Abstract
Down syndrome (DS) is a common cause of intellectual disability and is also associated with early age of onset of Alzheimer's disease (AD). Due to an extra copy of chromosome 21, most adults over 40 years old with DS have beta-amyloid plaques as a result of overexpression of the amyloid precursor protein. Cerebrovascular pathology may also be a significant contributor to neuropathology observed in the brains of adults with DS. This review describes the features of cardiovascular dysfunction and cerebrovascular pathology in DS that may be modifiable risk factors and thus targets for interventions. We will describe cerebrovascular pathology, the role of comorbidities, imaging studies indicating vascular pathology and the possible consequences. It is clear that our understanding of aging and AD in people with DS will benefit from further studies to determine the role that cerebrovascular dysfunction contributes to cognitive health.
Keywords: Beta-amyloid, Cerebral amyloid angiopathy, Hypertension, Hypotension, Microhemorrhages, Moyamoya, Sleep apnea, Stroke
1. Cerebrovascular disease and Alzheimer's disease
Vascular contribution to cognitive impairment and dementia (VCID) is widely considered to be the second most common cause of dementia after Alzheimer's disease (AD), accounting for 20–30% of cases [54]. In addition, VCID occurs as a co-morbidity with other common dementias including AD and is estimated to be co-morbid in as many as 40–50% of AD cases [12,46,50]. The most obvious, acute cause of VCID is a stroke. Dementia symptoms such as confusion, disorientation and trouble understanding speech can occur following a major stroke. However, most VCID cases are those that have a more-subtle pathophysiology. These pathophysiologies can include multiple small strokes, chronic cerebral hypoperfusion, cerebrovascular occlusions, cerebral microhemorrhages, and cerebral amyloid angiopathy (CAA) [54]. While VCID is clearly a significant cause of dementia, VCID remains understudied relative to other causes of dementia such as AD and frontotemporal dementia (FTD). This is in part due to a lack of in vitro or in vivo models and a lack of a single gene target or abnormal pathology [44].
Vascular factors are increasingly being recognized as a critical comorbidity that not only accelerates the age of onset of dementia but also can lead to a faster progression of the disease. White matter hyperintensities (WMH) can serve as a “second hit” necessary for clinical signs of dementia particularly when significant Aβ is present in the brain [76]. In addition, recent evidence from the Rotterdam Scan Study strongly suggests a link between white matter (WM) integrity (measured by fractional anisotropy — FA; lower FA is poorer WM integrity) and the number of cerebral microbleeds in older nondemented individuals over the age of 60 years old [3]; individuals with higher numbers of microbleeds had lower FA. Interestingly, previous studies show that ex vivo imaging of autopsy brain indicates an overlap between FA and WM lesions that reflect vascular deficits [6]. WMH, typically prominent in periventricular regions, lead to reduced FA [16].
Studies in mouse models of VCID suggest a critical role of inflammatory processes in the neurodegeneration and progression of the cerebrovascular pathology. Mouse models of CAA show a distinct inflammatory signature compared to animal models of parenchymal amyloid deposition [104]. Further, the hyperhomocysteinemia model of VCID that develops primarily impaired cerebral blood flow, microhemorrhages and WMH indicates a critical role for pro-inflammatory responses in the brain contributing to the progression and severity of cerebrovascular pathologies, as well as cognitive impairment [92]. Further support for a causal link between neuroinflammatory responses and WM integrity loss comes from a study of the permanent bilateral carotid artery occlusion rat model [29]. In this model significant microglial activation correlated with loss of myelin basic protein and oligodendrocyte density.
2. Down syndrome, aging and Alzheimer's disease
DS or trisomy 21 is one of the most common causes of intellectual disability. Improved medical care in DS has led to a significant lifespan extension (median life span is now estimated to be 60 years) and enhanced quality of life [9,33] but also has increased AD risk. Dementia incidence and prevalence increase substantially after 50 years old [85]. However, there is a subset of aged DS individuals who do not develop dementia at any age [38,39,85,112]. The reasons for a subset of older people with DS not developing dementia are as yet unknown and may be complex. A simple explanation may be that these individuals may have died before developing dementia. However, the underlying genetic cause for DS may also provide an explanation. For example, in a case study of a person with partial trisomy 21 (disomic for the amyloid precursor protein) lived into her 70s without dementia [75]. There may be other people who have a DS phenotype without genetic confirmation of trisomy 21 that may be in this group, including those with mosaicism. It may also be that lifestyle factors or other genes on chromosome 21 can be protective in some people with DS. These observations strongly suggest the need to follow larger groups of older adults with DS to clearly establish factors associated with a lack of development of dementia despite significant AD pathology. Mild cognitive impairment (MCI), a precursor to dementia in sporadic AD is of limited applicability to DS (at this time) and continues to be an important area to develop [48]. Virtually all DS adults have sufficient neuropathology for a diagnosis of AD by 40 years old [57,106,107], including senile plaques (SP-beta-amyloid (Aβ) protein) and neurofibrillary tangles (hyperphosphorylated tau protein). Aβ is derived from the β-amyloid precursor protein (APP), the gene for which is on chromosome 21 and overexpressed in DS [81]. Aβ accumulation in diffuse plaques does not appear to be consistently observed until after the age of 30 years old [57]. However, several interesting studies show significant diffuse plaque accumulation in much younger individuals such as the work by Lemere and colleagues and Leverenz and Raskind [51,53]. In particular, work by Lemere's group strongly suggests that more studies of younger individuals are necessary as the presence of diffuse plaques in children may as yet be underappreciated. Between the ages of 30 and 40 years old, neurofibrillary tangle and Aβ plaque neuropathology accumulate until they reach levels sufficient for a pathological diagnosis of AD [106]. Thus, there is a preclinical phase in DS when AD pathology accumulates (30–40 years) but dementia diagnosis may be delayed by up to a decade if not longer [49].
3. Cardiac abnormalities in Down syndrome
Reports of cardiac abnormalities in DS have shown a prevalence of 33% to 48% (cf. [30,87]) in children with DS with atrioventricular septal and ventricular septal defects appearing as the most common congenital heart defects. In a review of the heart and vascular system in DS, Vis and colleagues [100] described increased frequency of congenital heart defects along with factors associated with heart disease such as hypothyroidism, lipid levels, reduced homocysteine levels, decreased atherosclerosis and their association with chromosome 21 overexpression. Clearly, as noted by Vis and colleagues, DS affects both structure and function of the heart and vasculature; the resulting impact on the central nervous system has yet to be fully delineated. Several of these vascular risks are discussed below.
4. Cerebrovascular pathology in Down syndrome
Although cardiovascular disease (CVD) is a key contributor to sporadic AD, it has been virtually unexplored in DS. DS represents a unique opportunity to study the cerebrovascular features of aging and AD in a setting of more limited systemic vascular risk factors such as atherosclerosis, hypertension and hyperhomocysteinemia (as will be discussed later).
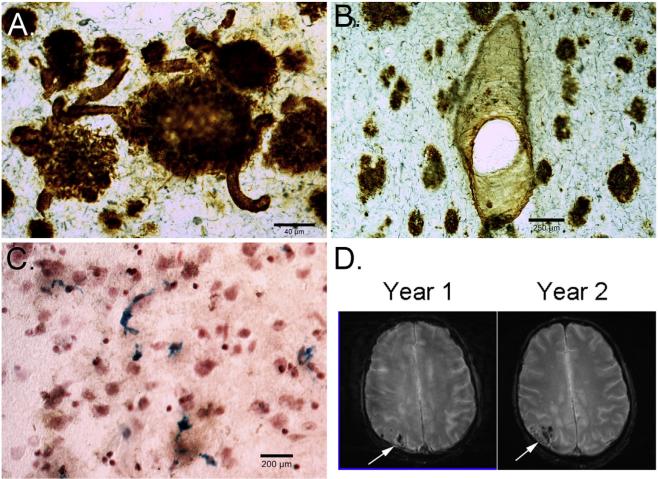
Adults with DS exhibit significant cerebral amyloid angiopathy (CAA), which is the term commonly used to define the deposition of amyloid in the walls of medium- and small-sized leptomeningeal and cortical arteries, arterioles and, less frequently, capillaries and veins. CAA can lead to micro- and macro-hemorrhages [99]. CAA is consistently observed in older individuals with DS over the age of 55 years old [7,40,49] (Fig. 1A, B) and also contains post-translationally modi-fied Aβ [31]. CAA in DS may be associated with extensive cerebrovascular hemorrhages or stroke [7,22,42,60,62,69] in some studies but not in others [40,49]. In our autopsy studies, we find a significant number of microhemorrhages in the DS brain (Fig. 1C). Aβ40 (typically associated with CAA — Fig. 1B) rises exponentially with age in the brain [15] and plasma [83] of people with DS and appears preferentially in the occipital cortex (Fig. 1D) but other areas are affected. Interestingly, there are no systematic studies in DS brain to evaluate the distribution of CAA with age, which could be accomplished by MR studies or by autopsy. With increased CAA and Aβ1-40 in DS, interestingly, VCID (or multi-infarct dementia as it was termed then) per se is rare with only one case report in the literature of a 55-year old woman with DS [19].
Fig. 1.
Cerebrovascular neuropathology in DS. (A) Beta-amyloid 1-42 immunostaining of the frontal cortex in 67-year old adult with DS and AD shows plaques and significant CAA in multiple small vessels (arrows). (B) Beta-amyloid 1-40 shows a different pattern with fewer plaques being labeled with CAA appearing more prominent, particularly in vessels (arrows). (C) Prussian blue staining of a 58-year old with DS and AD shows significant numbers of microhemorrhages (blue). T2* weighted MR images in a 60-year old male with DS, who is currently nondemented over a 2-year time interval, shows significant CAA in the occipital cortex that is progressively getting worse (white arrows) (images courtesy of Dr. David Powell, University of Kentucky).
5. Comorbidities associated with cerebrovascular disease or protection in DS
5.1. Atherosclerosis
In a previous study of 70 adults with DS ranging in age from 40 to 66 years old, blood pressure was lower and atheroma (accumulation of degenerative material in the tunica intima (inner layer) of artery walls) was absent compared to similarly aged adults without DS [66]. Other studies also report much lower frequency of atheroma [13,109]. Although less frequent in DS, atherosclerosis has been observed in two middle aged adults who had strokes [73] but it tends to be milder in adults with DS [110]. Indeed, death from atherosclerosis in adults with DS in a Swedish population was rare but may be more frequent than previously thought [26]. Possible mechanisms by which people with DS are protected from atherosclerosis may include reduced heart-type fatty acid binding protein, which is typically correlated with age-associated atherosclerosis in the non-DS population [98] as well as reduced brassicasterol (a plant sterol implicated with atheroprotective properties, which interestingly is reduced in DS plasma) [94] and C-reactive protein [34]. However, many serum proteins associated with increased atherosclerosis are also observed in DS including elevated triglycerides and total body fat [23,70].
Interestingly, chromosome 21 contains the cystathionine-beta-synthase gene that converts homocysteine to cysteine [1], suggesting lower homocysteine levels in DS [74], which is in turn thought to be associated with decreased coronary heart disease [11]. The observation of lower homocysteine in children with DS [74] suggests this may be protective, however, in a small study of older adults, higher levels were observed [55]. This interesting divergence suggests that homocysteine may be modified with age in people with DS.
5.2. Hypertension/hypotension
Lower blood pressure in children [79] and adults with DS [23, 24] has been consistently reported. Further, blood pressure does not rise with age in DS as it does in the general population [63]. Indeed, in one report, hypotension is a feature of aging and dementia in people with DS and hypertensive drugs were less likely to be prescribed [4]. In this large retrospective study, the incidence of hypertension was 0.58/100 person years and hypotension was 0.15/100 person years over the age of 30 years old. The overexpression of cystathionine β-synthase on chromosome 21 may be an underlying factor for these observations.
5.3. Moyamoya disease
Moyamoya is a chronic occlusive cerebrovascular disorder [93] of the carotid arteries and/or the arteries of the circle of Willis that can cause ischemic strokes in children with DS and hemorrhagic strokes in adults [8,20,21,68,71,73]. It is thought that moyamoya disease is more frequent in children with DS [32]. Further, there is up to a 26 fold greater prevalence of DS in patients with moyamoya [45]. The first report of moyamoya in DS was in 1977 by Schrager and colleagues where a child developed acute hemiplegia, cortical blindness, and a right paraventricular frontal infarct [82]. Subsequently, several case reports of moyamoya in children with DS were described [73,96]; in a study of 41 patients with DS, 4 had moyamoya [73]. Possible mechanisms underlying the higher frequency of moyamoya in DS may be linked to autoimmunity [52] or to genes on chromosome 21 associated with arterial physiology for example, superoxide dismutase 1, interferon gamma receptor, and cystathionine β-synthase [20].
5.4. Obesity/diabetes
Children and adults with DS show a higher frequency of being overweight or obese [18,61,67,78,80,89,97] with up to 60% of individuals being overweight. For example, in a study of 1600 Dutch children with DS, 25% of these healthy children were overweight with numbers reaching as high as 40% overweight or obese up to 18 years old [97]. This may be, in part, related to higher prevalence of hypothyroidism in addition to increased levels of circulating leptin [56]. Interestingly, postmenopausal obese women with DS perform significantly better on a test of verbal memory (but not other cognitive tests) than non-obese women with DS [72]. While this finding may be related to body mass index associated estrogen levels being higher, no differences were seen for serum estradiol levels between groups based on obesity and the association between estrogen levels and memory scores did not suggest a large effect size (R2 from .053 to .073). However, estrogen receptor variants (ESR1 polymorphisms) contribute a 2 to 3-fold increase in AD risk in women with DS [84,111] suggesting that the associations between memory, obesity, and estrogen levels in DS are likely complex and would not support the use of estrogen replacement to reduce AD risk in DS. Finally, obesity may suggest an increased propensity to develop type II diabetes in DS. There are few reports describing type II diabetes in DS however it could be lower than in the general population [27].
5.5. Sleep apnea
Given the higher prevalence of obesity in DS along with orofacial an-atomical variations (cf. [77]), it is not surprising that the frequency of obstructive sleep apnea is also high. The incidence of obstructive sleep apnea (OSA) in children 2 to 4 years old with DS is 57% based on polysomnography alone in one study [86]. Further OSA increased to 80% when the criterion also included an arousal index that was elevated in 61% of the children who were evaluated. Of note, Shott and colleagues did not find an association between OSA and body mass index (BMI) or presence of cardiovascular disease in their sample. In contrast, Trois and colleagues studied individuals with DS between the ages of 17 and 56 years old [95]. They reported that 94% of their sample had OSA of varying levels of severity with 69% in the severe range based on their apnea–hypopnea index. More importantly, they did not find an association between OSA and age but showed a correlation between BMI and apnea–hypopnea index.
The implications for the brain in OSA are well known in the general population and include elevated risk for dementia [10,108] and CVD (stroke, transient ischemic attack, small vessel disease, silent cerebral infarction), and are hypothesized to contribute to gray and white matter losses (cf., [14,17,47]). Durgan and Bryan [25] in a relatively recent review of the impact of OSA on CVD implicate multiple mechanisms such as inflammation, endothelial dysfunction, and oxidative stress. Given the prevalence of OSA in DS, it is possible that in addition to the impact of the genetic aspects of this disorder, OSA could readily contribute to VCID as well as AD as these individuals age.
6. Imaging studies in DS suggesting CVD
A key contributor to sporadic AD, the role of CVD, has been virtually unexplored in DS. As mentioned previously, the CVD contribution to AD is increasingly being recognized as a critical comorbidity that accelerates the age of onset of dementia and also leads to a faster progression of the disease [2,5,28,41]. Estimates of a mixed etiology of AD with CVD range from 5.7 to 45% in autopsy cases from the general population [43]. CVD can serve as a “second hit” necessary for clinical signs of dementia particularly when significant Aβ is present in the brain [76]. Several studies are underway that are evaluating the presence of cerebrovascular pathology in aging adults with DS by imaging approaches and in the coming years, more information will become available (e.g. Fig. 1D).
7. Consequences of CVD in DS — neuroinflammation
The rationale for interest in neuroinflammation stems from observations regarding decreased WM integrity and preliminary magnetic resonance (MR) imaging data showing CVD. Each of these pathologies can be caused by, or lead to, neuroinflammation. Interestingly, although neuroinflammation is widely accepted as a critical mediator in sporadic AD, little is known about the aging DS brain (recently reviewed in [101, 103]). Early studies by Dr. Griffin's and Lemere's group demonstrated some intriguing findings suggesting increased pro-inflammatory responses in the DS brain including increased IL-1b expression in the DS brain and increased complement activation [35–37,65,88,103]. This is particularly surprising given that chromosome 21 contains genes that are involved in pro- and anti-inflammatory processes [101].
Data from our current study support the systematic study of neuroinflammation in DS [105]. To explore the spectrum of neuroinflammatory responses, several laboratories have used markers associated with distinct macrophage phenotypes (reviewed in [102]). The macrophage nomenclature of M1, M2a, M2b and M2c has been used in the assessment of this neuroinflammatory spectrum [58]. M1 responses are the typical pro-inflammatory responses one expects to see with an immune stimulus such as lipopolysaccharide and interferon-gamma (IFNg). The M1 response is characterized by high IL-12, low IL-10, and high IL-1b, TNFa and I-6. The M2a response is associated stimulation by IL-4 or IL-13 and is characterized by the expression of high IL-12, arginase-1, chitinase-like proteins and IL-1 receptor antagonist. The primary role of this phenotype in the periphery is wound repair and matrix deposition. The M2b response is stimulated by the presence of immune complexes and the activation of the Fcg receptor signaling pathways. This response is characterized by high IL-10, low IL-12, and moderately high IL-1b, TNFa and IL-6. The exact functional consequence of this phenotype, beyond stimulating phagocytosis, is relatively unknown [59,64]. The M2c response is stimulated by IL-10 and is characterized by increased expression of TGFb1. The role of this phenotype is to actively downregulate potential M1 responses.
Using the M1–M2 spectrum of markers, we recently showed that DS brains, especially those with AD pathology, are characterized by the presence of a high M2b response. We have not previously found that human AD brains, even at the severe stages, show any M2b responses. In a previously published study of human autopsy tissue from early and late stage AD, we found expression of M1 and M2a associated markers, but not M2b [91,102]. This indicates to us that the DS brain has a different inflammatory response relative to sporadic AD. Based on our understanding of the M2b phenotype, and its dependence on stimulation by immune complexes [90], we hypothesize that the presence of this phenotype in the DS brain suggests a broad dysfunction of the blood–brain barrier (BBB) allowing significant leakage of IgG into the brain parenchyma generating sufficient immune complexes to promote this M2b response. Future studies will explore the potential for this mechanism and the dependence of the M2b response on cerebrovascular degeneration. The long term implications of this unique NI profile in individuals with DS are as yet unknown, but most likely will affect cognition, CVD, and WM integrity.
8. Summary and future studies
DS represents a unique opportunity to study the cerebrovascular features of aging and AD in a setting of more limited systemic vascular risk factors. Thus, adults with DS represent an important cohort to study CVD co-morbidities because of their unique characteristics: atheroma-free model and lower blood pressure but with significant CAA. Given that many of the co-morbidities associated with cerebrovascular dysfunction may be modifiable, it will be critical to determine if these may be targets for intervention that benefit this highly vulnerable group of adults. Thus, future studies that include biomarkers of cerebrovascular dysfunction as outcome measures will be important to consider in the design of longitudinal observational studies and clinical trials.
Acknowledgments
Study funding: Supported by Eunice Kennedy Shriver National Institute of Child Health and Development of the National Institutes of Health Grant Number — R01HD064993 and Alzheimer's Association grant DSADNIP-13-282631. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The authors appreciate the editorial assistance from Ms. Paula Thomason at the University of Kentucky.
Footnotes
This article is part of a Special Issue entitled: Vascular Contributions to Cognitive Impairment and Dementia edited by M. Paul Murphy, Roderick A. Corriveau and Donna M. Wilcock
Transparency document
The Transparency document associated with this article can be found in the online version.
References
- 1.Ait Yahya-Graison E, Aubert J, Dauphinot L, Rivals I, Prieur M, Golfier G, Rossier J, Personnaz L, Creau N, Blehaut H, Robin S, Delabar JM, Potier MC. Classification of human chromosome 21 gene-expression variations in Down syndrome: impact on disease phenotypes. Am. J. Hum. Genet. 2007;81:475–491. doi: 10.1086/520000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Akinyemi RO, Mukaetova-Ladinska EB, Attems J, Ihara M, Kalaria RN. Vascular risk factors and neurodegeneration in ageing related dementias: Alzheimer's disease and vascular dementia. Curr. Alzheimer Res. 2013;10:642–653. doi: 10.2174/15672050113109990037. [DOI] [PubMed] [Google Scholar]
- 3.Akoudad S, de Groot M, Koudstaal PJ, van der Lugt A, Niessen WJ, Hofman A, Ikram MA, Vernooij MW. Cerebral microbleeds are related to loss of white matter structural integrity. Neurology. 2013;81:1930–1937. doi: 10.1212/01.wnl.0000436609.20587.65. [DOI] [PubMed] [Google Scholar]
- 4.Alexander M, Petri H, Ding Y, Wandel C, Khwaja O, Foskett N. Morbidity and medication in a large population of individuals with Down syndrome compared to the general Ppopulation. Dev. Med. Child Neurol. 2015 doi: 10.1111/dmcn.12868. [DOI] [PubMed] [Google Scholar]
- 5.Attems J, Jellinger KA. The overlap between vascular disease and Alzheimer's disease—lessons from pathology. BMC Med. 2014;12:206. doi: 10.1186/s12916-014-0206-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Back SA, Kroenke CD, Sherman LS, Lawrence G, Gong X, Taber EN, Sonnen JA, Larson EB, Montine TJ. White matter lesions defined by diffusion tensor imaging in older adults. Ann. Neurol. 2011;70:465–476. doi: 10.1002/ana.22484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Belza MG, Urich H. Cerebral amyloid angiopathy in Down's syndrome. Clin. Neuropathol. 1986;5:257–260. [PubMed] [Google Scholar]
- 8.Berg JM, Armstrong D. On the association of moyamoya disease with Down's syndrome. J. Ment. Defic. Res. 1991;35(Pt 4):398–403. doi: 10.1111/j.1365-2788.1991.tb00412.x. [DOI] [PubMed] [Google Scholar]
- 9.Bittles AH, Bower C, Hussain R, Glasson EJ. The four ages of Down syndrome. Eur. J. Pub. Health. 2007;17:221–225. doi: 10.1093/eurpub/ckl103. [DOI] [PubMed] [Google Scholar]
- 10.Blackwell T, Yaffe K, Ancoli-Israel S, Redline S, Ensrud KE, Stefanick ML, Laffan A, Stone KL. Osteoporotic fractures in men study G. J. Am. Geriatr. Soc. 2011;59:2217–2225. doi: 10.1111/j.1532-5415.2011.03731.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Boushey CJ, Beresford SA, Omenn GS, Motulsky AG. A quantitative assessment of plasma homocysteine as a risk factor for vascular disease. JAMA. 1995;274:1049–1057. doi: 10.1001/jama.1995.03530130055028. [DOI] [PubMed] [Google Scholar]
- 12.Bowler JV, Munoz DG, Merskey H, Hachinski V. Fallacies in the pathological confirmation of the diagnosis of Alzheimer's disease. J. Neurol. Neurosurg. Psychiatry. 1998;64:18–24. doi: 10.1136/jnnp.64.1.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brattstrom L, Englund E, Brun A. Does Down syndrome support homocysteine theory of arteriosclerosis. Lancet. 1987;14:391–392. doi: 10.1016/s0140-6736(87)91772-7. [DOI] [PubMed] [Google Scholar]
- 14.Buterbaugh J, Wynstra C, Provencio N, Combs D, Gilbert M, Parthasarathy S. Cerebrovascular reactivity in young subjects with sleep apnea. Sleep. 2015;38:241–250. doi: 10.5665/sleep.4406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cenini G, Dowling AL, Beckett TL, Barone E, Mancuso C, Murphy MP, Levine H, 3rd, Lott IT, Schmitt FA, Butterfield DA, Head E. Association between frontal cortex oxidative damage and beta-amyloid as a function of age in Down syndrome. Biochim. Biophys. Acta. 2012;1822:130–138. doi: 10.1016/j.bbadis.2011.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chao LL, Decarli C, Kriger S, Truran D, Zhang Y, Laxamana J, Villeneuve S, Jagust WJ, Sanossian N, Mack WJ, Chui HC, Weiner MW. Associations between white matter hyperintensities and beta amyloid on integrity of projection, association, and limbic fiber tracts measured with diffusion tensor MRI. PLoS One. 2013;8:e65175. doi: 10.1371/journal.pone.0065175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cho ER, Kim H, Seo HS, Suh S, Lee SK, Shin C. Obstructive sleep apnea as a risk factor for silent cerebral infarction. J. Sleep Res. 2013;22:452–458. doi: 10.1111/jsr.12034. [DOI] [PubMed] [Google Scholar]
- 18.Chumlea WC, Cronk CE. Overweight among children with trisomy. J. Ment. Defic. Res. 1981;25(Pt 4):275–280. doi: 10.1111/j.1365-2788.1981.tb00118.x. [DOI] [PubMed] [Google Scholar]
- 19.Collacott RA, Cooper SA, Ismail IA. Multi-infarct dementia in Down's syndrome. J. Intellect. Disabil. Res. 1994;38(Pt 2):203–208. doi: 10.1111/j.1365-2788.1994.tb00375.x. [DOI] [PubMed] [Google Scholar]
- 20.Cramer SC, Robertson RL, Dooling EC, Scott RM. Moyamoya and Down syndrome. Stroke. 1996;27:2131–2135. doi: 10.1161/01.str.27.11.2131. [DOI] [PubMed] [Google Scholar]
- 21.de Borchgrave V, Saussu F, Depre A, de Barsy T. Moyamoya disease and Down syndrome: case report and review of the literature. Acta Neurol. Belg. 2002;102:63–66. [PubMed] [Google Scholar]
- 22.Donahue JE, Khurana JS, Adelman LS. Intracerebral hemorrhage in two patients with Down's syndrome and cerebral amyloid angiopathy. Acta Neuropathol. 1998;95:213–216. doi: 10.1007/s004010050789. [DOI] [PubMed] [Google Scholar]
- 23.Draheim CC, McCubbin JA, Williams DP. Differences in cardiovascular disease risk between nondiabetic adults with mental retardation with and without Down syndrome. Am. J. Ment. Retard. 2002;107:201–211. doi: 10.1352/0895-8017(2002)107<0201:DICDRB>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 24.Draheim CC, Geijer JR, Dengel DR. Comparison of intima-media thickness of the carotid artery and cardiovascular disease risk factors in adults with versus without the Down syndrome. Am. J. Cardiol. 2010;106:1512–1516. doi: 10.1016/j.amjcard.2010.06.079. [DOI] [PubMed] [Google Scholar]
- 25.Durgan DJ, Bryan RM., Jr. Cerebrovascular consequences of obstructive sleep apnea. J. Am. Heart Assoc. 2012;1:e000091. doi: 10.1161/JAHA.111.000091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Englund A, Jonsson B, Zander CS, Gustafsson J, Anneren G. Changes in mortality and causes of death in the Swedish Down syndrome population. Am. J. Med. Genet. A. 2013;161A:642–649. doi: 10.1002/ajmg.a.35706. [DOI] [PubMed] [Google Scholar]
- 27.Esbensen AJ. Health conditions associated with aging and end of life of adults with Down syndrome. Int. Rev. Res. Ment. Retard. 2010;39:107–126. doi: 10.1016/S0074-7750(10)39004-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Esiri M, Chance S, Joachim C, Warden D, Smallwood A, Sloan C, Christie S, Wilcock G, Smith AD. Cerebral amyloid angiopathy, subcortical white matter disease and dementia: literature review and study in OPTIMA. Brain Pathol. 2015;25:51–62. doi: 10.1111/bpa.12221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Farkas E, Donka G, de Vos RA, Mihaly A, Bari F, Luiten PG. Experimental cerebral hypoperfusion induces white matter injury and microglial activation in the rat brain. Acta Neuropathol. 2004;108:57–64. doi: 10.1007/s00401-004-0864-9. [DOI] [PubMed] [Google Scholar]
- 30.Freeman SB, Taft LF, Dooley KJ, Allran K, Sherman SL, Hassold TJ, Khoury MJ, Saker DM. Population-based study of congenital heart defects in Down syndrome. Am. J. Med. Genet. 1998;80:213–217. [PubMed] [Google Scholar]
- 31.Frost JL, Le KX, Cynis H, Ekpo E, Kleinschmidt M, Palmour RM, Ervin FR, Snigdha S, Cotman CW, Saido TC, Vassar RJ, St George-Hyslop P, Ikezu T, Schilling S, Demuth HU, Lemere CA. Pyroglutamate-3 amyloid-beta deposition in the brains of humans, non-human primates, canines, and Alzheimer disease-like transgenic mouse models. Am. J. Pathol. 2013;183:369–381. doi: 10.1016/j.ajpath.2013.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fukushima Y, Kondo Y, Kuroki Y, Miyake S, Iwamoto H, Sekido K, Yamaguchi K. Are Down syndrome patients predisposed to moyamoya disease? Eur. J. Pediatr. 1986;144:516–517. doi: 10.1007/BF00441756. [DOI] [PubMed] [Google Scholar]
- 33.Glasson EJ, Sullivan SG, Hussain R, Petterson BA, Montgomery PD, Bittles AH. The changing survival profile of people with Down's syndrome: implications for genetic counselling. Clin. Genet. 2002;62:390–393. doi: 10.1034/j.1399-0004.2002.620506.x. [DOI] [PubMed] [Google Scholar]
- 34.Goi G, Baquero-Herrera C, Licastro F, Dogliotti G, Corsi MM. Advanced oxidation protein products (AOPP) and high-sensitive C-reactive protein (hs-CRP) in an “atheroma-free model”: Down's syndrome. Int. J. Cardiol. 2006;113:427–429. doi: 10.1016/j.ijcard.2005.10.019. [DOI] [PubMed] [Google Scholar]
- 35.Griffin WS, Barger SW. Neuroinflammatory cytokines—the common thread in Alzheimer's pathogenesis. US Neurol. 2010;6:19–27. [PMC free article] [PubMed] [Google Scholar]
- 36.Griffin WS, Stanley LC, Ling C, White L, MacLeod V, Perrot LJ, White CL, Araoz C. Brain interleukin I and S-100 immunoreactivity are elevated in Down syndrome and Alzheimer's disease. Proc. Natl. Acad. Sci. U. S. A. 1989;86:7611–7615. doi: 10.1073/pnas.86.19.7611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Griffin WST, Sheng JG, McKenzie JE, Royston MC, Gentleman SM, Brumback RA, Cork LC, Del Bigio MR, Roberts GW, Mrak RE. Life-long overexpression of S100b in Down's syndrome: implications for Alzheimer pathogenesis. Neurobiol. Aging. 1998;19:401–405. doi: 10.1016/s0197-4580(98)00074-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Head E, Powell D, Gold BT, Schmitt FA. Alzheimer's disease in Down syndrome. European J. Neurodegen. Dis. 2012;1:353–364. [PMC free article] [PubMed] [Google Scholar]
- 39.Head E, Silverman W, Patterson D, Lott IT. Aging and Down syndrome. Curr. Gerontol. Geriatr. Res. 2012;2012a:412536. doi: 10.1155/2012/412536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ikeda S, Tokuda T, Yanagisawa N, Kametani F, Ohshima T, Allsop D. Variability of beta-amyloid protein deposited lesions in Down's syndrome brains. Tohoku J. Exp. Med. 1994;174:189–198. doi: 10.1620/tjem.174.189. [DOI] [PubMed] [Google Scholar]
- 41.James BD, Bennett DA, Boyle PA, Leurgans S, Schneider JA. Dementia from Alzheimer disease and mixed pathologies in the oldest old. JAMA. 2012;307:1798–1800. doi: 10.1001/jama.2012.3556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jastrzebski K, Kacperska MJ, Majos A, Grodzka M, Glabinski A. Hemorrhagic stroke, cerebral amyloid angiopathy, Down syndrome and the Boston criteria. Neurol. Neurochir. Pol. 2015;49:193–196. doi: 10.1016/j.pjnns.2015.04.006. [DOI] [PubMed] [Google Scholar]
- 43.Jellinger KA. Pathology and pathogenesis of vascular cognitive impairment—a critical update. Front. Aging Neurosci. 2013;5:17. doi: 10.3389/fnagi.2013.00017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jiwa NS, Garrard P, Hainsworth AH. Experimental models of vascular dementia and vascular cognitive impairment: a systematic review. J. Neurochem. 2010;115:814–828. doi: 10.1111/j.1471-4159.2010.06958.x. [DOI] [PubMed] [Google Scholar]
- 45.Kainth DS, Chaudhry SA, Kainth HS, Suri FK, Qureshi AI. Prevalence and characteristics of concurrent Down syndrome in patients with moyamoya disease. Neurosurgery. 2013;72:210–215. doi: 10.1227/NEU.0b013e31827b9beb. discussion 215. [DOI] [PubMed] [Google Scholar]
- 46.Kammoun S, Gold G, Bouras C, Giannakopoulos P, McGee W, Herrmann F, Michel JP. Immediate causes of death of demented and non-demented elderly. Acta Neurol. Scand. Suppl. 2000;176:96–99. doi: 10.1034/j.1600-0404.2000.00314.x. [DOI] [PubMed] [Google Scholar]
- 47.Kim H, Yun CH, Thomas RJ, Lee SH, Seo HS, Cho ER, Lee SK, Yoon DW, Suh S, Shin C. Obstructive sleep apnea as a risk factor for cerebral white matter change in a middle-aged and older general population. Sleep. 2013;36:709–715B. doi: 10.5665/sleep.2632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Krinsky-McHale SJ, Silverman W. Dementia and mild cognitive impairment in adults with intellectual disability: issues of diagnosis. Dev. Disabil. Res. Rev. 2013;18:31–42. doi: 10.1002/ddrr.1126. [DOI] [PubMed] [Google Scholar]
- 49.Lai F, Williams MD. A prospective study of Alzheimer disease in Down Syndrome. Arch. Neurol.-Chicago. 1989;46:849–853. doi: 10.1001/archneur.1989.00520440031017. [DOI] [PubMed] [Google Scholar]
- 50.Langa KM, Foster NL, Larson EB. Mixed dementia: emerging concepts and therapeutic implications. JAMA. 2004;292:2901–2908. doi: 10.1001/jama.292.23.2901. [DOI] [PubMed] [Google Scholar]
- 51.Lemere CA, Blusztajn JK, Yamaguchi H, Wisniewski T, Saido TC, Selkoe DJ. Sequence of deposition of heterogeneous amyloid beta-peptides and APOE in Down Syndrome: implications for initial events in amyloid plaque formation. Neurobiol. Dis. 1996;3:16–32. doi: 10.1006/nbdi.1996.0003. [DOI] [PubMed] [Google Scholar]
- 52.Leno C, Mateo I, Cid C, Berciano J, Sedano C. Autoimmunity in Down's syndrome: another possible mechanism of moyamoya disease. Stroke. 1998;29:868–869. doi: 10.1161/01.str.29.4.868. [DOI] [PubMed] [Google Scholar]
- 53.Leverenz JB, Raskind MA. Early amyloid deposition in the medial temporal lobe of young Down syndrome patients: a regional quantitative analysis. Exp. Neurol. 1998;150:296–304. doi: 10.1006/exnr.1997.6777. [DOI] [PubMed] [Google Scholar]
- 54.Levine DA, Langa KM. Vascular cognitive impairment: disease mechanisms and therapeutic implications. Neurotherapeutics. 2011;8:361–373. doi: 10.1007/s13311-011-0047-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Licastro F, Marocchi A, Penco S, Porcellini E, Lio D, Dogliotti G, C MM. Does Down's syndrome support the homocysteine theory of atherogenesis? Experience in elderly subjects with trisomy 21. Arch. Gerontol. Geriatr. 2006;43:381–387. doi: 10.1016/j.archger.2006.01.003. [DOI] [PubMed] [Google Scholar]
- 56.Magge SN, O'Neill KL, Shults J, Stallings VA, Stettler N. Leptin levels among pre-pubertal children with Down syndrome compared with their siblings. J. Pediatr. 2008;152:321–326. doi: 10.1016/j.jpeds.2007.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mann DMA, Esiri MM. The pattern of acquisition of plaques and tangles in the brains of patients under 50 years of age with Down's syndrome. J. Neurol. Sci. 1989;89:169–179. doi: 10.1016/0022-510x(89)90019-1. [DOI] [PubMed] [Google Scholar]
- 58.Mantovani A, Sica A, Sozzani S, Allavena P, Vecchi A, Locati M. The chemokine system in diverse forms of macrophage activation and polarization. Trends Immunol. 2004;25:677–686. doi: 10.1016/j.it.2004.09.015. [DOI] [PubMed] [Google Scholar]
- 59.Mantovani A, Sica A, Sozzani S, Allavena P, Vecchi A, Locati M. The chemokine system in diverse forms of macrophage activation and polarization. Trends Immunol. 2004;25:677–686. doi: 10.1016/j.it.2004.09.015. [DOI] [PubMed] [Google Scholar]
- 60.McCarron MO, Nicoll JA, Graham DI. A quartet of Down's syndrome, Alzheimer's disease, cerebral amyloid angiopathy, and cerebral haemorrhage: interacting genetic risk factors. J. Neurol. Neurosurg. Psychiatry. 1998;65:405–406. doi: 10.1136/jnnp.65.3.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Melville CA, Cooper SA, McGrother CW, Thorp CF, Collacott R. Obesity in adults with Down syndrome: a case–control study. J. Intellect. Disabil. Res. 2005;49:125–133. doi: 10.1111/j.1365-2788.2004.00616.x. [DOI] [PubMed] [Google Scholar]
- 62.Mendel T, Bertrand E, Szpak GM, Stepien T, Wierzba-Bobrowicz T. Cerebral amyloid angiopathy as a cause of an extensive brain hemorrhage in adult patient with Down's syndrome — a case report. Folia Neuropathol. 2010;48:206–211. [PubMed] [Google Scholar]
- 63.Morrison RA, McGrath A, Davidson G, Brown JJ, Murray GD, Lever AF. Low blood pressure in Down's syndrome, a link with Alzheimer's disease? Hypertension. 1996;28:569–575. doi: 10.1161/01.hyp.28.4.569. [DOI] [PubMed] [Google Scholar]
- 64.Mosser DM, Edwards JP. Exploring the full spectrum of macrophage activation. Nat. Rev. Immunol. 2008;8:958–969. doi: 10.1038/nri2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Mrak RE, Griffin WS. Trisomy 21 and the brain. J. Neuropathol. Exp. Neurol. 2004;63:679–685. doi: 10.1093/jnen/63.7.679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Murdoch JC, Rodger JC, Rao SS, Fletcher CD, Dunningham MG. Down's syndrome: an atheroma-free model? Br. Med. J. 1977;2:226–228. doi: 10.1136/bmj.2.6081.226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Myrelid A, Gustafsson J, Ollars B, Anneren G. Growth charts for Down's syndrome from birth to 18 years of age. Arch. Dis. Child. 2002;87:97–103. doi: 10.1136/adc.87.2.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Nagasaka T, Shiozawa Z, Kobayashi M, Shindo K, Tsunoda S, Amino A. Autopsy findings in Down's syndrome with cerebrovascular disorder. Clin. Neuropathol. 1996;15:145–149. [PubMed] [Google Scholar]
- 69.Naito KS, Sekijima Y, Ikeda S. Cerebral amyloid angiopathy-related hemorrhage in a middle-aged patient with Down's syndrome. Amyloid. 2008;15:275–277. doi: 10.1080/13506120802524981. [DOI] [PubMed] [Google Scholar]
- 70.Nishida Y, Akaoka I, Nishizawa T, Maruki M, Maruki K. Hyperlipidaemia in patients with Down's syndrome. Atherosclerosis. 1977;26:369–372. doi: 10.1016/0021-9150(77)90090-9. [DOI] [PubMed] [Google Scholar]
- 71.Park M, Raila FA, Russell WF. Moyamoya disease in an adult with Down syndrome: comparison of magnetic resonance angiography and conventional angiography. South. Med. J. 1996;89:89–92. doi: 10.1097/00007611-199601000-00018. [DOI] [PubMed] [Google Scholar]
- 72.Patel BN, Pang D, Stern Y, Silverman W, Kline JK, Mayeux R, Schupf N. Obesity enhances verbal memory in postmenopausal women with Down syndrome. Neurobiol. Aging. 2004;25:159–166. doi: 10.1016/s0197-4580(03)00089-7. [DOI] [PubMed] [Google Scholar]
- 73.Pearson E, Lenn NJ, Cail WS. Moyamoya and other causes of stroke in patients with Down syndrome. Pediatr. Neurol. 1985;1:174–179. doi: 10.1016/0887-8994(85)90060-8. [DOI] [PubMed] [Google Scholar]
- 74.Pogribna M, Melnyk S, Pogribny I, Chango A, Yi P, James SJ. Homocysteine metabolism in children with Down syndrome: in vitro modulation. Am. J. Hum. Genet. 2001;69:88–95. doi: 10.1086/321262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Prasher VP, Farrer MJ, Kessling AM, Fisher EM, West RJ, Barber PC, Butler AC. Molecular mapping of Alzheimer-type dementia in Down's syndrome. Ann. Neurol. 1998;43:380–383. doi: 10.1002/ana.410430316. [DOI] [PubMed] [Google Scholar]
- 76.Provenzano FA, Muraskin J, Tosto G, Narkhede A, Wasserman BT, Griffith EY, Guzman VA, Meier IB, Zimmerman ME, Brickman AM. I. Alzheimer's Disease Neuroimaging, White matter hyperintensities and cerebral amyloidosis: necessary and sufficient for clinical expression of Alzheimer disease? JAMA Neurol. 2013;70:455–461. doi: 10.1001/jamaneurol.2013.1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ramia M, Musharrafieh U, Khaddage W, Sabri A. Revisiting Down syndrome from the ENT perspective: review of literature and recommendations. Eur. Arch. Otorhinolaryngol. 2014;271:863–869. doi: 10.1007/s00405-013-2563-4. [DOI] [PubMed] [Google Scholar]
- 78.Rimmer JH, Yamaki K, Lowry BM, Wang E, Vogel LC. Obesity and obesity-related secondary conditions in adolescents with intellectual/developmental disabilities. J. Intellect. Disabil. Res. 2010;54:787–794. doi: 10.1111/j.1365-2788.2010.01305.x. [DOI] [PubMed] [Google Scholar]
- 79.Rodrigues AN, Coelho LC, Goncalves WL, Gouvea SA, Vasconcellos MJ, Cunha RS, Abreu GR. Stiffness of the large arteries in individuals with and without Down syndrome. Vasc. Health Risk Manag. 2011;7:375–381. doi: 10.2147/VHRM.S21273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Rubin SS, Rimmer JH, Chicoine B, Braddock D, McGuire DE. Overweight prevalence in persons with Down syndrome. Ment. Retard. 1998;36:175–181. doi: 10.1352/0047-6765(1998)036<0175:OPIPWD>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 81.Rumble B, Retallack R, Hilbich C, Simms G, Multhaup G, Martins R, Hockey A, Montgomery P, Beyreuther K, Masters CL. Amyloid A4 and its precursor in Down's syndrome and Alzheimer's disease. N. Engl. J. Med. 1989;320:1446–1462. doi: 10.1056/NEJM198906013202203. [DOI] [PubMed] [Google Scholar]
- 82.Schrager GO, Cohen SJ, Vigman MP. Acute hemiplegia and cortical blindess due to moyamoya disease: report of a case in a child with Down's syndrome. Pediatrics. 1977;60:33–37. [PubMed] [Google Scholar]
- 83.Schupf N, Zigman WB, Tang MX, Pang D, Mayeux R, Mehta P, Silverman W. Change in plasma Ass peptides and onset of dementia in adults with Down syndrome. Neurology. 2010;75:1639–1644. doi: 10.1212/WNL.0b013e3181fb448b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Schupf N, Lee JH, Wei M, Pang D, Chace C, Cheng R, Zigman WB, Tycko B, Silverman W. Estrogen receptor-alpha variants increase risk of Alzheimer's disease in women with Down syndrome. Dement. Geriatr. Cogn. Disord. 2008;25:476–482. doi: 10.1159/000126495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Schupf N, Sergievsky GH. Genetic and host factors for dementia in Down's syndrome. Br. J. Psychiatry. 2002;180:405–410. doi: 10.1192/bjp.180.5.405. [DOI] [PubMed] [Google Scholar]
- 86.Shott SR, Amin R, Chini B, Heubi C, Hotze S, Akers R. Obstructive sleep apnea: should all children with Down syndrome be tested? Arch. Otolaryngol. Head Neck Surg. 2006;132:432–436. doi: 10.1001/archotol.132.4.432. [DOI] [PubMed] [Google Scholar]
- 87.Stoll C, Alembik Y, Dott B, Roth MP. Study of Down syndrome in 238,942 consecutive births. Ann. Genet. 1998;41:44–51. [PubMed] [Google Scholar]
- 88.Stoltzner SE, Grenfell TJ, Mori C, Wisniewski KE, Wisniewski TM, Selkoe DJ, Lemere CA. Temporal accrual of complement proteins in amyloid plaques in Down's syndrome with Alzheimer's disease. Am. J. Pathol. 2000;156:489–499. doi: 10.1016/S0002-9440(10)64753-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Styles ME, Cole TJ, Dennis J, Preece MA. New cross sectional stature, weight, and head circumference references for Down's syndrome in the UK and Republic of Ireland. Arch. Dis. Child. 2002;87:104–108. doi: 10.1136/adc.87.2.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Sudduth TL, Greenstein A, Wilcock DM. Intracranial injection of gammagard, a human IVIg, modulates the inflammatory response of the brain and lowers Abeta in APP/PS1 mice along a different time course than anti-Abeta antibodies. J. Neurosci. 2013;33:9684–9692. doi: 10.1523/JNEUROSCI.1220-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Sudduth TL, Schmitt FA, Nelson PT, Wilcock DM. Neuroinflammatory phenotype in early Alzheimer's disease. Neurobiol. Aging. 2013;34:1051–1059. doi: 10.1016/j.neurobiolaging.2012.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Sudduth TL, Weekman EM, Brothers HM, Braun K, Wilcock DM. Beta-amyloid deposition is shifted to the vasculature and memory impairment is exacerbated when hyperhomocysteinemia is induced in APP/PS1 transgenic mice. Alzheimers Res. Ther. 2014;6:32. doi: 10.1186/alzrt262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Suzuki J, Takaku A. Cerebrovascular “moyamoya” disease. Disease showing abnormal net-like vessels in base of brain. Arch. Neurol. 1969;20:288–299. doi: 10.1001/archneur.1969.00480090076012. [DOI] [PubMed] [Google Scholar]
- 94.Tansley G, Holmes DT, Lutjohann D, Head E, Wellington CL. Sterol lipid metabolism in Down syndrome revisited: Down syndrome is associated with a selective reduction in serum brassicasterol levels. Curr. Gerontol. Geriatr. Res. 2012;2012:179318. doi: 10.1155/2012/179318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Trois MS, Capone GT, Lutz JA, Melendres MC, Schwartz AR, Collop NA, Marcus CL. Obstructive sleep apnea in adults with Down syndrome. J. Clin. Sleep Med. 2009;5:317–323. [PMC free article] [PubMed] [Google Scholar]
- 96.van Erven PM, Gabreels FJ, Thijssen HO, Renier WO. The Moya-moya syndrome: a report of two children. Clin. Neurol. Neurosurg. 1982;84:179–189. doi: 10.1016/0303-8467(82)90041-5. [DOI] [PubMed] [Google Scholar]
- 97.van Gameren-Oosterom HB, van Dommelen P, Schonbeck Y, Oudesluys-Murphy AM, van Wouwe JP, Buitendijk SE. Prevalence of overweight in Dutch children with Down syndrome. Pediatrics. 2012;130:e1520–e1526. doi: 10.1542/peds.2012-0886. [DOI] [PubMed] [Google Scholar]
- 98.Vianello E, Dogliotti G, Dozio E, Corsi Romanelli MM. Low heart-type fatty acid binding protein level during aging may protect Down syndrome people against atherosclerosis. Immun. Ageing. 2013;10:2. doi: 10.1186/1742-4933-10-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Vinters HV. Cerebral amyloid angiopathy. Stroke. 1987;18:311–324. doi: 10.1161/01.str.18.2.311. [DOI] [PubMed] [Google Scholar]
- 100.Vis JC, Duffels MG, Winter MM, Weijerman ME, Cobben JM, Huisman SA, Mulder BJ. Down syndrome: a cardiovascular perspective. J. Intellect. Disabil. Res. 2009;53:419–425. doi: 10.1111/j.1365-2788.2009.01158.x. [DOI] [PubMed] [Google Scholar]
- 101.Wilcock DM. Neuroinflammation in the aging Down syndrome brain; lessons from Alzheimer's disease. Curr. Gerontol. Geriatr. Res. 2012;2012:170276. doi: 10.1155/2012/170276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Wilcock DM. Neuroinflammatory phenotypes and their roles in Alzheimer's disease. Neurodegener. Dis. 2014;13:183–185. doi: 10.1159/000354228. [DOI] [PubMed] [Google Scholar]
- 103.Wilcock DM, Griffin WS. Down's syndrome, neuroinflammation, and Alzheimer neuropathogenesis. J. Neuroinflammation. 2013;10:84. doi: 10.1186/1742-2094-10-84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Wilcock DM, Zhao Q, Morgan D, Gordon MN, Everhart A, Wilson JG, Lee JE, Colton CA. Diverse inflammatory responses in transgenic mouse models of AD and the effect of immunotherapy on these responses. ASN Neuro. 2011 doi: 10.1042/AN20110018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Wilcock DM, Hurban J, Helman AM, Sudduth TL, McCarty KL, Beckett TL, Ferrell JC, Murphy MP, Abner EL, Schmitt FA, Head E. Down syndrome individuals with Alzheimer's disease have a distinct neuroinflammatory phenotype compared to sporadic Alzheimer's disease. Neurobiol. Aging. 2015;36:2468–2474. doi: 10.1016/j.neurobiolaging.2015.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Wisniewski K, Wisniewski H, Wen G. Occurrence of neuropathological changes and dementia of Alzheimer's disease in Down's syndrome. Ann. Neurol. 1985;17:278–282. doi: 10.1002/ana.410170310. [DOI] [PubMed] [Google Scholar]
- 107.Wisniewski K, Howe J, Williams G, Wisniewski HM. Precocious aging and dementia in patients with Down's syndrome. Biol. Psychiatry. 1978;13:619–627. [PubMed] [Google Scholar]
- 108.Yaffe K, Laffan AM, Harrison SL, Redline S, Spira AP, Ensrud KE, Ancoli-Israel S, Stone KL. Sleep-disordered breathing, hypoxia, and risk of mild cognitive impairment and dementia in older women. JAMA. 2011;306:613–619. doi: 10.1001/jama.2011.1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Yla-Herttuala S, Luoma J, Nikkari T, Kivimaki T. Down's syndrome and atherosclerosis. Atherosclerosis. 1989;76:269–272. doi: 10.1016/0021-9150(89)90110-x. [DOI] [PubMed] [Google Scholar]
- 110.Yla-Herttuala S, Luoma J, Nikkari T, Kivimaki T. Down's syndrome and atherosclerosis. Atherosclerosis. 1989;76:269–272. doi: 10.1016/0021-9150(89)90110-x. [DOI] [PubMed] [Google Scholar]
- 111.Zhao Q, Lee JH, Pang D, Temkin A, Park N, Janicki SC, Zigman WB, Silverman W, Tycko B, Schupf N. Estrogen receptor-beta variants are associated with increased risk of Alzheimer's disease in women with Down syndrome. Dement. Geriatr. Cogn. Disord. 2011;32:241–249. doi: 10.1159/000334522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Zigman WB. Atypical aging in Down syndrome. Dev. Disabil. Res. Rev. 2013;18:51–67. doi: 10.1002/ddrr.1128. [DOI] [PubMed] [Google Scholar]