Abstract
Elucidating the neurobiological mechanisms underlying individual differences in the extent to which reward cues acquire the ability to act as incentive stimuli may contribute to the development of successful treatments for addiction and related disorders. We used the sign-tracker/goal-tracker animal model to examine the role of dopamine D2 and D3 receptors in the propensity to attribute incentive salience to reward cues. Following Pavlovian training, wherein a discrete lever-cue was paired with food reward, rats were classified as sign- or goal-trackers based on the resultant conditioned response. We examined the effects of D2/D3 agonists, 7-OH-DPAT (0.01–0.32 mg/kg) or pramipexole (0.032–0.32 mg/kg), the D2/D3 antagonist raclopride (0.1 mg/kg), and the selective D3 antagonist, SB-277011A (6 or 24 mg/kg), on the expression of sign- and goal-tracking conditioned responses. The lever-cue acquired predictive value and elicited a conditioned response for sign- and goal-trackers, but only for sign-trackers did it also acquire incentive value. Following administration of either 7-OH-DPAT, pramipexole, or raclopride, the performance of the previously acquired conditioned response was attenuated for both sign- and goal-trackers. For sign-trackers, the D2/D3 agonist, 7-OH-DPAT, also attenuated the conditioned reinforcing properties of the lever-cue. The selective D3 antagonist did not affect either conditioned response. Alterations in D2/D3 receptor signaling, but not D3 signaling alone, transiently attenuate a previously acquired Pavlovian conditioned response, regardless of whether the response is a result of incentive motivational processes. These findings suggest activity at the dopamine D2 receptor is critical for a reward cue to maintain either its incentive or predictive qualities.
Keywords: Motivation, Sign-tracking, Goal-tracking, Dopamine, D2 receptor, D3 receptor
1. Introduction
There is general agreement in the literature that mesolimbic dopamine signaling is involved in reward-related processes, but its precise role remains controversial. Some have argued that dopamine encodes a reward-prediction error signal, necessary for learning cue-reward relationships [1–3]. In contrast, others have argued that mesolimbic dopamine facilitates the attribution of incentive salience to reward-paired cues [4–7]. Reward-paired stimuli in the environment that are attributed with incentive salience—incentive stimuli—become attractive and desired in their own right, and often gain inordinate control over behavior [7,8]. In fact, it has long been postulated that the undue attraction of drug cues in addicts arises from these incentive motivational processes [7,9]. Yet, it has been difficult to discern the neurobiological processes by which reward cues acquire incentive motivational properties, because it was previously assumed that if a stimulus acquired predictive value and was capable of evoking a conditioned response, then it also acquired incentive properties. However, using an animal model that captures individual variation in the extent to which incentive salience is attributed to reward-paired cues, we now know that these are two distinct processes that rely on different neural mechanisms [10–13].
Using a classical Pavlovian conditioning paradigm in which presentation of a lever-cue (conditioned stimulus, CS) predicts the delivery of a food reward in an adjacent food cup, we have shown that two distinct conditioned responses emerge [10]. Some animals, termed sign-trackers [14], approach and vigorously engage the lever-cue. Others, termed goal-trackers [15], first orient to the lever-cue and then enter the food cup upon cue presentation. There are three important points to note about this paradigm and the resultant behaviors: 1) no response is required for the delivery of the food reward, 2) all of the rats consume the reward, and 3) both groups of animals readily learn the association between the cue and reward. The lever-cue attains predictive qualities and elicits a conditioned response for both sign- and goal-trackers; but only for sign-trackers does the cue become an incentive stimulus, thus becoming a “motivational magnet” [16]. That is, for sign-trackers the cue is attractive, elicits approach [17] and supports the learning of an instrumental response as an effective conditioned reinforcer [10].
In recent years we, and others, have utilized the sign-tracker/goal-tracker animal model to further examine the neurobiology underlying the attribution of incentive salience to reward cues [11,12,18]. Thus far, a primary focus has been on the role of dopamine in these behaviors [13,19,20]. It has previously been shown that systemic antagonism of D1- and D2-type receptors with flupenthixol prevents the development of a sign-tracking, but not goal-tracking, conditioned response [13]. Further, using fast-scan cyclic voltammetry, it was shown that dopamine transmission in the nucleus accumbens core tracked the attribution of incentive, but not predictive value, to a reward-paired stimulus [13]. Additionally, local blockade of dopamine in the nucleus accumbens core with flupenthixol diminished the expression of a sign-tracking, but not goal-tracking, response [20]. Based on these findings, it appears that sign-tracking is dependent on the actions of dopamine for both its acquisition and expression; whereas the development of a goal-tracking response is dopamine independent.
While this previous work has demonstrated a critical role for dopamine in the attribution of incentive salience to reward cues, the mechanisms of these effects are not yet known. The pharmacological agents that were previously used to target the dopamine system in this animal model were non-specific, antagonizing both dopamine D1- and D2-type receptors. Recent work, however, has highlighted a role specifically for the D2-family of receptors, particularly D3 receptors, as a clinical target for the treatment of addiction-related behaviors [21,22]. As the sign-tracker/goal-tracker animal model captures the ability of cues, paired either with food or drugs of abuse, to motivate and control reward-seeking behaviors [23], we utilized this model to examine the role of D2 and D3 receptors in mediating these individual differences. Specifically, we conducted 4 experiments to assess the impact of the following pharmacological agents on the expression of sign- and goal-tracking behaviors: 1) the D2/D3 agonist, 7-OH-DPAT, 2) the D2/D3 agonist pramipexole, 3) the D2/D3 antagonist, raclopride, and 4) the selective D3 antagonist SB-277011A [24].
2. Methods
2.1 Ethics Statement
All experiments followed the principles of animal care published in the Guide for the Care and Use of Laboratory Animals: Eighth Edition, revised in 2011, published by the National Academy of Sciences. In addition, all procedures were approved by the University of Michigan’s University Committee on the Use and Care of Animals.
2.2 Subjects
Adult male Sprague-Dawley rats (n=204 in total) were housed in a temperature- and humidity-controlled room and maintained on a 12-h light/dark cycle (lights on at 07:00 hrs). Rats were obtained from multiple vendors, as it has been previously observed that rats from different vendors differ in their propensity to sign- versus goal-track [25], and we aimed to get equal numbers of each phenotype. In the current experiments, we were interested only in the extremes of the population, as this would allow us to assess the effects of dopaminergic agents on the expression of behaviors resulting from distinct forms of stimulus-reward learning [10]. Thus, rats that were classified as intermediate responders (n=92 in total) were eliminated from the study following Pavlovian conditioning and, out of the 204 rats that were initially screened, 112 were used for the following experiments. After arrival at our facilities, rats were given one week to acclimate before handling and experimental procedures began. Water and food were available ad libitum throughout the experiments.
2.3 Behavioral Apparatus
Behavioral testing occurred in standard Med Associates conditioning chambers (20.5 × 24.1 cm floor area, 29.2 cm high; St. Albans, VT) that were located in sound attenuating cabinets equipped with ventilating fans that provided background noise. For Pavlovian conditioning sessions each chamber had a food cup located in the center of one wall, approximately 3 cm above the grid floor, and was flanked 2.5 cm to the right or left by a retractable lever, located 6 cm above the grid floor. The location of the lever was counterbalanced. On the opposite wall, near the top of the chamber, was a white house light that was illuminated for the duration of each session. Levers required a 10-g force to deflect and each deflection was recorded as a “contact”. Operation of the pellet dispenser resulted in the delivery of one 45-mg banana-flavored pellet (F0059; Bio-Serv, Frenchtown, NJ) into the food cup.
2.4 Pavlovian Conditioned Approach
Pavlovian training procedures were similar to those described previously [11]. All sessions were conducted between the hours of 10:00–17:00. For the 2 days prior to training, rats were handled by experimenters and given a small amount of 45-mg banana-flavored pellets in their home cage, to acquaint the animals with the food to be used as the unconditioned stimulus (US). Animals then received two sessions of food cup pre-training in the conditioning chambers. Prior to each pre-training session, the food cups were primed with 3 pellets. The session then consisted of the delivery of 25 banana-flavored pellets on a variable interval (VI) 30 s schedule, averaging 12.5 minutes per session. All animals retrieved all of the pellets during food cup pre-training and Pavlovian conditioning procedures began the following day.
During Pavlovian conditioning sessions a trial consisted of insertion of the illuminated lever (conditioned stimulus, CS) into the chamber for 8 s, at which time it was retracted and immediately followed by delivery of a 45-mg banana-flavored pellet (unconditioned stimulus, US) into the adjacent food cup. Each Pavlovian conditioned approach (PCA) session consisted of twenty-five trials with a variable inter-trial interval (ITI) of 90 s (the period between CS presentations ranged from 30 s to 150 s), so each session lasted approximately 40 minutes. The following were recorded per trial using Med Associates software: 1) number of lever contacts, 2) latency to first lever contact, 3) number of food cup entries during CS presentation, 4) latency to first food cup entry during CS presentation, and 5) number of food cup entries during the ITI. In addition, the number of food pellets consumed was recorded after each session to ensure all animals were consuming their pellets. Using these metrics, sign- and goal-tracking behavior is quantified to examine an individual’s preference for the lever-cue or the food cup using a Pavlovian Conditioned Approach (PCA) Index score [26]. The PCA Index score accounts for three measures of approach behavior: 1) Response Bias: the ratio of lever presses and food cup entries in relation to the total number of responses, [(total lever-directed contacts – total food cup-directed contacts)/(sum of total contacts)], 2) Probability Difference: the difference between the probability of contacting the lever and the food cup, [probability of lever contact – probability of food cup contact], and 3) Latency Score: the difference between the latencies to contact the lever and the food cup, [(food cup entry latency – lever contact latency)/8]. These three values are then averaged together to give PCA Index scores range from -1.0 to 1.0. Those rats with an average PCA Index ranging from -1.0 to -0.3 were classified as goal-trackers (GTs) and those with a PCA Index ranging from 0.3 to 1.0 were classified as sign-trackers (STs). For all experiments, rats with a PCA Index between -0.29 and 0.29 were classified as intermediate responders and were not used further because we were interested in comparing rats that strongly differed in their attribution of incentive salience to reward-paired cues. Classifying rats in this manner splits the total population of rats roughly into thirds [12,27].
2.5 Drugs
(±)-7-hydroxy-2-(di-n-propylamino)tetralin hydrobromide (7-OH-DPAT) was received from the National Institutes of Mental Health Chemical Synthesis and Drug Supply Program. Pramipexole dihydrochloride and S-(−)-Raclopride were purchased from Sigma-Aldrich (A1237; R121; St. Louis, MO). 7-OH-DPAT, pramipexole, and raclopride were dissolved in 0.9% sterile physiological saline and administered subcutaneously. SB-277011A was obtained from MEGAPharma Kft (Budapest, Hungary) and dissolved in 25% w/v hydroxypropyl-β-cyclodextrin in sterile water and administered intraperitoneally. All solutions were made fresh daily and administered in a volume of 1 ml/kg.
2.6 Experiment 1: The Effect of D2/D3 Agonism by 7-OH-DPAT on Pavlovian Conditioned Approach Behaviors and Conditioned Reinforcement
2.6.1 Pavlovian Conditioned Approach Behavior
Male Sprague-Dawley rats (initial n=30; Harlan Laboratories, Indianapolis, IN) weighing 250–300 g were triple-housed upon arrival. Rats were classified as sign-trackers (STs, n=10) or goal-trackers (GTs, n=8) based on their average PCA Index score [26] from Sessions 6 and 7 of a 7-session Pavlovian training paradigm. Those rats with an average PCA Index ranging from -1.0 to -0.3 were classified as GTs (n=8) and those with a PCA Index ranging from 0.3 to 1.0 were classified as STs (n=10). On the eighth session all rats received injections of vehicle 15 minutes prior to session start. A within subjects design was used to examine the effects of 7-OH-DPAT on the expression of sign- and goal-tracking behaviors (see Fig. 1a for Experimental Design). Fifteen minutes prior to each session, rats received a single dose of 7-OH-DPAT, with doses escalating across sessions in the following order: 0.01, 0.032, 0.1, 0.2, and 0.32 mg/kg. These doses were selected to examine the effects of increased D2 relative to D3 stimulation; and also to avoid nonspecific effects on locomotor activity [28,29]. On the days following each drug injection rats received vehicle injections to prevent carry-over drug effects. Thus, 7-OH-DPAT was administered prior to Sessions 9,11,13,15, and 17 and vehicle was administered prior to Sessions 8,10,12,14 and 16 (see Fig. 1a) and for an additional 2 days (i.e. Sessions 18 and 19) following the last dose tested.
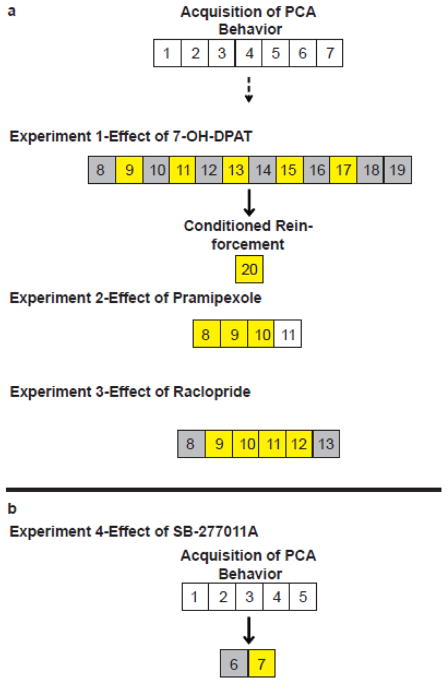
Fig. 1.
Schematic illustration of Experimental Design. Independent groups of rats were used for each experiment. Each numbered box indicates a session of Pavlovian conditioning. Yellow boxes illustrate sessions that experimental compounds were administered prior to session start. Grey boxes illustrate sessions where all subjects were administered vehicle. a) Following 7 sessions of Pavlovian conditioning rats were classified as sign- or goal-trackers. For Experiment 1 all animals underwent treatment according to a within subjects design. Following session 19 rats were split into balanced groups based on their performance in the initial 7 sessions of PCA training and proceeded to undergo a test of conditioned reinforcement. For Experiments 2 and 3 rats were split into balanced treatment and vehicle groups based on their average PCA Index score from sessions 6 and 7 prior to testing with pramipexole and raclopride. b) Following 5 sessions of Pavlovian conditioning rats were classified as sign- or goal-trackers and were split into balanced treatment groups based on their average PCA Index score from sessions 4 and 5. All rats then received vehicle prior to session 6, and then their respective treatment prior to session 7
2.6.2 Conditioned Reinforcement
The day after the last Pavlovian training session (i.e. Session 19), all subjects were split into vehicle (GT n=4, ST n=5) or treatment (GT n=4, ST n=5) groups to examine the effects of 7-OH-DPAT on the conditioned reinforcing properties of the lever-cue. Groups were counterbalanced based on their original index score from Sessions 6 and 7. Thus, both groups consisted of animals that had previously received drug during the PCA portion of the experiment. Rats in the treatment group received 0.032 mg/kg 7-OH-DPAT prior to the conditioned reinforcement test, while rats in the vehicle group received saline. This dose was chosen based on the effects observed during the PCA phase of the experiment.
The conditioning chambers were rearranged such that the food cup and pellet dispenser were removed and the retractable lever was placed in the center of the chamber. Two nose ports were located approximately 2.5 cm on either side of the lever and were located with the bottom of the port approximately 4 cm above the grid floor. The nose port placed opposite of the lever’s previous position was designated as the active nose port. Pokes in the active port resulted in presentation of the illuminated lever for 2 s on a fixed ratio (FR) 1 schedule, and pokes in the inactive port were without consequence. The session lasted for 40 minutes and the following were recorded using Med Associates software: 1) pokes in the active nose port, 2) pokes in the inactive nose port, and 3) contacts with the lever.
2.7 Experiment 2: The Effect of D2/D3 Agonism by Pramipexole on the Expression of Sign- and Goal-Tracking Behaviors
As the 7-OH-DPAT experiment was performed with ascending doses, we wished to compare the effects of a similar agent on behavior, yet in a different design to confirm the effects of D2/D3 agonism on these behaviors. Male Sprague-Dawley rats (initial n=34, Charles River Laboratories, Portage, MI; initial n=36, Taconic Biosciences, Germantown, NY) weighing 250–300 g were pair-housed upon arrival. Following 7 sessions of Pavlovian conditioning, animals were characterized as sign- or goal-trackers based on the average PCA Index score from Sessions 6 and 7 as described above. A between subjects design was then implemented such that rats received injections of vehicle (ST n=5, GT n=11) or descending doses of pramipexole (0.32, 0.1, and 0.032 mg/kg; ST n=7, GT n=10) on Sessions 8, 9 and 10 (see Fig. 1a for Experimental Timeline). These doses were selected based on previous studies showing that there are no effects on locomotor activity within this range [30]. All injections were given 10 min prior to the start of each session. An additional “drug free” session was conducted following the last treatment session to assess possible carry-over effects of treatment. Alterations in responding were compared between those receiving pramipexole and those receiving vehicle across the 3 experimental sessions.
2.8 Experiment 3: The Effect of D2/D3 Antagonism by Raclopride on the Expression of Sign- and Goal-Tracking Behaviors
Male Sprague-Dawley rats (initial n=44; Charles River Laboratories, Raleigh, NC) weighing 200–250 g were group-housed upon arrival. After 7 sessions of Pavlovian conditioning rats were characterized as sign- or goal-trackers based on the average PCA Index score from Sessions 6 and 7 as described above. Prior to Session 8 all rats received a vehicle injection. A between subjects design was then implemented such that rats received injections of vehicle (ST n=6, GT n=6) or 0.1 mg/kg raclopride (ST n=9, GT n=7) on Sessions 9–12 (see Fig. 1a for Experimental Design). This dose was selected as it does not produce locomotor impairment and it is believed to have equal affinity for both D2 and D3 receptors [31,32]. All injections were given 30 minutes prior to session start. Animals then received one additional injection of vehicle prior to the next session (i.e. Session 13) to assess possible carry-over effects of treatment. This experimental design allowed for comparing the effects of treatment within subjects to their own vehicle performance on Session 8, in addition to comparing between treatment groups on Sessions 9 through 12.
2.9 Experiment 4: The Effect of D3 Antagonism by SB-277011A on the Expression of Sign- and Goal-Tracking Behaviors
Male Sprague-Dawley rats (initial n=60; Charles River Laboratories, Raleigh, NC) weighing 150–200 g were pair-housed upon arrival. Following 5 sessions of Pavlovian conditioning rats were characterized as sign- or goal-trackers based on the average PCA Index score from Sessions 4 and 5, as we and others have adapted a quicker approach for classification of these phenotypes [27,33]. Thirty minutes prior to Session 6 all rats received a vehicle injection and then, 30 minutes prior to Session 7, rats received either vehicle (ST n=5, GT n=5), 6 mg/kg (ST n=6, GT n=7), or 24 mg/kg SB-277011A (ST n=5, GT n=5). These doses were selected based on previous preclinical studies and do not produce non-specific locomotor effects [34]. This design allowed for comparisons between treatment groups on Session 7, in addition to allowing comparisons of performance within subjects in each treatment group between Sessions 6 and 7 (see Fig. 1b for Experimental Design).
2.10 Statistical Analyses
All analyses were performed with SPSS 22.0 (IBM, Armonk, NY) and figures constructed in GraphPad Prism 6 (La Jolla, CA). Linear mixed-effects models (LMM) were used to assess the effects of treatment on the expression of Pavlovian conditioned approach behavior data from each of the 25 trials were compiled per session and Session was treated as a repeated independent variable. Phenotype and/or treatment group were also independent variables. The best-fit model of covariance was selected by the lowest Akaike information criteria score. Depending on the model selected, degrees of freedom were adjusted to a non-integer value. Following detection of significant interactions, Bonferroni comparisons were conducted.
Performance on the conditioned reinforcement test in Experiment 1 was assessed using a three-way analysis of variance (ANOVA) in which phenotype (ST vs. GT), treatment (vehicle vs. drug), and nose port (active vs. inactive) were the independent variables and the number of pokes was the dependent variable. A two-way ANOVA with phenotype and treatment as independent variables and lever contacts as the dependent variable was also used to examine group differences on the conditioned reinforcement test. Planned comparisons were conducted to assess the effect of treatment or nose port within each phenotype. Paired t-tests were used to assess whether treatment with raclopride reduced responding relative to initial training stages and whether treatment had lasting effects on performance. For all analyses significance levels were set at p<0.05.
3. Results
3.1 Experiment 1: Dopamine D2/D3 Agonism by 7-OH-DPAT Attenuates Both Sign- and Goal-Tracking Behaviors
3.1.1 Acquisition of Pavlovian Conditioned Approach Behavior
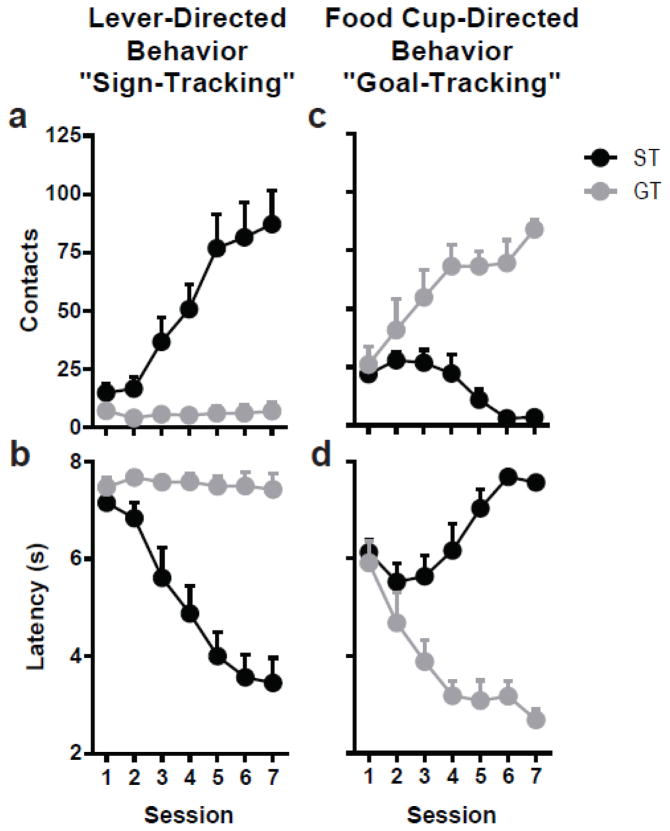
Figure 2 illustrates the differential acquisition of PCA behavior over the initial seven sessions of training for Experiment 1. As described above, rats were classified as STs or GTs using their PCA Index scores, with STs defined as those ranging from 1.0 to 0.3, and GTs as those with scores ranging from -0.3 to -1.0. By the end of the training period, STs were reliably approaching and manipulating the lever-cue upon its presentation (Fig. 2a), and did so with increasing rapidity (Fig. 2b) over the course of training. In contrast, GTs were reliably approaching and entering the food cup upon lever-cue presentation (Fig. 2c), and did so with increasing rapidity (Fig. 2d). Therefore, as seen in previous studies [17,26] the lever-cue evoked a conditioned response in both sign- and goal-trackers, serving as an equally effective predictor of reward delivery for both groups; yet only for STs did the lever-cue acquire incentive qualities to the extent that it was attractive and elicited approach.
Fig. 2.
Individual differences in Pavlovian conditioned approach behavior. Lever-directed behavior (sign-tracking; a–b) and food cup-directed behavior (goal-tracking; c–d) across 7 sessions of training prior to exposure to 7-OH-DPAT for rats classified as STs (n=10) or GTs (n=8). Error bars indicate SEM
3.1.2 Effects of 7-OH-DPAT on Sign-Tracking
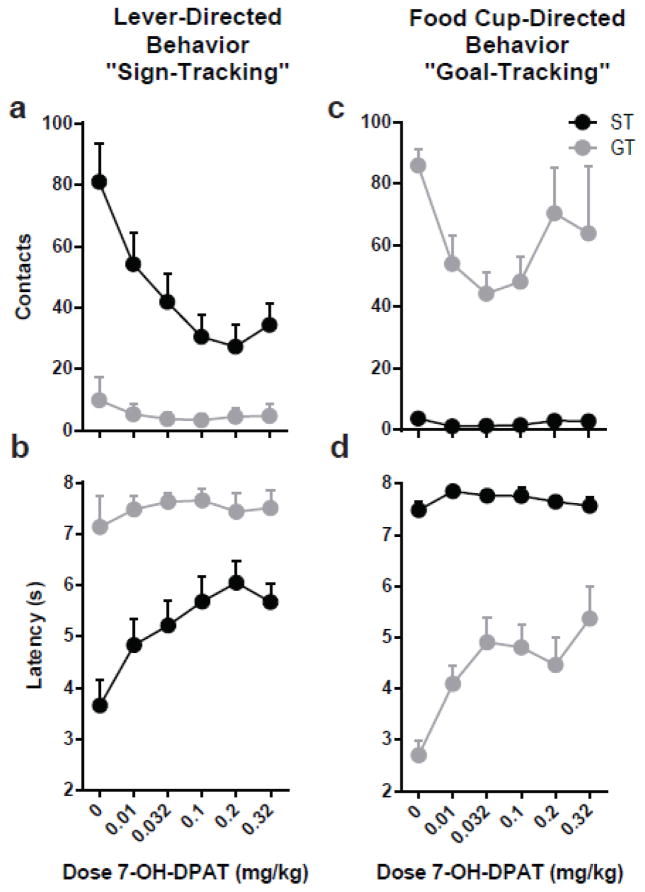
Following the initial 7 training sessions, the effects of 7-OH-DPAT on sign- and goal-tracking conditioned responses were assessed. Vehicle was administered on the days in between drug injections (see Fig. 1a for Experimental Design), and because there were no significant differences in behavior following the repeated vehicle injections (see Supplemental Results, Supplemental Fig. 1), the data from these sessions (i.e. 8,10,12,14,16,18,19) were averaged and collapsed into a single data point per phenotype and dose-response curves were analyzed with respect to this data point (i.e. 0 mg/kg; Fig. 3).
Fig. 3.
Effects of 7-OH-DPAT on Pavlovian conditioned responding for STs (n=10) or GTs (n=8). Treatment with 7-OH-DPAT selectively attenuated the performance of the previously acquired conditioned response, both in sign-trackers (a–b) and goal-trackers (c–d). Error bars indicate SEM
Treatment with 7-OH-DPAT attenuated lever-directed behavior for rats classified as STs. There was a significant interaction of Dose X Phenotype for the number of contacts with the lever (F5,16=5.21, p=0.005) and latency to approach the lever (F5,75=2.67, p=0.028) upon its presentation. Further analysis revealed that there was a significant Effect of Dose only for STs, for both lever contacts (Effect of Dose, F5,16=15.82, p<0.001) and latency to approach the lever (Effect of Dose, F5,75=8.26, p<0.001). Relative to vehicle performance, each dose of 7-OH-DPAT significantly reduced the number of contacts (p<0.001; Fig. 3a) and increased the latency to approach the lever (p<0.001; Fig. 3b) for STs. Given that the vehicle performance is illustrative of each “drug-off” session interspersed between the “drug-on” sessions, these data illustrate that the performance of the rats returned to baseline levels when the drug was not on board. It should also be noted that increasing doses of 7-OH-DPAT did not produce a greater deficit for STs, as there were no significant differences between the doses administered. Taken together, these findings demonstrate that 7-OH-DPAT attenuates lever-directed responses in STs.
3.1.3 Effects of 7-OH-DPAT on Goal-Tracking
Interestingly, treatment with 7-OH-DPAT also attenuated food cup-directed behaviors for rats classified as GTs. There was a significant Dose X Phenotype interaction for contacts with the food cup (F5,23=11.71, p<0.001) and latency to approach the food cup (F5,80=7.60, p<0.001) during lever-cue presentation. Furthermore, examining each phenotype separately revealed a significant Effect of Dose for both of these measures in GTs (food cup contacts, F5,36=23.34, p<0.001; food cup latency, F5,80=13.93, p<0.001), but not STs. The time it took for GTs to approach the food cup was increased relative to vehicle in response to all doses (p<0.01; Fig. 3d); whereas the number of contacts made during the CS period was decreased relative to vehicle in response to 0.01, 0.032, and 0.1 mg/kg (p<0.01; Fig. 3c). The lack of effect in response to 0.2 and 0.32 may be due to a non-specific increase in activity, as responding in the food cup during the intertrial interval also increased (for more information, see Supplemental Results, Supplemental Fig. 2a). Relative to STs, GTs showed a greater number of head entries into the food cup during the intertrial interval (Effect of Phenotype F1,16=5.10, p=0.038), and, although there is not a significant Phenotype X Dose interaction, this effect appears to be more pronounced at the two highest doses (Effect of Dose, F5,18=6.45, p=0.001; Supplemental Fig. 2a). Taken together, these data demonstrate a transient attenuation of goal-tracking behavior following treatment with moderate doses of 7-OH-DPAT for GTs, but not STs.
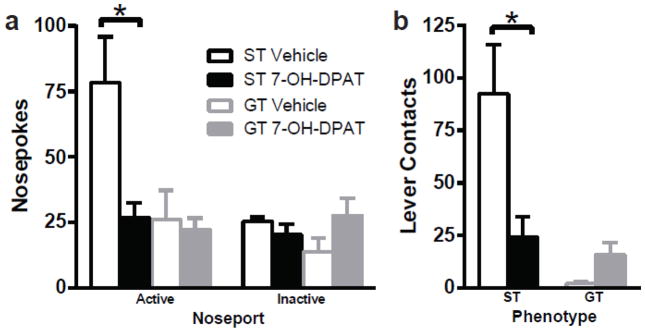
3.1.4 7-OH-DPAT Alters Motivation for Cue Presentation in Sign-Trackers
The effects of treatment with 7-OH-DPAT (0.032 mg/kg) on the motivation to work for presentation of the lever-cue, or the conditioned reinforcing properties of the cue are illustrated in Fig. 4a. Although there was not a significant Nose Port X Phenotype X Treatment interaction, there was a significant Phenotype X Treatment interaction (F=6.96, p=0.013) and a significant Nose Port X Treatment interaction (F=6.44, p=0.017) that justified further comparisons.
Fig. 4.
Effects of 7-OH-DPAT on Conditioned Reinforcement. Treatment with 7-OH-DPAT prevented the acquisition of a new instrumental learning process selectively in sign-trackers. Sign-trackers receiving 7-OH-DPAT (n=5) were significantly attenuated compared to vehicle controls (n=5) on active nose pokes for lever presentation (a) and vigor of responding following lever presentation (b) while goal-trackers were not affected by treatment on either measure. *p<0.05 relative to vehicle. Error bars indicate SEM
As expected based on previous studies [10], STs in the vehicle group showed a greater preference for the active nose port over the inactive port (Effect of Nose Port, F=19.80, p<0.001), but GTs did not discriminate between the two ports (p=0.364). Similar to GTs, STs who received 7-OH-DPAT did not show a preference for the active over the inactive nose port (p=0.594), and poked significantly less in the active port compared to STs in the vehicle group (p<0.001). STs that received 7-OH-DPAT were indistinguishable from GTs in either treatment group when comparing responses in both the active and inactive ports. There was no effect of treatment with 7-OH-DPAT on inactive nose pokes for either sign-trackers or goal-trackers.
The vigor of activity directed towards the lever-cue once it was presented during the conditioned reinforcement task was also analyzed. For lever contacts, there was a significant Effect of Phenotype (F=10.59, p=0.006) and Treatment (F=7.33, p=0.018), and a trend towards significance for a Phenotype X Treatment interaction (F=3.39, p=0.089). Although the interaction was not significant, the effect of treatment was examined within each phenotype, as our a priori hypothesis was that the drug would affect the behavior of STs and not GTs on this test. In agreement, Bonferroni comparisons revealed that sign-trackers made more contacts with the lever during its brief presentation compared to goal-trackers (p=0.006), and administration of 7-OH-DPAT significantly attenuated the number of lever contacts (p=0.006), selectively in sign-trackers (Fig. 4b). Taken together, these data suggest that treatment with 7-OH-DPAT impeded the ability of the lever-cue to serve as a conditioned reinforcer for STs, those that attribute incentive salience to the lever-cue.
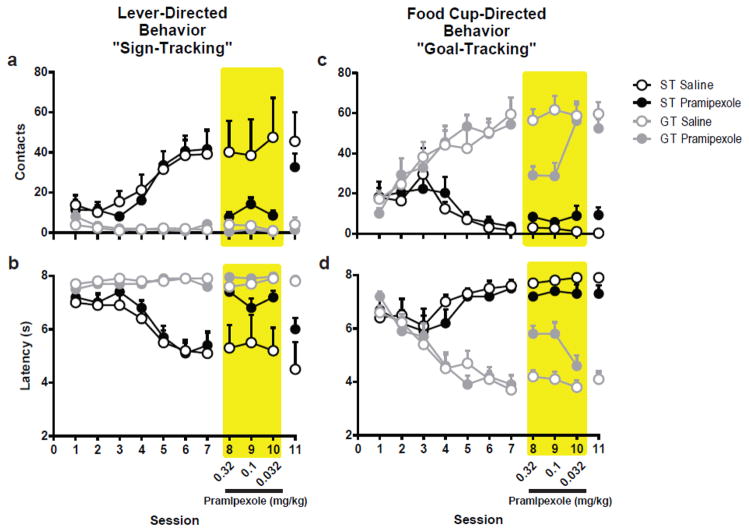
3.2 Experiment 2: Dopamine D2/D3 Agonism by Pramipexole Attenuates both Sign- and Goal-Tracking Behaviors
3.2.1 Pavlovian Conditioned Approach Behavior
Similar to Experiment 1, rats acquired sign- and goal-tracking conditioned responses, which appeared to be stable at the end of seven Pavlovian training sessions. Rats were classified based on the average PCA Index from Sessions 6 and 7. The impact of pramipexole on their propensity to interact with the lever-cue or food cup was examined on Sessions 8, 9 and 10, with decreasing doses across sessions (i.e. 0.32, 0.1 and 0.032 mg/kg, respectively). Behavior in the vehicle-treated groups was compared to the pramipexole-treated groups across these three sessions.
3.2.2 Effects of Pramipexole on Sign-Tracking
Similar to 7-OH-DPAT, pramipexole significantly attenuated lever-directed behaviors in sign-trackers. There was a significant Dose X Phenotype X Treatment interaction (F2,29=3.93, p=0.031; Fig. 5a) for the number of lever-cue contacts. Bonferroni comparisons confirmed that pramipexole reduced the amount of lever-cue contacts for STs (within group Effect of Treatment F1,29=12.77, p=0.001) and there was a significant difference between treatment groups at each of the three doses (p<0.02). In agreement, there was a significant Treatment X Phenotype interaction (F1,29=6.72, p=0.015) for the latency to lever contact, indicating that pramipexole administration increased the time it took for STs to contact the lever following its presentation (Effect of Treatment F1,29=13.51, p=0.001; Fig. 5b), regardless of dose (p=0.001). There were no significant effects of Treatment or Dose for measures of lever-directed behavior for GTs. Thus, stimulation of D2/D3 receptors by pramipexole selectively attenuated lever-directed behaviors in rats that exhibit a sign-tracking response.
Fig. 5.
Effects of Pramipexole on Pavlovian conditioned approach behavior. Following seven sessions of Pavlovian conditioning animals were classified as STs (n=12) or GTs (n=21) based on their propensity to approach the lever or food cup during the CS period. STs and GTs were then split into balanced treatment groups based on PCA Index score, with one group receiving vehicle (ST n=5; GT n=11) and the others receiving descending doses (0.32, 0.1, 0.032 mg/kg) of pramipexole (ST n=7; GT n=10) over the next three sessions (shaded region). On session 11 all rats were tested without vehicle or drug treatment. Pramipexole attenuated lever-directed behaviors in animals previously classified as STs (a–b) and food cup-directed behavior only in animals previously classified as GTs (c–d). Error bars indicate SEM
3.2.3 Effects of Pramipexole on Goal-Tracking
Similar to the effects of 7-OH-DPAT, only for GTs was there a significant attenuation of food cup contacts in response to treatment with pramipexole (Treatment X Phenotype interaction F1,30=6.26, p=0.018; within phenotype Effect of Treatment F1,30=11.02, p=0.002; Fig. 5c). In agreement, there was a significant Treatment X Phenotype interaction (F1,29 =11.60, p=0.002) for the latency to contact the food cup during the CS-period. Pramipexole increased the time it took to contact the food cup following CS presentation only for GTs (Effect of Treatment F1,29 =18.55, p<0.001; Fig. 5d). Although the data illustrated in Fig. 5c and d suggest an effect of dose on goal-tracking behaviors in response to pramipexole, the statistics reveal only a significant Treatment X Dose interaction (F2,48=3.97, p=0.025) for the number of contacts with the food cup and a Phenotype X Dose interaction (F2,58=4.01, p=0.023) for the latency measure. Thus, although there appears to be a differential effect in response to 0.032 mg/kg within GTs, the absence of a statistically significant Treatment X Dose X Phenotype interaction for either measure did not justify these comparisons.
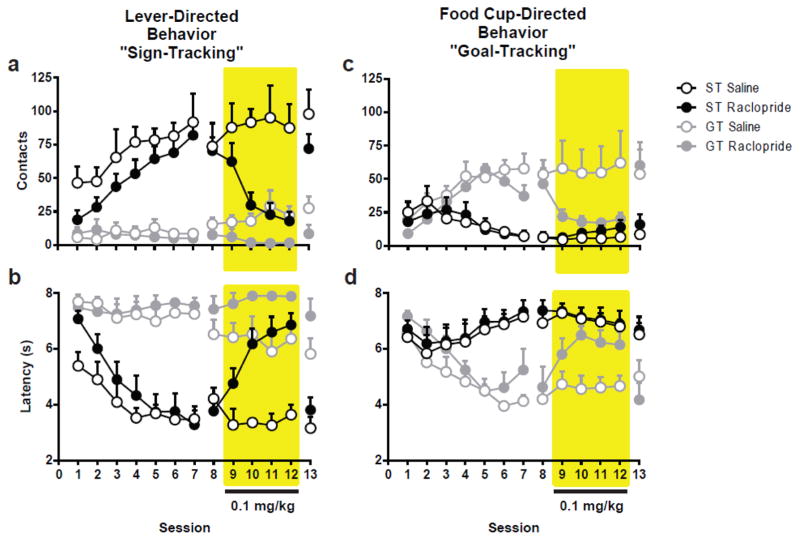
3.3 Experiment 3: Dopamine D2/D3 Antagonism by Raclopride Attenuates both Sign- and Goal-Tracking Behaviors
3.3.1 Pavlovian Conditioned Approach Behavior
Similar to Experiment 1, following seven Pavlovian conditioning sessions, animals were characterized as sign- or goal-trackers based on the conditioned response that emerged. Following characterization, all animals received a vehicle injection prior to Session 8, and treatment with either raclopride or vehicle occurred over Sessions 9–12. Behavior was compared between phenotypes and treatments groups across sessions 8–12, as indicated below.
3.3.2 Effects of Raclopride on Sign-Tracking
Examining the effects of raclopride treatment on lever contacts revealed a significant Session X Phenotype X Treatment interaction (F4,32=3.14, p=0.033; Fig. 6a). Bonferroni comparisons confirmed that raclopride attenuated lever-directed behavior only in STs as indicated by a within-phenotype Effect of Treatment (F1,24=12.17, p=0.002) and Session (F4,24=3.93, p=0.014). STs receiving raclopride showed significantly attenuated lever contacts compared to STs receiving vehicle on Sessions 10 through 12 (p<0.001). In addition, raclopride significantly attenuated lever contacts on Sessions 10–12 (p<0.02) in comparison to vehicle performance on Session 8 (Effect of Session F4,24=16.20, p<0.001) for STs receiving drug treatment. Raclopride treatment also altered the speed with which rats approached the lever-cue, as indicated by a significant Session X Phenotype X Treatment interaction (F4,60=3.45, p=0.013; Fig. 6b) for the latency measure. There was a significant Effect of Treatment (F1,25=14.19, p=0.001) in STs. On Sessions 9–12 STs treated with raclopride took longer to approach the lever-cue compared to vehicle-treated STs (all p<0.03). Additionally, there was an Effect of Session for raclopride-treated STs (F4,60=17.52, p<0.001), showing that repeated doses of raclopride caused an increasing deficit in latency to approach the lever-cue. Relative to their own vehicle performance, STs treated with raclopride were significantly slower to approach the lever-cue across Sessions 9–12 (all p<0.02). Bonferroni comparisons also indicated that the extent of attenuation on Sessions 10–12 was greater than on Session 9 (all p<0.001). Interestingly, while GTs showed no Effect of Treatment on the lever contacts, there was an Effect of Treatment for latency to approach the lever-cue (F1,25=5.73, p=0.025) in GTs. Comparing these effects between groups revealed vehicle-treated GTs were quicker to approach the lever-cue on Sessions 11 (p=0.006) and 12 (p=0.033) compared to raclopride-treated GTs. There was not an Effect of Session for GTs.
Fig. 6.
Effects of Raclopride on Pavlovian conditioned approach behavior. Following seven sessions of Pavlovian conditioning animals were classified as STs (n=15) or GTs (n=13) based on their propensity to approach the lever or food cup during the CS period. On session 8 all animals received vehicle injections, then were split into treatment groups with one group receiving vehicle injections (ST n=6; GT n=6) and the others receiving 0.1 mg/kg raclopride (ST n=9; GT n=7) over the next four sessions (shaded region). On session 13 all animals received vehicle treatment to reassess behavior following raclopride administration. Raclopride attenuated lever-directed behaviors in animals previously classified as STs (a–b) and food cup-directed behavior only in animals previously classified as GTs (c–d). Error bars indicate SEM
To determine the extent to which raclopride attenuated performance, the final session of treatment, Session 12, was compared to the very first session of Pavlovian conditioning. Treatment with raclopride over four sessions essentially eliminated any evidence of having learned the CS-US association, with levels of performance on Session 12 equivalent to those on Session 1 for treated STs (lever contacts, t8=-0.094, p=0.927; lever latency, t8=0.45, p=0.665). Importantly, treatment did not have lasting effects on the performance, as sign-tracking behavior off drug on Session 13 did not differ from that on Session 8 (lever contacts, t8=-0.165, p=0.873; lever latency, t8=-0.078, p=0.939). These results suggest that D2/D3 antagonism transiently resulted in a decrease in lever-directed behaviors, specifically in STs.
3.3.3 Effects of Raclopride on Goal-Tracking
Raclopride attenuated responding on measures of goal-tracking behavior, but did so only in rats previously classified as GTs. There was a significant Treatment x Phenotype interaction for the number of food cup contacts during the CS-period (F1,24=4.41, p=0.046; Fig. 6c). Raclopride significantly attenuated responding on this measure only for GTs, as indicated by a within phenotype Effect of Treatment (F1,24=6.73, p=0.016). Raclopride reduced GTs to levels of responding equivalent to STs, effectively eliminating phenotypic differences (Effect of Phenotype within vehicle groups, F1,24=15.89, p=0.001; Effect of Phenotype within raclopride groups, F1,24=1.89, p=0.182). Although a similar pattern was seen with the latency to approach the food cup, there was only a trend towards significance for an overall Effect of Treatment (F1,24=3.67, p=0.068) and the Treatment x Phenotype interaction was not significant
Although raclopride was able to reduce food cup-directed behaviors for GTs, their performance on the last day of treatment (i.e. Session 12) was still greater than their performance on Session 1 (food cup contacts t6=-2.885, p=0.028). Raclopride treatment had no carryover effects in GTs, as their performance on Session 13, after treatment ended, was similar to their performance on Session 8 (food cup contacts t6=-1.948, p=0.099). Taken together, D2/D3 antagonism produced an attenuation of food cup-directed behaviors in GTs, but this was a transient effect, apparent only during the course of treatment.
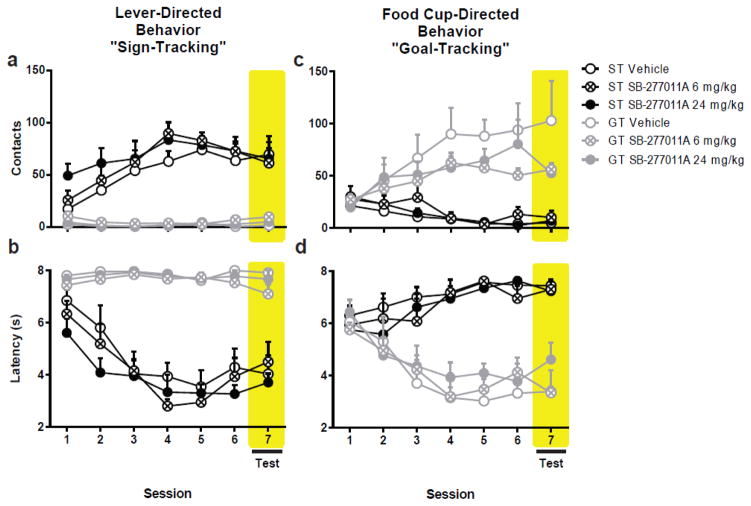
3.4 Experiment 4: Selective Antagonism of Dopamine D3 Receptors by SB-277011A Does Not Alter Sign- or Goal-Tracking Behaviors
Over the course of 5 sessions of Pavlovian conditioning, rats developed a sign- or goal-tracking conditioned response. Treatment with either 6 or 24 mg/kg SB-277011A failed to influence either conditioned response, as there was not an effect of D3 antagonism on any measure of lever- or food cup-directed behavior (Fig. 7). That is, treatment with SB-277011A did not alter performance on any behavioral measures when treated sign- and goal-trackers were compared to their vehicle counterparts; nor did SB-277011A administration affect performance relative to baseline (i.e. Session 6).
Fig. 7.
Effects of SB-277011A on Pavlovian conditioned approach behavior. Following five sessions of Pavlovian conditioning animals were classified as STs (n=16) or GTs (n=17). All animals received vehicle prior to session six. On session 7 animals received either vehicle (ST n=5; GT n=5), 6 mg/kg (ST n=6; GT n=7), or 24 mg/kg (ST n=5; GT n=5) SB-277011A. Treatment with SB-277011A did not affect sign-tracking (a–b) or goal-tracking (c–d). Shaded region indicates session when SB-277011A was administered. Error bars indicate SEM
4. Discussion
There is now ample evidence to suggest that dopamine facilitates the attribution of incentive salience to reward-associated cues (for review [5] and see also [13,20,35]). The present study builds upon these findings by examining the contributions of the dopamine D2 and D3 receptors in the expression of sign- and goal-tracking behavior. Following repeated pairings of a discrete lever-cue with delivery of a food reward into a nearby food cup, two distinct conditioned responses developed, as previously reported [26]. The goal-tracking CR consisted of cue-elicited approach directed towards the food cup; whereas the sign-tracking CR consisted of approach and manipulation of the lever-cue itself. Thus, the lever-cue served as a predictor and elicited a CR in both sign- and goal-trackers, but only for sign-trackers did the lever-cue become an incentive stimulus. This model then allowed us to examine the effects of D2-like agents on the expression of behaviors resulting from predictive or incentive Pavlovian learning processes.
We found that administration of either a D2/D3 agonist or antagonist attenuated the expression of both a sign- and goal-tracking CR; but only the CR that was previously acquired was affected for each phenotype. It is possible that an attenuation of goal-tracking behavior in STs, or sign-tracking behavior in GTs, was not evident because these alternate responses were already at a minimum value by definition of the phenotypes. However, given that these drugs did not appear to affect general activity levels (see Supplemental Fig. 2), we do not believe this is the case. In fact, based on other studies (e.g. [13]), with the attenuation of one conditioned response, we would have expected to see an increase in the alternate conditioned response, but this was not observed. These findings therefore suggest that no new learning of the CS-US association is occurring under the influence of these D2/D3 agents. Rather, these drugs are specifically and transiently reducing the ability of the reward-cue to reliably elicit the previously learned conditioned response, regardless of whether predictive or incentive learning processes were involved.
It could, however, be argued that the apparent reduction in the previously acquired value of the reward cue is due to extinction learning. In fact, previous studies have shown that disruption of dopamine transmission, either by treatment with dopamine antagonists or by 6-hydroxydopamine lesion, produces a gradual reduction in the performance of a learned response and potentially leads to new learning pertaining to the value of the CS [36–38]. Here, however, both D2/D3 agonists and antagonists generally impeded the performance of both a sign- and goal-tracking conditioned response on the first session of treatment, making these interpretations seem unlikely. Further, given a recent report demonstrating that GTs show faster and more complete extinction of conditioned responding relative to STs [33], if these drugs were indeed facilitating extinction, we would expect to see differential effects on sign- and goal-trackers, which were not readily apparent. The only drug that appeared to elicit differential effects in sign- vs. goal-trackers on the first session of treatment was raclopride; which significantly attenuated goal-tracking behavior and only some measures of sign-tracking behavior during the initial treatment session. Yet, by the second session of treatment, both STs and GTs were showing significantly impaired performance. It is also important to note that, for all experiments, rats continued to consume the reward throughout the sessions. In addition, the apparent effects were transient and not evident on subsequent sessions when the drugs were not administered. Thus, the observed effects of these dopaminergic agents do not appear to be due to extinction of the previously acquired CR.
Although we selectively chose doses and agents that have not previously caused general locomotor deficits [28,30,32], this is a potential concern in interpreting the current findings as we did not independently measure locomotor activity. Importantly, aside from the two highest doses of 7-OH-DPAT, which appeared to increase general activity, there were no significant differences for any of the drugs administered in the number of food cup entries during the intertrial interval (see Supplemental Fig. 2), a measure previously used as an index non-specific motor effects [13,21]. Additionally, nearly identical deficits as those reported above for the number of contacts were observed for the probability to approach the lever-cue or food cup (see Supplemental Results, Supplemental Fig. 3). Given that probability is calculated based on a single response during a trial, it is less likely to be impacted by changes in locomotor behavior. That is, if there was a more general locomotor deficit we would expect a “disconnect” between probability measures and the vigor of the response, as measured by the number of contacts. Further, following administration of 7-OH-DPAT, there were no significant differences between treatment groups for either phenotype on the number of responses in the inactive nose-port on the conditioned reinforcement test; nor were there any differences between treatment groups in the number of responses in the active port for GTs. Taken together, although we cannot completely rule out the potential impact of locomotor deficits in the current study, the reported effects of both the agonists and antagonists appear to be due to a specific reduction in the motivational value of the lever-cue.
The fact that agents targeting the D2/D3 receptors impaired the expression of both sign- and goal-tracking behaviors to a similar extent was somewhat surprising. We previously demonstrated that dopamine is involved in the acquisition of a sign-tracking, but not goal-tracking, response [13]. Yet, at doses comparable to those that blocked the acquisition of a sign-tracking CR, systemic administration of a non-specific dopamine antagonist, flupenthixol, attenuated the expression of both CRs after they were acquired [13]. Here, we expand these previous findings, demonstrating that the expression of both a sign- and goal-tracking CR similarly rely on proper dopamine signaling at the D2, and to a lesser extent D3, receptor. That is, manipulations of D3 signaling alone did not affect the expression of these conditioned responses, but given the effects of the D2/D3 agonists and antagonists, it is possible that synergistic activity at D2 and D3 receptors is involved in these behaviors. Furthermore, it is important to note that the role of these receptors in the expression of these behaviors may be quite different from their role in the acquisition of the conditioned responses; thus, ongoing studies are examining the impact of these agents prior to the development of the conditioned responses.
While the current study administered these dopaminergic agents systemically, it is possible that local administration would yield different effects. For example, studies by Saunders and Robinson (2012) showed that administration of flupenthixol directly into the core of the nucleus accumbens selectively attenuated the expression of a sign-tracking, but not goal-tracking CR. Administration of the dopaminergic agents used in the current study might yield similar results, affecting only sign-tracking behavior, if administered directly into the nucleus accumbens core. Dopamine in the core of the nucleus accumbens is necessary for the expression of a conditioned response following the attribution of incentive salience to environmental stimuli [20,27,35,39–41]. The mechanisms underlying the ability of dopamine in the core of the nucleus accumbens to evoke these responses have yet to be elucidated, but the D2-family of receptors may be involved. Likewise, local administration into other brain areas, such as prefrontal cortical regions might specifically affect goal-tracking behavior, as dopaminergic transmission in these regions is believed to be important in mediating goal-directed behaviors [42–45].
A previous report from Danna and Elmer (2010) [19] showed that systemic administration of antipsychotics, which act at the D2-family of receptors, was able to disrupt approach to a cue-light, indicative of sign-tracking, but not approach to the site of reward delivery, or goal-tracking. Although these results may seem inconsistent with the current findings, it should be noted that unlike the agents selected for the present study [24,29,31], antipsychotics act at a variety of receptors outside of the D2-family of receptors [46], which may have contributed to these disparate effects. Further, different training procedures were used in the Danna and Elmer (2010) study, where rats were trained with both a reward-associated (CS+) and non-reward-associated (CS-) light-cues, and all rats appeared to vacillate between approach to the cue and the site of reward delivery. That is, rats were not classified as sign- or goal-trackers per se. Thus, the seemingly discrepant findings between the Danna and Elmer (2010) study and the current results may be due to actions at the D2-family of receptors and their many targets, coupled with differences in experimental procedures.
A more recent report offers somewhat more congruent result to our own findings, but also with some notable differences [47]. Similar to the current findings, Lopez and colleagues (2015) demonstrated that both a D2/D3 agonist and antagonist impaired a sign-tracking CR. However, a goal-tracking CR was only impaired by a D2/D3 antagonist in the Lopez et al. study. Importantly, the D2/D3 agonist used by Lopez and colleagues has different affinities for D2 and D3 receptors than the two agonists used in the current study [29]. Further, in the present study, rats were performing asymptotically prior to classification as either sign- or goal-trackers, which we have previously reported takes anywhere from 5 to 7 sessions of training [11,26]; whereas Lopez and colleagues examined drug-effects following just 4 sessions of training. Thus, learning of the CS-US association might have still been occurring at the time of testing, meaning that the manipulations might have affected learning of each CR, rather than expression—which was explored in the current study. These and other experimental differences may explain the discrepant findings; but the congruent findings between the current study and that of Lopez and colleagues further validate the specific involvement of the D2 receptor in both predictive and Pavlovian incentive motivational processes.
The mechanism by which the dopaminergic agents used in the current study are eliciting their effects warrants further investigation as both D2/D3 receptor agonists and antagonists produced similar results. These receptors act pre-synaptically as autoreceptors by reducing dopamine release, and post-synaptically by inhibiting intracellular signaling [48–50]. Although both receptors are known to act as autoreceptors pre-synaptically, D2 receptors appear to be more predominant in this role [51]. Nonetheless, given that D2 and D3 receptors act both pre- and post-synaptically, one can speculate regarding the net effects of agonism or antagonism at these receptors. For example, it has been shown that raclopride elicits an increase in extracellular dopamine [52], likely due to antagonizing pre-synaptic and post-synaptic receptors. Thus, the large increase in extracellular dopamine may directly compete with raclopride post-synaptically, resulting in stimulation at post-synaptic receptor sites. Although mechanistic evidence is lacking for D2/D3 agonists in vivo, we speculate that pramipexole and 7-OH-DPAT stimulate autoreceptors, blunting dopamine release, while concomitantly producing high levels of post-synaptic activity. Therefore, it is possible the net effects of D2/D3 agonism or antagonism are similar in post-synaptic cells, which could, at least in part, explain the behavioral results reported here.
It is also arguable that the manipulations of D2 and D3 receptors altered signaling at post-synaptic D1-receptor expressing neurons and that the impairment in sign- and goal-tracking behavior is due to a disruption in D1 signaling. However, it is unlikely that this is the sole mechanism for the observed effects. Dalley and colleagues (2005) reported that D1 receptors in the nucleus accumbens core were important for the acquisition of a Pavlovian conditioned response, yet their contribution is negligible in later stages of training [53]. Clark and colleagues (2013) recently demonstrated that, as Pavlovian conditioned approach extends past asymptotic performance, phasic dopamine, which biases signaling towards D1-type receptors, is no longer necessary for the execution of the CR. Moreover, administration of a specific D1 receptor antagonist, SCH23390, was only able to impede conditioned approach in early pre-asymptotic stages, but not following acquisition of peak performance [54]. In agreement, we have shown that following a single PCA training session, animals exhibiting sign-tracking behavior have enhanced D1 mRNA in the core of the nucleus accumbens compared to those exhibiting food cup-directed responses, and following additional training this difference between phenotypes in D1 mRNA is no longer apparent [17]. During later stages of training, however, differences emerge in the expression of D2 mRNA in the nucleus accumbens, which may explain the ability of D2-like agents to impede performance; and further highlight a role for D2 in mediating these behaviors. It would be interesting to examine what contributions, if any, dopamine D1 receptors have to sign- and goal-tracking behaviors using a targeted pharmacological approach.
In contrast to the effects with the mixed D2/D3 agents, the selective D3 antagonist SB-277011A did not produce a deficit in either a sign- or goal-tracking CR. These results are in line with studies using second-order schedules of reinforcement which have consistently shown SB-277011A does not impair responding when food is used as the US [34]. There are, however, a number of studies supporting a role for D3 in responding to drug-associated cues [55–58]. Thus, the effects of a specific D3 antagonist on sign-tracking behavior in response to a drug-associated cue might be more reminiscent of the D2/D3 effects shown here. Further, as discussed above, we might expect different effects if we were to administer the dopamine D3 receptor antagonist locally, in specific regions known to be enriched with these receptors and implicated in Pavlovian learning processes, such as the paraventricular nucleus of the thalamus (e.g. see [11,59]) or the nucleus accumbens (e.g. see [21]). Nonetheless, the current study is the first to our knowledge to show that D3 receptors alone do not mediate conditioned responding for a food-cue in a purely associative paradigm.
In conclusion, we demonstrated that systemic manipulations of signaling at the D2 and D3 receptors combined were able to attenuate a previously acquired sign- or goal-tracking conditioned response. However, systemic D3 receptor antagonism alone was not sufficient to alter the performance of either conditioned response. Taken together these results suggest normal signaling at D2, perhaps in synergy with D3, receptors is necessary for proper expression of a conditioned response irrespective of incentive salience attribution.
Supplementary Material
Highlights.
Sign-trackers attribute greater incentive salience to a lever-cue than goal-trackers.
Agonism or antagonism of both D2/D3 receptors disrupts sign- and goal-tracking.
Selective antagonism of D3 receptors does not affect sign- or goal-tracking.
D2 receptor signaling is critical for the expression of sign- and goal-tracking.
Acknowledgments
The authors would like to thank Rebeca Kelly, Katie Long, Allison Lang and Tessa Shapiro for technical assistance with these studies. This work was supported by the National Institute on Drug Abuse Intramural Research Program (ELG) and external grants T32 DA007821 (JLH), F31 DA037680 (JLH), and P01 DA031656 (SBF); as well as internal funds from the Department of Psychiatry at the University of Michigan (SBF). We would also like to thank Brittany Kuhn and Drs. Terry Robinson, Aram Parsegian and Ignacio Rivero-Covelo for comments on earlier versions of the manuscript.
Funding
This work was supported by grants from the National Institute on Drug Abuse to J.L.H (T32 DA007821; F31 DA037680) and S.B.F (P01 DA031656) and by funding from the Intramural Research Program, National Institute on Drug Abuse to E.L.G.
Footnotes
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any financial or commercial relationships that could be construed as a potential conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Hollerman JR, Schultz W. Dopamine neurons report an error in the temporal prediction of reward during learning. Nat Neurosci. 1998;1:304–309. doi: 10.1038/1124. [DOI] [PubMed] [Google Scholar]
- 2.Schultz W, Dayan P, Montague PR. A Neural Substrate of Prediction and Reward. Science. 1997;275:1593–1599. doi: 10.1126/science.275.5306.1593. [DOI] [PubMed] [Google Scholar]
- 3.Steinberg EE, Keiflin R, Boivin JR, Witten IB, Deisseroth K, Janak PH. A causal link between prediction errors, dopamine neurons and learning. Nature Publishing Group. 2013;16:966–973. doi: 10.1038/nn.3413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Berridge KC. The debate over dopamine’s role in reward: the case for incentive salience. Psychopharmacology. 2007;191:391–431. doi: 10.1007/s00213-006-0578-x. [DOI] [PubMed] [Google Scholar]
- 5.Berridge KC. From prediction error to incentive salience: mesolimbic computation of reward motivation. Eur J Neurosci. 2012;35:1124–1143. doi: 10.1111/j.1460-9568.2012.07990.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Berridge KC. Reward Learning: Reinforcement, Incentives, and Expectations. In: Medin DL, editor. The Psychology of Learning and Motivation Advances in Research and Theory. Vol. 40. San Diego, CA: The Psychology of Learning and Motivation; 2001. pp. 223–278. [Google Scholar]
- 7.Robinson TE, Berridge KC. The neural basis of drug craving: an incentive-sensitization theory of addiction. Brain Research Reviews. 1993;18:247–291. doi: 10.1016/0165-0173(93)90013-p. [DOI] [PubMed] [Google Scholar]
- 8.Berridge KC, Robinson TE. Parsing reward. Trends in Neurosciences. 2003;26:507–513. doi: 10.1016/S0166-2236(03)00233-9. [DOI] [PubMed] [Google Scholar]
- 9.Stewart J, deWit H, Eikelboom R. Role of Unconditioned and Conditioned Drug Effects in the Self-Administration of Opiates and Stimulants. Psychological Review. 1984;91:251–268. [PubMed] [Google Scholar]
- 10.Robinson TE, Flagel SB. Dissociating the Predictive and Incentive Motivational Properties of Reward-Related Cues Through the Study of Individual Differences. BPS. 2009;65:869–873. doi: 10.1016/j.biopsych.2008.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Haight JL, Fraser KM, Akil H, Flagel SB. Lesions of the paraventricular nucleus of the thalamus differentially affect sign- and goal-tracking conditioned responses. Eur J Neurosci. 2015:1–39. doi: 10.1111/ejn.13031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Flagel SB, Cameron CM, Pickup KN, Watson SJ, Akil H, Robinson TE. A food predictive cue must be attributed with incentive salience for it to induce c-fos mRNA expression in cortico-striatal-thalamic brain regions. NSC. 2011;196:80–96. doi: 10.1016/j.neuroscience.2011.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Flagel SB, Clark JJ, Robinson TE, Mayo L, Czuj A, Willuhn I, et al. A selective role for dopamine in stimulus-reward learning. Nature. 2011;469:53–57. doi: 10.1038/nature09588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hearst E, Jenkins H. Monograph of the Psychonomic Society. Austin; 1974. Sign-tracking: The Stimulus-Reinforcer Relation and Directed Action. [Google Scholar]
- 15.Boakes RA. Performance on learning to associate a stimulus with positive reinforcement. In: Davis H, Hurwitz H, editors. Operant-Pavlovian Interactions. Hillsdale, NJ: 1977. pp. 67–97. [Google Scholar]
- 16.Berridge KC, Robinson TE, Aldridge JW. Dissecting components of reward: ‘liking’, ‘wanting’, and learning. Current Opinion in Pharmacology. 2009;9:65–73. doi: 10.1016/j.coph.2008.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Flagel SB, Watson SJ, Robinson TE, Akil H. Individual differences in the propensity to approach signals vs goals promote different adaptations in the dopamine system of rats. Psychopharmacology. 2007;191:599–607. doi: 10.1007/s00213-006-0535-8. [DOI] [PubMed] [Google Scholar]
- 18.Danna CL, Shepard PD, Elmer GI. The habenula governs the attribution of incentive salience to reward predictive cues. Frontiers in Human Neuroscience. 2013:1–7. doi: 10.3389/fnhum.2013.00781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Danna CL, Elmer GI. Disruption of conditioned reward association by typical and atypical antipsychotics. Pharmacology, Biochemistry and Behavior. 2010;96:40–47. doi: 10.1016/j.pbb.2010.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Saunders BT, Robinson TE. The role of dopamine in the accumbens core in the expression of Pavlovian-conditioned responses. Eur J Neurosci. 2012;36:2521–2532. doi: 10.1111/j.1460-9568.2012.08217.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Le Foll B, Di Ciano P. Neuronal circuitry underlying the impact of D3 receptor ligands in drug addiction. European Neuropsychopharmacology. 2014:1–9. doi: 10.1016/j.euroneuro.2014.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Volkow ND, Wang GJ, Fowler JS, Tomasi D, Telang F. Addiction: Beyond dopamine reward circuitry. Proc Natl Acad Sci USA. 2011;108:15037–15042. doi: 10.1073/pnas.1010654108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Robinson TE, Yager LM, Cogan ES, Saunders BT. On the motivational properties of reward cues: Individual differences. Neuropharmacology. 2014;76:450–459. doi: 10.1016/j.neuropharm.2013.05.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Reavill C, Taylor SG, Wood MD, Ashmeade T, Austin NE, Avenell KY, et al. Pharmacological Actions of a Novel, High-Affinity, and Selective Human Dopamine D3 Receptor Antagonist, SB-277011-A. Journal of Pharmacology and Experimental Therapeutics. 2000;294:1154–1165. [PubMed] [Google Scholar]
- 25.Fitzpatrick CJ, Gopalakrishnan S, Cogan ES, Yager LM, Meyer PJ, Lovic V, et al. Variation in the form of Pavlovian conditioned approach behavior among outbred male Sprague-Dawley rats from different vendors and colonies: sign-tracking vs. goal-tracking. PLoS ONE. 2013;8:e75042. doi: 10.1371/journal.pone.0075042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Meyer PJ, Lovic V, Saunders BT, Yager LM, Flagel SB, Morrow JD, et al. Quantifying Individual Variation in the Propensity to Attribute Incentive Salience to Reward Cues. PLoS ONE. 2012;7:e38987. doi: 10.1371/journal.pone.0038987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yager LM, Pitchers KK, Flagel SB, Robinson TE. Individual Variation in the Motivational and Neurobiological Effects of an Opioid Cue. Neuropsychopharmacology. 2014:1–9. doi: 10.1038/npp.2014.314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li S-M, Collins GT, Paul NM, Grundt P, Newman AH, Xu M, et al. Yawning and locomotor behavior induced by dopamine receptor agonists in mice and rats. Behavioural Pharmacology. 2010;21:171–181. doi: 10.1097/FBP.0b013e32833a5c68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Collins GT, Newman AH, Grundt P, Rice KC, Husbands SM, Chauvignac C, et al. Yawning and hypothermia in rats: effects of dopamine D3 and D2 agonists and antagonists. Psychopharmacology. 2007;193:159–170. doi: 10.1007/s00213-007-0766-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Collins GT, Truong YN-T, Levant B, Chen J, Wang S, Woods JH. Behavioral sensitization to cocaine in rats: evidence for temporal differences in dopamine D3 and D2 receptor sensitivity. Psychopharmacology. 2011;215:609–620. doi: 10.1007/s00213-010-2154-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Seeman P, Van Tol HHM. Dopamine receptor pharmacology. Trends in Pharmacological Sciences. 1994;15:264–270. doi: 10.1016/0165-6147(94)90323-9. [DOI] [PubMed] [Google Scholar]
- 32.Chausmer AL, Ettenberg A. A Role for D2, but not D1, Dopamine Receptors in the Response-Reinstating Effects of Food Reinforcement. Pharmacology, Biochemistry and Behavior. 1997;57:681–685. doi: 10.1016/s0091-3057(96)00388-7. [DOI] [PubMed] [Google Scholar]
- 33.Ahrens AM, Singer BF, Fitzpatrick CJ, Morrow JD, Robinson TE. Rats that sign-track are resistant to Pavlovian but not instrumental extinction. Behavioural Brain Research. 2015:1–45. doi: 10.1016/j.bbr.2015.07.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Di Ciano P, Underwood RJ, Hagan JJ, Everitt BJ. Attenuation of Cue-Controlled Cocaine-Seeking by a Selective D3 Dopamine Receptor Antagonist SB-277011-A. Neuropsychopharmacology. 2003;28:329–338. doi: 10.1038/sj.npp.1300148. [DOI] [PubMed] [Google Scholar]
- 35.Saunders BT, O’Donnell EG, Aurbach EL, Robinson TE. A Cocaine Context Renews Drug Seeking Preferentially in a Subset of Individuals. Neuropsychopharmacology. 2014:1–29. doi: 10.1038/npp.2014.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ettenberg A. Dopamine, Neuroleptics and Reinforced Behavior. Neuroscience and Biobehavioral Reviews. 1989;13:105–111. doi: 10.1016/s0149-7634(89)80018-1. [DOI] [PubMed] [Google Scholar]
- 37.Dowd E, Dunnett SB. Movement without dopamine: striatal dopamine is required to maintain but not to perform learned actions. Biochemical Society Transactions. 2007;35:428–432. doi: 10.1042/BST0350428. [DOI] [PubMed] [Google Scholar]
- 38.Wise RA, Spindler J, deWit H, Gerber GJ. Neuroleptic-Induced “Anhedonia” in Rats: Pimozide Blocks Reward Quality of Food. Science. 1978;201:262–264. doi: 10.1126/science.566469. [DOI] [PubMed] [Google Scholar]
- 39.Parkinson JA, Dalley JW, Cardinal RN, Bamford A, Fehnert B, Lachenal G, et al. Nucleus accumbens dopamine depletion impairs both acquisition and performance of appetitive Pavlovian approach behaviour: implications for mesoaccumbens dopamine function. Behavioural Brain Research. 2002;137:149–163. doi: 10.1016/s0166-4328(02)00291-7. [DOI] [PubMed] [Google Scholar]
- 40.Saunders BT, Yager LM, Robinson TE. Cue-Evoked Cocaine “Craving”: Role of Dopamine in the Accumbens Core. Journal of Neuroscience. 2013;33:13989–14000. doi: 10.1523/JNEUROSCI.0450-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Di Ciano P, Cardinal RN, Cowell RA, Little SJ, Everitt BJ. Differential Involvement of NMDA, AMPA/Kainate, and Dopamine Receptors in the Nucleus Accumbens Core in the Acquisition and Performance of Pavlovian Approach Behavior. Journal of Neuroscience. 2001;21:9471–9477. doi: 10.1523/JNEUROSCI.21-23-09471.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Floresco SB, Magyar O. Mesocortical dopamine modulation of executive functions: beyond working memory. Psychopharmacology. 2006;188:567–585. doi: 10.1007/s00213-006-0404-5. [DOI] [PubMed] [Google Scholar]
- 43.Le Moal M, Simon H. Mesocorticolimbic Dopaminergic Network: Functional and Regulatory Roles. Physiological Reviews. 1999;21:155–232. doi: 10.1152/physrev.1991.71.1.155. [DOI] [PubMed] [Google Scholar]
- 44.Robbins TW, Roberts AC. Differential Regulation of Fronto-Executive Function by the Monoamines and Acetylcholine. Cerebral Cortex. 2007;17:i151–i160. doi: 10.1093/cercor/bhm066. [DOI] [PubMed] [Google Scholar]
- 45.Ott T, Jacob SN, Nieder A. Dopamine Receptors Differentially Enhance Rule Coding in Primate Prefrontal Cortex Neurons. Neuron. 2014;84:1317–1328. doi: 10.1016/j.neuron.2014.11.012. [DOI] [PubMed] [Google Scholar]
- 46.Kroeze WK, Hufeisen SJ, Popadak BA, Renock SM, Steinberg S, Ernsberger P, et al. H1-Histamine Receptor Affinity Predicts Short-Term Weight Gain for Typical and Atypical Antipsychotic Drugs. Neuropsychopharmacology. 2003;28:519–526. doi: 10.1038/sj.npp.1300027. [DOI] [PubMed] [Google Scholar]
- 47.Lopez JC, Karlsson R-M, O’Donnell P. Dopamine D2 Modulation of Sign and Goal Tracking in Rats. Neuropsychopharmacology. 2015;40:2096–2102. doi: 10.1038/npp.2015.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gainetdinov RR, Sotnikova TD, Grekhova TV, Rayevsky KS. In vivo evidence for preferential role of dopamine D3 receptor in the presynaptic regulation of dopamine release but not synthesis. European Journal of Pharmacology. 1996;308:261–269. doi: 10.1016/0014-2999(96)00300-7. [DOI] [PubMed] [Google Scholar]
- 49.Joseph JD, Wang YM, Miles PR, Budygin EA, Picetti R, Gainetdinov RR, et al. Dopamine Autoreceptor Regulation of Release and Uptake in Mouse Brain Slices in the Absence of D3 Receptors. Neuroscience. 2002;112:39–49. doi: 10.1016/s0306-4522(02)00067-2. [DOI] [PubMed] [Google Scholar]
- 50.Sokoloff P, Giros B, Martres M-P, Bouthenet M-L, Schwartz J-C. Molecular cloning and characterization of a novel dopamine receptor (D3) as a target for neuroleptics. Nature. 1990;347:146–151. doi: 10.1038/347146a0. [DOI] [PubMed] [Google Scholar]
- 51.Zapata A, Witkin JM, Shippenberg TS. Selective D3 receptor agonist effects of (+)-PD 128907 on dialysate dopamine at low doses. Neuropharmacology. 2001;41:351–359. doi: 10.1016/s0028-3908(01)00069-7. [DOI] [PubMed] [Google Scholar]
- 52.Vander Weele CM, Porter-Stransky KA, Mabrouk OS, Lovic V, Singer BF, Kennedy RT, et al. Rapid dopamine transmission within the nucleus accumbens: Dramatic difference between morphine and oxycodone delivery. Eur J Neurosci. 2014;40:3041–3054. doi: 10.1111/ejn.12709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Dalley JW, Lääne K, Theobald DEH, Armstrong HC, Corlett PR, Chudasama Y, et al. Time-limited modulation of appetitive Pavlovian memory by D1 and NMDA receptors in thenucleus accumbens. Proc Natl Acad Sci USA. 2005;102:6189–6194. doi: 10.1073/pnas.0502080102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Clark JJ, Collins AL, Sanford CA, Phillips PEM. Dopamine Encoding of Pavlovian Incentive Stimuli Diminishes with Extended Training. Journal of Neuroscience. 2013;33:3526–3532. doi: 10.1523/JNEUROSCI.5119-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Khaled MA, Pushparaj A, Di Ciano P, Diaz J, Le Foll B. Dopamine D3 Receptors in the Basolateral Amygdala and the Lateral Habenula Modulate Cue-Induced Reinstatement of Nicotine Seeking. Neuropsychopharmacology. 2014;39:3049–3058. doi: 10.1038/npp.2014.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Song R, Zhang H-Y, Peng X-Q, Su R-B, Yang R-F, Li J, et al. Dopamine D3 receptor deletion or blockade attenuates cocaine-induced conditioned place preference in mice. Neuropharmacology. 2013;72:82–87. doi: 10.1016/j.neuropharm.2013.04.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Le Foll B, Frances H, Diaz J, Schwartz J-C, Sokoloff P. Role of the dopamine D3 receptor in reactivity to cocaine-associated cues in mice. Eur J Neurosci. 2002;15:2016–2026. doi: 10.1046/j.1460-9568.2002.02049.x. [DOI] [PubMed] [Google Scholar]
- 58.Le Foll B, Sokoloff P, Stark H, Goldberg SR. Dopamine D3 Receptor Ligands Block Nicotine-Induced Conditioned Place Preferences through a Mechanism that does not Involve Discriminative-Stimulus or Antidepressant-Like Effects. Neuropsychopharmacology. 2004:1–11. doi: 10.1038/sj.npp.1300622. [DOI] [PubMed] [Google Scholar]
- 59.Haight JL, Flagel SB. A potential role for the paraventricular nucleus of the thalamus in mediating individual variation in Pavlovian conditioned responses. Frontiers in Behavioral Neuroscience. 2014;8:1–10. doi: 10.3389/fnbeh.2014.00079. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.