Abstract
Chronic Obstructive Pulmonary Disease (COPD) is a devastating disease with no effective therapies. We investigated the role of the C-type lectin receptor, CLEC5A, in macrophage activation and pulmonary pathogenesis in a mouse model of COPD. We demonstrate that CLEC5A is expressed on alveolar macrophages in mice exposed long-term to cigarette smoke (CS) and in human smokers. We also show that CLEC5A-mediated activation of macrophages enhanced cytokine elaboration alone, and in combination with LPS or GM-CSF in CS-exposed mice. Furthermore, using Clec5a-deficient mice, we demonstrate that CS-induced macrophage responsiveness is mediated by CLEC5A and CLEC5A is required for the development of inflammation, proinflammatory cytokine expression and airspace enlargement. These findings suggest a novel mechanism that promotes airway inflammation and pathologies in response to CS exposure and identifies CLEC5A as a novel target for the therapeutic control of COPD pathogenesis.
Keywords: Chronic obstructive pulmonary disease, macrophage, CLEC5A, TNFα, GM-CSF
Introduction
COPD is a progressive disease of airway obstruction characterized by chronic inflammation, emphysema, and chronic bronchitis. Inflammatory processes are important drivers of COPD, and previous research has identified a role for virtually all leukocyte populations in response to cigarette smoke (CS) (1). The majority of studies have focused on macrophage- and neutrophil-derived proteases being causative factors in tissue destruction (2, 3). While many studies have detailed the effects of macrophage activation in the context of CS exposure and COPD, few data exist on the specific mechanisms whereby CS exposure activates macrophages. Although these studies are bountiful and informative on the general pathways of CS-induced stress and leukocyte effector functions, little is understood of specific pathways of leukocyte activation.
CLEC5A is a type II transmembrane protein with a short cytoplasmic tail and requires association with an adaptor protein (DAP12) to initiate signaling (4). CLEC5A receptor expression has been demonstrated on human peripheral blood monocytes, mouse bone-marrow-derived macrophages, and immature marrow-derived neutrophil precursors (5, 6). CLEC5A is implicated in the progression of multiple acute and chronic inflammatory diseases including hemorrhagic fever, lethal shock, and the development of autoimmune arthritis (5–7). These studies reveal exaggerated cytokine release from CLEC5A-expressing cells in respective pathologies, similar to features observed in macrophage in COPD. Along these lines, a recent study reported CS-exposure induced phosphorylation of DAP12 in Raw264.7 cells and increased expression of CLEC5A transcripts in alveolar macrophage of smokers (8).
Given the important role of alveolar macrophage in COPD pathogenesis, we postulated that CLEC5A activation contributes to CS-induced macrophage activation and pulmonary pathologies. We generated Clec5a-deficient mice and utilized a CLEC5A activating antibody (6) to examine the role of CLEC5A in a model of COPD. Our study reveals CLEC5A expression on alveolar macrophages in CS-exposed mice and human smokers. Further, we demonstrate that CLEC5A required for the development of CS-induced macrophage responsiveness, pulmonary inflammation, macrophage activation, and lung remodeling. These studies identify CLEC5A as a novel target for therapeutic inhibition of aberrant macrophage activation in COPD pathogenesis.
Methods
Mice
We generated Clec5a-deficient mice (Clec5a−/−) by homologous recombination using a Clec5a targeting vector that eliminated exon 2 at the University of Cincinnati Transgenic and Knockout Core Facility. Clec5a−/− mice were repeatedly crossed onto C57BL/6J mice. Mice used in studies were derived from heterozygote matings. Protocols were reviewed and approved by the Animal Care and Use Committee, University of Cincinnati Medical Center.
Cigarette smoke exposure
Mice were exposed to filtered, room air (FA) or cigarette smoke (CS) generated from 3R4F Kentucky Reference Cigarettes (University of Kentucky, Lexington, KY) for 4 h/d, 5 d/wk for indicated times as described (9).
Mouse macrophage isolation and leukocyte enumeration in BAL
Lungs were lavaged three times with 1 ml of 1× Hank’s balanced salt solution. Total cell counts were determined with a hemocytometer and differential counts were determined on Hemacolor-stained (EM Science, Gibbstown, NJ) slides. TNFα, IL-6, and IL12p40 levels were measured by ELISA (eBioscience).
Lung fixation, histology, MLI, immunostaining
Mean linear intercept (MLI), a measure of alveolar diameter, was determined on formalin-fixed, paraffin-embedded mouse lung tissue (10). Sections (3 per lung) were blinded to two reviewers and the measurements were averaged. CLEC5A was detected by α-CLEC5A antibody (clone 226402, R&D Systems). Primary antibody (1:400 dilution) was incubated overnight at 4°C and detected the following day with goat anti-rat AlexaFluor 568 (ThermoFisher Scientific). Nuclear counterstaining with 4’,6-diamidino-2-phenylindole (DAPI; Sigma Chemical, 1:2,000), facilitated identification of macrophage. Images were captured using a Nikon A1 Inverted confocal microscope (Nikon, Tokyo, Japan) at Cincinnati Children’s Hospital Medical Center Confocal Imaging Core.
Mouse CLEC5A expression on monocytes/macrophages
Mouse lung macrophages were isolated by BAL. Mouse peripheral blood mononuclear cells were isolated by cardiac puncture. CLEC5A expression on mouse macrophages was assessed using α-mouse CLEC5A (clone226402, R&D Systems), F4/80 (eBioscience), and Cd11b (eBioscience) to distinguish alveolar macrophages (F4/80hiCd11blo). Peripheral blood monocytes were identified as Cd11bhiLy6Glo (Ly6G, eBioscience) (11). Cells were analyzed on a FACSCalibur (BD Biosciences, San Jose, CA) using FlowJo software (Tree Star, Ashland, OR).
Human CLEC5A expression on monocytes/macrophages
Sputum was collected from never smokers (0 pack years, n=4) or current smokers (>10 pack years, n=4) (12). Sample collection was performed in accordance with NIH guidelines and approved by the Institutional Review Board of the University of Cincinnati. Sputum cells were primarily macrophages (>75%) with epithelial cells, neutrophils and lymphocytes as assessed by H&E-stained preparations. Cells were allowed to adhere to plastic culture plates to enrich (>90%) for macrophages (2 hr, 37°C, 5% CO2). CLEC5A was assessed with α-human CLEC5A antibody (clone 283834, R&D Systems) and α-CD45 (eBioscience) using a FACSCalibur and FlowJo software.
Macrophage stimulation ex vivo
BAL macrophage were suspended in RPMI 1640 containing 10% FBS, 1mM sodium pyruvate, 100 U/ml penicillin, 100 µg/ml Streptomycin, and 1× nonessential amino acids [MP Biomedicals, Solon, OH]). 5.0 × 104 cells in 100 µl were stimulated for 24 h with non-specific control IgG (Invivogen), α-CLEC5A activating mAb (DX163, Merck, Palo Alto, CA) (6) and/or LPS (10 ng/ml) (Invivogen) or GM-CSF (20ng/ml) (R&D Systems). Supernatants were harvested and assayed for TNFα, IL-6, and IL12p40 production by ELISA (eBioscience).
Quantitative real-time PCR
Total RNA was isolated from BAL macrophage with TRIzol Transcription Kit (Applied Biosystems). For each gene examined, quantitative RT-PCR was performed on an ABI 7300 System using pre-validated TaqMan Gene Expression Assays Reagent (Invitrogen) and subsequently converted into cDNA using the High Capacity cDNA Reverse according to manufacturer’s protocols (Applied Biosystems). Gene expression was normalized to the Hprt1 control gene, and relative quantitation of gene expression was calculated using the comparative Ct method (2–ΔΔCt) as described (10).
Statistics
Significant differences among groups were identified by t-test or one-way ANOVA wherever appropriate.
Results and Discussion
CLEC5A Receptor Expression
Previously, CLEC5A transcripts were shown to be elevated in macrophage obtained from the lungs of smokers compared to non-smokers(8). However, CLEC5A surface expression was not assessed on the macrophage. Here, we analyzed the expression of CLEC5A on mouse peripheral blood monocytes, lung macrophage from FA and CS-exposed mice, and lung macrophage from human smokers. CLEC5A immunostaining shows that alveolar macrophage from CS-exposed mice, but not FA-exposed mice, express CLEC5A (Fig 1A and 1B). Utilizing flow cytometry we show a small population of peripheral blood monocytes that express CLEC5A in naïve wild-type mice (Figure 1C). In the lung, there is a small population of alveolar macrophage (F4/80hiCD11blo cells) that express CLEC5A in FA-exposed mice, whereas most macrophage from CS-exposed mice express CLEC5A (Figure 1D–F). Next, we examined the effects of smoking on the expression of CLEC5A on human lung macrophage. Similar to CLEC5A expression in CS-exposed mice, we found that most macrophage from smokers are CLEC5A positive, whereas, few positive cells are detected on the macrophage of non-smokers (Figure 1G and 1H). These findings indicate that CLEC5A expression is induced by CS exposure in existing pulmonary macrophage or that CLEC5A expression is acquired by cells (monocytes) migrating to the lung in response to CS-mediated inflammatory signals.
Figure 1. CLEC5A Expression on Alveolar Macrophage.
Immunofluorescent analysis of CLEC5A in lungs of mice exposed to (A) FA or (B) CS for 6 months. Arrows indicate alveolar macrophage (representative of 4 mice). CLEC5A surface expression was measured on (C) peripheral blood monocytes and (D and E) alveolar macrophage (F4/80hiCD11blo) from FA- and CS- exposed mice. Dashed line represents isotype control, solid line represents CLEC5A (n=5 mice). (F) Average number of CLEC5A+ cells and median fluorescence intensity of CLEC5A on alveolar macrophage. (G) CLEC5A expression on lung macrophage of never smokers (NS) and current smoker (S) (n=4 subjects/group). (H) Average number of CLEC5A+ cells and median fluorescence intensity of CLEC5A on alveolar macrophage.
CLEC5A Activation and Macrophage Responsiveness in Mouse Model of COPD
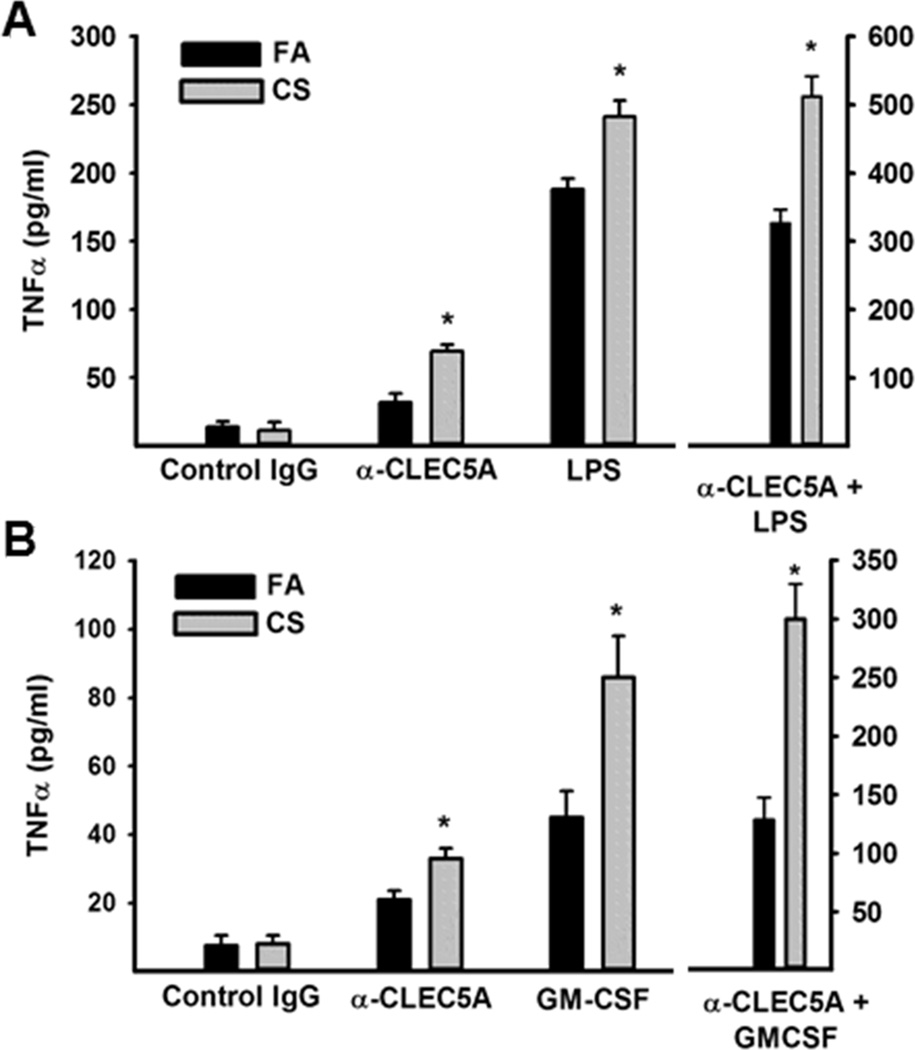
A physiological role for CLEC5A was first described by the interaction of the dengue virus with CLEC5A on human peripheral blood-derived macrophages and murine bone marrow-derived macrophages (5). Subsequently, we reported that CLEC5A regulates chronic inflammation associated with autoimmune arthritis (6) and acute inflammation during lethal shock (7). Next, we investigated the potential functions of CLEC5A in mediating macrophage activation in a mouse COPD model. We exposed mice to FA or CS for 6 months, isolated bronchoalveolar macrophage (~95% macrophage) and stimulated the cells with a CLEC5A agonist antibody alone and in combination with macrophage activating factors LPS or GM-CSF. Analysis of TNFα secretion reveal that CLEC5A activation, alone, stimulates macrophage and this effect is enhanced in macrophage from CS-exposed mice (Figure 2A and B). A similar, albeit stronger, stimulation is observed following LPS or GM-CSF treatment. Most intriguing, the combination of CLEC5A activation and LPS or GM-CSF treatment produces an exaggerated response that is amplified in macrophages from CS-exposed mice.
Figure 2. CLEC5A and LPS or GM-CSF co-stimulate macrophage from CS-exposed mice.
BAL macrophage (5×104) were stimulated with (A) control IgG, α-CLEC5A activating antibody (10 ug/ml), LPS (10 ng/ml), or both for 24hrs. Similarly, BAL macrophage (5×104) were stimulated with (B) control IgG, α-CLEC5A activating antibody (10 ug/ml), GM-CSF (20 ng/ml), or both for 24hrs. TNFα was measured by ELISA. Data are mean +/− sem of 3 experiments performed in duplicate. * p<0.05 compared to FA mice.
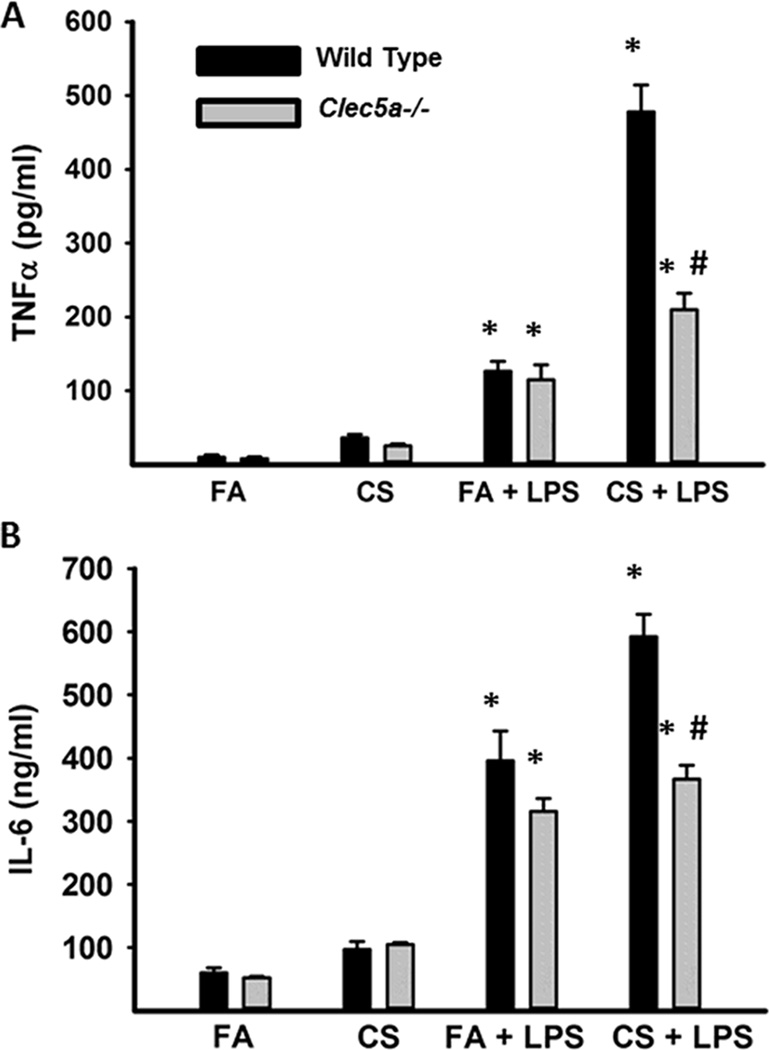
We further examined the requirement of CLEC5A in CS-induced macrophage responsiveness utilizing Clec5a−/− mice exposed to FA or CS for 6 months. Lung macrophage were stimulated with LPS and cytokine production was assessed. We observed an increased response to LPS in macrophage from CS-exposed mice (Figure 3A and 3B). More importantly, LPS responsiveness was slightly decreased in FA-exposed Clec5a−/− mice and markedly decreased in CS-exposed Clec5a−/− mice compared to wild-type mice. Multiple reports implicate the upregulation of various signaling pathways (13–17) in macrophage in response to smoking, suggesting the potential for enhanced signal integration via CLEC5A and multiple families of cell surface activating receptors including TLRs and cytokine/chemokine receptors. The culmination of these signals could lead to enhanced macrophage activation in response to endogenous and exogenous signals resulting in greater inflammation and tissue destruction.
Figure 3. CLEC5A is required for CS-induced macrophage hyper-responsiveness.
BAL macrophage (5×104) from FA- and CS-exposed mice (6 mos.) were stimulated with media or LPS (10 ng/ml × 24 hrs). TNFα and IL-6 levels were analyzed by ELISA. Data are mean +/− SEM from 3 experiments performed in triplicate. # p<0.05 compared to wild-type mice.
CLEC5A is Required for CS-Induced Inflammation and Pathology
To examine the role of CLEC5A in CS-induced inflammation and pathology, Clec5a−/− mice were exposed to FA or CS for up to six months. Significantly, Clec5a−/− mice exhibited decreased airspace enlargement compared to CS-exposed WT mice (Figures 4A and 4B). Additionally, Clec5a−/− mice showed less lung inflammation compared to wild-type mice following exposures as little as 2 weeks and up to 6 months (Figure 4C). The differential cell profiles in the BAL were not significantly different between CS-exposed wild-type and Clec5a−/− mice. All samples were >93% macrophage. Similarly, there no differences in peripheral blood leukocytes between wild-type and Clec5a−/− mice (not shown).
Figure 4. Reduced COPD Pathologies and proinflammatory markers in Clec5a−/− mice.
(A and B) WT and Clec5a−/− mice were exposed to CS for up to 6 months to evaluate lung remodeling as assessed by MLI (Data are mean +/− SEM, n=7–8). (C) Pulmonary inflammation was assessed by enumerating the total cells in BAL. (D) Cytokine levels in BAL of WT and Clec5a−/− mice (n=5–8). (E) mRNA expression levels in BAL macrophage of WT and Clec5a−/− mice (n=5–6). * p<0.05 compared to FA mice. # p<0.05 compared to wild-type mice.
Next, we examined the consequences of Clec5a deletion on the production of proinflammatory cytokines in the lung. Similar to the ex vivo studies with isolated macrophage, we found increased levels of TNFα and IL-6 in the BAL fluid of CS-exposed wild-type mice but no change in IL-12p40 (Figure 4D). TNFα and IL-6 were also increased in the BAL fluid of CS-exposed Clec5a−/− mice but the levels were significantly lower compared to wild-type mice (Figure 4D). To further explore differences in macrophage activation in wild-type and Clec5a−/− mice, we assessed macrophage mRNA expression of matrix metalloproteinases and adhesion molecules implicated in tissue destruction and macrophage activation, respectively. These results demonstrate a significant increase in Mmp12 expression in CS-exposed wild-type mice that is significantly lower in the Clec5a−/− mice (Figure 4E). There were no changes in Mmp2 or Mmp9 mRNA expression. CS exposure induced the expression of several macrophage adhesion molecules including Icam1, Itgav, and Itgb6 in both wild-type and knockout mice. However, only Itgav expression was reduced in the Clec5a−/− mice compared to wild-type.
These studies suggest that CLEC5A-mediated macrophage activation (i.e., cytokine elaboration, MMP and adhesion molecule expression) is important for the recruitment of inflammatory cells and subsequent macrophage–dependent tissue destruction. Given that there are fewer macrophage in the Clec5a−/− mice and that the macrophage from the Clec5a−/− mice are less activated, it is possible that migration, induction of CLEC5A expression, and macrophage activation are mechanistically undivided. Further studies into these processes will help define the bridge between local CS-induced lung injury and the magnitude of macrophage responsiveness to inflammatory signals.
Acknowledgments
The authors would like to thank Dr. Ralph Panos for providing the human sputum samples and helpful discussions.
Footnotes
This work was supported by National Institutes of Health Research Grants R01 HL119538 and R21 HL109635 (to M.T.B.), T32 ES016646 (to B.L.W.), and a Flight Attendant Medical Research Institute grant (to M.T.B.).
References
- 1.Cosio MG, Saetta M, Agusti A. Immunologic aspects of chronic obstructive pulmonary disease. N Engl J Med. 2009;360:2445–2454. doi: 10.1056/NEJMra0804752. [DOI] [PubMed] [Google Scholar]
- 2.Hautamaki RD, Kobayashi DK, Senior RM, Shapiro SD. Requirement for macrophage elastase for cigarette smoke-induced emphysema in mice. Science. 1997;277:2002–2004. doi: 10.1126/science.277.5334.2002. [DOI] [PubMed] [Google Scholar]
- 3.Russell RE, Thorley A, Culpitt SV, Dodd S, Donnelly LE, Demattos C, Fitzgerald M, Barnes PJ. Alveolar macrophage-mediated elastolysis: roles of matrix metalloproteinases, cysteine, and serine proteases. Am J Physiol Lung Cell Mol Physiol. 2002;283:L867–L873. doi: 10.1152/ajplung.00020.2002. [DOI] [PubMed] [Google Scholar]
- 4.Bakker AB, Baker E, Sutherland GR, Phillips JH, Lanier LL. Myeloid DAP12-associating lectin (MDL)-1 is a cell surface receptor involved in the activation of myeloid cells. Proc Natl Acad Sci U S A. 1999;96:9792–9796. doi: 10.1073/pnas.96.17.9792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen ST, Lin YL, Huang MT, Wu MF, Cheng SC, Lei HY, Lee CK, Chiou TW, Wong CH, Hsieh SL. CLEC5A is critical for dengue-virus-induced lethal disease. Nature. 2008;453:672–676. doi: 10.1038/nature07013. [DOI] [PubMed] [Google Scholar]
- 6.Joyce-Shaikh B, Bigler ME, Chao CC, Murphy EE, Blumenschein WM, Adamopoulos IE, Heyworth PG, Antonenko S, Bowman EP, McClanahan TK, Phillips JH, Cua DJ. Myeloid DAP12-associating lectin (MDL)-1 regulates synovial inflammation and bone erosion associated with autoimmune arthritis. J Exp Med. 2010;207:579–589. doi: 10.1084/jem.20090516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cheung R, Shen F, Phillips JH, McGeachy MJ, Cua DJ, Heyworth PG, Pierce RH. Activation of MDL-1 (CLEC5A) on immature myeloid cells triggers lethal shock in mice. J Clin Invest. 2011;121:4446–4461. doi: 10.1172/JCI57682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Koth LL, Cambier CJ, Ellwanger A, Solon M, Hou L, Lanier LL, Abram CL, Hamerman JA, Woodruff PG. DAP12 is required for macrophage recruitment to the lung in response to cigarette smoke and chemotaxis toward CCL2. J Immunol. 184:6522–6528. doi: 10.4049/jimmunol.0901171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Motz GT, Eppert BL, Sun G, Wesselkamper SC, Linke MJ, Deka R, Borchers MT. Persistence of Lung CD8 T Cell Oligoclonal Expansions upon Smoking Cessation in a Mouse Model of Cigarette Smoke-Induced Emphysema. J Immunol. 2008;181:8036–8043. doi: 10.4049/jimmunol.181.11.8036. [DOI] [PubMed] [Google Scholar]
- 10.Borchers MT, Wesselkamper SC, Curull V, Ramirez-Sarmiento A, Sanchez-Font A, Garcia-Aymerich J, Coronell C, Lloreta J, Agusti AG, Gea J, Howington JA, Reed MF, Starnes SL, Harris NL, Vitucci M, Eppert BL, Motz GT, Fogel K, McGraw DW, Tichelaar JW, Orozco-Levi M. Sustained CTL activation by murine pulmonary epithelial cells promotes the development of COPD-like disease. J Clin Invest. 2009;119:636–649. doi: 10.1172/JCI34462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Landsman L, Jung S. Lung macrophages serve as obligatory intermediate between blood monocytes and alveolar macrophages. J Immunol. 2007;179:3488–3494. doi: 10.4049/jimmunol.179.6.3488. [DOI] [PubMed] [Google Scholar]
- 12.Urbanowicz RA, Lamb JR, Todd I, Corne JM, Fairclough LC. Enhanced effector function of cytotoxic cells in the induced sputum of COPD patients. Respir Res. 11:76. doi: 10.1186/1465-9921-11-76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kent LM, Smyth LJ, Plumb J, Clayton CL, Fox SM, Ray DW, Farrow SN, Singh D. Inhibition of lipopolysaccharide-stimulated chronic obstructive pulmonary disease macrophage inflammatory gene expression by dexamethasone and the p38 mitogen-activated protein kinase inhibitor N-cyano-N'-(2-{[8-(2,6-difluorophenyl)-4-(4-fluoro-2-methylphenyl)-7-oxo-7,8-dihy dropyrido[2,3-d] pyrimidin-2-yl]amino}ethyl)guanidine (SB706504) J Pharmacol Exp Ther. 2009;328:458–468. doi: 10.1124/jpet.108.142950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Renda T, Baraldo S, Pelaia G, Bazzan E, Turato G, Papi A, Maestrelli P, Maselli R, Vatrella A, Fabbri LM, Zuin R, Marsico SA, Saetta M. Increased activation of p38 MAPK in COPD. Eur Respir J. 2008;31:62–69. doi: 10.1183/09031936.00036707. [DOI] [PubMed] [Google Scholar]
- 15.Gunella G, Bardelli C, Amoruso A, Viano I, Balbo P, Brunelleschi S. Macrophage-stimulating protein differently affects human alveolar macrophages from smoker and non-smoker patients: evaluation of respiratory burst, cytokine release and NF-kappaB pathway. Br J Pharmacol. 2006;148:478–489. doi: 10.1038/sj.bjp.0706751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mochida-Nishimura K, Surewicz K, Cross JV, Hejal R, Templeton D, Rich EA, Toossi Z. Differential activation of MAP kinase signaling pathways and nuclear factor-kappaB in bronchoalveolar cells of smokers and nonsmokers. Molecular medicine. 2001;7:177–185. [PMC free article] [PubMed] [Google Scholar]
- 17.Yang SR, Chida AS, Bauter MR, Shafiq N, Seweryniak K, Maggirwar SB, Kilty I, Rahman I. Cigarette smoke induces proinflammatory cytokine release by activation of NF-kappaB and posttranslational modifications of histone deacetylase in macrophages. Am J Physiol Lung Cell Mol Physiol. 2006;291:L46–L57. doi: 10.1152/ajplung.00241.2005. [DOI] [PubMed] [Google Scholar]