Abstract
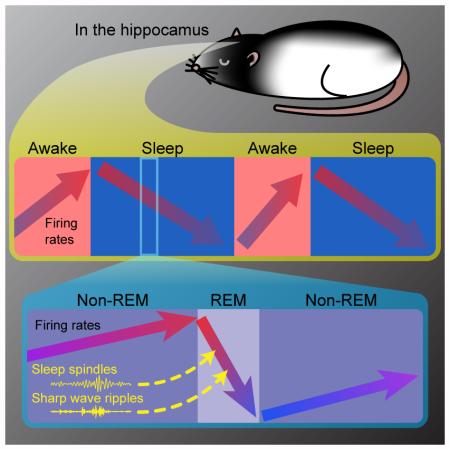
It has been hypothesized that waking leads to higher firing neurons, with increased energy expenditure, and sleep serves to return activity to baseline levels. Oscillatory activity patterns during different stages of sleep may play specific roles in this process, but consensus has been missing. To evaluate these phenomena in the hippocampus, we recorded from region CA1 neurons in rats across the 24-hr cycle and found that their firing increased upon waking, and decreased 11 % per hr across sleep. Waking and sleep also affected lower and higher firing neurons differently. Interestingly, the incidences of sleep spindles and sharp-wave ripples (SWRs), typically associated with cortical plasticity, were predictive of ensuing firing changes and more robustly than were other oscillatory events. Spindles and SWRs were initiated during non-REM sleep yet the changes were incorporated in the network over the following REM sleep epoch. These findings indicate an important role for spindles and SWRs and provide novel evidence of a symbiotic relationship between non-REM and REM stages of sleep in the homeostatic regulation of neuronal activity.
INTRODUCTION
Sleep is a biological necessity and strongly affects brain activity. Neuronal activity patterns during mammalian sleep vacillate between REM (rapid eye movement) and non-REM sleep. Each of these states features distinct oscillatory events that carry out specific functions. Slow waves, thalamocortical sleep spindles and hippocampal sharp-wave ripples (SWRs) during non-REM sleep and theta oscillations and pontine-waves during REM, have been separately and together associated with memory processes and synaptic plasticity [1-3]. Nevertheless, the functions of sleep states and related oscillations remain debated. Sleep’s role is also restorative for the body and the brain. We hypothesized that this would be evident in the rate of action potentials generated by neurons. We therefore investigated spiking activity changes over sleep and waking, and the potential mechanisms for effecting those changes.
Action potentials are costly due to the need to restore ions, vesicles, and neurotransmitters [4], resulting in cellular stress [5]. Additionally, firing rates are affected by and informative of the connectivity within circuits [6, 7]. Recently, Vyazovskiy et al. [8] reported decreased firing in rat barrel cortex across sleep in parallel with decreasing amplitude in slow waves. These slow waves are generated by alternations between UP (depolarized) and DOWN (hyperpolarized) population states during non-REM. Based on this and other evidence, it was argued that DOWN states may provide a mechanism by which synaptic connectivity is downscaled and results in decreased neuronal firing [5, 7]. However, firing changes within epochs were not investigated and no role was prescribed for REM sleep. In contrast, in a report based on the rat hippocampus but not controlled for circadian or sleep-history effects, neuronal firing increased within non-REM sleep epochs [9], but decreased within and following REM sleep. The firing decreases were correlated with the power of theta oscillations during REM. To reconcile these disparate observations, it was suggested that hippocampus and neocortex operate under different rules [7, 9], with REM and non-REM serving different roles depending on brain region. Interestingly, neither study investigated potential roles for spindles and sharp-wave ripples, the oscillatory events most associated with network plasticity [3].
During the course of a day, the brain and body undergo significant variations in sleep pressure, temperature, and plasticity [10]. Apparent differences in studies may arise from differences in time-of-day and sleep-history conditions. To overcome these confounds, we performed long-duration recordings over light and dark cycles in freely moving rats, and analyzed spiking and local field activity in hippocampal region CA1 populations along with neocortical surface electroencephalogram (EEG). We found evidence that hippocampal firing rates are regulated, with decreased firing across extended sleep comprised of sequential epochs of non-REM and REM sleep, and increased firing over time awake. However, in contrast with expectations, we observed relatively weak relationships between the neuronal firing decreases and both theta oscillations and slow waves. Rather, we found that the incidences of spindles and SWRs during non-REM sleep were strongly predictive of firing decreases, following an intervening epoch of REM. These novel findings suggest that the activities effecting homeostatic changes in neuronal circuits are triggered during non-REM but are implemented over REM sleep. Additionally, they point towards a previously unappreciated role in the regulation of cortical firing by oscillatory events frequently associated with learning and plasticity.
RESULTS
Hippocampal firing depends on sleep/wake state
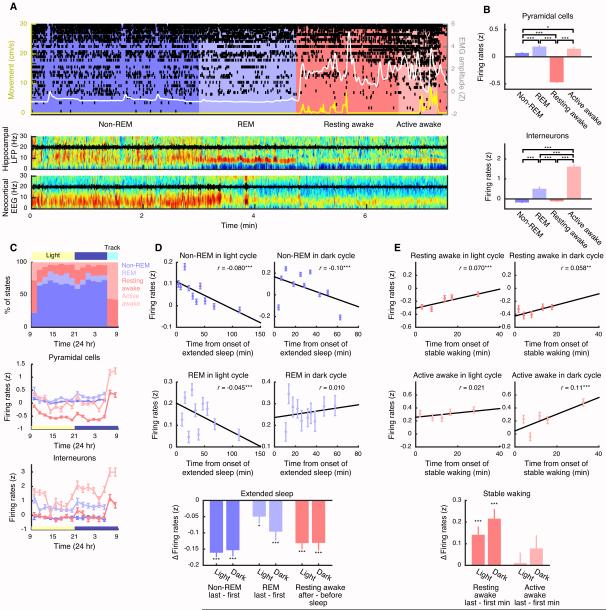
First, we investigated how hippocampal activity varies between different states. We identified REM, non-REM, resting awake, and active awake epochs, using pyramidal layer local field potential (LFP), nuchal electromyograph (EMG) and movement (Figure 1A; see Experimental Procedures and Supplemental Experimental Procedures), and detected and isolated CA1 units in multiple 6–12 hr sessions during dark (9 p.m.–9 a.m.) and light cycles (9 a.m.–9 p.m.) from four animals. We discarded units that did not meet strict criterion for stability (Figure S1), resulting in 1369 putative pyramidal cells (11–98, M=52.7 cells/session) and 160 putative interneurons (0–22, M=6.2 cells/session). We measured firing rates within and across alternating non-REM (lasting 465.9 ± 343.9 s) and REM (lasting 144.0 ± 61.0 s) sleep epochs and detected slow waves (0.5–4 Hz), spindles (9–18 Hz) and SWRs (130–230 Hz) in the LFP. Sleeping patterns and firing rates varied across animals and sessions (e.g. Figure S2A). Nevertheless, some consistent patterns were observed (Figure S2B-E). On average (Figure 1B), pyramidal cell firing was highest during active awake and REM sleep and lowest during resting awake, when the animal was awake but immobile, while interneuron firing was highest during active awake, and lowest during non-REM sleep (see also Figure S2B-F).
Figure 1. Hippocampal firing across different stages of behavior and sleep.
(A) Sample recording with spike rasters (top) from 55 neurons (black). Background colors indicate waking/sleep states determined from head movement (yellow, left axis) EMG (white, right axis), and spectrogram of CA1 pyramidal layer LFP (middle), along with neocortical EEG with raw traces superimposed (black, bottom). (B) Mean firing rates depended on brain states (non-REM (dark blue), REM (light blue), resting awake (dark pink), active awake (light pink); see also Figure S2) in pyramidal cells (n=1017 cells, top) and interneurons (n=116 cells, bottom). One-way ANOVA followed by Tukey-Kramer tests identified significant differences. (C) Fraction of time spent in each state (top) and mean firing rates (pyramidal cells [/interneurons], middle [/bottom]) in 90-min bins, calculated exclusively within each state (per color). Rats repeatedly performed on linear tracks 6 – 9 a.m. every day but otherwise slept and awoke spontaneously in the home cage. Light/dark cycles (yellow/blue) are indicated above or below x-axes. (D) (Top) Mean firing rates of pyramidal cells in non-REM (21449 [/10341] data points from 3892 [/2313] cells in 409 [/177] epochs over 70 [/39] extended sleeps in light [/dark] cycles (left [/right], separated into 10 equally sized groups, with mean and SEM plotted for each group). Regression line (black) was fit through all (ungrouped) data points. (Middle) Mean firing rates in REM over extended sleep (14956 [/5860] data points from 3475 [/1813] cells in 290 [/101] epochs in light [/dark]; left [/right]), separated into 10 equally sized groups. Note shorter extended sleeps during dark. (Bottom) Pairwise comparisons between the first and last epochs in extended sleep in non-REM (3892 [/2313] cells over 70 [/39] extended sleeps in light [/dark]), REM (3475 [/1813] cells), and resting awake immediately preceding and following extended sleep (3728 [/2284] cells). (E) (Top) Mean firing rates of pyramidal cells in resting awake increase with time (4692 [/2651] data points from 1419 [/772] cells in 120 [/42] epochs over 34 [/13] stable waking episodes in light [/dark]; left [/right], separated into 5 equally-sized groups). (Middle) Firing rates in active awake increase over stable waking (4390 [/2728] data points from 1249 [/615] cells in 110 [/43] epochs over 32 [/12] stable waking episodes in light [/dark]; left [/right], separated into 5 equally-sized groups). (Bottom) Pairwise comparisons between the first and last minutes of resting and active awake in stable waking (1419 [/772] cells in resting over 34 /[13] stable waking episodes and 1249 [/615] cells in active awake over 32 [/12] stable waking episodes in light [/dark]; not all stable waking contained active awake). *p < 0.05, ** p < 0.01, *** p < 0.001. Error bars indicate SEM.
Regulation of firing rates across extended sleep and stable waking
Our goal was to test whether sleep and waking produce respective firing decreases and increases, irrespective of time-of-day. Neuronal firing fluctuated during the day, (Figure 1C; e.g. apparent decrease at onset of light, following the track running session), but it was not possible to attribute these changes to either waking or sleep. We therefore focused on “extended sleep,” which we defined as continuous sequences of non-REM and REM epochs lasting in sum > 30 min without interruptions > 60 s. To avoid confounds from state-dependence (i.e. Figure 1B), we averaged and compared firing rates in pyramidal cells (which account for the majority of our units and the bulk of the brain’s energy expenditure [4]; results were similar for interneurons) within individual epochs. Firing rates in non-REM decreased across extended sleep during both light and dark cycles (Figure 1D), showing a significant negative correlation with time, and a significant pairwise decrease between first and last non-REM epochs in extended sleep. These decreases were consistently observed (Figure S2G-I), in both early and late sleep [11], across animals analyzed separately, for subsampled data (≤ 250 neurons/animal, not shown), for raw firing rate measured in Hz, and for epoch (as well as neuron) means (r=−0.136 [/−0.305], p=0.0057 [/3.5 ×10−4], in light [/dark]). Calculated for each neuron separately, decreases (regression slopes) averaged −10.8 ± 1.3% per hour (−14.7 [/−4.3]% in light [/dark]; Figure S2I). Firing rates in REM showed similar pairwise decreases across extended sleep during both light and dark cycles. A significant correlation with time was also observed during light but not dark cycles, likely due the combined effects of shorter extended sleep sequences during dark (Figure S2J) and greater variability during REM (Figure S2K). Significant decreases were further evident when comparing firing in resting awake epochs immediately before and after extended sleep.
In contrast, when animals awoke, firing rates showed a complementary increase (Figure 1E). We defined and detected “stable waking” episodes lasting > 15 min, involving spontaneous resting and active awake epochs in the home cage. Firing rates during resting awake increased significantly with time awake in both light and dark cycles. These increases (regression slopes) averaged 47.3% ± 16.5% per hour (48.6% [/45.2%] in light [/dark]; Figure S2I). Pairwise comparisons showed increased firing between first and last minutes of resting awake during stable waking episodes in both light and dark, and in 3 out of 4 animals evaluated separately (Figure S2H). A significant correlation was also observed between firing rates during active awake epochs and time in stable waking, but only in dark cycles (and not pairwise comparisons), likely due to the sporadic and infrequent timing and variable firing of awake activity in the home cage (Figure S2A,K). In summary, we found that hippocampal firing rates rise quickly during waking and decrease across sleep in both light and dark cycles. We next sought potential mechanisms for the decreases over sleep.
SWRs and spindles during non-REM predict decreased firing
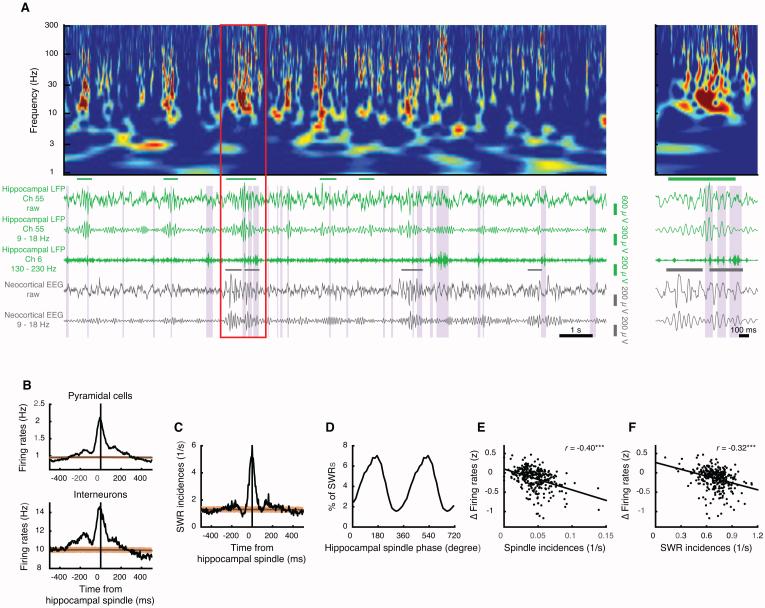
We hypothesized that SWRs and spindles are the most likely candidate mechanisms for producing changes in network firing. SWRs and spindles are well suited for driving plasticity [1, 12-14] and were observable in the hippocampal LFP (Figure 2A-B; [15]), modulating neuronal spiking (Figure S3A). SWRs coincided with spindles (Figure 2C) and were phase-locked to the trough of the spindle oscillation (Figure 2D). We therefore measured the incidence rates of spindles and SWRs and tested whether they predicted firing changes across non-REMi/REM/non-REMi+1 triplet sequences. Remarkably, we found that the incidence of spindles during non-REMi was highly predictive of the decrease in mean firing rates observed in the very next non-REMi+1 epoch (Figure 2E). This relationship remained significant in both light and dark cycles (Figure S3B) and in 3 out of 4 animals evaluated separately (the fourth showed a consistent trend). A similar predictive relationship was observed for the incidence of SWRs (Figure 2F), and in dark (but not light) cycles evaluated separately (Figure S3C). These relationships were not redundant; partial correlations between incidences and across-sleep firing changes were significant for spindles when controlling for SWRs, and vice versa (Figure S3D).
Figure 2. Hippocampal spindles and SWRs coupled with firing rate changes.
(A) Example non-REM wavelet power spectrum (top) of hippocampal LFP (green) alongside neocortical EEG (gray). Band pass filtered traces show channels used to detect spindles (9 – 18 Hz) and SWRs (130 – 230 Hz). Detected hippocampal [/neocortical] spindles are indicated with green [/gray] horizontal bars, and hippocampal SWRs in shaded vertical bands. The region marked with a red rectangle is expanded on the right.
(B) Spindle-peak triggered peri-event time histograms (PETH, 5-ms bins; 19 sessions) demonstrate increased spiking of pyramidal cells (top; 1017 cells) and interneurons (bottom; 116 cells) during spindles (see also Figure S3A). The brown line and bands indicate mean and 95% confidence intervals, obtained from 1-s random jitters of spindle timestamps. (C) Spindle-peak triggered PETH reveals temporal coincidence with SWRs.
(D) SWRs occurred preferentially near the trough (172.5 degrees) of the spindle oscillation (p < 10−300, Rayleigh test) (E,F) In non-REMi/REM/non-REMi+1 sequences, incidence rates of spindles (E) and SWRs (F) in non-REMi were predictive of subsequent changes in mean firing rate between non-REMi and non-REMi+1 (306 sequences in 19 sessions). *** p < 0.001.
To verify the robustness of these relationships, we performed a number of additional analyses. First, both predictive relations persisted even when we excluded the minority of spikes (31.5 ± 0.5 %) fired during spindles and SWRs (not shown). Interestingly, firing and participation rates strictly within these events increased, even while average firing rates decreased across sleep (Figure S3E). Nevertheless, incidence rates of spindles and SWRs covaried with firing rates (r=0.49, p=2.6×10−20 for spindles and r=0.51, p=8.5×10−22 for SWRs). To ensure that this covariation could not explain our observations, we calculated and confirmed significant partial correlations (Figure S3F,G) between both spindles (r=−0.26, p=4.0×10−6) and SWRs (r=−0.15, p=0.094) in non-REMi and firing rate changes when partialling out (controlling for) the spindle/SWR correlations with firing rate. In contrast, there was no significant partial correlation in the reverse direction, when i and i+1 were flipped (Figure S3F,G). Critically, spindles and SWRs predicted firing changes only between non-REM epochs separated by REM but not by waking (Figure S3H). In summary, our observation could not be simply explained by covariability of spindles/SWRs and firing rate or by firing within spindles and SWRs themselves.
We further reasoned that neurons that specifically activate during spindles and SWRs could display greater firing decreases. We compared correlations between the firing rate changes of each neuron and the incidence of spindles/SWRs in which that neuron fired vs. those in which it did not fire. In support of our hypothesis, we found stronger correlations in the former (i.e. fired) case than in the latter (not fired) case (r=−0.19 vs. −0.10, p=3.7×10−13 for spindles, and r=−0.12 vs. r=−0.09, p=0.008 for SWRs, Meng’s z-test).
Firing rates decrease following theta oscillations during REM
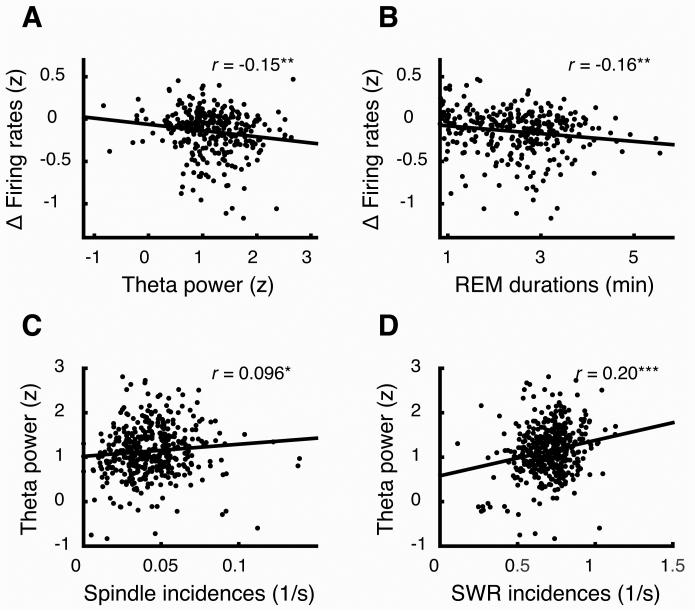
These observations suggest that the key mechanisms for homeostatic regulation of firing occur during non-REM sleep. However, consistent with an earlier study [9], we also found that firing rate decreases across non-REMi/REM/non-REMi+1 sequences correlated with the power of theta oscillations during the intervening REM epochs (r=−0.15, p=0.008; Figure 3A). When the data was split according to time-of-day, this correlation remained significant in light (r=−0.15, p=0.028) though not in dark cycles (r=−0.14, p=0.20; Figure S4A), likely because of diminished statistical power (lower sample size). Interestingly, we also found a significant correlation between the firing decreases and the durations of the REM epochs (r=−0.16, p=0.004; Figure 3B). This correlation was similarly significant in light (r=−0.19, p=0.006) but not in dark cycles (r=−0.14, p=0.19; Figure S4B).
Figure 3. Theta oscillations in REM correlate with activity in non-REM.
(A,B) Changes in firing rates between non-REMi and non-REMi+1 epochs were correlated with the mean theta power (A) and duration (B) in interleaving REM epochs (306 sequences). (C,D) The incidence rates of spindles (C) and SWRs (D) during non-REM were predictive of theta power in the immediately following REM epochs (421 sequences). See also Figure S4. * p < 0.05, *** p < 0.001.
We conjectured that some of these features of REM sleep might in fact be triggered in the preceding non-REM epoch. Indeed, we found that the incidence rates of spindles and SWRs during non-REM epochs were significantly predictive of theta power in the immediately following REM epochs (r=0.096, p=0.048 and r=0.20, p=2.5×10−5 for spindles and SWRs, respectively; Figure 3C,D), though not of the REM epoch durations (r=−0.03, p=0.54, and r=0.04, p=0.47, respectively). Mean firing rates in non-REM were also predictive of theta power during the following REM (r=0.22, p=7.6 ×10−5). Yet, unlike for spindles and SWRs, the partial correlation between theta power and firing changes was not significant when partialling out for mean firing rate (r=−0.07, p=0.20). Overall, these observations suggest that the mechanisms for sleep-dependent firing rate decreases originate in non-REM sleep, but REM may provide a window (the longer the better) for incorporating the changes within the network.
Progressive decrease of hippocampal slow wave activity over sleep
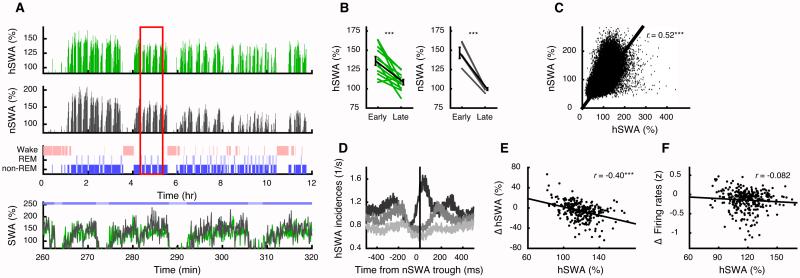
Slow waves, arising from 0.5–4 Hz transitions between UP and DOWN states, are another key feature of non-REM sleep. The amplitude of the neocortical slow wave has been proposed to reflect sleep pressure [16, 17] as well as firing rate homeostasis [8, 18]. While CA1 cells do not show bistable UP/DOWN membrane potentials [19, 20], recent work indicates that slow waves can propagate the hippocampus from the entorhinal cortex [19-21]. We therefore asked whether slow wave activity in the hippocampus (hSWA) shows similar patterns. In two animals, we detected slow wave activity in the neocortical EEG (nSWA) over the frontal lobe, and as expected, observed decreasing nSWA across the light cycle [16, 17] (Figure 4A,B). We then performed these same measurements on the concurrent hippocampal LFP and observed robust hSWA that similarly decreased across the light cycle (Figure 4A,B; see also Figure S5). hSWA was not volume-conducted, but reflected local neuronal spiking (Figure S5A-C). The timing of spindles and SWRs were likewise modulated [22, 23], reaching a maximum 20 ms following the slow wave peak (Figure S5D,E). Overall, hSWA and nSWA covaried (Figure 4A,C), with neocortex leading the hippocampus (Figure 4D). Within non-REM epochs, hSWA and nSWA increased, often reaching an asymptote and dropping during intervening REM and waking epochs. These patterns were well-described by the “process S” model [17](Figure 4E and Figure S5F), indicating that hSWA patterns are consistent with sleep regulation and, potentially, synaptic homeostasis [17, 18].
Figure 4. Hippocampal slow waves reflect sleep homeostasis but not firing changes.
(A) Slow wave amplitudes in hippocampus (hSWA; green) and neocortex (nSWA; gray), measured as a percentage of the 12-hr mean and averaged in 1-min bins, decrease across a sample light cycle. Third row shows the hypnogram. Red box is expanded below with 5-s bins. hSWA and nSWA show concomitant increases and decreases (colors above indicate sleep/wake states, as in Figure 1). (B) hSWA (left, 12 sessions) and nSWA (right, 5 sessions) decreased significantly across the light cycle. Mean amplitudes were compared from first hr in early sleep and last hr in late sleep. Error bars indicate SEM.
(C) Mean slow wave amplitudes were correlated between hippocampus and neocortex (5-s bins, r=0.52). (D) Hippocampal slow wave peaks were more likely to follow neocortical slow waves (low, middle, and high amplitude thirds in corresponding shades of gray; high amplitude peak at lag 25 ms). (E). Changes in hSWA across non-REMi/REM/non-REMi+1 sequences were correlated with the hSWA in non-REMi. (F) In contrast, hSWA was not correlated with firing rate changes between non-REMi and non-REMi+1. See also Figure S5. *** p < 0.001.
Firing rates decrease independently of slow wave activity
Slow waves, and DOWN states in particular, were proposed as a mechanism for downscaling cortical connectivity [5, 7, 24, 25], which could produce lower firing across sleep [6]. However, we failed to find a significant correlation between hSWA and firing rate changes between non-REMi+1 and non-REMi (Figure 4F). Neither were across-epoch firing rate decreases significantly predicted by the incidence, duration, nor fraction of DOWN states (periods > 50 ms with no unit spikes [8, 9]) during non-REMi (Figure S5G-I). We did, however, find a significant correlation with the mean power in the delta (< 4 Hz) band (r=−0.17, p=0.003; Figure S5J), an alternate measure of slow wave activity. However, this correlation was weaker than the ones for spindles (p=6.4 ×10−4, Meng’s z-test) or SWRs (p=0.008, Meng’s z-test).
SWRs and spindles were most predictive of firing decreases during sleep
To determine the most effective predictor of firing rate decreases, we performed multiple regression analyses with the difference in firing rate between non-REMi and non-REMi+1 as the dependent variable. Since the incidence rates of spindles and SWRs were correlated (r=0.42, p=1.1×10−14), we performed the analysis twice, with mean delta power, mean theta power, and either spindle incidence or SWR incidence as the independent variables. Both analyses were significantly predictive, with the model including spindle incidence (R2=0.18, p=8.5×10−13) providing greater predictive value over the model with SWRs (R2=0.11, p=2.1×10−7). Spindle incidence (b=−0.38, p=1.7×10−11) and SWR incidence (b=−0.29, p=7.8×10−6) explained a significant fraction of the variance in firing rates. Theta power explained a smaller but still significant fraction of the variance in the model with spindles (b=−0.11, p=0.03) but not in the model with SWRs (b=−0.08, p=0.15). On the other hand, delta power did not significantly explain the variance in either case (b=−0.054, p=0.31 and b=−0.009, p=0.88 for spindle and SWR models, respectively). We therefore conclude that spindles and SWRs during non-REM sleep were the most reliable predictors of hippocampal firing rate decreases across sleep.
Enhanced firing following prolonged waking
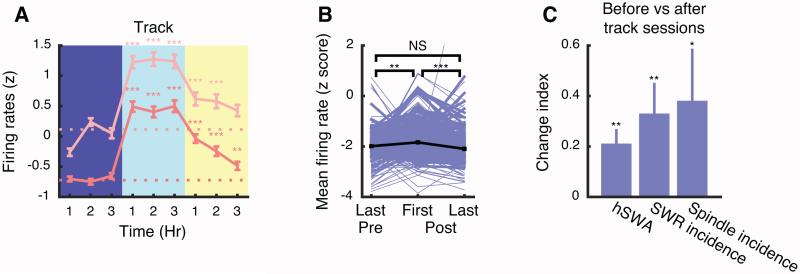
We saw that firing rates increased rapidly during spontaneous waking in the home cage (Figure 1E). We then tested whether firing would increase further after prolonged active waking behavior. We recorded seven sessions from three animals during extended track running at the end of the dark cycle (6–9 am) and clustered the units with 3 hrs in the home cage before and after (Figure S6). Firing rates were highest during track running, but reached a plateau. Nevertheless, this enhanced firing persisted during both active and resting awake after the track (Figure 5A), partially due to the stimulating effect of novel environments [26], even though movement was similar before and after the track (speed ΔM=0.77±0.64 cm/s, p=0.24; acceleration ΔM=0.55±0.59 cm/s2, p=0.36, paired t-test, during active awake). Firing rates, hSWA, and the incidence rates of SWRs and spindles showed similar increases in the first non-REM epochs after the track, but, importantly, firing subsequently dissipated (Figure 5B-C). SWRs were also seen outside of non-REM, during resting waking. However, we failed to find a significant correlation between the incidence of waking SWRs and firing rates changes over sequential [resting awake]i to [resting awake]i+1 epochs; Figure S3I) during either track sessions (r=−0.058, p=0.30) or stable waking in the home cage (r=−0.13, p=0.36) . These observations further demonstrate the excitable effects of waking and the homeostatic effects specific to sleep on neuronal spiking activity.
Figure 5. Extended activity enhances neuronal firing.
(A) Mean pyramidal cell firing rates in resting awake (dark pink) and active awake (light pink) epochs increased during prolonged track sessions and gradually decreased following post-track sleep (352 cells in 7 sessions from 3 animals; see examples in Figure S6). Bins in which firing rates were significantly higher than the pre-track 3-hr average (horizontal dotted lines) are marked,(Tukey–Kramer test). (B) Comparing non-REM sleep before and after the track session, we observed an increase in firing rates in first non-REM epoch after the track session compared to the last non-REM epoch before the track. This increase dissipated over the course of post-track sleep. Significant differences were tested with Tukey-Kramer following one-way ANOVA (p=7.9 ×10−7). (C) Comparing non-REM sleep before and after track sessions, mean hSWA, SWR incidence, and spindle incidence increased. * p < 0.05, ** p < 0.01, *** p < 0.001. Error bars indicate SEM.
Sleep and waking effects across the population of neurons
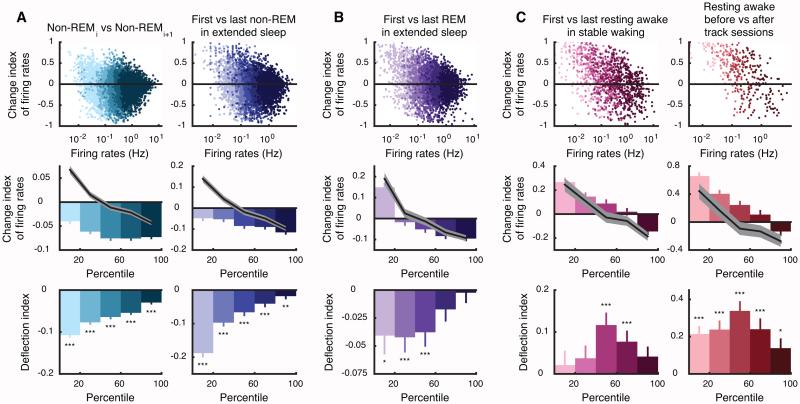
Finally, we asked how changes affect neurons with different initial firing rates. Since regression towards the mean affects lower and higher firing neurons differently, we created 10,000 surrogate “null” sleep sequences by shuffling before and after (i and i+1) indices. We measured changes relative to the surrogate mean (“deflection index”) within each rank-ordered quintile. Across sleep, all quintiles showed decreased firing. However, one-way ANOVA revealed that the amount of change between sequential non-REMi vs. non-REMi+1 epochs depended significantly on the firing rate quintile in non-REMi (p=1.2×10−56 one-way ANOVA; Figure 6A). Surprisingly, the lower firing cells appeared to show the largest relative firing decreases. A somewhat similar pattern was seen for firing rates within REM epochs (Figure 6B). Meanwhile, comparisons in the resting awake state (Figure 6C) showed increased firing across the population, with significances in the median quintile following stable waking and in all quintiles following 3-hour track sessions. ANOVA could not detect significant differences across quintiles for REM and resting awake, perhaps because of the higher variability in these states (Figure S2K). These findings indicate that the relative excitability of neurons is ever-changing over waking and sleep, with each state preferentially affecting different populations.
Figure 6. Differential effects of sleep and waking on firing rates.
(A) Top panels show the change indices of firing rates between non-REMi and non-REMi+1 (left, 16081 cells in 306 sequences) and between first and last non-REM epochs in extended sleep (right, 5906 cells in 109 extended sleeps) as functions of firing rates (color shades correspond to twenty percentile quintiles) during non-REMi (left) and the first non-REM (right). Mean change index within each quintile is shown in middle panels, with shuffled mean (black) and 95% confidence intervals gray). The deflection indices of firing rates (bottom panels; defined relative to shuffled data, see Experimental Procedures) showed non-uniform and significant decreases across bins (p=1.2×10−56 for non-REMi vs non-REMi+1, p=5.2×10-47 for first vs last non-REM, one-way ANOVA) with largest relative decrease in lowest firing cells. (B) Firing rates in REM showed smaller decreases that were still significant for most quintiles. (C) Following stable waking, firing rates increased in higher-firing neurons (left, 1820 cells in 38 stable wakings) and for all neurons following the track sessions (right, 352 cells in 7 sessions). * p < 0.05, ** p < 0.01, *** p < 0.001. Error bars indicate SEM.
DISCUSSION
The processes that take place during sleep significantly affect the functions of neurons, their firing dynamics, network connections, and related energy expenditures [5, 7, 27]. Here, we observed an overall 11% per hour decrease in firing during sleep, which has important consequences for the organism. Action potentials are energetically costly, resulting in as much adenosine triphosphate (ATP) consumption as an equal mass of leg muscle tissue during a marathon [4, 28]. Higher firing and vesicle release also translate into higher metabolic and respiration rates, producing greater oxidative stress, and potentially resulting in cellular damage, accelerated aging, neurodegeneration and cancer [5, 29-31]. Therefore, our findings have significant consequences both for energy expenditure and for the stability and integrity of cortical networks.
Based on apparently conflicting reports, it had been reasoned that the hippocampus and neocortex operate under different rules [7, 9] but our findings demonstrate that activities in these regions are consistent in multiple ways. First, slow waves and spindles are widely considered neocortical oscillations but we found them in the hippocampus as well. These oscillations showed similar across-sleep patterns in both neocortex and hippocampus. Given the absence of direct thalamic projections, except through reuniens, hippocampal spindles and slow waves are in fact likely inherited from the entorhinal cortex [15, 19, 20]. Additionally, the state-dependent firing patterns we observed were consistent with those in the barrel cortex [8]; putative interneurons comprised roughly half of recorded neurons in ref [8], and were weighed more due to their higher firing rates. In our study, interneurons fired most during active waking, decreased in firing from waking to non-REM and increased from non-REM to REM (Figure S2B,E), as was reported for merged populations in the barrel cortex [8]. Thus, our work reconciles previously conflicting accounts and suggests that similar mechanisms are at work throughout the (allo- and neo-) cortex.
Downscaling of synapses, driven by neuronal excitability [32], is a likely candidate for the decreased firing rates we observed [6, 7, 18, 32]. Our findings indicate that this process occurs during sleep, as predicted by the synaptic homeostasis hypothesis [7, 18]. Alternations between network UP and DOWN states were proposed as a mechanism for sleep-dependent regulation of neural activity [7, 24, 25]. Indeed, the ~1 Hz frequency of slow waves during non-REM sleep is consistent with long-term depression (LTD) [33, 34]. However, a predictive relationship between slow wave activity and neuronal firing rates had not been previously tested in large-scale neuronal recordings. The lack of correlation between either slow wave amplitudes or the incidence of DOWN states and the firing rate changes in our data argues against their direct involvement in downscaling neural activity. We did detect a significant effect of power in the delta band, but this effect was explained by covariation with spindles and SWRs. Thus, our data suggests that slow wave activity may serve to downscale synapses largely through coordination of spindles and SWRs [19, 22]. Nevertheless, since hippocampal CA1 neurons do not demonstrate bistable UP/DOWN membrane potentials [19], it remains possible that a weaker DOWN hyperpolarization in hippocampal cells prevented a detectable effect through this mechanism.
Importantly, our results point towards new mechanisms during non-REM sleep for initiating network downscaling, specifically thalamocortical sleep spindles and hippocampal SWRs. Both spindles [14] and SWRs [12, 13] are considered to trigger surges in intracellular Ca2+ levels suitable for synaptic plasticity and have been linked to memory improvements following sleep [3]. These and other observations have led to speculation that reactivation of neuronal ensembles during spindle/SWR complexes, coordinated by slow waves, plays a pivotal role in systems consolidation of memory [1, 3, 19, 22, 35] and may spare ensembles from synaptic downscaling [7, 36]. Our study suggests that they may also play a central role in the regulation of neuronal activity and connectivity.
How can sleep spindles and SWRs trigger decreases in neuronal firing? One possible explanation is that the similarity between reactivated and behavioral sequences decreases rapidly in sleep [37]. Thus, the bulk of sleep firing patterns may not be true “replay,” but noisy and unfaithful to waking, providing a means for net synaptic downscaling. Another possibility is that recurrent conduction delays combined with synchronous population bursts during SWRs produce depression at CA3 synapses [38] (but see [39]). A third possible scenario is that the biophysical mechanisms underlying SWRs serve to downscale synaptic connections, either through dendritic depolarization in the absence of postsynaptic spiking [12, 13, 40], or through the antidromic propagation of spikes [41]. Similarly, during spindles, Ca2+ influx coupled with shunted somatic spiking [14, 23] and an 8-16 Hz timescale [42, 43] are fully consistent with LTD. While additional research is necessary to understand the biophysical details surrounding these events, our findings indicate that mechanisms of memory consolidation and those of homeostatic regulation of neuronal firing may be intimately intertwined.
A possible caveat remains that the correlation between these events and the observed firing rate decreases may arise from a third agent affecting both variables through global excitability. However, we controlled for global excitability (i.e. mean firing rate) in our partial correlation analysis (Figure S3F,G) and we saw no significant partial correlations when before and after indices were flipped. Furthermore, it is unclear how and why this third agent would affect spindles/SWRs more strongly than other events, such as slow waves, a widely-used measure of sleep pressure [16, 17], or theta oscillations [9]. Moreover, none of the neuromodulatory systems [44] appear to follow the activity patterns we observed (Figure S2). Extracellular adenosine, another potential candidate, covaries with sleep pressure and inhibits synaptic transmission and neuronal excitability [45, 46]; but decreasing adenosine levels over sleep would be expected to increase (rather than decrease) excitability [46]. In sum, we find it unlikely that a global modulator simultaneously and coincidentally modifies spindles/SWRs in an epoch and then decreases firing in the subsequent epoch.
The question remains what, if any, role does REM sleep play in these homeostatic processes? Our partial correlation analysis indicated that theta power during REM was not a strong predictor of firing rate decreases. Moreover, the power of theta during REM was predicted by the rate of spindles in the preceding non-REM, indicating that the mechanisms for regulating firing were triggered in non-REM. However, these firing changes were not manifest until an interleaving epoch of REM sleep. The non-REM firing rate decrease across sleep were also correlated with the duration of REM, and firing rates during waking were lower following transitions out of REM sleep than out of non-REM (Figure S2). Our results therefore suggest that REM sleep may play a role in incorporating the processes initiated during non-REM sleep [47, 48].
Experimental Procedures
Animals and data collection
Four male Long-Evans rats were anesthetized with isoflurane and implanted with “Buzsaki 64” silicon probes (NeuroNexus, Ann Arbor, MI) targeting dorsal hippocampus region CA1. In three of the rats, 2 stainless steel wires were placed into the nuchal muscles to measure the electromyograph (EMG). In two of the rats, screws were inserted on the skull above the right frontal lobe for EEG. Movement was tracked with an overhead camera. In total, we analyzed eleven 12-hr sessions and one (interrupted) 6-hr session from the light cycle, seven 9-hr sessions during the dark cycle. In seven additional sessions, we concatenated the data from both light and dark cycles, in order to track neurons from before, during, and after extended track running sessions. Rats were maintained in a home cage except during the last 3 hrs of dark cycles, during which 3 of 4 were put on a linear track in the same room and given water rewards after every traversal. The remaining rat yielded data only during the light cycle. For further details see Supplemental Experimental Procedures.
Data Analysis
Sleep and waking were separated based on EMG and the animal’s movement. Sleep states with high hippocampal theta were classified as REM (rapid eye movement) and the remainder were classified as non-REM. Similarly, waking periods with high theta were labeled “active awake” and the remainder were labeled “resting awake”. In one rat in which EMG signals were not recorded, timing of sleep state transitions was determined based on slow waves, which are observed only in non-REM sleep, and REM was inferred from high theta epochs with no movement, sandwiched between non-REM epochs. Data from this animal was consistent with the rest. Epochs were defined by state transitions into and out of a given state. Interruptions < 10 s were ignored. Unit clustering and cell classification were performed as previously described [49]. Firing rate z-scores were calculated using means and standard deviations (SDs) in one-min bins. Similar results were obtained using non-normalized (Hz) and mean– normalized firing rate measures (e.g. Figure S2I). Unless explicitly stated, data were pooled across light and dark cycles.
Change and Deflection indices
“Change index” for variable X was defined as (X̄post – pre)/(post + pre). To account for regression to the mean, the “deflection index” was defined as the difference between the change index and the mean of 10,000 surrogates obtained by randomly flipping before/after indices. Significance was obtained from the confidence intervals of the surrogate distribution.
Oscillatory event detection
SWRs (130 – 230 Hz) with peak band-passed power greater than 5 SDs of the mean were detected following previously described methods [50]. Hippocampal LFP and neocortical EEG were again band-pass filtered (9 - 18 Hz) and candidate spindles were detected when amplitudes of the Hilbert transform exceeded 1.5 SDs above the mean for > 350 ms [15]. We applied a commonly used method [8, 18] to detect the amplitude of individual slow waves (referred to as slow wave activity) in the neocortical EEG (nSWA) and the hippocampal LFP (hSWA).
Supplementary Material
ACKNOWLEDGEMENTS
We thank Sen Cheng, Kenji Mizuseki, Alfonso Renart, Yuri Saalmann, Eran Stark and Laurel Watkins de Jong for helpful comments and discussions. This work was supported by NIH grants MH103775, NS088798 and MH109170 and the University of Wisconsin-Milwaukee Research Growth Initiative.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
AUTHOR CONTRIBUTIONS
H.M. and K.D. designed the experiments. H.M. carried out the experiments and analyzed the data. H.M. and K.D. wrote the manuscript.
REFERENCES
- 1.Buzsaki G. Two-stage model of memory trace formation: a role for "noisy" brain states. Neuroscience. 1989;31:551–570. doi: 10.1016/0306-4522(89)90423-5. [DOI] [PubMed] [Google Scholar]
- 2.Datta S. Avoidance task training potentiates phasic pontine-wave density in the rat: A mechanism for sleep-dependent plasticity. J Neurosci. 2000;20:8607–8613. doi: 10.1523/JNEUROSCI.20-22-08607.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rasch B, Born J. About sleep's role in memory. Physiol Rev. 2013;93:681–766. doi: 10.1152/physrev.00032.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Howarth C, Gleeson P, Attwell D. Updated energy budgets for neural computation in the neocortex and cerebellum. J Cereb Blood Flow Metab. 2012;32:1222–1232. doi: 10.1038/jcbfm.2012.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vyazovskiy VV, Harris KD. Sleep and the single neuron: the role of global slow oscillations in individual cell rest. Nat Rev Neurosci. 2013;14:443–451. doi: 10.1038/nrn3494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Olcese U, Esser SK, Tononi G. Sleep and synaptic renormalization: a computational study. J Neurophysiol. 2010;104:3476–3493. doi: 10.1152/jn.00593.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tononi G, Cirelli C. Sleep and the price of plasticity: from synaptic and cellular homeostasis to memory consolidation and integration. Neuron. 2014;81:12–34. doi: 10.1016/j.neuron.2013.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vyazovskiy VV, Olcese U, Lazimy YM, Faraguna U, Esser SK, Williams JC, Cirelli C, Tononi G. Cortical firing and sleep homeostasis. Neuron. 2009;63:865–878. doi: 10.1016/j.neuron.2009.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Grosmark AD, Mizuseki K, Pastalkova E, Diba K, Buzsaki G. REM sleep reorganizes hippocampal excitability. Neuron. 2012;75:1001–1007. doi: 10.1016/j.neuron.2012.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Herzog ED. Neurons and networks in daily rhythms. Nat Rev Neurosci. 2007;8:790–802. doi: 10.1038/nrn2215. [DOI] [PubMed] [Google Scholar]
- 11.Genzel L, Kroes MC, Dresler M, Battaglia FP. Light sleep versus slow wave sleep in memory consolidation: a question of global versus local processes? Trends Neurosci. 2014;37:10–19. doi: 10.1016/j.tins.2013.10.002. [DOI] [PubMed] [Google Scholar]
- 12.English DF, Peyrache A, Stark E, Roux L, Vallentin D, Long MA, Buzsaki G. Excitation and inhibition compete to control spiking during hippocampal ripples: intracellular study in behaving mice. J Neurosci. 2014;34:16509–16517. doi: 10.1523/JNEUROSCI.2600-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kamondi A, Acsady L, Buzsaki G. Dendritic spikes are enhanced by cooperative network activity in the intact hippocampus. J Neurosci. 1998;18:3919–3928. doi: 10.1523/JNEUROSCI.18-10-03919.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sejnowski TJ, Destexhe A. Why do we sleep? Brain Res. 2000;886:208–223. doi: 10.1016/s0006-8993(00)03007-9. [DOI] [PubMed] [Google Scholar]
- 15.Sullivan D, Mizuseki K, Sorgi A, Buzsaki G. Comparison of sleep spindles and theta oscillations in the hippocampus. J Neurosci. 2014;34:662–674. doi: 10.1523/JNEUROSCI.0552-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Achermann P, Dijk DJ, Brunner DP, Borbely AA. A model of human sleep homeostasis based on EEG slow-wave activity: quantitative comparison of data and simulations. Brain Res Bull. 1993;31:97–113. doi: 10.1016/0361-9230(93)90016-5. [DOI] [PubMed] [Google Scholar]
- 17.Borbely AA, Achermann P. Sleep homeostasis and models of sleep regulation. J Biol Rhythms. 1999;14:557–568. doi: 10.1177/074873099129000894. [DOI] [PubMed] [Google Scholar]
- 18.Vyazovskiy VV, Cirelli C, Tononi G. Electrophysiological correlates of sleep homeostasis in freely behaving rats. Prog Brain Res. 2011;193:17–38. doi: 10.1016/B978-0-444-53839-0.00002-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Isomura Y, Sirota A, Ozen S, Montgomery S, Mizuseki K, Henze DA, Buzsaki G. Integration and segregation of activity in entorhinal-hippocampal subregions by neocortical slow oscillations. Neuron. 2006;52:871–882. doi: 10.1016/j.neuron.2006.10.023. [DOI] [PubMed] [Google Scholar]
- 20.Hahn TT, Sakmann B, Mehta MR. Differential responses of hippocampal subfields to cortical up-down states. Proc Natl Acad Sci. 2007;104:5169–5174. doi: 10.1073/pnas.0700222104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wolansky T, Clement EA, Peters SR, Palczak MA, Dickson CT. Hippocampal slow oscillation: a novel EEG state and its coordination with ongoing neocortical activity. J Neurosci. 2006;26:6213–6229. doi: 10.1523/JNEUROSCI.5594-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sirota A, Csicsvari J, Buhl D, Buzsaki G. Communication between neocortex and hippocampus during sleep in rodents. Proc Natl Acad Sci U S A. 2003;100:2065–2069. doi: 10.1073/pnas.0437938100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Peyrache A, Battaglia FP, Destexhe A. Inhibition recruitment in prefrontal cortex during sleep spindles and gating of hippocampal inputs. Proc Natl Acad Sci U S A. 2011;108:17207–17212. doi: 10.1073/pnas.1103612108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tononi G, Cirelli C. Sleep function and synaptic homeostasis. Sleep Med Rev. 2006;10:49–62. doi: 10.1016/j.smrv.2005.05.002. [DOI] [PubMed] [Google Scholar]
- 25.Tononi G, Cirelli C. Sleep and synaptic homeostasis: a hypothesis. Brain Res Bull. 2003;62:143–150. doi: 10.1016/j.brainresbull.2003.09.004. [DOI] [PubMed] [Google Scholar]
- 26.Larkin MC, Lykken C, Tye LD, Wickelgren JG, Frank LM. Hippocampal output area CA1 broadcasts a generalized novelty signal during an object-place recognition task. Hippocampus. 2014;24:773–783. doi: 10.1002/hipo.22268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schmidt MH. The energy allocation function of sleep: A unifying theory of sleep, torpor, and continuous wakefulness. Neurosci Biobehav Rev. 2014;47C:122–153. doi: 10.1016/j.neubiorev.2014.08.001. [DOI] [PubMed] [Google Scholar]
- 28.Schonfeld P, Reiser G. Why does brain metabolism not favor burning of fatty acids to provide energy? Reflections on disadvantages of the use of free fatty acids as fuel for brain. J Cereb Blood Flow Metab. 2013;33:1493–1499. doi: 10.1038/jcbfm.2013.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lin MT, Beal MF. Mitochondrial dysfunction and oxidative stress in neurodegenerative diseases. Nature. 2006;443:787–795. doi: 10.1038/nature05292. [DOI] [PubMed] [Google Scholar]
- 30.Fang M, Shen Z, Huang S, Zhao L, Chen S, Mak TW, Wang X. The ER UDPase ENTPD5 promotes protein N-glycosylation, the Warburg effect, and proliferation in the PTEN pathway. Cell. 2010;143:711–724. doi: 10.1016/j.cell.2010.10.010. [DOI] [PubMed] [Google Scholar]
- 31.Coyle JT, Puttfarcken P. Oxidative stress, glutamate, and neurodegenerative disorders. Science. 1993;262:689–695. doi: 10.1126/science.7901908. [DOI] [PubMed] [Google Scholar]
- 32.Turrigiano G. Too many cooks? Intrinsic and synaptic homeostatic mechanisms in cortical circuit refinement. Annual review of neuroscience. 2011;34:89–103. doi: 10.1146/annurev-neuro-060909-153238. [DOI] [PubMed] [Google Scholar]
- 33.Massey PV, Bashir ZI. Long-term depression: multiple forms and implications for brain function. Trends Neurosci. 2007;30:176–184. doi: 10.1016/j.tins.2007.02.005. [DOI] [PubMed] [Google Scholar]
- 34.Nabavi S, Fox R, Proulx CD, Lin JY, Tsien RY, Malinow R. Engineering a memory with LTD and LTP. Nature. 2014;511:348–352. doi: 10.1038/nature13294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Siapas AG, Wilson MA. Coordinated interactions between hippocampal ripples and cortical spindles during slow-wave sleep. Neuron. 1998;21:1123–1128. doi: 10.1016/s0896-6273(00)80629-7. [DOI] [PubMed] [Google Scholar]
- 36.Nere A, Hashmi A, Cirelli C, Tononi G. Sleep-dependent synaptic down-selection (I): modeling the benefits of sleep on memory consolidation and integration. Frontiers in neurology. 2013;4:143. doi: 10.3389/fneur.2013.00143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kudrimoti HS, Barnes CA, McNaughton BL. Reactivation of hippocampal cell assemblies: effects of behavioral state, experience, and EEG dynamics. J Neurosci. 1999;19:4090–4101. doi: 10.1523/JNEUROSCI.19-10-04090.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lubenov EV, Siapas AG. Decoupling through synchrony in neuronal circuits with propagation delays. Neuron. 2008;58:118–131. doi: 10.1016/j.neuron.2008.01.036. [DOI] [PubMed] [Google Scholar]
- 39.Knoblauch A, Hauser F, Gewaltig MO, Korner E, Palm G. Does spike-timing-dependent synaptic plasticity couple or decouple neurons firing in synchrony? Front Comput Neurosci. 2012;6:55. doi: 10.3389/fncom.2012.00055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Christie BR, Magee JC, Johnston D. The role of dendritic action potentials and Ca2+ influx in the induction of homosynaptic long-term depression in hippocampal CA1 pyramidal neurons. Learn Mem. 1996;3:160–169. doi: 10.1101/lm.3.2-3.160. [DOI] [PubMed] [Google Scholar]
- 41.Bukalo O, Campanac E, Hoffman DA, Fields RD. Synaptic plasticity by antidromic firing during hippocampal network oscillations. Proc Natl Acad Sci U S A. 2013;110:5175–5180. doi: 10.1073/pnas.1210735110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Werk CM, Klein HS, Nesbitt CE, Chapman CA. Long-term depression in the sensorimotor cortex induced by repeated delivery of 10 Hz trains in vivo. Neuroscience. 2006;140:13–20. doi: 10.1016/j.neuroscience.2006.02.004. [DOI] [PubMed] [Google Scholar]
- 43.Rosanova M, Ulrich D. Pattern-specific associative long-term potentiation induced by a sleep spindle-related spike train. J Neurosci. 2005;25:9398–9405. doi: 10.1523/JNEUROSCI.2149-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Brown RE, Basheer R, McKenna JT, Strecker RE, McCarley RW. Control of sleep and wakefulness. Physiol Rev. 2012;92:1087–1187. doi: 10.1152/physrev.00032.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Halassa MM, Florian C, Fellin T, Munoz JR, Lee SY, Abel T, Haydon PG, Frank MG. Astrocytic modulation of sleep homeostasis and cognitive consequences of sleep loss. Neuron. 2009;61:213–219. doi: 10.1016/j.neuron.2008.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Halassa MM, Haydon PG. Integrated brain circuits: astrocytic networks modulate neuronal activity and behavior. Annual review of physiology. 2010;72:335–355. doi: 10.1146/annurev-physiol-021909-135843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Diekelmann S, Born J. The memory function of sleep. Nat Rev Neurosci. 2010;11:114–126. doi: 10.1038/nrn2762. [DOI] [PubMed] [Google Scholar]
- 48.Luo J, Phan TX, Yang Y, Garelick MG, Storm DR. Increases in cAMP, MAPK activity, and CREB phosphorylation during REM sleep: implications for REM sleep and memory consolidation. J Neurosci. 2013;33:6460–6468. doi: 10.1523/JNEUROSCI.5018-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Diba K, Buzsaki G. Hippocampal network dynamics constrain the time lag between pyramidal cells across modified environments. J Neurosci. 2008;28:13448–13456. doi: 10.1523/JNEUROSCI.3824-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Diba K, Amarasingham A, Mizuseki K, Buzsáki G. Millisecond timescale synchrony among hippocampal neurons. J Neurosci. 2014;34:14984–14994. doi: 10.1523/JNEUROSCI.1091-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.