Cervix cancer is a worldwide scourge. It is the second leading cause of cancer deaths in women worldwide with a rate of 728 deaths per day. 87% of cervix cancer deaths occur in low- and middle-income countries, with mortality rates varying 18-fold.1 Even in high-income nations, there has been little improvement in survival rates.2 The last major improvement in cervix cancer was published 15 years ago with the superiority of cisplatin-based chemoradiotherapy to radiotherapy alone.3,4
The Cervix Cancer Research Network (CCRN) developed within the Gynecologic Cancer Intergroup (GCIG) out of a realisation that cervical cancer trials were becoming scarce. While cervical cancer rates are declining in GCIG countries, low- and middle-income countries are coping with large disease burdens. This led to extending trial development beyond GCIG borders to involve a network of accredited, capable centres in other areas of the world. It was envisaged that this could harness local enthusiasm to raise standards of care. The CCRN would provide the infrastructure and support for high quality trials.
The CCRN recognized that many low- and middle-income countries would not be eligible to participate in trials since the degree of infrastructure required to participate would be lacking. The literature was evaluated for best practices for clinical trials within gynecologic cancers.5 The CCRN developed standard operating procedures to ensure adequate infrastructure. A pre-qualifying questionnaire was developed to evaluate potential sites addressing clinical activity, site resources, trials operation, radiation records, quality assurance, and clinical management information. Participation in a beam measurement program is required every two years to determine the stability of linear accelerators. The Imaging and Radiation Oncology Imaging Core (IROC) in Houston, Texas has partnered with CCRN to provide regular quality assurance.
Site visits are performed by an audit team, including a physician and clinical trial manager, to evaluate the appropriateness to participate in CCRN trials. The GCIG has grown to include 28 member groups, and worldwide interest in the CCRN is shown in Figure 1. To date, 17 different CCRN site visits have been performed. There are ten approved CCRN sites including Tata Memorial Hospital; Bangalore, India; Ramathibodi, Thailand; Trivandrum, India; Siriaj, Thailand; Pramongkutklao, Thailand; Ho Chi Minh, Vietnam; Blokhin Russian Cancer Research Center, the Hertzen Moscow Cancer Research Institute, and the Russian Scientific Center of Roentgenoradiology. Several additional sites have been approved with contingencies, including Cluj, Romania and Minsk, Belarus.
Figure 1.
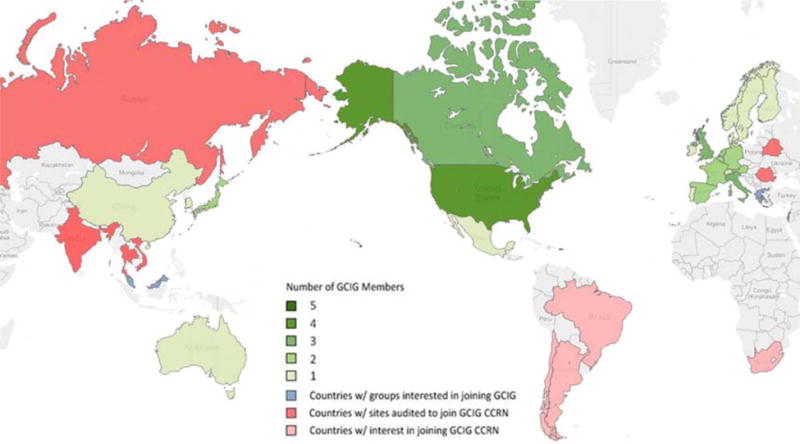
Worldwide GCIG and CCRN participation.
CCRN currently has four multinational publicly funded clinical trials that are open for enrollment in low-, middle-, and high-income countries. The TACO Trial, which was developed by investigators from the Korean Gynecologic Oncology Group (KGOG) and the Thai Cooperative Group, is comparing weekly cisplatin cancer to every-three-week chemotherapy for locally advanced cervical cancer. Cisplatin delivered every three weeks may be more effective by achieving a higher peak concentration.6 The OUTBACK Trial, sponsored by the Australia New Zealand Gynaecology Oncology Group (ANZGOG) with participation from NRG Oncology and other cooperative groups, is evaluating the efficacy of extended adjuvant chemotherapy compared to the standard weekly cisplatin chemoradiotherapy. The extended adjuvant chemotherapy consists of four cycles of carboplatin and paclitaxel. The INTERLACE Trial is headed by the National Cancer Research Institute (NCRI) from the United Kingdom. This trial is evaluating neoadjuvant chemotherapy prior to definitive chemoradiotherapy. Compliance with chemotherapy may be higher in a neoadjuvant approach than in an extended adjuvant approach. In the meta-analysis for neoadjuvant chemotherapy in locally advanced cervix cancer, a benefit was observed when the dose intensity of cisplatin was greater than 25 mg/m2 per week.8 The SHAPE Trial, led by the NCIC Clinical Trials Group in Canada, is a randomized trial evaluating radical hysterectomy versus simple hysterectomy in low risk, early-stage cervix cancer (lesions < 2 cm). The primary endpoint is pelvic relapse-free survival with secondary endpoints of quality of life, sexual health, and cost-effectiveness.9
The greatest burden of cervix cancer resides in low- and middle-income countries, most notably in Central and South America, sub-Saharan Africa, Eastern Europe, India, and parts of Asia. Simultaneously, the incidence of cervix cancer is declining in the high-income countries. Clinical research is an ongoing challenge, and requires collaboration amongst physicians, hospitals, and patients. In many countries, there are governmental barriers to trial participation and data sharing. The legislation and bureaucracy involved in clinical trials means there is significant cost associated with participation. Physicians and hospitals frequently donate their time to these important efforts, but even in developed nations governmental funding is not sufficient to cover all costs.
As technology improves, the information sharing and data collection are easier, but rigorous quality assurance is necessary to maintain the validity of the research question and the credibility of the results. Radiotherapy quality assurance in these trials is done via an internet-based approach. Many areas of the world have experienced relatively rapid economic growth and investment in health systems, which will provide more opportunity for conducting trials.
The mission of the CCRN is to promote high quality research performed in locations where there is significant need. In three years we have met with success in this endeavor: there are four fully funded cervix cancer trials; standard operating procedures; a large cadre of expert volunteer site visitors; 8 sites have been opened in 6 countries; and 50 patients have been accrued. The cost of clinical research is small compared with the cost of clinical care. Through these efforts, CCRN hopes to improve the care of women with cervical cancer worldwide. As CCRN expands, an additional goal is to provide formalized training to physicians, study staff, and patient navigators to ensure high quality care delivery and follow-up. This can be a motivational aspect for international collaboration and development of country-specific, comprehensive healthcare programs. The goal, to save more lives from cervical cancer, is one that must be achieved.
Footnotes
Conflict of Interest: None
References
- 1.Cervical Cancer Estimated Incidence, Mortality and Prevalence Worldwide in 2012. International Agency for Research on Cancer. World Health Organization Website: http://globocan.iarc.fr/old/FactSheets/cancers/cervix-new.asp. [Google Scholar]
- 2.Siegel R, Ma J, Zou Z, et al. Cancer statistics, 2014. CA Cancer J Clin. 2014;64(1):9–29. doi: 10.3322/caac.21208. [DOI] [PubMed] [Google Scholar]
- 3.Rose PG, Bundy BN, Watkins EB, et al. Concurrent cisplatin-based radiotherapy and chemotherapy forlocally advanced cervical cancer. N Engl J Med. 1999;340(15):1144–53. doi: 10.1056/NEJM199904153401502. [DOI] [PubMed] [Google Scholar]
- 4.Morris M, Eifel PJ, Lu J, et al. Pelvic radiation with concurrent chemotherapy compared with pelvic and para-aortic radiation for high-risk cervical cancer. N Engl J Med. 1999;340(15):1137–43. doi: 10.1056/NEJM199904153401501. [DOI] [PubMed] [Google Scholar]
- 5.Nag S, Dally M, de la Torre M, et al. Recommendations for implementation of high dose rate 192Ir brachytherapy in developing countries by the Advisory Group of International Atomic Energy Agency. Radiother Oncol. 2002;64(3):297–308. doi: 10.1016/s0167-8140(02)00166-4. [DOI] [PubMed] [Google Scholar]
- 6.Ryu S-Y, Lee W-M, Kim K, et al. Randomized clinical trial of weekly vs. triweekly cisplatin-based chemotherapy concurrent with radiotherapy in the treatment of locally advanced cervical cancer. Int J Radiat Oncol Biol Phys. 2011;81(4):577–81. doi: 10.1016/j.ijrobp.2011.05.002. [DOI] [PubMed] [Google Scholar]
- 7.Chemoradiotherapy for Cervical Cancer Meta-Analysis Collaboration. Reducing uncertainties about the effects of chemoradiotherapy for cervical cancer: a systematic review and meta-analysis of individual patient data from 18 randomized trials. J Clin Oncol. 2008;26(35):5802–12. doi: 10.1200/JCO.2008.16.4368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Neoadjuvant Chemotherapy for Locally Advanced Cervical Cancer Meta-analysis Collaboration. Neoadjuvant chemotherapy for locally advanced cervical cancer: a systemic review and meta-analysis of individual patient data from 21 randomised trials. Eur J Cancer. 2003;39(17):2470–86. doi: 10.1016/s0959-8049(03)00425-8. [DOI] [PubMed] [Google Scholar]
- 9.Plante M. Evolution in fertility-preserving options for early-stage cervical cancer: radical trachelectomy, simple trachelectomy, neoadjuvant chemotherapy. Int J Gynecol Cancer. 2013;23(6):982–9. doi: 10.1097/IGC.0b013e318295906b. [DOI] [PubMed] [Google Scholar]


