Abstract
Lysophosphatidic acid (LPA) plays a critical role in the migration and invasion of ovarian cancer cells. However, the downstream spatiotemporal signaling events involving specific G protein(s) underlying this process are largely unknown. In this report, we demonstrate that LPA signaling causes the translocation of Gαi2 into the invadopodia leading to its interaction with the tyrosine kinase Src and the Rac/CDC42-specific guanine nucleotide exchange factor, β-pix. Our results establish that Gαi2 activates Rac1 through a p130Cas-dependent pathway in ovarian cancer cells. Moreover, our report reveals that knockdown of Gαi2 leads to loss of β-pix and active-Rac association in the invadopodia. We also show that knockdown of Gαi2 leads to the complete loss of translocation to p130Cas to focal adhesions. Finally, when Gαi2 is knocked down, this led to the total distribution of Src being shifted primarily from invadopodia and the leading edge of the cells to the perinuclear region, suggesting that Src is inactive in the absence of Gαi2. Overall, our report provides tantalizing evidence that Gαi2 is a critical signaling component of a large signaling complex in the invadopodia that if disrupted could serve as an excellent target for therapy in ovarian and potentially other cancers.
Keywords: LPA, G protein, Cell migration, Gαi2, Src, Ovarian Cancer
1.) Introduction
Lysophosphatidic acid (LPA) is a simple phospholipid that has been implicated in the progression of ovarian cancer and other cancers [14, 32, 41]. Importantly, the involvement of LPA in migration and invasion of ovarian and other types of cancer has been well documented by our lab and others [3, 4, 18, 20, 22, 35, 40, 42]. Therefore, understanding how LPA mediates ovarian cancer progression is of critical importance for elucidating the biology of ovarian cancer and the contributions of LPA and G proteins in ovarian and potentially other cancers. LPA binds to and activates at least six different G protein-coupled receptors (GPCRs). All of these LPA-specific GPCRs activate both the Gi and Gq family of proteins. Furthermore, all of the LPA receptors, except for LPA3, also activate the G12/13 family of proteins [12].
To date there have been very little studies that have looked at the role of G proteins in migration and even fewer studies have studied the spatiotemporal dynamics of G protein signaling. Therefore, in this report we sought to characterize the cellular localization and contribution that Gαi2, also known as the gip2 proto-oncogene, has on ovarian cancer cell migration. We chose to utilize ovarian cancer cells since these cells migrate robustly to LPA stimulation. Thus, by utilizing LPA to stimulate Gαi2-dependent invasive migration, we report here that Gαi2 directly associates with Src and β-pix in invadopodia. We demonstrate further that this interaction of Src and β-pix led to activation of Rac. In addition, our results indicate that Gαi2 could activate Rac1 through a pathway involving the scaffold protein p130Cas. Thus, our current study defines the spatiotemporal localization of Gαi2 in response to LPA and unravels the mechanism through which its downstream effectors could orchestrate invasive migration of cancer cells.
2.) Materials and Methods
Reagents
The ovarian cancer cell lines HeyA8 were kindly provided by Dr. E. Premkumar Reddy (Mount Sinai School of Medicine, New York, NY) and SKOV3-ip cells were provided by Dr. Robert C. Bast (MD Anderson Cancer Center, Houston, TX). HeyA8 cells were maintained in Dulbecco’s modified Eagle’s medium (DMEM) and SKOV3-ip cells were maintained in Roswell Park Memorial Institute (RPMI) 1640 media (Mediatech, Manassas, VA) containing 10% FBS (Gemini Bio-Products, West Sacramento, CA), 50 U/mL penicillin, 50 μg/ml streptomycin Mediatech, Manassas, VA) at 37°C in a 5% CO2 incubator. For serum-starvation the media used was Dulbecco’s modified Eagle’s medium (DMEM) with 0.1% BSA Fraction V, heat-shock, fatty acid ultra-free (Roche, Indianapolis, IN), 50 U/mL penicillin and 50 mg/mL streptomycin (Mediatech). Lysophosphatidic acid (1-oleoyl-2-hydroxy-sn-glycero-3-phosphate) was obtained from Avanti Polar Lipids (Alabaster, AL) and dissolved into 10 mM stock solutions in PBS with 0.1% BSA and stored at −80°C until use. si-p130Cas (5′-GGUCGACAGUGGUGUGUAUUU-3′) and non-targeting siRNA ON-TARGETplus Non-targeting siRNA #1 were purchased from Dharmacon (Lafayette, CO). shRNA for Gαi2 (5′-GCATGAGAGCATGAAGCTATT-3′) and nonsense shRNA were purchased from Open Biosystems (Lafayette, CO). Peroxidase-conjugated anti-rabbit IgG was purchased from Promega (Madison, WI), and peroxidase-conjugated anti-mouse was purchased from GE Healthcare (Little Chalfont, UK). Gαi2 (sc-13534), WASP (sc-13139), Vinculin (sc-25336) and p130Cas (sc-20029) were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Rac-GTP antibody was purchased from NewEast Biosciences (King of Prussia, PA). Rac1 antibody was purchased from Millipore (Temecula, CA). Src (#2109) β-pix (#4515), and phospho-Rac (#2461) antibodies were purchased from Cell Signaling (Danvers, MA). Alexa 568 anti-mouse and Alexa 488 anti-rabbit were purchased from Invitrogen (Eugene, OR). Phalloidin was purchased from Life Technologies (Grand Island, NY) and used according to the manufactures instructions. DAPI was purchased from Life Technologies and used at a working concentration of 0.25 μg/mL.
Cell Imaging
HeyA8 cells were plated at density of 1 × 105 in 6-well plates with glass coverslips at the bottom. The cells were allowed to adhere overnight in a 37°C incubator with 5% CO2. The following day the cells were washed 3× with sterile PBS and then serum-starved for 18 hours. After serum-starvation, the cells were treated with 20 μM of LPA for the indicated time points. After LPA treatment, the cells were washed with ice-cold PBS one time and then treated with 4% paraformaldehyde for 15 minutes while rocking. The cells were then washed with PBS 1× and then stored at 4°C until they were stained.
To stain the cells they were first permeablized with 0.25% Triton X-100 for 10 minutes and then washed with PBS 3×. The coverslips were then blocked with 1% BSA in PBS for 30 minutes at room temperature while rocking. After blocking, the coverslips were washed with PBS 1×. After washing, the primary antibody was applied at the appropriate dilution and rocked for 10 minutes at room temperature. The coverslips were then transferred to 4°C and incubated overnight while rocking. The following day the primary antibody was removed and the coverslips were washed 3× for 5 minutes each. After washing, the coverslips were incubated with secondary antibody for 45 minutes at room temperature while rocking and covered with aluminum foil. After incubation with the secondary antibody, the coverslips were washed 1× with PBS and then stained with DAPI for 5 minutes. The coverslips were then washed 3× with PBS for 5 minutes each wash and then allowed to dry. Once dry, the coverslips were mounted with ProLong Gold antifade from Life Technologies (Grand Island, NY) on glass slides. The coverslips were allowed to dry overnight at room temperature in the dark and then imaged the following day with a Nikon Eclipse Ni-U (Melville, NY) at 600×.
Immunoprecipitation and Western Blotting
All cells were lysed with RIPA buffer (PBS pH 7.4, 1% Nonidet P-40, 0.5% sodium deoxycholate, 0.1% sodium dodecyl sulfate, 50 mM sodium fluoride, 1 mM phenylmethylsulfonyl fluoride, 0.2 mM sodium vanadate, 2 mg/mL leupeptin, 2 mg/mL pepstatin A, and 4 mg/mL aprotinin) and 500 μg of protein was incubated with the indicated antibody overnight at 4°C while rotating in a sealed 1.5 mL plastic tube. The following day the immunoprecipitated lysates were incubated with Protein A/G for 4 hours and subsequently separated by 10% SDS/PAGE and immunoblotted with the indicated antibody. Immunoblot analysis with the indicated antibodies were carried out following previously published procedures [25, 40] and developed with a Kodak Image Station 4000MM.
Collagen-1 migratory invasion assay
The Collagen-1 migratory invasion assay was performed as previously published [40]. Collagen type 1 was coated overnight onto 8-mm pore transwells placed into 24-well plates at 4°C. The following day, the collagen-coated cell culture inserts were washed and placed in a separate 24-well plate. The collagen-coated inserts were seeded with 2.5 × 104 SKOV3-ip cells that were suspended in 200 mL serum-free media. Each well contained 500 mL media containing serum-free media control or serum-free media containing 20 μM of LPA. The cells were incubated for 20 hours. Non-migrating cells on the proximal side of the inserts were removed with a cotton swab, and the migrated cells on the distal side of the inserts were fixed and stained with Hemacolor (EMD Chemicals). Images were obtained at 200× in 5 random fields for each group. The experiments were repeated 3 times. SKOV3-ip cells were transfected with the indicated plasmid (sh-RNA) and plated into 6-well plates for a total of 48 hours [40]. Twenty-four hours after transfection, the cells were serum starved for an additional 24 hours, trypsinized, counted, and placed into the transwell.
Statistical analysis
Statistical analysis was carried out with GraphPad Prism by a 2-tailed Student t test with Welch correction. All depicted error bars represent the standard error of the mean.
Cell Transfection
All cells were transfected with a Nucleofector II system from Lonza (Allendale, NJ) using the provided transfection protocol forcells [40]. HeyA8 cells were trypsinized and counted using a Countess automated cell counter (Life Technologies). 2 × 106 cells per transfection cuvette were transfected with either non-targeting siRNA (100 nM) or with p130Cas-targeting siRNA (100 nM) as indicated and/or transfected with Gαi2QL (5 μg) or pcDNA3 (5 μg; vector) as indicated. After transfection, the cells were plated on 60 mm plates and allowed to adhere overnight. The following day the media was changed and the cells were allowed to grow until the end of the day. The cells were then were replated at a density of 500,000 cells per 100 mm plate and allowed to adhere overnight. If the cells were not to be stimulated with LPA, the following day the cells were lysed. For transfected cells that were to be stimulated with LPA, they were serum-starved after they were counted and allowed to adhere overnight for 18 hours. After serum-starvation the cells were treated with 20 μM for the indicated time point and lysed. For stable transfection, SKOV3-ip were transfected as above with sh-Gαi2 or control, nonsense sh-RNA and selected for expression of sh-Gαi2 or the nonsense vector with puromycin as described previously [20].
3.) Results
3.1) LPA stimulates the translocation of Gαi2 to the leading edge of ovarian cancer cells
To confirm our recent study [40] that indicated Gαi2 is necessary for LPA-meditated invasive migration we determined the effect of silencing Gαi2 on LPA-mediated invasive migration of SKOV3-ip cells. As shown in Figure 1A, the ovarian cancer cells that had Gαi2 stably silenced had significantly diminished migration. Our recent study has established that the Gαi2-Src-p130Cas pathway is directly involved in inducing LPA-stimulated migration of ovarian cancer cells [40]. However, the spatiotemporal localization of Gαi2 in relation to Src and p130Cas in transmitting the signals from LPA towards cell migration remained to be elucidated. Therefore, to define the spatiotemporal signaling events involving Gαi2, we stimulated the ovarian cancer cell line HeyA8 with 20 μM of LPA for a time course of 60 minutes. As shown in Figure 1B, as early as 5 minutes Gαi2 was localized into the leading edge of cells and continued in this location out to 60 minutes. Under further evaluation, we observed that there were two distinct populations of Gαi2 in cells stimulated with LPA (Figure 1B, Panel B). The first population was localized in the leading edge of cells, whereas the second population was localized in a diffused pattern in the perinuclear and nuclear region of the cell.
Figure 1. LPA-mediated migration of ovarian cancer cells require Gαi2.
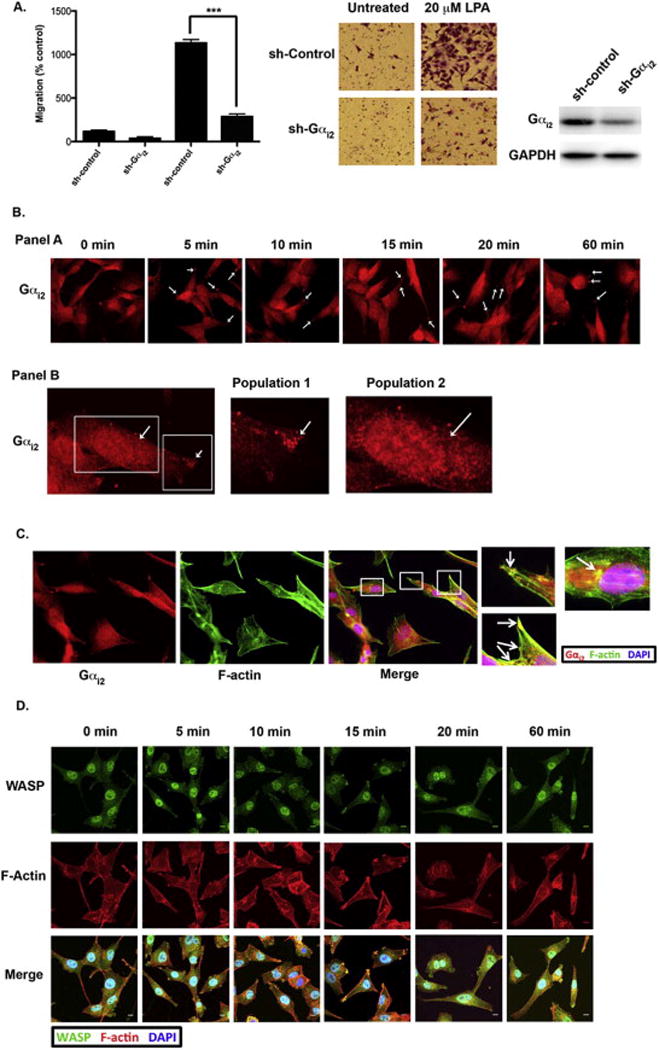
(A) We confirmed our previous results [40] that silencing Gαi2 attenuated LPA-stimulated invasive migration of ovarian cancer cells. SKOV3IP cells (2 × 106) were stably transfected with Gαi2-specific shRNA or nonsense shRNA and the silencing of Gαi2 in these cells was verified by immunoblot analysis. The transwell assay was carried out as described previously [40]. Representative images from each experimental group are shown. *** = p < 0.001 (B) Panel A: Stimulation of HeyA8 cells with LPA causes Gαi2 to translocated to punctate spots in the leading edge of the cells. HeyA8 cells were plated on glass slides, serum-starved overnight and stimulated with 20 μM of LPA for the indicated time points. Punctate spots (arrows) were seen at the leading edge of cells for all the time points. Panel B: Closer analysis revealed that Gαi2 is localized to two separate populations in LPA-stimulated cells. Panel B shows a representative zoomed in image of HeyA8 cells treated with 20 μM LPA for 10 minutes. The white boxes indicate the two areas where Gαi2 was concentrated. Population 1 was seen at the tip of the leading edge in structures that resembled invadopodia. Conversely, population 2 of Gαi2 was seen in the perinuclear (arrow) and nuclear region. (C) Gαi2 is localized to areas rich in F-actin and WASP indicating these structures are invadopodia. HeyA8 cells were stimulated with 20 μM LPA and stained with an antibody against Gαi2 and fluorescently tagged phalloidin indicated that Gαi2 was localized in structures that were rich in F-actin mainly in leading edge. Similarly, Gαi2 was co-localized to the same structures that contained the podosome/invadopodia marker WASP [33] (D) Co-staining HeyA8 cells with phalloidin and an antibody against WASP confirmed the actin-rich structures were invadopodia. These structures were seen mainly in the leading edge of the cell; however, there was some localization of WASP and F-actin in the perinuclear region (arrows). HeyA8 cells were stimulated with 20 μM LPA for the indicated time points and stained with fluorescently tagged phalloidin and anti-WASP antibodies, confirming that these actin-rich structures that contain Gαi2 were invadopodia since WASP is a well characterized marker of invadopodia and podosomes [33].
It has been shown previously that cell migration and invasion are dependent on actin-rich structures that have been termed podosomes or invadopodia that form direct contact with the underlying extracellular matrix (ECM) substrate [28, 33]. To determine if Gαi2 was interacting with these actin-rich structures in the leading edge, we stimulated HeyA8 cells with 20 μM of LPA and stained the cells with both phalloidin and anti-Gαi2. As shown in Figure 1C, Gαi2 and actin are clearly co-localizing in these cells. To further confirm that these structures are indeed invadopodia we stimulated HeyA8 cells with 20 μM of LPA from 5 minutes to 60 minutes. As shown in Figure 1D, these actin rich structures were also highly enriched with WASP, a marker of invadopodia [33], confirming these actin-rich structures were invadopodia. On closer analysis, we found that the majority of the actin-rich structures that contained WASP were localized to the leading edge of the cell. However, there was a small population of cells that had F-actin and WASP co-localization in distinct punctate spots in the perinuclear region (Figure 1D, Panel B).
3.2) Gαi2 co-localizes with Src in invadopodia
Recently, we have shown that Src is a direct downstream effector of Gαi2 signaling in LPA-mediated migration of ovarian cancer cells [40]. A previous report has indicated that the tyrosine kinase Src could directly interact with the α-subunits of Gi family of proteins leading to activation of Src by inducing a conformational change in Src [29]. It has also been noted that Src is localized in podosomes and invadopodia of cancer cells that show invasive migration [6, 19, 33]. Therefore, we sought to determine if Src was co-localizing with Gαi2 in invadopodia in these cells.
Using immunofluorescent microscopy, we determined the localization of Src in HeyA8 cells treated with 20 μM of LPA during a 60-minute time course. As with Gαi2, Src was also recruited to the leading edge of HeyA8 cells as early as 5 minutes and remained there to 60 minutes (Figure 2A). Identical to Gαi2, Src also co-localized with F-actin in distinct actin-rich circular structures at the leading edge of LPA-stimulated HeyA8 cells (Figure 2B). Furthermore, our results indicated the co-localization of Src and Gαi2 in invadopodia (Figure 2C). Finally, co-immunoprecipitation of Gαi2 and Src demonstrated that stimulation with 20 μM of LPA caused direct interaction of these two proteins (Figure 2C, Panel B).
Figure 2. LPA stimulates the translocation of Src to invadopodia.
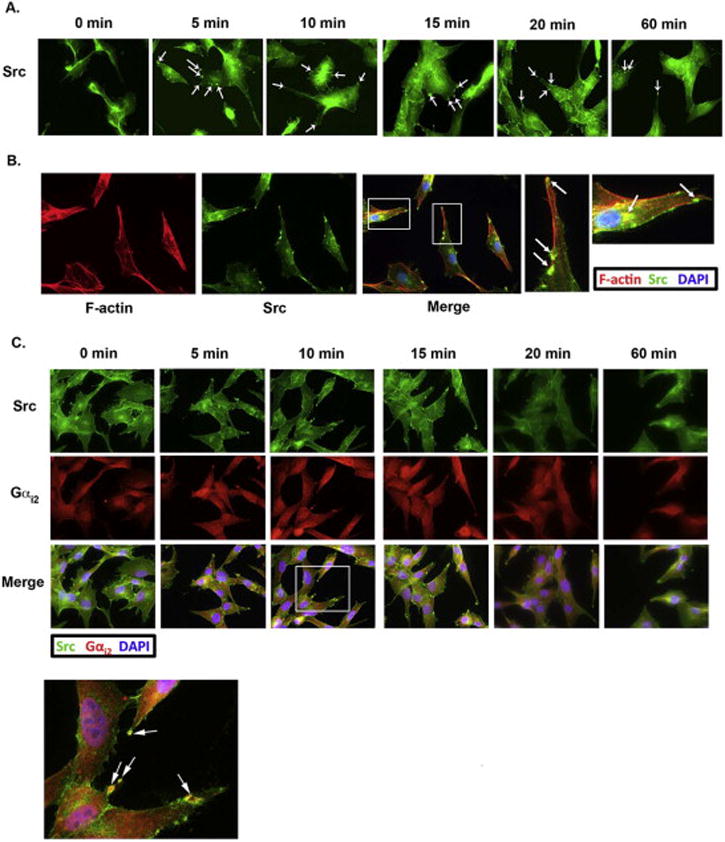
(A) Stimulation of HeyA8 cells with LPA caused Src to translocate to punctate spots in the leading edge of cells (arrows). HeyA8 cells were stimulated with 20 μM of LPA for the indicated time points and stained with an antibody directed against Src. (B) Src is also localized into actin-rich invadopodia after LPA stimulation. HeyA8 cells were plated onto glass slides, serum-starved and then stimulated with 20 μM of LPA for 10 minutes. The plated cells were then fixed and stained with fluorescently-tagged phalloidin and anti-Src. Src co-localized with F-actin mainly in the leading edge of cells and to a lesser degree in the perinuclear region, similar to the localization of F-actin and Gαi2. (C) Src and Gαi2 co-localize and directly interact in invadopodia. Panel A: To determine if Gαi2 and Src interact in invadopodia we treated HeyA8 with LPA for the indicated time points and stained the cells with anti-Src and anti-Gαi2 antibodies. Gαi2 and Src were found to co-localize to the same structures for all the time points tested. Panel B: Gαi2 and Src directly interact. HeyA8 cells were serum starved and treated with 20 μM of LPA for 10 minutes or left untreated and the lysates were immunoprecipitated for Gαi2 and immunoblotted for Src. Alternatively, the same cell lysates were immunoprecipitated for Src and immunoblotted for Gαi2. Representative images are shown for three separate experiments.
3.3) Activation of Rac1 by Gαi2 requires p130Cas
Previously we have shown that Gαi2 stimulates the phosphorylation of p130Cas via a pathway involving Src [40]. It has been shown by others that p130Cas is critical in initiating protrusion of the leading edge and in focal adhesion assembly and disassembly through its ability to stimulate the activation of Rac [11, 24, 30]. In addition, it has also been determined that p130Cas is a component of podosomes and invadopodia. Therefore, it can be reasoned that Gαi2 interaction with Src would involve p130Cas and subsequent activation of Rac1.
First, we determined whether Gαi2 alone was sufficient to activate Rac1. As shown in Figure 3A, transfection of HeyA8 cells with Gαi2QL, a constitutively active form of Gαi2, led to activation of Rac1 in the absence of exogenous LPA. These data indicate that Gαi2 is sufficient to directly activate Rac1 in ovarian cancer cells. Next, to determine if p130Cas is required for Gαi2-dependent activation of Rac1, we again transfected HeyA8 cells with Gαi2QL and in one group of these cells we also knocked-down p130Cas with siRNA. As shown in Figure 3B, knockdown of p130Cas significantly attenuated Gαi2QL-mediated activation of Rac1, indicating that p130Cas is required for Gαi2-mediated activation of Rac1. Finally, to confirm that LPA could activate Rac1 and that p130Cas was necessary for Rac activation in the context of LPA-mediated signaling, we monitored the activation of Rac in response to LPA in the presence or absence of p130Cas. As shown in Figure 3C, stimulation of HeyA8 cells with 20 μM of LPA for 10 minutes led to activation of Rac1. More significantly, when the expression of p130Cas was silenced in HeyA8 cells, there was little or no activation of Rac1 in response to LPA.
Figure 3. Activation of Rac by Gαi2 requires p130Cas.
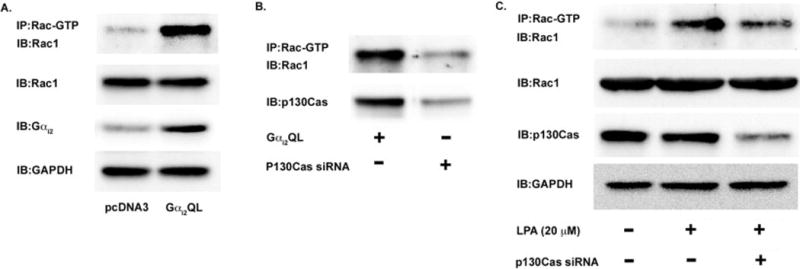
(A) Gαi2 activation is sufficient to lead to the activation of Rac1 in ovarian cancer cells. HeyA8 cells were transfected with either Gαi2QL, a constitutively active form of Gαi2, or pcDNA3 (vector). The cells were grown in normal media and not stimulated with exogenous LPA. After 48 hours, the cells were lysed and the lysates were immunoprecipitated with an antibody for the active form of Rac (Rac-GTP) overnight. The following day, the immunoprecipitated lysate was subjected to 10% SDS-PAGE and immunoblotted with an antibody for Rac1. 25 μg of lysate was subjected to 10% SDS-PAGE and immunoblotted for Gαi2 to demonstrate overexpression of the Gαi2QL vector. (B) Activation of Rac1 via Gαi2 requires the scaffold protein p130Cas. HeyA8 cells were transfected with Gαi2QL and with either non-targeting siRNA or siRNA directed against p130Cas as indicated. The cells were allowed to grow for 48 hours and the lysates were immunoprecipitated for active Rac for 24 hours. After 24 hours, the lysates were separated via 10% SDS-PAGE and immunoblotted for Rac1. To confirm knockdown of p130Cas, 25 μg of lysate was separated via 10% SDS-PAGE and immunoblotted for p130Cas. (C) p130Cas is required for Rac1 activation via LPA signaling. HeyA8 cells were transfected with either non-targeting siRNA or siRNA directed against p130Cas as indicated. After 24 hours, the transfected cells were serum-starved for 18 hours and then treated with 20 μM LPA for 20 minutes. The cells were then lysed and the lysates were immunoprecipitated with active Rac antibodies for 24 hours. The immunoprecipitated lysate was then subjected to 10% SDS-PAGE and immunoblotted with anti-Rac1 antibody. To confirm knockdown of p130Cas, 25 μg of lysate was subjected to 10% SDS-PAGE and immunoblotted for p130Cas. (D) p130Cas is localized to focal adhesions. HeyA8 cells were plated on glass slides and allowed to adhere overnight. The following day, the cells were serum-starved overnight and then stimulated with 20 μM of LPA for 5 to 60 minutes. The cells were then fixed and imaged for p130Cas and vinculin, a marker of focal adhesions. A representative image from 10 minutes is shown for p130Cas and vinculin co-localization and. (E.) p130Cas localizes to focal adhesions in a complex with activated Rac. Using the same treatment as Figure 3D, it was found that p130Cas and activated rac were localized to focal adhesions when cells were treated with 20 μM of LPA. Thus, it appears that p130Cas is localized mainly to focal adhesions and a sub-population of activated Rac is co-localized and directly interacting with p130Cas in the focal adhesions.
Finally, we wanted to determine if p130Cas was localizing to invadopodia and/or to focal adhesions [2, 38]. As shown in Figure 3D, stimulation of HeyA8 cells with 20 μM of LPA resulted in p130Cas mainly being recruited to focal adhesions, which was shown by the co-localization of p130Cas with the focal adhesion marker vinculin [31]. Since we had found that Rac1 and p130Cas were directly interacting, we wanted to determine if activated Rac was localized to focal adhesions with p130Cas. Indeed, as shown in Figure 3E, p130Cas co-localized with activated Rac. Therefore, it appears that p130Cas is recruited mainly to focal adhesions and its presence in focal adhesions is required for the activation of Rac, which is in line with previous studies [2, 38].
3.4) Gαi2 interacts with β-pix in the leading edge of ovarian cancer cells
Previous studies have shown that β-pix, a CDC42/Rac-specific guanine nucleotide exchange factor, is important in recruitment and the activation of Rac1 in the leading edge of cells [9]. In addition, it has been shown that Src can activate the GEF-activity of β-pix by phosphorylating Tyr-442 of β-pix [16]. It has also been shown that the α-subunits of different G proteins including Gαi3 [9], Gαq/11 [10], and Gα12 [10], co-immunoprecipitate with β-pix. Therefore, we wanted to determine if β-pix was also co-localizing with Gαi2 in LPA stimulated cells. To address this question, we carried out a 60-minute time course experiment with LPA stimulation and monitored the localization of Gαi2 and β-pix. As shown in the top panel of Figure 4A, Gαi2 and β-pix showed co-localization in cells stimulated with LPA throughout the entire duration of the experiment. Interestingly, we observed that β-pix, in addition to interacting with Gαi2 in invadopodia, were also seen in filament-like structures on the leading edge of the cell (Figure 4A; bottom panel) that appeared to be focal adhesions. Furthermore, in a small population of cells Gαi2 seemed to interact with β-pix in the focal adhesions. Finally, there appeared to be another population of β-pix and Gαi2 co-interacting in the perinuclear region.
Figure 4. Gαi2 directly interacts with β-pix in invadopodia.
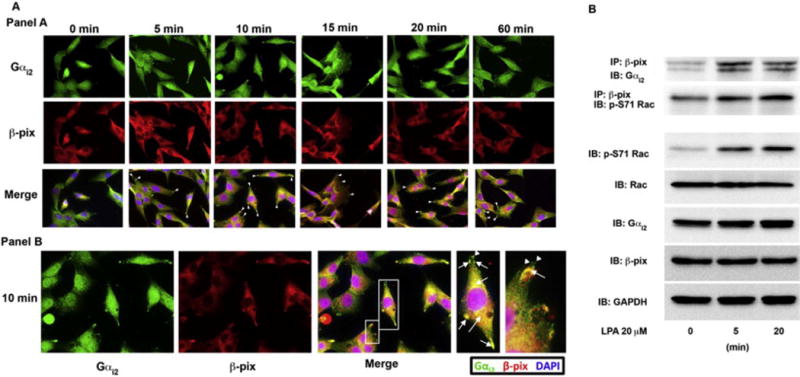
(A) Panel A: Gαi2 co-localizes with β-pix in invadopodia. To determine if Gαi2 and β-pix interact in invadopodia we treated HeyA8 with LPA for the indicated time points and stained the cells with anti-β-pix and anti-Gαi2 antibodies. Gαi2 and β-pix were found to co-localize in the leading edge during all time points tested. Panel B: The 10-minute image from panel A is shown. Zoomed in image of merged image for the 10-minute time point (white boxes) showing co-localization of Gαi2 and β-pix in invadopodia (arrows) both in the leading edge and in the perinuclear region. β-pix was also found to also localize to focal adhesions (arrowhead) without Gαi2. (B) Gαi2 and β-pix were shown to directly interact after LPA stimulation of 5 minutes and 20 minutes. β-pix and phosphorylated Rac were also shown to interact at the same time points. Phosphorylated Rac correlated with Rac activation seen by stimulation of these cells with LPA in Figure 3C and in the subsequent imaging studies. HeyA8 cells were plated and serum-starved for 18 hours. The cells were then left untreated or stimulated with 20 μM of LPA for 10 minutes, lysed and 500 μg of protein from the lysate was immunoprecipitated for β-pix for 24 hours. After immunoprecipitation, the lysate was separated by 10% SDS-PAGE and immunoblotted for Gαi2 and activated Rac, which was phosphorylated at Ser-71 using specific antibodies (p-S71 Rac). Total cell lysates were analyzed for phosphorylated Rac (p-S71 Rac), Rac1, β-pix, and Gαi2 along with GAPDH as a loading control.
To further establish the endogenous interaction between Gαi2 and β-pix in ovarian cancer cells, we carried out a co-immunoprecipitation analysis. HeyA8 cells were stimulated with 20 μM of LPA for 5 and 20 minutes and the lysates were subjected to immunoprecipitation with Gαi2 and probed for the presence of β-pix by immunoblot analysis. As shown in Figure 4B, Gαi2 and β-pix directly interacted with each other when Gαi2 is activated by LPA signaling. Furthermore, we immunoprecipitated β-pix and probed for the presence of the phosphorylated form of Rac that has been shown to represent its active configuration{Schwarz, 2012 #3638}. As shown in Figure 4B, when the cells were stimulated with LPA this resulted in the direct interaction of β-pix and phosphorylated Rac. Additionally, when we probed the lysate from these same cells, we found that there was an increase in phosphorylated Rac, which matched well with our data showing that Rac is activated in the presence of LPA.
Since we found β-pix localized to both focal adhesion and to invadopodia in LPA-treated cells (Figure 4A), we wanted to determine whether Rac was activated in both of these areas or only in the invadopodia that contained Gαi2 when ovarian cancer cells were stimulated with LPA. As shown in Figure 5A, β-pix and active Rac co-localize in the leading edge of the cell in large punctate structures that appear to be invadopodia. Similar to our results in Figure 4A, we found there was a second population of β-pix localized to filament-like structures at the very tip of cells, which appeared to be focal adhesions. Interestingly, in all the time points observed there rarely was active Rac localized to focal adhesions with β-pix. Additionally, we observed β-pix in focal adhesions even in un-stimulated cells. To determine if the fibrous structures at the tip of cells were indeed focal adhesions we stimulated cells with LPA for various time points and stained the cells with vinculin, a marker of focal adhesions. As shown in Figure 5B, the same filamentous staining pattern was seen with vinculin staining as with β-pix staining indicating that these structures are indeed focal adhesions. Furthermore, we also co-stained the same cells with Src to indicate the location of invadopodia. As shown in Figure 5B, the invadopodia and focal adhesions are distinct structures; however, there does seem to be some overlap between the two structures at times, such as seen in the first panel in the upper right of the leading edge of the cell. To confirm that active Rac was found in invadopodia we stimulated HeyA8 cells with LPA for 5 to 60 minutes and stained these cells with phalloidin and anti-active Rac.
Figure 5. Activated Rac colocalizes with β-pix in invadopodia.
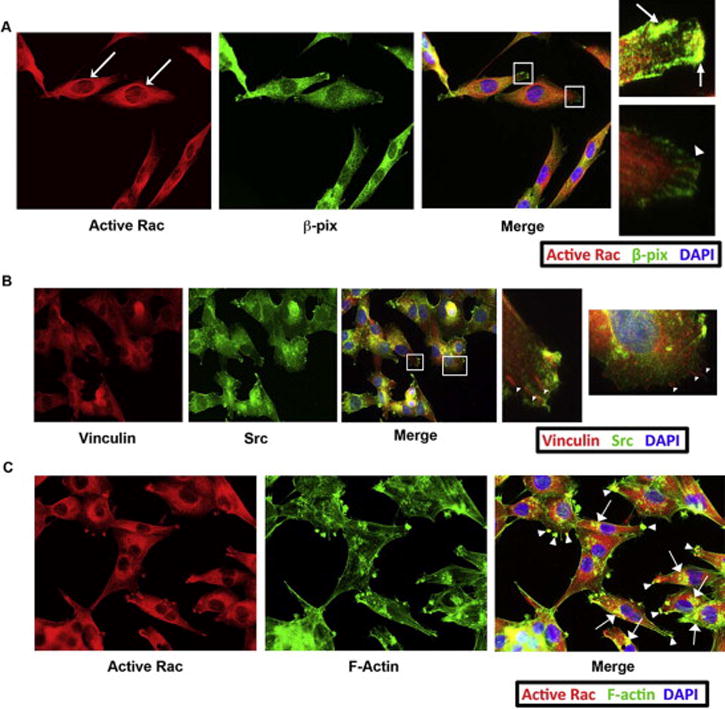
(A) Activated Rac co-localizes with β-pix in invadopodia but not focal adhesions. HeyA8 cells were plated, serum-starved and treated with 20 μM of LPA for 10 minutes. The fixed cells were stained with antibodies that recognized active Rac and β-pix. Zoomed in images (white boxes) from the merged image showed that active Rac and β-pix co-localize in punctate structures that resemble invadopodia (arrows). Additionally, β-pix was also found to localize to structures that resembled focal adhesions with little or no localization with active Rac (arrowheads). Finally, active Rac was found in strongly-staining circular structures of unknown importance directly surrounding the nucleus in LPA stimulated cells at all time points (dashed arrow). (B) The filamentous structures at the leading edge of the cell were confirmed to be focal adhesions. HeyA8 cells were treated as in part A and stained for vinculin (a marker of focal adhesions) and Src (a marker of invadopodia). The filamentous staining seen with β-pix and p130Cas was the same as with vinculin, indicating these structures were focal adhesions. Src was found to be primarily located in structures that were separate from the focal adhesions, further indicating that Src is localized to invadopodia. (C) Active Rac is localized primarily to actin-rich invadopodia in both the leading edge (arrowheads) and in the perinuclear region (arrows). HeyA8 cells were plated and treated with LPA as indicated in part A and B and stained for active Rac and with fluorophore-tagged phalloidin.
3.5) Silencing of Gαi2-expression inhibits localization of key signaling mediators of migration
Next, we wanted to determine if Gαi2 is required for recruitment of Src and β-pix and subsequent activation of Rac in invadopodia. As shown in Figure 6A, cells in which the expression of Gαi2 was silenced, the localization of Src in the invadopodia is reduced. With the silencing of Gαi2, Src could be observed to be localized in the periphery of the nucleus of the cell, indicating the inactivation of Src [6]. Similarly, we previously showed that knockdown of Gαi2 impaired the ability of LPA to active Src [40]. Therefore, without the presence of Gαi2, LPA signaling is unable to activate Src and activate Rac in these cells.
Figure 6. Silencing of Gαi2 alters the translocation of Src, β-pix, p190Cas and active-Rac.
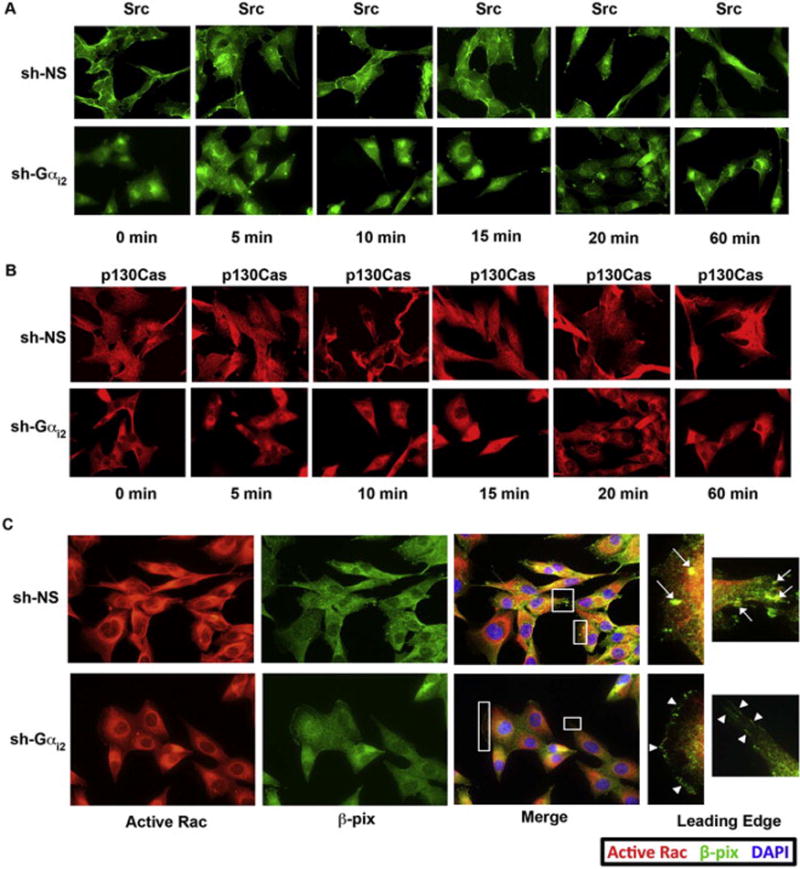
(A) Knockdown of Gαi2 caused Src to be localized in a diffuse pattern in the perinuclear region, which is indicate of inactive Src [19]. HeyA8 cells were transfected with either non-sense shRNA or with shRNA specific for Gαi2. After 48 hours post-transfection these cells were stimulated with 20 μM of LPA for a time course of 60 minutes and stained with antibody directed against Src. A representative image is shown from the 10-minute time point of each group. The findings were similar in all the time points stimulated with LPA. (B) Knockdown of Gαi2 resulted in the loss of p130Cas translocation to focal adhesions. HeyA8 cells were transfected with non-sense shRNA or with shRNA targeting Gαi2 and treated with LPA for a 60-minute time course. A representative image is shown from the 10-minute time point of each group. The findings were similar in all the time points stimulated with LPA. Knockdown of Gαi2 led to a dramatic loss of p130Cas localizing to focal adhesions as compared to the control (arrowheads). (C) Knockdown of Gαi2 led to a loss of active Rac in the leading edge. HeyA8 cells were transfected with either non-sense shRNA or with shRNA specific for Gαi2. After 48 hours post-transfection these cells were stimulated with 20 μM of LPA for 10 minutes and co-stained with antibodies directed against active Rac and β-pix. There was a loss of the formation of invadopodia with β-pix present in the Gαi2-silenced cells. Interestingly, there was a shift for β-pix to be found solely in focal adhesions, reminiscent of untreated cells. Additionally, there was a reduction of the ring-like swirls of active Rac around the nucleus of Gαi2-silenced cells.
Our previous studies have shown that LPA stimulates the phosphorylation of p130Cas via Src and this pathway could lead to the activation of Rac1 [40]. This is substantiated by the findings that the highly conserved p130Cas-Crk-DOCK-ELMO pathways is involved in the activation of Rac1. Furthermore, p130Cas has been shown to be located transiently in focal adhesions [13] and in nascent integrin adhesion sites of migrating cells [7, 17]. Therefore, we investigated whether the silencing Gαi2 disrupts the localization of p130Cas and the subsequent activation of Rac1.
To determine the localization of p130Cas in LPA-stimulated cells we first stimulated HeyA8 cells with 20 μM of LPA over a 60-minute time course that resulted in recruitment of p130Cas to the leading edge of cells in small filamentous strands. For clarity and ease of observation, in Figure 6B we show a representative image from the 10 minute time point, since each time point had similar results. From our previous vinculin staining (Figure 3D), it is clear that these structures are focal adhesions. Alternatively, in cells that were transfected with shRNA specifically targeting Gαi2 there was a complete loss of p130Cas recruitment to focal adhesions (Figure 6B; right panel).
Next, we investigated the localization of activated Rac in response to LPA in Gαi2-silenced Hey8A cells. As shown in Figure 6C, silencing of Gαi2 led to an overall decrease in the levels of activated-Rac in response to LPA. More significantly, the localization of activated-Rac1 is drastically reduced in the leading edge and invadopodia. Subsequently, we investigated the effect of silencing Gαi2 in the localization of β-pix in response to LPA. As shown in Figure 6C, the silencing of Gαi2 reduced the levels of β-pix co-localized with activated Rac in the invadopodia. However, the localization of β-pix at the leading focal adhesions in the Gαi2-silenced cells was similar to unstimulated cells (Figure 4A; 0 min). Therefore, it appears that β-pix is recruited to invadopodia in actively migrating cells, whereas it is localized to focal adhesions in cells that are not actively migrating but are adherent to the underlying substratum.
4.) Discussion
To date there has been little work done on how each specific G protein individually contributes to migration of cells. Importantly, our current study shows that, Gαi2 is localized in invadopodia and that it directly interacts with Src and β-pix to activate Rac. Taken together with the previous findings that Src stimulates the phosphorylation of β-pix, which in turn activates Rac1 [15], it appears that Gαi2 plays an active role in directly initiating the invasive migration of ovarian cancer cells in response to LPA. In this context, it is worth noting that our report is the first to conclusively demonstrate that Src, Gαi2, and β-pix directly interact with each other in invadopodia and that knockdown of Gαi2 inhibits translocation of Src and β-pix to invadopodia. Finally, our report demonstrates that knockdown of Gαi2 inhibits recruitment of p130Cas to focal adhesions. Paired with our recent work [40] on Gαi2 we have shown that inhibition of Gαi2 and its subsequent localization to invadopodia and activation of Src leads to loss of migration and invasion of LPA-stimulated ovarian cancer cells.
From the findings in this report, it appears that Gαi2 in response to LPA directly interacts with Src in invadopodia leading to the recruitment of β-pix and subsequent activation of Rac. Most importantly, it appears that Gαi2 and Src make up a large signaling complex that is found specifically in invadopodia and that localization of Gαi2 to this structure is a critical event in mediating the formation of invadopodia and activating cellular cascades involved in mediating migration of cells.
Although previous studies have shown that Gαi3 [10] can interact with β-pix, this is the first report to show that LPA stimulates an active signaling complex consisting of Gαi2, β-pix, and Src that result in the activation of Rac. Similarly, we report for the first time the co-localization of Gαi2, Src, β-pix, and activate Rac in invadopodia. Importantly, the loss of localization of these proteins to the invadopodia upon silencing of Gαi2 points to a critical role for Gαi2 in initiating the recruitment of these proteins to the invadopodia. When our current findings are paired with our previous observation that LPA stimulates the phosphorylation of Src via Gαi2 in ovarian cancer cells [40] and the finding that Src phosphorylates β-pix to activate it [15, 16] and that β-pix directly interacts with Rac to activate it [15, 27, 36] this unravels a novel Gαi2-regulated signaling pathway underlying the invasive migration of ovarian cancer cells.
Another set of interesting findings we have reported here is the Gαi2-dependent localization of p130Cas in focal adhesions. Our previous study [40] indicated that LPA-stimulates activation of Src and via p130Cas this pathway led to the activation of Rac. Our present findings suggest the possibility that the signaling through this pathway may involve the activation of Rac required for the formation of nascent focal adhesions involved in cell migration. Since p130Cas-mediated activation of Rac1 involves the DOCK-ELMO complex and p130Cas can be activated by Src, it appears that Gαi2 activates Rac through different mechanisms in two different but interrelated signaling milieus.
Previous reports have shown that Src can also be found in focal adhesions [5, 33]. Accordingly, it is a possibility that Gαi2 and Src can also form a complex, similar to the ones we observed in the invadopodia, which is localized to focal adhesions. This complex could also participate in the phosphorylation of p130Cas. However, this may require a more sensitive method of imaging than was used in our current study. Thus, a critical question that remains is whether there is a small population of active Src that localizes to focal adhesions to phosphorylate p130Cas or if p130Cas is phosphorylated in invadopodia and then translocated to the focal adhesions or vice versa. Further investigation should elucidate the mechanism(s) through which Gαi2 spatiotemporally regulates these pathways through Src in a non-overlapping manner.
Interestingly, we observed that there may be a second population of Gαi2, Src, and active Rac that is localized in punctate spots in the perinuclear region when ovarian cancer cells are stimulated with LPA. Several recent reports have shown that proteins involved in invadopodia do translocate to the perinuclear region and these actin-rich structures may be involved in the inactivation of the proteins found in the invadopodia of the leading edge [23, 26, 37]. It is also possible that these structures are part of a recycling process. Further analyses should define the role of these perinuclear structures in the migration of ovarian cancer cells. Nevertheless, studies from several laboratories including ours suggest that the invadopodia that are present in the leading edge are structures that are important in mediating invasive migration of ovarian cancer cells.
From our results in Figure 6A, and in similar studies [6, 19, 23], it could be argued that when Src is completely inactivated, it is localized to the perinuclear region in a diffuse staining pattern (Figure 6A, 60 minute time point) [6] in contrast to the punctate structures seen during the earlier time points following LPA-stimulation. This, it is more likely that Gαi2, Src and Rac are recycled to the perinuclear region for inactivation and recruited back later for reactivation as the cell moves forward. Such a hypothetical model would allow the cells to move forward by inactivating Rac and dissolving the focal adhesions and invadopodia to allow the cell to lift up and push forward. As the cycle continues, these components could then be translocated back to the leading edge for the perinuclear regions where they could be activated again to allow the cell to grasp the substrate. One piece of supporting evidence for this model is the observation that Src located in the perinuclear region is typically inactivated [6]. However, this hypothetical model needs to be substantiated with further analyses.
In this report, we have shown that Src and Gαi2 interact in distinct foci in ovarian cancer cells. Furthermore, we have shown that the recruitment of Gαi2 to invadopodia leads to the activation of Rac. This is the first report to demonstrate that LPA stimulates the interaction of Gαi2 with β-pix facilitating the recruitment and activation of Rac in invadopodia. In addition, we provide indirect evidence that Gαi2 can activate Rac through p130Cas in foci distinct from that of β-Pix. Results from this study in conjunction with our previous work [40], clearly points out that LPA through specific LPA-receptors 1 and 3 activate Src via Gαi2. Src, in turn stimulates Rac via β-pix and p130Cas-DOCK-ELMO pathways in two spatially distinct, but functionally interrelated signaling complexes involved in cell migration. This is consistent with previous reportsthat LPA receptors 1 and 3 are involved in activating Rac-dependent migration pathways via Gi signaling [21, 39]. However, it has also been shown that LPA-mediated migration in ovarian cancer cells involves Gα13 [3]. In addition, we have previously shown that Gα13 can activate Rac in fibroblasts [34]. Therefore, future studies should define the contribution of the interaction between Gα13 and Gαi2 signaling pathways in LPA-induced migration of ovarian cancer cells. It is quite likely that these pathways form a complex signaling network that involves different cytoskeletal proteins, signaling and adaptor molecules, and kinases in mediating invasive migration of ovarian cancer cells in response to LPA. Further defining of the critical nodes in this signaling network will have important implications for cancer therapy.
Figure 7. Schematic representation of Gαi2-Src signaling in LPA-mediated invasive cell migration.

Stimulation of LPA receptors activates Gαi2. Once activated, Gαi2 is recruited to invadopodia where it binds and activates Src and β-pix, which are also recruited to the invadopodia in a Gαi2-dependent fashion. In the invadopodia, the Gαi2-Src-β-pix complex causes the activation of Rac. In a spatially distinct population, activation of Gαi2 leads to the translocation of p130Cas to focal adhesions where p130Cas could participate in the activation of Rac1 through DOCK180-Elmo complex. While the pathways highlighted above appear to be spatially distinct, they are more likely to be temporally and functionally coordinated along with other signaling proteins during cell migration.
Acknowledgments
This work was supported by grants from the National Institutes of Health (CA116984, CA123233). OUHSC Flow Cytometry and Imaging Core is acknowledged for the use of confocal and epifluorescence microscopy. We thank Stephenson Cancer Center at the University of Oklahoma, Oklahoma City, OK and an Institutional Development Award (IDeA) from the National Institute of General Medical Sciences of the National Institutes of Health under grant number P20 GM103639 for the immunostaining service.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of Interest Statement:
The authors have no conflicts of interests and declare no competing financial interests.
References
- 1.Artym VV, Matsumoto K, Mueller SC, Yamada KM. Dynamic membrane remodeling at invadopodia differentiates invadopodia from podosomes. European journal of cell biology. 2011;90:172–180. doi: 10.1016/j.ejcb.2010.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barrett A, Pellet-Many C, Zachary IC, Evans IM, Frankel P. p130Cas: a key signalling node in health and disease. Cellular signalling. 2013;25:766–777. doi: 10.1016/j.cellsig.2012.12.019. [DOI] [PubMed] [Google Scholar]
- 3.Bian D, Mahanivong C, Yu J, Frisch SM, Pan ZK, Ye RD, Huang S. The G12/13-RhoA signaling pathway contributes to efficient lysophosphatidic acid-stimulated cell migration. Oncogene. 2006;25:2234–2244. doi: 10.1038/sj.onc.1209261. [DOI] [PubMed] [Google Scholar]
- 4.Bian D, Su S, Mahanivong C, Cheng RK, Han Q, Pan ZK, Sun P, Huang S. Lysophosphatidic Acid Stimulates Ovarian Cancer Cell Migration via a Ras-MEK Kinase 1 Pathway. Cancer research. 2004;64:4209–4217. doi: 10.1158/0008-5472.CAN-04-0060. [DOI] [PubMed] [Google Scholar]
- 5.Block MR, Badowski C, Millon-Fremillon A, Bouvard D, Bouin AP, Faurobert E, Gerber-Scokaert D, Planus E, Albiges-Rizo C. Podosome-type adhesions and focal adhesions, so alike yet so different. European journal of cell biology. 2008;87:491–506. doi: 10.1016/j.ejcb.2008.02.012. [DOI] [PubMed] [Google Scholar]
- 6.Boateng LR, Huttenlocher A. Spatiotemporal regulation of Src and its substrates at invadosomes. European journal of cell biology. 2012;91:878–888. doi: 10.1016/j.ejcb.2012.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brabek J, Constancio SS, Shin NY, Pozzi A, Weaver AM, Hanks SK. CAS promotes invasiveness of Src-transformed cells. Oncogene. 2004;23:7406–7415. doi: 10.1038/sj.onc.1207965. [DOI] [PubMed] [Google Scholar]
- 8.Burridge K, Wennerberg K. Rho and Rac take center stage. Cell. 2004;116:167–179. doi: 10.1016/s0092-8674(04)00003-0. [DOI] [PubMed] [Google Scholar]
- 9.Chahdi A, Sorokin A. Endothelin-1 induces p66Shc activation through EGF receptor transactivation: Role of beta(1)Pix/Galpha(i3) interaction. Cellular signalling. 2010;22:325–329. doi: 10.1016/j.cellsig.2009.09.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chahdi A, Sorokin A. The role of beta(1)Pix/caveolin-1 interaction in endothelin signaling through Galpha subunits. Biochemical and biophysical research communications. 2010;391:1330–1335. doi: 10.1016/j.bbrc.2009.12.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cho SY, Klemke RL. Purification of pseudopodia from polarized cells reveals redistribution and activation of Rac through assembly of a CAS/Crk scaffold. The Journal of cell biology. 2002;156:725–736. doi: 10.1083/jcb.200111032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Choi JW, Herr DR, Noguchi K, Yung YC, Lee CW, Mutoh T, Lin ME, Teo ST, Park KE, Mosley AN, Chun J. LPA receptors: subtypes and biological actions. Annual review of pharmacology and toxicology. 2010;50:157–186. doi: 10.1146/annurev.pharmtox.010909.105753. [DOI] [PubMed] [Google Scholar]
- 13.Donato DM, Ryzhova LM, Meenderink LM, Kaverina I, Hanks SK. Dynamics and mechanism of p130Cas localization to focal adhesions. The Journal of biological chemistry. 2010;285:20769–20779. doi: 10.1074/jbc.M109.091207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fang X, Gaudette D, Furui T, Mao M, Estrella V, Eder A, Pustilnik T, Sasagawa T, Lapushin R, Yu S, Jaffe RB, Wiener JR, Erickson JR, Mills GB. Lysophospholipid growth factors in the initiation, progression, metastases, and management of ovarian cancer. Annals of the New York Academy of Sciences. 2000;905:188–208. doi: 10.1111/j.1749-6632.2000.tb06550.x. [DOI] [PubMed] [Google Scholar]
- 15.Feng Q, Baird D, Peng X, Wang J, Ly T, Guan JL, Cerione RA. Cool-1 functions as an essential regulatory node for EGF receptor- and Src-mediated cell growth. Nature cell biology. 2006;8:945–956. doi: 10.1038/ncb1453. [DOI] [PubMed] [Google Scholar]
- 16.Feng Q, Baird D, Yoo S, Antonyak M, Cerione RA. Phosphorylation of the cool-1/beta-Pix protein serves as a regulatory signal for the migration and invasive activity of Src-transformed cells. The Journal of biological chemistry. 2010;285:18806–18816. doi: 10.1074/jbc.M109.098079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fonseca PM, Shin NY, Brabek J, Ryzhova L, Wu J, Hanks SK. Regulation and localization of CAS substrate domain tyrosine phosphorylation. Cellular signalling. 2004;16:621–629. doi: 10.1016/j.cellsig.2003.10.004. [DOI] [PubMed] [Google Scholar]
- 18.Gardner JA, Ha JH, Jayaraman M, Dhanasekaran DN. The gep proto-oncogene Galpha13 mediates lysophosphatidic acid-mediated migration of pancreatic cancer cells. Pancreas. 2013;42:819–828. doi: 10.1097/MPA.0b013e318279c577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gimona M, Buccione R, Courtneidge SA, Linder S. Assembly and biological role of podosomes and invadopodia. Curr Opin Cell Biol. 2008;20:235–241. doi: 10.1016/j.ceb.2008.01.005. [DOI] [PubMed] [Google Scholar]
- 20.Goldsmith ZG, Ha JH, Jayaraman M, Dhanasekaran DN. Lysophosphatidic Acid Stimulates the Proliferation of Ovarian Cancer Cells via the gep Proto-Oncogene Galpha(12) Genes & cancer. 2011;2:563–575. doi: 10.1177/1947601911419362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hama K, Aoki J, Fukaya M, Kishi Y, Sakai T, Suzuki R, Ohta H, Yamori T, Watanabe M, Chun J, Arai H. Lysophosphatidic acid and autotaxin stimulate cell motility of neoplastic and non-neoplastic cells through LPA1. The Journal of biological chemistry. 2004;279:17634–17639. doi: 10.1074/jbc.M313927200. [DOI] [PubMed] [Google Scholar]
- 22.Hasegawa Y, Murph M, Yu S, Tigyi G, Mills GB. Lysophosphatidic acid (LPA)-induced vasodilator-stimulated phosphoprotein mediates lamellipodia formation to initiate motility in PC-3 prostate cancer cells. Molecular oncology. 2008;2:54–69. doi: 10.1016/j.molonc.2008.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hauck CR, Hsia DA, Ilic D, Schlaepfer DD. v-Src SH3-enhanced interaction with focal adhesion kinase at beta 1 integrin-containing invadopodia promotes cell invasion. The Journal of biological chemistry. 2002;277:12487–12490. doi: 10.1074/jbc.C100760200. [DOI] [PubMed] [Google Scholar]
- 24.Klemke RL, Leng J, Molander R, Brooks PC, Vuori K, Cheresh DA. CAS/Crk coupling serves as a “molecular switch” for induction of cell migration. The Journal of cell biology. 1998;140:961–972. doi: 10.1083/jcb.140.4.961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kumar RN, Ha JH, Radhakrishnan R, Dhanasekaran DN. Transactivation of platelet-derived growth factor receptor alpha by the GTPase-deficient activated mutant of Galpha12. Mol Cell Biol. 2006;26:50–62. doi: 10.1128/MCB.26.1.50-62.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lee MS, Kim S, Kim BG, Won C, Nam SH, Kang S, Kim HJ, Kang M, Ryu J, Song HE, Lee D, Ye SK, Jeon NL, Kim TY, Cho NH, Lee JW. Snail1 induced in breast cancer cells in 3D collagen I gel environment suppresses cortactin and impairs effective invadopodia formation. Biochimica et biophysica acta. 2014;1843:2037–2054. doi: 10.1016/j.bbamcr.2014.05.007. [DOI] [PubMed] [Google Scholar]
- 27.Li Z, Hannigan M, Mo Z, Liu B, Lu W, Wu Y, Smrcka AV, Wu G, Li L, Liu M, Huang CK, Wu D. Directional sensing requires G beta gamma-mediated PAK1 and PIX alpha-dependent activation of Cdc42. Cell. 2003;114:215–227. doi: 10.1016/s0092-8674(03)00559-2. [DOI] [PubMed] [Google Scholar]
- 28.Linder S. The matrix corroded: podosomes and invadopodia in extracellular matrix degradation. Trends in cell biology. 2007;17:107–117. doi: 10.1016/j.tcb.2007.01.002. [DOI] [PubMed] [Google Scholar]
- 29.Ma YC, Huang J, Ali S, Lowry W, Huang XY. Src tyrosine kinase is a novel direct effector of G proteins. Cell. 2000;102:635–646. doi: 10.1016/s0092-8674(00)00086-6. [DOI] [PubMed] [Google Scholar]
- 30.Meenderink LM, Ryzhova LM, Donato DM, Gochberg DF, Kaverina I, Hanks SK. P130Cas Src-binding and substrate domains have distinct roles in sustaining focal adhesion disassembly and promoting cell migration. PloS one. 2010;5:e13412. doi: 10.1371/journal.pone.0013412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mierke CT. The role of vinculin in the regulation of the mechanical properties of cells. Cell biochemistry and biophysics. 2009;53:115–126. doi: 10.1007/s12013-009-9047-6. [DOI] [PubMed] [Google Scholar]
- 32.Mills GB, Moolenaar WH. The emerging role of lysophosphatidic acid in cancer, Nature reviews. Cancer. 2003;3:582–591. doi: 10.1038/nrc1143. [DOI] [PubMed] [Google Scholar]
- 33.Murphy DA, Courtneidge SA. The ‘ins’ and ‘outs’ of podosomes and invadopodia: characteristics, formation and function, Nature reviews. Molecular cell biology. 2011;12:413–426. doi: 10.1038/nrm3141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Radhika V, Onesime D, Ha JH, Dhanasekaran N. Galpha13 stimulates cell migration through cortactin-interacting protein Hax-1. The Journal of biological chemistry. 2004;279:49406–49413. doi: 10.1074/jbc.M408836200. [DOI] [PubMed] [Google Scholar]
- 35.Shida D, Kitayama J, Yamaguchi H, Okaji Y, Tsuno NH, Watanabe T, Takuwa Y, Nagawa H. Lysophosphatidic acid (LPA) enhances the metastatic potential of human colon carcinoma DLD1 cells through LPA1. Cancer research. 2003;63:1706–1711. [PubMed] [Google Scholar]
- 36.Shin EY, Woo KN, Lee CS, Koo SH, Kim YG, Kim WJ, Bae CD, Chang SI, Kim EG. Basic fibroblast growth factor stimulates activation of Rac1 through a p85 betaPIX phosphorylation-dependent pathway. The Journal of biological chemistry. 2004;279:1994–2004. doi: 10.1074/jbc.M307330200. [DOI] [PubMed] [Google Scholar]
- 37.Smith-Pearson PS, Greuber EK, Yogalingam G, Pendergast AM. Abl kinases are required for invadopodia formation and chemokine-induced invasion. The Journal of biological chemistry. 2010;285:40201–40211. doi: 10.1074/jbc.M110.147330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tikhmyanova N, Little JL, Golemis EA. CAS proteins in normal and pathological cell growth control, Cellular and molecular life sciences. CMLS. 2010;67:1025–1048. doi: 10.1007/s00018-009-0213-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Van Leeuwen FN, Olivo C, Grivell S, Giepmans BN, Collard JG, Moolenaar WH. Rac activation by lysophosphatidic acid LPA1 receptors through the guanine nucleotide exchange factor Tiam1. The Journal of biological chemistry. 2003;278:400–406. doi: 10.1074/jbc.M210151200. [DOI] [PubMed] [Google Scholar]
- 40.Ward JD, Dhanasekaran DN. LPA Stimulates the Phosphorylation of p130Cas via Galphai2 in Ovarian Cancer Cells. Genes Cancer. 2012;3:578–591. doi: 10.1177/1947601913475360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Xu Y, Fang XJ, Casey G, Mills GB. Lysophospholipids activate ovarian and breast cancer cells. The Biochemical journal. 1995;309(Pt 3):933–940. doi: 10.1042/bj3090933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yu S, Murph MM, Lu Y, Liu S, Hall HS, Liu J, Stephens C, Fang X, Mills GB. Lysophosphatidic acid receptors determine tumorigenicity and aggressiveness of ovarian cancer cells. Journal of the National Cancer Institute. 2008;100:1630–1642. doi: 10.1093/jnci/djn378. [DOI] [PMC free article] [PubMed] [Google Scholar]


