Summary
Flagellation in polar flagellates is one of the rare biosynthetic processes known to be numerically regulated in bacteria. Polar flagellates must spatially and numerically regulate flagellar biogenesis to create flagellation patterns for each species that are ideal for motility. FlhG ATPases numerically regulate polar flagellar biogenesis, yet FlhG orthologs are diverse in motif composition. We discovered that Campylobacter jejuni FlhG is at the center of a multipartite mechanism that likely influences a flagellar biosynthetic step to control flagellar number for amphitrichous flagellation, rather than suppressing activators of flagellar gene transcription as in Vibrio and Pseudomonas species. Unlike other FlhG orthologs, the FlhG ATPase domain was not required to regulate flagellar number in C. jejuni. Instead, two regions of C. jejuni FlhG that are absent or significantly altered in FlhG orthologs are involved in numerical regulation of flagellar biogenesis. Additionally, we found that C. jejuni FlhG influences FlhF GTPase activity, which may mechanistically contribute to flagellar number regulation. Our work suggests that FlhG ATPases divergently evolved in each polarly flagellated species to employ different intrinsic domains and extrinsic effectors to ultimately mediate a common output – precise numerical control of polar flagellar biogenesis required to create species-specific flagellation patterns optimal for motility.
Introduction
Bacteria are capable of spatially regulating biosynthesis of some organelles and macromolecular complexes. However, other than chromosomal and plasmid DNAs, the extent of numerical regulation of biosynthesis of other components in the bacterial cell is unclear. The flagellum is one organelle that is numerically regulated in polarly flagellated bacteria (Kazmierczak and Hendrixson, 2013; Schuhmacher et al., 2015b). Each polar flagellate is genetically programmed to produce a specific number of flagella per cell, in addition to spatially regulating biosynthesis of the organelles so that they are only produced at a pole. For example, Vibrio cholerae, Vibrio alginolyticus, Pseudomonas aeruginosa and Shewanella putrefaciens are monotrichous bacteria that produce exactly one flagellum at a single pole of a cell. Campylobacter jejuni is amphitrichous, which is defined by a single flagellum synthesized at both poles. Helicobacter pylori is lophotrichous, resulting in the production of a tuft of flagella (commonly 2–6 organelles) at a single pole. In contrast, peritrichous organisms tend to produce more flagella on the cell surface, but existence of a definitive mechanism to regulate flagellar number in these species is less clear.
The FlhG ATPase has been implicated as a major mediator of numerical control of flagellar biosynthesis in polar flagellates. Mutation of flhG (referred to as fleN in Pseudomonas species) in P. aeruginosa, V. cholerae, V. alginolyticus, C. jejuni and S. putrefaciens causes an increase in the number of hyperflagellated cells (i.e., those that produce more than one flagellum at a pole) in the population (Dasgupta et al., 2000; Dasgupta and Ramphal, 2001; Correa et al., 2005; Kusumoto et al., 2006; Balaban and Hendrixson, 2011; Schuhmacher et al., 2015a). Spatial regulation of flagellation is preserved, with flagella restricted to poles in these hyperflagellated flhG mutants. In V. cholerae and P. aeruginosa, FlhG/FleN represses the activity or expression of a master transcriptional regulator, which is thought to restrict flagellar gene expression to a level that supports production of a single flagellum per cell (Dasgupta et al., 2000; Dasgupta and Ramphal, 2001; Correa et al., 2005; Baraquet and Harwood, 2013). C. jejuni lacks a master transcriptional regulator for flagellar gene expression, suggesting that C. jejuni FlhG (Cjj81176_0101 in the C. jejuni 81–176 genome) exerts numerical control of flagellation by an alternative mechanism. S. putrefaciens FlhG interacts with flagellar C ring proteins that form the rotor and switch complex at the cytoplasmic base of the flagellum, but it is unclear whether this potential in vivo interaction contributes to regulation of flagellar number (Schuhmacher et al., 2015a).
FlhG is a member of the MinD family of ATPases (Leipe et al., 2002). MinD is one component of the Min system, which functions in many bacteria to spatially regulate division so that the divisome forms at the midpoint for symmetrical division rather than at the poles (for reviews, see Rothfield et al., 2005; Lutkenhaus, 2007). We found that C. jejuni ΔflhG has a division defect similar to Escherichia coli and Bacillus subtilis minD mutants, exemplified by the production of minicells (Adler et al., 1967; Van Alstyne and Simon, 1971; Davie et al., 1984; de Boer et al., 1988; 1989; Levin et al., 1992; Varley and Stewart, 1992; Balaban and Hendrixson, 2011). Minicells are generated when the divisome inappropriately forms near a bacterial pole rather than at the cellular midpoint for subsequent symmetrical division. Due to lack of Min components encoded in the C. jejuni genome, we proposed that FlhG may dually function in numerically regulating flagellar biogenesis (consistent with other FlhG proteins) and preventing division at polar regions by a MinD-like activity. Furthermore, we found that flagellar proteins associated with the inner membrane and cytoplasm – the FliF MS ring protein, the FliM and FliN switch proteins of the C ring and the FlhF GTPase – are required with FlhG to prevent division at polar regions (Balaban and Hendrixson, 2011). These findings led us to propose that the amphitrichous flagellation pattern of C. jejuni creates polar flagella that assist FlhG to directly or indirectly inhibit division at each pole so that viable daughter cells are produced by proper symmetrical division.
In the genomes of many polar flagellates, flhG is often located next to flhF. FlhF generally functions as a positive factor for polar flagellar biogenesis. However, FlhF activities and the net effect of FlhF on flagellation phenotypes appear to be different among polar flagellates (Kazmierczak and Hendrixson, 2013). For example, Pseudomonas flhF mutants produce a single flagellum, but its location occurs at a non-polar site (Pandza et al., 2000; Murray and Kazmierczak, 2006). Furthermore, the GTPase activity of P. aeruginosa FlhF appears to affect flagellar rotation but not polar flagellar biogenesis (Schniederberend et al., 2013). In contrast, FlhF is required for WT levels of flagellar gene expression in V. cholerae, C. jejuni and H. pylori (Hendrixson and DiRita, 2003; Niehus et al., 2004; Correa et al., 2005; Balaban et al., 2009); ΔflhF mutants in these bacteria rarely produce flagella. However, the GTPase activity of C. jejuni FlhF (Cjj81176_0102 in the C. jejuni 81–176 genome) is not required for flagellar gene transcription. Instead, disrupting FlhF GTPase activity increased the frequency of hyperflagellated, laterally flagellated or aflagellated cells in the population (Balaban et al., 2009). More molecular evidence for a role of FlhF in spatial placement of flagella was found in V. cholerae where FlhF is required for polar localization of the FliF MS ring protein, which is one of the first structures formed in a nascent flagellum (Green et al., 2009).
Although FlhF and FlhG are absent in peritrichous E. coli and Salmonella species, B. subtilis is an example of a peritrichous bacterium that produces both proteins. B. subtilis FlhF and FlhG function together to spatially organize flagella in a defined grid-like pattern around the cellular midpoint, but not at polar regions (Guttenplan et al., 2013). Disruption of flhF causes flagella to be positioned more closely to poles; mutation of flhG causes aggregation of flagellar basal bodies and a lack of dispersion of flagella across the surface. FlhG does not appear to affect numerical regulation of flagellar biogenesis in B. subtilis. Instead, flagellar numbers are increased by preventing the degradation of the SwrA master transcriptional regulator for flagellar gene expression (Mukherjee et al., 2015). Structural and biochemical analyses showed that the N-terminal region of B. subtilis FlhG interacts with FlhF and is required to stimulate the GTPase activity of FlhF (Bange et al., 2007; 2011). Like S. putrefaciens, B. subtilis FlhG interacts with C ring proteins and is speculated to assist in forming the C ring in vivo (Schuhmacher et al., 2015a). However, it is unknown whether FlhG interactions with C ring proteins or FlhG stimulation of FlhF GTPase activity affects the spatial patterning of flagellation in B. subtilis.
We pursued a detailed analysis in C. jejuni to understand how FlhG facilitates numerical control of flagellar biosynthesis and whether any potential extrinsic factors may function with FlhG to mediate this regulation. Our analysis revealed that different FlhG regions and activities are required to regulate flagellar number in C. jejuni compared with other polar flagellates. Among our findings was the correlation between a region of FlhG that influences the GTPase activity of FlhF with flagellar number control. Together, our findings indicate that C. jejuni FlhG is at the center in a mechanism to influence a flagellar biosynthetic step, rather than a transcriptional process, to tightly control flagellar number. Our work shows that FlhG proteins have converged for precise numerical control of flagellation in different bacterial species despite employing diverse intrinsic domains and extrinsic proteins for this regulatory process.
Results
C. jejuni FlhG employs different domains relative to other FlhG proteins to numerically regulate flagellar biogenesis
A few studies from different bacterial species highlighted important regions of FlhG to mediate numerical control of flagellar biogenesis, but it was unknown whether C. jejuni FlhG would function similarly. MinD and FlhG belong to a common family of structurally similar ATPases, but the proteins exert different biological functions in bacteria (Leipe et al., 2002; Bange and Sinning, 2013; Kazmierczak and Hendrixson, 2013). From a structural perspective relative to MinD, FlhG proteins possess a similar ATPase domain, with key residues conserved for ATP binding and hydrolysis (Fig. 1). In addition, some FlhG proteins also possess an amphipathic helix at the C-terminus like MinD that is termed the ‘membrane targeting sequence’ (MTS; Szeto et al., 2002). This region of MinD interacts with membranes to assist MinD-MinC complexes in inhibiting division at poles (Szeto et al., 2002; Hu and Lutkenhaus, 2003; Zhou and Lutkenhaus, 2003). The C-terminus of S. putrefaciens, C. jejuni and H. pylori FlhG proteins are predicted to possess an amphipathic helix, and a weak amphipathic helix is predicted in V. cholerae FlhG (Fig. 1). This amphipathic helix appears to be missing from the C-terminus of P. aeruginosa FleN. Unlike MinD, most FlhG orthologs possess an additional N-terminal extension with a DQAxxLR motif containing a conserved glutamine (Q4 in C. jejuni FlhG; Fig. 1). The V. cholerae FlhG N-terminus shows variation in length across strains, but a potentially equivalent glutamine can be identified. However, this N-terminal extension is absent from P. aeruginosa FleN.
Fig. 1.

Domains of MinD and FlhG proteins across bacterial species. MinD and FlhG proteins contain a homologous ATPase domain (red box) with conserved lysine and aspartate residues that are involved in ATP binding and hydrolysis, respectively. E. coli MinD and B. subtilis FlhG also possess a C-terminal membrane-targeting sequence (MTS; black box) that contains an amphipathic helix to interact with the inner membrane in an ATP-dependent manner. The FlhG proteins from some bacterial species have a C-terminus predicted to form an amphipathic helix (gray box), which may function as an MTS. For many FlhG proteins, the ATPase domain is preceded by a short N-terminal region (blue box) that contains a conserved glutamine residue that has been implicated in some bacteria to stimulate the GTPase activity of FlhF.
In previous studies, some of these domains and motifs of FlhG proteins from other bacterial species were discovered to impact flagellation. The conserved glutamine at the N-terminus of B. subtilis FlhG is required to stimulate the in vitro GTPase activity of FlhF (Bange et al., 2011). In addition, the ATPase domain and the C-terminal MTS of B. subtilis FlhG are required for interactions with C ring proteins (Schuhmacher et al., 2015a). However, whether these FlhG domains and activities impact flagellar biogenesis in B. subtilis is unknown. Mutation of the ATPase and proposed C-terminal MTS in S. putrefaciens FlhG causes hyperflagellation, although a molecular mechanism for how these domains contribute to numerical regulation of flagellar biogenesis is unknown (Schuhmacher et al., 2015a). For P. aeruginosa FleN, ATP-binding is important for FleN to interact with the FleQ master transcriptional regulator and repress its activity so that flagellar gene transcription is maintained at a low level to ensure only one flagellum per cell is formed (Dasgupta and Ramphal, 2001; Baraquet and Harwood, 2013).
Based on these previous studies defining some requirements of FlhG for biological activity, we generated mutations within C. jejuni FlhG and characterized the mutants for defects in regulation of flagellar number. For analysis of the N-terminus, we removed residues 4–24 (FlhGΔ4–24) or changed the conserved glutamine residue to an alanine (FlhGQ4A). For the ATPase domain, we analyzed the previously constructed C. jejuni flhGD61A mutant (a predicted ATP hydrolysis mutant; Balaban and Hendrixson, 2011) and created C. jejuni flhGK37A that is predicted to have reduced ATP binding. Although we have yet to demonstrate an in vitro ATPase activity for FlhG, FlhGD61A causes a gross elongation of C. jejuni cell bodies (Balaban and Hendrixson, 2011). This elongation phenotype is similar to an E. coli minD mutant that has an alteration of the same conserved aspartate in the ATPase domain, which allows for ATP binding but not hydrolysis (Wu et al., 2011). Thus, we postulate that C. jejuni FlhG likely does have an in vivo ATPase activity. We also constructed many mutations within the putative C-terminal amphipathic helix that may serve as an MTS. We replaced each hydrophobic residue (F280, F281, I284, I285 or F288) at the hydrophobic face of the putative amphipathic helix with a charged glutamate residue. We also created a quadruple mutant (FlhG4E) that contained four glutamate mutations (F281E, I284E, I285E and F288E), which we speculated would have a more severe disruption of the hydrophobicity of the amphipathic helix.
Due to the inability to generate specific antiserum to detect native C. jejuni FlhG, we constructed plasmids for in trans constitutive expression of WT FlhG and mutant proteins with an N-terminal FLAG-tag from a chloramphenicol acetyltransferase (cat) promoter in C. jejuni ΔflhG. Complementation of ΔflhG with a plasmid expressing FLAG-WT FlhG restored proper regulation of flagellar number (Fig. S1). Most FLAG-tagged mutant FlhG proteins were produced at similar levels as FLAG-WT FlhG in whole-cell lysates of C. jejuni (Fig. 2; data not shown). However, FLAG-FlhGD61A was produced at reduced levels and FLAG-FlhGF280E was not detected and likely unstable (Fig. 2 and data not shown). FlhGF280E was not analyzed further in this study. We then replaced WT flhG at the native locus on the C. jejuni chromosome with flhG mutant alleles encoding untagged versions of the respective mutant proteins for functional analysis of regulation of flagellar number.
Fig. 2.

Compartmental localization of WT FlhG and FlhG mutant proteins in C. jejuni. C. jejuni ΔflhG was used to express FLAG WT-FlhG or FLAG-FlhG mutant proteins with the indicated mutations. Proteins from whole-cell lysates (WCL), cytoplasmic fractions, or inner membrane fractions were obtained for each strain and then analyzed by immunoblotting analysis with a rabbit α-FLAG antibody to detect FlhG proteins. Detection of RpoA and CmeB by specific antisera served as controls for fractionation procedures with RpoA present mainly in the cytoplasm and CmeB present both in the cytoplasm and inner membrane. Loading of samples for each protein was normalized to the density of each culture to ensure equal amounts of total protein were analyzed.
By electron microscopy analysis, approximately 90% of WT C. jejuni cells produced a single flagellum at one or both poles, which we classified as the WT amphitrichous phenotype (Fig. 3A and B). Only 1.5% of cells produced two or more flagella at a pole and 8.5% were aflagellated (Fig. 3A). Approximately 46% of the cells in the ΔflhG population were hyperflagellated with another 46% producing the correct number of flagella for the WT amphitrichous flagellation pattern, which is similar to our previous observations (Fig. 3A, C and D; Balaban and Hendrixson, 2011). C. jejuni producing FlhGK37A or FlhGD61A, which are predicted to lack ATP binding or hydrolysis respectively, displayed a similar population of cells with amphitrichous flagellation as WT C. jejuni (Fig. 3A, E and F). In contrast, mutation of either the N- or C-terminal regions of FlhG affected regulation of flagellar number. Deletion of the N-terminus of FlhG (FlhGΔ4–24) or alteration of the conserved glutamine (FlhGQ4A) within this domain resulted in a hyperflagellated population of cells similar to C. jejuni ΔflhG (Fig. 3A, G and H). C. jejuni FlhG C-terminal mutants displayed a greater hyperflagellated population relative to WT C. jejuni but at a lower level than the FlhG N-terminal mutants. Production of FlhG4E, which altered all four hydrophobic residues (F281E, I284E, I285E and F288E) within the amphipathic helix, caused an increase in the percentage of cells producing two or more flagella at a pole from 1.5% to 17.8% (Fig. 3A). This phenotype could be replicated with a single alteration of the hydrophobic residues I285 or F288 to a glutamate, but not by altering only F281 or I284 (Fig. 3A). The finding that the N-and C-termini of C. jejuni FlhG, but not the ATPase domain, is required for numerical regulation of flagellar biogenesis demonstrates that C. jejuni FlhG utilizes a different collection of domains to regulate flagellar number compared with other characterized FlhG proteins of polar flagellates.
Fig. 3.
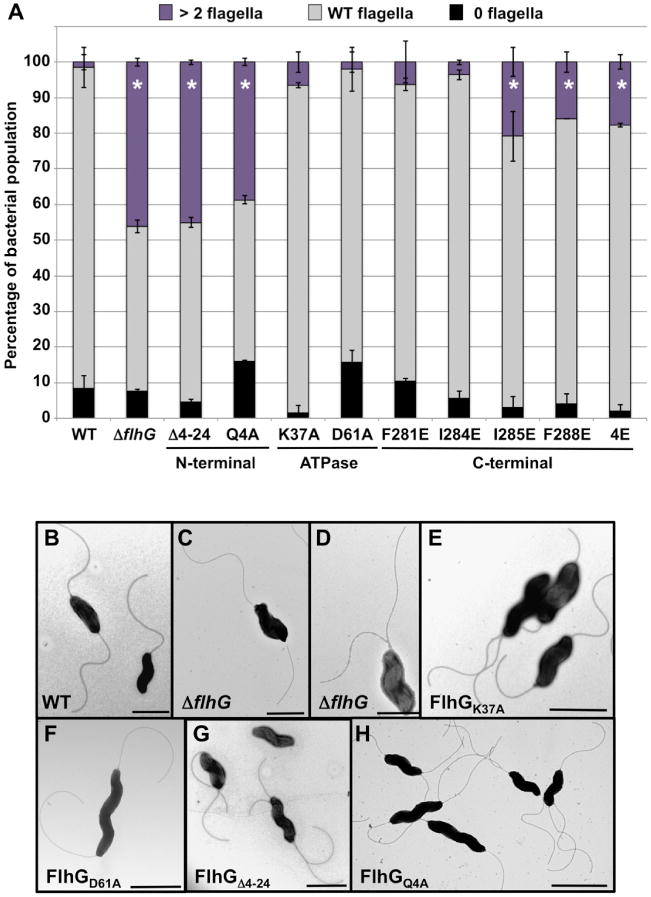
Analysis of domains of C. jejuni FlhG required for flagellar number control.
A. Quantification of flagellar numbers in WT C. jejuni and flhG mutants. Individual bacteria were analyzed for the number of flagella produced at each pole. Data are reported as the percentage of bacterial populations with the following numerical patterns: > 2 flagella (hyperflagellated), a bacterium producing two or more flagella at least at one pole (purple); WT flagella, producing a single flagellum at both poles or a flagellum at one pole with the other pole aflagellated (gray); and 0 flagella, aflagellated bacteria (black). Data represent the average of two experiments in which at least 100 individual cells for each strain were examined per an experiment. Bars represent standard deviations. Asterisks (*) indicate flhG mutants with a statistically significant higher population of bacteria producing > 2 flagella (hyperflagellated) at a pole relative to the WT strain (P < 0.05).
B–H. Electron micrographs demonstrating flagellar number phenotypes of WT C. jejuni and isogenic flhG mutants. (B) WT C. jejuni, (C and D) ΔflhG, (E) flhGK37A, (F) flhGD61A, (G) flhGΔ4–24, and (H) flhGQ4A. For (B–H), bars = 1 μm.
C. jejuni FlhG localizes with both the cytoplasm and the inner membrane
For MinD and B. subtilis FlhG, ATP-binding and the MTS are required for dimerization and interaction with lipids for transient localization to the inner membrane (Hu and Lutkenhaus, 2001; 2003; Hu et al., 2002; Lackner et al., 2003; Schuhmacher et al., 2015a). To begin to understand how the N-terminus and C-terminus may contribute to the ability of FlhG to numerical regulate flagellar biogenesis, we analyzed the intracellular location of WT and mutant FlhG proteins in C. jejuni. For this analysis, we isolated cytoplasmic and inner membrane proteins from C. jejuni ΔflhG expressing FLAG-tagged WT and mutant versions of FlhG. We observed FLAG-WT FlhG associated with both the cytoplasm and inner membrane (Fig. 2). Similarly, the N- or C-terminal FlhG mutants that resulted in significant hyperflagellation phenotypes were found to associate with the inner membrane at similar levels as WT FlhG. The only exception was FlhG4E, which has an alteration of four hydrophobic residues of the C-terminal putative amphipathic helix. This mutant protein appeared less abundantly associated with the C. jejuni inner membrane, indicating that the C-terminus of FlhG may contain an amphipathic helix to serve as an MTS for some degree of membrane association. However, FlhGI285E, FlhGF288E and FlhG4E all produced a similar level of hyperflagellated cells in a population (Fig. 3A). Because FlhGI285E and FlhGF288E did not show a similar reduction in inner membrane localization as FlhG4E, we conclude that the C-terminus of FlhG may serve another function besides as a MTS to assist in proper regulation of flagellar number.
As controls for our fractionation analysis, we detected the membrane protein CmeB in both the cytoplasm (presumably as a nascently translated protein) and the inner membrane. We detected the RNA polymerase α subunit (RpoA) primarily in the cytoplasm with a very minor amount in the inner membrane. In previous studies in which we analyzed the localization of various inner membrane and cytoplasmic proteins in different C. jejuni mutant strains, we consistently observed RpoA only associated with the cytoplasm (Bingham-Ramos and Hendrixson, 2008; Balaban et al., 2009). In these studies, all C. jejuni strains contain WT FlhG and thus produce few minicells. In the fractionation analysis performed in this study, all C. jejuni strains are in the ΔflhG background, which has a severe minicell phenotype. Upon expression of WT FlhG in ΔflhG, the division defect is reduced, but minicells are still modestly evident (Balaban and Hendrixson, 2011). We suspect that the minor amount of RpoA associated with the inner membrane preparations is due to the resultant higher than normal minicells produced by the C. jejuni ΔflhG strains complemented with different FLAG-tagged WT or FlhG mutant proteins that are difficult to completely remove via centrifugation during our fractionation procedures. Keeping this caveat in mind, we interpret our observations to suggest that FlhG can associate with the inner membrane of C. jejuni and also has a presence in the cytoplasm, which is consistent with the localizations of MinD and other FlhG proteins.
In additional analysis, we observed that FlhGK37A, which alters a residue predicted to be involved in ATP binding and is not defective for regulation of flagellar number, was localized to the inner membrane at WT levels (Fig. 2). This finding is in contrast to MinD and B. subtilis FlhG, which require ATP-binding to associate with the inner membrane. Interpretation of the localization FlhGD61A, which is predicted to be deficient in ATP hydrolysis, is less clear. This protein showed less localization to the inner membrane than WT FlhG, but FlhGD61A is also produced at lower levels (Fig. 2). Our previous work demonstrated that C. jejuni FlhGD61A has enhanced division inhibition and elongated cell phenotype, similar to an equivalent aspartate mutation in E. coli MinD that is locked into an ATP-bound state, hydrolysis deficient and able to interact with membranes (Balaban and Hendrixson, 2011; Wu et al., 2011). Thus, like the MinD mutant, we suspect that lowering the ATP hydrolysis activity of C. jejuni FlhG does not impair membrane localization.
Influence of C. jejuni FlhG mutations on polar localization
In our previous work, we noted that FlhG-GFP is polarly localized in C. jejuni when overexpressed from a plasmid (Balaban and Hendrixson, 2011). For better assessment, we aimed to analyze FlhG polar localization under more native expression conditions and without the use of GFP chimeras. Because FLAG-WT FlhG restored proper numerical regulation of flagellar biogenesis to C. jejuni ΔflhG (Fig. S1), we developed an immunofluorescent microscopy assay to analyze the spatial distribution of N-terminally FLAG-tagged WT FlhG and FlhG mutants using an antibody against the FLAG tag epitope in C. jejuni ΔflhG. Upon assessment of the distribution of FLAG-WT FlhG, we classified FlhG localization into one of five possible categories (as shown in Fig. S2): polar (strict localization to one or both poles); diffuse (general localization throughout the cell body with no specific punctum); non-polar (a single punctum not localized to either pole); multiple (two or more puncta with at least one punctum not polarly localized); and other (including cells with mixed staining such as diffuse and one or more puncta).
We observed that WT FlhG was strictly polarly localized in approximately 70% of cells, with alternative localization including diffuse, non-polar punctae or multiple punctae ranging from 6% to 15% in the remaining population (Table 1). In general, mutation of the N-terminus (either by deleting most of the region or by creating the Q4A mutation) or the C-terminus significantly altered the pattern of FlhG distribution relative to WT FlhG. Specifically, we observed that the percentage of cells with exclusive polar localization of FlhG was decreased by 30–40%, and the percentage of cells with multiple puncta of FlhG was increased three- to four-fold. The exception was FlhGF288E, which showed an increase only in multiple punctae relative to WT FlhG without a significant decrease in polar localization, but the overall pattern of FlhG distribution was still significantly altered (Table 1). These data possibly suggest that both the N-terminal and C-terminal regions of FlhG assist in spatial organization of the protein, which may influence the ability of FlhG to regulate flagellar number at poles in C. jejuni.
Table 1.
Spatial localization of WT FlhG and FlhG mutants in C. jejuni.
| Spatial | Percentage of the bacterial population expressing:a | |||||
|---|---|---|---|---|---|---|
|
|
||||||
| Pattern | WT FlhG | FlhGΔ4–24* | FlhGQ4A* | FlhGI285E* | FlhGF288E* | FlhG4E* |
| Polarb | 70.7 ± 5.0 | 49.3 ± 0.6# | 37.7 ± 5.7# | 45.3 ± 1.5# | 62.6 ± 8.0 | 42.0 ± 7.0# |
| Diffusec | 15.0 ± 5.0 | 12.0 ± 7.8 | 6.7 ± 2.1# | 19.3 ± 8.7 | 8.0 ± 6.6 | 20.0 ± 1.7 |
| Non-polard | 6.7 ± 2.3 | 8.3 ± 3.5 | 6.6 ± 0.2# | 6.7 ± 2.1 | 9.0 ± 4.4 | 12.0 ± 6.2 |
| Multiplee | 6.3 ± 1.5 | 25.0 ± 6.0# | 39.7 ± 14.0# | 26.3 ± 9.5# | 19.0 ± 2.7# | 24.3 ± 4.0# |
| Otherf | 1.0 ± 1.7 | 1.7 ± 2.1 | 0.3 ± 0.6 | 2.3 ± 1.1 | 1.3 ± 1.5 | 1.7 ± 2.9 |
FlhG proteins were fused to a N-terminal FLAG tag and expressed from a plasmid for detection by immunofluorescence with a monoclonal FLAG-tag antibody in C. jejuni ΔflhG. At least 100 cells from three independent samples were analyzed and averaged with indicated standard deviations.
Defined by strict localization to one or both poles.
Localization throughout the cell without puncta.
One punctum not localized to a cell pole.
Two or more puncta with at least one punctum not at a pole.
Displaying a pattern of localization not defined by other patterns (e.g., both diffuse and polar).
Asterisk indicates statistically significant alteration of pattern of localization of FLAG-tagged FlhG mutant proteins relative to FLAG-WT FlhG as calculated by χ2 analysis with four degrees of freedom. The χ2 value for each mutant protein are: 439.2 (FlhGΔ4–24), 1143.0 (FlhGQ4A), 414.2 (FlhGI285E), 230.8 (FlhGF288E) and 171.2 (FlhG4E). These values correlated with a P < 0.01.
Individual pattern of localization of the mutant FlhG protein was significantly reduced or increased compared with the respective pattern of localization of WT FlhG as determined by the individual χ2 component exceeding the χ2 critical value (9.488) for significance at P < 0.05.
C. jejuni FlhG does not regulate flagellar number by controlling flagellar gene transcription or protein production
In V. cholerae and P. aeruginosa, FlhG and FleN numerically control polar flagellar biogenesis indirectly by reducing the expression or activity of the FlrA/FleQ master transcriptional regulator. FlhG/FleN-dependent repression of these regulators that are atop the respective transcriptional hierarchies for flagellar genes is thought to ensure a low level of transcription of all flagellar genes that supports only a single flagellum per cell (Dasgupta et al., 2000; Dasgupta and Ramphal, 2001; Jyot et al., 2002; Correa et al., 2005; Baraquet and Harwood, 2013).
Campylobacter jejuni lacks a similar master transcriptional regulator for flagellar gene transcription. To rule out the possibility that FlhG targets transcription of flagellar genes as a means to regulate flagellar number in C. jejuni, we compared the level of expression of different flagellar genes in WT C. jejuni and the ΔflhG mutant. By semi-quantitative real-time RT-PCR (qRT-PCR), we did not detect any significant changes in expression of genes encoding flagellar type III secretion system (T3SS) proteins (flhA, flhB, fliP, fliQ or fliR), the MS ring protein (fliF) or C ring proteins (e.g., fliM) that surround the T3SS to form a functional secretion system for flagellar protein export (Fig. 4). Furthermore, expression of σ54 (rpoN) or the flagellar two-component regulatory system (flgS and flgR), which with the T3SS and FliF, are required for expression of most flagellar rod and hook genes was not increased (Hendrixson and DiRita, 2003; Joslin and Hendrixson, 2008; 2009; Boll and Hendrixson, 2011; 2013). Expression of the flgFG operon, flgD or flaA (encoding distal flagellar rod proteins, hook-associated proteins or the major flagellin, respectively) was mildly increased but only the fourfold increase in expression of the flgD met statistical significance (Fig. 4). In addition, expression of flhF, encoding the GTPase that positively influences flagellar biogenesis and σ54-dependent flagellar gene expression, was not enhanced in ΔflhG (Fig. 4; (Hendrixson and DiRita, 2003; Balaban et al., 2009).
Fig. 4.

Flagellar gene expression in WT C. jejuni and C. jejuni ΔflhG. Semi-quantitative real time RT-PCR (qRT-PCR) analysis of expression of a broad range of flagellar genes in WT C. jejuni 81–176 SmR (gray) and an isogenic ΔflhG mutant (black). Expression of each gene in ΔflhG is relative to the expression in WT C. jejuni, which was set to 1. All strains were examined in triplicate and the error bars indicate the standard error. Statistically significant differences in gene expression between WT C. jejuni and ΔflhG are indicated (* P-value < 0.05).
The levels of protein encoded by the genes within operons with enhanced expression in ΔflhG were compared with those of WT C. jejuni, with RpoA serving as an endogenous control. The only protein with a significant increase in levels in ΔflhG relative to WT C. jejuni was the FlgD hook cap protein with a 3.7-fold increase (Table 2). FlgD is required to trap FlgE hook proteins as they are secreted so that the hook polymerizes on the tip of the rod (Ohnishi et al., 1994; Moriya et al., 2011). FlgD, other hook proteins, flagellins and other filament proteins require the flagellar T3SS, MS ring, C ring and rod proteins for secretion, but those genes or proteins were not expressed at greater levels in ΔflhG. Therefore, we conclude that C. jejuni FlhG does not globally repress transcription of flagellar genes as a mechanism to regulate flagellar number, unlike FlhG/FleN proteins in other polar flagellates.
Table 2.
Protein levels of select flagellar regulatory, rod, hook or flagellin proteins in WT C. jejuni and C. jejuni ΔflhG.
| Protein | Relative level of proteina | Average ratio of protein | |
|---|---|---|---|
|
|
|
||
| WT | ΔflhG | ΔflhG/WTb | |
| FlgS | 0.87 ± 0.21 | 1.17 ± 0.30 | 1.34 |
| FlhF | 0.71 ± 0.20 | 0.37 ± 0.05 | 0.52 |
| FlgG | 0.74 ± 0.20 | 0.48 ± 0.12 | 0.65 |
| FlgD | 0.40 ± 0.18 | 1.49 ± 0.09* | 3.74 |
| FlaA | 0.22 ± 0.09 | 0.05 ± 0.02 | 0.21 |
Relative level of protein compared with RpoA in lysates of WT C. jejuni or ΔflhG as determined by quantitative immunoblot analysis. Each protein was analyzed in three independent samples. Levels were averaged and presented with standard deviations.
Asterisk indicates statistically significant differences in amount of a protein compared with WT C. jejuni (P < 0.05).
Ratio of protein levels in ΔflhG compared with WT C. jejuni.
A correlation between FlhG-mediated stimulation of FlhF GTPase activity and flagellar number control
As a GTPase, FlhF has been postulated to toggle between active and inactive states similar to monomeric G proteins and GTPases activated by dimerization (GADs) (Bourne et al., 1990; Gasper et al., 2009; Bange and Sinning, 2013). If this switching of molecular activities occurs with FlhF in GTP- and GDP-bound states in C. jejuni, it is possible that FlhF•GTP is in an active (or ‘ON’ state) state that positively affects flagellar biogenesis or placement, whereas FlhF•GDP is in a non-functional, inactive (or ‘OFF’) state with opposing effects on flagellation, or vice versa. In B. subtilis, FlhG stimulates FlhF GTP hydrolysis in vitro and the conserved Q8 residue at the N-terminus is required for this stimulation (Bange et al., 2011), suggesting that FlhG may assist in switching FlhF from active to inactive states. However, it is unclear what consequences this activity has on flagellar biogenesis in B. subtilis.
Whereas C. jejuni FlhF is required for WT levels of expression of flagellar genes, its GTPase activity is not (Hendrixson and DiRita, 2003; Balaban et al., 2009). We previously observed heterogeneity in the flagellation patterns and numbers of C. jejuni producing FlhFR324A, which alters a residue that is required for GTP hydrolysis, but not binding in other GTPases. About a third of the population of C. jejuni flhFR324A cells are aflagellated (Balaban et al., 2009). In comparing only the flagellated populations, seven-fold more C. jejuni flhFR324A cells are hyperflagellated compared with WT C. jejuni (Table 3). In addition, more C. jejuni flhFR324A cells produce lateral flagella. These observations suggest that some aspect of nucleotide binding and/or GTP hydrolysis by FlhF influences flagellar number and polar placement (Table 3).
Table 3.
Flagellar numbers of the flagellated cell populations of WT C. jejuni and C. jejuni flhFR324A.
| Strain | Percentage of the flagellated populationa | ||
|---|---|---|---|
|
| |||
| WT flagellationb | ≥ 2 flagella at a polec | Lateral flagellad | |
| WT C. jejuni | 99.1 ± 1.2 | 1.4 ± 0.5 | 0.5 ± 0.7 |
| flhFR324A | 63.1 ± 2.9* | 9.8 ± 0.5* | 27.1 ± 3.4* |
At least 70 individual cells from two independent samples of C. jejuni strains were counted, averaged and presented with standard deviations.
Asterisk indicates statistically significant differences compared with WT C. jejuni (P < 0.05).
Bacteria producing a single flagellum at both poles or a flagellum at one pole and the other aflagellated.
Bacteria with two or more flagella at least at one pole.
Bacteria with at least one flagellum at a non-polar location.
Like C. jejuni ΔflhG, the flhGΔ4–24, flhGQ4A and flhFR324A mutants produced a significant hyperflagellation population (Fig. 3A and Table 3). Both FlhGΔ4–24 and FlhGQ4A lack or alter Q4, which is equivalent to the active residue of B. subtilis FlhG that is required to stimulate FlhF GTPase activity. We reasoned that hyperflagellation in these flhG mutants could be due to a deficiency in stimulation of FlhF GTPase activity by the mutant FlhG proteins. Similarly, WT FlhG may ineffectively stimulate the GTPase activity of FlhFR324A in C. jejuni to result in a longer GTP-bound state that results in a hyperflagellated population.
To provide evidence for this possibility, we analyzed whether C. jejuni FlhG can stimulate the GTPase activity of C. jejuni FlhF in vitro. By high-pressure liquid chromatography (HPLC), we observed recombinant WT FlhF or FlhFR324A alone exhibited weak GTP hydrolysis (3.6 to 4.1 nmol/h; Fig. 5). Addition of WT FlhG stimulated the GTPase activity of WT FlhF by 450%, but only increased the GTPase activity of FlhFR324A by 50% (Fig. 5). These data may indicate that FlhFR324A in vivo exists temporally longer in a GTP-bound state without stimulation by WT FlhG to cause an alteration in flagellar number and localization. Importantly, the ability of FlhG to stimulate the GTPase activity of WT FlhF was dependent on the conserved Q4 residue. Addition of FlhGQ4A to WT FlhF or FlhFR324A only increased GTP hydrolysis by 50% (Fig. 5). These data are consistent with the hypothesis that FlhF GTP hydrolysis and its nucleotide-bound state may be controlled by FlhG in C. jejuni. As such, FlhF may reside longer in a GTP-bound, active state in C. jejuni mutants lacking FlhG or producing FlhGQ4A to affect a step in flagellar biogenesis to result in hyperflagellation.
Fig. 5.

In vitro stimulation of the GTPase activity of WT and mutant C. jejuni FlhF proteins by FlhG. Recombinant WT or mutant FlhF or FlhG proteins were mixed at a final concentration of 50 μM and incubated for 1 h. Total GTP hydrolysis is shown as a mean value with standard deviation of three independent measurements. Recombinant FlhF (WT FlhF or FlhFR324A) and FlhG (WT FlhG or FlhGQ4A) proteins were used in the reactions as indicated.
Discussion
Bacterial flagella promote swimming motility and are similar in structure across bacterial species. However, how these organelles are presented on the surface vary among motile bacteria. Polar flagella are excellent specimens for analysis to understand how bacteria spatially and numerically regulate biogenesis of macromolecular complexes in the cell. FlhG proteins clearly are major determinants in the numerical regulation of flagellar biogenesis. Although molecular mechanisms for how FlhG regulates flagellar number is lacking for many polar flagellates, cumulative observations from existing studies suggest variations in how numerical regulation of flagellar biogenesis is achieved among these bacteria. Our work has revealed a diversity of requirements for FlhG to regulate flagellar number in C. jejuni relative to other polar flagellates. Considering the FlhG proteins investigated to date, it appears that these proteins have evolved within each species to employ a diverse set of intrinsic features and extrinsic proteins to facilitate a common output – tight numerical control of polar flagellar biogenesis that is ideal for each species to promote motility. B. subtilis has adapted FlhG to assist in spatial patterning of flagellation rather than numerical control of flagellar biogenesis (Guttenplan et al., 2013), further demonstrating how an FlhG protein has evolved to influence peritrichous flagellation.
For V. cholerae and P. aeruginosa, FlhG/FleN regulates flagellar number by controlling the expression or activity of a master transcriptional regulator (Dasgupta et al., 2000; Dasgupta and Ramphal, 2001; Correa et al., 2005; Baraquet and Harwood, 2013). With P. aeruginosa FleN, the reduction in activity of the master transcriptional regulator is dependent upon ATP and the ATPase domain of FleN (Dasgupta and Ramphal, 2001; Baraquet and Harwood, 2013). P. aeruginosa FleN lacks the N-terminal extension common to many FlhG proteins and the C-terminal amphipathic helix that functions as an MTS in MinD and B. subtilis FlhG. In this work, we did not find that the C. jejuni FlhG ATPase domain was involved in numerical regulation of flagellar biogenesis. Instead, we found that the N- and C-terminal regions of C. jejuni FlhG are involved in controlling flagellar number. In addition, we found no evidence that FlhG influences the level of transcription of a wide range of flagellar genes or production of respective proteins. Thus, C. jejuni FlhG not only utilizes different domains to regulate flagellar number but likely influences flagellar number independently of affecting the level of transcription of flagellar genes.
By comparing the hyperflagellation phenotypes of some C. jejuni flhG mutants and the FlhF GTPase mutant (flhFR324A) with in vitro biochemical analysis of FlhF GTPase activity, we acquired evidence that flagellar number in C. jejuni may be controlled via FlhG influencing the transition of FlhF between GTP- and GDP-bound states. Mutations in the C. jejuni FlhG N-terminus demonstrated the most dramatic defects in numerical regulation of polar flagellar biogenesis. The degree of hyperflagellation in these mutants was comparable with C. jejuni ΔflhG. The N-terminal domain – specifically the Q4 residue – was essential for the in vitro stimulation of the GTPase activity of FlhF. If FlhG stimulates FlhF GTP hydrolysis in C. jejuni, then FlhG could influence a switch in FlhF activity from a GTP-bound, active form to a GDP-bound, inactive form that impacts a step in flagellation. In a C. jejuni mutant without FlhG or producing FlhGQ4A, FlhF may remain temporally longer in an active FlhF•GTP state. Similarly, FlhFR324A has a defective GTPase activity that is not stimulated by WT FlhG and may also remain temporally longer in a GTP-bound, active state in this C. jejuni mutant. Either of these situations could cause FlhF to repeatedly promote a step in flagellar biogenesis that would produce multiple flagella at a single pole.
Currently, the exact roles of FlhF and its GTPase activity in flagellation are unknown in large part due to difficulty in creating FlhF mutants that can be assured to exist exclusively in GTP-bound or GDP-bound states in bacteria and then correlating each state to a specific effect on flagellation. However, considering that altering the GTPase activity of FlhF caused a significant proportion of the cells to mislocalize flagellar biogenesis or increase flagellar number (Balaban et al., 2009), we suspect that FlhF functions at an early stage of flagellation in C. jejuni. One such stage could be the formation of the flagellar secretory system, which is required for secretion of most structural proteins that compose a flagellum. The flagellar secretory system is an attractive candidate to target for regulation as factors that could affect its positioning or the number of secretory systems formed would likely alter the spatial and numerical presentation of flagella in polar flagellates. There is precedence for FlhF influencing an early flagellar biogenesis step in V. cholerae. In this bacterium, FlhF influences the polar localization of FliF, which forms the MS ring surrounding the T3SS to create a functional secretory system (Green et al., 2009).
Although a number of molecular details undoubtedly remain to be elucidated, we can begin to present a possible model for how FlhF and FlhG may function together to properly produce a single flagellum at a pole in C. jejuni. After division of an amphitrichously flagellated C. jejuni cell, a daughter cell lacks a flagellum at the new pole. We suspect that FlhF may be able to localize to the new pole by an unknown mechanism. If FlhF is in a GTP-bound, active form, it could facilitate a process required to generate a single flagellum at the new pole. Subsequent FlhG localization to this pole, either by an intrinsic ability to localize to the inner membrane of the new pole or via other proteins, could trigger nucleotide hydrolysis by FlhF. This stimulation would convert FlhF to a GDP-bound, inactive state that is no longer able to support synthesis of a second flagellum at the pole. Further in vivo analysis of the temporal interactions between FlhF and FlhG in relation to flagellar biogenesis and delineation of the exact role of C. jejuni FlhF in flagellation will assist in providing a deeper understanding of how numerical regulation of flagellar biogenesis is achieved in C. jejuni.
We also provided strong evidence that C. jejuni FlhG localizes to poles and can associate with the inner membrane, which are features common to MinD and some other FlhG proteins. However, it is less clear if the C-terminal amphipathic helix serves as a bona fide MTS. Indeed, FlhG4E, which has an alteration of four hydrophobic residues in the putative C-terminal amphipathic helix that serves as an MTS in MinD and B. subtilis FlhG showed a reduction in association with the inner membrane. However, C jejuni flhG4E produced a similar level of hyperflagellated cells as C. jejuni producing FlhGI285E and FlhGF288E that only contained an alteration of one of the hydrophobic residues and localized to the inner membrane at similar levels as WT FlhG. Thus, it appears that the FlhG C-terminus likely influences flagellar number due to an effect that is independent of any membrane interactions that may be facilitated by this region of the protein. Additionally, all the FlhG mutants that caused defects in regulating flagellar number also tended to appear as multiple punctae in C. jejuni, rather than showing more strict polar localization that was observed with WT FlhG. Currently, it is unclear whether polar or membrane localization is required for FlhG to regulate flagellar number and if FlhG can mediate both types of localization alone or requires other proteins. We suspect that accurate targeting of FlhG to at least polar regions contributes to regulation of flagellar number, perhaps by positioning FlhG near FlhF so that it can impact the activity of FlhF on flagellation by controlling its nucleotide-bound state.
The MinD and FlhG ATPases are a fascinating group of proteins that mediate different processes in bacteria. Although some FlhG proteins and MinD share MTS and ATPase domains, recent analyses indicate that other regions of the proteins have diversified to interact with different effector partners to mediate outputs. Whereas MinD interacts with MinC and MinE to spatially regulate division in E. coli, B. subtilis and S. putrefaciens FlhG uses a similar region to interact with flagellar C ring proteins, which likely impacts flagellar biogenesis in these organisms (Guttenplan et al., 2013; Schuhmacher et al., 2015a). Furthermore, our previous findings suggest that FlhG functions with some C ring proteins (FliM and FliN) to prevent division at polar regions in C. jejuni, but it is unknown whether C. jejuni FlhG interacts with these proteins directly (Balaban and Hendrixson, 2011). We propose that FlhG proteins of polar flagellates have evolved and diversified intrinsic domains in each species to interact with different extrinsic effectors to mediate varied strategies for a common output – exquisite species-specific flagellar number control for polar flagellation.
Experimental procedures
Bacterial strains and plasmids
All strains and plasmids used in this study are listed in Tables 4 and 5. C. jejuni strain 81–176 was originally isolated from a patient with gastroenteritis (Korlath et al., 1985). C. jejuni was typically grown in microaerobic conditions (85% N2, 10% CO2, 5% O2) on Mueller Hinton (MH) agar at 37°C. As required, antibiotics were added to the MH media at the following concentrations: 10 μgml−1 trimethoprim (TMP), 15 μgml−1 chloramphenicol, 50 μg ml−1 kanamycin, 0.5, 1, 2 or 5 mg ml−1 streptomycin. All C. jejuni strains were stored at −80°C in a solution of 85% MH broth and 15% glycerol. For routine growth to perform most experiments, C. jejuni strains were grown from frozen stocks for 48 h in microaerobic conditions at 37°C, then restreaked onto MH agar and grown for an additional 16 h in identical conditions. E. coli DH5α, XL1-Blue and BL21 strains were grown on Luria-Bertani (LB) agar or in LB broth containing the following concentrations of antibiotics when appropriate: 100 μg ml−1 ampicillin, 50 μg ml−1 kanamycin or 15 μg ml−1 chloramphenicol. All E. coli strains were stored at −80°C in a solution of 80% LB broth and 20% glycerol.
Table 4.
Bacterial strains used in this study.
| Strain | Genotype | Source/reference |
|---|---|---|
| DH5α | E. coli supE44 ΔlacU169 (φ80lacZDM15) hsdR17 recA1 endA1 gyrA96 thi-1 relA1 | Invitrogen |
| DH5α/RK212.1 | DH5α with conjugation transfer element | Figurski and Helinski (1979) |
| BL21 (DE3) | E. coli fhuA2 [lon] ompTgal (λ DE3) [dcm] ΔhsdS λ DE3 = λ sBamHIo ΔEcoRI-B int∷(lacI∷PlacUV5∷T7 gene1) i21 Δnin5 | New England Biolabs |
| DRH212 | 81–176 rpsLSm | Hendrixson et al. (2001) |
| SMS368 | DRH212 flhG∷cat-rpsL | Balaban et al. (2009) |
| SMS370 | DRH212 flhG∷cat-rpsL | Balaban et al. (2009) |
| MB361 | DRH212 flhGK37A | This study |
| MB628 | DRH212 flhFR324A | Balaban et al. (2009) |
| MB770 | DRH212 ΔflhG | Balaban et al. (2009) |
| MB1040 | DRH212 flhGD61A | Balaban et al. (2009) |
| CRG348 | MB770 with pDAR964 | This study |
| CRG354 | MB770 with pCRG309 | This study |
| CRG374 | MB770 with pCRG315 | This study |
| CRG377 | MB770 with pCRG329 | This study |
| CRG402 | MB770 with pCRG380 | This study |
| CRG479 | DRH212 with pDAR964 | This study |
| CRG507 | MB770 with pCRG306 | This study |
| CRG513 | MB770 with pCRG320 | This study |
| CRG525 | DRH212 flhGΔ4–24 | This study |
| CRG526 | DRH212 flhGQ4A | This study |
| CRG527 | DRH212 flhGI284E | This study |
| CRG568 | DRH212 flhGF281E | This study |
| CRG571 | DRH212 flhGI285E | This study |
| CRG836 | MB770 with pCRG746 | This study |
| CRG1042 | MB770 with pCRG1024 | This study |
| CRG1165 | DRH212 flhG4E | This study |
| CRG1324 | MB770 with pCRG746 | This study |
| CRG1343 | DRH212 flhGF288E | This study |
Table 5.
Plasmids used in this study.
| Plasmid | Genotype/description | Source/reference |
|---|---|---|
| pUC19 | AmpR | New England Biolabs |
| pET24d | AmpR | Novagen |
| pGEX-4T-2 | AmpR | GE Healthcare |
| pQE30 | AmpR | Qiagen |
| pRY108 | KanR; E. coli-C. jejuni shuttle vector | Yao et al. (1993) |
| pRY109 | pUC19∷cat | Yao et al. (1993) |
| pRY112 | CatR; E. coli-C. jejuni shuttle vector | Yao et al. (1993) |
| pDAR964 | CatR; pRY112 with cat promoter, with in frame N-terminal FLAG sequence | This study |
| pDAR1627 | KanR; pRY108 with cat promoter, with in frame N-terminal FLAG sequence | This study |
| pDRH2270 | pQE30∷flhF | Balaban et al. (2009) |
| pDRH4503 | pET24d∷6XHisflhGQ4A | This study |
| pCRG1317 | pET24d∷6XHisflhG | This study |
| pSMS248 | pUC19∷flhG | Balaban and Hendrixson (2011) |
| pMB169 | pSMS248 with a point mutation to generate flhGK37A | This study |
| pMB681 | pQE30∷flhFR324A | Balaban et al. (2009) |
| pCRG131 | pSMS248 with a point mutation to generate flhGF281E | This study |
| pCRG132 | pSMS248 with a point mutation to generate flhGI284E | This study |
| pCRG133 | pSMS248 with a point mutation to generate flhGI285E | This study |
| pCRG134 | pSMS248 with a point mutation to generate flhGF288E | This study |
| pCRG143 | pSMS248 with a point mutation to generate flhGΔN4-24 | This study |
| pCRG306 | pDAR964 with the coding sequence of flhGI285E from the second codon to the stop codon cloned into the BamHI site | This study |
| pCRG309 | pDAR964 with the coding sequence of flhGΔ4–24 from the second codon to the stop codon cloned into the BamHI site | This study |
| pCRG315 | pDAR964 with the coding sequence of flhGD61A from the second codon to the stop codon cloned into the BamHI site | This study |
| pCRG320 | pDAR964 with the coding sequence of flhGF288E from the second codon to the stop codon cloned into the BamHI site | This study |
| pCRG329 | pDAR964 with the coding sequence of flhGK37A from the second codon to the stop codon cloned into the BamHI site | This study |
| pCRG370 | pSMS248 with a point mutation to generate flhGQ4A | This study |
| pCRG380 | pDARH964 with the coding sequence flhGQ4A from the second codon to the stop codon cloned into the BamHI site | This study |
| pCRG563 | pET24d with the coding sequence of flhGQ4A from the second codon to the stop codon cloned into the BamHI and NcoI sites to generate 6XHis-FlhGQ4A | This study |
| pCRG746 | pDAR964 with the coding sequence of flhG from the second codon to the stop codon cloned into the BamHI site | This study |
| pCRG1024 | pDAR1627 with the coding sequence of flhG4E from the start to penultimate codons cloned into the BamHI site | This study |
| pCRG1131 | pSMS248 with a point mutation to generate flhG4E | This study |
Construction of C. jejuni mutants
Campylobacter jejuni mutants were constructed by electroporation following previously described methods (Hendrixson et al., 2001). PCR-mediated mutagenesis was used to introduce point mutations or small domain deletions in the coding sequence of flhG in pSMS248 to result in the following plasmids containing the respective mutations: pMB169 (flhGK37A), pCRG131 (flhGF281E), pCRG132 (flhGI284E), pCRG133 (flhGI285E), pCRG134 (flhGF288E), pCRG143 (flhGΔ4–24), pCRG370 (flhGQ4A) and pCRG1131 (flhG4E). These plasmids were electroporated into SMS368 or SMS370 (81–176 SmR flhG∷cat-rpsL) to replace flhG∷cat-rpsL on the chromosome with genes encoding the mutant proteins. Transformants were recovered on MH agar containing 0.5, 1, 2 or 5 mg ml-1 streptomycin and screened for chloramphenicol sensitivity. Putative transformants were verified by colony PCR and sequencing to result in the following isogenic 81–176 SmR ΔflhG mutants: MB361 (flhGK37A), CRG525 (flhGΔ4–24), CRG526 (flhGQ4A), CRG527 (flhG1284E), CRG568 (flhGF281E), CRG571 (flhGI285E), CRG1165 (flhG4E), CRG1343 (flhGF288E).
Construction of plasmids for expression of FLAG-tagged FlhG proteins and complementation studies
We constructed two different plasmids for expression of FLAG-tagged FlhG proteins in C. jejuni. For each one, the cat promoter through the start codon from pRY109 was amplified with primers in which one primer added a 5′ XbaI restriction site upstream of the promoter and another that added in frame to the start codon those for a FLAG tag followed immediately in frame by a BamHI restriction site. These identical cat promoter fragments were cloned into XbaI- and BamHI-digested pRY112 or pRY108 to generate pDAR964 and pDAR1627 respectively. The coding sequence of WT flhG or flhG mutants with point mutations or domain deletions from the second codon to the stop codon were amplified with primers containing in frame 5′ BamHI sites from the respective plasmids harboring these genes. These fragments were then cloned into BamHI-digested pDAR964 or pDAR1627 and then sequenced for verification (Table 5). Upon expression, these plasmids produce a FLAG-tag separated by two amino acids encoded by the BamHI site fused to the second amino acid of the FlhG proteins. The plasmids were then conjugated into MB770 (81–176 SmR ΔflhG).
Generation of antisera production
For production of rabbit antiserum against C. jejuni, WT C. jejuni 81–176 was grown and resuspended from plates in 5 ml of PBS, washed twice with PBS and resuspended in 10 ml of 0.4% formalin in water. After overnight agitation at 4°C, cells were collected by centrifugation, washed twice in PBS and resuspended in 1.25 ml of PBS. A 100 μl aliquot of the suspension was spread on MH plates to confirm that the preparation contained non-viable bacteria. Rabbits were immunized with this preparation to generate polyclonal anti-serum against bacterial components by a commercial vendor (Cocalico Biologicals). All use of animals in experimentation has been approved by IACUC at the University of Texas Southwestern Medical Center.
Electron microscopy analysis
After growth, strains were resuspended from MH agar into PBS, pelleted for 3 min at 13 200 r.p.m. in a microcentrifuge, resuspended in 2% gluteraldehye, and then incubated on ice for 1 h. Samples were then stained with 2% uranyl acetate and visualized with an FEI Technai G2 Spirit Bio TWIN transmission electron microscope. Flagellar numbers were counted from at least 100 individual cells and averaged from two biological replicates to determine the proportion of bacterial populations producing different flagellation phenotypes: hyperflagellated (a bacterium producing at two or more flagella at least at one pole); wild-type, (producing a single flagellum at both poles or a flagellum at one pole with the other pole aflagellated); or aflagellated (lacking a flagellum). After averaging, the standard deviation for each population was calculated. The Student's t-test was used to evaluate statistical significance of the hyperflagellation phenotype in flhG mutants relative to WT FlhG.
Fluorescence microscopy analysis
After growth, strains were suspended from MH agar plates into PBS and diluted to OD600 1.0. Fifty microliters of this suspension was added to poly-L-lysine coated chamber slides and 80 μl of PLP buffer (75 mM NaPO4 pH 7.4, 2.5 mM NaCl, 2% paraformaldehyde) was added dropwise to each sample. After 10 min at room temperature, the chambers were washed with PBS three times. Sixty-five microliters of blocking buffer (3% BSA and 0.1% Triton X-100 in PBS) was applied to each sample for 10 min and then removed by aspiration. Seventy microliters of a mixture containing a 1:50 dilution of the appropriate primary antibody in buffer 2 (3% BSA, 1% saponin, 0.1% Triton X-100,0.02% sodium azide in PBS) was applied to each sample and incubated overnight 4°C (for the monoclonal murine α-FLAG-tag M2 antibody; Sigma) or for 30 min at room temperature (for the rabbit α-C. jejuni antiserum UT527). Samples were then washed three times with blocking buffer. Seventy microliters of a mixture containing a 1:200 dilution of the appropriate secondary antibody (goat Texas Red-conjugated anti-rabbit antibody or goat fluorescin-conjugated anti-mouse antibody, Santa Cruz Biotechnology) was added to the sample for 30 min at room temperature. Samples were washed three times with blocking buffer, chamber gaskets were then removed, and one drop of ProLong Gold antifade reagent with DAPI (Molecular Probes) was added followed by a coverslip. Slides were cured at room temperature overnight before imaging on an Applied Precision PersonalDV deconvolution microscope with an Olympus 100× objective lens and a Cool-SNAP_HQ2 camera. Images were adjusted for brightness and contrast using ImageJ software. Individual cells were placed into one of five categories based on the pattern of fluorescence: polar (fluorescent signal observed only at one or both poles); diffuse (fluorescent signal throughout the cell); multiple (two or more puncta with at least one punctum at a non-polar region); non-polar (a single fluorescent puncta at a non-polar region); or other (a signal pattern not described by others such as a polar puncta with diffuse fluorescence). Three separate samples were prepared for each strain, and at least 100 cells were counted for each sample. After averaging, a χ2 analysis with four degrees of freedom was performed to determine if the pattern of localization for each FlhG mutant was significantly different from the pattern of cellular distribution of WT FlhG.
Immunoblotting analysis of C. jejuni proteins
Campylobacter jejuni proteins from different cellular compartments were recovered for immunoblotting analysis based on previously described procedures (Bingham-Ramos and Hendrixson, 2008). Briefly, C. jejuni strains were suspended in PBS after growth on MH agar and diluted to an OD600 0.8. For whole-cell lysates (WCL), 1 ml of each strain was pelleted in a microcentrifuge at 13 200 r.p.m. for 3 min and washed once with 1 ml PBS. For isolation of inner membrane proteins, 5 ml of each culture was pelleted at 6000 r.p.m. by centrifugation and washed once with 1 ml of 10 mM HEPES (pH 7.4). After resuspension in 1 ml of 10 mM HEPES, strains were sonicated. Unbroken cells were initially removed by centrifugation at 13 200 r.p.m. for 3 min in a microcentrifuge. Then, inner and outer membranes were recovered by centrifugation at 13 200 r.p.m. for 30 min in a microcentrifuge. Membranes were suspended in 10 mM HEPES with 1% N-lauroylsarcosine and incubated at room temperature for 30 min. The insoluble outer membrane proteins were separated from the soluble inner membrane proteins by centrifugation at 13 200 r.p.m. at 30 min in a microcentrifuge. For isolation of cytoplasmic proteins, 20 ml of each culture was centrifuged at 6000 r.p.m. for 10 min and then washed twice with 2 ml PBSG (PBS containing 0.1% gelatin). After these washes, the pellets were resuspended in 2 ml PBSG containing 20 mg ml−1 polymixin B sulfate (Sigma) to compromise the outer membranes and generate spheroplasts. The spheroplasts were recovered by centrifugation in a microcentrifuge for 30 min at 13 200 r.p.m. After resuspension in 1 ml PBSG, spheroplasts were sonicated, and inner membranes components were removed by centrifugation at 13 200 for 30 min in a microcentrifuge.
For WCL, inner membrane and cytoplasmic fractions, protein samples were loaded to represent the proteins recovered from 400 μl (to detect FLAG-FlhG proteins), 200 μl (to detect CmeB) or 100 μl (to detect RpoA) of bacterial cultures, which had been normalized to the same density (OD600 0.8). Immunoblotting analysis was performed with specific primary antiserum at the following dilutions: rabbit α-FLAG (Sigma), 1:500; murine α-RpoA M60 or M251, 1:2000–1:5000 (Joslin and Hendrixson, 2009); and rabbit α-CmeB 1:10 000 (Lin et al., 2002). Secondary goat α-murine or α-rabbit HRP-conjugated antibodies were used at 1:5000 to 1:10 000 dilutions.
For quantitative immunoblot analysis, independent WCL were prepared in triplicate for each strain, and immunoblotting procedures were performed as described above, with detection of RpoA as a control in each sample. After addition of Western Lightning Plus ECL reagent (Perkin-Elmer), blots were visualized using a Synoptics 4.0 MP camera (Syngene, Cambridge, UK). The intensity of each protein band was quantified using GeneSys Software (version 1.4.3.0) (Syngene, Cambridge, UK) and normalized to the intensity of the RpoA band for each sample.
Real-time RT-PCR analysis
Campylobacter jejuni strains WT 81–176 SmR (DRH212) and 81–176 SmR ΔflhG (MB770) were grown on MH agar, suspended into MH broth, and total RNA was extracted with Trizol reagent (Invitrogen). RNA was then treated with DNase prior to analysis. A final concentration of 50 ng μl−1 of RNA was used in a Sybr green PCR master mix with specific forward and reverse primers for each gene analyzed. Realtime reverse transcription-PCR (RT-PCR) was performed using a 7500 real-time PCR system (Applied Biosystems). Detection of mRNA for 16S rRNA served as an endogenous control, and the transcript levels of each gene in ΔflhG was compared with those of WT C. jejuni. Transcription of all genes in both strains was analyzed in triplicate and then averaged. The Student's t-test was used to evaluate statistical significance of changes in gene expression between WT C. jejuni and C. jejuni ΔflhG.
Purification of recombinant FlhF and FlhG proteins and in vitro GTPase assays
WT flhG and flhGQ4A were amplified respectively from the WT C. jejuni 81–176 chromosome or CRG526 (81–176 rpsLSm flhGQ4A) with primers containing 5′ BamHI or NcoI sites for cloning into pET24d to generate 6XHis-tagged proteins. The resulting plasmids (pCRG563 and pDRH4503) were verified by sequencing. E. coli BL21(DE3) (New England BioLabs) cells carrying pDRH2270, pMB681, pCRG563 and pDRH4503 were grown in LB supplemented with 100 μg ml−1 ampicillin and 12.5 g l−1 D(+)-lactose-monohydrate for 16 h at 30°C with shaking at 150 r.p.m. Cells were harvested by centrifugation at 3500 × g for 20 min at 4°C and resuspended in lysis buffer (20 mM HEPES-Na, pH 8.0, 250 mM NaCl, 40 mM imidazole, 20 mM MgCl2 and 20 mM KCl). Cells were lysed with the M-110 l Microfluidizer (Microfluidics). After centrifugation at 47 850 × g for 20 min at 4°C, the cleared supernatant was loaded on a 1 ml HisTrap column (GE Healthcare) equilibrated with 10 column volumes (CV) of lysis buffer. After washing with 10 CV lysis buffer, FlhF or FlhG proteins were eluted with 15 ml elution buffer, which consisted of lysis buffer containing 500 mM imidazole. Each protein was concentrated to approximately 15 mg ml−1 using an Amicon Ultracel-10K (Millipore). The concentrated samples were applied to size-exclusion chromatography (HiLoad 26/600 Superdex 200 pg, GE Healthcare) equilibrated with SEC-buffer (20 mM HEPES-Na, pH 7.5, 200 mM NaCl 20 mM MgCl2 and 20 mM KCl). Protein concentration was determined by a spectrophotometer (NanoDrop Lite, Thermo Scientific).
GTPase activity of FlhF was monitored by high-pressure liquid-chromatography (HPLC). Reactions contained combinations of WT FlhF, FlhFR324A, WT FlhG or FlhGQ4A at a final concentration of 50 μM in 50 μl total volumes as indicated. After incubation with 5 mM GTP in SEC-buffer for 1 h at 37°C, reactions were stopped by flash-freezing with liquid nitrogen and stored at −20°C until measurement. HPLC measurements were performed with an Agilent 1100 Series HPLC system (Agilent Technologies) and a C18 column (EC 250/4.6 Nucleodur HTec 3 μm; Macherey-Nagel). GDP and GTP were eluted with a buffer containing 50 mM KH2PO4, 50 mM K2HPO4, 10 mM tetrapentylammonium bromide and 15% (v/v) acetonitrile at 0.8 ml min−1 flow rate and detected at a wavelength of 253 nm in agreement with standards. GDP originating from non-enzymatic hydrolysis of GTP was determined by triplicate measurement of 5 mM GTP treated similar as the enzymatic reactions and subtracted from the quantified GDP.
Supplementary Material
Acknowledgments
This work was supported by Public Health NIH grants R01AI065539, R21AI103643 and R21AI115362. C.J.G was supported by NIH training grant T32 AI007520. The LOEWE program of the state of Hesse (Germany) supported this work (to G. B.). G.B. and C.K. thank Wieland Steinchen (Synmikro) for his advice during HPLC measurements.
Footnotes
Supporting information: Additional supporting information may be found in the online version of this article at the publisher's web-site.
References
- Adler HI, Fisher WD, Cohen A, Hardigree AA. Miniature Escherichia coli cells deficient in DNA. Proc Natl Acad Sci USA. 1967;57:321–326. doi: 10.1073/pnas.57.2.321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balaban M, Hendrixson DR. Polar flagellar biosynthesis and a regulator of flagellar number influence spatial parameters of cell division in Campylobacter jejuni. PLoS Pathog. 2011;7:e1002420. doi: 10.1371/journal.ppat.1002420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balaban M, Joslin SN, Hendrixson DR. FlhF and its GTPase activity are required for distinct processes in flagellar gene regulation and biosynthesis in Campylobacter jejuni. J Bacteriol. 2009;191:6602–6611. doi: 10.1128/JB.00884-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bange G, Sinning I. SIMIBI twins in protein targeting and localization. Nat Struct Mol Biol. 2013;20:776–780. doi: 10.1038/nsmb.2605. [DOI] [PubMed] [Google Scholar]
- Bange G, Petzold G, Wild K, Parlitz RO, Sinning I. The crystal structure of the third signal-recognition particle GTPase FlhF reveals a homodimer with bound GTP. Proc Natl Acad Sci USA. 2007;104:13621–13625. doi: 10.1073/pnas.0702570104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bange G, Kummerer N, Grudnik P, Lindner R, Petzold G, Kressler D, et al. Structural basis for the molecular evolution of SRP-GTPase activation by protein. Nat Struct Mol Biol. 2011;18:1376–1380. doi: 10.1038/nsmb.2141. [DOI] [PubMed] [Google Scholar]
- Baraquet C, Harwood CS. Cyclic diguanosine monophosphate represses bacterial flagella synthesis by interacting with the Walker A motif of the enhancer-binding protein FleQ. Proc Natl Acad Sci USA. 2013;110:18478–18483. doi: 10.1073/pnas.1318972110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bingham-Ramos LK, Hendrixson DR. Characterization of two putative cytochrome c peroxidases of Campylobacter jejuni involved in promoting commensal colonization of poultry. Infect Immun. 2008;76:1105–1114. doi: 10.1128/IAI.01430-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Boer PA, Crossley RE, Rothfield LI. Isolation and properties of minB, a complex genetic locus involved in correct placement of the division site in Escherichia coli. J Bacteriol. 1988;170:2106–2112. doi: 10.1128/jb.170.5.2106-2112.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Boer PA, Crossley RE, Rothfield LI. A division inhibitor and a topological specificity factor coded for by the minicell locus determine proper placement of the division septum in E. coli. Cell. 1989;56:641–649. doi: 10.1016/0092-8674(89)90586-2. [DOI] [PubMed] [Google Scholar]
- Boll JM, Hendrixson DR. A specificity determinant for phosphorylation in a response regulator prevents in vivo cross-talk and modification by acetyl phosphate. Proc Natl Acad Sci USA. 2011;108:20160–20165. doi: 10.1073/pnas.1113013108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boll JM, Hendrixson DR. A regulatory checkpoint during flagellar biogenesis in Campylobacter jejuni initiates signal transduction to activate transcription of flagellar genes. mBio. 2013;4:e00432–13. doi: 10.1128/mBio.00432-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourne HR, Sanders DA, McCormick F. The GTPase superfamily: a conserved switch for diverse cell functions. Nature. 1990;348:125–132. doi: 10.1038/348125a0. [DOI] [PubMed] [Google Scholar]
- Correa NE, Peng F, Klose KE. Roles of the regulatory proteins FlhF and FlhG in the Vibrio cholerae flagellar transcription heirarchy. J Bacteriol. 2005;187:6324–6332. doi: 10.1128/JB.187.18.6324-6332.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dasgupta N, Ramphal R. Interaction of the antiactivator FleN with the transcriptional activator FleQ regulates flagellar number in Pseudomonas aeruginosa. J Bacteriol. 2001;183:6636–6644. doi: 10.1128/JB.183.22.6636-6644.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dasgupta N, Arora SK, Ramphal R. fleN, a gene that regulates flagellar number in Pseudomonas aeruginosa. J Bacteriol. 2000;182:357–364. doi: 10.1128/jb.182.2.357-364.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davie E, Sydnor K, Rothfield LI. Genetic basis of minicell formation in Escherichia coli K-12. J Bacteriol. 1984;158:1202–1203. doi: 10.1128/jb.158.3.1202-1203.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Figurski DH, Helinski DR. Replication of an origin-containing derivative of plasmid RK2 dependent on a plasmid function provided in trans. Proc Natl Acad Sci USA. 1979;76:1648–1652. doi: 10.1073/pnas.76.4.1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasper R, Meyer S, Gotthardt K, Sirajuddin M, Wittinghofer A. It takes two to tango: regulation of G proteins by dimerization. Nat Rev Mol Cell Biol. 2009;10:423–429. doi: 10.1038/nrm2689. [DOI] [PubMed] [Google Scholar]
- Green JC, Kahramanoglou C, Rahman A, Pender AM, Charbonnel N, Fraser GM. Recruitment of the earliest component of the bacterial flagellum to the old cell division pole by a membrane-associated signal recognition particle family GTP-binding protein. J Mol Biol. 2009;391:679–690. doi: 10.1016/j.jmb.2009.05.075. [DOI] [PubMed] [Google Scholar]
- Guttenplan SB, Shaw S, Kearns DB. The cell biology of peritrichous flagella in Bacillus subtilis. Mol Microbiol. 2013;87:211–229. doi: 10.1111/mmi.12103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendrixson DR, DiRita VJ. Transcription of σ54-dependent but not σ28-dependent flagellar genes in Campylobacter jejuni is associated with formation of the flagellar secretory apparatus. Mol Microbiol. 2003;50:687–702. doi: 10.1046/j.1365-2958.2003.03731.x. [DOI] [PubMed] [Google Scholar]
- Hendrixson DR, Akerley BJ, DiRita VJ. Transposon mutagenesis of Campylobacter jejuni identifies a bipartite energy taxis system required for motility. Mol Microbiol. 2001;40:214–224. doi: 10.1046/j.1365-2958.2001.02376.x. [DOI] [PubMed] [Google Scholar]
- Hu Z, Lutkenhaus J. Topological regulation of cell division in E. coli. spatiotemporal oscillation of MinD requires stimulation of its ATPase by MinE and phospholipid. Mol Cell. 2001;7:1337–1343. doi: 10.1016/s1097-2765(01)00273-8. [DOI] [PubMed] [Google Scholar]
- Hu Z, Lutkenhaus J. A conserved sequence at the C-terminus of MinD is required for binding to the membrane and targeting MinC to the septum. Mol Microbiol. 2003;47:345–355. doi: 10.1046/j.1365-2958.2003.03321.x. [DOI] [PubMed] [Google Scholar]
- Hu Z, Gogol EP, Lutkenhaus J. Dynamic assembly of MinD on phospholipid vesicles regulated by ATP and MinE. Proc Natl Acad Sci USA. 2002;99:6761–6766. doi: 10.1073/pnas.102059099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joslin SN, Hendrixson DR. Analysis of the Campylobacter jejuni FlgR response regulator suggests integration of diverse mechanisms to activate an NtrC-like protein. J Bacteriol. 2008;190:2422–2433. doi: 10.1128/JB.01827-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joslin SN, Hendrixson DR. Activation of the Campylobacter jejuni FlgSR two-component system is linked to the flagellar export apparatus. J Bacteriol. 2009;191:2656–2667. doi: 10.1128/JB.01689-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jyot J, Dasgupta N, Ramphal R. FleQ, the major flagellar gene regulator in Pseudomonas aeruginosa, binds to enhancer sites located either upstream or atypically downstream of the RpoN binding site. J Bacteriol. 2002;184:5251–5260. doi: 10.1128/JB.184.19.5251-5260.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kazmierczak BI, Hendrixson DR. Spatial and numerical regulation of flagellar biosynthesis in polarly flagellated bacteria. Mol Microbiol. 2013;88:655–663. doi: 10.1111/mmi.12221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korlath JA, Osterholm MT, Judy LA, Forfang JC, Robinson RA. A point-source outbreak of campylobacteriosis associated with consumption of raw milk. J Infect Dis. 1985;152:592–596. doi: 10.1093/infdis/152.3.592. [DOI] [PubMed] [Google Scholar]
- Kusumoto A, Kamisaka K, Yakushi T, Terashima H, Shinohara A, Homma M. Regulation of polar flagellar number by the flhF and flhG genes in Vibrio alginolyticus. J Biochem. 2006;139:113–121. doi: 10.1093/jb/mvj010. [DOI] [PubMed] [Google Scholar]
- Lackner LL, Raskin DM, de Boer PA. ATP-dependent interactions between Escherichia coli Min proteins and the phospholipid membrane in vitro. J Bacteriol. 2003;185:735–749. doi: 10.1128/JB.185.3.735-749.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leipe DD, Wolf YI, Koonin EV, Aravind L. Classification and evolution of P-loop GTPases and related ATPases. J Mol Biol. 2002;317:41–72. doi: 10.1006/jmbi.2001.5378. [DOI] [PubMed] [Google Scholar]
- Levin PA, Margolis PS, Setlow P, Losick R, Sun D. Identification of Bacillus subtilis genes for septum placement and shape determination. J Bacteriol. 1992;174:6717–6728. doi: 10.1128/jb.174.21.6717-6728.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin J, Michel LO, Zhang Q. CmeABC functions as a multidrug efflux system in Campylobacter jejuni. Antimicrob Agents Chemother. 2002;46:2124–2131. doi: 10.1128/AAC.46.7.2124-2131.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutkenhaus J. Assembly dynamics of the bacterial MinCDE system and spatial regulation of the Z ring. Annu Rev Biochem. 2007;76:539–562. doi: 10.1146/annurev.biochem.75.103004.142652. [DOI] [PubMed] [Google Scholar]
- Moriya N, Minamino T, Imada K, Namba K. Genetic analysis of the bacterial hook-capping protein FlgD responsible for hook assembly. Microbiology. 2011;157:1354–1362. doi: 10.1099/mic.0.047100-0. [DOI] [PubMed] [Google Scholar]
- Mukherjee S, Bree AC, Liu J, Patrick JE, Chien P, Kearns DB. Adaptor-mediated Lon proteolysis restricts Bacillus subtilis hyperflagellation. Proc Natl Acad Sci USA. 2015;112:250–255. doi: 10.1073/pnas.1417419112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray TS, Kazmierczak BI. FlhF is required for swimming and swarming in Pseudomonas aeruginosa. J Bacteriol. 2006;188:6995–7004. doi: 10.1128/JB.00790-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niehus E, Gressmann H, Ye F, Schlapbach R, Dehio M, Dehio C, et al. Genome-wide analysis of transcriptional hierarchy and feedback regulation in the flagellar system of Helicobacter pylori. Mol Microbiol. 2004;52:947–961. doi: 10.1111/j.1365-2958.2004.04006.x. [DOI] [PubMed] [Google Scholar]
- Ohnishi K, Ohto Y, Aizawa S, Macnab RM, Iino T. FlgD is a scaffolding protein needed for flagellar hook assembly in Salmonella typhimurium. J Bacteriol. 1994;176:2272–2281. doi: 10.1128/jb.176.8.2272-2281.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandza S, Baetens M, Park CH, Au T, Keyhan M, Matin A. The G-protein FlhF has a role in polar flagellar placement and general stress response induction in Pseudomonas putida. Mol Microbiol. 2000;36:414–423. doi: 10.1046/j.1365-2958.2000.01859.x. [DOI] [PubMed] [Google Scholar]
- Rothfield L, Taghbalout A, Shih YL. Spatial control of bacterial division-site placement. Nat Rev Microbiol. 2005;3:959–968. doi: 10.1038/nrmicro1290. [DOI] [PubMed] [Google Scholar]
- Schniederberend M, Abdurachim K, Murray TS, Kazmierczak BI. The GTPase activity of FlhF is dispensable for flagellar localization, but not motility, in Pseudomonas aeruginosa. J Bacteriol. 2013;195:1051–1060. doi: 10.1128/JB.02013-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuhmacher JS, Rossmann F, Dempwolff F, Knauer C, Altegoer F, Steinchen W, et al. MinD-like ATPase FlhG effects location and number of bacterial flagella during C-ring assembly. Proc Natl Acad Sci USA. 2015a;112:3092–3097. doi: 10.1073/pnas.1419388112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuhmacher JS, Thormann KM, Bange G. How bacteria maintain location and number of flagella? FEMS Microbiol Rev. 2015b;39:812–822. doi: 10.1093/femsre/fuv034. [DOI] [PubMed] [Google Scholar]
- Szeto TH, Rowland SL, Rothfield LI, King GF. Membrane localization of MinD is mediated by a C-terminal motif that is conserved across eubacteria, archaea, and chloroplasts. Proc Natl Acad Sci USA. 2002;99:15693–15698. doi: 10.1073/pnas.232590599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Alstyne D, Simon MI. Division mutants of Bacillus subtilis: isolation and PBS1 transduction of division-specific markers. J Bacteriol. 1971;108:1366–1379. doi: 10.1128/jb.108.3.1366-1379.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varley AW, Stewart GC. The divIVB region of the Bacillus subtilis chromosome encodes homologs of Escherichia coli septum placement (minCD) and cell shape (mreBCD) determinants. J Bacteriol. 1992;174:6729–6742. doi: 10.1128/jb.174.21.6729-6742.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu W, Park KT, Holyoak T, Lutkenhaus J. Determination of the structure of the MinD-ATP complex reveals the orientation of MinD on the membrane and the relative location of the binding sites for MinE and MinC. Mol Microbiol. 2011;79:1515–1528. doi: 10.1111/j.1365-2958.2010.07536.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao R, Alm RA, Trust TJ, Guerry P. Construction of new Campylobacter cloning vectors and a new mutational cat cassette. Gene. 1993;130:127–130. doi: 10.1016/0378-1119(93)90355-7. [DOI] [PubMed] [Google Scholar]
- Zhou H, Lutkenhaus J. Membrane binding by MinD involves insertion of hydrophobic residues within the C-terminal amphipathic helix into the bilayer. J Bacteriol. 2003;185:4326–4335. doi: 10.1128/JB.185.15.4326-4335.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


