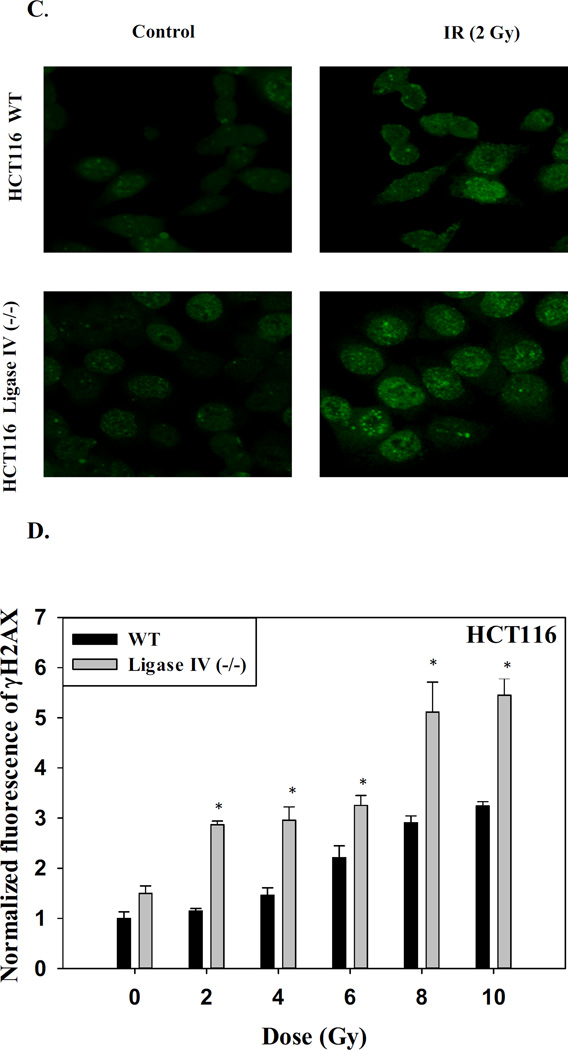
The difference in sensitivity of the two cell lines to radiation is likely to be a consequence of the extent and persistence of DNA damage [42–43]. Figure 1C (staining with γH2AX antibody) and Figure 1D (H2AX phosphorylation) indicate that the number of DNA damage foci in HCT116 Ligase IV (−/−) cells was increased compared to the Ligase IV proficient cells. That is, over a range of radiation doses, the extent of residual damage (i.e., γH2AX staining) at 96 h was significantly higher in the Ligase IV-deficient HCT116 cells than in the Ligase IV-proficient cells.
As an additional confirmation of the increased DNA damage, results of Comet assay experiments presented in Figure 1E show more extensive formation of tails in the HCT116 Ligase IV (−/−) cells compared to the HCT116 WT cells at low dose of radiation (2 Gy). Furthermore, cell cycle analyses indicated that nearly 45% of the HCT116 Ligase IV (−/−) cells were arrested at the G2/M phase in comparison to 20% of the HCT116 WT cells 72 hours after exposure to 2 Gy of radiation (Figure 1F).
3.2 Induction of autophagy and senescence by ionizing radiation in Ligase IV-proficient HCT-116 and Ligase IV-deficient HCT-116 cell lines
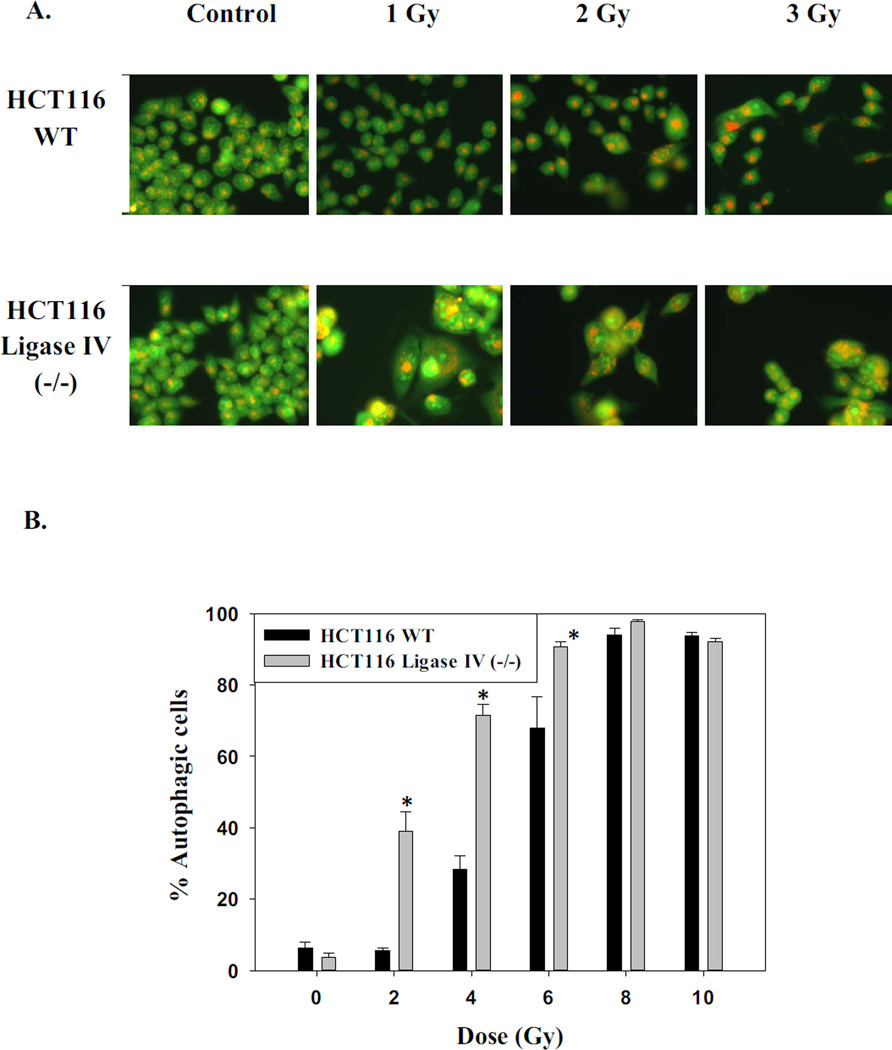
We and others have reported that a primary response of tumor cells to radiation is autophagy [44–47]. Figures 2A presents images of irradiated cells stained with acridine orange, which is indicative of autophagy while Figures 2B provides quantification of the extent of autophagy over a range of radiation doses. While the extent of autophagy was significantly greater in the Ligase IV deficient cells compared to parental cells at lower doses of radiation, essentially the entire cell population had entered a state of autophagy for both cell lines at the higher doses.
Figure 2. Promotion of autophagy and senescence by radiation in HCT116 cells.
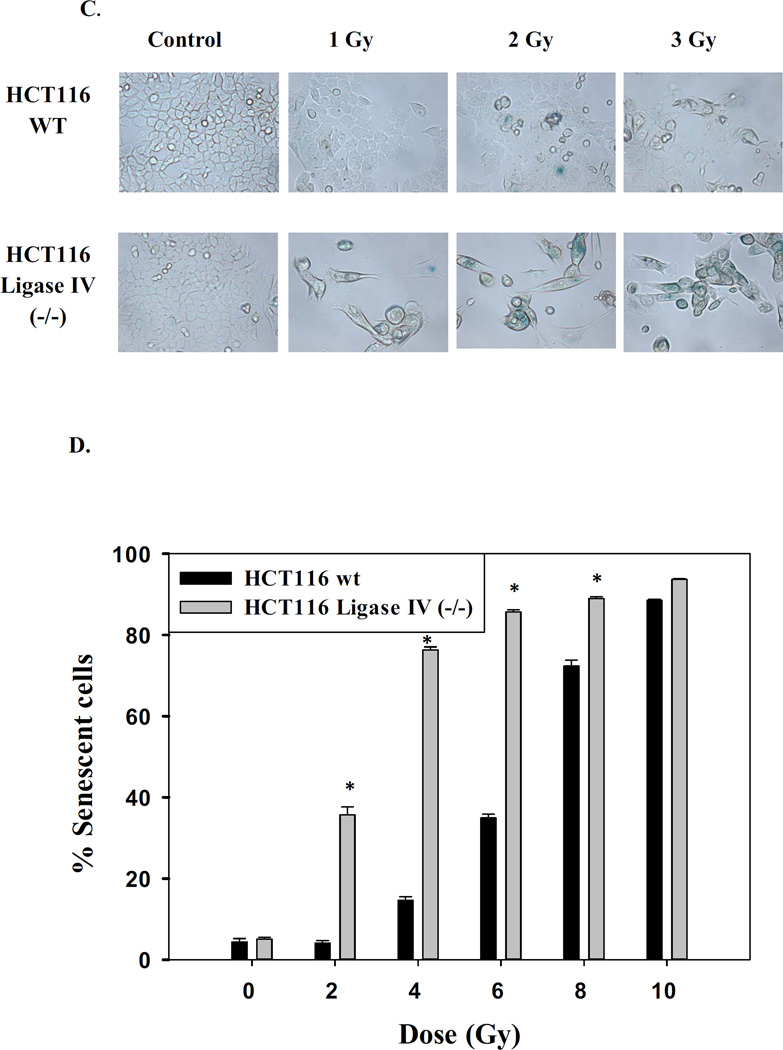
A. Acridine orange staining of HCT116 wt and HCT116 Ligase IV-deficient cells 96 hrs post treatment. Images shown are representative of three replicate studies. B. Quantification of autophagy by acridine orange flow cytometry 96 hr post treatment. Error bars represent standard error (* p<0.05 compared to the corresponding dose of radiation in HCT116 wt cells) (n=3). C. Promotion of senescence based on β-galactosidase staining. D. Quantification of β-galactosidase by flow cytometry at 96 hr (n=3). Error bars represent standard error. * in B and D indicates p<0.05 compared to the corresponding effect at a similar dose of radiation in the HCT116 wt cells) (n=3).
As senescence has been closely associated with autophagy in a number of studies [31, 48], the induction of senescence by radiation was also monitored. Both cell lines demonstrated physiological markers of senescence such as granulation, flattening, and spreading as well as β-galactosidase staining, a hallmark of senescence (Figure 2C). In parallel with the findings relating to autophagy, senescence was more pronounced in the Ligase IV deficient cells compared to the Ligase IV proficient cells at the lower doses of radiation while higher doses yielded maximal senescent populations in both cell lines (Figure 2D).
Although radiation-induced autophagy, senescence and persistent H2AX phosphorylation were greater in the HCT116 Ligase IV-deficient cells than in Ligase IV-proficient cells at the lower doses of radiation, the fraction of cells showing autophagy and senescence, at any given level of γH2AX, was very similar for the two cell lines (Supplementary Figures 1A and 1B). Supplementary Figure 1C also indicates a direct correspondence between the extent of autophagy and senescence (as a function of the dose of radiation) in both HCT116 tumor cell lines. Thus, both senescence and autophagy correlate with, and are likely triggered by, persistent double-strand breaks.
3.3 The relationship between autophagy and senescence in irradiated cells
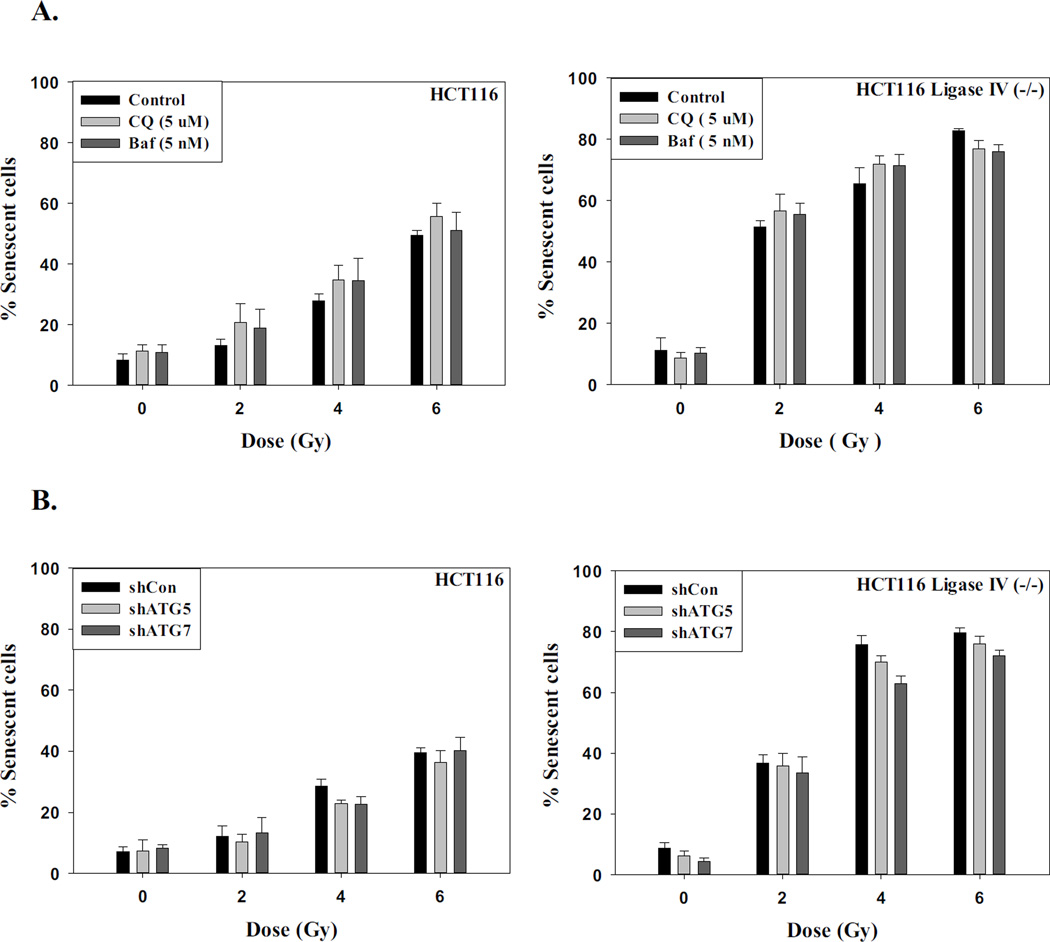
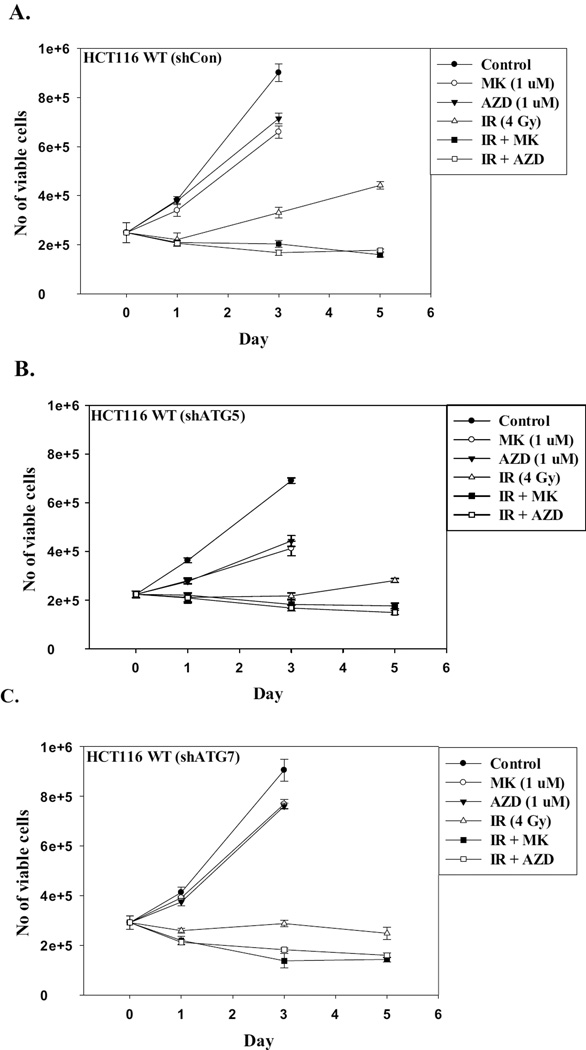
Our studies are indicative of a close correspondence between the induction of autophagy and senescence by radiation in both the Ligase IV deficient and the Ligase IV proficient cell lines (Supplementary Figure 1C), which is also the case for oncogene and chemotherapy-induced autophagy and senescence [31, 48]. To more rigorously investigate the potential association between autophagy and senescence in response to radiation, both cell lines were either pre-incubated with the pharmacological inhibitors of autophagy, chloroquine (5 µM) and bafilomycin (5 nM), for 3 h prior to irradiation, or infected with lentivirus to induce a knockdown of the essential autophagy factors ATG5 and ATG7. (Supplementary Figure 2A). Supplementary Figures 2B and 2C confirm that autophagy has been inhibited by chloroquine and bafilomycin in both cell lines based on the interference with radiation-induced degradation of p62/SQSTM1. Similarly, Supplementary Figures 2D and 2E confirm that autophagy has been inhibited by the genetic silencing approaches. Figure 3 indicates that pharmacological and genetic inhibition of autophagy had no effect on the promotion of radiation-induced senescence in these cell lines, as the extent of senescence was essentially identical in the absence and presence of functional autophagy, strongly indicating that autophagy and senescence in response to radiation are dissociable. This has, in fact, proven to be the case for both oncogene-induced senescence and senescence induced by doxorubicin [31, 48].
Figure 3. Inhibition of autophagy fails to suppress radiation-induced senescence.
A. HCT116 cells and HCT116 Ligase IV-deficient cells were pretreated with chloroquine (5 µM) or Bafilomycin (5 nM) for 3 hr prior to irradiation and maintained in the presence of the autophagy inhibitors for an additional 24 hr. Senescence was assessed after 96 h by flow cytometry (n=3). B. HCT116 cells and HCT116 Ligase IV-deficient cells with silencing of ATG5 or ATG7 were exposed to the indicated doses of radiation and senescence was assessed after 96 h by flow cytometry (n=3).
3.5 Cells induced to undergo autophagy/senescence by irradiation retain the capacity for proliferative recovery and are capable of repairing DNA double strand breaks (DSBs)
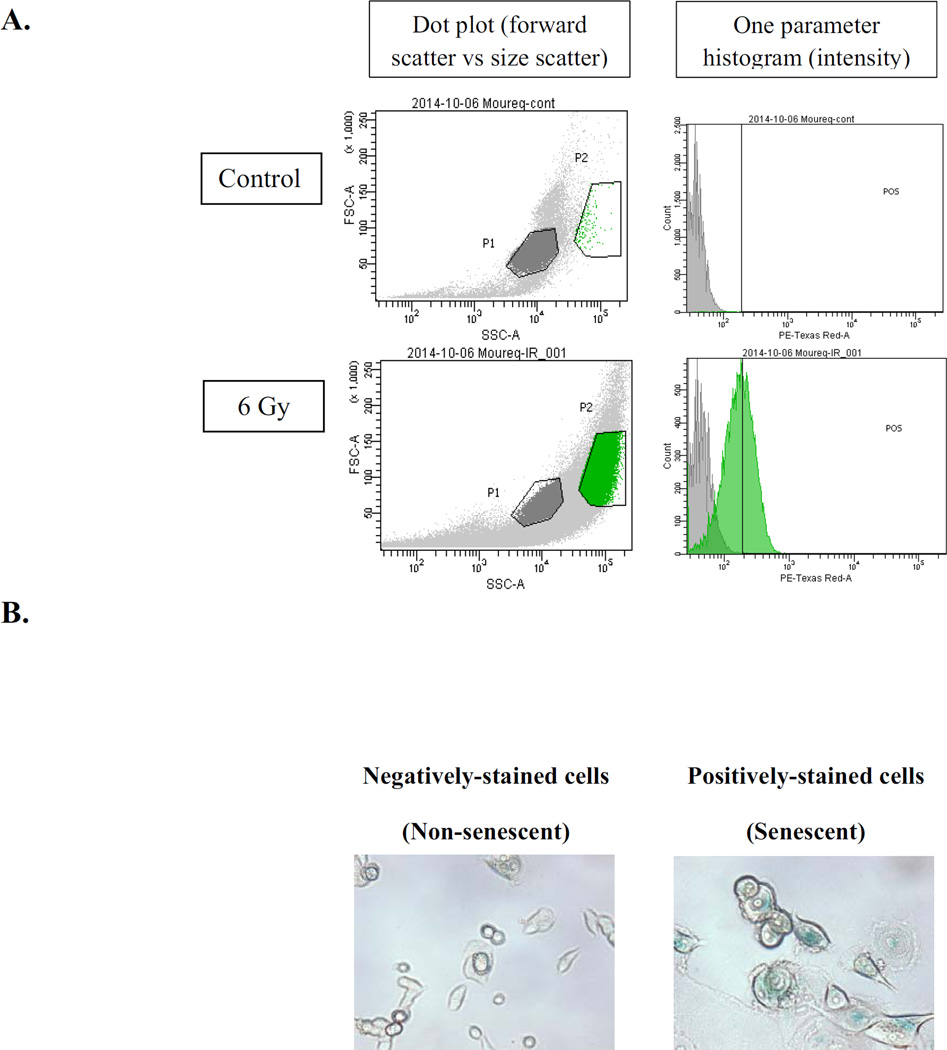
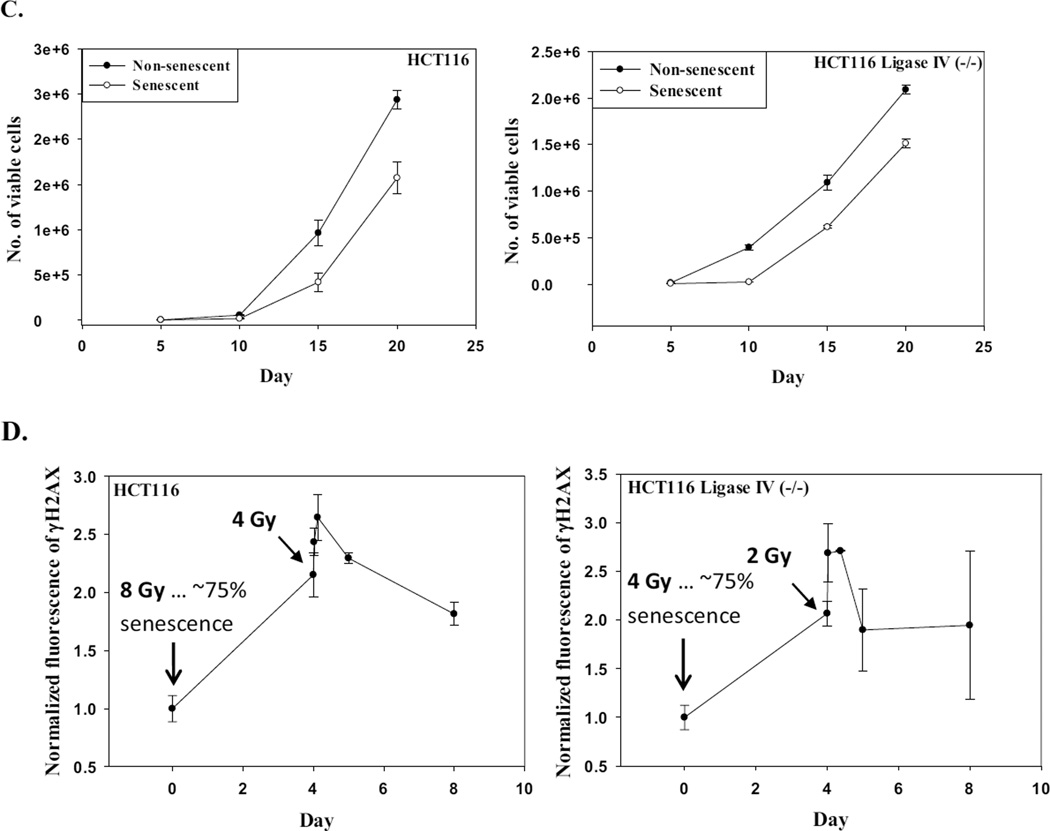
We have shown proliferative recovery after induction of senescence by radiation as well as doxorubicin in breast tumor cells [16, 31, 44, 47]. The HCT116 Ligase IV proficient and HCT116 Ligase IV deficient cells were exposed to radiation doses of 6 Gy and 3 Gy, respectively; cells were sorted based on staining with the senescence marker, C12FDG, 96 hours post-radiation by flow cytometry (Figure 4A and Figure 4B). Both sub-populations (i.e., positively stained and negatively stained cells) were replated at subconfluent density. Figure 4C confirms that recovery occurs after radiation-induced autophagy and senescence in both the HCT116 Ligase IV-proficient and Ligase IV-deficient cells. These findings are consistent with studies where proliferative recovery was observed after irradiation of MCF-7 breast tumor cells [16, 44].
Figure 4. DNA repair capacity in senescent cells.
A. HCT116 cells were stained with β-galactosidase substrate (C12FDG) 96 hours post-treatment, and subjected for sorting by flow cytometry at excitation/emission wavelengths of 490/514 nm. Left panels show gating of cells based on forward scatter vs side scatter; right panels show gating applied to data from 488–610/20 channel to detect β-Gal fluorescence. B. Both subpopulations were stained with β-galactosidase to ensure that cells were successfully sorted according to size and fluorescence. C. Senescent and non-senescent sub-populations were replated separately in 6-well plates, and viable cell number was monitored at the indicated time points by trypan blue exclusion. D. HCT116 cells and HCT116 Ligase IV (−/−) cells were exposed to 8 Gy or 4 Gy radiation followed by a 96 h interval for DNA repair and subsequent re-exposure to 4 Gy or 2 Gy radiation, respectively. Intensity of γH2AX fluorescence was measured by flow cytometry at the indicated time points (n=3).
The capacity for proliferative recovery suggests that DNA repair is likely to be functional in the autophagy/senescent cells. To address this question, HCT116 cells were exposed to a dose of radiation (8 Gy) that induces ~75% of both autophagy and senescence; the cells were then allowed to undergo repair for 4 days, followed by re-irradiation with 4 Gy. Repair intensity was measured after 30 min, 3 h, 24 h, and 4 days based on γH2AX intensity determined by flow cytometry. Four days after the first dose of radiation (8 Gy), the level of γH2AX remained high. At the second dose of radiation (4 Gy), the intensity of γH2AX was further elevated for 3 h. However, the intensity of γH2AX was reduced by 24 and 96 h after the second dose, suggesting that, despite the persistence of initial DNA damage, these cells were still generally proficient in DNA repair capacity (Figure 4D, left panel).
Similarly, HCT116 Ligase IV (−/−) were treated initially with 4 Gy, a dose that induces ~75% of senescence and autophagy, followed four days late by 2 Gy of radiation. Figure 4D, right panel indicates that even these ostensibly repair-incompetent cells show the capacity to repair the newly induced DNA damage.
3.6 Radiosensitization by PARP inhibitors correlates with increased autophagy and senescence, but not apoptosis
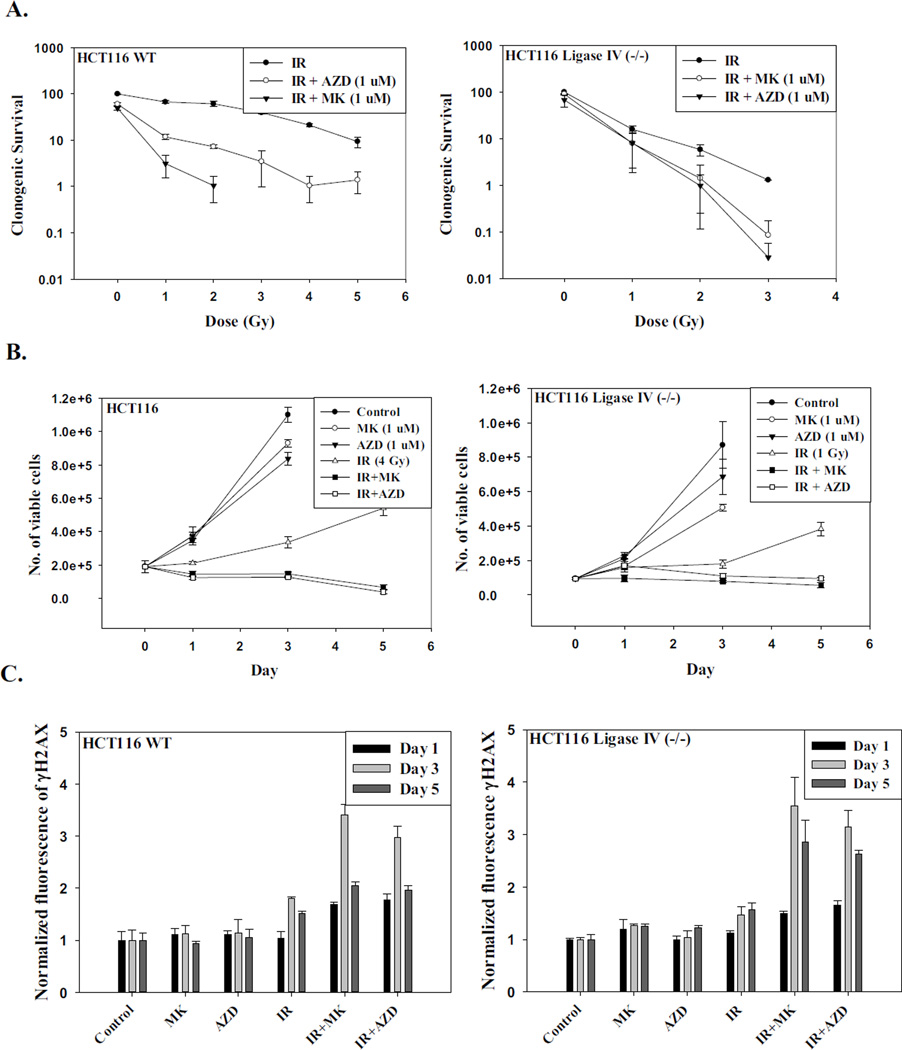
There has been a great deal of interest in utilizing DNA repair inhibitors in combination with chemotherapeutic drugs and radiation to enhance the efficacy of cancer therapy. In the context of this work, it has been reported that radiation sensitization by PARP inhibitors is accompanied by increased senescence [30, 49]. Given the evidence for correspondence between autophagy and senescence in the current work, we proceeded to investigate whether sensitization by PARP inhibitors could be mediated through the promotion of autophagy as well as senescence. Two different PARP inhibitors, AZD-2281 (Olaparib) and MK-4827 (Niraparib), were utilized to investigate whether the PARP inhibitors could sensitize both Ligase IV deficient cells and Ligase IV proficient HCT116 cells to radiation. Figure 5A shows that PARP inhibitors conferred profound radiation sensitization in the Ligase IV-proficient HCT116 cells. However, while Ligase IV deficient cells were also sensitized, the degree of sensitization was clearly less than in the Ligase IV proficient cells. Temporal response data (Figure 5B) also showed a more pronounced radiosensitization in Ligase IV proficient than in the Ligase IV deficient cell lines when using equitoxic doses of radiation. As would have been expected, this sensitization was associated with an increase in DNA damage based on the intensity of γH2AX formation (Figure 5C) and the increased DNA content in the comet tails by the Comet assay (Supplementary Figures 3A and 3B).
Figure 5. Cell survival, DNA damage, autophagy and senescence in irradiated cells exposed to PARP inhibitors.
HCT116 WT and HCT116 Ligase IV deficient cells were incubated with AZD-2281 (1 µM) or MK-4827 (1 µM) for 3 hr prior to irradiation and maintained in the presence of the inhibitors for an additional 24 hr. A. Number of colonies was determined after 14 days (n=3). B. Number of viable cells was counted at the indicated time points (n=5). C. γH2AX intensity was measured at the indicated time points by flow cytometry in both cell lines (n=3). D. Quantification of senescence by flow cytometry at the indicated time points (n=3). E. Quantification of autophagy by flow cytometry at the indicated time points (n=3).
Sensitization to radiation by the PARP inhibitors is also associated with an increase in senescence. Quantification of the intensity of β-galactosidase staining by flow cytometry indicated that between 55–60% of the Ligase IV proficient HCT116 cells had entered a state of senescence when the PARP inhibitors were used in combination with radiation, whereas radiation alone induced ~20% senescence (Figures 5D, left panel, and Supplementary Figure 3C). Similarly, the HCT116 Ligase IV deficient cells showed an increase in the senescent population from less than 20% to between 45–55% when the PARP inhibitor was administered along with radiation (Figure 5D, right panel, and Supplementary Figure 3D). Consistent with the increased senescence, cell cycle analysis results demonstrated that ~ 45% of the population in both cell lines underwent growth arrest at the G2/M phase when cells were treated with the combination compared to ~ 20% when cells were exposed to radiation alone (Supplementary Figure 3E).
The combination of AZD-2281 or MK-4827 with radiation also resulted in increased autophagy. Quantification of the intensity of autophagy by flow cytometry showed an increase in the number of autophagic cells to 70–80% of the population for the combination treatment in the Ligase IV-proficient cells, whereas radiation alone promoted approximately 30% autophagy (Figure 5E, left panel, and Supplementary Figures 4A and 4B). Similarly, the HCT116 Ligase IV-deficient cells show an increase in the autophagic population to 75% when the PARP inhibitor was administered along with radiation compared to 35% when exposed to radiation alone (Figure 5E, right panel and Supplementary Figure 4C).
Overall, the PARP inhibitors appear to produce comparable enhancement of H2AX phosphorylation, autophagy and senescence in wild-type and Ligase IV-deficient cells, but less radiosensitization of Ligase IV-deficient cells, particularly as measured by clonogenic survival.
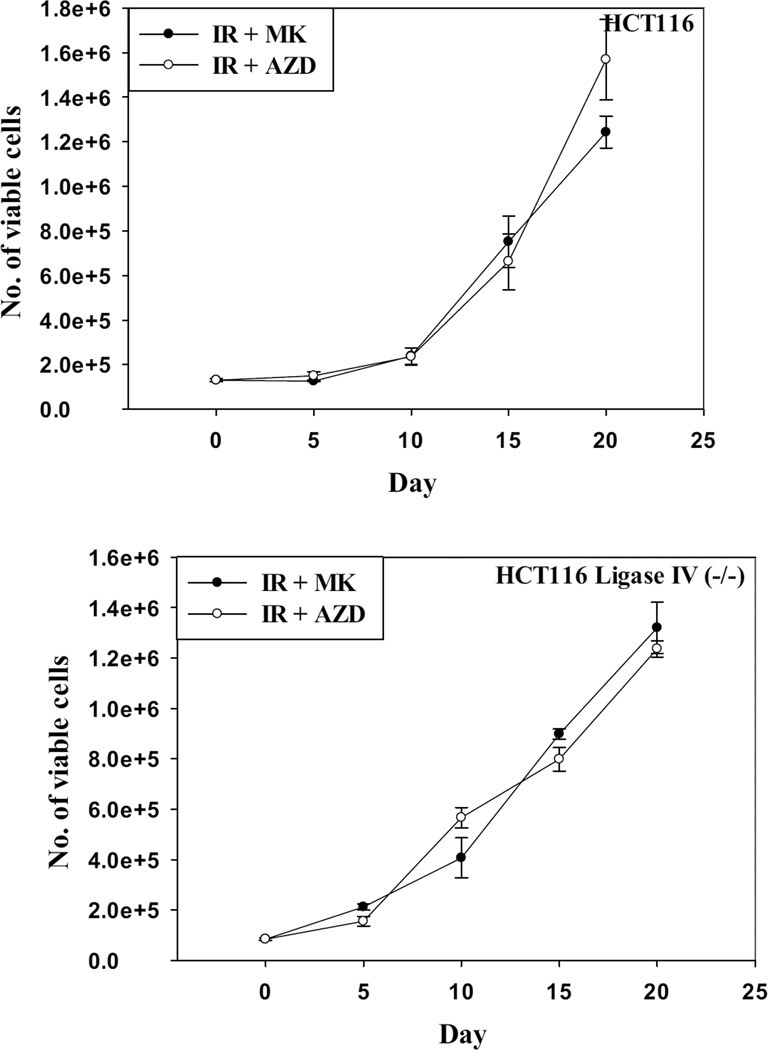
Although co-treatment with PARP inhibitors enhanced the radio-sensitivity of HCT116 cells and HCT116 Ligase IV-deficient cells, it was critical to determine whether the cells would retain their proliferative recovery after the exposure to the combination treatment by monitoring cell viability over an extended period post treatment. Figure 6 demonstrates that both cell lines recovered proliferative capacity on days 10, 15, and 20 post-treatment when radiation was combined with the PARP inhibitors.
Figure 6. Proliferative recovery after radiation and PARP inhibition.
HCT116 wt and HCT116 Ligase IV-deficient cells were incubated with AZD-2281 (1 µM) or MK-4827 (1 µM) for 3 h before exposure to radiation doses of 4 Gy and 1 Gy, respectively, and maintained in the presence of the inhibitors for an additional 24 hr. Viable cell number was monitored over a period of 20 days.
3.7 Lack of involvement of apoptosis in sensitization by PARP inhibitors in HCT116 cells
Use of PARP inhibitors has generally been shown to radiosensitize cells through the induction of senescence, but not apoptosis [30, 49]. To rule out the potential involvement of apoptosis in radiation sensitization, apoptotic cell death was monitored by Annexin V staining. Supplementary Figure 5A indicates that apoptosis is unlikely to be involved in sensitization of both cell lines to radiation by PARP inhibition as apoptosis was minimal and not increased by the PARP inhibitors. The minimal involvement of apoptosis in radiosensitization by PARP inhibition was confirmed by assessment of apoptosis 72 h post-treatment using the TUNEL assay (Supplementary Figures 5B and 5C). To further confirm these results, irradiated HCT116 cells were treated using the Pan Caspase Inhibitor Z-VAD-FMK (10 µM) and viable cell numbers were monitored over a period of five days. Supplementary Figure 5D shows that interference with apoptosis via inhibition of caspases did not interfere with radio-sensitization by the PARP inhibitors, indicating that apoptosis does not appear to be involved in mediating the observed effects. Consistent with these observations, cell cycle analysis demonstrated that administering PARP inhibitors along with radiation does not increase the sub-G1 population (data not shown), confirming that apoptosis is not occurring in cells exposed to radiation + PARP inhibitors.
3.8 Effects of autophagy inhibition on radiosensitization by PARP inhibition
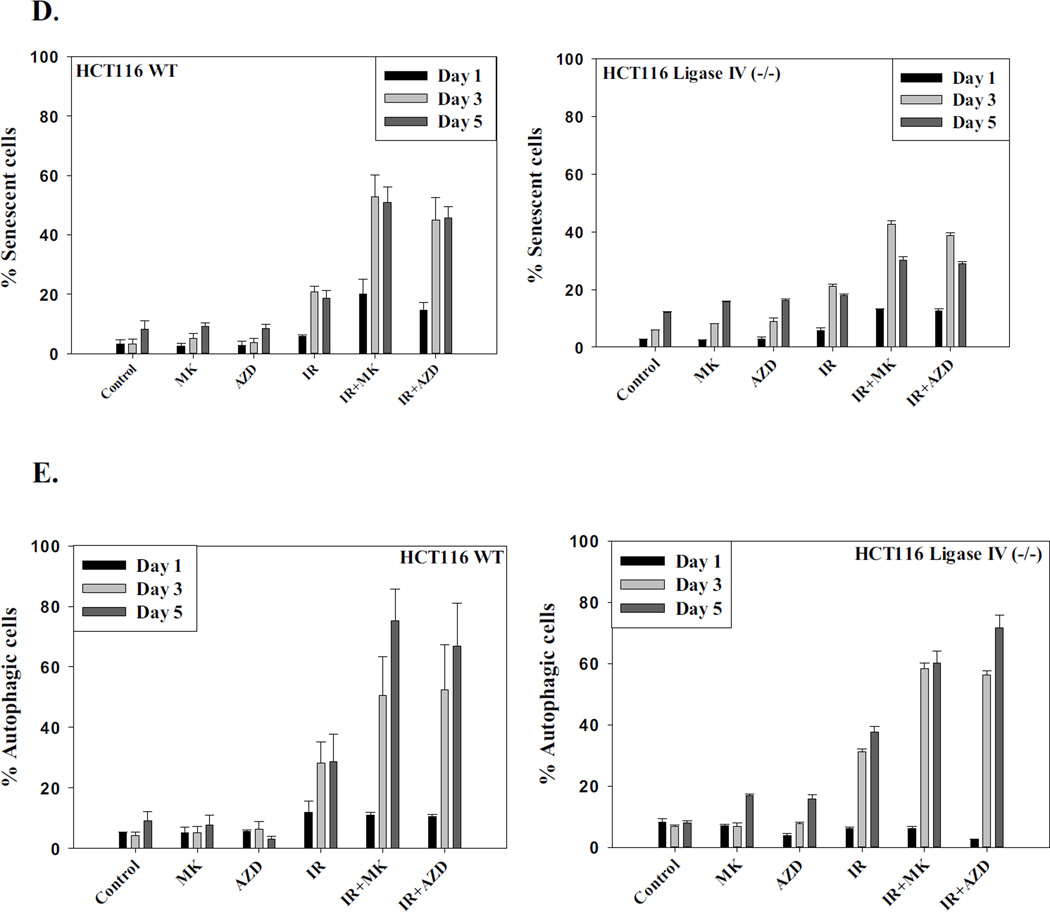
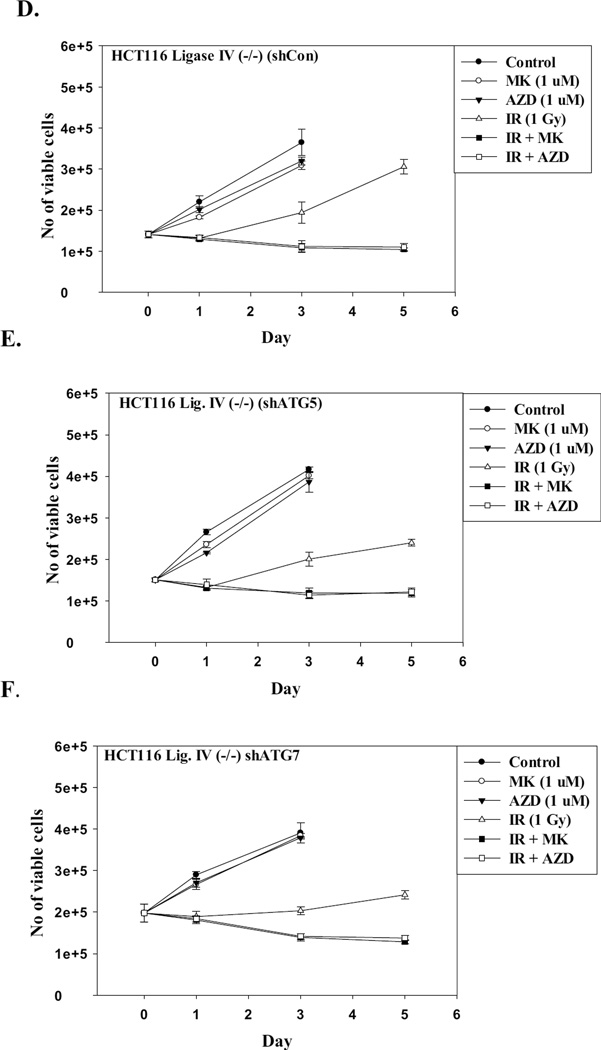
The studies presented above clearly show that autophagy was increased in association with senescence when PARP inhibitors are used in combination with radiation. Several studies, including our own, have demonstrated that autophagy can act as a cytotoxic or cytostatic process through which cells die or undergo prolonged growth arrest [44, 47, 50–51]. To address whether inhibition of autophagy would interfere with the radiosensitization by PARP inhibitors, HCT116 cell lines where autophagy was genetically silenced or pretreated with choroquine were exposed to radiation in the absence and presence of the PARP inhibitors. Figures 7A–7F demonstrate that genetic interference with autophagy does not rescue either of the HCT-116 cell lines from radiosensitization by PARP inhibition. These findings are supported by the data presented in Supplementary Figure 6 where the autophagy inhibitor, chloroquine, also failed to interfere with radiosensitization by the PARP inhibitors in HCT116 cells as well as H460 non-small cell lung cancer cells, indicating that radiosensitization does not occur via the promotion of autophagy.
Figure 7. Inhibition of autophagy does not alter sensitization to radiation by PARP inhibition.
Autophagy regulated genes were silenced in the HCT116 wt and HCT116 Ligase IV deficient cell lines using short hairpin RNA (shRNA) for ATG5 and ATG7. A–C. Autophagy proficient HCT116 cells were irradiated with and without exposure to the PARP inhibitors. D–F. Autophagy-deficient HCT116 Ligase IV cells were irradiated with and without exposure to the PARP inhibitors.
4. Discussion
4.1. DNA damage, autophagy and senescence induced by radiation
Although radiotherapy is one of the most widely used cancer therapies, the effectiveness of radiation may vary widely according to tumor type. For example, radiotherapy significantly reduces recurrence and improves outcomes in breast and head and neck cancer, respectively [52–53], but is less effective in the treatment of glioblastoma and lung cancer [54–56]. Consequently, decades of preclinical efforts have been devoted to the development of strategies to sensitize malignancies to radiation therapy.
While it is generally agreed that radiation kills tumor cells by generating double-strand breaks, these breaks as well as other DNA damage elicit a complex cascade of responses that can influence the repair and persistence of DNA damage as well as the consequences of unrepaired damage - factors which together determine whether or not an irradiated tumor cell will ultimately resume proliferation. Among these responses, radiation can induce cells to enter states of senescence as well as autophagy [5, 57]. Because autophagy can function either as a pro-survival mechanism or as pro-death mechanism [58–59], depending on the agents used and the experimental systems, it provides an especially attractive target for pharmacological manipulations that could selectively increase radiosensitivity of tumor cells but not normal cells.
The relationship between autophagy and DNA repair is unclear, but autophagy can alter the cellular response to DNA damaging agents. Disruption of autophagy by bafilomycin A1, an autophagy inhibitor, sensitized glioma cells to the alkylating agent telmozolamide by inducing apoptosis [11]. Similarly, 6-thioguanine-induced autophagy enhanced the survival of human colorectal and endometrial cells, indicating in both studies that autophagy may play a protective role against DNA damage [12]. Robert et al. have recently found that autophagy and protein acetylation are important in DNA damage repair via activation of cell cycle check points, influencing homologous recombination repair (HRR) [13]. Another study has shown that PARP-1 might link DNA damage to autophagy through the depletion of ATP and (NAD+), which may indicate that a cytoprotective function of autophagy was promoted to supply the cell with energy [14]. Interference with autophagy by knocking out FIP200, an essential player in mammalian autophagy, resulted in impaired DNA repair in mouse embryonic fibroblast (MEF) treated with DNA damaging agents [15]. On the other hand, human malignant glioma cells undergo autophagic cell death upon the inhibition of the DNA dependent protein kinase catalytic subunit (DNA–PKcs), a protein involved in non-homologous end joining [60]. In related studies, inhibition of DNA-PKcs was found to radiosenitize radioresistant prostate cancer cells by inducing autophagy [61]. It therefore appears likely that autophagy has an important function in the enhancement of DNA repair in cells during exposure to genotoxic stress, but that the role of autophagy may differ according to the status of DNA repair.
Senescence is also induced upon exposure to a DNA-damaging agent such as radiation [16–17, 57]. Moreover, similar to the telomere-associated foci of replicative senescence, radiation-induced senescence is associated with persistent DNA damage foci [62–63], presumably unrepaired double-strand breaks that can remain for months and may be essential for maintaining the long-term growth arrest that characterizes the senescent cell.
In this work, we sought to identify whether autophagy and senescence play major roles in either facilitating or antagonizing DNA repair, using two isogenic cell lines, HCT116 and HCT116 Ligase IV (−/−) cells. The Ligase IV mutation completely inactivates repair of double-strand breaks by classical nonhomologous end joining [64]. Thus, as expected, at 1 to 2 Gy, the Ligase IV deficient cell line demonstrated lower survival than parental cells and higher levels of persistent γH2AX foci. At each dose of radiation this increased damage was accompanied by higher levels of both senescence and autophagy than in parental cells. Moreover, when senescence or autophagy were plotted as a function of γH2AX intensity at 96 hr postirradiation, the plots for the two cell lines were very similar. Thus, autophagy and senescence appear to correlate with the level of persistent double-strand breaks, suggesting that the persistent breaks and associated repair foci are primarily responsible for promoting and sustaining both senescence and autophagy. These data are consistent with studies showing that DNA damage can also induce cells to undergo a state of senescence associated with autophagy [31].
4.2 Relationship between radiation-induced autophagy and senescence
As we observed a direct correlation between the fraction of autophagic and of senescent cells in response to DNA damage in both cell lines, it was noteworthy to determine whether the functions of autophagy and senescence were interlinked. The relationship between autophagy and senescence is still debatable. While the induction of senescence has been reported to be, at least in part, dependent on autophagy [31, 48], other studies concluded that senescence is independent of autophagy [65–66]. Despite the close correspondence between autophagy and senescence in parental and Ligase IV−/− HCT116 cells, pharmacological and genetic inhibition of autophagy did not seem to affect the promotion of senescence even at high doses of radiation, indicating that promotion of senescence is independent of autophagy in this experimental model.
4.3 Effect of PARP inhibition on radiation sensitivity, autophagy and senescence
Given the observation that irradiated cells appear to undergo proliferative recovery after a period of growth arrest, we sought to sensitize both cell lines to radiation via interfering with DNA repair to combat the recovery. PARP inhibitors are considered one of the promising radio-sensitizing agents that have been tested in clinical trials [67–72]. The poly (ADP-ribose) polymerase (PARP) enzyme is involved in repair of single-strand breaks (SSBs), and lack of this enzyme in knockout mice enhanced sensitivity to radiation and alkalyting agents [73]. PARP inhibition converts SSBs to DSBs, which in turn leads to the activation of HRR [74]. In cells lacking the BRCA1 protein, which is a critical component of the HRR repair pathway, PARP inhibition can be lethal even in the absence of exogenous DNA damaging agents [75]. Due to microsatellite instability in colorectal cancer cells, the expression of MRE11, another protein involved in the HRR pathway, is reduced [76]. Thus, co-administration of PARP inhibitors with radiation was anticipated to lead to radiosensitization in HCT116 cells.
PARP inhibitors increased the intensity of γH2AX and the number of irradiated cells undergoing autophagy and senescence, but not apoptosis. These data were consistent with our findings that both autophagy and senescence are directly correlated with induced DNA damage. In parental HCT116 cells, this increased DNA damage was associated with a dramatic reduction in clonogenic survival of irradiated cells, especially with MK. Radiosensitization of the already radiosensitive Ligase IV-deficient cells was less robust, despite similarly elevated levels of γH2AX, autophagy and senescence. This result is consistent with a model wherein radiosensitization results at least in part from inappropriate channeling of replication-associated one-sided double-strand breaks into NHEJ; thus, when NHEJ is absent, radiosensitization is diminished. This mechanism has been invoked previously to explain the similar dependence of PARP inhibitor sensitivity on the absence of NHEJ in BRCA1-deficient cells [77], except that in those cells the initial single-strand breaks would be spontaneous rather than radiation-induced. In contrast to our results, a previous study reported that Ligase IV-deficient mouse fibroblasts were radiosensitized by a PARP inhibitor [olaparib] at least as much as wild-type cells, and in that case radiosensitization was attributed to inhibition of a backup “Alt-NHEJ” pathway that is PARP-dependent and Ligase IV-independent [78]. Intriguingly, however, those Ligase IV−/− cells, but not the normal cells, were also p53−/−, due to the inviability of p53+/+ Ligase IV−/− mice, whereas HCT116 and its derivatives are p53+/+. An alternative explanation for the relative lack of sensitization of the Ligase IV−/− HCT116 cells is that these cells, after extended propagation in culture, have acquired upregulated HRR or Alt-NHEJ functions [79], rendering these repair systems less susceptible to PARP inhibitors.
In any case, apoptosis does not seem to be involved in the cytotoxicity of combination therapy in either cell line, indicating that the radiosensitization of these cell lines by PARP inhibitors might be mediated by promoting autophagy and senescence. Interestingly, inhibition of autophagy also did not interfere with PARP inhibitor-mediated radiosensitization, as judged by the temporal response assay. Taking into the consideration that radiation-induced senescence and radiation-induced autophagy are not linked in our system, these findings support the premise that radiation sensitization is likely to be occurring via the promotion of senescence.
A recent study showed that PARP-1 is involved in a newly identified back up pathway named PARP1-dependent end joining (PARP1-EJ) [80]. This new finding adds another aspect of the lethality of our combination therapy in HCT116 cell lines when main repair pathways are blocked, i.e., it could account for the residual radio-sensitizing effect of PARP inhibitors in the cells that lack Ligase IV. However, another NHEJ-like back-up repair mechanism, called the mutagenic NHEJ pathway or A-NHEJ, can be activated when PARP1- mediated and HR pathways are inactivated [81].
The relationship between DNA damage, autophagy and senescence is likely to be quite complex and our goals were primarily to establish whether sensitization through PARP inhibitors could occur through senescence, as postulated by the Weichselbaum laboratory [30, 49], whether the senescence might be dependent on autophagy and whether autophagy might also play a role in sensitization. Our studies rule out the involvement of autophagy in the radiosensitization and furthermore dissociate radiation-induced autophagy from senescence. With regard to mechanistic questions, it has been demonstrated that DNA damage can lead to senescence and autophagy, possibly via induction of ATM. The up-regulation of ATM leads to the activation of its downstream target p53, which then promotes senescence via the p21-pRb pathway [82–83]. Also, p53 can activate the autophagy promoter AMP-activated protein kinase during the genotoxic stress, which in turn phosphorylates tuberous sclerosis (TSC) complex proteins TSC1 and TSC2 [84–86]. Both proteins TSC1 and TSC2 downregulate mTOR, which eventually lead to the promotion of autophagy [87–88]. It is worth mentioning that HCT116 cells have a frameshift mutation in p16 [89], however, a number of reports, including our own, have indicated that senescence could be upregulated in a p16-independent manner [5, 90–91]. Our data suggests that a persistent DNA damage response (DDR) may upregulate ATM and induce p53. It is possible that the pathways diverge at p53 wherein for senescence the pathway could involve the sequence of p53-p21-pRb, whereas in autophagy the pathway may reflect actions at the level of p53-AMPK-TSC-mTOR. We anticipate that future studies will address these questions.
4.4 Conclusions
There is no consensus as to whether senescence induced by radiation or chemotherapy is reversible [92–93]. We demonstrate, both in the case of radiation alone and in the studies combining PARP inhibition with radiation, that growth arrest is followed by proliferative recovery. In this context, the studies by Chitkova et al in an apoptosis-deficient cell line [94] support our findings that senescence may be reversible. These observations clearly suggest that tumor cells that enter a state of autophagy/senescence have the capacity to re-emerge into a proliferative state. If these findings can be extrapolated to clinical cancer, this may explain why radiation is not fully effective in the treatment of some types of malignancies. Furthermore, it is likely that the use of PARP inhibitors will result in only transient radiosensitization.
We conclude that the extent of radiation-induced DNA damage is accompanied with an increase of autophagy and senescence. The extent of autophagy and senescence induced at different doses of radiation was more pronounced in the ligase IV deficient cells, which is correlated with increased levels of DNA damage. Autophagic/senescent HCT116 cells demonstrated the ability to repair the newly formed DSBs. These data may indicate that promoting senescence alone would not have an effect on overall DNA repair system efficiency, which may explain why even the radiosensitized cells ultimately recover proliferative capacity. Current therapeutic regimens such as radiotherapy generally fail to completely eradicate the tumor cell population; this could be due, in part, to the induction of autophagy and senescence, which may be permissive for DNA repair as well as proliferative recovery that occurs even with the inclusion of PARP inhibitors, which may therefore not interfere with disease recurrence.
Supplementary Material
Acknowledgments
This work was supported by grants from the National Cancer Institute CA40615 (LFP), CA166264 (LFP), CA154461 (EAH), CA190492 (EAH), from the National Institutes of General Medical Sciences, GM088351 (EAH) and by the Office of the Assistant Secretary of Defense for Health Affairs through the Breast Cancer Research Program under Award No. W81XWH-14-1-0088 (DAG).
Contributor Information
Moureq Alotaibi, Department of Pharmacology and Toxicology, Virginia Commonwealth University; Department of Pharmacology and Toxicology, College of Pharmacy, King Saud University, P.O. Box 2457, Riyadh, 11451, Kingdom of Saudi Arabia.
Khushboo Sharma, Department of Pharmacology and Toxicology, Virginia Commonwealth University.
Tareq Saleh, Department of Pharmacology and Toxicology, Virginia Commonwealth University.
Lawrence F. Povirk, Department of Pharmacology and Toxicology, Virginia Commonwealth University
Eric A. Hendrickson, Department of Biochemistry, Molecular Biology and Biophysics, University of Minnesota, Minneapolis MN 55455
David A. Gewirtz, Department of Pharmacology and Toxicology, Virginia Commonwealth University Department of Medicine, Massey Cancer Center.
References
- 1.Vignard J, Mirey G, Salles B. Ionizing-radiation induced DNA double-strand breaks: a direct and indirect lighting up. Radiother Oncol. 2013;108:362–369. doi: 10.1016/j.radonc.2013.06.013. [DOI] [PubMed] [Google Scholar]
- 2.Tounekti O, Kenani A, Foray N, et al. The ratio of single- to double-strand DNA breaks and their absolute values determine cell death pathway. Br J Cancer. 2001;84:1272–1279. doi: 10.1054/bjoc.2001.1786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jackson SP. Sensing and repairing DNA double-strand breaks. Carcinogenesis. 2002;23:687–696. doi: 10.1093/carcin/23.5.687. [DOI] [PubMed] [Google Scholar]
- 4.Arthur CR, Gupton JT, Kellogg GE, et al. Autophagic cell death, polyploidy and senescence induced in breast tumor cells by the substituted pyrrole JG-03-14, a novel microtubule poison. Biochem Pharmacol. 2007;74:981–991. doi: 10.1016/j.bcp.2007.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gewirtz DA, Holt SE, Elmore LW. Accelerated senescence: an emerging role in tumor cell response to chemotherapy and radiation. Biochem Pharmacol. 2008;76:947–957. doi: 10.1016/j.bcp.2008.06.024. [DOI] [PubMed] [Google Scholar]
- 6.Adams JM, Cory S. The Bcl-2 apoptotic switch in cancer development and therapy. Oncogene. 2007;26:1324–1337. doi: 10.1038/sj.onc.1210220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kouroku Y, Fujita E, Tanida I, et al. ER stress (PERK/eIF2alpha phosphorylation) mediates the polyglutamine-induced LC3 conversion, an essential step for autophagy formation. Cell Death Differ. 2007;14:230–239. doi: 10.1038/sj.cdd.4401984. [DOI] [PubMed] [Google Scholar]
- 8.Jain MV, Paczulla AM, Klonisch T, et al. Interconnections between apoptotic, autophagic and necrotic pathways: implications for cancer therapy development. J Cell Mol Med. 2013;17:12–29. doi: 10.1111/jcmm.12001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gewirtz DA. Autophagy, senescence and tumor dormancy in cancer therapy. Autophagy. 2009;5:1232–1234. doi: 10.4161/auto.5.8.9896. [DOI] [PubMed] [Google Scholar]
- 10.Gewirtz DA. The autophagic response to radiation: relevance for radiation sensitization in cancer therapy. Radiat Res. 2014;182:363–367. doi: 10.1667/RR13774.1. [DOI] [PubMed] [Google Scholar]
- 11.Kanzawa T, Germano IM, Komata T, et al. Role of autophagy in temozolomide-induced cytotoxicity for malignant glioma cells. Cell Death Differ. 2004;11:448–457. doi: 10.1038/sj.cdd.4401359. [DOI] [PubMed] [Google Scholar]
- 12.Zeng X, Yan T, Schupp JE, et al. DNA mismatch repair initiates 6-thioguanine--induced autophagy through p53 activation in human tumor cells. Clin Cancer Res. 2007;13:1315–1321. doi: 10.1158/1078-0432.CCR-06-1517. [DOI] [PubMed] [Google Scholar]
- 13.Robert T, Vanoli F, Chiolo I, et al. HDACs link the DNA damage response, processing of double-strand breaks and autophagy. Nature. 2011;471:74–79. doi: 10.1038/nature09803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Munoz-Gamez JA, Rodriguez-Vargas JM, Quiles-Perez R, et al. PARP-1 is involved in autophagy induced by DNA damage. Autophagy. 2009;5:61–74. doi: 10.4161/auto.5.1.7272. [DOI] [PubMed] [Google Scholar]
- 15.Bae H, Guan JL. Suppression of autophagy by FIP200 deletion impairs DNA damage repair and increases cell death upon treatments with anticancer agents. Mol Cancer Res. 2011;9:1232–1241. doi: 10.1158/1541-7786.MCR-11-0098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jones KR, Elmore LW, Jackson-Cook C, et al. p53-Dependent accelerated senescence induced by ionizing radiation in breast tumour cells. Int J Radiat Biol. 2005;81:445–458. doi: 10.1080/09553000500168549. [DOI] [PubMed] [Google Scholar]
- 17.te Poele RH, Okorokov AL, Jardine L, et al. DNA damage is able to induce senescence in tumor cells in vitro and in vivo. Cancer Res. 2002;62:1876–1883. [PubMed] [Google Scholar]
- 18.Ding Z, Wu CJ, Jaskelioff M, et al. Telomerase reactivation following telomere dysfunction yields murine prostate tumors with bone metastases. Cell. 2012;148:896–907. doi: 10.1016/j.cell.2012.01.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chakradeo S, Elmore LW, Gewirtz DA. Is senescence reversible? Curr Drug Targets. 2015 doi: 10.2174/1389450116666150825113500. [DOI] [PubMed] [Google Scholar]
- 20.Kavanagh JN, Currell FJ, Timson DJ, et al. Antiproton induced DNA damage: proton like in flight, carbon-ion like near rest. Sci Rep. 2013;3:1770. doi: 10.1038/srep01770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Staaf E, Brehwens K, Haghdoost S, et al. Gamma-H2AX foci in cells exposed to a mixed beam of X-rays and alpha particles. Genome Integr. 2012;3:8. doi: 10.1186/2041-9414-3-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Roberson RS, Kussick SJ, Vallieres E, et al. Escape from therapy-induced accelerated cellular senescence in p53-null lung cancer cells and in human lung cancers. Cancer Res. 2005;65:2795–2803. doi: 10.1158/0008-5472.CAN-04-1270. [DOI] [PubMed] [Google Scholar]
- 23.Elmore LW, Di X, Dumur C, et al. Evasion of a single-step, chemotherapy-induced senescence in breast cancer cells: implications for treatment response. Clin Cancer Res. 2005;11:2637–2643. doi: 10.1158/1078-0432.CCR-04-1462. [DOI] [PubMed] [Google Scholar]
- 24.Roninson IB, Broude EV, Chang BD. If not apoptosis, then what? Treatment-induced senescence and mitotic catastrophe in tumor cells. Drug Resist Updat. 2001;4:303–313. doi: 10.1054/drup.2001.0213. [DOI] [PubMed] [Google Scholar]
- 25.Dent RA, Lindeman GJ, Clemons M, et al. Phase I trial of the oral PARP inhibitor olaparib in combination with paclitaxel for first- or second-line treatment of patients with metastatic triple-negative breast cancer. Breast Cancer Res. 2013;15:R88. doi: 10.1186/bcr3484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Plummer R, Lorigan P, Steven N, et al. A phase II study of the potent PARP inhibitor, Rucaparib (PF-01367338, AG014699), with temozolomide in patients with metastatic melanoma demonstrating evidence of chemopotentiation. Cancer Chemother Pharmacol. 2013;71:1191–1199. doi: 10.1007/s00280-013-2113-1. [DOI] [PubMed] [Google Scholar]
- 27.Gunderson CC, Moore KN. Olaparib: an oral PARP-1 and PARP-2 inhibitor with promising activity in ovarian cancer. Future Oncol. 2015;11:747–757. doi: 10.2217/fon.14.313. [DOI] [PubMed] [Google Scholar]
- 28.Scott CL, Swisher EM, Kaufmann SH. Poly (ADP-ribose) polymerase inhibitors: recent advances and future development. J Clin Oncol. 2015;33:1397–1406. doi: 10.1200/JCO.2014.58.8848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Azad A, Bukczynska P, Jackson S, et al. Co-targeting deoxyribonucleic acid-dependent protein kinase and poly(adenosine diphosphate-ribose) polymerase-1 promotes accelerated senescence of irradiated cancer cells. Int J Radiat Oncol Biol Phys. 2014;88:385–394. doi: 10.1016/j.ijrobp.2013.10.043. [DOI] [PubMed] [Google Scholar]
- 30.Barreto-Andrade JC, Efimova EV, Mauceri HJ, et al. Response of human prostate cancer cells and tumors to combining PARP inhibition with ionizing radiation. Mol Cancer Ther. 2011;10:1185–1193. doi: 10.1158/1535-7163.MCT-11-0061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Goehe RW, Di X, Sharma K, et al. The autophagy-senescence connection in chemotherapy: must tumor cells (self) eat before they sleep? J Pharmacol Exp Ther. 2012;343:763–778. doi: 10.1124/jpet.112.197590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gewirtz DA. Autophagy and senescence: a partnership in search of definition. Autophagy. 2013;9:808–812. doi: 10.4161/auto.23922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Singh K, Matsuyama S, Drazba JA, et al. Autophagy-dependent senescence in response to DNA damage and chronic apoptotic stress. Autophagy. 2012;8:236–251. doi: 10.4161/auto.8.2.18600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hamstra DA, Johnson SB, Daignault S, et al. The impact of numeracy on verbatim knowledge of the longitudinal risk for prostate cancer recurrence following radiation therapy. Med Decis Making. 2015;35:27–36. doi: 10.1177/0272989X14551639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hein AL, Ouellette MM, Yan Y. Radiation-induced signaling pathways that promote cancer cell survival (review) Int J Oncol. 2014;45:1813–1819. doi: 10.3892/ijo.2014.2614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Patel NR, Lanciano R, Sura K, et al. Stereotactic body radiotherapy for re-irradiation of lung cancer recurrence with lower biological effective doses. J Radiat Oncol. 2015;4:65–70. doi: 10.1007/s13566-014-0175-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chao C, Williams SG, Xu L, et al. Statin therapy is not associated with prostate cancer recurrence among patients who underwent radiation therapy. Cancer Lett. 2013;335:214–218. doi: 10.1016/j.canlet.2013.02.017. [DOI] [PubMed] [Google Scholar]
- 38.Oh S, Wang Y, Zimbric J, et al. Human LIGIV is synthetically lethal with the loss of Rad54B-dependent recombination and is required for certain chromosome fusion events induced by telomere dysfunction. Nucleic Acids Res. 2013;41:1734–1749. doi: 10.1093/nar/gks1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bampton ET, Goemans CG, Niranjan D, et al. The dynamics of autophagy visualized in live cells: from autophagosome formation to fusion with endo/lysosomes. Autophagy. 2005;1:23–36. doi: 10.4161/auto.1.1.1495. [DOI] [PubMed] [Google Scholar]
- 40.Katsube T, Mori M, Tsuji H, et al. Differences in sensitivity to DNA-damaging Agents between XRCC4- and Artemis-deficient human cells. J Radiat Res. 2011;52:415–424. doi: 10.1269/jrr.10168. [DOI] [PubMed] [Google Scholar]
- 41.Kuhne M, Riballo E, Rief N, et al. A double-strand break repair defect in ATM-deficient cells contributes to radiosensitivity. Cancer Res. 2004;64:500–508. doi: 10.1158/0008-5472.can-03-2384. [DOI] [PubMed] [Google Scholar]
- 42.Allalunis-Turner MJ, Zia PK, Barron GM, et al. Radiation-induced DNA damage and repair in cells of a radiosensitive human malignant glioma cell line. Radiat Res. 1995;144:288–293. [PubMed] [Google Scholar]
- 43.Trucco C, Oliver FJ, de Murcia G, et al. DNA repair defect in poly(ADP-ribose) polymerase-deficient cell lines. Nucleic Acids Res. 1998;26:2644–2649. doi: 10.1093/nar/26.11.2644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bristol ML, Di X, Beckman MJ, et al. Dual functions of autophagy in the response of breast tumor cells to radiation: cytoprotective autophagy with radiation alone and cytotoxic autophagy in radiosensitization by vitamin D 3. Autophagy. 2012;8:739–753. doi: 10.4161/auto.19313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bristol ML, Emery SM, Maycotte P, et al. Autophagy inhibition for chemosensitization and radiosensitization in cancer: do the preclinical data support this therapeutic strategy? J Pharmacol Exp Ther. 2013;344:544–552. doi: 10.1124/jpet.112.199802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chakradeo S, Sharma K, Alhaddad A, et al. Yet Another Function of p53: The Switch that Determines Whether Radiation-Induced Autophagy Will be Cytoprotective or Nonprotective. Implications for Autophagy Inhibition as a Therapeutic Strategy. Mol Pharmacol. 2015 doi: 10.1124/mol.114.095273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sharma K, Goehe RW, Di X, et al. A novel cytostatic form of autophagy in sensitization of non-small cell lung cancer cells to radiation by vitamin D and the vitamin D analog, EB 1089. Autophagy. 2014;10:2346–2361. doi: 10.4161/15548627.2014.993283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Young AR, Narita M, Ferreira M, et al. Autophagy mediates the mitotic senescence transition. Genes Dev. 2009;23:798–803. doi: 10.1101/gad.519709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Efimova EV, Mauceri HJ, Golden DW, et al. Poly(ADP-ribose) polymerase inhibitor induces accelerated senescence in irradiated breast cancer cells and tumors. Cancer Res. 2010;70:6277–6282. doi: 10.1158/0008-5472.CAN-09-4224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chakradeo S, Sharma K, Alhaddad A, et al. Yet another function of p53--the switch that determines whether radiation-induced autophagy will be cytoprotective or nonprotective: implications for autophagy inhibition as a therapeutic strategy. Mol Pharmacol. 2015;87:803–814. doi: 10.1124/mol.114.095273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wilson EN, Bristol ML, Di X, et al. A switch between cytoprotective and cytotoxic autophagy in the radiosensitization of breast tumor cells by chloroquine and vitamin D. Horm Cancer. 2011;2:272–285. doi: 10.1007/s12672-011-0081-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Darby S, McGale P, Correa C, et al. Effect of radiotherapy after breast-conserving surgery on 10-year recurrence and 15-year breast cancer death: meta-analysis of individual patient data for 10,801 women in 17 randomised trials. Lancet. 2011;378:1707–1716. doi: 10.1016/S0140-6736(11)61629-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Krstevska V. Radiotherapy and chemotherapy in locally advanced head and neck squamous cell carcinoma. J BUON. 2009;14:361–373. [PubMed] [Google Scholar]
- 54.Rosenzweig KE, Fox JL, Yorke E, et al. Results of a phase I dose-escalation study using three-dimensional conformal radiotherapy in the treatment of inoperable nonsmall cell lung carcinoma. Cancer. 2005;103:2118–2127. doi: 10.1002/cncr.21007. [DOI] [PubMed] [Google Scholar]
- 55.Okawara G, Mackay JA, Evans WK, et al. Management of unresected stage III non-small cell lung cancer: a systematic review. J Thorac Oncol. 2006;1:377–393. [PubMed] [Google Scholar]
- 56.Mannino M, Chalmers AJ. Radioresistance of glioma stem cells: intrinsic characteristic or property of the 'microenvironment-stem cell unit'? Mol Oncol. 2011;5:374–386. doi: 10.1016/j.molonc.2011.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gewirtz DA. Autophagy and senescence in cancer therapy. J Cell Physiol. 2014;229:6–9. doi: 10.1002/jcp.24420. [DOI] [PubMed] [Google Scholar]
- 58.Gewirtz DA. The four faces of autophagy: implications for cancer therapy. Cancer Res. 2014;74:647–651. doi: 10.1158/0008-5472.CAN-13-2966. [DOI] [PubMed] [Google Scholar]
- 59.Sharma K, Le N, Alotaibi M, et al. Cytotoxic autophagy in cancer therapy. Int J Mol Sci. 2014;15:10034–10051. doi: 10.3390/ijms150610034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Daido S, Yamamoto A, Fujiwara K, et al. Inhibition of the DNA-dependent protein kinase catalytic subunit radiosensitizes malignant glioma cells by inducing autophagy. Cancer Res. 2005;65:4368–4375. doi: 10.1158/0008-5472.CAN-04-4202. [DOI] [PubMed] [Google Scholar]
- 61.Yu L, Tumati V, Tseng SF, et al. DAB2IP regulates autophagy in prostate cancer in response to combined treatment of radiation and a DNA-PKcs inhibitor. Neoplasia. 2012;14:1203–1212. doi: 10.1593/neo.121310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Rodier F, Coppe JP, Patil CK, et al. Persistent DNA damage signalling triggers senescence-associated inflammatory cytokine secretion. Nat Cell Biol. 2009;11:973–979. doi: 10.1038/ncb1909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Fumagalli M, Rossiello F, Clerici M, et al. Telomeric DNA damage is irreparable and causes persistent DNA-damage-response activation. Nat Cell Biol. 2012;14:355–365. doi: 10.1038/ncb2466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Riballo E, Doherty AJ, Dai Y, et al. Cellular and biochemical impact of a mutation in DNA ligase IV conferring clinical radiosensitivity. J Biol Chem. 2001;276:31124–31132. doi: 10.1074/jbc.M103866200. [DOI] [PubMed] [Google Scholar]
- 65.Filippi-Chiela EC, Bueno ESMM, Thome MP, et al. Single-cell analysis challenges the connection between autophagy and senescence induced by DNA damage. Autophagy. 2015:0. doi: 10.1080/15548627.2015.1009795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Mar FA, Debnath J, Stohr BA. Autophagy-independent senescence and genome instability driven by targeted telomere dysfunction. Autophagy. 2015;11:527–537. doi: 10.1080/15548627.2015.1017189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hunter JE, Willmore E, Irving JA, et al. NF-kappaB mediates radio-sensitization by the PARP-1 inhibitor, AG-014699. Oncogene. 2012;31:251–264. doi: 10.1038/onc.2011.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Godon C, Cordelieres FP, Biard D, et al. PARP inhibition versus PARP-1 silencing: different outcomes in terms of single-strand break repair and radiation susceptibility. Nucleic Acids Res. 2008;36:4454–4464. doi: 10.1093/nar/gkn403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Guillot C, Favaudon V, Herceg Z, et al. PARP inhibition and the radiosensitizing effects of the PARP inhibitor ABT-888 in in vitro hepatocellular carcinoma models. BMC Cancer. 2014;14:603. doi: 10.1186/1471-2407-14-603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Calabrese CR, Almassy R, Barton S, et al. Anticancer chemosensitization and radiosensitization by the novel poly(ADP-ribose) polymerase-1 inhibitor AG14361. J Natl Cancer Inst. 2004;96:56–67. doi: 10.1093/jnci/djh005. [DOI] [PubMed] [Google Scholar]
- 71.Noel G, Godon C, Fernet M, et al. Radiosensitization by the poly(ADP-ribose) polymerase inhibitor 4-amino-1,8-naphthalimide is specific of the S phase of the cell cycle and involves arrest of DNA synthesis. Mol Cancer Ther. 2006;5:564–574. doi: 10.1158/1535-7163.MCT-05-0418. [DOI] [PubMed] [Google Scholar]
- 72.Bridges KA, Toniatti C, Buser CA, et al. Niraparib (MK-4827), a novel poly(ADP-Ribose) polymerase inhibitor, radiosensitizes human lung and breast cancer cells. Oncotarget. 2014;5:5076–5086. doi: 10.18632/oncotarget.2083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Burkle A, Brabeck C, Diefenbach J, et al. The emerging role of poly(ADP-ribose) polymerase-1 in longevity. Int J Biochem Cell Biol. 2005;37:1043–1053. doi: 10.1016/j.biocel.2004.10.006. [DOI] [PubMed] [Google Scholar]
- 74.Lord CJ, Ashworth A. Targeted therapy for cancer using PARP inhibitors. Curr Opin Pharmacol. 2008;8:363–369. doi: 10.1016/j.coph.2008.06.016. [DOI] [PubMed] [Google Scholar]
- 75.Bryant HE, Schultz N, Thomas HD, et al. Specific killing of BRCA2-deficient tumours with inhibitors of poly(ADP-ribose) polymerase. Nature. 2005;434:913–917. doi: 10.1038/nature03443. [DOI] [PubMed] [Google Scholar]
- 76.Giannini G, Ristori E, Cerignoli F, et al. Human MRE11 is inactivated in mismatch repair-deficient cancers. EMBO Rep. 2002;3:248–254. doi: 10.1093/embo-reports/kvf044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Patel AG, Sarkaria JN, Kaufmann SH. Nonhomologous end joining drives poly(ADP-ribose) polymerase (PARP) inhibitor lethality in homologous recombination-deficient cells. Proc Natl Acad Sci U S A. 2011;108:3406–3411. doi: 10.1073/pnas.1013715108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Loser DA, Shibata A, Shibata AK, et al. Sensitization to radiation and alkylating agents by inhibitors of poly(ADP-ribose) polymerase is enhanced in cells deficient in DNA double-strand break repair. Mol Cancer Ther. 2010;9:1775–1787. doi: 10.1158/1535-7163.MCT-09-1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Chatterjee P, Choudhary GS, Alswillah T, et al. The TMPRSS2-ERG Gene Fusion Blocks XRCC4-Mediated Nonhomologous End-Joining Repair and Radiosensitizes Prostate Cancer Cells to PARP Inhibition. Mol Cancer Ther. 2015;14:1896–1906. doi: 10.1158/1535-7163.MCT-14-0865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kotter A, Cornils K, Borgmann K, et al. Inhibition of PARP1-dependent end-joining contributes to Olaparib-mediated radiosensitization in tumor cells. Mol Oncol. 2014;8:1616–1625. doi: 10.1016/j.molonc.2014.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Metzger MJ, Stoddard BL, Monnat RJ., Jr PARP-mediated repair, homologous recombination, and back-up non-homologous end joining-like repair of single-strand nicks. DNA Repair (Amst) 2013;12:529–534. doi: 10.1016/j.dnarep.2013.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Afshari CA, Nichols MA, Xiong Y, et al. A role for a p21-E2F interaction during senescence arrest of normal human fibroblasts. Cell Growth Differ. 1996;7:979–988. [PubMed] [Google Scholar]
- 83.Benson EK, Mungamuri SK, Attie O, et al. p53-dependent gene repression through p21 is mediated by recruitment of E2F4 repression complexes. Oncogene. 2014;33:3959–3969. doi: 10.1038/onc.2013.378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Budanov AV, Karin M. p53 target genes sestrin1 and sestrin2 connect genotoxic stress and mTOR signaling. Cell. 2008;134:451–460. doi: 10.1016/j.cell.2008.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Feng Z, Hu W, de Stanchina E, et al. The regulation of AMPK beta1, TSC2, and PTEN expression by p53: stress, cell and tissue specificity, and the role of these gene products in modulating the IGF-1-AKT-mTOR pathways. Cancer Res. 2007;67:3043–3053. doi: 10.1158/0008-5472.CAN-06-4149. [DOI] [PubMed] [Google Scholar]
- 86.Feng Z, Zhang H, Levine AJ, et al. The coordinate regulation of the p53 and mTOR pathways in cells. Proc Natl Acad Sci U S A. 2005;102:8204–8209. doi: 10.1073/pnas.0502857102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Jung CH, Ro SH, Cao J, et al. mTOR regulation of autophagy. FEBS Lett. 2010;584:1287–1295. doi: 10.1016/j.febslet.2010.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Shang L, Wang X. AMPK and mTOR coordinate the regulation of Ulk1 and mammalian autophagy initiation. Autophagy. 2011;7:924–926. doi: 10.4161/auto.7.8.15860. [DOI] [PubMed] [Google Scholar]
- 89.Okamoto A, Demetrick DJ, Spillare EA, et al. Mutations and altered expression of p16INK4 in human cancer. Proc Natl Acad Sci U S A. 1994;91:11045–11049. doi: 10.1073/pnas.91.23.11045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Prieur A, Besnard E, Babled A, et al. p53 and p16(INK4A) independent induction of senescence by chromatin-dependent alteration of S-phase progression. Nat Commun. 2011;2:473. doi: 10.1038/ncomms1473. [DOI] [PubMed] [Google Scholar]
- 91.Duan J, Zhang Z, Tong T. Senescence delay of human diploid fibroblast induced by antisense p16INK4a expression. J Biol Chem. 2001;276:48325–48331. doi: 10.1074/jbc.M104814200. [DOI] [PubMed] [Google Scholar]
- 92.Beausejour CM, Krtolica A, Galimi F, et al. Reversal of human cellular senescence: roles of the p53 and p16 pathways. EMBO J. 2003;22:4212–4222. doi: 10.1093/emboj/cdg417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Boquoi A, Arora S, Chen T, et al. Reversible cell cycle inhibition and premature aging features imposed by conditional expression of p16Ink4a. Aging Cell. 2015;14:139–147. doi: 10.1111/acel.12279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Chitikova ZV, Gordeev SA, Bykova TV, et al. Sustained activation of DNA damage response in irradiated apoptosis-resistant cells induces reversible senescence associated with mTOR downregulation and expression of stem cell markers. Cell Cycle. 2014;13:1424–1439. doi: 10.4161/cc.28402. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.