Abstract
Effects of nicotinic receptor agonists (epibatidine and nicotine) on mechano-sensitive bladder afferent nerve (MS-BAN) activity were studied in an in vitro bladder-pelvic afferent nerve preparation. MS-BAN activity was induced by isotonic distention of the bladder at pressures of 10–40 cmH2O. The effect of epibatidine varied according to the concentration, route of administration and the intravesical pressure stimulus. Epibatidine (300–500 nM) administered in the perfusate to the serosal surface of the bladder decreased distension evoked afferent firing by 30%–50% depending on the bladder pressure. However these concentrations also produced an immediate increase in tonic afferent firing in the empty bladder. Lower concentrations (50–100 nM) elicited weaker and more variable effects. The inhibitory effects were blocked by bath application of mecamylamine (150 μM) a nicotinic receptor antagonist. Bath application of nicotine (20 μM) elicited similar effects. Intravesical administration of epibatidine (500 nM) significantly increased MS-BAN firing by 15–30%; while lower concentrations (200–300 nM) were ineffective. This facilitatory effect of epibatidine was blocked by intravesical administration of mecamylamine (250 μM). Electrical stimulation on the surface of the bladder elicited action potentials (AP) in BAN. Bath application of epibatidine (300 nM) or nicotine (20 μM) did not change either the voltage threshold or the area of evoked AP. These results indicate that nicotinic agonists: (1) enhance MS-BAN activity originating at afferent receptors near the urothelium, (2) inhibit MS-BAN activity originating at afferent receptors located at other sites in the bladder, (3) directly excite unidentified afferents, (4) do not alter afferent axonal excitability.
Keywords: Neuronal nicotinic receptors, Bladder afferent nerves, Urothelium, Epibatidine, Nicotine
1. Introduction
Intravesical administration of nicotinic (Beckel et al., 2006; Beckel and Birder, 2012; Kontani et al., 2009; Masuda et al., 2006) or muscarinic (Kullmann et al., 2008b; Matsumoto et al., 2010, 2012) cholinergic receptor agonists increases or decreases the frequency of reflex voiding in rats depending on the concentration of the agonist and/or the type of receptor activated (de Groat et al., 2015). These observations raise the possibility that sensory pathways in the urinary bladder are sensitive to cholinergic modulatory mechanisms. The modulation may occur as a result of a direct action on afferent nerves which express nicotinic and muscarinic receptors (Kontani et al., 2009; Masuda et al., 2006; Nandigama et al., 2010; 2013; Yu and de Groat, 2010) or indirectly via an action on urothelial cells which also express these receptors (Beckel and Birder, 2012; Kullmann et al., 2008a) and release neurotransmitters such as ATP, ACh and NO (Birder et al., 1998; Ferguson et al., 1997; Hanna-Mitchell et al., 2007; Lips et al., 2007; Silva et al., 2015; Yoshida et al., 2010) that can influence the excitability of adjacent afferent nerves (Birder and Andersson, 2013; Cockayne et al., 2000; de Groat and Yoshimura, 2009; Ford et al., 2015; Kullmann et al., 2008b).
In anesthetized rats intravesical administration of oxotremorine, a non-selective muscarinic receptor agonist, reduces voiding frequency in low concentrations and increases voiding frequency in high concentrations (Kullmann et al., 2008b). The former is suppressed by a NOS inhibitor and the latter is partially reduced by a purinergic receptor antagonist (PPADS) indicating that NO and ATP contribute to the inhibitory and facilitatory effects, respectively.
Intravesical administration of neuronal nicotinic receptor (NNR) agonists elicits similar mixed effects on voiding frequency (Beckel et al., 2006). Nicotine or choline reduce voiding frequency; an effect blocked by a specific α7 NNR antagonist (methyllycaconitine); whereas cytisine, an α3 NNR agonist increases voiding frequency; an effect blocked by PPADS (Beckel and Birder, 2012). Cytisine increases release of ATP from cultured urothelial cells, whereas choline suppresses release suggesting that the effects of the NNR agonists on voiding are mediated in part by activation of receptors in the urothelium leading to changes in urothelial-afferent interactions and in turn changes in bladder afferent input to the spinal cord (Beckel and Birder, 2012).
Combined immunohistochemical and RT-PCR analysis has revealed that bladder afferent neurons in mouse lumbosacral dorsal root ganglia express multiple subtypes of NNRs (Nandigama et al., 2013). The majority of afferent neurons express α3 NNRs which are present in at least two distinct populations of bladder afferents: (1) mucosal high-threshold mechanoreceptors with chemosensitivity and (2) fast-conducting mechanosensors.
In the present experiments we used an in vitro bladder-pelvic afferent nerve preparation (Yu and de Groat, 2008) to examine the effects of nicotinic receptor agonists (epibatidine or nicotine) and antagonists (mecamylamine or hexamethonium) on the activity of mechano-sensitive bladder afferents induced by bladder distension. The results indicate that activation of nicotinic receptors enhances the activity of mechano-sensitive afferents with receptors near the urothelium, inhibits the activity of mechano-sensitive afferents with receptors at other sites in the bladder and excites unidentified afferent nerves.
2. Results
2.1. Effects of bath application of nicotinic receptor agonists on bladder afferent firing
Multiunit afferent activity in the pelvic nerve was induced by isotonic distension of the bladder with Krebs solution at 10, 20, 30 and 40 cmH2O pressures for 30 sec at 30 sec intervals to evaluate the pressure-response relationship of mechano-sensitive afferent nerve activity (Fig. 1). Afferent firing reached a maximum within 5–10 sec after isotonic bladder distention and then declined indicating some adaptation at a constant intravesical pressure. Peak firing ranged between 20–80 spikes/sec (Fig. 1) and increased with increasing pressures (Fig. 2A). Multiple applications of the series of pressure steps after 10–15 min recovery periods elicited similar stimulus-response curves (Fig. 2A).
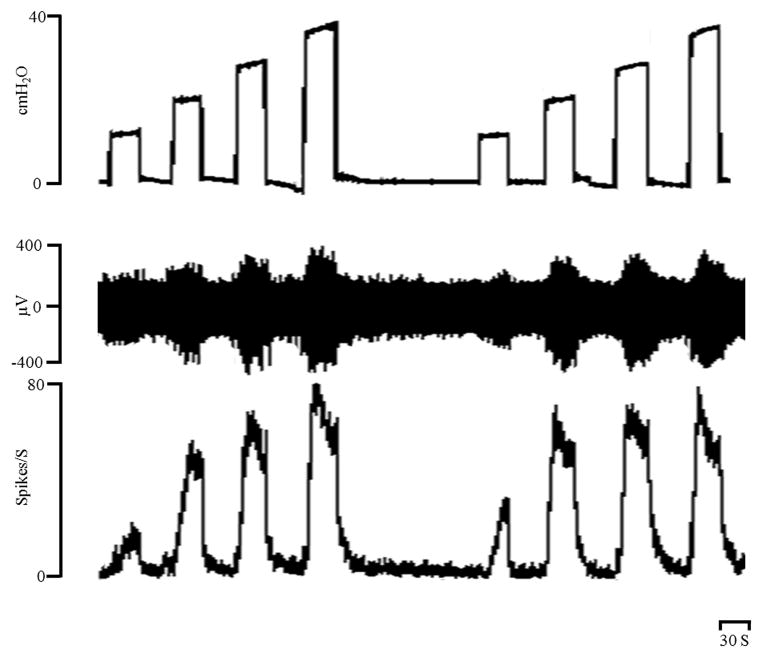
Figure 1.
Increases in bladder afferent firing evoked by 10, 20, 30 and 40 cm H2O intravesical pressures applied for 30 sec at 30 sec intervals. Top trace: intravesical pressure (cm H2O), middle trace afferent firing (μV), bottom trace rate meter recording of afferent firing (Spikes/S). Horizontal calibration bar represents 30 sec.
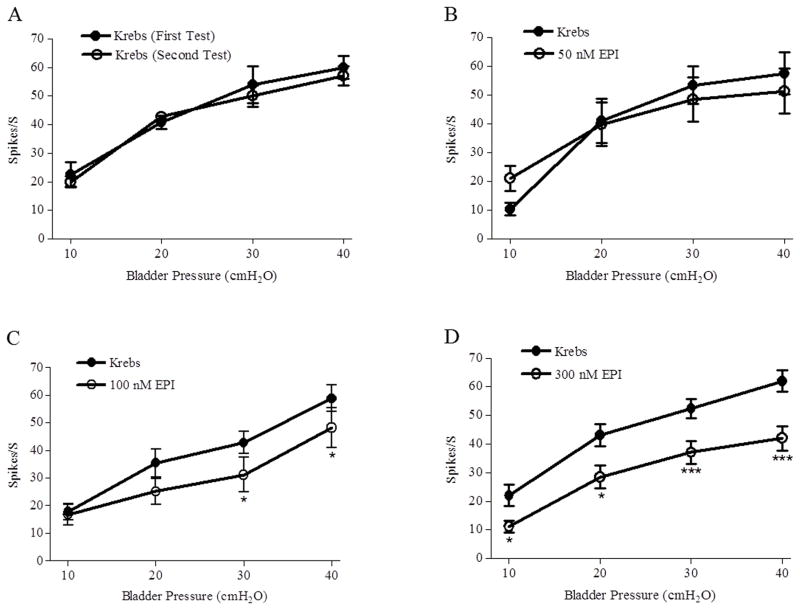
Figure 2.
Effects of bath application of epibatidine (EPI, 50, 100 and 300 nM) on peak bladder afferent nerve firing (Spikes/S) induced by isotonic distention of the bladder at 10, 20, 30, and 40 cmH2O for 30 sec at 30 sec intervals. A: Two trials of bladder distention elicit reproducible responses (n=4 preparations). B: EPI (50 nM) does not alter afferent firing (p>0.05, n=7). C: EPI (100 nM) does not alter afferent firing elicited by 10 and 20 cmH2O bladder pressures, however significantly decreases afferent firing (* p<0.05, n=11) elicited by 30 and 40 cmH2O bladder pressures. D: EPI (300 nM) significantly decreases afferent firing (* p<0.05, *** p<0.001, n=12) elicited by 10–40 cmH2O bladder pressures.
Concentrated epibatidine solutions injected into the bathing solution to produce final concentrations ranging from 50–500 nM were tested on afferent firing induced by isotonic distention of the bladder at pressures ranging from 10 to 40 cmH2O (Figs. 2B–D and 3B). High concentrations of epibatidine (300 nM, n=12, Figs 2D and 3B or 500 nM, n=4, not shown in the figures) significantly (p<0.05) decreased firing between 30–50% depending on the pressure stimulus (Figs. 2D and 3B), while 100 nM decreased the firing induced by 30 and 40 cmH2O pressures but not by 10 and 20 cmH2O pressures (n=11, Figure 2C). Epibatidine (50 nM) did not change the afferent firing (n=5, Fig 2B, p >0.05),
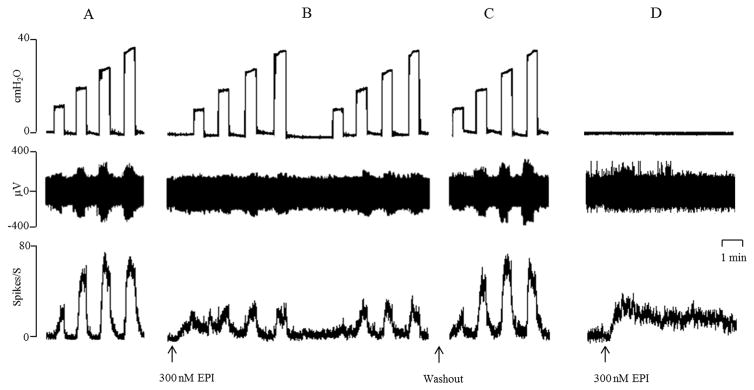
Figure 3.
Bath application of epibatidine (EPI, 300 nM at arrows) suppresses bladder afferent firing evoked by increases in bladder pressure (10–40 cm H2O) (A–C) applied for 30 sec at 30 sec intervals. Top trace: intravesical pressure (cm H2O), middle trace afferent firing (μV), bottom trace rate meter recording of afferent firing (Spikes/S). A: Control. B: EPI suppresses afferent firing. Note EPI also elicits a small increase in tonic firing between bladder distensions. C: Recovery of firing after washout (arrow) of EPI for 30 min. D: With the bladder empty bath application of EPI (300 nM, at arrow) increases afferent firing. All records are from one preparation. Horizontal calibration bar represents 1 min.
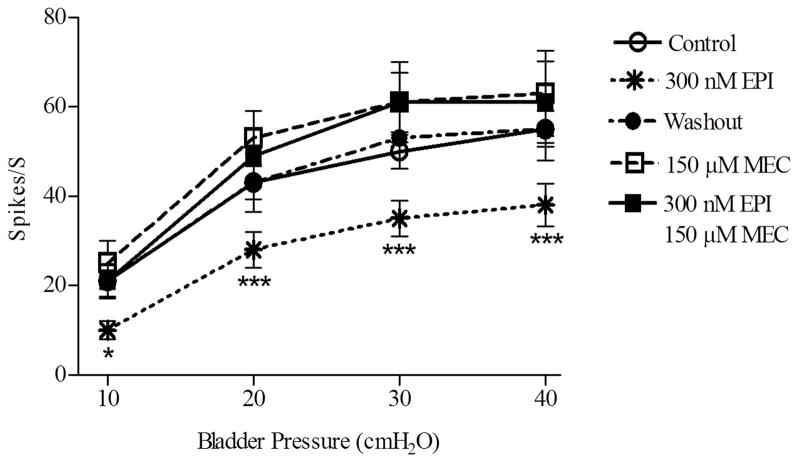
The highest concentrations of epibatidine (300–500 nM) also induced tonic afferent firing that was evident at 0 cmH2O bladder pressure between the isotonic bladder distensions (Fig. 3B and D; Fig. 4B). The tonic firing ranged from 10–30 spikes/sec in different experiments and persisted for 5–10 min after the application of the drug. The inhibitory effects as well as the tonic afferent firing induced by epibatidine (300 nM and 500 nM) were suppressed by bath application of 150 μM mecamylamine, a neuronal nicotinic receptor blocking agent (Fig. 4E and Fig 5, n=5). Application of 150 μM mecamylamine in the absence of epibatidine did not significantly alter the afferent firing induced by isotonic distension of the bladder at 10–40 cmH2O pressures (Fig. 4E and Fig. 5). The inhibitory effect of epibatidine recovered 30–40 min after washout of mecamylamine (Fig. 4F).
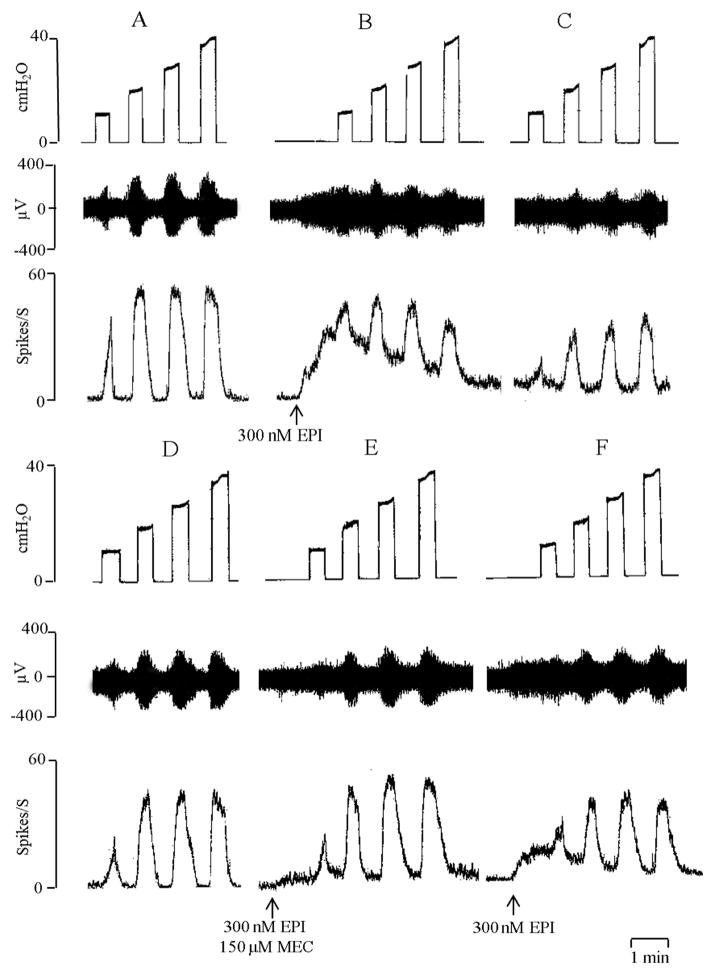
Figure 4.
Bath application of epibatidine (EPI, 300 nM, at arrows) evokes tonic firing and suppresses bladder afferent nerve firing induced by isotonic distention of the bladder at 10–40 cmH2O. All records are from one preparation. Top traces are intravesical pressure in cmH2O; middle traces show afferent nerve firing and bottom traces represent rate meter recording of afferent firing. A: Afferent firing evoked by distention of the bladder before application of EPI. B: Bath application of EPI (300 nM) elicits tonic firing and decreases bladder distension evoked afferent firing. C: The inhibitory effect persists for 20 min in the presence of EPI at a time when the EPI evoked tonic firing is markedly reduced. Time between records B and C is 20 min. D: After washout of EPI the distension evoked afferent firing returned to control. E: Inhibitory effect of EPI on distension evoked afferent firing and the tonic firing induced by EPI is blocked by bath administration of mecamylamine (150 μM) a nicotinic receptor antagonist. Time between D and E is 15 min. F: 30 min after washout of mecamylamine, EPI induced tonic firing and inhibition of distension evoked afferent firing partially recovers. Horizontal calibration represents 1 min.
Figure 5.
Summary of the effect of bath application of epibatidine (EPI, 300 nM) on bladder afferent firing (Spikes/S) induced by isotonic distention of the bladder at pressures of 10–40 cmH2O. EPI decrease in afferent firing (n=5 experiments, ***p<0.001, * p<0.05) is reversed 30 min after washout and is blocked by 150 μM mecamylamine (MEC) (n=5). MEC alone does not alter bladder afferent firing.
The effect of nicotine on bladder afferent firing was tested using a similar experimental protocol and bladder distention at pressures of 10–40 cmH2O. Bath application of 5 μM (n=4) or 10 μM (n=8) nicotine did not change distension evoked or basal afferent firing. However, 20 μM nicotine (n=5) elicited an initial increase in tonic firing to 10–20 spikes/sec within a few seconds after injection into the bath when the bladder was empty (data not shown). This concentration of nicotine also significantly decreased by 26–31% (p<0.05, n=5) the afferent firing induced by isotonic distention of the bladder at pressures of 20, 30 and 40 cmH2O. The peak firing at 10 cmH2O pressure was not significantly reduced (n=5, p>0.05). The inhibitory effects of nicotine on the bladder afferent nerve firing were completely blocked by bath application of hexamethonium (250 μM), a neuronal nicotinic receptor blocking agent (data not shown).
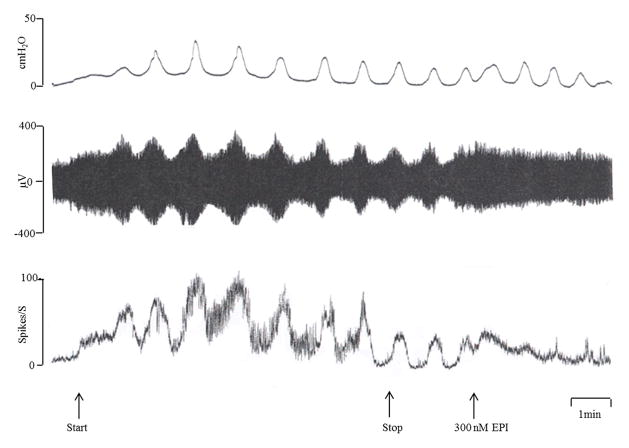
Additional experiments were conducted to examine the mechanisms underlying the increase in tonic afferent firing evoked by high concentrations of epibatidine or nicotine. Nicotinic receptor agonists can excite autonomic ganglion cells or postganglionic nerve terminals and thereby evoke the release of ACh or ATP that could in turn elicit bladder contractions leading to an increase tension in the bladder wall and induce mechano-sensitive afferent firing. This possibility was evaluated by filling the bladder with Krebs solution at the rate of 0.04 ml/min for 3 or 8 min (Fig. 6) and then measuring afferent firing and intravesical pressure under isovolumetric conditions following bath application of 300 nM epibatidine (Fig. 6). In some experiments (n=3) epibatidine did not induce a rise in baseline bladder pressure or a change in the phasic bladder contractions. However afferent firing transiently increased in these experiments (Fig. 6). In other experiments (n=6) epibatidine (300 nM) injected into bath did increase bladder pressure and firing (Fig. 7A). In the majority of these experiments (5 out of 6) the afferent firing increased 5–15 sec before a detectable increase in pressure (Fig. 7A); and the firing peaked (range, 30–60 spikes/sec) and began to decline prior to the peak in bladder pressure. On the other hand, afferent firing evoked by spontaneous contractions (Fig. 6) in the distended bladder or evoked by isotonic distension of the bladder (Fig. 1) correlated well with the onset and peak of the increases in pressure.
Figure 6.
Effects of bath application of epibatidine (EPI, 300 nM) on bladder afferent nerve firing induced by intravesical infusion of Krebs solution at the rate of 0.04 ml/min for 8 min. The top traces represent bladder contractile activity measured as intravesical pressure in cmH2O, the middle traces represent afferent nerve firing (μV) and the bottom traces represent rate meter recording of afferent firing (Spikes/S). Arrows represent start and stop of infusion of Krebs solution and bath application of EPI. Bladder distension induces rhythmic bladder contractions (recorded under isovolumetric conditions) that are accompanied by bursts of afferent activity. EPI induces an initial increase in tonic afferent firing and a later occurring decrease in the firing associated with bladder contractions. Horizontal calibration represents 1 min.
Figure 7.
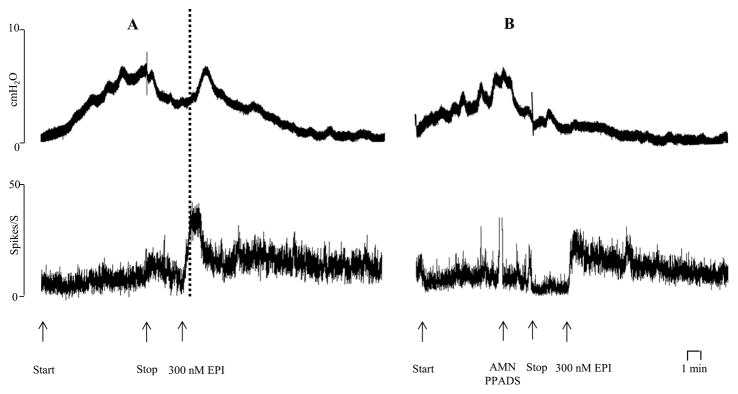
Effects of bath application of epibatidine (EPI, 300 nM) on bladder activity induced by intravesical infusion of Krebs solution at 0.04 ml/min for 8 min. Arrows indicate start and stop of the infusion. Records A and B are from the same preparation. A: Under isovolumetric conditions EPI induces a bladder contraction, an increase in bladder pressure (top trace) and afferent firing (bottom trace). The dotted line shows the afferent firing increases before a detectable increase in bladder pressure. EPI was washed out of the bath during the time (30 min) between records A and B. B: After application of atropine methyl nitrate (AMN, 5 μM) and PPADS (30 μM) to block muscarinic and purinergic receptors bath application of EPI (300 nM) still increases afferent firing but does not elicit a bladder contraction and an increase in bladder pressure. Horizontal calibration represents 1 min.
To determine the mechanism underlying the epibatidine evoked contractions and the relationship between the contractions and afferent firing, we administered a combination of atropine methyl nitrate (AMN, 5 uM) and PPADS (30 uM) into the bath prior to epibatidine to block post-junctional muscarinic cholinergic and purinergic receptors that could mediate smooth muscle contractions elicited by excitatory transmitters released from parasympathetic postganglionic nerves. This treatment blocked the epibatidine evoked contractions (Fig. 7B) but only reduced the afferent firing by 32.7% (p=0.03, n=5 experiments) from an average peak of 39.8 ± 9.2 spikes/sec to 26.8 ± 7.6 spikes/sec. These experiments indicate that the contractions were due to release of excitatory transmitters from efferent nerves and that only a minor component of the afferent firing was triggered by the contractions and the rise in intravesical pressure.
After pretreatment with the combination of AMN and PPADS epibatidine (300 nM) still depressed (maximal depression ranging from 30–70%, n=3 experiments) the afferent firing evoked by bladder distension (10–40 cmH2O pressures).
2.2 Effects of intravesical infusion of epibatidine on bladder afferent nerve firing
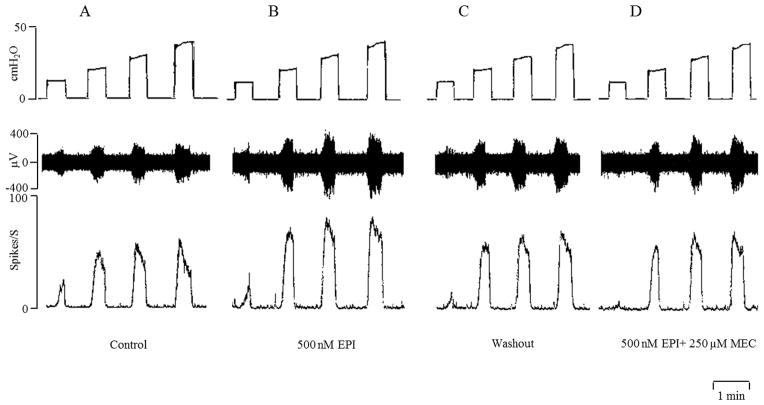
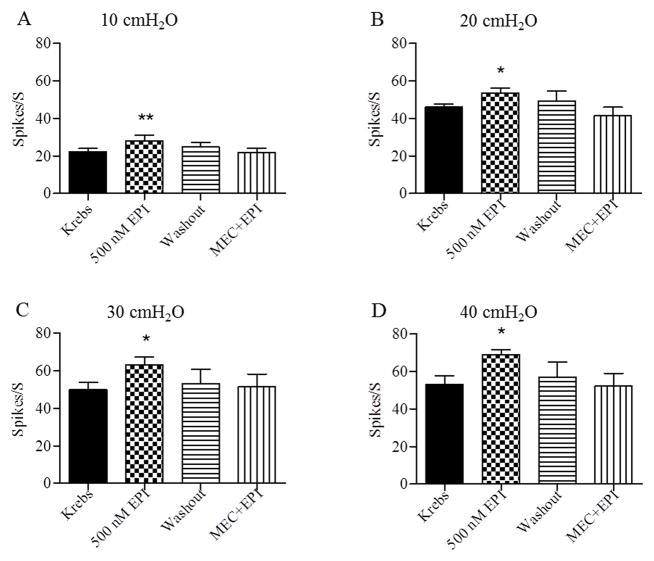
Intravesical administration of 200 nM (n=5) or 300 nM (n=3) epibatidine did not alter the afferent nerve activity elicited by isotonic distension of the bladder at 10–40 cmH2O pressures for 30 sec at 30 sec intervals. However, intravesical administration of a higher concentration (500 nM) of epibatidine significantly increased the firing evoked by bladder distentions at 10–40 cmH2O pressures (Fig 8 and Fig. 9, n=7). This concentration of epibatidine did not induce tonic afferent firing at 0 cmH2O bladder pressure. Similar responses to intravesical administration of 500 nM epibatidine were obtained during a second series of isotonic distensions of the bladder at 10–40 cmH2O pressures after washout with Krebs solution and a 30–60 min recovery period. The facilitatory effects of epibatidine on afferent firing were completely blocked by intravesical administration of mecamylamine (250 μM) (Fig. 8D and Fig. 9, n=6). However intravesical application of mecamylamine alone (n=6) in the absence of epibatidine did not significantly alter afferent firing induced by bladder distension at 10–40 cmH2O pressures.
Figure 8.
Intravesical infusion of epibatidine (EPI, 500 nM) increases bladder afferent nerve firing induced by isotonic distention of the bladder at 10, 20, 30, and 40 cmH2O. Top traces are intravesical pressure in cmH2O; middle traces show afferent nerve firing and bottom traces represent rate meter recording of afferent firing. A: Distention of the bladder with Krebs solution. B: Intravesical administration of EPI increases distension evoked afferent firing. C: Afferent firing returns to control 30 min after washing EPI out of the bladder with Krebs solution. D: The facilitatory effect of EPI on afferent firing is suppressed by intravesical administration of mecamylamine (MEC, 250 μM). Horizontal calibration represents 1 min.
Figure 9.
Summary of the effects of intravesical administration of epibatidine (EPI, 500 nM) on bladder afferent nerve firing induced by isotonic distention of the bladder with Krebs solution at 10 to 40 cmH2O intravesical pressures. EPI increased afferent firing (n=7, ** p<0.01, * p<0.05). The facilitatory effect of EPI is blocked by intravesical administration of mecamylamine (MEC, 250 μM).
2.3. Effects of nicotinic receptor agonists on evoked action potentials
To determine if bladder afferent axons are directly affected by nicotinic agonists, nerves on the serosal surface of the bladder close to the bladder neck were electrically stimulated with bipolar electrodes after filling the bladder with Krebs solution at the rate of 0.04 ml/min for 8 min and evoked compound action potentials were recorded on the pelvic nerve. The voltage threshold for evoked action potentials with the bladder filled with Krebs solution was 7.3 ± 0.4 V (pulse duration 0.15 ms) (n=4). Action potentials were characterized by short and long latency components corresponding to axonal conduction velocities ranging from 10 to 0.3 m/s at 27 C. The largest amplitude action potential in the recording occurred at short latencies and was biphasic. Lower amplitude potentials occurred at slower conduction velocities ranging from 1.0–0.3 m/s.
After bath application of epibatidine (300 nM) the threshold for evoked compound action potentials was not significantly changed (7.3 ± 0.4 V in Krebs solution and 7.8 ± 0.5 V in 300 nM epibatidine) (n=4, p>0.05). Additionally the area of action potentials including the short and long latency potentials evoked at a submaximal stimulus intensity (80 V, 0.15 ms duration) was not changed (5.9 ± 0.5 μV•ms in Krebs solution and 5.6 ± 1.0 μV•ms after epibatidine). Intravesical administration of epibatidine (500 nM) also did not change the threshold (6.4 ± 0.5 V in Krebs solution and 7.0 ± 0.2 V after 500 nM epibatidine) or the area of the evoked action potentials (6.9 ± 0.5 μV•msec in Krebs solution and 6.2 ± 0.8 μV•msec after 500 nM epibatidine, n=4, p>0.05) induced by submaximumal stimulus intensity.
A similar study conducted with bath application of nicotine (20 μM) did not reveal any change in the threshold for evoked action potentials (6.3 ± 0.9 V in control with Krebs solution and 6.0 ± 1.2 V, n=6, p>0.05 after nicotine) or the area of evoked action potentials (4.9 ± 1.0 μV•ms in Krebs solution and 5.8 ± 0.8 μV•ms after nicotine, n=6, p>0.05) elicited by submaximal stimulus intensity (80 V, 0.15 msec).
3. Discussion
The present study revealed that epibatidine or nicotine, non-selective neuronal nicotinic receptor (NNR) agonists, applied to the serosal surface of the bladder inhibit afferent firing induced by bladder distension but also elicit firing in the undistended bladder or in the bladder distended under isovolumetric conditions. On the other hand epibatidine applied intravesically to the lumenal surface of the bladder enhances distension evoked afferent firing. These results raise the possibility that cholinergic nicotinic mechanisms modulate the activity of at least three populations of bladder afferents: (1) suburothelial mechano-sensitive afferents that exhibit facilitatory responses to NNR agonists (designated Type 1 in this paper), (2) mechano-sensitive afferents located in the bladder muscle or serosa that are inhibited by NNR agonists (Type 2) and (3) unidentified afferents that are excited by NNR agonists (Type 3). These data are consistent with previous reports based on immunohistochemical and RT-PCR analysis of mouse bladder afferents that multiple populations of bladder afferents express NNR (Nandigama et al., 2013). The agonist induced responses of all three types of afferents identified in the present experiments are abolished by mecamylamine, a non-selective NNR channel blocker. However application of mecamylamine alone did not alter afferent firing induced by bladder distension indicating that the afferent modulatory functions of NNR are inactive under the conditions of our experiments.
The effect of epibatidine to facilitate firing of Type 1 afferents and excite Type 3 afferents could be due to a direct action on the afferent receptors because capsaicin-sensitive bladder afferent neurons dissociated from lumbosacral dorsal root ganglia of rats exhibit an inward current in response to nicotine (Masuda et al., 2006). Additionally the increase in voiding frequency induced by intravesical administration of nicotine (1–10 mM) (Beckel et al., 2006; Masuda et al., 2006) is suppressed after desensitization of TRPV1 receptors by treating animals with capsaicin. This indicates that the facilitatory effects of nicotine on reflex bladder activity are due to activation of capsaicin-sensitive, unmyelinated, bladder afferents (Masuda et al., 2006).
The initiation of the NNR agonist induced firing of Type 3 afferents could occur at multiple sites because acetylcholine and other NNR agonists are known to excite unmyelinated afferent axons in addition to exciting afferent nerve terminals. Lang et al 2003 who used the threshold tracking method to characterize functional NNRs in unmyelinated axons in the human sural nerve reported that epibatidine and nicotine reduced the threshold current for generating action potentials and that this effect was blocked by mecamylamine (Lang et al., 2003). They also showed with immunohistochemical techniques that the NNRs in the nerves are composed of α3, α5 and β4, but not α4, β2 or α7 subunits. In view of these results we were surprised to find in our experiments that epibatidine did not change the electrical threshold or the magnitude of the pelvic nerve compound action potentials evoked by electrical stimulation of axons on the serosal surface of the bladder. These data suggest that bladder and cutaneous afferent axons are different in their response to NNR agonists and that the direct excitatory effects of the agonists on Type 3 bladder afferents are due to stimulation of intramural nerve terminals and not axons on the bladder serosal surface.
Epibatidine and nicotine could also influence afferent activity indirectly by modulating the release of neurotransmitters in the bladder because NNRs are expressed at various sites in the bladder-pelvic nerve preparation including efferent nerve terminals, urothelial cells and autonomic ganglion cells located in the extrinsic nerves innervating the bladder (Birder and Andersson, 2013; Birder et al., 2014; de Groat and Yoshimura, 2009; de Groat et al., 2015; Hisayama et al., 1988; Shinkai et al., 1991; Somogyi and de Groat, 1992). Indirect effects on afferent excitability due to stimulation of efferent pathways and release of acetylcholine or ATP were examined because in the majority of experiments bath application of epibatidine elicited bladder contractions. These contractions must have been mediated by release of transmitters from parasympathetic efferent nerves because they were blocked by a combination of a muscarinic receptor antagonist (atropine methyl nitrate) and a non-selective but not universal P2 purinergic receptor blocking agent (PPADS) (Ralevic and Burnstock, 1998) (Fig. 7B). However it is unlikely that the increase in intravesical pressure produced by the contractions is the major factor underlying the epibatidine induced firing of Type 3 afferents because the firing preceded the rise in intravesical pressure (Fig. 7A) and was reduced by only 32.7% by the combination of muscarinic and purinergic receptor antagonists that blocked the evoked contractions (Fig. 7B). Additionally the antagonists did not alter the epibatidine inhibition of distension evoked afferent firing, thus eliminating the contribution of certain efferent neurotransmitter mechanisms. However, it is still possible that other transmitters released by efferent nerves (e.g., nitric oxide and norepinephrine) that are known to suppress afferent nerve excitability (Aizawa et al., 2012; 2015a; Ozawa et al., 1999; Yoshimura et al., 2001; Yu and de Groat, 2013) might contribute to the NNR agonist induced inhibition of Type 2 afferent firing.
The enhancement of distension evoked firing of suburothelial Type 1 afferents by intravesical administration of epibatidine could also be due to direct effects or indirect effects on the afferents mediated by the release of chemical mediators from the urothelium. Various NNRs are expressed in the urothelium (Beckel et al., 2006) and stimulation of urothelial α3 NNR with cytisine releases ATP (Beckel and Birder, 2012) which has an excitatory effect on bladder afferents (Ralevic and Burnstock, 1998; Yu and de Groat, 2008). In anesthetized rats intravesical administration of cytisine facilitates reflex micturition, an effect suppressed by PPADS, a purinergic receptor antagonist, suggesting that activation of NNRs near the lumenal surface of the bladder sensitizes afferents in part via an indirect purinergic mechanism (Beckel et al., 2006; Beckel and Birder, 2012). Previous studies (Aizawa et al., 2015b; Kullmann et al., 2008b; Yu and de Groat, 2010) revealed that intravesical administration of a muscarinic receptor agonist also enhances the firing of unmyelinated bladder afferents and facilitates reflex bladder activity by releasing ATP in the bladder.
The high intravesical concentration (500 nM) of epibatidine required to facilitate type 1 afferent firing however, raises questions about the role of urothelial α3 NNR and urothelial ATP release in afferent facilitation. Epibatidine is an ultrapotent agonist at α3 type NNR (EC50, 10 nM) (Gerzanich et al., 1995) and therefore should be effective in a low concentration to stimulate NNR in superficial urothelial cells when administered intravesically. The high concentration (500 nM) of epibatidine required to facilitate afferent firing suggests that it does not act directly on α3 type NNR in umbrella cells at the lumenal surface of the bladder but rather may activate a subtype of NNR on the urothelial cells that is relatively insensitive to α3 agonists or may diffuse through the urothelial barrier to act on targets below the barrier, i.e. basal/intermediate urothelial cells or suburothelial afferents. One of these mechanisms could account for the low potency of epibatidine in enhancing the distension evoked firing of Type 1 bladder afferents.
When administered to the serosal surface of the bladder, epibatidine was effective at a considerably lower concentration (100 nM) to suppress distension evoked Type 2 afferent firing. Nicotine elicited a similar inhibitory effect but was less potent (threshold concentration: 20 μM). The different effect of epibatidine when administered to the serosal and lumenal surfaces of the bladder suggests that when administered at these two sites the drug targets different populations of afferents and different subtypes of NNR,. For example bath application of the NNR agonists might target a population of mechano-sensitive afferent nerves in the muscle layer located close to the serosal surface that are not accessible to the drug administered intravesically.
Activation of another NNR subtype (α7) in the urothelium has been implicated in the inhibition of reflex bladder activity by NNR agonists. Activation of α7 receptors in cultured urothelial cells with choline suppresses ATP release; and intravesical administration of choline in anesthetized rats decreases the frequency of reflex voiding presumably by decreasing afferent excitability (Beckel et al., 2006; Beckel and Birder, 2012; de Groat et al., 2015). These effects are reduced by administration of a selective α 7 antagonist (methyllcaconitine, MLA). Although the present experiments revealed inhibitory effects of NNR agonists on Type 2 afferent firing it did not provide any evidence for inhibition mediated by α7 NNR receptors because α3 NNR antagonists (mecamylamine or hexamethonium) blocked both the inhibitory and excitatory responses. Furthermore, suppression of the facilitatory effects of intravesical epibatidine with mecamylamine did not unmask inhibitory effects that might have been obscured by more prominent facilitatory effects that occurred with the high concentration of epibatidine used in our experiments. Thus we would not expect the effect of epibatidine on afferent firing to be altered by MLA.
In conclusion these results indicate that activation of nicotinic receptors in the bladder: (1) facilitates the firing of mechano-sensitive afferents with terminals located near the bladder lumen, (2) inhibits the firing of mechano-sensitive afferents located near the serosal surface or in the muscle layers of the bladder, (3) excites unidentified- afferents, (4) does not alter afferent axonal excitability. To determine the mechanism of epibatidine induced inhibition of bladder afferent firing, it will be important in future experiments to test the effect of α7 and α3 receptor antagonists on the inhibitory and excitatory effects, respectively, of epibatidine. However, based on the present data showing that mecamylamine, a non-selective NNR antagonist, with 10 fold greater affinity for α3 than α7 receptors (Papke et al., 2001) is equally effective in suppressing the excitatory and inhibitory effects of epibatidine on distension evoked bladder afferent firing it is possible that both effects are mediated by an α3 NNR subtype.
4. Experimental Procedures
4.1. Animals and surgical procedures
The urinary bladder, urethra, prostate gland, seminal vesicles and innervation including the pelvic nerves and the major pelvic ganglia were removed from ketamine (50 mg/kg, i.m.) anesthetized male Sprague Dawley rats (n=64, body weight 100–170g) and placed in a 20 ml bath that was perfused (1 ml/min) with Krebs solution at a temperature of 27°C and continuously bubbled with 95% O2 and 5% CO2. A temperature below body temperature was used to prolong the survival of the in vitro preparation. The Krebs solution had the following composition (mM): NaCl 128, KCl 1.8, NaHCO3 22, KH2PO4 1.5, MgSO4 1.3, glucose 10, CaCl2.2H2O 0.4 and H2O2 0.4 at pH 7.4. A catheter (PE50) was inserted into the bladder through the urethra and then connected to an infusion pump to infuse solutions intravesically and to a pressure transducer to monitor bladder activity. The duration of the experiments ranged from 4 to 7 hours.
4.2. Nerve stimulation and recording
The pelvic nerve on one side was placed in an adjacent chamber filled with paraffin oil and positioned on silver bipolar electrodes (inter-electrode distance: approximately 3 mm) for recording multiunit afferent nerve activity. Standard electrophysiological methods were used to amplify and analyze the afferent nerve activity (Yu and de Groat, 2008). Afferent firing was elicited by intravesical infusion of Krebs solution at the rate of 0.04 ml/min for 8 min which also evoked rhythmic contractions of the bladder smooth muscle. After bladder filling was stopped multiunit afferent activity was measured (spikes/sec) for 10 min using a pulse height discriminator rate meter and displayed on a rectilinear paper recorder and also recorded on a VCR and a digital computer for later off-line analysis. Afferent activity was also evoked by isotonic distension of the bladder with Krebs solution for 30 sec periods at pressures of 10, 20, 30 and 40 cmH2O. Nicotinic agonists (epibatidine or nicotine) or antagonists (mecamylamine or hexamethonium) were administered to the serosal surface of the bladder by injection into the bath solution or to the lumenal surface of the bladder by intravesical infusion. Reversibility of drug effects was evaluated by flushing the bath or bladder with Krebs solution and repeatability was tested by additional applications at intervals of 30 to 60 min.
Multiunit compound action potentials were also elicited by electrical stimulation (0.15 ms pulse duration) using a pair of silver wire electrodes (diameter: 0.25 mm, inter-electrode distance: 2–3 mm) positioned on the serosal surface close to the neck of the bladder (Yu and de Groat, 2008). Individual responses were averaged with a computer (Lab View program, National Instrument Company). Based on the latencies of evoked action potentials in the pelvic nerve and the distance between stimulus and recording sites the conduction velocities of the afferent nerves were calculated. The distance between stimulating and recording electrodes ranged between 11–13 mm. Changes in the electrically evoked action potentials were studied after intravesical administration or bath application of epibatidine or nicotine.
4.3. Drugs
Stock solutions of epibatidine dihydrochloride (0.1 mM) mecamylamine hydrochloride (5 mM), nicotine tartrate (10 mM) and hexamethonium chloride (50 mM) atropine methyl nitrate, pyridoxal-phosphate-6-azophenyl-2′,4′-disulfonate were prepared in distilled water and diluted in Krebs solution prior to application to the preparation. All drugs were obtained from Sigma Aldrich Inc. Mecamylamine (150 μM) and hexamethonium (250 μM) were applied in concentrations shown to block nicotinic receptors in other tissues or isolated cells (Imamura et al., 2015; Ren et al., 2015; Suzuki and Volle, 1979).
4.4. Statistics
Analysis of Data: Multiunit recordings of afferent activity are presented as peak firing frequency in spikes/sec recorded under isovolumetric or isotonic conditions. The Lab View program (National Instrument Company) was used to analyze the area of evoked action potentials. All data are expressed as mean ± SE. Results were evaluated using two-way ANOVA followed by Bonferroni post tests using Prism 4 program (GraphPad software Inc, San Diego, CA).
Highlights.
Nicotinic agonists modulate firing of mechano-sensitive bladder afferent nerves (BAN)
Agonists applied to the bladder lumen enhance mechano-sensitive BAN firing
Agonists applied to the bladder serosal surface suppress mechano-sensitive BAN firing
Agonists applied to the bladder serosal surface also elicit BAN firing
Mecamylamine, a nicotinic antagonist blocks the effects of agonists
Acknowledgments
This work was supported by National Institutes of Health Grants DK-091253 and DK-093424.
Abbreviations
- ACh
acetylcholine
- AMN
atropine methyl nitrate
- AP
action potential
- EPI
epibatidine
- BAN
bladder afferent nerve
- MEC
mecamylamine
- NNR
neuronal nicotinic receptors
- NO
nitric oxide
- NOS
nitric oxide synthase
- PPADS
pyridoxal-phosphate-6-azophenyl-2′,4′-disulfonate
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aizawa N, Homma Y, Igawa Y. Effects of mirabegron, a novel beta3-adrenoceptor agonist, on primary bladder afferent activity and bladder microcontractions in rats compared with the effects of oxybutynin. Eur Urol. 2012;62:1165–1173. doi: 10.1016/j.eururo.2012.08.056. [DOI] [PubMed] [Google Scholar]
- Aizawa N, Homma Y, Igawa Y. Effects of L-arginine, mirabegron, and oxybutynin on the primary bladder afferent nerve activities synchronized with reflexic, rhythmic bladder contractions in the rat. Neurourol Urodyn. 2015a;34:368–374. doi: 10.1002/nau.22571. [DOI] [PubMed] [Google Scholar]
- Aizawa N, Ito H, Sugiyama R, Fujimura T, Suzuki M, Fukuhara H, Homma Y, Igawa Y. Selective inhibitory effect of imidafenacin and 5-hydroxymethyl tolterodine on capsaicin sensitive C fibers of the primary bladder mechanosensitive afferent nerves in the rat. J Urol. 2015b;193:1423–1432. doi: 10.1016/j.juro.2014.09.005. [DOI] [PubMed] [Google Scholar]
- Beckel JM, Kanai A, Lee SJ, de Groat WC, Birder LA. Expression of functional nicotinic acetylcholine receptors in rat urinary bladder epithelial cells. Am J Physiol Renal Physiol. 2006;290:F103–F110. doi: 10.1152/ajprenal.00098.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckel JM, Birder LA. Differential expression and function of nicotinic acetylcholine receptors in the urinary bladder epithelium of the rat. J Physiol. 2012;590:1465–1480. doi: 10.1113/jphysiol.2011.226860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birder L, Andersson KE. Urothelial signaling. Physiol Rev. 2013;93:653–680. doi: 10.1152/physrev.00030.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birder LA, Apodaca G, De Groat WC, Kanai AJ. Adrenergic- and capsaicin-evoked nitric oxide release from urothelium and afferent nerves in urinary bladder. Am J Physiol. 1998;275:F226–F229. doi: 10.1152/ajprenal.1998.275.2.F226. [DOI] [PubMed] [Google Scholar]
- Birder LA, Andersson KE, Kanai AJ, Hanna-Mitchell AT, Fry CH. Urothelial mucosal signaling and the overactive bladder-ICI-RS 2013. Neurourol Urodyn. 2014;33:597–601. doi: 10.1002/nau.22604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cockayne DA, Hamilton SG, Zhu QM, Dunn PM, Zhong Y, Novakovic S, Malmberg AB, Cain G, Berson A, Kassotakis L, Hedley L, Lachnit WG, Burnstock G, McMahon SB, Ford AP. Urinary bladder hyporeflexia and reduced pain-related behaviour in P2X3-deficient mice. Nature. 2000;407:1011–1015. doi: 10.1038/35039519. [DOI] [PubMed] [Google Scholar]
- de Groat WC, Yoshimura N. Afferent nerve regulation of bladder function in health and disease. In: Canning BJ, Spina D, editors. Sensory Nerves Handb Exp Pharmacol. Vol. 194. Springer; Berlin Heidelberg, Berlin: 2009. pp. 91–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Groat WC, Griffiths D, Yoshimura N. Neural control of the lower urinary tract. Compr Physiol. 2015;5:327–396. doi: 10.1002/cphy.c130056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson DR, Kennedy I, Burton TJ. ATP is released from rabbit urinary bladder epithelial cells by hydrostatic pressure changes--a possible sensory mechanism? J Physiol. 1997;505(Pt 2):503–511. doi: 10.1111/j.1469-7793.1997.503bb.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford AP, Undem BJ, Birder LA, Grundy D, Pijacka W, Paton JF. P2X3 receptors and sensitization of autonomic reflexes. Auton Neurosci. 2015;191:16–24. doi: 10.1016/j.autneu.2015.04.005. [DOI] [PubMed] [Google Scholar]
- Gerzanich V, Peng X, Wang F, Wells G, Anand R, Fletcher S, Lindstrom J. Comparative pharmacology of epibatidine: a potent agonist for neuronal nicotinic acetylcholine receptors. Mol Pharmacol. 1995;48:774–782. [PubMed] [Google Scholar]
- Hanna-Mitchell AT, Beckel JM, Barbadora S, Kanai AJ, de Groat WC, Birder LA. Non-neuronal acetylcholine and urinary bladder urothelium. Life Sci. 2007;80:2298–2302. doi: 10.1016/j.lfs.2007.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hisayama T, Shinkai M, Takayanagi I, Toyoda T. Mechanism of action of nicotine in isolated urinary bladder of guinea-pig. Br J Pharmacol. 1988;95:465–472. doi: 10.1111/j.1476-5381.1988.tb11667.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imamura O, Arai M, Dateki M, Ogata T, Uchida R, Tomoda H, Takishima K. Nicotinic acetylcholine receptors mediate donepezil-induced oligodendrocyte differentiation. J Neurochem. 2015;135:1086–1098. doi: 10.1111/jnc.13294. [DOI] [PubMed] [Google Scholar]
- Kontani H, Okamura T, Kimura S, Ishida K, Takeno S. Effect of nicotine on the pelvic afferent nerve activity and bladder pressure in rats. Int J Urol. 2009;16:692–698. doi: 10.1111/j.1442-2042.2009.02327.x. [DOI] [PubMed] [Google Scholar]
- Kullmann FA, Artim D, Beckel J, Barrick S, de Groat WC, Birder LA. Heterogeneity of muscarinic receptor-mediated Ca2+ responses in cultured urothelial cells from rat. Am J Physiol Renal Physiol. 2008a;294:F971–F981. doi: 10.1152/ajprenal.00313.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kullmann FA, Artim DE, Birder LA, de Groat WC. Activation of muscarinic receptors in rat bladder sensory pathways alters reflex bladder activity. J Neurosci. 2008b;28:1977–1987. doi: 10.1523/JNEUROSCI.4694-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang PM, Burgstahler R, Sippel W, Irnich D, Schlotter-Weigel B, Grafe P. Characterization of neuronal nicotinic acetylcholine receptors in the membrane of unmyelinated human C-fiber axons by in vitro studies. J Neurophysiol. 2003;90:3295–3303. doi: 10.1152/jn.00512.2003. [DOI] [PubMed] [Google Scholar]
- Lips KS, Wunsch J, Zarghooni S, Bschleipfer T, Schukowski K, Weidner W, Wessler I, Schwantes U, Koepsell H, Kummer W. Acetylcholine and molecular components of its synthesis and release machinery in the urothelium. Eur Urol. 2007;51:1042–1053. doi: 10.1016/j.eururo.2006.10.028. [DOI] [PubMed] [Google Scholar]
- Masuda H, Hayashi Y, Chancellor MB, Kihara K, de Groat WC, de Miguel F, Yoshimura N. Roles of peripheral and central nicotinic receptors in the micturition reflex in rats. J Urol. 2006;176:374–379. doi: 10.1016/S0022-5347(06)00581-7. [DOI] [PubMed] [Google Scholar]
- Matsumoto Y, Miyazato M, Furuta A, Torimoto K, Hirao Y, Chancellor MB, Yoshimura N. Differential roles of M2 and M3 muscarinic receptor subtypes in modulation of bladder afferent activity in rats. Urology. 2010;75:862–867. doi: 10.1016/j.urology.2009.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto Y, Miyazato M, Yokoyama H, Kita M, Hirao Y, Chancellor MB, Yoshimura N. Role of M2 and M3 muscarinic acetylcholine receptor subtypes in activation of bladder afferent pathways in spinal cord injured rats. Urology. 2012;79:1184, e15–20. doi: 10.1016/j.urology.2012.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nandigama R, Bonitz M, Papadakis T, Schwantes U, Bschleipfer T, Kummer W. Muscarinic acetylcholine receptor subtypes expressed by mouse bladder afferent neurons. Neuroscience. 2010;168:842–850. doi: 10.1016/j.neuroscience.2010.04.012. [DOI] [PubMed] [Google Scholar]
- Nandigama R, Ibanez-Tallon I, Lips KS, Schwantes U, Kummer W, Bschleipfer T. Expression of nicotinic acetylcholine receptor subunit mRNA in mouse bladder afferent neurons. Neuroscience. 2013;229:27–35. doi: 10.1016/j.neuroscience.2012.10.059. [DOI] [PubMed] [Google Scholar]
- Ozawa H, Chancellor MB, Jung SY, Yokoyama T, Fraser MO, Yu Y, de Groat WC, Yoshimura N. Effect of intravesical nitric oxide therapy on cyclophosphamide-induced cystitis. J Urol. 1999;162:2211–2216. doi: 10.1016/S0022-5347(05)68161-X. [DOI] [PubMed] [Google Scholar]
- Papke RL, Sanberg PR, Shytle RD. Analysis of mecamylamine stereoisomers on human nicotinic receptor subtypes. J Pharmacol Exp Ther. 2001;297:646–656. [PubMed] [Google Scholar]
- Ralevic V, Burnstock G. Receptors for purines and pyrimidines. Pharmacol Rev. 1998;50:413–492. [PubMed] [Google Scholar]
- Ren ZJ, Mummalaneni S, Qian J, Baumgarten CM, DeSimone JA, Lyall V. Nicotinic acetylcholine receptor (nAChR) dependent chorda tympani taste nerve responses to nicotine, ethanol and acetylcholine. PLoS One. 2015;10:e0127936. doi: 10.1371/journal.pone.0127936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinkai M, Takayanagi I, Kato T. Contrasting effects of tachykinins and guanethidine on the acetylcholine output stimulated by nicotine from guinea-pig bladder. Br J Pharmacol. 1991;103:1191–1195. doi: 10.1111/j.1476-5381.1991.tb12322.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva I, Ferreirinha F, Magalhaes-Cardoso MT, Silva-Ramos M, Correia-de-Sa P. Activation of P2Y6 receptors facilitates nonneuronal adenosine triphosphate and acetylcholine release from urothelium with the lamina propria of men with bladder outlet obstruction. J Urol. 2015;194:1146–1154. doi: 10.1016/j.juro.2015.05.080. [DOI] [PubMed] [Google Scholar]
- Somogyi GT, de Groat WC. Evidence for inhibitory nicotinic and facilitatory muscarinic receptors in cholinergic nerve terminals of the rat urinary bladder. J Auton Nerv Syst. 1992;37:89–97. doi: 10.1016/0165-1838(92)90237-b. [DOI] [PubMed] [Google Scholar]
- Suzuki T, Volle RL. Nicotinic, muscarinic and adrenergic receptors in a parasympathetic ganglion. J Pharmacol Exp Ther. 1979;211:252–256. [PubMed] [Google Scholar]
- Yoshida M, Masunaga K, Nagata T, Yono M, Homma Y. The forefront for novel therapeutic agents based on the pathophysiology of lower urinary tract dysfunction: pathophysiology and pharmacotherapy of overactive bladder. J Pharmacol Sci. 2010;112:128–134. doi: 10.1254/jphs.09r12fm. [DOI] [PubMed] [Google Scholar]
- Yoshimura N, Seki S, de Groat WC. Nitric oxide modulates Ca(2+) channels in dorsal root ganglion neurons innervating rat urinary bladder. J Neurophysiol. 2001;86:304–311. doi: 10.1152/jn.2001.86.1.304. [DOI] [PubMed] [Google Scholar]
- Yu Y, de Groat WC. Sensitization of pelvic afferent nerves in the in vitro rat urinary bladder-pelvic nerve preparation by purinergic agonists and cyclophosphamide pretreatment. Am J Physiol Renal Physiol. 2008;294:F1146–F1156. doi: 10.1152/ajprenal.00592.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu Y, de Groat WC. Effects of stimulation of muscarinic receptors on bladder afferent nerves in the in vitro bladder-pelvic afferent nerve preparation of the rat. Brain Res. 2010;1361:43–53. doi: 10.1016/j.brainres.2010.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu Y, de Groat WC. Nitric oxide modulates bladder afferent nerve activity in the in vitro urinary bladder-pelvic nerve preparation from rats with cyclophosphamide induced cystitis. Brain Res. 2013;1490:83–94. doi: 10.1016/j.brainres.2012.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]