Abstract
Studies with animal models implicate the plasma proteases factor XIIa (FXIIa) and α-kallikrein in arterial and venous thrombosis. As congenital deficiencies of factor XII (FXII) or prekallikrein (PK), the zymogens of FXIIa and α-kallikrein respectively, do not cause bleeding disorders, inhibition of these enzymes may have therapeutic benefit without compromising hemostasis. The relative contributions of FXIIa and α-kallikrein to thrombosis in animal models are not clear. We compared mice lacking FXII or PK to wild type mice in established models of arterial thrombosis. Wild type mice developed carotid artery occlusion when the vessel was exposed to a 3.5% solution of ferric chloride (FeCl3). FXII-deficient mice were resistant to occlusion at 5% FeCl3 and partially resistant at 10% FeCl3. PK-deficient mice were resistant at 3.5% FeCl3 and partially resistant at 5% FeCl3. Mice lacking high molecular weight kininogen, a cofactor for PK activation and activity, were also partially resistant to thrombosis at 5% FeCl3. Induction of carotid artery thrombosis with Rose Bengal was delayed in FXII-deficient mice compared to wild type or PK-deficient animals. In human plasma supplemented with silica, DNA or collagen to induce contact activation, an antibody to the FXIIa active site was more effective at preventing thrombin generation than an antibody to the α-kallikrein active site. Similarly, the FXIIa antibody was more effective at reducing fibrin formation in human blood flowing through collagen coated-tubes. The findings suggest that inhibitors of FXIIa will have more potent anti-thrombotic effects than inhibitors of α-kallikrein.
Keywords: Factor XII, Factor XIIa, Prekallikrein, α-kallikrein, thrombosis
INTRODUCTION
Factor XIIa (FXIIa) is a plasma protease that contributes to a number of host-defense processes, including thrombin generation and inflammation [1–4]. Conversion of zymogen factor XII (FXII) to factor XIIa may be initiated when blood is exposed to a variety of compounds and surfaces including polyanions released from activated platelets and damaged tissues [5–8], cell walls and membranes of microbial pathogens [9], and materials used in medical devices [10,11]. Such substances appear to function as cofactors that promote FXII activation through two main mechanisms. FXII can undergo autoactivation [12,13]. This may be initiated by traces of FXIIa in plasma, or perhaps by a conformational change upon surface binding that confers activity on the zymogen [14]. FXII is also activated by α-kallikrein, the protease form of the plasma zymogen prekallikrein (PK), in a reaction enhanced by high molecular weight kininogen (HK) [15–17]. The relative contributions of the two mechanisms for FXII activation likely depend on a number of factors including the nature of the initiating substance/surface.
Work over the past ten years has established that FXII plays a key role in occlusive thrombus formation in animal models [5,18–22], and there is considerable interest in developing FXIIa inhibitors to prevent or treat thromboembolic disorders [23,24]. Given that FXIIa is not required for hemostasis at a sit of blood vessel injury [25], therapies targeting this protease should be safer than currently used anticoagulants. More recently, PK has also been shown to contribute to thrombosis in mice [26], perhaps in part through its capacity to activate FXII. It is difficult to estimate the relative importance of FXII and PK to thrombosis from an assessment of the animal work, as models performed in different laboratories may differ in ways that affect sensitivity to a protein of interest. Here, we present a comparison of the effects of FXII or PK deficiency on arterial thrombosis in mice, and the effects of inhibitory antibodies to FXIIa and α-kallikrein on thrombin generation and thrombus formation in human blood.
MATERIALS AND METHODS
Antibodies
IgGs 559C-X181-D06 (D06) against human FXIIa and 559A-M202-H03 (H03) against human kallikrein were isolated from a human antibody phage display library [27]. mHK1 affinity-purified rabbit anti-kininogen IgG recognizes a polypeptide sequence specific to the product of the mouse Kng1 gene [28]. Goat anti-human FXII and sheep anti-human PK IgG conjugated to horseradish peroxidase (HRP) were from Affinity Biologicals (Ancaster, ON). The biotinylated monoclonal anti-mouse factor XI IgG 14E11 has been described [20].
Proteins and reagents
Human FXII, FXIIa, PK, α-kallikrein and plasmin were from Enzyme Research Laboratory (South Bend, IN). Human factor XIa was from Haematologic Technologies (Burlington, VT). Type I fibrillar collagen was from Chrono-Log (Havertown, PA). Anhydrous iron (III) chloride (FeCl3, molecular mass 160.20 Daltons) and delipidated bovine serum albumin (BSA) was from Sigma-Aldrich. Phosphatidylcholine:phosphatidylserine (PC/PS) vesicles were form Avanti Polar Lipids (Alabaster, Alabama). S-2366 (L-pyro-Glu-L-Pro-L-Arg-p-nitroanilide) and S2302 (H-D-prolyl-L-phenylalanyl-L-arginine-p-nitroaniline dihydrochloride.) were from DiaPharma (West Chester, OH). Z-Gly-Gly-Arg-AMC was from Bachem (Torrance, CA). PTT A silica-based activated partial thromboplastin time (aPTT) reagent was from Diagnostic Stago (Parsippany, NJ). Human genomic DNA was isolated from blood leukocytes by conventional phenol:chloroform extraction.
Western blots
Mouse plasma (1 µl) was size fractionated on 10% polyacylamide-SDS gels then transferred to nitrocellulose. Blots were incubated with 10 µg/ml HRP-conjugated IgG to FXII, HRP-conjugated IgG to PK, anti-kininogen IgG mHK1, or biotinylated anti-factor XI IgG 14E11. Anti-FXII and anti-PK IgGs were detected by chemiluminescence, mHK1 was detected with goat-anti rabbit IgG-HRP/chemiluminescence, and 14E11 was detected by streptavidin-HRP/chemiluminescence.
Mouse arterial thrombosis models
Procedures were approved by the Vanderbilt University Institutional Animal Care and Use Committee. C57Bl/6 mice deficient in FXII (fXII−/−) [18], PK (Klkb1−/−) [26] or HK (Kng1−/−) [28] were back-crossed >10 generations to wild type C57Bl/6 mice from Jackson Laboratory (Bar Harbor, ME). Mice were anesthesthetized with 50 mg/kg IP pentobarbital. The right common carotid artery was exposed and fitted with a Doppler probe (Model 0.5 VB, Transonic System, Ithaca, NY). Thrombus formation was induced by applying two 1 × 1.5 mm filter papers (GB003, Schleicher & Schuell, Keene, NH) saturated with FeCl3 (2.5 to 15% solutions [equivalent to 0.16 to 0.93 M solutions]) to opposite sides of the artery for three min and flow was monitored for 30 min. In a separate study, Rose Bengal (75mg/kg) was infused into the internal jugular vein, and the carotid artery was illuminated with a 1.5 mW 540 nm laser (Melles Griot, Carlsbad, CA) positioned 6 cm from the artery. Flow was monitored for 120 min. Mice were sacrificed by pentobarbital overdose after conclusion of the experiment.
IgG inhibition of FXIIa and α-kallikrein cleavage of small chromogenic substrates
FXIIa (100 nM), α-kallikrein (5 nM) or FXIa (5 nM) were incubated at RT for 3 min with vehicle (C) or a 10-fold molar excesses of D06 or H03, in 20 mM HEPES, pH 7.4, 100 mM NaCl, 0.1% PEG-8000, 10 µM ZnCl2. S-2302 (FXIIa and α-kallikrein) or S-2366 (FXIa) was added to a concentration of 200 µM and absorbance change at 405 nM was followed on a microplate reader.
IgG inhibition of macromolecular substrate activation by FXIIa and α-kallikrein
FXII (200 nM) in 20 mM HEPES, pH 7.4, 100 mM NaCl, 0.1% PEG-8000 was incubated with vehicle, 50 nM H03; 5 nM α-kallikrein, or 5 nM α-kallekrein and 50 nM H03 for 60 min at 37 °C. Aliquots were mixed with stop solution (0.1 mg/ml Polybrene and 50 nM H03, final concentrations) and S-2302 was added to 500 uM. Change in OD at 405 nm was monitored and FXIIa generated was determined by comparison to a control curve made with pure FXIIa. PK (60 nM) was incubated with vehicle; 5 nM D06; 0.5 nM FXIIa or 0.5 nM FXIIa and 5 nM DO6 for 30 min at 37 °C. Aliquots were mixed with stop solution (0.1 mg/ml Polybrene and 5 nM D06, final concentrations) and S-2302 was added to 100 uM. Change in OD at 405 nm was monitored and α-kallikrein generated was determined by comparison to a control curve made with pure α-kallikrein.
IgG inhibition of reciprocal activation of FXII and PK
Human FXII (200 nM) and PK (300 nM) in 20 mM HEPES, pH 7.4, 100 mM NaCl, 0.1% PEG-8000, 10 µM ZnCl2 was incubated at 37 °C in the presence or absence of human genomic DNA (100 µg/ml). Reactions were run in the presence of vehicle or 100 nM D06 or H03. At various times, 10 µl reactions were mixed with 4 µl of reducing SDS-sample buffer. Samples were size fractionated by SDS-PAGE (12% acrylamide) and then transferred to nitrocellulose membranes. Duplicate blots for each reaction were probed with HRP-conjugated polyclonal antibody to FXII or PK. Antibody detection was with streptavidin-HRP/chemiluminescence.
Thrombin Generation Assays
Plasma thrombin generation was measured at 37 °C on a Thrombinoscope® in 96-well polypropylene plates [29,20]. Plasma prepared from blood collected from healthy volunteers into 0.32% sodium citrate (final concentration) was supplemented with 415 µM Z-Gly-Gly-Arg-AMC; 5 µM PC:PS vesicles; 100 µg/ml D06, H03 or vehicle; and 1% (final concentration) silica aPTT reagent, leukocyte DNA (100 µg/ml) or fibrillar collagen (100 µg/ml). Supplemented plasma (100 µl) was mixed with 20 µl of 20 mM HEPES pH 7.4, 100 mM CaCl2, 6% BSA and fluorescence (excitation λ 390 nm, emission λ 460 nm) was monitored. Each set of conditions was tested in duplicate. Peak thrombin generation and area under the curve (Endogenous Thrombin Potential, ETP) were calculated using Thrombinoscope Analysis software, version 3.0.
Human blood ex vivo flow model
Blood collected from healthy volunteers into 0.32% sodium citrate was supplemented with Alexa-594-labeled fibrinogen (5 µg/ml). Cylindrical glass capillary tubes (0.3 mm diameter, Drummond Scientific, Broomall, PA) were coated with 50 µg/ml type I fibrillar collagen overnight at 4°C, then blocked with 0.5% BSA. Blood was perfused through tubes at initial shear rates of 300 s−1 using a syringe pump for 10 minutes [21,31]. Prior to entering the capillary tube, blood was mixed with 20 mM Tris-HCl pH 7.4, 154 mM NaCl with 37.5 mM CaCl2, 18.8 mM MgCl2 via a second pump in a coiled 15 cm mixing tube. Blood is diluted ~20% by this step, with estimated final free [Ca2+] and [Mg2+] ~2.5 and 1.2 mM, respectively. At the end of the run the tubes were perfused for 2 min with PBS at 0.3 ml/min. The tube contents were transferred into 0.1 ml RIPA buffer containing 25 µg/ml human plasmin, and incubated overnight at 37° C. Suspensions were transferred to a 96-well plate and fluorescence was measured on a platelet reader (excitation λ 580 nm, emission λ 620 nm).
Statistical analysis
Differences between groups of mice in the FeCl3 thrombosis model were assessed with Fisher’s exact test. For all analyses a p value of <0.05 was considered significant.
RESULTS
Contributions of contact factors to arterial thrombosis in mice
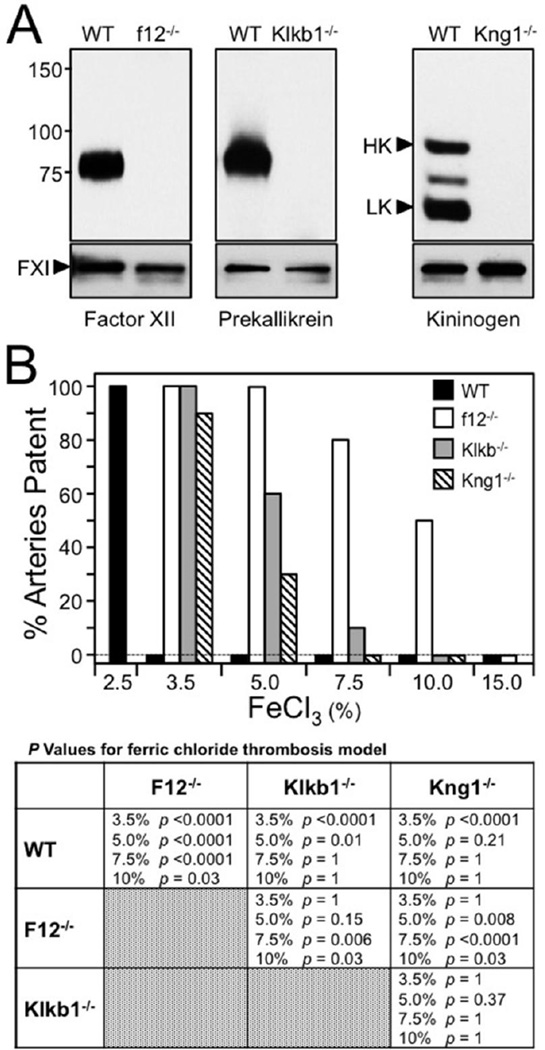
Mice homozygous for null disruptions of the FXII (fXII−/−), PK (Klkb1−/−), or kininogen 1 (Kng1−/−) gene lacked the corresponding antigens in their plasmas (Figure 1A). Humans have a single kininogen gene that encodes both HK and low-molecular weight kininogen (LK). Mice, in contrast, have two similar kininogen genes (Kng1 and Kng2). Most, if not all of the HK and LK in plasma appear to be products of the Kng1 gene [28].
Figure 1. Thrombosis in contact factor deficient mice.
(A) Western blots of mouse plasma. Western blots of plasma from wild type (WT) mice, and mice with total deficeincy of FXII (f12−/−), PK (Klb1−/−) or kininogen (Kng1). Blots were developed with polyclonal antibodies to the proteins indicated at the bottom of each blot. Small control blots in the lower panels where developed with antibody to factor XI (FXI). Positions of molecular mass standards (in kiloDaltons) are indicated on the left. For the kininogen blot, the positions of high molecular weight kininogen (HK) and low molecular weight kininogen (LK) are indicated by black arrows. (B) Ferric chloride-induced carotid artery occlusion. Carotid artery occlusion was induced in C57Bl/6 mice using varying concentrations of FeCl3 (bottom of graph). The percent of animals with patent arteries 30 minutes after FeCl3 exposure is shown. Wild type (WT-black bars), f12−/− (white), Klb1−/− (gray) or Kng1−/− (cross-hatched) mice (n = 10 for each bar) were studied. Groups were compared with Fisher exact test (table at bottom). The different concentrations of FeCl3 are shown as % solutions. p values <0.5 were conisdered significant.
Applying FeCl3 to the exterior of the carotid artery causes changes in vascular endothelium that lead to vessel occlusion by platelet-rich thrombi [32]. Different FeCl3 concentrations were used to induce occlusion in wild type, fXII−/−, Klkb1−/− and Kng1−/− C57Bl/6 mice (Figure 1B) [20,33]. Consistent with published data [33], vessel occlusion occurs consistently in wild type mice treated with 3.5% FeCl3, but not 2.5% FeCl3. For comparison, a 200 U/kg dose of unfractionated heparin prevents occlusion at 3.5% FeCl3, but is relatively ineffective at 5% FeCl3 [33]. All fXII−/− mice were resistant to occlusion at 5% FeCl3 (p <0.0001 compared to wild type mice), 80% were resistant at 7.5% FeCl3 (p <0.0001), and 50% were resistant at 10% FeCl3 (p = 0.03). Klkb1−/− mice were resistant to occlusion at 3.5% FeCl3 (p <0.0001 compared to wild type mice), and 60% did not occlude at 5% FeCl3 (p = 0.01). The differences between fXII−/− and Klkb1−/− mice were not significant at 5.0% FeCl3 (p =0.15), but were significant at 7.5% (p = 0.06) and FeCl3 10% (p = 0.03). Results with Kng1−/− mice roughly mirrored those for Klkb1−/− mice. The results for the two lines were not significantly different at 5.0% (p = 0.37) or 7.5% (p = 1) FeCl3. FXII and PK deficient mice were tested in a second model where carotid artery occlusion was induced by illuminating the vessel with a laser after infusion of the dye Rose Bengal [20,34]. Endothelial injury is caused by free-radical production when the dye is exposed to light. Time to occlusion was longer in FXII deficient mice (94 +/− 12 min) than in wild type mice (59 ± 21 min) or PK deficient mice (54 ± 18 min).
Antibody inhibition of reciprocal FXII and PK activation
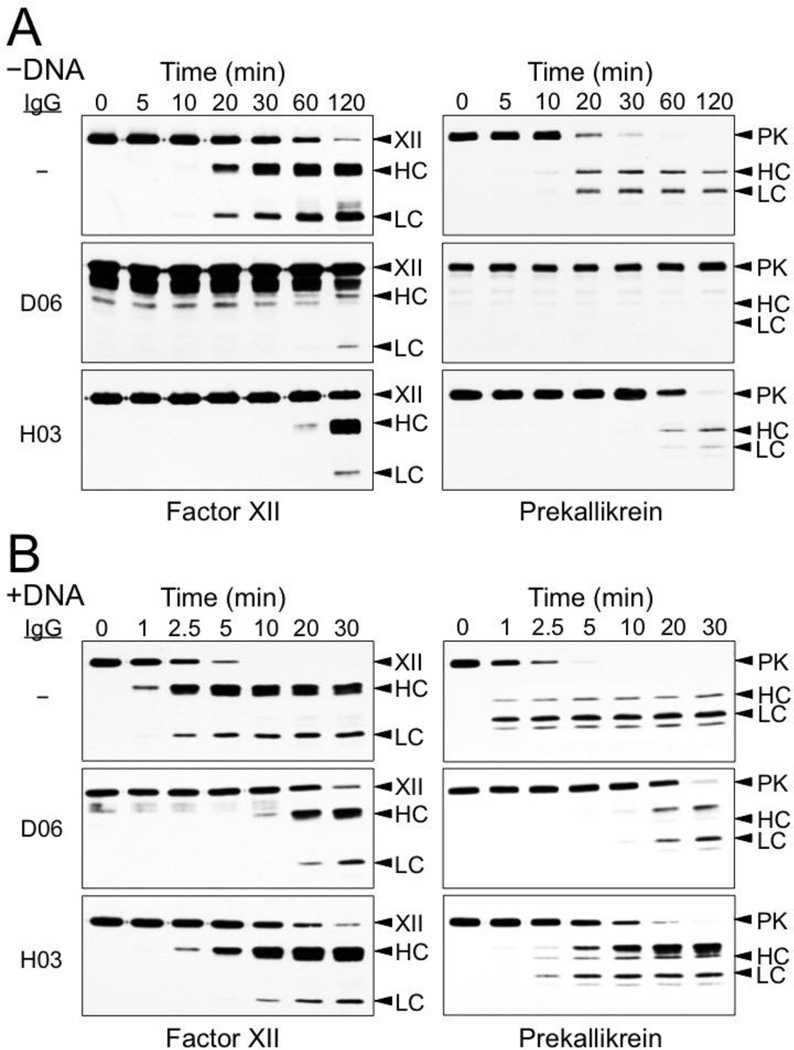
Mixing FXII and PK at plasma concentrations leads to generation of FXIIa and α-kallikrein by reciprocal activation (Figure 2A). DNA has been shown to promote FXII activation [7,8], and addition of leukocyte DNA to FXII/PK reaction enhances activation of both zymogens 5 to 10-fold (Figure 2B) compared with the reaction without DNA (Figure 2A).
Figure 2. Reciprocal activation of FXII and PK.
(A) Activation without DNA (−DNA). FXII (200 nM) and PK (300 nM) fXII were incubated in 20 mM HEPES, pH 7.4, 100 mM NaCl, 0.1% PEG-8000, 10 µM ZnCl2 at 37 °C. At the indicated time points (top) aliquots were removed into reducing SDS-sample buffer, followed by SDS-PAGE and Western Blot analysis for FXII/XIIa (left column) and PK (right). (B) Activation with DNA (+ DNA). FXII (200 nM) and PK (300 nM) fXII were incubated in 20 mM HEPES, pH 7.4, 100 mM NaCl, 0.1% PEG-8000, 10 µM ZnCl2 and 100 µg/ml leukocyte DNA at 37 °C. At the indicated time points (top) aliquots were removed into reducing SDS-sample buffer, followed by SDS-PAGE and Western Blot analysis for FXII/XIIa (left column) and PK (right). Extra bands appear on the Western blots of samples containing D06 that were developed with the polyclonal anti-FXII antibody. The bands are more prominent in the experiment in the absence of FNA, and may represent cross-reactivity between the anti-FXII antibody used to develop the blot and component of the D06 preparation. for left column: XII - zymogen FXII, HC – FXIIa heavy chain, LC – FXIIa light chain. Abbreviation for right colum: PK - zymogen PK, HC – α-kallikrein heavy chain, LC – α-kallikrein light chain.
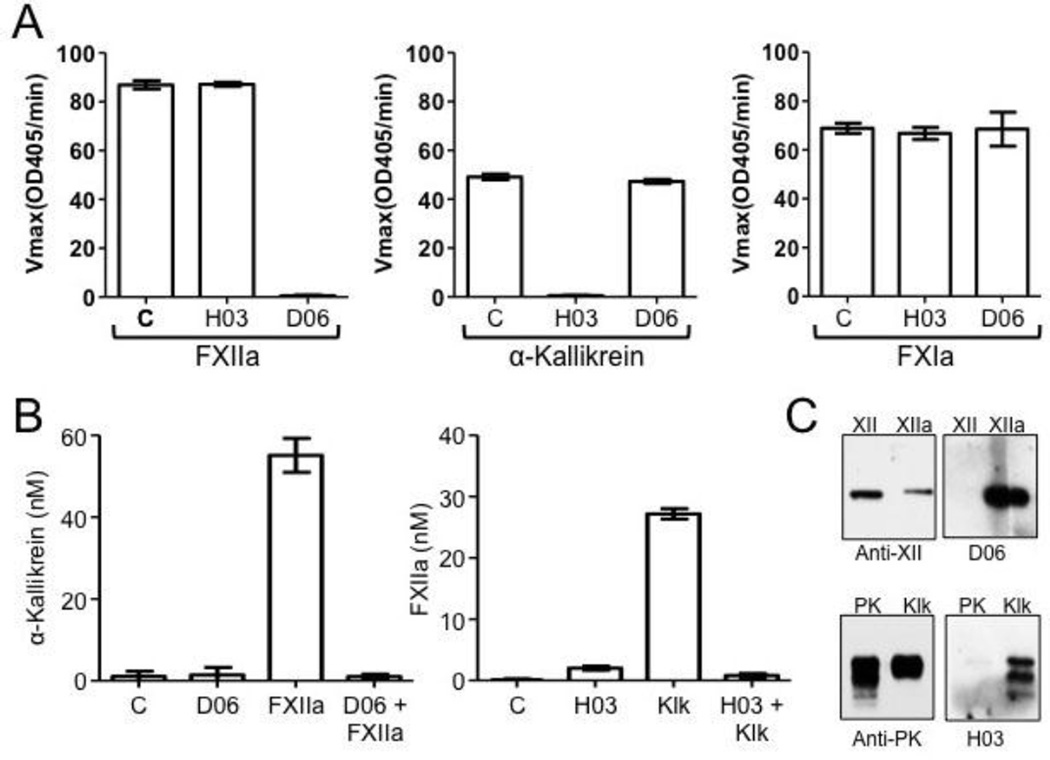
The Antibodies D06 and H03 [27] specifically bind to the active sites of human FXIIa and α-kallikrein, respectively, blocking protease cleavage of small peptide (Figure 3A) and macromolecular (Figure 3B) substrates. They do not recognize zymogen FXII or PK on western blots (Figure 3C) or in binding assays (data not shown). Both antibodies have a strong inhibitory effect on FXII and PK activation in the reciprocal reaction in the absence of DNA (Figure 2A). While both antibodies also slow FXII and PK activation in the presence of DNA (Figure 2B), D06 is more potent than H03. This is consistent with the hypothesis that FXII should be able to undergoing autoactivation in the presence of DNA, and convert PK to α-kallikrein, even in the presence of a strong α-kallikrein inhibitor such as H03.
Figure 3. Antibodies to FXIIa and kallikrein.
(A) IgG Specificity. FXIIa (100 nM), α-kallikrein (5 nM) or factor XIa (5 nM) were incubated at room temperature for 3 min with control vehicle (C) or a 10-fold molar excess of anti-FXIIa IgG D06 or anti-kallikrein antibody H03. Residual protease activity was measured by chromogenic substrate assay as described under Materials and Methods. Error bars are +/− one standard deviation. (B) Effects of antibodies on macromolecular substrate cleavage. Left panel - PK (60 nM) was incubated with vehicle; 5 nM D06; 0.5 nM FXIIa or 0.5 nM FXIIa and 5 nM DO6 for 30 min at 37 °C. After reactions were stopped, kallikrein activity was determined by chromogenic substrate assay as described under Materials and Methods. Right Panel - FXII (200 nM) was incubated with vehicle, 50 nM H03; 5 nM α-kallekrein, or 5 nM α-kallekrein and 50 nM H03 for 60 min at 37 °C. After reactions were stopped, kallikrein activity was determined by chromogenic substrate assay as described under Materials and Methods. Error bars are +/− one standard deviation. (C) Western blots of zymogens and proteases. FXII, FXIIa, PK and α-kallikrein were size-fractionated on 10% polyacrylamide-SDS gels. Blots were developed with polyclonal IgGs to FXII or PK (left) or D06 or H03 (right). Note that D06 and H03 only recognize the active protease form of each protein.
Antibody inhibition of thrombin generation
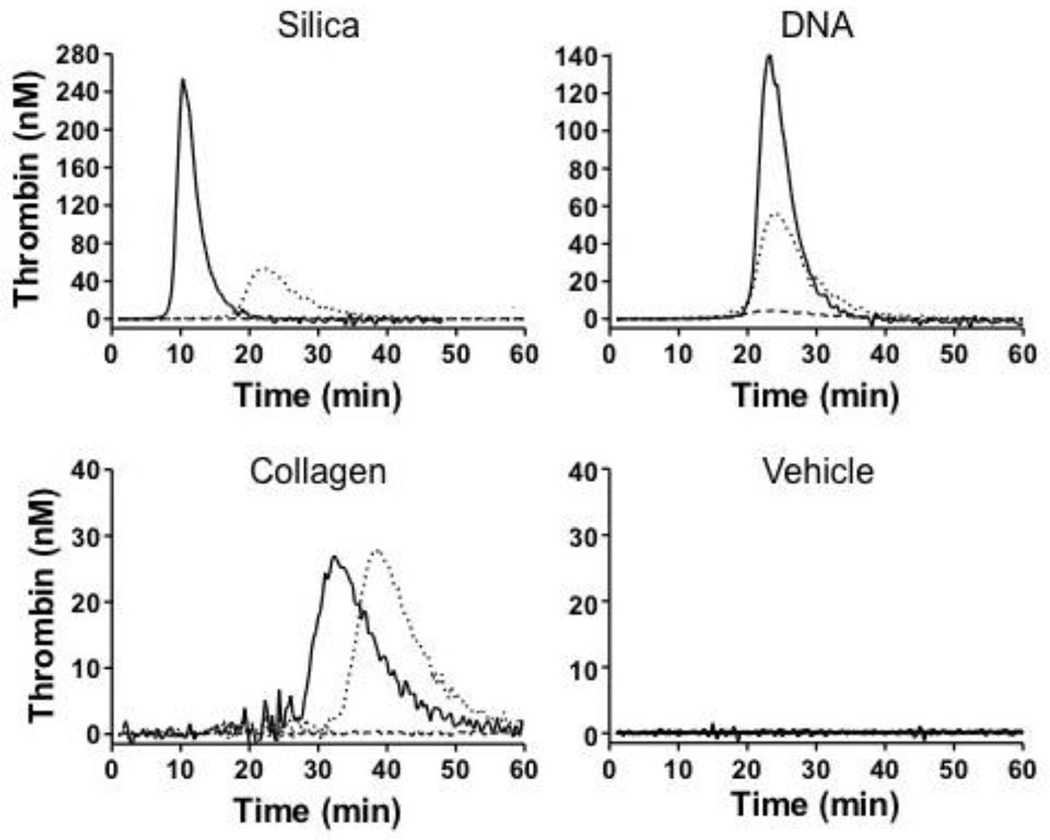
Thrombin generation was induced in human plasma by addition of a silica-based aPTT reagent (ETP 995 nM.min, peak 254 nM), genomic human DNA (ETP 884 nM.min, peak 140 nM) or collagen (ETP 364 nM.min, peak 27 nM) (Figure 4). In each case thrombin generation was blocked by the anti-FXIIa IgG D06. Similar results were obtained with an antibody to factor XI (data not shown), consistent with FXIIa driving thrombin generation through factor XI activation in this system. Anti-kallikrein IgG H03 reduced, but did not completely block, thrombin generation induced by silica (ETP 545 nM.min, peak 53 nM,) or DNA (ETP 537 nM.min, peak 56 nM), and had relatively little effect on collagen-induced thrombin generation (ETP 374 nM.min, peak 28 nM). The data are most consistent with a mechanism in which α-kallikrein contributes to FXII activation in the presence of silica or DNA, but that FXII autoactivation still promotes thrombin generation in the absence of α-kallikrein. In reactions with collagen, α-kallikrein appears to play a minimal role in FXII activation. This is consistent with observations that the contribution of PK to plasma coagulation in the aPTT assay varies depending on the substance used to initiate FXII activation [25].
Figure 4. Thrombin Generation.
Shown are representative curves for thrombin generation in recalcified normal human plasma supplemented with (control vehicle), a silica-based aPTT reagent, leukocyte DNA (100 µg/ml), or fibrilar collagen (100 µg/ml) in the presence of vehicle (solid line), 100 µg/ml D06 (dashed line) or 100 µg/ml H03 (dotted line). Each curve represents averages for two experiments.
Antibody inhibition of fibrin formation in flowing blood
Previously, we showed that antibodies to FXII or factor XI reduced fibrin formation in recalcified human blood perfused through collagen-coated capillary tubes [21,31]. Recent work by Zhu et al. confirmed that, in the absence of tissue factor, thrombin generation and fibrin formation are largely FXII-dependent in such flow systems [35]. D06 produced a dose-dependent inhibition of fibrin formation when human blood flows through collagen-coated tubes at a shear rate of 300 sec−1, approaching background levels at a plasma concentration of 100 µg/ml (~650 nM) (Figure 5). Possible activators of FXII in this system include the collagen coating the capillary tube and polyphosphate released from platelet dense granules. IgG H03 inhibited fibrin formation, but was not as potent as D06. Under the experimental conditions, the results support the data from the studies with mice showing that the contribution of FXIIa to thrombus formation is greater than that of α-kallikrein,
Figure 5. Fibrin deposition in flow blood.
Blood from a healthy donor anticoagulated with 0.32% (final concentration) sodium citrate was supplemented with Alexa-594-labeled fibrinogen and varying concentrations of D06 (◇) or H03 (◆). Blood was supplemented with CaCl2 and MgCl2, then pumped through collagen-coated capillary tubes at an initial shear shear rate of 300 sec−1. Fibrin deposition in the tubes, as reflected by changes in relative fluorescence in the tube contents, was determined as described under Materials and Methods. Error bars are +/− one standard deviation.
DISCUSSION
The zymogens FXII and PK and the non-enzymatic cofactor HK comprise the plasma kallikrein-kinin system [1–4]. FXII was identified as a plasma constituent missing in a patient with a defect in surface-induced plasma coagulation, but with no symptoms of a bleeding disorder [25]. It is now clear that FXII is not required for hemostasis [1–4,25]. While relatively few patients with PK or HK deficiency have been described, these conditions, like FXII deficiency, are not associated with a bleeding diathesis [25,36,37]. The discoveries that FXII [5,10,18–21], and more recently PK [26,38] and HK [28], contribute to thrombosis in animal models raises the prospect that drugs targeting the kallikrein-kinin system could produce beneficial therapeutic effects without the risk of serious bleeding that accompanies use of anticoagulants targeting thrombin and/or factor Xa. The work presented here investigated the relative contributions of FXII and PK to thrombosis in mouse models and in clotting human blood. The head-tohead comparisons were designed to address the issue that subtle variations in models performed in different laboratories render comparisons between published studies difficult.
Components of the kallikrein-kinin system may become activated when blood is exposed to certain surfaces or substances (often carrying a net negative charge) through a process called contact activation. Contact activation is initiated when FXII binds to a surface/substance that supports its autoactivation [2,4]. FXIIa then convert PK to α-kallikrein, which amplifies the process by activating additional FXII to FXIIa. HK enhances the process by facilitating PK and α-kallikrein binding to the contact surface. FXIIa ultimately promotes thrombin generation by activating factor XI. Naturally occurring polymers such as polyphosphate, DNA and RNA enhance FXII activation in vitro. It has been proposed that extracellular forms of these polyanions may be physiologic enhancers of FXII activation in vivo, and they have been implicated in the pathogenesis of thrombosis in animal models [5–8]. PK can also be converted to α-kallikrein by a FXII-independent mechanism involving the lysosomal enzyme prolylcarboxypeptidase isoform 1 (PRCP1) [39]. While it seems reasonable to postulate that α-kallikrein generated through this mechanism could contribute to FXII activation, and subsequently thrombosis, a recent analysis suggests otherwise. Adams et al. observed that PRCP1-deficient mice have evidence of vascular dysfunction, perhaps secondary to increased production of reactive oxygen species, and an increased propensity to develop arterial thrombosis [40]. While this raises the possibility that PRCP1 activation of PK actually contributes to a protective anti-thrombotic effect, the results may reflect PK-independent processes, and the importance of PRCP1-mediated PK activation to thrombus formation in mice remains uncertain.
FXII and PK appear to undergo reciprocal activation at a basal rate in healthy animals. Antisense oligonucleotide (ASO) knockdown of expression of either zymogen in mice results in reduced activation of the other [19]. Hypothetically, cofactors or conditions that enhance reciprocal FXII-PK activation could contribute to thrombosis. While the reciprocal reactions could hypothetically be down-regulated by inhibiting either FXIIa or α-kallikrein, it seems reasonable to postulate that FXIIa inhibition would have a greater effect, if FXII autoactivation is contributing significantly to FXIIa generation. Our results showing that FXII deficiency had a greater effect than PK or HK deficiency on arterial thrombus formation in mice support this premise, as do the experiments demonstrating that an anti-FXIIa antibody is more effective than an antibody to α-kallikrein at blocking surface-induced thrombin generation and fibrin formation in human blood. Taken as a whole the data support the conclusion that FXIIa is a major driver of thrombus formation in the mouse models, with α-kallikrein serving a supporting role.
The data make a stronger case for developing FXIIa inhibitors than α-kallikrein inhibitors as a therapeutic approach to thrombosis prevention; however, a few points should be considered. First, the effect of PK deficiency on FeCl3-induced thrombosis actually compares favorably with published data on the effects of unfractionated heparin in this model [33]. Second, a therapeutic approach targeting both FXIIa and α-kallikrein may be more effective at reducing reciprocal FXII-PK activation than targeting only one of the proteases. Finally, recent work by Stavrou et al. indicates that the anti-thrombotic effect of PK deficiency is partly FXII-independent. In their analysis, they noted that blood vessels of Klkb1−/− mice had reduced tissue factor expression and increased prostacyclin secretion, possibly as a consequence of reduced bradykinin production [38]. While short-term therapy with an α-kallikrein inhibitor would not be expected to produce such changes, longer duration therapy might do so, and could produce a synergistic effect with a FXIIa inhibitor.
Our results with the Rose Bengal-laser injury model require comment. We observed that FXII deficiency prolonged time to vessel occlusion compared to wild type mice, while PK deficiency did not. This result conflicts partly with the recent report from Stavrou et al describing a significant prolongation of time to vessel occlusion in Klkb1−/− mice compared to wild type mice using this model [38]. We suspect that minor differences in the two assays explain this situation. For example, differences in the anesthetic used, the dose of Rose Bengal, and the incident angle of the laser to the blood vessel could affect the sensitivity of the assay to the absence of PK. The discrepant results drive home the importance of comparing animals with different genotypes in a head-to-head fashion.
The plasma zymogen factor XI is a homolog of PK and a major proteolytic target of FXIIa [24]. Recently, Büller et al reported that ASO-induced reduction of factor XI was more effective than standard low molecular weight heparin therapy at preventing deep vein thrombosis in patients undergoing knee replacement [41]. In this study, patients treated with anti-factor XI ASO did not experience excessive bleeding despite factor XI levels consistently less than 20% of the normal plasma level at the time of surgery. It remains to be determined if targeting FXII or PK will be effective antithrombotic strategies in humans; however, the experience with factor XI reduction supports the premise that therapies targeting the kallikrein-kinin system should not compromise hemostasis and, therefore may be applicable to a wider range of clinical scenarios than are currently used anticoagulants.
HIGHLIGHTS.
Factor XII (FXII) and prekallikrein (PK) contribute to thrombosis in mice.
FXII deficient mice are more resistant to thrombosis than are PK deficient mice.
inhibiting FXII or PK affects reciprocal FXII-PK activation.
FXII inhibition has a greater effect on coagulation than PK inhibition in plasma.
Acknowledgments
The authors wish to acknowledge support from awards HL81326 and HL58837 (D. Gailani) and HL089796 to (K. McCrae) from the National Heart, Lung and Blood Institute.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
BIBLIOGRAPHY
- 1.Schmaier AH. Physiologic activities of the contact activation system. Thromb Res. 2014;133(Suppl 1):S41–S44. doi: 10.1016/j.thromres.2014.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wu Y. Contact pathway of coagulation and inflammation. Thromb J. 2015;13:17. doi: 10.1186/s12959-015-0048-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Björkqvist J, Nickel KF, Stavrou E, Renné T. In vivo activation and functions of the protease factor XII. Thromb Haemost. 2014;112:868–875. doi: 10.1160/TH14-04-0311. [DOI] [PubMed] [Google Scholar]
- 4.de Maat S, Tersteeg C, Herczenik E, Maas C. Tracking down contact activation – from coagulation in vitro to inflammation in vivo. Int J Lab Hematol. 2014;36:374–381. doi: 10.1111/ijlh.12222. [DOI] [PubMed] [Google Scholar]
- 5.Müller F, Mutch NJ, Schenk WA, Smith SA, Esterl L, Spronk HM, Schmidbauer S, Gahl WA, Morrissey JH, Renné T. Platelet polyphosphates are proinflammatory and procoagulant mediators in vivo. Cell. 2009;139:1143–1156. doi: 10.1016/j.cell.2009.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kannemeier C, Shibamiya A, Nakazawa F, Trusheim H, Ruppert C, Markart P, Song Y, Tzima E, Kennerknecht E, Niepmann M, von Bruehl ML, Sedding D, Massberg S, Günther A, Engelmann B, Preissner KT. Extracellular RNA constitutes a natural procoagulant cofactor in blood coagulation. Proc Natl Acad Sci U S A. 2007;104:6388–6393. doi: 10.1073/pnas.0608647104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fuchs TA, Brill A, Duerschmied D, Schatzberg D, Monestier M, Myers DD, Jr, Wrobleski SK, Wakefield TW, Hartwig JH, Wagner DD. Extracellular DNA traps promote thrombosis. Proc Natl Acad Sci U S A. 2010;107:15880–15885. doi: 10.1073/pnas.1005743107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gould TJ, Vu TT, Swystun LL, Dwivedi DJ, Mai SH, Weitz JI, Liaw PC. Neutrophil extracellular traps promote thrombin generation through platelet-dependent and platelet-independent mechanisms. Arterioscler Thromb Vasc Biol. 2014;34:1977–1984. doi: 10.1161/ATVBAHA.114.304114. [DOI] [PubMed] [Google Scholar]
- 9.Frick IM, Björck L, Herwald H. The dual role of the contact system in bacterial infectious disease. Thromb Haemost. 2007;98:497–502. [PubMed] [Google Scholar]
- 10.Larsson M, Rayzman V, Nolte MW, Nickel KF, Björkqvist J, Jämsä A, Hardy MP, Fries M, Schmidbauer S, Hedenqvist P, Broomé M, Pragst I, Dickneite G, Wilson MJ, Nash AD, Panousis C, Renné T. A factor XIIa inhibitory antibody provides thromboprotection in extracorporeal circulation without increasing bleeding risk. Sci Transl Med. 2014;6:222ra17. doi: 10.1126/scitranslmed.3006804. [DOI] [PubMed] [Google Scholar]
- 11.Jaffer IH, Fredenburgh JC, Hirsh J, Weitz JI. Medical device-induced thrombosis: what causes it and how can we prevent it? J Thromb Haemost. 2015;13(Suppl 1):S72–S81. doi: 10.1111/jth.12961. [DOI] [PubMed] [Google Scholar]
- 12.Dunn JT, Silverberg M, Kaplan AP. The cleavage and formation of activated human Hageman factor by autodigestion and by kallikrein. J Biol Chem. 1982;257:1779–1784. [PubMed] [Google Scholar]
- 13.Tans G, Rosing J, Griffin JH. Sulfatide-dependent autoactivation of human blood coagulation Factor XII (Hageman Factor) J Biol Chem. 1983;258:8215–8222. [PubMed] [Google Scholar]
- 14.Engel R, Brain CM, Paget J, Lionikiene AS, Mutch NJ. Single-chain factor XII exhibits activity when complexed to polyphosphate. J Thromb Haemost. 2014;12:1513–1522. doi: 10.1111/jth.12663. [DOI] [PubMed] [Google Scholar]
- 15.Griffin JH. Role of surface in surface-dependent activation of Hageman factor (blood coagulation factor XII) Proc Natl Acad Sci USA. 1978;75:1998–2002. doi: 10.1073/pnas.75.4.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Revak SD, Cochrane CG, Griffin JH. The binding and cleavage characteristics of human Hageman factor during contact activation. A comparison of normal plasma with plasmas deficient in factor XI, prekallikrein, or high molecular weight kininogen. J Clin Invest. 1977;59:1167–1175. doi: 10.1172/JCI108741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Meier HL, Pierce JV, Colman RW, Kaplan AP. Activation and function of human Hageman factor. The role of high molecular weight kininogen and prekallikrein. J Clin Invest. 1977;60:18–31. doi: 10.1172/JCI108754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Renné T, Pozgajová M, Grüner S, Schuh K, Pauer HU, Burfeind P, Gailani D, Nieswandt B. Defective thrombus formation in mice lacking coagulation factor XII. J Exp Med. 2005;202:271–281. doi: 10.1084/jem.20050664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Revenko AS, Gao D, Crosby JR, Bhattacharjee G, Zhao C, May C, Gailani D, Monia BP, MacLeod AR. Selective depletion of plasma prekallikrein or coagulation factor XII inhibits thrombosis in mice without increased risk of bleeding. Blood. 2011;118:5302–5311. doi: 10.1182/blood-2011-05-355248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cheng Q, Tucker EI, Pine MS, Sisler I, Matafonov A, Sun MF, White-Adams TC, Smith SA, Hanson SR, McCarty OJ, Renné T, Gruber A, Gailani D. A role for factor XIIa-mediated factor XI activation in thrombus formation in vivo. Blood. 2010;116:3981–3989. doi: 10.1182/blood-2010-02-270918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Matafonov A, Leung PY, Gailani AE, Grach SL, Puy C, Cheng Q, Sun MF, McCarty OJ, Tucker EI, Kataoka H, Renné T, Morrissey JH, Gruber A, Gailani D. Factor XII inhibition reduces thrombus formation in a primate thrombosis model. Blood. 2014;123:1739–1746. doi: 10.1182/blood-2013-04-499111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yau JW, Liao P, Fredenburgh JC, Stafford AR, Revenko AS, Monia BP, Weitz JI. Selective depletion of factor XI or factor XII with antisense oligonucleotides attenuates catheter thrombosis in rabbits. Blood. 2014;123:2102–2107. doi: 10.1182/blood-2013-12-540872. [DOI] [PubMed] [Google Scholar]
- 23.Key NS. Epidemiologic and clinical data linking factor XI and factor XII to thrombosis. Hematology Am Soc Hematol Educ Program. 2014:66–70. doi: 10.1182/asheducation-2014.1.66. [DOI] [PubMed] [Google Scholar]
- 24.Gailani D, Bane CE, Gruber A. Factor XI and contact activation as targets for antithrombotic therapy. J Thromb Haemost. 2015;13:1383–1395. doi: 10.1111/jth.13005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gailani D, Neff AT. Rare coagulation factor deficiencies. In: Hoffman R, Nenz EJ, Silberstein LE, Heslop HE, Weitz JI, Anastasi J, editors. Hematology: basic principles and practice. 6th. Saunders-Elsevier; 2010. pp. 1939–1952. [Google Scholar]
- 26.Bird JE, Smith PL, Wang X, Schumacher WA, Barbera F, Revelli JP, Seiffert D. Effects of plasma kallikrein deficiency on haemostasis and thrombosis in mice: murine ortholog of the Fletcher trait. Thromb Haemost. 2012;107:1141–1150. doi: 10.1160/th-11-10-0682. [DOI] [PubMed] [Google Scholar]
- 27.Kenniston JA, Faucette RR, Martik D, Comeau SR, Lindberg AP, Kopacz KJ, Conley GP, Chen J, Viswanathan M, Kastrapeli N, Cosic J, Mason S, DiLeo M, Abendroth J, Kuzmic P, Ladner RC, Edwards TE, TenHoor C, Adelman BA, Nixon AE, Sexton DJ. Inhibition of plasma kallikrein by a highly specific active site blocking antibody. J Biol Chem. 2014;289:23596–23608. doi: 10.1074/jbc.M114.569061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Merkulov S, Zhang WM, Komar AA, Schmaier AH, Barnes E, Zhou Y, Lu X, Iwaki T, Castellino FJ, Luo G, McCrae KR. Deletion of murine kininogen gene 1 (mKng1) causes loss of plasma kininogen and delays thrombosis. Blood. 2008;111:1274–1281. doi: 10.1182/blood-2007-06-092338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kravtsov DV, Matafonov A, Tucker EI, Sun MF, Walsh PN, Gruber A, Gailani D. Factor XI contributes to thrombin generation in the absence of factor XII. Blood. 2009;114:452–458. doi: 10.1182/blood-2009-02-203604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Matafonov A, Sarilla S, Sun MF, Sheehan JP, Serebrov V, Verhamme IM, Gailani D. Activation of factor XI by products of prothrombin activation. Blood. 2011;118:437–445. doi: 10.1182/blood-2010-10-312983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tucker EI, Marzec UM, White TC, Hurst S, Rugonyi S, McCarty OJ, Gailani D, Gruber A, Hanson SR. Prevention of vascular graft occlusion and thrombus-associated thrombin generation by inhibition of factor XI. Blood. 2009;113:936–944. doi: 10.1182/blood-2008-06-163675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Barr JD, Chauhan AK, Schaeffer GV, Hansen JK, Motto DG. Red blood cells mediate the onset of thrombosis in the ferric chloride murine model. Blood. 2013;121:3733–3741. doi: 10.1182/blood-2012-11-468983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang X, Cheng Q, Xu L, Feuerstein GZ, Hsu MY, Smith PL, Seiffert DA, Schumacher WA, Ogletree ML, Gailani D. Effects of factor IX or factor XI deficiency on ferric chloride-induced carotid artery occlusion in mice. J Thromb Haemost. 2005;3:695–702. doi: 10.1111/j.1538-7836.2005.01236.x. [DOI] [PubMed] [Google Scholar]
- 34.He L, Vicente CP, Westrick RJ, Eitzman DT, Tollefsen DM. Heparin cofactor II inhibits arterial thrombosis after endothelial injury. J Clin Invest. 2002;109:213–219. doi: 10.1172/JCI13432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhu S, Travers RJ, Morrissey JH, Diamond SL. FXIa and platelet polyphosphate as therapeutic targets during human blood clotting on collagen/tissue factor surfaces under flow. Blood. 2015;126:1494–1502. doi: 10.1182/blood-2015-04-641472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Girolami A, Scarparo P, Candeo N, Lombardi AM. Congenital prekallikrein deficiency. Exp Rev Hematol. 2010;3:685–695. doi: 10.1586/ehm.10.69. [DOI] [PubMed] [Google Scholar]
- 37.Fukushima N, Itamura H, Wada H, Ikejiri M, Igarashi Y, Masaki H, Sano M, Komiyama Y, Ichinohe T, Kimura S. A novel frameshift mutation in exon 4 causing a deficiency of high-molecular-weight kininogen in a patient with splenic infarction. Intern Med. 2014;53:253–257. doi: 10.2169/internalmedicine.53.0737. [DOI] [PubMed] [Google Scholar]
- 38.Stavrou EX, Fang C, Merkulova A, Alhalabi O, Grobe N, Antoniak S, Mackman N, Schmaier AH. Reduced thrombosis in Klkb1−/− mice is mediated by increased Mas receptor, prostacyclin, Sirt1, and KLF4 and decreased tissue factor. Blood. 2015;125:710–719. doi: 10.1182/blood-2014-01-550285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shariat-Madar Z, Mahdi F, Schmaier AH. Identification and characterization of prolylcarboxypeptidase as an endothelial cell prekallikrein activator. J Biol Chem. 2002;277:17962–17969. doi: 10.1074/jbc.M106101200. [DOI] [PubMed] [Google Scholar]
- 40.Adams GN, LaRusch GA, Stavrou E, Zhou Y, Nieman MT, Jacobs GH, Cui Y, Lu Y, Jain MK, Mahdi F, Shariat-Madar Z, Okada Y, D'Alecy LG, Schmaier AH. Murine prolylcarboxypeptidase depletion induces vascular dysfunction with hypertension and faster arterial thrombosis. Blood. 2011;117:3929–3937. doi: 10.1182/blood-2010-11-318527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Büller HR, Bethune C, Bhanot S, Gailani D, Monia BP, Raskob GE, Segers A, Verhamme P, Weitz JI FXI-ASO TKA Investigators. Factor XI antisense oligonucleotides for prevention of venous thrombosis. N Engl J Med. 2015;372:232–240. doi: 10.1056/NEJMoa1405760. [DOI] [PMC free article] [PubMed] [Google Scholar]