Abstract
Background
While an increased risk of thyroid cancer from post-Chernobyl exposure to Iodine-131 (I-131) in children and adolescents has been well-documented, risks of other cancers or leukemia as a result of residence in radioactively contaminated areas remain uncertain.
Methods
We studied non-thyroid cancer incidence in a cohort of about 12,000 individuals from Belarus exposed under age of 18 years to Chernobyl fallout (median age at the time of Chernobyl accident of 7.9 years). During 15 years of follow-up from1997 through 2011, 54 incident cancers excluding thyroid were identified in the study cohort with 142,968 person-years at risk. We performed Standardized Incidence Ratio (SIR) analysis of all solid cancers excluding thyroid (n=42), of leukemia (n=6) and of lymphoma (n=6).
Results
We found no significant increase in the incidence of non-thyroid solid cancer (SIR=0.83, 95% Confidence Interval [CI]: 0.61; 1.11), lymphoma (SIR=0.66, 95% CI: 0.26; 1.33) or leukemia (SIR=1.78, 95% CI: 0.71; 3.61) in the study cohort as compared with the sex-, age- and calendar-time-specific national rates. These findings may in part reflect the relatively young age of study subjects (median attained age of 33.4years), and long latency for some radiation-related solid cancers.
Conclusions
We found no evidence of statistically significant increases in solid cancer, lymphoma and leukemia incidence 25 years after childhood exposure in the study cohort; however, it is important to continue follow-up non-thyroid cancers in individuals exposed to low-level radiation at radiosensitive ages.
Keywords: standardized incidence ratio (SIR), solid cancer, leukemia, lymphoma, Chernobyl
Introduction
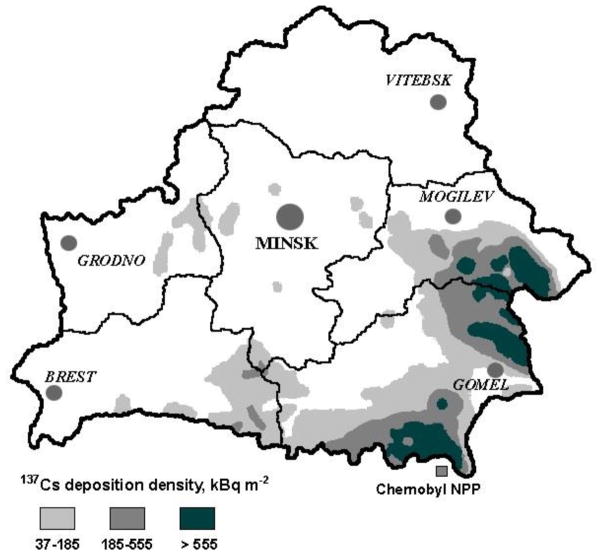
The 1986 accident at the Chernobyl nuclear power plant in Ukraine, about 10 km away from the border of Belarus, caused massive releases of a mixture of radionuclides into the surrounding territories, including short-lived iodine-131 (131I, half-life 8 days) and long-lived cesium-134 (134Cs, half-life 2 years) and cesium-137 (137Cs, half-life 30 years) (UNSCEAR, 2010). A large number of people residing in contaminated territories received internal (mainly from ingestion of radioactively contaminated products) and external (from radionuclides deposited on the ground) irradiation. Radioactive iodine exposure is primarily to the thyroid gland, while radioactive cesium exposure can involve all body organs. In Belarus, the highest densities of 137Cs ground deposition occurred in Gomel oblast (Figure), where the area residents could have received average effective whole-body doses of about 10 millisievert (mSv) accrued during the period of 1986 – 2005 (Drozdovitch et al., 2007).
Figure.
Map of the Republic of Belarus with indication of Caesium-137 (137Cs) deposition density
Health consequences, especially long-term cancer effects, of radiation exposure, among young, and ostensibly sensitive groups of exposed residents are of special concern. While an increased risk of thyroid cancer from post-Chernobyl exposure to 131I in children and adolescents has been well-documented (Brenner et al., 2011; Cardis et al., 2005; Ivanov et al., 2006; Tronko et al., 2006; Zablotska et al., 2011), risks of other cancers or leukemia as a result of residence in radioactively contaminated areas remain uncertain (Davis et al., 2006; Ivanov et al., 1998; Noshchenko et al., 2010; Parkin et al., 1996; Petridou et al., 1996; Tondel et al., 1996). Some studies have reported an increased risk of infant leukemia following in utero exposure (Petridou et al., 1996), others of childhood leukemia following exposure at age of 0 – 5 years (Noshchenko et al., 2010), but most studies found no evidence of an association between leukemia risk and environmental radiation exposure (Davis et al., 2006; Ivanov et al., 1993; Ivanov et al., 1998; Ivanov et al., 2003; Parkin et al., 1996; Steiner et al., 1998; Tondel et al., 1996). Inability to detect radiation-associated cancer risk following environmental exposures could reflect a low dose, insufficient statistical power, or long latency of radiation-related cancers (Cardis et al., 2006). Leukemia and solid cancer risk among individuals exposed in childhood to Chernobyl fallout remain a substantial public concern and require continued monitoring as this group ages in order to evaluate the pattern of radiation-related cancer risks over time.
We have been conducting cancer follow-up of two parallel screening cohorts of ~ 25,000 individuals exposed to Chernobyl fallout as children and adolescents in Ukraine and Belarus (Stezhko et al., 2004). A Standardized Incidence Ratio (SIR) analysis of non-thyroid solid cancer, leukemia and lymphoma in the Ukraine cohort found no significant increased risks although the SIR for leukemia, based on 5 cases, was elevated (SIR=1.92) (Hatch et al., 2015). Here we report on an SIR analysis of solid and hematopoietic cancer incidence rates, excluding thyroid cancer, in a cohort of individuals who were under 18 years of age at the time of the Chernobyl accident, resided in various contaminated territories of Belarus. The study objective was to assess risks of cancer other than thyroid by calculating SIRs using the national sex-, age- and period-specific cancer incidence rates for the period of 01.01.1997 through 31.12.2011.
The study was reviewed and approved by the institutional review boards of the participating organizations in Belarus and the United States.
Materials and Methods
The study cohort included 11,970 individuals from Belarus exposed to radioactive fallout from the Chernobyl accident under age of 18 years. A detailed description of study participants’ recruitment and follow-up is published elsewhere (Stezhko et al., 2004). Between 1996 and 2007, the cohort was regularly screened for thyroid cancer and non-cancer thyroid diseases using standardized clinical, instrumental and laboratory procedures. We excluded 123 of 11,970 screened individuals because they were outside the eligible age range (n=114) or did not reside in Belarus at the time of the accident (n = 9).
We identified incident non-thyroid cancers through linkage of the cohort with the Belarus National Cancer Registry (BNCR) using individual identifying information. The BNCR began operation in 1973 and includes individual information on newly diagnosed cancer cases nationwide since 1978 (Curado et al., 2009; Okeanov et al., 2004). The BNCR adheres to the principles and methods developed by the International Agency for Research on Cancer for cancer registration (Jensen et al., 1991). Information on each newly diagnosed case of malignant tumor is reported by the physician who established the diagnosis, within three days of diagnosis date, to an oncology dispensary at a regional level using a special notification form. At the end of each calendar year computerized individual data from regional oncology dispensaries are sent to the N.N. Alexandrov National Cancer Center of Belarus in Minsk. Since 1991 all oncology dispensaries have used a computerized system of cancer registration allowing continuous data collection. Information collected for each cancer case includes the patient’s last, first and patronymic names, date of birth, sex, place of residence, date and place of cancer diagnosis, methods used for diagnosis verification, cancer diagnosis and its code according to International Classification of Diseases (ICD) and ICD-O for morphological classification. The proportion of microscopically verified cases for all cancer sites except for non-melanoma skin is 91.5% in men and 94.% in women.
For linkage with the BNCR, we used a computerized deterministic record linkage technique with probabilistic elements which has proved to be a reliable tool for cohort studies requiring linkage with a continuously updated registry (Howe, 1998). The technique incorporates a set of comparison functions based on the value of each identifier on each record. Through these computerized comparisons, a probability is estimated that matched records are a true link. We used individuals’ gender, full name (last, first, patronymic), date of birth (day, month and year), and complete residential address as link identifiers. For each pair of matched records, the values of computerized comparison functions were summed up to establish empirically a threshold value which prevents missing true links. All links with a threshold value suggesting that the matched records were a possible link were reviewed manually by independent experts. Only after manual reviews was a link determined to be ‘true’ or ‘false’.
We calculated person-years at risk for each study subject starting from January 1, 1997 through December 31, 2011, date of first primary cancer diagnosis or date of death, whatever came first. We chose 01.01.1997 as the beginning of follow-up because this is when a full-scale screening of the cohort began; we chose 31.12.2011 as the end of follow-up because this is when the most complete cancer incidence data for the cohort became available.
We stratified person-years by sex, oblast [an administrative subdivision similar to a state or province] of residence at the time of screening examination (Gomel, Minsk, Mogilev and other), attained age (5-year categories from 5 to 45 years), age at exposure (5-year categories from 0 to 20 years), and calendar period (1997 – 2003 – 2004 – 2007 – 2008 – 2011). We calculated standardized incidence ratios (SIR) by comparing the number of observed cases with the number of expected cases using national cancer incidence statistics stratified by sex, age and calendar period (Curado et al., 2009; Parkin et al., 1997; Parkin et al., 2002). All analyses were performed using Epicure software (Preston et al., 1993) assuming Poisson distribution of the data. Significance tests and 95% confidence intervals (CI) were determined directly from maximum likelihood analysis. All p-values were derived from two-sided tests, with p ≤ 0.05 considered to be statistically significant. We performed SIR analysis for all solid cancer excluding thyroid, and separately for leukemia, one of the most radiosensitive malignancies, and lymphoma.
Results
Men comprised about 49% of the study cohort (Table 1). Sixty-one percent of the cohort (n=7,275) resided in Gomel oblast which had the highest levels of radioactive contamination. About 30% of the study cohort resided in Minsk oblast including Minsk city. Sixty-two percent of the cohort (n=7,359) were less than 10 years old at the time of the accident, and median attained age of study subjects at the end of follow-up was 33.4 years. During 15 years of follow-up with 142,968 person-years at risk, 54 malignancies, excluding thyroid cancer, were identified in the cohort: 42 solid cancers, six lymphomas, and six leukemias. The most frequent sites of solid cancer were female genital organs (12 cancers of cervix uteri, two ovarian cancers and cancer of corpus uteri), central nervous system [CNS] (three brain cancers, one cancer of eye and two cancers of other parts of CNS), female breast (five cases), and digestive tract (four stomach and one colon cancers). Four of six lymphomas were Hodgkin lymphomas. Leukemias included three myeloid (acute myeloblastic, acute promyelocytic, and chronic myeloid leukemia), one T-cell large granular lymphocytic leukemia, one acute megakaryoblastic leukaemia, and one leukaemic reticuloendotheliosis.
Table 1.
Main characteristics of a cohort of individuals exposed under age of 18 years in Belarus to the Chernobyl fallout, follow-up 01.01.1997 – 31.12.2011
| Characteristic | # of subjects | % | PYR |
|---|---|---|---|
| Sex | |||
| Men | 5,752 | 48.6 | 69,521 |
| Women | 6,095 | 51.4 | 73,447 |
| Oblast of residence at the time of screening | |||
| Gomel | 7,275 | 61.4 | 87,486 |
| Minsk including Minsk city | 3,500 | 29.6 | 42,417 |
| Mogilev | 613 | 5.2 | 7,467 |
| Other | 459 | 3.9 | 5,598 |
| Age at the time of the accident, years | |||
| 0 – 9 | 7,359 | 62.1 | 89,679 |
| 10 – 14 | 3,111 | 26.3 | 37,025 |
| 15 – 18 | 1,377 | 11.6 | 16,264 |
| Median age at the time of the accident, years (IQR) | 7.9 (8.4) | ||
| Median attained age, years (IQR) | 33.4 (8.5) | ||
| Total | 11,847 | 100.0 | 142,968 |
PYR, person-years at risk; IQR, interquartile range
After stratification by sex, attained age and time period, the SIR estimates for solid cancer excluding thyroid and for lymphoma were 17% and 34%, respectively, lower than the corresponding national rates (SIR=0.83, 95% CI: 0.61 – 1.11 for non-thyroid solid cancers and SIR=0.66, 95% CI: 0.26 – 1.33 for lymphoma), although not statistically significant (Table 2). The leukemia SIR was almost two times higher than the corresponding national rates (SIR=1.78, 95% CI: 0.71 – 3.61), but statistically non-significant.
Table 2.
Non-thyroid cancer Standardized Incidence Ratio (SIR) estimates by selected characteristics in a cohort of individuals exposed under age of 18 years in Belarus to the Chernobyl fallout, follow-up 01.01.1997 – 31.12.2011
| Characteristic | Solid cancer, except thyroid (C00–C80 except C73) | Lymphoma (C81–C90) | Leukemia (C91–C95) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Cases | SIR, 95%CI | Cases | SIR, 95%CI | Cases | SIR, 95%CI | ||||
| Obs | Exp | Obs | Exp | Obs | Exp | ||||
| Overall | 42 | 50.4 | 0.83 (0.61 – 1.11) | 6 | 9.2 | 0.66 (0.26 – 1.33) | 6 | 3.4 | 1.78 (0.71 – 3.61) |
| Sex | |||||||||
| Men | 12 | 18.2 | 0.66 (0.35 – 1.11) | 2 | 4.1 | 0.49 (0.08 – 1.51) | 3 | 1.8 | 1.66 (0.41 – 4.31) |
| Women | 30 | 32.2 | 0.93 (0.64 – 1.31) | 4 | 5.1 | 0.79(0.25 – 1.83) | 3 | 1.6 | 1.93 (0.48 – 5.00) |
| P for heterogeneity1, df = 1 | 0.30 | 0.57 | 0.86 | ||||||
| Age at the time of the accident, yrs | |||||||||
| 0 – 9 | 15 | 18.4 | 0.82 (0.47 – 1.30) | 4 | 5.8 | 0.69 (0.22 – 1.61) | 2 | 1.97 | 1.01 (0.17 – 3.13) |
| 10 – 14 | 15 | 18.9 | 0.79 (0.46 – 1.27) | 2 | 2.4 | 0.83 (0.14 – 2.57) | 3 | 0.9 | 3.26 (0.81 – 8.45) |
| 15 – 18 | 12 | 13.1 | 0.92 (0.49 – 1.54) | 0 | 1.0 | 0.0 (0.0 – 1.95) | 1 | 0.5 | 2.12 (0.12 – 9.34) |
| P for trend2, df=1 | 0.80 | 0.70 | 0.46 | ||||||
| Attained age, yrs | |||||||||
| 11 – 19 | 2 | 1.3 | 1.50 (0.25 ; 4.64) | 0 | 0.9 | 0.0 (0.0 – 2.19) | 0 | 0.5 | 0.0 (0.0 – 4.38) |
| 20 – 29 | 11 | 14.9 | 0.74 (0.38 – 1.26) | 4 | 5.0 | 0.80 (0.25 – 1.87) | 3 | 1.6 | 1.87 (0.47 – 4.86) |
| 30 – 42 | 29 | 34.1 | 0.85 (0.58 – 1.20) | 2 | 3.3 | 0.61 (0.10 – 1.87) | 3 | 1.3 | 2.27 (0.56 – 5.88) |
| P for trend, df=1 | 0.30 | 0.47 | 0.89 | ||||||
| Oblast of residency at screening | |||||||||
| Gomel | 27 | 32.4 | 0.83 (0.56 – 1.19) | 3 | 5.6 | 0.54 (0.13 – 1.39) | 2 | 2.1 | 0.96 (0.16 – 2.97) |
| Minsk including Minsk city | 11 | 13.7 | 0.80 (0.42 – 1.37) | 2 | 2.7 | 0.73 (0.12 – 2.26) | 4 | 1.0 | 4.09 (1.27 – 9.50) |
| Mogilev | 2 | 2.4 | 0.84 (0.14 – 2.58) | 1 | 0.5 | 2.10 (0.12 – 9.26) | 0 | 0.2 | 0.0 (0.0 – 11.05) |
| Other | 2 | 1.9 | 1.08 (0.18 – 3.33) | 0 | 0.4 | 0.0 (0.0 – 5.39) | 0 | 0.1 | 0.0 (0.0 – 14.68) |
| P for heterogeneity, df = 3 | 0.99 | 0.67 | 0.24 | ||||||
| Calendar time | |||||||||
| 1997 – 2003 | 11 | 9.6 | 1.14 (0.59 – 1.96) | 1 | 3.1 | 0.32 (0.02 – 1.42) | 2 | 1.1 | 1.77 (0.29 – 5.48) |
| 2004 – 2007 | 16 | 15.5 | 1.03 (0.61 – 1.63) | 1 | 3.1 | 0.32 (0.02 – 1.43) | 4 | 1.1 | 3.80 (1.18 – 8.83) |
| 2008 – 2011 | 15 | 25.3 | 0.59 (0.34 – 0.95) | 4 | 3.0 | 1.35 (0.42 – 3.14) | 0 | 1.2 | 0.0 (0.0 – 1.62) |
| P for trend, df =1 | 0.04 | 0.07 | 0.21 | ||||||
SIR, standardized incidence ratio; CI, confidence intervals; Obs, number of observed cases; Exp, number of expected cases; df, degrees of freedom.
p for heterogeneity estimated for a model using categories of age at exposure, attained age and calendar year.
p for trend estimated for a model using age at exposure, attained age or calendar year as continuous variables.
95% CI estimated from the maximum likelihood.
We did not observe statistically significant variation of SIR estimates by sex, age at the time of the accident, attained age, or oblast of residence at the time of screening examination for any study outcome (Table 2). We observed a lower non-thyroid solid cancer SIR in 2008 – 2011 as compared with the estimates in 2004 – 2007 and 1997 – 2003 (SIR=0.59 vs. SIR=1.03 and 1.14, respectively) with p for linear trend = 0.04; however the test for heterogeneity was not statistically significant (p for heterogeneity = 0.16). For leukemia, no single case was observed in 2008 – 2011, while SIRs for 2004 – 2007 and 1997 – 2003 were elevated (SIR=0 vs. SIR=3.88 and SIR=1.77, respectively) with the test of heterogeneity (p for heterogeneity = 0.05), but not linear trend (p for linear trend = 0.21) being statistically significant. Four of six leukemias were registered in Minsk oblast including Minsk city at the time of screening, while the remaining two were in Gomel oblast, resulting in the SIRs of 4.09 (95% CI: 1.27 – 9.50) and 0.96 (95% CI: 0.16 – 2.97), respectively; the test of heterogeneity by oblast was not significant (p for heterogeneity = 0.24).
Discussion
Twenty-five years after the accident, we found no evidence of an increase in incidence of all solid cancers excluding thyroid, of lymphoma or of leukemia in the cohort of almost 12,000 Belarusian residents exposed to Chernobyl fallout in childhood or adolescence. Our results were based on a comparison of sex-, age- and calendar time-specific cancer rates in the study cohort with the respective Belarus national cancer rates through SIR estimation. This cohort and the parallel cohort in Ukraine are both characterized by high thyroid doses of radiation resulting from internal exposure to 131I, which has been linked to an exceedingly high increased risk of thyroid cancer (Brenner et al., 2011; Tronko et al., 2006; Zablotska et al., 2011). The focus of the present analysis was on malignancies other than thyroid cancer, with the main concern being possible effects of exposure to other radionuclides, especially 137Cs.
Belarus along with Ukraine and parts of the Russian Federation was seriously affected by the accident as evidenced by high levels of 137Cs ground contamination (UNSCEAR, 2010). Having a half-life of 30 years, 137Cs is the most important contributor to cumulative radiation doses of the Belarus population through external irradiation from contaminated ground and internal irradiation through consumption of locally produced contaminated food. Because of the even distribution of 137Cs throughout the body, resulting organ doses are relatively uniform. An estimation of effective whole-body dose due to 137Cs exposure at the population-based level suggested low levels of exposure in general (Drozdovitch et al., 2007). For the study cohort participants the mean external dose for the period from 1986 through 2006 was estimated to be 10 mGy and the mean internal dose due to 137Cs incorporation to be 3 mGy (Drozdovitch et al., 2013).
In agreement with the non-thyroid cancer SIR results in the Ukrainian cohort of 13,203 individuals (Hatch et al., 2015), we did not observe a significantly increased SIR for the three major cancer groups, nor did we find statistically significant variation of SIRs by sex, age at exposure, attained age, or oblast of residence for any studied outcome. There was some suggestion of difference in SIRs for both leukemia and non-thyroid solid cancer over time, but the results were inconsistent that could be due to the relatively small number of cancer cases in the study. Specifically, based on six observed cases, there was a suggestive evidence of heterogeneity in the leukemia SIRs over calendar time, but the test for linear trend was not statistically significant. In contrast, there was a suggestion of a significant linear decrease in non-thyroid solid cancers SIR with calendar time, but the test for heterogeneity of the estimates was not statistically significant.
Some increase in solid cancer and leukemia mortality in 1950 – 1990, although not statistically significant, was reported for atomic bomb survivors exposed at age of 0 – 14 years at colon doses below 5 mSv as compared with respective mortality rates in the entire Japanese population (Goto et al., 2012). For those exposed at colon dose from 5 to < 100 mSv, a significant elevation in estimates of standardized mortality ratio (SMR) for all cancers and for solid cancer was observed only in men, with SMRs of 1.25 (p=0.013) and 1.31 (p=0.004), respectively, while leukemia SMRs were less than one both in men (SMR=0.70, p=0.53) and women (SMR=0.61, p=0.52) (Goto et al., 2012).
Leukemia is considered one of the most radiosensitive cancers, with a relatively short latency period after exposure to ionizing radiation (UNSCEAR, 2010). Following the Chernobyl accident there were a few reports of increased risk of infant leukemia following exposure in utero (Petridou et al., 1996), although no clear association with ground contamination levels was observed. Similarly, no evidence of increased leukemia rates after exposure in childhood has been reported by studies conducted in other affected countries (Davis et al., 2006; Ivanov et al., 1998; Ivanov et al., 2003). It bears mentioning that we have observed comparable non-significant elevations in the SIRs for leukemia in both Belarus and Ukraine cohorts (SIR=1.78, 95%CI: 0.71; 3.61 and SIR=1.92, 95%CI: 0.69; 4.13, respectively), based on small numbers of cases (n=6 and n=5). When leukemia data in these two cohorts are combined, the SIR is 1.84 (95% CI: 0.96; 3.16). Given the consistent elevations in rates of leukemia, the most radiosensitive site, there is a need for careful continued monitoring of leukemia trends. No convincing evidence of increased risks of solid cancers other than thyroid following exposure to Chernobyl fallout in childhood has been found in this or other studies (Michaelis et al., 1996; Tondel et al., 1996).
The Belarusian cohort remains young, with a mean age at the end of follow-up of 33.6 years, as about 60% of cohort members were < 10 years old at the time of the accident. The amount of person-years over the follow-up period from 1.1.1997 through 31.12.2011 was 142,986, with 42 non-thyroid solid cancers, six leukemias and six lymphomas. To account for possible losses in the cohort due to death, we performed SIR analysis using person-years adjusted for sex-, age- and calendar time-specific survival rates based on the Belarus national statistics data (http://apps.who.int/gho/data/?theme=main&vid=60140). Because of low mortality rates at the age range of 10 – 45 years, neither the amount of adjusted person-years (141,784 vs. original 142,986) nor the SIRs changed meaningfully. We did not adjust person-years for possible losses due to migration because as was shown in our paper on non-thyroid cancer SIRs in Ukraine, follow-up losses due to migration are very low (< 2%); and because there has been a national cancer registry in Belarus since 1978.
We consider this well-established cohort of individuals exposed at the most radiosensitive ages and followed over time to be a major strength of our study. Linkage with the BNCR, that is a longstanding nationwide registry, ensures complete and reliable cancer ascertainment. The high quality of the BNCR has been recognized by its inclusion in the several editions of IARC’s “Cancer in Five Continents,” a compilation of data from population-based registries worldwide.
We consider lack of individual dose estimates to be the main limitation of our study. Without dose-response analyses, interpretation of risk estimates is challenging. Another limitation is that we excluded from our analysis data on solid cancers and leukemia in the first decade following the accident, when radiation-related increase in leukemia would be likely to occur. It was done because we wished to avoid selection bias as the cohort screening examination started in 01.01.1997. In addition, the young age of study participants and relatively small number of observed cancers to date indicate the need for further follow-up to assess the long-term cancer risks after exposure to Chernobyl fallout. The failure to detect excess cancers in this radiation-exposed cohort could reflect insufficient statistical power, given the presumed low doses and relatively small cohort size. An additional reason is the long latency of many radiation-related cancers, as well as the young age of the cohort. Because leukemia and solid cancer risk among individuals exposed in childhood to Chernobyl fallout remain a substantial public concern, we consider it important to continue monitoring cohorts exposed at sensitive ages in order to evaluate the pattern of radiation-related cancer risks over time.
We monitor cancers in a Belarusian cohort of exposed as children due to Chernobyl
No increase in solid cancer rates was found as compared to the national rates
An elevation of leukemia rates was detected, although statistically insignificant
Results are consistent with those in a cohort of exposed as children in Ukraine
Further monitoring of cancer situation in this cohort is warranted
Acknowledgments
This research was supported by the Intramural Research Program of the NIH, National Cancer Institute. The funding agency had no influence on study design, conduct, or reporting. We acknowledge the contribution of Olga Shcherbina (Republican Scientific and Practical Center for Medical Technologies, Informatization, Administration and Management of Health, Minsk, Belarus) to the study execution.
Footnotes
Conflict of interest statement
None declared.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Evgenia Ostroumova, Email: ostroumovae@iarc.fr.
Maureen Hatch, Email: hatchm@mail.nih.gov.
Alina Brenner, Email: brennera@mail.nih.gov.
Eldar Nadyrov, Email: nadyrov2006@rambler.ru.
Ilya Veyalkin, Email: veyalkin@mail.ru.
Olga Polyanskaya, Email: polyanskaya@tut.by.
Vasilina Yauseyenka, Email: yaus@mail.ru.
Semion Polyakov, Email: spolyakov@belcmt.by.
Leonid Levin, Email: llevin@omr.med.by.
Lydia Zablotska, Email: lydia.zablotska@ucsf.edu.
Alexander Rozhko, Email: rcrm@tut.by.
Kiyohiko Mabuchi, Email: mabuchik@mail.nih.gov.
References
- Brenner AV, Tronko MD, Hatch M, Bogdanova TI, Oliynik VA, Lubin JH, et al. I-131 dose response for incident thyroid cancers in Ukraine related to the Chornobyl accident. Environ Health Perspect. 2011;119(7):933–939. doi: 10.1289/ehp.1002674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardis E, Howe G, Ron E, Bebeshko V, Bogdanova T, Bouville A, et al. Cancer consequences of the Chernobyl accident: 20 years on. J Radiol Prot. 2006;26(2):127–140. doi: 10.1088/0952-4746/26/2/001. [DOI] [PubMed] [Google Scholar]
- Cardis E, Kesminiene A, Ivanov V, Malakhova I, Shibata Y, Khrouch V, et al. Risk of thyroid cancer after exposure to 131I in childhood. J Natl Cancer Inst. 2005;97(10):724–732. doi: 10.1093/jnci/dji129. [DOI] [PubMed] [Google Scholar]
- Curado MP, Edwards B, Shin HR, Storm H, Ferlay J, Heanue M, et al. IARC Scientific Publications N. IX. WHO Press; Geneva: 2009. Cancer incidence in five continents; p. 160. [Google Scholar]
- Davis S, Day RW, Kopecky KJ, Mahoney MC, McCarthy PL, Michalek AM, et al. Childhood leukaemia in Belarus, Russia, and Ukraine following the Chernobyl power station accident: results from an international collaborative population-based case-control study. Int J Epidemiol. 2006;35(2):386–396. doi: 10.1093/ije/dyi220. [DOI] [PubMed] [Google Scholar]
- Drozdovitch V, Bouville A, Chobanova N, Filistovic V, Ilus T, Kovacic M, et al. Radiation exposure to the population of Europe following the Chernobyl accident. Radiat Prot Dosimetry. 2007;123(4):515–528. doi: 10.1093/rpd/ncl528. [DOI] [PubMed] [Google Scholar]
- Drozdovitch V, Minenko V, Khrouch V, Leshcheva S, Gavrilin Y, Khrutchinsky A, et al. Thyroid dose estimates for a cohort of Belarusian children exposed to radiation from the Chernobyl accident. Radiat Res. 2013;179(5):597–609. doi: 10.1667/RR3153.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goto H, Watanabe T, Miyao M, Fukuda H, Sato Y, Oshida Y. Cancer mortality among atomic bomb survivors exposed as children. Environ Health Prev Med. 2012;17(3):228–234. doi: 10.1007/s12199-011-0246-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatch M, Ostroumova E, Brenner A, Federenko Z, Gorokh Y, Zvinchuk O, et al. Non-thyroid cancer in Northern Ukraine in the post-Chernobyl period: Short report. Cancer Epidemiol. 2015;39(3):279–283. doi: 10.1016/j.canep.2015.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howe GR. Use of computerized record linkage in cohort studies. Epidemiol Rev. 1998;20(1):112–121. doi: 10.1093/oxfordjournals.epirev.a017966. [DOI] [PubMed] [Google Scholar]
- Ivanov EP, Tolochko G, Lazarev VS, Shuvaeva L. Child leukaemia after Chernobyl. Nature. 1993;365(6448):702. doi: 10.1038/365702a0. [DOI] [PubMed] [Google Scholar]
- Ivanov EP, Tolochko GV, Shuvaeva LP, Ivanov VE, Iaroshevich RF, Becker S, et al. Infant leukemia in Belarus after the Chernobyl accident. Radiat Environ Biophys. 1998;37(1):53–55. doi: 10.1007/s004110050092. [DOI] [PubMed] [Google Scholar]
- Ivanov VK, Gorski AI, Tsyb AF, Maksioutov MA, Tumanov KA, Vlasov OK. Radiation-epidemiological studies of thyroid cancer incidence among children and adolescents in the Bryansk oblast of Russia after the Chernobyl accident (1991–2001 follow-up period) Radiat Environ Biophys. 2006;45(1):9–16. doi: 10.1007/s00411-006-0039-2. [DOI] [PubMed] [Google Scholar]
- Ivanov VK, Gorskii AI, Tsyb AF, Khaut SE. Incidence of post-Chernobyl leukemia and thyroid cancer in children and adolescents in the Briansk region: evaluation of radiation risks. Vopr Onkol. 2003;49(4):445–449. [PubMed] [Google Scholar]
- Jensen OM, Parkin DM, MacLennan R, Muir CS, Skeet R. Cancer registration: principles and methods. WHO Press; Geneva: 1991. [Google Scholar]
- Michaelis J, Haaf HG, Zollner J, Kaatsch P, Krummenauer F, Berthold F. Case control study of neuroblastoma in west-Germany after the Chernobyl accident. Klin Padiatr. 1996;208(4):172–178. doi: 10.1055/s-2008-1046469. [DOI] [PubMed] [Google Scholar]
- Noshchenko AG, Bondar OY, Drozdova VD. Radiation-induced leukemia among children aged 0–5 years at the time of the Chernobyl accident. Int J Cancer. 2010;127(2):412–426. doi: 10.1002/ijc.24834. [DOI] [PubMed] [Google Scholar]
- Okeanov AE, Sosnovskaya EY, Priatkina OP. National cancer registry to assess trends after the Chernobyl accident. Swiss Med Wkly. 2004;134(43–44):645–649. doi: 10.4414/smw.2004.10221. [DOI] [PubMed] [Google Scholar]
- Parkin DM, Clayton D, Black RJ, Masuyer E, Friedl HP, Ivanov E, et al. Childhood leukaemia in Europe after Chernobyl: 5 year follow-up. Br J Cancer. 1996;73(8):1006–1012. doi: 10.1038/bjc.1996.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parkin DM, Whelan SL, Ferlay J, Raymond L, Young J. IARC Scientific Publications N. VII. WHO Press; Geneva: International Agency for Research on Cancer; 1997. Cancer incidence in five continents; p. 143. [Google Scholar]
- Parkin M, Whealn SL, Ferlay J, Teppo L, Thomas DB. IARC Scientific Publications N. VIII. WHO Press; Geneva: International Agency for Research on Cancer; 2002. Cancer incidence in five continents; p. 155. [Google Scholar]
- Petridou E, Trichopoulos D, Dessypris N, Flytzani V, Haidas S, Kalmanti M, et al. Infant leukaemia after in utero exposure to radiation from Chernobyl. Nature. 1996;382(6589):352–353. doi: 10.1038/382352a0. [DOI] [PubMed] [Google Scholar]
- Preston DL, Lubin JH, Pierce DA, McConney ME. Epicure user’s guide. Hirosoft International Corporation; Seattle, WA: 1993. [Google Scholar]
- Steiner M, Burkart W, Grosche B, Kaletsch U, Michaelis J. Trends in infant leukaemia in West Germany in relation to in utero exposure due to Chernobyl accident. Radiat Environ Biophys. 1998;37(2):87–93. doi: 10.1007/s004110050099. [DOI] [PubMed] [Google Scholar]
- Stezhko VA, Buglova EE, Danilova LI, Drozd VM, Krysenko NA, Lesnikova NR, et al. A cohort study of thyroid cancer and other thyroid diseases after the Chornobyl accident: objectives, design and methods. Radiat Res. 2004;161(4):481–492. doi: 10.1667/3148. [DOI] [PubMed] [Google Scholar]
- Tondel M, Carlsson G, Hardell L, Eriksson M, Jakobsson S, Flodin U, et al. Incidence of neoplasms in ages 0–19 y in parts of Sweden with high 137Cs fallout after the Chernobyl accident. Health Phys. 1996;71(6):947–950. doi: 10.1097/00004032-199612000-00012. [DOI] [PubMed] [Google Scholar]
- Tronko MD, Howe GR, Bogdanova TI, Bouville AC, Epstein OV, Brill AB, et al. A cohort study of thyroid cancer and other thyroid diseases after the chornobyl accident: thyroid cancer in Ukraine detected during first screening. J Natl Cancer Inst. 2006;98(13):897–903. doi: 10.1093/jnci/djj244. [DOI] [PubMed] [Google Scholar]
- UNSCEAR. Sources and effects of ionizing radiation : United Nations Scientific Committee on the Effects of Atomic Radiation : UNSCEAR 2008 report to the General Assembly, with scientific annexes. United Nations; New York: 2010. [Google Scholar]
- Zablotska LB, Ron E, Rozhko AV, Hatch M, Polyanskaya ON, Brenner AV, et al. Thyroid cancer risk in Belarus among children and adolescents exposed to radioiodine after the Chornobyl accident. Br J Cancer. 2011;104(1):181–187. doi: 10.1038/sj.bjc.6605967. [DOI] [PMC free article] [PubMed] [Google Scholar]