Abstract
Significant progress has been made in the understanding of embryonic competence and endometrial receptivity since the inception of Assisted Reproductive Technologies (ART). The endometrium is a highly dynamic tissue that plays a crucial role in the establishment and maintenance of normal pregnancy. In response to steroid sex hormones, the endometrium undergoes marked changes during the menstrual cycle that are critical for acceptance of the nascent embryo. There is also a wide body of literature on systemic factors that impact ART outcomes. Patient prognosis is impacted by an array of factors that tip the scales in her favor or against success. Recognizing the local and systemic factors will allow clinicians to better understand and optimize the maternal environment at the time of implantation. This review will address the current literature on endometrial and systemic factors related to impaired implantation and highlight recent advances in this area of reproductive medicine.
Keywords: implantation, endometrium, thyroid, vitamin D, immune factors, IVF
IMPAIRED EXPRESSION OF ENDOMETRIAL FACTORS CORRELATES WITH REDUCED IMPLANTATION
Introduction
The human endometrium is a hormone responsive mucosa that lines the uterine cavity and undergoes cyclic proliferation and differentiation to support embryo implantation (1). During the proliferative phase, the endometrium grows in response to estrogen, arising from the remaining basalis layer that remains after menstruation. A dynamic transition from proliferation to a secretory morphology occurs after ovulation (2), orchestrated directly and indirectly by the sex steroids estrogen and progesterone (1) and is further mediated by a complex array of secondary autocrine and paracrine factors including cytokines and chemokines and their receptors and second messengers (3, 4).
Endometrial development after ovulation normally culminates with a defined period of endometrial receptivity. The secretory phase is divided into three recognized stages. The early secretory phase from post-ovulatory days 1 to 5, is characterized histologically by initiation of secretory products and characterized by the presence of sub-nuclear vacuoles that traverse the cells by post-ovulatory day 6 (5). The mid-secretory phase, representing the window of implantation and time of maximal endometrial receptivity, occurs from post-ovulatory day 6 to 10. During this period stromal cells are undergoing pseudo-decidualization reactions and epithelial cells develop specialized structures known as pinopodes (6) and cell adhesion molecules (7–9). The third phase in non-conception cycles represents the late luteal phase (post-ovulatory days 11–14), during which preparation for menstruation occurs. In the absence of the nidatory hCG signal from the embryo, endometrial break down occurs associated with apoptosis and an orchestrated inflammatory response that leads to an orderly and brief episode of menstrual shedding in anticipation of the next cycle (10). When pregnancy occurs, decidualization of the endometrial stroma transforms into a specialized epithelialized mesenchymal structure, essential for pregnancy (11, 12).
The mid-secretory phase coincides with the entry into the uterine cavity of the pre-implantation blastocyst, with the differentiation of trophectoderm by post-ovulatory day 5. A defined period of endometrial receptivity during the mid-secretory phase also corresponds well to prime responsiveness of the corpus luteum to hCG (13, 14). In fact, evidence from the 1999 Wilcox study shows that late implanting embryos are at higher risk for miscarriage than those that implant during the window of implantation (WOI), between post ovulatory days 6 to 10 (15). One interpretation for these interesting findings is that a sustained rescue of the corpus luteum occurs best at the time of normal implantation. This hypothesis is supported by early studies that examined CL rescue in response to early or late administration of hCG (13). The CL has a more robust response to hCG administered on post-ovulatory days 8–10 compared to post-ovulatory days 11–14, and progesterone may fall more quickly in early losses or implantation failure than after pregnancy is established (16). Our intention in this review will be to focus, however, not on the CL, but rather on uterine factors that contribute to a delay in implantation that then contributes to both pregnancy loss and implantation failure.
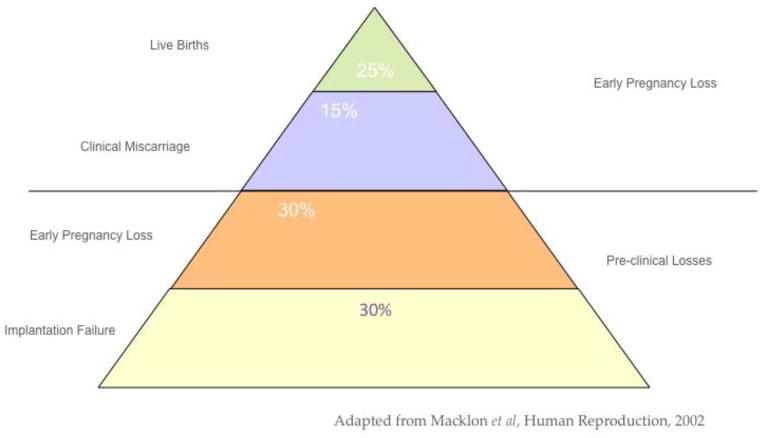
The efficiency of human reproduction is relatively low compared to other mammalian species. As summarized by Macklon (17) (Figure 1), there are many more implantation failures and early clinical and pre-clinical losses than successful pregnancies. While this is obvious to the clinician who treats infertility, our understanding of the basis for defects in endometrial receptivity has remained fragmented. A failed pregnancy can be the result of many diverse factors, including chromosomal defects in the nascent embryo, mechanical causes in the reproductive tract or inflammatory changes associated with disease. Assigning cause and effect in terms of the embryo or endometrial defects has been problematic. In this era of preimplantation genetic screening (PGS), answers may be forthcoming. In a report on a large series of euploid blastocysts, the proportion of euploid embryos failing to implant was approximately 40% (18). For those who study endometrial receptivity defects, those data may be a smoking gun regarding the importance of the endometrium.
Figure 1.
The hidden impact of implantation failure.
Do Endometrial Receptivity Defects Exist?
Historically, Georgianna Seegar Jones might have been the first investigator to show that defects in the endometrial histology could be associated with infertility (19). Using the then newly identified morphological changes in the secretory phase endometrium (5), she noted for the first time that women with infertility could have a lag in predicted endometrial histological development, a term she coined as “luteal phase deficiency” or LPD. It is worth noting that the existence and impact of LPD has come under question (20, 21). Nevertheless, the concept of a shifting WOI has been shown to have continued importance. In a landmark study by Wilcox et al., in 1999 (15) it was noted that women who implant beyond the normal window had an increasing chance for pregnancy loss. Biochemical defects have also been described that support a concept of a delayed WOI and retarded histology, including the use of placenta protein-14 (PP14; aka Glycodelin), integrins, MUC-1, pinopods, leukemia inhibitory factor and many others (22–26). In addition, cycles without histological lag have been described that display defects in key biomarkers of endometrial receptivity as well (27, 28).
Regulation of Endometrial Receptivity
The endometrium undergoes well-defined and regulated gene expression in preparation for implantation (1). The timing of endometrial receptivity coincides with the down-regulation of epithelial estrogen receptor alpha (ESR1) in normal mid-secretory endometrium (29), as seen in other mammals studied at the time of implantation (30). Progesterone and its receptor (PR) is essential for successful embryo implantation, but there is a shift in PR out of the epithelium to the stromal compartment that also occurs during the WOI (29). Persistence of ESR1 and PR in the glandular epithelium is associated with infertility and suspected implantation defects (31, 32). Aberrant over-expression of ESR1 and PR at the time of implantation is a sign of progesterone resistance, as progesterone normally down-regulates both endometrial ESR1 and its own receptor (PR) (33). P-resistance is associated with luteal phase deficiency, pregnancy loss or infertility due to endometriosis.
Progesterone also limits estrogen action through the induction of 17β-hydroxysteroid dehydrogenase-type 2 (HSD17βII) in the endometrium which converts estradiol to the less active estrone (34). Through these complex mechanisms of induction and inhibition of gene expression, there is a shift during the WOI from direct actions of progesterone (endocrine factors) to indirect actions (via paracrine and autocrine factors) (3, 35, 36). Failure to make this transition is likely a cause of implantation failure.
Disorders associated with implantation failure
Individual uterine factors associated with some implantation failures in the setting of infertility, recurrent loss and IVF have previously been reported. These include mechanical, inflammatory, and systemic factors (26, 37, 38). Mechanical factors encompass both congenital uterine anomalies and acquired intracavitary conditions. Congenital uterine anomalies including uterine septae have been linked to early miscarriages. One study comparing IVF outcomes between women with untreated septate uteri versus women who had undergone hysteroscopic metroplasty found that untreated women had worse IVF outcomes (39). Acquired intracavitary conditions such as submucosal fibroids, endometrial polyps, and intrauterine adhesions depending on size and location have also been linked to poor obstetric outcomes and may also contribute to recurrent implantation failure (RIF) (40). Available evidence suggests that surgical correction of these intrauterine pathologies may improve pregnancy outcomes (41, 42).
Inflammatory factors associated with implantation failure include endometriosis, adenomyosis, hydrosalpinges, and endometritis (1). A meta-analysis in 2002 of IVF success rates in patients with endometriosis found not only pregnancy rates were decreased compared to control patients, but fertilization rates, implantation rates, and number of oocytes retrieved were significantly reduced (43). Hydrosalpinx, or a blocked fallopian tube is also associated with implantation failure (44), with improvement noted after salpingectomy (45–48). Endometritis, an inflammation of the endometrium, is also associated with infertility and obstetrical complications (49). Endometritis is associated with aberrant inflammatory cytokine expression and has been associated with endometriosis (50). Polycystic ovary syndrome, a common cause of infertility and the most common endocrinopathy affecting reproductive-aged women, is associated with reduced endometrial receptivity (51, 52). Progesterone resistance, which is associated with inflammatory changes in the endometrium (36), has been observed in both endometriosis and PCOS by DNA microarray analysis (53, 54) and both conditions exhibit increased estrogen receptor dominance during the secretory phase (32, 55). Collectively, all of these conditions share an inflammatory component, increasingly considered to be a root cause of impaired implantation (38, 56).
Endometrial Factors as Biomarkers of Receptivity
Pinopodes are protrusions of the endometrial epithelium first identified in mice in 1958 (57) and later identified in human endometrium by electron microscopy (EM) (58). Since that time, pinopodes have been identified as markers of endometrial receptivity (59), due to their putative expression coinciding with the WOI (6). Blastocyst attachment has been shown to occur at the site of endometrial pinopode expression in vitro (60), and pinopodes are the site of expression of uterine receptivity including ανβ3 integrin and osteopontin (9, 24). Although the detection of pinopodes have been employed for assessment of uterine receptivity, clinical usefulness is limited by technical factors due to the need for EM, the brief time of expression, and the subjective nature of scoring them (61). In addition, three prospective studies have failed to confirm a precise association between the temporal timing of pinopode expression and the WOI (23, 62, 63), raising substantive doubts related to this endometrial feature as a biomarker of receptivity in humans.
Numerous molecular mediators of early feto-maternal interface have been identified in the literature. These include adhesion molecules, cytokines, growth factors, lipids, and other factors (37, 38). One of the better-described endometrial biomarkers associated with the WOI is the ανβ3 integrin (7, 24, 64). Integrins are a class of cell-adhesion molecules (CAMs) that interact with extracellular matrix (ECM) ligands, other CAMs, and matrix metalloproteinases (MMPs). Studies have documented how integrin expression is aberrant in many of the same inflammatory conditions associated with implantation failure, including endometriosis, hydrosalpinges, PCOS (27, 46, 65, 66) and endometritis (unpublished results). Reduced ανβ3 integrin expression has been associated with unexplained IVF failure (67, 68), whereas positive integrin expression has been found to predict future IVF success (69). Additional CAMs have been investigated to play a role in endometrial receptivity including CD 44 (70), trophinin (71), and cadherin-11 (72).
Glycodelin, formerly referred to as placental protein 14 (PP14), is a major secretory protein from the glandular endometrium expressed during and after the window of implantation (1). It is an immune modulator with a putative role in prevention of maternal immune rejection of the fetal allograft (73). Glycodelin has been investigated as a marker of endometrial receptivity with conflicting results (74–76).
Mucin 1 (MUC-1) is another glycoprotein localized to the luminal surface epithelium of the receptive endometrium. In primates and mice, MUC-1 appears to function as a barrier to implantation during the non-receptive phase and must be removed at the time of implantation (77, 78). In humans, MUC-1 localizes on the luminal surface, but is excluded from cells with pinopodes suggesting the anti-adhesive molecule may allow the blastocyst to preferentially attach to these specialized structures on the apical surface (79).
Several cytokines and growth factors have been identified whose expression in the endometrium is temporal with implantation and have been suggested as biomarkers for uterine receptivity. These include leukemia inhibitory factor (LIF), heparin binding-epidermal growth factor-like factor (HB-EGF), insulin-like growth factor II (IGF-II) (1). LIF appears to play a role in events between the endometrium and the blastocyst and is expressed in the endometrium at the time of implantation. In mouse models, female homozygote mice with a LIF null mutation demonstrate complete lack of implantation (80, 81). Normal appearing blastocysts were found within the uteri of these mice lacking LIF, but successfully implanted when placed into LIF positive controls. Interestingly, administration of exogenous LIF resulted in a partial reversal of the defect, demonstrating the implantation abnormalities resulted from a defect of the endometrial protein and not the blastocyst. Examination of LIF in human samples suggests LIF maintains importance in the human endometrium as well (82, 83). HB-EGF and IGF-II are expressed during the window of implantation and appear to play an important role in successful implantation (84, 85). Other potential markers include calcitonin (86, 87), HOXA-10 transcription factor (88, 89) and L-selectin and L-selectin ligand (8).
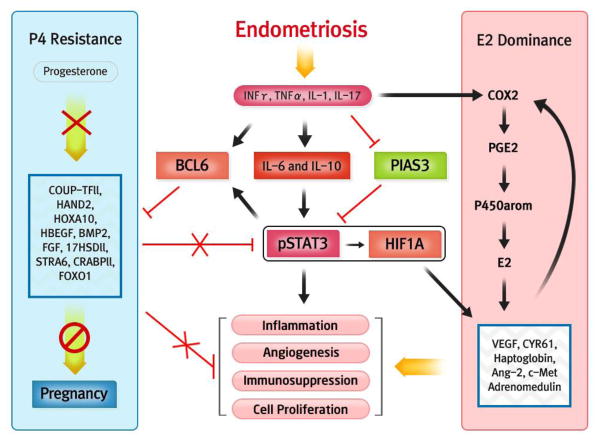
Aromatase (p450arom) is over-expressed in inflammatory conditions involving the endometrium, including endometriosis (90). Aromatase over-expression shows promise as an important predictor of implantation failure in ART cycles (68, 91). An over-expression of this enzyme coupled with a decreased expression of the estrogen metabolizing enzyme (17-hydroxysteroid dehydrogenase II) (90, 92, 93), increases bioavailable estrogen in the endometrium, potentially accounting for aberrantly high ESR1 and proliferation (32, 34, 94, 95). Estrogen is a potent inhibitor of endometrial ανβ3 integrin (96), a prime cell adhesion molecule involved in embryo attachment and invasion (7, 97–100). Alterations in eutopic endometrial metabolism of estrogen in endometriosis is regulated by complex changes in autocrine and paracrine signaling associated with inflammation (36, 94, 101–104), driven in part by prostaglandin E2 (PGE2) (105, 106), produced in response to estrogen-regulated cycylooyxgenase 2 (COX-2) (107) and hypoxia induced factor-1 (HIF1α) (108). HIF1α is stabilized by activation of STAT3 that we recently showed are both over-expressed in women with endometriosis and infertility (109). COX-2 and STAT3 expression have been linked to inflammatory cytokines such as IL-17 and IL-6 (109–111), which are also elevated in women with endometriosis (109, 112). Inhibitors of aromatase can reverse the negative effects of endometriosis in general (113) and improves outcomes in IVF for patients with suspected defects in endometrial receptivity (68).
While STAT5 is central to progesterone-mediated signaling (114), STAT3 appears central to progesterone resistance (115). One of the proteins induced by activated STAT3 is B-cell lymphoma protein 6 (BCL6), while is also inhibited by STAT5 (116). BCL6 appears to be a reliable single biomarker for the detection of endometriosis (117). BCL6 targets GLI1(118), a signaling factor involved in the Indian Hedgehog pathway, making it a prime candidate driving the progesterone resistance observed in endometriosis. This relationship between inflammatory changes, estrogen dominance and progesterone resistance represents a unifying theory of the link between inflammation, estrogen dominance and progesterone resistance (Figure 2).
Figure 2.
Schematic of inflammatory influences on the balance of estrogen and progesterone action in the endometrium of women with infertility and endometriosis.
Progesterone resistance is a hallmark of implantation failure and associated with measurable changes in endometrial gene expression (115, 119). With the advent of transcriptome microarrays, the signatures of gene expression throughout the menstrual cycle have been well-documented in normal women (120, 121) and in those with gynecological disorders (53, 54, 122, 123). New panels of selected biomarkers are now becoming available with the potential to screen for a receptive and non-receptive endometrium. A commercialized test based on transcriptomics (Endometrial Receptivity Assay or ERA) has been offered from the IVI group in Valencia, Spain (124–127). Interestingly, while the ERA test is accurate at assigning histological stage (128), this array of biomarkers in aggregate does not differ significantly in women with endometriosis (129), a known cause of endometrial receptivity defects and progesterone resistance (54).
Individual tests for endometrial receptivity, including the Etegrity test based on integrin expression (EtegrityTest.Com), are also available. As discussed above, the presence or absence of the ανβ3 integrin indicates potential defects in endometrial receptivity. This specific integrin is highly specific to the initiation of the window of implantation on post-ovulatory day 5 to 6, and is always absent in histologically delayed endometrium prior to the opening of the WOI (19). This test could be complemented by additional uterine factors that do not depend as heavily on histology, including LIF (25).
Another commercialized endometrial function test (EFT) from Yale University, is based on alterations in cyclin E, and p27 expression (130, 131). These biomarkers are associated with cell proliferation, as seen in eutopic endometrium of women with endometriosis (95) and therefore, reacting to estrogen dominance. In aggregate, the evidence showing benefit or utility for any of these tests remains relatively weak, and validation of these and future tests as predictors of IVF outcomes or implantation failure need to be rigorously studied in prospective, randomized trials to fully evaluate their performance and reliability.
Future Directions
Implantation concerns arise frequently in couples with infertility, especially in the setting of ART cycles. Implantation rates have been relatively stagnant over the past 10 years (www.sart.org), suggesting that progress in solving implantation problems may have slowed. IVF failure in more than half of all cases in women across all age groups appears to be concentrated in specific subgroups of patients, including those with unexplained causes. Future directions are now focused on identifying new biomarkers that alone or together reliably predict implantation success or failure. In an era where the underlying causes of infertility are increasingly not being identified or surgically addressed, availability of such biomarkers could be a key to identifying and better treating these women. Until such a time when reliable endometrial receptivity tests are available and adequately tested, that subset of women with implantation failure will continue to go largely unrecognized. In the future, our goal should be to make repetitive implantation failure an exceedingly rare occurrence.
THE EFFECT OF SYSTEMIC FACTORS ON IMPLANTATION AFTER IN VITRO FERTILIZATION
Introduction
Implantation rates after IVF have increased over the past 30 years as a result of advances in the basic understanding of reproductive science and the implementation of new technologies and practices. However, the primary focus of research aimed at improving IVF outcomes has focused on two areas: assessment of embryonic competence and optimization of endometrial receptivity. Research in embryonic competence has led to advanced diagnostics that have enhanced embryo selection and substantially improved implantation rates (132). Investigative efforts focused on the endometrium have uncovered the concept of embryo-endometrial synchrony, and led to the characterization of the transcriptomic signature of the receptive endometrium (133).
However, despite our progress, a substantial portion of patients fail to become pregnant following IVF. Many of these patients fail even after the transfer of a euploid embryo into a seemingly receptive endometrium. A portion of these failures undoubtedly reflects the limitation of current diagnostic tools to select the most competent embryo. However, many of these failures are due to systemic factors that affect the maternal environment and negatively impact an embryo’s ability to implant. While research into these systemic factors has received less focus than the preimplantation embryo and the perinidatory endometrium, many have been clearly demonstrated to affect IVF success. While a factor in isolation may not preclude a successful pregnancy, the combination of deleterious effects decreases the chance that an individual embryo transfer results in a pregnancy. Thus, it is essential these factors are optimized to give each patient the best chance at success.
Thyroid Dysfunction
Thyroid hormones influence the feto-maternal interface through interactions with thyroid hormone receptors and thyroid stimulating hormone (TSH) receptors present in the endometrium and trophoblast during implantation. This interaction is mediated by a variety of downstream effects – including altered transcription and translation of essential cellular proteins during implantation (134). Thyroid dysfunction has been mostly studied in the context of ART, in terms of pregnancy success as well as miscarriage. However, the threshold TSH values that confer implantation success and those that predispose patients to adverse outcomes may be different. Thus, when attempting to isolate the effect of thyroid function on implantation the threshold values used in the study must be noted.
The upper limit of the reference range for TSH levels was established by the National Health and Nutritional Examination Survey to be 4.5 – 5 mIU/L (135). Thus, the classical definition of subclinical hypothyroidism (SCH) is a TSH level greater than 4.5 mIU/L, with normal free thyroxine (T4) levels. Using this definition, Kim et al. (136) performed a randomized controlled trial of 64 patients to assess the effect of levothyroxine on IVF patients with SCH. In this study, patients were randomized to either levothyroxine 50mcg or no treatment. The implantation rate was significantly higher in the treatment arm than the control group (26.9% vs. 14.9%, p=0.044). A similar study used a TSH cutoff of 4.2 mIU/L to diagnose SCH, and randomized 70 patients to levothyroxine (50–100mcg daily) or placebo. In this study, the clinical pregnancy rate was significantly higher in the treatment group (35% vs. 10%, p = 0.02) (137). Thus, there is high quality data demonstrating that untreated subclinical hypothyroidism negatively impacts the implantation rate following ART.
In practice, most ART programs use 2.5 mIU/L as a threshold for initiating levothyroxine treatment. Green et al, by evaluating TSH levels under 2.5 with 1599 euploid transfers, determined that no level under that cut-off is more favorable (138).This strategy follows recommendations of the Endocrine Society to maintain TSH levels below 2.5 mIU/L during the first trimester of pregnancy. However, no studies have evaluated whether treatment of TSH levels between 2.5 mIU/L and the upper limit of normal impacts implantation rates or miscarriage following IVF, although in one study levels between 2.5 and 5 mIU/L in the first 11 weeks of pregnancy were associated with a significant increase in pregnancy loss (6.1 vs 3.6%, p = 0.006) (139). However, this investigation did not control for the chromosomal status of the embryo. As a result, it is possible that lower hCG levels associated with aneuploid gestations may have contributed to the failure of TSH to fall below 2.5 mIU/L in this group. Thus, the ideal TSH level within the normal range for optimizing implantation success is unclear, but levels over 2.5 mIU/L during early pregnancy appear to increase miscarriage.
Additionally, multiple studies have assessed the effect of thyroid autoimmunity (either anti-thyroperoxidase or anti-thyroglobulin antibodies) on IVF success. A meta-analysis of seven studies including 330 thyroid antibody positive patients and 1430 controls, demonstrated no difference in clinical pregnancy rate following IVF (OR = 0.67, 95% CI 0.36–1.4, p=0.67) (134). One prospective, randomized controlled trial evaluated empiric treatment with levothyroxine in euthyroid patients with evidence of thyroid autoimmunity. In that study, there was no difference in clinical pregnancy rates between the treated and untreated patients (56% vs. 49%, p=0.71), however, transfer order was not reported, limiting the conclusion (140). Available evidence does not support the notion that thyroid autoimmunity significantly impacts implantation, although a systematic review and a randomized study of levothyroxine therapy suggested that thyroid autoimmunity increased miscarriage and premature delivery (134) (141), which could be prevented by replacement therapy (141). If a decision is made to not treat women with TSH levels between 2.5 and 5.0 mIU/L, it may be prudent to measure thyroid peroxidase antibodies in those women and to treat if positive.
Vitamin D deficiency
The current Vitamin D (25OHD) deficiency epidemic in the developed world has led to increased interest in the role of 25OHD in ART. This interest is based on evidence that calcitriol, the active form of 25OHD, is secreted by the endometrium and regulates expression of target genes that are essential for implantation. In an attempt to control for the effect of 25OHD on the oocyte and resultant embryo, multiple studies have examined the association between 25OHD levels in donor oocyte recipients, with conflicting results. Rudick et. al. (142) performed a retrospective cohort study of 99 recipients and found that clinical pregnancy rates were lower among 25OHD deficient patients than 25OHD replete patients (37% vs. 78%, p = 0.004). A subsequent study by Fabris et. al. (143) retrospectively examined 267 oocyte donation cycles and found no difference in implantation rate among 25OHD replete, deficient, or insufficient patients (61% vs. 63.4% vs. 65.2%, p=0.894).
The largest analysis was performed by Franasiak, et al. (144) and controlled for the chromosomal status of embryos by analyzing euploid transfers. In this study, the average serum 25OHD level was no different between women with and without ongoing pregnancies. A multivariate logistic regression demonstrated no association between 25OHD levels and pregnancy rates. Thus, while more attention to the 25OHD deficiency epidemic in reproductive age women is warranted given its impact on a general health, it does not appear to be a significant determinant of success following IVF.
Prolactin
Circulating levels of prolactin (PRL) are elevated during ovarian stimulation cycles in some women. Limited investigations regarding PRL levels and IVF outcomes have reported associations of higher PRL levels with an improved ovarian response and pregnancy (145) (146), while others have failed to demonstrate this association (147) (148) (149) (150). Doldi et al (151) treated a group of women with dopamine agonists and observed a higher ovarian response and improved oocyte morphology and fertilization in an untreated control group, suggesting a beneficial effect of PRL on the ovary. Jinno et al (152) applied a “bromocriptine rebound” to elevate PRL levels and observed an increase in follicles, fertilized oocytes, embryo quality, clinical pregnancy and live birth. These investigations are important, due to evidence of decidual production of prolactin and the suggestion that it may mediate events associated with implantation. However, none of the above noted studies have sought to isolate the effect of prolactin levels on implantation rates. Future studies may benefit from measuring prolactin levels in the endometrial secretome, as the local effects of prolactin production may be more relevant in determining implantation success than circulating levels of the hormone.
Inflammatory Bowel Disease
Inflammatory bowel disease (IBD) has been linked to both endometriosis and subfertility. It is unclear whether this effect manifests itself as diminished ovarian reserve or an increased risk for implantation failure. To better characterize this association, Oza, et al. (153) published a retrospective cohort study comparing IVF outcomes in 120 patients with IBD to 470 age-matched controls. While implantation rate was not calculated, the mean number of embryos transferred (two) was the same for each group. There was no difference in clinical pregnancy rate in the first cycle for each patient (40.9% in non-IBD patients vs. 46.7% in IBD patients, p=0.18). Furthermore, the cumulative live birth rate after up to 6 IVF cycles was equivalent between the groups (63% vs. 53%, p=0.13). Thus, while further research is needed, there is no current data to suggest that IBD negatively impacts implantation.
Obesity
The incidence of obesity in the United States has increased substantially since the inception of ART 35 years ago. Today, over 35% of reproductive age women are obese (body mass index [BMI] ≥ 30 kg/m2). Obese women are more likely to be infertile and have poor obstetric outcomes. Thus, obesity is a common and modifiable risk factor for poor pregnancy rates and maternal and neonatal morbidity following IVF.
The association between obesity and IVF outcomes has been widely studied. The most effective study design to isolate obesity’s effect on implantation rates following IVF examines donor oocyte recipients. Two large retrospective reviews have utilized this design. The largest examined the 2008–2010 SART Registry, and included 22,317 donor oocyte cycles (154). Recipients with BMIs between 30–34.9 kg/m2 had a lower implantation rate than normal range (BMI 18.5–24.9 kg/m2) patients (42.6% vs. 49.3%, p<0.001). However, this study did not provide information on the BMI of the oocyte donors, limiting the ability to isolate obesity’s impact on implantation. In contrast, Bellver et. al. (155) examined the effect of increasing recipient BMI on IVF outcomes utilizing only oocyte donors with BMI <25 kg/m2. In this study, the implantation rate for recipients with BMI ≥30 kg/m2 was significantly lower than those <30 kg/m2 (30.9 % vs. 40 % , p< 0.001). Thus, while prospective studies are needed to better characterize this phenomenon, the available evidence suggests that obesity negatively impacts the ability of good prognosis embryos to implant.
Cigarette Smoking
Cigarette smoking represents another modifiable factor that substantially impacts reproductive success. Although the incidence of cigarette smoking has dropped substantially, one in five adults between the age of 25 and 44 still smoke cigarettes (156). The negative impact of cigarette smoking on ovarian function among smokers is well established. A number of studies have also addressed the effect of cigarette smoking on implantation following IVF.
Most studies that have examined the effect of cigarette use on ART outcomes are confounded by the positive correlation between cigarette use and maternal age. However, in their meta-analysis, Waylen et al. (157) identified 9 studies that controlled for maternal age. This pooled analysis of 1480 patients demonstrated that the odds of a clinical pregnancy following IVF were significantly lower for smokers than nonsmokers (OR = 0.51, 95% CI 0.32–0.79, p<0.0001). In order to isolate cigarette smoking’s effect on endometrial receptivity, Soares et. al. (158) utilized the donor oocyte recipient model. In this retrospective study of 785 recipients, the authors compared heavy smoking recipients (>10 cigarettes/day) to light smokers and nonsmokers after controlling for the tobacco use of the oocyte donors. The clinical pregnancy rate was significantly lower for heavy smoking recipients (34.1% vs. 52.2%, p=0.02).
Interestingly, the negative impact of cigarette smoke extends to nonsmokers who are exposed to secondhand smoke. Benedict at al. (159) found cotinine, a nicotine metabolite, to be present in the follicular fluid of 1909 nonsmoking women during IVF treatment. These patients had a 52% increased risk of implantation failure when compared to cotinine negative nonsmokers. Thus, chronic exposure to secondhand smoke also results in decreased implantation efficiency and patients should be counseled to avoid any exposure.
Autoimmunity
The potential role of autoimmune disorders in limiting ART outcomes has been extensively investigated. The established relationship with second trimester loss and antiphospholipid antibodies (APLAs) led some investigators to evaluate a potential role for these antibodies in failed implantation and early clinical losses. A meta-analysis of multiple studies demonstrated that the presence of APLAs does not impact pregnancy rates (160). Thus, clinical screening of APLAs in patients whose clinical diagnosis is infertility is not indicated.
Natural killer (NK) cells are prominent in the perinidatory and post-implantation endometrium. It is almost intuitive that abnormalities in NK cell activity might impact clinical outcomes. Unfortunately, the literature has been confused by efforts to measure NK cells in the peripheral circulation as a way of prognosticating NK cell density or function in the endometrium. There is no physiologic reason to assume that any such relationship exists (161). In fact, NK cell numbers in the peripheral circulation and the endometrium are unrelated (162). Prospective clinical studies have failed to demonstrate meaningful relationships with ART outcome and clinical screening of NK cells (either peripheral or endometrial) is not indicated (163).
While simple studies of NK cell concentration have not been clinically useful, studies evaluating variation in their function are more intriguing. NK cells are involved in early remodeling of the maternal stromal and vascular compartments and play important roles in villous formation (164). The NK cells are activated by HLA-C which is expressed on the surface of the invading trophoblast. Combinations of killer immunoglobin receptor (KIR’s) types and the nature of HLA-C expression have been associated with increased risk of clinical pregnancy loss and placental insufficiency in the third trimester (165). Recent data have extended these findings to suggest that adverse combinations of the maternal KIR genotype and embryonic HLA-C genotypes prognosticate reduced outcomes in oocyte donation cycles (166). Clinical screening is not indicated at this time, but this remains an area of active investigation.
No marker of autoimmune dysfunction evaluated to date has demonstrated clinical value. However, some investigators remain concerned that impaired immune function adversely impacts clinical outcomes. Trials of empiric treatments using anticoagulants, intralipid, or IVIg have produced mixed but generally negative results (167) (168) (169). Empiric treatment is not indicated at this time.
Conclusions
Significant progress has been made in understanding many significant embryonic and endometrial factors that mediate ART success. While much work remains in optimizing embryo selection, we must not lose sight of the systemic factors that modulate the perinidatory environment. Even the transfer of a euploid embryo into a synchronous endometrium will fail in an inhospitable maternal environment. Thus, as clinicians, we must reduce the negative impact of systemic factors that decrease the odds of a given embryo implanting and progressing to a healthy delivery. As investigators, more research is needed to identify epidemiologic factors that affect IVF success and to better understand the molecular mechanisms that govern these relationships.
Acknowledgments
This work was supported by the NIH R01 HD067721 (to S.L.Y and B.A.L) and NIH R01 HD057873 (to J.W.J.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Lessey BA, Young SL. The structure, function, and evaluation of the female reproductive tract. In: Strauss JF III, Barbieri RL, editors. Reproductive Endocrinology: Physiology, pathophysiology, and clinical management. Philadelphia, PA: Saunders Elsevier; 2012. pp. 192–235. [Google Scholar]
- 2.Allen WM, Corner GW. Physiology of the corpus luteum. III. Normal growth and implantation of embryos after very early ablation of the ovaries, under the influence of extracts of the corpus luteum. AmJPhysiol. 1929;88:340. [Google Scholar]
- 3.Large MJ, Demayo FJ. The regulation of embryo implantation and endometrial decidualization by progesterone receptor signaling. Molecular and cellular endocrinology. 2012;358:155–65. doi: 10.1016/j.mce.2011.07.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Singh M, Chaudhry P, Asselin E. Bridging endometrial receptivity and implantation: Network of hormones, cytokines, and growth factors. The Journal of endocrinology. 2011 doi: 10.1530/JOE-10-0461. [DOI] [PubMed] [Google Scholar]
- 5.Noyes RW, Hertig AI, Rock J. Dating the endometrial biopsy. Fertility and sterility. 1950;1:3–25. doi: 10.1016/j.fertnstert.2019.08.079. [DOI] [PubMed] [Google Scholar]
- 6.Nikas G, Drakakis P, Loutradis D, Mara-Skoufari C, Koumantakis E, Michalas S, et al. Uterine pinopodes as markers of the ‘nidation window’ in cycling women receiving exogenous oestradiol and progesterone. Hum Reprod. 1995;10:1208–13. doi: 10.1093/oxfordjournals.humrep.a136120. [DOI] [PubMed] [Google Scholar]
- 7.Lessey BA, Castelbaum AJ, Buck CA, Lei Y, Yowell CW, Sun J. Further characterization of endometrial integrins during the menstrual cycle and in pregnancy. Fertility and sterility. 1994;62:497– 506. [PubMed] [Google Scholar]
- 8.Genbacev OD, Prakobphol A, Foulk RA, Krtolica AR, Ilic D, Singer MS, et al. Trophoblast L-selectin-mediated adhesion at the maternal-fetal interface. Science. 2003;299:405–8. doi: 10.1126/science.1079546. [DOI] [PubMed] [Google Scholar]
- 9.Apparao KB, Murray MJ, Fritz MA, Meyer WR, Chambers AF, Truong PR, et al. Osteopontin and its receptor alphavbeta(3) integrin are coexpressed in the human endometrium during the menstrual cycle but regulated differentially. The Journal of clinical endocrinology and metabolism. 2001;86:4991– 5000. doi: 10.1210/jcem.86.10.7906. [DOI] [PubMed] [Google Scholar]
- 10.Evans J, Salamonsen LA. Inflammation, leukocytes and menstruation. Reviews in endocrine & metabolic disorders. 2012;13:277–88. doi: 10.1007/s11154-012-9223-7. [DOI] [PubMed] [Google Scholar]
- 11.Takano M, Lu Z, Goto T, Fusi L, Higham J, Francis J, et al. Transcriptional cross talk between the forkhead transcription factor forkhead box O1A and the progesterone receptor coordinates cell cycle regulation and differentiation in human endometrial stromal cells. Mol Endocrinol. 2007;21:2334–49. doi: 10.1210/me.2007-0058. [DOI] [PubMed] [Google Scholar]
- 12.Gellersen B, Brosens IA, Brosens JJ. Decidualization of the human endometrium: mechanisms, functions, and clinical perspectives. Seminars in reproductive medicine. 2007;25:445–53. doi: 10.1055/s-2007-991042. [DOI] [PubMed] [Google Scholar]
- 13.Tay PY, Lenton EA. The optimum time for exogenous human chorionic gonadotropin to rescue the corpus luteum. Journal of assisted reproduction and genetics. 1999;16:495–9. doi: 10.1023/A:1020507217897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tay PYS, Lenton EA. Corpus luteum response to exogenous HCG during the mid-luteal phase of the menstrual cycle. ClinEndocrinol(Oxf) 2000;53:345. doi: 10.1046/j.1365-2265.2000.01075.x. [DOI] [PubMed] [Google Scholar]
- 15.Wilcox AJ, Baird DD, Wenberg CR. Time of implantation of the conceptus and loss of pregnancy. The New England journal of medicine. 1999;340:1796–9. doi: 10.1056/NEJM199906103402304. [DOI] [PubMed] [Google Scholar]
- 16.Nakajima ST, Nason FG, Badger GJ, Gibson M. Progesterone production in early pregnancy. Fertility and sterility. 1991;55:516–21. [PubMed] [Google Scholar]
- 17.Macklon NS, Geraedts JP, Fauser BC. Conception to ongoing pregnancy: the 'black box' of early pregnancy loss. Human reproduction update. 2002;8:333–43. doi: 10.1093/humupd/8.4.333. [DOI] [PubMed] [Google Scholar]
- 18.Harton GL, Munne S, Surrey M, Grifo J, Kaplan B, McCulloh DH, et al. Diminished effect of maternal age on implantation after preimplantation genetic diagnosis with array comparative genomic hybridization. Fertility and sterility. 2013;100:1695–703. doi: 10.1016/j.fertnstert.2013.07.2002. [DOI] [PubMed] [Google Scholar]
- 19.Jones GS. Some newer aspects of management of infertility. JAMA : the journal of the American Medical Association. 1949;141:1123–9. doi: 10.1001/jama.1949.02910160013004. [DOI] [PubMed] [Google Scholar]
- 20.Fritz MA, Lessey BA. Defective luteal function. In: Fraser IS, Jansen RPS, Lobo RA, Whitehead MI, editors. Estrogens and progestogens in clinical practice. Vol. 1. London: Churchhill Livingstone; 1998. pp. 437–53. [Google Scholar]
- 21.Practice Committee of American Society for Reproductive M. Current clinical irrelevance of luteal phase deficiency: a committee opinion. Fertility and sterility. 2015;98:111207. doi: 10.1016/j.fertnstert.2014.12.128. [DOI] [PubMed] [Google Scholar]
- 22.Creus M, Balasch J, Ordi J, F†bregues F, Casamitjana R, Quinto L, et al. Integrin expression in normal and out-of-phase endometria. Hum Reprod. 1998;13:3460–8. doi: 10.1093/humrep/13.12.3460. [DOI] [PubMed] [Google Scholar]
- 23.Acosta AA, Elberger L, Borghi M, Calamera JC, Chemes H, Doncel GF, et al. Endometrial dating and determination of the window of implantation in healthy fertile women. Fertility and sterility. 2000;73:788–98. doi: 10.1016/s0015-0282(99)00605-6. [DOI] [PubMed] [Google Scholar]
- 24.Lessey BA, Damjanovich L, Coutifaris C, Castelbaum A, Albelda SM, Buck CA. Integrin adhesion molecules in the human endometrium. Correlation with the normal and abnormal menstrual cycle. The Journal of clinical investigation. 1992;90:188–95. doi: 10.1172/JCI115835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tulppala M, Julkunen M, Tiitinen A, Stenman UH, Seppälä M. Habitual abortion is accompanied by low serum levels of placental protein 14 in the luteal phase of the fertile cycle. Fertility and sterility. 1995;63:792. doi: 10.1016/s0015-0282(16)57483-4. [DOI] [PubMed] [Google Scholar]
- 26.Ilesanmi AO, Hawkins DA, Lessey BA. Immunohistochemical markers of uterine receptivity in the human endometrium. Microscopy research and technique. 1993;25:208–22. doi: 10.1002/jemt.1070250304. [DOI] [PubMed] [Google Scholar]
- 27.Lessey BA, Castelbaum AJ, Sawin SW, Buck CA, Schinnar R, Bilker W, et al. Aberrant integrin expression in the endometrium of women with endometriosis. The Journal of clinical endocrinology and metabolism. 1994;79:643–9. doi: 10.1210/jcem.79.2.7519194. [DOI] [PubMed] [Google Scholar]
- 28.Franasiak J, Holoch K, Yuan L, Fritz MA, Young S, Lessey BA. Leukemia inhibitory factor and anb3 integrin expression in normal and abnormal endometrium. Fertility and sterility. 2012 In preparation. [Google Scholar]
- 29.Lessey BA, Killam AP, Metzger DA, Haney AF, Greene GL, McCarty KS., Jr Immunohistochemical analysis of human uterine estrogen and progesterone receptors throughout the menstrual cycle. The Journal of clinical endocrinology and metabolism. 1988;67:334–40. doi: 10.1210/jcem-67-2-334. [DOI] [PubMed] [Google Scholar]
- 30.Tan J, Paria BC, Dey SK, Das SK. Differential uterine expression of estrogen and progesterone receptors correlates with uterine preparation for implantation and decidualization in the mouse. Endocrinology. 1999;140:5310–21. doi: 10.1210/endo.140.11.7148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lessey BA, Yeh I, Castelbaum AJ, Fritz MA, Ilesanmi AO, Korzeniowski P, et al. Endometrial progesterone receptors and markers of uterine receptivity in the window of implantation. Fertility and sterility. 1996;65:477–83. [PubMed] [Google Scholar]
- 32.Lessey BA, Palomino WA, Apparao KB, Young SL, Lininger RA. Estrogen receptor-alpha (ER-alpha) and defects in uterine receptivity in women. Reproductive biology and endocrinology : RB&E. 2006;4(Suppl 1):S9. doi: 10.1186/1477-7827-4-S1-S9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Young SL, Lessey BA. Progesterone function in human endometrium: clinical perspectives. Seminars in reproductive medicine. 2010;28:5–16. doi: 10.1055/s-0029-1242988. [DOI] [PubMed] [Google Scholar]
- 34.Bulun SE, Zeitoun KM, Takayama K, Sasano H. Estrogen biosynthesis in endometriosis: molecular basis and clinical relevance. JMolEndocrinol. 2000;25:35. doi: 10.1677/jme.0.0250035. [DOI] [PubMed] [Google Scholar]
- 35.Lessey BA. Two pathways of progesterone action in the human endometrium: implications for implantation and contraception. Steroids. 2003;68:809–15. doi: 10.1016/j.steroids.2003.09.004. [DOI] [PubMed] [Google Scholar]
- 36.Li X, Large MJ, Creighton CJ, Lanz RB, Jeong JW, Young SL, et al. COUP-TFII Regulates Human Endometrial Stromal Genes Involved in Inflammation. Mol Endocrinol. 2013;27:2041–54. doi: 10.1210/me.2013-1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Achache H, Revel A. Endometrial receptivity markers, the journey to successful embryo implantation. Human reproduction update. 2006;12:731–46. doi: 10.1093/humupd/dml004. [DOI] [PubMed] [Google Scholar]
- 38.Lessey BA, Lebovic DI, Taylor RN. Eutopic endometrium in women with endometriosis: ground zero for the study of implantation defects. Seminars in reproductive medicine. 2013;31:109–24. doi: 10.1055/s-0032-1333476. [DOI] [PubMed] [Google Scholar]
- 39.Lavergne N, Aristizabal J, Zarka V, Erny R, Hedon B. Uterine anomalies and in vitro fertilization: what are the results? European journal of obstetrics, gynecology, and reproductive biology. 1996;68:29– 34. doi: 10.1016/0301-2115(96)02459-1. [DOI] [PubMed] [Google Scholar]
- 40.Coughlan C, Ledger W, Wang Q, Liu F, Demirol A, Gurgan T, et al. Recurrent implantation failure: definition and management. Reproductive biomedicine online. 2014;28:14–38. doi: 10.1016/j.rbmo.2013.08.011. [DOI] [PubMed] [Google Scholar]
- 41.Bosteels J, Weyers S, Puttemans P, Panayotidis C, Van Herendael B, Gomel V, et al. The effectiveness of hysteroscopy in improving pregnancy rates in subfertile women without other gynaecological symptoms: a systematic review. Human reproduction update. 2010;16:1–11. doi: 10.1093/humupd/dmp033. [DOI] [PubMed] [Google Scholar]
- 42.Dawood A, Al-Talib A, Tulandi T. Predisposing factors and treatment outcome of different stages of intrauterine adhesions. Journal of obstetrics and gynaecology Canada : JOGC = Journal d'obstetrique et gynecologie du Canada : JOGC. 2010;32:767–70. doi: 10.1016/s1701-2163(16)34618-7. [DOI] [PubMed] [Google Scholar]
- 43.Barnhart K, Dunsmoor-Su R, Coutifaris C. Effect of endometriosis on in vitro fertilization. Fertility and sterility. 2002;77:1148–55. doi: 10.1016/s0015-0282(02)03112-6. [DOI] [PubMed] [Google Scholar]
- 44.Strandell A, Lindhard A, Waldenstrîm U, Thorburn J, Janson PO, Hamberger L. Hydrosalpinx and IVF outcome: a prospective, randomized multicentre trial in Scandinavia on salpingectomy prior to IVF. Hum Reprod. 1999;14:2762–9. doi: 10.1093/humrep/14.11.2762. [DOI] [PubMed] [Google Scholar]
- 45.Strandell A, Lindhard A, Waldenstrîm U, Thorburn J. Hydrosalpinx and IVF outcome: cumulative results after salpingectomy in a randomized controlled trial. Hum Reprod. 2001;16:2403–10. doi: 10.1093/humrep/16.11.2403. [DOI] [PubMed] [Google Scholar]
- 46.Meyer WR, Castelbaum AJ, Somkuti S, Sagoskin AW, Doyle M, Harris JE, et al. Hydrosalpinges adversely affect markers of endometrial receptivity. Hum Reprod. 1997;12:1393–8. doi: 10.1093/humrep/12.7.1393. [DOI] [PubMed] [Google Scholar]
- 47.Shelton KE, Butler L, Toner JP, Oehninger S, Muasher SJ. Salpingectomy improves the pregnancy rate in in-vitro fertilization patients with hydrosalpinx. Hum Reprod. 1996;11:523–5. doi: 10.1093/humrep/11.3.523. [DOI] [PubMed] [Google Scholar]
- 48.Vandromme J, Chasse E, Lejeune B, Van Rysselberge M, Delvigne A, Leroy F. Hydrosalpinges in in-vitro fertilization: an unfavourable prognostic feature. Hum Reprod. 1995;10:576–9. doi: 10.1093/oxfordjournals.humrep.a135992. [DOI] [PubMed] [Google Scholar]
- 49.Kitaya K, Matsubayashi H, Yamaguchi K, Nishiyama R, Takaya Y, Ishikawa T, et al. Chronic Endometritis: Potential Cause of Infertility and Obstetric and Neonatal Complications. Am J Reprod Immunol. 2015 doi: 10.1111/aji.12438. [DOI] [PubMed] [Google Scholar]
- 50.Takebayashi A, Kimura F, Kishi Y, Ishida M, Takahashi A, Yamanaka A, et al. The association between endometriosis and chronic endometritis. PloS one. 2014;9:e88354. doi: 10.1371/journal.pone.0088354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Dor J, Itzkowic DJ, Mashiach S, Lunenfeld B, Serr DM. Cumulative conception rates following gonadotropin therapy. American journal of obstetrics and gynecology. 1980;136:102–5. doi: 10.1016/0002-9378(80)90574-8. [DOI] [PubMed] [Google Scholar]
- 52.Cermik D, Selam B, Taylor HS. Regulation of HOXA-10 expression by testosterone in vitro and in the endometrium of patients with polycystic ovary syndrome. The Journal of clinical endocrinology and metabolism. 2003;88:238–43. doi: 10.1210/jc.2002-021072. [DOI] [PubMed] [Google Scholar]
- 53.Savaris RF, Groll JM, Young SL, DeMayo FJ, Jeong JW, Hamilton AE, et al. Progesterone resistance in PCOS endometrium: a microarray analysis in clomiphene citrate-treated and artificial menstrual cycles. The Journal of clinical endocrinology and metabolism. 2011;96:1737–46. doi: 10.1210/jc.2010-2600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Burney RO, Talbi S, Hamilton AE, Vo KC, Nyegaard M, Nezhat CR, et al. Gene expression analysis of endometrium reveals progesterone resistance and candidate susceptibility genes in women with endometriosis. Endocrinology. 2007;148:3814–26. doi: 10.1210/en.2006-1692. [DOI] [PubMed] [Google Scholar]
- 55.Gregory CW, Wilson EM, Apparao KB, Lininger RA, Meyer WR, Kowalik A, et al. Steroid receptor coactivator expression throughout the menstrual cycle in normal and abnormal endometrium. The Journal of clinical endocrinology and metabolism. 2002;87:2960–6. doi: 10.1210/jcem.87.6.8572. [DOI] [PubMed] [Google Scholar]
- 56.Bruner-Tran KL, Herington JL, Duleba AJ, Taylor HS, Osteen KG. Medical management of endometriosis: emerging evidence linking inflammation to disease pathophysiology. Minerva ginecologica. 2013;65:199–213. [PMC free article] [PubMed] [Google Scholar]
- 57.Nilsson O. Ultrastructure of mouse uterine surface epithelium under different estrogenic influences. 5. Continuous administration of estrogen. J Ultrastruct Res. 1959;2:342–51. doi: 10.1016/s0022-5320(59)80006-x. [DOI] [PubMed] [Google Scholar]
- 58.Psychoyos A, Mandon P. Etude de la surface de l'epithelium uterin au microscope electronique a balayage. CRHebdSeances AcadSciParis. 1971;272:2723–5. [PubMed] [Google Scholar]
- 59.Psychoyos A, Nikas G. Uterine pinopodes as markers of uterine receptivity. Assisted Reproduction Reviews. 1994;4:26. [Google Scholar]
- 60.Bentin-Ley U. Relevance of endometrial pinopodes for human blastocyst implantation. Hum Reprod. 2000;15(Suppl 6):67–73. [PubMed] [Google Scholar]
- 61.Donaghay M, Lessey BA. Uterine receptivity: alterations associated with benign gynecological disease. Seminars in reproductive medicine. 2007;25:461–75. doi: 10.1055/s-2007-991044. [DOI] [PubMed] [Google Scholar]
- 62.Quinn CE, Casper RF. Pinopodes: a questionable role in endometrial receptivity. Human reproduction update. 2009;15:229–36. doi: 10.1093/humupd/dmn052. [DOI] [PubMed] [Google Scholar]
- 63.Usadi RS, Lessey BA, Bagnell RC, Meyer WR, Fritz MA. Distribution of pinopods in the secretory phase: a prospective, randomized assessment in healthy, fertile women. Fertility and sterility. 2001;76:S39. [Google Scholar]
- 64.Tabibzadeh S. Patterns of expression of integrin molecules in human endometrium throughout the menstrual cycle. Hum Reprod. 1992;7:876–82. doi: 10.1093/oxfordjournals.humrep.a137753. [DOI] [PubMed] [Google Scholar]
- 65.Lessey BA, Castelbaum AJ, Sawin SW, Sun J. Integrins as markers of uterine receptivity in women with primary unexplained infertility. Fertility and sterility. 1995;63:535–42. [PubMed] [Google Scholar]
- 66.Apparao KBC, Lovely LP, Gui YT, Lining RA, Lessey BA. Elevated endometrial androgen receptor expression in women with polycystic ovarian syndrome. Biology of reproduction. 2002;66:297–304. doi: 10.1095/biolreprod66.2.297. [DOI] [PubMed] [Google Scholar]
- 67.Tei C, Maruyama T, Kuji N, Miyazaki T, Mikami M, Yoshimura Y. Reduced expression of alphavbeta3 integrin in the endometrium of unexplained infertility patients with recurrent IVF-ET failures: improvement by danazol treatment. Journal of assisted reproduction and genetics. 2003;20:13– 20. doi: 10.1023/A:1021254620888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Miller PB, Parnell BA, Bushnell G, Tallman N, Forstein DA, Higdon HL, 3rd, et al. Endometrial receptivity defects during IVF cycles with and without letrozole. Hum Reprod. 2012;27:881–8. doi: 10.1093/humrep/der452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Thomas K, Thomson AJ, Wood SJ, Kingsland CR, Vince G, Lewis-Jones DI. Endometrial integrin expression in women undergoing IVF and ICSI: a comparison of the two groups and fertile controls. Hum Reprod. 2003;18:364–9. doi: 10.1093/humrep/deg104. [DOI] [PubMed] [Google Scholar]
- 70.Yaegashi N, Fujita N, Yajima A, Nakamura M. Menstrual cycle dependent expression of CD44 in normal human endometrium. Human pathology. 1995;26:862–5. doi: 10.1016/0046-8177(95)90008-x. [DOI] [PubMed] [Google Scholar]
- 71.Fukuda MN, Sato T, Nakayama J, Klier G, Mikami M, Aoki D, et al. Trophinin and tastin, a novel cell adhesion molecule complex with potential involvement in embryo implantation. Genes Dev. 1995;9:1199–210. doi: 10.1101/gad.9.10.1199. [DOI] [PubMed] [Google Scholar]
- 72.MacCalman CD, Furth EE, Omigbodun A, Bronner M, Coutifaris C, Strauss JF., 3rd Regulated expression of cadherin-11 in human epithelial cells: a role for cadherin-11 in trophoblast-endometrium interactions? Developmental dynamics : an official publication of the American Association of Anatomists. 1996;206:201–11. doi: 10.1002/(SICI)1097-0177(199606)206:2<201::AID-AJA9>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 73.Clark GF, Oehninger S, Patankar MS, Koistinen R, Dell A, Morris HR, et al. A role for glycoconjugates in human development: the human feto-embryonic defence system hypothesis. Hum Reprod. 1996;11:467–73. doi: 10.1093/humrep/11.3.467. [DOI] [PubMed] [Google Scholar]
- 74.Tulppala M, Julkunen M, Tiitinen A, Stenman UH, Seppala M. Habitual abortion is accompanied by low serum levels of placental protein 14 in the luteal phase of the fertile cycle. Fertility and sterility. 1995;63:792–5. doi: 10.1016/s0015-0282(16)57483-4. [DOI] [PubMed] [Google Scholar]
- 75.Klentzeris LD, Bulmer JN, Seppala M, Li TC, Warren MA, Cooke ID. Placental protein 14 in cycles with normal and retarded endometrial differentiation. Hum Reprod. 1994;9:394–8. doi: 10.1093/oxfordjournals.humrep.a138515. [DOI] [PubMed] [Google Scholar]
- 76.Batista MC, Bravo N, Cartledge TP, Loriaux DL, Merriam GR, Nieman LK. Serum levels of placental protein 14 do not accurately reflect histologic maturation of the endometrium. Obstetrics and gynecology. 1993;81:439–43. [PubMed] [Google Scholar]
- 77.Surveyor GA, Gendler SJ, Pemberton L, Spicer AP, Carson DD. Differential expression of Muc-1 at the apical cell surface of mouse uterine epithelial cells. FASEB J. 1993;7:1151a. [Google Scholar]
- 78.Hild-Petito S, Fazleabas AT, Julian J, Carson DD. Mucin (Muc-1) expression is differentially regulated in uterine luminal and glandular epithelia of the baboon ( Papio anubis ) Biology of reproduction. 1996;54:939. doi: 10.1095/biolreprod54.5.939. [DOI] [PubMed] [Google Scholar]
- 79.Carson DD, Bagchi I, Dey SK, Enders AC, Fazleabas AT, Lessey BA, et al. Embryo implantation. Developmental biology. 2000;223:217–37. doi: 10.1006/dbio.2000.9767. [DOI] [PubMed] [Google Scholar]
- 80.Bhatt H, Brunet LJ, Stewart CL. Uterine expression of leukemia inhibitory factor coincides with the onset of blastocyst implantation. ProcNatlAcad SciUS A. 1991;88:11408–12. doi: 10.1073/pnas.88.24.11408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Stewart CL, Kaspar P, Brunet LJ, Bhatt H, Gadi I, Kontgen F, et al. Blastocyst implantation depends on maternal expression of leukaemia inhibitory factor. Nature. 1992;359:76–9. doi: 10.1038/359076a0. [DOI] [PubMed] [Google Scholar]
- 82.Cullinan EB, Abbondanzo SJ, Anderson PS, Pollard JW, Lessey BA, Stewart CL. Leukemia inhibitory factor (LIF) and LIF receptor expression in human endometrium suggests a potential autocrine/paracrine function in regulating embryo implantation. Proceedings of the National Academy of Sciences of the United States of America. 1996;93:3115–20. doi: 10.1073/pnas.93.7.3115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Franasiak JM, Holoch KJ, Yuan L, Schammel DP, Young SL, Lessey BA. Prospective assessment of midsecretory endometrial leukemia inhibitor factor expression versus alphanubeta3 testing in women with unexplained infertility. Fertility and sterility. 2014 doi: 10.1016/j.fertnstert.2014.02.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Yoo HJ, Barlow DH, Mardon HJ. Temporal and spatial regulation of expression of heparin-binding epidermal growth factor-like growth factor in the human endometrium: a possible role in blastocyst implantation. Developmental genetics. 1997;21:102–8. doi: 10.1002/(SICI)1520-6408(1997)21:1<102::AID-DVG12>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 85.Lim JJ, Lee DR, Song HS, Kim KS, Yoon TK, Gye MC, et al. Heparin-binding epidermal growth factor (HB-EGF) may improve embryonic development and implantation by increasing vitronectin receptor (integrin alphanubeta3) expression in peri-implantation mouse embryos. Journal of assisted reproduction and genetics. 2006 doi: 10.1007/s10815-006-9021-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kumar S, Zhu LJ, Polihronis M, Cameron ST, Baird DT, Schatz F, et al. Progesterone induces calcitonin gene expression in human endometrium within the putative window of implantation. The Journal of clinical endocrinology and metabolism. 1998;83:4443. doi: 10.1210/jcem.83.12.5328. [DOI] [PubMed] [Google Scholar]
- 87.Zhu LJ, Cullinan-Bove K, Polihronis M, Bagchi MK, Bagchi IC. Calcitonin is a progesterone-regulated marker that forecasts the receptive state of endometrium during implantation. Endocrinology. 1998;139:3923. doi: 10.1210/endo.139.9.6178. [DOI] [PubMed] [Google Scholar]
- 88.Taylor HS, Arici A, Olive D, Igarashi P. HOXA10 is expressed in response to sex steroids at the time of implantation in the human endometrium. The Journal of clinical investigation. 1998;101:1379–84. doi: 10.1172/JCI1597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Ma L, Benson GV, Lim HJ, Dey SK, Maas RL. Abdominal B (AbdB) Hoxa genes: Regulation in adult uterus by estrogen and progesterone and repression in Mullerian duct by the synthetic estrogen diethylstilbestrol (DES) Developmental biology. 1998;197:141. doi: 10.1006/dbio.1998.8907. [DOI] [PubMed] [Google Scholar]
- 90.Noble LS, Simpson ER, Johns A, Bulun SE. Aromatase expression in endometriosis. The Journal of clinical endocrinology and metabolism. 1996;81:174–9. doi: 10.1210/jcem.81.1.8550748. [DOI] [PubMed] [Google Scholar]
- 91.Brosens J, Verhoeven H, Campo R, Gianaroli L, Gordts S, Hazekamp J, et al. High endometrial aromatase P450 mRNA expression is associated with poor IVF outcome. Hum Reprod. 2004;19:352–6. doi: 10.1093/humrep/deh075. [DOI] [PubMed] [Google Scholar]
- 92.Bulun SE, Noble LS, Takayama K, Michael MD, Agarwal V, Fisher C, et al. Endocrine disorders associated with inappropriately high aromatase expression. The Journal of steroid biochemistry and molecular biology. 1997;61:133–9. [PubMed] [Google Scholar]
- 93.Bulun SE, Zeitoun K, Takayama K, Noble L, Michael D, Simpson E, et al. Estrogen production in endometriosis and use of aromatase inhibitors to treat endometriosis. Endocrine-related cancer. 1999;6:293–301. doi: 10.1677/erc.0.0060293. [DOI] [PubMed] [Google Scholar]
- 94.Bulun SE, Cheng YH, Pavone ME, Xue Q, Attar E, Trukhacheva E, et al. Estrogen receptor-beta, estrogen receptor-alpha, and progesterone resistance in endometriosis. Seminars in reproductive endocrinology. 2010;28:36–43. doi: 10.1055/s-0029-1242991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Park JS, Lee JH, Kim M, Chang HJ, Hwang KJ, Chang KH. Endometrium from women with endometriosis shows increased proliferation activity. Fertility and sterility. 2009;92:1246–9. doi: 10.1016/j.fertnstert.2009.04.025. [DOI] [PubMed] [Google Scholar]
- 96.Somkuti SG, Yuan L, Fritz MA, Lessey BA. Epidermal growth factor and sex steroids dynamically regulate a marker of endometrial receptivity in Ishikawa cells. The Journal of clinical endocrinology and metabolism. 1997;82:2192–7. doi: 10.1210/jcem.82.7.4102. [DOI] [PubMed] [Google Scholar]
- 97.Damsky CH, Librach C, Lim KH, Fitzgerald ML, McMaster MT, Janatpour M, et al. Integrin switching regulates normal trophoblast invasion. Development. 1994;120:3657–66. doi: 10.1242/dev.120.12.3657. [DOI] [PubMed] [Google Scholar]
- 98.Zhou Y, Fisher SJ, Janatpour M, Genbacev O, Dejana E, Wheelock M, et al. Human cytotrophoblasts adopt a vascular phenotype as they differentiate - A strategy for successful endovascular invasion? The Journal of clinical investigation. 1997;99:2139–51. doi: 10.1172/JCI119387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Illera MJ, Cullinan E, Gui Y, Yuan L, Beyler SA, Lessey BA. Blockade of the alpha(v)beta(3) integrin adversely affects implantation in the mouse. Biology of reproduction. 2000;62:1285–90. doi: 10.1095/biolreprod62.5.1285. [DOI] [PubMed] [Google Scholar]
- 100.Illera MJ, Lorenzo PL, Gui YT, Beyler SA, Apparao KB, Lessey BA. A role for alphavbeta3 integrin during implantation in the rabbit model. Biology of reproduction. 2003;68:766–71. doi: 10.1093/biolreprod/68.3.766. [DOI] [PubMed] [Google Scholar]
- 101.Bulun SE, Monsavais D, Pavone ME, Dyson M, Xue Q, Attar E, et al. Role of estrogen receptor-beta in endometriosis. Seminars in reproductive medicine. 2012;30:39–45. doi: 10.1055/s-0031-1299596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Bulun SE, Utsunomiya H, Lin Z, Yin P, Cheng YH, Pavone ME, et al. Steroidogenic factor-1 and endometriosis. Molecular and cellular endocrinology. 2009;300:104–8. doi: 10.1016/j.mce.2008.12.012. [DOI] [PubMed] [Google Scholar]
- 103.Pavone ME, Dyson M, Reirstad S, Pearson E, Ishikawa H, Cheng YH, et al. Endometriosis expresses a molecular pattern consistent with decreased retinoid uptake, metabolism and action. Hum Reprod. 2011;26:2157–64. doi: 10.1093/humrep/der172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Pavone ME, Reierstad S, Sun H, Milad M, Bulun SE, Cheng YH. Altered retinoid uptake and action contributes to cell survival in endometriosis. The Journal of clinical endocrinology and metabolism. 2010;95:E300–9. doi: 10.1210/jc.2010-0459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Noble LS, Takayama K, Zeitoun KM, Putman JM, Johns DA, Hinshelwood MM, et al. Prostaglandin E2 stimulates aromatase expression in endometriosis-derived stromal cells. The Journal of clinical endocrinology and metabolism. 1997;82:600–6. doi: 10.1210/jcem.82.2.3783. [DOI] [PubMed] [Google Scholar]
- 106.Wu MH, Lu CW, Chuang PC, Tsai SJ. Prostaglandin E2: the master of endometriosis? Exp Biol Med (Maywood) 2010;235:668–77. doi: 10.1258/ebm.2010.009321. [DOI] [PubMed] [Google Scholar]
- 107.Tamura M, Sebastian S, Yang S, Gurates B, Ferrer K, Sasano H, et al. Up-regulation of cyclooxygenase-2 expression and prostaglandin synthesis in endometrial stromal cells by malignant endometrial epithelial cells. A paracrine effect mediated by prostaglandin E2 and nuclear factor-kappa B. The Journal of biological chemistry. 2002;277:26208–16. doi: 10.1074/jbc.M201347200. [DOI] [PubMed] [Google Scholar]
- 108.Kim KH, Kim HY, Kim HH, Lee KS, Cheong J. Hypoxia induces expression of COX-2 through the homeodomain transcription factor CDX1 and orphan nuclear receptor SHP in human endometrial cells. Molecular human reproduction. 2011;17:710–9. doi: 10.1093/molehr/gar036. [DOI] [PubMed] [Google Scholar]
- 109.Kim BG, Yoo JY, Kim TH, Shin JH, Langenheim JF, Ferguson SD, et al. Aberrant activation of signal transducer and activator of transcription-3 (STAT3) signaling in endometriosis. Hum Reprod. 2015;30:1069–78. doi: 10.1093/humrep/dev050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Hirata T, Osuga Y, Takamura M, Saito A, Hasegawa A, Koga K, et al. Interleukin-17F increases the secretion of interleukin-8 and the expression of cyclooxygenase 2 in endometriosis. Fertility and sterility. 2011;96:113–7. doi: 10.1016/j.fertnstert.2011.04.060. [DOI] [PubMed] [Google Scholar]
- 111.Bukulmez O, Hardy DB, Carr BR, Word RA, Mendelson CR. Inflammatory status influences aromatase and steroid receptor expression in endometriosis. Endocrinology. 2008;149:1190–204. doi: 10.1210/en.2007-0665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Ahn SH, Edwards AK, Singh SS, Young SL, Lessey BA, Tayade C. IL-17A Contributes to the Pathogenesis of Endometriosis by Triggering Proinflammatory Cytokines and Angiogenic Growth Factors. J Immunol. 2015;195:2591–600. doi: 10.4049/jimmunol.1501138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Pavone ME, Bulun SE. Aromatase inhibitors for the treatment of endometriosis. Fertility and sterility. 2012;98:1370–9. doi: 10.1016/j.fertnstert.2012.08.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Maruyama T, Yoshimura Y. Molecular and cellular mechanisms for differentiation and regeneration of the uterine endometrium. Endocrine journal. 2008;55:795–810. doi: 10.1507/endocrj.k08e-067. [DOI] [PubMed] [Google Scholar]
- 115.Lessey BA, Young SL. Homeostasis imbalance in the endometrium of women with implantation defects: the role of estrogen and progesterone. Seminars in reproductive medicine. 2014;32:365–75. doi: 10.1055/s-0034-1376355. [DOI] [PubMed] [Google Scholar]
- 116.Walker SR, Nelson EA, Yeh JE, Pinello L, Yuan GC, Frank DA. STAT5 outcompetes STAT3 to regulate the expression of the oncogenic transcriptional modulator BCL6. Molecular and cellular biology. 2013;33:2879–90. doi: 10.1128/MCB.01620-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Evans-Hoeker E, Lessey BA, Yuan L-Y, Savaris RF, Jeong J-W, Schammel DP, et al. Endometrial BCL6 Overexpression in Eutopic Endometrium of Women with Endometriosis. Reprod Sciences. 2016 doi: 10.1177/1933719116649711. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Tiberi L, Bonnefont J, van den Ameele J, Le Bon SD, Herpoel A, Bilheu A, et al. A BCL6/BCOR/SIRT1 Complex Triggers Neurogenesis and Suppresses Medulloblastoma by Repressing Sonic Hedgehog Signaling. Cancer cell. 2014;26:797–812. doi: 10.1016/j.ccell.2014.10.021. [DOI] [PubMed] [Google Scholar]
- 119.Bulun SE, Cheng YH, Yin P, Imir G, Utsunomiya H, Attar E, et al. Progesterone resistance in endometriosis: link to failure to metabolize estradiol. Molecular and cellular endocrinology. 2006;248:94– 103. doi: 10.1016/j.mce.2005.11.041. [DOI] [PubMed] [Google Scholar]
- 120.Kao LC, Tulac S, Lobo S, Imani B, Yang JP, Germeyer A, et al. Global gene profiling in human endometrium during the window of implantation. Endocrinology. 2002;143:2119–38. doi: 10.1210/endo.143.6.8885. [DOI] [PubMed] [Google Scholar]
- 121.Talbi S, Hamilton AE, Vo KC, Tulac S, Overgaard MT, Dosiou C, et al. Molecular phenotyping of human endometrium distinguishes menstrual cycle phases and underlying biological processes in normo-ovulatory women. Endocrinology. 2006;147:1097–121. doi: 10.1210/en.2005-1076. [DOI] [PubMed] [Google Scholar]
- 122.Kao LC, Germeyer A, Tulac S, Lobo S, Yang JP, Taylor RN, et al. Expression profiling of endometrium from women with endometriosis reveals candidate genes for disease-based implantation failure and infertility. Endocrinology. 2003;144:2870–81. doi: 10.1210/en.2003-0043. [DOI] [PubMed] [Google Scholar]
- 123.Tamaresis JS, Irwin JC, Goldfien GA, Rabban JT, Burney RO, Nezhat C, et al. Molecular classification of endometriosis and disease stage using high-dimensional genomic data. Endocrinology. 2014;155:4986–99. doi: 10.1210/en.2014-1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Blesa D, Ruiz-Alonso M, Simon C. Clinical management of endometrial receptivity. Seminars in reproductive medicine. 2014;32:410–3. doi: 10.1055/s-0034-1376360. [DOI] [PubMed] [Google Scholar]
- 125.Diaz-Gimeno P, Ruiz-Alonso M, Blesa D, Simon C. Transcriptomics of the human endometrium. The International journal of developmental biology. 2014;58:127–37. doi: 10.1387/ijdb.130340pd. [DOI] [PubMed] [Google Scholar]
- 126.Gomez E, Ruiz-Alonso M, Miravet J, Simon C. Human Endometrial Transcriptomics: Implications for Embryonic Implantation. Cold Spring Harbor perspectives in medicine. 2015;5:a022996. doi: 10.1101/cshperspect.a022996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Ruiz-Alonso M, Blesa D, Simon C. The genomics of the human endometrium. Biochimica et biophysica acta. 2012;1822:1931–42. doi: 10.1016/j.bbadis.2012.05.004. [DOI] [PubMed] [Google Scholar]
- 128.Diaz-Gimeno P, Ruiz-Alonso M, Blesa D, Bosch N, Martinez-Conejero JA, Alama P, et al. The accuracy and reproducibility of the endometrial receptivity array is superior to histology as a diagnostic method for endometrial receptivity. Fertility and sterility. 2013;99:508–17. doi: 10.1016/j.fertnstert.2012.09.046. [DOI] [PubMed] [Google Scholar]
- 129.Garcia-Velasco JA, Fassbender A, Ruiz-Alonso M, Blesa D, D'Hooghe T, Simon C. Is endometrial receptivity transcriptomics affected in women with endometriosis? A pilot study. Reproductive biomedicine online. 2015 doi: 10.1016/j.rbmo.2015.07.014. [DOI] [PubMed] [Google Scholar]
- 130.Dubowy RL, Feinberg RF, Keefe DL, Doncel GF, Williams SC, McSweet JC, et al. Improved endometrial assessment using cyclin E and p27. Fertility and sterility. 2003;80:146–56. doi: 10.1016/s0015-0282(03)00573-9. [DOI] [PubMed] [Google Scholar]
- 131.Kliman HJ, Honig S, Walls D, Luna M, McSweet JC, Copperman AB. Optimization of endometrial preparation results in a normal endometrial function test (EFT) and good reproductive outcome in donor ovum recipients. Journal of assisted reproduction and genetics. 2006;23:299–303. doi: 10.1007/s10815-006-9061-1. [DOI] [PubMed] [Google Scholar]
- 132.Scott RT, Jr, Upham KM, Forman EJ, Hong KH, Scott KL, Taylor D, et al. Blastocyst biopsy with comprehensive chromosome screening and fresh embryo transfer significantly increases in vitro fertilization implantation and delivery rates: a randomized controlled trial. Fertility and sterility. 2013;100:697–703. doi: 10.1016/j.fertnstert.2013.04.035. [DOI] [PubMed] [Google Scholar]
- 133.Diaz-Gimeno P, Horcajadas JA, Martinez-Conejero JA, Esteban FJ, Alama P, Pellicer A, et al. A genomic diagnostic tool for human endometrial receptivity based on the transcriptomic signature. Fertility and sterility. 2011;95:50–60. e1–15. doi: 10.1016/j.fertnstert.2010.04.063. [DOI] [PubMed] [Google Scholar]
- 134.van den Boogaard E, Vissenberg R, Land JA, van Wely M, van der Post JA, Goddijn M, et al. Significance of (sub)clinical thyroid dysfunction and thyroid autoimmunity before conception and in early pregnancy: a systematic review. Hum Reprod Update. 2011;17:605–19. doi: 10.1093/humupd/dmr024. [DOI] [PubMed] [Google Scholar]
- 135.Hollowell JG, Staehling NW, Flanders WD, Hannon WH, Gunter EW, Spencer CA, et al. Serum TSH, T(4), and thyroid antibodies in the United States population (1988 to 1994): National Health and Nutrition Examination Survey (NHANES III) The Journal of clinical endocrinology and metabolism. 2002;87:489–99. doi: 10.1210/jcem.87.2.8182. [DOI] [PubMed] [Google Scholar]
- 136.Kim CH, Ahn JW, Kang SP, Kim SH, Chae HD, Kang BM. Effect of levothyroxine treatment on in vitro fertilization and pregnancy outcome in infertile women with subclinical hypothyroidism undergoing in vitro fertilization/intracytoplasmic sperm injection. Fertility and sterility. 2011;95:1650–4. doi: 10.1016/j.fertnstert.2010.12.004. [DOI] [PubMed] [Google Scholar]
- 137.Laboratory support for the diagnosis of thyroid disease. Vol. 13 Washington DC: The National Academy of Clinical Biochemistry; 2002. guidelines NAoCBLmp. [Google Scholar]
- 138.Green KA, Werner MD, Franasiak JM, Juneau CR, Hong KH, Scott RT., Jr Investigating the optimal preconception TSH range for patients undergoing IVF when controlling for embryo quality. J Assist Reprod Genet. 2015;32:1469–76. doi: 10.1007/s10815-015-0549-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Negro R, Schwartz A, Gismondi R, Tinelli A, Mangieri T, Stagnaro-Green A. Increased pregnancy loss rate in thyroid antibody negative women with TSH levels between 2.5 and 5. 0 in the first trimester of pregnancy. The Journal of clinical endocrinology and metabolism. 2010;95:E44–8. doi: 10.1210/jc.2010-0340. [DOI] [PubMed] [Google Scholar]
- 140.Negro R, Mangieri T, Coppola L, Presicce G, Casavola EC, Gismondi R, et al. Levothyroxine treatment in thyroid peroxidase antibody-positive women undergoing assisted reproduction technologies: a prospective study. Human reproduction. 2005;20:1529–33. doi: 10.1093/humrep/deh843. [DOI] [PubMed] [Google Scholar]
- 141.Negro R, Formoso G, Mangieri T, Pezzarossa A, Dazzi D, Hassan H. Levothyroxine treatment in euthyroid pregnant women with autoimmune thyroid disease: effects on obstetrical complications. The Journal of clinical endocrinology and metabolism. 2006;91:2587–91. doi: 10.1210/jc.2005-1603. [DOI] [PubMed] [Google Scholar]
- 142.Rudick BJ, Ingles SA, Chung K, Stanczyk FZ, Paulson RJ, Bendikson KA. Influence of vitamin D levels on in vitro fertilization outcomes in donor-recipient cycles. Fertility and sterility. 2014;101:447–52. doi: 10.1016/j.fertnstert.2013.10.008. [DOI] [PubMed] [Google Scholar]
- 143.Fabris A, Pacheco A, Cruz M, Puente JM, Fatemi H, Garcia-Velasco JA. Impact of circulating levels of total and bioavailable serum vitamin D on pregnancy rate in egg donation recipients. Fertility and sterility. 2014;102:1608–12. doi: 10.1016/j.fertnstert.2014.08.030. [DOI] [PubMed] [Google Scholar]
- 144.Franasiak JM, Molinaro TA, Dubell EK, Scott KL, Ruiz AR, Forman EJ, et al. Vitamin D levels do not affect IVF outcomes following the transfer of euploid blastocysts. Am J Obstet Gynecol. 2015;212:315, e1–6. doi: 10.1016/j.ajog.2014.09.029. [DOI] [PubMed] [Google Scholar]
- 145.Mendes MC, Ferriani RA, Sala MM, Moura MD, Carrara HH, de Sa MF. Effect of transitory hyperprolactinemia on in vitro fertilization of human oocytes. J Reprod Med. 2001;46:444–50. [PubMed] [Google Scholar]
- 146.Gonen Y, Casper RF. Does transient hyperprolactinemia during ovarian hyperstimulation interfere with conception or pregnancy outcome? Fertility and sterility. 1989;51:1007–10. doi: 10.1016/s0015-0282(16)60734-3. [DOI] [PubMed] [Google Scholar]
- 147.Hofmann GE, Denis AL, Scott RT, Muasher SJ. The incidence of transient hyperprolactinemia in gonadotropin-stimulated cycles for in vitro fertilization and its effect on pregnancy outcome. Fertility and sterility. 1989;52:622–6. doi: 10.1016/s0015-0282(16)60975-5. [DOI] [PubMed] [Google Scholar]
- 148.Pattinson HA, Taylor PJ, Fleetham JA, Servis SA. Transient hyperprolactinemia has no effect on endocrine response and outcome in in vitro fertilization (IVF) J In Vitro Fert Embryo Transf. 1990;7:89– 93. doi: 10.1007/BF01135580. [DOI] [PubMed] [Google Scholar]
- 149.Meldrum DR, Cedars MI, Hamilton F, Huynh D, Wisot A, Marr B. Leuprolide acetate elevates prolactin during ovarian stimulation with gonadotropins. J Assist Reprod Genet. 1992;9:251–3. doi: 10.1007/BF01203823. [DOI] [PubMed] [Google Scholar]
- 150.Balasch J, Creus M, Fabregues F, Carmona F, Casamitjana R, Penarrubia J, et al. Hormonal profiles in successful and unsuccessful implantation in IVF-ET after combined GnRH agonist/gonadotropin treatment for superovulation and hCG luteal support. Gynecological endocrinology : the official journal of the International Society of Gynecological Endocrinology. 1995;9:51–8. doi: 10.3109/09513599509160191. [DOI] [PubMed] [Google Scholar]
- 151.Doldi N, Papaleo E, De Santis L, Ferrari A. Treatment versus no treatment of transient hyperprolactinemia in patients undergoing intracytoplasmic sperm injection programs. Gynecological endocrinology : the official journal of the International Society of Gynecological Endocrinology. 2000;14:437–41. doi: 10.3109/09513590009167716. [DOI] [PubMed] [Google Scholar]
- 152.Jinno M, Katsumata Y, Hoshiai T, Nakamura Y, Matsumoto K, Yoshimura Y. A therapeutic role of prolactin supplementation in ovarian stimulation for in vitro fertilization: the bromocriptine-rebound method. The Journal of clinical endocrinology and metabolism. 1997;82:3603–11. doi: 10.1210/jcem.82.11.4349. [DOI] [PubMed] [Google Scholar]
- 153.Oza SS, Pabby V, Dodge LE, Moragianni VA, Hacker MR, Fox JH, et al. In Vitro Fertilization in Women With Inflammatory Bowel Disease Is as Successful as in Women From the General Infertility Population. Clin Gastroenterol Hepatol. 2015;13:1641–6. e3. doi: 10.1016/j.cgh.2015.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Provost MP, Acharya KS, Acharya CR, Yeh JS, Steward RG, Eaton JL, et al. Pregnancy outcomes decline with increasing recipient body mass index: an analysis of 22,317 fresh donor/recipient cycles from the 2008–2010 Society for Assisted Reproductive Technology Clinic Outcome Reporting System registry. Fertility and sterility. 2015 doi: 10.1016/j.fertnstert.2015.10.015. [DOI] [PubMed] [Google Scholar]
- 155.Bellver J, Pellicer A, Garcia-Velasco JA, Ballesteros A, Remohi J, Meseguer M. Obesity reduces uterine receptivity: clinical experience from 9,587 first cycles of ovum donation with normal weight donors. Fertility and sterility. 2013;100:1050–8. doi: 10.1016/j.fertnstert.2013.06.001. [DOI] [PubMed] [Google Scholar]
- 156.prevention CfDCa. Current Cigarette Smoking Among Adults - United States, 2005–2013. Morbidity and Mortality Weekly Report. 2014;63:1108–12. [PMC free article] [PubMed] [Google Scholar]
- 157.Waylen AL, Metwally M, Jones GL, Wilkinson AJ, Ledger WL. Effects of cigarette smoking upon clinical outcomes of assisted reproduction: a meta-analysis. Hum Reprod Update. 2009;15:31–44. doi: 10.1093/humupd/dmn046. [DOI] [PubMed] [Google Scholar]
- 158.Soares SR, Simon C, Remohi J, Pellicer A. Cigarette smoking affects uterine receptiveness. Human reproduction. 2007;22:543–7. doi: 10.1093/humrep/del394. [DOI] [PubMed] [Google Scholar]
- 159.Benedict MD, Missmer SA, Vahratian A, Berry KF, Vitonis AF, Cramer DW, et al. Secondhand tobacco smoke exposure is associated with increased risk of failed implantation and reduced IVF success. Human reproduction. 2011;26:2525–31. doi: 10.1093/humrep/der226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Hornstein MD, Davis OK, Massey JB, Paulson RJ, Collins JA. Antiphospholipid antibodies and in vitro fertilization success: a meta-analysis. Fertility and sterility. 2000;73:330–3. doi: 10.1016/s0015-0282(99)00498-7. [DOI] [PubMed] [Google Scholar]
- 161.Moffett-King A. Natural killer cells and pregnancy. Nature reviews Immunology. 2002;2:656–63. doi: 10.1038/nri886. [DOI] [PubMed] [Google Scholar]
- 162.Rai R, Sacks G, Trew G. Natural killer cells and reproductive failure--theory, practice and prejudice. Human reproduction. 2005;20:1123–6. doi: 10.1093/humrep/deh804. [DOI] [PubMed] [Google Scholar]
- 163.Moffett A, Shreeve N. First do no harm: uterine natural killer (NK) cells in assisted reproduction. Human reproduction. 2015;30:1519–25. doi: 10.1093/humrep/dev098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Kieckbusch J, Gaynor LM, Moffett A, Colucci F. MHC-dependent inhibition of uterine NK cells impedes fetal growth and decidual vascular remodelling. Nat Commun. 2014;5:3359. doi: 10.1038/ncomms4359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Hiby SE, Apps R, Sharkey AM, Farrell LE, Gardner L, Mulder A, et al. Maternal activating KIRs protect against human reproductive failure mediated by fetal HLA-C2. The Journal of clinical investigation. 2010;120:4102–10. doi: 10.1172/JCI43998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Alecsandru D, Garrido N, Vicario JL, Barrio A, Aparicio P, Requena A, et al. Maternal KIR haplotype influences live birth rate after double embryo transfer in IVF cycles in patients with recurrent miscarriages and implantation failure. Human reproduction. 2014;29:2637–43. doi: 10.1093/humrep/deu251. [DOI] [PubMed] [Google Scholar]
- 167.Kaandorp SP, Goddijn M, van der Post JA, Hutten BA, Verhoeve HR, Hamulyak K, et al. Aspirin plus heparin or aspirin alone in women with recurrent miscarriage. The New England journal of medicine. 2010;362:1586–96. doi: 10.1056/NEJMoa1000641. [DOI] [PubMed] [Google Scholar]
- 168.Shreeve N, Sadek K. Intralipid therapy for recurrent implantation failure: new hope or false dawn? J Reprod Immunol. 2012;93:38–40. doi: 10.1016/j.jri.2011.11.003. [DOI] [PubMed] [Google Scholar]
- 169.Practice Committee of the American Society for Reproductive M. Intravenous immunoglobulin (IVIG) and recurrent spontaneous pregnancy loss. Fertility and sterility. 2006;86:S226–7. doi: 10.1016/j.fertnstert.2006.08.047. [DOI] [PubMed] [Google Scholar]