Abstract
Background
Gestational diabetes mellitus (GDM) is one of most common complications of pregnancy, with incidence rates varying by maternal age, race/ethnicity, obesity, parity, and family history. Given its increasing prevalence in recent decades, co-variant environmental and sociodemographic factors may be additional determinants of GDM occurrence.
Objectives
We hypothesized that environmental risk factors, in particular measures of the food environment, may be a diabetes contributor. We employed geospatial modeling in a populous U.S. county to characterize the association of the relative availability of fast food restaurants and supermarkets to GDM.
Study Design
Utilizing a perinatal database with over 4900 encoded antenatal and outcome variables inclusive of zip code data, 8912 consecutive pregnancies were analyzed for correlations between GDM and food environment based on county-wide food permit registration data. Linkage between pregnancies and food environment was achieved on the basis of validated 5 digit zip code data. The prevalence of supermarkets and fast food restaurants per 100,000 inhabitants for each zip code were gathered from publicly available food permit sources. In order to independently authenticate our findings with objective data, we measured hemoglobin A1c (HbA1c) levels as a function of geospatial distribution of food environment in a matched subset (n=80).
Results
Residence in neighborhoods with a high prevalence of fast food restaurants (fourth quartile) was significantly associated with an increased risk of developing GDM (relative to first quartile, aOR: 1.63 [95% CI 1.21–2.19]). In multivariate analysis, this association held true after controlling for potential confounders (p=0.002). Measurement of HbA1c levels in a matched subset were significantly increased in association with residence in a zip code with a higher fast food/supermarket ratio (n=80, r=0.251 p<0.05).
Conclusions
As demonstrated by geospatial analysis, a relationship of food environment and risk for gestational diabetes was identified.
Keywords: food environment, geospatial analysis, gestational diabetes, neighborhood, pregnancy outcome
INTRODUCTION
Gestational diabetes mellitus (GDM), defined as glucose intolerance first detected during pregnancy, is a prevalent pregnancy co-morbidity and strong predictor of Type II DM in both the mother and her offspring (1–5). The incidence of GDM amongst women in the United States has increased in recent decades (1–4) with higher prevalence in Hispanic and African-American populations (5–12). While there are a number of recognized risk factors, the occurrence of GDM increases by virtue of maternal age, obesity (BMI>30), race/ethnicity, smoking, and familial history. In addition, early initial studies have shown that potentially modifiable independent factors such as physical activity, diet, and low socioeconomic status are associated with the development of gestational diabetes (1–4,6,8–12). With respect to diet and of interest to our work reported herein, the influence of distance to food establishments on the diet quality of gravidae (independent of diabetes) has been described (5–14). In the non-pregnant population, there is early and initial evidence suggesting an association between food environment and later in life diabetes among non-pregnant subjects (3,13–17). However, to date no one has linked food availability in pregnancy with the occurrence of GDM.
In order to analyze the potential influence of the food environment on GDM, we performed geospatial modeling in Harris County (the third most populous U.S. county) using subject-specific zip code data. Geospatial modeling utilizes multi-modal databases in order to assess the prevalence of environmental factors in specific geographic areas with the co-occurrence of disease. When integrated with robust perinatal databases, geospatial modeling serves as an approach to investigate and evaluate previously not considered risk factors, as a study on smoking during pregnancy and antenatal care within spatial clusters has shown (3,18). In our study, we focused on the risk factors of population density, availability of healthy and unhealthy food and examined their potential relationship with GDM.
The objective of our study was to employ geospatial modeling to characterize the association of the relative availability of food choices (supermarkets and fast food restaurants) with GDM and elevated hemoglobin A1c (HbA1c) values.
MATERIALS AND METHODS
Subjects and specimens
This study population is part of an ongoing perinatal database and biobank (PeriBank) at Baylor College of Medicine. IRB approval for this study has been given by Baylor College of Medicine (H-26364). Subjects were recruited by trained PeriBank study personnel who approach eligible gravidae at the time of admission to labor and delivery. After consent was obtained, over 4,900 variables of clinical information were directly extracted from the electronic medical record and accompanying prenatal records alongside directed subject questioning. A subset of charts are routinely audited by a board-certified maternal-fetal medicine physician scientist (K.A.) to ensure quality of the data (19). For this study, extracted and analyzed clinical data include socioeconomic background (income and education), patient history (prior diseases, vaccination record, current and past smoking status, substance abuse) and residential information coded to the patient’s five-digit zip code by trimester of pregnancy for where they live and where they work. Routine screening for GDM was performed between 24 and 28 weeks of pregnancy with a 50g-Oral Glucose Challenge Test (cutoff value of 140mg/dl), followed by a 100g-Oral Glucose Tolerance Test. Blood samples were collected under a uniform protocol and stored under high integrity conditions at −80°C. Over the interval of this study, PeriBank enrolled 90.8%% of all gravidae delivering at the Baylor College of Medicine affiliated hospital, Ben Taub General Hospital (Harris Health System, Houston, TX).
Inclusion criteria
We included all singleton pregnancies enrolled between August 2011 and December 2014 (n=10208). The exclusion criteria were lack of validated zip code information (n=648), residential zip code outside of Harris County (n=533), hospitalization without delivery (n=32) and diabetes mellitus type I (n=83). Data extraction was performed for key aspects of maternal demographic and pregnancy history as described above.
Data Sources for geospatial analysis
Publicly available data sources including the 2011 and 2012 United States Census Bureau were utilized to determine the population density (number of food establishments per 100,000 inhabitants) and the availability of food establishments (here defined as the entity of fast food restaurants, supermarkets and other grocery stores) on zip code basis: ‘ZIP Code Business Patterns’ were utilized to assess economic activity of every registered establishment within zip code areas of Harris County. The United States Census Bureau databank does not include specific information on the sales volume nor affiliation to a certain chain. Food establishments were coded by the North American Industry Classification System (NAICS). We used the code 445110 for “Supermarket and other grocery (except convenience stores) stores” extraction, simply referred to as “supermarkets” throughout this manuscript. Additionally code 445120 was used for extraction of information on “convenience stores” and code 722513 was used for the extraction of numbers of “Limited Service Restaurants”, being referred to as “fast food restaurants” throughout the text. The terminus “food environment” refers to food establishments within a defined residential area.
Harris County, which includes several smaller cities besides the city of Houston (Bellaire, Pasadena, Spring, Katy, etc.) has 230 annotated zip codes, of which only 132 reflect residential areas (remainder are: P.O. box or company zip codes). The proportion of food establishments to the population within each zip code was calculated in order to obtain density specification per 100,000 inhabitants.
Assumptions that zip code data may not reflect sole exposure to food environment is justified, as inhabitants cross zip code area borders for shopping and dining, in transit to and from work, and lack of supermarkets in several less populous zip codes. To overcome this potential limitation, we employed the technique of creating ‘buffers’ (or buffer zone) around every single zip code in accordance with previously validated means (3,20,21). A buffer zone was defined as the entity of multiple neighboring zip codes that entirely surrounded one central zip code of interest. The range of zip codes in a buffer was 3–11. These buffer zones were generated by manual curation, based on current zip code maps of Harris County. In succession we approximated the density of food establishments within the corresponding ‘neighborhood’ (here defined as central zip code plus buffer zone) of a single zip code where the patients most likely conducted their grocery shopping and eating out during pregnancy. Density of food establishments in each neighborhood was calculated using a weighted mean approach (sum of food establishments in central zip code plus ‘buffer zone’ / sum of inhabitants within the central zip code plus sum of inhabitants within the ‘buffer zone’). The ‘buffer’ approach is an established method of defining neighborhoods and creating a basis for calculating density of establishments within reach of residence (3,20). Nevertheless there is no compulsory criteria available regarding appropriate buffer determination in the current literature (1,3,21). For each pregnancy zip code and neighborhood food establishment density was assigned by first trimester residential zip code. If not available, second or third trimester zip code information was utilized instead.
Correlation of gestational HbA1c levels with food environmental factors
Maternal HbA1c and total hemoglobin levels were measured in 80 serum specimens from maternal term blood samples. Specifically, HbA1c was uniformly measured in extracted specimens obtained from PeriBank using commercially available ELISA kits (CUSABIO Human glycated hemoglobin A1c ELISA Kit, CSG-E08139h, Wuhan, China) following the manufacturers’ protocols. Per measurement, 50μl of serum was used. Percentage of HbA1c was calculated according to the DCCT (Diabetes Control and Complications Trial) using the following formula: HgbA1c [%] = HgbA1c [mmol/mol Hgb]/10.929+2.15 (1,22).
Statistical Analysis
Clinical and demographic variables for all pregnancies were extracted from the PeriBank database. Subsequent handling of the data as well as statistical testing was performed using SPSS software (version 22, IBM). Descriptive statistics (frequency, median and interquartile range) were used to characterize the demographic, clinical and environmental variables of the dataset. Since all continuous variables were found to be non-normally distributed on Kolmogorov-Smirnov tests, non-parametric statistical tests were utilized for bivariate analyses. Associations between gestational diabetes occurrence and food establishment density were tested using Spearman’s Rank correlation. Prior to entering into the regression models, non-normally distributed variables were log-transformed. Multiple logistic regression analysis was performed to assess the associations between gestational diabetes and food establishment density, adjusting for race/ethnicity, education, gravidity, income, maternal age and BMI. In addition, odds ratios for GDM occurrence were calculated with stratified environmental variables on the basis of median and quartiles (above vs. below median, fourth quartile vs. first quartile). Adjusted odds ratios were calculated by correcting for the same variables as described above. Correlation between HbA1c level and food environment was assessed using Spearman’s Rank correlation, followed by multilinear regression analysis. Across all statistical analysis, p< 0.05 was accepted as significant. Values are given as count (percentage) or median (interquartile range, IQR) in the manuscript, except as indicated otherwise.
RESULTS
Study population
This is a nested cohort analysis of clinical and environmental data of 8912 gravidae with validated zip code data enrolled during the defined study period (August 2011 until December 2014). Demographic data is summarized in Table 1. The median age of our study population was 29.3 years (IQR: 24.3–33.9 years) with the majority being white Hispanic (68%), followed by white, non-Hispanic (20%), African American (9%), Asian (3%), Hawaiian/Pacific Islander (0.2%) and Native American (0.1%). The majority of our patients earned less than $34,999 per year and only a minority earned above a familial low-income level ($35,000–74,000: 2.8% and > $75,000: 1.4%). The majority of subjects (58.6%) had not completed a high school degree, were multigravida (median gravidity of 3), and were overweight in early pregnancy (median 27.3 kg/m2, 23.6–31.6) with sufficient or excessive weight gain (24.9% and 46.6%, respectively) (23). Consistent with national and regional estimates in our cohort, 12.9% of subjects carried a diagnosis of GDM, while 3.6% of our patients had preexisting diabetes mellitus type II. Delivery routes include spontaneous vaginal (72.3%), cesarean (26.3%) and operative vaginal delivery (1.2%, Table 2). With respect to newborn data of interest, 7.3% were macrosomic.
Table 1.
Demographic Characteristics of the Study Population
Table shows major demographic characteristics of our study population including diabetes associated parameters (i.e. macrosomia, delivery route).
| Demographics | Total | GDM | No GDM | GDM including Diabetes Mellitus Type II | Diabetes Mellitus Type II (no GDM) | GDM versus No GDM | GDM including Diabetes Mellitus Type II versus No GDM |
|---|---|---|---|---|---|---|---|
| Median (IQR) or % | Median (IQR) or % | Median (IQR) or % | Median (IQR) or % | Median (IQR) or % | Qui-Square or Kruskal-Wallis/Mann- Whitney-U Test | Qui-Square or Kruskal-Wallis/Mann- Whitney-U Test | |
| Number | 8912 | 1152 | 7442 | 1308 | 162 | 8594 | 8750 |
| Age | 29.29 (24.29–33.92) | 32.57 (28.54–36.34) | 28.48 (23.69–33.12) | 32.73 (28.63–36.48) | 33.49 | p <0.001 | p <0.001 |
| Gravidity | 3 (2–4) | 3 (2–5) | 3 (2–4) | 3 (2–5) | 3 (2–5) | p <0.001 | p <0.001 |
| BMI (1st trimester) | 27.29 (23.64–31.61) | 30.07 (26.58–34) | 26.6 (23.21–30.71) | 30.27 (26.72–34.15) | 30.7 (27.51–35.93) | p <0.001 | p <0.001 |
| Ethnicity | |||||||
| Hispanic | 80.2% | 86.6% | 79.0% | 86.5% | 86.4% | p < 0.001 | p < 0.001 |
| Non-Hispanic | 19.8% | 13.4% | 21.0% | 13.5% | 13.6% | ||
| Education | |||||||
| less than 12th grade | 58.6% | 62.6% | 57.6% | 62.4% | 71.8% | p = 0.005 | p = 0.003 |
| High School/GED | 27.5% | 26.2% | 27.8% | 26.6% | 20.9% | ||
| Some College | 6.4% | 6.3% | 6.5% | 6.1% | 4.5% | ||
| College Degree | 5.6% | 4.2% | 6.0% | 3.9% | 1.8% | ||
| Masters Degree | 1.1% | 0.7% | 1.1% | 1.0% | 0.9% | ||
| Doctoral/Professional | 0.8% | 0.1% | 0.9% | 0.1% | 0% | ||
| Income | |||||||
| < $34,999 | 97.2% | 96.9% | 95.5% | 97.0% | 97.8% | p = 0.028 | p = 0.019 |
| $35,000–74,999 | 2.8% | 2.5% | 3.1% | 2.5% | 2.2% | ||
| ≥ $75,000 | 1.4% | 0.5% | 1.8% | 0.5% | 0% | ||
BMI: body mass index.
Table 2.
Clinical Characteristics Stratified by Gestational Diabetes Occurence
Overview of relevant pregnancy characteristics.
| Clinical Characteristics | Total | GDM | No GDM | GDM including Diabetes Mellitus Type II | Diabetes Mellitus Type II (no GDM) | GDM versus No GDM | GDM including Diabetes Mellitus Type II versus No GDM |
|---|---|---|---|---|---|---|---|
| Median (IQR) or % | Median (IQR) or % | Median (IQR) or % | Median (IQR) or % | Median (IQR) or % | Qui-Square or Kruskal- Wallis/Mann- Whitney-U Test | Qui-Square or Kruskal- Wallis/Mann- Whitney-U Test | |
| Number | 8912 | 1152 | 7442 | 1308 | 162 | 8594 | 8750 |
| Weight gain (lbs) | 23 (13–33) | 18.0 (9–27) | 24 (15–33) | 16 (9–26) | 19 (11–30) | p < 0.001 | p < 0.001 |
| Weight gain category# | |||||||
| Insufficient | 28.5% | 37.7% | 26.8% | 37.9% | 28.8% | p < 0.001 | p < 0.001 |
| Goal | 24.9% | 25.0% | 24.8% | 25.1% | 28.8% | ||
| Excessive | 46.6% | 37.3% | 48.8% | 37.0% | 42.4% | ||
| Macrosomia | 7.3% | 9.6% | 6.7% | 9.8% | 17.5% | p < 0.001 | p < 0.001 |
| Birthweight (g) | 3295 (2977–3615) | 3325 (2995–3640) | 3294 (2972–3615) | 3330 (2995–3646) | 3252 (2902–3771) | p=0.080 | p=0.065 |
| IUGR | 0.7% | 0.7% | 0.7% | 0.6% | 0.6% | p = 0.869 | p = 0.615 |
| Gestational Age | 39 (38–40) | 38.9 (37.6–39.3) | 39.1 (38.1–40.1) | 38.9 (37.5–39.1) | 38.3 (37.0–39.0) | p<0.001 | p<0.001 |
| Preterm Birth | 10.3% | 13.4% | 9.3% | 14.2% | 21.6% | p<0.001 | p<0.001 |
| Delivery Route | |||||||
| Spontaneous Vaginal | 72.3% | 71.9% | 72.4% | 72.5% | 65.4% | p = 0.201 | p = 0.203 |
| Operative Vaginal | 1.2% | 1.8% | 1.1% | 1.8% | 2.5% | ||
| Cesarean Delivery | 26.3% | 26.2% | 26.3% | 25.7% | 32.1% | ||
| Chorioamnionitis | 0.2% | 0.3% | 0.2% | 0.4% | 0% | p = 0.173 | p = 0.094 |
| Poorly Controlled Diabetes* | 1.8% | 9.0% | 0.2% | 9.6% | 11.1% | p < 0.001 | p < 0.001 |
| Prior GDMA1 | 2.8% | 12.6% | 1.3% | 11.2% | 3.1% | p < 0.001 | p < 0.001 |
| Prior GDMA2 | 3.8% | 19.5% | 1.3% | 17.8% | 5.6% | p < 0.001 | p < 0.001 |
IUGR: intrauterine growth restriction, GDMA: gestational diabetes mellitus, *Definition: >50% of blood glucose levels persistently elevated (across multiple visits), # weight gain category as calculated based on the formula from (23).
Food density measures
Detailed fast food restaurant and supermarket information was obtained for all 132 residential zip codes in Harris County as specified in the Methods. We found that the average number of fast food restaurants per 100,000 inhabitants was 87.7 (range 62.0–121.3) across all zip codes, while the average number of supermarkets per 100,000 inhabitants was 32.4 (range 20.8–55.1) across all zip codes (average zip code population: 21,487 [15,262–27,989], Table 3). While fast food restaurants were allocated within every zip code in Harris County, eight zip codes contained no supermarkets. Calculation of fast food and supermarket density using zip code clusters reduced the variability within the dataset (fast food restaurants: 102.7 [75.5–132.5] per 100,000 inhabitants; supermarkets: 35.1 [23.3–45.9] per 100,000 inhabitants). After buffering, every neighborhood contained at least one supermarket. Overall, our study population was distributed across 130 of 132 zip code areas (median: 22.5 [10–57] patients per zip code).
Table 3.
Density of Food Choices within Zip Code Areas and Defined Neighborhoods
Density of fast food restaurants, supermarkets and fast food restaurant per supermarket ratios stratified by GDM occurrence.
| Food environmental variables | Total | GDM | No GDM | GDM including Diabetes Mellitus Type II |
|---|---|---|---|---|
| Median (IQR) or % | Median (IQR) or % | Median (IQR) or % | Median (IQR) or % | |
| Fast food restaurants per 100,000 inhabitants | ||||
| individual zip codes | 87.7 (62.0–121.3) | 84.3 (66.1–119.8) | 87.7 (62.0–121.3) | 87.7 (66.1–121.3) |
| neighborhoods | 102.7 (75.5–132.5) | 105.2 (77.5–132.5) | 100.9 (75.5–132.5) | 105.2 (77.5–132.5) |
| Supermarkets per 100,000 inhabitants | ||||
| individual zip codes | 32.4 (20.8–55.1) | 34.7 (21.0–65.6) | 32.4 (20.8–55.1) | 34.7 (20.9–65.6) |
| neighborhoods | 35.1 (23.3–45.9) | 36.2 (24.4–46.7) | 35.2 (23.3–45.9) | 36.0 (24.7–46.2) |
| Fast Food Restaurant per Supermarket ratio | ||||
| individual zip codes | 5.1 (3.1–7.3) | 5.1 (3.1–7.3) | 5.1 (3.1–7.3) | 5.1 (3.1–7.3) |
| neighborhoods | 6.0 (4.3–7.7) | 6.0 (4.6–8.0) | 6.0 (4.3–7.7) | 6.0 (4.6–8.0) |
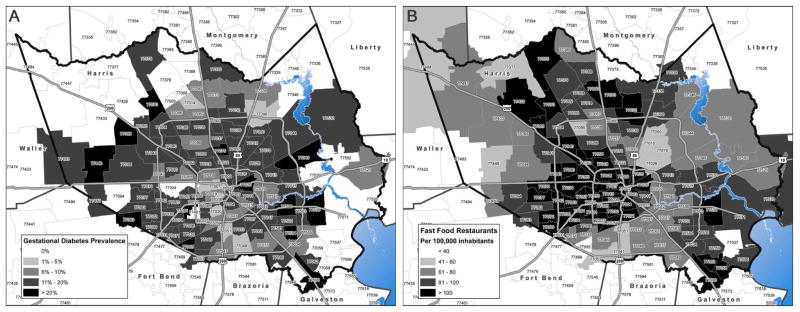
Association between the food environment and GDM
Geographical distribution of GDM prevalence (Figure 1A) and fast food restaurant density (Figure 1B) on zip code level has been visualized on a map of Harris County. The associations of GDM with clinical and demographic parameters of interest were quantitatively analyzed by bivariate statistics (Tables 1, 2). Consistent with multiple other studies to date, GDM correlated positively with gravidity, maternal age, pre or early pregnancy BMI, macrosomia of the newborn, gestational age, preterm birth as well as with history of prior GDM. Conversely, other sociodemographic variables inclusive of education attainment and household income revealed a negative correlation with GDM diagnosis.
Figure 1. Geographical distribution of gestational diabetes prevalence and fast food restaurant density within Harris County.
A) Map illustrates gestational diabetes prevalence in zip code areas of Harris County B) Map reveals fast food restaurant density (per 100,000 inhabitants) in different zip code areas of Harris County, assembled by ArcGIS 10.3 Desktop Advanced
Gestational diabetes was shown to correlate with the density of fast food restaurants (number per 100,000 inhabitants), calculated on neighborhood level (r=0.034, p=0.001) in bivariate statistics, as well as after correction for gravidity, ethnicity, education, income, maternal age, BMI, gestational age, preterm birth, gestational weight gain and prior gestational diabetes (Table 4). In order to investigate the odd’s ratios for GDM of a significant number of patients living within neighborhoods of low or high fast food restaurant densities, we compared patients living within neighborhoods of fast food densities above and below the median, as well as the highest (fourth) and lowest (first) quartiles. The odds ratios for having GDM and living in a neighborhood with a fast food density above the median versus below the median was calculated to be 1.25 (95% CI 1.11–1.40), with notable increases among those in the fourth quartile (OR 1.33, 95% CI 1.13–1.56) when comparing residency in a neighborhood with a fast food density lying in the first quartile. Adjusted odds ratios after controlling for potential confounders were aOR 1.39 (95% CI 1.12–1.73) and aOR 1.63 (95% CI 1.21–2.19) for stratification based on median and quartiles, respectively. When we alternately analyzed by neighborhood supermarket density (calculated as number of supermarkets per 100,000 inhabitants), we again observed a significant association (r= 0.054, p<0.001) in Spearman’s Rank correlation, as well by multivariate regression analysis (r=0.010, p=0.023). The density of convenience stores in zip code areas and neighborhoods was not associated with GDM (p=0.207 and p=0.458). Adjusted OR for living within a neighborhood of Harris County with a supermarket density above versus below median was 1.22 (95% CI 0.97–1.52), again with significant variation amongst the fourth quartile (aOR 1.56, 95% CI 1.15–2.13).
Table 4.
Associations Between Food Availability and GDM
Spearman’s Rank correlation and multilogistic regression analysis reveal correlations between food environment (fast food restaurants and supermarkets) and GDM.
|
|
|
|
||||||
|---|---|---|---|---|---|---|---|---|
| Food Environmental Variables | Bivariate Correlation Analysis | Multivariate Regression Analysis | Odds Ratio | |||||
| Spearman’s Rank Correlation | Multilogistic Regression | unadjusted OR (95% CI) | adjusted OR (95% CI) # | |||||
| r | Significance | r | Significance | > median vs. < median | 4th quartile vs. 1st quartile | > median vs. < median | 4th quartile vs. 1st quartile | |
| Fast food restaurants per 100,000 inhabitants | ||||||||
| individual zip codes | 0.008 | p = 0.441 | 0.001 | p = 0.032 | 0.90 (0.80–1.02) | 1.11 (0.93–1–31) | 1.04 (0.84–1.30) | 1.32 (0.97–1.79) |
| neighborhoods | 0.034 | p = 0.001 | 0.004 | p = 0.004 | 1.25 (1.11–1.40) | 1.33 (1.13–1.56) | 1.39 (1.12–1.73) | 1.63 (1.21–2.19) |
| Supermarkets per 100,000 inhabitants | ||||||||
| individual zip codes | 0.045 | p < 0.001 | 0.004 | p = 0.020 | 1.22 (1.08–1.37) | 1.35 (1.14–1.58) | 1.04 (0.84–1.30) | 1.44 (1.06–1.95) |
| neighborhoods | 0.054 | p < 0.001 | 0.010 | p = 0.023 | 1.29 (1.15–1.46) | 1.46 (1.24–1.71) | 1.22 (0.97–1.52) | 1.56 (1.15–2.13) |
| Fast Food Restaurant per Supermarket ratio | ||||||||
| individual zip codes | 0.010 | p = 0.344 | 0.000 | p = 0.970 | 1.08 (0.95–1.22) | 0.98 (0.83–1.16) | 1.14 (0.91–1.44) | 1.09 (0.79–1.49) |
| neighborhoods | 0.024 | p = 0.022 | 0.082 | p = 0.005 | 1.22 (1.08–1.37) | 1.24 (1.10–1.41) | 1.47 (1.17–1.84) | 1.42 (1.25–1.69) |
Odds Ratios (OR) demonstrate how being exposed to certain fast food restaurant and supermarket densities influences the likelihood of developing gestational diabetes.
Results of multilogistic regression analysis and adjusted odds ratios (aOR) were corrected for gravidity, ethnicity, education, income, maternal age, BMI, gestational age, preterm birth, gestational weight gain and prior gestational diabetes.
Given the risk of GDM occurrence in association with both the fourth quartile supermarket and fast food restaurant values, we calculated ratios of fast food restaurants per supermarkets by zip code and buffer. Adjusted OR for living in areas with low or high fast food restaurants per supermarket ratios were found to be 1.47 (95% CI 1.17–1.84) and 1.42 (95% CI 1.25–1.69) for stratification based on median and quartiles, respectively.
Validation by HbA1c levels
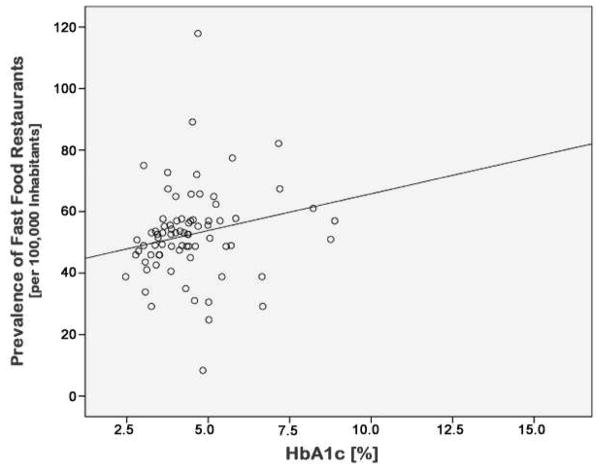
In order to independently validate the detected correlation of fast food restaurant density with gestational diabetes using objective data not potentially biased by disease diagnosis and classification, a random subset of subjects from across representative zip codes were assayed for HbA1c levels. The demographics and prevalence of GDM in this subset were similar to the total study population (data not shown). HbA1c levels were positively correlated with fast food restaurant density (Spearman’s Rank r=0.290, p=0.009, Figure 2) and fast food restaurant to supermarket ratios (r=0.247, p=0.027). While the correlation between fast food restaurant density and HbA1c levels did not persist after correction for gravidity, ethnicity, education, income, maternal age and BMI, the latter association persisted (r=0.251, r2= 0.063, p=0.025). As our aim was to demonstrate association with a higher ratio of fast food restaurants, and not to diagnose GDM nor DM per se, a correlation across the range rather than by diagnostic cut off (i.e., 6.5% HbA1c) was chosen.
Figure 2. Correlation of HbA1c levels with fast food restaurant density.
Scatterplot showing the correlation of HbA1c levels with fast-food restaurant density (Spearman’s Rank r=0.290, p=0.009).
COMMENT
Gestational diabetes is a common co-morbidity with adverse outcomes for both mother and fetus. While traditional risk factors have been extensively studied, the etiology is likely multifactorial including significant environmental influences. In our study, we focused on the influence of fast food restaurant and supermarket density on the incidence of GDM. We found that gravidae living in an area with high fast food restaurant density exhibited significantly higher GDM rates and also had a higher aOR for GDM (Table 4). These associations were further confirmed by measurement of HbA1c levels, which is commonly used as a marker for persistently elevated blood glucose levels (glycated hemoglobin). We found that HbA1c levels of subjects correlated with the fast food restaurant to supermarket ratio as well as fast food restaurant density on bivariate statistics (Figure 2). The correlation for fast food restaurant to supermarket ratio persisted after correction for gravidity, ethnicity, education, income, maternal age and BMI. The described correlation between GDM and fast food restaurant density was not seen on zip code level. Considering that zip code areas do not reflect the environmental exposure of individuals appropriately, we grant greater importance to our analysis on a neighborhood level (buffer approach)(3).
Early initial studies have shown that modifiable factors such as diet, physical activity and low socioeconomic status are independently associated with the development of GDM (6–8,10–12,24). An individual’s food environment has an influence on specific health risks. It has been reported that availability of fast food is an influencing factor on diet quality and obesity (1,25,26). Others have shown that diminished supermarket availability and proximity was found to be associated with low diet quality during pregnancy (14,25). Our study expands upon these findings, revealing that food environment, specifically the ratio of fast food restaurants to grocery stores, is a potential risk factor in the susceptibility to GDM and independent measures of HbA1c. Of interest, our study results indicate that sufficient availability of supermarkets does not necessarily correlate with a reduced number of GDM in densely populated areas like Harris County, where population exhibits high mobility (average of 2.27 vehicles per household according to DISCOS - District and County Statistics, Texas Department of Transportation: Finance Division, 2012). We understand that our patients may not only make healthy food choices at supermarkets but also purchase goods with negative impact on diet quality, which are commonly offered in a supermarket (i.e. sugary drinks, sweets, ready-to-serve meals). However, our data suggests that a high relative density of fast food restaurants is adversely associated with diet quality, as indicated by HbA1c levels, as well as higher incidence of GDM.
While we have included a significant sample size from a population-based cohort, our study does have limitations. Our study population primarily consisted of Hispanic women with a low level of education and income. Because Hispanics are a high-risk population for the development of GDM (8,27), this study is reflective of a traditionally underserved population with unique pregnancy risks. Therefore our results might not be applicable to different populations or geographic areas. Also, our most detailed patient information on residency was at the zip code level, as per our approved IRB protocol. Therefore we were not able to apply proximity approaches as a geospatial measurement from the home to the food source. However, previous research has shown that quantification of distances in the context of neighborhood research lacks precision (28) and a review of 22 studies measuring proximity to food stores and its relation to diet quality as a health outcome did not find any associations between both parameters in the majority of study results (1). Further, a study on shopping behavior revealed that residency of low-income, comparable to our own study population, do not necessarily shop at the closest food stores (29). Also, there was no information on individual food choices available within our database. Taken together, this is consistent with the validity of our geospatial modeling approach as presented herein.
In sum, we have taken an approach towards examining the association of fast food and supermarket density within residential areas at-risk for the occurrence of GDM. We have shown that relative increases in fast food density and relative availability of fast food is associated with risk of GDM, as well as elevated HbA1c levels. These data suggest that density of fast food restaurants and supermarkets may serve as unique and modifiable risk factors, and provides evidence that geospatial modeling for the purpose of analyzing food environments may offer an opportunity to identify additional community-based risk factors among gravidae. While further research is needed in order to understand the underlying mechanisms driving our observed influence of food density on GDM, these data provide early opportunities for public health and community-minded interventions.
Acknowledgments
Financial support: This work was funded by MSD/Merck Sharp & Dohme to MKK and with NIH with grant support to K.M.A. (NR014792 and DK089201).
The authors would like to thank all of our study participants for participating in our project. Also, we would like to thank the PeriBank staff for coding patient information and for the collection of blood samples.
Footnotes
Disclosure Statement: The authors report no conflicts of interest.
Paper presentation information: This work was presented as abstract #508 at the 35th Annual Scientific Meeting of the Society of Maternal Fetal Medicine; 2015 February 2-7, San Diego, California.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ni Mhurchu C, Vandevijvere S, Waterlander W, Thornton LE, Kelly B, Cameron AJ, et al. Monitoring the availability of healthy and unhealthy foods and non-alcoholic beverages in community and consumer retail food environments globally. Obesity Reviews. 2013 Sep 17;14:108–19. doi: 10.1111/obr.12080. [DOI] [PubMed] [Google Scholar]
- 2.Rosenberg TJ, Garbers S, Lipkind H, Chiasson MA. Maternal Obesity and Diabetes as Risk Factors for Adverse Pregnancy Outcomes: Differences Among 4 Racial/Ethnic Groups. Am J Public Health. 2005 Sep;95(9):1545–51. doi: 10.2105/AJPH.2005.065680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Charreire H, Casey R, Salze P, Simon C, Chaix B, Banos A, et al. Measuring the food environment using geographical information systems: a methodological review. Public Health Nutr. 2010 Apr 21;13(11):1773–85. doi: 10.1017/S1368980010000753. [DOI] [PubMed] [Google Scholar]
- 4.Hartling L, Dryden DM, Guthrie A, Muise M, Vandermeer B, Donovan L. Benefits and harms of treating gestational diabetes mellitus: a systematic review and meta-analysis for the U.S. Preventive Services Task Force and the National Institutes of Health Office of Medical Applications of Research. Ann Intern Med. 2013 Jul 16;159(2):123–9. doi: 10.7326/0003-4819-159-2-201307160-00661. [DOI] [PubMed] [Google Scholar]
- 5.Frank LD, Saelens BE, Chapman J, Sallis JF, Kerr J, Glanz K, et al. Objective Assessment of ObesogenicEnvironments in Youth. Am J Prev Med Elsevier Inc. 2012 May 1;42(5):e47–e55. doi: 10.1016/j.amepre.2012.02.006. [DOI] [PubMed] [Google Scholar]
- 6.Harizopoulou VC, Kritikos A, Papanikolaou Z, Saranti E, Vavilis D, Klonos E, et al. Maternal physical activity before and during early pregnancy as a risk factor for gestational diabetes mellitus. Acta Diabetol. 2009 Jul 18;47(S1):83–9. doi: 10.1007/s00592-009-0136-1. [DOI] [PubMed] [Google Scholar]
- 7.Bardenheier BH, Elixhauser A, Imperatore G, Devlin HM, Kuklina EV, Geiss LS, et al. Variation in Prevalence of Gestational Diabetes Mellitus Among Hospital Discharges for Obstetric Delivery Across 23 States in the United States. Diabetes Care. 2013 Apr 23;36(5):1209–14. doi: 10.2337/dc12-0901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hedderson MM, Darbinian JA, Ferrara A. Disparities in the risk of gestational diabetes by race-ethnicity and country of birth. Paediatr Perinat Epidemiol. 2010 Jul 7;24(5):441–8. doi: 10.1111/j.1365-3016.2010.01140.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Reece EA, Leguizamón G, Wiznitzer A. SeminarGestational diabetes: the need for a common ground. The Lancet Elsevier Ltd. 2009 May 23;373(9677):1789–97. doi: 10.1016/S0140-6736(09)60515-8. [DOI] [PubMed] [Google Scholar]
- 10.Zhang C, Ning Y. Effect of dietary and lifestyle factors on the risk of gestational diabetes: review of epidemiologic evidence. American Journal of Clinical Nutrition. 2011 Dec 5;94(6_Suppl):1975S–1979S. doi: 10.3945/ajcn.110.001032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sampson L, Dasgupta K, Ross NA. The association between socio-demographic marginalization and plasma glucose levels at diagnosis of gestational diabetes. Diabet Med. 2014 Jul 15;31(12):1563–7. doi: 10.1111/dme.12529. [DOI] [PubMed] [Google Scholar]
- 12.Bouthoorn SH, Silva LM, Murray SE, Steegers EAP, Jaddoe VWV, Moll H, et al. Low-educated women have an increased risk of gestational diabetes mellitus: the Generation R Study. Acta Diabetol. 2014 Oct 26; doi: 10.1007/s00592-014-0668-x. [DOI] [PubMed] [Google Scholar]
- 13.Block JP, Scribner RA, DeSalvo KB. Fast food, race/ethnicity, and income. Am J Prev Med. 2004 Oct;27(3):211–7. doi: 10.1016/j.amepre.2004.06.007. [DOI] [PubMed] [Google Scholar]
- 14.Laraia BA, Siega-Riz AM, Kaufman JS, Jones SJ. Proximity of supermarkets is positively associated with diet quality index for pregnancy. Prev Med. 2004 Nov;39(5):869–75. doi: 10.1016/j.ypmed.2004.03.018. [DOI] [PubMed] [Google Scholar]
- 15.Bodicoat DH, Carter P, Comber A, Edwardson C, Gray LJ, Hill S, et al. Is the number of fast-food outlets in the neighbourhood related to screen-detected type 2 diabetes mellitus and associated risk factors? Public Health Nutr. 2014 Oct 31;:1–8. doi: 10.1017/S1368980014002316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jago R, Baranowski T, Baranowski JC, Cullen KW, Thompson D. Distance to food stores & adolescent male fruit and vegetable consumption: mediation effects. Int J Behav Nutr Phys Act. 2007;4:35. doi: 10.1186/1479-5868-4-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bodor JN, Rice JC, Farley TA, Swalm CM, Rose D. The Association between Obesity and Urban Food Environments. J Urban Health. 2010 May 11;87(5):771–81. doi: 10.1007/s11524-010-9460-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chong S, Nelson M, Byun R, Harris L, Eastwood J, Jalaludin Bin. Geospatial analyses to identify clusters of adverse antenatal factors for targeted interventions. Int J Health Geogr International Journal of Health Geographics. 2013 Oct 24;12(1):1–1. doi: 10.1186/1476-072X-12-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Aagaard K, Ma J, Antony KM, Ganu R, Petrosino J, Versalovic J. The placenta harbors a unique microbiome. Sci Transl Med. 2014 May 21;6(237):237ra65. doi: 10.1126/scitranslmed.3008599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Urban LE, Roberts SB, Fierstein JL, Gary CE, Lichtenstein AH. Temporal Trends in Fast-Food Restaurant Energy, Sodium, Saturated Fat, and TransFat Content, United States, 1996–2013. Prev Chronic Dis. 2014 Dec 31;11:140202. doi: 10.5888/pcd11.140202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schlosser E. FAST FOOD NATION. 2012 Jan 11;:1–164. [Google Scholar]
- 22.Andersen CD, Bennet L, Nyström L, Lindblad U, Lindholm E, Groop L, et al. Worse glycaemic control in LADA patients than in those with type 2 diabetes, despite a longer time on insulin therapy. Diabetologia. 2012 Oct 25;56(2):252–8. doi: 10.1007/s00125-012-2759-y. [DOI] [PubMed] [Google Scholar]
- 23.Johnson J, Clifton RG, Roberts JM, Myatt L, Hauth JC, Spong CY, et al. Pregnancy Outcomes With Weight Gain Above or Below the 2009 Institute of Medicine Guidelines. Obstetrics & Gynecology. 2013 May;121(5):969–75. doi: 10.1097/AOG.0b013e31828aea03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Abouzeid M, Versace VL, Janus ED, Davey M-A, Philpot B, Oats J, et al. Socio-Cultural Disparities in GDM Burden Differ by Maternal Age at First Delivery. In: Iozzo P, editor. PLoS ONE. 2. Vol. 10. 2015. Feb 13, p. e0117085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Janevic T, Borrell LN, Savitz DA, Herring AH, Rundle A. Neighbourhood food environment and gestational diabetes in New York City. Paediatr Perinat Epidemiol. 2010 Apr 8;24(3):249–54. doi: 10.1111/j.1365-3016.2010.01107.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Burgoine T, Forouhi NG, Griffin SJ, Wareham NJ, Monsivais P. Associations between exposure to takeaway food outlets, takeaway food consumption, and body weight in Cambridgeshire, UK: population based, cross sectional study. BMJ. 2014 Mar 13;348(5):g1464–4. doi: 10.1136/bmj.g1464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fujimoto W, Samoa R, Wotring A. Gestational Diabetes in High-Risk Populations. Clinical Diabetes. 2013 Apr 9;31:1–5. [Google Scholar]
- 28.Apparicio P, Abdelmajid M, Riva M, Shearmur R. Comparing alternative approaches to measuring the geographical accessibility of urban health services: Distance types and aggregation-error issues. Int J Health Geogr. 2008;7(1):7. doi: 10.1186/1476-072X-7-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hillier A, Cannuscio CC, Karpyn A, McLaughlin J, Chilton M, Glanz K. How Far Do Low-Income Parents Travel to Shop for Food? Empirical Evidence from Two Urban Neighborhoods. Urban Geography. 2013 May 16;32(5):712–29. [Google Scholar]