Abstract
Context
Despite reported success, surgery for pharmacoresistant seizures is often seen as a last resort. Patients are typically referred for surgery after 20 years of seizures, often too late to avoid significant disability and premature death.
Objective
We sought to determine whether surgery soon after failure of 2 antiepileptic drug (AED) trials is superior to continued medical management in controlling seizures and improving quality of life (QOL).
Design, Setting, and Participants
The Early Randomized Surgical Epilepsy Trial (ERSET) is a multicenter, controlled, parallel-group clinical trial performed at 16 US epilepsy surgery centers. The 38 participants (18 men and 20 women; aged ≥ 12 years) had mesial temporal lobe epilepsy (MTLE) and disabling seizues for no more than 2 consecutive years following adequate trials of 2 brand-name AEDs. Eligibility for anteromesial temporal resection (AMTR) was based on a standardized presurgical evaluation protocol. Participants were randomized to continued AED treatment or AMTR 2003–2007, and observed for 2 years. Planned enrollment was 200, but the trial was halted prematurely due to slow accrual.
Intervention
Receipt of continued AED treatment (n=23) or a standardized AMTR plus AED treatment (n = 15). In the medical group, 7 participants underwent AMTR prior to the end of follow-up and 1 participant in the surgical group never received surgery.
Main Outcome Measures
The primary outcome variable was freedom from disabling seizures during year 2 of follow-up. Secondary outcome variables were health-related QOL (measured primarily by the 2-year change in the Quality of Life in Epilepsy 89 [QOLIE-89] overall T-score), cognitive function, and social adaptation.
Results
Zero of 23 participants in the medical group and 11 of 15 in the surgical group were seizure free during year 2 of follow-up (odds ratio=∞; 95% CI, 11.8 to ∞;P <.001). In an intention-to-treat analysis, the mean improvement in QOLIE-89 overall T-score was higher in the surgical group than in the medical group but this difference was not statistically significant (12.6 vs 4.0 points; treatment effect = 8.5; 95% CI, −1.0 to 18.1; P =.08). When data obtained after surgery from participants in the medical group were excluded, the effect of surgery on QOL was significant (12.8 vs 2.8 points; treatment effect=9.9; 95% CI, 2.2 to 17.7; P =.01). Memory decline (assessed using the Rey Auditory Verbal Learning Test) occurred in 4 participants (36%) after surgery, consistent with rates seen in the literature; but the sample was too small to permit definitive conclusions about treatment group differences in cognitive outcomes. Adverse events included a transient neurologic deficit attributed to a magnetic resonance imaging–identified postoperative stroke in a participant who had surgery and 3 cases of status epilepticus in the medical group.
Conclusions
Among patients with newly intractable disabling MTLE, resective surgery plus AED treatment resulted in a lower probability of seizures during year 2 of follow-up than continued AED treatment alone. Given the premature termination of the trial, the results should be interpreted with appropriate caution.
Epilepsy is a Worldwide Serious health concern, accounting for 1% of the global burden of disease, equivalent to lung cancer in men and breast cancer in women.1 The 20% to 40% of patients who have medically intractable2 epilepsy account for 80% of the cost of epilepsy.3 Temporal lobe epilepsy (TLE) is the most common cause of drug-resistant seizures,4–7 but it can be treated surgically.8–10 The American Academy of Neurology practice parameter recommends surgery as the treatment of choice for medically intractable TLE,9 based in part on a single-center Canadian randomized controlled trial (RCT) that demonstrated the efficacy of surgical treatment in patients with longstanding TLE.10
Nevertheless, surgical treatment for epilepsy is delayed and underutilized.8 Patients who are referred for surgery have had epilepsy for an average of 22 years, more than 10 years after failure of 2 antiepileptic drugs (AEDs),11 the international definition of medical intractability.12 Since the publication of the Canadian RCT (2001) and practice parameter (2003), the time to surgical referral has not decreased.13 Because earlier surgery could prevent significant morbidity1 and premature death, the practice parameter recommended an RCT to evaluate the efficacy of early surgical intervention in newly intractable patients with TLE.9 The primary purpose of the National Institute of Neurological Disorders and Stroke (NINDS)–funded Early Randomized Surgical Epilepsy Trial (ERSET)14,15 was to compare outcomes of surgery with those of continued pharmacotherapy at a time when adverse psychological and social consequences of disabling seizures might be minimal and seizures might conceivably still respond to further trials of AEDs.
METHODS
Study Design
A detailed description of the multicenter, controlled, parallel-group RCT design has been published elsewhere.15 Participants were randomized to receive continued pharmacotherapy or surgery plus pharmacotherapy and observed for 24 months. Participants were males and females aged 12 years or older with mesial TLE (MTLE)16,17 and disabling seizures that had persisted for no more than 2 consecutive years following adequate trials of 2 brand-name AEDs. Participants had to be considered candidates for anteromesial temporal resection based on a standardized presurgical evaluation protocol.15
The study was approved by the institutional review board at each site. All participants provided written informed consent to participate. Details concerning the informed consent process, eligibility criteria, and randomization procedure are given in the supplementary online material (eAppendix, available at http://www.jama.com).
All sites utilized the same anteromesial temporal resection, which consisted of en bloc resection of the anterior 3.5 to 4 cm (in the dominant and nondominant hemispheres, respectively) of the lateral temporal lobe, sparing the superior temporal gyrus, followed by removal of the mesial structures including en bloc resection of the hippocampus and resection of parahippocampal gyrus and part of the amygdala.18
The objectives of pharmacotherapy were to achieve and maintain a seizure-free state and minimize adverse effects. The same protocol was used for both treatment groups and reflected current practice used in most US epilepsy centers. It consisted of 4 stages: (1) monotherapy; (2) ditherapy; (3) optional treatment with rarely used AEDs; and (4) treatment with multiple AEDs. Generic drugs were not permitted. Pharmacotherapy for seizures for both study groups was monitored by an independent panel of AED clinical pharmacology experts who were blinded to treatment assignment.
Follow-up Evaluations
Participants were seen at the study site every 3 months for 2 years. Adverse events were recorded and seizure logs were collected at every visit. All other outcome variables were collected at baseline and at either 6- or 12-month intervals. Race and ethnicity information, with categories defined using National Institutes of Health guidelines, was obtained from the participant by the investigator during the screening process.
Primary Outcome Variable
The primary outcome variable was freedom from disabling seizures during the second follow-up year. A blinded central seizure adjudication committee reviewed all seizure types recorded in participant diaries (eAppendix), determined whether the seizures should be considered epileptic, and if so, classified them. Disabling seizures were defined as simple partial seizures with impairment (clear consciousness but noticeable by an observer or interfering with function), complex partial seizures, or secondarily generalized seizures. Simple partial seizures without impairment (auras) were not considered disabling. Participants free of disabling seizures were considered to be seizure free.
Secondary Outcome Variables
Seizures
Data from seizure diaries were summarized as seizure frequencies by seizure type and overall.
Quality of Life
This was assessed at baseline and at months 6, 12, 18, and 24 using the self-administered Quality of Life in Epilepsy 89 (QOLIE-89)19 for adults (aged ≥17 years) and Quality of Life in Epilepsy 48 (QOLIE AD-48)20 for adolescents (aged 12–16 years).
Cognitive Function
This was assessed at baseline and at months 12 and 24 using clinical neuropsychological measures of visual and auditory attention, motor speed/ dexterity, verbal and visuospatial memory, and word finding.15
Ancillary Outcome
Employment or educational status, number of hours per week worked, number of sick days in the past 3 months, driving status, number of hospitalizations in the past 3 months, and number of days per month spent socializing with family or friends were assessed at baseline and at months 12 and 24.
Statistical Analysis
A sample size of 200 participants was originally planned for ERSET; a justification of this sample size is given in the eAppendix. Due to the difficulties encountered in recruiting study participants, the data and safety monitoring board (DSMB) appointed by the NINDS recommended termination of the study after only 38 participants had been randomized. At the time of this decision, the DSMB had access to the results by treatment group for the primary outcome variable for participants who had thus far completed follow-up, but not for any other efficacy outcomes. The DSMB stated that its recommendation to halt the trial was based solely on feasibility of recruitment and not at all on efficacy data. If the DSMB had considered these data in their decision making, some degree of inflation of type I error and bias would have been possible. Given the large magnitude of the treatment effect on the primary outcome variable that was observed, however, the effect of this on the trial results would have been minimal.
The primary statistical analyses were performed in accordance with the intention-to-treat principle and included all available data from all randomized participants. Fisher exact test was used to compare the treatment groups with respect to the primary outcome variable. For the primary analysis, participants who did not have complete follow-up during year 2 were considered to not be seizure free; the analyses were repeated after omitting participants who did not have complete follow-up during year 2 unless they had reported disabling seizures in year 2. An additional sensitivity analysis of the primary outcome variable was performed using multiple imputation for the 6 participants with missing data for the primary outcome variable; details concerning the imputation model and other aspects of this analysis are provided in the eAppendix. For continuous outcome variables (QO-LIE-89 scores), repeated measures analysis of covariance models were used to estimate treatment effects (surgical vs medical) at each time point with effects at month 24 being of primary interest. All available data from all adult participants (aged ≥ 17 years) were included in the analyses.
Secondary analyses were performed that omitted data obtained after the time of surgery for participants in the medical group who had surgery prior to the month-24 visit.
The primary analyses of the data on cognitive function compared the 2 treatment groups with respect to memory and nonmemory measures using the O’Brien nonparametric global multivariate test.21 Further details concerning the primary and secondary analyses of the cognitive function data and other secondary outcome variables can be found in the eAppendix. A significance level of 5% (2-tailed) was used for hypothesis testing. All analyses were performed using SAS statistical software version 9.2.
RESULTS
Patients
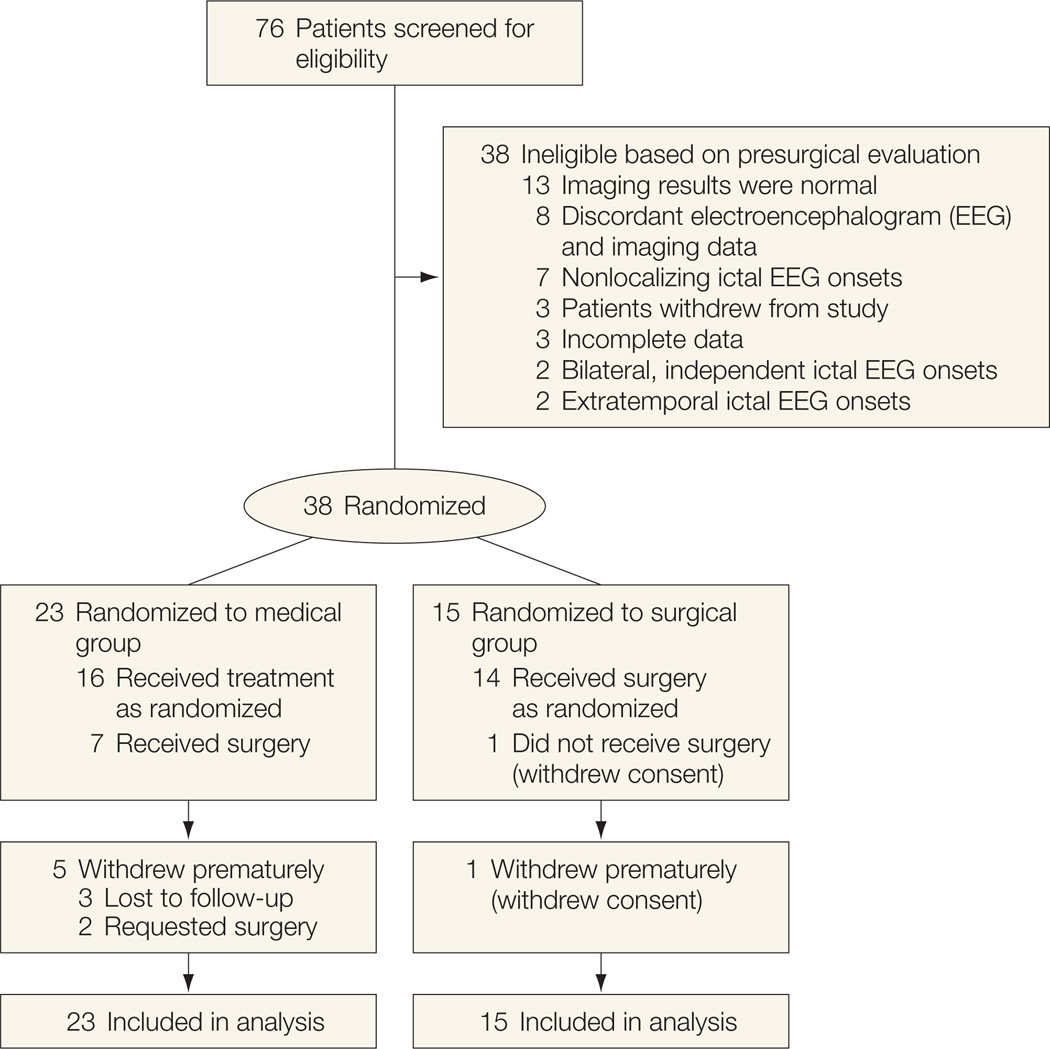
Seventy-six patients at 18 centers provided informed consent over a 2-year period. Only 38 of these patients (from 16 centers) were deemed surgical candidates following presurgical evaluation. Reasons for failure of the presurgical evaluation are noted in Figure 1. Twenty-three participants were randomized to the medical group and 15 to the surgical group. Baseline characteristics (Table 1) were comparable between the 2 treatment groups except for sex and age. Magnetic resonance imaging (MRI) and positron-emission tomography (PET) were both diagnostic in all participants. The only 2 adolescents enrolled were in the medical group.
Figure 1.
Participant Flow
Table 1.
Baseline Characteristics by Treatment Group
| Medical (n = 23) |
Surgical (n = 15) |
|
|---|---|---|
| Age, mean (SD), y | 30.9 (10.1) | 37.5 (11.1) |
| Age <17 y, No. (%) | 2 (8.7) | 0 |
| Male Sex, No. (%) | 14 (60.9) | 4 (26.7) |
| Race, No. (%) Whitea |
18 (78.3) | 11 (73.3) |
| Black | 3 (13.0) | 3 (20.0) |
| Other | 2 (8.7) | 1 (6.7) |
| Duration of epilepsy, median (IQR), y | 5.3 (2.8–13.4) | 5.2 (3.2–15.8) |
| Left side of ictal onset, No. (%) | 14 (60.9) | 9 (60.0) |
| Focal onset, No. (%) | 19 (82.6) | 14 (93.3) |
| Antiepileptic drugs used, mean (SD) | 1.9 (0.9) | 1.6 (0.7) |
| Employment status, No. (%) Full-time employed |
10 (43.5) | 6 (40.0) |
| Part-time employed | 3 (13.0) | 1 (6.7) |
| Full-time student | 4 (17.4) | 1 (6.7) |
| Other | 6 (26.1) | 7 (46.7) |
| Driving, No. (%) | 7 (30.4) | 1 (6.7) |
| Estimated IQ, mean (SD) | 96.5 (11.3) | 98.5 (13.9) |
| No. of seizures/mo, median (IQR) Simple partial |
0.0 (0.0–1.0) | 0.0 (0.0–0.0) |
| Complex partial | 3.0 (1.0–7.0) | 2.0 (1.0–3.0) |
| Secondary generalized | 0.0 (0.0–0.0) | 0.0 (0.0–0.3) |
| Total | 3.0 (2.0–7.0) | 2.0 (1.0–3.3) |
| QOLIE-89 raw scores, mean (SD)b Overall |
61.0 (15.4) | 53.7 (19.4) |
| Mental health factor | −0.20 (0.91) | −0.72 (1.08) |
| Epilepsy-targeted factor | −0.66 (0.78) | −0.81 (1.00) |
| Cognitive factor | −0.15 (0.89) | −0.47 (1.07) |
| Physical health factor | −0.31 (0.93) | −0.70 (1.05) |
| QOLIE-89 T-scores, mean (SD)b Overall |
45.7 (10.0) | 41.3 (12.2) |
| Mental health factor | 47.8 (9.7) | 42.3 (11.5) |
| Epilepsy-targeted factor | 42.6 (8.7) | 41.0 (11.2) |
| Cognitive factor | 48.4 (9.7) | 44.9 (11.7) |
| Physical health factor | 46.4 (10.7) | 41.9 (12.0) |
| SF-36, mean (SD)b Physical component summary |
46.4 (7.6) | 43.7 (11.6) |
| Mental component summary | 43.9 (10.6) | 38.9 (13.8) |
| Brief Symptom Inventory Global Severity Index, mean (SD) | 56.5 (11.3) | 59.3 (8.5) |
Abbreviations: IQR, interquartile range; QOLIE-89, Quality of Life in Epilepsy 89; SF-36, Short Form 36.
Hispanic ethnicity was reported as the ethnic background for 1 patient in the medical group and 1 patient in the surgical group, both of whom were also racially indentified as white.
The ranges of possible scores for QOLIE-89 overall raw score, 0 to 100; QOLIE-89 raw factor scores, −3.8 to 2.0 (mental health), −3.2 to 1.9 (epilepsy targeted), −3.6 to 1.8 (cognitive), and −4.5 to 2.3 (physical health); QOLIE-89 T-scores, 6 to71; SF-36 summary scores, 0 to 100; and Brief Symptom Inventory, 20 to 80. Data on QOLIE-89 and SF-36 scores include only adult participants (n=36); the 2 children who were excluded were both assigned to the medical group.
Reasons for participant withdrawal are provided in Figure 1. Fourteen of 15 participants randomized to the surgical group received surgery according to protocol. One surgical participant withdrew consent and never received surgery. Another surgical participant was observed for 2 years after randomization but did not have surgery until 5 months after randomization, and thus had incomplete follow-up (only 19 months) after surgery. All other participants had surgery 13 to 43 days after randomization.
Five of 23 participants in the medical group withdrew from the trial prior to the 2-year visit, 3 of these at or before the 1-year visit. Seven participants in the medical group received surgery prior to the 2-year visit, 2 prior to the 2-year visit. Five of these participants continued to be observed for 2 years.
The mean (SD) number of AEDs used was comparable in the 2 groups at baseline (1.9 [0.9] in the medical group and 1.6 [0.7] in the surgical group; Table 1) and remained stable in both groups at the final visit (1.8 [1.0] in the medical group and 1.5 [0.7] in the surgical group). The specific AEDs that were used by participants at the baseline and final visits are summarized by treatment group in Table 2.
Table 2.
Antiepileptic Medication Use at the Baseline and Final Visits by Treatment Group
| No. (%) |
||||
|---|---|---|---|---|
| Baseline |
Final Visit |
|||
| Medication | Medical | Surgical | Medical | Surgical |
| Carbamazepine | 7 (30) | 7 (50) | 1 (5) | 6 (43) |
| Clonazepam | 0 | 0 | 1 (5) | 0 |
| Diazepam | 0 | 0 | 1 (5) | 0 |
| Divalproex sodium | 0 | 0 | 2 (9) | 0 |
| Gabapentin | 2 (9) | 0 | 0 | 0 |
| Lamotrigine | 5 (22) | 5 (36) | 11 (50) | 4 (29) |
| Levetiracetam | 9 (39) | 3 (21) | 8 (36) | 4 (29) |
| Lorazepam | 2 (9) | 0 | 0 | 0 |
| Oxcarbazepine | 7 (30) | 2 (14) | 8 (36) | 1 (7) |
| Phenobarbital | 1 (4) | 0 | 2 (9) | 0 |
| Phenytoin | 2 (9) | 1 (7) | 1 (5) | 1 (7) |
| Pregabalin | 0 | 0 | 0 | 1 (7) |
| Topiramate | 6 (26) | 4 (29) | 3 (14) | 4 (29) |
| Zonisamide | 2 (9) | 0 | 1 (5) | 0 |
Seizures
Seizure Freedom in the Second Year of Follow-up
In the medical group, 19 participants provided seizure logs and all had seizures recorded during year 2. Four participants who withdrew provided no seizure logs for year 2. One participant in the medical group (who did not have surgery) was seizure free for the last 50 weeks of follow-up. In the surgical group, 14 participants provided seizure logs and 2 participants had seizures during year 2. In the primary analysis, which considered participants who did not have complete follow-up during year 2 as not seizure free, 0 of 23 in the medical group and 11 of 15 in the surgical group (73%) were seizure free (odds ratio [OR]=∞; 95% CI, 11.8 to ∞; P<.001). Analysis of only those participants who provided complete data in year 2 (or reported seizures in year 2) showed that 0 of 19 in the medical group (0%) vs 11 of 13 in the surgical group (85%) were seizure free (OR=∞; 95% CI, 14.8 to ∞; P< .001). The sensitivity analysis using multiple imputation yielded an estimated OR of 12.4 (95% CI, 2.6–59.2; P = .002).
Seizure Frequency
Seizure frequency over the 24 months of follow-up is shown in Table 3. Nine of the 11 participants in the surgical group who became free of disabling seizures never experienced a seizure after surgery; the other 2 participants last reported seizures 4 and 21 days after surgery Seizure-free participants were also free of auras. One participant in the surgical group last reported a seizure 10 months after surgery but was only observed for 7 months in the second postoperative year. The 2 participants in the surgical group who continued to have seizures in year 2 experienced substantial improvement in seizure frequency (Table 3).
Table 3.
Seizure Frequency During the 2-Year Study Period and Participant Disposition
| No. of Seizures by 3-mo Intervala |
Event, mo |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Participants | Baseline | 1–3 | 4–6 | 7–9 | 10–12 | 13–15 | 16–18 | 19–21 | 22–24 | Withdrawal | Surgery |
| Medical group (n = 23) | |||||||||||
| 101 | 9 | 10 | 21 | 10 | 9 | 2 | 0 | 2 | 0 | ||
| 109 | 3 | 7 | 1 | 0 | 2 | 1 | 0 | 0 | 0 | 15 | |
| 113 | 15 | 12 | 16 | 10 | 13 | 7 | 19 | 28 | 18 | ||
| 145 | 21 | 0 | 0 | 4 | 6 | 9 | 5 | 14 | 5 | ||
| 157 | 3 | 5 | 4 | 2 | 2 | 17 | |||||
| 173 | 3 | 90 | 31 | 9 | 12 | 12 | |||||
| 185 | 4 | 15 | 16 | 10 | 15 | 14 | 8 | 0 | 0 | 18 | |
| 189 | 6 | 4 | 0 | 0 | 2 | 0 | 8 | 4 | 1 | 22 | |
| 191 | 21 | 1 | 0 | 1 | 0 | 5 | 3 | 0 | 0 | ||
| 205 | 9 | 2 | 8 | 6 | 1 | 2 | 0 | 0 | 0 | ||
| 207 | 9 | 2 | 0 | 0 | 0 | 2 | 1 | 3 | 1 | ||
| 209 | 6 | 33 | 46 | 65 | 52 | 40 | 104 | 137 | 97 | ||
| 221 | 15 | 58 | 121 | 136 | 153 | 214 | 179 | 1 | 20 | 17 | |
| 237 | 63 | 18 | 14 | 4 | 9 | 12 | 4 | 212 | 7 | ||
| 253 | 21 | 11 | 22 | 22 | 17 | 11 | 6 | 0 | 0 | 16 | |
| 257 | 36 | 20 | 40 | 24 | 26 | 18 | 21 | 16 | 13 | ||
| 273 | 12 | 12 | 4 | 1 | 0 | 1 | 0 | 0 | 2 | ||
| 285 | 30 | 10 | 24 | 24 | 10 | 10 | |||||
| 321 | 4 | 25 | 8 | 0 | 1 | 1 | 0 | 2 | 5 | 22 | 5 |
| 338 | 15 | 13 | 13 | 3 | 4 | 13 | 13 | 7 | 9 | ||
| 350 | 9 | 1 | 29 | 2 | 2 | 1 | 2 | 3 | 0 | ||
| 352 | 3 | ||||||||||
| 381 | 9 | 0 | 2 | 0 | 0 | 0 | 1 | 0 | 2 | ||
| Surgical group (n = 15) | |||||||||||
| 110 | 6 | 1 | 1 | 1 | 1 | 0 | 4 | 3 | 1 | ||
| 114 | 6 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | ||
| 190 | 32 | 2 | |||||||||
| 206 | 3 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | ||
| 225b | 9 | 0 | 1 | 4 | 2 | 0 | 0 | 0 | 5c | ||
| 258 | 279 | 4 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | ||
| 259 | 6 | 0 | 0 | 0 | 0 | 0 | 1 | 2 | 3 | ||
| 269 | 3 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | ||
| 317 | 5 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | ||
| 333 | 3 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | ||
| 337 | 6 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | ||
| 349 | 10 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | ||
| 351 | 84 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | ||
| 382 | 9 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | ||
| 417 | 3 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | ||
Values are counts of disabling seizures over each 3-month period, with the time origin being randomization for the medical group and surgery for the surgical group. Unshaded table cells showing a value of zero indicate no seizures; cells shaded in gray indicate 1 to 4 seizures per month; cells shaded in blue indicate more than 4 seizures per month; and empty cells indicate no data collected on the seizure log.
Participant 225 had surgery delayed until month 5 and consequently was not observed for 2 full years after surgery.
Indicates a late surgery.
Quality of Life
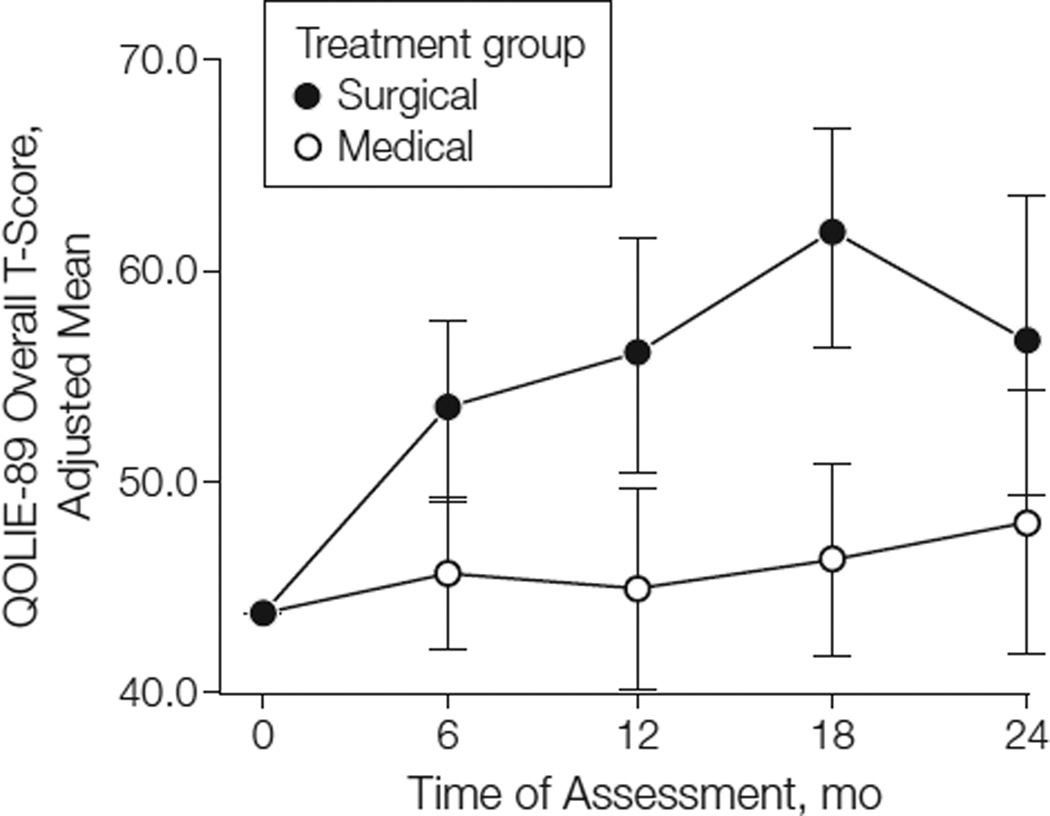
QOLIE-89 data were available for 36 participants who were at least 17 years old. Adjusted mean overall T-scores at each follow-up visit are shown by treatment group in Figure 2. In the intention-to-treat analyses, participants in the surgical group had significantly higher increases in health-related QOL than those in the medical group at months 6, 12, and 18 (P< .009; Figure 2), but not at month 24 (P = .08; Table 4). When excluding data obtained after surgery from participants in the medical group (n = 6), the effect of surgery on overall QOL was statistically significant at month 24 (P =.01). The effect of surgery on overall QOL was reflected in the Mental Health, Epilepsy-Targeted, and Cognitive subscales of the QOLIE-89, but not the Physical Health subscale. In exploratory analyses that further adjusted for age (continuous) and sex, the results were almost identical.
Figure 2.
Adjusted Mean QOLIE-89 Overall T-Score Over Time by Treatment Group
Bars indicate 95% CIs for the adjusted means. The adjusted means were obtained from a repeated-measures analysis of covariance model that included side of ictal onset and baseline Quality of Life in Epilepsy 89 (QOLIE-89) overall T-score as covariates.
Table 4.
Treatment Effects on Quality of Life Outcomes at Month 24a
| Variable | Mean Change from Baseline |
Treatment Effect (95% CI) |
P Value | |
|---|---|---|---|---|
| Medical | Surgical | |||
| QOLIE-89b Overall |
4.0 | 12.6 | 8.5 (−1.0 to 18.1) | .08 |
| Mental health | 1.9 | 11.1 | 9.2 (0.6 to 17.9) | .04 |
| Epilepsy targeted | 5.8 | 15.1 | 9.3 (0.2 to 18.3) | .04 |
| Cognitive | 0.4 | 7.8 | 7.4 (−1.0 to 15.9) | .08 |
| Physical health | 4.7 | 8.4 | 3.7 (−3.6 to 11.0) | .31 |
| QOLIE-89c Overall |
2.8 | 12.8 | 9.9 (2.2 to 17.7) | .01 |
| Mental health | 1.7 | 11.4 | 9.8 (2.7 to 16.9) | .009 |
| Epilepsy targeted | 5.1 | 15.5 | 10.4 (1.9 to 18.9) | .02 |
| Cognitive | 0.1 | 7.8 | 7.8 (0.9 to 14.7) | .03 |
| Physical health | 4.1 | 8.5 | 4.4 (−1.9 to 10.7) | .16 |
Abbreviations: QOLIE-89, Quality of Life in Epilepsy 89.
Values are mean changes from baseline adjusted for side of ictal onset and the baseline value of the outcome variable using a repeated-measures analysis of covariance model; see “Statistical Analysis.” Treatment effect refers to the difference in adjusted mean change between the surgical and medical groups.
All available data included, intention-to-treat analysis.
Data obtained after surgery excluded for medical group participants.
Cognitive Function
There were no significant treatment group differences with respect to the primary memory and nonmemory measures (eTable 1), although participants in the medical group tended to perform better on memory measures, particularly at month 24 (P =.08) (eTable 2). When individual tests were examined, the surgical group had lower performance than the medical group on the WMS-R22 Logical Memory Immediate (P =.01) and Delayed Recall (P =.02) tests at month 24. No significant treatment group differences were observed on the nonmemory measures. A higher percentage of participants in the surgical group than in the medical group had statistically reliable declines at month 12 in delayed verbal recall (RAVLT Delayed Recall23; 36% vs 0%; P =.03) and naming (Boston Naming Test24; 55% vs 7%; P =.02). Detailed neuropsychological test results are presented in the eTables 1–4
Ancillary Outcomes
At baseline, 7 of 23 participants in the medical group (30%) and 1 of 15 participants in the surgical group (7%) were driving by self-report, presumably against medical advice. Despite this imbalance, 5 of 23 participants in the medical group (22%) compared with 12 of 15 participants in the surgical group (80%) were driving at month 24 (OR, 14.4; 95% CI, 2.37–102.9; P< .001, Fisher exact test). For the 29 participants who contributed data at both baseline and month 24, participants in the surgical group reported a significant increase in the number of days per month socializing with friends (median increase of 6.5 days; interquartile range, 1–20 days) relative to the medical group (median decrease of 1.0 day; interquartile range, −6 to 2 days); the estimated treatment effect was 10 days (95% CI, 4–20 days; P =.002, Wilcoxon rank sum test). No treatment group differences were apparent with respect to employment status, hours per week worked, sick days reported, or socialization with family.
Adverse Events
A total of 13 serious adverse events (SAEs) (7 in the medical group and 6 in the surgical group) occurred in 9 participants (4 in the medical group 5 in the surgical group) during the study. Of the 7 SAEs in the medical group, 2 were considered unrelated to the study or to seizures (tonsillectomy and dehydration related to gastritis). Seizures were the underlying cause for the 5 remaining medical group SAEs (3 participants), including 3 cases of status epilepticus (2 participants). All of the medical group SAEs resolved without enduring sequelae.
Of the 6 SAEs that occurred in the surgical group, 3 were findings on postoperative MRI suggestive of ischemic changes, but only 1 was a cerebral infarction with clinical manifestations (mild impairment in naming and receptive language that fully resolved during the follow-up period). Although the other 2 MRI changes were distant from the surgical site, relation to the surgical procedures cannot be ruled out. One of these participants had medical risk factors for cerebral ischemia with multifocal subcortical infarctions on the preoperative MRI. Two other SAEs were postoperative complications (postoperative vomiting due to worsening of a preexisting esophageal dysmotility that required a gastrostomy and a communicating hydrocephalus attributed to resection-related bleeding into the sub-arachnoid space that resulted in placement of a ventriculoperitoneal shunt). One SAE was a shoulder dislocation and fracture due to a seizure during video-EEG monitoring.
COMMENT
Surgery was superior to pharmacotherapy for MTLE with respect to seizure outcome, and the data strongly suggested that surgery also improved QOL and ability and access to driving and socialization, despite the small number of participants. These results agree with the findings of the only other RCT of epilepsy surgery10 in which participants were more heterogeneous, had longstanding epilepsy, and underwent randomization prior to presurgical evaluation. We found that the benefit of surgery in newly intractable epilepsy is very large (allowing it to be demonstrated in a small randomized trial) and that patients who continue pharmacotherapy at this early stage of intractability have a very low likelihood of being seizure free during the second year, potentially increasing their risk for adverse psychological and social consequences and death. Seizure outcome was better than in the previous RCT10 and the meta-analysis contained in the American Academy of Neurology practice parameter,9 perhaps because our participants were a relatively homogeneous sample identified as surgical candidates prior to randomization. Our results, therefore, are not necessarily generalizable to patients with TLE who do not meet the strict inclusion criteria of this trial or who do not have surgery at level 4 epilepsy centers.25 Because only 2 participants in this study were younger than 17 years, and none were in the surgical group, it is unknown whether these results apply to adolescents with MTLE.
Early surgical treatment for pharmacoresistant epileptic seizures has been recommended to reduce the deleterious effects associated with these disabling events,26–31 including the risk of premature death.32,33 Although the mean age at enrollment for this study (34.3 years) was only slightly lower than in the Canadian RCT (35.0 years)10 and the Multicenter Study of Epilepsy Surgery (37.8 years, the largest and most representative sample of epilepsy surgery patients in the United States,34 the majority of whom had temporal lobe resections11), the mean duration of epilepsy in the current study (10.9 years) is considerably shorter than in those studies (19.7 and 22 years, respectively10,11). Thus, our cohort would appear to differ from the general population of MTLE surgical referrals only by their older age at the time of intractability, which likely reflects difficulties in recruitment of younger patients for surgical RCTs.15 The mean duration of epilepsy of 10.9 years in the current study fits well with prior data indicating that it takes an average of 9 years for 2 trials of AEDs to fail in patients,11 and that participants were enrolled within 2 years after failure of 2 AED trials.
There is concern that surgery early in the course of MTLE could produce cognitive deficits in otherwise cognitively intact patients. Conversely, continued epileptic seizures have a negative effect on cognitive function28–30 so deficits may develop later without surgery in any event, but this has not been adequately established. Given the important role of the hippocampus in memory, verbal memory deficits are to be expected following resection of the language-dominant mesial temporal lobe of participants with normal presurgical memory.35 There was no statistically significant treatment effect in the primary analysis of the memory outcomes, but the sample size was too small to permit a definitive conclusion that early surgery does not present a greater risk for cognitive disturbances than continued pharmacotherapy. Indeed, observed percentages of participants in the surgical group exhibiting reliable decline in verbal recall (36%) and naming (55%) were greater than those observed in participants in the medical group and were generally consistent with postsurgical rates reported in a recent meta-analysis.36
In keeping with other prospective studies, health-related QOL (HRQOL) improved early after surgery (month 6) and the improvement appeared to endure throughout the 2-year evaluation period,10,37 although the difference in HRQOL outcomes at month 24 was only significant when postsurgical data from participants in the medical group were excluded. The effect of surgery on HRQOL was mainly in the cognitive and psychosocial domains rather than physical function, ie, the domains most effected by epilepsy.
The treatment group differences in ancillary outcomes bode well for long-term psychosocial outcome in surgically treated patients. Lack of reliance on others for transport is a significant benefit for many patients.19 Having a driver’s license has been associated with improved employment outcomes after surgery.38 The lack of group differences in employment status and other outcomes could reflect the relatively short postoperative observational period, the early timing in the course of illness, the small sample size, or the absence of a true effect.
Patients are rarely referred to epilepsy centers soon after failure of 2 AED trials.4,33 Obtaining referrals from the community for this study was difficult, and half of those referred were excluded after the screening evaluation, which led to premature termination of the trial. Thirteen were excluded because of normal MRI and PET results. Because MTLE appears to be a progressive disorder,39 many patients with newly intractable limbic seizures, who might otherwise be considered surgical candidates, could have underlying lesions that cannot yet be identified on routine neuroimaging. Under standard protocols for presurgical evaluation, most of the patients who were excluded after screening in this study would undergo invasive monitoring with intracranial electrodes40; however, it was considered unethical to subject patients to an invasive investigation and then randomize them to the medical group for 2 additional years. Patients who present with findings consistent with a diagnosis of MTLE, have normal neuroimaging, and undergo invasive monitoring are likely to demonstrate a localized epileptogenic region, benefit from surgical resection, and have structural abnormalities in the resected tissue.40–42
The adverse events of surgery reported here were greater in number than has been reported in the literature,9 as would be expected in a prospective study. However, the small number of participants makes it difficult to assess the importance of this observation. Most of these were not associated with neurologic deficits and perhaps were only detected because the follow-up in this study was more detailed than might be the standard of practice. Of the 2 small infarctions seen on postoperative MRIs that were distant from the site of surgery, one occurred in the context of known cerebrovascular disease. The intracarotid amobarbital procedure also presents a risk of cerebral infarction. These SAEs underscore the need to continue to improve surgical technique, to more carefully consider the need for intracarotid amobarbital procedure, and to identify patients preoperatively who are at higher risk for stroke.
A limitation of this trial is that the outcomes of principal interest, seizures and QOL, were reported by the participants who could not be blinded to the intervention that they had received. We attempted to minimize bias by including a seizure adjudication committee (to assess whether or not events should be classified as epileptic) and a pharmacotherapy committee (to monitor the appropriateness of pharmacotherapy for each participant) that were both blinded to treatment assignment.
Only a small percentage of patients with medically intractable epilepsy are ever referred to an epilepsy center that offers surgery, and they are often referred too late for successful surgery to prevent serious disability.2,9,26–31 The reasons for this remain obscure.2,33,43 The data presented here reinforce the view that surgery soon after failure of 2 AED trials offers the best chance of preventing a lifetime of disability.9,26,27 The results of this study support the conclusions of the American Academy of Neurology practice parameter,9 namely that all patients with epilepsy should be referred to an epilepsy center as soon as trials of 2 AEDs fail, and surgery should be performed if patients meet criteria for an AMTR.
Supplementary Material
Acknowledgments
Drs Engel, Stern, Sperling, and Mintzer and Mss Gardiner and Jacobs report receipt of an institutional grant from the National Institutes of Health (NIH); and Drs McDermott, Langfitt, and Fried report receipt of an institutional grant from the National Institute of Neurological Disorders and Stroke (NINDS). Dr Engel reports receipt of consultancy fees from Medtronics, Valeant, Acorda, the US Food and Drug Administration, Best Doctors; receipt of fees to the institution for expert testimony; receipt of lecture/speakers bureau fees from Esai, Johnson & Johnson, Novartis, Lippincott; receipt of patent fees (WO2009/123734A1 and WO2009/ 123735A1); receipt of royalties from Wolters Kluwer, Wiley Blackwell, Elsevier, MedLink; and receipt of fees for travel accommodations/meeting expenses from UCB Pharmaceuticals. Dr McDermott reports receipt of fees to the institution for travel accommodations/ meeting expenses for this study from NINDS, fees to the institution for consultancy from Boehringer-Ingelheim and Pfizer; receipt of consultancy fees from Teva Pharmaceutical Industries, Smith and Nephew, Synosia, Impax Pharmaceuticals; grant support or pending grant support to the institution from Medivation, NeuroSearch Sweden AB, Boehringer-Ingelheim, Pfizer. Dr Wiebe reports grant support from NINDS, fees for travel accommodations/meeting expenses from NINDS; employment with the University of Calgary; grant support or pending grant support from the Canadian Institutes of Health Research; fees for travel accommodations/meeting expenses from the International League Against Epilepsy. Dr Langfitt reports receipt of fees for travel accommodations/meeting expenses from NIH; consultancy fees from NINDS, UCSF/ Elekta, Northern Illinois University, University of Cincinnatti, NIH CSR (Center for Scientific Review); grant support or pending grant support from NINDS and the Agency for Healthcare Research and Quality; fees to the institution for development of educational presentations from NIH. Dr Stern reports receipt of fees for travel accommodations/meeting expenses from NIH. Dr Sperling reports receipt of consultancy fees from UCB, Vertex, Sunovion; grant support or pending grant support from NIH, UCP, Vertex, Eisai, Sunovion, Medtronics, Neuropace, SK Life Sciences, Novartis, Neuronex; lecture/speakers bureau fees from UCB; other fees as associate editor from Epilepsia.
Funding/Support: This study was supported by the National Institutes of Health (grant numbers R21 NS37897 and U01 NS42372).
Role of the Sponsor: This study was funded as a cooperative agreement with the National Institute of Neurological Disorders and Stroke, whose representative (Ms Jacobs) was involved in the design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, and approval of the manuscript. The study’s biostatistician (Dr McDermott) from the University of Rochester performed or supervised, and is responsible for, all statistical analyses for this study.
Appendix
ERSET Study Group: David Blum, MD, Steven Chung, MD, and David Treiman, MD, Barrow Neurological Institute, Phoenix, AZ; Erasmo A. Passaro, MD, Bayfront Medical Center, St Petersburg, FL; David R. Browne, MD, School of Medicine, Boston University, Boston, MA; Nancy Foldvary-Schaefer, DO, MS, Cleveland Clinic Neurological Institute, Cleveland, OH; Robert Goodman, MD, PhD, W. Allen Hauser, MD, and Martha J. Morrell, MD, Columbia University, New York, NY; Thomas R. Henry, MD, Raghuveer Krishna Halkar, MD, and Patricia A. Hudgins, MD, FACR, Emory University, Atlanta, GA; Andrew J. Cole, MD, FRCP(C), and Daniel B. Hoch, MD, PhD, Harvard Medical School and Massachusetts General Hospital, Boston, MA; Steven C. Schachter, MD, Harvard Medical School and Beth Israel Deaconess Medical Center, Boston, MA; Vicenta Salanova, MD, FAAN, Indiana University, Indianapolis; Gregory L. Krauss, MD, Johns Hopkins University, Baltimore, MD; Thaddeus S. Walczak, MD, MINCEP Epilepsy Care, Minneapolis, MN; Prashanthi Boppana, MD, Northwestern University, Evanston, IL; L. James Willmore, MD, St Louis University, St Louis, MO; John J. Barry, MD, Robert S. Fisher, MD, PhD, and Michael W. Risinger, MD, Stanford University, Stanford, CA; Robert Beach, MD, PhD, FAAN, State University of New York, Syracuse; Diana A. Kraemer, MD, and David G. Vossler, MD, FAAN, Swedish Medical Center, Seattle, WA; Rochelle Caplan, MD, William E. Cunningham, MD, PhD, Faustino Lopez-Rodriguez, MD, PhD, Gary Mathern, MD, W. Donald Shields, MD, Jason R. Soss, MD, Karleen Swarztrauber, MD, PhD, and Barbara G. Vickrey, MD, MPH, University of California, Los Angeles; Henry A. Buchtel, PhD, Daniela N. Minecan, MD, and Oren Sagher, MD, University of Michigan, Ann Arbor; Jerry J. Shih, MD, University of New Mexico, Albuquerque; Jacqueline A. French, MD, University of Pennsylvania, Philadelphia; Michel Berg, MD, James Burchfiel, PhD, Catherine Covert, MA, Arthur Watts, BS, and Earl Westerlund, University of Rochester, Rochester, NY; Paul C. Van Ness, MD, University of Texas Southwestern Medical Center, Dallas; Nathan B. Fountain, MD, University of Virginia, Charlottesville; Bassel Abou-Khalil, MD, Vanderbilt University Medical Center, Nashville, TN; John M. ( Jack) Pellock, MD, Virginia Commonwealth University, Richmond; Frank Gilliam, MD, MPH, Washington University, St Louis, MO; and Dennis Spencer, MD, Yale University School of Medicine, New Haven, CT.
Footnotes
Author Contributions: Dr Engel had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Engel, McDermott, Wiebe, Langfitt, Dewar, Sperling, Gardiner, Erba, Fried, Jacobs, Kieburtz.
Acquisition of data: Engel, Wiebe, Stern, Dewar, Sperling, Fried, Vinters, Mintzer.
Analysis and interpretation of data: Engel, McDermott, Wiebe, Langfitt, Stern, Sperling, Fried, Mintzer, Kieburtz.
Drafting of the manuscript: Engel, Wiebe, Langfitt, Stern, Sperling, Fried, Kieburtz.
Critical revision of the manuscript for important intellectual content: Engel, McDermott, Wiebe, Langfitt, Stern, Dewar, Sperling, Gardiner, Erba, Fried, Jacobs, Vinters, Mintzer, Kieburtz.
Statistical analysis: McDermott, Wiebe, Langfitt.
Obtained funding: Engel, Langfitt, Jacobs, Vinters, Kieburtz.
Administrative, technical, or material support: Engel, Stern, Dewar, Gardiner, Fried, Jacobs, Kieburtz.
Study supervision: Engel, Wiebe, Dewar, Erba, Fried, Kieburtz.
Conflict of Interest Disclosures: All authors have completed and submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Mss Gardiner and Dewar and Dr Erba report no disclosures.
Online-Only Material: eAppendix, eTables 1–4, and eReferences are available at http://www.jama.com.
Additional Contributions: Analyses of the cognitive outcomes were completed by Neil Osuch, MS, in partial fulfillment of a master’s degree in medical statistics at the University of Rochester. Hua He, PhD, performed the sensitivity analysis of the primary outcome variable using multiple imputation. These individuals were not compensated for their contributions to this article.
REFERENCES
- 1.Murray GJL, Lopez AD. Global Comparative Assessments in the Health Sector: Disease Burden, Expenditure, Intervention Packages. Geneva, Switzerland: World Health Organization; 1994. [Google Scholar]
- 2.Berg AT. Understanding the delay before epilepsy surgery: who develops intractable focal epilepsy and when? CNS Spectr. 2004;9(2):136–144. doi: 10.1017/s109285290000849x. [DOI] [PubMed] [Google Scholar]
- 3.Begley CE, Famulari M, Annegers JF, et al. The cost of epilepsy in the United States: an estimate from population-based clinical and survey data. Epilepsia. 2000;41(3):342–351. doi: 10.1111/j.1528-1157.2000.tb00166.x. [DOI] [PubMed] [Google Scholar]
- 4.Berg AT, Shinnar S, Levy SR, Testa FM. Newly diagnosed epilepsy in children: presentation at diagnosis. Epilepsia. 1999;40(4):445–452. doi: 10.1111/j.1528-1157.1999.tb00739.x. [DOI] [PubMed] [Google Scholar]
- 5.Jallon P, Loiseau P, Loiseau J. Newly diagnosed unprovoked epileptic seizures: presentation at diagnosis in CAROLE study: Coordination Active du Réseau Observatoire Longitudinal de l’ Epilepsie. Epilepsia. 2001;42(4):464–475. doi: 10.1046/j.1528-1157.2001.31400.x. [DOI] [PubMed] [Google Scholar]
- 6.Semah F, Picot M-C, Adam C, et al. Is the underlying cause of epilepsy a major prognostic factor for recurrence? Neurology. 1998;51(5):1256–1262. doi: 10.1212/wnl.51.5.1256. [DOI] [PubMed] [Google Scholar]
- 7.Engel J., Jr Etiology as a risk factor for medically refractory epilepsy: a case for early surgical intervention. Neurology. 1998;51(5):1243–1244. doi: 10.1212/wnl.51.5.1243. [DOI] [PubMed] [Google Scholar]
- 8.Engel J, Jr, Shewmon DA. Overview: who should be considered a surgical candidate? In: Engel J Jr, editor. Surgical Treatment of the Epilepsies. 2nd. New York, NY: Raven Press; 1993. pp. 23–34. [Google Scholar]
- 9.Engel J, Jr, Wiebe S, French J, et al. Quality Standards Subcommittee of the American Academy of Neurology; American Epilepsy Society; American Association of Neurological Surgeons. Practice parameter: temporal lobe and localized neocortical resections for epilepsy: report of the Quality Standards Subcommittee of the American Academy of Neurology, in association with the American Epilepsy Society and the American Association of Neurological Surgeons. Neurology. 2003;60(4):538–547. doi: 10.1212/01.wnl.0000055086.35806.2d. [DOI] [PubMed] [Google Scholar]
- 10.Wiebe S, Blume WT, Girvin JP, Eliasziw M. Effectiveness Efficiency of Surgery for Temporal Lobe Epilepsy Study Group. A randomized, controlled trial of surgery for temporal-lobe epilepsy. N Engl J Med. 2001;345(5):311–318. doi: 10.1056/NEJM200108023450501. [DOI] [PubMed] [Google Scholar]
- 11.Berg AT, Langfitt J, Shinnar S, et al. How long does it take for partial epilepsy to become intractable? Neurology. 2003;60(2):186–190. doi: 10.1212/01.wnl.0000031792.89992.ec. [DOI] [PubMed] [Google Scholar]
- 12.Kwan P, Arzimanoglou A, Berg AT, et al. Definition of drug-resistant epilepsy: consensus proposal by the ad hoc task force of the ILAE Commission on Therapeutic Strategies. Epilepsia. 2010;51(6):1069–1077. doi: 10.1111/j.1528-1167.2009.02397.x. [DOI] [PubMed] [Google Scholar]
- 13.Haneef Z, Stern J, Dewar S, Engel J., Jr Referral pattern for epilepsy surgery after evidence-based recommendations: a retrospective study. Neurology. 2010;75(8):699–704. doi: 10.1212/WNL.0b013e3181eee457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Engel J., Jr The goal of epilepsy therapy: no seizures, no side effects, as soon as possible. CNS Spectr. 2004;9(2):95–97. doi: 10.1017/s1092852900008452. [DOI] [PubMed] [Google Scholar]
- 15.Engel J, Jr, McDermott MP, Wiebe S, et al. Early Randomized Surgical Epilepsy Trial (ERSET) Study Group. Design considerations for a multicenter randomized controlled trial of early surgery for mesial temporal lobe epilepsy. Epilepsia. 2010;51(10):1978–1986. doi: 10.1111/j.1528-1167.2010.02641.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Engel J, Jr, Williamson PD, Wieser HG. Mesial temporal lobe epilepsy with hippocampal sclerosis. In: Engel J Jr, Pedley TA, editors. Epilepsy: a Comprehensive Textbook. 2nd. Philadelphia, PA: Lippincott Williams & Wilkins; 2008. pp. 2479–2486. [Google Scholar]
- 17.Wieser HG. ILAE Commission on Neurosurgery of Epilepsy ILAE Commission Report: mesial temporal lobe epilepsy with hippocampal sclerosis. Epilepsia. 2004;45(6):695–714. doi: 10.1111/j.0013-9580.2004.09004.x. [DOI] [PubMed] [Google Scholar]
- 18.Spencer DD, Spencer SS, Mattson RH, Williamson PD, Novelly RA. Access to the posterior medial temporal lobe structures in the surgical treatment of temporal lobe epilepsy. Neurosurgery. 1984;15(5):667–671. doi: 10.1227/00006123-198411000-00005. [DOI] [PubMed] [Google Scholar]
- 19.Devinsky O, Vickrey BG, Cramer J, et al. Development of the Quality of Life in Epilepsy Inventory. Epilepsia. 1995;36(11):1089–1104. doi: 10.1111/j.1528-1157.1995.tb00467.x. [DOI] [PubMed] [Google Scholar]
- 20.Cramer JA, Westbrook LE, Devinsky O, Perrine K, Glassman MB, Camfield C. Development of the Quality of Life in Epilepsy Inventory for Adolescents: the QOLIE-AD-48. Epilepsia. 1999;40(8):1114–1121. doi: 10.1111/j.1528-1157.1999.tb00828.x. [DOI] [PubMed] [Google Scholar]
- 21.O’Brien PC. Procedures for comparing samples with multiple endpoints. Biometrics. 1984;40(4):1079–1087. [PubMed] [Google Scholar]
- 22.Wechsler D. Wechsler Memory Scale-Revised. San Antonio, TX: Psychological Corp; 1987. [Google Scholar]
- 23.Schmidt M. Rey Auditory Verbal Learning Test. Los Angeles, CA: Western Psycholgical Services; 1996. [Google Scholar]
- 24.Kaplan E, Goodglass H, Weintraub S. The Boston Naming Test. 2nd. Philadelphia, PA: Lippincott Williams & Wilkins; 2001. [Google Scholar]
- 25.Gumnit RJ, Walczak TS. National Association of Epilepsy Centers Guidelines for essential services, personnel, and facilities in specialized epilepsy centers in the United States. Epilepsia. 2001;42(6):804–814. doi: 10.1046/j.1528-1157.2001.08701.x. [DOI] [PubMed] [Google Scholar]
- 26.Engel J., Jr Finally, a randomized, controlled trial of epilepsy surgery. N Engl J Med. 2001;345(5):365–367. doi: 10.1056/NEJM200108023450510. [DOI] [PubMed] [Google Scholar]
- 27.Engel J., Jr Surgical treatment for epilepsy: too little, too late? JAMA. 2008;300(21):2548–2550. doi: 10.1001/jama.2008.756. [DOI] [PubMed] [Google Scholar]
- 28.Gilliam FG, Albertson B. Identifying epilepsy surgery candidates in the outpatient clinic. Epilepsy Behav. 2011;20(2):156–159. doi: 10.1016/j.yebeh.2010.12.022. [DOI] [PubMed] [Google Scholar]
- 29.Jacoby A, Baker GA, Steen N, Potts P, Chadwick DW. The clinical course of epilepsy and its psychosocial correlates: findings from a UK community study. Epilepsia. 1996;37(2):148–161. doi: 10.1111/j.1528-1157.1996.tb00006.x. [DOI] [PubMed] [Google Scholar]
- 30.Jokeit H, Ebner A. Effects of chronic epilepsy on intellectual functions. Prog Brain Res. 2002;135:455–463. doi: 10.1016/S0079-6123(02)35042-8. [DOI] [PubMed] [Google Scholar]
- 31.Choi H, Sell RL, Lenert L, et al. Epilepsy surgery for pharmacoresistant temporal lobe epilepsy: a decision analysis. JAMA. 2008;300(21):2497–2505. doi: 10.1001/jama.2008.771. [DOI] [PubMed] [Google Scholar]
- 32.Sperling MR. The consequences of uncontrolled epilepsy. CNS Spectr. 2004;9(2):98–101. doi: 10.1017/s1092852900008464. 106ȓ109. [DOI] [PubMed] [Google Scholar]
- 33.Sperling MR, Feldman H, Kinman J, Liporace JD, O’Connor MJ. Seizure control and mortality in epilepsy. Ann Neurol. 1999;46(1):45–50. doi: 10.1002/1531-8249(199907)46:1<45::aid-ana8>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 34.Berg AT, Vickrey BG, Langfitt JT, et al. Multicenter Study of Epilepsy Surgery. The multicenter study of epilepsy surgery: recruitment and selection for surgery. Epilepsia. 2003;44(11):1425–1433. doi: 10.1046/j.1528-1157.2003.24203.x. [DOI] [PubMed] [Google Scholar]
- 35.Seidenberg M, Hermann B, Wyler AR, Davies K, Dohan FC, Jr, Leveroni C. Neuropsychological outcome following anterior temporal lobectomy in patients with and without the syndrome of mesial temporal lobe epilepsy. Neuropsychology. 1998;12(2):303–316. doi: 10.1037//0894-4105.12.2.303. [DOI] [PubMed] [Google Scholar]
- 36.Sherman EM, Wiebe S, Fay-McClymont TB, et al. Neuropsychological outcomes after epilepsy surgery: systematic review and pooled estimates. Epilepsia. 2011;52(5):857–869. doi: 10.1111/j.1528-1167.2011.03022.x. [DOI] [PubMed] [Google Scholar]
- 37.Spencer SS, Berg AT, Vickrey BG, et al. Multi-center Study of Epilepsy Surgery. Health-related quality of life over time since resective epilepsy surgery. Ann Neurol. 2007;62(4):327–334. doi: 10.1002/ana.21131. [DOI] [PubMed] [Google Scholar]
- 38.Reeves AL, So EL, Evans RW, et al. Factors associated with work outcome after anterior temporal lobectomy for intractable epilepsy. Epilepsia. 1997;38(6):689–695. doi: 10.1111/j.1528-1157.1997.tb01238.x. [DOI] [PubMed] [Google Scholar]
- 39.Cendes F. Progressive hippocampal and extra-hippocampal atrophy in drug-resistant epilepsy. Curr Opin Neurol. 2005;18(2):173–177. doi: 10.1097/01.wco.0000162860.49842.90. [DOI] [PubMed] [Google Scholar]
- 40.Spencer SS, Sperling MR, Shewmon DA, et al. In-tracranial electrodes. In: Engel J Jr, Pedley TA, editors. Epilepsy: a Comprehensive Textbook. 2nd. Philadelphia, PA: Lippincott Williams & Wilkins; 1791–1815. [Google Scholar]
- 41.Alarcoón G, Valentín A, Watt C, et al. Is it worth pursuing surgery for epilepsy in patients with normal neuroimaging? J Neurol Neurosurg Psychiatry. 2006;77(4):474–480. doi: 10.1136/jnnp.2005.077289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Immonen A, Jutila L, Muraja-Murro A, et al. Long-term epilepsy surgery outcomes in patients with MRI-negative temporal lobe epilepsy. Epilepsia. 2010;51(11):2260–2269. doi: 10.1111/j.1528-1167.2010.02720.x. [DOI] [PubMed] [Google Scholar]
- 43.Swarztrauber K, Dewar S, Engel J., Jr Patient attitudes about treatments for intractable epilepsy. Epilepsy Behav. 2003;4(1):19–25. doi: 10.1016/s1525-5050(02)00687-x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.