Abstract
Background
Fetal exposure to nicotine is not limited to maternal tobacco smoke, as electronic cigarettes have an increased prevalence of use among reproductive aged women. Animal models have shown that nicotine exposure in utero is associated with increased risk of asthma and cognitive deficits, as well as increased expression of the hippocampal glucocorticoid receptor. We hypothesized that in utero nicotine exposure is associated with epigenetic changes in the offspring lung and brain which may contribute to a memory of this exposure.
Methods
Sprague-Dawley rat dams received either saline or 2mg/kg of nicotine by intraperitoneal injection once daily from embryonic day 6 (e6) to e22. Pups were sacrificed on day 1 of life, and brain and lung tissues were harvested (N=3/ group).
Results
We found that nicotine exposed offspring have altered histone modifications in the brain. Dimethylation of lysine 9 of histone H3 is decreased (0.43-fold, p=0.03) while acetylation is increased (1.79-fold, p=0.031). Histone deacetylase (HDAC) activity is significantly decreased with nicotine exposure in brain and lung (0.11-fold, p<0.001; 0.12-fold, p<0.001, respectively). Expression of splice variant 1.7 of the glucocorticoid receptor is reduced in the nicotine exposed offspring lung (0.25-fold, p=0.038).
Conclusions
We conclude that nicotine exposure is associated with epigenetic alterations in the offspring and may lead to susceptibility to adult disease,. Our finding that in utero exposure to nicotine is associated with inhibition of HDAC activity in the brain of offspring is of importance as a similar inhibition has been suggested as a mechanism for the potentiation of addiction.
Keywords: nicotine, epigenetics, developmental origins of adult disease
INTRODUCTION
Fetal exposure to nicotine is a major public health concern since more than 10% of women may smoke at some point during pregnancy (Martin et. al., 2007, Andres, 2000, Suter, 2012). Maternal cigarette smoking has long been known as a modifiable risk factor for the prevention of intrauterine growth restriction (IUGR) (Andres, 2000, Suter, 2012) and childhood asthma (Burke, 2012). Because women are encouraged to stop smoking during pregnancy, nicotine replacement therapy (NRT) is now more commonly used by pregnant women (Clark, 2011, Forinash, 2010). Furthermore, there has been an increase in electronic cigarette use by women of reproductive age (Arrazola, 2014). Electronic cigarettes are battery operated nicotine delivery systems which do not require the combustion of tobacco. Studies interrogating the effects of prenatal nicotine exposure on the fetus are of increasing relevance in perinatal health.
Because tobacco smoke contains over 4000 chemicals, animal models have helped our understanding of the specific effects of nicotine exposure on the offspring. Using a rat model of prenatal nicotine exposure, we have previously shown that nicotine can alter the developmental programming of the fetal lung (Krebs, 2010, Rehan, 2007) and that nicotine exposed offspring are susceptible to cigarette smoke exposure induced asthma (Liu, 2013). Nicotine exposure in utero has also been associated with metabolic, neurological, and behavioral consequences (Ernst, 2001, Gao, 2008, Gao, 2005, Muneoka, 2001, Seidler, 1992). Additionally, we have also shown that the asthma phenotype in nicotine-exposed pups in utero is transgenerational (Rehan, 2012). When F1 offspring were exposed to nicotine in utero and were mated, their offspring had a similar asthma phenotype, despite lack of in utero nicotine exposure.
Such transgenerational inheritance suggests an epigenetic mechanism (Leslie, 2013, Rehan, 2012). Epigenetic modifications refer to changes in chromatin structure that do not involve a change to the underlying DNA sequence. Posttranslational modifications to histone proteins, including acetylation and methylation, are potential epigenetic modifications which may contribute to maintaining a “memory” of an in utero exposure. In general, histone acetylation is associated with permissive or open chromatin structure. For example, acetylation of lysine 9 of histone H3 (H3K9ac) is enriched in the promoters of active genes (Cui, 2007, Regha, 2007). Di- and trimethylation of lysine 9 of histone H3 (H3K9me2 and H3K9me3, respectively) are enriched in the promoters of repressed genes (Le Gac, 2006, McGarvey, 2006, Regha, 2007).
Prior work has shown that the F1 generation individuals exposed to nicotine in utero have epigenetic changes in their lung, ovaries and testes (Rehan, 2012). Global DNA methylation is altered in ovary and testes, but not in lung. In the lung, H3 acetylation is increased while H4 acetylation decreased (Rehan, 2012). Such changes may act as a memory of the in utero nicotine exposure. Epigenetic changes in the brain of exposed offspring are of upmost importance. In a mouse model, nicotine administration is associated with an increased behavioral response to cocaine and increased histone H3 and H4 acetylation in the brain (Levine, 2011). Chronic nicotine administration has been found to reduce histone deacetylase (HDAC) activity (Levine, 2011).
Prenatal exposure to nicotine also has been shown to alter the hypothalamic-pituitary-adrenal (HPA) axis in offspring (Liu, 2012). Activation of the glucocorticoid receptor (GR) activates a negative feedback loop inhibiting the stress response of the HPA (Jacobson, 1991), and different splice variants of the GR show a tissue-specific distribution (Turner and Muller, 2005). . GR gene expression is increased with prenatal nicotine exposure in the hippocampus in offspring (Xu, 2012). An alteration in GR expression and splice variant specificity appears to be regulated by an epigenetic mechanism in the hypothalamus in IUGR offspring (Ke, 2010). However, it is unknown if GR expression is altered in the offspring lung with prenatal nicotine exposure.
In this study, we sought to determine which specific epigenetic changes occur in the offspring brain and lung with in utero exposure to nicotine. We found that in brain H3K9ac is increased and H3K9me2 is decreased in the nicotine exposed offspring. In both brain and lung, HDAC activity is significantly decreased with nicotine treatment. Analysis of the GR splice variants in both brain and lung revealed that splice variant 1.7 is significantly decreased in lung with nicotine exposure in the postnatal offspring.
MATERIALS AND METHODS
Animal Model
Timed mated Sprague-Dawley rat dams received either saline or 2mg/kg of nicotine by intraperitoneal injection in 100μl volumes once daily from embryonic day 6 (e6) to e22 (Krebs, 2010). Male pups were sacrificed on day 1 of life following spontaneous term delivery, and tissue was harvested (N=3 pups/ group. Each pup was from a separate dam). Control and nicotine treated dams were pair-fed, had free access to food and water, and were subjected to 12H:12H light and dark cycles. All experiments were performed in accordance with IACUC approval from Los Angeles Biomedical Research Institute and the University of Utah.
Histone Isolation and Western Blotting
Histones were isolated from day 1 lung and whole brain tissue by acid extraction as previously described (Aagaard-Tillery, 2008). Histone concentrations were determined using a BCA protein assay (Pierce Biotechnology, IL). 20mg of histones were separated on 15% SDS-PAGE gels and transferred to PVDF membranes. Blocking was carried out with freshly prepared TBS-Tween plus 3% non-fat milk. After washing, the membrane was incubated overnight with diluted primary antibodies: anti-H3K9ac (1:400; Cell Signaling, Beverly MA), anti-H3K14ac (1:5000), anti-H3KI8ac (1:500), and anti-H3 (1:2000; Millipore, Billerica, MA). Appropriate secondary antibodies conjugated with horse radish peroxidase were incubated for 1 hour at room temperature. Signals were detected using ECL. All modifications were normalized to total histone H3, and a Student's T-test was performed for each modification to determine significance.
qPCR
qPCR was carried out as previously described (Ke, 2010). RNA was extracted from tissue using the Machery Nagel kit. cDNA was made using Superscript III from Invitrogen. Primers used have been previously described, with GAPDH utilized for normalization (Ke, 2010) Student's T-tests were performed for statistical analysis to determine significance.
HDAC Assay
HDAC assays were performed as previously described (Aagaard-Tillery, 2008). Nuclear extracts were made from frozen tissue and quantified by a BCA assay (Pierce Biotechnology, IL). Approximately 80mg was used for each reaction. A commercially available HDAC activity kit from Biomol was used which measures the activity of Class I and Class II HDACs, and manufacturer's instructions were followed. For each assay, samples were assayed in triplicate. All components of the kit were defrosted and then stored on ice until use. Substrate and developer were prepared fresh according to manufacturer's instructions. Assay buffer, sample and substrate were added to each well to a final volume of 50μL with a final concentration of substrate of 1mM. The plate was incubated at 37°C for one hour. Developer was added to each well and the plate was incubated at 37°C for 15 minutes. Absorbance was read at 405nm. A Student's T-test was utilized to determine significance.
RESULTS
Prenatal nicotine exposure alters histone H3 lysine 9 methylation and acetylation in the offspring brain
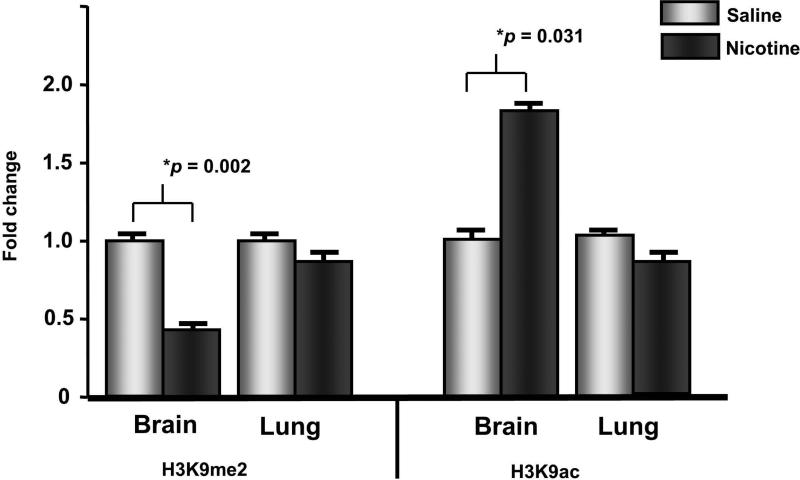
We hypothesized that in utero nicotine exposure would alter the offspring brain and lung epigenome. In order to determine which site specific modifications are altered with prenatal nicotine exposure, we utilized Western blotting on acid extracted histones from brain and lung tissue. Levels of H3K4me2, H3K4me3, H3K9me3, H3K14ac, H3K18ac and H3K27ac remained unchanged in both tissues in control and nicotine exposed offspring (Table 1). However, in the brain, levels of H3K9me2 were decreased (0.425-fold, p=0.002) while H3K9ac levels were increased (1.8-fold, p = 0.031; Figure 1) in the nicotine-exposed offspring. These modifications were not altered in the lung tissue of either controls or nicotine exposed offspring.
Table 1.
Epigenetic modifications in nicotine exposed offspring
| Modification | Tissue | Fold change | p-value |
|---|---|---|---|
| H3K4me2 | Lung | 0.943 | 0.772 |
| Brain | 1.092 | 0.656 | |
| H3K4me3 | Lung | 1.310 | 0.410 |
| Brain | 0.940 | 0.860 | |
| H3K9me2 | Lung | 0.874 | 0.370 |
| Brain | 0.425 | 0.002 | |
| H3K9me3 | Lung | 0.800 | 0.490 |
| Brain | 0.678 | 0.270 | |
| H3K14ac | Lung | 0.588 | 0.233 |
| Brain | 0.730 | 0.308 | |
| H3K18ac | Lung | 0.706 | 0.409 |
| Brain | 0.863 | 0.624 | |
| H3K9ac | Lung | 0.804 | 0.583 |
| Brain | 1.786 | 0.031 | |
| H3K27ac | Lung | 0.924 | 0.845 |
| Brain | 1.567 | 0.078 |
Figure 1. Prenatal nicotine exposure alters the brain epigeneome in postnatal offspring.
In the brain, levels of H3K4me2 are decreased while H3K9ac levels are increased with nicotine exposure compared with control. Neither modification significantly differs in offspring lung tissue. Error bars represent standard error of the mean.
HDAC activity is reduced in offspring lung and brain with prenatal nicotine exposure
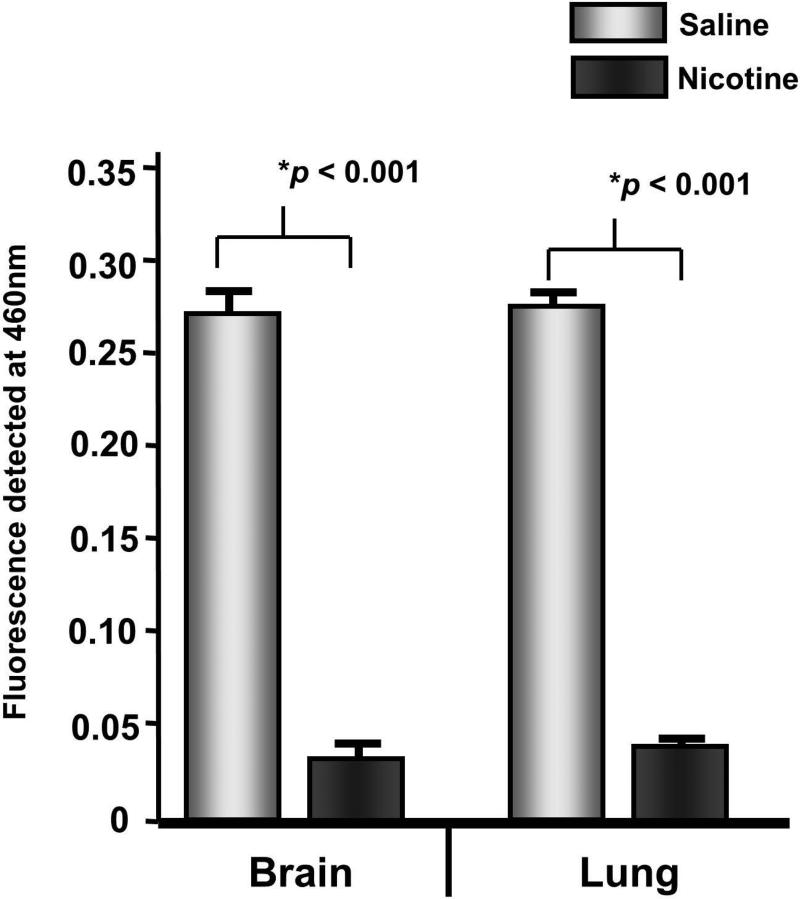
Because we observed an increase in H3K9ac in the offspring brain, we hypothesized that a decrease in HDAC activity may contribute to this increase. Using a commercially available kit, we measured HDAC activity on nuclear extracts from brain and lung tissue (Aagaard-Tillery, 2008, Suter, 2012). HDAC activity in the brain is reduced in the nicotine exposed offspring compared with control brain (0.11-fold, p<0.001) (Figure 2). In the lung, a similar reduction in HDAC activity also is observed in the nicotine exposed offspring compared to control (0.12-fold, p<0.001).
Figure 2. HDAC activity is decreased in brain and lung with nicotine exposure.
Nuclear extracts from brain and lung of nicotine exposed offspring reveals a significant decrease in HDAC activity compared with control offspring. Error bars represent standard error of the mean.
Splice variant 1.7 of the glucocorticoid receptor is significantly reduced in the offspring lung
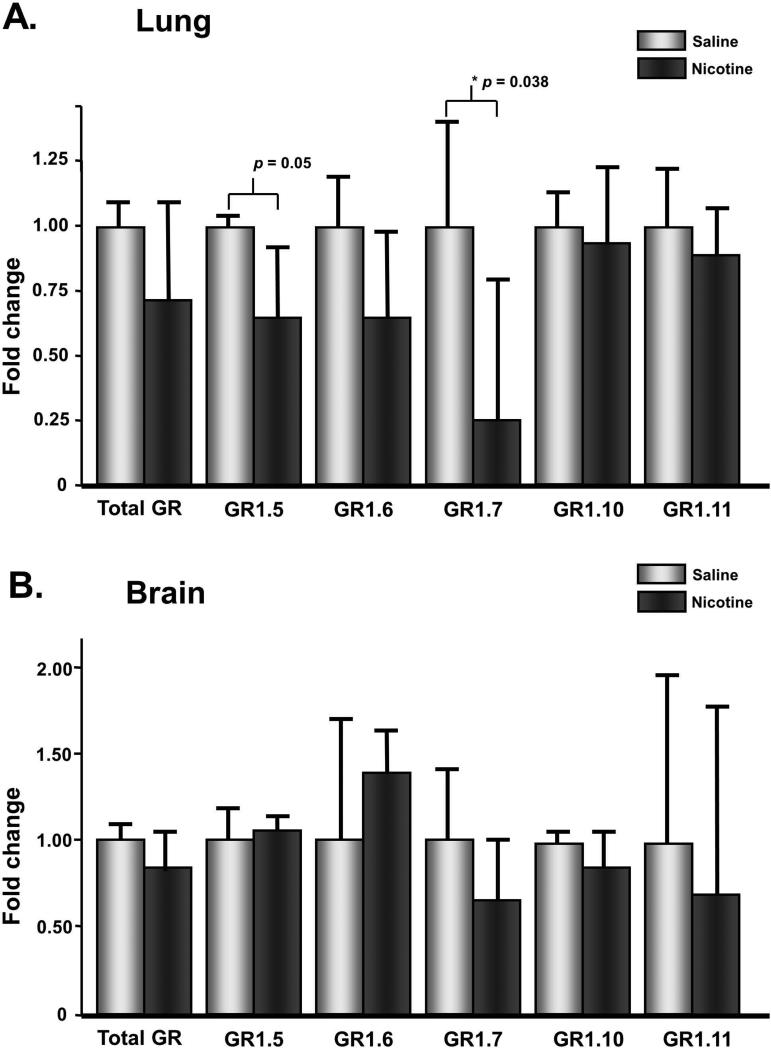
It has been previously shown that the glucocorticoid receptor has various splice variants that are associated with epigenetic changes in the GR promoter region (Ke, 2010). Differential expression of these variants has been associated with IUGR, a condition associated with prenatal tobacco smoke exposure. In order to determine if prenatal administration of nicotine alone alters GR splice variant expression, qPCR was utilized to measure levels in both brain and lung tissue. While levels did not change in the brain (Figure 3B), we found that levels of splice variant 1.7 were significantly down-regulated in lung tissue in the nicotine exposed offspring compared with control offspring (Figure 3A).
Figure 3. Levels of the splice variant 1.7 of the glucocorticoid receptor are decreased with nicotine exposure in the offspring lung.
Expression levels of various GR splice variants revealed that while there is no change in expression in the brain of nicotine exposed offspring, GR1.7 shows a significant decrease in expression in the lung compared with control animals. Error bars represent standard error of the mean, calculated from the ΔCT levels after normalizing to GAPDH.
COMMENT
In this study we have found that in utero nicotine exposure is associated with epigenetic changes in the offspring lung and brain. We utilized daily IP injections of nicotine, which is believed to cause a spike in nicotine levels similar to those seen in smokers (Mantella et. al, 2013). In the brain of 1 day old pups, H3K9me2 is decreased while H3K9ac is increased with nicotine exposure. In both the brain and lung, HDAC activity is significantly decreased with nicotine exposure. These epigenetic alterations are associated with a change in expression of the GR splice variant 1.7 in the lung.
These data lend clarity to the effects of nicotine on the offspring. While the effects of maternal smoking on the offspring have long been studied, the mechanisms by which they occur remain poorly understood. Given that combustible tobacco smoke contains more than 4000 known chemicals, it is impossible to ascribe specific effects to the direct action of nicotine in tobacco exposed infants. With the use of nicotine replacement therapies by pregnant women and the emerging popularity of electronic cigarettes among women of a reproductive age, a better understanding of the specific effects of nicotine alone on the offspring is important for determining strategies to improve public health.
The developmental origins of adult disease hypothesis postulates that in utero exposures can predispose the offspring to diseases later in life. Animal models of prenatal nicotine exposure confirm that offspring exposed in utero are more likely to suffer from asthma (Maritz, 2013) as well as memory and learning deficits later in life (Ernst, 2001). Thus, the study of epigenetic changes which occur in utero in the lung and brain with nicotine exposure is essential because it will help with the development of strategies to prevent epigenetic changes.. Equally important is how and if these modifications contribute to a memory of the exposure and are associated with the adult onset of disease.
We have found that offspring exposed to nicotine in utero have increased H3K9ac and decreased H3K9me2 in the brain. These results expand upon our previous findings that H3 acetylation is increased in the lungs and testes of offspring with nicotine exposure (Rehan, 2012). The consequences of these epigenetic alterations are currently unknown. However, in a mouse model, administration of nicotine acts to inhibit histone deacetylase activity in the brain and increase global histone acetylation in the striatum (Levine, 2011). These epigenetic alterations were shown to be associated with a behavioral alteration, specifically, a primed response to cocaine compared with controls who had not been administered nicotine.
The idea that prenatal nicotine exposure may be priming the fetal brain for other addictive drugs is an intriguing one deserving of further investigation. The social, psychological and economic burden of narcotic addiction is a major public health issue (Degenhardt, 2012). Nicotine may act as a gateway drug by virtue of molecular changes in the brain which set the stage for an enhanced response to narcotics. With in utero nicotine exposure, the gateway drug (nicotine) is taken by the mother. However, the offspring may suffer the consequences through an epigenetic reprogramming of the brain, increasing the preference for narcotics and enhancing the potential for addiction.
Concomitant with the increase in histone acetylation, we also observed a decrease in HDAC activity in both brain and lung tissue. Again, these findings are in line with the observation that nicotine administration inhibits HDAC activity in a mouse model (Levine, 2011). Because nicotine may act as an HDAC inhibitor systemically, epigenetic alterations including increased histone acetylation may be occurring in many other tissues in the exposed offspring. Rat models of prenatal nicotine exposure have shown that offspring have increased blood pressure, increased perivascular adipose tissue, and increased beta cell apoptosis (Gao, 2008, Gao, 2005, Holloway, 2005) Similarly, epigenetic modifications may play a role in these tissues after nicotine exposure.
Epigenetic modifications have also been associated with expression of specific splice variants of GR in association with IUGR in a rat model (Ke, 2010). In this model, we have shown that IUGR is associated with a global increase in H3K9ac in the brain, similar to what we have observed with nicotine exposure, as well as a decrease in expression of HDAC1 at day 0 (Ke, 2006). We also observed that there was an increase in hippocampal total GR and GR1.7 expression which was associated with an increase in H3K9ac in the promoter region at day 0 (Ke, 2010). Because nicotine has been shown to down-regulate GR expression in cell culture (Sun, 2012), and given the epigenetic changes found associated with GR expression in the IUGR model, we determined whether the various GR splice variants were altered with nicotine exposure in either brain or lung. While none of the variants were altered in the brain, nicotine exposure was associated with a down-regulation of splice variant 1.7 in the postnatal lung. While the role of the specific splice variants is unknown, it is possible that the decreased HDAC activity in the lung is altering the lung epigenome, leading to altered splicing of this gene. It is tempting to postulate that other genes may also undergo differential splicing with nicotine exposure.
While others have revealed a change in GR expression in the nicotine exposed offspring brain, they have reported these changes in a region- specific fashion. It is possible that because we utilized whole brain homogenates for these experiments, we would not be able to detect the changes which happen only in a specific brain region.
In conclusion, our findings support a model where prenatal nicotine exposure alters the fetal epigenome. These modifications may prime the offspring for adult-onset disease or a heightened response to illicit drugs, perhaps favoring addiction. As electronic cigarettes, which deliver noncombustible nicotine, become more popular among pregnant women, a better understanding of the fetal and lifelong potential effects of in utero nicotine exposure is of paramount importance.
ACKNOWLEDGEMENTS
The authors would like to thank the members of the Aagaard and Hawkins labs for their input.
Financial support: This work was funded by NIH Directors New Innovator Award DP2120OD001500-01.
Footnotes
Disclosure Statement: The authors report no conflicts of interest.
REFERENCES
- Aagaard-Tillery KM, Grove K, Bishop J, Ke X, Fu Q, McKnight R, Lane R. Developmental origins of disease and determinants of chromatin structure: maternal diet modifies the primate fetal epigenome. J Mol Endocrinol. 2008;41:91–102. doi: 10.1677/JME-08-0025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andres RL, Day M. Perinatal complications associated with maternal tobacco use. Semin Neonatol. 2000;5:231–41. doi: 10.1053/siny.2000.0025. [DOI] [PubMed] [Google Scholar]
- Arrazola RA, Kuiper NM, Dube S. Patterns of current use of tobacco products among U.S. high school students for 2000-2012--findings from the National Youth Tobacco Survey. J Adolesc Health. 2014;54:54–60 e9. doi: 10.1016/j.jadohealth.2013.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke H, Leonardi-Bee J, Hashim A, Pine-Abata H, Chen Y, Cook DG, Britton JR, McKeever T. Prenatal and passive smoke exposure and incidence of asthma and wheeze: systematic review and meta-analysis. Pediatrics. 2012;129:735–44. doi: 10.1542/peds.2011-2196. [DOI] [PubMed] [Google Scholar]
- Clark SM, Nakad R. Pharmacotherapeutic management of nicotine dependence in pregnancy. Obstet Gynecol Clin North Am. 2011;38:297–311, x.. doi: 10.1016/j.ogc.2011.02.017. [DOI] [PubMed] [Google Scholar]
- Cui L, Miao J, Furuya T, Li X, Su X. PfGCN5-mediated histone H3 acetylation plays a key role in gene expression in Plasmodium falciparum. Eukaryot Cell. 2007;6:1219–27. doi: 10.1128/EC.00062-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Degenhardt L, Hall W. Extent of illicit drug use and dependence, and their contribution to the global burden of disease. Lancet. 2012;379:55–70. doi: 10.1016/S0140-6736(11)61138-0. [DOI] [PubMed] [Google Scholar]
- Ernst M, Moolchan ET, Robinson M. Behavioral and neural consequences of prenatal exposure to nicotine. J Am Acad Child Adolesc Psychiatry. 2001;40:630–41. doi: 10.1097/00004583-200106000-00007. [DOI] [PubMed] [Google Scholar]
- Forinash AB, Pitlick JM, Clark K, Alstat V. Nicotine replacement therapy effect on pregnancy outcomes. Ann Pharmacother. 2010;44:1817–21. doi: 10.1345/aph.1P279. [DOI] [PubMed] [Google Scholar]
- Gao YJ, Holloway AC, Su LY, Takemori K, Lu C, Lee R. Effects of fetal and neonatal exposure to nicotine on blood pressure and perivascular adipose tissue function in adult life. Eur J Pharmacol. 2008;590:264–8. doi: 10.1016/j.ejphar.2008.05.044. [DOI] [PubMed] [Google Scholar]
- Gao YJ, Holloway AC, Zeng ZH, Lim GE, Petrik JJ, Foster WG, Lee R. Prenatal exposure to nicotine causes postnatal obesity and altered perivascular adipose tissue function. Obes Res. 2005;13:687–92. doi: 10.1038/oby.2005.77. [DOI] [PubMed] [Google Scholar]
- Holloway AC, Lim GE, Petrik JJ, Foster WG, Morrison KM, Gerstein H. Fetal and neonatal exposure to nicotine in Wistar rats results in increased beta cell apoptosis at birth and postnatal endocrine and metabolic changes associated with type 2 diabetes. Diabetologia. 2005;48:2661–6. doi: 10.1007/s00125-005-0022-5. [DOI] [PubMed] [Google Scholar]
- Jacobson L, Sapolsky R. The role of the hippocampus in feedback regulation of the hypothalamic-pituitary-adrenocortical axis. Endocr Rev. 1991;12:118–34. doi: 10.1210/edrv-12-2-118. [DOI] [PubMed] [Google Scholar]
- Ke X, Lei Q, James SJ, Kelleher SL, Melnyk S, Jernigan S, Yu X, Wang L, Callaway CW, Gill G, Chan GM, Albertine KH, McKnight RA, Lane RH. Uteroplacental insufficiency affects epigenetic determinants of chromatin structure in brains of neonatal and juvenile IUGR rats. Physiol Genomics. 2006;25:16–28. doi: 10.1152/physiolgenomics.00093.2005. [DOI] [PubMed] [Google Scholar]
- Ke X, Schober ME, McKnight RA, O'Grady S, Caprau D, Yu X, Callaway CW, Lane R. Intrauterine growth retardation affects expression and epigenetic characteristics of the rat hippocampal glucocorticoid receptor gene. Physiol Genomics. 2010;42:177–89. doi: 10.1152/physiolgenomics.00201.2009. [DOI] [PubMed] [Google Scholar]
- Krebs M, Sakurai R, Torday JS, Rehan V. Evidence for in vivo nicotine-induced alveolar interstitial fibroblast-to-myofibroblast transdifferentiation. Exp Lung Res. 2010;36:390–8. doi: 10.3109/01902141003714023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Gac G, Esteve PO, Ferec C, Pradhan S. DNA damage-induced down-regulation of human Cdc25C and Cdc2 is mediated by cooperation between p53 and maintenance DNA (cytosine-5) methyltransferase 1. J Biol Chem. 2006;281:24161–70. doi: 10.1074/jbc.M603724200. [DOI] [PubMed] [Google Scholar]
- Leslie FM. Multigenerational epigenetic effects of nicotine on lung function. BMC Med. 2013;11:27. doi: 10.1186/1741-7015-11-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine A, Huang Y, Drisaldi B, Griffin EA, Jr., Pollak DD, Xu S, Yin D, Schaffran C, Kandel DB, Kandel ER. Molecular mechanism for a gateway drug: epigenetic changes initiated by nicotine prime gene expression by cocaine. Sci Transl Med. 2011;3:107ra109. doi: 10.1126/scitranslmed.3003062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Naeem E, Tian J, Lombardi V, Kwong K, Akbari O, Torday JS, Rehan V. Sex-specific perinatal nicotine-induced asthma in rat offspring. Am J Respir Cell Mol Biol. 2013;48:53–62. doi: 10.1165/rcmb.2011-0344OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L, Liu F, Kou H, Zhang BJ, Xu D, Chen B, Chen LB, Magdalou J, Wang H. Prenatal nicotine exposure induced a hypothalamic-pituitary-adrenal axis-associated neuroendocrine metabolic programmed alteration in intrauterine growth retardation offspring rats. Toxicol Lett. 2012;214:307–13. doi: 10.1016/j.toxlet.2012.09.001. [DOI] [PubMed] [Google Scholar]
- Maritz GS. Perinatal exposure to nicotine and implications for subsequent obstructive lung disease. Paediatr Respir Rev. 2013;14:3–8. doi: 10.1016/j.prrv.2012.03.006. [DOI] [PubMed] [Google Scholar]
- Martin JA, Hamilton BE, Sutton PD, Ventura SJ, Menacker F, Kirmeyer S, Munson M. Births: final data for 2005. Natl Vital Stat Rep. 2007;56:1–103. [PubMed] [Google Scholar]
- Mantella NM, Kent PF, Youngentob S. Fetal nicotine exposure increases preference for nicotine odor in early postnatal and adolescent, but not adult, rats. PLoS One. 2013;8:e84989. doi: 10.1371/journal.pone.0084989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGarvey KM, Fahrner JA, Greene E, Martens J, Jenuwein T, Baylin S. Silenced tumor suppressor genes reactivated by DNA demethylation do not return to a fully euchromatic chromatin state. Cancer Res. 2006;66:3541–9. doi: 10.1158/0008-5472.CAN-05-2481. [DOI] [PubMed] [Google Scholar]
- Muneoka K, Ogawa T, Kamei K, Mimura Y, Kato H, Takigawa M. Nicotine exposure during pregnancy is a factor which influences serotonin transporter density in the rat brain. Eur J Pharmacol. 2001;411:279–82. doi: 10.1016/s0014-2999(00)00925-0. [DOI] [PubMed] [Google Scholar]
- Regha K, Sloane MA, Huang R, Pauler FM, Warczok KE, Melikant B, Radolf M, Martens JH, Schotta G, Jenuwein T, Barlow D. Active and repressive chromatin are interspersed without spreading in an imprinted gene cluster in the mammalian genome. Mol Cell. 2007;27:353–66. doi: 10.1016/j.molcel.2007.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rehan VK, Liu J, Naeem E, Tian J, Sakurai R, Kwong K, Akbari O, Torday J. Perinatal nicotine exposure induces asthma in second generation offspring. BMC Med. 2012;10:129. doi: 10.1186/1741-7015-10-129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rehan VK, Wang Y, Sugano S, Santos J, Patel S, Sakurai R, Boros LG, Lee WP, Torday J. In utero nicotine exposure alters fetal rat lung alveolar type II cell proliferation, differentiation, and metabolism. Am J Physiol Lung Cell Mol Physiol. 2007;292:L323–33. doi: 10.1152/ajplung.00071.2006. [DOI] [PubMed] [Google Scholar]
- Seidler FJ, Levin ED, Lappi SE, Slotkin T. Fetal nicotine exposure ablates the ability of postnatal nicotine challenge to release norepinephrine from rat brain regions. Brain Res Dev Brain Res. 1992;69:288–91. doi: 10.1016/0165-3806(92)90170-2. [DOI] [PubMed] [Google Scholar]
- Sun LC, Lin JT, Li W, Zhang L, Zhou TL, Zhang X. Nicotine inhibits histone deacetylase 6 activity and chaperone-dependent activation of the glucocorticoid receptor in A549 cells. Chin Med J (Engl) 2012;125:662–6. [PubMed] [Google Scholar]
- Suter MA, Aagaard K. What changes in DNA methylation take place in individuals exposed to maternal smoking in utero? Epigenomics. 2012;4:115–8. doi: 10.2217/epi.12.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suter MA, Chen A, Burdine MS, Choudhury M, Harris RA, Lane RH, Friedman JE, Grove KL, Tackett AJ, Aagaard K. A maternal high-fat diet modulates fetal SIRT1 histone and protein deacetylase activity in nonhuman primates. Faseb J. 2012;26:5106–14. doi: 10.1096/fj.12-212878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner JD, Muller C. Structure of the glucocorticoid receptor (NR3C1) gene 5’ untranslated region: identification, and tissue distribution of multiple new human exon 1. J Mol Endocrinol. 2005;35(2):283–92. doi: 10.1677/jme.1.01822. [DOI] [PubMed] [Google Scholar]
- Xu D, Liang G, Yan YE, He WW, Liu YS, Chen LB, Magdalou J, Wang H. Nicotine-induced over-exposure to maternal glucocorticoid and activated glucocorticoid metabolism causes hypothalamic-pituitary-adrenal axis-associated neuroendocrine metabolic alterations in fetal rats. Toxicol Lett. 2012;209:282–90. doi: 10.1016/j.toxlet.2012.01.006. [DOI] [PubMed] [Google Scholar]