Abstract
The extent of age-related changes in glutamate and other neurometabolites in the anterior cingulate cortex (ACC) in individuals with schizophrenia remain unclear. Magnetic resonance spectroscopy (MRS) at 7 Tesla, which yields precise measurements of various metabolites and can distinguish glutamate from glutamine, was used to determine levels of ACC glutamate and other metabolites in 24 individuals with schizophrenia and 24 matched controls. Multiple regression analysis revealed that ACC glutamate decreased with age in patients but not controls. No changes were detected in levels of glutamine, N-acetylaspartate, myo-inositol, GABA, glutathione or other metabolites. These results suggest that age may be an important modifier of ACC glutamate in schizophrenia.
Keywords: schizophrenia, glutamate, anterior cingulate cortex, magnetic resonance spectroscopy, 7T
1. INTRODUCTION
Imaging, post-mortem pathology, and genetics studies of schizophrenia have shown disruption of the glutamatergic system, yet the specifics remain unclear (Wijtenburg et al 2015). The components of the glutamate system have considerable potential as biomarkers of disease progression, though much is unknown, including the brain regions where measurement may yield the most useful information. The anterior cingulate cortex (ACC) is a region of particular interest in schizophrenia because of its involvement in emotion, attention, and cognition (Benes 2009, Reid et al 2010) and anatomic evidence for disruption in schizophrenia (Roberts et al 2015, Fornito et al 2009). Glutamatergic dysfunction may be modulated by hypofunction of the N-methyl-D-aspartate receptor (NMDAR), leading to increased glutamate release in the ACC, neurodegeneration, and subsequent decreased ACC glutamate levels (Plitman et al 2014). In humans, NMDAR antagonists lead to increased glutamate release and psychotic symptoms (Stone et al 2012; Moghaddam and Javitt 2012). Chronic NMDAR antagonist administered to rats leads to patterns of neurodegeneration similar to those found in schizophrenia, including in the ACC (Olney and Farber 1995).
Severity of symptoms in schizophrenia often decreases with advancing age, and the role of the glutamatergic system in these changes is not understood (Jeste et al 2011). Investigations of glutamate changes using magnetic resonance spectroscopy (MRS), which non-invasively quantifies steady-state concentrations of metabolites in specific brain regions, have reached inconsistent findings. This inconsistency is likely due to a combination of variation in voxel location, different methods of data acquisition and analysis (Wijtenburg et al 2015, Rowland et al 2013, Chang et al 2007), and differences in patient populations including age, disease duration, medication use, and included/excluded co-morbidities. A meta-analysis of studies using MRS at field strengths of 1.5 to 4T revealed lower levels of glutamate in the medial prefrontal region in older patients (Marsman et al 2013). Separating glutamate and glutamine at lower field strengths has proved challenging, despite promising new approaches (Zhang and Shen 2015, Ramadan et al 2013). Studies have shown improved precision of glutamate measurement, distinguishing it from glutamine, at 7T compared to lower field strengths (Mekle et al 2009, Pradhan et al 2015, Tkáč et al 2009). In the current study, therefore, the enhanced spectral sensitivity and resolution of 7T MRS was used to explore differences in ACC glutamate with age in patients with schizophrenia and healthy control subjects.
2. METHODS
2.1 Study Population
27 patients and 27 matched controls were recruited from Johns Hopkins clinics and the surrounding community. Inclusion criteria was diagnosis of schizophrenia or schizoaffective disorder. Exclusion criteria included intellectual disability, history of another central nervous system disorder, history of head injury resulting in loss of consciousness > 20 minutes, alcohol or substance dependence in past 6 months, recent marijuana use, abnormal movements sufficiently severe to interfere with MRI quality, and any contraindication for 7T MRI. All patients were clinically stable on antipsychotic medicines, including aripiprazole, clozapine, fluphenazine, haloperidol, risperidone, quetiapine, olanzapine, and ziprasidone. Four patients were also on low dose benzodiazepines at bedtime.
Controls, recruited from the local community, were matched for age, sex, and education level, and screened to ensure that neither they nor any first degree relative had a history of psychotic illness. The study was approved by the Johns Hopkins Medicine Institutional Review Board, and informed consent was obtained from all participants.
On the day of imaging, all subjects were assessed with the “Mini” Psychiatric Interview, the Montreal Cognitive Assessment (MOCA), the Edinburg Handedness Inventory, the Hopkins Adult Reading Test to estimate premorbid IQ, the Hamilton Depression Rating Scale, the Brief Psychiatric Rating Scale, and an MRI safety screening questionnaire. Data from three patients and three controls were excluded due to difficulties with data collection or processing or late discovery of an exclusion criterion. Data from 24 patients and 24 controls were included in the analysis.
2.2 MRI and MRS Acquisition
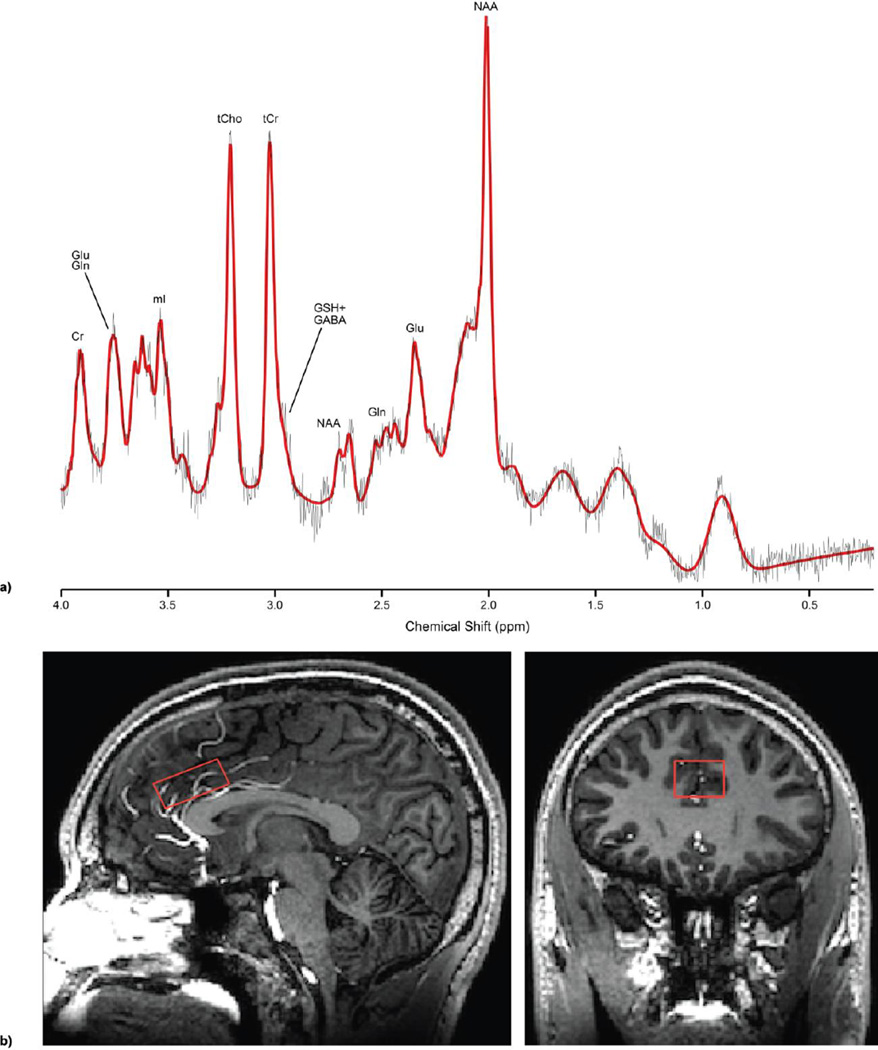
Scanning was performed at the F.M. Kirby Center for Functional Brain Imaging on a 7T whole-body scanner (‘Achieva’, Philips Healthcare LLC, Cleveland, OH) equipped with a 32-channel receive head coil (Nova Medical, Wilmington, MA). A high resolution T1-weighted MPRAGE structural image was used for voxel placement and tissue segmentation. MRS data were acquired using a stimulated echo acquisition mode (STEAM) sequence with TE 14ms, TR 3s, TE 28ms and 112 averages (scan time 5min 36s). VAPOR water suppression was used, along with a separate non-water suppressed acquisition for eddy-current correction and quantitation purposes. A 30 × 20 × 12 mm3 (7.2 mL) voxel was placed in the dorsal ACC, positioned just superior to the genu of the corpus callosum (Fig 1a).
Figure 1.
a) Representative LCModel spectral fit in red with metabolites of interest labeled, b) Location of MRS voxel used in the anterior cingulate cortex region
2.3 Spectroscopy Data Processing
The ‘LCModel’ program (Provencher 1993) was used to analyze the spectra with a custom basis set that included 22 metabolites (simulated using chemical shifts and coupling constants from the literature) (Govindaraju et al 2000) (Fig 1b). The default macromolecule spectra along with the spline baseline from LCModel were used to fit the baseline with an added constraint of 0.2 ppm knot spacing. Glutamate (Glu), glutamine (Gln), N-acetyl aspartate (NAA), N-acetylaspartylglutamic acid (NAAG), myo-inositol (mI), GABA, glutathione (GSH), total creatine (tCr), and total choline (tCh) were quantified relative to the ACC water signal. Only metabolites with Cramer-Rao lower bounds (CRLB) 20% or lower were included in the final analysis, except for NAAG (inclusion at CRLB of 50% or lower). Consequently, 4 NAAG concentrations were excluded from the final analysis.
2.4 Partial Voluming Correction
Metabolite concentrations were corrected for voxel tissue fraction. A voxel mask was created using the in-house program ‘SVMask’. The T1-weighted images were segmented using FSL5.0 (Zhang et al 2001) to determine the voxel fractions of white matter, gray matter, and CSF. Corrected metabolite concentrations were calculated by normalizing the metabolite levels by the total tissue fraction for each participant.
2.5 Statistical Analysis
Students’ t-test, Fisher’s exact test, and multiple regression analyses were performed using Small Stata 14.1 for Mac. Current chlorpromazine (CPZ) equivalents were used as a proxy for lifetime antipsychotic exposure, and patients were divided into high and low CPZ groups by median CPZ equivalents (Andreasen et al 2010).
3. RESULTS
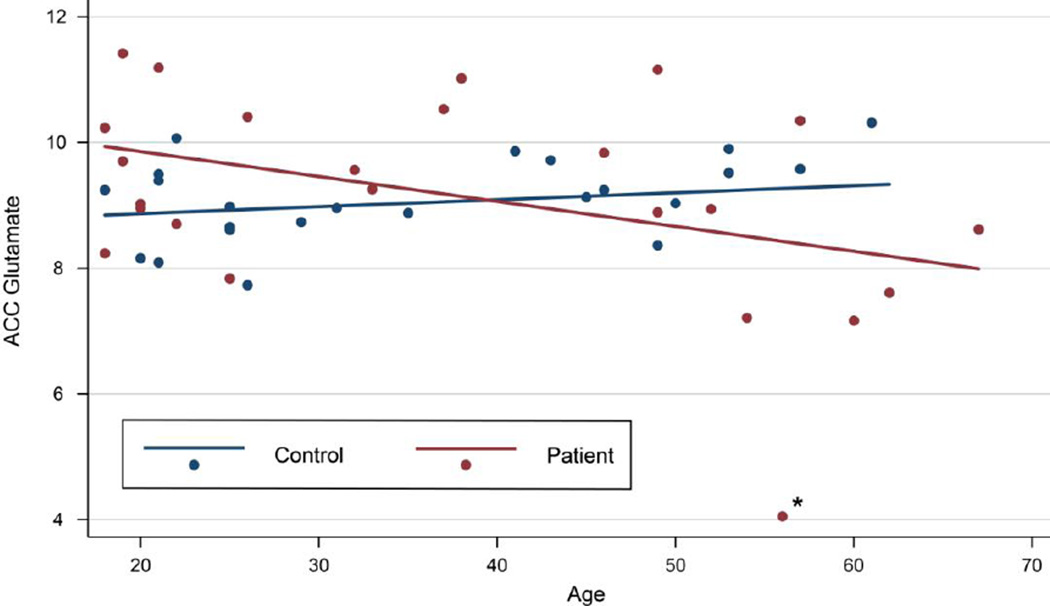
Demographic information is presented in Table 1. There were no significant differences in levels of ACC glutamate, the ratio of glutamate/glutamine, or any other metabolite studied (Table 2) when comparing all patients to all controls. ACC glutamate inversely correlated with age for patients and not for controls, independent of smoking status (Fig 2). For participants under age 40, there was a significant difference in ACC glutamate between patients (M = 9.72, sd = 0.30) and controls (M = 8.84, sd = 0.18); t(25) = −2.47, p = 0.021). For participants over age 40, the lower level of ACC glutamate in patients (M= 8.38, sd = 0.64) compared to controls (M = 9.29, sd = 0.23) did not reach statistical significance (t(11.34) = 1.35, p = 0.20). ACC glutamate/glutamine was not correlated with age for patients or controls. There were no significant relationships between other metabolites and age or smoking status for patients or controls (Supplementary Table). There were no significant differences in ACC glutamate levels between patients with high and low antipsychotic dosages. Excluding patients who were on antipsychotics for less than five years did not affect the results. There were no significant correlations between metabolite levels and MOCA score or between age and MOCA score.
Table 1. Demographic and neuropsychological information for controls and schizophrenia patients.
Demographics and neuropsychological testing scores for controls and schizophrenia patients. There were no significant differences in mean age, years of education, or premorbid IQ (HART FSIQ), as measured by independent sample t-tests. As measured by Fisher’s exact test, patients and controls were equally likely to be male or female and smokers or non-smokers. MOCA scores were significantly lower in patients than controls (p = 0.003). Data are presented as mean (standard deviation). A cutoff of p < 0.05 was used for significance.
| Control | Patient | p - Value | |
|---|---|---|---|
| Male/Female | 20/4 | 19/5 | n.s. |
| Age (y) | 36.6 (14.6) | 37.5 (16.7) | n.s. |
| Education (y) | 12.7 (1.5) | 12.6 (1.7) | n.s. |
| Smoker/Non-smoker | 9/15 | 14/10 | n.s. |
| MOCA | 26 (3.3) | 23.1 (3.2) | 0.003 |
| HART FSIQ | 103.6 (9.8) | 100.4 (8.8) | n.s. |
Table 2. Metabolite concentrations for controls and schizophrenia patients.
ACC metabolite concentrations for 24 controls and 24 patients with schizophrenia. Data are presented as mean (standard deviation). A cutoff of p < 0.05 was used for significance.
| Control | Patient | t Test (p) | |
|---|---|---|---|
| Glu | 9.05 (0.72) | 9.16 (1.66) | n.s. |
| Gln | 2.42 (0.51) | 2.32 (0.64) | n.s. |
| Gln/Glu | 0.27 (0.06) | 0.25 (0.01) | n.s. |
| NAA | 7.87 (0.76) | 7.33 (1.83) | n.s. |
| NAAG | 0.68 (0.24) | 0.87 (0.52) | n.s. |
| mI | 6.69 (0.88) | 6.36 (1.34) | n.s. |
| GABA | 2.24 (0.31) | 2.36 (0.41) | n.s. |
| GSH | 1.73 (0.22) | 1.74 (0.31) | n.s. |
| tCr | 6.44 (0.58) | 6.21 (1.15) | n.s. |
| tCh | 2.16 (0.29) | 2.10 (0.47) | n.s. |
Figure 2. Glutamate levels versus age in control (blue) and patient (red) data sets.
By multiple regression (R2 = 0.16), when covarying for age and smoking status, patients showed higher levels of ACC glutamate (β=1.89, p=0.047) than controls. ACC glutamate decreases with age for patients but not controls (β=−0.050, p=0.036). Smoking (β=0.37, p=0.34) did not contribute significantly to the model. When the patient indicated by the asterisk was excluded from the model, patients had significantly higher glutamate than controls (β=1.68, p=0.036), and there was a trend of glutamate decreasing with age for patients (β=−0.037, p=0.065). The intersection between patients and controls occurs at age 40.
4. DISCUSSION
Using 7T, we demonstrate an age-dependent difference in ACC glutamate between patients and controls. ACC glutamate was higher in younger patients than younger controls, and decreased with age in patients but not controls. This finding is consistent with a meta-analysis of MRS studies at 4T and below in the medial prefrontal cortex (Marsman et al 2013). No difference was found in ACC glutamate when comparing all patients and all controls without controlling for age, consistent with other MRS and postmortem studies of the glutamatergic system (Wood et al 2007, Reid et al 2010, Kraguljac et al 2012, Barksdale et al 2014).
Decreased ACC glutamate with age may reflect an interaction between aging and disease, longer disease duration, or chronic medication use. The temporal changes in glutamate are consistent with a glutamatergic excitotoxicity hypothesis; increased glutamatergic activity may occur early in the disease, leading to neurodegeneration and decreased ACC glutamate later in the course. There have been discrepancies in the literature on the effect of smoking on ACC glutamate (Durazzo et al 2015, Mennecke et al 2014, Gallinat and Schubert 2007). Co-varying for smoking had no effect on our results.
MRS quantifies all glutamate within the localized voxel, including both ‘metabolic’ and ‘neuronal’ glutamate. The implications of changes in this entire pool of glutamate on neuronal function are not clear (Wijtenburg et al 2015). Given the close metabolic relationship of glutamate and NAA (two biosynthetic steps through aspartate), changes in glutamate levels that are not accompanied by NAA are notable. Similarly, glutamate and glutamine are metabolically coupled, as synaptic glutamate is taken up by astrocytes and converted to glutamine (Wijtenburg et al 2015). Our finding of an age-related decrease of glutamate in schizophrenia, without associated changes in NAA or glutamine, could indicate an age related glutamate specific process, such as dysfunction of Glu-Gln cycling or uncoupling of ACC glutamate and NAA (Coughlin et al 2015), perhaps in a specific neuronal subtype.
The study is limited by a modest sample size. Gender in particular may be an important modifier of ACC glutamate that we could not assess given the inclusion of few women. Another important limitation is the universal treatment of patients with antipsychotic medications. Several MRS studies have shown decreased glutamate or glutamine+glutamate in brain, including in the dorsolateral prefrontal cortex, associative striatum, and medial prefrontal cortex, after the use of antipsychotic medications (Poels et al 2014, de la Fuente-Sandoval et al 2013, Kegeles et al 2012). However, this finding is not consistent, as changes in glutamate in the ACC or thalamus were not detected in other studies when examining antipsychotic treatment over 30 or 80 months (Théberge et al 2007, Aoyama et al 2011, Wijtenburg 2015 et al). We did not find an effect of antipsychotic treatment on ACC glutamate levels in this study, though alternative methods for estimating antipsychotic exposure may have yielded different results.
In conclusion, 7T MRS revealed decreased ACC glutamate with age in schizophrenia patients treated with antipsychotics, suggesting a potential role of 7T as a powerful tool for establishing biomarkers for schizophrenia. Larger scale studies of both younger and older unmedicated individuals, as well as longitudinal assessment of specific individuals, will serve to validate the findings here.
Supplementary Material
Acknowledgments
We thank all study participants. We thank Terri Brawner, Ivana Kusevic, and Kathleen Kahl of the F.M. Kirby Research Center for their technical assistance. We thank Brian Caffo for his statistical assistance. We thank Mr. Jose Brito for his generous support.
Equipment used in the study was manufactured by Philips. Peter C. M. van Zijl receives grant support from Philips, is a paid lecturer for Philips, and is the inventor of technology that is licensed to Philips.
Role of funding source:
This work was supported by NIH grants R01MH096263 and P41EB015909, and a generous donation from Mr. Jose Brito.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest:
This arrangement has been approved by Johns Hopkins in accordance with its conflict of interest policies.
Contributors:
Allison Brandt conducted data collection, data analysis and interpretation, drafting of the article, revision of the article, and final approval.
Paul Unschuld contributed to data collection and revision of the article.
Subechhya Pradhan conducted data processing and contributed to data analysis and revision of the article.
Issel Anne Lim contributed to study design and data collection.
Gregory Churchill contributed to data collection and subject assessment.
Ashley Harris contributed to data analysis, data interpretation, and revision of the article.
Jun Hua contributed to data analysis and interpretation.
Peter Barker contributed to data interpretation and revision of the article.
Christopher A. Ross contributed to study design and data interpretation.
Peter van Zijl contributed to study design, data interpretation, and revision of the article.
Richard Edden contributed to study design, data analysis, data interpretation, and revision of the article.
Russell L Margolis contributed to study design, data analysis, data interpretation, drafting of the article, revision of the article, and final approval.
REFERENCES
- Andreasen NC, Pressler M, Nopoulos P, Miller D, Ho BC. Antipsychotic dose equivalents and dose-years: a standardized method for comparing exposure to different drugs. Biol Psychiatry. 2010;67(3):255–262. doi: 10.1016/j.biopsych.2009.08.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aoyama N, Théberge J, Drost DJ, Manchanda R, Northcott S, Neufeld RWJ, Menon RS, Rajakumar N, Pavlosky WF, Densmore M, Schaefer B, Williamson PC. Grey matter and social functioning correlates of glutamatergic metabolite loss in schizophrenia. Br J Psychiatry. 2011;198(6):448–456. doi: 10.1192/bjp.bp.110.079608. [DOI] [PubMed] [Google Scholar]
- Barksdale KA, Lahti AC, Roberts RC. Synaptic proteins in the postmortem anterior cingulate cortex in schizophrenia: Relationship to treatment and treatment response. Neuropsychopharmacology. 2014;39(9):2095–2103. doi: 10.1038/npp.2014.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benes FM. Amygdalocortical Circuitry in Schizophrenia: From Circuits to Molecules. Neuropsychopharmacology. 2009;35(1):239–257. doi: 10.1038/npp.2009.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benes FM. Emerging principles of altered neural circuitry in schizophrenia. Brain Res Rev. 2000;31(2–3):251–269. doi: 10.1016/s0165-0173(99)00041-7. [DOI] [PubMed] [Google Scholar]
- Breier A, Malhotra AK, Pinals DA, Weisenfeld NI, Pickar D. Association of ketamine-induced psychosis with focal activation of the prefrontal cortex in healthy volunteers. Am J Psychiatry. 1997;154(6):805–811. doi: 10.1176/ajp.154.6.805. [DOI] [PubMed] [Google Scholar]
- Bustillo J, Barrow R, Paz R, Tang J, Seraji-Bozorgzad N, Moore GJ, Bolognani F, Lauriello J, Perrone-Bizzozero N, Galloway MP. Long-term treatment of rats with haloperidol: lack of an effect on brain N-acetyl aspartate levels. Neuropsychopharmacology. 2006;31(4):751–756. doi: 10.1038/sj.npp.1300874. [DOI] [PubMed] [Google Scholar]
- Bustillo JR, Chen H, Gasparovic C, Mullins P, Caprihan A, Qualls C, Apfeldorf W, Lauriello J, Posse S. Glutamate as a marker of cognitive function in schizophrenia: a proton spectroscopic imaging study at 4 Tesla. Biol Psychiatry. 2011;69(1):19–27. doi: 10.1016/j.biopsych.2010.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bustillo JR, Chen H, Jones T, Lemke N, Abbott C, Qualls C, Canive J, Gasparovic C. Increased glutamine in patients undergoing long-term treatment for schizophrenia: a proton magnetic resonance spectroscopy study at 3 T. JAMA Psychiatry. 2014;71(3):265–272. doi: 10.1001/jamapsychiatry.2013.3939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bustillo JR, Rowland LM, Mullins P, Jung R, Chen H, Qualls C, Hammond R, Brooks WM, Lauriello J. 1H-MRS at 4 Tesla in minimally treated early schizophrenia. Molecular psychiatry. 2010;15(6):629–636. doi: 10.1038/mp.2009.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang L, Friedman J, Ernst T, Zhong K, Tsopelas ND, Davis K. Brain metabolite abnormalities in the white matter of elderly schizophrenic subjects: implication for glial dysfunction. Biol Psychiatry. 2007;62(12):1396–1404. doi: 10.1016/j.biopsych.2007.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coughlin JM, Tanaka T, Marsman A, Wang H, Bonekamp S, Kim PK, Higgs C, Varvaris M, Edden RAE, Pomper M, Schretlen D, Barker PB, Sawa A. Decoupling of N-acetyl-aspartate and Glutamate Within the Dorsolateral Prefrontal Cortex in Schizophrenia. Current molecular medicine. 2015;15(2):176–183. doi: 10.2174/1566524015666150303104811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coyle JT. Glutamate and schizophrenia: beyond the dopamine hypothesis. Cell Mol Neurobiol. 2006;26(4–6):365–384. doi: 10.1007/s10571-006-9062-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de la Fuente-Sandoval C, Leon-Ortiz P, Azcarraga M, Stephano S, Favila R, Diaz-Galvis L, Alvarado-Alanis P, Ramirez-Bermudez J, Graff-Guerrero A. Glutamate levels in the associative striatum before and after 4 weeks of antipsychotic treatment in first-episode psychosis: a longitudinal proton magnetic resonance spectroscopy study. JAMA Psychiatry. 2013;70(10):1057–1066. doi: 10.1001/jamapsychiatry.2013.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durazzo TC, Meyerhoff DJ, Mon A, Abé C, Gazdzinski S, Murray DE. Chronic Cigarette Smoking in Healthy Middle-Aged Individuals Is Associated with Decreased Regional Brain N-acetylaspartate and Glutamate Levels. Biological Psychiatry. 2015 doi: 10.1016/j.biopsych.2015.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egerton A, Brugger S, Raffin M, Barker GJ, Lythgoe DJ, McGuire PK, Stone JM. Anterior cingulate glutamate levels related to clinical status following treatment in first-episode schizophrenia. Neuropsychopharmacology. 2012;37(11):2515–2521. doi: 10.1038/npp.2012.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falkenberg LE, Westerhausen R, Craven AR, Johnsen E, Kroken RA, EM LB, Specht K, Hugdahl K. Impact of glutamate levels on neuronal response and cognitive abilities in schizophrenia. Neuroimage Clin. 2014;4:576–584. doi: 10.1016/j.nicl.2014.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fornito A, Yucel M, Dean B, Wood SJ, Pantelis C. Anatomical abnormalities of the anterior cingulate cortex in schizophrenia: bridging the gap between neuroimaging and neuropathology. Schizophr Bull. 2009;35(5):973–993. doi: 10.1093/schbul/sbn025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallinat J, Schubert F. Regional cerebral glutamate concentrations and chronic tobacco consumption. Pharmacopsychiatry. 2007;40(2):64–67. doi: 10.1055/s-2007-970144. [DOI] [PubMed] [Google Scholar]
- Goff DC, Coyle JT. The emerging role of glutamate in the pathophysiology and treatment of schizophrenia. Am J Psychiatry. 2001;158(9):1367–1377. doi: 10.1176/appi.ajp.158.9.1367. [DOI] [PubMed] [Google Scholar]
- Govindaraju V, Young K, Maudsley AA. Proton NMR chemical shifts and coupling constants for brain metabolites. NMR Biomed. 2000;13(3):129–153. doi: 10.1002/1099-1492(200005)13:3<129::aid-nbm619>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- Gruber O, Chadha Santuccione A, Aach H. Magnetic resonance imaging in studying schizophrenia, negative symptoms, and the glutamate system. Front Psychiatry. 2014;5:32. doi: 10.3389/fpsyt.2014.00032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermann D, Frischknecht U, Heinrich M, Hoerst M, Vollmert C, Vollstadt-Klein S, Tunc-Skarka N, Kiefer F, Mann K, Ende G. MR spectroscopy in opiate maintenance therapy: association of glutamate with the number of previous withdrawals in the anterior cingulate cortex. Addict Biol. 2012;17(3):659–667. doi: 10.1111/j.1369-1600.2010.00290.x. [DOI] [PubMed] [Google Scholar]
- Holcomb HH, Lahti AC, Medoff DR, Cullen T, Tamminga CA. Effects of noncompetitive NMDA receptor blockade on anterior cingulate cerebral blood flow in volunteers with schizophrenia. Neuropsychopharmacology. 2005;30(12):2275–2282. doi: 10.1038/sj.npp.1300824. [DOI] [PubMed] [Google Scholar]
- Jeste DV, Wolkowitz OM, Palmer BW. Divergent trajectories of physical, cognitive, and psychosocial aging in schizophrenia. Schizophr Bull. 2011;37(3):451–455. doi: 10.1093/schbul/sbr026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaiser LG, Schuff N, Cashdollar N, Weiner MW. Age-related glutamate and glutamine concentration changes in normal human brain: 1H MR spectroscopy study at 4 T. Neurobiol Aging. 2005;26(5):665–672. doi: 10.1016/j.neurobiolaging.2004.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kegeles LS, Mao X, Stanford AD, Girgis R, Ojeil N, Xu X, Gil R, Slifstein M, Abi-Dargham A, Lisanby SH, Shungu DC. Elevated prefrontal cortex gamma-aminobutyric acid and glutamate-glutamine levels in schizophrenia measured in vivo with proton magnetic resonance spectroscopy. Arch Gen Psychiatry. 2012;69(5):449–459. doi: 10.1001/archgenpsychiatry.2011.1519. [DOI] [PubMed] [Google Scholar]
- Kraguljac NV, Reid MA, White DM, den Hollander J, Lahti AC. Regional decoupling of N-acetyl-aspartate and glutamate in schizophrenia. Neuropsychopharmacology. 2012;37(12):2635–2642. doi: 10.1038/npp.2012.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindquist DM, Dunn RS, Cecil KM. Long term antipsychotic treatment does not alter metabolite concentrations in rat striatum: an in vivo magnetic resonance spectroscopy study. Schizophr Res. 2011;128(1–3):83–90. doi: 10.1016/j.schres.2011.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lisman JE, Coyle JT, Green RW, Javitt DC, Benes FM, Heckers S, Grace AA. Circuit-based framework for understanding neurotransmitter and risk gene interactions in schizophrenia. Trends Neurosci. 2008;31(5):234–242. doi: 10.1016/j.tins.2008.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Gil X, Babot Z, Amargos-Bosch M, Sunol C, Artigas F, Adell A. Clozapine and haloperidol differently suppress the MK-801-increased glutamatergic and serotonergic transmission in the medial prefrontal cortex of the rat. Neuropsychopharmacology. 2007;32(10):2087–2097. doi: 10.1038/sj.npp.1301356. [DOI] [PubMed] [Google Scholar]
- Luby ED. Study of a New Schizophrenomimetic Drug—Sernyl. Archives of Neurology And Psychiatry. 1959;81(3):363. doi: 10.1001/archneurpsyc.1959.02340150095011. [DOI] [PubMed] [Google Scholar]
- Maddock R, Buonocore M. In: MR Spectroscopic Studies of the Brain in Psychiatric Disorders. Carter CS, Dalley JW, editors. Springer Berlin Heidelberg; 2012. pp. 199–251. [DOI] [PubMed] [Google Scholar]
- Marsman A, Mandl RC, Klomp DW, Bohlken MM, Boer VO, Andreychenko A, Cahn W, Kahn RS, Luijten PR, Hulshoff Pol HE. GABA and glutamate in schizophrenia: a 7 T (1)HMRS study. Neuroimage Clin. 2014;6(0):398–407. doi: 10.1016/j.nicl.2014.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsman A, van den Heuvel MP, Klomp DW, Kahn RS, Luijten PR, Hulshoff Pol HE. Glutamate in schizophrenia: a focused review and meta-analysis of (1)H-MRS studies. Schizophr Bull. 2013;39(1):120–129. doi: 10.1093/schbul/sbr069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mashhoon Y, Janes AC, Jensen JE, Prescot AP, Pachas G, Renshaw PF, Fava M, Evins AE, Kaufman MJ. Anterior cingulate proton spectroscopy glutamate levels differ as a function of smoking cessation outcome. Prog Neuropsychopharmacol Biol Psychiatry. 2011;35(7):1709–1713. doi: 10.1016/j.pnpbp.2011.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mekle R, Mlynárik V, Gambarota G, Hergt M, Krueger G, Gruetter R. MR spectroscopy of the human brain with enhanced signal intensity at ultrashort echo times on a clinical platform at 3T and 7T. Magnetic Resonance in Medicine. 2009;61(6):1279–1285. doi: 10.1002/mrm.21961. [DOI] [PubMed] [Google Scholar]
- Mennecke A, Gossler A, Hammen T, Dorfler A, Stadlbauer A, Rosch J, Kornhuber J, Bleich S, Dolken M, Thurauf N. Physiological effects of cigarette smoking in the limbic system revealed by 3 tesla magnetic resonance spectroscopy. J Neural Transm (Vienna) 2014;121(10):1211–1219. doi: 10.1007/s00702-014-1190-6. [DOI] [PubMed] [Google Scholar]
- Merritt K, McGuire P, Egerton A. Relationship between Glutamate Dysfunction and Symptoms and Cognitive Function in Psychosis. Front Psychiatry. 2013;4:151. doi: 10.3389/fpsyt.2013.00151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moghaddam B, Adams BW. Reversal of phencyclidine effects by a group II metabotropic glutamate receptor agonist in rats. Science. 1998;281(5381):1349–1352. doi: 10.1126/science.281.5381.1349. [DOI] [PubMed] [Google Scholar]
- Moghaddam B, Javitt D. From revolution to evolution: the glutamate hypothesis of schizophrenia and its implication for treatment. Neuropsychopharmacology. 2012;37(1):4–15. doi: 10.1038/npp.2011.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohrmann P, Kugel H, Bauer J, Siegmund A, Kolkebeck K, Suslow T, Wiedl KH, Rothermundt M, Arolt V, Pedersen A. Learning potential on the WCST in schizophrenia is related to the neuronal integrity of the anterior cingulate cortex as measured by proton magnetic resonance spectroscopy. Schizophr Res. 2008;106(2–3):156–163. doi: 10.1016/j.schres.2008.08.005. [DOI] [PubMed] [Google Scholar]
- Olney JW, Farber NB. Glutamate receptor dysfunction and schizophrenia. Arch Gen Psychiatry. 1995;52(12):998–1007. doi: 10.1001/archpsyc.1995.03950240016004. [DOI] [PubMed] [Google Scholar]
- Oni-Orisan A, Kristiansen LV, Haroutunian V, Meador-Woodruff JH, McCullumsmith RE. Altered vesicular glutamate transporter expression in the anterior cingulate cortex in schizophrenia. Biol Psychiatry. 2008;63(8):766–775. doi: 10.1016/j.biopsych.2007.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paz RD, Tardito S, Atzori M, Tseng KY. Glutamatergic dysfunction in schizophrenia: from basic neuroscience to clinical psychopharmacology. Eur Neuropsychopharmacol. 2008;18(11):773–786. doi: 10.1016/j.euroneuro.2008.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plitman E, Nakajima S, de la Fuente-Sandoval C, Gerretsen P, Chakravarty MM, Kobylianskii J, Chung JK, Caravaggio F, Iwata Y, Remington G, Graff-Guerrero A. Glutamate-mediated excitotoxicity in schizophrenia: a review. Eur Neuropsychopharmacol. 2014;24(10):1591–1605. doi: 10.1016/j.euroneuro.2014.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poels EM, Kegeles LS, Kantrowitz JT, Javitt DC, Lieberman JA, Abi-Dargham A, Girgis RR. Glutamatergic abnormalities in schizophrenia: a review of proton MRS findings. Schizophr Res. 2014;152(2–3):325–332. doi: 10.1016/j.schres.2013.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poels EM, Kegeles LS, Kantrowitz JT, Slifstein M, Javitt DC, Lieberman JA, Abi-Dargham A, Girgis RR. Imaging glutamate in schizophrenia: review of findings and implications for drug discovery. Mol Psychiatry. 2014;19(1):20–29. doi: 10.1038/mp.2013.136. [DOI] [PubMed] [Google Scholar]
- Pradhan S, Bonekamp S, Gillen JS, Rowland LM, Wijtenburg SA, Edden RAE, Barker PB. Comparison of single voxel brain MRS AT 3 T and 7 T using 32-channel head coils. Magnetic Resonance Imaging. 2015;33(8):1013–1018. doi: 10.1016/j.mri.2015.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Provencher SW. Estimation of metabolite concentrations from localized in vivo proton NMR spectra. Magn Reson Med. 1993;30(6):672–679. doi: 10.1002/mrm.1910300604. [DOI] [PubMed] [Google Scholar]
- Rae CD. A guide to the metabolic pathways and function of metabolites observed in human brain 1H magnetic resonance spectra. Neurochem Res. 2014;39(1):1–36. doi: 10.1007/s11064-013-1199-5. [DOI] [PubMed] [Google Scholar]
- Ramadan S, Lin A, Stanwell P. Glutamate and glutamine: a review of in vivo MRS in the human brain. NMR Biomed. 2013;26(12):1630–1646. doi: 10.1002/nbm.3045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid MA, Stoeckel LE, White DM, Avsar KB, Bolding MS, Akella NS, Knowlton RC, den Hollander JA, Lahti AC. Assessments of function and biochemistry of the anterior cingulate cortex in schizophrenia. Biol Psychiatry. 2010;68(7):625–633. doi: 10.1016/j.biopsych.2010.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts RC, Barksdale KA, Roche JK, Lahti AC. Decreased synaptic and mitochondrial density in the postmortem anterior cingulate cortex in schizophrenia. Schizophr Res. 2015;168(1–2):543–553. doi: 10.1016/j.schres.2015.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross CA, Margolis RL, Reading SA, Pletnikov M, Coyle JT. Neurobiology of schizophrenia. Neuron. 2006;52(1):139–153. doi: 10.1016/j.neuron.2006.09.015. [DOI] [PubMed] [Google Scholar]
- Rowland LM, Kontson K, West J, Edden RA, Zhu H, Wijtenburg SA, Holcomb HH, Barker PB. In vivo measurements of glutamate, GABA, and NAAG in schizophrenia. Schizophr Bull. 2013;39(5):1096–1104. doi: 10.1093/schbul/sbs092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schubert F, Gallinat J, Seifert F, Rinneberg H. Glutamate concentrations in human brain using single voxel proton magnetic resonance spectroscopy at 3 Tesla. Neuroimage. 2004;21(4):1762–1771. doi: 10.1016/j.neuroimage.2003.11.014. [DOI] [PubMed] [Google Scholar]
- Segovia G, Porras A, Del Arco A, Mora F. Glutamatergic neurotransmission in aging: a critical perspective. Mech Ageing Dev. 2001;122(1):1–29. doi: 10.1016/s0047-6374(00)00225-6. [DOI] [PubMed] [Google Scholar]
- Stone JM, Dietrich C, Edden R, Mehta MA, De Simoni S, Reed LJ, Krystal JH, Nutt D, Barker GJ. Ketamine effects on brain GABA and glutamate levels with 1H-MRS: relationship to ketamine-induced psychopathology. Mol Psychiatry. 2012;17(7):664–665. doi: 10.1038/mp.2011.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi H, Higuchi M, Suhara T. The role of extrastriatal dopamine D2 receptors in schizophrenia. Biol Psychiatry. 2006;59(10):919–928. doi: 10.1016/j.biopsych.2006.01.022. [DOI] [PubMed] [Google Scholar]
- Tayoshi S, Nakataki M, Sumitani S, Taniguchi K, Shibuya-Tayoshi S, Numata S, Iga J, Ueno S, Harada M, Ohmori T. GABA concentration in schizophrenia patients and the effects of antipsychotic medication: a proton magnetic resonance spectroscopy study. Schizophr Res. 2010;117(1):83–91. doi: 10.1016/j.schres.2009.11.011. [DOI] [PubMed] [Google Scholar]
- Tayoshi S, Sumitani S, Taniguchi K, Shibuya-Tayoshi S, Numata S, Iga J, Nakataki M, Ueno S, Harada M, Ohmori T. Metabolite changes and gender differences in schizophrenia using 3-Tesla proton magnetic resonance spectroscopy (1H-MRS) Schizophr Res. 2009;108(1–3):69–77. doi: 10.1016/j.schres.2008.11.014. [DOI] [PubMed] [Google Scholar]
- Théberge J, Williamson KE, Aoyama N, Drost DJ, Manchanda R, Malla AK, Northcott S, Menon RS, Neufeld RW, Rajakumar N, Pavlosky W, Densmore M, Schaefer B, Williamson PC. Longitudinal grey-matter and glutamatergic losses in first-episode schizophrenia. Br J Psychiatry. 2007;191:325–334. doi: 10.1192/bjp.bp.106.033670. [DOI] [PubMed] [Google Scholar]
- Théberge J, Al-Semaan Y, Williamson PC, Menon RS, Neufeld RWJ, Rajakumar N, Schaefer B, Densmore M, Drost DJ. Glutamate and Glutamine in the Anterior Cingulate and Thalamus of Medicated Patients With Chronic Schizophrenia and Healthy Comparison Subjects Measured With 4.0-T Proton MRS. American Journal of Psychiatry. 2003;160(12):2231–2233. doi: 10.1176/appi.ajp.160.12.2231. [DOI] [PubMed] [Google Scholar]
- Théberge J, Bartha R, Drost DJ, Menon RS, Malla A, Takhar J, Neufeld RW, Rogers J, Pavlosky W, Schaefer B, Densmore M, Al-Semaan Y, Williamson PC. Glutamate and Glutamine Measured With 4.0 T Proton MRS in Never-Treated Patients With Schizophrenia and Healthy Volunteers. American Journal of Psychiatry. 2002;159(11):1944–1946. doi: 10.1176/appi.ajp.159.11.1944. [DOI] [PubMed] [Google Scholar]
- Théberge J, Williamson KE, Aoyama N, Drost DJ, Manchanda R, Malla AK, Northcott S, Menon RS, Neufeld RW, Rajakumar N, Pavlosky W, Densmore M, Schaefer B, Williamson PC. Longitudinal grey-matter and glutamatergic losses in first-episode schizophrenia. Br J Psychiatry. 2007;191(4):325–334. doi: 10.1192/bjp.bp.106.033670. [DOI] [PubMed] [Google Scholar]
- Tkáč I, Andersen P, Adriany G, Merkle H, Uǧurbil K, Gruetter R. In vivo 1H NMR spectroscopy of the human brain at 7 T. Magnetic Resonance in Medicine. 2001;46(3):451–456. doi: 10.1002/mrm.1213. [DOI] [PubMed] [Google Scholar]
- Tkáč I, Öz G, Adriany G, Uğurbil K, Gruetter R. In vivo 1H NMR spectroscopy of the human brain at high magnetic fields: Metabolite quantification at 4T vs. 7T. Magnetic Resonance in Medicine. 2009;62(4):868–879. doi: 10.1002/mrm.22086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai G, Passani LA, Slusher BS, Carter R, Baer L, Kleinman JE, Coyle JT. Abnormal excitatory neurotransmitter metabolism in schizophrenic brains. Arch Gen Psychiatry. 1995;52(10):829–836. doi: 10.1001/archpsyc.1995.03950220039008. [DOI] [PubMed] [Google Scholar]
- Wijtenburg SA, Yang S, Fischer BA, Rowland LM. In vivo assessment of neurotransmitters and modulators with magnetic resonance spectroscopy: application to schizophrenia. Neurosci Biobehav Rev. 2015;51:276–295. doi: 10.1016/j.neubiorev.2015.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams M, Pearce RK, Hirsch SR, Ansorge O, Thom M, Maier M. Fibrillary astrocytes are decreased in the subgenual cingulate in schizophrenia. Eur Arch Psychiatry Clin Neurosci. 2014;264(4):357–362. doi: 10.1007/s00406-013-0482-4. [DOI] [PubMed] [Google Scholar]
- Wood SJ, Yucel M, Wellard RM, Harrison BJ, Clarke K, Fornito A, Velakoulis D, Pantelis C. Evidence for neuronal dysfunction in the anterior cingulate of patients with schizophrenia: a proton magnetic resonance spectroscopy study at 3 T. Schizophr Res. 2007;94(1–3):328–331. doi: 10.1016/j.schres.2007.05.008. [DOI] [PubMed] [Google Scholar]
- Zhang YY, Brady M, Smith S. Segmentation of brain MR images through a hidden Markov random field model and the expectation-maximization algorithm. IEEE Transactions on Medical Imaging. 2001;20(1):45–57. doi: 10.1109/42.906424. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Shen J. Simultaneous quantification of glutamate and glutamine by J-modulated spectroscopy at 3 Tesla. Magnetic Resonance in Medicine. 2015 doi: 10.1002/mrm.25922. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.