Abstract
Host-derived angiogenic and inflammatory tumor supportive microenvironment regulates progression and metastasis, but the molecular mechanism(s) underlying host-tumor interactions remains unclear. Tumor expression of CXCR2 and its ligands have been shown to regulate angiogenesis, invasion, tumor growth, and metastasis. In this report, we hypothesized that host-derived Cxcr2-dependent signaling plays an important role in breast cancer growth and metastasis. Two mammary tumor cell lines Cl66 and 4T1 cells were orthotopically implanted into the mammary fat pad of wild-type and Cxcr2−/− female BALB/c mice. Tumor growth and spontaneous lung metastasis were monitored. Immunohistochemical analyses of the tumor tissues were performed to analyze proliferation, angiogenesis, apoptosis and immune cell infiltration. Our results demonstrated that knock-down of host Cxcr2 decreases tumor growth and metastasis by reducing angiogenesis, proliferation and enhancing apoptosis. Host Cxcr2 plays an important role in governing the pro-inflammatory response in mammary tumors as evaluated by decreased Gr1+ tumor-associated granulocytes, F4/80+ tumor associated macrophages, and CD11b+Gr1+ myeloid derived suppressor cells in Cxcr2−/− mice as compared to control wild-type mice. Together, these results demonstrate that host Cxcr2-dependent signaling regulates mammary tumor growth and metastasis by promoting angiogenesis and pro-inflammatory responses.
Keywords: CXCR2, Angiogenesis, Metastasis, Inflammatory response, Chemokines
Introduction
Despite improvement in current therapeutic regimens, breast cancer still remains the second most common cause of cancer death among women [1]. The vast majority of these deaths are due to therapy resistance, disease progression and metastasis [2]. The molecular mechanism(s) underlying breast cancer growth and invasion have been extensively examined; however, most of these studies are focused on malignant cells. The outcome of tumor progression and metastasis depends on both intrinsic properties of tumors and responses of the host [3–5].
Recent reports from our laboratory and others demonstrated increased expression of pro-inflammatory chemokines in various cancers and documented that they have an important role in the tumor microenvironment [6–8]. Chemokines have been shown to regulate the inflammatory response in multiple tumor types [9, 10]. The host immune response regulates tumor growth and progression through favorable host homeostatic mechanisms stimulating migration and interrupting these mechanisms may inhibit cancer metastasis [4, 5, 10]. CXCR2 and its ligands are known to be pro-inflammatory and angiogenic, supporting tumor growth and metastasis in an autocrine and paracrine manner [9, 11–15]. Importantly the ligands, CXCL8 and CXCL1, have been observed to influence breast tumor growth, chemoresistance and metastasis [6–8, 16, 17]. In addition, CXCR2 expressed by endothelial cells binds to its angiogenic ELR+ (Glu-Leu-Arg) ligands secreted by tumor cells and facilitates angiogenesis in breast tumors [11, 12]. Similarly, neutrophils, bone marrow-derived myeloid cells (BMDCs) and myeloid suppressor cells (MDSC) express CXCR2 and aid in tumor growth [17–19]. Neutrophils once recruited to the tumor site help establish a niche for inflammatory cells via production of cytokines [15, 20]. BMDCs on the other hand mature to M2 type macrophages and instead of eradicating cancer cells provide growth benefits to cancer cells [9, 21]. Our lab has shown that inhibiting CXCR2 expression in tumor cells decreases metastasis, angiogenesis, proliferation and increases apoptosis of mammary tumor cells. Moreover, the functional role of tumor CXCR2 and its ligands in the regulation of the malignant phenotype is well established [13, 22], however, the role of host CXCR2 dependent signaling in breast cancer remains unclear. In this part of the project, we demonstrate that host Cxcr2 dependent signaling plays an important role in mammary tumor growth, angiogenesis and metastasis.
Materials and methods
Animals
BALB/c mice heterozygous for Cxcr2 (Cxcr2+/−) were obtained from Jackson Laboratory (Bar Harbor, ME). Mice that lack an intact mIL-8Rh (mouse homologue of human IL-8 receptor/Cxcr2) gene, were originally developed by gene targeting with a vector constructed by deleting the single exon containing the 350-amino acid open reading frame of the murine IL-8 receptor [which has 68 and 71 % amino acid identity with human IL-8 receptors A (CXCR1) and B (CXCR2)] [23]. We generated Cxcr2−/− mice following crosses between BALB/c mice Cxcr2 heterozygous female and Cxcr2 homozygous male. Mice were housed and handled according to protocols approved by the University of Nebraska Medical Center Institutional Animal Care and Use Committee.
Mice were genotyped using DNA from their tail and amplifying it for Cxcr2tm1Mwm using the primers 5′-GGT CGT ACT GCG TAT CCT GCC TCA G-3′ and 5′-TAG CCA TGA TCT TGA GAAGTC CAT G-3′ which amplified a 360 bp fragment of the wild-type allele and the primers 5′-CTT GGG TGG AGA GGC TAT TC-3′ and 5′-AGG TGA GAT GAC AGG AGA TC-3′ which amplified a 280 bp fragments of the inserted neomycin gene (Fig. 1a, b).
Fig. 1.
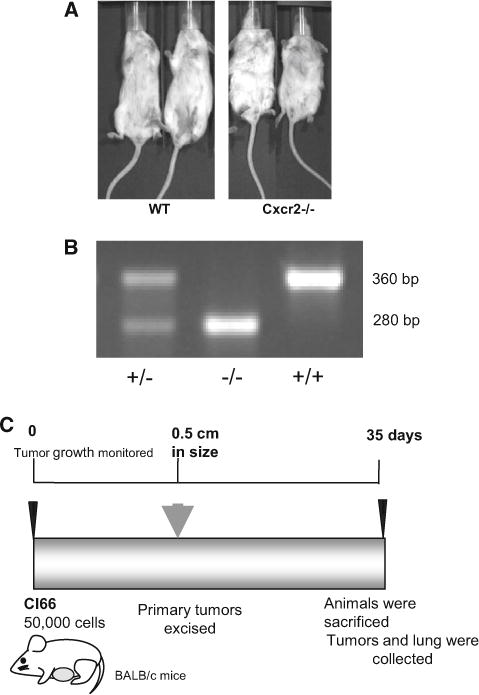
Genotyping of mice: a Representative images of wild type and Cxcr2−/− mice depicting the phenotype differences. b Genotyping of genomic DNA by PCR to determine Cxcr2 status of the mice. c Schematic diagram representing experimental strategy for tumor implantation and end point analysis for metastasis
Cell lines
Two murine mammary adenocarcinoma cell lines differing in their metastatic potential, 4T1 (highly metastatic) and Cl66 (moderately metastatic) [24, 25] were used. Cell lines were maintained in Dulbecco’s Modified Eagle Media (DMEM) (Mediatech, Hendon, VA) with 5 % serum supreme (Biowhitaker, Walkersville, MD), 1 % vitamins, 1 % L-glutamine (Mediatech Inc. Manassas, VA) and 0.08 % gentamycin (Invitrogen, Carlsbad, CA). All cultures were free of mycoplasma and pathogenic murine viruses. Cultures were maintained for no longer than 4 weeks after recovery from frozen stocks.
Tumor growth and metastasis
Cl66 and 4T1 cells (50,000 in 50 μl of Hank’s Balanced Salt Solution) were orthotopically implanted in mammary fat pad (MFP) to study tumor growth and spontaneous metastasis in wild-type or Cxcr2−/− female mice. Tumor growth was measured twice a week. Tumor volume was calculated using the formula π/6 × (smaller diameter)2 × (larger diameter). Tumors resected from mice were fixed in formalin, embedded in paraffin and processed for histopathological evaluation and immunohistochemistry.
For spontaneous metastasis, primary tumors were surgically removed when the tumors reached 0.5 cm3 in size and mice were monitored for metastases. Mice were sacrificed at 35 days after implantation. Lungs were collected and fixed in Bouin’s fixative. Metastatic lung nodules were then manually counted using a dissecting microscope.
Immunohistochemistry and immunofluorescence staining
Immunohistochemical analysis was performed to determine proliferation, micro-vessel density, apoptosis and immune infiltration as previously described [26]. In brief, 6-μm thick tumor sections were de-paraffinized by xylenes and ethanol and blocked for 30 min. Tumor sections were incubated overnight in a humid chamber with the following primary antibodies: monoclonal mouse anti-PCNA (1:40; Santa Cruz Biotechnology) or monoclonal rabbit antibody against cleaved caspase-3 (Cell Signaling, 1:200) or monoclonal biotin-conjugated rat anti-mouse Ly-6G and Ly-6C (Gr-1) (BD Biosciences, 1:50) or rabbit anti-CD31 (Abcam, 1:50) or monoclonal rat anti-F4/80 (Abcam, 1:100). Corresponding biotinylated secondary antibody was used (except for Gr-1) at room temperature. Immunoreactivity was detected using the ABC Elite kit and DAB substrate (Vector Laboratories) as per the manufacturer’s instructions. A reddish brown precipitate indicated a positive reaction. Negative controls had all reagents except antibody. The number of positive cells was quantitated microscopically with a 5 × 5 reticle grid (Klarmann Rulings, Litchfield, NH) using 400× objective (250 μm total area).
For flow cytometric analysis to determine MDSC recruitment, single cell suspension of tumor associated leukocytes from Cl66 tumors derived from Cxcr2−/− mice and wild type mice were prepared as described earlier [27] and stained for CD11b and Gr1 antibodies (BD Bioscience). Cells were analyzed using FACScan Plus (BD Biosciences).
Enzyme linked immunosorbant assay
Serum from Cxcr2−/− and wild type tumor bearing mice were collected and analyzed for CXCL-1, -2, -5, and -7 using methods described earlier [8]. Standards (recombinant proteins) and samples were added 100 μl/well in duplicate. After incubation, plates were washed and then incubated with biotinylated secondary antibody 100 μl/well (at concentrations recommended by manufacturers; R&D Systems Inc). After washing strepavidin-horseradish peroxidase (1:20000) was added and 3,3′,5,5′-tetramethyl-benzidine substrate (100 μl/well)was used. Reactions were stopped and plates were read at 450 nm using an ELx800 (Bio-Teck) plate reader. Concentrations were normalized to total protein concentrations.
Statistical analysis
In vivo analysis was performed using the Mann–Whitney U-test and paired t test using Sigma Plot 11. All the values are expressed, as mean ± SEM. p ≤ 0.05 was considered statistically significant.
Results
Host Cxcr2 knockout inhibits tumor growth and metastasis
To assess the effect of host Cxcr2, we used an orthotropic syngenic model for breast cancer. We injected Cl66 and 4T1 murine mammary tumor cells in the MFP of wild type and Cxcr2−/− female mice (Fig. 1). Tumor growth was examined by measuring the tumor size twice a week for 3 weeks (Fig. 1). Tumor incidence was lower in Cxcr2−/− mice when compared to wild type mice implanted with Cl66 tumors. Furthermore, we observed a decrease in tumor growth in Cxcr2−/− mice when compared to wild type mice suggesting that host Cxcr2 has a crucial role in suppressing the tumor growth of Cl66 tumors. However, in mice with 4T1 tumor cells, we did not observe any change in the in vivo tumor growth kinetics in Cxcr2 knockout (Cxcr2−/−) mice as compared to wild-type mice (Figs. 2a and 3a).
Fig. 2.
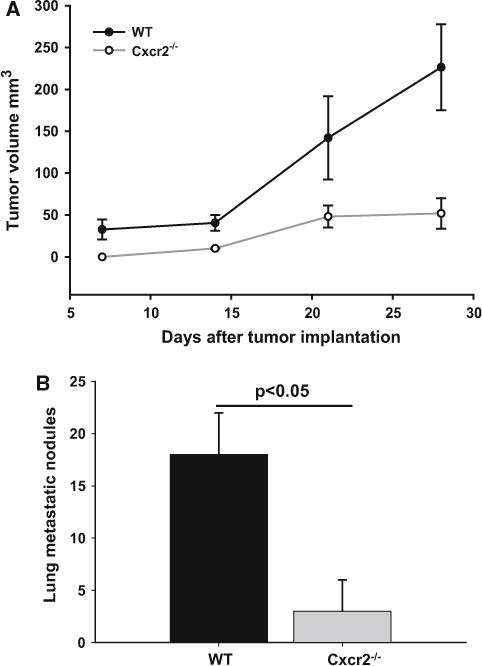
Tumor growth in wild type and Cxcr2 null mice: a Growth kinetics of tumors formed by Cl66 cells in wild type and Cxcr2−/−mice (n = 5, p = 0.036). b Spontaneous lung metastasis in wild type and Cxcr2−/− mice. Lung metastatic nodules were counted manually for both groups (n = 5) and the mean was calculated. Mean ± SEM was graphed for each group and p < 0.05 was considered significant
Fig. 3.
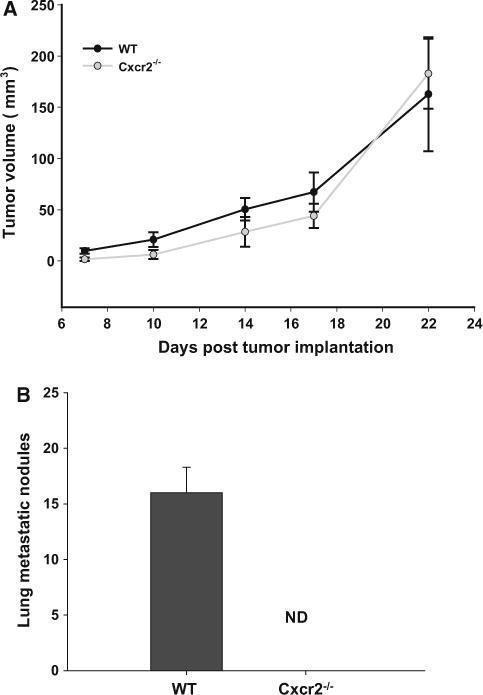
Tumor growth by 4T1 cells in mice with different Cxcr2 status: a Growth kinetics of tumors formed by 4T1 cells in wild type and Cxcr2−/− mice (n = 5). b Spontaneous lung metastasis in wild type and Cxcr2−/− mice. Lung nodules were counted for both groups (n = 5) and mean ± SEM was plotted p < 0.05
We next investigated the role of host Cxcr2 on spontaneous lung metastasis of murine mammary tumor. Primary tumors were surgically removed when reached to 0.5 cm3 in size and animals were monitored for spontaneous metastasis (Fig. 1c). Mice were sacrificed and metastatic lung nodules were counted in all groups. We found a significant reduction in metastatic lung nodules in Cxcr2−/− tumor-bearing mice as compared to wild-type tumor-bearing mice (Figs. 2b and 3b). Although, 4T1 tumor bearing mice had no significant difference in tumor growth, there was a significant difference in metastatic lung nodules with wild-type mice having more nodules than Cxcr2−/− mice (Fig. 3b).
Host Cxcr2 knockdown decreased in situ cell proliferation, angiogenesis and increased apoptosis
To evaluate the mechanism of tumor regression in Cxcr2−/− mice, we immunostained tumor tissues using PCNA, cleaved caspase-3 and CD31 antibodies. We observed decreased in situ tumor cell proliferation in Cxcr2−/− compared to wild-type mice (p ≤ 0.01) (Fig. 4a, c). To further investigate the role of host Cxcr2 in tumor progression, we analyzed microvessel density and detected a significant reduction in the number of blood vessels in tumor tissues obtained from Cxcr2−/− mice (p ≤ 0.001) as compared to wild-type mice (Fig. 4b, d). Similarly, there was a significant increase in cleaved caspase-3 staining in tumor tissues from Cxcr2−/− mice (p ≤ 0.001) in comparison to wild-type mice (Fig. 5a, b). Taken together, our cell proliferation and neo-vascularization data along with the apoptosis results demonstrate that host Cxcr2-dependent signaling in the tumor microenvironment is an important regulator of progression and metastasis.
Fig. 4.
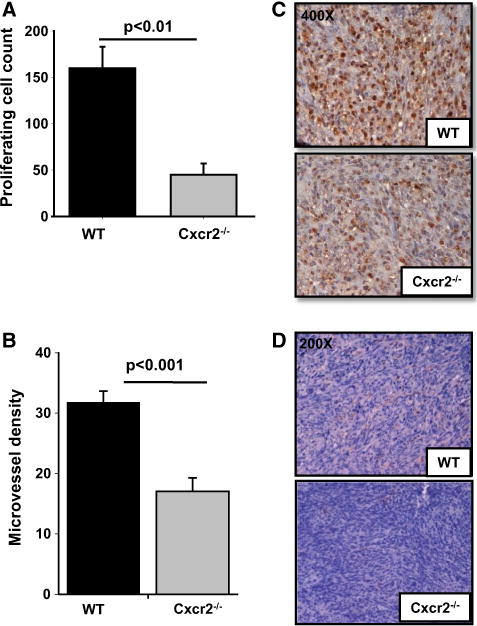
Immunohistochemical analysis of tumors formed by Cl66 cells in wild type and Cxcr2−/− mice for proliferation and angiogenesis: a and b Quantification of PCNA (a) and CD31 (b) staining. Five fields were counted from each tumor for PCNA and CD31 positive cells (n = 5). The mean was calculated and mean ± SEM was graphed for each group. Significant differences are represented with p values. c and d Microscopic images of representative tumor sections from wild type or Cxcr2−/− mice showing PCNA and CD31 staining
Fig. 5.
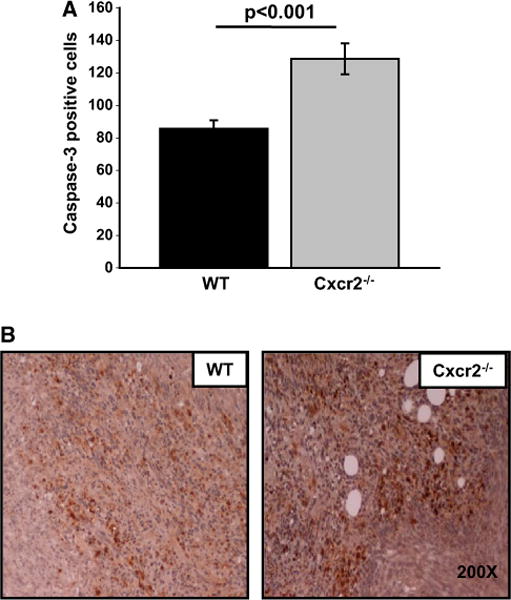
Apoptosis in Cl66 tumors formed in wild type and Cxcr2 null mice: a Quantification of cleaved caspase-3 staining. Five fields were counted from each tumor for caspase positive cells (n = 5). The mean was calculated and mean ± SEM was graphed for each group. b Microscopic image of a representative section of tumor showing caspase-3 staining in tumors from wild type or Cxcr2−/− mice
Diminished pro-inflammatory response in Cxcr2 knockout mice
Immune inflammatory cells in the tumor microenvironment have been linked with tumor growth, angiogenesis and metastasis [5, 28–30]. Tumor-associated neutrophils and macrophages have also been documented to express CXCR2 [10, 15]. To analyze the inflammatory response (neutrophils) in wild-type and Cxcr2−/− mice, tumor tissue sections were stained using an anti-Gr-1 (Ly-6G/C) antibody. Tumors from Cxcr2−/− mice contained a lower frequency of Gr-1+cells as compared to tumors obtained from wild type mice (Fig. 6a, b) suggesting that Cxcr2 receptor status of the host influences neutrophil infiltration in mammary tumors. In addition, we analyzed the frequency of CD11b+Gr1+ MDSCs in primary tumors. We observed a significant difference in frequency of MDSCs in tumors derived from Cxcr2−/− mice as compared to wild type mice (Fig. 6c).
Fig. 6.
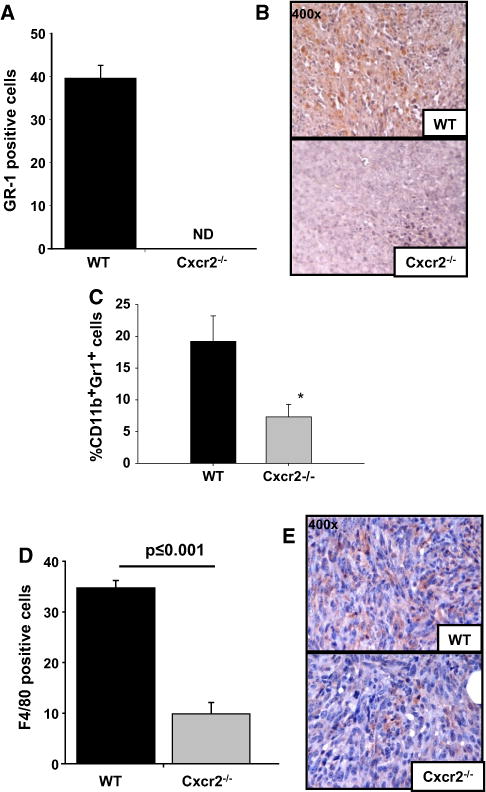
Tumor infiltration of inflammatory cells: a & b Quantification of Gr-1 (a), CD11b+Gr1+ MDSc (c) and F4/80 (d) staining to evaluate neutrophil and macrophage infiltration in tumors. Five fields were counted from each tumor for Gr-1 or F/480 positive cells (n = 5). Flow cytometric analysis was performed using CD11b and Gr1 antibodies (n = 5). The mean was calculated and mean ± SEM was graphed for each group. b and e Microscopic image of a representative section of tumor showing Gr-1 (b) or F4/80 (e) staining in tumors formed in wild-type or Cxcr2−/− mice
To further evaluate whether host Cxcr2 receptor modification modulates the inflammatory macrophage response in mammary tumors, we examined tumors for F4/80+ cells by immunohistochemistry. Tumors from Cxcr2−/− mice showed a significantly (p ≤ 0.001) lower frequency of F4/80+ cells than tumors from wild-type mice (Fig. 6b, d), indicating that knockdown of host Cxcr2 affects macrophage recruitment to tumors. These findings suggest that infiltration of pro-inflammatory immune cells play a role in mammary tumor growth.
Higher expression of Cxcr2 ligands in Cxcr2−/− tumor bearing mice
We examined the levels of circulating Cxcr2 ligands in Cxcr2−/− and wild type tumor bearing mice. We observed undetectable levels of CXCL-1 and CXCL-3 in both wild type and Cxcr2−/− mice (data not shown). Interestingly, the serum levels of CXCL-2 and CXCL-5 was significantly higher in Cxcr2−/− tumor bearing mice as compared to wild type tumor bearing mice (Fig. 7). The level of CXCL-7 was high in wild type and Cxcr2−/− tumor bearing mice. These data suggest an elevation of Cxcr2 ligands in Cxcr2−/− mammary tumor bearing mice.
Fig. 7.
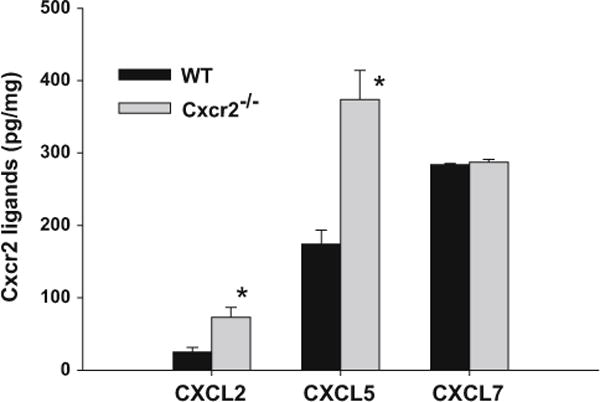
Cxcr2 ligands expression in wild type and Cxcr2−/− tumor bearing mice. The serum levels of CXCL-2, -5 and -7 was analyzed using ELISA. The levels of ligands were normalized to total serum protein levels. The values are mean ± SEM of triplicate samples (n = 4)
Discussion
We investigated the role of host Cxcr2 in mammary tumor progression and metastasis. Our data demonstrated that knockdown of host Cxcr2 reduces tumor metastasis. Host Cxcr2 influenced angiogenesis, spontaneous metastasis, proliferation, apoptosis and the inflammatory response in mammary tumor models. Our earlier reports demonstrate that CXCR2 knockdown in malignant tumor cells significantly decreases spontaneous lung metastasis both in breast cancer and melanoma tumor models [13, 31]. Similar observations were made in our earlier report demonstrating that host Cxcr2 regulates melanoma growth and experimental lung metastasis [31]. Our present data show that mammary tumors from Cxcr2−/− mice were significantly smaller compared to tumors in wild type mice using Cl66 cells. Although we didn’t observe any difference in tumor growth with 4T1 cells there was a significant decrease in the spontaneous lung metastasis. An earlier report has demonstrated that CXCR2 antagonist treatment reduced the number of PyVMT/TGFBR2MGKO metastases to the lung and this was coincident with the inhibition of recruitment of MDSCs to the lung [17]. However there was little effect of the CXCR2 antagonist on the growth of the very aggressive primary tumor [17]. In addition, 4T1 cells are more aggressive than Cl66 based on their 6-thioguanine resistance [32] which might explain the difference in primary tumor growth. Also, injecting less cell number for 4T1 may better evaluate the difference in tumor growth with respect to host CXCR2 expression. Furthermore, we have observed similar tumor growth kinetics and metastasis pattern with Cl66 cells selected for Doxyrubicine (Dox) resistance (personal communication). We are currently investigating the the significance of cancer stem cell like cells and/or modulation of metastatic niche in Cxcr2 deficient mice using 4T1 and Dox resistant Cl66 tumor cells.
CXC chemokines having ELR motifs, particularly CXCL1 in the mice have been documented to recruit macrophages and neutrophils during inflammation and cancer [15, 20, 33, 34]. CXCL1 binds to its receptor CXCR2, which is also present on the surface of endothelial cells and has been shown to enhance angiogenesis [33]. We observed higher serum levels of CXCL-2, -5 and -7 in Cxcr2−/− mice as compared to wild type mice. This observation is similar to earlier finding that Cxcr2 acts as scavenger to its ligands and lack of Cxcr2 leads to increased ligand accumulation [35]. Despite higher levels of the ligands, we observed a significant decrease in microvessel density in tumors from Cxcr2−/− mice as compared to wild type mice.
This study also evaluated the significance of host CXCR2 in modulating the host inflammatory response to mammary tumor growth. We observed that absence of Cxcr2 in mice reduced macrophage, neutrophil, and MDSC recruitment to the tumor site. These observations are in accordance with an earlier report where they observed a decrease in inflammation-driven and spontaneous tumorigenesis in both benign and malignant intestinal adenomas [36] and promote colitis-associated tumorigenesis [19].
Reduced tumor angiogenesis and in situ cell proliferation in Cxcr2−/− mice provide a plausible explanation for the smaller tumor size observed in these mice. Moreover, increased apoptosis in tumor tissues from Cxcr2−/− mice also favors the smaller tumor size. Data from our previous studies with CXCR2 knockdown tumor cells also demonstrated increased apoptosis, reduced angiogenesis and proliferation in tumors [13]. These studies together suggest that the absence of CXCR2 expressing host-derived cells (neutrophils, macrophages and endothelial cells) in the tumor microenvironment might prevent tumor cells from responding to the chemokine storm, preventing their migration to various organs resulting in decreased metastasis.
Together our data suggest that host Cxcr2 expression plays a critical role in mammary tumor growth, proliferation and angiogenesis. Although we observed that Cxcr2 signaling has a significant role in mammary tumors, Cxcr1 another CXC receptor, which binds some of the same Cxcr2 ligands, may also affect tumor growth or metastasis. Our present study does not evaluate the status of Cxcr1. However, previous studies from our lab have shown that in melanoma both CXCR1/2 are expressed by tumor cells but expression of CXCR2 increases with aggressiveness of tumor cells and is involved during metastasis [26]. Moreover, data from other laboratories has shown that the CXCR1/CXCL8 axis plays an important role in selecting cancer stem cells in breast tumors [37] and that CXCR1/2 mediated signaling can be targeted to significantly reduce breast cancer stem cell activity [38]. Furthermore, our lab has reported previously that both tumor and host expression of CXCR2 modulate melanoma growth, angiogenesis, proliferation, apoptosis and metastasis [31, 39, 40]. Also, CXCR2 knockdown in tumor cells alone or in combination with chemotherapeutic drugs reduces angiogenesis and enhances apoptosis in mammary tumors [8, 13]. These studies along with our current data substantiate the role of CXCR2 in mammary tumor growth and metastasis.
In summary, our data suggest that targeting CXCR2 in breast cancer may provide a novel therapeutic alternative for treating breast cancer. Preclinical data in prostate cancer showed that blocking this signaling using CXCR1/2 antagonist inhibits prostate cancer indicating the possibility of blocking this signaling in various cancers [41]. Our lab has also shown in melanoma that when given orally CXCR1/2 antagonists inhibit tumor growth and metastasis in preclinical settings [40]. The CXCR2 antagonists are in phase II clinical trials for chronic obstructive pulmonary disease (COPD) [42] and there are no side effects of CXCR2 antagonists in patients with severe asthma [43] suggesting its safe usage. Ongoing developments of these agents for other diseases provide essential data regarding the efficacy and safety profile, which provide an important basis for accelerated development as a novel therapeutic modality for breast cancer.
Supplementary Material
Acknowledgments
This work was supported in part by Susan G. Komen for the Cure Grant KG090860 COBRE Grant RR021937 (Nebraska Center for Nanomedicine), and U54CA163120 and Cancer Center Support Grant (P30CA036727) from National Cancer Institute, National Institutes of Health. Bhawna Sharma was supported through University of Nebraska Medical Center Graduate Student Fellowship/Assistantship. R. K. Singh Grandly Supported by Susan G. Komen for the Cure grant KG090860.
Footnotes
Electronic supplementary material The online version of this article (doi:10.1007/s10585-014-9691-0) contains supplementary material, which is available to authorized users.
Conflict of interest Authors disclose no conflict of interest.
Contributor Information
Bhawna Sharma, Department of Medicine, University of Wisconsin Carbone Cancer Center, University of Wisconsin School of Medicine and Public Health, Madison, WI, USA.
Kalyan C. Nannuru, Regeneron Pharmaceuticals Inc., Tarrytown, NY, USA
Michelle L. Varney, Department of Pathology and Microbiology, University of Nebraska Medical Center, 985900 Nebraska Medical Center, Omaha, NE 68198-5900, USA
Rakesh K. Singh, Email: rsingh@unmc.edu, Department of Pathology and Microbiology, University of Nebraska Medical Center, 985900 Nebraska Medical Center, Omaha, NE 68198-5900, USA.
References
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013;17:10. doi: 10.3322/caac.21166. [DOI] [PubMed] [Google Scholar]
- 2.Jones SE. Metastatic breast cancer: the treatment challenge. Clin Breast Cancer. 2008;8(3):224–233. doi: 10.3816/CBC.2008.n.025. [DOI] [PubMed] [Google Scholar]
- 3.Fidler IJ. Seed and soil revisited: contribution of the organ microenvironment to cancer metastasis. Surg Oncol Clin N Am. 2001;10(2):257–269. [PubMed] [Google Scholar]
- 4.Langley RR, Fidler IJ. Tumor cell-organ microenvironment interactions in the pathogenesis of cancer metastasis. Endocr Rev. 2007;28(3):297–321. doi: 10.1210/er.2006-0027. [DOI] [PubMed] [Google Scholar]
- 5.Liotta LA, Kohn EC. The microenvironment of the tumour-host interface. Nature. 2001;411(6835):375–379. doi: 10.1038/35077241. [DOI] [PubMed] [Google Scholar]
- 6.Benoy IH, Salgado R, Van DP, Geboers K, Van ME, Scharpe S, Vermeulen PB, Dirix LY. Increased serum interleukin-8 in patients with early and metastatic breast cancer correlates with early dissemination and survival. Clin Cancer Res. 2004;10(21):7157–7162. doi: 10.1158/1078-0432.CCR-04-0812. [DOI] [PubMed] [Google Scholar]
- 7.Kozlowski L, Zakrzewska I, Tokajuk P, Wojtukiewicz MZ. Concentration of interleukin-6 (IL-6), interleukin-8 (IL-8) and interleukin-10 (IL-10) in blood serum of breast cancer patients. Rocz Akad Med Bialymst. 2003;48:82–84. [PubMed] [Google Scholar]
- 8.Sharma B, Nawandar DM, Nannuru KC, Varney ML, Singh RK. Targeting CXCR2 enhances chemotherapeutic response, inhibits mammary tumor growth, angiogenesis and lung metastasis. Mol Cancer Ther. 2013;12(5):799–808. doi: 10.1158/1535-7163.MCT-12-0529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Balkwill F. Cancer and the chemokine network. Nat Rev Cancer. 2004;4(7):540–550. doi: 10.1038/nrc1388. [DOI] [PubMed] [Google Scholar]
- 10.Lazennec G, Richmond A. Chemokines and chemokine receptors: new insights into cancer-related inflammation. Trends Mol Med. 2010;16(3):133–144. doi: 10.1016/j.molmed.2010.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Addison CL, Daniel TO, Burdick MD, Liu H, Ehlert JE, Xue YY, Buechi L, Walz A, Richmond A, Strieter RM. The CXC chemokine receptor 2, CXCR2, is the putative receptor for ELR + CXC chemokine-induced angiogenic activity. J Immunol. 2000;165(9):5269–5277. doi: 10.4049/jimmunol.165.9.5269. [DOI] [PubMed] [Google Scholar]
- 12.Heidemann J, Ogawa H, Dwinell MB, Rafiee P, Maaser C, Gockel HR, Otterson MF, Ota DM, Lugering N, Domschke W, Binion DG. Angiogenic effects of interleukin 8 (CXCL8) in human intestinal microvascular endothelial cells are mediated by CXCR2. J Biol Chem. 2003;278(10):8508–8515. doi: 10.1074/jbc.M208231200. [DOI] [PubMed] [Google Scholar]
- 13.Nannuru KC, Sharma B, Varney ML, Singh RK. Role of chemokine receptor CXCR2 expression in mammary tumor growth, angiogenesis and metastasis. J Carcinog. 2011;10:40. doi: 10.4103/1477-3163.92308. Epub@2011 Dec 31.:40-3163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Singh S, Wu S, Varney M, Singh AP, Singh RK. CXCR1 and CXCR2 silencing modulates CXCL8-dependent endothelial cell proliferation, migration and capillary-like structure formation. Microvasc Res. 2011;82(3):318–325. doi: 10.1016/j.mvr.2011.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhou SL, Dai Z, Zhou ZJ, Wang XY, Yang GH, Wang Z, Huang XW, Fan J, Zhou J. Overexpression of CXCL5 mediates neutrophil infiltration and indicates poor prognosis for hepatocellular carcinoma. Hepatology. 2012;56(6):2242–2254. doi: 10.1002/hep.25907. [DOI] [PubMed] [Google Scholar]
- 16.Acharyya S, Oskarsson T, Vanharanta S, Malladi S, Kim J, Morris PG, Manova-Todorova K, Leversha M, Hogg N, Seshan VE, Norton L, Brogi E, et al. A CXCL1 paracrine network links cancer chemoresistance and metastasis. Cell. 2012;150(1):165–178. doi: 10.1016/j.cell.2012.04.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yang L, Huang J, Ren X, Gorska AE, Chytil A, Aakre M, Carbone DP, Matrisian LM, Richmond A, Lin PC, Moses HL. Abrogation of TGF beta signaling in mammary carcinomas recruits Gr-1 + CD11b + myeloid cells that promote metastasis. Cancer Cell. 2008;13(1):23–35. doi: 10.1016/j.ccr.2007.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu L, Li M, Spangler LC, Spear C, Veenstra M, Darnall L, Chang C, Cotleur AC, Ransohoff RM. Functional defect of peripheral neutrophils in mice with induced deletion of CXCR2. Genesis. 2013;51(8):587–595. doi: 10.1002/dvg.22401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Katoh H, Wang D, Daikoku T, Sun H, Dey SK, Dubois RN. CXCR2-expressing myeloid-derived suppressor cells are essential to promote colitis-associated tumorigenesis. Cancer Cell. 2013;24(5):631–644. doi: 10.1016/j.ccr.2013.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gregory AD, Houghton AM. Tumor-associated neutrophils: new targets for cancer therapy. Cancer Res. 2011;71(7):2411–2416. doi: 10.1158/0008-5472.CAN-10-2583. [DOI] [PubMed] [Google Scholar]
- 21.Pollard JW. Tumour-educated macrophages promote tumour progression and metastasis. Nat Rev Cancer. 2004;4(1):71–78. doi: 10.1038/nrc1256. [DOI] [PubMed] [Google Scholar]
- 22.Sharma B, Singh RK. Emerging candidates in breast cancer stem cell maintenance, therapy resistance and relapse. J Carcinog. 2011;10:36. doi: 10.4103/1477-3163.91119. Epub@2011 Dec 22.:36-3163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cacalano G, Lee J, Kikly K, Ryan AM, Pitts-Meek S, Hultgren B, Wood WI, Moore MW. Neutrophil and B cell expansion in mice that lack the murine IL-8 receptor homolog. Science. 1994;265(5172):682–684. doi: 10.1126/science.8036519. [DOI] [PubMed] [Google Scholar]
- 24.Aslakson CJ, Miller FR. Selective events in the metastatic process defined by analysis of the sequential dissemination of subpopulations of a mouse mammary tumor. Cancer Res. 1992;52(6):1399–1405. [PubMed] [Google Scholar]
- 25.Wilson TJ, Nannuru KC, Futakuchi M, Sadanandam A, Singh RK. Cathepsin G enhances mammary tumor-induced osteolysis by generating soluble receptor activator of nuclear factor-{kappa}B ligand. Cancer Res. 2008;68(14):5803–5811. doi: 10.1158/0008-5472.CAN-07-5889. [DOI] [PubMed] [Google Scholar]
- 26.Varney ML, Johansson SL, Singh RK. Distinct expression of CXCL8 and its receptors CXCR1 and CXCR2 and their association with vessel density and aggressiveness in malignant melanoma. Am J Clin Pathol. 2006;125(2):209–216. doi: 10.1309/VPL5-R3JR-7F1D-6V03. [DOI] [PubMed] [Google Scholar]
- 27.Abe F, Dafferner AJ, Donkor M, Westphal SN, Scholar EM, Solheim JC, Singh RK, Hoke TA, Talmadge JE. Myeloid-derived suppressor cells in mammary tumor progression in FVB Neu transgenic mice. Cancer Immunol Immunother. 2010;59(1):47–62. doi: 10.1007/s00262-009-0719-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Brigati C, Noonan DM, Albini A, Benelli R. Tumors and inflammatory infiltrates: friends or foes? Clin Exp Metastasis. 2002;19(3):247–258. doi: 10.1023/a:1015587423262. [DOI] [PubMed] [Google Scholar]
- 29.Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420(6917):860–867. doi: 10.1038/nature01322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mantovani A, Allavena P, Sica A, Balkwill F. Cancer-related inflammation. Nature. 2008;454(7203):436–444. doi: 10.1038/nature07205. [DOI] [PubMed] [Google Scholar]
- 31.Singh S, Varney M, Singh RK. Host CXCR2-dependent regulation of melanoma growth, angiogenesis, and experimental lung metastasis. Cancer Res. 2009;69(2):411–415. doi: 10.1158/0008-5472.CAN-08-3378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Aslakson CJ, Miller FR. Selective events in the metastatic process defined by analysis of the sequential dissemination of subpopulations of a mouse mammary tumor. Cancer Res. 1992;52(6):1399–1405. [PubMed] [Google Scholar]
- 33.Strieter RM, Burdick MD, Mestas J, Gomperts B, Keane MP, Belperio JA. Cancer CXC chemokine networks and tumour angiogenesis. Eur J Cancer. 2006;42(6):768–778. doi: 10.1016/j.ejca.2006.01.006. [DOI] [PubMed] [Google Scholar]
- 34.Reutershan J, Morris MA, Burcin TL, Smith DF, Chang D, Saprito MS, Ley K. Critical role of endothelial CXCR2 in LPS-induced neutrophil migration into the lung. J Clin Invest. 2006;116(3):695–702. doi: 10.1172/JCI27009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cardona AE, Sasse ME, Liu L, Cardona SM, Mizutani M, Savarin C, Hu T, Ransohoff RM. Scavenging roles of chemokine receptors: chemokine receptor deficiency is associated with increased levels of ligand in circulation and tissues. Blood. 2008;112(2):256–263. doi: 10.1182/blood-2007-10-118497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jamieson T, Clarke M, Steele CW, Samuel MS, Neumann J, Jung A, Huels D, Olson MF, Das S, Nibbs RJ, Sansom OJ. Inhibition of CXCR2 profoundly suppresses inflammation-driven and spontaneous tumorigenesis. J Clin Invest. 2012;122(9):3127–3144. doi: 10.1172/JCI61067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ginestier C, Liu S, Diebel ME, Korkaya H, Luo M, Brown M, Wicinski J, Cabaud O, Charafe-Jauffret E, Birnbaum D, Guan JL, Dontu G, et al. CXCR1 blockade selectively targets human breast cancer stem cells in vitro and in xenografts. J Clin Invest. 2010;120(2):485–497. doi: 10.1172/JCI39397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Singh JK, Farnie G, Bundred NJ, Simoes BM, Shergill A, Landberg G, Howell SJ, Clarke RB. Targeting CXCR1/2 Significantly Reduces Breast Cancer Stem Cell Activity and Increases the Efficacy of Inhibiting HER2 via HER2-Dependent and -Independent Mechanisms. Clin Cancer Res. 2013;19(3):643–656. doi: 10.1158/1078-0432.CCR-12-1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Singh S, Nannuru KC, Sadanandam A, Varney ML, Singh RK. CXCR1 and CXCR2 enhances human melanoma tumourigenesis, growth and invasion. Br J Cancer. 2009;100(10):1638–1646. doi: 10.1038/sj.bjc.6605055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Singh S, Sadanandam A, Nannuru KC, Varney ML, Mayer-Ezell R, Bond R, Singh RK. Small-molecule antagonists for CXCR2 and CXCR1 inhibit human melanoma growth by decreasing tumor cell proliferation, survival, and angiogenesis. Clin Cancer Res. 2009;15(7):2380–2386. doi: 10.1158/1078-0432.CCR-08-2387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Liu X, Peng J, Sun W, Yang S, Deng G, Li F, Cheng JW, Gordon JR. G31P, an antagonist against CXC chemokine receptors 1 and 2, inhibits growth of human prostate cancer cells in nude mice. Tohoku J Exp Med. 2012;228(2):147–156. doi: 10.1620/tjem.228.147. [DOI] [PubMed] [Google Scholar]
- 42.Allegretti M, Cesta MC, Garin A, Proudfoot AE. Current status of chemokine receptor inhibitors in development. Immunol Lett. 2012;145(1–2):68–78. doi: 10.1016/j.imlet.2012.04.003. [DOI] [PubMed] [Google Scholar]
- 43.Nair P, Gaga M, Zervas E, Alagha K, Hargreave FE, O’Byrne PM, Stryszak P, Gann L, Sadeh J, Chanez P. Safety and efficacy of a CXCR2 antagonist in patients with severe asthma and sputum neutrophils: a randomized, placebo-controlled clinical trial. Clin Exp Allergy. 2012;42(7):1097–1103. doi: 10.1111/j.1365-2222.2012.04014.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


