Abstract
Merkel cell carcinoma (MCC) is a highly aggressive neuroendocrine skin cancer with profound but poorly understood resistance to chemotherapy, which poses a significant barrier to clinical MCC treatment. Here we show that ATP–binding cassette member B5 (ABCB5) confers resistance to standard-of-care MCC chemotherapeutic agents and provide proof-of-principle that ABCB5 blockade can inhibit human MCC tumor growth through sensitization to drug-induced cell cytotoxicity. ABCB5 expression was detected in both established MCC lines and clinical MCC specimens at levels significantly higher than those in normal skin. Carboplatin and etoposide-resistant MCC cell lines exhibited increased expression of ABCB5, along with enhanced ABCB1 and ABCC3 transcript expression. ABCB5-expressing MCC cells in heterogeneous cancers preferentially survived treatment with carboplatin and etoposide in vitro and in human MCC xenograft-bearing mice in vivo. Moreover, MCC patients also exhibited enhanced ABCB5 positivity following carboplatin- and etoposide-based chemotherapy, pointing to clinical significance of this chemoresistance mechanism. Importantly, ABCB5 blockade reversed MCC drug resistance and impaired tumor growth in xenotransplantation models in vivo. Our results establish ABCB5 as a chemoresistance mechanism in MCC and suggest utility of this molecular target for improved MCC therapy.
Introduction
Merkel cell carcinoma (MCC) is a highly aggressive neuroendocrine cancer of the skin that, on a case-by-case basis, is more deadly than melanoma (Poulsen, 2004). Although MCC is relatively rare, its incidence has tripled over the past two decades and continues to rise by 8% annually (Hodgson, 2005). Current treatment modalities are local excision surgery of primary lesions and chemo- and radiation-therapy of metastatic disease (Eng et al., 2007). Although combination therapy with carboplatin and etoposide, the first-line chemotherapeutic agents utilized in advanced-stage MCC, yields initial response rates of up to 60%, most patients experience disease relapse, usually with fatal outcomes (Tai et al., 2000). Due to the limited availability of human MCC cell lines and patient samples from this rare form of cancer (Becker, 2010), mechanisms of MCC chemotherapy resistance are largely understudied, and treatment of patients with advanced disease poses a significant challenge. Thus, elucidating the mechanisms underlying MCC therapeutic resistance is of critical importance for improving patient survival.
We have previously cloned and characterized ABCB5 (Frank et al., 2005; Frank et al., 2003; Frank et al., 2011; Ksander et al., 2014; Lin et al., 2013; Schatton et al., 2008; Schatton et al., 2010; Schatton et al., 2015; Wilson et al., 2014; Wilson et al., 2011), which has been shown to serve as a clinically relevant multidrug resistance (MDR) mediator in human malignant melanoma (Chartrain et al., 2012; Frank et al., 2005; Wilson et al., 2014), colorectal cancer (Wilson et al., 2011) and hepatocellular carcinoma (Cheung et al., 2011). Moreover, ABCB5 expression correlates with tumor virulence and clinical cancer progression in these malignancies (Cheung et al., 2011; Gazzaniga et al., 2010; Schatton et al., 2008; Sharma et al., 2010; Wilson et al., 2011). Given its role in MDR of multiple malignancies, we hypothesized that ABCB5 might also identify therapy-refractory tumor populations in MCC.
The herein reported results establish ABCB5 expression in MCC and show that ABCB5 marks therapy-refractory tumor subpopulations following standard-of-care carboplatin and etoposide–based combination chemotherapy in MCC patients. Similarly, in MCC xenotransplantation models, ABCB5+ tumor cells also preferentially survive carboplatin- and etoposide-induced cytotoxicity. Moreover, antibody-mediated ABCB5 blockade sensitizes MCC cells to carboplatin- and etoposide-mediated cell killing concomitant with significantly enhanced inhibition of MCC xenograft growth.
Results and Discussion
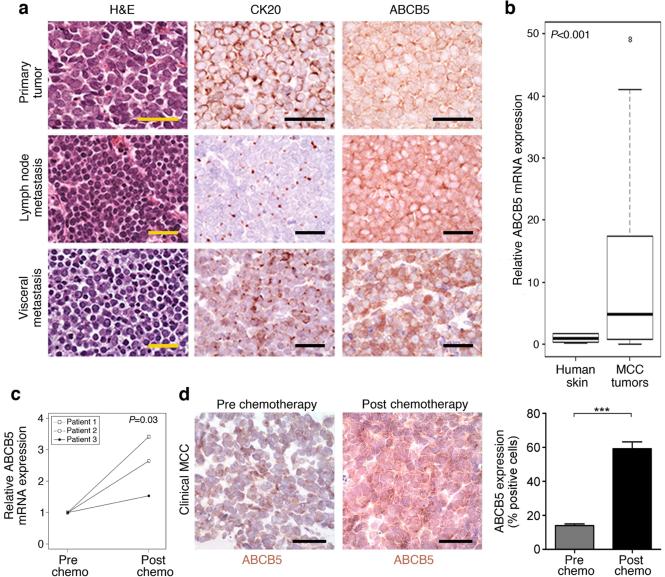
In healthy human skin, ABCB5 is expressed only on rare subsets of cells (Frank et al., 2003; Schatton et al., 2015). Similarly, in human malignant melanoma, ABCB5 expression is confined to relatively rare tumorigenic minority populations (Schatton et al., 2008). Clinical human MCC specimens obtained at various stages of disease progression (n = 85), on the other hand, demonstrated marked ABCB5 membrane expression by cytokeratin 20 (CK20)-positive MCC cells (Fig. 1a). Although cell-cell membrane apposition made it difficult to enumerate numbers of positive cells and specimens displayed heterogeneity for ABCB5 expression, ABCB5 immunoreactivity was typically observed in the majority of tumor cells. Table S1 summarizes the clinical parameters for all clinical MCC specimens analyzed. Aggregate quantitative RTPCR-based analysis of all tissue specimens showed significantly higher (P<0.001) ABCB5 mRNA expression in MCC patient biopsies (n = 85) compared with normal human skin (n = 10) (Fig. 1b). Based on the previously described correlation of ABCB5 frequency with disease progression in melanoma (Schatton et al., 2008; Setia et al., 2012) and other cancers (Cheung et al., 2011), we next examined ABCB5 expression levels in MCC samples obtained before and after first-line chemotherapy from three patients afflicted by this extraordinarily rare orphan disease with availability of this unique biopsy material. Analysis of patient-matched pre- and post-chemotherapy MCC specimens revealed significantly increased ABCB5 mRNA expression in post-chemotherapy local recurrences compared to pre-chemotherapy biopsy specimens, both at the mRNA (Fig. 1c) and immunoreactive protein levels (cell frequency 59.2 ± 4.1% vs. 14.0 ± 1.0%, mean±s.e.m., respectively, P<0.001) (Fig. 1d). These findings in patient specimens were consistent with the possibility that ABCB5+ MCC cells are preferentially resistant to treatment with the first-line chemotherapeutic agents, carboplatin and etoposide.
Figure 1. ABCB5 expression in patients with MCC pre and post first-line chemotherapy.
(a) Representative H&E, CK20, and ABCB5 immunohistochemistry of a primary MCC tumor, a lymph node and visceral metastasis. (b) Relative ABCB5 mRNA expression in normal human skin (n=10) versus clinical MCC specimens (n=85), as determined by quantitative RT-PCR. (c) Relative ABCB5 mRNA expression and (d) immunohistochemically-determined ABCB5 protein expression (mean±s.e.m.) by patient-matched pre- and post-chemotherapy MCC biospecimens (n=3, respectively). Size bars, 50 μm. (NS: not significant, *** P<0.001, as determined by the unpaired (b) or paired (c, d) Student's t test).
Based on previous studies implicating ABCB5 expression in conferring chemotherapeutic resistance in several human cancers (Chartrain et al., 2012; Cheung et al., 2011; Frank et al., 2005; Wilson et al., 2014; Wilson et al., 2011), we next examined the potential functional contribution of ABCB5 to carboplatin and/or etoposide resistance in MCC. We first demonstrated ABCB5 mRNA expression in the established human MCC cell lines, MKL-1, MKL-2, MS-1, and WaGa (Guastafierro et al., 2013; Houben et al., 2010; Rodig et al., 2012; Rosen et al., 1987), by RT-PCR amplification and sequencing (Fig. 2a). All four MCC lines also showed ABCB5 surface protein expression, as determined by immunofluorescence staining (Fig. 2b) and by flow cytometric analysis, with ABCB5+ cell frequencies (mean ± s.e.m.) averaging 10.0 ± 1.8% for MKL-1, 9.1 ± 2.4% for MKL-2, 8.3 ± 1.5% for MS-1 and 16.9 ± 5.4% for WaGa cells (Fig. 2c). To explore the potential role of ABCB5 in MCC refractoriness to first-line chemotherapy, we next investigated ABCB5 expression in control (wildtype) versus MCC-lines rendered drug-resistant via continuous exposure to carboplatin- or etoposide over a 2-month period. First, we confirmed preferential survival of carboplatin- and etoposide-resistant compared to wildtype MCC cells for the respective drugs (Fig. S1a). Subsequent qPCR analyses revealed markedly increased ABCB5 mRNA expression levels in both carboplatin- and etoposide-resistant MKL-1, MKL-2, MS-1 and WaGa lines compared to the respective wildtype cell lines (Fig. 2d). At the protein level, exposure to cytotoxic levels of carboplatin or etoposide resulted in significantly increased ABCB5 expression among viable MKL-1, MKL-2, MS-1, and WaGa cells compared to vehicle-treated controls, respectively (Fig. 2e and 2f). While the percentage of ABCB5+ cells was markedly enhanced in chemorefractory MCC cell lines, we also noted that a significant proportion of carboplatin- and etoposide-resistant cells did not display ABCB5 expression.
Figure 2. ABCB5 expression in response to in vitro carboplatin and etoposide treatment.
(a) ABCB5 mRNA expression by MKL-1, MKL-2, MS-1, and WaGa MCC cell lines, as determined by RT-PCR (positive control: G3361 melanoma cells). (b) Representative ABCB5 immunofluorescence staining (red) of a cytospin preparation of WaGa cells (inset: isotype control antibody staining). Nuclei are counterstained with DAPI (blue). Size bars, 50 μm. (c) Representative flow cytometry of ABCB5 protein expression by MKL-1, MKL-2, MS-1, and WaGa cells (n=6 independent experiments). (d) Relative ABCB5 mRNA expression (mean±s.e.m.) by MCC cells resistant to 50-150 μM carboplatin or 1-3 μM etoposide compared to that in wildtype MCC cells, as determined by quantitative RT-PCR (n=6-9 each, representative of 2-3 independent experiments). (e) Flow cytometrically determined ABCB5 protein expression (% live cells, mean±s.e.m.) in wildtype versus carboplatin- or etoposide-resistant MCC cells (n=3-8 independent experiments, respectively). (f) Representative flow cytometry of ABCB5 protein expression (MKL-1) in experimental groups as in (e) and of (g) cell viability (calcein AM positivity) of wildtype ABCB5+ versus ABCB5− MCC cells cultured in the presence of cytotoxic carboplatin (250 μM) or etoposide (5 μM) concentrations. (* P<0.05, ** P<0.01, *** P<0.001, as determined by the unpaired Student's t test).
To directly demonstrate that ABCB5+ tumor cell subsets preferentially survive carboplatin- and etoposide-induced cytotoxicity, we compared the viability of ABCB5+ versus ABCB5− MKL-1 and WaGa cells grown in the presence of cytotoxic carboplatin or etoposide levels. We found that ABCB5+ cells cultured under these conditions demonstrated increased viability compared to ABCB5− MCC populations (Fig. 2g), indicating that ABCB5+ MCC subsets preferentially survive drug-induced cell killing. However, we cannot entirely exclude the possibility of induction of ABCB5 expression as opposed to preferential survival. Because other ABC transporters, including ABCB1, ABCC3, and ABCG2, are known mediators of carboplatin and etoposide resistance in other cancers (Dean et al., 2001), we examined whether drug-resistant MCC lines also expressed high levels of these ABC transporters, in addition to ABCB5. With the exception of etoposide-resistant MKL-1 cells, all drug-resistant MCC cell lines examined showed a significant increase in ABCB1 and ABCC3 but not ABCG2 transcript expression compared to the respective wildtype cell lines (Fig. S1b), raising the possibility that several ABC transporters, in addition to ABCB5, might contribute to chemoresistance in drug-induced MCC cell lines. Together, these results suggested a direct relationship between therapeutic resistance to both carboplatin and etoposide treatment and ABCB5 expression in the MCC lines evaluated and further suggest the potential contribution of additional ABC transporters (ABCB1 and ABCC3) to MCC chemoresistance.
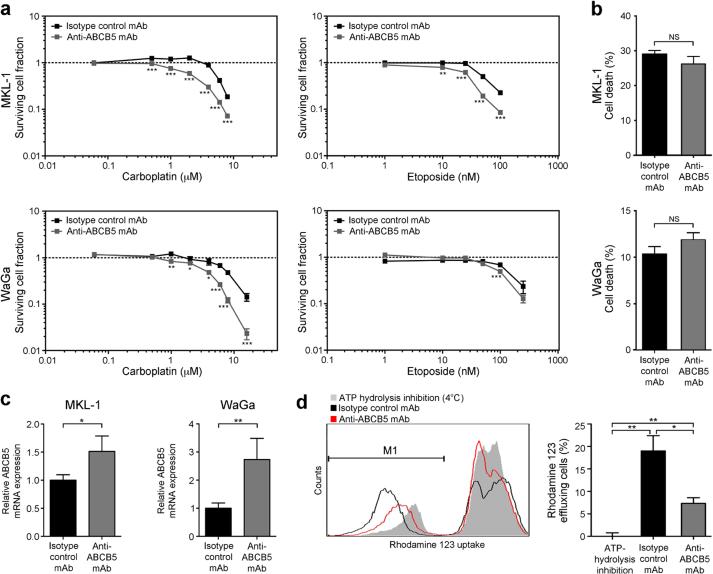
To explore the potential role of ABCB5 as a carboplatin- and/or etoposide resistance mediator in MCC, we evaluated cell viability in MCC cultures exposed to increasing concentrations of carboplatin or etoposide in the presence of an anti-ABCB5 blocking monoclonal antibody (mAb) (Frank et al., 2005; Frank et al., 2003; Ksander et al., 2014; Schatton et al., 2008; Wilson et al., 2014) versus isotype control mAb. ABCB5 blockade reversed carboplatin and etoposide resistance of both MKL-1 and WaGa cells (Fig. 3a), resulting in significantly enhanced cell killing at all carboplatin concentrations greater than 1 μM versus controls and at etoposide concentrations as low as 10 nM in MKL-1 cells, and reductions of the LD50 in both MKL-1 (LD50 (carboplatin) 2.3 μM vs. 5.9 μM and LD50 (etoposide) 25.0 nM vs. 62.4 nM, respectively) and WaGa cells (LD50 (carboplatin) 3.1 μM vs. 7.6 μM and LD50 (etoposide) 98.4 nM vs. 130.4 nM, respectively) (Fig. 3a). Drug-induced selection for ABCB5+ MCC cells (Figs. 2d-g) might hereby explain why the ABCB5 blocking mAb mediates carboplatin and etoposide-induced cytotoxicity in proportions of MCC cells that exceeded those frequencies observed in native cell lines (Fig. 2c), especially at higher drug concentrations. Treatment with the anti-ABCB5 mAb alone had no significant effect on in vitro cell survival (Fig. 3b), but, in line with blocking specificity, induced compensatory increases in ABCB5 mRNA expression compared to isotype control mAb treatment (Fig. 3c). To more rigorously demonstrate that the anti-ABCB5 mAb used in our study blocks ABCB5 function in MCC, we next examined whether it blocked cellular efflux of the green fluorescent dye and known ABCB5 substrate (Frank et al., 2003; Lin et al., 2013), rhodamine 123 (Rh123) in MCC cells. Compared to isotype control mAb-treated MCC cells, of which a subpopulation of 19.0%±2.8% (mean±s.e.m., n = 3) effluxed Rh123 over a 120 minute incubation period at 37°C, mAb-mediated ABCB5 blockade significantly (P<0.05) inhibited Rh123 efflux by >60% (Fig. 3d). The effect of ABCB5 blockade on Rh123 efflux was assessed compared to incubation of MCC cells for 120 minutes at 4°C (Fig. 3d), which blocks ATP hydrolysis and hence ABC transport function (Frank et al., 2003; Lin et al., 2013). Together, these results established that ABCB5 is expressed as a functional xenobiotic efflux transporter in MCC and that ABCB5 blockade reverses carboplatin and etoposide resistance in this malignancy.
Figure 3. Chemoresistance reversal of human MCC cells by ABCB5 inhibition.
(a) Effects of anti-ABCB5 versus isotype control antibody on carboplatin- or etoposide-induced MKL-1 and WaGa cell killing, determined by the MTT assay. Surviving cell fractions are plotted against drug concentration (n=6 each, representative of n=3 independent experiments). (b) Flow cytometric assessment of cell death (percent Annexin-V+/7AAD+ cells, mean±s.e.m., n=3) and (c) relative ABCB5 mRNA expression (mean±s.e.m.) in isotype control- versus anti-ABCB5 antibody-treated MCC cultures (n=3-9, respectively). (d) Representative histogram plot and percent rhodamine 123-effluxing wildtype MKL-1 cells (mean±s.e.m.) under conditions of isotype control or ABCB5-blocking antibody treatment (37°C), or with incubation at 4°C to block ATP-dependent efflux (n=3 independent experiments). (NS: not significant, * P<0.05, ** P<0.01, *** P<0.001, as determined by the unpaired Student's t test).
To investigate the relationship between ABCB5 and first-line chemotherapy resistance in MCC in vivo, we also examined ABCB5 expression and carboplatin or etoposide treatment response of ABCB5+ cancer cell populations in an established model system (Lezcano et al., 2014) utilizing NOD/SCID IL-2Rγ−/− (NSG) mice bearing MKL-1 or WaGa xenografts. MKL-1 and WaGa tumors grew at similar rates (Fig. S2a) and exhibited CK20 and ABCB5 expression profiles similar to those found in patient MCC samples (Fig. S2b). We found that the carboplatin and etoposide doses (Fichtner et al., 2008) administered to NSG mice for six consecutive days resulted in significant volume reduction of pre-established MKL-1 and WaGa xenograft tumors, whereas vehicle-treated controls showed continued tumor growth (Fig. 4a). Quantitative RTPCR analysis revealed greater than 8-fold elevation of ABCB5 mRNA expression by tumor xenograft tissue harvested from carboplatin- and etoposide-treated versus vehicle control-treated MKL-1 (P<0.001) or WaGa xenograft-bearing mice (P<0.05), respectively (Fig. 4b). Compared to tumor xenografts of vehicle control-treated mice, residual MKL-1 and WaGa specimens resected from NSG mice treated with carboplatin or etoposide also showed 2.0-4.3-fold enhanced (P<0.001, respectively) ABCB5 protein expression by viable MCC cells (Fig. 4c), consistent with our in vitro results (Fig. 2). In line with our in vitro findings, we also found that ABCB1 and ABCC3 transcript levels tended to be elevated in both MKL-1 and WaGa tumors resected from NSG mice treated with carboplatin or etoposide compared to those treated with vehicle control (Fig. S3).
Figure 4. Response of ABCB5 expression to carboplatin and etoposide treatment in MCC tumor xenografts.
(a) Tumor growth kinetics (mean±s.d.) of vehicle control-, carboplatin- (75 mg/kg/d) or etoposide-treated (10 mg/kg/d) MKL-1 and WaGa xenografts in NSG mice (n=6-8, respectively). Arrows indicate days of carboplatin or etoposide administration. (b) Relative ABCB5 mRNA expression (mean±s.e.m.) determined by quantitative RT-PCR and (c)immunohistochemically determined ABCB5 protein expression (mean±s.e.m.) by MCC xenografts (n=6-8, respectively) harvested 40 days post inoculation to NSG mice, in experimental groups as in (a). Representative images of ABCB5 immunohistochemistry are shown on the left. Size bars, 50 μm. (NS: not significant, * P<0.05, ** P<0.01, *** P<0.001, as determined by two-way ANOVA followed by the Bonferroni correction (a) or by the unpaired Student's t test (b, c)).
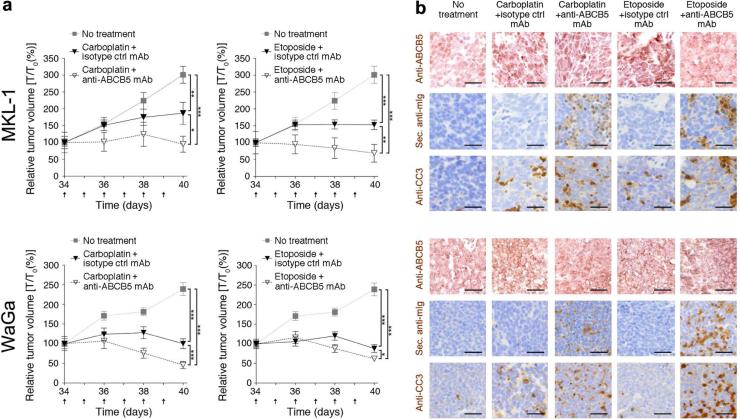
To determine if ABCB5 blockade in the context of carboplatin- or etoposide-based monotherapy has an additive inhibitory effect on MCC growth in vivo, we administered submaximal doses of carboplatin (30 mg/kg) or etoposide (5 mg/kg) in combination with anti-ABCB5 mAb (Frank et al., 2005; Frank et al., 2003; Ksander et al., 2014; Lin et al., 2013; Schatton et al., 2008; Schatton et al., 2015; Wilson et al., 2014) or isotype control mAb to MKL-1 or WaGa tumor xenograft-bearing NSG mice. Compared to the respective vehicle control groups, low-dose carboplatin or etoposide treatment resulted in significantly (P<0.01) attenuated growth of both MKL-1 and WaGa tumor xenografts (Fig. 5a). Importantly, combination therapies involving anti-ABCB5 mAb plus carboplatin or anti-ABCB5 mAb plus etoposide resulted in significantly greater inhibition of tumor growth compared to treatment with either chemotherapeutic alone (P<0.05, respectively) (Fig. 5a). Immunohistochemical analysis of serial sections of resected MKL-1 and WaGa specimens revealed binding of in vivo-administered mAb to tumor target tissue, which coincided with regions of ABCB5 positivity, in anti-ABCB5 mAbco-treated, but not isotype control mAb-co-treated carboplatin or etoposide treatment groups (Fig. 5b), supporting the notion of a direct anti-ABCB5 mAb effect on ABCB5+ MCC target cells. While MKL-1 and WaGa xenografts of NSG mice treated with either carboplatin or etoposide plus isotype control mAb showed only moderate apoptotic cell death compared to vehicle controls (determined by cleaved caspase 3 (CC3) immunostaining, (Fig. 5b)), the combination therapies involving carboplatin or etoposide plus anti-ABCB5 mAb resulted in enhanced MCC apoptosis, in tumor areas that also show binding of anti-ABCB5 mAb to MCC cells (Fig. 5b). Without concurrent administration of carboplatin or etoposide, anti-ABCB5 mAb administration to MKL-1 or WaGa tumor xenograft-bearing NSG mice at the equivalent observation endpoint, did neither result in significant differences in tumor growth (Fig. S4a) nor in changes in apoptotic tumor target cell death, compared to isotype control mAb treatment alone (Fig. S4b).
Figure 5. Effect of ABCB5 blockade on MCC tumor xenograft growth and chemotherapy-induced cytotoxicity.
(a) Tumor growth kinetics (mean ± s.d.) and (b) ABCB5, secondary antibody (sec. anti-mIg staining, recognizing in vivo-administered anti-ABCB5 antibody) and cleaved caspase 3 (CC3) immunohistochemical staining of MKL-1 (top, n = 6-8) and WaGa (bottom, n = 8-9) xenografts dissected from vehicle control-, carboplatin- (30 mg/kg/d) or etoposide-treated (5 mg/kg/d) NSG mice also treated with anti-human ABCB5- or isotype control antibody (500 μg/d), respectively. Arrows in panel (a) indicate days of carboplatin or etoposide and isotype control or anti-ABCB5 antibody administration. Size bars, 50 μm. (* P<0.05, ** P<0.01, *** P<0.001, as determined by two-way ANOVA followed by the Bonferroni correction).
In this study, based on previously established roles of ABCB5 as a clinically relevant chemoresistance mechanism in cutaneous melanoma (Chartrain et al., 2012; Frank et al., 2005; Wilson et al., 2014) and other cancers (Cheung et al., 2011; Wilson et al., 2011), we analyzed ABCB5 expression and function in MCC, through study of MCC tumor biospecimens (n = 85) obtained from 66 patients, healthy human skin specimens from 10 individuals, and 4 established MCC cell lines. Our results provide evidence that ABCB5 is highly expressed in MCC and that its expression levels markedly surpass those in healthy human skin. Importantly, we show that ABCB5 identifies carboplatin- and etoposide-resistant MCC subsets in vitro and in tumor xenografts and clinical specimens in vivo. Additional ABC transporters, namely ABCB1 and ABCC3, were also overexpressed in carboplatin- and etoposide-resistant MCC cell lines, raising the possibility of multiple ABC transporter involvement in MCC chemoresistance. Moreover, we cannot exclude the possibility that more pronounced cellular quiescence in chemorefractory MCC cells may have contributed to resistance to the agents used. While ABCB5 may function as an MCC efflux transporter, as evidenced by our findings of ABCB5 mAb-mediated MCC cell retention of the ATP transporter substrate rhodamine 123, the question of whether ABCB5 directly effluxes the standard-of-care clinical MCC therapeutic agents, carboplatin and etoposide, as a mechanism of resistance, or if alternative, efflux-independent ABCB5 functions (Ksander et al., 2014; Wilson et al., 2014) might be primarily responsible for MCC chemoresistance, requires further investigation.
The finding that ABCB5 serves as a chemoresistance mediator in MCC is of potential high clinical significance, since emergence of resistance to first-line chemotherapy is a major impediment to successful MCC treatment (Eng et al., 2007; Tai et al., 2000). Indeed, because addition of an ABCB5 blocking mAb to carboplatin or etoposide treatment resulted in enhanced tumor cell apoptosis and significant inhibition of tumor xenograft growth, our results establish initial proof-of-principle that ABCB5 can be targeted in MCC to attenuate resistance to clinically relevant chemotherapeutic agents. In summary, our findings provide a clear rationale to translate ABCB5-targeted chemoresistance reversal strategies to the clinic in order to enhance the efficacy of currently available systemic MCC therapies.
Materials and Methods
Clinical specimens, Merkel cell carcinoma (MCC) cell lines, culture methods, and generation of drug-resistant MCC lines
MCC biospecimens and healthy human skin were obtained from patients and healthy volunteers in accordance with protocols approved by the IRBs of Partners Health Care Research Management and the Dana-Farber Cancer Institute and assurances filed with and approved by the U.S. Department of Health and Human Services. Written informed consent was obtained from all subjects. Authenticated human MCC cell lines were obtained from Dr. James DeCaprio of the Dana-Farber Cancer Institute, Boston, MA (Rodig et al., 2012) and were cultured fewer than 6 months in RPMI 1640 medium supplemented with 20% (v/v) FBS and 1% (v/v) penicillin/streptomycin (Gibco, Life Technologies, Grand Island, NY). MCC cell lines were rendered drug resistant to carboplatin or etoposide by incubation in increasing doses of up to 150 μM carboplatin or of up to 3 μM etoposide over the course of 2 months, respectively.
RNA extraction, RT-PCR, and real-time quantitative PCR
Human ABCB5 was amplified and sequenced following reverse transcription of total RNA using ABCB5-specific primer pairs. Relative ABCB5, ABCB1, ABCC3 and ABCG2 transcript levels were determined by real-time quantitative RT-PCR and calculated using the 2(−ΔΔCt) method (Schatton et al., 2008; Schatton et al., 2010). Please see supplemental information for primer sequences.
Immunohistochemistry and immunofluorescence staining
Immunofluorescence labeling for ABCB5 in cytospin preparations of MCC cell lines, conventional histology, and immunohistochemical analysis of ABCB5, CK20, and/or cleaved caspase 3 (CC3) expression by tumor biospecimens obtained from MCC patients or human MCC tumor xenografts, and of in vivo anti-ABCB5 mAb binding to MCC tumor xenograft tissue were carried out as described (Schatton et al., 2008; Schatton et al., 2010). ABCB5 immunoreactivity was quantified using ImageJ software analysis, as described previously (Wilson et al., 2011).
Flow cytometry and cell viability measurements
ABCB5 surface protein expression by established wildtype, drug-exposed or drug-resistant MCC lines with or without concurrent counterstaining with the viability dye, calcein AM, was analyzed by single- or dual-color flow cytometry (Schatton et al., 2008; Schatton et al., 2010). Assessment of cell viability in anti-ABCB5 versus isotype control mAb-treated MCC cells was carried out by annexin V/7-amino-actinomycin D (7-AAD) staining and subsequent flow cytometric analysis, as described (Schatton et al., 2010).
MTT cytotoxicity assays
To confirm preferential chemosensitivity of wildtype versus drug-resistant MCC lines and determine the effect of ABCB5 mAb blockade on carboplatin- or etoposide-induced cell killing, MCC cells were exposed to a range of concentrations of carboplatin or etoposide in the presence or absence of anti-ABCB5 mAb or isotype control mAb over a course of 7 days. Subsequently, in vitro growth kinetics of cells were assayed using the TACS 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) cell proliferation assay kit.
Rhodamine 123 efflux transport assays
Efflux transport capacity for the green fluorescent dye, rhodamine 123, was assessed by flow cytometry for MCC cells incubated for 120 min at 37°C with the 3C2-1D12 anti-ABCB5 blocking mAb (Frank et al., 2005; Frank et al., 2003; Ksander et al., 2014; Lin et al., 2013; Schatton et al., 2008; Wilson et al., 2011) versus isotype control mAb, as described previously (Frank et al., 2003; Lin et al., 2013).
Human MCC xenotransplantation, in vivo carboplatin and etoposide treatment, and anti-ABCB5 mAb targeting
NSG mice were maintained and experiments performed in accordance with IACUC-approved experimental protocols. For tumorigenicity studies, MCC cells were injected subcutaneously (1 × 107 cells/inoculum) into the flanks of recipient NSG mice (Schatton et al., 2008). At day 34 post tumor cell inoculation, mice were randomized to carboplatin, etoposide or vehicle control treatment groups and carboplatin (75 mg/kg) or etoposide (10 mg/kg) were administered daily by intraperitoneal injection for 6 consecutive days, as previously described (Fichtner et al., 2008). Control animals were given vehicle at equal volumes. For in vivo ABCB5 targeting experiments, human MCC cells were grafted, mice randomized to experimental treatment groups, and animals were injected intraperitoneally with anti-ABCB5 mAb or control mAb daily (500 μg per injection, respectively) for 9 consecutive days with or without concurrent administration of carboplatin (30 mg/kg) or etoposide (5 mg/kg) for 6 consecutive days starting 72 hours post initial antibody treatment, respectively. Tumor volumes were measured daily for the duration of the treatment and tumor volumes were calculated as described (Schatton et al., 2008).
Statistics
Analyses were performed using the PRISM software (version 5 for Macintosh, GraphPad Inc.) and R version 3.02. Statistical hypotheses were tested using the two-tailed Student's t test, the nonparametric Mann-Whitney test, or two-way ANOVA followed by the Bonferroni correction. Data was tested for normal distribution using the D'Agostino and Pearson omnibus normality test. A two-sided value of P<0.05 was considered statistically significant.
See also the Supplementary Materials and Methods.
Supplementary Material
Acknowledgments
The authors thank Dr. James DeCaprio for providing Merkel cell carcinoma cell lines for our study. This work was supported by Brigham and Women's Hospital, Department of Dermatology funding for new investigators and a Fund to Sustain Research Excellence from the Brigham Research Institute (to T.S.), NIH/NCI grants 1R01CA113796 and 1R01CA138231 (to M.H.F.), and 1R01CA158467 (to M.H.F. and G.F.M.). T.S. is the recipient of a Research Career Development award from the Dermatology Foundation. N.L. is the recipient of a Medical Student Grant from the American Skin Association.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Supplemental Information
Supplemental data includes four figures, one table, and supplementary materials and methods, and can be found with this article online at www.nature.com/JID/supplemental-data.
Conflict of Interest
M.H.F. is inventor or co-inventor of US and international patents assigned to Brigham and Women's Hospital and/or Boston Children's Hospital, Boston, MA, and licensed to Ticeba GmbH (Heidelberg, Germany) and Rheacell GmbH & Co. KG (Heidelberg, Germany). M.H.F. serves as a scientific advisor to Ticeba GmbH and Rheacell GmbH & Co. KG. and participates in corporate sponsored research collaborations with Rheacell GmbH & Co. KG.
References
- Becker JC. Merkel cell carcinoma. Ann Oncol. 2010;21(Suppl 7):vii81–5. doi: 10.1093/annonc/mdq366. [DOI] [PubMed] [Google Scholar]
- Chartrain M, Riond J, Stennevin A, Vandenberghe I, Gomes B, Lamant L, et al. Melanoma chemotherapy leads to the selection of ABCB5-expressing cells. PLoS One. 2012;7:e36762. doi: 10.1371/journal.pone.0036762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung ST, Cheung PF, Cheng CK, Wongg NC, Fan ST. Granulin-epithelin precursor and ATP-dependent binding cassette (ABC)B5 regulate liver cancer cell chemoresistance. Gastroenterology. 2011;140:344–55. doi: 10.1053/j.gastro.2010.07.049. [DOI] [PubMed] [Google Scholar]
- Dean M, Rzhetsky A, Allikmets R. The human ATP-binding cassette (ABC) transporter superfamily. Genome Res. 2001;11:1156–66. doi: 10.1101/gr.184901. [DOI] [PubMed] [Google Scholar]
- Eng TY, Boersma MG, Fuller CD, Goytia V, Jonew WE, Joyner M, et al. A comprehensive review of the treatment of Merkel cell carcinoma. Am J Clin Oncol. 2007;30:624–36. doi: 10.1097/COC.0b013e318142c882. [DOI] [PubMed] [Google Scholar]
- Fichtner I, Rolff J, Soong R, Hoffmann J, Hammer S, Sommer A, et al. Establishment of patient-derived non-small cell lung cancer xenografts as models for the identification of predictive biomarkers. Clin Cancer Res. 2008;14:6456–68. doi: 10.1158/1078-0432.CCR-08-0138. [DOI] [PubMed] [Google Scholar]
- Frank NY, Margaryan A, Huang Y, Schatton T, Waaga-Gasser AM, Gasser M, et al. ABCB5-mediated doxorubicin transport and chemoresistance in human malignant melanoma. Cancer Res. 2005;65:4320–33. doi: 10.1158/0008-5472.CAN-04-3327. [DOI] [PubMed] [Google Scholar]
- Frank NY, Pendse SS, Lapchak PH, Margaryan A, Shlain D, Doeing C, et al. Regulation of progenitor cell fusion by ABCB5 P-glycoprotein, a novel human ATP-binding cassette transporter. J Biol Chem. 2003;278:47156–65. doi: 10.1074/jbc.M308700200. [DOI] [PubMed] [Google Scholar]
- Frank NY, Schatton T, Kim S, Zhan Q, Wilson BJ, Ma J, et al. VEGFR-1 expressed by malignant melanoma-initiating cells is required for tumor growth. Cancer Res. 2011;71:1474–85. doi: 10.1158/0008-5472.CAN-10-1660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gazzaniga P, Cigna E, Panasiti V, Devirgillis V, Bottoni U, Vincenzi B, et al. CD133 and ABCB5 as stem cell markers on sentinel lymph node from melanoma patients. Eur J Surg Oncol. 2010;36:1211–4. doi: 10.1016/j.ejso.2010.05.001. [DOI] [PubMed] [Google Scholar]
- Guastafierro A, Feng H, Thant M, Kirkwood JM, Chang Y, Moore PS, et al. Characterization of an early passage Merkel cell polyomavirus-positive Merkel cell carcinoma cell line, MS-1, and its growth in NOD scid gamma mice. J Virol Methods. 2013;187:6–14. doi: 10.1016/j.jviromet.2012.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodgson NC. Merkel cell carcinoma: changing incidence trends. J Surg Oncol. 2005;89:1–4. doi: 10.1002/jso.20167. [DOI] [PubMed] [Google Scholar]
- Houben R, Shuda M, Weinkam R, Schrama D, Feng H, Chang Y, et al. Merkel cell polyomavirus-infected Merkel cell carcinoma cells require expression of viral T antigens. J Virol. 2010;84:7064–72. doi: 10.1128/JVI.02400-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ksander BR, Kolovou PE, Wilson BJ, Saab KR, Guo Q, Ma J, et al. ABCB5 is a limbal stem cell gene required for corneal development and repair. Nature. 2014;511:353–7. doi: 10.1038/nature13426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lezcano C, Kleffel S, Lee N, Larson AR, Zhan Q, DoRosario A, et al. Merkel cell carcinoma expresses vasculogenic mimicry: demonstration in patients and experimental manipulation in xenografts. Lab Invest. 2014;94:1092–102. doi: 10.1038/labinvest.2014.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin JY, Zhang M, Schatton T, Wilson BJ, Alloo A, Ma J, et al. Genetically determined ABCB5 functionality correlates with pigmentation phenotype and melanoma risk. Biochem Biophys Res Commun. 2013;436:536–42. doi: 10.1016/j.bbrc.2013.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poulsen M. Merkel-cell carcinoma of the skin. Lancet Oncol. 2004;5:593–9. doi: 10.1016/S1470-2045(04)01593-1. [DOI] [PubMed] [Google Scholar]
- Rodig SJ, Cheng J, Wardzala J, DoRosario A, Scanlon JJ, Laga AC, et al. Improved detection suggests all Merkel cell carcinomas harbor Merkel polyomavirus. J Clin Invest. 2012;122:4645–53. doi: 10.1172/JCI64116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen ST, Gould VE, Salwen HR, Herst CV, Le Beau MM, Lee I, et al. Establishment and characterization of a neuroendocrine skin carcinoma cell line. Lab Invest. 1987;56:302–12. [PubMed] [Google Scholar]
- Schatton T, Murphy GF, Frank NY, Yamaura K, Waaga-Gasser AM, Gasser M, et al. Identification of cells initiating human melanomas. Nature. 2008;451:345–9. doi: 10.1038/nature06489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schatton T, Schutte U, Frank NY, Zhan Q, Hoerning A, Robles SC, et al. Modulation of T-cell activation by malignant melanoma initiating cells. Cancer Res. 2010;70:697–708. doi: 10.1158/0008-5472.CAN-09-1592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schatton T, Yang J, Kleffel S, Uehara M, Barthel SR, Schlapbach C, et al. ABCB5 Identifies Immunoregulatory Dermal Cells. Cell Rep. 2015;12:1564–74. doi: 10.1016/j.celrep.2015.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Setia N, Abbas O, Sousa Y, Garb JL, Mahalingam M. Profiling of ABC transporters ABCB5, ABCF2 and nestin-positive stem cells in nevi, in situ and invasive melanoma. Mod Pathol. 2012;25:1169–75. doi: 10.1038/modpathol.2012.71. [DOI] [PubMed] [Google Scholar]
- Sharma BK, Manglik V, Elias EG. Immuno-expression of human melanoma stem cell markers in tissues at different stages of the disease. J Surg Res. 2010;163:e11–5. doi: 10.1016/j.jss.2010.03.043. [DOI] [PubMed] [Google Scholar]
- Tai PT, Yu E, Winquist E, Hammond A, Stitt L, Tonita J, et al. Chemotherapy in neuroendocrine/Merkel cell carcinoma of the skin: case series and review of 204 cases. J Clin Oncol. 2000;18:2493–9. doi: 10.1200/JCO.2000.18.12.2493. [DOI] [PubMed] [Google Scholar]
- Wilson BJ, Saab KR, Ma J, Schatton T, Putz P, Zhan Q, et al. ABCB5 Maintains Melanoma-Initiating Cells through a Proinflammatory Cytokine Signaling Circuit. Cancer Res. 2014;74:4196–207. doi: 10.1158/0008-5472.CAN-14-0582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson BJ, Schatton T, Zhan Q, Gasser M, Ma J, Saab KR, et al. ABCB5 identifies a therapy-refractory tumor cell population in colorectal cancer patients. Cancer Res. 2011;71:5307–16. doi: 10.1158/0008-5472.CAN-11-0221. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.