Jang et al. show that eosinophils in the small intestine can suppress Th17 cell differentiation through the secretion of the IL-1 receptor antagonist.
Abstract
Eosinophils play proinflammatory roles in helminth infections and allergic diseases. Under steady-state conditions, eosinophils are abundantly found in the small intestinal lamina propria, but their physiological function is largely unexplored. In this study, we found that small intestinal eosinophils down-regulate Th17 cells. Th17 cells in the small intestine were markedly increased in the ΔdblGATA-1 mice lacking eosinophils, and an inverse correlation was observed between the number of eosinophils and that of Th17 cells in the small intestine of wild-type mice. In addition, small intestinal eosinophils suppressed the in vitro differentiation of Th17 cells, as well as IL-17 production by small intestinal CD4+ T cells. Unlike other small intestinal immune cells or circulating eosinophils, we found that small intestinal eosinophils have a unique ability to constitutively secrete high levels of IL-1 receptor antagonist (IL-1Ra), a natural inhibitor of IL-1β. Moreover, small intestinal eosinophils isolated from IL-1Ra−deficient mice failed to suppress Th17 cells. Collectively, our results demonstrate that small intestinal eosinophils play a pivotal role in the maintenance of intestinal homeostasis by regulating Th17 cells via production of IL-1Ra.
The small intestinal lamina propria (LP) contains a variety of immune cells. These include Th17 cells, a subset of activated CD4+ T cells characterized by the production of IL-17A, IL-17F, IL-21, and IL-22 (Korn et al., 2009). Th17 cells have the potential to protect or damage the intestinal tissue environment, so their activity must be tightly regulated (O’Connor et al., 2009; Morrison et al., 2011). Several cytokines are known to promote the development of Th17 cells; IL-6 and TGF-β are required for the differentiation of Th17 cells from naive CD4+ T cells, and IL-1β and IL-23 are critical for the maintenance of Th17 cells, as well as their differentiation (Zhou et al., 2007; Chung et al., 2009). Commensal bacteria contribute to the generation of small intestinal Th17 cells in the steady state (Atarashi et al., 2008, 2015; Ivanov et al., 2009). In particular, commensal-induced IL-1β production by intestinal macrophages is required for the development of Th17 cells (Shaw et al., 2012).
IL-1β is a proinflammatory cytokine primarily produced by activated macrophages and acts as a key mediator in various inflammatory diseases, including inflammatory bowel disease and rheumatoid arthritis (Sims and Smith, 2010). Consequently, mice deficient for IL-1 receptor antagonist (IL-1Ra), which competes with IL-1β for receptor binding, spontaneously develop arthritis with a marked increase in Th17 cells (Nakae et al., 2003; Koenders et al., 2008). In humans, a decrease in the IL-1Ra to IL-1 ratio has been linked to inflammatory bowel disease (Casini-Raggi et al., 1995). IL-1Ra secreted by intestinal epithelial cells upon TLR5 activation reduces tissue damage (Carvalho et al., 2011), and treatment with recombinant IL-1Ra ameliorates intestinal graft-versus-host disease by inhibiting Th17 responses (Jankovic et al., 2013). Thus, the balance between IL-1β and IL-1Ra is critical for controlling Th17 cells and maintaining intestinal immune homeostasis.
Eosinophils are commonly known as proinflammatory cells, mediating the host responses against helminth infections, as well as the pathogenesis of various allergic diseases and gastrointestinal disorders (Rothenberg and Hogan, 2006). However, recent studies found that eosinophils also play various roles in maintaining homeostasis, such as supporting glucose homeostasis by sustaining alternatively activated macrophages in adipose tissue (Wu et al., 2011) and promoting the generation and survival of plasma cells (Chu et al., 2014b; Jung et al., 2015). Under steady-state conditions, eosinophils develop in the bone marrow and migrate primarily to the gastrointestinal tract (Mishra et al., 1999; Rothenberg and Hogan, 2006). Small intestinal eosinophils have unique phenotypes and extended life spans (Carlens et al., 2009; Verjan Garcia et al., 2011). However, their function under healthy homeostatic conditions remains to be fully elucidated.
In this study, we show that small intestinal eosinophils down-regulate Th17 cells by constitutively secreting a large amount of IL-1Ra. We found a decrease in serum IL-1Ra and a concomitant increase in small intestinal Th17 cells in ΔdblGATA-1 mice, which lack eosinophil-lineage cells (Yu et al., 2002). In WT mice, the number of Th17 cells in the small intestine was inversely correlated with that of eosinophils. Furthermore, eosinophils isolated from the small intestine of WT mice, but not of IL-1Ra–deficient mice, inhibited the Th17 cells. Our findings demonstrate a hitherto unappreciated role of small intestinal eosinophils to regulate intestinal homeostasis by controlling Th17 cells.
RESULTS
Small intestinal Th17 cells are increased in eosinophil-deficient mice
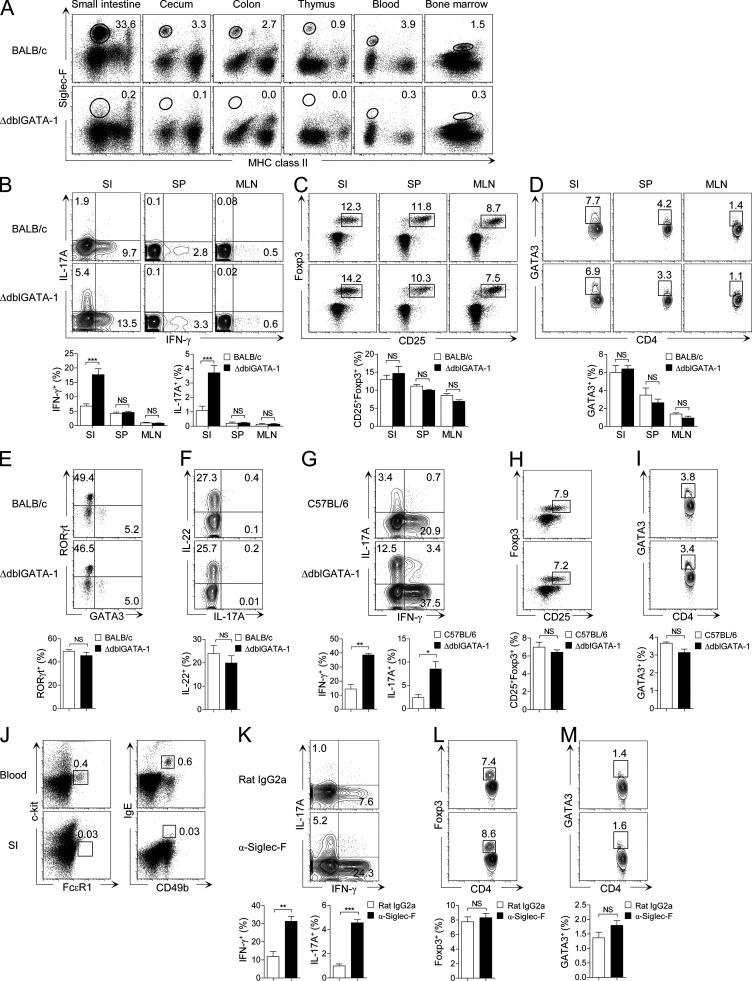
Eosinophils accumulate most abundantly in the small intestine under steady-state conditions and are absent in ΔdblGATA-1 mice (Fig. 1 A). To explore the role of eosinophils in the small intestinal immune system, we analyzed T cells in ΔdblGATA-1 mice. As shown in Fig. 1 B, Th1 and Th17 cells in the small intestine, but not in the spleen or mesenteric LNs (MLNs), were significantly increased in ΔdblGATA-1 mice on a BALB/c background. On the other hand, CD25+Foxp3+ regulatory T (T reg) cells, GATA3+ Th2 cells, and RORγt+ innate lymphoid cells (ILC3s) were not affected by the absence of eosinophils (Fig. 1, C–F). We also observed the same phenomena in the ΔdblGATA-1 mice on a C57BL/6 background (Fig. 1, G–I).
Figure 1.
Small intestinal Th17 and Th1 cells are increased in eosinophil-deficient mice. (A) Flow cytometry analysis of the frequency of MHC class IIneg/low Siglec-Fhigh eosinophils in various tissues from WT BALB/c or eosinophil-deficient (ΔdblGATA-1) mice. (B–D) Flow cytometry analysis of IFN-γ+ and IL-17A+ (B), CD25+Foxp3+ (C), and GATA3+ (D) CD4+ T cells in the small intestine (SI), spleen (SP), and MLN from ΔdblGATA-1 or littermate control mice (BALB/c). FACS plots show cells gated on TCRβ+CD4+ cells. Data in B and C are representative of at least 10 independent experiments (n = 3–5 mice per group per experiment), and data in D are representative of four independent experiments (n = 3–5 mice per group per experiment). (E and F) Flow cytometry analysis of RORγt+ and GATA3+ (E) and IL-22+ (F) innate lymphoid cells in the small intestine of ΔdblGATA-1 or littermate control mice (BALB/c). FACS plots show cells gated on Thy1.2+lineage− (CD3−CD4−CD8α−CD11b−CD11c−CD19−B220−TER119−Gr-1−NK1.1−) cells and are representative of four independent experiments (n = 2–4 mice per group per experiment). (G–I) Frequency of IFN-γ+ and IL-17A+ (G), CD25+Foxp3+ (H), and GATA3+ (I) CD4+ T cells in the small intestine of ΔdblGATA-1 or littermate control mice (C57BL/6). ΔdblGATA-1 mice backcrossed with C57BL/6 for at least 10 generations were used. FACS plots show cells gated on TCRβ+CD4+ cells and are representative of at least three independent experiments (n = 3–4 mice per group per experiment). (J) Flow cytometry analysis of the frequency of c-kit−FcεR1+ and IgE+CD49b+ basophils in peripheral blood and the small intestine. (K–M) Frequency of IFN-γ+ and IL-17A+ (K), Foxp3+ (L), and GATA3+ (M) CD4+ T cells in the small intestine of C57BL/6 mice administered every second day with Siglec-F–specific antibodies or isotype control (Rat IgG2a) for 2 wk. FACS plots show cells gated on TCRβ+CD4+ cells and are representative of two independent experiments (n = 4 mice per group per experiment). Bar graphs show the mean ± SEM. *, P < 0.05; **, P < 0.01; ***, P < 0.001. NS, not significant (unpaired Student’s t test).
A recent publication suggested that ΔdblGATA-1 mice also showed defects in the generation and function of basophils (Nei et al., 2013). This is unlikely to account for the aforementioned findings, as basophils constitute a rare population (<0.03%) of leukocytes in the small intestinal LP under steady-state conditions (Fig. 1 J). Nonetheless, to rule out potential effects mediated by noneosinophil defects in ΔdblGATA-1 mice, we specifically depleted eosinophils in WT C57BL/6 mice by injecting them with Siglec-F–specific antibodies every other day for 2 wk (Chu et al., 2014b; Griseri et al., 2015). We found an increase in small intestinal Th1 and Th17 cells (Fig. 1 K), but not T reg or Th2 cells (Fig. 1, L and M), in these mice, mirroring our observations in ΔdblGATA-1 mice. Collectively, these results indicate that eosinophils negatively influence Th1 and Th17 cell homeostasis in the small intestine.
Small intestinal eosinophils suppress Th17 cells
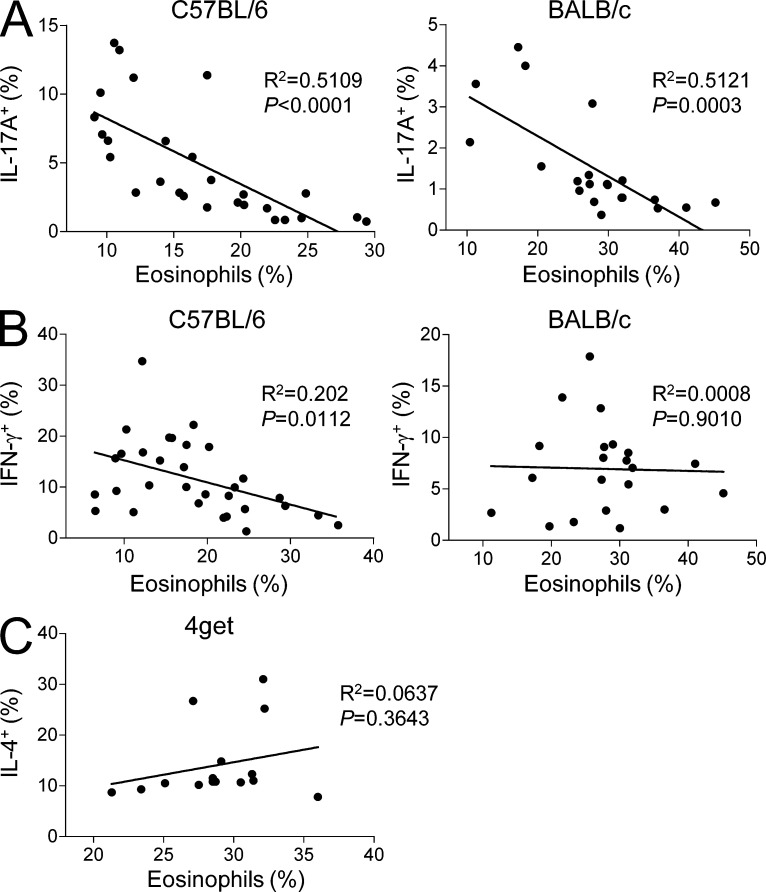
To further investigate a possible connection between eosinophils and small intestinal Th1/Th17 cells, we analyzed for a correlation between the frequency of eosinophils and that of Th1/Th17 cells in the small intestine of individual WT C57BL/6 and BALB/c mice. Strikingly, we found a strong inverse correlation between the frequency of eosinophils and that of Th17 cells in both mouse strains (Fig. 2 A). The frequency of Th1 cells was also weakly inverse-correlated with eosinophils in C57BL/6 mice, but not in BALB/c mice (Fig. 2 B). We also performed correlational analysis between the frequency of eosinophils and that of Th2 cells in the small intestine using IL-4/GFP reporter (4get) mice and found no statistically significant correlation between them under steady-state conditions (Fig. 2 C).
Figure 2.
Eosinophils are inversely correlated with Th17 cells in the small intestine. (A and B) Graphs show the correlation between the percentages of eosinophils and IL-17A+ Th17 (A) and IFN-γ+ Th1 cells (B) in the small intestine from C57BL/6 or BALB/c mice (n = 27 of C57BL6 [A] and 31 of C57BL/6 [B], and 21 of BALB/c [A] and 22 of BALB/c [B], respectively). (C) Graph shows the correlation between the percentages of IL-4+ Th2 cells and eosinophils in the small intestine from 4get mice (n = 15). The correlation coefficient was calculated by Prism software (GraphPad Software). Each dot represents one mouse. Mice with various ages (6–20 wk) and sex (female and male) were examined.
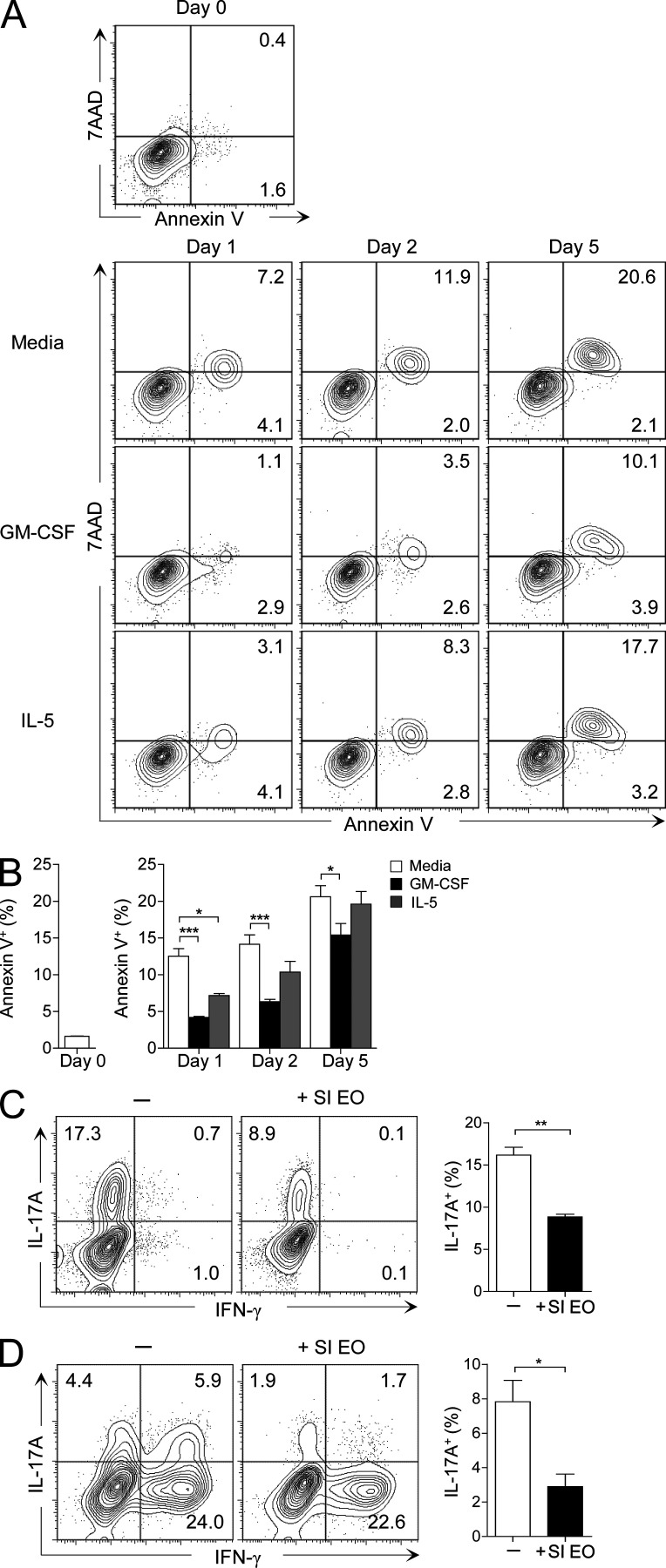
To determine whether small intestinal eosinophils suppress Th17 cell development, we induced in vitro differentiation of Th17 cells from naive CD4+ T cells in the presence or absence of eosinophils purified from the small intestine (Fig. S1). Along with TGF-β, IL-6, IL-1β, and IL-23, which are conventionally used to induce Th17 cell differentiation, we added granulocyte macrophage colony-stimulating factor (GM-CSF) to promote eosinophil survival during the culture. GM-CSF was more effective than IL-5 in maintaining the small intestinal eosinophils alive (Fig. 3, A and B). As shown in Fig. 3 C, the addition of small intestinal eosinophils significantly inhibited the Th17 cell differentiation in vitro. Furthermore, small intestinal eosinophils suppressed IL-17A production by small intestinal CD4+ T cells in vitro (Fig. 3 D). These data suggest that small intestinal eosinophils suppress the differentiation and/or maintenance of Th17 cells.
Figure 3.
Small intestinal eosinophils suppress Th17 cells in vitro. (A) Small intestinal LP cells were cultured for 1, 2, and 5 d with or without GM-CSF (10 ng/ml) or IL-5 (10 ng/ml), and then stained with 7AAD and Annexin V for analysis of cell viability. FACS plots show eosinophils (MHCII−Siglec-F+ cells) and are representative of two independent experiments. (B) Bar graphs show the mean ± SEM of the percentage of Annexin V+ eosinophils. *, P < 0.05; ***, P < 0.001 (two-way ANOVA followed by Bonferroni post-test). (C) LN-derived naive CD4+ T cells (105) and splenic DCs (104) were cultured with or without small intestinal eosinophils (SI EO; 105) in the presence of soluble anti-CD3/CD28 (5 and 2 µg/ml, respectively), neutralizing anti–IFN-γ (5 µg/ml), neutralizing anti–IL-4 (5 µg/ml), neutralizing anti–IL-2 (2.5 µg/ml), GM-CSF (10 ng/ml), TGF-β (5 ng/ml), IL-6 (20 ng/ml), IL-1β (20 ng/ml), and IL-23 (10 ng/ml). On day 5, the cells were collected and intracellular cytokine staining for IFN-γ and IL-17A in CD4+ T cells was analyzed by flow cytometry. (D) Small intestinal CD4+CD25− T cells (105) and splenic DCs (104) were cultured with or without small intestinal eosinophils (105), as in C, except for the omission of IL-6. On day 5, the cells were analyzed for the production of IFN-γ and IL-17A, as in C. FACS plots are gated on CD4+ T cells and are representative of at least three similar experiments. Bar graphs show the mean ± SEM. *, P < 0.05; **, P < 0.01 (unpaired Student’s t test).
Small intestinal eosinophils secrete high levels of IL-1Ra
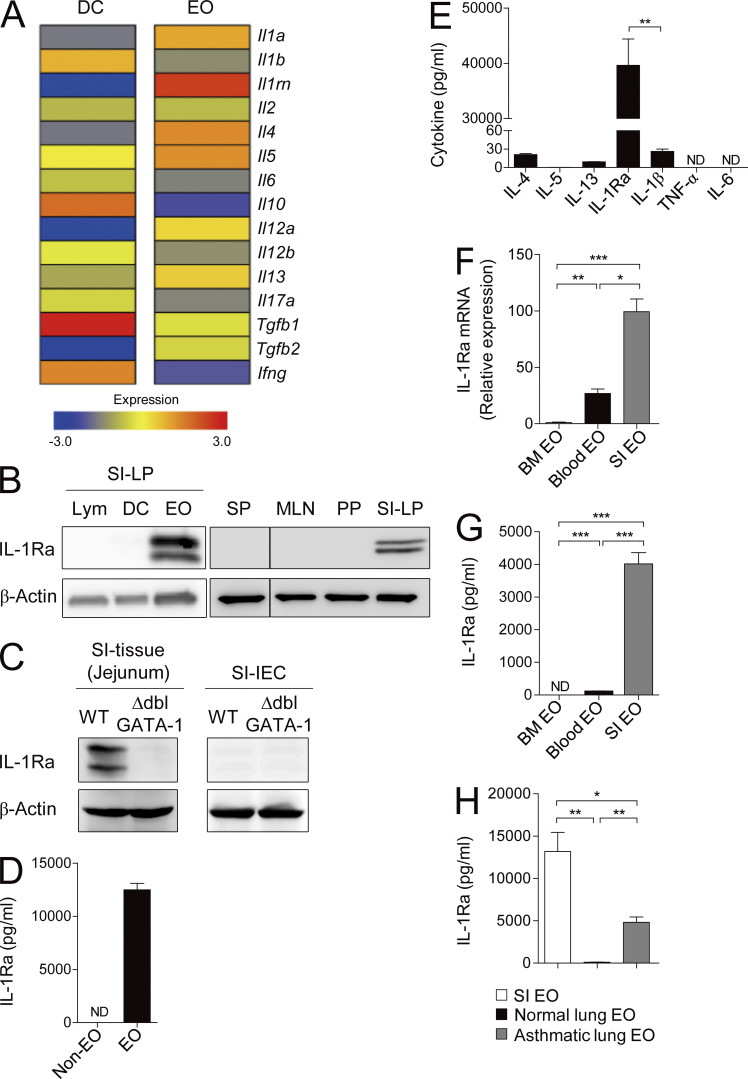
To explore the factors mediating the suppressive function of small intestinal eosinophils, we compared gene expression profiles of eosinophils and DCs from the small intestine by conducting a genome-wide cDNA microarray analysis. Surprisingly, we found that small intestinal eosinophils, but not DCs, strongly express IL-1Ra, which is encoded by Il1rn (Fig. 4 A). IL-1Ra antagonizes IL-1α/β by competitively binding to their receptor IL-1R and is known to be secreted mainly by epithelial cells and mononuclear cells (Garlanda et al., 2013). We confirmed the expression of IL-1Ra in small intestinal eosinophils using immunoblotting and ELISA. In contrast to eosinophils, IL-1Ra was undetectable in lymphocytes and DCs from small intestine and in leukocytes from spleen or other gut-associated lymphoid tissues (Fig. 4, B and D). IL-1Ra protein was extremely scarce in the small intestinal tissue of ΔdblGATA mice and was undetectable in small intestinal epithelial cells (Fig. 4 C), supporting that it is predominantly produced by eosinophils in the small intestine. During in vitro culture, small intestinal eosinophils secreted IL-1Ra at extremely high levels, much higher than other well-known effector cytokines of eosinophils, including IL-1β, IL-4, IL-5, IL-6, IL-13, and TNF (Rothenberg and Hogan, 2006; Jung et al., 2015; Fig. 4 E). Unlike small intestinal eosinophils, eosinophils from the bone marrow and blood scarcely produced IL-1Ra as determined by RT-PCR and ELISA (Fig. 4, F and G). We found that eosinophils isolated from asthmatic lung tissue, unlike the ones from the normal lung, also produce IL-1Ra, but at a much lower level compared with small intestinal eosinophils, suggesting that IL-1Ra expression could be inducible under certain conditions (Fig. 4 H).
Figure 4.
Small intestinal eosinophils express high levels of the IL-1Ra. (A) Microarray analysis of DCs and eosinophils (EO) from the small intestine of WT C57BL/6 mice. (B) Western blot analysis of IL-1Ra protein in cell lysates of lymphocytes (Lym), DCs, and eosinophils sorted from the small intestinal LP (left). (Right) IL-1Ra protein detected from whole-cell lysates of spleen (SP), MLNs, PPs, and small intestinal LP (SI-LP). (C) Western blot analysis of IL-1Ra protein in lysates of total small intestinal tissue (jejunum) and small intestinal epithelial cells (SI-IEC) purified from BALB/c (WT) or ΔdblGATA-1 (KO) mice. (D) IL-1Ra secreted by eosinophils (106) or other cells (Non-EO; 106) in small intestine during a 24-h culture was measured by ELISA. (E) Various cytokines secreted by sorted small intestinal eosinophils (106) during a 24-h culture were measured by ELISA. (F) Quantitative RT-PCR analysis of IL-1Ra in eosinophils isolated from bone marrow, blood, or small intestine. (G) IL-1Ra in culture supernatants of eosinophils (4 × 104) sorted from bone marrow, blood, or small intestine was measured by ELISA. (H) IL-1Ra secreted by eosinophils (105) sorted from small intestine, normal lung, or allergic asthma-induced lung during a 24-h culture was measured by ELISA. Data are representative of two (E and H) or at least three (B–D, F, and G) experiments. Bar graphs show the mean ± SEM. ND, not detected. *, P < 0.05; **, P < 0.01; ***, P < 0.001 (unpaired Student’s t test).
Collectively, these findings show that small intestinal eosinophils secrete a large amount of IL-1Ra under steady-state conditions, which could be responsible for the suppression of Th17 cells.
GM-CSF promotes small intestinal eosinophils to produce IL-1Ra
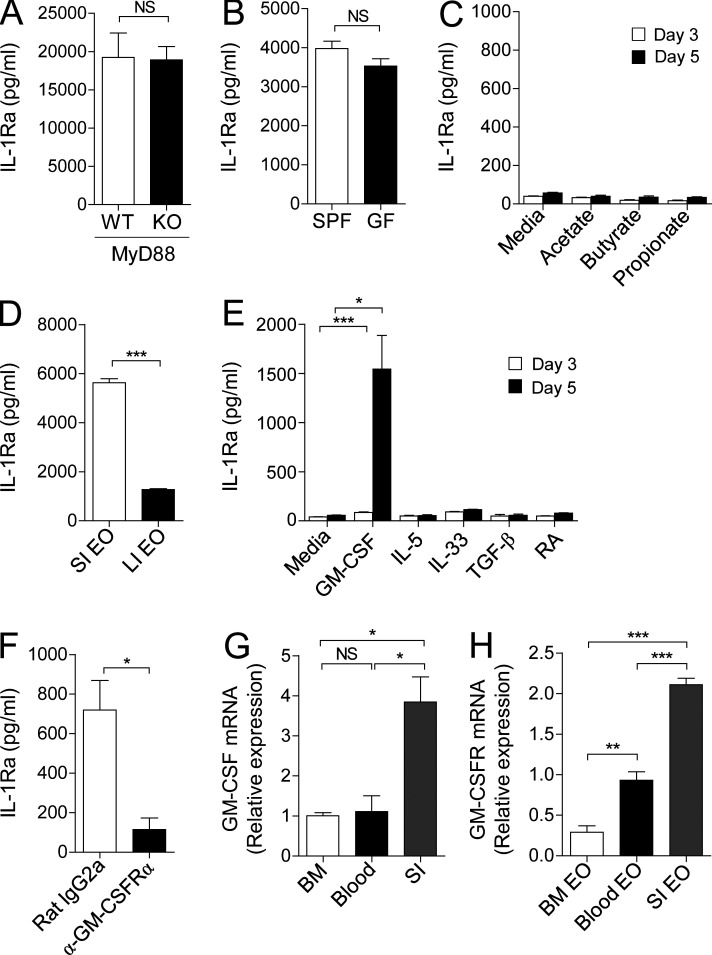
Next, to determine gut-specific signals inducing expression of IL-1Ra in intestinal eosinophils, we first examined involvement of commensal microbiota. Intestinal epithelial cells are known to secrete IL-1Ra upon TLR5 activation (Carvalho et al., 2011). In contrast, small intestinal eosinophils spontaneously secrete IL-1Ra in a MyD88-independent manner (Fig. 5 A). Also, there was no significant difference between small intestinal eosinophils from specific pathogen–free (SPF) and germ-free (GF) mice in the ability to produce IL-1Ra (Fig. 5 B), indicating independence from gut microbiota for this activity. In this regard, stimulation of bone marrow eosinophils with short-chain fatty acids, which are major end products of gut microbial fermentation in the intestine (Kim et al., 2013), did not induce IL-1Ra secretion (Fig. 5 C). Furthermore, we found that large intestinal eosinophils produce a smaller amount of IL-1Ra compared with small intestinal eosinophils (Fig. 5 D). These data indicate that small intestine–specific stimulus other than gut microbiota may play a crucial role in IL-1Ra expression by eosinophils.
Figure 5.
Eosinophils secrete IL-1Ra upon GM-CSF stimulation. (A and B) IL-1Ra in the culture supernatant of small intestinal eosinophils sorted from MyD88-deficient (KO; 105) or littermate control (WT; 105) mice (A) and SPF (5 × 104) or GF (5 × 104) mice (B) after a 24-h culture was measured by ELISA. (C) IL-1Ra in the culture supernatant of bone marrow eosinophils (2 × 104) after a 3 or 5 d culture with acetate (1 mM), butyrate (1 mM), or propionate (1 mM) was measured by ELISA. (D) IL-1Ra in culture supernatant of eosinophils (5 × 104) sorted from small intestine or large intestine was measured by ELISA. (E) IL-1Ra in the culture supernatant of bone marrow eosinophils (2 × 104) after a 3 or 5 d culture with GM-CSF (10 ng/ml), IL-5 (10 ng/ml), IL-33 (10 ng/ml), TGF-β (5 ng/ml), or retinoic acid (RA; 10 mM) was measured by ELISA. (F) IL-1Ra in the culture supernatant of bone marrow eosinophils (1.5 × 104) after a 5 d culture with GM-CSF (10 ng/ml) in the presence of GM-CSF receptor α blocking antibodies (α-GM-CSFRα; 8 µg/ml) or isotype control (Rat IgG2a; 8 µg/ml) was measured by ELISA. (G) Quantitative RT-PCR analysis of GM-CSF in bone marrow, blood, or jejunum (SI). (H) Quantitative RT-PCR analysis of GM-CSF receptor in eosinophils sorted from bone marrow, blood, or small intestine. All data are representative of at least three independent experiments. Bar graphs show the mean ± SEM. *, P < 0.05, ***, P < 0.001; NS, not significant (unpaired Student’s t test).
To further elucidate the factors that promote eosinophils to express IL-1Ra, we stimulated bone marrow eosinophils with a variety of cytokines (GM-CSF, IL-5, IL-33, and TGF-β) or retinoic acid, both of which are abundantly present in the intestine (Mowat and Agace, 2014; Peterson and Artis, 2014). Bone marrow eosinophils, which do not normally express IL-1Ra, were found to produce IL-1Ra only when cultured with GM-CSF, but not with other cytokines (Fig. 5 E). This response was blocked by the neutralizing monoclonal antibody against GM-CSF receptor α chain (GM-CSFRα; Fig. 5 F). GM-CSF is constitutively secreted by the intestinal epithelial cells (Egea et al., 2010) and promotes the activation and survival of eosinophils (Esnault and Malter, 2002; Hamilton, 2008). We confirmed that GM-CSF is highly expressed in the small intestine compared with bone marrow and blood (Fig. 5 G). Furthermore, small intestinal eosinophils expressed highly elevated levels of GM-CSF receptor compared with eosinophils in the bone marrow and blood (Fig. 5 H). These findings suggest that small intestinal eosinophils produce high levels of IL-1Ra, mostly likely in a GM-CSF−dependent manner.
IL-1Ra mediates the suppressive effect of small intestinal eosinophils on Th17 cells
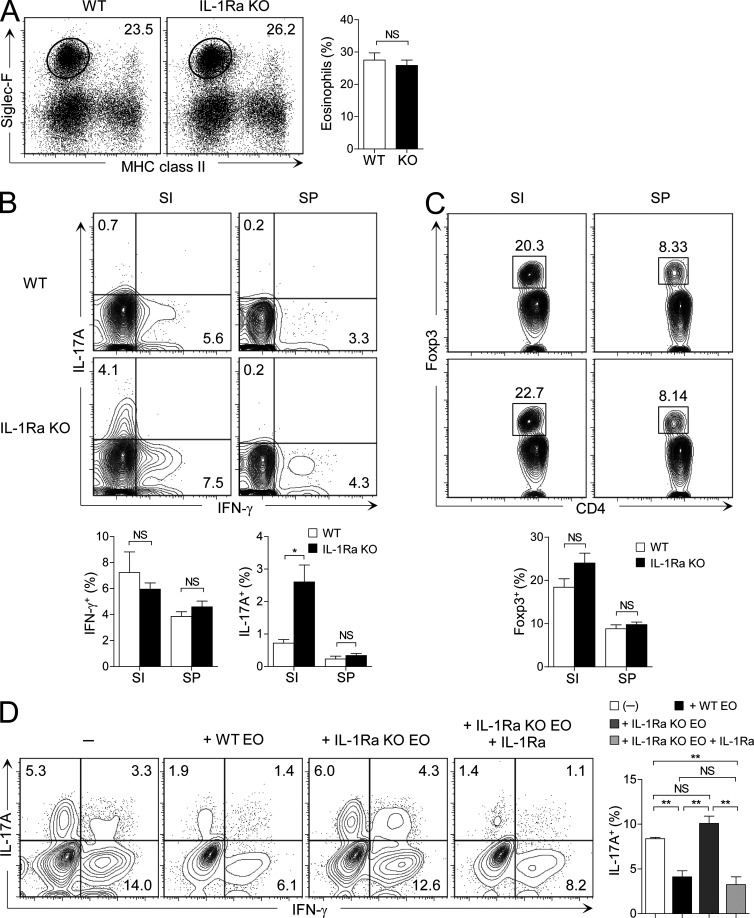
IL-1Ra–deficient mice develop spontaneous inflammatory arthritis (Nakae et al., 2003; Koenders et al., 2008). However, little is known about the alteration of intestinal immune system in these mice. We found that the frequency of eosinophils in the small intestine was not affected by the absence of IL-1Ra (Fig. 6 A). However, Th17 cells were significantly increased in the small intestine of IL-1Ra–deficient mice compared with littermate control WT mice (Fig. 6 B). In contrast to the small intestinal Th17 cells, no significant changes were found in the frequency of Th17 cells in the spleen. Th1 and T reg cells were not affected both in the small intestine and spleen of the IL-1Ra–deficient mice (Fig. 6, B and C). These results indicate that IL-1Ra plays an important role in limiting the number of Th17 cells in the small intestine.
Figure 6.
Small intestinal eosinophils suppress Th17 cells by producing IL-1Ra. (A) Flow cytometry analysis of the frequency of eosinophils in IL-1Ra–deficient (KO) or littermate control BALB/c mice (WT). FACS plots are representative of three independent experiments (n = 3 mice per group per experiment). Bar graphs show the mean ± SEM of pooled data. (B and C) Frequency of IFN-γ+ and IL-17A+ (B) and Foxp3+ (C) CD4+ T cells in the small intestine (SI) or spleen (SP) from IL-1Ra–deficient or littermate control BALB/c mice. FACS plots show cells gated on TCRβ+CD4+ cells and are representative of three independent experiments (n = 3−5 mice per group per experiment). Bar graphs show the mean ± SEM of pooled data. (D) Small intestinal CD4+CD25− T cells (105) and splenic DCs (104) were cultured with small intestinal eosinophils (105) from WT BALB/c or IL-1Ra–deficient mice in the presence of anti-CD3/CD28, neutralizing anti–IFN-γ, neutralizing anti–IL-4, neutralizing anti–IL-2, GM-CSF, TGF-β, IL-1β, and IL-23. IL-1Ra was added to certain cultures. After 5 d of culture, cells were collected and intracellular cytokine staining for IFN-γ and IL-17A in CD4+ T cells was analyzed by flow cytometry. FACS plots show cells gated on CD4+ T cells and are representative of four similar experiments. Bar graphs show the mean ± SEM. *, P < 0.05; **, P < 0.01. NS, not significant (unpaired Student’s t test).
To test whether small intestinal eosinophils suppress Th17 cells by secreting IL-1Ra, we co-cultured small intestinal CD4+ T cells along with eosinophils purified from the small intestine of WT or IL-1Ra–deficient mice. As shown in Fig. 6 D, small intestinal eosinophils from IL-1Ra−deficient mice completely failed to suppress Th17 cells, and addition of the recombinant IL-1Ra nicely restored the suppressive effect of IL-1Ra–deficient eosinophils. These data demonstrate that IL-1Ra secreted by small intestinal eosinophils down-regulates Th17 cells in the small intestine.
Eosinophils attenuate inflammation by producing IL-1Ra
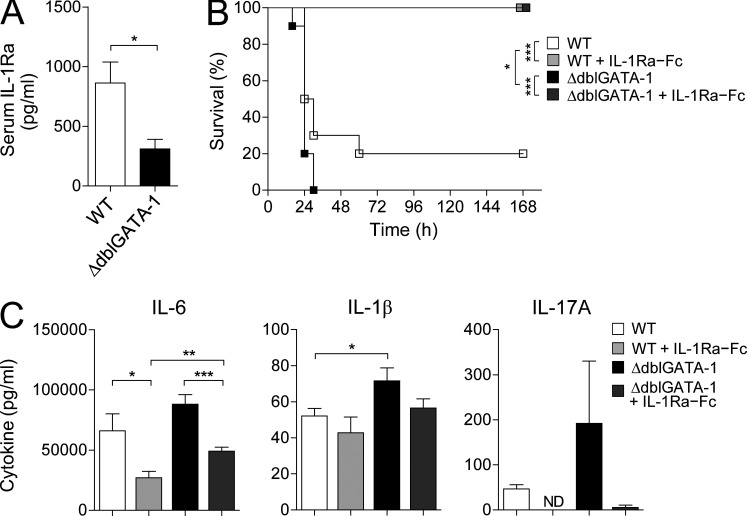
Serum IL-1Ra was significantly reduced in ΔdblGATA-1 mice, suggesting that eosinophils, especially the ones in the small intestine, are the major sources of systemic IL-1Ra in steady-state conditions (Fig. 7 A). To test whether eosinophils indeed inhibit inflammatory responses, we induced acute inflammation in WT BALB/c or ΔdblGATA-1 mice by intraperitoneal injection with LPS and monitored the survival of mice. LPS challenge stimulates massive production of proinflammatory cytokines, such as IL-1β, IL-6, and IL-17A, through damaging the intestinal barrier and consequently causes lethality in mice (Wang et al., 1998; Li et al., 2011). As shown in Fig. 7 B, ΔdblGATA-1 mice were more susceptible to LPS-induced lethality than WT mice, and administration of recombinant IL-1Ra–Fc completely rescued both WT and ΔdblGATA-1 mice from lethality. As expected from the mortality rate, the levels of proinflammatory cytokines, including IL-1β and IL-17A, were much higher in ΔdblGATA-1 mice than in WT mice at 6 h after LPS injection (Fig. 7 C). Administration of IL-1Ra–Fc suppressed the elevation of proinflammatory cytokines, more so in ΔdblGATA-1 mice. These results suggest that eosinophils can play an antiinflammatory role by producing IL-1Ra.
Figure 7.
An antiinflammatory function of eosinophils. (A) IL-1Ra in serum from ΔdblGATA-1 or littermate control mice (WT) was measured by ELISA. Results shown are representative of at least three independent experiments (n ≥ 4 mice per group per experiment). Bar graphs show the mean ± SEM. *, P < 0.05 (unpaired Student’s t test). (B) Mice were intraperitoneally injected with 15 mg/kg of LPS. IL-1Ra–hybrid Fc fusion protein (IL-1Ra–Fc; 25 mg/kg) was injected 4 h before and 20 min after the injection of LPS. Thereafter, mice were treated four times (every 3 h) with IL-1Ra–Fc. Survival of mice was monitored for 7 d (WT, n = 10; WT + IL-1Ra–Fc, n = 9; ΔdblGATA-1, n = 10; ΔdblGATA-1 + IL-1Ra–Fc, n = 10). Data are representative of three similar experiments. Statistical curve comparison was analyzed by Log-rank (Mantel-Cox) test. *, P < 0.05; ***, P < 0.001. (C) Serum samples of mice in B were collected at 6 h after LPS injection and cytokines were measured. Data are representative of two independent experiments. Bar graphs show the mean ± SEM. ND, not detected. *, P < 0.05; **, P < 0.01; ***, P < 0.001 (unpaired Student’s t test).
DISCUSSION
There are several mechanisms for regulating Th17 cells in the small intestine, reflecting the importance of maintaining their numbers in the right balance (Bettelli et al., 2006; Esplugues et al., 2011). This study for the first time shows that eosinophils play a crucial role in controlling Th17 cells in the small intestine. We found that eosinophil-deficient ΔdblGATA-1 mice, regardless of genetic background, had much higher frequency of Th17 cells in the small intestine. Also, depletion of eosinophils using a Siglec-F–specific monoclonal antibody greatly enhanced Th17 cells in the small intestine of WT mice. Our results herein suggest that small intestinal eosinophils regulate Th17 cell homeostasis by constitutively secreting a copious amount of IL-1Ra, a cytokine that inhibits the activity of IL-1β and consequently suppresses the differentiation and maintenance of Th17 cells.
Although less marked than the effects on Th17 cells, we found that the absence of eosinophils in ΔdblGATA-1 mice was also correlated with an increase in Th1 cells (Fig. 1). Supporting this finding, the extract of Ulmus davidiana var. japonica Nakai up-regulates eosinophils and reduces the frequencies of Th1 as well as Th17 cells in the small intestine (Lee et al., 2013). Contrary to eosinophil-deficient ΔdblGATA-1 mice, IL-1Ra–deficient mice did not show a significant increase of Th1 cells in the small intestine (Fig. 6 B), therefore, small intestinal eosinophils might suppress Th1 cells by secreting other, unidentified cytokines.
IL-1Ra is mainly expressed in mononuclear cells and epithelial cells under inflammatory conditions (Sims and Smith, 2010), but small intestinal eosinophils produce IL-1Ra even under homeostatic conditions. Such IL-1Ra production under steady-state conditions seems to be restricted to the eosinophils in the small intestine because eosinophils purified from the bone marrow and blood scarcely secreted IL-1Ra (Fig. 4 G). After developing and maturing in the bone marrow, eosinophils are released into the blood circulation and finally migrate to various tissues (Mishra et al., 1999; Rothenberg and Hogan, 2006). Through a series of events during trafficking, eosinophils are thought to be educated by the local tissue-specific microenvironments. The intestine is in continuous contact with diverse microbiota and food antigens and is exposed to a vast array of metabolites derived from both microbiota and specialized host cells (Hooper et al., 2012). Such unique intestinal microenvironment likely imprints small intestinal eosinophils to produce a high level of IL-1Ra. However, based on our data that small intestinal eosinophils from both SPF and GF mice secrete similar levels of IL-1Ra and that metabolites such as retinoic acid and short-chain fatty acids fail to induce IL-1Ra production in bone marrow eosinophils, commensal microbiota and those metabolites do not seem to be crucial for IL-1Ra production by small intestinal eosinophils under steady-state conditions (Fig. 5 B and C). Instead, our study identified GM-CSF as a key factor for inducing IL-1Ra secretion (Fig. 5 E and F). GM-CSF is known to stimulate development, survival, and effector function of eosinophils (Esnault and Malter, 2002; Geering et al., 2013). However, its role of inducing eosinophils to secrete an antiinflammatory cytokine has not been previously appreciated.
GM-CSF induced high expression of IL-1Ra in the bone marrow eosinophils, which normally do not produce the cytokine. We also found that basal expression of GM-CSF in the bone marrow and blood was low, whereas it was very high in the small intestine. In the small intestine, Paneth cells, among epithelial cells, have been reported to constitutively produce GM-CSF (Egea et al., 2010). In addition, small intestinal eosinophils expressed a high level of GM-CSFR mRNA, unlike bone marrow and blood eosinophils. The high basal level of GM-CSF in the small intestine combined with high expression GM-CSFR in the small intestinal eosinophils explains the unique ability of the small intestinal eosinophils to continuously secrete a large amount IL-1Ra at steady-state conditions. Apart from the intestine, GM-CSF levels are highly elevated in the lung of OVA-induced allergic asthma model (Hamilton, 2008; Su et al., 2008). Accordingly, we found that eosinophils purified from asthmatic lung were able to secrete IL-1Ra, in contrast to eosinophils from normal lung.
IL-17 enhances the activity of eosinophils either directly or indirectly, and consequently induces or aggravates the eosinophilic inflammatory responses in allergic diseases (Cheung et al., 2008; Wakashin et al., 2008; Dias and Banerjee, 2013). In addition, Th17 cells activated by IL-1 or IL-23 were reported to produce GM-CSF (El-Behi et al., 2011; Griseri et al., 2012). Therefore, it is plausible that eosinophils activated by Th17 cells under inflammatory milieu could acquire the ability of IL-1Ra production through GM-CSF/GM-CSFR signaling and facilitate a negative feedback control of Th17 cell homeostasis. Further studies will be required to determine whether GM-CSF/GM-CSFR signaling indeed directly induces IL-1Ra expression by the eosinophils in vivo, for example by using eosinophil-specific deletion of GM-CSFR.
Under physiological conditions, few eosinophils exist in most tissues. Apart from the small intestine, the uterus is another organ with high numbers of tissue eosinophils, and uterine eosinophils were shown to regulate the estrous cycle (Robertson et al., 2000; Gouon-Evans and Pollard, 2001). Eosinophils in the small intestine and the uterus both express CD11c, unlike the eosinophils found in blood and lung, and have a markedly longer life span (Carlens et al., 2009). These findings imply that steady-state tissue-resident eosinophils are phenotypically and, most likely, functionally different from eosinophils recruited to the tissues under nonhomeostatic conditions. Interestingly, although eosinophils are distributed throughout the gastrointestinal tract, the frequency of eosinophils in the large intestine at steady state is very low, and large intestinal eosinophils show a distinctive expression pattern of surface proteins, including CD11c, ST2, and Ly6C, as compared with small intestinal ones (Chu et al., 2014a). Moreover, we found that large intestinal eosinophils produce much lower levels of IL-1Ra than small intestinal ones (Fig. 5 D). Thus, eosinophils in the large intestine seem to be more closely related to the proinflammatory eosinophils rather than to other tissue-resident eosinophils, such as small intestinal and uterine eosinophils that play homeostatic roles. Consistent with this idea, other groups have reported that eosinophils infiltrating the colon promote colitis (Ahrens et al., 2008; Waddell et al., 2011; Griseri et al., 2015). Numbers and properties of eosinophils found in various tissues are likely controlled by local milieu. For full characterization of eosinophils in various tissues and conditions, it would be useful to establish the surface markers that can discriminate homeostatic and proinflammatory eosinophils.
In summary, our findings in this study show that eosinophils play a key role in regulating Th17 cells in the small intestine and contribute to the maintenance of local intestinal immune homeostasis, highlighting a novel immune regulatory function of eosinophils, once considered solely as proinflammatory cells.
MATERIALS AND METHODS
Mice
C57BL/6, BALB/c, ΔdblGATA-1, and IL-4−IRES−eGFP (4get) mice were purchased from the Jackson Laboratory. MyD88-deficient mice were purchased from the Oriental Bio Service, and IL-1Ra–deficient mice were provided by Y. Iwakura (University of Tokyo, Tokyo, Japan). For generation of C57BL/6 background ΔdblGATA-1 mice, BALB/c background ΔdblGATA-1 mice were backcrossed >10 generations with C57BL/6 mice. The mice were maintained in SPF facilities at the Pohang University of Science and Technology (POSTECH) and Research Institute for Microbial Diseases of Osaka University. A colony of GF C57BL/6 mice was established at POSTECH from breeders obtained from A. Macpherson (Bern University, Bern, Switzerland). GF mice were maintained in sterile flexible film isolators (Class Biological Clean Ltd.), and GF status was monitored monthly by anaerobic and aerobic culture of cecal contents. All procedures were approved by the Animal Care and Use Committee of POSTECH and the Animal Research Committee of Osaka University.
Cell preparation
To isolate LP cells from the intestine, fat tissues and Peyer’s patches (PPs) were removed, and the intestine was opened longitudinally, washed in PBS, and cut into 1-cm sections. The epithelial cell layer was removed by vigorous stirring in FACS buffer (PBS containing 3% FBS, 20 mM Hepes, 100 U/ml penicillin, 100 µg/ml streptomycin, 1 mM sodium pyruvate, and 10 mM EDTA) for 20 min at 37°C. The intestinal fragments were shaken in PBS, minced, and digested in RPMI-1640 containing 3% FBS, 20 mM Hepes, 100 U/ml penicillin, 100 µg/ml streptomycin, 1 mM sodium pyruvate, 1 mM nonessential amino acids, 400 U/ml collagenase D (Roche), and 100 µg/ml DNase I (Roche) at 37°C for 45 min with continuous stirring. EDTA was added to a final concentration of 10 mM, and the cell suspension was incubated for an additional 5 min at 37°C. The cell suspension was then filtered through a 100-µm cell strainer and pelleted by centrifugation for 5 min at 1,300 rpm. The pellet was subjected to 40/75% Percoll (GE Healthcare) density-gradient centrifugation for 20 min at 2,000 rpm at room temperature. Cells at the interface were collected, washed, and used for analysis and culture. To isolate epithelial cells from the intestine, intestinal fragments were shaken in FACS buffer containing 1 mM EDTA for 30 min at 37°C, the cell suspension was filtered through a 100-µm cell strainer, and epithelial cells were enriched by 25/40% Percoll density-gradient centrifugation. Blood samples were lysed with ACK (Ammonium-Chloride-Potassium) lysis buffer on ice and used for FACS analysis. Thymi were minced using curved scissors and digested the same way as the intestinal fragments, and the cells were then spun through a 17.5% Accudenz (Accurate Chemical & Scientific Co.) solution. Cells at the interface were collected, washed, and used for FACS analysis. To isolate DCs from spleen, the organs were minced and digested in RPMI-1640 containing collagenase D and DNase I as described in intestinal LP cell isolation section. The cells were spun through a 17.5% Accudenz (Accurate Chemical & Scientific Co.) solution and separated by density-gradient centrifugation for 20 min at 2,000 rpm at room temperature. Cells at the interface were collected, washed, and used for analysis and culture. To isolate lymphocytes, spleens were mechanically minced through a 100-µm cell strainer in the presence of FACS buffer, and RBCs were lysed using ACK lysis buffer. The cells were washed and used for analysis and culture.
Flow cytometry
Fc receptors were blocked with anti–mouse CD16/CD32 (2.4G2; BD), and the cells were stained using the following antibodies: anti-CD11b (M1/70; BD), anti-CD11c (HL3; BD), anti-MHC class II (M5/114.15.2; eBioscience), anti-CCR3 (83103; BD), anti–Siglec-F (E50-2440; BD), anti–c-kit (2B8; BD), anti-FcεR1 (MAR-1; eBioscience), anti-CD49b (DX5; BioLegend), anti-IgE (RME-1; BioLegend), 7AAD (BD), Annexin V (BD), anti-CD3e (145-2C11; BD), anti-CD8α (53-6.7; BD), anti-CD19 (1D3; BD), anti-B220 (RA3-6B2; BD), anti-TER119/Ly-76 (TER-119; BD), anti–Gr-1 (RB6-8C5; BD), anti-NK1.1 (PK136; eBioscience), anti-Thy1.2 (53-2.1; eBioscience), anti-TCRβ (H57-597; BioLegend), anti-CD25 (PC61.5; eBioscience), anti-CD4 (RM4-5, eBioscience), anti–IFN-γ (XMG1.2; eBioscience), anti–IL-13 (eBio13A; eBioscience), anti–IL-17A (TC11-18H10; BD), anti-RORγt (B2D; eBioscience), anti-GATA3 (TWAJ; eBioscience), anti-Foxp3 (FJK-16s; eBioscience), and streptavidin (BD). For intracellular transcription factor staining, Foxp3/transcription factor staining buffer set (eBioscience) was used. For intracellular cytokine staining, cells were stimulated with phorbol 12-myristate 13-acetate (PMA; 50 ng/ml; Sigma-Aldrich) and ionomycin (750 ng/ml; Sigma-Aldrich), or recombinant mouse (rm) IL-1β (40 ng/ml; R&D Systems) and rm IL-23 (20 ng/ml; R&D Systems) in the presence of monensin (GolgiStop; BD) for 4 h, and stained using a Cytofix/Cytoperm staining kit (BD). Data were acquired on an LSRFortessa (BD) and analyzed with FlowJo software (Tree Star).
Cell sorting
LN cells were stained with anti-CD4, anti-CD25, anti-CD44 (IM7; BD), and anti-MHC class II to isolate MHCII−CD4+CD25−CD44− naive CD4+ T cells. Splenocytes were stained with anti-CD11c and anti-MHC class II to isolate MHCII+CD11c+ DCs. Blood cells were stained with anti-MHC class II, anti-CCR3, and anti–Siglec-F, and bone marrow cells were stained with anti–MHC class II, anti-CD11b, and anti–Siglec-F to isolate MHCII−CCR3highSiglec-Fhigh and MHCII−CD11bintSiglec-Fhigh eosinophils, respectively. Small intestinal epithelial cell preparation was stained with anti-CD45 (30-F11; eBioscience) and anti-EpCAM (G8.8; BioLegend) to isolate CD45−EpCAM+ intestinal epithelial cells. Small intestinal LP cells were stained with anti-CD4, anti-CD25, anti–MHC class II, and anti–Siglec-F; MHCII−Siglec-F+ eosinophils and MHCII−Siglec-F−CD4+CD25− T cells were sorted on a MoFlo Astrios or MoFlo XDP (Beckman Coulter).
Real-time PCR
Total RNA was extracted using TRIzol reagent (Invitrogen), and cDNA was synthesized with QuantiTect Reverse Transcription kit (QIAGEN) according to the manufacturer’s protocol. Real-time PCR was performed using SYBR Premix Ex Taq (Takara Bio Inc.) and ViiA 7 system (Applied Biosystems). The following primer pairs were used: GM-CSF (Csf2), 5′-GCATGTAGAGGCCATCAAAGA-3′ and 5′-CGGGTCTGCACACATGTTA-3′; GM-CSFRα, 5′-GCGGGCGACACGAGGATGAAGCAC-3′ and 5′-CTAGGGCTGCAGGAGGTCCTTCCT-3′; IL-1Ra, 5′-TACTGTGGACACCACCCTCA-3′ and 5′-TCTGCCTCCAAGCAAAAGAT-3′; HPRT, 5′-TCAACAATCAAGACATTCTTTCCA-3′ and 5′-CAGACTGAAGAGCTACTGTAATGATCA-3′.
Microarray analysis
Total RNA was extracted from small intestinal LP DCs and eosinophils using TRIzol reagent (Invitrogen) and RNeasy kit (QIAGEN), and cDNA was synthesized with a SuperScript First-Strand Synthesis System for RT-PCR (Invitrogen) according to the manufacturer’s protocol. The cDNA was hybridized to Murine Genome 430 2.0 microarray chips (Affymetrix) according to the manufacturer’s instructions. Hybridized chips were stained, washed, and scanned with a GeneArray Scanner (Affymetrix). Microarray Suite (Version 5.0; Affymetrix) and GeneSpring (Silicon Genetics) software were used for data analysis. All datasets have been deposited at the National Center for Biotechnology Information Gene Expression Omnibus under accession no. GSE77545.
Immunoblotting
Whole-cell lysates were prepared from the spleen, MLN, PPs, and small intestinal LP. Lysates were also prepared from small intestinal tissues (jejunum), FACS-sorted small intestinal epithelial cells, LP lymphocytes, LP DCs, and LP eosinophils isolated as described above. Blots were probed with anti–IL-1Ra (BAF-480; R&D Systems) using anti–β-Actin (sc-47778; Santa Cruz Biotechnology, Inc.) as a control. Streptavidin-conjugated horseradish peroxidase (DY998; R&D Systems) was used as a secondary antibody, and band images were visualized with ImageQuant LAS4000 (GE Healthcare).
Cytokine measurements in cell culture supernatants and serum
FACS-sorted eosinophils were cultured in RPMI-1640 containing 10% FBS, 10 mM Hepes, 100 U/ml penicillin, 100 µg/ml streptomycin, 50 µg/ml gentamicin, 1 mM sodium pyruvate, 55 µM 2-mercaptoethanol, 1 mM nonessential amino acids, and 2 mM l-Glutamine at 37°C. Mouse ELISA kits (IL-1Ra/IL-1F3; R&D Systems; IL-1β, IL-4, IL-5, IL-6, IL-13, IL-12p40, and IL-17A; eBioscience; TNF; BD) were used according to the manufacturer’s instructions to measure these cytokine levels in mouse serum or cell culture supernatants.
In vitro co-culture
CD4+ T cells (105) were sorted from LNs or small intestinal LP and cultured with splenic DCs (104) in the presence or absence of small intestinal eosinophils (105) from WT or IL-1Ra–deficient mice. The following antibodies and cytokines were included in the culture: anti-CD3e (5 µg/ml; 145-2C11; BD), anti-CD28 (2 µg/ml; 37.51; BD), neutralizing anti–IFN-γ (5 µg/ml; XMG1.2; eBioscience), neutralizing anti–IL-4 (5 µg/ml; 11B11; eBioscience), neutralizing anti–IL-2 (2.5 µg/ml; S4B6; BD), recombinant human TGF-β (5 ng/ml; R&D Systems), rm IL-1β (20 ng/ml; R&D Systems), rm IL-6 (20 ng/ml; R&D Systems), rm IL-23 (10 ng/ml; R&D Systems), rm GM-CSF (10 ng/ml; R&D Systems), and rm IL-1Ra (200 ng/ml; R&D Systems). On day 5, the cells were stimulated with PMA (50 ng/ml) and ionomycin (750 ng/ml) in the presence of monensin for 4 h, and the cells producing IFN-γ and IL-17A were analyzed by flow cytometry.
Depletion of eosinophils
C57BL/6 mice were injected six times with 25 µg of Siglec-F–specific antibodies (R&D Systems) or isotype control (Rat IgG2a; R&D Systems) for 2 wk (every second day) and analyzed 3 d later.
Murine model of allergic asthma
BALB/c mice were sensitized by an intraperitoneal injection of 100 µg OVA (Sigma-Aldrich) plus 2 mg aluminum hydroxide (Sigma-Aldrich) on days 0 and 14, after which the mice were challenged by intranasal administration of 75 µg OVA on days 21, 22, 28, and 29. Pulmonary inflammation was evaluated and cells were isolated from lung tissues at 48 h after the final allergen challenge (on day 31).
LPS challenge
BALB/c or ΔdblGATA-1 mice were intraperitoneally injected with 15 mg/kg of LPS (Escherichia coli 055:B5; Sigma-Aldrich). 25 mg/kg of IL-1Ra–hybrid Fc fusion protein (IL-1Ra–Fc; Handok Pharmaceuticals Co.) was injected 4 h before and 20 min after the injection of LPS. Thereafter, mice were treated four times (every 3 h) with IL-1Ra–Fc. Survival of mice was monitored for 7 d.
Statistical analysis
Statistical analysis was performed with Prism 5 software (GraphPad Software). For statistical comparisons between groups, unpaired Student’s t test, two-way ANOVA or log-rank (Mantel-Cox) test was performed when indicated. P-values <0.05 were considered statistically significant (*, P < 0.05; **, P < 0.01; ***, P < 0.001).
Online supplemental information
Fig. S1 shows the gating strategy for small intestinal eosinophils. Online supplemental material is available at http://www.jem.org/cgi/content/full/jem.20141388/DC1.
Supplementary Material
ACKNOWLEDGMENTS
We thank Dr. Young-Chul Sung and Handok Pharmaceuticals Co., Ltd for kindly providing the IL-1Ra–hybrid Fc fusion proteins, Jin-Myung Bae for technical assistance, Hae-Jin Jung for assistance with cell sorting, and the staff of the animal facility at Pohang University of Science and Technology and Osaka University for excellent animal care.
This work was supported by the Institute for Basic Science (IBS) project IBS-R005-S1-2015-a00 and IBS-R005-D1-2015-a00, the National Research Foundation, Korea Ministry of Science, Information/Communication Technology and Future Planning, and a grant from the National Research Foundation of Korea funded by the Ministry of Education (NRF-2015R1A2A2A04003471). M.H. Jang was supported by the World Class Universities project, the National Research Foundation of Korea, the Ministry of Education (R31-10105), and the Ministry of Education, Culture, Sports, Science and Technology of Japan (18790338 and 19041044).
The authors declare no competing financial interests.
Footnotes
Abbreviations used:
- GF
- germ-free
- GM-CSF
- granulocyte macrophage colony-stimulating factor
- GM-CSFR
- GM-CSF receptor
- LP
- lamina propria
- MLN
- mesenteric LN
- PP
- Peyer’s patch
- rm
- recombinant mouse
- SPF
- specific pathogen–free
References
- Ahrens R., Waddell A., Seidu L., Blanchard C., Carey R., Forbes E., Lampinen M., Wilson T., Cohen E., Stringer K., et al. 2008. Intestinal macrophage/epithelial cell-derived CCL11/eotaxin-1 mediates eosinophil recruitment and function in pediatric ulcerative colitis. J. Immunol. 181:7390–7399. 10.4049/jimmunol.181.10.7390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atarashi K., Nishimura J., Shima T., Umesaki Y., Yamamoto M., Onoue M., Yagita H., Ishii N., Evans R., Honda K., and Takeda K.. 2008. ATP drives lamina propria TH17 cell differentiation. Nature. 455:808–812. 10.1038/nature07240 [DOI] [PubMed] [Google Scholar]
- Atarashi K., Tanoue T., Ando M., Kamada N., Nagano Y., Narushima S., Suda W., Imaoka A., Setoyama H., Nagamori T., et al. 2015. Th17 cell induction by adhesion of microbes to intestinal epithelial cells. Cell. 163:367–380. 10.1016/j.cell.2015.08.058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bettelli E., Carrier Y., Gao W., Korn T., Strom T.B., Oukka M., Weiner H.L., and Kuchroo V.K.. 2006. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature. 441:235–238. 10.1038/nature04753 [DOI] [PubMed] [Google Scholar]
- Carlens J., Wahl B., Ballmaier M., Bulfone-Paus S., Förster R., and Pabst O.. 2009. Common γ-chain-dependent signals confer selective survival of eosinophils in the murine small intestine. J. Immunol. 183:5600–5607. 10.4049/jimmunol.0801581 [DOI] [PubMed] [Google Scholar]
- Carvalho F.A., Aitken J.D., Gewirtz A.T., and Vijay-Kumar M.. 2011. TLR5 activation induces secretory interleukin-1 receptor antagonist (sIL-1Ra) and reduces inflammasome-associated tissue damage. Mucosal Immunol. 4:102–111. 10.1038/mi.2010.57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casini-Raggi V., Kam L., Chong Y.J., Fiocchi C., Pizarro T.T., and Cominelli F.. 1995. Mucosal imbalance of IL-1 and IL-1 receptor antagonist in inflammatory bowel disease. A novel mechanism of chronic intestinal inflammation. J. Immunol. 154:2434–2440. [PubMed] [Google Scholar]
- Cheung P.F., Wong C.K., and Lam C.W.. 2008. Molecular mechanisms of cytokine and chemokine release from eosinophils activated by IL-17A, IL-17F, and IL-23: implication for Th17 lymphocytes-mediated allergic inflammation. J. Immunol. 180:5625–5635. 10.4049/jimmunol.180.8.5625 [DOI] [PubMed] [Google Scholar]
- Chu D.K., Jimenez-Saiz R., Verschoor C.P., Walker T.D., Goncharova S., Llop-Guevara A., Shen P., Gordon M.E., Barra N.G., Bassett J.D., et al. 2014a Indigenous enteric eosinophils control DCs to initiate a primary Th2 immune response in vivo. J. Exp. Med. 211:1657–1672. 10.1084/jem.20131800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu V.T., Beller A., Rausch S., Strandmark J., Zänker M., Arbach O., Kruglov A., and Berek C.. 2014b Eosinophils promote generation and maintenance of immunoglobulin-A-expressing plasma cells and contribute to gut immune homeostasis. Immunity. 40:582–593. 10.1016/j.immuni.2014.02.014 [DOI] [PubMed] [Google Scholar]
- Chung Y., Chang S.H., Martinez G.J., Yang X.O., Nurieva R., Kang H.S., Ma L., Watowich S.S., Jetten A.M., Tian Q., and Dong C.. 2009. Critical regulation of early Th17 cell differentiation by interleukin-1 signaling. Immunity. 30:576–587. 10.1016/j.immuni.2009.02.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dias P.M., and Banerjee G.. 2013. The role of Th17/IL-17 on eosinophilic inflammation. J. Autoimmun. 40:9–20. 10.1016/j.jaut.2012.07.004 [DOI] [PubMed] [Google Scholar]
- Egea L., Hirata Y., and Kagnoff M.F.. 2010. GM-CSF: a role in immune and inflammatory reactions in the intestine. Expert Rev. Gastroenterol. Hepatol. 4:723–731. 10.1586/egh.10.73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Behi M., Ciric B., Dai H., Yan Y., Cullimore M., Safavi F., Zhang G.X., Dittel B.N., and Rostami A.. 2011. The encephalitogenicity of TH17 cells is dependent on IL-1- and IL-23-induced production of the cytokine GM-CSF. Nat. Immunol. 12:568–575. 10.1038/ni.2031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esnault S., and Malter J.S.. 2002. GM-CSF regulation in eosinophils. Arch. Immunol. Ther. Exp. (Warsz.). 50:121–130. [PubMed] [Google Scholar]
- Esplugues E., Huber S., Gagliani N., Hauser A.E., Town T., Wan Y.Y., O’Connor W. Jr, Rongvaux A., Van Rooijen N., Haberman A.M., et al. 2011. Control of TH17 cells occurs in the small intestine. Nature. 475:514–518. 10.1038/nature10228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garlanda C., Dinarello C.A., and Mantovani A.. 2013. The interleukin-1 family: back to the future. Immunity. 39:1003–1018. 10.1016/j.immuni.2013.11.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geering B., Stoeckle C., Conus S., and Simon H.U.. 2013. Living and dying for inflammation: neutrophils, eosinophils, basophils. Trends Immunol. 34:398–409. 10.1016/j.it.2013.04.002 [DOI] [PubMed] [Google Scholar]
- Gouon-Evans V., and Pollard J.W.. 2001. Eotaxin is required for eosinophil homing into the stroma of the pubertal and cycling uterus. Endocrinology. 142:4515–4521. 10.1210/endo.142.10.8459 [DOI] [PubMed] [Google Scholar]
- Griseri T., McKenzie B.S., Schiering C., and Powrie F.. 2012. Dysregulated hematopoietic stem and progenitor cell activity promotes interleukin-23-driven chronic intestinal inflammation. Immunity. 37:1116–1129. 10.1016/j.immuni.2012.08.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griseri T., Arnold I.C., Pearson C., Krausgruber T., Schiering C., Franchini F., Schulthess J., McKenzie B.S., Crocker P.R., and Powrie F.. 2015. Granulocyte macrophage colony-stimulating factor-activated eosinophils promote interleukin-23 driven chronic colitis. Immunity. 43:187–199. 10.1016/j.immuni.2015.07.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton J.A. 2008. Colony-stimulating factors in inflammation and autoimmunity. Nat. Rev. Immunol. 8:533–544. 10.1038/nri2356 [DOI] [PubMed] [Google Scholar]
- Hooper L.V., Littman D.R., and Macpherson A.J.. 2012. Interactions between the microbiota and the immune system. Science. 336:1268–1273. 10.1126/science.1223490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivanov I.I., Atarashi K., Manel N., Brodie E.L., Shima T., Karaoz U., Wei D., Goldfarb K.C., Santee C.A., Lynch S.V., et al. 2009. Induction of intestinal Th17 cells by segmented filamentous bacteria. Cell. 139:485–498. 10.1016/j.cell.2009.09.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jankovic D., Ganesan J., Bscheider M., Stickel N., Weber F.C., Guarda G., Follo M., Pfeifer D., Tardivel A., Ludigs K., et al. 2013. The Nlrp3 inflammasome regulates acute graft-versus-host disease. J. Exp. Med. 210:1899–1910. 10.1084/jem.20130084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung Y., Wen T., Mingler M.K., Caldwell J.M., Wang Y.H., Chaplin D.D., Lee E.H., Jang M.H., Woo S.Y., Seoh J.Y., et al. 2015. IL-1β in eosinophil-mediated small intestinal homeostasis and IgA production. Mucosal Immunol. 8:930–942. 10.1038/mi.2014.123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim M.H., Kang S.G., Park J.H., Yanagisawa M., and Kim C.H.. 2013. Short-chain fatty acids activate GPR41 and GPR43 on intestinal epithelial cells to promote inflammatory responses in mice. Gastroenterology. 145:396–406. 10.1053/j.gastro.2013.04.056 [DOI] [PubMed] [Google Scholar]
- Koenders M.I., Devesa I., Marijnissen R.J., Abdollahi-Roodsaz S., Boots A.M., Walgreen B., di Padova F.E., Nicklin M.J., Joosten L.A., and van den Berg W.B.. 2008. Interleukin-1 drives pathogenic Th17 cells during spontaneous arthritis in interleukin-1 receptor antagonist-deficient mice. Arthritis Rheum. 58:3461–3470. 10.1002/art.23957 [DOI] [PubMed] [Google Scholar]
- Korn T., Bettelli E., Oukka M., and Kuchroo V.K.. 2009. IL-17 and Th17 cells. Annu. Rev. Immunol. 27:485–517. 10.1146/annurev.immunol.021908.132710 [DOI] [PubMed] [Google Scholar]
- Lee H.S., Jang M.S., Kim J.H., Hong C.P., Lee E.J., Jeun E.J., Kim C., Kim E.K., Ahn K.S., Yang B.G., et al. 2013. Ulmus davidiana var. japonica Nakai upregulates eosinophils and suppresses Th1 and Th17 cells in the small intestine. PLoS One. 8:e76716 10.1371/journal.pone.0076716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H.M., Wang Y.Y., Wang H.D., Cao W.J., Yu X.H., Lu D.X., Qi R.B., Hu C.F., and Yan Y.X.. 2011. Berberine protects against lipopolysaccharide-induced intestinal injury in mice via alpha 2 adrenoceptor-independent mechanisms. Acta Pharmacol. Sin. 32:1364–1372. 10.1038/aps.2011.102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishra A., Hogan S.P., Lee J.J., Foster P.S., and Rothenberg M.E.. 1999. Fundamental signals that regulate eosinophil homing to the gastrointestinal tract. J. Clin. Invest. 103:1719–1727. 10.1172/JCI6560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison P.J., Ballantyne S.J., and Kullberg M.C.. 2011. Interleukin-23 and T helper 17-type responses in intestinal inflammation: from cytokines to T-cell plasticity. Immunology. 133:397–408. 10.1111/j.1365-2567.2011.03454.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mowat A.M., and Agace W.W.. 2014. Regional specialization within the intestinal immune system. Nat. Rev. Immunol. 14:667–685. 10.1038/nri3738 [DOI] [PubMed] [Google Scholar]
- Nakae S., Saijo S., Horai R., Sudo K., Mori S., and Iwakura Y.. 2003. IL-17 production from activated T cells is required for the spontaneous development of destructive arthritis in mice deficient in IL-1 receptor antagonist. Proc. Natl. Acad. Sci. USA. 100:5986–5990. 10.1073/pnas.1035999100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nei Y., Obata-Ninomiya K., Tsutsui H., Ishiwata K., Miyasaka M., Matsumoto K., Nakae S., Kanuka H., Inase N., and Karasuyama H.. 2013. GATA-1 regulates the generation and function of basophils. Proc. Natl. Acad. Sci. USA. 110:18620–18625. 10.1073/pnas.1311668110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Connor W. Jr., Kamanaka M., Booth C.J., Town T., Nakae S., Iwakura Y., Kolls J.K., and Flavell R.A.. 2009. A protective function for interleukin 17A in T cell-mediated intestinal inflammation. Nat. Immunol. 10:603–609. 10.1038/ni.1736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson L.W., and Artis D.. 2014. Intestinal epithelial cells: regulators of barrier function and immune homeostasis. Nat. Rev. Immunol. 14:141–153. 10.1038/nri3608 [DOI] [PubMed] [Google Scholar]
- Robertson S.A., Mau V.J., Young I.G., and Matthaei K.I.. 2000. Uterine eosinophils and reproductive performance in interleukin 5-deficient mice. J. Reprod. Fertil. 120:423–432. 10.1530/reprod/120.2.423 [DOI] [PubMed] [Google Scholar]
- Rothenberg M.E., and Hogan S.P.. 2006. The eosinophil. Annu. Rev. Immunol. 24:147–174. 10.1146/annurev.immunol.24.021605.090720 [DOI] [PubMed] [Google Scholar]
- Shaw M.H., Kamada N., Kim Y.G., and Núñez G.. 2012. Microbiota-induced IL-1β, but not IL-6, is critical for the development of steady-state TH17 cells in the intestine. J. Exp. Med. 209:251–258. 10.1084/jem.20111703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sims J.E., and Smith D.E.. 2010. The IL-1 family: regulators of immunity. Nat. Rev. Immunol. 10:89–102. 10.1038/nri2691 [DOI] [PubMed] [Google Scholar]
- Su Y.C., Rolph M.S., Hansbro N.G., Mackay C.R., and Sewell W.A.. 2008. Granulocyte-macrophage colony-stimulating factor is required for bronchial eosinophilia in a murine model of allergic airway inflammation. J. Immunol. 180:2600–2607. 10.4049/jimmunol.180.4.2600 [DOI] [PubMed] [Google Scholar]
- Verjan Garcia N., Umemoto E., Saito Y., Yamasaki M., Hata E., Matozaki T., Murakami M., Jung Y.J., Woo S.Y., Seoh J.Y., et al. 2011. SIRPα/CD172a regulates eosinophil homeostasis. J. Immunol. 187:2268–2277. 10.4049/jimmunol.1101008 [DOI] [PubMed] [Google Scholar]
- Waddell A., Ahrens R., Steinbrecher K., Donovan B., Rothenberg M.E., Munitz A., and Hogan S.P.. 2011. Colonic eosinophilic inflammation in experimental colitis is mediated by Ly6C(high) CCR2(+) inflammatory monocyte/macrophage-derived CCL11. J. Immunol. 186:5993–6003. 10.4049/jimmunol.1003844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakashin H., Hirose K., Maezawa Y., Kagami S., Suto A., Watanabe N., Saito Y., Hatano M., Tokuhisa T., Iwakura Y., et al. 2008. IL-23 and Th17 cells enhance Th2-cell-mediated eosinophilic airway inflammation in mice. Am. J. Respir. Crit. Care Med. 178:1023–1032. 10.1164/rccm.200801-086OC [DOI] [PubMed] [Google Scholar]
- Wang Q., Wang J.J., Boyce S., Fischer J.E., and Hasselgren P.O.. 1998. Endotoxemia and IL-1 beta stimulate mucosal IL-6 production in different parts of the gastrointestinal tract. J. Surg. Res. 76:27–31. 10.1006/jsre.1998.5288 [DOI] [PubMed] [Google Scholar]
- Wu D., Molofsky A.B., Liang H.E., Ricardo-Gonzalez R.R., Jouihan H.A., Bando J.K., Chawla A., and Locksley R.M.. 2011. Eosinophils sustain adipose alternatively activated macrophages associated with glucose homeostasis. Science. 332:243–247. 10.1126/science.1201475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu C., Cantor A.B., Yang H., Browne C., Wells R.A., Fujiwara Y., and Orkin S.H.. 2002. Targeted deletion of a high-affinity GATA-binding site in the GATA-1 promoter leads to selective loss of the eosinophil lineage in vivo. J. Exp. Med. 195:1387–1395. 10.1084/jem.20020656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou L., Ivanov I.I., Spolski R., Min R., Shenderov K., Egawa T., Levy D.E., Leonard W.J., and Littman D.R.. 2007. IL-6 programs TH-17 cell differentiation by promoting sequential engagement of the IL-21 and IL-23 pathways. Nat. Immunol. 8:967–974. 10.1038/ni1488 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.