Abstract
Importance
Human immunodeficiency virus (HIV) diagnoses continue to increase among young men who have sex with men (YMSM). Many YMSM living with HIV engage in sexual risk behaviors, and those who have a detectable viral load can transmit HIV to sex partners. Understanding factors that are related to sexual risk taking among virologically detectable (VL+) YMSM can inform prevention and treatment efforts.
Objectives
To describe differences between virologically suppressed (VL−) and VL+ YMSM living with HIV and to identify correlates of condomless anal intercourse (CAI) and serodiscordant CAI among VL+ YMSM.
Design, Setting, and Participants
In this cross-sectional survey conducted from December 1, 2009, through June 30, 2012, we studied 991 HIV-infected YMSM 15 to 26 years of age at 20 adolescent HIV clinics in the United States. Data analysis was conducted December 1, 2013, through July 31, 2015.
Main Outcomes and Measures
Demographic, behavioral, and psychosocial assessments obtained using audio computer-assisted self-interviews. Viral load information was obtained via blood draw or medical record abstraction.
Results
Of the 991 participants, 688 (69.4%) were VL+ and 458 (46.2%) reported CAI, with 310 (31.3%) reporting serodiscordant CAI in the past 3 months. The VL+ YMSM were more likely than the VL− YMSM to report CAI (detectable, 266 [54.7%]; suppressed, 91 [44.4%]; P = .01) and serodiscordant CAI (detectable, 187 [34.9%]; suppressed, 57 [25.0%]; P < .01). Multivariable analyses indicated that among VL+ YMSM, those reporting problematic substance use were more likely to report CAI (adjusted odds ratio [AOR], 1.46; 95% CI, 1.02-2.10) and serodiscordant CAI (AOR, 1.45; 95% CI, 1.06-1.99). Black VL+ YMSM were less likely to report CAI (AOR, 0.63; 95% CI, 0.44-0.90) or serodiscordant CAI (AOR, 0.66; 95% CI, 0.46-0.94) compared with other VL+ YMSM. In addition, VL+ YMSM who disclosed their HIV status to sex partners were more likely to report CAI compared with nondisclosing YMSM (AOR, 1.35; 95% CI, 1.01-1.81). Transgender participants were less likely to report CAI than cisgender participants (AOR, 0.35; 95% CI, 0.14-0.85). Last, VL+ YMSM who reported currently being employed were less likely to report serodiscordant CAI than those who were unemployed (AOR,0.74; 95% CI, 0.55-0.99).
Conclusions and Relevance
Targeted multilevel interventions are needed to reduce HIV transmission risk behaviors among YMSM living with HIV, particularly among those who are VL+.
Human immunodeficiency virus(HIV) continues to disproportionately affect men who have sex with men (MSM) in the United States. The most recent seroprevalence data available from the US Centers for Disease Control and Prevention suggest that the HIV epidemic is increasing among MSM, whereas it is relatively stable in heterosexual populations.1,2 Young MSM (YMSM) (13-29 years of age) are particularly vulnerable to HIV infection. More than one-quarter of new infections in the United States occur in YMSM,1 and this population constitutes more than 70% of new HIV infections among youths.3 Black YMSM and young transgender women are subgroups disproportionately affected by HIV. In 2009, black YMSM accounted for 61% of all new infections in the United States.1 Likewise, HIV prevalence among transgender women in the United States has been estimated to be more than 20%.3
It is critical to implement biomedical and behavioral strategies to reduce the likelihood of transmission via male-to-male sexual contact, the primary mode through which HIV is transmitted among MSM.4 In addition to condom use, chemoprophylaxis, and routine screening for HIV, treatment of HIV-infected individuals to improve their immune status and to suppress viral load drastically reduces the odds of transmission.5 A landmark 6-year, global research study5 found that early treatment and consistent adherence to antiretroviral treatment (ART) among HIV-infected individuals reduced transmission of HIV to uninfected sex partners by 96%. An ongoing observational trial of serodiscordant gay and heterosexual couples provides additional support for the efficacy of treating HIV-infected people to reduce onward transmission6 and suggests that the paradigm of treatment as prevention has great potential as a strategy to reduce HIV among MSM.
The success of treatment as prevention in reducing the number of new infections among YMSM in the United States relies on increasing levels of HIV testing, ART uptake, adherence, and viral suppression among HIV-infected YMSM. However, challenges persist in keeping YMSM engaged in care and adherent to ART. For example, one review study7 suggested that only 51% of HIV-infected youths achieve viral suppression. Among MSM, rates of suppression are even lower. A modeling study8 found that only 34% of HIV-infected white MSM and 16% of HIV-infected black MSM achieve viral suppression. Other research has highlighted racial disparities in access to HIV care and uptake of treatment among HIV-infected MSM9 and notably among YMSM.10
Those HIV-infected YMSM who are receiving care have perhaps the greatest chance among YMSM to be virally suppressed, although a recently conducted study11 of HIV-infected youths and adolescents in care suggested that, among behaviorally infected youths, only 50% were currently receiving ART and only 27.1% were virally suppressed. Moreover, the study found that HIV-infected young men and gay- or bisexual-identified HIV-infected youths were less likely than HIV-infected young women and heterosexual HIV-infected youth, respectively, to be receiving ART.
Behavioral approaches to improve engagement in care and adherence to medication specifically may need to occur in tandem with interventions to reduce sexual risk behaviors, such as condomless anal intercourse (CAI). Numerous studies12-15 have found that many HIV-infected YMSM engage in risky sexual behaviors after their diagnosis. Young MSM who are not virally suppressed may be at particular risk to engage in sexual risk behaviors that serve to perpetuate HIV transmission.
Demographic factors, such as age, sex, race/ethnicity, and socioeconomic factors, and psychosocial factors, such as disclosure to sex partners, substance use, and poor mental health, are related to enhanced sexual risk among MSM.9,16-18 However, few studies have explored these factors specifically among HIV-infected YMSM who are virologically detectable.
The current study has 2 aims: (1) to describe differences in demographic and psychosocial factors between virologically suppressed (VL−) and virologically detectable (VL+) HIV-infected YMSM who are currently in care and (2) to identify psychosocial factors associated with any CAI and serodiscordant CAI in the past 3 months among VL+ HIV-infected YMSM.
Methods
Sample
From December 1,2009, through June 30, 2012, a total of 2225 HIV-infected youths linked to care at clinics associated with the Adolescent Medicine Trials Network for HIV/AIDS Interventions (ATN) were recruited to participate in a cross-sectional survey. The 20 clinics were located in major US cities and Puerto Rico (see Acknowledgments section for specific locations of clinics). Data analysis was conducted December 1, 2013, through July 31, 2015.
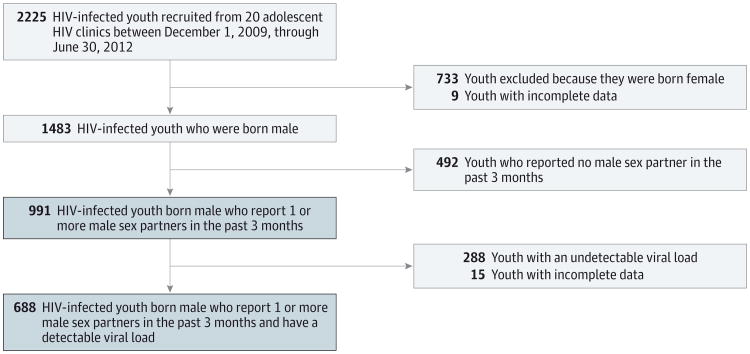
To be eligible, youths had to be (1) 12 through 26 years of age, (2) living with HIV/AIDS, (3) aware they were HIV infected, (4) linked to care in one of the ATN's clinical sites or affiliates (eg, had at least one clinic visit during the enrollment period), and (5) able to understand English or Spanish. The study was approved by the institutional review boards at all participating sites and those from the members of the protocol team. The current study focuses on a subsample of participants whose birth sex was male (regardless of their current gender identity) and who reported sexual behavior (ie, oral or anal intercourse) with another male in the 3 months before the survey (991 [44.5%] of the total sample). The Figure shows the flow of the participants throughout the study.
Figure. Participant Flow Diagram.
Blue shaded boxes represent subsamples examined in the current study. HIV indicates human immunodeficiency virus.
Research staff approached all youth who met eligibility criteria during one of their scheduled clinic visits. After a thorough explanation of the study, staff obtained signed written informed consent or assent from youths agreeing to participate. Within 2 weeks of providing consent or assent, participants completed audio computer-assisted self-interviews to assess psychosocial and health factors, which took approximately 45 to 90 minutes. Health-related data, including most recent viral load, were also abstracted from participants' medical records. In a few cases in which recent (within the 6 months before study enrollment) viral load data were not available, blood samples were taken for measurement. Participants were given a small incentive determined by the sites' institutional review boards as compensation for their time.
Measures
The assessment measured 3 primary constructs: (1) substance use, (2) mental health, and (3) sexual behavior. The assessment also obtained demographic information, including age, birth sex, current gender identity, race/ethnicity, sexual orientation, route of HIV infection, housing status, incarceration history, and disclosure of HIV status to sexual partners. Health-related data abstracted from medical records included whether the participant was prescribed ART (dichotomous variable), the date ART was prescribed (dichotomized into 2 groups: ≤6 and >6 months since starting ART), the date of confirmatory HIV diagnosis (dichotomized into 2 groups: ≤6 and >6 months since diagnosis), and most recent viral load. Specific information on the measures and viral load assessments used follows.
Substance Use
The Alcohol, Smoking, and Substance Involvement Screening Test (ASSIST)19 was used to measure substance use behaviors, and the Car, Relax, Alone, Forget, Friends, Trouble (CRAFFT)20 was used to assess problematic substance use. Eight items on the ASSIST questionnaire were used to assess the frequency and consequences of substance use. The current study examined daily use of alcohol and marijuana because these were the main substances study participants reported using. CRAFFT is a 6-item measure designed to assess the consequences of alcohol and/or marijuana use by adolescents and young adults in clinical settings. Scores of 2 or higher are suggestive of problem substance use, abuse, and/or dependence.
Mental Health
Mental health issues were assessed with the Brief Symptom Inventory (BSI),21 which yields 9 primary symptom scales, and a Global Severity Index (GSI), which has norms based on age (ie, adolescent and adult) and sex. The GSI reflects an overall evaluation of a respondent's psychopathological status. The BSI depression and anxiety subscales and the GSI were used in the current analyses.
Sexual Behavior
A 38-item questionnaire was used to assess sexual behaviors during the past 90 days. Participants reported the number of male and female sex partners and the frequency of condomless oral, vaginal, and anal sexual activity with HIV-infected, HIV-negative, and unknown-status male and female partners.
Viral Load
Plasma HIV RNAandCD4+ cell count data obtained within the last 6 months and type of assay were abstracted from medical records. Because of the variability in type of assay used across the study sites (ie, Versant HIV-1 RNA 3.0 [Siemens Diagnostics]; Amplicor HIV-1 Monitor – Standard/Ultrasensitive [F. Hoffmann-La Roche Ltd]; COBAS AmpliPrep/COBAS Taqman HIV-1 Test, versions 1.0 and 2.0 [F. Hoffmann-La Roche Ltd]; and RealTime HIV-1 Assay [Abbott Laboratories]), we used the corresponding assay cutoff for the lower limit of HIV RNA detection (all were <200 copies/mL; see Kahana et al11 for sensitivity analysis) and created a dichotomous variable to differentiate VL− and VL+ participants.
Statistical Analysis
Demographic, health-related (except for BSI subscales), and substance use variables were primarily nominal or ordinal and were transformed to dichotomous variables for analytical purposes. These variables were compared between VL− and VL+ participants using standard descriptive statistics. Given the time needed to achieve viral suppression after starting ART, YMSM who had been receiving ART for 6 months or less (175 [17.7%] of the sample) were excluded from the analyses comparing VL+ and VL− YMSM.
Logistic regression analyses were conducted to explore factors related to any CAI and serodiscordant CAI among the subsample of VL+ YMSM. We used generalized estimating equations with robust SE estimates to account for the correlation due to clustering of participants within clinics. Analyses occurred in 2 steps. Variables were examined in univariable logistic regression models to test associations between each independent variable and the 2 CAI outcome variables. Factors that were observed to be statistically significant (P ≤ .10) in univariable models were included in separate generalized estimating equation models predicting the 2 CAI outcome variables. Because of multicollinearity, the BSI depression and anxiety and GSI variables were each examined in separate multivariable models with other variables observed to be significant in the univariable analyses. Estimates for non-BSI variables included in the multivariable models did not differ among models with different BSI subscale variables; thus, estimates for non-BSI variables are presented from multivariable models that included the GSI variable. All analyses were conducted using SPSS statistical software, version 22.0 (SPSS Inc).
Results
Frequencies for variables included in the analyses are presented in Table 1. Just more than half (52.1%) of YMSM reported currently being prescribed ART. Nearly half (46.2%) of YMSM reported CAI in the past 3 months,whereas310(31.3%) reported serodiscordant CAI.
Table 1. Variable Frequencies for Full Sample and Comparison Between VL− and VL+ Subsamples of 991 HIV-Infected YMSMa.
| Variable | Full Sample (N = 991) | VL− Subsample (n = 233) | VL+ Subsample (n = 549) | P Value |
|---|---|---|---|---|
| Age ≥21 y | 352 (35.5) | 61 (26.2) | 216 (39.5) | <.01 |
| Current employment | 437 (44.1) | 121 (52.2) | 217 (39.6) | <.01 |
| Post–high school educational level | 410 (41.4) | 124 (53.4) | 190 (34.7) | <.01 |
| Transgender | 54 (5.4) | 8 (3.4) | 33 (6.0) | .14 |
| Black race | 628 (63.4) | 144 (62.3) | 348 (63.5) | .76 |
| Hispanic ethnicity | 219 (22.1) | 56 (24.0) | 126 (23.1) | .77 |
| Ever incarcerated | 358 (36.1) | 72 (30.9) | 215 (39.2) | .03 |
| Current unstable housing | 54 (5.4) | 9 (3.9) | 32 (6.0) | .25 |
| Prescribed ART | 516 (52.1) | 225 (96.6) | 89 (16.2) | <.01 |
| Receiving ART for ≤6 months | 175 (17.7) | |||
| Disclosure to sex partners | 464 (46.8) | 108 (50.9) | 246 (50.5) | .92 |
| CRAFFT, mean (SD) | 2.7 (1.8) | 2.7 (1.8) | 2.7 (1.8) | .79 |
| Daily alcohol use | 59 (6.0) | 9 (4.1) | 37 (7.3) | .10 |
| Daily marijuana use | 265 (26.7) | 55 (31.8) | 155 (36.4) | .29 |
| BSI depression subscale score, mean (SD) | 7.0 (6.1) | 6.3 (5.7) | 7.3 (6.2) | .03 |
| BSI anxiety subscale score, mean (SD) | 5.4 (5.4) | 4.7 (5.1) | 5.7 (5.4) | .02 |
| BSI GSI score, mean (SD)b | 1.0 (0.8) | 0.9 (0.8) | 1.1 (0.8) | .03 |
| CAI | 458 (46.2) | 91 (44.4) | 266 (54.7) | .01 |
| Serodiscordant CAI | 310 (31.3) | 57 (25.0) | 187 (34.9) | .01 |
Abbreviations: ART, antiretroviral treatment; BSI, Brief Symptom Inventory; CAI, condomless anal Intercourse; CRAFFT, Car, Relax, Alone, Forget, Friends, Trouble (keywords in a screening questionnaire to identify at-risk teen substance abusers); GSI, Global Severity Index; HIV, human immunodeficiency virus; VL−, virologically suppressed; VL+, virologically detectable; YMSM, young men who have sex with men.
Data are presented as number (percentage) unless otherwise indicated.
The BSI GSI criteria for clinically indicative symptoms are as follows: males 19 years or younger with GSI scores of 1.71 or higher, females 19 years or younger with GSI scores of 1.59 or higher, males 20 years or older with GSI scores of 0.58 or higher, and females 20 years or older with GSI scores of 0.78 or higher.
Demographic and Psychosocial Differences Between VL− and VL+ HIV-Infected YMSM
In total, 288 YMSM (29.1%) were VL−, whereas 688 (69.4%) were VL+; data were not available for 15 participants (1.5%). Excluding YMSM who were receiving ART for 6 months or less, 233 (29.3%) were VL− and 549 (69.0%) were VL+. Table 1 gives the differences between VL− and VL+ YMSM on study variables. Compared with YMSM who were VL−, those who were VL+ were more likely to be 21 years or older and to have ever been incarcerated. The VL+ YMSM were less likely to be currently employed and to report a post–high school educational level compared with VL− YMSM. In addition, VL+ YMSM had significantly higher scores than VL− YMSM on the BSI depression and anxiety subscales and the GSI. The VL+ YMSM were also more likely to report any CAI and serodiscordant CAI compared with VL− YMSM.
Factors Associated With CAI Among VL+ HIV-Infected YMSM
Univariable analyses revealed several factors associated with any CAI and serodiscordant CAI during the past 3 months among VL+ YMSM (eTable in the Supplement). Variables that were negatively related to CAI included transgender gender identity and black or African American race, whereas those that were positively associated with CAI included disclosure of HIV status to sex partners, problematic substance use, daily alcohol use, and BSI depression and anxiety subscale and GSI scores. In addition, factors that were negatively associated with serodiscordant CAI included current employment and black or African American race, whereas those that were positively related to serodiscordant CAI included problematic substance use, daily alcohol use, and BSI depression and anxiety subscale and GSI scores.
Table 2 and Table 3 contain findings from the multivariable analyses. Transgender gender identity (adjusted odds ratio [AOR], 0.35; 95% CI, 0.14-0.85) and black or African American race (AOR, 0.64; 95% CI, 0.48-0.86) were negatively associated with CAI. Disclosure of HIV status to sex partners (AOR, 1.45; 95% CI, 1.08-1.94) and problematic substance use (AOR, 1.14; 95% CI, 1.04-1.25) were positively associated with CAI. Variables observed to be negatively associated with serodiscordant CAI were current employment (AOR, 0.74; 95% CI, 0.55-0.99) and black or African American race (AOR, 0.63; 95% CI, 0.47-0.84). Problematic substance use was positively associated with serodiscordant CAI (AOR, 1.10; 95% CI, 1.01-1.20).
Table 2. Multivariable Analysis Predicting Condomless Anal Intercourse in Past 3 Months Among 688 VL+ HIV-Infected YMSM.
| Variable | Odds Ratio (95% CI) |
|---|---|
| Transgender | 0.35 (0.14-0.85)a |
| Black race | 0.35 (0.14-0.85)a |
| Disclosure to sex partners | 0.63 (0.44-0.90)b |
| CRAFFT score ≥2 | 1.35 (1.01-1.81)a |
| Daily alcohol use | 1.46 (1.02-2.10)a |
| BSI depression subscale scorec | 1.86 (0.90-3.83) |
| BSI anxiety subscale scorec | 0.98 (0.96-1.01) |
| BSI GSI scorec | 0.98 (0.95-1.02) |
Abbreviations: BSI, Brief Symptom Inventory; CRAFFT, Car, Relax, Alone, Forget, Friends, Trouble (keywords in a screening questionnaire to identify at-risk teen substance abusers); GSI, Global Severity Index; HIV, human immunodeficiency virus; VL+, virologically detectable; YMSM, young men who have sex with men.
P ≤ .05.
P ≤ .01.
The BSI depression and anxiety and GSI variables were tested in separate multivariate models because of multicollinearity.
Table 3. Multivariable Analysis Predicting Serodiscordant Condomless Anal Intercourse in Past 3 Months Among 688 VL+ HIV-Infected YMSM.
| Variable | Odds Ratio (95% CI) |
|---|---|
| Current employment | 0.76 (0.58-0.99)a |
| Black race | 0.66 (0.46-0.94)a |
| CRAFFT score ≥2 | 1.45 (1.06-1.99)a |
| Daily alcohol use | 1.52 (0.92-2.53) |
| BSI depression subscale scoreb | 0.98 (0.95-1.00) |
| BSI anxiety subscale scoreb | 0.99 (0.97-1.02) |
| BSI GSI scoreb | 0.90 (0.74-1.09) |
Abbreviations: BSI, Brief Symptom Inventory; CRAFFT, Car, Relax, Alone, Forget, Friends, Trouble (keywords in a screening questionnaire to identify at-risk teen substance abusers); GSI, Global Severity Index; HIV, human immunodeficiency virus; VL+, virologically detectable; YMSM, young men who have sex with men.
P ≤.05.
The BSI depression and anxiety and GSI variables were tested in separate multivariate models because of multicollinearity.
Discussion
This study is among the first to examine factors associated with sexual risk behaviors among VL+ YMSM. Condomless anal intercourse occurred with a relatively high level of frequency among YMSM in our sample. Furthermore, VL+ YMSM were more likely to report sexual risk behaviors than VL− YMSM. Taken together, findings suggest that to achieve the full promise offered by treatment as prevention for YMSM living with HIV, it is critical to develop behavioral interventions to address sexual risk behavior.2
It was particularly troubling that in a sample of YMSM linked to care fewer than 3 in 10 (29.1%) were VL−. This estimate is on the lowest end of the range observed in observational studies included in a recently conducted review7 of research examining the continuum of care for HIV-infected youth (ie, viral suppression rates ranged from 30% to 70%), suggesting stark disparities in viral suppression affecting HIV-infected YMSM.
Previous research22 has found disparities in ART uptake and adherence affecting youth living with HIV. Demographic (ie, age, educational level) and structural (ie, unemployment, incarceration) factors were related to being VL+ among the YMSM in our sample. Likewise, mental health problems and engagement in sexual risk behaviors were associated with being VL+ among participants. Research has suggested that a confluence of structural, psychological, and behavioral factors enhance vulnerability to poor health outcomes among MSM, notably those who are YMSM and racial/ethnic minority MSM.18,23-25
Univariable analyses revealed that a number of demographic, psychological, behavioral, and structural factors were associated with CAI in the subsample of YMSM with a detectable viral load. However, multivariable analyses revealed the independent positive associations of problematic substance use, nonblack race, unemployment, cisgender identity, and HIV status disclosure to sexual risk behaviors (ie, any CAI and/or serodiscordant CAI) among VL+ YMSM. Previous research has found that substance use is associated with sexual risk behavior among YMSM26,27 and also disrupts adherence and impedes uptake of ART.28,29 Our findings suggest that substance use must continue to be a target of health promotion interventions targeting HIV-infected youth.
Findings from the study also illustrate the paradox described by Millet et al9 in which black MSM have lower levels of sexual risk behaviors yet are the most highly affected by HIV in the United States. In our study, VL+ black YMSM had a lower likelihood of engaging in sexual risk behaviors compared with VL+ YMSM of other racial/ethnic groups. This finding underscores the role that social network and structural factors play in explaining enhanced HIV vulnerability among black YMSM.9 Our finding that transgender participants were less likely to report CAI compared with cisgender participants highlights the need for more research on the factors associated with high prevalence of HIV among transgender women. Similar to black YMSM, network-level factors, and not solely individual-level behaviors, may influence heightened risk for HIV in this population.3
The finding that YMSM who disclosed their HIV status to sex partners were more likely to report any CAI than those not disclosing their status is consistent with other research17 and may suggest that many YMSM in the sample engaged in serosorting as a harm reduction strategy, given the significant association of disclosure to any CAI but not to serodiscordant CAI. Last, our findings suggest that socioeconomic factors, such as unemployment, are associated with increased HIV transmission risk behaviors. More research is needed to examine the mechanisms through which structural-level factors, such as joblessness, are related to sexual risk. For example, socioeconomic stress resulting from unemployment and related factors may lead to externalizing symptoms, such as sensation seeking and the use of sex to avoid or alleviate negative affect, which have been linked to sexual risk behavior among sexually active MSM.18,30,31
Our study is limited in that we used a cross-sectional approach that does not permit for causal inference. As noted, the sample includes only HIV-infected YMSM who are linked to care and thus limits the generalizability of the findings. In addition, our sample included a small number of transgender participants. Future research is needed to explore the unique risk factors for poor health outcomes among HIV-infected transgender women. Despite these limitations, this research makes valuable contributions to our understanding of factors associated with viral detectability among HIV-infected YMSM and with sexual risk behavior among VL+ YMSM.
Conclusions
Our findings highlight the inadequacies of treatment as prevention as the sole risk reduction method among HIV-infected YMSM in the United States and speak to a greater need for behavioral approaches to improve ART uptake and ultimately achieve higher rates of viral suppression in this population. Combination HIV prevention and treatment interventions, which include behavioral, biomedical, and structural strategies to increase viral suppression and reduce HIV transmission risk behaviors, that target HIV-infected YMSM are needed. To truly curb HIV incidence among YMSM, we cannot solely rely on one strategy to prevent and treat HIV.
Supplementary Material
At a Glance.
A total of 688 (69.4%) of the human immunodeficiency virus (HIV)–infected young men who have sex with men (YMSM) in the sample had a detectable viral load.
The HIV-infected YMSM with a detectable viral load were more likely to report sexual risk behavior compared with those who were virally suppressed. More than half (266 [54.7%]) of detectable YMSM reported condomless anal intercourse (CAI), whereas 91 (44.4%) of those who were suppressed reported CAI (P = .01). Likewise, 187 (34.9%) of the detectable YMSM reported CAI with an HIV-negative partner, whereas only 57 (25.0%) of suppressed YMSM reported serodiscordant CAI (P < .01).
Factors related to CAI among virally detectable YMSM included problematic substance use, nonblack race, disclosure of HIV to sex partners, cisgender identity, and current unemployment.
Acknowledgments
Funding/Support: This work was supported by grants U01 HD 040533 and U01 D 040474 from the ATN of the National Institutes of Health through the National Institute of Child Health and Human Development, with supplemental funding from the National Institute on Drug Abuse and the National Institute on Mental Health.
Role of the Funder/Sponsor: The funding sources had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Additional Contributions: The study was scientifically reviewed by the Adolescent Medicine Trials Network for HIV/Aids Intervention's (ATN) Behavioral Leadership Group. Network, scientific, and logistical support was provided by the ATN Coordinating Center at University of Alabama at Birmingham. Network operations and data management support were provided by the ATN Data and Operations Center at Westat Inc. We acknowledge the contribution of the investigators and staff at the following sites that participated in this study: University of South Florida, Children's Hospital of Los Angeles, Children's National Medical Center, Children's Hospital of Philadelphia, John H. Stroger, Jr. Hospital of Cook County and the Ruth M. Rothstein CORE Center, University of Puerto Rico, Montefiore Medical Center, Mount Sinai Medical Center, University of California, San Francisco, Tulane University Health Sciences Center, University of Maryland, University of Miami School of Medicine, Children's Diagnostic and Treatment Center, St. Jude's Children's Research Hospital, Children's Memorial Hospital, Baylor College of Medicine, Wayne State University, John Hopkins University School of Medicine, The Fenway Institute–Boston, and University of Colorado Denver. The investigators are grateful to the members of the local youth community advisory boards for their insight and counsel and are particularly indebted to the youth who participated in this study.
Footnotes
Author Contributions: Dr P. A. Wilson had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: P. A. Wilson, Kahana, Fernandez, C. M. Wilson, Hightow-Weidman.
Acquisition, analysis, or interpretation of data: All authors.
Drafting of the manuscript: P. A. Wilson, Kahana, Fernandez, Harper, Mayer, C. M. Wilson, Hightow-Weidman.
Critical revision of the manuscript for important intellectual content: P. A. Wilson, Kahana, Fernandez, Mayer, C. M. Wilson, Hightow-Weidman.
Statistical analysis: P. A. Wilson, Mayer.
Administrative, technical, or material support: Kahana, Fernandez, Harper, C. M. Wilson.
Study supervision: Fernandez, Mayer, C. M. Wilson.
Conflict of Interest Disclosures: None reported.
Disclaimer: The views and opinions expressed in this report are those of the authors and should not be construed to represent the views of the National Institute on Drug Abuse, the Eunice Kennedy Shriver National Institute of Child Health and Human Development, any of the sponsoring organizations or agencies, or the US government.
Additional Information: Clinics were located in the following cities: Los Angeles, California; San Francisco, California; Washington, DC; Baltimore, Maryland; Boston, Massachusetts; Chicago, Illinois; Philadelphia, Pennsylvania; New York City (Bronx and Manhattan), New York; San Juan, Puerto Rico; New Orleans, Louisiana; Memphis, Tennessee; Miami, Florida; Tampa, Florida; Ft. Lauderdale, Florida; Detroit, Michigan; Denver, Colorado; and Houston, Texas.
Contributor Information
Patrick A. Wilson, Mailman School of Public Health, Columbia University, New York, New York.
Shoshana Y. Kahana, Division of Epidemiology, Services, and Prevention Research, National Institute on Drug Abuse, National Institutes of Health, Bethesda, Maryland.
Maria Isabel Fernandez, College of Osteopathic Medicine, Nova Southeastern University, Ft Lauderdale, Florida.
Gary W. Harper, School of Public Health, University of Michigan, Ann Arbor.
Kenneth Mayer, The Fenway Institute, Harvard Medical School, Boston, Massachusetts.
Craig M. Wilson, School of Public Health, University of Alabama at Birmingham, Birmingham.
Lisa B. Hightow-Weidman, Division of Infectious Diseases, University of North Carolina at Chapel Hill, Chapel Hill (Hightow-Weidman).
References
- 1.Prejean J, Song R, Hernandez A, et al. HIV Incidence Surveillance Group. Estimated HIV incidence in the United States, 2006-2009. PloS One. 2011;6(8):e17502. doi: 10.1371/journal.pone.0017502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Johnson AS, Hall HI, Hu X, Lansky A, Holtgrave DR, Mermin J. Trends in diagnoses of HIV infection in the United States, 2002-2011. JAMA. 2014;312(4):432–434. doi: 10.1001/jama.2014.8534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baral SD, Poteat T, Strömdahl S, Wirtz AL, Guadamuz TE, Beyrer C. Worldwide burden of HIV in transgender women: a systematic review and meta-analysis. Lancet Infect Dis. 2013;13(3):214–222. doi: 10.1016/S1473-3099(12)70315-8. [DOI] [PubMed] [Google Scholar]
- 4.Centers for Disease Control and Prevention (CDC) Vital signs: HIV infection, testing, and risk behaviors among youths—United States. MMWR Morb Mortal Wkly Rep. 2012;61(47):971–976. [PubMed] [Google Scholar]
- 5.Cohen MS, Chen YQ, McCauley M, et al. HPTN 052 Study Team. Prevention of HIV-1 infection with early antiretroviral therapy. N Engl J Med. 2011;365(6):493–505. doi: 10.1056/NEJMoa1105243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rodger A, Bruun T, Cambiano V, et al. HIV transmission risk through condomless sex if HIV+ partner on suppressive ART: PARTNER study. Proceedings from the Conference on Retroviruses and Opportunistic Infections (CROI 2014); March 3-6, 2014; Boston, MA: Abstract 153LB. [Google Scholar]
- 7.Zanoni BC, Mayer KH. The adolescent and young adult HIV cascade of care in the United States: exaggerated health disparities. AIDS Patient Care STDS. 2014;28(3):128–135. doi: 10.1089/apc.2013.0345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rosenberg ES, Millett GA, Sullivan PS, Del Rio C, Curran JW. Understanding the HIV disparities between black and white men who have sex with men in the USA using the HIV care continuum: a modeling study. Lancet HIV. 2014;1(3):e112–e118. doi: 10.1016/S2352-3018(14)00011-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Millett GA, Peterson JL, Flores SA, et al. Comparisons of disparities and risks of HIV infection in black and other men who have sex with men in Canada, UK, and USA: a meta-analysis. Lancet. 2012;380(9839):341–348. doi: 10.1016/S0140-6736(12)60899-X. [DOI] [PubMed] [Google Scholar]
- 10.Magnus M, Jones K, Phillips G, II, et al. YMSM of color Special Projects of National Significance Initiative Study Group. Characteristics associated with retention among African American and Latino adolescent HIV-positive men: results from the outreach, care, and prevention to engage HIV-seropositive young MSM of color special project of national significance initiative. J AcquirImmune Defic Syndr. 2010;53(4):529–536. doi: 10.1097/QAI.0b013e3181b56404. [DOI] [PubMed] [Google Scholar]
- 11.Kahana SY, Fernandez MI, Wilson PA, et al. Rates and correlates of antiretroviral therapy use and virologic suppression among perinatally and behaviorally HIV-infected youth linked to care in the United States. J Acquir Immune Defic Syndr. 2015;68(2):169–177. doi: 10.1097/QAI.0000000000000408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kalichman SC. HIV transmission risk behaviors of men and women living with HIV/AIDS: prevalence, predictors, and emerging clinical interventions. Clin Psychol-Sci Pr. 2000;7(1):32–47. [Google Scholar]
- 13.Murphy DA, Durako SJ, Moscicki AB, et al. Adolescent Medicine HIV/AIDS Research Network. No change in health risk behaviors over time among HIV infected adolescents in care: role of psychological distress. J Adolesc Health. 2001;29(3 suppl):57–63. doi: 10.1016/s1054-139x(01)00287-7. [DOI] [PubMed] [Google Scholar]
- 14.Tanney MR, Naar-King S, Murphy DA, Parsons JT, Janisse H ATN 004 Protocol Team. Multiple risk behaviors among youth living with human immunodeficiency virus in five U.S. cities. J Adolesc Health. 2010;46(1):11–16. doi: 10.1016/j.jadohealth.2009.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Valleroy LA, MacKellar DA, Karon JM, et al. Young Men's Survey Study Group. HIV prevalence and associated risks in young men who have sex with men. JAMA. 2000;284(2):198–204. doi: 10.1001/jama.284.2.198. [DOI] [PubMed] [Google Scholar]
- 16.Boone MR, Cook SH, Wilson P. Substance use and sexual risk behavior in HIV-positive men who have sex with men: an episode-level analysis. AIDS Behav. 2013;17(5):1883–1887. doi: 10.1007/s10461-012-0167-4. [DOI] [PubMed] [Google Scholar]
- 17.Cook SH, Valera P, Wilson PA Adolescent Trials Network for HIV/AIDS Interventions. HIV status disclosure, depressive symptoms, and sexual risk behavior among HIV-positive young men who have sex with men. J Behav Med. 2015;38(3):507–517. doi: 10.1007/s10865-015-9624-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wilson PA, Stadler G, Boone MR, Bolger N. Fluctuations in depression and well-being are associated with sexual risk episodes among HIV-positive men. Health Psychol. 2014;33(7):681–685. doi: 10.1037/a0035405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.WHO ASSIST Working Group. The Alcohol, Smoking and Substance Involvement Screening Test (ASSIST): development, reliability and feasibility. Addiction. 2002;97(9):1183–1194. doi: 10.1046/j.1360-0443.2002.00185.x. [DOI] [PubMed] [Google Scholar]
- 20.Knight JR, Sherritt L, Shrier LA, Harris SK, Chang G. Validity of the CRAFFT substance abuse screening test among adolescent clinic patients. Arch Pediatr Adolesc Med. 2002;156(6):607–614. doi: 10.1001/archpedi.156.6.607. [DOI] [PubMed] [Google Scholar]
- 21.Derogatis L, Spencer M. The Brief Symptom Inventory (BSI): Administration, Scoring, and Procedures Manual 1. Baltimore, MD: Johns Hopkins University School of Medicine, Clinical Psychometrics Research Unit; 1982. [Google Scholar]
- 22.Agwu AL, Jang SS, Korthuis PT, Araneta MRG, Gebo KA. Pregnancy incidence and outcomes in vertically and behaviorally HIV-infected youth. JAMA. 2011;305(5):468–470. doi: 10.1001/jama.2011.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Halkitis PN, Wolitski RJ, Millett GA. A holistic approach to addressing HIV infection disparities in gay, bisexual, and other men who have sex with men. Am Psychol. 2013;68(4):261–273. doi: 10.1037/a0032746. [DOI] [PubMed] [Google Scholar]
- 24.Balaji AB, Bowles KE, Le BC, Paz-Bailey G, Oster AM NHBS Study Group. High HIV incidence and prevalence and associated factors among young MSM, 2008. AIDS. 2013;27(2):269–278. doi: 10.1097/QAD.0b013e32835ad489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dyer TP, Shoptaw S, Guadamuz TE, et al. Application of syndemic theory to black men who have sex with men in the Multicenter AIDS Cohort Study. J Urban Health. 2012;89(4):697–708. doi: 10.1007/s11524-012-9674-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bruce D, Kahana S, Harper GW, Fernández MI ATN. Alcohol use predicts sexual risk behavior with HIV-negative or partners of unknown status among young HIV-positive men who have sex with men. AIDS Care. 2013;25(5):559–565. doi: 10.1080/09540121.2012.720363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mustanski BS, Newcomb ME, Du Bois SN, Garcia SC, Grov C. HIV in young men who have sex with men: a review of epidemiology, risk and protective factors, and interventions. J Sex Res. 2011;48(2-3):218–253. doi: 10.1080/00224499.2011.558645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hinkin CH, Barclay TR, Castellon SA, et al. Drug use and medication adherence among HIV-1 infected individuals. AIDS Behav. 2007;11(2):185–194. doi: 10.1007/s10461-006-9152-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nugent NR, Brown LK, Belzer M, Harper GW, Nachman S, Naar-King S Adolescent Trials Network for HIV/AIDS Interventions. Youth living with HIV and problem substance use: elevated distress is associated with nonadherence and sexual risk. J Int Assoc Physicians AIDS Care (Chic) 2010;9(2):113–115. doi: 10.1177/1545109709357472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Alvy LM, McKirnan DJ, Mansergh G, et al. Project MIX Study Group. Depression is associated with sexual risk among men who have sex with men, but is mediated by cognitive escape and self-efficacy. AIDS Behav. 2011;15(6):1171–1179. doi: 10.1007/s10461-010-9678-z. [DOI] [PubMed] [Google Scholar]
- 31.Mustanski B, Garofalo R, Herrick A, Donenberg G. Psychosocial health problems increase risk for HIV among urban young men who have sex with men: preliminary evidence of a syndemic in need of attention. Ann Behav Med. 2007;34(1):37–45. doi: 10.1080/08836610701495268. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.