Abstract
Cytoglobin (Cygb) is a hexa-coordinated hemoprotein with yet to be defined physiological functions. The iron coordination and spin state of the Cygb heme group are sensitive to oxidation of two cysteine residues (Cys38/Cys83) and/or the binding of free fatty acids. However, the roles of redox vs lipid regulators of Cygb’s structural rearrangements in the context of the protein peroxidase competence are not known. Searching for physiologically relevant lipid regulators of Cygb, here we report that anionic phospholipids, particularly phosphatidylinositolphosphates, affect structural organization of the protein and modulate its iron state and peroxidase activity both conjointly and/or independently of cysteine oxidation. Thus, different anionic lipids can operate in cysteine-dependent and cysteine-independent ways as inducers of the peroxidase activity. We establish that Cygb’s peroxidase activity can be utilized for the catalysis of peroxidation of anionic phospholipids (including phosphatidylinositolphosphates) yielding mono-oxygenated molecular species. Combined with the computational simulations we propose a bipartite lipid binding model that rationalizes the modes of interactions with phospholipids, the effects on structural re-arrangements and the peroxidase activity of the hemoprotein.
Keywords: Cytoglobin, Phosphatidylinositolphosphates, lipid binding, peroxidase activity
1. INTRODUCTION
The abilities of iron-containing hemoproteins to bind vitally important small molecules (O2, CO, NO, H2S) as well as to transfer electrons and catalyze a variety of oxidation reactions have defined their essential and evolutionary conserved functions. Specific involvement and competence in these respective processes is dependent on coordination of heme-iron whereby small ligand binding and electron transport are optimally accommodated by the penta- and hexa-coordinated organizations, respectively [1-3]. While catalytic peroxidase (oxygenase) functions commonly require penta-coordination of heme-iron, hexa-coordinated proteins can also effectively participate in these reactions via re-arrangements of their protein structure thus facilitating their dual functions in either electron transfer or peroxidase processes [1, 2].
An intermembrane space hemoprotein, cytochrome c (Cyt c), normally operates as a hexa-coordinated electron shuttle between mitochondrial respiratory complexes III and IV [4] but can adopt a peroxidase function essential for the execution of the apoptotic program [5]. This conversion occurs upon its binding to an anionic mitochondria-specific phospholipid, cardiolipin [6], which triggers partial unfolding of the protein resulting in realization of its penta-coordinated form capable of catalyzing peroxidation of cardiolipin [5]
Two recently discovered hemoproteins – neuroglobin and cytoglobin (Cygb) – are generally found in a hexa-coordinated form [7, 8]. However, the hexa- and penta-coordinated forms are present in a dynamic equilibrium, and both proteins can stabilize their penta-coordinated states upon oxidation of their Cys residues to an internal disulfide [9-12]. Recent work has established that, similarly to Cyt c, Cygb can also stabilize the penta-coordinate species by lipid binding, and thus hypothetically promote its peroxidase activity [13]. The significance of this effect remains obscure as the transition was induced by supra-physiological concentrations of oleic acid (OA) [13]. However, the ability of an anionic lipid, OA, to elicit this transition also suggests that there may be other, yet to be established, lipid regulators capable of causing this transformation of Cygb under physiological conditions. With this in mind, we explored the effectiveness of several classes of major phospholipids, including several anionic phospholipids, in triggering peroxidase function of Cygb. Here we report that highly negatively charged anionic phospholipids (but not free fatty acids) are capable of activating both thiol-reduced and disulfide forms of Cygb into a peroxidase. The peroxidase activity can operate on exogenous phenolic substrates but also catalyze peroxidation of these polyunsaturated anionic phospholipids. Molecular dynamics studies revealed that disulfide formation can create a hydrophobic pocket enabling lipid binding. A bipartite binding model, with specific sites for the hydrophobic and the polar portions of the phospholipids can explain the observed effects.
2. MATERIALS AND METHODS
2.1. Reagents
Reagents were purchased from Sigma-Aldrich (St. Louis, MO) unless stated otherwise. Amplex Red (N-acetyl-3,7-dihydroxyphenoxazine) was obtained from Molecular Probes (Eugene, OR). DOPC, TOCL, PIP2, PIP3, PA, SAPA, SAPIP3 were purchased from Avanti Polar Lipids (Alabaster, AL).
2.2. Cloning, expression and purification of recombinant cytoglobin
Recombinant human Cygb was purified from E. coli as described [14]. In summary, the region encoding the human Cygb protein was cloned from the Origene (Rockville, MD) cDNA clone SC321813 (NM_134268) and inserted into the NcoI/HindIII restriction sites of the pET28a plasmid (Novagen) generating the pET28-HsaCygb plasmid. The plasmid was transformed into SoluBL21 E. coli cells (Genlantis). The truncations Δ1-18, Δ1-15, Δ174-190 and Δ1-18 Δ174-190, and the Cys38Ser/Cys83Ser mutations were introduced in the pET28-HsaCygb plasmid using the Quikchange mutagenesis kit (Stratagene) with adequate primers. The mutations were confirmed by DNA sequencing at the Genomics Research Core of the University of Pittsburgh.
Small scale (50-200 ml) overnight cultures in LB media were used to inoculate 4 liter flasks containing 1 l of TB (50 ml per liter of TB). The cultures were grown at 37 °C and induced at an OD of 0.8 at 600 nm. The induction was carried out by adding 1 mM IPTG; δ-aminolevulenic acid (400 μM) was also added to support bacterial heme synthesis. The proteins were purified by chromatography in Ni-NTA resin (Qiagen) according to the manufacturer instructions. An elution gradient of 10-200 mM Imidazole in the working buffer (50 mM sodium phosphate, pH 8.0, 300 mM NaCl) was used. The samples containing the Cygb, as monitored by the absorbance at 410 nm, were pooled and concentrated using Amicon ultra centrifugal devices with a 10 kDa MW cutoff (Millipore, Billerica, MA).
2.3. Lipid binding studies
The binding of OA to Cygb was assessed by differential spectroscopy as previously reported [13, 14]. The measurements were carried out in a Cary 50 spectrophotometer (Agilent Technologies, Palo Alto, CA). Experiments were carried out in 100 mM Sodium phosphate buffer, pH 7.4, at 25 °C. OA stocks of 200 μM were prepared in buffer from a 50 mM stock of OA in water right before use. The difference spectra were obtained by adding aliquots of the 200 μM OA stock to a solution of ferric Cygb (4-15 μM). The change in absorbance was fit to a 1:1 binding model according to the equation:
| (1) |
Where Δε is the change in the extinction coefficient between the initial, unbound species and the complex; P is the total concentration of protein; L is the total concentration of ligand, and KD is the dissociation constant of the protein-ligand complex.
2.4. Small unilamellar liposomes
Individual phospholipids, stored in chloroform, were mixed and dried under nitrogen. Then lipids were mixed in vortex in HEPES buffer (20 mM, pH 7.4) and sonicated five times for 30 s on ice. Liposomes were used immediately after preparation.
2.5. Peroxidase Activity measurements
Peroxidase activity of Cygb was assessed using a phenolic substrate, Amplex Red, by measuring the fluorescence of its oxidation product, resorufin, in the presence of hydrogen peroxide. Cygb (0.5 μM) was incubated with FAs or liposomes containing phospholipids. In the experiments where the protein was pretreated with DTT, the excess DTT was removed using spin-column Zeba Spin Desalting Columns (Thermo Scientific, Grand Island, NY). The thiol- reducing agents are able to reduce the heme iron as well, but due to the fast autoxidation rates of cytoglobin (k = 0.107 min-1; ferrous oxy (FeII-O2) species half-life ≈ 7 min at 25 °C [14]) the heme iron is quickly oxidized back to the ferric form once the reducing agent is removed. The reduced thiols oxidize at a much slower rate (See Supplementary Figure 2 and Supplementary Methods). Therefore the predominant state of the protein when thiol-reducing agents are used is ferric heme/reduced thiol. The reaction was started by addition of Amplex Red (100 μM) and H2O2 (100 μM) and was followed for 2 minutes. The reaction rate was linear in the entire time interval. Fluorescence signal was measured using a Shimadzu RF5301-PC spectrofluorometer. The excitation and emission wavelengths were 570 and 585 nm, respectively. Reactions were performed in HEPES buffer (20 mM, pH 7.4) in the presence of 100 μM DTPA at room temperature.
2.6. EPR Spectroscopy
EPR spectra of etoposide phenoxyl radical were recorded on a JEOL-REIX spectrometer with 100 kHz modulation (JEOL, Kyoto, Japan) at room temperature in gas permeable Teflon tubing (inner diameter, 0.8 mm; thickness, 0.013; Alpha Wire Corp., Elizabeth, NJ). The tubing was filled with 60 μL of sample, double-folded, and placed in an open 3.0 mm (internal diameter) EPR quartz tube. The spectra were recorded under the following EPR conditions: center field, 3350 G; sweep width, 50 G; microwave power, 10 mW; field modulation, 0.5 G; receiver gain, 2×103; time constant, 0.03 s; scan time, 8 min. The change of etoposide radical EPR signals was obtained by repeated scanning of the same part of the spectrum (3350 G centered field; 2.5 G sweep width; other instrumental conditions were the same). The time course was measured 1 min after the addition of 100 μM H2O2 to the 5 μM Cygb/phospholipid and 200 μM etoposide solution.
2.7. NEM treatment
Phosphate-buffered saline (PBS) containing 0.1M phosphate, 0.15M sodium chloride; pH 7.0 was used as a buffer. Cygb (150 μM) dissolved in PBS was incubated with 8 mM DTT for 1h at room temperature and excess of DTT was removed using Zeba Spin Desalting Columns (Thermo Scientific, Grand Island, NY). Eluent obtained after centrifugation through spin column was separated on two fractions. One fraction was used in experiments as DTT-treated Cygb, another part was used for further treatment with NEM. Alkylation with NEM (850 μM) was performed for 2 hours at room temperature and excess of NEM was removed by spin column. Concentration of native, DTT-treated and NEM-treated Cygb was assessed by absorbance at 413 nm.
2.8. LC-MS analysis of lipid oxidation products
The products of the reaction of Cygb with selected lipids in the presence of H2O2 were assessed by LC-MS as follows. 50 μl of a reaction mixture containing 50 μM lipid, 2 μM Cygb and 150 μM H2O2 were incubated for 1 hr in HEPES buffer (20 mM, pH 7.4) in the presence of 100 μM DTPA). LC-MS/MS analysis was performed using a Dionex Ultimate 3000 RSLC nano system coupled on-line to a Q-Exactive hybrid quadrupole-orbitrap mass spectrometer (ThermoFisher Scientific) using a C18 column (XTerra 2.5 μm, 125 Å, 50 × 2 mm (Waters)). For SAPIP3 analysis, an isocratic solvent system that consisted of acetonitrile/water/piperidine, 95/4.5/0.5 v/v, was used with the following flow rates; 0-2 minutes constant at 200 μl min−1, 2.0-2.5 minutes linearly increased from 200 to 500 μl min−1, 2.5–9.0 minutes constant at 500 μl min−1, 9-10 minutes linearly decreased to 200 μl min−1. For the analysis of SAPA and SAPC an isocratic solvent system that consisted of acetonitrile/isopropanol/water, 60/35/5 v/v with 10 mM ammonium acetate was used at the following flow rates; 0.0-8.0 minutes constant at 400 μl min−1, 8.0-8.5 minutes linearly increased to 500 μl min−1, 8.5-12.0 minutes constant at 500 μl min−1, 12.0-12.5 minutes linearly decreased to 400 μl min−1. Spectra were acquired in negative-ion mode using data-dependent mode with an inclusion list. The reaction mixtures were diluted 10 times by the corresponding solvent and 5 μl of the sample was utilized for the LC-MS/MS analysis.
2.9. Molecular Dynamics Simulations
To model the formation of the Cygb Cys38-Cys83 disulfide bond we used a Steered Molecular Dynamics (SMD) simulation to bring the thiol of the cysteine side chains together using the ferrous-CO Cygb crystal as the starting state (PDB ID: 3AG0). SMD simulations place a harmonic constraint on the distance between two specified atoms, in this case the sulfur atoms of the cysteine side chains. The center of the distance constraint changes throughout the course of the 10 ns simulation from a starting (5.3 Å) to an ending distance (1.5 Å). The final frame of this short simulation was used to start a new unconstrained simulation after forming the disulfide bond with tleap [15].
Both MD simulations were conducted with the pmemd.cuda [16] module of AMBER14 [15], using the force fields AMBER ff14SB and gaff (general AMBER force field) [17]. An octahedral TIP3P water box was constructed with 12 Å from the edge of the box to the solute and the total system charge was neutralized by adding chloride ions. The non-bonded cutoff was specified at 10 Å. In the first energy minimization run, the solute was held fixed and the solvent was relaxed through 500 cycles of steepest descent followed by 500 cycles of conjugate gradient minimization. Subsequently, the system was minimized again with no constraints through 2,000 cycles of steepest descent followed by 3,000 cycles of conjugate gradient minimization. Following the energy minimization, a 50,000 step MD simulation was used to raise the system temperature to 300 K while holding the solute fixed with weak (10.0 kcal/mol) restraints on the solute atoms. The bonds involving hydrogens were held at a fixed length and an integration step of 2 fs was used. This simulation was followed by a second equilibration simulation at constant pressure for 50,000 steps. The final MD simulation of this equilibrated structure was run with no constraints for 100 ns.
2.10. Molecular Docking
The binding mode of the lipids (OA, DOPA, PIP2 and PIP3) were modeled to snapshots of the unconstrained simulation of the modeled Cygb structure (with intramolecular disulfide bond) using the program smina [18]. The docking box was set to be larger than the size of the protein, i.e. global docking. Due to the much higher flexibility of the lipids being studied compared to typical small molecule inhibitors, the ‘exhaustiveness’ parameter, which controls the number of trial poses tested, was set to 50 (default: 8) and the number of returned poses (the ‘num_modes’ parameter) was also increased to 50 (default: 9).
3. RESULTS
3.1. Protein expression and purification
It has been proposed that the N-and C-terminal extensions of Cygb, not present in other globins, may play a role in lipid binding [13, 19]. In order to test this hypothesis, we produced mutant Cygbs including deletions of these putative lipid binding segments. According to the available crystal structures of Cygb [20, 21] we defined these fragments as aminoacids 1 to 18 (N-terminus) and 174-190 (C-terminus). We purified human wild-type and mutants of Cygb from E. coli cultures. Deletion of the 1-18 segment (Cygb Δ1-18) did not produce soluble protein, however the deletion of residues 1-15 (Cygb Δ1-15) was tolerated resulting in a soluble protein. Deletion of the residues 174-190 (Cygb Δ174-190) produced soluble protein when expressed alone or combined with the 1-15 deletion (Cygb Δ1-18 Δ174-190).
Previous studies indicate that the redox state of the human Cygb thiols - Cys38 and Cys83 - and the oligomerization state of the protein can influence lipid binding. To determine the oxidation state of the thiol groups and the oligomerization state of the protein we conducted gel filtration and electrophoresis studies (Supplementary Figures 1 and 2). The gel filtration profiles indicate that the proteins are present mostly in a monomeric state as shown by the peak around 15.5 ml (Supplementary Figure 1). To determine the oxidation state of the thiol groups and the presence or absence of the disulfide bond between Cys38 and Cys83 we ran the Cygb samples untreated or after incubation with increasing amounts of DTT. As shown in Supplementary Figure 2, the Cygb monomer forms two discernible bands that we ascribe to a difference in the thiol reduction state, in agreement with previous reports [11, 12]. The upper band corresponds to the protein with the reduced thiols, whereas the protein with the intramolecular disulfide bond formed gives a band at a lower molecular weight. The addition of DTT causes a shift in the Cygb sample from the lower band to the upper band that is almost complete after incubation with 5 mM DTT. Supplementary Figure 2. Altogether we conclude that our isolated Cygb was represented mostly by the monomeric protein with an intra-molecular disulfide bond; these protein preparations were used in experiments without further purification.
3.2. Oleic acid binding to wild type and truncated Cygbs
Given the postulated role of the N- and C- terminal extensions of Cygb in lipid binding [13, 19], we used the Cygb deletion mutants to assess their necessity and/or sufficiency in binding of OA (C18:1). Differential spectroscopy revealed that binding of lipids to the ferric Cygb induces a transition from the hexa-coordinate form (with His81 and His113 as distal ligands) to the penta-coordinate protein (with His81 displaced by a water molecule) [13]. We monitored the binding of OA to the wild type and to the truncated Cygb. In the case of the wild-type Cygb (Figure 1A, 1C), we observed a differential spectra similar to that reported by Reeder et al. [13], with a dissociation constant of 2.3 ± 1.0 μM. This is in reasonable agreement with the published values of 0.7 ± 0.03 μM. For the truncated protein without the N- and C-terminal extensions (Cygb Δ1-15 Δ174-190), very similar difference spectra virtually identical to the wild-type protein were obtained (Figure 1B, 1D). Our calculations indicate a dissociation constant of 3.0 ± 1.8 μM. We therefore conclude that the N- and C-terminal segments do not contribute significantly to lipid binding and may be involved in other interactions
Figure 1. Oleic acid binding to Cygb WT and Cygb Δ1-15Δ174-190.
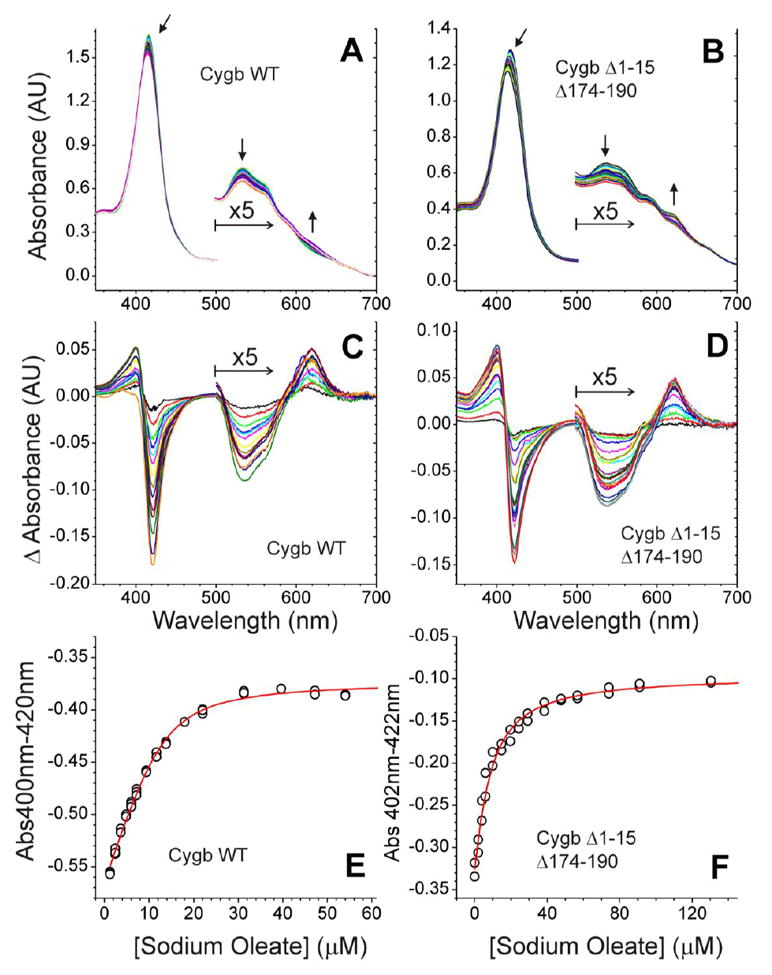
Panels A and B show the spectra after sequential addition of OA to 13 μM Cygb WT (A) or 11 μM Cygb Δ1-15Δ174-190 (B). The arrows indicate the direction of the spectral changes. Panels C and D show the difference spectra along the titration for Cygb WT (C) and Cygb Δ1-15Δ174-190 (D). The 500-700nm region of the spectra is enlarged for clarity. Panels E and F show the fit of the data to a 1:1 binding model (see methods for details). The averaged KD values calculated are 2.3 ± 1.0 μM for Cygb WT (E) and 3.0 ± 1.8 μM for Cygb Δ1-15Δ174-190 (F).
3.3. Effect of lipids and thiol oxidation on the peroxidase activity of cytoglobin
Earlier studies have not found noticeable differences in the binding affinities of OA and TOCL to Cygb [13]. This suggests that the binding may be regulated mostly by the FA chains rather than by the polar head-groups of the molecules. Assuming that physiologically relevant phospholipids with higher densities of negative charges may be more effective in altering the heme-iron coordination, we monitored the peroxidase activity of Cygb by the oxidation of Amplex Red. We compared the effects of two free FA (AA, OA) with diverse anionic phospholipids (DOPA, TOCL, PIP2, PIP3) using DOPC as a non-charged phospholipid (Figure 2). We found that Cygb peroxidase activity was modulated in a broad range by the interaction with different lipids revealing strong dependence on Cygb:lipid ratio (Figure 2). Unexpectedly, free FAs, including OA - the most studied Cygb lipid regulator [11, 13, 14] - appeared to be very weak activators of the peroxidase activity. Similar to AA and OA, the response to non-charged zwitter-ionic DOPC was low, with the peroxidase activities similar to that in the absence of lipids. Conversely, anionic phospholipids appeared to be strong activators of the Cygb peroxidase activity. For a 1:25 Cygb:lipid ratio, we observed many-fold increases in the peroxidase activity (up to ~5-fold in the case of PIP3). The increase in the activity correlated with the negative charges of the phospholipid and in the order (net charge in parentheses): DOPC (0) < DOPA (-1) < TOCL (-2) < PIP2 (-3) < PIP3 (-4). For the anionic phospholipids the effect was concentration dependent and increased with a greater negative charge. For a 1:25 Cygb:lipid ratio, the oxidation rate increased by a factor of 1.6 for DOPA, 2.3 for TOCL, 3.5 for PIP2 and 4.9 for PIP3 (Figure 2).
Figure 2. Effect of lipids on Amplex red oxidation by cytoglobin.
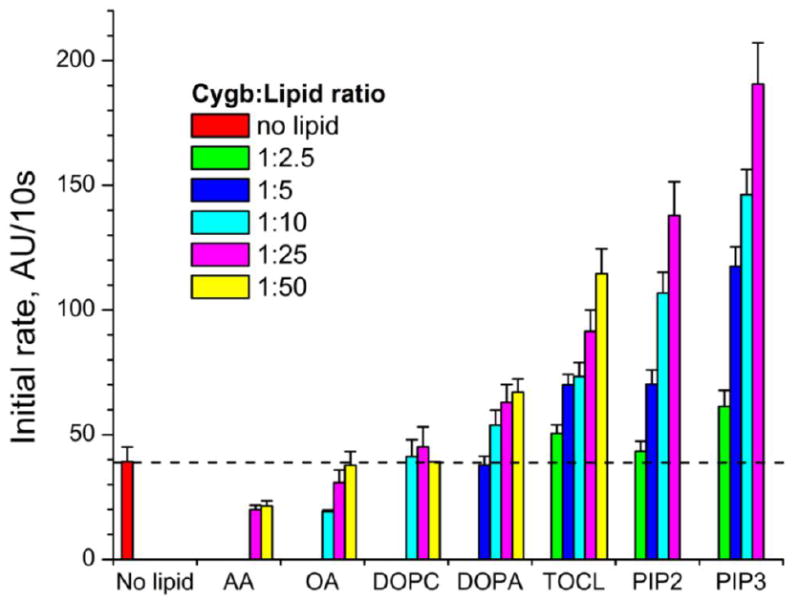
The peroxidase activity of Cygb was studied in the presence of free FAs (AA, OA) or liposomes containing neutral (DOPC) or anionic (DOPA, TOCL, PIP2, PIP3) phospholipids. The oxidation of the peroxidation substrate Amplex red was monitored following the fluorescence of its oxidation product resorufin. 0.5 μM Cygb was incubated with lipids in Cygb/lipid ratios from 1:2.5 to 1:50.
The role of a disulfide bond between Cys38 and Cys83 and lipid activation of Cygb is controversial: while it has been claimed essential for the binding of OA [11], around 25% of the oxidase activity of the wild-type protein, assayed as diene formation, was detected in the Cys38Arg mutant [11]. Therefore, we studied the effect of both disulfide bond reduction and anionic phospholipids on the peroxidase activity of Cygb (Figure 3). Treatment of Cygb with varying concentrations of DTT (0.3, 1.5 and 5 mM) yielded proportional amounts of Cygb species with no disulfide bond (~50%, 80% and 95%, respectively, Supplementary figure 2) and decreased peroxidase activity (Figure 3). In the absence of phospholipids, the thiol-reduced protein still retained ~ 20% of the peroxidase activity. Anionic phospholipids - DOPA and TOCL - were able to activate the fully thiol-reduced Cygb and caused a 2.5-fold (DOPA) and 4.4-fold (TOCL) increase in the rate of Amplex Red oxidation.
Figure 3. Effect of cytoglobin thiol reduction on Amplex red oxidation.
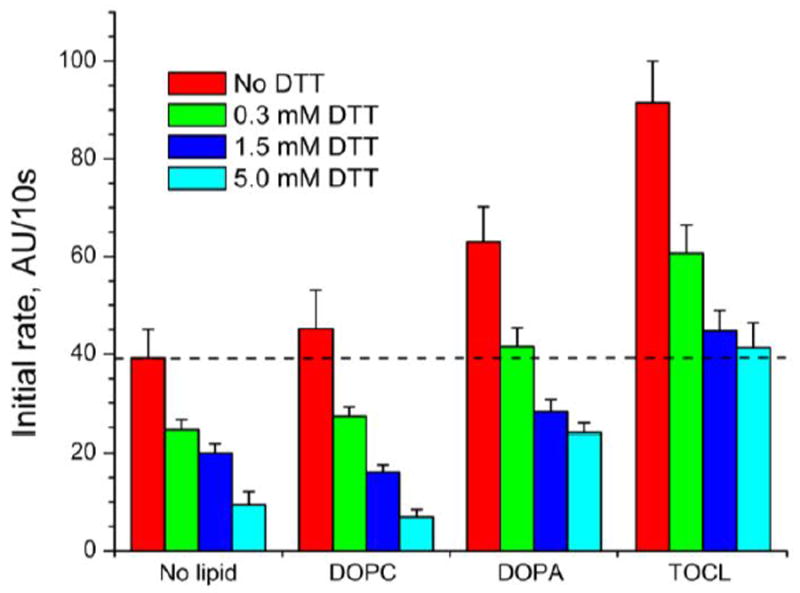
Cygb was pretreated with different concentrations of DTT to generate increasing amounts of the thiol-reduced protein. Excess DTT was removed before the reaction. The peroxidase activity of Cygb was studied in the absence of lipids or in the presence of DOPC, DOPA or TOCL. The oxidation of the peroxidation substrate Amplex red was monitored following the fluorescence of its oxidation product resorufin. 0.5 μM Cygb was incubated with 12.5 μM of each lipid.
To get further insights in the mechanisms of Cys38/Cys83 redox state-dependent peroxidase activation, we compared the rates of Amplex Red oxidation by native Cygb, thiol-reduced Cygb, NEM-treated Cygb and Cys38Ser/Cys83Ser mutant of Cygb (Figure 4, see also Supplementary Figures 3, 4 and Supplementary Material for details). Elimination of the disulfide bond by chemical reduction (5 mM DTT), covalent thiol modification (NEM) or site-directed mutagenesis (C38C/C83S) leads in all cases to a decrease in the peroxidase activity. This is in line with the data of Beckerson et al. on the role of the disulfide bond in lipid binding [11]. Anionic phospholipids were also able to increase the peroxidase activity of thiol-reduced Cygb (Figure 4).
Figure 4. Effect of thiol-modifying agents on Amplex red oxidation by cytoglobin.
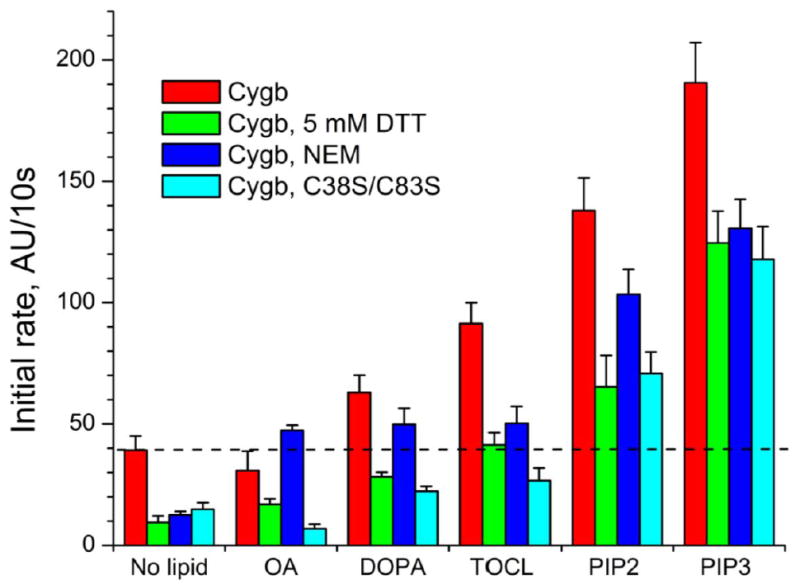
The peroxidase activity of Cygb with an intramolecular disulfide bond (WT, native), with reduced thiols (Cygb, 5mM DTT), with NEM-blocked thiols (Cygb, NEM), or a mutant Cygb without cysteine groups (Cygb, C38S/C83S) was studied using Amplex red as substrate. The reaction was monitored in the absence of lipids or in the presence of OA or anionic phospholids (DOPA, TOCL, PIP2, PIP3). 0.5 μM Cygb was incubated with 12.5 μM of each lipid.
Interestingly, DTT reduction and Cys38Ser/Cys83Ser mutations showed similar patterns of responses (Figure 4). The NEM-treated protein had a higher activity likely due to the conformational changes induced by the bulky NEM-moieties of alkylated Cys38 and Cys83 resulting in a weakening of the Heme-His81 (E7) interaction in Cygb. In summary, our results provide additional evidence about the role of the Cys38-Cys83 disulfide bond on lipid binding. It is also remarkable that in zebrafish globins, that do not conserve this cysteine couple, lipid binding is not detected at low concentrations. This result further stress the importance of the disulfide bond in the modulation of lipid binding [14].
3.4. Assessment of high-spin iron state
Unfolding of Cygb by anionic phospholipids and the transition of hexa-coordinated heme iron configuration into penta-coordinated state should cause the appearance of high-spin heme iron. Similarly to other hemoproteins such as Mb, Hb and Cyt c the presence of high spin iron in Cygb can be detected by the formation of a new relatively weak band at ca. 630 nm, a slight intensity increase at ca. 495 nm, and a more-pronounced shoulder at ca. 560 nm. [11, 13, 22-25].
In binding experiments, absorbance changes indicating lipid binding to the reduced thiol form can be observed for TOCL and PIP3, although the different shape of the difference spectra, especially in the Soret region, suggests that the binding mode is different in these conditions. Indeed, increasing amounts of TOCL and PIP3 caused spectral changes characteristic of high spin iron (Supplementary figure 5). Notably, PIP3 was more effective as an inducer of the high-spin iron formation than TOCL. Both lipids caused more robust formation of high-spin iron in Cygb treated with DTT. Interestingly, TOCL at low ratios to Cygb (5:1 and 10:1) increased oxidation of Amplex Red, without causing a detectable transition of iron to the high spin state.
3.5. EPR analysis of the peroxidase activity of Cygb
Peroxidase reactive intermediates, Compounds I or II, readily oxidize phenolic compounds via one-electron formation of phenoxyl radicals [26]. We further performed EPR spectroscopy of etoposide phenoxyl radicals generated from a typical phenolic substrate, etoposide, to independently assess the responses of Cygb to thiol oxidation/reduction and anionic phospholipids (Figure 5). We observed a markedly stronger increase (3.5-fold) in the generation of etoposide phenoxyl radicals by Cygb with the intramolecular disulfide bond as compared to the thiol-reduced protein (Figure 5C).
Figure 5. EPR assessments of the cytoglobin peroxidase activity in the presence of TOCL.
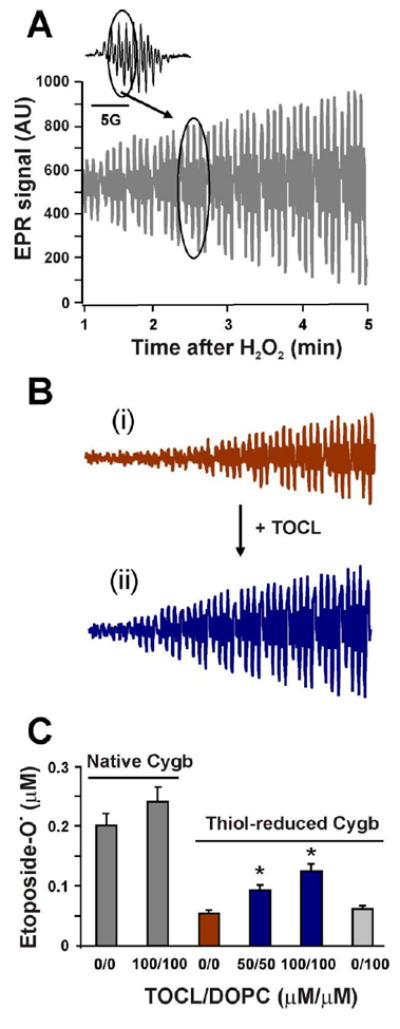
The peroxidase activity of Cygb with an intramolecular disulfide bond (Panel A) or after DTT-treatment (Panel B) was monitored by the formation of etoposide phenoxyl radicals. The inset shows the typical EPR signal of etoposide phenoxyl radicals. The encircled part of the spectrum was recorded repeatedly to obtain the time course of etoposide radical formation. Panel B, the EPR signal of thiol-reduced Cygb in the absence (i) or presence (ii) of TOCL is shown. Panel C, effect of lipids on etoposide radical generation by native or thiol-reduced Cygb measured 3 min after the addition of H2O2. 4 μM Cygb was incubated with different concentrations of lipids in 20 mM HEPES, 100 μM DTPA, pH 7.4 for 5 minutes. Measurements of the time course of etoposide radical formation were started 1 minute after the addition of H2O2 to the incubation mixture.
TOCL enhanced peroxidase activity of Cygb of both thiol-oxidized and thiol-reduced protein whereby the effect was significantly stronger in the latter than in the former (Figures 5B-C). To quantify the levels of etoposide radical formation we compared the integral intensity of the EPR spectrum of etoposide radicals formed in the peroxidase reaction (inset, Figure 5A) with that of the EPR signal from a stable nitroxide radical (tetramethyl-piperidine oxide (TEMPO), 2 μM) [26]. This evaluation showed that, under our experimental conditions, 100 AU (arbitrary units) of EPR signal corresponds to ~6.5×10-2 μM etoposide radicals.
3.6. Analysis of Cygb-dependent phospholipid oxidation by liquid chromatography/mass spectrometry (LC/MS)
In the absence of artificial exogenous substrates, peroxidases are known to utilize as their substrates either closely associated phospholipids or amino acid residues in the proximity of their catalytic site [27, 28]. Therefore, we further examined the effectiveness of Cygb in peroxidizing three biologically relevant phospholipids, SAPC, SAPA and SAPIP3. For comparison, we utilized three phospholipids with differently charged head groups but identical FA residues (stearic/arachidonic; net charge in parentheses): (SAPC (0) > SAPA (-1) > SAPIP3 (-4)). LC-MS/MS analysis demonstrated the accumulation of mono-, di-, tri-, and tetra-oxygenated species (Figure 6). The activity was many-fold higher with anionic phospholipids than with a zwitterionic non-charged phospholipid resulting in the accumulation of 5.6 ± 0.1 and 7.5 ± 0.6 picomoles of oxidation products for SAPA and SAPIP3, respectively, whereas only 0.2 ± 0.08 picomoles of oxidation products were formed from SAPC. The identity of lipid oxidation products was confirmed by fragmentation analysis (Figure 6 B-C).
Figure 6. LC-MS/MS analysis and quantitation of phospholipid oxidation by Cygb/H2O2 system.
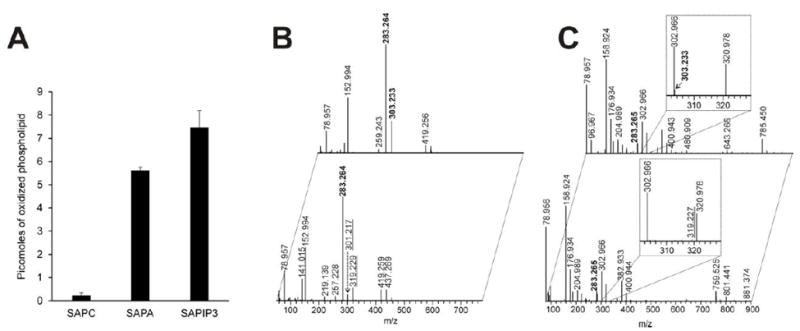
Panel A, the bar graph shows the amount of phospholipid oxidized by the Cygb/H2O2 system. In the presented values, control experiments (without Cygb) were subtracted (mean ± SD from three experiments). Panel B, confirmation of SAPA (top spectrum) and the oxidized SAPA (bottom spectrum) by MS2 fragmentation. The bold labelled m/z indicates FA fragments (283.264-stearoyl, 303.233-arachidonoyl). The underlined m/z indicates an oxidized arachidonoyl fragment (319.227) and a fragment representing a lost water molecule from the oxidized arachidonoyl fragment (301.127). Panel C, confirmation of SAPIP3 (top spectrum) and oxidized SAPIP3 (bottom spectrum) by MS2 fragmentation. Labels are as indicated for panel B.
Mono-oxygenation of arachidanoyl residues (m/z 302.966) in SAPA and SAPIP3 was documented by the fragments with m/z of either 319.227 or 301.127 (resulting from water loss). Thus, we show that Cygb can directly and effectively peroxidize anionic lipids.
3.7. Molecular modeling of the cytoglobin-lipid interaction
To date, the location of the lipid binding site in Cygb is unknown. As we have discussed above, previous studies hypothesized a role of the N- and C-terminal extension in lipid binding [13, 19]. Our studies with the truncated Cygb Δ1-15Δ174-190 indicate that this is not the case.
On the other hand, our data suggests that the binding site should be within the globin core, for which structural information is available. However, most Cygb structures have been solved for cysteine free Cygb mutants. The structure of Cygb with an intramolecular disulfide bridge has not been yet described, although a model has been reported [29]. We thus used molecular dynamics (MD) to model the Cygb structure with the intramolecular disulfide bridge.
The MD model for the Cygb monomer with an intramolecular disulfide bond is shown in Figure 7. The most striking feature of this structure is the large displacement of the E helix, breaking the His81(E7)-Fe interaction and leading to a penta-coordinated heme. A similar, although less dramatic change was observed in the Cygb crystal structure [21] where one of the monomers shows a hexa-coordinated structure, whereas the other is penta–coordinated showing a slight displacement of the E-helix with a separation of the His81 from the heme. Although the ferric state of Cygb is in general hexa-coordinated at neutral pH [8, 13], this structure can represent the penta-coordinate structure of the ferric protein with a disulfide bond. Differential spectroscopy studies indicate that the binding of lipids to Cygb leads to a change from hexa-coordinate to a penta-coordinate structure [11, 13, 14]. Thus we considered that the MD model was a reasonable substrate to model the binding of lipids to Cygb.
Figure 7. Formation of disulfide bond between Cys38 and Cys83 induces the bending of the E helix and an increase of the distance between E and F helices.
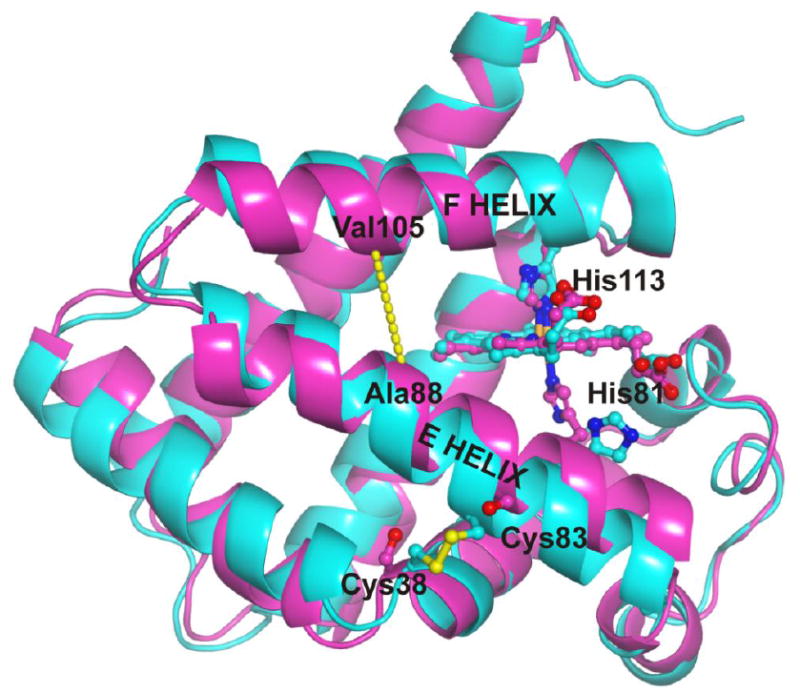
The figure shows the overlay of the hexa-coordinated crystal structure of the C38S/C83S mutant of human cytoglobin (purple, PDB ID: 1UT0) with a model of the penta-coordinated, ferric wild-type structure (cyan). The yellow dashed line indicates the distance between the alpha carbons of Ala88 (E helix) and Val105 (F helix). Molecular dynamics simulations indicate that the formation of the disulfide bond causes an increase in this interhelical distance (8.04 ± 0.64 Å vs 7.2 Å, respectively), allowing for the binding of hydrophobic chains in the hydrophobic core of Cygb.
The lower energy models for the binding of OA, DOPA and PIP3 to Cygb are shown in Figure 8. The models indicate that when the intramolecular disulfide bond is formed, the rearrangement of the E helix creates an internal cavity in the globin hydrophobic core can be accessed from the protein surface. Thus the FA moiety can access this hydrophobic site, making the penta-coordinate state more stable. The three lipids can be docked with their FA chains occupying this cavity in very similar orientations (Figure 8). In addition, the polar head of the lipid can contribute other interactions that may lead to lipid specific effects. For OA, the carboxylate group can interact with the solvent in the protein surface, while making a hydrogen bond with the Thr91 residue (Figures 8A-B). In the case of DOPA and PIP3, additional interactions with the protein are formed. The phosphate group of DOPA can be stabilized by interactions with Arg84 and Thr91 (Figures 8C-D). For PIP3, the residues Arg84 and Thr91 also provide interactions with oxygen atoms, whereas the phosphate groups can be stabilized by Lys111 and Lys116 (Figures 8E-F). We also note that the structural differences between PIP2 and PIP3 are due to the phosphate group stabilized by Lys116, which can explain the different effects of both phospholipids.
Figure 8. Binding models for cytoglobin and OA, DOPA and PIP3.
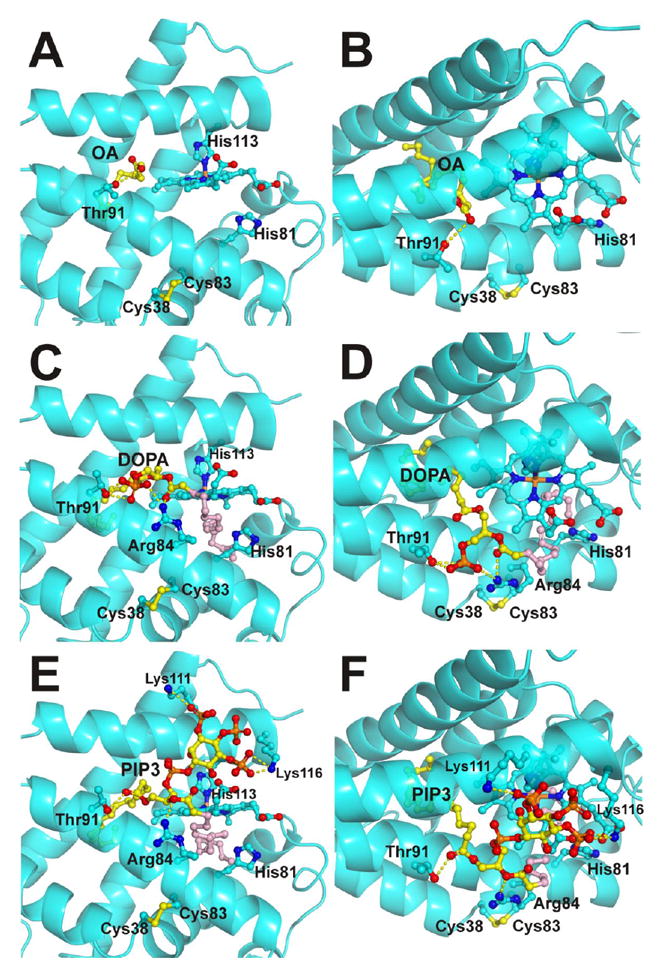
Panels A and B, predicted binding model for OA. The aliphatic chain extends into the hydrophobic core of the protein; the carboxylate group interacts with the Thr91 side chain. Panel A shows the view from the heme plane. Panel B shows a top view. Panels C and D, predicted binding model for DOPA. Panel C, view from the heme plane; Panel D, top view. The model shows Thr91 and Arg84 stabilizing the phosphate group of DOPA; one oleic chain extends into the hydrophobic core of the protein (yellow) whereas the other hydrophobic chain is either interacting with the solvent or occupying a secondary binding site in the distal heme pocket (pink). Panels E and F, predicted binding model for PIP3. Panel E, view from the heme plane; Panel F, top view. The phosphate groups of PIP3 are shown interacting with Lys111 and Lys116. Arg84 and Thr91 interact with the ester groups from the FA chains. The phosphate group missing in PIP2 is the phosphate group interacting with Lys 116. As observed in the DOPA models, one oleic chain extends into the hydrophobic core of the protein (yellow) whereas the other hydrophobic chain is either interacting with the solvent or occupying a secondary binding site in the distal heme pocket (pink). The lipids, heme group and selected Cygb residues are shown as ball and sticks. Lipid carbon atoms are shown in yellow/pink, heme and Cygb side chain carbon atoms are shown in cyan.
We also observed the presence of a secondary binding site for the FA in the heme distal pocket (shown in pink in Figure 8C-F). Our molecular docking models yield a lower support for this secondary site. However, the location of this site in close proximity to the heme iron makes it interesting in terms of lipid oxidation processes. As OA binding experiments are consistent with a 1:1 stoichiometry [13] (Figure 1), there are two possible interpretations for a two-site model: i) the sites have very different binding affinities, or ii) one of the sites may not induce spectral changes large enough to be detectable spectroscopically.
In the absence of thiol-disulfide formation, the FA binding was not detected. However, the anionic moieties of PIP2 and PIP3 can establish contacts with Cygb in a very similar fashion to that observed for Cygb when the disulfide bond is formed, in agreement with the experimental results (Supplementary Figure 6).
4. DISCUSSION
This work describes, for the first time, that anionic phospholipids, at physiologically relevant concentrations, can awaken the dormant peroxidase activity of Cygb, i.e. can act as regulators of this important redox function of the protein. This effect of anionic lipids is realized via triggering structural re-arrangements of the protein leading to its transition from the hexa-coordinated to penta-coordinated heme-iron state. Since the discovery of Cygb in 2001, several studies have documented the importance of the thiol to disulfide conversions of Cys38/Cys83 in controlling heme coordination and the binding of diatomic ligands [9, 12, 30] as well as lipids [10, 11], thus emphasizing possible redox mechanisms of its regulation. Recently, the peroxidase activity of Cygb has been described [31], albeit at supra-physiological concentrations of H2O2 [31]. Furthermore, OA binding is dependent on the redox status of Cys38/Cys83 and occurs only in the protein with intra-molecularly cross-linked disulfide with already pre-existing significant peroxidase activity of Cygb [13, 31, 32]. Essentially, this indicates that OA may act only as a co-activator of the peroxidase function of Cygb. Our data demonstrate that – in addition to this effect – lipids can enhance the peroxidase activity of Cygb, and in particular highly negatively charged anionic phospholipids are capable of peroxidase activation of the protein independently of the redox status of Cys38/Cys83, i.e., act as activators at physiologically relevant concentrations, probably through interaction with both reduction states of Cygb.
In the absence of structural information on the lipid binding to Cygb, the relationship between the formation of the Cys38/Cys83 disulfide and OA binding has been puzzling. Moreover, the difficulties to crystallize Cygb with an intact disulfide bond make it complex to address the issue by structural techniques. Here we present a computational model that can explain satisfactorily the long range effects of the disulfide bond on lipid binding, and provides a link between lipid binding and the hexa-coordinate to penta-coordinate transition. This model predicts the insertion of the FA moiety in the hydrophobic core of Cygb, mediated by the formation of the disulfide bond. The binding models for OA, DOPA and PIP3 suggest that lipid binding may follow a bipartite model, where the FA chain and the anionic portion of the phospholipid can bind in a partially independent fashion. The binding of the FA chain is dependent on some flexibility of the globin core, which is in turn regulated by the formation of the intramolecular disulfide bond. The location of the FA chain inside the hydrophobic core of the Cygb, but in close proximity of the heme, opens the possibility of a direct peroxidation of the bound lipid. Although there is no structural data available for the monomeric, intramolecular disulfide-bond cytoglobin structure, a model was recently reported [29]. The structural changes in the E helix due to disulfide bond formation are not large in the model but that may be related to the energy minimization methods used, which limit the structural movement. However, it is interesting that even after small structural changes the authors did observe the formation of new cavities close to the heme in both distal and proximal sides [29]. The location of these new cavities appears to be consistent with part of the primary and secondary binding sites observed in our lipid binding models.
Interestingly, the shift to a high spin state did not seem to correlate with the lipid-associated increase in peroxidase activity of the protein. Indeed, OA induced the changes in the spin state at concentrations that were ineffective in changing the peroxidase activity. Conversely, TOCL-dependent induction of the peroxidase activity was not accompanied by the transition to the high spin state of the heme iron. In contrast, PIP3 induced both the increase in peroxidase activity and the transition to high spin iron of Cygb. This lack of connection between the electronic structure and redox enzymatic activity of the protein can be rationalized within the context of the “hyper-oxidized” oxoferryl state of iron by the insertion of oxygen upon interaction of the hemoprotein with H2O2 [33]. Thus there are distinct differences in the requirements for the access to a source of oxidizing equivalents (H2O2) and for the necessary rearrangements of the electronic structure of the hemoprotein.
Our experimental data and the model indicate that the polar portion of the lipid can bind independently of the disulfide bond formation. This binding is apparently weaker and leads to a lesser activation of the peroxidase activity (Figure 9). Remarkably, the binding model for PIP3 is different from the common patterns of protein interaction employed by this ligand. While the general binding of PIP3 involves a number of interactions between the inositol moiety and the receptor, usually a pleckstrin homology domain [34, 35], the mode of binding presented here, using both the inositol ring and the FA chain, would be a unique interaction mode for phosphatidyl inositides.
Figure 9. Bipartite binding model for cytoglobin-lipid binding.
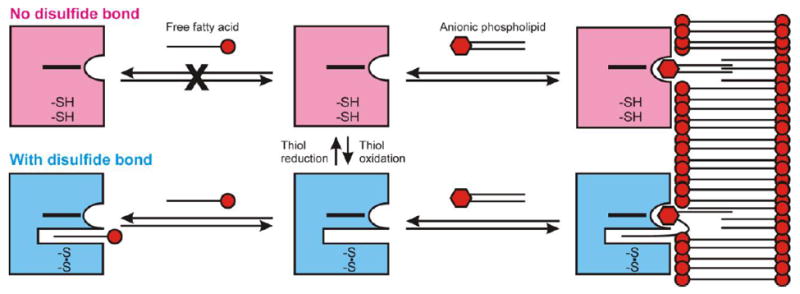
The binding of aliphatic chains to Cygb is regulated by the formation of the disulfide bridge between Cys38 and Cys83. The state of the disulfide bond is regulated by the cell environment and can shift between the free thiol conformations (pink) and the disulfide bond state (blue). Free FAs may bind to Cygb only when the disulfide bond is formed (bottom left). Anionic phospholipids may bind to the cytoglobin surface independently of the formation of the disulfide bond (right side). The formation of the disulfide bond allows for the additional binding of one phospholipid FA chain, increasing the affinity for the lipid.
The large increase of the Cygb peroxidase activity by PIP2 and PIP3 poses new alternatives for the yet unknown physiological roles of Cygb. Although PIP2 and PIP3 constitute less than 1% of the total membrane lipids, they are critical regulators of a number of cellular processes [36, 37]. PIP2 and PIP3 are critical components of the Phosphoinositol 3-kinase/ Protein kinase B (PI3K/Akt) signaling pathway, a main regulator of cell proliferation and survival, and a conspicuous pathway in the survival of cancer cells [38]. Oxidative stress increases the amount of PIP3 and the PIP3/PIP2 ratio, both by decreasing PIP3 dephosphorylation by PTEN [39] and increasing the rate of PIP2 phosphorylation [40]. Hypoxia also increases PI3K activity [41]. Similar trends have been observed for Cygb levels in cells, with increases in Cygb expression under hypoxia [42] and H2O2-induced oxidative stress [43]. Thus a possible signaling through a Cygb/PIP3 interaction would be amplified under stress conditions. In the light of the growing evidence that links Cygb and cancer [44-47], the possible relevance of Cygb in the PI3K/Akt pathway through lipid peroxidation or other mechanisms deserves further study
It is also notable that this bipartite binding could work as a mechanism to effectively target Cygb to the cell membrane, and work as a palmitoylation or myristoylation. As Cygb is generally located in either the cytoplasm or the nucleus [48, 49] we hypothesize that the cytoplasmic fraction would be the main target of such lipid-mediated regulatory mechanism. At the same time that the Cygb is interacting with the anionic phospholipid head group, the lipid would be still anchored to the membrane by one or two FA chains, effectively mobilizing the Cygb to the membrane (Figure 9). Other globins evolutionary related to Cygb, such as globin X, have N-terminal acylation motifs [50, 51]. In turn, it has been proposed that the common ancestor to the globins in the Cygb/Ngb/Mb/Hb family may have been a membrane-bound globin [52]. However, these palmitoylation/myristoylation motifs are not present in the human cytoglobin sequence as assessed from bioinformatics analysis (Myristoylator server [53], CSS-Palm 4.0 server [54]).
The binding of lipids by globins is infrequent but several examples have been reported. Bacterial flavohemoglobins (flavoHbs) can bind not only FAs but also phospholipids [55]. These interactions can mediate membrane binding [56]. It is notable that lipid binding in flavoHb also involves a large cavity in the distal side of the heme pocket. Interestingly, our model is comparable with the reported structure of the Ralstonia eutropha (A. eutrophus) flavoHb. In the latter case, two fatty acid chains occupy two sites in the distal side of the heme pocket, with one of the FA chains in close proximity to the heme iron. These two sites resemble the two FA binding sites observed in our models (Supplementary Figure 7) [55]. Importantly, the structures of R. eutropha flavoHb in the presence of azole inhibitors bound to the heme iron show a single FA chain at the site farther from the iron, indicating that these sites can be occupied independently [57] . In the case of mammalian globins, it has been reported that myoglobin can bind palmitic acid and OA with low affinity [58]. Although crystal structures are not available, a computational model of the binding of palmitic acid to myoglobin has been recently reported. In this model, palmitic acid occupies a site in the distal pocket and interacts with the heme-bound oxygen molecule [59]. In summary, we note that without imposing any homology constraints on the lipid binding modeling, we have achieved a solution that is consistent with the experimental phospholipid binding model reported for other globins.
Arachidonoyl-containing phosphatidylinositols are well known as biosynthetic precursors of lipid mediators, particularly eicosanoids [60, 61]. This pathway includes sequential hydrolysis by one of Ca2+-dependent phospholipases A2 followed by the oxygenation of the released arachidonic acid by cyclooxygenases or lipoxygenases. Recently, an alternative Ca2+-independent process has been proposed whereby the oxygenation stage occurs within the phospholipid molecule that subsequently undergoes hydrolysis by a Ca2+-independent phospholipase A2 to yield eicosanoids [27, 28]. It has been documented that this mechanism can be realized in mitochondria via the oxygenation of polyunsaturated cardiolipins. The initiating stage is catalyzed by the mitochondrial hemoprotein Cyt c [5]. Normally, Cyt c is present in the hexa-coordinated form and functions as an electron shuttle between the respiratory complexes III and IV [2]. Upon binding of a mitochondria specific anionic phospholipid, cardiolipin (CL), the hemoprotein undergoes partial unfolding causing the weakening of the iron liganding with Met80 and conversion into the penta-coordinated state [62]. These structural re-arrangements confer the peroxidase catalytic competence on the protein resulting - in the presence of sources of oxidizing equivalents - in oxygenation of CL [5]. It is tempting to speculate that anionic phospholipids, particularly PIPs, by triggering similar hexa-penta coordinate transitions in Cygb will convert the hemoprotein into a cytosolic peroxidase with a possible role in generating oxygenated lipid mediators. In this case, the necessary hydrolysis stage can be fulfilled by one of Ca2+-independent cytosolic phospholipases A2 (iPLA2 or platelet activating factor hydrolase [63]. Further studies will unravel the validity and contribution of this proposed mechanism into the overall production of lipid mediators under conditions facilitating expression of Cygb in the cytosol.
Supplementary Material
HIGHLIGHTS.
Peroxidase activity of cytoglobin is modulated by lipids.
Anionic phospholipids interact with cytoglobin independently of cysteine oxidation.
Anionic phospholipids cause the largest increases in cytoglobin peroxidase activity.
A computational model for the binding of anionic lipids to cytoglobin is proposed.
Acknowledgments
We want to thank Venkata Ragireddy and Rheanne Gatmaitan for excellent technical support. This work was supported by funding from the Institute for Transfusion Medicine and the Hemophilia Center of Western Pennsylvania (to M.T.G.) and by National Institutes of Health Grants GM097082 (to C.J.C), NS076511, NS061817, U19AI068021 (to H.B.), P01 HL114453, U19AI068021, ES020693; NS076511, CA165065 (to V.E.K.), and the Human Frontier Science Program (HFSP-RGP0013/2014) (to V.E.K.).
ABBREVIATIONS
- AA
arachidonic acid
- Cygb
cytoglobin
- Cyt c
cytochrome c
- DOPA
dioleyl phosphatidic acid (1,2- dioleoyl-sn-glycero-3-phosphate)
- DOPC
dioleyl phosphatidyl choline (1,2 Dioleoyl-sn-glycero-3-phosphocholine)
- DTPA
diethylenetriaminepentaacetic acid
- DTT
dithiotreitol
- FA
fatty acid
- HEPES
4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid
- MD
molecular dynamics
- NEM
N-Ethylmaleimide
- Ngb
neuroglobin
- OA
oleic acid
- PIP2
dioleyl phosphatidyl inositol 4,5-bisphosphate (1,2-dioleoyl-sn-glycero-3-phosphoinositol 4,5-bisphosphate)
- PIP3
dioleyl phosphatidylinositol 3,4,5-trisphosphate (1,2-dioleoyl-sn-glycero-3-phosphoinositol 3,4,5-trisphosphate)
- SAPA
stearoyl-arachidonoyl phosphatidic acid (1-stearoyl-2-arachidonoyl-sn-glycero-3-phosphate)
- SAPC
stearoyl-arachidonoyl phosphatidyl choline (1-stearoyl-2-arachidonoyl-sn-glycero-3-phosphocholine)
- SAPIP3
stearoyl-arachidonoyl phosphatidylinositol (3,4,5)-trisphosphate (1-stearoyl-2-arachidonoyl-sn-glycero-3-phosphoinositol 3,4,5-trisphosphate)
- SDS
sodium dodecyl sulphate
- TEMPO
2,2,6,6-tetramethylpiperidinyl-1-oxy
- TOCL
tetraoleyl cardiolipin (1,1′,2,2′-tetraoleoylcardiolipin)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Reeder BJ. The redox activity of hemoglobins: from physiologic functions to pathologic mechanisms. Antioxid Redox Signal. 2010;13:1087–123. doi: 10.1089/ars.2009.2974. [DOI] [PubMed] [Google Scholar]
- 2.Kagan VE, Bayir HA, Belikova NA, Kapralov O, Tyurina YY, Tyurin VA, Jiang J, Stoyanovsky DA, Wipf P, Kochanek PM, Greenberger JS, Pitt B, Shvedova AA, Borisenko G. Cytochrome c/cardiolipin relations in mitochondria: a kiss of death. Free Radic Biol Med. 2009;46:1439–53. doi: 10.1016/j.freeradbiomed.2009.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pesce A, De Sanctis D, Nardini M, Dewilde S, Moens L, Hankeln T, Burmester T, Ascenzi P, Bolognesi M. Reversible hexa- to penta-coordination of the heme Fe atom modulates ligand binding properties of neuroglobin and cytoglobin. IUBMB Life. 2004;56:657–64. doi: 10.1080/15216540500078830. [DOI] [PubMed] [Google Scholar]
- 4.Genova ML, Lenaz G. Functional role of mitochondrial respiratory supercomplexes. Biochim Biophys Acta. 2014;1837:427–43. doi: 10.1016/j.bbabio.2013.11.002. [DOI] [PubMed] [Google Scholar]
- 5.Kagan VE, Tyurin VA, Jiang J, Tyurina YY, Ritov VB, Amoscato AA, Osipov AN, Belikova NA, Kapralov AA, Kini V, Vlasova II, Zhao Q, Zou M, Di P, Svistunenko DA, Kurnikov IV, Borisenko GG. Cytochrome c acts as a cardiolipin oxygenase required for release of proapoptotic factors. Nat Chem Biol. 2005;1:223–32. doi: 10.1038/nchembio727. [DOI] [PubMed] [Google Scholar]
- 6.Belikova NA, Vladimirov YA, Osipov AN, Kapralov AA, Tyurin VA, Potapovich MV, Basova LV, Peterson J, Kurnikov IV, Kagan VE. Peroxidase activity and structural transitions of cytochrome c bound to cardiolipin-containing membranes. Biochemistry. 2006;45:4998–5009. doi: 10.1021/bi0525573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dewilde S, Kiger L, Burmester T, Hankeln T, Baudin-Creuza V, Aerts T, Marden MC, Caubergs R, Moens L. Biochemical characterization and ligand binding properties of neuroglobin, a novel member of the globin family. J Biol Chem. 2001;2763:8949–55. doi: 10.1074/jbc.M106438200. [DOI] [PubMed] [Google Scholar]
- 8.Trent JT, 3rd, Hargrove MS. A ubiquitously expressed human hexacoordinate hemoglobin. J Biol Chem. 2002;2771:9538–45. doi: 10.1074/jbc.M201934200. [DOI] [PubMed] [Google Scholar]
- 9.Hamdane D, Kiger L, Dewilde S, Green BN, Pesce A, Uzan J, Burmester T, Hankeln T, Bolognesi M, Moens L, Marden MC. The redox state of the cell regulates the ligand binding affinity of human neuroglobin and cytoglobin. J Biol Chem. 2003;2785:1713–21. doi: 10.1074/jbc.M309396200. [DOI] [PubMed] [Google Scholar]
- 10.Beckerson P, Reeder BJ, Wilson MT. Coupling of disulfide bond and distal histidine dissociation in human ferrous cytoglobin regulates ligand binding. FEBS Lett. 2015;589:507–12. doi: 10.1016/j.febslet.2015.01.010. [DOI] [PubMed] [Google Scholar]
- 11.Beckerson P, Wilson MT, Svistunenko DA, Reeder BJ. Cytoglobin ligand binding regulated by changing haem-co-ordination in response to intramolecular disulfide bond formation and lipid interaction. Biochem J. 2015;465:127–37. doi: 10.1042/BJ20140827. [DOI] [PubMed] [Google Scholar]
- 12.Tsujino H, Yamashita T, Nose A, Kukino K, Sawai H, Shiro Y, Uno T. Disulfide bonds regulate binding of exogenous ligand to human cytoglobin. J Inorg Biochem. 2014;135:20–7. doi: 10.1016/j.jinorgbio.2014.02.011. [DOI] [PubMed] [Google Scholar]
- 13.Reeder BJ, Svistunenko DA, Wilson MT. Lipid binding to cytoglobin leads to a change in haem co-ordination: a role for cytoglobin in lipid signalling of oxidative stress. Biochem J. 2011;434:483–92. doi: 10.1042/BJ20101136. [DOI] [PubMed] [Google Scholar]
- 14.Corti P, Ieraci M, Tejero J. Characterization of zebrafish neuroglobin and cytoglobins 1 and 2: Zebrafish cytoglobins provide insights into the transition from six-coordinate to five-coordinate globins. Nitric Oxide. 2016;53:22–34. doi: 10.1016/j.niox.2015.12.004. [DOI] [PubMed] [Google Scholar]
- 15.Case DA, Babin V, Berryman JT, Betz RM, Cai Q, Cerutti DS, Cheatham TE, Darden TA, Duke RE, Gohlke H, Gotz AW, Gusarov S, Homeyer N, Janoski P, Kaus J, Kolossvary I, Kovalenko A, Lee TS, LeGrand S, Luchko T, Luo R, Madej B, Merz KM, Paesani F, Roe DR, Roitberg A, Sagui C, Salomon-Ferrer R, Seabra G, Simmerling C, Smith W, Swails J, Walker RC, Wang J, Wolf RM, Wu X, Kollman PA. AMBER. Vol. 14. University of California; San Francisco: 2014. [Google Scholar]
- 16.Gotz AW, Williamson MJ, Xu D, Poole D, Le Grand S, Walker RC. Routine Microsecond Molecular Dynamics Simulations with AMBER on GPUs. 1. Generalized Born. Journal of chemical theory and computation. 2012;8:1542–1555. doi: 10.1021/ct200909j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang J, Wolf RM, Caldwell JW, Kollman PA, Case DA. Development and testing of a general amber force field. Journal of computational chemistry. 2004;25:1157–74. doi: 10.1002/jcc.20035. [DOI] [PubMed] [Google Scholar]
- 18.Koes DR, Baumgartner MP, Camacho CJ. Lessons learned in empirical scoring with smina from the CSAR 2011 benchmarking exercise. Journal of chemical information and modeling. 2013;53:1893–904. doi: 10.1021/ci300604z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ascenzi P, Marino M, Polticelli F, Coletta M, Gioia M, Marini S, Pesce A, Nardini M, Bolognesi M, Reeder BJ, Wilson MT. Non-covalent and covalent modifications modulate the reactivity of monomeric mammalian globins. Biochim Biophys Acta. 2013;1834:1750–6. doi: 10.1016/j.bbapap.2013.02.012. [DOI] [PubMed] [Google Scholar]
- 20.Makino M, Sugimoto H, Sawai H, Kawada N, Yoshizato K, Shiro Y. High-resolution structure of human cytoglobin: identification of extra N- and C-termini and a new dimerization mode. Acta Crystallogr D Biol Crystallogr. 2006;62:671–7. doi: 10.1107/S0907444906013813. [DOI] [PubMed] [Google Scholar]
- 21.de Sanctis D, Dewilde S, Pesce A, Moens L, Ascenzi P, Hankeln T, Burmester T, Bolognesi M. Crystal structure of cytoglobin: the fourth globin type discovered in man displays heme hexa-coordination. J Mol Biol. 2004;336:917–27. doi: 10.1016/j.jmb.2003.12.063. [DOI] [PubMed] [Google Scholar]
- 22.Munro OQ, Marques HM. Heme-Peptide Models for Hemoproteins. 1. Solution Chemistry of N-Acetylmicroperoxidase-8. Inorg Chem. 1996;35:3752–3767. doi: 10.1021/ic9502842. [DOI] [PubMed] [Google Scholar]
- 23.Antonini E, Brunori M. Chapter 3: The derivatives of ferric hemoglobin and myoglobin in Hemoglobin and Myoglobin in their reactions with ligands. North Holland, Amsterdam: 1971. pp. 40–54. [Google Scholar]
- 24.Carraway AD, McCollum MG, Peterson J. Characterization of N-Acetylated Heme Undecapeptide and Some of Its Derivatives in Aqueous Media: Monomeric Model Systems for Hemoproteins. Inorg Chem. 1996;35:6885–6891. doi: 10.1021/ic960434o. [DOI] [PubMed] [Google Scholar]
- 25.Jiang J, Bakan A, Kapralov AA, Silva KI, Huang Z, Amoscato AA, Peterson J, Garapati VK, Saxena S, Bayir H, Atkinson J, Bahar I, Kagan VE. Designing inhibitors of cytochrome c/cardiolipin peroxidase complexes: mitochondria-targeted imidazole-substituted fatty acids. Free Radic Biol Med. 2014;71:221–30. doi: 10.1016/j.freeradbiomed.2014.02.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tyurina YY, Kini V, Tyurin VA, Vlasova II, Jiang J, Kapralov AA, Belikova NA, Yalowich JC, Kurnikov IV, Kagan VE. Mechanisms of cardiolipin oxidation by cytochrome c: relevance to pro- and antiapoptotic functions of etoposide. Mol Pharmacol. 2006;70:706–17. doi: 10.1124/mol.106.022731. [DOI] [PubMed] [Google Scholar]
- 27.Tyurina YY, Poloyac SM, Tyurin VA, Kapralov AA, Jiang J, Anthonymuthu TS, Kapralova VI, Vikulina AS, Jung MY, Epperly MW, Mohammadyani D, Klein-Seetharaman J, Jackson TC, Kochanek PM, Pitt BR, Greenberger JS, Vladimirov YA, Bayir H, Kagan VE. A mitochondrial pathway for biosynthesis of lipid mediators. Nature chemistry. 2014;6:542–52. doi: 10.1038/nchem.1924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Delporte C, Van Antwerpen P, Vanhamme L, Roumeguere T, Zouaoui Boudjeltia K. Low-density lipoprotein modified by myeloperoxidase in inflammatory pathways and clinical studies. Mediators of inflammation. 2013;2013:971579. doi: 10.1155/2013/971579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Astudillo L, Bernad S, Derrien V, Sebban P, Miksovska J. Reduction of the internal disulfide bond between Cys 38 and 83 switches the ligand migration pathway in cytoglobin. J Inorg Biochem. 2013;129:23–9. doi: 10.1016/j.jinorgbio.2013.08.006. [DOI] [PubMed] [Google Scholar]
- 30.Astudillo L, Bernad S, Derrien V, Sebban P, Miksovska J. Probing the role of the internal disulfide bond in regulating conformational dynamics in neuroglobin. Biophys J. 2010;99:L16–8. doi: 10.1016/j.bpj.2010.04.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Beckerson P, Svistunenko D, Reeder B. Effect of the distal histidine on the peroxidatic activity of monomeric cytoglobin. 2015;4:87. doi: 10.12688/f1000research.5971.1. [v1; ref status: indexed http://f1000r.es/4xg] F1000Research. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kawada N, Kristensen DB, Asahina K, Nakatani K, Minamiyama Y, Seki S, Yoshizato K. Characterization of a stellate cell activation-associated protein (STAP) with peroxidase activity found in rat hepatic stellate cells. J Biol Chem. 2001;2762:5318–23. doi: 10.1074/jbc.M102630200. [DOI] [PubMed] [Google Scholar]
- 33.Svistunenko DA. Reaction of haem containing proteins and enzymes with hydroperoxides: the radical view. Biochim Biophys Acta. 2005;1707:127–55. doi: 10.1016/j.bbabio.2005.01.004. [DOI] [PubMed] [Google Scholar]
- 34.Hurley JH, Meyer T. Subcellular targeting by membrane lipids. Current opinion in cell biology. 2001;13:146–52. doi: 10.1016/s0955-0674(00)00191-5. [DOI] [PubMed] [Google Scholar]
- 35.Newton AC. Lipid activation of protein kinases. Journal of lipid research. 2009;50(Suppl):S266–71. doi: 10.1194/jlr.R800064-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Czech MP. PIP2 and PIP3: complex roles at the cell surface. Cell. 2000;100:603–6. doi: 10.1016/s0092-8674(00)80696-0. [DOI] [PubMed] [Google Scholar]
- 37.Cantley LC. The phosphoinositide 3-kinase pathway. Science. 2002;296:1655–7. doi: 10.1126/science.296.5573.1655. [DOI] [PubMed] [Google Scholar]
- 38.Engelman JA. Targeting PI3K signalling in cancer: opportunities, challenges and limitations. Nature reviews Cancer. 2009;9:550–62. doi: 10.1038/nrc2664. [DOI] [PubMed] [Google Scholar]
- 39.Lee SR, Yang KS, Kwon J, Lee C, Jeong W, Rhee SG. Reversible inactivation of the tumor suppressor PTEN by H2O2. J Biol Chem. 2002;277:20336–42. doi: 10.1074/jbc.M111899200. [DOI] [PubMed] [Google Scholar]
- 40.Mehdi MZ, Pandey NR, Pandey SK, Srivastava AK. H2O2-induced phosphorylation of ERK1/2 and PKB requires tyrosine kinase activity of insulin receptor and c-Src. Antioxid Redox Signal. 2005;7:1014–20. doi: 10.1089/ars.2005.7.1014. [DOI] [PubMed] [Google Scholar]
- 41.Mazure NM, Chen EY, Laderoute KR, Giaccia AJ. Induction of vascular endothelial growth factor by hypoxia is modulated by a phosphatidylinositol 3-kinase/Akt signaling pathway in Ha-ras-transformed cells through a hypoxia inducible factor-1 transcriptional element. Blood. 1997;90:3322–31. [PubMed] [Google Scholar]
- 42.Fordel E, Geuens E, Dewilde S, Rottiers P, Carmeliet P, Grooten J, Moens L. Cytoglobin expression is upregulated in all tissues upon hypoxia: an in vitro and in vivo study by quantitative real-time PCR. Biochem Biophys Res Commun. 2004;319:342–8. doi: 10.1016/j.bbrc.2004.05.010. [DOI] [PubMed] [Google Scholar]
- 43.Li D, Chen XQ, Li WJ, Yang YH, Wang JZ, Yu AC. Cytoglobin up-regulated by hydrogen peroxide plays a protective role in oxidative stress. Neurochem Res. 2007;32:1375–80. doi: 10.1007/s11064-007-9317-x. [DOI] [PubMed] [Google Scholar]
- 44.Oleksiewicz U, Liloglou T, Tasopoulou KM, Daskoulidou N, Bryan J, Gosney JR, Field JK, Xinarianos G. Cytoglobin has bimodal: tumour suppressor and oncogene functions in lung cancer cell lines. Human molecular genetics. 2013;22:3207–17. doi: 10.1093/hmg/ddt174. [DOI] [PubMed] [Google Scholar]
- 45.Shivapurkar N, Stastny V, Okumura N, Girard L, Xie Y, Prinsen C, Thunnissen FB, Wistuba II, Czerniak B, Frenkel E, Roth JA, Liloglou T, Xinarianos G, Field JK, Minna JD, Gazdar AF. Cytoglobin, the newest member of the globin family, functions as a tumor suppressor gene. Cancer Res. 2008;68:7448–56. doi: 10.1158/0008-5472.CAN-08-0565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Thuy le TT, Morita T, Yoshida K, Wakasa K, Iizuka M, Ogawa T, Mori M, Sekiya Y, Momen S, Motoyama H, Ikeda K, Yoshizato K, Kawada N. Promotion of liver and lung tumorigenesis in DEN-treated cytoglobin-deficient mice. Am J Pathol. 2011;179:1050–60. doi: 10.1016/j.ajpath.2011.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Thuy le TT, Matsumoto Y, Thuy TT, Hai H, Suoh M, Urahara Y, Motoyama H, Fujii H, Tamori A, Kubo S, Takemura S, Morita T, Yoshizato K, Kawada N. Cytoglobin deficiency promotes liver cancer development from hepatosteatosis through activation of the oxidative stress pathway. Am J Pathol. 2015;185:1045–60. doi: 10.1016/j.ajpath.2014.12.017. [DOI] [PubMed] [Google Scholar]
- 48.Schmidt M, Gerlach F, Avivi A, Laufs T, Wystub S, Simpson JC, Nevo E, Saaler-Reinhardt S, Reuss S, Hankeln T, Burmester T. Cytoglobin is a respiratory protein in connective tissue and neurons, which is up-regulated by hypoxia. J Biol Chem. 2004;279:8063–9. doi: 10.1074/jbc.M310540200. [DOI] [PubMed] [Google Scholar]
- 49.Singh S, Canseco DC, Manda SM, Shelton JM, Chirumamilla RR, Goetsch SC, Ye Q, Gerard RD, Schneider JW, Richardson JA, Rothermel BA, Mammen PP. Cytoglobin modulates myogenic progenitor cell viability and muscle regeneration. Proc Natl Acad Sci U S A. 2014;111:E129–38. doi: 10.1073/pnas.1314962111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ertas B, Kiger L, Blank M, Marden MC, Burmester T. A membrane-bound hemoglobin from gills of the green shore crab Carcinus maenas. J Biol Chem. 2011;286:3185–93. doi: 10.1074/jbc.M110.160341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Blank M, Wollberg J, Gerlach F, Reimann K, Roesner A, Hankeln T, Fago A, Weber RE, Burmester T. A membrane-bound vertebrate globin. PLoS One. 2011;6:e25292. doi: 10.1371/journal.pone.0025292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Blank M, Burmester T. Widespread occurrence of N-terminal acylation in animal globins and possible origin of respiratory globins from a membrane-bound ancestor. Mol Biol Evol. 2012;29:3553–61. doi: 10.1093/molbev/mss164. [DOI] [PubMed] [Google Scholar]
- 53.Bologna G, Yvon C, Duvaud S, Veuthey AL. N-Terminal myristoylation predictions by ensembles of neural networks. Proteomics. 2004;4:1626–32. doi: 10.1002/pmic.200300783. [DOI] [PubMed] [Google Scholar]
- 54.Ren J, Wen L, Gao X, Jin C, Xue Y, Yao X. CSS-Palm 2.0: an updated software for palmitoylation sites prediction. Protein engineering, design & selection : PEDS. 2008;21:639–44. doi: 10.1093/protein/gzn039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ollesch G, Kaunzinger A, Juchelka D, Schubert-Zsilavecz M, Ermler U. Phospholipid bound to the flavohemoprotein from Alcaligenes eutrophus. Eur J Biochem. 1999;262:396–405. doi: 10.1046/j.1432-1327.1999.00381.x. [DOI] [PubMed] [Google Scholar]
- 56.Bonamore A, Farina A, Gattoni M, Schinina ME, Bellelli A, Boffi A. Interaction with membrane lipids and heme ligand binding properties of Escherichia coli flavohemoglobin. Biochemistry. 2003;42:5792–801. doi: 10.1021/bi0206311. [DOI] [PubMed] [Google Scholar]
- 57.El Hammi E, Warkentin E, Demmer U, Limam F, Marzouki NM, Ermler U, Baciou L. Structure of Ralstonia eutropha flavohemoglobin in complex with three antibiotic azole compounds. Biochemistry. 2011;50:1255–64. doi: 10.1021/bi101650q. [DOI] [PubMed] [Google Scholar]
- 58.Sriram R, Kreutzer U, Shih L, Jue T. Interaction of fatty acid with myoglobin. FEBS Lett. 2008;582:3643–9. doi: 10.1016/j.febslet.2008.09.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Chintapalli SV, Bhardwaj G, Patel R, Shah N, Patterson RL, van Rossum DB, Anishkin A, Adams SH. Molecular dynamic simulations reveal the structural determinants of Fatty Acid binding to oxy-myoglobin. PLoS One. 2015;10:e0128496. doi: 10.1371/journal.pone.0128496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.D’Souza K, Epand RM. Enrichment of phosphatidylinositols with specific acyl chains. Biochim Biophys Acta. 2014;1838:1501–8. doi: 10.1016/j.bbamem.2013.10.003. [DOI] [PubMed] [Google Scholar]
- 61.Kuwata H, Yoshimura M, Sasaki Y, Yoda E, Nakatani Y, Kudo I, Hara S. Role of long-chain acyl-coenzyme A synthetases in the regulation of arachidonic acid metabolism in interleukin 1beta-stimulated rat fibroblasts. Biochim Biophys Acta. 2014;1841:44–53. doi: 10.1016/j.bbalip.2013.09.015. [DOI] [PubMed] [Google Scholar]
- 62.Kapralov AA, Kurnikov IV, Vlasova II, Belikova NA, Tyurin VA, Basova LV, Zhao Q, Tyurina YY, Jiang J, Bayir H, Vladimirov YA, Kagan VE. The hierarchy of structural transitions induced in cytochrome c by anionic phospholipids determines its peroxidase activation and selective peroxidation during apoptosis in cells. Biochemistry. 2007;46:14232–44. doi: 10.1021/bi701237b. [DOI] [PubMed] [Google Scholar]
- 63.Ramanadham S, Ali T, Ashley JW, Bone RN, Hancock WD, Lei X. Calcium-independent phospholipases A2 and their roles in biological processes and diseases. Journal of lipid research. 2015;56:1643–68. doi: 10.1194/jlr.R058701. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


