Abstract
Aim
The aim of this article is to cross-sectionally compare objectively measured physical activity (PA) levels and their association with migraine characteristics in obese women with and without migraine.
Methods
Obese women seeking weight loss treatment were divided into migraine (n = 25) and control (n = 25) groups matched by age and body mass index (BMI). Participants wore the SenseWear Armband monitor for seven days to objectively evaluate daily light-(LPA) and moderate-to-vigorous intensity PA (MVPA). Migraine diagnosis was confirmed by a neurologist using ICHD-3-beta criteria. Migraine characteristics were tracked daily using a smartphone-based diary over a four-week period immediately preceding the objective PA assessment.
Results
Migraine participants spent 57.9 fewer minutes/day in LPA (141.1 ± 56.4 vs. 199.1 ± 87.7, p = 0.019) and 24.5 fewer minutes/day in MVPA (27.8 ± 17.0 vs. 52.3 ± 26.0, p <0.001), compared to controls. Migraine participants reported 4.8 ± 3.1 migraine days/month (mean duration = 17.1 ± 8.9 hours; mean maximum pain severity = 6.4 ± 1.7 on a 0–10 scale). Higher BMI (p <0.05), but not migraine characteristics, were related to lower total PA. Additionally, total objectively measured PA was not associated with how often PA was reported to exacerbate migraine attacks during the four-week diary assessment.
Conclusions
Obese women with migraine spent nearly 1.5 hours/day less in PA compared to controls; however, lower PA was not related to migraine characteristics. Further research is needed to identify PA barriers and effective interventions in obese women with migraine.
Keywords: Obesity, migraine, headache, physical activity, smartphone
Introduction
Evidence for an association between obesity and migraine continues to accumulate. Multiple studies have shown that obesity increases the risk for episodic and chronic migraine (1–5). Moreover, a number of plausible mechanisms have been proposed that give further support to the epidemiological link between obesity and migraine (6,7), including shared behavioral risk factors (8,9).
One of the overlapping behavioral mechanisms of migraine and obesity may be low physical activity (PA). The role of low PA in the development and persistence of obesity is well documented (10). Additionally, the recent advent of accelerometers and multi-sensor monitors for objective quantification of movement and related energy expenditure has contributed to a more sophisticated understanding of the relationship between weight status and free-living PA patterns. Research using these objective measures indicates that obese individuals take fewer steps per day, spend fewer minutes in light and moderate-to-vigorous PA, and are less likely to achieve public health guidelines than those who are overweight or normal weight (11–13).
Although the role of low PA in migraine has received far less attention, population-based studies suggest that low PA levels are associated with higher prevalence and frequency of migraine and other headaches (14–17). Conversely, intervention and observational research has shown that higher PA levels are associated with reductions in migraine headache frequency and with less migraine-related disability (18–21). Additionally, PA is associated with reversion of chronic migraine into episodic migraine (22). Findings from the above studies, however, are limited by the use of retrospective self-report physical activity questionnaires. These tools are prone to measurement error and bias, and are often unable to provide detailed information on PA levels and patterns in daily life (23). Moreover, while previous research suggests that lower PA levels are associated with migraine, no study has directly compared whether individuals with migraine have lower PA levels than individuals without migraine. Additionally, most of the epidemiological studies evaluating PA in people with migraine did not assess migraine prospectively over time, thereby limiting ability to obtain reliable information about patterns in migraine attack frequency, severity, duration, and related clinical parameters. Consequently, the free-living habitual PA patterns of individuals with migraine and the association of these patterns with migraine characteristics remains unclear. Moreover, no study, including those examining the effect of aerobic exercise on migraine (18–20), has examined these associations within the context of obesity.
The primary purpose of the current study was twofold: 1) to compare objectively measured typical daily patterns of light- and moderate-to-vigorous intensity PA over a seven-day period in obese women with migraine and in obese women without migraine matched by age and body mass index (BMI); and 2) to examine the association between total volume of PA and migraine characteristics (i.e. attack frequency, duration, and severity), measured prospectively in real time over a four-week period via a daily smartphone-based headache diary that immediately preceded the seven-day objective PA assessment. We hypothesized that: 1) participants with migraine would spend fewer minutes per day in light- and moderate-to-vigorous intensity PA compared to control participants without migraine; and 2) greater migraine frequency, severity, and duration would be associated with lower total daily PA minutes. Finally, we also explored associations of daily PA with degree of obesity and depression within the migraine and control groups separately.
Methods
Design, participants and procedures
This study employed a cross-sectional, case (i.e. obese women with migraine)-control (obese women without migraine) design. Migraine participants (n = 25) were women, age 18–50 years old, who were obese (BMI = 30.0–49.9 kg/m2), had a neurologist-confirmed diagnosis of migraine with or without aura according to International Classification for Headache Disorders third edition beta (ICHD-III-beta) criteria (24), and were seeking behavioral treatment to lose weight and treat their migraine attacks as part of the Women’s Health and Migraine (WHAM) trial (25).
Non-migraine control participants (n = 25) were selected from a sample of obese women who were seeking behavioral treatment to lose weight at the same research center as part of another study designed for the general overweight/obese adult population and that was unrelated to migraine. Individuals were deemed as not having migraine if they indicated on a questionnaire that they had never been diagnosed with migraine and reported no migraine headaches based on ICHD-III-beta criteria during the past month. Non-migraine status based on evaluation of control participants’ questionnaire responses was determined by the primary author, who was trained by a study neurologist familiar with ICHD-III-beta criteria. Control participants were matched with migraine participants on age (±2 years) and BMI (±1 kg/m2 unit).
Participants were recruited from the community via advertisements for behavioral weight loss treatment (control participants) or behavioral weight loss and migraine treatment (migraine participants) on Internet sites, social media outlets (i.e. Facebook and Twitter), newspapers, and direct mailings between January 2013 and April 2014. Individuals interested in participating were asked to contact the research center by calling a provided telephone number or visiting a Web site. Individuals were screened by telephone to determine eligibility. Those individuals deemed initially eligible were invited to a study orientation visit during which informed consent procedures, height and weight measurements, and demographics and psychological questionnaires were completed. Additionally, control participants completed the questionnaire to confirm non-migraine status. Participants with migraine were evaluated by the study neurologist to confirm migraine diagnosis and given a smartphone-based diary to monitor headache activity for 28 consecutive days. All participants were given a PA monitor to wear for seven consecutive days once during a baseline assessment period prior to study randomization and initiation of treatment. Participants in the migraine group wore the activity monitor during the week immediately after they completed the four-week migraine diary. The study protocol was approved by the Rhode Island Hospital Institutional Review Board, Providence, RI, USA.
Measures
Objective physical activity assessment
The SenseWear Mini Armband monitor (SWA, BodyMedia Inc, Pittsburgh, PA) was used to objectively measure daily time spent in PA of both a light- and moderate-to-vigorous intensity. The SWA is a wireless multi-sensor monitor worn over the upper left triceps muscle that simultaneously integrates motion data from a triaxial accelerometer, physiologic metrics from multiple sensors (i.e. heat flux, galvanic skin response, and skin and near-body temperatures), and sex, age, body weight, and height information to estimate energy expenditure and intensity of activities using proprietary software algorithms (SenseWear Professional Software, version 7.0). The SWA has been shown to accurately measure daily energy expenditure when evaluated against indirect calorimetry and doubly labeled water criterion measures (26,27) and provides estimates of time spent engaged in light and moderate-to-vigorous PA comparable to other objective monitors (28,29).
Participants were asked to wear the SWA during all waking hours for seven consecutive days. The number of minutes participants spent in activities of different intensities was determined using metabolic equivalent (MET) values, with activities 1.5–2.9 METs, activities ≥3 METs, and activities ≥1.5 METs classified as light, moderate-to-vigorous, and total PA, respectively. For data to be considered valid, participants needed to have worn the SWA for ≥8 hours/day on ≥4 days during the seven-day assessment period. All participants met the criteria, wearing the SWA for an average of 13.5 ± 1.7 hours/day on 6.7 ± 1.6 days.
Prospective measurement of migraine characteristics
Participants in the migraine group reported their headache activity for 28 consecutive days using a smart-phone and accompanying Web-based headache diary application at the end of each day (25). Participants used this device to record migraine attack occurrence, severity, and duration, as well as number of attacks with related clinical features, including exacerbation by PA. This smartphone-based monitoring approach enabled reporting of migraine activity in real time, thereby promoting optimal compliance and reducing bias inherent to use of paper-and-pencil diaries (23,25).
Demographic, anthropometric, and psychological characteristics
Demographic characteristics (age, race, level of education, and marital status) were assessed via questionnaire. Weight was measured in light street clothing, without shoes, and on a calibrated scale. BMI was calculated using the formula: BMI (kg/m2) = weight (kg)/(height (m))2. Depressive mood and symptoms during the previous week were assessed using the 10-item short version of the Center for Epidemiologic Studies-Depression Scale (CES-D) (30). Higher scores indicate greater symptoms and a cutoff score of ≥10 indicates clinically relevant symptoms of depression (31).
Statistical analysis
Descriptive statistics (mean and SD) were calculated for all continuous variables. Categorical variables were represented using counts and proportions (%). T-test and chi-square were used to compare demographic, anthropometric, and psychological characteristics between the migraine and control groups. Analysis of covariance (ANCOVA) was used to compare differences between the migraine and control groups in minutes per day spent in light, moderate-to-vigorous, and total PA, adjusting for differences in daily SWA monitor wear time. Separate linear regression models were used to examine the relationship of light, moderate-to-vigorous, and total PA minutes per day with migraine frequency (number of headache days per 28 days), average attack duration, and average maximum pain severity on a 0 (no pain)–10 (extreme pain) scale, adjusting for age and BMI. Linear regression analyses were also used to examine the relationship between degree of obesity and daily time spent in light, moderate-to-vigorous and total PA, adjusting for age, in control participants. Pearson’s r correlation coefficient was used to examine associations of daily light, moderate-to-vigorous, and total PA with the reported proportion of migraine attacks exacerbated by PA in the migraine group, and with depression symptoms in both the migraine and control groups. All analyses were performed using SPSS Statistics for Windows (version 20.0; SPSS, IBM Corp, Armonk, NY). All tests of statistical significance were two tailed, with α = 0.05, unless otherwise noted.
Results
Characteristics of obese women participants with and without migraine
Table 1 shows demographic and weight characteristics of obese women participants in the migraine (n = 25) and control (n = 25) groups. On average, the groups were equivalent on age (42.1 ± 6.6 years) and BMI (37.5 ± 4.8 kg/m2) as per design. Additionally, the groups did not differ on race, educational level, and marital status. On average, participants with migraine had worse depressive symptoms than controls. However, the proportion of participants in the migraine and control groups that met the threshold for clinically significant depressive symptoms was not significantly different (44.0% vs. 24.0%, p = 0.14).
Table 1.
Characteristics of obese women with and without migraine.
| Obese women with migraine (n = 25) | Obese women without migraine (n = 25) | p | |
|---|---|---|---|
| Age, mean (±SD), years | 42.0 (6.1) | 42.2 (7.3) | 0.92 |
| Body mass index, mean (±SD), kg/m2 | 37.8 (5.0) | 37.3 (4.6) | 0.71 |
| Race (%) | 0.41 | ||
| White | 92.0 | 92.0 | |
| African American | 4.0 | 0.0 | |
| Native Hawaiian or Pacific Islander | 0.0 | 4.0 | |
| Other | 4.0 | 0.0 | |
| Mixed race | 0.0 | 4.0 | |
| Education (%) | 0.10 | ||
| High school | 4.0 | 20.0 | |
| Vocational training | 0.0 | 8.0 | |
| Some college | 40.0 | 16.0 | |
| College/university degree | 36.0 | 28.0 | |
| Graduate or professional education | 20.0 | 28.0 | |
| Marital status (%) | 0.42 | ||
| Never married | 12.0 | 20.0 | |
| Not married, living with partner | 4.0 | 0.0 | |
| Married | 56.0 | 52.0 | |
| Divorced/separated/widowed | 28.0 | 28.0 | |
| Depressive symptoms, mean (±SD) | 9.5 (6.3) | 5.8 (5.1) | 0.03 |
| Migraine characteristics, mean (±SD) | |||
| Number of migraine attacks | 3.5 (2.7) | – | – |
| Number of migraine days | 4.8 (3.2) | – | – |
| Attack duration, hours | 17.1 (8.9) | – | – |
| Average maximum pain severity | 6.4 (1.7) | – | – |
Participants with migraine reported on average approximately five migraine days and four attacks with average duration of 17 hours and average maximum pain severity of 6.4 on a 0 (no pain)–10 (pain as bad as you can imagine) scale during the four-week smartphone diary assessment.
Comparison of daily PA between obese women participants with and without migraine
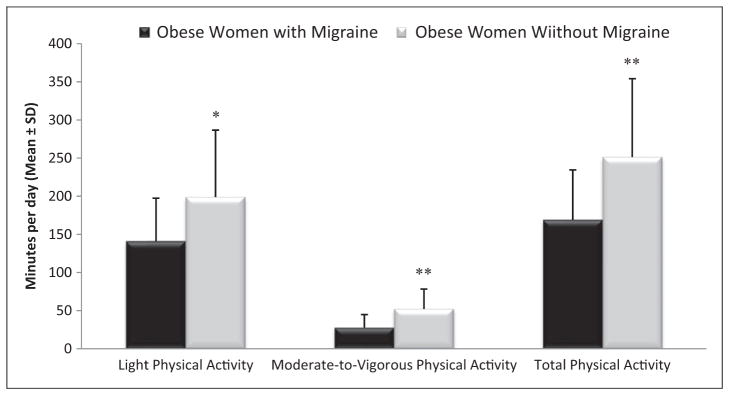
There was no statistically significant difference between the migraine and control groups in the amount of time participants wore the SWA monitor each day (13.1 ± 1.9 vs. 13.8 ± 1.3 hours/day, p = 0.16). Figure 1 shows differences between the migraine and control groups in minutes per day spent in light, moderate-to-vigorous, and total PA. Participants with migraine spent an average of 57.9 fewer minutes per day in light PA (141.1 ± 56.4 vs. 199.0 ± 87.7 minutes/day, p = 0.019) and 24.5 fewer minutes per day in moderate-to-vigorous PA (27.8 ± 17.0 vs. 52.3 ± 26.0 minutes/day, p <0.001), totaling 82.4 fewer minutes of PA per day (169.0 ± 65.6 vs. 251.4 ± 102.8 minutes/day, p = 0.004), compared to controls.
Figure 1.
Comparison of daily physical activity in obese women with and without migraine.
*= p <0.05, ** p <0.001. Adjusted for daily monitor (i.e. SenseWear Mini Armband) wear time.
Associations between total daily PA and migraine characteristics in obese women participants with migraine
Results of linear regression analysis models predicting total daily PA minutes from number of migraine days (β= −0.22, p= 0.27), average attack duration (β= 0.13, p= 0.49), and average maximum pain severity (β= −0.03, p= 0.88) were all non-significant when controlling for age and BMI. However, higher BMI was associated with lower amount of total daily PA in the models in which migraine days (β= −0.68, p= 0.004), migraine duration (β= −0.53, p= 0.01), and maximum pain severity (β= −0.57, p= 0.009) were used to predict total daily PA minutes.
Physical activity was reported to be an exacerbating factor in nearly half (48.9±40.5%) of migraine attacks, and the majority (68.0%) of participants indicated that PA exacerbated at least one attack. However, daily time spent in light (r= −0.18, p= 0.40), moderate-to- vigorous (r= −0.23, p= 0.26) and total (r= −0.21, p= 0.31) PA were not associated with the number of attacks in which PA was cited as an exacerbating factor.
Associations between obesity and daily PA in obese women participants without migraine
Results of linear regression analyses examining associations of total daily PA minutes with BMI indicated that greater degree of obesity was associated with fewer minutes per day spent in light (β= −0.70, p<0.001), moderate-to-vigorous (β= −0.54, p= 0.007), and total (β= −0.73, p<0.001) PA, controlling for age.
Associations between depression and daily PA in obese women participants with and without migraine
Correlational analyses indicated that depression was not related to light (r= 0.004, p= 0.99), moderate-to-vigorous (r= −0.31, p= 0.14), and total (r= −0.08, p= 0.72) daily PA in migraine participants. Similar relationships between depression and light (r= 0.09, p= 0.67), moderate-to-vigorous (r= 0.27, p= 0.19), and total (r= 0.15, p= 0.49) daily PA were shown in control participants.
Discussion
Recent years have witnessed a burgeoning interest in the relationship between obesity and migraine (6–9). However, little research has been conducted on putative mechanisms linking these two conditions, particularly behaviors such as low PA (8). The current study is the first to evaluate the relationship between objectively measured daily physical activity patterns and migraine in women within the context of obesity. Results showed obese women with migraine on average spent nearly 1.5 hours less per day in PA compared to obese women without migraine matched by age and BMI. These findings suggest that migraine is associated, above and beyond that of obesity, with lower PA levels.
We also found that greater degree of obesity was associated with lower PA in both the migraine and control groups. However, contrary to our hypothesis, in participants with migraine, only higher BMI, and not higher attack frequency, duration, or severity, was significantly related to lower PA. These findings taken together with those described above suggest that the presence of a migraine diagnosis may be associated with lower PA, but that the pattern of migraine symptoms (i.e. frequency, duration, and severity of attacks) may have only a weak association with overall accumulation of daily PA. However, the reason for this is unclear. One potential explanation is that patients with migraine in this study avoided PA because they believe that it can trigger or exacerbate a migraine attack (32). These beliefs could arise from personal experience or advice. While laboratory and intervention studies suggest that PA does not reliably provoke or exacerbate attacks in most people with migraine (19,33), these personal beliefs may persist and could still contribute to an overall reduction in PA. It is also possible that variables may be involved for which we did not account or are unaware. For example, given the debilitating nature of migraine and its impact on health-related quality of life (34), individuals with migraine may have lower perceived control over their health in general, which could contribute to less engagement in PA and possibly other health behaviors. It is also possible that obesity may further elevate already higher levels of inflammation in women with migraine (6–9), which in turn could contribute to lower engagement in PA levels due to factors such as greater fatigue and poorer physical function (35,36). Depression could confound the association between migraine and PA in obese women; however, we did not find this to be the case in the current study. Additional research is needed to identify PA barriers and appropriate interventions to counter such barriers in obese migraineurs. This is particularly the case in light of findings from both the current study and those from a recent randomized, controlled trial that showed PA to be as equally effective of a migraine treatment option compared to relaxation training and daily topiramate use (20).
This study possesses several important strengths and innovations that build on previous research that has evaluated the relationship between migraine and PA. It is the first study to: 1) utilize both an objective measure of PA and a smartphone-based diary to measure PA and headache activity prospectively in real time in the natural environment; 2) compare PA levels of women with and without migraine matched on demographic and anthropometric characteristics; and 3) examine associations between PA and migraine within the context of obesity. Additionally, the number of days that participants in both groups wore the objective PA monitor has been shown to accurately estimate typical PA behavior patterns (37). Finally, migraine and control participants were both recruited from the same geographical location via similar approaches to participate in studies at the same research center and used the same activity monitor to objectively assess PA.
This study also has certain limitations. This study included a sample of predominantly white women with obesity and migraine who were seeking behavioral weight loss treatment. Thus, the extent to which our findings can be generalized to people with migraine who are not white, obese, women, and seeking weight loss treatment is unclear. While we found that among individuals with migraine, the attack frequency, duration, and severity was not associated with total PA, we did not measure migraine attacks and PA at the same time. Thus, we cannot determine whether PA levels were affected during a migraine attack, which remains a very real possibility. Future research that measures migraine attacks and PA concurrently will be needed to determine the acute effect of migraine attacks on PA. Finally, while control participants did not have migraine headaches, it is possible that some had tension-type headaches. While theoretically this may have led to less robust findings, it is notable that the association between obesity and tension-type headache is lower than that of migraine, if present at all (3,38).
Conclusions
Considerable epidemiologic evidence supports a relationship between obesity and migraine; however, no research has been conducted on putative behavioral mechanisms that overlap between these conditions, such as low PA. In the current study, we found that obese women with migraine were significantly less active than obese women without migraine, performing nearly 1.5 hours less of PA per day according to objective measures. In the women with obesity and migraine, PA levels decreased with increasing weight, but not with increasing migraine attack frequency, duration, or maximum severity. Thus, migraine rather than specific migraine characteristics appears to be associated with lower PA in women with obesity. Studies to increase PA in women with obesity and migraine and that examine the effect of objectively measured increases in PA on migraine frequency and other characteristics are clearly needed.
Clinical implications.
Migraine is comorbid with obesity, but there has been little study of putative behavioral mechanisms, such as physical activity. Moreover, no previous study has directly evaluated whether individuals with migraine have lower physical activity levels than those without migraine, and within the context of obesity.
The current study shows that obese women with migraine are significantly less active than obese women without migraine matched by age and body mass index.
Additionally, higher degree of obesity, but not higher attack frequency, severity, and duration, is significantly associated with lower accumulation of daily physical activity in obese women with migraine.
In obese women, migraine diagnosis appears to be associated with lower physical activity levels, above and beyond obesity alone.
Additional research is needed to identify physical activity barriers and effective physical activity interventions in obese women with migraine.
Acknowledgments
Funding
This work was supported by grants from the National Institute of Neurological Disorders and Stroke (R01 NS077925, PI: DSB) and the National Institute of Diabetes and Digestive and Kidney Diseases (R01 DK095779, PI: JGT).
The authors wish to acknowledge Tiffany LeBlond, Krystal DeFaria, and Marie Kearns for their assistance with data collection.
Footnotes
This study originated from trials registered at clinicaltrials.gov as NCT01197196 and NCT01724632.
Conflict of interest
None declared.
References
- 1.Bigal ME, Liberman JN, Lipton RB. Obesity and migraine: A population study. Neurology. 2006;66:545–550. doi: 10.1212/01.wnl.0000197218.05284.82. [DOI] [PubMed] [Google Scholar]
- 2.Bigal ME, Lipton RB. Obesity is a risk factor for transformed migraine but not chronic tension-type headache. Neurology. 2006;67:252–257. doi: 10.1212/01.wnl.0000225052.35019.f9. [DOI] [PubMed] [Google Scholar]
- 3.Bigal ME, Tsang A, Loder E, et al. Body mass index and episodic headaches: A population-based study. Arch Intern Med. 2007;167:1964–1970. doi: 10.1001/archinte.167.18.1964. [DOI] [PubMed] [Google Scholar]
- 4.Fava A, Pirritano D, Consoli D, et al. Chronic migraine in women is associated with insulin resistance: A cross-sectional study. Eur J Neurol. 2014;21:267–272. doi: 10.1111/ene.12289. [DOI] [PubMed] [Google Scholar]
- 5.Peterlin BL, Rosso AL, Williams MA, et al. Episodic migraine and obesity and the influence of age, race, and sex. Neurology. 2013;81:1314–1321. doi: 10.1212/WNL.0b013e3182a824f7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bigal ME, Lipton RB, Holland PR, et al. Obesity, migraine, and chronic migraine: Possible mechanisms of interaction. Neurology. 2007;68:1851–1861. doi: 10.1212/01.wnl.0000262045.11646.b1. [DOI] [PubMed] [Google Scholar]
- 7.Peterlin BL, Rapoport AM, Kurth T. Migraine and obesity: Epidemiology, mechanisms, and implications. Headache. 2010;50:631–648. doi: 10.1111/j.1526-4610.2009.01554.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bond DS, Roth J, Nash JM, et al. Migraine and obesity: Epidemiology, possible mechanisms, and the potential role of weight loss treatment. Obes Rev. 2011;12:e362–e371. doi: 10.1111/j.1467-789X.2010.00791.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chai CN, Bond DS, Moghekar A, et al. Obesity and headache: Part II—potential mechanisms and treatment considerations. Headache. 2014;54:459–471. doi: 10.1111/head.12297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jensen MD, Ryan DH, Apovian CM, et al. 2013 AHA/ACC/TOS guideline for the management of overweight and obesity in adults: A report of the American College of Cardiology, American Heart Association Task Force on Practice Guidelines and The Obesity Society. Circulation. 2014;129(25 Suppl 2):S102–138. doi: 10.1161/01.cir.0000437739.71477.ee. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bond DS, Jakicic JM, Vithiananthan S, et al. Objective quantification of physical activity in bariatric surgery candidates and normal-weight controls. Surg Obes Relat Dis. 2010;6:72–78. doi: 10.1016/j.soard.2009.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tudor-Locke C, Brashear MM, Johnson WD, et al. Accelerometer profiles of physical activity and inactivity in normal-weight, overweight, and obese U.S. men and women. Int J Behav Nutr Phys Act. 2010;7:60. doi: 10.1186/1479-5868-7-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hansen BH, Holme I, Anderssen SA, et al. Patterns of objectively measured physical activity in normal weight, overweight and obese individuals (20–85 years): A cross-sectional study. PLoS One. 2013;8:e53044. doi: 10.1371/journal.pone.0053044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Queiroz LP, Peres MFP, Piovesan EJ, et al. A nationwide population-based study of migraine in Brazil. Cephalalgia. 2009;29:642–649. doi: 10.1111/j.1468-2982.2008.01782.x. [DOI] [PubMed] [Google Scholar]
- 15.Varkey E, Hagen K, Swart JA, et al. Physical activity and headache: Results from the Nord-Trøndelag Health Study (HUNT) Cephalalgia. 2008;28:1292–1297. doi: 10.1111/j.1468-2982.2008.01678.x. [DOI] [PubMed] [Google Scholar]
- 16.Wöber C, Brannath W, Schmidt K, et al. Prospective analysis of factors related to migraine attacks: The PAMINA study. Cephalalgia. 2007;27:304–314. doi: 10.1111/j.1468-2982.2007.01279.x. [DOI] [PubMed] [Google Scholar]
- 17.Robberstad L, Dyb G, Hagen K, et al. An unfavorable lifestyle and recurrent headaches among adolescents: The HUNT Study. Neurology. 2010;75:712–717. doi: 10.1212/WNL.0b013e3181eee244. [DOI] [PubMed] [Google Scholar]
- 18.Koseoglu E, Akboyraz A, Soyeur A, et al. Aerobic exercise and plasma beta endorphin levels in patients with migrainous headache without aura. Cephalalgia. 2003;23:972–976. doi: 10.1046/j.1468-2982.2003.00624.x. [DOI] [PubMed] [Google Scholar]
- 19.Varkey E, Cider A, Carlsson J, et al. A study to evaluate the feasibility of an aerobic exercise program in patients with migraine. Headache. 2009;49:563–570. doi: 10.1111/j.1526-4610.2008.01231.x. [DOI] [PubMed] [Google Scholar]
- 20.Varkey E, Cider A, Carlsson J, et al. Exercise as migraine prophylaxis: A randomized study using relaxation and topiramate as controls. Cephalalgia. 2011;31:1428–1438. doi: 10.1177/0333102411419681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Domingues RB, Teixeira AL, Domingues SA. Physical practice is associated with less functional disability in medical students with migraine. Arq Neuropsiquiatr. 2011;69:39–43. doi: 10.1590/s0004-282x2011000100009. [DOI] [PubMed] [Google Scholar]
- 22.Seok JI, Cho HI, Chung SS. From transformed migraine to episodic migraine: Reversion factors. Headache. 2006;46:1186–1190. doi: 10.1111/j.1526-4610.2006.00509.x. [DOI] [PubMed] [Google Scholar]
- 23.Thomas JG, Bond DS, Sarwer DB, et al. Technology for behavioral assessment and intervention in bariatric surgery. Surg Obes Relat Dis. 2011;7:548–557. doi: 10.1016/j.soard.2011.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Headache Classification Committee of the International Headache Society (IHS) The International Classification of Headache Disorders: 3rd edition (beta version) Cephalalgia. 2013;33:629–808. doi: 10.1177/0333102413485658. [DOI] [PubMed] [Google Scholar]
- 25.Bond DS, O’Leary KC, Thomas JG, et al. Can weight loss improve migraine headaches in obese women? Rationale and design of the Women’s Health and Migraine (WHAM) randomized controlled trial. Contemp Clin Trials. 2013;35:133–144. doi: 10.1016/j.cct.2013.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jakicic JM, Marcus M, Gallagher KI, et al. Evaluation of the SenseWear Pro Armband to assess energy expenditure during exercise. Med Sci Sports Exerc. 2004;36:897–904. doi: 10.1249/01.mss.0000126805.32659.43. [DOI] [PubMed] [Google Scholar]
- 27.Johannsen DL, Calabro MA, Stewart J, et al. Accuracy of armband monitors for measuring daily energy expenditure in healthy adults. Med Sci Sports Exerc. 2010;42:2134–2140. doi: 10.1249/MSS.0b013e3181e0b3ff. [DOI] [PubMed] [Google Scholar]
- 28.Unick JL, Bond DS, Jakicic JM, et al. Comparison of two objective monitors for assessing physical activity and sedentary behaviors in bariatric surgery patients. Obes Surg. 2012;22:347–352. doi: 10.1007/s11695-011-0491-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wetten AA, Batterham M, Tan SY, et al. Relative validity of 3 accelerometer models for estimating energy expenditure during light activity. J Phys Act Health. 2014;11:638–647. doi: 10.1123/jpah.2011-0167. [DOI] [PubMed] [Google Scholar]
- 30.Radloff LS. The CES-D Scale: A self-report depressive scale for research in the general population. J Appl Psychol Meas. 1977;1:385–401. [Google Scholar]
- 31.Andresen EM, Malmgren JA, Carter WB, et al. Screening for depression in well older adults: Evaluation of a short-form of the CES-D (Center for Epidemiologic Studies Depression Scale) Am J Prev Med. 1994;10:77–84. [PubMed] [Google Scholar]
- 32.Kelman L. The triggers or precipitants of the acute migraine attack. Cephalalgia. 2007;27:394–402. doi: 10.1111/j.1468-2982.2007.01303.x. [DOI] [PubMed] [Google Scholar]
- 33.Hougaard A, Amin F, Hauge AW, et al. Provocation of migraine with aura using natural trigger factors. Neurology. 2013;80:428–431. doi: 10.1212/WNL.0b013e31827f0f10. [DOI] [PubMed] [Google Scholar]
- 34.Lipton RB, Liberman JN, Kolodner KB, et al. Migraine headache disability and health-related quality of life: A population-based case-control study from England. Cephalalgia. 2003;23:441–450. doi: 10.1046/j.1468-2982.2003.00546.x. [DOI] [PubMed] [Google Scholar]
- 35.Raison CL, Lin JM, Reeves WC. Association of peripheral inflammatory markers with chronic fatigue in a population-based sample. Brain Behav Immun. 2009;23:327–337. doi: 10.1016/j.bbi.2008.11.005. [DOI] [PubMed] [Google Scholar]
- 36.Hart T, Swartz AM, Cashin SE, et al. How many days of monitoring predict physical activity and sedentary behaviour in older adults? Int J Behav Nutr Phys Act. 2011;8:62. doi: 10.1186/1479-5868-8-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Brinkley TE, Leng X, Miller ME, et al. Chronic inflammation is associated with low physical function in older adults across multiple comorbidities. J Gerontol A Biol Sci Med Sci. 2009;64:455–461. doi: 10.1093/gerona/gln038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chai NC, Scher AL, Moghekar A, et al. Obesity and headache: Part I—a systematic review of the epidemiology of obesity and headache. Headache. 2014;54:219–234. doi: 10.1111/head.12296. [DOI] [PMC free article] [PubMed] [Google Scholar]