Abstract
Linoleic acid (LA) is known to activate G-protein coupled receptors and connexin hemichannels (Cx HCs) but possible interlinks between these two responses remain unexplored. Here, we evaluated the mechanism of action of LA on the membrane permeability mediated by Cx HCs in MKN28 cells. These cells were found to express connexins, GPR40, GPR120, and CD36 receptors. The Cx HC activity of these cells increased after 5 min of treatment with LA or GW9508, an agonist of GPR40/GPR120; or exposure to extracellular divalent cation-free solution (DCFS), known to increase the open probability of Cx HCs, yields an immediate increase in Cx HC of similar intensity and additive with LA-induced change. Treatment with a CD36 blocker or transfection with siRNA-GPR120 maintain the LA-induced Cx HC activity. However, cells transfected with siRNA-GPR40 did not show LA-induced Cx HC activity but activity was increased upon exposure to DCFS, confirming the presence of activatable Cx HCs in the cell membrane. Treatment with AKTi (Akt inhibitor) abrogated the LA-induced Cx HC activity. In HeLa cells transfected with Cx43 (HeLa-Cx43), LA induced phosphorylation of surface Cx43 at serine 373 (S373), site for Akt phosphorylation. HeLa-Cx43 but not HeLa-Cx43 cells with a S373A mutation showed a LA-induced Cx HC activity directly related to an increase in cell surface Cx43 levels. Thus, the increase in membrane permeability induced by LA is mediated by an intracellular signaling pathway activated by GPR40 that leads to an increase in membrane levels of Cx43 phosphorylated at serine 373 via Akt.
Keywords: polyunsaturated fatty acid (PUFA), FFAR1, connexon (hemichannel), transmembrane permeability, protein phosphorylation, Akt kinase, G protein-coupled receptor (GPCR)
1. INTRODUCTION
Connexin (Cx) proteins are encoded by a gene family composed by 21 and 20 members in human and mouse, respectively [1, 2], and they are assembled into hexamers to form hemichannels (HCs) or connexons, and dodecamers to form gap junction channels (GJCs) connecting two cells. HCs can act as relatively non-selective channels and are found at the cell surface of most vertebrate cells [1, 3-5]. HCs are often involved in paracrine and autocrine cellular signaling, being membrane pathways for releasing signaling molecules (e.g., ATP, NAD+ and glutamate) to the extracellular space [3]. Different tissues express different Cxs depending on their developmental and physiologic state, and they are named alphanumerically with the prefix Cx followed by the molecular mass of the human family member in kilodaltons (e.g., Cx43) [1, 6].
Experimental evidence suggests that functional HCs and GJCs are involved in physiological and pathophysiological cell responses. In the gastrointestinal system, they might participate as paracellular permeability pathway in intestinal epithelial cells [7] or intestinal innate immune defense [8]. Moreover, in tanycytes and skeletal muscle, Cx43 HCs or pannexin 1 channels (Panx1 Chs), respectively, allow diffusional entry of molecules such as glucose [9, 10], so they could be thought of as membrane pathways for cellular uptake of nutrients. At least 7 different Cx variants have been described in the human gastrointestinal system [11], with the three Cxs found in gastric cells being connexin 26 (Cx26), Cx32 and Cx43 [12]. The functional roles of these Cxs are poorly understood but one proposed by Guttman et al. [13], postulated that Cx43 HCs present in intestine are involved in transport of water, at least under pathological conditions such as in diarrhea caused by bacterial infections. Also, the expression of Cx26 is associated with intestinal type-gastric cancer and Cx32 and Cx43 with Helicobacter pylori-associated gastric tumorigenesis [14, 15].
In the gastrointestinal tract, free fatty acids (FFAs) are essential nutrients but they also play important roles as signaling molecules [17, 18] in various physiological processes [19, 20]. However, knowledge about the regulation of HCs (or GJCs) by FFAs in the epithelium of the gastrointestinal tract is very limited and is incompletely understood in other cell types [21-24].
FFAs exert at least some of their biological signaling effect through the G-protein coupled receptor (GPCR) family [25, 26] of which at least 5 genes (GPR40, GPR41, GPR43, GPR84 and GPR120) and one pseudo gene (GPR42) have been described [27]. These receptors have different affinities for different FFAs with those having medium and long aliphatic chains being the best agonists for GPR40 (or FFAR1) and/or GPR120 [27, 28]. In addition, CD36 (a ubiquitous membrane glycoprotein) has been shown to mediate responses to FFAs, by acting as a lipid sensor [29, 30]. These three types of receptors (i.e. GPR40, GPR120 and CD36) are activated by long chain fatty acids [27, 28, 31]. Probably the two most well studied FFAs are linoleic and α-linolenic acids, both of which are precursors of the longer aliphatic chains ω-6 and ω-3 fatty acids, respectively, and are essential nutrients in the human diet [32-35].
Linoleic acid (LA) has been shown to modulate the functional state of Cx46 HCs [21]. Moreover, in HeLa cells transfected with different Cxs, our group reported that LA increases Cx26, Cx43 and Cx45 HC activity, and this effect is associated with elevated intracellular free Ca2+ concentration and activation of the PI3K/Akt-dependent pathway [24]. However, the molecular mechanisms involved in this response remain unknown and as does the generality of the response of cell types that have actual contact with FFAs. Hence, we evaluated the mechanism of action of LA on the functional state of Cx43 HCs in cells derived from human gastric epithelium (MKN28 cells). To further understand the mechanism of action, we also used HeLa cells transfected with Cx43 or Cx43 lacking an Akt phosphorylation site. We found that LA induces an increase in Cx43 HC activity by increasing the number of hemichannels in the cell membrane through an extracellular Ca2+ independent mechanism that sequentially involves activation of GPR40 and Akt followed by phosphorylation of Cx43 at serine 373.
2. MATERIAL AND METHODS
2.1. Reagents
Linoleic acid (LA), GW9508, ethidium (Etd+) bromide and lanthanum (La3+) chloride were obtained from Sigma-Aldrich (St. Louis, MO, USA); Akt inhibitor VIII (AKTi) from Calbiochem (Merck KGaA, Darmstadt, Germany); GPR40 and Negative control siRNA (Neg siRNA) were obtained from Qiagen (Valencia, CA, USA), polyclonal antibodies anti-GPR40 and GPR120 from Abcam (Cambridge, MA, USA). A custom made previously described anti-pS373 Cx43 antibody was used [36]. Monoclonal antibody anti-α-tubulin was obtained from Sigma-Aldrich, and anti-mouse and anti-rabbit secondary antibodies conjugated to horseradish peroxidase were from Santa Cruz Biotechnology Inc (Santa Cruz, CA, USA).
2.2. Cell culture
HeLa-Parental cells were obtained from ATCC (CCL-2; ATCC, Rockville, MD) and HeLa cells stably transfected with rat Cx26 cDNA were kindly provided by Dr. Bruce Nicholson, Department of Biochemistry at the University of San Antonio, USA. HeLa cells stably transfected with mouse Cx32 or Cx43 cDNA were kindly provided by Dr. Klaus Willecke from the Limus Institute, Bonn University, Germany. MKN28 cells (cell line derived from human gastric epithelium) were obtained from American Type Culture Collection (ATCC, Manassas, VA, USA). HeLa cells were cultured in DMEM-10% fetal bovine serum (GIBCO) with 100 U/ml penicillin, 100 μg/ml streptomycin sulfate. Puromycin (10 μg/ml) or Geneticin (250 μg/ml) were used to select transfected cells. Untransfected HeLa-Parental cells were used as controls. MKN28 cells were cultured in RPMI-10% fetal bovine serum with 100 U/ml penicillin, 100 μg/ml streptomycin sulfate. Cells were used 36 – 48 h after seeded.
2.3. RT-PCR assay
Total RNA was isolated from MKN28 or HeLa cells using Trizol® Reagent following the manufacturer instructions (Ambion, USA). Aliquots of 2 μg of total RNA were reversed transcribed into cDNA using MMLV-reverse transcriptase (Fermentas, USA) and mRNA levels were evaluated by PCR amplification (GoTaq® Flexi DNA Polymerase; Promega, USA). The primers used were GAPDH sense 5′-ACCACAGTCCATGCCATCAC-3′, antisense 5′-TCCACCACCCTGTTGCTGTA-3′; GPR40 sense 5′-TGGCCCACTTCTTCCCACTC-3′, antisense 5′-CAGGAGAGAGAGGCTGAAGC-3′, GPR120 sense 5′-TTGCACACTGATTTGGCCCA-3′, antisense 5′-CTTCCACTCATTCCTGCACA-3′; Cx32 sense 5′-CAGCTCATCCTAGTTTCCACC-3′, 5′-ACCACCTCGGCCACATTGA-3′; and Cx43 sense 5′- GTCCCTGGCCTTGAATATCA-3′, antisense 5′-TCTGGTTATCATCGGGGAAA-3′. For Cx26 was previously described [14]. The expected size products were: GPR40, 317 bp; GPR120, 463 bp; Cx43, 371 bp; Cx32 392 bp and GAPDH: 452 bp.
2.4. Western blot assays
Cells were washed twice with ice-cold PBS (pH 7.4), harvested by scraping with a rubber policeman in 80 μl of solution containing protease and phosphatase inhibitor cocktail (Thermo Scientific, Pierce, Rockford, IL, USA), placed on ice and then sonicated (Ultrasonic cell disrupter, Microson, Ultrasons, Annemasse, France). Blots were incubated overnight with polyclonal rabbit immunopurified anti-GPR40, GPR120, Cx26 or Cx43 antibodies diluted with 5% non-fat milk in TBS. Then, they were rinsed with TBS and incubated for 1 h at room temperature with horse radish peroxidase-conjugated goat anti-rabbit IgG antibodies at the appropriate dilution in PBS with 5% non-fat milk. After repeated rinses, immunoreactive proteins were detected using ECL reagents (Pierce biotechnology, Rockford, IL) according to the manufacturer’s instructions.
2.5 Indirect immunofluorescence analysis
Cells grown on glass coverslips were washed three times with PBS (pH 7.4) and fixed with 4% paraformaldehyde for 5 min at room temperature. Cells were then washed three times, and permeabilized and blocked in PBS containing 1% BSA and 0.25% Triton X-100. Cells were incubated for 1 hour at room temperature with primary anti-GPR40 or anti-GPR120 antibodies previously dissolved in the washing solution containing 0.2% BSA in PBS and 0.025% Triton X-100. Cells were then washed three times to remove the unbound antibody. Afterwards, cells were incubated for 1 hour at room temperature with anti-rabbit secondary antibody labeled with Cy2 (Jackson Immuno Research Laboratories Inc., West Grove, PA, USA), then washed three times and mounted with DABCO mounting medium (Gelvatol, Sigma Aldrich, St. Louis, MO, USA) containing 1:2,000 4,6-diamidino-2-phenylindole dihydrochloride (DAPI, Sigma Aldrich, St. Louis, MO, USA) and imaged using the immunofluorescence microscopy. Secondary antibodies alone were used as negative controls.
2.6. Dye uptake
The HC activity was measured using a published dye uptake method [9]. In brief, the cells were plated onto glass coverslips and after 36–48 h they were washed twice with Locke′s buffer (in mM: 154 NaCl, 5.4 KCl, 2.3 CaCl2, 1.5 MgCl2, 5 glucose, 5 Hepes, pH 7.4) containing 5 μM ethidium (Etd+), a membrane-impermeant cationic dye, and images were recorded (at regions of interest in different cells) every 30 s during 30 min using a Nikon Eclipse Ti inverted microscope (Japan) and NIS-Elements software. Basal fluorescence signal was recorded (during 5 min) in cells only in the presence of Locke′s buffer that contained divalent cations. Then, LA was added to the same buffer and recorded for ~15 min, and at the end of the experiment La3+ was added. Image analysis was performed with ImageJ 1.46r software (NIH, USA).
2.7. siRNA Transfection
MKN28 and HeLa cells were transfected with GPR40 or control (Neg) siRNAs (Qiagen) at a final concentration of 100 nM using Lipofectamine LTX and PLUS Reagents as described in the manufacturer’s instructions (Invitrogen) for the 35 mm dish format. pEGFP-N1 vector was included to determine transfection efficiency. After 36–48 h of transfection, cells were used for Etd+ uptake, Ca2+ signal evaluation and immunofluorescence experiments.
2.8. Levels of Cx43 HCs at the cell surface
Cell surface proteins were biotinylated as described previously [37]. In brief, cell cultures were grown in 100 mm-dishes, washed 3 times with ice-cold Hank’s saline solution pH 7.4 and incubated (30 min, at 4°C) in 3 ml of 0.5 mg/ml sulfo-NHS-SSbiotin (Pierce, Rockford, IL). The cells were washed 3 times with a saline solution containing 15 mM glycine pH 8.0 to neutralize unreacted biotin and finally harvested in saline solution with a cocktail of protease and phosphatase inhibitors. Then cells were sonicated on ice and protein concentration was determined. Immobilized NeutrAvidin (Pierce) was added (1 ml of NeutrAvidin per 3 g of biotinylated protein) and the mixture was incubated for 1 h at 4°C. Then 1 ml of washing buffer (saline solution, pH 7.4, 0.1% SDS and 1% NP-40) was added, the mix was centrifuged (2 min, 14,000 rpm, 4°C), and the supernatant was discarded. After performing this procedure 3 times, 40 μl Hanks solution buffer at pH 2.8 containing 0.1 M glycine was added to the pellet to release the proteins bound to biotin. The pellet was resuspended and centrifuged. The supernatant was transferred to a new 1.5 ml Eppendorf tube, and the pH was adjusted immediately by adding 10 μl of 1 M Tris pH 7.4. Finally, relative levels of protein were evaluated by Western blot.
2.9. Data Analysis
For each group of data, results were expressed as mean ± SEM, and n represents the number of independent experiments or the number of cells analyzed as indicated. For Etd+ uptake experiments, each mean corresponds to the average of at least 20 cells. Data sets were compared by one-way analysis of variance (ANOVA) followed by a Bonferroni’s post-test. Differences were considered significant at p≤0.05. The analyses were performed with GraphPad Prism 5 software for Windows (1992–2007, GraphPad Software).
3. RESULTS
3.1 Human gastric epithelial cells present Cx HC activity
Connexin expression has been previously demonstrated in different sections of the gastrointestinal tract [6, 7, 11]. Here we show that the MKN28 cell line, derived from human gastric epithelium [38], expresses the mRNA for Cx26, Cx32 and Cx43 (Figure 1A). Since these Cxs can form hemichannels (HCs) in cell membrane [1], we tested to see if MKN28 cells express functional Cx HCs. Under basal conditions, most Cx HCs remain in the closed state (i.e., show low open probability). However, their presence can be demonstrated by increasing their open probability, for example, by drastically reducing the extracellular concentration of divalent cations [39, 40]. To this end, cells were bathed with divalent cation-free solution (DCFS), which has been shown to promote HC opening [41-43]. The functional state of Cx HCs was monitored by measuring the Etd+ uptake over time (Fig. 1B, C). Etd+ is a membrane-impermeant cationic dye that becomes fluorescent when intercalated in nucleic acid strands [44]. A rapid increase in fluorescence intensity was observed upon DCFS application that was drastically reduced by lanthanum ions (La3+, 200 μM) (Figure 1C, D), a known Cx HC blocker [42, 43]. The quantification of Etd+ uptake rate revealed a ~3-fold increase in DCFS, and this response was blocked by La3+ to values even lower than that recorded under control conditions (Figure 1D). These results indicate there is likely a low level of Cx HC activity even under basal conditions.
Figure 1. Gastric epithelial cells show connexin expression and hemichannel activity.
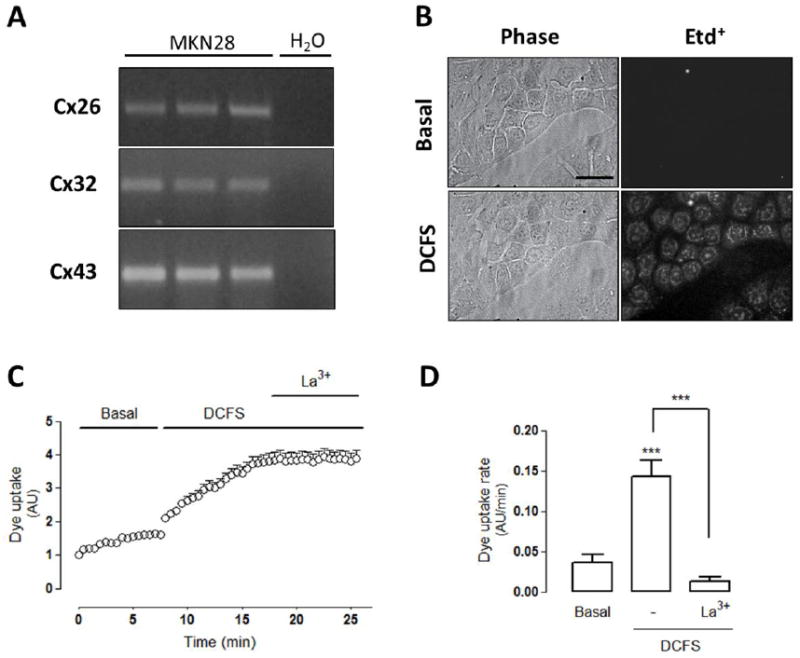
(A) The mRNAs of connexins (Cxs) 26, 32 and 43 mRNA were detected by PCR (shown in triplicate). Negative H2O controls are also shown. (B) Phase contrast images (left panels) show cells exposed to ethidium (5 μM Etd+) for 5 min under (Top) basal conditions or (bottom) after exposure to divalent cation free solution (DCFS) for 5 min. Right panels show fluorescence of Etd+ (Scale bar: 50 μm). (C) Representative Dye uptake curve under basal conditions and after exposure to DCFS followed by the application of lanthanum ions (La3+, 200 μM). (D) Dye uptake rate of cells under the same conditions indicated in (C). Statistical significance versus basal conditions ***p<0.001 and versus DCFS ***p<0.001, n=3-6.
3.2. Linoleic acid (LA) increases the Cx HC activity through a mechanism independent of extracellular divalent cations
Since MKN28 cells were found to express Cx HCs, we then tested if the activity of these hemichannels is affected by linoleic acid (LA). Approximately 5 min after the application of 100 μM LA, Etd+ uptake increased several-fold and this response was completely blocked by La3+ (Figure 2). Notably, the intensity of this effect was similar to that induced by DCFS though the DCFS-induced rise did not show the initial 5 min lag in uptake, suggesting different mechanisms of activation for these treatments. To further study this possibility, HeLa-Cx43 cells were first exposed to DCFS and 10 min later treated with 100 μM LA for 10 min (Figure 2C) or vice versa (Figure 2D). In both cases, after 20 min, 200 μM La3+ was applied (Figure 2C and D). The LA concentration used is known to induce its maximal effect on Cx HC [24] and complete replacement of the media with DCFS should yield the maximal open probability of Cx HCs via this mechanism. Since DCFS treatment after LA application, and vice versa show further increases in HC activity, it seems likely that these two treatments increase hemichannel activity via independent mechanisms.
Figure 2. Linoleic acid increases hemichannel activity on MKN28 cells.
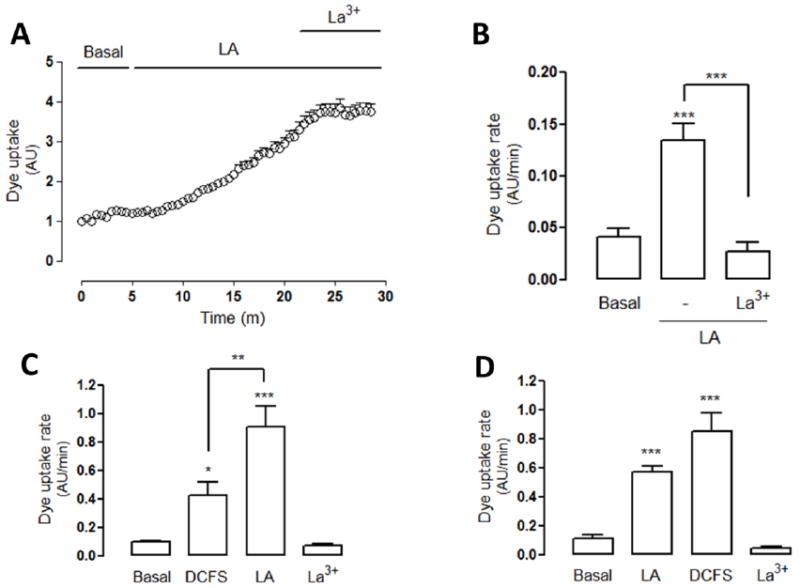
(A) Representative dye (Etd+) uptake curve of MKN28 cells exposed to linoleic acid (LA, 100 μM): ethidium uptake was increased ~5 min after treatment with LA. Dye uptake was blocked by lanthanum ions (La3+, 200 μM). (B) Quantification of dye uptake rate of cells under basal conditions and treated with LA. The increase was sensitive to La3+. (C) Dye uptake rate of HeLa-Cx43 cells exposed first to divalent cation-free solution (DCFS) for 10 min followed by 100 μM LA for additional 10 min and to lanthanum ions (La3+, 200 μM) for 5 more minutes. (D) Dye uptake rate of HeLa-Cx43 cells exposed first to LA, then to DCFS, and finally to La3+ for the same periods of time as in (C). Statistical significance versus basal conditions *p<0.05, ***p<0.001, versus LA ***p<0.001 and versus DCFS **p<0.01, n=3-4.
3.3. The LA-induced Cx HC activity requires G-protein receptor 40 (GPR40)
Diverse effects of different FFAs have been linked to G-protein coupled receptors (GPCRs) [25, 26]. In particular, the effects of medium and long chain FFAs were found associated with two different receptors: GPR40/ FFAR1 (Free Fatty Acids Receptor 1) and GPR120 [26, 45]. Also, another possible candidate for FFA receptor is the broadly expressed membrane glycoprotein CD36 [46]. Although the effect of LA, a ω-6 long chain FFA, has been associated with GPR40 [25, 45] to our knowledge the membrane transduction of FFA in MKN28 cells has not been described. We found that MKN28 cells express the mRNA for GPR40, GPR120 mRNA (Figure 3A), and CD36 (Supp Figure 1A). Moreover, the application of GW9508 (40 μM), an agonist of GPR40 and GPR120 [19, 47], increased the Etd+ uptake after a delay of about 5 min (Figure 3B). The increase in Etd+ uptake rate was ~3-fold and the application of La3+ reduced the Etd+ uptake to values lower than that of control cells (Figure 3C). The possible participation of CD36 in the LA-induced Cx HC activity in MKN28 cells was discarded because treatment with 100 μM sulfosuccinimidyl oleate, a CD36 inhibitor, did not affect the LA-induced Etd+ uptake of MKN28 cells (Supp Figure 1B, 1C).
Figure 3. MKN28 cells express GPR40 and GPR120 and their activation increases hemichannel activity.
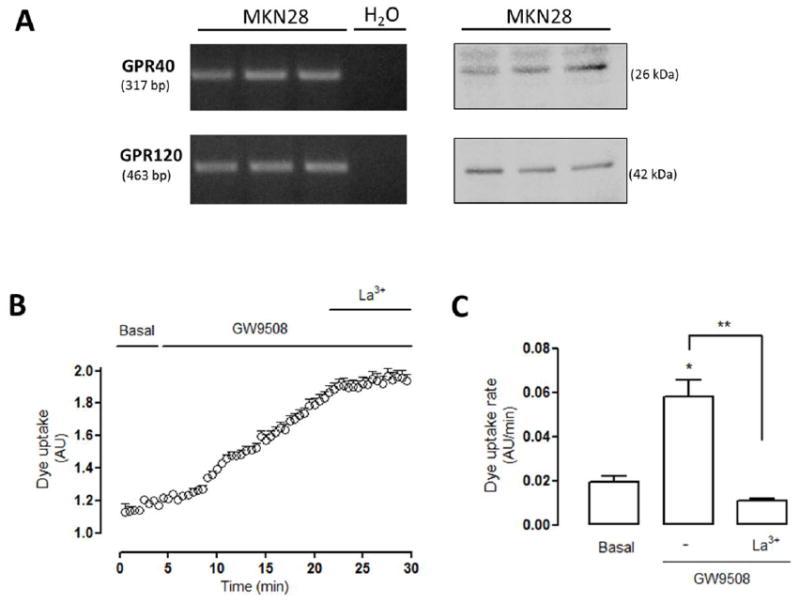
(A) In total cellular homogenate of MKN28 cells the presence of GPR40 and GPR120 mRNA were detected by RT-PCR (H2O was used as a negative control). Also, GPR40 and GPR120 proteins were detected by western blot analysis. The expected size of the amplicons and molecular weight of the proteins are indicated in base pairs (bp) and kilodaltons (kDa), respectively. (B) MKN28 cells were exposed to ethidium solution (5 μM Etd+). Representative Dye (5 μM Etd+) uptake curve of MKN28 cells exposed to 40 μM GW9508, agonist by GPR40 and GPR120 receptors, followed by the application of lanthanum ions (200 μM La3+). (C) Dye uptake rate under basal conditions and after consecutive application of GW9508 and La3+ as shown in (B). Statistical significance versus basal conditions *p<0.05 and versus GW9508 **p<0.01, n=3-4.
To further confirm the involvement of GRP40 in the LA-induced increase in membrane permeability, MKN28 cells were transfected with a siRNA for GPR40. The knock-down of GPR40 was confirmed by RT-PCR and immunofluorescence analyses, respectively, but use of a control siRNA (Neg siRNA) did not (Figure 4A). In MKN28 cells transfected with GPR40-siRNA, LA (100 μM) did not affect Etd+ uptake, but cells transfected with Neg-siRNA showed an evident increase in Etd+ uptake and this response was blocked with La3+ (200 μM) (Figure 4B, 4Ca). Nevertheless, we did not know if the absence of response to LA found in cells transfected with siRNA-GPR40 was due to knock-down of GPR40 or down regulation of Cx HC activity induced by the GPR40 knock-down procedure. To test for reduced Cx HCs, cells were transfected cells with Neg- or GPR40- siRNA and treated with DCFS. Both preparations showed similar increases in dye uptake and inhibition by La3+ (Figure 4Cb), indicating that the amount of Cx HCs found in the cell surface was unaffected by the procedure and thus, the lack of response was due to the absence of effective GPR40-mediated signal transduction. Moreover, the effect of LA over Cx HC activity in MKN28 cells apparently is independent of GPR120 activity, because cells transfected with siRNA-GPR120 showed drastic reduction in GPR120 reactivity and levels of GPR120 mRNA but presented similar increase in Etd+ uptake induced by 100 μM LA (Supp Figure 2A y 2B).
Figure 4. Knock-down of GPR40 receptor abrogates the hemichannel activity induced by linoleic acid.
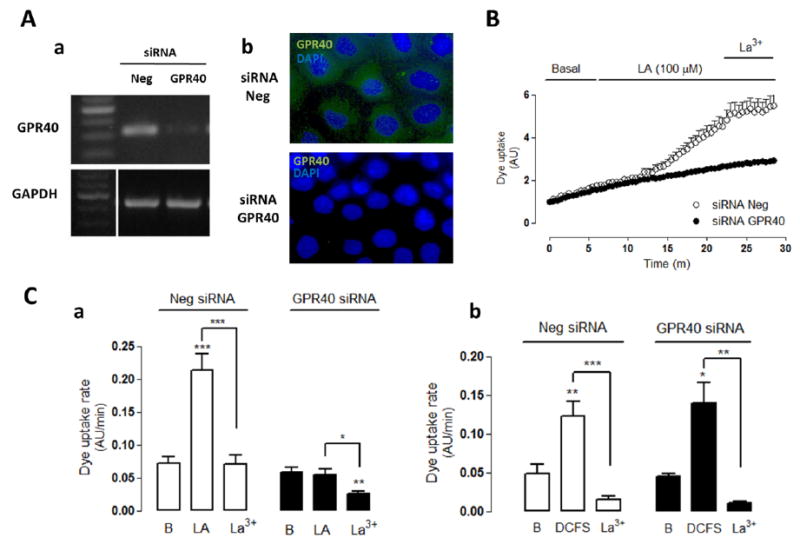
MKN28 cells were transfected with GPR40 siRNA or random sequence (Neg siRNA) and analyzed 40-48 h later. (A) The knock-down of GPR40 was confirmed by PCR (Aa) and immunofluorescence analyses (Ab). (B) Representative dye uptake (5 μM Etd+) curve. In cells transfected with Neg siRNA the increased of dye uptake by linoleic acid (LA, 100 μM) was abrogated by GPR40 knock-down (GPR40 siRNA). Under both transfection conditions, the LA effect was blocked by lanthanum ions (La3+, 200 μM). (Ca) Dye uptake rate in cells transfected with Neg or GPR40 siRNA under basal conditions and sequentially treated with LA and lanthanum ions (La3+). (Cb) Dye uptake rate of cells transfected with Neg or GPR40 siRNA under basal conditions or exposed to divalent cation-free solution (DCFS) and La3+ as indicated. Statistical significance versus basal (B) conditions ***p<0.001, versus LA *p<0.05, ***p<0.001 and versus DCFS n=4-5.
Previously, a report found that LA increases the hemichannel activity in HeLa cells transfected with Cx26, Cx43 or Cx45 [24], but the possible involvement of GPR40 remained unknown. Here, we show that HeLa cells transfected with Cx26, Cx32, Cx43 or Cx45 express GPR40 (Figure 5A) and the LA-induced dye uptake was reduced in cells transiently transfected with siRNA-GPR40 (Figure 5B and C). Similarly, the increase in dye uptake rate of HeLa cells transfected with Cx26 or Cx32 or Cx43 was completely abrogated by transfection with siRNA-GPR40 (Figure 5C). In all transfectant cells, Etd+ uptake was inhibited by La3+ (Figure 5B and C).
Figure 5. Linoleic acid induces GPR40-dependent hemichannel activity in connexin HeLa cells transfectants.
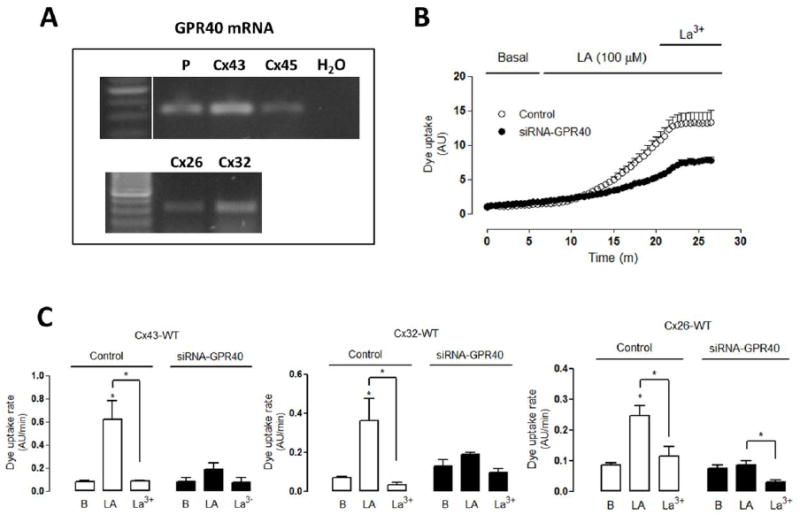
HeLa cells stably transfected with connexins 43 (Cx43), Cx32 or Cx26 were used. Sister cultured cells were transiently transfected (40–48 h) with GPR40-siRNA. (A) The mRNA of GPR40 was evaluated in HeLa parental cells (P) or in HeLa cell transfectants (Cx43, Cx45, Cx26, Cx32). H2O was used as negative control. (B) Representative Dye (5 μM Etd+) uptake curve of control HeLa-Cx43 cells and HeLa-Cx43 transfected with GPR40 siRNA treated with linoleic acid (LA, 100 μM) followed by treatment with 200 μM La3+. (C) Dye uptake rate of HeLa-Cx43, -Cx32 or -Cx26 cells under control conditions or transfected with GPR40-siRNA under basal conditions, followed by LA and La3+ treatment. Statistical significance versus basal (B) conditions *p<0.05 and versus LA*p<0.05, n=3-4.
3.4. The LA-induced increase in membrane permeability requires a Cx43 Akt phosphorylation site
The LA-induced increase in Cx HC activity has been shown to involve the activation of Akt [24]. Hence, we wanted to determine the involvement of Akt in the Cx HC membrane permeability of MKN28 cells treated with LA. We found that LA-induced dye uptake was completely abrogated in MKN28 cells preincubated for 30 min with 10 μM AKTi, an Akt kinase inhibitor [37] (Figure 6A). Quantification of the Etd+ uptake rate revealed that AKTi treatment apparently lead to inhibition below control values even in cells not treated with LA (Figure 6B), suggesting basal Cx HC activity depends on basal Akt activity and possibly phosphorylation of Cx43. Cx43 have been shown to be phosphorylated by Akt primarily at serine 373 [36, 48, 49]. To study the possible participation of Cx43 phosphorylation via Akt, we studied the effect of LA on Cx HCs in HeLa-Cx43-S373A cells. As shown above, HeLa cells transfected with wild type Cx43 (Cx43-WT) showed an evident LA-induced increase in Etd+ uptake rate (Figure 5C). However, dye uptake of HeLa cells transfected with Cx43-S373A was not modified by LA (Figure 6C). HeLa-Cx43-S373A cells bathed with DCFS showed rapid activation of Cx HCs that was blocked with La3+ (200 μM). Thus, the effect of Cx43 mutation at serine 373 appears to be specific to the LA-induced pathway as the mutation modifies neither the amount of available Cx HC in the cell membrane nor their sensitivity to low concentration of divalent cations.
Figure 6. The linoleic acid-induced activation of connexin hemichannels is downstream of Akt.
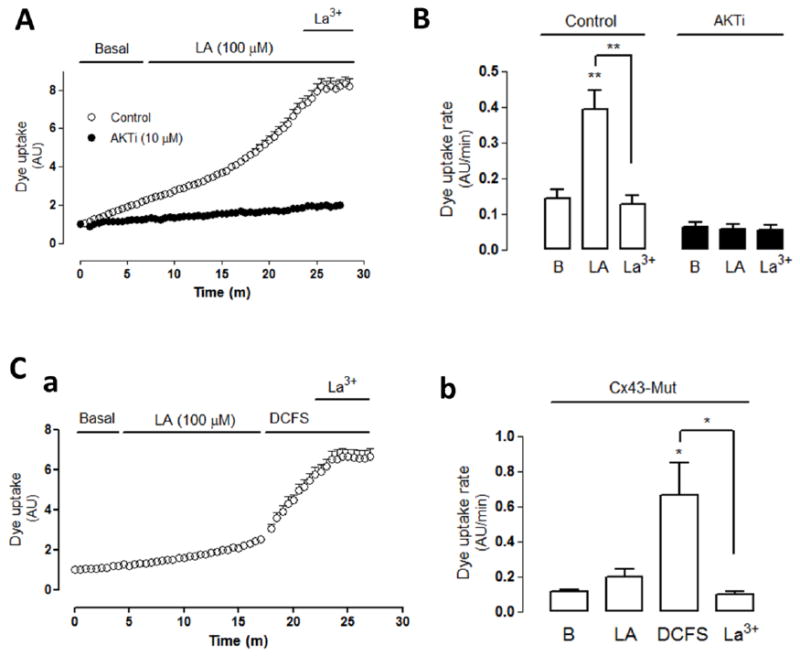
(A) Representative dye (5 μM Etd+) uptake curve of control cells treated with 100 μM linoleic acid (LA) or cells pretreated with 10 μM AKTi (Akt inhibitor) and then treated with LA. After 15 min with LA, cells were treated with 100 μM lanthanum ions (La3+) as indicated by the black traces on top of the graph. (B) Quantification of dye uptake rate under control conditions or after pretreatment with AKTi under basal conditions and sequentially treated with LA and LA plus La3+. (C) Connexin43 with S373A mutation (Cx43-Mut) stably transfected into HeLa cells. (Ca) Dye uptake curve of cells exposed first to LA and then to divalent cation-free solution (DCFS) and DCFS plus 200 μM La3+. (Cb) Dye uptake rate of cells (Cx43-Mut) under each of the conditions described in (a). Statistical significance versus basal (B) conditions *p<0.05, **p<0.01, versus LA **p<0.01 and versus DCFS *p<0.05, n=3-4.
The increase in Cx HC activity induced by LA could result from an increase open probability of HCs already present in the cell membrane and/or from an increase in amount of available Cx HCs in the cell surface. Here, we tested the latter possibility using biotinylation of cell surface membrane proteins followed by precipitation and Western blot analysis of Cx43. Cx43 was readily detected on the surface of the HeLa–Cx43 cells (Figure 7A). The levels of cell surface Cx43 in cells under control conditions was not significantly affected by 30 min incubation with 10 μM AKTi. Moreover, the application of 100 μM LA for 15 min approximately doubled the amount of cell surface Cx43 and that increase was abrogated in cells preincubated with 10 μM AKTi for 30 min (Figure 7). Also, phosphorylation of Cx43 at S373 occurred only after application of LA and this response was completely prevented by AKTi (Figure 7A).
Figure 7. Linoleic acid increases the number of connexin43 hemichannels at the cell surface in HeLa cells.
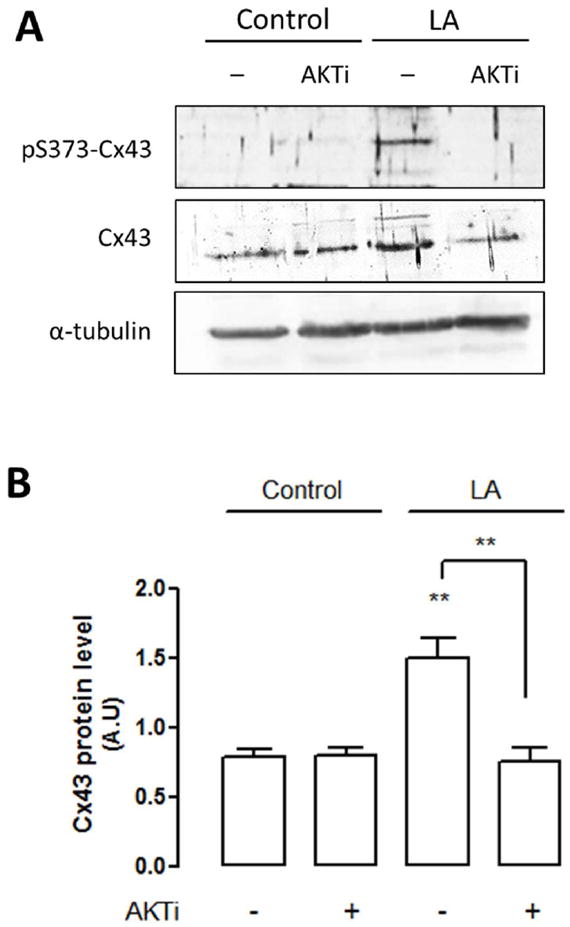
The relative amount of Cx43 at the cell surface in HeLa-Cx43 cells was determined by biotinylation of cell surface proteins followed by western blot assays. (A) Representative image of the relative amount of Cx43 at the cell surface in HeLa-Cx43 cells, in absence (control) or the presence of linoleic acid (LA, 100 μM), and with and without AKTi (10 μM). The biotinylation was followed by western blot analysis using antibodies against Cx43 (Cx43) (repeated 3 times) or Cx43 phosphorylated at S373 (pS373-Cx43) (repeated 2 times). α-tubulin corresponds to the total protein fraction, before purification of surface hemichannels and was used as a loading control. (B) Quantification of cell surface Cx43 levels in control HeLa-Cx43 cells, in absence (control) or the presence of LA, and with and without AKTi or cells exposed to AKTi (10 μM) in the presence or absence (control) of linoleic acid (LA, 100 μM). Statistical significance compared to control in the absence of AKTi. **p<0.01 and versus LA (without AKTi) **p<0.01, n= 2-3.
4. DISCUSSION
Here, we show that linoleic acid (LA), an essential unsaturated fatty acid, increases the permeability of the plasma membrane by increasing the activity of Cx HCs in gastric epithelial cells through a mechanism that involves a membrane receptor (GPR40) and activation of an Akt-dependent intracellular signaling pathway that involves phosphorylation of Cx43 at serine residue 373. Thus, LA causes an increase amount of Cx HCs at the cell surface and an increase in the Cx HC activity allowing more diffusion of Cx HC permeant ions and molecules across the cell membrane.
LA had previously been shown to increase the Cx HC activity after about 5 min [24], suggesting the involvement of signaling and/or metabolic steps that take time to occur. We found that LA requires the presence of a membrane transducer, GPR40, an Akt-dependent intracellular signaling pathway, and increased levels of Cx43 HCs in the plasma membrane, steps which could take some time to occur. The relevance of GPR40 is supported by three findings: 1) GW9508, an agonist of GPR40, also triggered an increase in Etd+ uptake that presented with a delay of ~5 min, suggesting the participation of the same pathway activated by LA, 2) the participation of other two receptors that can response to FFAs, GPR120 and CD36, was discarded, and 3) cells transfected with siRNA for GPR40 did not show LA-induced dye uptake. The latter result was specific because the Neg siRNA for GPR40 was ineffective at reducing the level of GPR40 or the LA-induced dye uptake. Interestingly, the effects of LA, a ω-6 long chain FFA, have been associated with GPR40 [25, 45]. In MKN28 cells, we found expression of GPR40 and GPR120, and CD36, but LA is known to be a GPR40 endogenous ligand that induces activation through the Gq protein [19], which could explain the complete inhibition of LA on Cx HC activity in cells transfected with siRNA for GPR40, where we know that GPR120 expression was unchanged by siRNA-GPR40 (data not shown). Also, it is worth mentioning that the different receptors elicit differential response to FFAs, and in particular for GPR120 and CD36 it has been reported that they have no overlapping roles in intracellular signaling induces by FFAs [31].
The increase in Cx HC activity induced by LA was previously proposed to be PI3K- and Akt-dependent but independent of p38 kinase [24]. In the present work, we first confirmed that AKTi prevents completely the LA-induced dye uptake. Then, we found that serine 373 located in the carboxyl terminal of Cx43 is required for the LA-induced Cx HC response because HeLa-Cx43-S373A did not respond. Thus, Akt is likely to catalyze a later step in the GPR40 activation intracellular signaling pathway leading to changes in Cx43 HCs. Notably, it has been previously found that the size of gap junctions are greatly influenced by phosphorylation at Akt sites on Cx43 [36, 48, 49], suggesting that hemichannels and gap junctions share some aspects of their regulation. Interestingly, inhibition of Akt with AKTi in HeLa-Cx43 cells drastically reduced the Cx43 HC activity below basal level but did not reduce the levels of Cx43 in the cell membrane compared to control, suggesting that Akt-mediated phosphorylation might increase Cx43 plasma membrane levels and regulate other Cx43 HC features such as permeability and/or conductance. The fast opening of Cx HCs in cells exposed to DCFS is consistent with the involvement of a gating mechanism that increases the open probability of Cx HCs [40] without affecting Cx levels in the membrane [44]. In contrast, The LA-induced dye uptake required the participation of membrane and cytoplasmic events that increase the number of Cx HCs at the cell surface as shown by the increased levels of Cx43 detected in biotinylation of cell surface proteins followed by Western blot analysis.
The LA-induced increase in Cx HCs activity has been shown to be preceded by a small, transient increase in intracellular Ca2+ concentration ([Ca2+]i) independent of Cx HCs, followed by a progressive increase in [Ca2+]i that paralleled the Cx HC activity change [25]. In addition, the increase in [Ca2+]i was prevented by chelation with intracellular BAPTA. However, it was not shown if the absence of divalent cations affects the LA-induced Cx HC activity. Here, we found that the LA-induced Cx HC activity is at least partially independent of the effect of reduced extracellular [Ca2+] since their effects at saturation were additive. Accordingly, regulation of Cx HCs by extracellular divalent cations has been shown to depend on amino acid residues located in Cx domains facing the extracellular milieu [4] while regulation by LA depends upon an intracellular transduction mechanism and modification of an amino acid residue located intracellularly in the C-terminal domain. The Etd+ uptake induced by LA reached maximal values within 5 min making increased Cx43 protein synthesis an unlikely explanation for the change. Alternatively, the increase in Cx43 level in the cell membrane could result from increased trafficking of Cx43 from intracellular Cx43 stores, or less removal of Cx43 from the cell membrane or different distribution in raft membrane fraction. These mechanisms could lead to an increase in open probability due to the presence of more hemichannels. It remains to be demonstrated whether LA also affects the intrinsic open probability of hemichannels already present in the membrane.
Since LA increased the activity of hemichannels composed of Cx26 or Cx32 in HeLa cell transfectants and these Cxs were also found in MKN28 cells, it is likely that in the latter cells the Akt-dependent transduction mechanism that acts on Cx43 HCs also affects the activity of other Cx HCs. In support of this interpretation, knock down of GPR40 prevented the LA-induced increase in Cx26 and Cx32 HC activity. Moreover, the LA-induced Cx HC activity was completely abrogated in MKN28 cells pretreated with AKTi. Cx26 and Cx32 have been shown to be phosphoproteins [51-53], but it remains to be demonstrated whether phosphorylation of these Cxs is mediated directly by Akt or other protein kinases downstream of Akt. In cells that co-express Cx43 with other connexins (e.g., Cx26 and/or Cx32), it is possible that Cx43 HC opening promotes signal transduction pathways or alters the transmembrane ionic gradients triggering Cx26 HC and/or Cx32 HC opening.
In diverse cell types, increases in Cx HC activity have been associated with physiological and pathophysiological conditions [3], but epithelial cells of the gastrointestinal tract remained unexplored. Epithelial gastric cells likely see varying levels of FFA, including LA, thus it is likely that the activity of Cx HCs changes throughout the day. This regulation of Cx HCs might be important because they would favor the diffusional uptake of diverse small nutrient molecules that permeate hemichannels. Given the different properties of different connexins, the presence of different Cxs could allow the formation of homomeric (HC composed of one Cx type) and heterometic hemichannels (Cx26 and Cx32 intermixed in a HC) with different permeability properties possibly favoring the diffusional exchange of different sets of small molecules [54]. However, the effects of LA have been associated mainly with inflammatory conditions [35, 55]. Since the cells expressing Cx43-S373A do not respond to metabolic inhibition, a proinflammatory condition that mimics hypoxia-reoxygenation [37], most likely the physiological or pathophysiological outcome of LA on cells via Cx HCs will depend on the intensity and duration of the stimulus; high concentrations or long term action of an effective LA concentration could lead to Ca2+ overload and thus degenerative mechanisms that activate an inflammatory response.
Our results lead us to propose that LA increases the Cx43 HC activity through activation of a signaling pathway that involves the membrane receptor GPR40 and AKT kinase activity. Then, the phosphorylation of Cx43 in serine 373 by AKT promotes an increase in the number of Cx43 HC at the cell surface, which increases the open probability of Cx43 HCs via a mechanism that is independent of extracellular [Ca2+].
Supplementary Material
Highlights.
Linoleic acid increases the plasma membrane permeability of gastric epithelial cells
Activation of GPR40 receptor induces phosphorylation of connexin 43 at serine 373
Linoleic acid, via Akt, induces an increase in the amount of Cx43 at the cell surface
Acknowledgments
We thank to Ms. Teresa Vergara and Ms. Paola Fernández for their technical support. This work was partially supported by Fondo Nacional de Desarrollo Científico y Tecnológico (FONDECYT): Grant 3130662 (to CP); 1150291 (to JCS), ICM-Economía P09-022-F Centro Interdisciplinario de Neurociencias de Valparaíso (to JCS) and a Grant from the US National Institutes of Health (NIH): GM55632 (PDL).
Abbreviations used
- Cx
connexin
- DCFS
divalent cation-free solution
- Etd+
ethidium
- FFA
free fatty acid
- GPCR
G-protein coupled receptor
- GPR40
G-protein receptor 40
- HC
hemichannel
- LA
linoleic acid
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Bedner P, Steinhäuser C, Theis M. Functional redundancy and compensation among members of gap junction protein families? Biochim Biophys Acta. 2012;1818:1971–1984. doi: 10.1016/j.bbamem.2011.10.016. [DOI] [PubMed] [Google Scholar]
- 2.Söhl G, Willecke K. Gap junctions and the connexin protein family. Cardiovasc Res. 2004;62:228–232. doi: 10.1016/j.cardiores.2003.11.013. [DOI] [PubMed] [Google Scholar]
- 3.Sáez JC, Retamal MA, Basilio D, Bukauskas FF, Bennett MV. Connexin-based gap junction hemichannels: gating mechanisms. Biochim Biophys Acta. 2005;1711:215–224. doi: 10.1016/j.bbamem.2005.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.De Vuyst E, Decrock E, De Bock M, Yamasaki H, Naus CC, Evans WH, Leybaert L. Connexin hemichannels and gap junction channels are differentially influenced by lipopolysaccharide and basic fibroblast growth factor. Mol Biol Cell. 2007;18:34–46. doi: 10.1091/mbc.E06-03-0182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Retamal MA, Froger N, Palacios-Prado N, Ezan P, Sáez PJ, Sáez JC, Giaume C. Cx43 hemichannels and gap junction channels in astrocytes are regulated oppositely by proinflammatory cytokines released from activated microglia. J Neurosci. 2007;27:13781–13792. doi: 10.1523/JNEUROSCI.2042-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rackauskas M, Neverauskas V, Skeberdis VA. Diversity and properties of connexin gap junction channels. Medicina (Kaunas) 2010;46:1–12. [PubMed] [Google Scholar]
- 7.Morita H, Katsuno T, Hoshimoto A, Hirano N, Saito Y, Suzuki Y. Connexin 26-mediated gap junctional intercellular communication suppresses paracellular permeability of human intestinal epithelial cell monolayers. Exp Cell Res. 2004;298:1–8. doi: 10.1016/j.yexcr.2004.03.046. [DOI] [PubMed] [Google Scholar]
- 8.Ey B, Eyking A, Gerken G, Podolsky DK, Cario E. TLR2 mediates gap junctional intercellular communication through connexin-43 in intestinal epithelial barrier injury. J Biol Chem. 2009;284:22332–22343. doi: 10.1074/jbc.M901619200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Orellana JA, Sáez PJ, Cortés-Campos C, Elizondo RJ, Shoji KF, Contreras-Duarte S, Figueroa V, Velarde V, Jiang JX, Nualart F, Sáez JC, García MA. Glucose increases intracellular free Ca(2+) in tanycytes via ATP released through connexin 43 hemichannels. Glia. 2012;60:53–68. doi: 10.1002/glia.21246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Riquelme MA, Cea LA, Vega JL, Boric MP, Monyer H, Bennett MV, Frank M, Willecke K, Sáez JC. The ATP required for potentiation of skeletal muscle contraction is released via pannexin hemichannels. Neuropharmacology. 2013;75:594–603. doi: 10.1016/j.neuropharm.2013.03.022. [DOI] [PubMed] [Google Scholar]
- 11.Maes M, Cogliati B, Crespo-Yanguas S, Willebrords J, Vinken M. Roles of connexins and pannexins in digestive homeostasis. Cell Mol Life Sci. 2015a;72:2809–2821. doi: 10.1007/s00018-015-1961-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Maes M, Crespo-Yanguas S, Willebrords J, Cogliati B, Vinken M. Connexin and pannexin signaling in gastrointestinal and liver disease. Transl Res. 2015b doi: 10.1016/j.trsl.2015.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Guttman JA, Lin AE, Li Y, Bechberger J, Naus CC, Vogl AW, Finlay BB. Gap junction hemichannels contribute to the generation of diarrhoea during infectious enteric disease. Gut. 2010;59:218–226. doi: 10.1136/gut.2008.170464. [DOI] [PubMed] [Google Scholar]
- 14.Liu X, Furuya T, Li D, Xu J, Cao X, Li Q, Xu J, Xu Z, Sasaki K, Liu X. Connexin 26 expression correlates with less aggressive phenotype of intestinal type-gastric carcinomas. Int J Mol Med. 2010;25:709–716. doi: 10.3892/ijmm_00000395. [DOI] [PubMed] [Google Scholar]
- 15.Wang Y, Huang LH, Xu CX, Xiao J, Zhou L, Cao D, Liu XM, Qi Y. Connexin 32 and 43 promoter methylation in Helicobacter pylori-associated gastric tumorigenesis. World J Gastroenterol. 2014;20:11770–11779. doi: 10.3748/wjg.v20.i33.11770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Reinehr T, Roth CL. The gut sensor as regulator of body weight. Endocrine. 2015;49:35–50. doi: 10.1007/s12020-014-0518-1. [DOI] [PubMed] [Google Scholar]
- 17.Aikawa S, Hashimoto T, Kano K, Aoki J. Lysophosphatidic acid as a lipid mediator with multiple biological actions. J Biochem. 2015;157:81–89. doi: 10.1093/jb/mvu077. [DOI] [PubMed] [Google Scholar]
- 18.Glatz JF. Lipids and lipid binding proteins: a perfect match. Prostaglandins Leukot Essent Fatty Acids. 2015;93:45–49. doi: 10.1016/j.plefa.2014.07.011. [DOI] [PubMed] [Google Scholar]
- 19.Hara T, Hirasawa A, Ichimura A, Kimura I, Tsujimoto G. Free fatty acid receptors FFAR1 and GPR120 as novel therapeutic targets for metabolic disorders. J Pharm Sci. 2011;100:3594–3601. doi: 10.1002/jps.22639. [DOI] [PubMed] [Google Scholar]
- 20.Milligan G, Ulven T, Murdoch H, Hudson BD. G-protein-coupled receptors for free fatty acids: nutritional and therapeutic targets. Br J Nutr. 2014;111:S3–S7. doi: 10.1017/S0007114513002249. [DOI] [PubMed] [Google Scholar]
- 21.Retamal MA, Evangelista-Martínez F, León-Paravic CG, Altenberg GA, Reuss L. Biphasic effect of linoleic acid on connexin 46 hemichannels. Pflügers Arch. 2011;461:635–643. doi: 10.1007/s00424-011-0936-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kiec-Wilk B, Czech U, Janczarska K, Knapp A, Goralska J, Cialowicz U, Malecki MT, Dembinska-Kiec A. Connexin 43 and metabolic effect of fatty acids in stressed endothelial cells. Genes Nutr. 2012;7:257–263. doi: 10.1007/s12263-011-0247-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Marandykina A, Palacios-Prado N, Rimkut L, Skeberdis VA, Bukauskas FF. Regulation of connexin36 gap junction channels by n-alkanols and arachidonic acid. J Physiol. 2013;591:2087–2101. doi: 10.1113/jphysiol.2013.250910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Figueroa V, Sáez PJ, Salas JD, Salas D, Jara O, Martínez AD, Sáez JC, Retamal MA. Linoleic acid induces opening of connexin26 hemichannels through a PI3K/Akt/Ca(2+)-dependent pathway. Biochim Biophys Acta. 2013;1828:1169–1179. doi: 10.1016/j.bbamem.2012.12.006. [DOI] [PubMed] [Google Scholar]
- 25.Itoh Y, Kawamata Y, Harada M, Kobayashi M, Fujii R, Fukusumi S, Ogi K, Hosoya M, Tanaka Y, Uejima H, Tanaka H, Maruyama M, Satoh R, Okubo S, Kizawa H, Komatsu H, Matsumura F, Noguchi Y, Shinohara T, Hinuma S, Fujisawa Y, Fujino M. Free fatty acids regulate insulin secretion from pancreatic beta cells through GPR40. Nature. 2003;422:173–176. doi: 10.1038/nature01478. [DOI] [PubMed] [Google Scholar]
- 26.Hirasawa A, Tsumaya K, Awaji T, Katsuma S, Adachi T, Yamada M, Sugimoto Y, Miyazaki S, Tsujimoto G. Free fatty acids regulate gut incretin glucagon-like peptide-1 secretion through GPR120. Nat Med. 2005;11:90–94. doi: 10.1038/nm1168. [DOI] [PubMed] [Google Scholar]
- 27.Ichimura A, Hirasawa A, Hara T, Tsujimoto G. Free fatty acid receptors act as nutrient sensors to regulate energy homeostasis. Prostaglandins Other Lipid Mediat. 2009;89:82–88. doi: 10.1016/j.prostaglandins.2009.05.003. [DOI] [PubMed] [Google Scholar]
- 28.Hirasawa A, Hara T, Katsuma S, Adachi T, Tsujimoto G. Free fatty acid receptors and drug discovery. Biol Pharm Bull. 2008;31:1847–1851. doi: 10.1248/bpb.31.1847. [DOI] [PubMed] [Google Scholar]
- 29.Martin C, Chevrot M, Poirier H, Passilly-Degrace P, Niot I, Besnard P. CD36 as a lipid sensor. Physiol Behav. 2011;105(1):36–42. doi: 10.1016/j.physbeh.2011.02.029. [DOI] [PubMed] [Google Scholar]
- 30.Naville D, Duchampt A, Vigier M, Oursel D, Lessire R, Poirier H, Niot I, Bégeot M, Besnard P, Mithieux G. Link between intestinal CD36 ligand binding and satiety induced by a high protein diet in mice. PLoS One. 2012;7(1):e30686. doi: 10.1371/journal.pone.0030686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ozdener MH, Subramaniam S, Sundaresan S, Sery O, Hashimoto T, Asakawa Y, Besnard P, Abumrad NA, Khan NA. CD36- and GPR120-mediated Ca2+ signaling in human taste bud cells mediates differential responses to fatty acids and is altered in obese mice. Gastroenterology. 2014;146:995–1005. doi: 10.1053/j.gastro.2014.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Innis SM. Human milk: maternal dietary lipids and infant development. Proc Nutr Soc. 2007;66:397–404. doi: 10.1017/S0029665107005666. [DOI] [PubMed] [Google Scholar]
- 33.Schmitz G, Ecker J. The opposing effects of n-3 and n-6 fatty acids. Prog Lipid Res. 2008;47:147–155. doi: 10.1016/j.plipres.2007.12.004. [DOI] [PubMed] [Google Scholar]
- 34.Barceló-Coblijn G, Murphy EJ. Alpha-linolenic acid and its conversion to longer chain n-3 fatty acids: benefits for human health and a role in maintaining tissue n-3 fatty acid levels. Prog Lipid Res. 2009;48:355–374. doi: 10.1016/j.plipres.2009.07.002. [DOI] [PubMed] [Google Scholar]
- 35.Yang Z, Huffman SL. Modelling linoleic acid and α-linolenic acid requirements for infants and young children in developing countries. Matern Child Nutr. 2013;9(Suppl 1):72–77. doi: 10.1111/j.1740-8709.2012.00448.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dunn CA, Lampe PD. Injury-triggered Akt phosphorylation of Cx43: a ZO-1-driven molecular switch that regulates gap junction size. J Cell Sci. 2014;127:455–464. doi: 10.1242/jcs.142497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Salas D, Puebla C, Lampe PD, Lavandero S, Sáez JC. Role of Akt and Ca2+ on cell permeabilization via connexin43 hemichannels induced by metabolic inhibition. Biochim Biophys Acta. 2015;1852:1268–1277. doi: 10.1016/j.bbadis.2015.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wroblewski LE, Shen L, Ogden S, Romero-Gallo J, Lapierre LA, Israel DA, Turner JR, Peek RM., Jr Helicobacter pylori dysregulation of gastric epithelial tight junctions by urease-mediated myosin II activation. Gastroenterology. 2009;136:236–246. doi: 10.1053/j.gastro.2008.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Li H, Liu TF, Lazrak A, Peracchia C, Goldberg GS, Lampe PD, Johnson RG. Properties and regulation of gap junctional hemichannels in the plasma membranes of cultured cells. J Cell Biol. 1996;134:1019–1030. doi: 10.1083/jcb.134.4.1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gómez-Hernández JM, de Miguel M, Larrosa B, González D, Barrio LC. Molecular basis of calcium regulation in connexin-32 hemichannels. Proc Natl Acad Sci U S A. 2003;100:16030–16035. doi: 10.1073/pnas.2530348100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ye ZC, Wyeth MS, Baltan-Tekkok S, Ransom BR. Functional hemichannels in astrocytes: a novel mechanism of glutamate release. J Neurosci. 2003;23:3588–3596. doi: 10.1523/JNEUROSCI.23-09-03588.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sánchez HA, Orellana JA, Verselis VK, Sáez JC. Metabolic inhibition increases activity of connexin-32 hemichannels permeable to Ca2+ in transfected HeLa cells. Am J Physiol Cell Physiol. 2009;297:C665–C678. doi: 10.1152/ajpcell.00200.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Orellana JA, Díaz E, Schalper KA, Vargas AA, Bennett MV, Sáez JC. Cation permeation through connexin 43 hemichannels is cooperative, competitive and saturable with parameters depending on the permeant species. Biochem Biophys Res Commun. 2011;409:603–609. doi: 10.1016/j.bbrc.2011.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Schalper KA, Palacios-Prado N, Orellana JA, Sáez JC. Currently used methods for identification and characterization of hemichannels. Cell Commun Adhes. 2008;15:207–218. doi: 10.1080/15419060802014198. [DOI] [PubMed] [Google Scholar]
- 45.Briscoe CP, Tadayyon M, Andrews JL, Benson WG, Chambers JK, Eilert MM, Ellis C, Elshourbagy NA, Goetz AS, Minnick DT, Murdock PR, Sauls HR, Jr, Shabon U, Spinage LD, Strum JC, Szekeres PG, Tan KB, Way JM, Ignar DM, Wilson S, Muir AI. The orphan G protein-coupled receptor GPR40 is activated by medium and long chain fatty acids. J Biol Chem. 2003;278:11303–11311. doi: 10.1074/jbc.M211495200. [DOI] [PubMed] [Google Scholar]
- 46.Febbraio M, Silverstein RL. CD36: implications in cardiovascular disease. Int J Biochem Cell Biol. 2007;39(11):2012–2030. doi: 10.1016/j.biocel.2007.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Swaminath G. Fatty acid binding receptors and their physiological role in type 2 diabetes. Arch Pharm (Weinheim) 2008;341:753–761. doi: 10.1002/ardp.200800096. [DOI] [PubMed] [Google Scholar]
- 48.Solan JL, Lampe PD. Specific Cx43 phosphorylation events regulate gap junction turnover in vivo. FEBS Lett. 2014;588:1423–1429. doi: 10.1016/j.febslet.2014.01.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Park DJ, Wallick CJ, Martyn KD, Lau AF, Jin C, Warn-Cramer BJ. Akt phosphorylates connexin43 on Ser373, a ‘‘mode-1’’ binding site for 14-3-3. Cell Commun Adhes. 2007;14:211–226. doi: 10.1080/15419060701755958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Fasciani I, Temperán A, Pérez-Atencio LF, Escudero A, Martínez-Montero P, Molano J, Gómez-Hernández JM, Paino CL, González-Nieto D, Barrio LC. Regulation of connexin hemichannel activity by membrane potential and the extracellular calcium in health and disease. Neuropharmacology. 2013;75:479–490. doi: 10.1016/j.neuropharm.2013.03.040. [DOI] [PubMed] [Google Scholar]
- 51.Locke D, Koreen IV, Harris AL. Isoelectric points and post-translational modifications of connexin26 and connexin32. FASEB J. 2006;20:1221–1223. doi: 10.1096/fj.05-5309fje. [DOI] [PubMed] [Google Scholar]
- 52.Locke D, Bian S, Li H, Harris AL. Post-translational modifications of connexin26 revealed by mass spectrometry. Biochem J. 2009;424:385–398. doi: 10.1042/BJ20091140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Traub O, Look J, Dermietzel R, Brümmer F, Hülser D, Willecke K. Comparative characterization of the 21-kD and 26-kD gap junction proteins in murine liver and cultured hepatocytes. J Cell Biol. 1989;108:1039–1051. doi: 10.1083/jcb.108.3.1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hansen DB, Braunstein TH, Nielsen MS, MacAulay N. Distinct permeation profiles of the connexin 30 and 43 hemichannels. FEBS Lett. 2014;588:1446–1457. doi: 10.1016/j.febslet.2014.01.036. [DOI] [PubMed] [Google Scholar]
- 55.Weiss LA, Chambers CD, Gonzalez V, Hagey LR, Jones KL. The omega-6 fatty acid linoleic acid is associated with risk of gastroschisis: a novel dietary risk factor. Am J Med Genet A. 2012;158A:803–807. doi: 10.1002/ajmg.a.35204. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


