Abstract
Mitochondrial dysfunction plays a key role in the progression of Alzheimer’s disease (AD). The accumulation of amyloid-beta peptide (Aβ) in the brains of AD patients is thought to be closely related to neuronal mitochondrial dysfunction and oxidative stress. Therefore, protecting mitochondria from Aβ-induced neurotoxicity is an effective strategy for AD therapeutics. In a previous study, we found that geniposide, a pharmacologically active compound purified from gardenia fruit, has protective effects on oxidative stress and mitochondrial dysfunction in AD transgenic mouse models. However, whether geniposide has a protective effect on Aβ-induced neuronal dysfunction remains unknown. In the present study, we demonstrate that geniposide protects cultured primary cortical neurons from Aβ-mediated mitochondrial dysfunction by recovering ATP generation, mitochondrial membrane potential (MMP), and cytochrome c oxidase (CcO) and caspase 3/9 activity; by reducing ROS production and cytochrome c leakage; as well as by inhibiting apoptosis. These findings suggest that geniposide may attenuate Aβ-induced neuronal injury by inhibiting mitochondrial dysfunction and oxidative stress.
Introduction
Alzheimer’s disease (AD) is a progressive neurodegenerative disorder characterized by the clinical manifestation of severe memory impairment, cognitive deficits and personality changes [1–5]. The pathologic characteristics of AD are amyloid-beta peptide (Aβ) deposition, neurofibrillary tangle (NFT) formation and neuronal loss [6, 7].
To meet the high energy demands of brain, mitochondria play an important role in central nervous system neurons. Moreover, it is reported that molecular indices of mitochondrial dysfunction occur early in AD and worsen with progression [8–11]. Recently, increasing evidence suggested that Aβ is the key factor contributing to mitochondrial dysfunction [7, 12–14]. There are several ways for Aβ to damage neuronal mitochondria, cell membrane, cellular matrix, intermembrane space, outer/inner mitochondrial membrane, and the matrix. Among them, a transmembrane receptor of the immunoglobulin super family, receptor for advanced glycation end products (RAGE) play an important role in mediating Aβ-induced mitochondrial dysfunction [15].
The binding of Aβ and membranal RAGE can activate NADPH oxidase which is one of the major source of ROS [16]. Over-producted ROS may cause mitochondrial dysfunction due to lowered ETC enzyme activities. In addition, RAGE mediate the tranlocation of Aβ across the cytomembrane from extracellular to intracellular [17]. Aβ and amyloid precursor protein (APP) proteins were found localizing and accumulating in the mitochondrial membrane and matrix, which directly cause a series of terrible outcomes, such as opening the mitochondrial permeability transition pore (mtPTP), disrupting the electron transport chain (ETC), and reducing cytochrome c oxidase (CcO) activity. These problems may result in loss of mitochondrial membrane potential (MMP), decreased ATP production, increased levels of reactive oxygen species (ROS) and impaired calcium homeostasis, leading to neuronal apoptosis and the aggravating pathology changes of AD [13, 14, 18–23]. Therefore, protecting mitochondria against the structural and functional damage of Aβ is an effective strategy for AD therapeutics.
Geniposide is an iridoid glucoside isolated from the gardenia fruit (Gardenia jasminoides Ellis, Rubiaceae) and has diverse pharmacological capabilities including anti-inflammatory [24], anti-oxidation [25] and anti-tumour [26] effects, as well as neurotrophic and neuroprotective properties. As we previously reported, geniposide could inhibit both oxidative stress and mitochondrial dysfunction and could improve cognition in an Alzheimer’s disease mouse model [27, 28]. Furthermore, our studies suggest that geniposide inhibits the interaction of Aβ and RAGE [29]. In addition, the ability to cross the blood-brain barrier (BBB) allows geniposide to enter the central nervous system and act on neurons or other brain cells [30, 31]. Therefore, we wonder whether geniposide has any neuroprotective effect on Aβ-induced mitochondrial dysfunction. In the present study, by cultured primary cortical neurons, we investigated the effects of geniposide on oligomeric Aβ-induced mitochondrial dysfunction related to neurotoxicity and apoptosis. Our results show that geniposide treatment significantly attenuates neuronal apoptosis by ameliorating mitochondrial dysfunction.
Material and Methods
Reagents
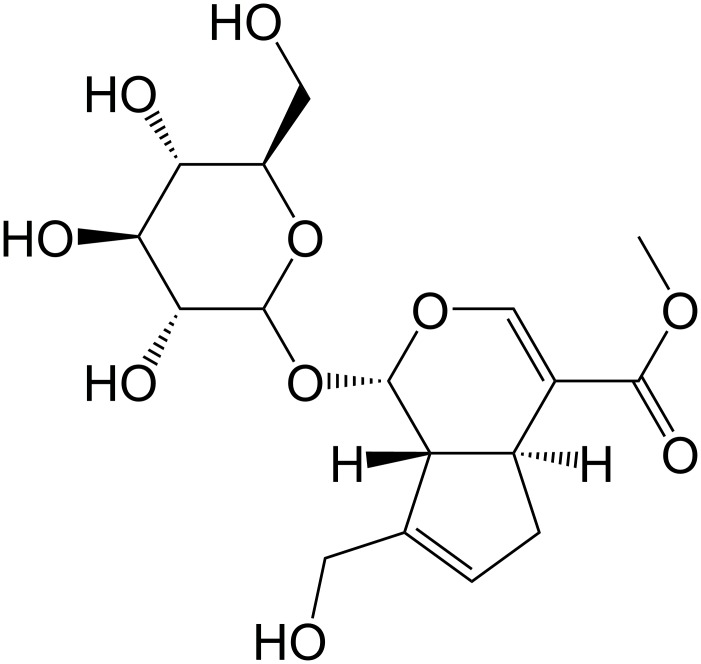
Geniposide (Purity: > 98%, Fig 1) was purchased from the National Institute for the Control of Pharmaceutical and Biological Products (Beijing, China) and was free of endotoxin. 1,1,1,3,3,3-Hexafluoro-2-propanal (HFIP), 3-(4, 5-dimethylthiazol-2-yl)-2,5-diphenyltetrazo-lium (MTT) and penicillin/streptomycin were from Sigma (St. Louis, MO, USA). Fetal bovine serum (FBS), B27, and Neurobasal medium were from Gibco. Monoclonal mouse antibody against cytochrome c (1:1000 in PBST containing 0.1% Tween-20 and 5% skimmed milk, Cat# ab110325) [32] and cytochrome c oxidase (CcO, 1:500 in PBST containing 0.1% Tween-20 and 5% skimmed milk, Cat# ab110259) [33] were purchased from Abcam (Cambridge, UK). Monoclonal mouse antibodies against Bcl-2 (B-cell lymphoma-2, 1:500 in PBST containing 0.1% Tween-20 and 5% skimmed milk, Cat# SC-509) [34], Bax (Bcl-2 associated X protein, 1:500 in PBST containing 0.1% Tween-20 and 5% skimmed milk, Cat# SC-20067) [35] and β-actin (1:2000 in PBST containing 0.1% Tween-20 and 5% skimmed milk, Cat# SC-47778) [36] were purchased from Santa Cruz (CA, USA). Monoclonal goat anti-mouse (1:10000 in PBST containing 0.1% Tween-20 and 5% skimmed milk) secondary antibody were provided by GE Healthcare (Buckinghamshire, UK). The kits employed to isolate the mitochondria and to detect the levels of MDA, caspase 3/9 and the degree of mitochondrial oxidative damage were purchased from Shanghai GENMED Pharmaceutical Technology Co., Ltd. (Shanghai, China). The kits employed to detect the level of CcO activity and ATP were purchased from Sigma (St. Louis, MO, USA) and Roche (Indianapolis, USA), respectively. 2’,7’-Dichlorodihydrofluorescein diacetate (DCFH-DA) was purchased from Sigma Chemical Co. (St. Louis, MO, USA). All other chemicals were reagent grade.
Fig 1. Chemical structure of geniposide.
Oligomer Aβ preparation
Oligomeric Aβ1–42 was prepared from commercially available synthetic peptides (Sigma Chemical Co., St. Louis, MO, USA) as described [37, 38]. Briefly, lyophilized peptide was resuspended in cold 1,1,1,3,3,3-hexafluoro-2-propanol (HFIP) at a concentration of 1 mg/mL and aliquoted into microcentrifuge tubes to obtain 0.1 mg stocks quickly. The stocks were stored at room temperature and protected from light for 2–24 hours before removing HFIP under gentle vacuum, leaving a thin transparent film of peptides at the internal surface of the tube, and then stored at -20°C. For the aggregation protocols, HFIP-treated peptide was dissolved in anhydrous dimethyl sulphoxide (DMSO) at 5 mM and diluted to 100 μM in PBS, pH 7.4. Diluted peptides were then incubated at 4°C for 24 hours to obtain oligomeric Aβ1–42.
Cortical neuron culture and treatment
All animal procedures were carried out in accordance with the National Institute of Health Guide for the Care and Use of Laboratory Animals (NIH Publications No. 80–23, revised 1996), and the Beijing Normal University Laboratory Animals Care and Use Committee approved the study protocol. Animals were sacrificed by cervical dislocation, All efforts were made to minimize the number of animals used and their suffering.
Male and female C57BL/6 mice, aged 6–8 weeks and purchased from Beijing HFK Bio-Technology. Co. Ltd., were housed 2 per cage at an ambient temperature of 23 ± 1°C and relative humidity of 55 ± 5% under a 12 h light/dark cycle. Animals had free access to water and food.
Primary cortical neurons were prepared from the cortices of 1-day-old newborn pups. Briefly, the cortices were dissected in cold PBS. Tissues were collected and washed in PBS, and 0.05% (v/v) trypsin was added for digestion at 37°C for 15 minutes. The digestion was stopped by the addition of fetal bovine serum to a final concentration of 10% (v/v). Cells were collected by centrifugation at 800 x g for 10 minutes to remove the PBS and were resuspended in Neurobasal medium supplemented with 2% (v/v) B27.
Cells were plated onto pre-coated poly-D-lysine (10 μg/ml) 6-, 24- or 96-well plates (~ 5×104 cells/ml) for different analyses. Cells were cultured at 37°C and 5% CO2 until use. Half of the initial medium was removed at day 2 and replaced with fresh medium. After seven days, the medium was replaced with Neurobasal medium without serum and phenol red (which affects the aggregation of Aβ). To probe the cytotoxicity of Aβ aggregates, neurons were incubated with the indicated concentrations of Aβ1–42 aggregates for 24 h, and cell viability was determined using the MTT assay. To explore the protection of geniposide on Aβ1–42 aggregate-treated neurons, primary cultured cortical neurons were pre-incubated for 1 h in the absence or presence of geniposide (2.5, 5, and 10 μM) before the addition of oligomeric Aβ1–42 (5 μM) for 24 h.
Identification of cultured cortical neurons
Neurons plated on Greiner CELLview dish were rinsed with PBS three times and then fixed in 4% paraformaldehyde for 25 min at 4°C. Fixed cells were incubated in 0.1% (v/v) sodium citrate contain 0.1% (v/v) TritonX-100 for 2 min on ice, and then neurons were washed twice with PBS and incubated with 300 μl Anti-NeuN (1:500 in PBS containing 10% goat serum, Cat# ab177487) monoclonal primary rabbit antibody for 2 hr at 37°C. An Alexa Fluor® 594-conjugated goat anti-rabbit IgG (1/1000) was used as the secondary antibody. DAPI (4',6-diamidino-2-phenylindole) was added for 10 min at room temperature followed by PBS washing to fluorescently label nuclei. Samples were photographed using a fluorescence microscope (LEICA DMI3000, Japan) and analysed using the Leica application suite. Image J software was used for counting cells. Three independent experiments were performed, and cells were counted in three fields per well. Data were expressed as the ratio of NeuN labelled neurons to total neurons.
MTT
To explore the cytotoxicity of oligomeric Aβ1–42 and geniposide, as well as the effect of geniposide on cell viability induced by oligomeric Aβ1–42, cell viability was determined by the MTT colourimetric assay after the cells were treated. Cells were incubated for 4 h at 37°C with MTT (0.5 mg/ml final concentration) and dissolved in fresh complete medium in which metabolically active cells reduced the dye to purple formazan. Formazan crystals were dissolved with DMSO, and the absorbance was measured on a microplate reader (Thermo Multiskan MK3, USA) using a reference wavelength of 630 nm and a test wavelength of 570 nm.
Assessment of Apoptosis
Apoptosis of Aβ1–42-treated neurons was detected with the In Situ Cell Death Detection Kit, Fluorescein (Roche, USA) according to the manufacturer's protocol. Briefly, neurons were rinsed with PBS three times and then fixed in 4% paraformaldehyde for 25 min at 4°C. Fixed cells were incubated in 0.1% (v/v) sodium citrate contain 0.1% (v/v) TritonX-100 for 2 min on ice, and then neurons were washed twice with PBS and incubated with 50 μl TUNEL reaction mixture for 60 min at 37°C in a humidified chamber in the dark. DAPI was added for 10 min at room temperature followed by PBS washing to fluorescently label nuclei. Negative controls were performed by adding 50 μl label solution at the same time. Samples were photographed using a fluorescence microscope (LEICA DMI3000, Japan) and analysed using the Leica application suite. Image J software was used for counting cells. Three independent experiments were performed, and cells were counted in three fields per well. Data were expressed as the ratio of apoptotic neurons to total neurons.
ROS Measurement
Reactive oxygen species (ROS) were measured with 2’,7’-dichlorodihydrofluorescein diacetate (DCFH-DA), which detects most ROS including hydrogen peroxide, peroxyl radicals, and peroxynitrite anions. The cell-permeable DCFH-DA crosses into the cytoplasm, where it is de-esterified by cellular esterase resulting in DCFH. In turn, DCFH is converted upon oxidation to the highly fluorescent DCF. By measuring the fluorescence, we were able to quantify the levels of ROS. After incubation with Aβ1–42 for varying lengths of time, neurons were washed with PBS and incubated with 10 μM non-fluorescent DCFH-DA for 30 min at 37°C in the incubator with 5% CO2. The fluorescence intensity of DCF was measured with 485 nm excitation and 528 nm emission on a fluorescence microplate reader (BioTek Senergy HT, Vermont, USA). ROS levels are presented as arbitrary fluorescence units (AFU).
Measurement of MDA
The levels of malondialdehyde (MDA) were measured using commercially available assay kits according to the instructions recommended by the manufacturers. In brief, cells were washed with PBS and collected with the assay buffer provided in the kits and then further homogenized by sonication using 5 pulses of 30 seconds each on ice. The homogenates were centrifuged at 3000 x g for 20 min at 4°C to obtain the supernatant. All protein concentrations of cell homogenate samples were determined by the BCA method, and then samples were diluted with the appropriate buffer solution to determine the level of MDA. The optical density (OD) of the microplate was read at 532 nm.
Mitochondria Membrane Potential
Loss of mitochondrial membrane potential was determined using the JC-1 mitochondrial transmembrane potential detection kit (Cell Technology, Inc.) according to the manufacturer’s instructions. Briefly, cells were plated and cultured in glass-bottom dishes with four chambers. After cells were washed with PBS, 0.5 mL of 1× JC-1 reagent was added to each chamber. Then, cells were incubated at 37°C in a 5% CO2 incubator for 20 min. The cells were then washed with assay buffer (1×) and measured with a laser-scanning confocal microscope. Red fluorescence (excitation 550 nm, emission 600 nm) and green fluorescence (excitation 485 nm, emission 535 nm) were measured. The ratio of red to green fluorescence, an indicator for membrane potential, was determined. The ratio was decreased in dead cells and in cells undergoing apoptosis compared to healthy cells.
Measurement of Cytochrome c Oxidase (CcO) activity
CcO activity was measured according to a previously published method [39]. Briefly, neuronal cultures in 6-well plates were washed with ice-cold PBS, and cells were harvested, centrifuged, and suspended in 50 μl of isolation buffer containing 250 mM sucrose, 20 mM HEPES, pH 7.2 and 1 mM EDTA. Cell suspensions (containing ~3–4 mg of protein/ml) were added to a cuvette containing 0.95 ml of 1× assay buffer (10 mM Tris-HCl, pH 7.0, and 120 mM KCl), and the reaction volume was brought to 1.05 ml with 1× enzyme dilution buffer (10 mM Tris-HCl, pH 7.0). The reaction was started by the addition of 50 μl of ferrocytochrome c substrate solution (0.22 mM) (from Sigma). The change in absorbance of cytochrome c at 550 nm was measured using a UV-2450 spectrophotometer (Shimadzu Company, JAPAN). The reading was recorded every 10 seconds during the first 3 min. Background levels were measured without cell suspensions.
ATP Measurement
ATP levels were determined using a commercial kit (Roche, Indianapolis, USA). Briefly, neurons were treated with geniposide and/or oligomeric Aβ1–42 for different lengths of time. Neurons were harvested, centrifuged and diluted at a concentration of 1 × 104 cells/ml. The same volume of cell lysis reagent was added to the samples and then incubated for 5 min at 25°C. An appropriate volume of luciferase reagents was added to the samples, and readings were recorded consecutively from 1 to 10 seconds with an interval of 1 second using the fluorescence microplate reader (BioTek Senergy HT, Vermont, USA). The changes between different groups were compared.
Isolation of mitochondrial and cytosolic fractions
The Mitochondrial Fractionation Kit (Active Motif, Carlsbad, CA, USA) was used to isolate mitochondrial and cytosolic fractions from cells according to the manufacturer’s instructions. Briefly, the treated neurons were scraped and centrifuged twice at 600 × g for 5 min. Ice-cold 1× cytosolic buffer was added, and the cell pellet was resuspended and incubated on ice for 15 min. Cells were homogenized, and the lysate was centrifuged twice at 800 × g for 20 min. The resultant supernatant contained the cytosol, including mitochondria; the supernatant was centrifuged at 10,000 × g for 20 min to pellet the mitochondria. The mitochondrial pellet was washed and centrifuged with 1× cytosolic buffer at 10,000 × g for 10 min and then lysed by adding Complete Mitochondria Buffer from the kit, followed by incubation on ice for 15 min, the result of which was the mitochondrial fraction. At the same time, the supernatant was centrifuged at 16,000 × g for 25 min. The centrifuged supernatant was the cytosolic fraction. The protein concentration was measured by the BCA method. After isolation of the mitochondria and cytosolic fractions, the western blot assay was used to measure the level of cytochrome c.
Caspase-3/9 activity assay
Caspase-3 and -9 activities were independently assessed using the Caspase-3 Assay Kit and the Caspase-9 Assay Kit according to the instructions recommended by the manufacturers. Caspase-3 activity was assessed by reading excitation at 380 nm and emission at 460 nm in a microplate fluorometer (BioTek Senergy HT, Vermont, USA). Caspase-9 activity was assessed with excitation at 485 nm and emission at 528 nm.
Western blot analysis
Neurons were prepared in RIPA lysis buffer containing a cocktail of complete protease inhibitors (Roche Diagnostics). After the determination of protein concentration by the BCA method, proteins were analysed by western blot according to standard protocols. In brief, equal amounts of protein were loaded and separated by SDS-PAGE and transferred to nitrocellulose membranes (Millipore). Membranes were blocked in PBST buffer (20 mM Tris-HCl, 150 mM NaCl, 0.1% Tween-20) containing 5% skimmed milk (Santa Cruz) for 1 h at room temperature on a rotary shaker and then incubated and gently shaken overnight (at 4°C) with primary antibodies against CcO (1:500), cytochrome c (1:1000), Bax (1:500), Bcl-2 (1:500) or β-actin (1:2000) in PBST containing 5% skimmed milk; this was followed by incubation with the corresponding secondary antibody for 1 h at room temperature. The blots were washed twice with PBST and developed with an Infrared Imaging System (Odyssey). The intensity of blots was analysed and compared using the NIH Image J program.
Statistical analyses
The results were processed for statistical analysis using SPSS (version 13.0 for Windows). The results are presented as the mean ± SEM. Biochemical data were analysed using one-way ANOVA followed by independent single t-tests. The significance level was set at p < 0.05.
Results
Identification of cultured cortical neurons
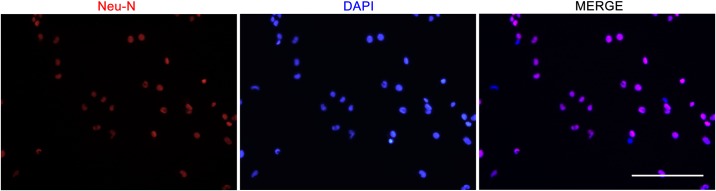
In the described culture condition, the purity of cultured mouse cortical neuron was 93.00% ± 1.23% at 7 days in vitro (Fig 2).
Fig 2. Immunocytochemsitry analysis of neurons labelling nuclei with NeuN.
Neuronal nuclei were labelled with specific neuronal marker-NeuN (red), while all cells’ nuclei were stained in blue.
Geniposide protected against Aβ1-42-induced cell viability loss in neurons
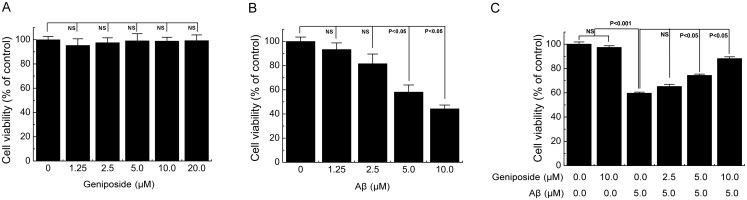
First, the neurons were treated with various concentrations (1.25, 2.5, 5, 10, and 20 μM) of geniposide for 24 h to determine whether geniposide is toxic to neurons. Cell viability was assessed by the MTT assay. The results showed that geniposide exhibited no significant neurotoxicity to the neurons (Fig 3A). Next, to determine the appropriate toxic concentration of Aβ1–42, the neurons were incubated with four different concentrations (1.25, 2.5, 5, and 10 μM) of Aβ1–42 for 24 h, and the cell viability was assessed by the MTT assay. The results showed that oligomeric Aβ1–42 peptides induced neurotoxicity in a dose-dependent manner (Fig 3B). Given these results, 5 μM Aβ1–42 was selected to incubate the primary cortical neurons for 24 h in the further experiments. Third, we detected the effect of geniposide on Aβ1-42-induced cell viability loss in neurons. Neurons were pre-treated for 24 h with various concentrations (2.5, 5, and 10 μM) of geniposide, followed by treatment with 5 μM Aβ1–42 for 24 h. As shown in Fig 3C, cell viability decreased to 59.59% ± 0.94% after exposure to 5 μM Aβ1–42 for 24 h, and pre-treatment with geniposide (2.5, 5, and 10 μM) for 24 h could enhance cell viability to 65.14% ± 1.81%, 74.29% ± 1.10%, and 88.12% ± 1.52% (Fig 3C), respectively. Therefore, 2.5, 5, and 10 μM geniposide were selected for all of the subsequent experiments.
Fig 3. Geniposide prevents the cytotoxicity of oligomeric Aβ1–42 in neurons.
A, Effects of geniposide on the cell viability in neurons. Cells were treated with the indicated doses of geniposide for 24 h, and the cell viability was determined with MTT. B, Effects of oligomeric Aβ1–42 on the cell viability in the primary cultured cortical neurons. After cells were treated with the indicated doses of Aβ1–42 for 24 h, the cell viability was determined with MTT. C, Geniposide increases the cell viability of neurons induced by Aβ1–42. Cells were pre-incubated with geniposide at 2.5–10 μM for 24h, and then exposed to oligomeric Aβ1–42 at 5.0 μM for 24 h in the presence and absence of geniposide. The cell viability was measured by MTT. NS: non significance. N = 6 per group of cells. Studies were repeated four times and data were expressed as means ± SEM of percentage of vehicle-treated cells.
Geniposide suppressed Aβ1-42-induced apoptosis in neurons
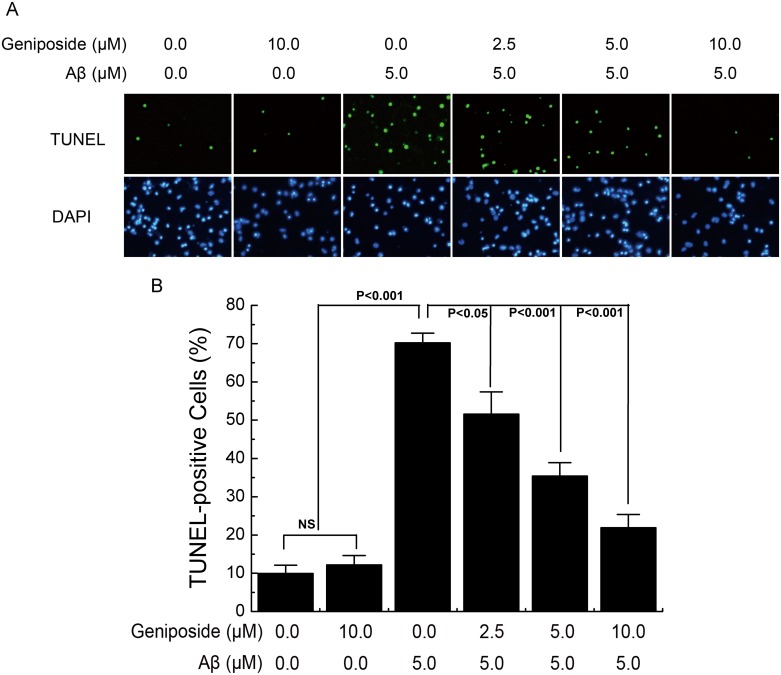
To investigate the protective effect of geniposide on Aβ-induced apoptosis, we applied TUNEL staining to detect the number of apoptotic neurons. Primary cultured neurons were pre-incubated for 24 h in the absence or presence of geniposide (2.5, 5 and 10 μM) before addition of oligomeric Aβ1–42 (5 μM) for 24 h and then stained by TUNEL and analysed under a fluorescence microscope. As shown in Fig 4A, TUNEL-labelled cells (green) were significantly increased in Aβ-treated neurons while precious little numbers of TUNEL-positive neurons were found in the vehicle-treated group. Treatment with geniposide significantly decreased the number of TUNEL-labelled cells in a dose-dependent manner. Statistical result of TUNEL staining (Fig 4B) suggested that Aβ observably increased the apoptosis rate (70.21%, p<0.001). By contrast, different concentrations of geniposide (2.5, 5 and 10 μM) reversed the Aβ-induced apoptosis(51.56%, P<0.05; 35.40%, P<0.001; 21.91%, P<0.001). Treatment with geniposide alone at 10μM showed no evident effect on the apoptosis rate, indicating that geniposide is not harmful to the neurons.
Fig 4. The effect of geniposide on Aβ1-42-induced apoptosis.
A, Photomicrographs of TUNEL and DAPI fluorescence staining. Neurons were cultured in the presence of 5 μM oligomeric Aβ1–42 and in the presence and absence of geniposide (2.5 μM, 5 μM, 10 μM) for 24 h. TUNEL-positive cells were stained in green while all cells’ nuclei were stained in blue. B, Quantitative analysis the ratio of TUNEL-positive cells to total neuron number. The ratio of TUNEL-labelled neurons was significantly increased in neurons treated with oligomeric Aβ1–42 for 24 hours while the geniposide treatment dramatically reduced the Aβ-induced TUNEL-positive cells in a dose-dependent manner. NS: non significance. N = 6 per group of cells. Studies were repeated thrice and data were expressed as mean ± SEM of percentage of vehicle-treated cells.
Geniposide attenuated Aβ1-42-induced ROS production in neurons
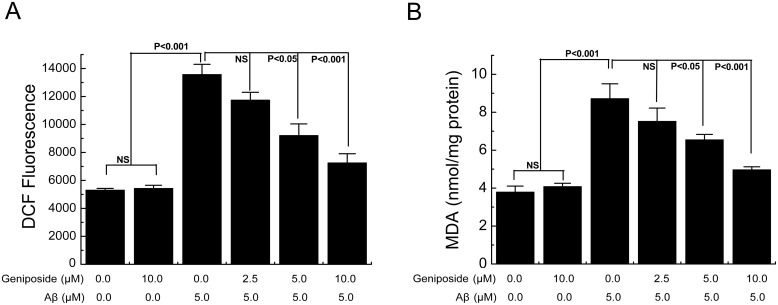
Mitochondria are major sources for the generation and accumulation of reactive oxygen species (ROS), and mitochondrial dysfunction causes increased radical production. To examine whether geniposide could suppress cell apoptosis via its antioxidant effects, we determined the effect of geniposide on Aβ1-42-induced oxidative stress. The levels of intracellular ROS and a marker of lipid peroxidation (malondialdehyde, MDA) were examined simultaneously, both of which were significantly increased in neurons exposed to Aβ1–42. The level of ROS in Aβ1-42-treated neurons was significantly increased (p<0.001) compared with the control group. However, pre-treatment with geniposide (5 and 10 μM) decreased the level of ROS compared with the Aβ1-42-treated group (p<0.05, p<0.001) (Fig 5A). The level of MDA in Aβ1-42-treated neurons was significantly increased (p<0.001) compared with the control group. However, pre-treatment with geniposide (5 and 10 μM) could decrease the level of MDA (p<0.05, p<0.001) (Fig 5B). These results suggest that geniposide plays a protective role against oxidative stress by decreasing the generation of ROS and MDA, which suggests that the antioxidant effects of geniposide may be involved in the neuroprotection mechanism.
Fig 5. Effects of geniposide on the ROS and MDA levels in primary cultured cortical neurons.
The levels of DCF fluorescence intensity (an indicator of ROS generation (A)) and MDA production (B) were significantly elevated in Aβ1-42-treated neurons compared to vehicle-treated neurons, and partially restored in the geniposide-treated neurons in a dose-dependent manner. NS: non significance. N = 6 per group of cells. Studies were repeated four times and data were expressed as mean ± SEM of percentage of vehicle-treated cells.
Geniposide protected against Aβ1-42-induced mitochondrial dysfunction
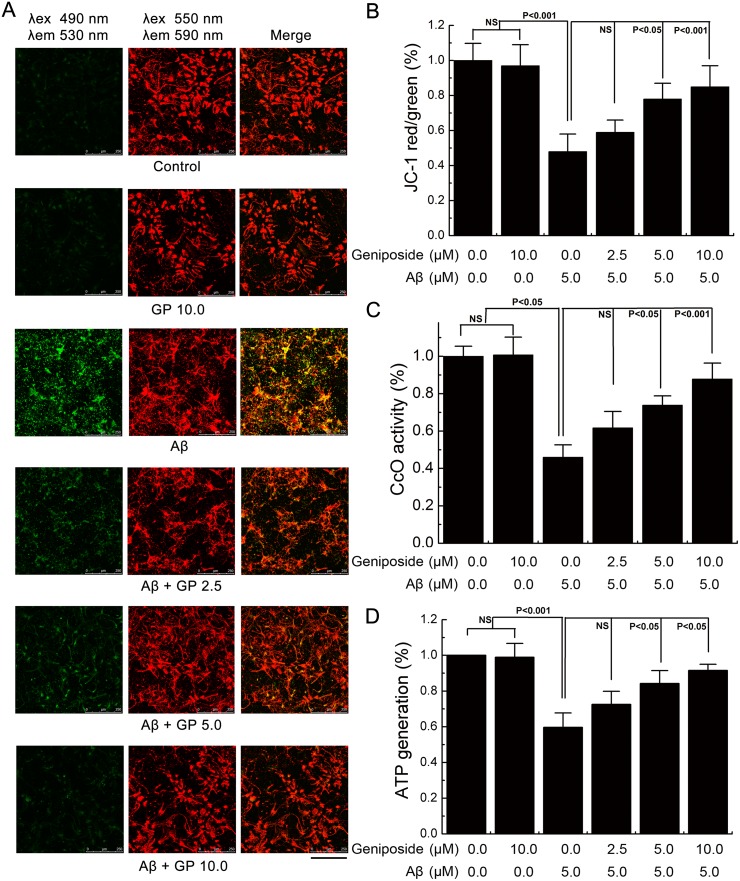
To examine whether geniposide could preserve mitochondrial function, mitochondrial membrane potential (ΔΨM), ATP levels and cytochrome c oxidase (CcO) were assessed. Neurons were pre-treated with varying doses of geniposide for 24 h and co-incubated with or without oligomeric Aβ1–42 for 24 h and then collected for further analysis. As shown in Fig 6A and 6B, a significant loss of ΔΨM was observed in neurons treated with Aβ1–42 (p<0.001). However, pre-treatment with varying doses of geniposide (2.5, 5, 10 μM) showed a dose-dependent increase in ΔΨM compared to neurons treated with Aβ1–42 alone.
Fig 6. The effects of geniposide on Aβ1-42-induced mitochondrial abnormalities.
A, Mitochondrial membrane potential. Cells were labeled with JC-1 dye and analyzed by laser-scanning confocal microscope per the manufacturer’s protocol. In the live non-apoptotic cells, the mitochondria appearred following aggregation of JC-1 reagent. In the apoptotic cells, the dye remains in its monomeric form and appears green. Scalebar = 250 μm. B, Quantitative analysis of the Δψm among groups. The ratio of red to green fluorescence in A was used as the indicator for mitochondrial membrane potential. C, The ATP level was determined by fluorescence microplate reader. D, CcO activity was tested by spectrophotometer. NS: non significance. N = 6 per group of cells. Studies were repeated four times and data were expressed as mean ± SEM of percentage of vehicle-treated cells.
Next, we measured ATP levels. Decreases in ATP levels correlated with the changes in ΔΨM. As shown in Fig 6C, a significant decrease in ATP levels (59.67% compared to control, p<0.001) was observed in neurons treated with Aβ1–42, which could be alleviated by geniposide treatment in a dose-dependent manner.
Third, we analysed mitochondrial respiratory function by measuring CcO activity. Defects in CcO activity have been shown in early stage AD brains, AD mouse models, and Aβ-treated neurons. As shown in Fig 6D, CcO activity was significantly decreased (46.02% compared to control, p<0.001) in Aβ1-42-treated neurons. However, geniposide(2.5, 5 and 10 μM) treatment significantly increased CcO activity in a dose-dependent manner(61.70%, NS; 73.89%, P<0.05; 87.82%, P<0.001).
The effect of geniposide on mitochondrial pathways in Aβ1-42-induced neuronal apoptosis
To determine whether the protective effect of geniposide on Aβ1-42-induced mitochondrial dysfunction correlated with neurotoxicity, we assessed changes in cytochrome c release, caspase-3/9 activity and Bcl-2 and Bax expression.
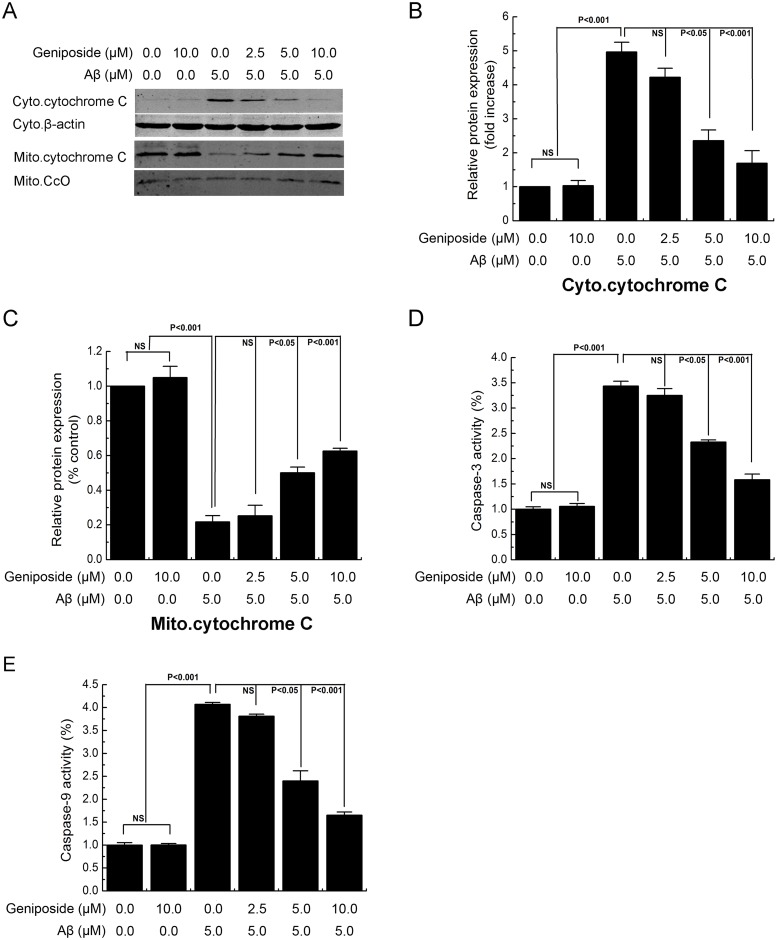
Mitochondrial dysfunction can also induce the release of cytochrome c from the mitochondria into the cytosol. Our results showed that Aβ1–42 caused a translocation of cytochrome c from the mitochondria into the cytosol after 24 h of treatment (Fig 7A, 7B and 7C). Pre-treatment with geniposide (2.5–10 μM) significantly attenuated the release of cytochrome c into the cytosol in a dose-dependent manner, showing that the mitochondrial dysfunction induced by Aβ1–42 was rescued by geniposide.
Fig 7. The effects of geniposide on Aβ1-42-induced release of mitochondrial cytochrome c and caspase-3/9 activity.
Neurons were cultured in the presence of 5 μM oligomeric Aβ1–42 and geniposide (2.5 μM, 5 μM, 10 μM) for 24 h, harvested and the levels of specific proteins were assessed by western blot analysis (A). Levels of cytochrome c in the cytosolic fraction (B) or mitochondrial fractions (C), Geniposide attenuated mitochondrial cytochrome c releasing to cytosolic fraction in neurons induced by oligomeric Aβ1–42 at 5 μM. Caspase-3/9 activity was assessed by microplate fluorometer. The increased activity of caspase-3/9 was attenuated (d, e) by the present of geniposide in a dose-independent manner. NS: non significance. N = 6 per group of cells. Studies were repeated four times and data were expressed as mean ± SEM of percentage of vehicle-treated cells.
The release of cytochrome c from mitochondria can activate caspase-9 and caspase-3. Activation of either caspase can cleave poly ADP-ribose polymerase (PARP) and trigger chromosomal DNA fragmentation. The results (Fig 7D and 7E) showed that both caspase-3 and caspase-9 activities were increased by the 24 h application of Aβ1–42. Treatment with geniposide significantly inhibited caspase-9 and caspase-3 activities in a dose-dependent manner.
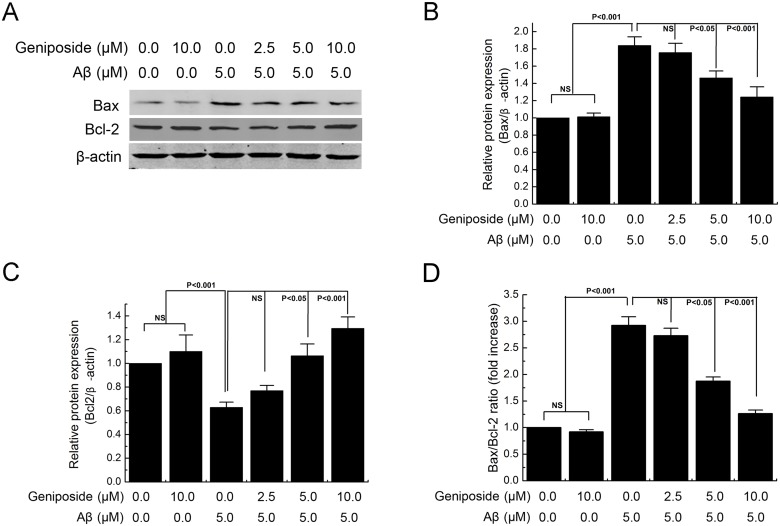
Because translocation of the Bcl-2 family of proteins from the mitochondria to the cytosol is known to play a key role in mitochondrial-mediated apoptosis induced by a variety of apoptotic stimuli, western blot analysis was performed to assess the effect of geniposide on the expression of Bcl-2 (anti-apoptotic) and Bax (pro-apoptotic) proteins (Fig 8A). The results showed that treatment of primary cortical neurons with Aβ1–42 alone caused a significant decrease in the level of Bcl-2 (p<0.001) and a significant increase in the level of Bax (p<0.001) compared with the control group. However, pre-treatment of primary cortical neurons with geniposide (5 and 10 μM) could upregulate Bcl-2 expression (p<0.05, p<0.001) (Fig 8B) and downregulate Bax expression (p<0.05, p<0.001) (Fig 8C) induced by Aβ1–42. Thus, the Bax/Bcl-2 ratio significantly decreased (p<0.05, p<0.001) (Fig 8D).
Fig 8. The effect of geniposide on Aβ1-42-induced Bax and Bcl-2 proteins abnormally expression level.
Neurons were cultured in the presence of 5 μM oligomeric Aβ1–42 and geniposide (2.5 μM, 5 μM, 10 μM) for 24 h, harvested and the levels of specific proteins were assessed by immunoblot analysis (A). Pretreatment of primary cortical neurons with geniposide (2.5–10 μM) could upregulate Bcl-2 expression (B), downregulate Bax expression (C) and decrease the Bax/Bcl-2 ratio (D) induced by Aβ1–42. NS: non significance. N = 6 per group of cells. Studies were repeated four times and data were expressed as mean ± SEM of percentage of vehicle-treated cells.
Discussion
Recent studies suggest that either intramitochondrial or extramitochondrial Aβ may directly cause mitochondrial dysfunction [15]. The dysfunction of mitochondria can lead to decreased ATP and MMP levels, increased ROS levels, the release of mitochondrial cytochrome c, elevated caspase-3 activity, and ultimately result in neuronal apoptosis.
According to the relevant articles [16, 40, 41] and our previous study [27–29], we hold the opinion that the binding of Aβ and membranal RAGE caused activation of NADPH oxidase and followed by generation of ROS is the major reason of mitochondrial dysfunction. In our previous studies [29], we proved that geniposide inhibits the interaction between Aβ and RAGE. So we thought geniposide may ameliorate mitochondrial dysfunction by inhibits the interaction between Aβ and membranal RAGE. Furthermore, Aβ can mediate its effect via other Aβ receptors. Murine PirB (paired immunoglobulin-like receptor B) and its human ortholog LilrB2 (leukocyte immunoglobulin-like receptor B2) act as receptors for oligomeric Aβ1–42 in human brains, mediated loss of synaptic plasticity and memory deficits [42]. Cellular prion protein (PrPC) and ephrin type B receptor 2 (EphB2) binds oligomeric Aβ and alter the function of NMDAR (NMDA receptor) in neurons, result in synaptic dysfunction [43–45]. However, whether geniposide has an effect on these receptors remains to be further investigated.
In addition, there is evidence to suggest that RAGE mediates transport of Aβ peptides across the cytomembrane from extracellular to intracellular [17, 46, 47]. Therefore, the application of geniposide may decrease the content of intracellular Aβ through inhibit the interaction of Aβ and RAGE. The accumulation of Aβ in the brain is a critical, early event that leads to neuronal dysfunction and memory impairment in AD pathogenesis. Mitochondria are the targets for both amyloid precursor protein (APP) and Aβ, which interact with several proteins inside mitochondria [15, 48]. Aβ may interact with respiratory chain complexes and affect the activity of several mitochondrial enzymes, such as the pyruvate dehydrogenase complex (PDH) and ketoglutarate dehydrogenase complex (KGDH) [49], as well as ETC enzyme complexes, especially complex IV (CcO), disrupting hydrogen transport across the mitochondrial intermembrane space and thus encouraging excessive MMP depolarization [50, 51].
One of the apoptotic factors, cytochrome c, transports electrons from complex III to IV along the ETC while protons are pumped from the matrix into the intermembrane space to form an electrochemical gradient (MMP) [15, 48]. Anti-apoptotic protein Bcl-2 and pro-apoptotic Bax are involved in the mitochondria-mediated apoptosis pathway. Bcl-2 binding to the mitochondrial membrane, competitive binding with Bax and the formation of the Bcl-2/Bax heterodimer lead to the closing of mtPTP and prevent the release of cytochrome c, while the binding of the Bax/Bax homodimer to the mitochondrial membrane and the formation of permeable channels lead to the release of cytochrome c, which couples with destroyed MMP and also activates the caspase-related apoptosis cascade reaction [52–55]. Our results showed that geniposide attenuated the level of increase of Bax/Bcl-2 and caspase-3/9 activity, the release of cytochrome c and the decrease in MMP induced by Aβ.
In addition, Recent studies shown that Aβ interaction with mitochondrial and plasma membrane voltage-dependent anion channel 1 (VDAC1) is involved in the extrinsic apoptotic pathway, and VDAC1 is overexpressed in post-mortem brains of Alzheimer disease (AD) patients, suggest that VDAC1 may serve as a potential target for AD treatment [56–58]. However, the mechanisms underlying it remains to be further investigated.
Plenty of evidence points to the fact that Aβ reduced the activity of the ETC enzyme complex IV (CcO) in isolated mitochondria or whole cells exposed to Aβ protein and in AD transgenic mice [59, 60]. Furthermore, CcO dysfunction may increase ROS production and reduce ATP levels, which in turn may impair the function of other mitochondrial enzymes by stalling electron transfer [50]. However, the protection mechanism of geniposide on CcO activity reduction needs further investigation.
AD brains experience significant neuron loss, which likely contributes to the progressive decline of memory and cognitive functions [51]. While some neuron loss is due to necrosis, the remainder is likely due to or invokes aspects of apoptosis, which is a tightly regulated form of programmed cell death [50]. DNA fragmentation, a common hallmark of apoptosis, is typically assessed by TUNEL staining. There was evidence that neurons in AD brains display increased DNA fragmentation compared to control brains [61, 62]. The present studies provide evidence of geniposide protection of neurons from Aβ-induced apoptosis.
In summary, the present study demonstrates that geniposide, the main active component of gardenia fruit, has protective effects against Aβ-mediated neurotoxicity in primary cultured mouse cortical neurons, via the recovery of ATP generation, MMP levels, CcO and caspase-3/9 activities, reduction of ROS production, as well as the inhibition of apoptosis. In addition, there is evidence demonstrating that geniposide could cross the BBB and produce protective properties in the brain [30]. Because AD has been considered to be a severe impairment of the brain, the detailed mechanisms underlying Aβ toxicity and the neuroprotective effects of geniposide required further investigation. Our results may contribute to provide support for potential applications of geniposide in AD treatment.
Acknowledgments
The authors are sincerely thankful to Fang Fang for providing advice and writing assistance in the preparation of this manuscript. All authors read and approved the final manuscript.
Data Availability
All relevant data are within the paper.
Funding Statement
This work was supported by grants by the National Natural Science Foundation of China (No. 81274118, http://www.nsfc.gov.cn/, WZ); The Key New Drug Creation and Development Program of China (No. 2012ZX09103-201, http://www.nmp.gov.cn/, WZ); and The National Key Technology R & D Program (No. 2012BAI29B08, http://www.most.gov.cn/kjgh/lskjgh/, WZ). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Hardy J, Selkoe DJ. The amyloid hypothesis of Alzheimer's disease: progress and problems on the road to therapeutics. Science. 2002;297(5580):353–6. [DOI] [PubMed] [Google Scholar]
- 2.Lesne S, Koh MT, Kotilinek L, Kayed R, Glabe CG, Yang A, et al. A specific amyloid-beta protein assembly in the brain impairs memory. Nature. 2006;440(7082):352–7. [DOI] [PubMed] [Google Scholar]
- 3.Mattson MP. Pathways towards and away from Alzheimer's disease. Nature. 2004;430(7000):631–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bertram L, Tanzi RE. The genetic epidemiology of neurodegenerative disease. The Journal of clinical investigation. 2005;115(6):1449–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ferri CP, Prince M, Brayne C, Brodaty H, Fratiglioni L, Ganguli M, et al. Global prevalence of dementia: a Delphi consensus study. Lancet. 2005;366(9503):2112–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Forlenza OV, Diniz BS, Gattaz WF. Diagnosis and biomarkers of predementia in Alzheimer's disease. BMC medicine. 2010;8:89 10.1186/1741-7015-8-89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gotz J, Eckert A, Matamales M, Ittner LM, Liu X. Modes of Abeta toxicity in Alzheimer's disease. Cellular and molecular life sciences: CMLS. 2011;68(20):3359–75. 10.1007/s00018-011-0750-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.de la Monte SM, Wands JR. Molecular indices of oxidative stress and mitochondrial dysfunction occur early and often progress with severity of Alzheimer's disease. Journal of Alzheimer's disease: JAD. 2006;9(2):167–81. [DOI] [PubMed] [Google Scholar]
- 9.Lee HP, Pancholi N, Esposito L, Previll LA, Wang X, Zhu X, et al. Early induction of oxidative stress in mouse model of Alzheimer disease with reduced mitochondrial superoxide dismutase activity. PloS one. 2012;7(1):e28033 10.1371/journal.pone.0028033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hauptmann S, Scherping I, Drose S, Brandt U, Schulz KL, Jendrach M, et al. Mitochondrial dysfunction: an early event in Alzheimer pathology accumulates with age in AD transgenic mice. Neurobiology of aging. 2009;30(10):1574–86. 10.1016/j.neurobiolaging.2007.12.005 [DOI] [PubMed] [Google Scholar]
- 11.Schon EA, Przedborski S. Mitochondria: the next (neurode)generation. Neuron. 2011;70(6):1033–53. 10.1016/j.neuron.2011.06.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Eckert A, Hauptmann S, Scherping I, Meinhardt J, Rhein V, Drose S, et al. Oligomeric and fibrillar species of beta-amyloid (A beta 42) both impair mitochondrial function in P301L tau transgenic mice. Journal of molecular medicine. 2008;86(11):1255–67. 10.1007/s00109-008-0391-6 [DOI] [PubMed] [Google Scholar]
- 13.Wang X, Su B, Siedlak SL, Moreira PI, Fujioka H, Wang Y, et al. Amyloid-beta overproduction causes abnormal mitochondrial dynamics via differential modulation of mitochondrial fission/fusion proteins. Proceedings of the National Academy of Sciences of the United States of America. 2008;105(49):19318–23. 10.1073/pnas.0804871105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Manczak M, Anekonda TS, Henson E, Park BS, Quinn J, Reddy PH. Mitochondria are a direct site of A beta accumulation in Alzheimer's disease neurons: implications for free radical generation and oxidative damage in disease progression. Human molecular genetics. 2006;15(9):1437–49. [DOI] [PubMed] [Google Scholar]
- 15.Pagani L, Eckert A. Amyloid-Beta interaction with mitochondria. International journal of Alzheimer's disease. 2011;2011:925050 10.4061/2011/925050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wautier MP, Chappey O, Corda S, Stern DM, Schmidt AM, Wautier JL. Activation of NADPH oxidase by AGE links oxidant stress to altered gene expression via RAGE. American journal of physiology Endocrinology and metabolism. 2001;280(5):E685–94. [DOI] [PubMed] [Google Scholar]
- 17.Takuma K, Fang F, Zhang WS, Yan SQ, Fukuzaki E, Du H, et al. RAGE-mediated signaling contributes to intraneuronal transport of amyloid-beta and neuronal dysfunction. Proceedings of the National Academy of Sciences of the United States of America. 2009;106(47):20021–6. 10.1073/pnas.0905686106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Caspersen C, Wang N, Yao J, Sosunov A, Chen X, Lustbader JW, et al. Mitochondrial Abeta: a potential focal point for neuronal metabolic dysfunction in Alzheimer's disease. FASEB journal: official publication of the Federation of American Societies for Experimental Biology. 2005;19(14):2040–1. [DOI] [PubMed] [Google Scholar]
- 19.Du H, Guo L, Fang F, Chen D, Sosunov AA, McKhann GM, et al. Cyclophilin D deficiency attenuates mitochondrial and neuronal perturbation and ameliorates learning and memory in Alzheimer's disease. Nature medicine. 2008;14(10):1097–105. 10.1038/nm.1868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dragicevic N, Mamcarz M, Zhu Y, Buzzeo R, Tan J, Arendash GW, et al. Mitochondrial amyloid-beta levels are associated with the extent of mitochondrial dysfunction in different brain regions and the degree of cognitive impairment in Alzheimer's transgenic mice. Journal of Alzheimer's disease: JAD. 2010;20 Suppl 2:S535–50. 10.3233/JAD-2010-100342 [DOI] [PubMed] [Google Scholar]
- 21.Yan SD, Stern DM. Mitochondrial dysfunction and Alzheimer's disease: role of amyloid-beta peptide alcohol dehydrogenase (ABAD). International journal of experimental pathology. 2005;86(3):161–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen JX, Yan SS. Role of mitochondrial amyloid-beta in Alzheimer's disease. Journal of Alzheimer's disease: JAD. 2010;20 Suppl 2:S569–78. 10.3233/JAD-2010-100357 [DOI] [PubMed] [Google Scholar]
- 23.Cavallucci V, Ferraina C, D'Amelio M. Key role of mitochondria in Alzheimer's disease synaptic dysfunction. Current pharmaceutical design. 2013;19(36):6440–50. [DOI] [PubMed] [Google Scholar]
- 24.Koo HJ, Lim KH, Jung HJ, Park EH. Anti-inflammatory evaluation of gardenia extract, geniposide and genipin. Journal of ethnopharmacology. 2006;103(3):496–500. [DOI] [PubMed] [Google Scholar]
- 25.Liu J, Yin F, Zheng X, Jing J, Hu Y. Geniposide, a novel agonist for GLP-1 receptor, prevents PC12 cells from oxidative damage via MAP kinase pathway. Neurochem Int. 2007;51(6–7):361–9. [DOI] [PubMed] [Google Scholar]
- 26.Peng CH, Huang CN, Hsu SP, Wang CJ. Penta-acetyl geniposide-induced apoptosis involving transcription of NGF/p75 via MAPK-mediated AP-1 activation in C6 glioma cells. Toxicology. 2007;238(2–3):130–9. [DOI] [PubMed] [Google Scholar]
- 27.Lv C, Liu X, Liu H, Chen T, Zhang W. Geniposide attenuates mitochondrial dysfunction and memory deficits in APP/PS1 transgenic mice. Curr Alzheimer Res. 2014;11(6):580–7. [DOI] [PubMed] [Google Scholar]
- 28.Lv C, Wang L, Liu X, Yan S, Yan SS, Wang Y, et al. Multi-faced neuroprotective effects of geniposide depending on the RAGE-mediated signaling in an Alzheimer mouse model. Neuropharmacology. 2014;89C:175–84. [DOI] [PubMed] [Google Scholar]
- 29.Lv C, Wang L, Liu X, Cong X, Yan SS, Wang Y, et al. Geniposide attenuates oligomeric Abeta(1–42)-induced inflammatory response by targeting RAGE-dependent signaling in BV2 cells. Curr Alzheimer Res. 2014;11(5):430–40. [DOI] [PubMed] [Google Scholar]
- 30.Liu J, Zhang Y, Deng X, Yin F. Geniposide decreases the level of Abeta1-42 in the hippocampus of streptozotocin-induced diabetic rats. Acta biochimica et biophysica Sinica. 2013;45(9):787–91. 10.1093/abbs/gmt069 [DOI] [PubMed] [Google Scholar]
- 31.Gao C, Liu Y, Jiang Y, Ding J, Li L. Geniposide ameliorates learning memory deficits, reduces tau phosphorylation and decreases apoptosis via GSK3beta pathway in streptozotocin-induced alzheimer rat model. Brain pathology. 2014;24(3):261–9. 10.1111/bpa.12116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mennes E, Dungan CM, Frendo-Cumbo S, Williamson DL, Wright DC. Aging-associated reductions in lipolytic and mitochondrial proteins in mouse adipose tissue are not rescued by metformin treatment. The journals of gerontology Series A, Biological sciences and medical sciences. 2014;69(9):1060–8. 10.1093/gerona/glt156 [DOI] [PubMed] [Google Scholar]
- 33.Chin D, Hagl S, Hoehn A, Huebbe P, Pallauf K, Grune T, et al. Adenosine triphosphate concentrations are higher in the brain of APOE3- compared to APOE4-targeted replacement mice and can be modulated by curcumin. Genes & nutrition. 2014;9(3):397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kunnumakkara AB, Sung B, Ravindran J, Diagaradjane P, Deorukhkar A, Dey S, et al. {Gamma}-tocotrienol inhibits pancreatic tumors and sensitizes them to gemcitabine treatment by modulating the inflammatory microenvironment. Cancer research. 2010;70(21):8695–705. 10.1158/0008-5472.CAN-10-2318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chen YH, Yeh CW, Lo HC, Su SL, Hseu YC, Hsu LS. Generation of reactive oxygen species mediates butein-induced apoptosis in neuroblastoma cells. Oncology reports. 2012;27(4):1233–7. 10.3892/or.2012.1632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Han L, Masani S, Hsieh CL, Yu K. DNA ligase I is not essential for mammalian cell viability. Cell reports. 2014;7(2):316–20. 10.1016/j.celrep.2014.03.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dahlgren KN, Manelli AM, Stine WB, Baker LK, Krafft GA, LaDu MJ. Oligomeric and fibrillar species of amyloid-beta peptides differentially affect neuronal viability. Journal of Biological Chemistry. 2002;277(35):32046–53. [DOI] [PubMed] [Google Scholar]
- 38.Yin F, Liu J, Ji X, Wang Y, Zidichouski J, Zhang J. Silibinin: a novel inhibitor of Abeta aggregation. Neurochem Int. 2011;58(3):399–403. 10.1016/j.neuint.2010.12.017 [DOI] [PubMed] [Google Scholar]
- 39.Li Z, Li H, Zhao C, Lv C, Zhong C, Yan SS, et al. Protective effect of Notoginsenoside R1 on an APP/PS1 mouse model of Alzheimer's disease by up-regulating insulin degrading enzyme and inhibiting Abeta accumulation. CNS & neurological disorders drug targets. 2015. [DOI] [PubMed] [Google Scholar]
- 40.Yan SD, Chen X, Fu J, Chen M, Zhu H, Roher A, et al. RAGE and amyloid-beta peptide neurotoxicity in Alzheimer's disease. Nature. 1996;382(6593):685–91. [DOI] [PubMed] [Google Scholar]
- 41.Daffu G, del Pozo CH, O'Shea KM, Ananthakrishnan R, Ramasamy R, Schmidt AM. Radical roles for RAGE in the pathogenesis of oxidative stress in cardiovascular diseases and beyond. International journal of molecular sciences. 2013;14(10):19891–910. 10.3390/ijms141019891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kim T, Vidal GS, Djurisic M, William CM, Birnbaum ME, Garcia KC, et al. Human LilrB2 is a beta-amyloid receptor and its murine homolog PirB regulates synaptic plasticity in an Alzheimer's model. Science. 2013;341(6152):1399–404. 10.1126/science.1242077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Um JW, Nygaard HB, Heiss JK, Kostylev MA, Stagi M, Vortmeyer A, et al. Alzheimer amyloid-beta oligomer bound to postsynaptic prion protein activates Fyn to impair neurons. Nature neuroscience. 2012;15(9):1227–35. 10.1038/nn.3178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lauren J, Gimbel DA, Nygaard HB, Gilbert JW, Strittmatter SM. Cellular prion protein mediates impairment of synaptic plasticity by amyloid-beta oligomers. Nature. 2009;457(7233):1128–32. 10.1038/nature07761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cisse M, Halabisky B, Harris J, Devidze N, Dubal DB, Sun B, et al. Reversing EphB2 depletion rescues cognitive functions in Alzheimer model. Nature. 2011;469(7328):47–52. 10.1038/nature09635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Candela P, Gosselet F, Saint-Pol J, Sevin E, Boucau MC, Boulanger E, et al. Apical-to-basolateral transport of amyloid-beta peptides through blood-brain barrier cells is mediated by the receptor for advanced glycation end-products and is restricted by P-glycoprotein. Journal of Alzheimer's disease: JAD. 2010;22(3):849–59. 10.3233/JAD-2010-100462 [DOI] [PubMed] [Google Scholar]
- 47.Deane R, Du Yan S, Submamaryan RK, LaRue B, Jovanovic S, Hogg E, et al. RAGE mediates amyloid-beta peptide transport across the blood-brain barrier and accumulation in brain. Nature medicine. 2003;9(7):907–13. [DOI] [PubMed] [Google Scholar]
- 48.Spuch C, Ortolano S, Navarro C. New insights in the amyloid-Beta interaction with mitochondria. Journal of aging research. 2012;2012:324968 10.1155/2012/324968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Eckert A, Schmitt K, Gotz J. Mitochondrial dysfunction—the beginning of the end in Alzheimer's disease? Separate and synergistic modes of tau and amyloid-beta toxicity. Alzheimer's research & therapy. 2011;3(2):15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Silva DF, Selfridge JE, Lu J, E L, Cardoso SM, Swerdlow RH. Mitochondrial abnormalities in Alzheimer's disease: possible targets for therapeutic intervention. Advances in pharmacology. 2012;64:83–126. 10.1016/B978-0-12-394816-8.00003-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Reddy PH, Tripathi R, Troung Q, Tirumala K, Reddy TP, Anekonda V, et al. Abnormal mitochondrial dynamics and synaptic degeneration as early events in Alzheimer's disease: implications to mitochondria-targeted antioxidant therapeutics. Biochimica et biophysica acta. 2012;1822(5):639–49. 10.1016/j.bbadis.2011.10.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kirkland RA, Franklin JL. Bax, reactive oxygen, and cytochrome c release in neuronal apoptosis. Antioxidants & redox signaling. 2003;5(5):589–96. [DOI] [PubMed] [Google Scholar]
- 53.Lalier L, Cartron PF, Juin P, Nedelkina S, Manon S, Bechinger B, et al. Bax activation and mitochondrial insertion during apoptosis. Apoptosis: an international journal on programmed cell death. 2007;12(5):887–96. [DOI] [PubMed] [Google Scholar]
- 54.Renault TT, Floros KV, Elkholi R, Corrigan KA, Kushnareva Y, Wieder SY, et al. Mitochondrial shape governs BAX-induced membrane permeabilization and apoptosis. Molecular cell. 2015;57(1):69–82. 10.1016/j.molcel.2014.10.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Robinson KS, Clements A, Williams AC, Berger CN, Frankel G. Bax inhibitor 1 in apoptosis and disease. Oncogene. 2011;30(21):2391–400. 10.1038/onc.2010.636 [DOI] [PubMed] [Google Scholar]
- 56.Thinnes FP. Neuroendocrine differentiation of LNCaP cells suggests: VDAC in the cell membrane is involved in the extrinsic apoptotic pathway. Molecular genetics and metabolism. 2009;97(4):241–3. 10.1016/j.ymgme.2009.04.010 [DOI] [PubMed] [Google Scholar]
- 57.Prezma T, Shteinfer A, Admoni L, Raviv Z, Sela I, Levi I, et al. VDAC1-based peptides: novel pro-apoptotic agents and potential therapeutics for B-cell chronic lymphocytic leukemia. Cell death & disease. 2013;4:e809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Smilansky A, Dangoor L, Nakdimon I, Ben-Hail D, Mizrachi D, Shoshan-Barmatz V. The Voltage-dependent Anion Channel 1 Mediates Amyloid beta Toxicity and Represents a Potential Target for Alzheimer Disease Therapy. The Journal of biological chemistry. 2015;290(52):30670–83. 10.1074/jbc.M115.691493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Devi L, Ohno M. Mitochondrial dysfunction and accumulation of the beta-secretase-cleaved C-terminal fragment of APP in Alzheimer's disease transgenic mice. Neurobiology of disease. 2012;45(1):417–24. 10.1016/j.nbd.2011.09.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Swerdlow RH. Alzheimer's disease pathologic cascades: who comes first, what drives what. Neurotoxicity research. 2012;22(3):182–94. 10.1007/s12640-011-9272-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Broe M, Shepherd CE, Milward EA, Halliday GM. Relationship between DNA fragmentation, morphological changes and neuronal loss in Alzheimer's disease and dementia with Lewy bodies. Acta neuropathologica. 2001;101(6):616–24. [DOI] [PubMed] [Google Scholar]
- 62.Colurso GJ, Nilson JE, Vervoort LG. Quantitative assessment of DNA fragmentation and beta-amyloid deposition in insular cortex and midfrontal gyrus from patients with Alzheimer's disease. Life sciences. 2003;73(14):1795–803. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All relevant data are within the paper.