Abstract
Flaxseed has been studied for decades for its health benefits that include anti-cancer, cardioprotective, anti-diabetic, anti-inflammatory properties. The biologically active components that mediate these effects are the omega-3 fatty acids and the lignan, secoisolaricirescinol diglucoside. We have previously shown that whole flaxseed supplemented diet decreases the severity and incidence of ovarian cancer while a 15% dose of flaxseed is most protective against inflammation and estrogen-induced chemical and genotoxicity. The objective of this study was to dissect the independent effects of the two flaxseed components on estrogen signaling and metabolism. Two and half year old hens were fed either a control diet, 15% whole flaxseed diet, defatted flax meal diet or 5% flax oil diet for 3 months after which the animals were sacrificed and blood and tissues were harvested. Whole flaxseed diet caused a decrease in expression of ERα. ERα target gene expression was assessed using RT2 profiler PCR array. Some targets involved in the IGF/insulin signaling pathway (IRS1, IGFBP4, IGFBP5) were downregulated by flaxseed and its components. Flaxseed diet also downregulated AKT expression. A number of targets related to NF-κB signaling were altered by flaxseed diet including a series of targets implicated in cancer. Whole flaxseed diet also affected E2 metabolism by increasing CYP1A1 expression with a corresponding increase in the onco-protective E2 metabolite, 2-methoxyestradiol.
The weak anti-estrogens, enterolactone, enterodiol and 2-methoxyestradiol, might be working synergistically to generate a protective effect on the ovaries from hens on whole flaxseed diet by altering the estrogen signaling and metabolism.
Keywords: estrogen, flaxseed, ovarian cancer, hen
1. INTRODUCTION
Flaxseed is mainly cultivated as an oil crop and is a rich source of the omega-3 fatty acid, alpha-linolenic acid. Other than its high oil content, flaxseed is also a source of dietary fiber, plant lignans and protein. This makes flaxseed a very rich source of nutrition [1]. For many years, scientists have been intrigued by the specific health benefits associated with the biologically active components of flaxseed. The two main biologically active components of flaxseed are the polyunsaturated omega-3 fatty acid, alpha linolenic acid, which makes up 59% of the oil content, and the lignan, [2] secoisolaricirescinol diglucoside (SDG) that comprises about 0.7%–1.9% of the whole flaxseed [3]. SDG is metabolized by the gut flora to enterolactone (EL) and enterodiol (ED).
The omega-3 fatty acids (OM-3FAs) have been reported to have chemo-preventative effects [4]. There is extensive evidence regarding the anti-inflammatory [5, 6], anti-arrhythmic [7], anti-thrombotic [8], anti-atheromatous [9] and hypolipidemic [10] effects of the OM-3FAs. The lignan, SDG, its aglycone form secoisolaricirescinol (SECO), and their metabolites, have hydroxyl radical scavenging properties which make them potent anti-oxidants [11]. The SDG metabolites, EL and ED have been shown to antagonize E2 signaling [12]. Flaxseed and its components are beneficial in reducing symptoms of benign prostate hyperplasia [13], decreasing cholesterol levels [14] and decreasing skin sensitivity while improving skin texture [15]. We have shown that flaxseed diet causes an increase in the 2-hydroxyestradiol/16-α hydroxyestradiol metabolite ratio in urine and serum [16, 17], which has been shown to decrease the risk of developing cancer in postmenopausal women [18]. Flaxseed and its components have protective effects against a myriad of cancers including breast cancer [19–21], prostate cancer [22, 23] and melanoma [24].
We have previously demonstrated that flaxseed supplemented diet reduces the incidence and severity of ovarian cancer in chickens [25, 26]. This effect corresponded with a decrease in COX-1 and COX-2 enzyme expression in parallel with decrease in prostaglandin E2 (PGE2) levels in the ovary [27]. The decrease in PGE2 levels could be a result of the high alpha linoleic acid content of flaxseed oil which is partially converted to EPA (eicosapentanoic acid) and DHA (docosahexanoic acid) in the cells. EPA and DHA can get incorporated into the cell membrane and shift the equilibrium of PG (prostaglandin) synthesis to a less inflammatory type (PGE3) [28] as previously demonstrated by our group [29]. In addition, E-cadherin, a marker characteristically upregulated in human epithelial ovarian cancers as well as in the hen ovarian tumor [30], is significantly downregulated in ovarian tumors from hens fed a flaxseed supplemented diet [31]. Flaxseed lignan metabolites, EL and ED, have been shown to decrease estrogen dependent receptor activation in breast cancer cells and flaxseed diet also results in decreased ERα expression in the chicken ovary[17]. This is significant because E2 increases tumor aggressiveness and it has been demonstrated that treatment with E2 resulted in increased rate of distant metastasis in the ovarian cancer xenograft mouse model [32].
Our group has previously shown that flaxseed diet can alter a myriad of markers implicated in ovarian cancer including estrogen receptor alpha, 2-hydroxyestradiol/16-α-hydroyxestradiol ratio, PGE2, COX-1 and COX-2 enzymes, E-cadherin, CYP1B1, FOXA2, PAX2, EN-1, MSX2 etc. [17, 26, 30, 31, 33], but it is important to determine the precise pathways targeted by the biologically active components of flaxseed that affect the expression of a wide range of cancer targets.
The objective of this study was to understand the independent effects of the individual components of the flaxseed on estrogen signaling. This is essential in order to attribute the changes in specific E2 targets to a particular biomolecule and its downstream pathway. By unraveling the molecular targets altered by flaxseed components we can design a better preventative and therapeutic strategy against ovarian cancer.
2. MATERIALS AND METHODS
2.1 Reagents
All the reagents are as described in [17]. The AKT (9272S) and pAKT (4060S) antibodies were from Cell Signaling Technology, Beverly, MA. The ERα (sc-73479),ERα (sc-543) antibodies and CYP1A1(sc-20772) antibody were from Santa Cruz Biotechnology, Dallas, TX. The Custom Chicken RT2 Profiler PCR array was designed by SABiosciences, Qiagen, Venlo, Netherlands. The dual luciferase reporter assay (e1910) was from Promega and lipofectamine 3000 from Invitrogen. E2 coat-a-count RIA kit from Siemens (TKE21). HEK 293 cells were obtained from ATCC (ATCC CRL-1573).
2.2 Diet composition
The diet compositions have been described in Table 1. The 4 diets included were control diet, whole flaxseed supplemented diet, defatted flax meal (DFM) supplemented diet (lignan rich) and flax oil supplemented diet (omega 3 fatty acid rich). All the diets were isocaloric. To balance the high protein and high fiber content of the flaxseed meal and whole flax diets, the flax oil and control diets were supplemented with corn gluten meal and solka floc (International Fiber Corporation), respectively. To balance for the fat content of the control and flax oil diets, the whole flax and flax meal diets were supplemented with the suitable amount of qual fat (DarPro). The diets were formulated to differentiate the effects of the flax lignan from the flax oil as illustrated in Table 1.
Table 1.
Formulation of diets and diet compositions
| Diet | Control | 5% flax oil | 15% DFM | 15% whole flaxseed |
|---|---|---|---|---|
| Enriched with: | ---- | ALA | SDG | ALA + SDG |
| Ingredient | ||||
| Corn | 67.40 | 52.00 | 51.90 | 47.58 |
| Flaxseed (whole) | 15.00 | |||
| SBM | 18.30 | 18.30 | 18.30 | 18.30 |
| Corn Gluten Meal | 3.00 | 5.00 | ||
| Flax Oil | 5.00 | |||
| Qual Fat | 3.80 | 2.50 | ||
| Defatted Flax Meal | 15.00 | |||
| Solka Floc | 0.30 | 8.70 | 5.62 | |
| Limestone | 8.75 | 8.75 | 8.75 | 8.75 |
| Dical | 1.50 | 1.50 | 1.50 | 1.50 |
| Salt | 0.30 | 0.30 | 0.30 | 0.30 |
| Vitamin Mix | 0.20 | 0.20 | 0.20 | 0.20 |
| Mineral Mix | 0.15 | 0.15 | 0.15 | 0.15 |
| DL-Met | 0.10 | 0.10 | 0.10 | 0.10 |
| Calculated Analysis | ||||
| CP, % | 16.56 | 16.49 | 18.49 | 16.50 |
| TME, kcal/kg | 2,816 | 2,815 | 2,816 | 2,815 |
| Calcium, % | 3.73 | 3.73 | 3.77 | 3.75 |
| Phosphorus, % | 0.38 | 0.37 | 0.40 | 0.38 |
| Met + Cys, % | 0.67 | 0.67 | 0.72 | 0.64 |
2.3 Animals
Two and a half year old hens (Gallus domesticus) were either fed control diet or diet supplemented with individual components of flaxseed for a period of 3 months (Table 2). Each diet group had 30 birds. Hens were exposed to a photoperiod of 17 h light: 7 h dark, with lights turned on at 05:00 h and turned off at 22:00 h. Animal management and procedures were reviewed and approved by the Institutional Animal Care and Use Committees at the University of Illinois at Urbana-Champaign and Southern Illinois University at Carbondale. Blood was collected at different time points throughout the study by wing vein puncture and tissues were harvested at the end of the study after sacrificing the hens. Tissue collection and processing was done as described previously [34].
Table 2.
Genes altered in the estrogen receptor target gene PCR array
| GENE NAME | FUNCTION | FOLD CHANGE |
DIET GROUPS WITH ALTERED EXPRESSION |
|---|---|---|---|
| GENES UPREGULATED | |||
| BDNF | Brain derived neurotrophic factor-involved in learning and memory; decrease in levels can increase risk of Alzheimer’s. | 4.8, 2.98 | Whole flax and Flax oil |
| GPER | G-protein coupled estrogen receptor that can mediate E2 effects. | 1.48 | Whole flax |
| FOS | Generally upregulated in cancers but downregulation of Fos has been associated with decreased progression free survival in OvCa. It is proapoptotic and higher expression is associated with low grade and low malignancy potential tumors.[71, 74–77] | 10.22 | Defatted flax meal |
| LTBP1 | It associates TGFβ to the extracellular matrix. | 1.45 | Flax oil |
| PHB2 | Acts as an estrogen repressor and as a tumor suppressor. [72] | 4.89 | Whole flax |
| NRIP1 | It interacts with nuclear receptors and promotes target gene transcription. | 4.48 | Defatted flax meal |
| NFKB1A | It is the inhibitor of the NF-κB that is constitutively active in a number of autoimmune and inflammatory diseases. | 1.33 | Flax oil |
| WISP2 | This gene is downstream of WNT 1 signaling pathway is associated with malignant transformation of breast cancer cells [78]. | 2.27 | Defatted flax meal |
| AHR | It is a ligand activated nuclear receptor regulates the expression of cytochrome p450 enzymes. | 1.66,1.37 | Defatted flax meal and whole flax |
| GENES DOWNREGULATED | |||
| IGFBP4 | It is known to increase the half-life of IGFs and also alter the interaction between IGFs and their cell surface receptors. Its expression is stimulated by estrogen receptor alpha. | 0.13, 0.36 | Whole flax and defatted flax meal |
| IGFBP5 | It can regulate metastasis of breast cancer[79] and involved in progression of prostate cancer [80] and thyroid cancer[81]. | 0.42, 0.7 | Whole flax and defatted flax meal |
| MAFF | Transcription factor involved in a number of biological processes. It is also upregulated in multiple myeloma[69] and breast cancer[68] patients. | 0.37 | Whole flax |
| RARA | Nuclear receptor, which on activation can induce targets involved in the TGF beta pathway. Also induces targets involved in cell proliferation and metastasis. Involved in promoting EMT [82] | 0.5 | Whole flax |
| NCOA1 | Overexpression promotes breast cancer metastasis [83]. Acts as a transcriptional repressor for pro-apoptotic proteins in turn promoting survival. [84] | 0.52 | Whole flax |
| NCOR2 | Increased expression of splice variant is associated with Tamoxifen resistance in breast cancer. [85] Over expression is associated with poor prognosis as it inhibits apoptosis and promotes cell progression. [86] | 0.74 | Whole flax |
| CTGF | It is an extracellular matrix associated protein that is involved in cell proliferation, migration. It is upregulated in a myriad of cancers [87, 88] | 0.59, 0.61 | Whole flax and Flax oil |
| BCAR1 | It is a scaffold protein that controls cell motility, migration and survival [66]. | 0.39 | Whole flax |
| BCL2L1 | Anti-apoptotic protein belonging to the BCL-2 family of proteins. | 0.57 | Whole flax |
| APBB1 | Nuclear adapter protein that interacts with amyloid precursor protein. | 0.4 | Whole flax |
| IRS1 | It is a docking protein important in intracellular signaling of the IR and IGF1R. It can contribute towards tumor progression [89] | 0.5, 0.41 | Whole flax and Flax oil |
| NR3C1 | Glucocorticoid receptor can act as a transcription factor and regulate inflammatory responses, proliferation and differentiation. | 0.55 | Whole flax |
2.4 RNA isolation and cDNA synthesis
Total RNA was extracted from ovarian tissue using Trizol reagent as described previously [34, 35]. RNA was quantified by measuring the sample absorbance at A260. RNA quality was assessed by using Experion RNA StdSens Analysis System (Biorad). RNA samples were then treated with RQ1 RNase-free DNase (Promega, Madison, WI) prior to reverse transcription reaction. cDNA was synthesized using the Applied Biosystems High-Capacity cDNA Reverse Transcription Kit (Life Technologies, Carlsbad, CA). This cDNA was used to analyze gene expression using real time qPCR and PCR array.
2.5 PCR array
Changes in the expression of estrogen target genes were assessed by using the SABiosciences RT2 PCR array. The array was specifically designed for analyzing E2 targets in the chicken tissue. Ninety-six target genes were analyzed for 6 samples from each of the 4 diet groups using 6 arrays. cDNA was synthesized from the RNA samples isolated from the ovary tissues using the RT2 First Strand kit (Qiagen). Real time qPCR was performed using the RT2 SYBR Green qPCR Mastermix (Qiagen) and CFX384 Touch Real Time PCR detection system (Biorad). Target genes were normalized to 5 housekeeping genes (ACTB, HMBS, H6PD, RPL4 and UBC) that were pre-selected for the array. PCR array data was analyzed by using the RT2 Profiler PCR Array Data Analysis version 3.5 on the SABiosciences website.
2.6 Real time qPCR
Target gene mRNA levels were analyzed with Real time quantitative PCR using the CFX384 Real Time System. Gene specific primers were used for target as well as reference gene amplification (Supplemental Table 2). GAPDH and 18s rRNA were used as housekeeping genes to normalize target gene expression. Amplification conditions: 95°C for 30s followed by 40 cycles of 95°C for 10s and 60°C for 15s with melt curve measured at 65 °C to 95 °C every 0.5 °C gradient for 5s. A non-template control was run for every target gene amplified.
2.7 Western blotting
Proteins were isolated from the ovary samples as described previously [34]. Twenty five micrograms of total protein was separated using a SDS-PAGE gel with 10% acrylamide/SDS separating gels and transferred to nitrocellulose membranes. Membranes were blocked using the SeaBlock blocking buffer (Pierce) followed by overnight incubation at 4° C with the target primary antibodies (CYP1A1, ER alpha, pAKT, AKT). Following the overnight incubation, blots were washed using 1X TBS with 0.01% Tween 20. Blots were further incubated with a DyLight™ 680 conjugated goat anti-mouse IgG antibody (H&L) and DyLight™ 800 conjugated goat anti-rabbit IgG antibody (H&L) for an hour at room temperature. After 3 washes with 1X TBS with 0.01% Tween 20, the blots were scanned for infrared signal using Odyssey CLx imaging system (LI-COR Biosciences). All the targets were normalized to β-Actin expression.
2.8 Immunohistochemistry
Tissues were collected, fixed and embedded as described in [34]. Five micrometer thick sections were cut and mounted on SuperFrost Plus microscope slides. Following deparaffinization, slides were rehydrated through xylene and graded ethanol solutions. Antigen retrieval was performed by using 0.9% Antigen unmasking solution (Vector Laboratories) and pressure cooked at 20 psi for 5 min. Slides were allowed to cool and sections were blocked with 5% normal horse serum. Sections were incubated with anti-estrogen receptor antibody overnight at 4° C. After washing with 1X PBS with 0.01% Tween 20, sections probed with anti-estrogen receptor antibody (sc-543) were incubated with Alexa 488 conjugated anti-mouse IgG (Jackson laboratories) for an hour at room temperature, washed with 1X PBS with 0.01% Tween 20 and mounted using Dapi Fluoromount G (Southern Biotech). Sections were visualized using a Leica DM5500Q microscope and images were captured using a Leica DFC365 FX camera. Images taken from the A4 (Dapi) and L5 (Alexa 488) channels were superimposed using the Leica Application Suite-Advanced fluorescence version 2.6.0.7266 software.
2.9 Liquid Chromatography Tandem Mass Spectrometry for Enterodiol and Enterolactone analysis
Levels of ED and EL were analyzed in the liver tissue by using LC MS/MS analysis. Extraction and analysis on the liver samples were performed as described previously [17].
2.10 High performance liquid chromatography for SDG analysis
SDG levels from the 3 different batches of diet were analyzed across all the diet groups. SDG was extracted and analyzed as described previously [17].
2.11 Gas chromatography for fatty acid analysis
Fatty acids were extracted from the ovary samples and levels of OM-3FAs and omega-6 fatty acids (OM-6FAs) were analyzed using gas chromatography as described previously [17, 29].
2.12 Statistics
All the surrogate endpoints were analyzed using 4–8 biological replicates and 2 technical replicates of each sample. Effect of the different flaxseed diets was assessed by normalizing to control diet. For experiments involving real time qPCR, western blotting, ELISA, RIA and in vitro reporter assay, statistical calculations were done using GraphPad Instat software by employing one-way ANOVA analysis followed by Tukey’s range test. For the PCR array, data analysis was performed by the RT2 profiler PCR array data analysis version 3.5 software available on the Qiagen website. The results that have been presented in the figures have been analyzed using the one way ANOVA. A p < 0.05 was considered significant while a p < 0.01 was considered highly significant.
3. RESULTS
3.1 Analysis of SDG and OM-3FA content in the diets using LC and GC
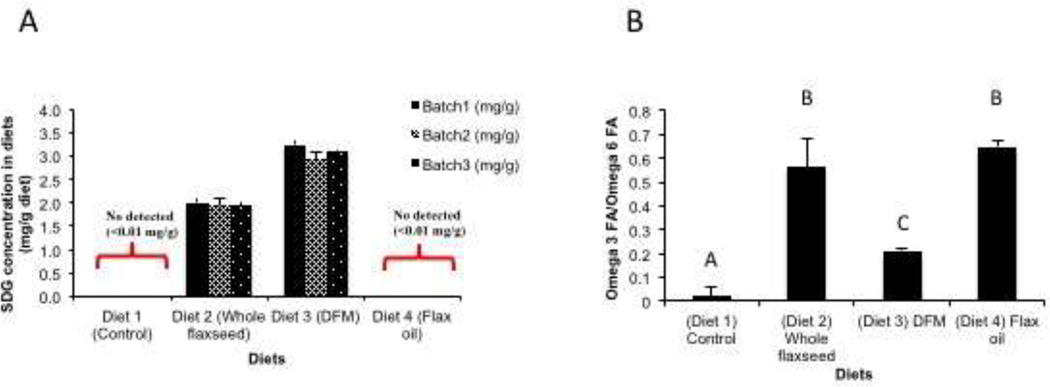
LCMS analysis indicated that SDG was only detected in the whole flaxseed and defatted flax meal diets while the control diet and flax oil supplemented diets did not have any detectable levels of SDG (Figure 1A). The SDG content of the defatted flax meal diet was 1.5 times more than that in the whole flaxseed supplemented diet (Figure 1A). Further calculations suggested that excess flax lignan got added while formulating the defatted flax meal diet. Levels of OM-3FAs and OM-6FAs were determined by GC analysis. Ratio of OM-3FAs to OM-6FAs was significantly higher in the whole flaxseed and flax oil supplemented diets in comparison to the defatted flax meal supplemented diet. Control diet had comparable levels of OM-3FAs and OM-6FAs (Figure 1B).
Figure 1.
Levels of SDG and omega fatty acids in the different diets. A, SDG was only detected in the whole flaxseed and defatted flax meal supplemented diet when analyzed by LC/MS. B, Ratio of omega 3 fatty acid to omega 6 fatty acid in the ovary tissue was significantly high in whole flaxseed and flax oil supplemented diets in comparison to defatted flax meal diet as analyzed by gas chromatography. Control diet had comparable levels of omega 3 and omega 6 fatty acids.
3.2 Levels of Enterolactone and Enterodiol in the liver tissue
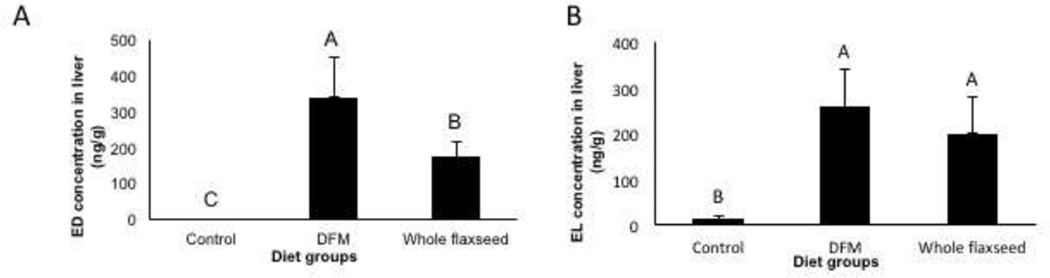
ED and EL were only detected in the tissue of the hens that were fed either whole flaxseed or defatted flax meal diets (Figure 2). No ED or EL was detected in the hens fed flax oil supplemented diet and hens fed control diet (data not shown).
Figure 2.
Levels of ED and EL in the liver as analyzed by LCMS, n=6. A, ED was only detected in the hens that were fed defatted flax meal and whole flaxseed supplemented diets. Control vs DFM (p<0.001), Control vs whole flaxseed (p<0.001), DFM vs whole flaxseed (p<0.01). B, EL was only detected in the hens that were fed defatted flax meal and whole flaxseed supplemented diets. Control vs DFM (p<0.001), Control vs whole flaxseed (p<0.001). Letters on the error bar shared among groups indicate no significant difference.
3.3 Estrogen receptor alpha expression decreases with whole flaxseed supplemented diet
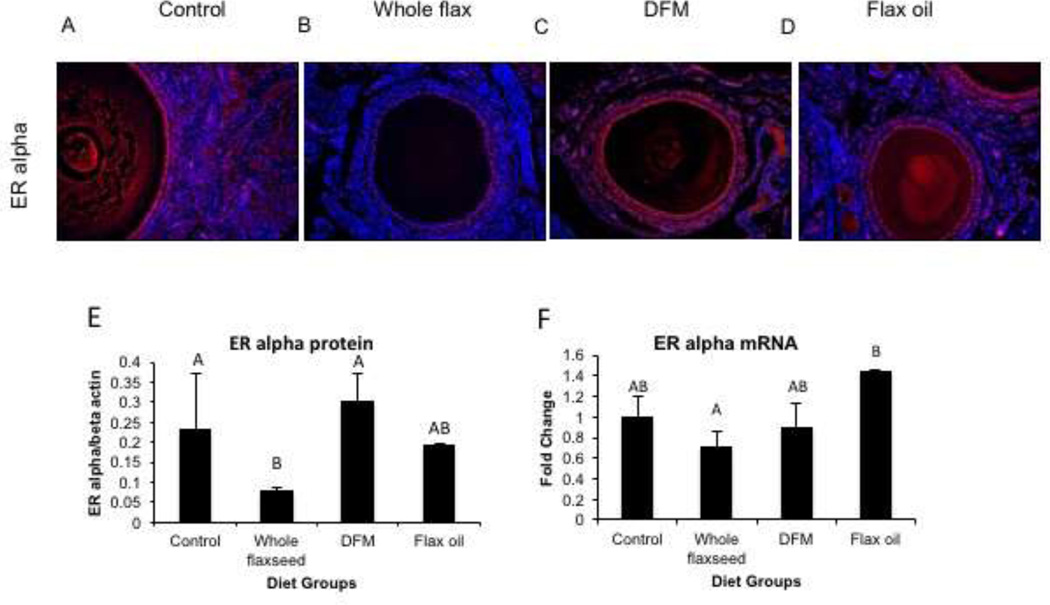
Estrogen receptor alpha is predominantly expressed in the granulosa cells of the hen ovary and to some extent in the ovarian surface epithelium. Immunofluorescence staining suggested that ERα protein expression decreased in the granulosa cells of hens that were fed a diet supplemented with whole flaxseed (Figure 3C) but not in either of the other diets supplemented with its components viz. flax oil (Figure 3D) and defatted flax meal (Figure 3B). Western blot analysis corroborated the immunofluorescence results and confirmed that the hens that were given whole flaxseed supplemented diet had decreased ERα protein expression in their ovaries (Figure 3E). Real time qPCR analysis also suggested that whole flaxseed supplemented diet causes a decrease in ERα mRNA levels (Figure 3F). The decrease in ER α expression seemed to have led to a compensatory increase in ovarian CYP19 expression (Supplementary Figure 2B) along with serum E2 levels in the whole flaxseed diet hens (Supplementary Figure 2A).
Figure 3.
Estrogen receptor alpha expression in the ovary. C, ERα expression was decreased in the granulosa cells of the ovaries of hens that were fed whole flaxseed supplemented diet in comparison to A, control fed or B, defatted flax meal supplemented or D, flax oil supplemented diets, as observed by immunofluorescence, n=3, total magnification=200x. E, ERα protein expression was decreased in the ovary tissue of whole flaxseed supplemented diet hens, assessed by western blotting, n=4. Control vs whole flaxseed (p<0.05), whole flaxseed vs DFM (p<0.05). F, mRNA levels were also downregulated in the ovaries of whole flaxseed diet fed hens in comparison to flax oil supplemented diet hens, when analyzed by qPCR n=8. Whole flaxseed vs flax oil (p<0.05). Letters on the error bar shared among groups indicate no significant difference.
3.4 Flaxseed and its components increase 2-methoxyestradiol levels in the serum with a corresponding increase in liver CYP1A1
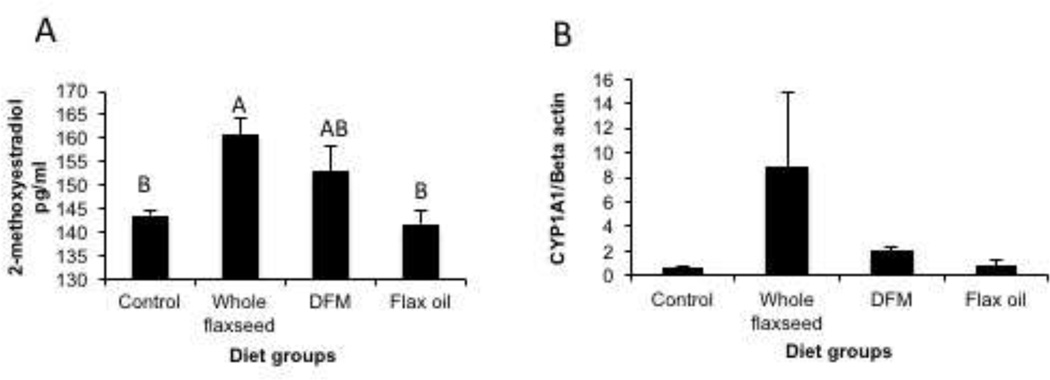
The enzyme CYP1A1 converts E2 to 2-hydroxyestradiol, which is in turn a substrate for Catechol-O-Methyltransferase (COMT) that results in the formation of 2-methoxyestradiol. ELISA analysis for 2-methoxyestradiol on the chicken serum samples suggested that hens that were fed whole flaxseed supplemented and defatted flax meal supplemented diets had higher levels of 2-methoxyestradiol (Figure 4A). Whole flaxseed supplemented diet dependent upregulation of CYP1A1 enzyme substantiates the observed increase in the levels of the anti-angiogenic and pro-apoptotic 2-methoxyestradiol in the serum (Figure 4B).
Figure 4.
Serum levels of 2-methoxyestradiol and CYP1A1 expression in the liver. A, Levels of 2-methoxyestradiol were upregulated in the serum from the hens that were fed whole flaxseed supplemented diet, when analyzed using the Cayman Chemicals EIA assay, n=6. Control vs whole flaxseed (p<0.05), whole flaxseed vs flax oil (p<0.05), B, CYP1A1 enzyme expression increased significantly in the hens that were fed whole flaxseed supplemented diet, analyzed by western blotting, n=5. Letters on the error bar shared among groups indicate no significant difference.
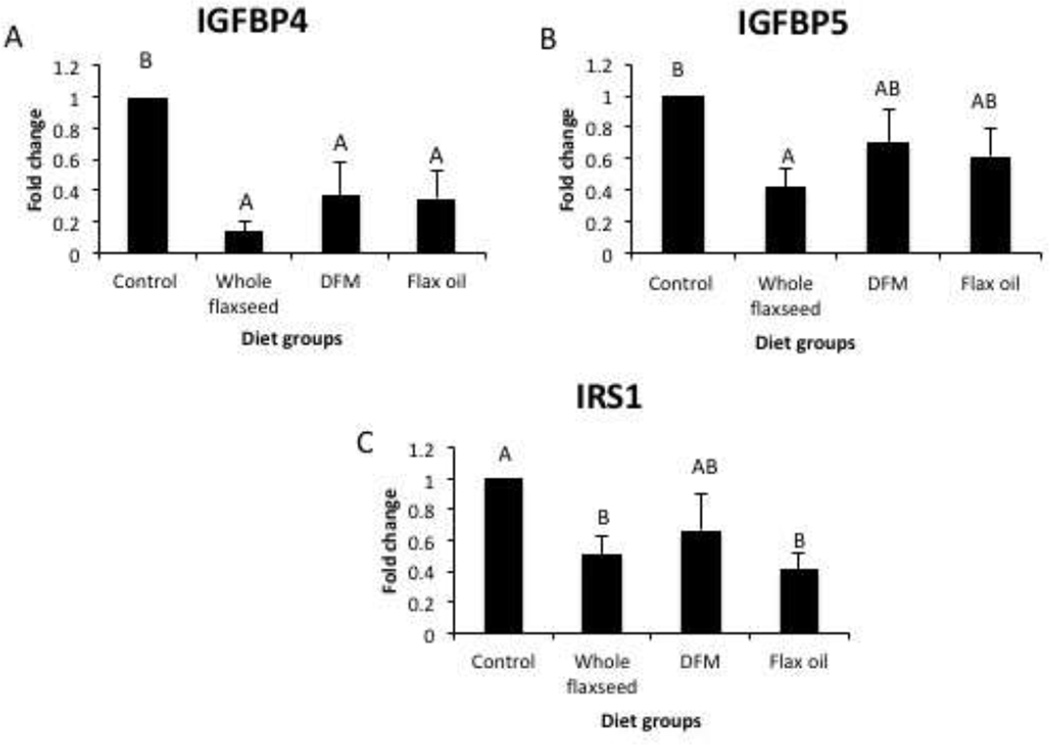
3.5 Effect of flaxseed supplemented diet on estrogen targets involved in the IGF signaling pathway
PCR array analysis suggested that the whole flaxseed supplemented diet resulted in a decrease in the mRNA expression of IGFBP4 and IGFBP5 (Figure 5A and 5B), which are known to stabilize IGF-1. Diets supplemented with flaxseed as well its components resulted in a decrease in IRS1 mRNA expression (Figure 5C).
Figure 5.
Expression of ER targets involved in the IGF signaling pathway analyzed by PCR array in the ovarian tissue, n=6. A, mRNA expression of IGFBP4 decreased with whole flaxseed supplemented diet, defatted flax meal supplemented diet and flax oil supplemented diet, control vs. whole flax (p<0.05), control vs. whole flax (p<0.05), control vs. flax oil (p<0.05). B, mRNA expression of IGFBP5 decreased with whole flaxseed supplemented diet (p<0.05) C, mRNA expression of IRS1 decreased with diets supplemented with whole flaxseed and flax oil (p<0.05). Letters on the error bar shared among groups indicate no significant difference.
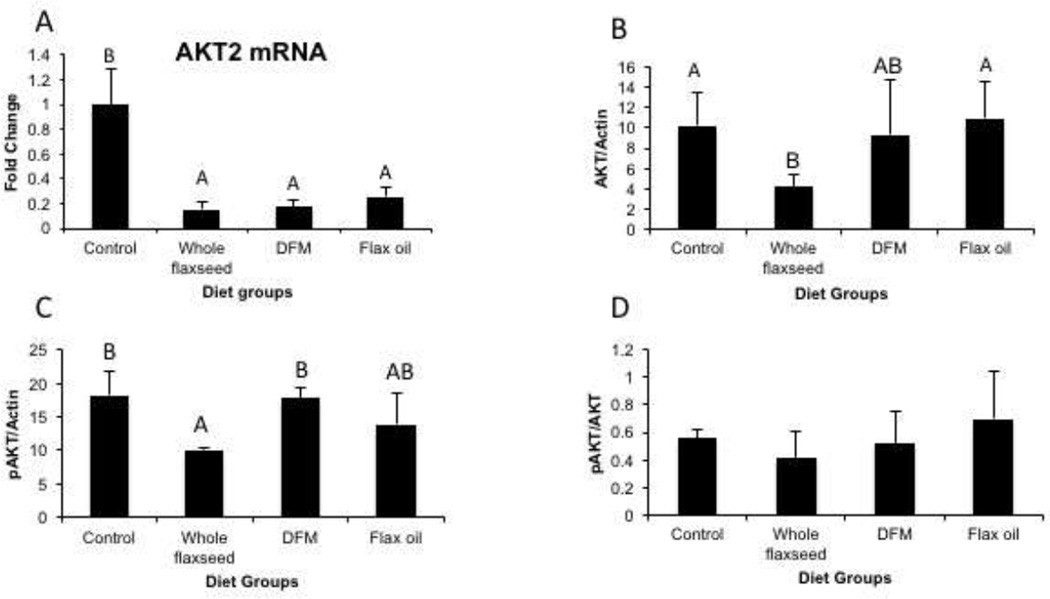
3.6 Flaxseed supplemented diet decreases AKT expression in the ovary
Since AKT2 is predominantly involved in glucose homeostasis we analyzed the effect of flaxseed diet on the mRNA expression of AKT2 and also examined the effect of diet on AKT activation. qPCR analysis revealed that mRNA expression of AKT2, predominantly involved in glucose homeostasis, was downregulated by diets supplemented with whole flaxseed and its components (Figure 6A). Western blotting analysis suggested that levels of total AKT protein and phosphorylated AKT protein decreased with whole flaxseed supplemented diet, when normalized to beta-actin (Figure 6B and 6C). However, the ratio of pAKT/AKT remained unaffected by flaxseed or its components (Figure 6D).
Figure 6.
Effect of flaxseed supplemented diet on AKT expression and phosphorylation. A, Real time qPCR suggested decrease in AKT2 mRNA expression by diets supplemented with whole flaxseed and its components, n=6, control vs. whole flaxseed (p<0.01), control vs. DFM (p<0.01), control vs. flax oil (p<0.01). B and C, western blotting revealed decreased expression of total AKT, control vs. whole flaxseed (p<0.05), whole flaxseed vs. flax oil (p<0.05) and phosphorylated AKT in the whole flaxseed supplemented diet, control vs. whole flaxseed (p<0.05), whole flaxseed vs. DFM (p<0.05). D, Ratio of phosphorylated Akt to total Akt remained unaltered with diet, n=3. Letters on the error bar shared among groups indicate no significant difference.
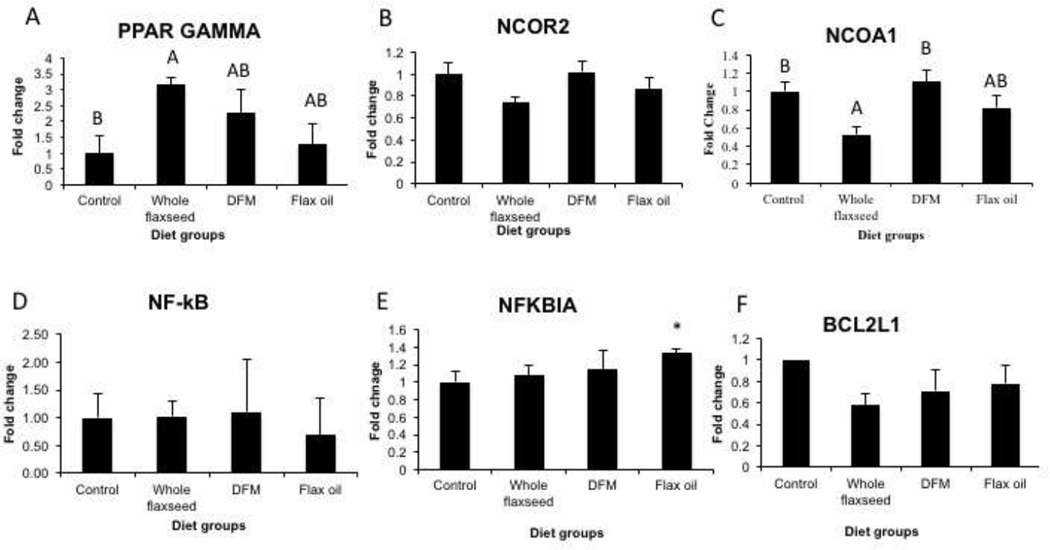
3.7 Whole flaxseed and its components may abrogate NF-κB activation
qPCR analysis on the ovary tissue indicated that hens that were fed whole flaxseed supplemented diet showed increased expression of PPAR γ mRNA, possibly due to a strong trend in NCOR2 downregulation with whole flaxseed supplemented diet (Figure 7A and 7B). Since PPAR γ can regulate NF-κB expression, we performed qPCR, which revealed that diet did not affect NF-κB mRNA expression (Figure 7D). Flax oil diet did lead to an upregulation of the NF-κB inhibitor, NFKBIA expression (Figure 7E). NFKBIA prevents nuclear translocation of NF-κB thus preventing its transcription activity. Interestingly, we observe a decrease in the expression of anti-apoptotic gene BCL-2L1, a direct target of NF-κB, in the whole flaxseed diet group (Figure 7F). NCOA1, a cofactor that regulates NF-κB’s transcriptional activity was also downregulated by whole flaxseed diet (Figure 7C).
Figure 7.
Effect of flaxseed supplemented diet on targets involved in the NF-κB pathway in the ovary, n=6. A, whole flaxseed supplemented diet increased the mRNA levels of PPAR gamma in comparison to control diet when analyzed using qPCR, control vs whole flaxseed (p<0.05), B, expression of transcriptional repressor, NCOR2 was also downregulated with whole flaxseed diet. C, whole flaxseed supplemented diet decreased the mRNA expression of NCOA1, control vs whole flax (p<0.05), whole flax vs. defatted flax meal (p<0.05). D, NF-κB mRNA expression was not significantly altered by the any diet group, E, mRNA expression of NFkBIA was upregulated in the flax oil diet. F, expression of pro-apoptotic protein BCL2L1 was also downregulated in the whole flaxseed supplementary diet. Letters on the error bar shared among groups indicate no significant difference.
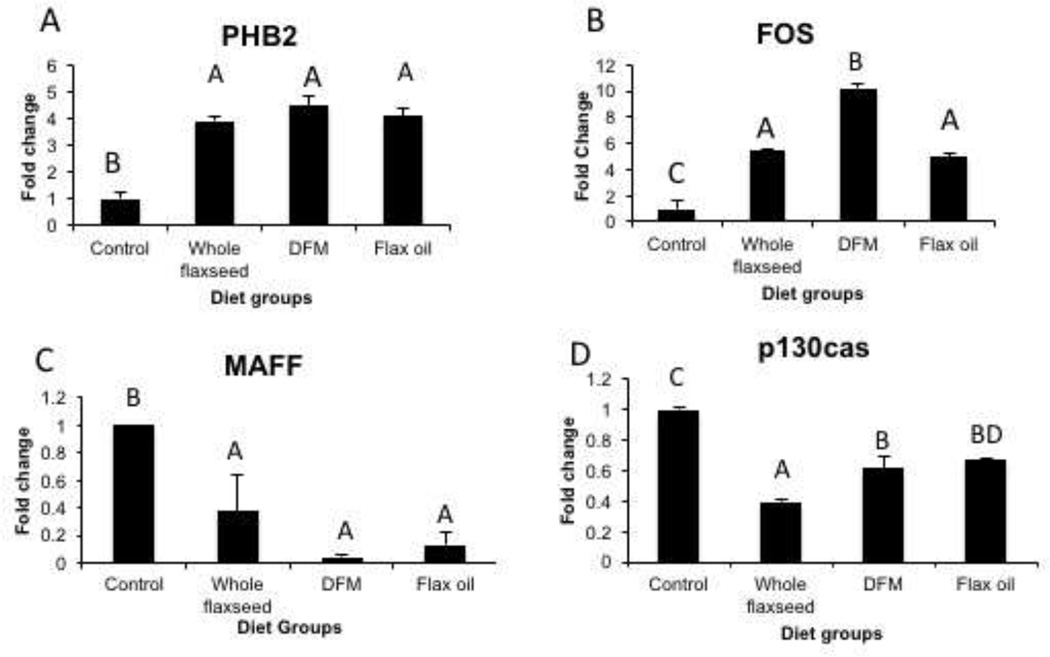
3.8 Flaxseed and its components alter ovarian expression of genes implicated in cancer
PCR array analysis of E2 targets revealed that mRNA levels PHB2 were upregulated in whole flaxseed, defatted flax meal and flax oil supplemented diets (Figure 8A) while mRNA levels of c-FOS were upregulated only with defatted flax meal (Figure 8B). mRNA levels of transcription factor, MAFF decreased with whole flaxseed, defatted flax meal and flax oil supplemented diets (Figure 8C) while mRNA levels of scaffold protein, p130cas decreased with whole flaxseed diet (Figure 8D). Other targets altered by the diets and analyzed by the PCR array are listed in Table 2.
Figure 8.
Flaxseed diet and its components alter the ovarian expression of a series of genes implicated in cancer as assessed by PCR array, n=6. A mRNA expression of estrogen repressor, PHB2 increases with whole flaxseed, defatted flax meal and flax oil supplemented diets, control vs whole flaxseed (p<0.05), control vs DFM (p<0.05), control vs. flax oil (p<0.05). B, c-FOS mRNA expression increased with whole flaxseed, defatted flax meal and flax oil supplemented diet, control vs whole flaxseed (p<0.001), control vs DFM (p<0.001), control vs flax oil (p<0.001), whole flax vs. DFM (p<0.01), DFM vs. Flax oil (p<0.001). C, whole flaxseed supplemented diet, defatted flax meal supplemented diet and flax oil supplemented diet decreases mRNA expression of MAFF, control vs whole flaxseed (p<0.05), control vs. DFM (p<0.05), control vs. flax oil (p<0.05). E, Whole flaxseed diet, DFM diet and flax oil diet decreases the mRNA expression of p130cas, control vs whole flaxseed (p<0.001), control vs DFM (p<0.001), control vs flax oil (p<0.001), whole flax vs. DFM (p<0.01), whole flax vs. Flax oil (p<0.001). Letters on the error bar shared among groups indicate no significant difference.
4. DISCUSSION
The objective of the current study was to dissect the individual effects of the biologically active components of flaxseed through dietary intervention. We have already established that a diet supplemented with flaxseed is effective in reducing the severity and incidence of ovarian cancer in chickens [25]. We have further demonstrated that the effects exhibited by flaxseed are dose dependent by showing that a dose of 15% flaxseed is the most protective against inflammation and estrogen-induced chemical and genotoxicity [17]. Based on these data, we designed a study to incorporate diets enriched with either whole flaxseed, defatted flax meal or flax oil and compared their effects to control diet (Table 1). SDG was present in the whole flaxseed and defatted flax meal diets while OM-3FAs were only present in the whole flaxseed and flax oil diets. Our data indicated that the whole flaxseed supplemented diet yielded the best results in terms of decreasing ERα expression and altering the expression of a series ERα targets genes involved in the IGF/insulin signaling pathway, cell proliferation, cancer metastasis as well as altering E2 metabolism.
LC MS/MS analysis on the liver tissue indicated that ED and EL were only detected in the tissues of the hens that were fed a diet supplemented with either whole flaxseed or defatted flax meal (Figure 2). ED and EL are metabolites of SDG, which was only present in the whole flaxseed and defatted flax meal supplemented diets (Figure 1). It has been proposed that ED and EL are ERα antagonists in the presence of E2 and weak agonists in the absence of E2 [12]. This was tested in our in vitro model with HEK293 cells. A high concentration of EL was able to decrease ERα activation in presence of E2 as measured by a luciferase reporter assay (Supplementary figure 1).
2-methoxyestradiol, a natural metabolite of estradiol has been shown to exhibit anti-tumorigenic and anti-angiogenic activity in vivo as well as in vitro [36]. 2-methoxyestradiol is formed by the action of COMT on 2-hydroxyestradiol, which is predominantly derived by the action of the cytochrome P450 enzyme CYP1A1. Similar to ED and EL, 2-methoxyestradiol binds weakly to the estrogen receptor and does not elicit strong estrogenic responses [37]. 2-methoxyestradiol can also induce apoptosis in cancer cells by upregulating p53 [38]. There is also evidence suggesting that 2-methoxyestradiol induces its apoptotic effects by interfering with the microtubule dynamics and by inhibition of the mitochondrial electron transport complex 1, leading to the generation of reactive oxygen species [39]. It has been suggested that the growth inhibitory effects mediated by 2-methoxyestradiol could be due to an intracellular effector or a specific receptor [40], distinct from its effects on ERα. Analysis of 2-methoxyestradiol levels suggested that the whole flaxseed supplemented diet and the defatted flax meal supplemented diet resulted in an increase in serum levels of 2-methoxyestradiol (Figure 4A). Concurrently, there was also an increase in CYP1A1 protein level in the livers of hens fed the whole flaxseed diet (Figure 4B). The whole flaxseed diet preferentially facilitates the 2-hydroxylation of E2, in turn making less E2 available for 4-hydroxylation or 16-hydroxylation. This indicates that whole flaxseed diet has a protective effect against the genotoxic 4-hydroxyestradiol and highly estrogenic 16-hydroxyestradiol [41, 42]. In addition, due to its weak affinity for the ER, 2-methoxyestradiol can act as an E2 antagonist similar to ED and EL.
The PCR array suggested that AHR mRNA levels were upregulated in defatted flax meal and whole flaxseed supplemented diets. The aryl hydrocarbon receptor (AHR) and its nuclear translocator (ARNT) are activated in presence of polyaromatic hydrocarbons (PAHs). Some plant derived compounds like resveratrol, isoflavones like diadzein, hesperetin etc. activate AHR [43]. Since flaxseed lignan metabolites ED and EL have a similar polycyclic structure, they might be stimulating AHR expression in our model. On activation, they promote the expression of cytochrome p450 enzymes like CYP1A1 and CYP1B1, which metabolize the PAHs [44]. It has been shown that AHR can regulate CYP1A1 and CYP1B1 differentially, based on the cell type and the ligand involved in activation [45]. We did not observe any significant change in CYP1B1 expression in the ovary or liver but CYP1A1 (undetectable in chicken ovary) expression was upregulated in the liver of whole flaxseed-fed birds (Figure 4B). In addition, it has been very well established that AHR can promote ER α destabilization by targeting it for proteasomal degradation by ubiquitin ligase [46].
We observed that ERα expression was down regulated in the ovaries of the hens that were fed a diet supplemented with 15% whole flaxseed (Figure 3). The ERα gene is E2 responsive; as a result, the presence of ED and EL could be responsible for downregulating ERα gene. Effects of E2 are predominantly mediated through ERα in the ovary. E2 is critical for maintaining normal ovarian function including egg development, tissue homeostasis etc. Flaxseed diets do not impact the egg laying frequency in these hens [17, 27] indicating that the normal function of the ovary was maintained. We analyzed the E2 levels in the serum samples and found that in fact the whole flaxseed diet and the flax oil diets had higher levels of E2 (Supplementary figure 2A). Cyp19/aromatase catalyzes the conversion of testosterone to estradiol. Assessing the mRNA levels of CYP19 in the ovary revealed that it was significantly upregulated in the whole flaxseed diet (Supplementary figure 2B). Downregulation of ER could lead to a compensatory increase in E2, likely the mechanism through which E2 maintains its normal functions in the ovary.
To test the effect of ERα downregulation on the expression of its target genes, we did a PCR array for assessing the expression of 96 ERα target genes, qPCR analysis and western blotting (Table 2). Genes involved in the IGF/insulin signaling pathway and the NF-κB pathway were altered by the whole flaxseed and defatted flax meal supplemented diets. Insulin and IGF are known to regulate cell cycle progression, growth, proliferation, metabolism and over all cell survival. There is strong evidence of IGF and insulin pathway dysregulation in several cancers including breast [47, 48], prostate, colon and pancreatic cancers [49]. Several therapies have been directed towards inhibiting IGF-1R signaling with the objective of preventing cancer progression [50]. In the last two decades, there have been reports of ovarian cancer cells over-expressing IGFs, IGFBPs, IGF-1R and other components of the IGF/insulin signaling pathway [51]. We found that the mRNA levels of IGFBP4, IGFBP5 and IRS-1 were downregulated by whole flaxseed supplemented diet (Figures 5A, 5B and 5C respectively). Studies with human ovarian and breast cancer cells have established that E2 upregulates, while the anti-estrogen, ICI downregulates IGFBP4 expression [52, 53]. Since we hypothesize that flaxseed is decreasing signaling thorough ERα, these results corroborate our theory. Wang et al. have shown that IGFBP5 was upregulated specifically in high grade ovarian serous carcinomas when its expression was analyzed on a tissue microarray that included a variety of normal and cancer samples[54]. Similarly, decrease in IRS-1 mRNA levels indicate a decrease in propagation of the signal stimulated by insulin/IGF-1 as IRS-1 is an important adaptor molecule activated downstream of IGF-1R. Insulin and IGF-1 are regulated via the MAP kinase/ERK1/2 and AKT/mTOR pathways. The MAP kinase ERK1/2 pathway mainly promotes cell survival. The AKT/mTOR pathway is involved in transcription of anti-apoptotic proteins, translation of targets involved in cell cycle progression, angiogenesis and regulating glucose metabolism. AKT can increase surface GLUT1 expression and regulate the activation of the rate limiting enzyme phosphofructokinase-1 [55]. AKT plays a role in oocyte maturation, granulosa cell development and activation of primary follicles [56]. We observed a decrease in the ovarian expression of AKT2 by supplementing the diet with whole flaxseed, defatted flax meal or flax oil (Figure 6D) but neither whole flaxseed nor its components had an effect on AKT phosphorylation in the ovary of hens (Figure 6E). We have also observed that flaxseed and its components downregulate total and phosphorylated AKT expression in hen ovarian tumors (manuscript in preparation). It is known that in endometrioid type ovarian cancer, the PI3K/AKT pathway is dysregulated due to mutation in the PTEN gene [57]. Endometrioid ovarian cancer is the most common histotype found in primary ovarian tumors of hens. As a result, alterations in the AKT pathway may be protective against cancers driven by the mutational landscape of endometrioid ovarian cancer. This suggests that flaxseed and its components regulate AKT expression at a transcriptional level and decrease carcinogenic potential of the ovary without affecting its normal function.
It has been demonstrated that E2 downregulates PPAR γ expression via the estrogen receptor [58]. We found that PPAR γ mRNA expression was upregulated by whole flaxseed supplemented diet further validating the possible decrease in signaling via ERα (Figure 7A). The Peroxisome Proliferator Activator Receptor (PPAR) family of nuclear receptors play a role in glucose metabolism and lipid homeostasis. PPAR γ is expressed in the granulosa cells of the normal ovary and plays a role in ovulation, inducing hormonal responses and tissue maintenance [59]. The anti-proliferative and pro-differentiation actions of PPAR γ render it anti-oncogenic. Transcriptional repressor cofactors like NCORs inhibit PPAR γ expression and in turn the transactivation and transrepression of PPAR γ target genes [60, 61]. As NCOR2 is an ERα target gene, flaxseed dependent downregulation of NCOR2, a PPAR γ transrepressor (Figure 7B), could be responsible for the observed increase in the expression of PPAR γ in the whole flaxseed supplemented diet. PPAR γ s also known to downregulate COX-2 expression [62] and decrease PGE2 levels in ovarian cancer cells [63], suggesting that it has anti-inflammatory effects. Analysis of PGE2 levels in the ovary revealed that supplementation with whole flaxseed and flax oil led to a decrease in PGE2 levels (data not shown).
PCR array analysis also revealed that the flax oil diet led to an upregulation of NF-κB inhibitor IkB (NFKBIA). IkBα prevents nuclear translocation of the NF-κB subunits by sequestering them in a complex in the cytoplasm [64]. Further, the array showed that the whole flaxseed diet resulted in downregulation of NCOA1 mRNA expression. NCOA1 (SRC 1) belongs to the p160 steroid receptor co-activator family and regulates NF-κB’s transcription activity. We also observed that the expression of anti-apoptotic protein, BCL-2L1 was downregulated in the whole flaxseed diet. BCL-2 is a major target of NF-κB and facilitates its pro-survival responses [65]. Although its target genes were downregulated, qPCR analysis of NF-κB expression did not suggest any change in NF-κB mRNA levels with diet. Since NFkB’s transcriptional activity depends on its nuclear translocation, flaxseed diet could be mediating its actions by sequestering NFkB in the cytoplasm. This definitely warrants further investigation. These data suggest that whole flaxseed and its components might decrease NF-κB mediated cellular growth, survival and inflammatory responses by decreasing NF-κB transcriptional activation in turn affecting its target gene expression.
Several other ER targets are also modulated with flaxseed diet (Table 2). P130cas (BCAR1) was downregulated in whole flaxseed supplemented diet while AP1 transcription factor family member, MAFF was downregulated in whole flaxseed, defatted flax meal and flax oil diets. P130cas is an adhesion protein that is upregulated in breast cancer [66], promotes cell migration and induces tamoxifen resistance [67]. E2 exposure leads to upregulation of Maf transcription factors in breast cancer [68] and promotes distant metastasis [69]. C-maf is commonly upregulated in multiple myeloma[70] and promotes the tumor-stroma interaction [70]. C-FOS and estrogen repressor PHB2 were upregulated with flaxseed and its components, in our study. Downregulation of FOS is considered to be a bad prognosis in ovarian and other cancers. Since FOS is believed to have a pro-apoptotic function, loss of FOS leads to a more invasive and metastatic tumor phenotype [71]. PHB2 is a tumor suppressor that on activation has been shown to prevent the progression of ERα positive breast cancer [72]. Because of its anti-estrogenic effects, it abrogates the signaling of nuclear as well as membrane associated ERα [73]. These data indicate that flaxseed is effective in altering the expression of genes implicated in a number of cancers. Since most of these genes are involved in steroid receptor regulation, they can be targeted for therapeutic strategies in steroid dependent cancers.
Our current study suggests that surrogate end points were differentially affected by the components of flaxseed. A series of ER targets were altered by whole flaxseed and its components due to direct alterations in ERα signaling and expression. Target genes that were downregulated by flaxseed include potent oncogenes, repressors that target cytokine inhibitory receptors, important signaling mediators of growth, proliferation and anti-apoptotic factors and steroid receptors that are upregulated in cancer. Some targets that were upregulated include estrogen receptor repressor, receptors with antiinflammatory actions, etc. Besides ER target genes, flaxseed diet also increased levels of 2-methoxyestradiol in turn corroborating our previous observations where we showed that flaxseed results in upregulation of 2-hydroxyestradiol (precursor of 2-methoxyestradiol).
In conclusion, the whole flaxseed supplemented diet was more effective than the defatted flax meal and the flax oil diets, in altering molecular targets involved in inflammation, glucose metabolism and apoptosis while whole flaxseed, defatted flax meal and flax oil diets were equally potent in altering the expression of genes involved in carcinogenesis. The weak anti-estrogens; enterolactone, enterodiol and 2-methoxyestradiol might be working synergistically to generate a protective effect in the ovaries from whole flaxseed fed hens by altering estrogen signaling and metabolism.
Supplementary Material
Table 3.
Genes examined in the PCR array. (Qiagen RT2 Profiler PCR array-PAGG-005Z)
| Symbol | Description |
|---|---|
| ADORA1 | Adenosine A1 receptor |
| AHR | Aryl hydrocarbon receptor |
| AKAP1 | A kinase (PRKA) anchor protein 1 |
| APBB1 | Amyloid beta (A4) precursor protein-binding, family B, member 1 (Fe65) |
| ATF3 | Activating transcription factor 3 |
| BCAR1 | Breast cancer anti-estrogen resistance 1 |
| BCL2L1 | BCL2-like 1 |
| BDNF | Brain-derived neurotrophic factor |
| BMP4 | Bone morphogenetic protein 4 |
| BMP7 | Bone morphogenetic protein 7 |
| BRCA1 | Breast cancer 1, early onset |
| C3 | Complement component 3 |
| CCND1 | Cyclin D1 |
| CITED2 | Cbp/p300-interacting transactivator, with Glu/Asp-rich carboxy-terminal domain, 2 |
| CKB | Creatine kinase, brain |
| CST3 | Cystatin C |
| CTGF | Connective tissue growth factor |
| CTSD | Cathepsin D |
| CYP19A1 | Cytochrome P450, family 19, subfamily A, polypeptide 1 |
| CYP1A1 | Cytochrome P450, family 1, subfamily A, polypeptide 1 |
| EBAG9 | Estrogen receptor binding site associated, antigen, 9 |
| EFNA5 | Ephrin-A5 |
| ERBB2 | V-erb-b2 erythroblastic leukemia viral oncogene homolog 2, neuro/glioblastoma derived oncogene homolog (avian) |
| ERBB3 | V-erb-b2 erythroblastic leukemia viral oncogene homolog 3 (avian) |
| ESR1 | Estrogen receptor 1 |
| ESR2 | Estrogen receptor 2 (ER beta) |
| FOS | FBJ murine osteosarcoma viral oncogene homolog |
| FOSL2 | FOS-like antigen 2 |
| FOXA1 | Forkhead box A1 |
| FST | Follistatin |
| GPER | G protein-coupled estrogen receptor 1 |
| HSP90AA1 | Heat shock protein 90kDa alpha (cytosolic), class A member 1 |
| IGF1 | Insulin-like growth factor 1 (somatomedin C) |
| IGFBP4 | Insulin-like growth factor binding protein 4 |
| IGFBP5 | Insulin-like growth factor binding protein 5 |
| IL10 | Interleukin 10 |
| IRS1 | Insulin receptor substrate 1 |
| L1CAM | Neuron-glia cell adhesion molecule (Ng-CAM) |
| LGALS1 | Lectin, galactoside-binding, soluble, 1 |
| LPL | Lipoprotein lipase |
| LTBP1 | Latent transforming growth factor beta binding protein 1 |
| MAFF | V-maf musculoaponeurotic fibrosarcoma oncogene homolog F (avian) |
| MED1 | Mediator complex subunit 1 |
| MMP9 | Matrix metallopeptidase 9 (gelatinase B, 92kDa gelatinase, 92kDa type IV collagenase) |
| MTA1 | Metastasis associated 1 |
| MUC1 | Mucin 1, cell surface associated |
| MYC | V-myc myelocytomatosis viral oncogene homolog (avian) |
| NCOA1 | Nuclear receptor coactivator 1 |
| NCOA2 | Nuclear receptor coactivator 2 |
| NCOA3 | Nuclear receptor coactivator 3 |
| NCOR1 | Nuclear receptor co-repressor 1 |
| NCOR2 | Nuclear receptor co-repressor 2 |
| NFKBIA | Nuclear factor of kappa light polypeptide gene enhancer in B-cells inhibitor, alpha |
| NOV | Nephroblastoma overexpressed gene |
| NR0B1 | Nuclear receptor subfamily 0, group B, member 1 |
| NR0B2 | Nuclear receptor subfamily 0, group B, member 2 |
| NR3C1 | Nuclear receptor subfamily 3, group C, member 1 (glucocorticoid receptor) |
| NR5A2 | Nuclear receptor subfamily 5, group A, member 2 |
| NRIP1 | Nuclear receptor interacting protein 1 |
| NRP1 | Neuropilin 1 |
| PDZK1 | PDZ domain containing 1 |
| PGR | Progesterone receptor |
| PHB2 | Prohibitin 2 |
| PTCH1 | Patched 1 |
| PTGS2 | Prostaglandin-endoperoxide synthase 2 (prostaglandin G/H synthase and cyclooxygenase) |
| RARA | Retinoic acid receptor, alpha |
| S100A6 | S100 calcium binding protein A6 |
| SAFB | Scaffold attachment factor B2 |
| SLC2A1 | Solute carrier family 2 (facilitated glucose transporter), member 1 |
| SLC9A3R1 | Solute carrier family 9 (sodium/hydrogen exchanger), member 3 regulator 1 |
| SMAD3 | SMAD family member 3 |
| SNAI1 | Snail homolog 1 (Drosophila) |
| SOCS3 | Suppressor of cytokine signaling 3 |
| SPP1 | Secreted phosphoprotein 1 (osteopontin, bone sialoprotein I, early T-lymphocyte activation 1) |
| TGFA | Transforming growth factor, alpha |
| TGFB3 | Transforming growth factor, beta 3 |
| THBS1 | Thrombospondin 1 |
| THRSP | Thyroid hormone responsive (SPOT14 homolog, rat) |
| VDR | Vitamin D (1,25- dihydroxyvitamin D3) receptor |
| VEGFA | Vascular endothelial growth factor A |
| WISP2 | WNT1 inducible signaling pathway protein 2 |
| WNT4 | Wingless-type MMTV integration site family, member 4 |
| WNT5A | Wingless-type MMTV integration site family, member 5A |
| XBP1 | X-box binding protein 1 |
| ACTB | Actin, beta |
| H6PD | Hexose-6-phosphate dehydrogenase (glucose 1-dehydrogenase) |
| HMBS | Hydroxymethylbilane synthase |
| RPL4 | Ribosomal protein L4 |
| UBC | Ubiquitin C |
| GGDC | Chicken Genomic DNA Contamination |
| RTC | Reverse Transcription Control |
| RTC | Reverse Transcription Control |
| RTC | Reverse Transcription Control |
| PPC | Positive PCR Control |
| PPC | Positive PCR Control |
| PPC | Positive PCR Control |
Highlights.
Flaxseed and its components differentially alter estrogen signaling and metabolism
Flaxseed was more effective than its components in altering inflammation and apoptosis targets.
All the diets were equally potent in targeting genes involved in carcinogenesis.
Flaxseed and its components generate a protective environment in the pre-neoplastic hen ovaries
E2 mediated ER alpha activation was abrogated by purified Enterolactone in vitro.
Acknowledgments
We are grateful to the poultry farm management that includes Chet Utterback, Pam Utterback, Shelby Reed and Brandon Zech, and Carl Parsons for helping us with the diet formulations. Dr. Richard van Breeman and his mass spectrometry lab members helped us with the enterolactone and enterodiol analysis at the University of Illinois, Chicago. Lacey Gibson performed the AKT2 mRNA qPCR analysis. We also thank Dr. Janice Bahr for her continued guidance. We want to thank Dr. Kenneth Korach at NIEHS who kindly provided the pGL3/3xERE Luc plasmid, Dr. Philip Jensik at Southern Illinois University Carbondale for the RSV-Renilla vector and Dr.Wei Xu at University of Wisconsin at Madison for the pCMX/ERα expression plasmid. We thank Omega Nutrition, Vancouver, BC, for providing the defatted flaxmeal and flax oil. We also want to thank Dr. Sheree Speckman and Dr. Stephanie Eastwood for their suggestions. We are very grateful for the NIH funding RO1AT005295.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure statement: the authors have nothing to disclose.
Conflict of interest: The authors have no conflict of interest.
REFERENCE
- 1.Ganorkar P, Jain R. Flaxseed-a nutritional punch. International Food Research Journal. 2013;20:519–525. [Google Scholar]
- 2.Touré A, Xueming X. Flaxseed Lignans: Source, Biosynthesis, Metabolism, Antioxidant Activity, Bio - Active Components, and Health Benefits. Comprehensive reviews in food science and food safety. 2010;9:261–269. doi: 10.1111/j.1541-4337.2009.00105.x. [DOI] [PubMed] [Google Scholar]
- 3.Morris DH. Flax: A health and nutrition primer. Flax Council of Canada. 2007 [Google Scholar]
- 4.Larsson SC, Kumlin M, Ingelman-Sundberg M, Wolk A. Dietary long-chain n- 3 fatty acids for the prevention of cancer: a review of potential mechanisms. The American journal of clinical nutrition. 2004;79:935–945. doi: 10.1093/ajcn/79.6.935. [DOI] [PubMed] [Google Scholar]
- 5.Lee TH, Hoover RL, Williams JD, Sperling RI, Ravalese J, III, Spur BW, Robinson DR, Corey E, Lewis RA, Austen KF. Effect of dietary enrichment with eicosapentaenoic and docosahexaenoic acids on in vitro neutrophil and monocyte leukotriene generation and neutrophil function. New Engl. J. Med. 1985;312:1217–1224. doi: 10.1056/NEJM198505093121903. [DOI] [PubMed] [Google Scholar]
- 6.Kremer JM, Lawrence DA, Jubiz W. Dietary ω3 and ω6 Fatty Acids. Springer; 1989. Different Doses of Fish-Oil Fatty Acid Ingestion in Active Rheumatoid Arthritis: A Prospective Study of Clinical and Immunological Parameters; pp. 343–350. [Google Scholar]
- 7.Charnock JS. Antiarrhythmic effects of fish oils. World review of nutrition and dietetics. 1991 doi: 10.1159/000419298. [DOI] [PubMed] [Google Scholar]
- 8.Barcelli U, Glas-Greenwalt P, Pollak VE. Enhancing effect of dietary supplementation with ω-3 fatty acids on plasma fibrinolysis in normal subjects. Thrombosis research. 1985;39:307–312. doi: 10.1016/0049-3848(85)90226-9. [DOI] [PubMed] [Google Scholar]
- 9.Davis H, Bridenstine R, Vesselinovitch D, Wissler R. Fish oil inhibits development of atherosclerosis in rhesus, monkeys, Arteriosclerosis, Thrombosis and Vascular Biology. 1987;7:441–449. doi: 10.1161/01.atv.7.5.441. [DOI] [PubMed] [Google Scholar]
- 10.Sanders T, Hochland MC. A comparison of the influence on plasma lipids and platelet function of supplements of ω3 and ω6 polyunsaturated fatty acids. British Journal of Nutrition. 1983;50:521–529. doi: 10.1079/bjn19830123. [DOI] [PubMed] [Google Scholar]
- 11.Prasad K. Hydroxyl radical-scavenging property of secoisolariciresinol diglucoside (SDG) isolated from flax-seed. Molecular and cellular biochemistry. 1997;168:117–123. doi: 10.1023/a:1006847310741. [DOI] [PubMed] [Google Scholar]
- 12.Mueller SO, Simon S, Chae K, Metzler M, Korach KS. Phytoestrogens and their human metabolites show distinct agonistic and antagonistic properties on estrogen receptor α (ERα) and ERβ in human cells. Toxicological Sciences. 2004;80:14–25. doi: 10.1093/toxsci/kfh147. [DOI] [PubMed] [Google Scholar]
- 13.Zhang W, Wang X, Liu Y, Tian H, Flickinger B, Empie MW, Sun SZ. Effects of dietary flaxseed lignan extract on symptoms of benign prostatic hyperplasia. Journal of medicinal food. 2008;11:207–214. doi: 10.1089/jmf.2007.602. [DOI] [PubMed] [Google Scholar]
- 14.Fukumitsu S, Aida K, Shimizu H, Toyoda K. Flaxseed lignan lowers blood cholesterol and decreases liver disease risk factors in moderately hypercholesterolemic men. Nutr Res. 2010;30:441–446. doi: 10.1016/j.nutres.2010.06.004. [DOI] [PubMed] [Google Scholar]
- 15.Neukam K, De Spirt S, Stahl W, Bejot M, Maurette J, Tronnier H, Heinrich U. Supplementation of flaxseed oil diminishes skin sensitivity and improves skin barrier function and condition. Skin pharmacology and physiology. 2010;24:67–74. doi: 10.1159/000321442. [DOI] [PubMed] [Google Scholar]
- 16.Thompson LU, Chen JM, Li T, Strasser-Weippl K, Goss PE. Dietary flaxseed alters tumor biological markers in postmenopausal breast cancer. Clinical Cancer Research. 2005;11:3828–3835. doi: 10.1158/1078-0432.CCR-04-2326. [DOI] [PubMed] [Google Scholar]
- 17.Dikshit A, Gomes Filho MA, Eilati E, McGee S, Small C, Gao C, Klug T, Hales DB. Flaxseed reduces the pro-carcinogenic micro-environment in the ovaries of normal hens by altering the PG and oestrogen pathways in a dose-dependent manner. British Journal of Nutrition. 2015:1–12. doi: 10.1017/S000711451500029X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Haggans CJ, Hutchins AM, Olson BA, Thomas W, Martini MC, Slavin JL. Effect of flaxseed consumption on urinary estrogen metabolites in postmenopausal women. Nutrition and cancer. 1999;33:188–195. doi: 10.1207/S15327914NC330211. [DOI] [PubMed] [Google Scholar]
- 19.Jungeström MB, Thompson LU, Dabrosin C. Flaxseed and its lignans inhibit estradiol-induced growth, angiogenesisand secretion of vascular endothelial growth factor in human breast cancer xenografts in vivo. Clinical Cancer Research. 2007;13:1061–1067. doi: 10.1158/1078-0432.CCR-06-1651. [DOI] [PubMed] [Google Scholar]
- 20.Chen J, Hui E, Ip T, Thompson LU. Dietary flaxseed enhances the inhibitory effect of tamoxifen on the growth of estrogen-dependent human breast cancer (mcf-7) in nude mice. Clinical Cancer Research. 2004;10:7703–7711. doi: 10.1158/1078-0432.CCR-04-1130. [DOI] [PubMed] [Google Scholar]
- 21.Chen J, Stavro PM, Thompson LU. Dietary flaxseed inhibits human breast cancer growth and metastasis and downregulates expression of insulin-like growth factor and epidermal growth factor receptor. Nutrition and cancer. 2002;43:187–192. doi: 10.1207/S15327914NC432_9. [DOI] [PubMed] [Google Scholar]
- 22.Demark-Wahnefried W, Polascik TJ, George SL, Switzer BR, Madden JF, Ruffin MT, Snyder DC, Owzar K, Hars V, Albala DM. Flaxseed supplementation (not dietary fat restriction) reduces prostate cancer proliferation rates in men presurgery. Cancer Epidemiology Biomarkers & Prevention. 2008;17:3577–3587. doi: 10.1158/1055-9965.EPI-08-0008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Demark-Wahnefried W, Price DT, Polascik TJ, Robertson CN, Anderson EE, Paulson DF, Walther PJ, Gannon M, Vollmer RT. Pilot study of dietary fat restriction flaxseed supplementation in men with prostate cancer before surgery: exploring the effects on hormonal levels prostate-specific antigen, and histopathologic features. Urology. 2001;58:47–52. doi: 10.1016/s0090-4295(01)01014-7. [DOI] [PubMed] [Google Scholar]
- 24.Yan L, Yee JA, Li D, McGuire MH, Thompson LU. Dietary flaxseed supplementation and experimental metastasis of melanoma cells in mice. Cancer letters. 1998;124:181–186. doi: 10.1016/s0304-3835(97)00470-9. [DOI] [PubMed] [Google Scholar]
- 25.Eilati E, Bahr JM, Hales DB. Long Term Consumption of Flaxseed Enriched Diet Decreased Ovarian Cancer Incidence and Prostaglandin E2 in Hens. Gynecologic Oncology. 2013;3:620–628. doi: 10.1016/j.ygyno.2013.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ansenberger K, Zhuge Y, Richards C, Barua A, Luborsky JL, Bahr JM, Hales DB. Decreased severity of ovarian cancer and increased survival in hens fed a flaxseed enriched diet for one year. Gynecologic Oncology. 2010;117:341–347. doi: 10.1016/j.ygyno.2010.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Eilati E, Hales K, Zhuge Y, Ansenberger Fricano K, Yu R, van Breemen RB, Buchanan Hales D. Flaxseed enriched diet-mediated reduction in ovarian cancer severity is correlated to the reduction of prostaglandin E< sub> 2</sub> in laying hen ovaries. Prostaglandins, Leukotrienes and Essential Fatty Acids (PLEFA) 2013 doi: 10.1016/j.plefa.2013.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Simopoulos A. Human requirement for N-3 polyunsaturated fatty acids. Poultry science. 2000;79:961–970. doi: 10.1093/ps/79.7.961. [DOI] [PubMed] [Google Scholar]
- 29.Eilati E, Small CC, McGee SR, Kurrey NK, Hales DB. Anti-inflammatory effects of fish oil in ovaries of laying hens target prostaglandin pathways. Lipids in health and disease. 2013;12:152. doi: 10.1186/1476-511X-12-152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ansenberger K, Zhuge Y, Lagman JA, Richards C, Barua A, Bahr JM, Hales DB. E-cadherin expression in ovarian cancer in the laying hen Gallus domesticus compared to human ovarian cancer. Gynecol Oncol. 2009;113:362–369. doi: 10.1016/j.ygyno.2009.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hales KH, Speckman SC, Kurrey NK, Hales DB. Uncovering molecular events associated with the chemosuppressive effects of flaxseed: a microarray analysis of the laying hen model of ovarian cancer. BMC genomics. 2014;15:709. doi: 10.1186/1471-2164-15-709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Spillman MA, Manning NG, Dye WW, Sartorius CA, Post MD, Harrell JC, Jacobsen BM, Horwitz KB. Tissue-specific pathways for estrogen regulation of ovarian cancer growth and metastasis. Cancer Research. 2010;70:8927–8936. doi: 10.1158/0008-5472.CAN-10-1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhuge Y, Lagman JAJ, Ansenberger K, Mahon CJ, Daikoku T, Dey SK, Bahr JM, Hales DB. CYP1B1 expression in ovarian cancer in the laying hen Gallusdomesticus. Gynecologic Oncology. 2009;112:171–178. doi: 10.1016/j.ygyno.2008.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Eilati E, Pan L, Bahr JM, Hales DB. Age dependent increase in prostaglandin pathway coincides with onset of ovarian cancer in laying hens Prostaglandins. Leukotrienes and Essential Fatty Acids. 2012;87:177–184. doi: 10.1016/j.plefa.2012.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Eilati E, Hales K, Zhuge Y, Ansenberger Fricano K, Yu R, van Breemen RB, Buchanan Hales D. Flaxseed enriched diet-mediated reduction in ovarian cancer severity is correlated to the reduction of prostaglandin E2 in laying hen ovaries Prostaglandins. Leukotrienes and Essential Fatty Acids (PLEFA) 2013;89:179–187. doi: 10.1016/j.plefa.2013.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fostis T, Zhang Y, Pepper M, Adlercreutz H, Montesano R, Nawroth P, Schweigere L. The endogenous oestrogen metabolite 2-methoxyestradiol inhibits angiogensin and suppresses tumor growth. Nature. 1994;17:237–239. doi: 10.1038/368237a0. [DOI] [PubMed] [Google Scholar]
- 37.Parl FF. Estrogens, estrogen receptor, and breast cancer. Ios Press; 2000. [Google Scholar]
- 38.Seegers JC, Lottering M-L, Grobler CJ, van Papendorp DH, Habbersett RC, Shou Y, Lehnert BE. The mammalian metabolite, 2-methoxyestradiol, affects P53 levels and apoptosis induction in transformed cells but not in normal cells. The Journal of Steroid Biochemistry and Molecular Biology. 1997;62:253–267. doi: 10.1016/s0960-0760(97)00043-5. [DOI] [PubMed] [Google Scholar]
- 39.Chua YS, Chua YL, Hagen T. Structure activity analysis of 2-methoxyestradiol analogues reveals targeting of microtubules as the major mechanism of antiproliferative and proapoptotic activity. Molecular cancer therapeutics. 2010;9:224–235. doi: 10.1158/1535-7163.MCT-09-1003. [DOI] [PubMed] [Google Scholar]
- 40.Zhu BT, Conney AH. Is 2-methoxyestradiol an endogenous estrogen metabolite that inhibits mammary carcinogenesis? Cancer Research. 1998;58:2269–2277. [PubMed] [Google Scholar]
- 41.Liehr JG, Ricci MJ. 4-Hydroxylation of estrogens as marker of human mammary tumors. Proceedings of the National Academy of Sciences. 1996;93:3294–3296. doi: 10.1073/pnas.93.8.3294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bradlow H, Hershcopf R, Martucci C, Fishman J. 16α - Hydroxylation of Estradiol: A Possible Risk Marker for Breast Cancer. Annals of the New York Academy of Sciences. 1986;464:138–151. doi: 10.1111/j.1749-6632.1986.tb16001.x. [DOI] [PubMed] [Google Scholar]
- 43.Amakura Y, Tsutsumi T, Nakamura M, Kitagawa H, Fujino J, Sasaki K, Toyoda M, Yoshida T, Maitani T. Activation of the aryl hydrocarbon receptor by some vegetable constituents determined using in vitro reporter gene assay. Biological and Pharmaceutical Bulletin. 2003;26:532–539. doi: 10.1248/bpb.26.532. [DOI] [PubMed] [Google Scholar]
- 44.Kress S, Greenlee WF. Cell-specific regulation of human CYP1A1 and CYP1B1 genes. Cancer Research. 1997;57:1264–1269. [PubMed] [Google Scholar]
- 45.Spink DC, Spink BC, Cao JQ, DePasquale JA, Pentecost BT, Fasco MJ, Li Y, Sutter TR. Differential expression of CYP1A1 and CYP1B1 in human breast epithelial cells and breast tumor cells. Carcinogenesis. 1998;19:291–298. doi: 10.1093/carcin/19.2.291. [DOI] [PubMed] [Google Scholar]
- 46.Tiong CT, Chen C, Zhang SJ, Li J, Soshilov A, Denison MS, Lee LS-U, Tam VH, Wong SP, Xu HE. A novel prenylflavone restricts breast cancer cell growth through AhR-mediated destabilization of ERα protein. Carcinogenesis. 2012;33:1089–1097. doi: 10.1093/carcin/bgs110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Frasca F, Pandini G, Vigneri R, Goldfine ID. Insulin and hybrid insulin/IGF receptors are major regulators of breast cancer cells. Breast disease. 2002;17:73–89. doi: 10.3233/bd-2003-17108. [DOI] [PubMed] [Google Scholar]
- 48.Sachdev D. Regulation of breast cancer metastasis by IGF signaling. Journal of mammary gland biology and neoplasia. 2008;13:431–441. doi: 10.1007/s10911-008-9105-5. [DOI] [PubMed] [Google Scholar]
- 49.Weiss JM, Huang WY, Rinaldi S, Fears TR, Chatterjee N, Chia D, Crawford ED, Kaaks R, Hayes RB. IGF - 1 IGFBP - 3: Risk of prostate cancer among men in the Prostate, Lung, Colorectal and Ovarian Cancer Screening Trial. Int. J. Cancer. 2007;121:2267–2273. doi: 10.1002/ijc.22921. [DOI] [PubMed] [Google Scholar]
- 50.Sachdev D, Yee D. Disrupting insulin-like growth factor signaling as a potential cancer therapy. Molecular cancer therapeutics. 2007;6:1–12. doi: 10.1158/1535-7163.MCT-06-0080. [DOI] [PubMed] [Google Scholar]
- 51.Gotlieb WH, Bruchim I, Gu J, Shi Y, Camirand A, Blouin M-J, Zhao Y, Pollak MN. Insulinlike growth factor receptor I targeting in epithelial ovarian cancer. Gynecologic Oncology. 2006;100:389–396. doi: 10.1016/j.ygyno.2005.09.048. [DOI] [PubMed] [Google Scholar]
- 52.Sheikh M, Shao Z, Hussain A, Chen J, Roberts C, LeRoith D, Fontana J. Retinoic acid and estrogen modulation of insulin-like growth factor binding protein-4 gene expression and the estrogen receptor status of human breast carcinoma cells. Biochemical and Biophysical Research Communications. 1993;193:1232–1238. doi: 10.1006/bbrc.1993.1757. [DOI] [PubMed] [Google Scholar]
- 53.Pratt SE, Pollak MN. Estrogen and antiestrogen modulation of MCF7 human breast cancer cell proliferation is associated with specific alterations in accumulation of insulin-like growth factor-binding proteins in conditioned media. Cancer Research. 1993;53:5193–5198. [PubMed] [Google Scholar]
- 54.Wang H, Rosen DG, Wang H, Fuller GN, Zhang W, Liu J. Insulin-like growth factor-binding protein 2 and 5 are differentially regulated in ovarian cancer of different histologic types. Modern pathology. 2006;19:1149–1156. doi: 10.1038/modpathol.3800637. [DOI] [PubMed] [Google Scholar]
- 55.Simons AL, Orcutt KP, Madsen JM, Scarbrough PM, Spitz DR. Oxidative Stress in Cancer Biology and Therapy. Springer; 2012. The role of Akt pathway signaling in glucose metabolism and metabolic oxidative stress; pp. 21–46. [Google Scholar]
- 56.Makker A, Goel MM, Mahdi AA. PI3K/PTEN/Akt and TSC/mTOR signaling pathways, ovarian dysfunction, and infertility: an update. J. Molec. Endocrinol. 2014;53:R103–R118. doi: 10.1530/JME-14-0220. [DOI] [PubMed] [Google Scholar]
- 57.Djordjevic B, Hennessy BT, Li J, Barkoh BA, Luthra R, Mills GB, Broaddus RR. Clinical assessment of PTEN loss in endometrial carcinoma: immunohistochemistry outperforms gene sequencing. Modern pathology. 2012;25:699–708. doi: 10.1038/modpathol.2011.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lin Y-M, Velmurugan BK, Yeh Y-L, Tu CC, Ho T-J, Lai TY, Tsai C-H, Tsai FJ, Tsai C-H, Huang C-Y. Activation of estrogen receptors with E2 downregulates peroxisome proliferator-activated receptor γ in hepatocellular carcinoma. Oncology reports. 2013;30:3027–3031. doi: 10.3892/or.2013.2793. [DOI] [PubMed] [Google Scholar]
- 59.Komar CM. Peroxisome proliferator-activated receptors (PPARs), ovarian function-implications for regulating steroidogenesis differentiation and tissue remodeling. Reproductive Biology and Endocrinology. 2005;3:41. doi: 10.1186/1477-7827-3-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Li P, Fan W, Xu J, Lu M, Yamamoto H, Auwerx J, Sears DD, Talukdar S, Oh D, Chen A. Adipocyte NCoR knockout decreases PPARγ phosphorylation and enhances PPARγ activity and insulin sensitivity. Cell. 2011;147:815–826. doi: 10.1016/j.cell.2011.09.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Picard F, Kurtev M, Chung N, Topark-Ngarm A, Senawong T, De Oliveira RM, Leid M, McBurney MW, Guarente L. Sirt1 promotes fat mobilization in white adipocytes by repressing PPAR-γ. Nature. 2004;429:771–776. doi: 10.1038/nature02583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sakamoto A, Yokoyama Y, Umemoto M, Futagami M, Sakamoto T, Bing X, Mizunuma H. Clinical implication of expression of cyclooxygenase-2 and peroxisome proliferator activated-receptor γ in epithelial ovarian tumours. British Journal Of Cancer. 2004;91:633–638. doi: 10.1038/sj.bjc.6602009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Berry EB, Keelan JA, Helliwell RJ, Gilmour RS, Mitchell MD. Nanomolar micromolar effects of 15-deoxy-δ12 14-prostaglandin J2 on amnion-derived WISH epithelial cells: differential roles of peroxisome proliferator-activated receptors γ and δ and nuclear factor κB. Molecular pharmacology. 2005;68:169–178. doi: 10.1124/mol.104.009449. [DOI] [PubMed] [Google Scholar]
- 64.Baeuerle PA, Baltimore D. I kappa B: a specific inhibitor of the NF-kappa B transcription factor. Science. 1988;242:540–546. doi: 10.1126/science.3140380. [DOI] [PubMed] [Google Scholar]
- 65.Catz SD, Johnson JL. Transcriptional regulation of bcl-2 by nuclear factor kappa B and its significance in prostate cancer. Oncogene. 2001;20:7342–7351. doi: 10.1038/sj.onc.1204926. [DOI] [PubMed] [Google Scholar]
- 66.Bisaro B, Montani M, Konstantinidou G, Marchini C, Pietrella L, Iezzi M, Galiè M, Orso F, Camporeale A, Colombo SM. p130Cas/Cyclooxygenase-2 axis in the control of mesenchymal plasticity of breast cancer cells. Breast Cancer Res. 2012;14:137. doi: 10.1186/bcr3342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Van Der Flier S, Brinkman A, Look MP, Kok EM, Meijer-van Gelder ME, Klijn JG, Dorssers LC, Foekens JA. Bcar1/p130Cas protein and primary breast cancer: prognosis and response to tamoxifen treatment. Journal of the National Cancer Institute. 2000;92:120–127. doi: 10.1093/jnci/92.2.120. [DOI] [PubMed] [Google Scholar]
- 68.Huan J, Wang L, Xing L, Qin X, Feng L, Pan X, Zhu L. Insights into significant pathways and gene interaction networks underlying breast cancer cell line MCF-7 treated with 17β-Estradiol (E2) Gene. 2014;533:346–355. doi: 10.1016/j.gene.2013.08.027. [DOI] [PubMed] [Google Scholar]
- 69.Pavlovic M, Arnal-Estapé A, Rojo F, Bellmunt A, Tarragona M, Guiu M, Planet E, Garcia-Albéniz X, Morales M, Urosevic J. Enhanced MAF Oncogene Expression and Breast Cancer Bone Metastasis. Journal of the National Cancer Institute. 2015;107:pjv256. doi: 10.1093/jnci/djv256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hurt EM, Wiestner A, Rosenwald A, Shaffer A, Campo E, Grogan T, Bergsagel PL, Kuehl WM, Staudt LM. Overexpression of c-maf is a frequent oncogenic event in multiple myeloma that promotes proliferation and pathological interactions with bone marrow stroma. Cancer Cell. 2004;5:191–199. doi: 10.1016/s1535-6108(04)00019-4. [DOI] [PubMed] [Google Scholar]
- 71.Mahner S, Baasch C, Schwarz J, Hein S, Wölber L, Jänicke F, Milde-Langosch K. C-Fos expression is a molecular predictor of progression and survival in epithelial ovarian carcinoma. British Journal Of Cancer. 2008;99:1269–1275. doi: 10.1038/sj.bjc.6604650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Yoshimaru T, Komatsu M, Tashiro E, Imoto M, Osada H, Miyoshi Y, Honda J, Sasa M, Katagiri T. Xanthohumol suppresses oestrogen-signalling in breast cancer through the inhibition of BIG3-PHB2 interactions. Scientific reports. 2014;4 doi: 10.1038/srep07355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Yoshimaru T, Komatsu M, Matsuo T, Chen Y-A, Murakami Y, Mizuguchi K, Mizohata E, Inoue T, Akiyama M, Yamaguchi R. Targeting BIG3–PHB2 interaction to overcome tamoxifen resistance in breast cancer cells. Nature communications. 2013;4 doi: 10.1038/ncomms3443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Appierto V, Villani M, Cavadini E, Lotan R, Vinson C, Formelli F. Involvement of c-Fos in fenretinide-induced apoptosis in human ovarian carcinoma cells. Cell Death & Differentiation. 2003;11:270–279. doi: 10.1038/sj.cdd.4401349. [DOI] [PubMed] [Google Scholar]
- 75.Welsh JB, Zarrinkar PP, Sapinoso LM, Kern SG, Behling CA, Monk BJ, Lockhart DJ, Burger RA, Hampton GM. Analysis of gene expression profiles in normal and neoplastic ovarian tissue samples identifies candidate molecular markers of epithelial ovarian cancer. Proceedings of the National Academy of Sciences. 2001;98:1176–1181. doi: 10.1073/pnas.98.3.1176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Meinhold-Heerlein I, Bauerschlag D, Hilpert F, Dimitrov P, Sapinoso LM, Orlowska-Volk M, Bauknecht T, Park T-W, Jonat W, Jacobsen A. Molecular and prognostic distinction between serous ovarian carcinomas of varying grade and malignant potential. Oncogene. 2004;24:1053–1065. doi: 10.1038/sj.onc.1208298. [DOI] [PubMed] [Google Scholar]
- 77.Oliveira-Ferrer L, Rößler K, Haustein V, Schröder C, Wicklein D, Maltseva D, Khaustova N, Samatov T, Tonevitsky A, Mahner S. c-FOS suppresses ovarian cancer progression by changing adhesion. British Journal Of Cancer. 2013;110:753–763. doi: 10.1038/bjc.2013.774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Banerjee S, Saxena N, Sengupta K, Tawfik O, Mayo MS, Banerjee SK. WISP-2 gene in human breast cancer: estrogen and progesterone inducible expression and regulation of tumor cell proliferation. Neoplasia. 2003;5:63–73. doi: 10.1016/s1476-5586(03)80018-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Akkiprik M, Feng Y, Wang H, Chen K, Hu L, Sahin A, Krishnamurthy S, Ozer A, Hao X, Zhang W. Multifunctional roles of insulin-like growth factor binding protein 5 in breast cancer. Breast Cancer Res. 2008;10:212. doi: 10.1186/bcr2116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Miyake H, Pollak M, Gleave ME. Castration-induced up-regulation of insulin-like growth factor binding protein-5 potentiates insulin-like growth factor-I activity and accelerates progression to androgen independence in prostate cancer models. Cancer Research. 2000;60:3058–3064. [PubMed] [Google Scholar]
- 81.Stolf BS, Carvalho AF, Martins WK, Runza FB, Brun M, Hirata R, Neves EJ, Soares FA, Postigo-Dias J, Kowalski LP. Differential expression of IGFBP-5 two human ESTs in thyroid glands with goiter, adenoma and papillary or follicular carcinomas. Cancer letters. 2003;191:193–202. doi: 10.1016/s0304-3835(02)00679-1. [DOI] [PubMed] [Google Scholar]
- 82.Doi A, Ishikawa K, Shibata N, Ito E, Fujimoto J, Yamamoto M, Shiga H, Mochizuki H, Kawamura Y, Goshima N. Enhanced expression of retinoic acid receptor alpha induces epithelial-to-mesenchymal transition and disruption of mammary acinar structures. Molecular Oncology. 2014 doi: 10.1016/j.molonc.2014.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Qin L, Wu Y-L, Toneff MJ, Li D, Liao L, Gao X, Bane FT, Tien JC, Xu Y, Feng Z. NCOA1 Directly Targets M-CSF1 Expression to Promote Breast Cancer Metastasis. Cancer Research. 2014 doi: 10.1158/0008-5472.CAN-13-2639. canres. 2639.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Walsh CA, Bolger JC, Byrne C, Cocchiglia S, Hao Y, Fagan A, Qin L, Cahalin A, McCartan D, McIlroy M. Global gene repression by the steroid receptor coactivator SRC-1 promotes oncogenesis. Cancer Research. 2014;74:2533–2544. doi: 10.1158/0008-5472.CAN-13-2133. [DOI] [PubMed] [Google Scholar]
- 85.Zhang L, Gong C, Lau SL, Yang N, Wong OG, Cheung AN, Tsang JW, Chan KY, Khoo U-S. SpliceArray profiling of breast cancer reveals a novel variant of NCOR2/SMRT that is associated with tamoxifen resistance and control of ERα transcriptional activity. Cancer Research. 2013;73:246–255. doi: 10.1158/0008-5472.CAN-12-2241. [DOI] [PubMed] [Google Scholar]
- 86.Blackmore JK, Karmakar S, Gu G, Chaubal V, Wang L, Li W, Smith CL. The SMRT Coregulator Enhances Growth of Estrogen Receptor-α-Positive Breast Cancer Cells by Promotion of Cell Cycle Progression and Inhibition of Apoptosis. Endocrinology. 2014;155:3251–3261. doi: 10.1210/en.2014-1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Braig S, Wallner S, Junglas B, Fuchshofer R, Bosserhoff A. CTGF is overexpressed in malignant melanoma and promotes cell invasion and migration. British Journal Of Cancer. 2011;105:231–238. doi: 10.1038/bjc.2011.226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Zhen Y, Ye Y, Yu X, Mai C, Zhou Y, Chen Y, Yang H, Lyu X, Song Y, Wu Q. Reduced CTGF expression promotes cell growth, migration, and invasion in nasopharyngeal carcinoma. 2013 doi: 10.1371/journal.pone.0064976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Reiss K, Del Valle L, Lassak A, Trojanek J. Nuclear IRS - 1 and cancer. Journal of cellular physiology. 2012;227:2992–3000. doi: 10.1002/jcp.24019. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.