Abstract
Vascular insults can initiate a cascade of molecular events leading to neurodegeneration, cognitive impairment and dementia. Here, we review the cellular and molecular mechanisms in cerebral blood vessels and the pathophysiological events leading to cerebral blood flow dysregulation and disruption of the neurovascular unit and the blood-brain barrier, which all may contribute to the onset and progression of dementia and Alzheimer’s disease (AD). Particularly, we examine the link between neurovascular dysfunction and neurodegeneration including the effects of AD genetic risk factors on cerebrovascular functions and clearance of Alzheimer’s amyloid-β peptide toxin, and the impact of vascular risk factors, environment and lifestyle on cerebral blood vessels, which in turn may affect synaptic, neuronal and cognitive functions. Finally, we examine potential experimental treatments for dementia and AD based on the neurovascular model, and discuss some critical questions to be addressed by future studies.
Keywords: Alzheimer’s disease, dementia, neurovascular unit, blood-brain barrier, vascular factors, neurovascular medicine
Graphical abstract
1. Introduction
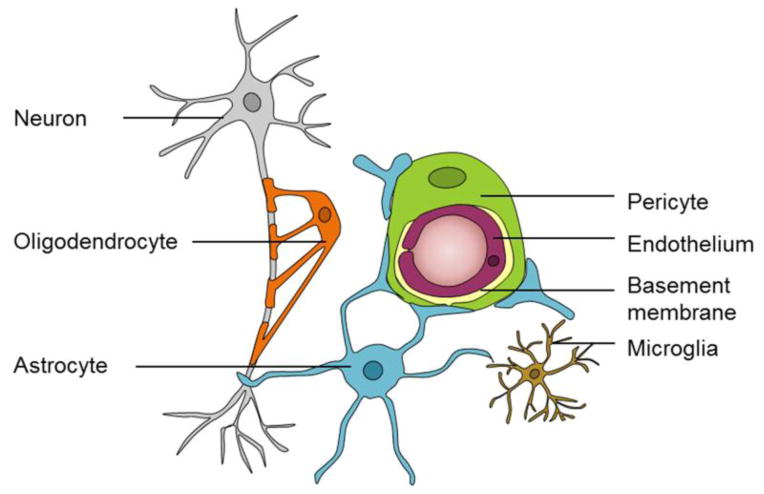
Blood vessels in the brain deliver essential nutrients to and remove metabolic waste products from the central nervous system (CNS) [1]. Although the human brain makes up only 2% of total body mass, it consumes 20% of the body’s oxygen and glucose supply [2]. Proper regulation of cerebral blood flow (CBF) is critical for brain health and survival. Loss of CBF halts brain functions in seconds and causes permanent brain damage within minutes [3]. CBF is precisely regulated by the neurovascular unit (NVU) to assure that brain energy demands are met [1,4]. The NVU is comprised of several cell types including vascular cells (endothelial cells, pericytes and vascular smooth muscle cells (VSMCs)), glia (astrocytes, microglia and oligodendrocytes) and neurons [1]. A schematic representation of the NVU at the level of brain capillaries is shown in Fig. 1.
Figure 1.
The neurovascular unit (NVU) at the level of brain capillary is comprised of vascular cells (pericytes and endothelial cells), glia (astrocytes, oligodendrocytes, and microglia) and neurons.
The blood-brain barrier (BBB) is part of the NVU that prevents uncontrolled entry of blood-derived products and pathogens into the brain, and mediates the exchange of molecules into and out of the brain parenchyma via a specialized substrate-specific transport system expressed in brain endothelium [1,5]. The BBB is quite unique when compared to the highly permeable systemic capillaries that permit the transport of larger molecules across the vessels and into peripheral tissues [6]. Brain capillary endothelial cells are connected by tight and adherens junction proteins (e.g., claudin, occludin, junction adhesion molecules, and cytoplasmic accessory proteins including zonula occludens (ZO)-1, 2, 3, cinguilin and others), forming a continuous endothelial monolayer. This anatomical barrier permits the passage of only small circulating lipid-soluble molecules (< 400 kDa) with less than nine hydrogen bonds into the brain, and allows the free exchange of oxygen and carbon dioxide [1]. The BBB isolates brain interstitial fluid (ISF) and cerebrospinal fluid (CSF) compartments from the plasma compartment, and thus prevents the entry of many blood-derived molecules into brain parenchyma [4]. The movement of large molecules across the BBB including proteins and peptides is only possible via highly specialized transport systems expressed in brain endothelium [7–9]. In the human brain there is an estimated 400 miles total length of capillaries with about 20 m2 of microvascular surface area available for molecular transport [10]. This makes the BBB the largest exchange transport surface area in the brain and the major clearance pathway for potentially neurotoxic molecules that are produced and/or accumulate in the brain, as for example, Alzheimer’s toxin amyloid-β peptide (Aβ) [11].
It is becoming increasingly apparent that cerebrovascular dysfunction contributes to dementia and AD [1,12–16], as well as other neurodegenerative disorders such as amyotrophic lateral sclerosis (ALS) [17], Parkinson’s disease (PD) [18] and Huntington’s disease (HD) [19]. NVU disruption and BBB breakdown can lead to entry of circulating neurotoxic molecules into the brain, faulty clearance of neurotoxic molecules from brain to blood, improper energy metabolite and nutrient delivery, and abnormal expression of growth factors, matrix molecules and vascular receptors, which all may eventually lead to synaptic and neuronal dysfunction [4]. Here, we review the cellular and molecular mechanisms in cerebral blood vessels leading to neurodegeneration including the effects of genetic risk factors for AD on cerebrovascular functions and Aβ clearance, and the impact of vascular risk factors, environment and lifestyle on cerebral blood vessels. Finally, we discuss potential experimental treatments for dementia and AD based on the neurovascular model, and review some critical questions in the field to be addressed by future studies.
2. Alzheimer’s disease
AD is the most common form of dementia that currently affects 5.3 million Americans [20]. The majority of cases are sporadic late-onset, and < 5% of cases are early-onset autosomal-dominant AD (ADAD) [21]. Pathological hallmarks of AD include elevated brain parenchymal and vascular Aβ, hyperphosphorylated neurofibrillary tau tangles, gliosis and neuronal loss [1]. Mounting evidence demonstrates that vascular dysfunction contributes to dementia and AD [1,14,15]. Vascular risk factors (e.g., hypertension, diabetes) and some major genetic risk factors for AD (e.g., apolipoprotein E ε4 (APOE4)) lead to cerebrovascular damage [1,4,20], and cerebrovascular disorders associated with AD. Elucidating the mechanisms underlying vascular pathophysiology to aid in identifying novel therapeutic targets for AD has been declared a national research priority [14,15].
2.1. The two-hit vascular hypothesis
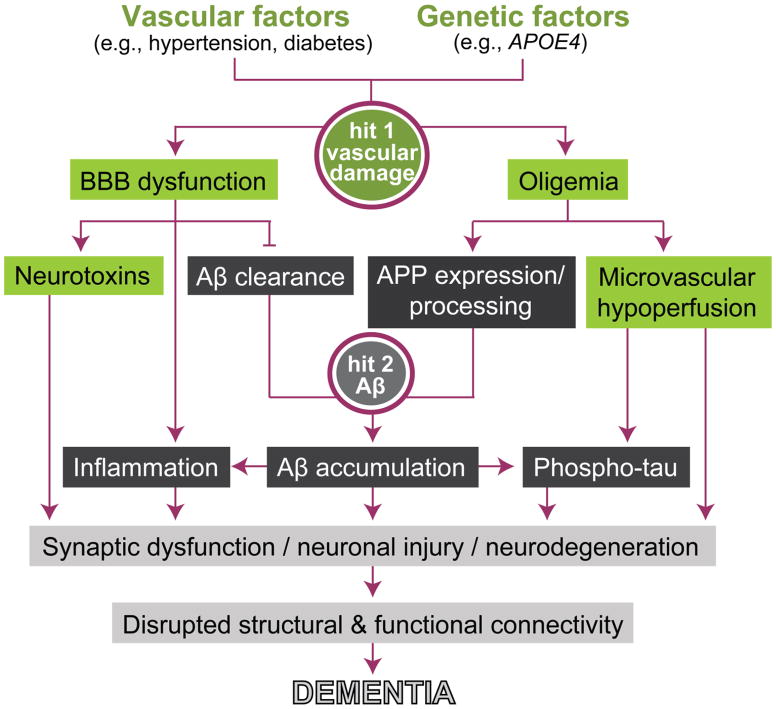
The two-hit vascular hypothesis of AD states that cerebrovascular damage (hit 1) is an initial insult that is self-sufficient to initiate neuronal injury and neurodegeneration, but can also promote accumulation of Alzheimer’s Aβ toxin in the brain (hit 2) (Fig. 2) [22–25,1,16]. Cerebrovascular disruption including BBB breakdown and resting CBF reductions can lead to accumulation of neurotoxic circulating molecules (e.g., thrombin, plasminogen, fibrinogen) and hypoperfusion in the brain, respectively, that can directly initiate neuronal injury [1,23]. Vascular dysfunction can also influence the amyloidogenic pathway to diminish Aβ clearance and increase Aβ production leading to elevated Aβ levels in the brain [1,11]. Thus, the Aβ-independent and Aβ-dependent pathways interact and can independently and/or synergistically lead to the onset and progression of AD dementia. Importantly, both pathways are impacted by vascular, genetic, environment and lifestyle risk factors.
Figure 2.
The two-hit vascular model of Alzheimer’s disease (AD) dementia. Vascular factors, such as hypertension and diabetes, and/or genetic risk factors for AD, such as apolipoprotein E ε4 (APOE4), can all lead to cerebrovascular damage (hit 1; green boxes). Within the amyloid-β peptide (Aβ)-independent pathway, cerebrovascular damage leads to blood-brain barrier (BBB) dysfunction and accumulation of blood-derived neurotoxic molecules in the brain, from one hand, and oligemia or reduced cerebral blood volume, from the other. Additionally, within the amyloidogenic Aβ pathway, BBB dysfunction can disrupt Aβ clearance across the BBB and oligemia leads to overexpression and enhanced processing of Aβ precursor protein (APP), which both can promote Aβ accumulation in the brain (hit 2; black boxes). The converging Aβ-independent and Aβ-dependent pathways can each independently and/or synergistically lead to synaptic and neuronal dysfunction, neurodegeneration, and disruption of brain structural and functional connectivity that ultimately leads to dementia.
2.2. AD genetic risk factors with vascular links
Age is the number one risk factor for AD, but genetic risk factors also have a significant effect on the AD disease process. Vascular implications are now known for several of AD genetic risk factors and are emerging for additional risk genes, as discussed below.
2.2.1. APOE4
APOE is a 34 kDa glycoprotein that is primarily produced by astrocytes in the brain and by the liver in the periphery, and is important for lipid metabolism [26]. In humans, there are three alleles of APOE: APOE2, APOE3 and APOE4. APOE4 is the strongest and most highly replicated risk factor for late-onset sporadic AD [21,27]. An individual’s risk of AD is increased 3.7 times in carriers of a single APOE4 allele and 12 times in carriers of two APOE4 alleles, compared to APOE3 carriers [28].
APOE4 increases BBB damage, cerebral amyloid angiopathy (CAA) and fibrinogen deposits in human brain [29–33]. Furthermore, young APOE4 carriers with no memory impairment have reduced cerebrovascular reactivity in response to a memory task and carbon dioxide inhalation [34]. APOE4 carriers have accelerated pericyte degeneration and BBB breakdown, compared to APOE3 carriers, that is likely related to the activation of cyclophilin A (CypA)-matrix metalloproteinase-9 (MMP-9) pathway in blood vessels, particularly in pericytes [28,29]. In agreement with these findings, APOE4 transgenic mice exhibit activation of the CypA-NFκB-MMP-9 pathway in pericytes that was demonstrated to result in degradation of BBB tight junction and basement membrane proteins causing BBB disruption [35]. However, APOE2 and APOE3 expressing transgenic mice have an intact BBB compared to APOE4 mice [35,36], and are able to suppress the CypA-NFκB-MMP-9 BBB-degrading pathway in pericytes [35].
Furthermore, APOE isoforms differentially affect binding and clearance of Aβ, where APOE2-Aβ > APOE3-Aβ complexes bind to a major Aβ clearance receptor the low-density lipoprotein receptor-related protein 1 (LRP1) at the abluminal side of the BBB and are rapidly cleared across the BBB into circulation, whereas APOE4-Aβ complexes interact poorly with LRP1 and are removed from the brain by the much slower and less efficient very low density lipoprotein receptor (VLDLR) clearance mechanism [37,38]. Thus, impaired Aβ clearance by APOE4 relative to APOE3 or APOE2 may ultimately accelerate Aβ accumulation in the brain.
2.2.2. PICALM
PICALM encodes the phosphatidylinositol-binding clathrin assembly protein that is involved in endocytosis and internalization of cell receptors and intracellular trafficking of endocytic proteins [39–41]. PICALM is highly expressed in the brain capillary endothelium lining the BBB [41]. Approximately 20 single nucleotide polymorphisms (SNPs) in the noncoding region of PICALM have been associated with AD in several genome wide association studies (GWAS) [21,42,43]. Recently, the molecular mechanism by which PICALM normally influences Aβ clearance across the BBB has been elucidated in an in vitro human model of the BBB [41]. Briefly, it has been shown that Aβ binding to LRP1 at the abluminal side of the BBB recruits PICALM and initiates rapid PICALM/clathrin-dependent endocytosis of Aβ-LRP1 complexes. PICALM then continues to guide trafficking of Aβ-containing endocytic vesicles across brain endothelium fusing sequentially with Rab5 and Rab11 and ultimately leading to intracellular transport and exocytosis of Aβ, respectively, at the luminal side of the BBB thus completing Aβ transcellular transport and clearance across the BBB [41]. Importantly, PICALM levels are reduced in AD brain endothelium, which correlates with elevated Aβ levels, Braak stage and the level of cognitive decline as shown by a recent neuropathological study [41]. Furthermore, induced pluripotent stem cell (iPSC)-derived human endothelial cells carrying the protective allele of the rs3851179 PICALM variant have higher PICALM expression and increased Aβ clearance [41]. Similarly, mice deficient in brain endothelial Picalm develop accelerated Aβ accumulation in the brain and behavioral deficits, which all can be reversed by endothelial-specific expression of Picalm [41]. Altogether, these data support the view that PICALM controls Aβ transport and clearance across the BBB and therefore could have a strong vascular link to AD.
2.2.3. CLU
Clusterin (CLU), also known as apolipoprotein J (APOJ), is a disulfide linked heterodimeric glycoprotein composed of an α- and β-subunit that aids in Aβ clearance [37]. Recent GWAS studies have identified a SNP within CLU on chromosome 8p21.1, rs11136000, that is significantly associated with sporadic AD [21,42,44]. The C allele is considered an AD risk factor; whereas the minor T allele has a protective effect and reduces the risk of AD by 16% [42,44]. Individuals with the CLU rs11136000 risk C allele exhibit reduced white matter integrity and hyperactivation of the prefrontal and limbic areas during working memory tasks, as determined recently via diffusion tensor imaging magnetic resonance imaging (DTI-MRI) and functional MRI, respectively [45]. CLU binds to several different proteins including Aβ, and has been shown to prevent aggregation and promote clearance of Aβ peptides across the BBB [37]. More specifically, Aβ42 bound to CLU can be cleared rapidly across the BBB via LRP2 as shown in a murine model [37,46].
2.2.4. PSEN1
Presenilin 1 (PSEN1) and PSEN2 genes encode presenilins 1 and 2, respectively, that act as aspartyl proteases to facilitate γ-secretase cleavage of Aβ precursor protein (APP) to produce Aβ [21]. Many PSEN mutations have been identified in ADAD including 185 mutations reported in PSEN1 and 13 mutations in PSEN2 [21]. Most of these mutations increase the ratio of Aβ42:Aβ40 in the brain [21]. However, PSEN1 mutations also lead to major cerebrovascular pathology in humans including disruption of small cerebral blood vessels, degeneration of pericytes and mural cells, BBB breakdown, and Aβ deposits in small cerebral arteries, arterioles and capillaries [47,48]. Similarly, prominent cerebrovascular pathology with BBB breakdown, characterized by microhemorrhages, thinned and irregular microvessels, string vessels, a thickened basement membrane and a reduction in hippocampal microvascular density, have been shown in PSEN1 transgenic models [49,50].
2.2.5. APP
There are 24 reported mutations in APP in ADAD subjects and, like PSEN mutations, mostly result in an increased Aβ42:Aβ40 ratio in the brain [21]. Patients with hereditary CAA of the Dutch, Iowa, Arctic, Flemish, Italian or Piedmont L34V vasculotropic mutations in APP have degeneration of VSMCs leading to hemorrhagic strokes and dementia [1,4,16]. Furthermore, duplication of the gene encoding APP causes ADAD with CAA and intracerebral bleeding [1].
2.2.6. MEOX2
Mesenchyme homeobox gene 2 (MEOX2), a transcription factor expressed solely in the vasculature in brain, regulates vascular cell differentiation and remodeling [51]. A GWAS study using high-resolution array comparative genomic hybridization found a copy number variant in MEOX2 in ADAD subjects [52]. Furthermore, brain endothelial cells from AD subjects express very low levels of MEOX2 which leads to vessel regression, LRP1 proteosomal degradation and reduced Aβ clearance across the BBB, all of which were reversed by restoring MEOX2 endothelial expression [53]. Transgenic mice with a single allele of Meox2 develop cerebral endothelial hypoplasia with reduced brain perfusion and impaired Aβ efflux caused by reduced LRP1 expression [53].
3. Cerebrovascular dysfunction in AD
Vascular damage and dysfunction are frequently associated with neurodegeneration and contribute to AD [1,2,12,15,54,55]. Some indicators of BBB breakdown include accumulation of blood-derived proteins and cells in the brain and CSF, loss of pericytes and endothelial tight junction molecules, microbleeds, and alterations in BBB Aβ transporter expression (e.g., receptor for advanced glycation endproducts (RAGE) and LRP1). Some key pathophysiological, cellular and molecular mechanisms leading to cerebrovascular dysfunction in AD are discussed below.
3.1. Diminished CBF and neurovascular uncoupling
The brain microvasculature regulates its local capillary blood flow by maintaining a delicate balance between the anti-thrombotic and pro-thrombotic pathways within the capillary network, which normally prevents intra-vascular coagulation and allows blood to flow [56]. Regional CBF responses are normally controlled by local neuronal activity within the NVU modules, a process known as neurovascular coupling [2]. A number of studies have shown reductions in resting CBF in aged cognitively normal individuals that are at risk of developing AD [1,2,57] and in AD patients [1,2,58], as well as CBF dysregulation [2,54]. The CBF reductions may occur prior to cognitive impairment in humans [57], and were also found in APP transgenic models of AD [59] and pericyte deficient murine models [60].
VSMCs derived from small cerebral penetrating arteries critically regulate CBF to the brain [2]. Recent studies have shown a hypercontractile phenotype of VSMCs derived from small arteries in AD and AD models characterized with accumulation of several contractile proteins directed by elevated activity of the serum response factor (SRF)/myocardin tandem of transcription factors caused by hypoxic changes [61]. Arterial hypercontractility and impaired ability to relax can in turn diminish arterial supply of blood to the brain, which reduces CBF and creates a chronic brain hypoperfusion state that may contribute to dementia by several pathways as discussed below.
In addition to the hypercontractile phenotype, arterial VSMCs in AD also exhibit a greatly reduced ability to clear Aβ, which leads to accumulation of Aβ in the vessel wall causing CAA [62] that in turn further aggravates CBF reductions and may promote cerebral microbleeds. Recent experimental studies have suggested that in addition to a major role of VSMCs in controlling CBF [2,63], CBF can also be regulated at the capillary level by pericytes, particularly during experimental cerebral ischemia that leads to constriction of pericytes obliterating capillary blood flow during the reperfusion phase [64]. This eventually can lead to pericyte cell death and degeneration, resembling pericyte degeneration seen in AD and other neurodegenerative disorders [17–19]. However, whether pericytes play a similar role in human disease as shown in animal models remains to be addressed by future studies.
Mild chronic hypoperfusion, known as oligemia, can lead to a deleterious chain of events in neurons by impairing neuronal protein synthesis that is required for synaptic plasticity, a process critically involved in learning and memory [65]. Additionally, CBF reductions diminish oxygen delivery to brain that favors anaerobic brain metabolism, which in turn diminishes ATP synthesis required for the maintenance of the sodium pump or Na+, K+-ATPase activity that regulates ion distribution in neurons necessary for proper firing of action potentials and normal neuronal excitability [2]. Hypoperfusion also leads to imbalances in pH, electrolytes and water gradients which lead to edema, white matter lesions and ultimately neuronal death [1].
3.2. BBB breakdown
Regional BBB permeability in the living human brain during normal aging and in mild dementia patients was recently assessed quantitatively using an advanced dynamic contrast-enhanced (DCE)-MRI method [12]. This study has shown that BBB breakdown in the hippocampus increases in an age-dependent manner, worsens in individuals with mild dementia [12], and corresponds with increases in CSF levels of pericyte injury marker, soluble platelet-derived growth factor receptor-β (sPDGFRβ) [12,66]. Reflective of BBB breakdown, the CSF to plasma albumin ratio is also increased in AD patients particularly in those with vascular risk factors [67], as well as in individuals with mild dementia or mild cognitive impairment (MCI) [12] and in cognitively normal APOE4 carriers at genetic risk for AD [29].
In post-mortem studies, an accumulation of blood-derived proteins including fibrinogen, thrombin, plasminogen, immunoglobulin G and albumin were found in the hippocampus and cortex of AD subjects [30–32,68,69], which was associated with pericyte degeneration [30,69–73]. MRI studies reveal microbleeds and iron accumulation in the brain of patients with preclinical AD [74] and AD [33,75,76], especially in the hippocampus [77]. In a cohort of 174 patients, lobar microbleeds were identified in 40% of AD subjects and 24.3% of MCI patients using susceptibility-weighted imaging (SWI) MRI [74]. The incidence of lobar microbleeds was positively associated with age, APOE4 carrier status, and Aβ burden, as measured by (11)C-labeled Pittsburgh Compound-B positron emission tomography (PIB-PET) [74]. Additionally, multiple lobar microbleeds were associated with lacunar infarctions and severity of white matter hyperintensities [74]. Also, in a recent study that included 148 AD patients, 44.6% of the patients exhibited brain microbleeds [75]. Furthermore, the patients with a high number of microbleeds had several associated vascular risk factors including diabetes and hypertension [75]. Microbleeds measured by SWI-MRI and/or T2*-weighted MRI were also found in 22% of 1,504 studied dementia patients [76]. The microbleeds positively correlate with age, sex (microbleeds occur more frequently in males), and hypertension, and negatively with Mini-Mental State Examination (MMSE) cognitive score [76].
Pericyte injury and/or loss has been identified by post-mortem analysis in many neurodegenerative diseases including AD [30,69,71,72] as well as by CSF analysis of living MCI patients [12]. Degenerating pericytes accumulate intracellular inclusions, pinocytic vesicles, and large lipid granules, and show mitochondrial abnormalities [71,72]. These microstructural changes in pericytes correlate with capillary reductions, dilation of vessels and the appearance of tortuous vessels [71]. Simplified schematics of normal BBB and BBB breakdown with neurovascular pathways leading to neurodegenerative changes are illustrated in Fig. 3.
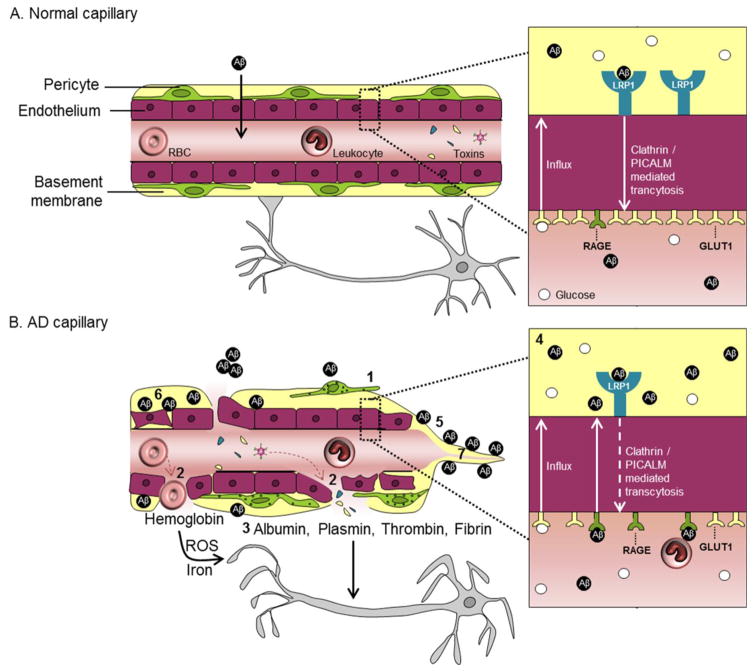
Figure 3.
Blood-brain barrier (BBB) pathways to neurodegeneration in dementia and Alzheimer’s disease (AD).
A. In the normal capillary, there is an intact BBB composed of tightly joined endothelial cells and supported by mural pericytes, as shown in this simplified schematic. The BBB normally selectively regulates the passage of molecules from blood to brain and vice versa, and restricts entry of blood-derived products and toxins into the brain. There are many transporters and receptors along the BBB that permit molecules to cross the BBB via substrate-specific transport systems, some of which are particularly relevant to AD pathophysiogenesis, as illustrated in the inset. For example, the normal BBB has high expression of the glucose transporter (GLUT1), moderate expression of low density lipoprotein receptor related protein-1 (LRP1) and minimal expression of receptor for advanced glycation end-products (RAGE).
B. In the AD capillary, there is a vicious cascade of events that can lead to neurodegeneration, as shown in this schematic and described as follows. 1. Pericytes degenerate and detach. 2. The BBB becomes leaky. 3. Blood-derived molecules like fibrinogen, thrombin and plasminogen leak from vessels and are directly toxic to neurons and can further induce BBB damage. Erythrocyte extravasation induces accumulation of hemoglobin derived-iron which causes generation of reactive oxygen species (ROS) and oxidative stress to neurons, and albumin promotes local tissue edema. 4. BBB transporter expression is altered, e.g., LRP1 and GLUT1 expression are significantly reduced, whereas RAGE expression is increased. The alterations in LRP1 and RAGE reduce the clearance and increase the uptake of Aβ into the brain, respectively, leading to Aβ accumulation in the brain. Also, normal cerebrovascular functions are disrupted by vascular pathologies including 5. Cerebral amyloid angiopathy, 6. Damaged and thickening of the basement membrane, and 7. String vessels.
3.3. Reduced glucose utilization
Glucose transporter 1 (GLUT1) on cerebral microvessels is decreased in post-mortem AD brain tissue [78]. GLUT1, encoded by SLC2A1, is the major transporter of glucose across the BBB from the blood to the brain [1,4,79]. Cognitively normal individuals with genetic risk for AD or positive AD family history [80] and mild or no cognitive impairment that later develop AD [81] all have reduced glucose utilization in the hippocampus, parietotemporal cortex and/or posterior cingulate cortex measured by 2-[18F]-fluoro-2-deoxy-D-glucose (FDG)-PET, which occurs prior to brain atrophy and neuronal dysfunction [80]. Brain glucose uptake correlates with the level of GLUT1 on cerebral microvessels [82]. In addition to glucose transport, GLUT1 is also critical for the maintenance of proper brain capillary networks, CBF, and BBB integrity, as demonstrated in humans with SLC2A1 mutations [83] and Slc2a1 transgenic mice [79] which contributes to neurodegeneration and behavioral deficits in murine model of AD and GLUT1 deficiency [79]. See Fig. 3.
3.4. Aβ clearance
In sporadic AD, faulty Aβ clearance, rather than increased Aβ production, is thought to promote elevated brain Aβ levels [84]. Experimental studies in multiple animal models have shown that under physiological conditions, 70–85% of Aβ is cleared from the brain primarily by transvascular clearance across the BBB and a small portion is removed by ISF bulk flow [11,85,86]. The primary receptor mediating BBB transcytosis of Aβ is the LRP1, that is expressed mainly at the abluminal side of the BBB [1,4,11,41,86,87]. Aβ bound to endothelial LRP1 is rapidly internalized by PICALM at the endothelium, which mediates PICALM/clathrin-dependent endocytosis of Aβ-LRP1 complexes by endothelium and guides trafficking of Aβ-containing endocytic vesicles towards exocytosis ultimately leading to Aβ transcytosis [41], as discussed above. Additionally, a soluble form of LRP1 (sLRP1) is generated by proteolytic cleavage of LRP1 by β-secretase and circulates in the plasma binding and sequestering free Aβ40 and Aβ42 and transporting them via blood to the liver and kidney for removal from systemic circulation [11,86]. See Fig. 3A inset.
3.4.1. LRP1
In normal aging and AD, there is a significant reduction in LRP1 expression in brain endothelial cells [88] and VSMCs [62]. A decrease of LRP1 on microvessels negatively correlates with an increase of Aβ in brain [88]. See Fig. 3B inset. High levels of SRF and myocardin in VSMCs [61,62] lead to elevated expression of sterol response element binding protein 2 (SREBP2), a major LRP1 transcriptional suppressor, that leads to LRP1 depletion and thereby reduced Aβ clearance across the BBB [24]. Oxidation of surface LRP1 and sLRP1 prevents them from binding Aβ and thus limits the efficient efflux of Aβ from the brain [89,90].
3.4.2. RAGE
RAGE is the major Aβ influx receptor at the BBB that transports Aβ from the blood into the brain [4,11,91]. RAGE is normally expressed at low levels at the BBB, but its expression is increased in normal aging and in AD brain endothelium and is associated with increased cerebrovascular and brain accumulation of Aβ [91–93]. See Fig. 3B inset.
3.5. Concomitant vascular abnormalities
CAA, basement membrane abnormalities, string vessel formation, microinfarcts, arteriosclerosis, and/or atherosclerosis, are frequently observed in MCI and AD patients [94] and in post-mortem AD brain tissue [95]. Brain microvasculature is structurally altered in AD and other neurodegenerative diseases and some of these vascular abnormalities are discussed below.
3.5.1. Cerebral amyloid angiopathy
CAA is the deposition of amyloid along the cerebrovasculature that occurs in both familial ADAD and sporadic AD (Fig. 3B). Patients with hereditary Dutch, Iowa, Arctic, Flemish, Italian or Piedmont L34V vasculotropic mutations develop CAA followed by rupture of blood vessels and hemorrhagic strokes in midlife [1,4,16]. CAA is known to worsen AD pathology and occurs in 80% of AD patients [1]. CAA likely develops as a result of the ineffective transvascular and perivascular clearance of Aβ [11,96], as well as poor Aβ clearance by arterial VSMCs [62]. It was recently reported that microvascular rather than parenchymal Aβ deposits are associated with early behavioral deficits in AD transgenic mice [97]. Individuals with CAA carrying APOE4 allele(s) have accelerated vascular pathology that can modulate Aβ accumulation [31]. Human post-mortem studies recently reported that AD patients that are APOE4 carriers had increased fibrinogen deposits with increased CAA disease severity compared to non-carriers, and the fibrinogen deposits associated with microvascular Aβ accumulation [31].
3.5.2. Basement membrane abnormalities
The basement membrane of the vessel wall has been shown to thicken, split, and develop abnormal inclusions (e.g., accumulation of collagen and perlecans) in the aging brain that is increased in AD as shown by post-mortem human brain tissue studies [1,98].
3.5.3. String vessels
String vessels are thin connective tissue strands composed of basement membrane that are remnants of capillaries without endothelial cells or blood flow [99]. The number of string vessels is significantly increased in AD post-mortem brain tissue [99,100] and in APOE4 carriers compared to non-carriers [100]. Preliminary studies found that Aβ accumulates substantially along string vessels [101].
4. Vascular risk factors, lifestyle and environment
In addition to genetic risk factors discussed above that can trigger neurovascular damage and cognitive decline, vascular conditions such as hypertension, diabetes, atherosclerosis, and hyperhomocysteinemia can also contribute to dementia and influence one’s risk for AD. Additionally, every person is differentially affected by lifestyle (e.g., education, sleep, diet and exercise) and environment (e.g., pollution/nanoparticles and environmental enrichment) that can further modify one’s risk for dementia. It is estimated that nearly half of AD cases might be attributed to modifiable risk factors and that effective control of vascular factors, pre-existing disease(s) and psychological condition(s), as well as lifestyle-based interventions may offer promising preventative strategies to delay disease onset and/or progression [102]. Below, we detail some of these factors related to AD.
4.1. Hypertension
Hypertension is one of the vascular risk factors for dementia. Several epidemiological studies indicate association of midlife hypertension with late-life dementia [103,104]. A meta-analysis of six longitudinal studies showed significant association of hypertension with increased risk and incidence of vascular dementia [105]. Hypertension causes regional CBF reduction and impairs cerebrovascular reactivity [54]. Compelling evidence suggests that chronic hypertension fuels atherosclerosis in cerebral arteries, and also increases the risk of microvascular damage, white matter lesions, ischemic stroke and intracranial hemorrhage in the presence of CAA [106–108]. In animal models, hypertension caused BBB impairment, induced brain Aβ accumulation, enhanced Aβ-induced neurovascular dysfunction and neurodegeneration [109–112]. Circulating plasma levels of markers of endothelial activation including soluble intercellular adhesion molecule-1 (sICAM-1), soluble vascular cell adhesion molecule-1 (sVCAM-1) and endothelin-1 (ET-1), an endothelial cell-derived potent vasoconstrictor, are increased in hypertensive subjects suggesting vascular injury [107,113,114]. Anti-hypertensive agents have been shown to reduce the risk of dementia and cognitive decline [115,116], thus proper management of hypertension is an important protective strategy against cognitive impairment.
4.2. Diabetes
Increasing evidence suggests that type 2 diabetes mellitus (T2DM) is a major vascular risk factor contributing to the development of AD [117–119]. Evidence of vascular alterations and increased BBB permeability have been reported in T2DM patients [120,121] and in several experimental models of diabetes [122–125]. Multiple mechanisms may explain the link between T2DM and pathological changes in brain microvasculature, neurons and glial cells including increased levels of advanced glycation end-products (AGEs) and RAGE, vascular inflammation, oxidative stress, reduced insulin transport across the BBB, impaired insulin signaling, insulin resistance and endoplasmic reticulum stress [126,127].
4.3. Atherosclerosis
Atherosclerosis is hardening and narrowing of the inner wall of arteries due to the buildup of plaques composed of fatty deposits, cholesterol and other blood derived substances. Atherosclerosis has been implicated in the pathogenesis of dementia and AD, and it has several overlapping risk factors including diabetes, hypercholesterolemia and aging [128]. Recently, a large cohort autopsy found that 77% of 410 AD subjects had grossly apparent circle of Willis atherosclerosis, which occurred more often than in non-AD subjects [129]. In non-demented patients, the carotid intima-media thickness, a marker of atherosclerosis, was found to be inversely related to annual cognitive measures of executive function [130]. A large population-based study of 4,371 stoke-free middle-aged patients found that atherosclerosis could predict future lower cognitive scores after 7 years [131]. Furthermore, a longitudinal study of 1,651 participants found that intima-media thickness was associated with a higher risk of developing cognitive impairment in a 10 year follow up study [132].
4.4. Hyperhomocysteinemia
Elevated plasma homocysteine, known as hyperhomocysteinemia, has been reported in subjects with atherosclerosis [133], stroke [134], cerebral small vessel disease [135,136], and AD [134,136]. Cerebrovascular damage, endothelial dysfunction, oxidative stress, demethylation, and thrombosis have been proposed to elucidate the link between hyperhomocysteinemia and AD [135,137,138]. Diet induced hyperhomocysteinemia in wild-type [139] and APP/PS1 AD [140] mice leads to spatial memory deficits, microhemorrhages, neuroinflammation and increased activity of MMP-2 and MMP-9.
4.5. Traumatic brain injury
Traumatic brain injury (TBI) increases the production of Aβ and also heightens the risk of developing AD [141]. Like AD, TBI leads to BBB leakage and vascular breakdown involving NVU impairment [141]. Experimental studies in rats have demonstrated that TBI leads to focal microbleeds, BBB and endothelial damage, gliosis, macrophage-mediated inflammation and myelin loss [142].
4.6. Sleep
Sleep inadequacy and daytime sleepiness increase the risk of AD and development of Aβ pathology [143]. Sleep-wake cycle alterations in AD subjects contribute to “sundowning syndrome,” when AD patients become agitated, confused, anxious and aggressive in the late afternoon and evening. Patterns of sleep are also affected, for instance rapid eye movement sleep duration is reduced in AD, but not in normal aging, and has been proposed to be a biomarker for AD [144]. Recent studies found an increased clearance rate of Aβ in sleeping mice compared to awake mice following intracerebral injection of 125I-Aβ40 into the frontal cortex [145]. This increased Aβ clearance was mediated 40% by perivascular ISF-to-CSF bulk flow and the remaining 60% was likely due to increased transvascular transport across the BBB [86,145].
Not only is sleep impaired in AD, but it has also been proposed that disturbances in sleep can lead to AD. There is mounting evidence that obstructive sleep apnea (OSA) could contribute to AD [146]. Severe OSA leads to sleep fragmentation, intermittent hypoxia, oxidative stress and increased production of Aβ via hypoxia-inducible factor-1α and β-secretase 1 [146]. Following intermittent hypoxia, reoxygenation results in damage to blood vessels and endothelial cells, and oxidative stress [146]. Furthermore, mice undergoing chronic sleep restriction have decreased expression and function of GLUT1, impaired tight junction expression and BBB transport, and suppressed vascular reactivity with reduced inducible nitric oxide synthase (iNOS), endothelial NOS (eNOS) and ET-1 [147]. Also, rats subjected to sleep deprivation exhibited BBB breakdown and leakage [148].
4.7. Pollution/nanoparticles
Air pollution has been shown to increase the risk of AD and AD-like brain pathologies [149,150]. Young residents of the Mexico City Metropolitan Area (MCMA) exposed to air pollution display cognitive impairment, BBB disruption, Aβ42 plaques and hyperphosphorylated tau accumulation [151], which are exacerbated in APOE4 carriers [152]. Furthermore, children from the MCMA have increased serum autoantibodies against neuronal proteins, likely due to compromised brain immunity and BBB breakdown [153]. In mouse experimental studies, aerosolized nickel nanoparticles caused a rapid and drastic increase in Aβ40 and Aβ42 [154]. Also, APOE null mice exposed to mixed vehicle exhaust have accelerated BBB breakdown, decreased expression of tight junction proteins (e.g., occludin, claudin-5) and increased generation of reactive oxygen species activity [155].
Many types of nanomaterials are emerging in medical science and research for their potential as biosensors, biomaterials, tissue engineering, DNA modification or drug delivery [156]. The sources of nanoparticles that humans are exposed to are numerous and include nanoscaled debris from hip replacements, prostheses, cosmetics, sunscreen, and many others. Unfortunately, nanoparticles have proven to be toxic in a number of host systems [156]. Highly active nanoparticles (e.g., silica coated to be hydrophilic, hydrophobic or amphiphilic) can be taken up by cellular membranes, including the BBB, and cross the membrane passively or by carrier-mediated endocytosis [156]. Experimental studies in rodents have shown that silver, copper or aluminum/aluminum oxide nanoparticles disrupt the BBB, reduce the expression of endothelial tight junctions, decrease CBF, and induce edema, synaptic dysfunction and neurodegeneration [157–160]. Interestingly, silver and copper nanoparticle exposure exacerbated BBB dysfunction when accompanying sleep deprivation [148] or diabetes [158].
4.8. Peripheral inflammation
Peripheral inflammation is being considered a possible risk factor for AD [161] and dementia [162]. Infectious agents including pneumonia, B. burgdorferi, Helicobacter pylori and herpes simplex virus 1 (HSV-1) have been identified in AD post-mortem brain tissue [163]. Interestingly, HSV-1 infections are found more often in APOE4 carriers [164], which have increased BBB permeability [30]. Additionally, dementia patients have a two-fold increased mortality rate from pneumonia [165], and pneumonia patients have elevated MMP-9 levels in their serum [166], which is known to be linked to BBB breakdown [29]. Furthermore, poor oral hygiene, oral inflammation and tooth loss worsen with age and are risk factors for AD [167]. Recently, fungus was identified in post-mortem brain tissue from AD subjects [168], and was found to localize around blood vessels in AD brain tissue [169]. In AD, infectious agents likely enter the brain through a leaky, disrupted BBB and cause more detrimental effects than they would normally if the BBB were intact.
4.9. Alcohol and substance abuse
Alcohol abuse increases CBF and blood pressure and disrupts the BBB causing vascular remodeling by oxidative stress, endothelial tight junction phosphorylation and changes in expression of BBB-degrading gelatinases MMP-2 and MMP-9 [170,171]. Likewise, drug abuse has been shown to have detrimental effects on the BBB. Psychostimulant drugs of abuse including methamphetamine, ecstasy/MDMA (3,4-methylenedioxymethamphetamine), cocaine and nicotine cause increased BBB permeability through alterations in tight junction protein expression and conformation, glial activation, enzyme activation related to BBB cytoskeletal remodeling, and induction of neuroinflammation [172–174]. In addition to increased BBB permeability, experimental studies have found that even a single acute exposure to methamphetamine causes profound CBF reductions [175]. Also, recent preliminary studies have found that heroin use leads to microvascular damage and microcirculation dysfunction, collapse and fracture of the myelin sheath and vacuole formation in white matter regions around microvessels, as identified in post-mortem human brain tissue [176,177].
5. Vasculoprotective approaches
With the substantial amount of evidence supporting the vascular link to AD and related dementias, using approaches to repair neurovascular damage is intuitive and may hold promise to delay and/or slow down cognitive decline. Likely, the best approach for treatment will be multi-targeted and potentially individualized, patient-specific, since AD, CAA and vascular dementia present complex and heterogeneous etiologies. Below, we focus on some approaches that could potentially restore neurovascular functions and/or protect the neurovascular system from pathophysiological changes seen in dementia and AD.
5.1. Restoring BBB Aβ transport machinery
5.1.1. LRP1 upregulation
LRP1 expression in cerebral vessels is reduced in AD [11,84], and therefore increasing microvascular LRP1 expression levels may increase Aβ clearance to thereby prevent development of Aβ-dependent pathologies. Future studies should investigate novel therapeutic drugs and/or gene therapy vectors to restore LRP1 levels in endothelial cells and pericytes at the BBB. In addition to increasing LRP1 on cerebral microvessels, restoration or selective enhancement of LRP1 by gene therapy in the liver could function as a systemic “vacuum” to increase the concentration gradient driving Aβ out of the brain by creating a peripheral “sink” [87]. In general, gene therapy targeting neurological disorders remains challenging due to the restricted entry of vectors across the BBB into the brain. However, targeting cell surface (e.g., GLUT1, LRP1) or cytoplasmic (e.g., PICALM) proteins whose expression is reduced in AD brain endothelium (Fig. 3B) would likely be more achievable due to the favorable anatomical position, as endothelial cells are directly accessible to therapeutic interventions by intravenous administration. Importantly, studies have used in vivo phage panning to identify modifiable epitopes on adeno-associated virus type 2 (AAV2) vectors to enable endothelial-specific gene therapy delivery [178,179].
Soluble LRP1 and/or its wild-type recombinant cluster IV, WT-LRPIV, bind to free Aβ aiding in its peripheral clearance and preventing it from reentering the brain via the RAGE receptor [180]. Recently, a mutant of LRPIV (LRPIV-D3674) was developed to preferentially and selectively bind neurotoxic Aβ compared to other LRP1 ligands [181]. LRPIV-D3674 is ~25% more effective at replacing oxidized sLRP1 and clearing endogenous Aβ in hippocampus and cortex in AD transgenic mice [181]. Therefore, LRPIV-D3674 has a therapeutic potential in AD to restore reduced or oxidized sLRP1, regulate peripheral Aβ levels and reestablish the Aβ homeostatic peripheral “sink” [180].
5.1.2. PICALM upregulation
As described above, PICALM critically regulates transcytosis and clearance of Aβ across the BBB and its levels are reduced in AD brain endothelium, as well as in individuals carrying non-protective PICALM alleles [41]. To date, there are no known drugs or gene therapy vectors that can increase PICALM expression levels. Future studies are needed to determine the benefit of increasing PICALM levels in AD models and AD.
5.1.3. GLUT1 upregulation
GLUT1 levels at the BBB correlate with brain glucose uptake [82,182] and also are critical for maintaining BBB integrity [79]. Importantly, the levels of GLUT1 in cerebral microvessels are reduced in AD [78,183]. Only a few studies have investigated drugs to upregulate GLUT1 expression. For example, one study found that ginsenoside Rb1, an active component of ginseng, can activate the insulin signaling and AKT pathways to increase expression of GLUT1 and GLUT4 in adipocytes of diabetic (db/db) mice [184]. Studies in rats found that cerebrolysin, an approved neurotrophic peptide for vascular dementia treatment in humans, increased GLUT1 expression and improved hippocampal learning and memory [185]. Although these few studies demonstrate increased GLUT1 expression, the molecular mechanisms underlying GLUT1 reductions in AD remain unknown. Furthermore, it is not known whether manipulating GLUT1 expression at the BBB in dementia and in AD patients will have an effect on BBB permeability, cognitive performance and Aβ pathology as shown in an animal model of AD and GLUT1 deficiency [79].
5.1.4. Blocking RAGE
Two highly specific, low molecular weight, high affinity RAGE inhibitors, FPS2 and FPS-ZM1, have been recently reported [91]. FPS-ZM1, a novel multimodal RAGE blocker that crosses the BBB, reduced influx of Aβ into the brain, improved CBF responses, downregulated β-secretase activity, decreased amyloid load, suppressed neuroinflammatory responses, and reduced behavioral deficits in a mouse model of AD [91]. Similarly, in a rodent model of hypertension, RAGE inhibition by FPS-ZM1 significantly reduced hypertension-induced AD pathology as shown by improved cognitive performance and reduced parenchymal Aβ deposition [110]. RAGE inhibitors are currently being examined in Phase 3 clinical trials for efficacy in mild to moderate AD (NCT02080364) [186,187]. See Fig. 3B.
5.2. Microvascular stabilization
5.2.1. Activated protein C (APC)
APC is a vasculoprotective protease that promotes cytoprotective signaling in injured and ischemic brain endothelium at the BBB [188,189]. APC also stimulates post-ischemic brain angiogenesis, vascular repair and neurogenesis [188–191]. In the CNS endothelium, APC activates protease activated receptor-1 (PAR-1), which elicits protective signaling by enhancing the BBB integrity via Rac1-dependent cytoskeletal stabilization, suppression of cerebrovascular MMP-9 activity, and inhibition of proinflammatory cytokines expression and apoptosis [188,189,192–194]. A genetically engineered variant of APC, 3K3A-APC, with normal cell signaling and greatly reduced anticoagulant activity has an excellent safety profile in primates and humans [195,196] and is currently being studied as a neuroprotective agent in a Phase 2a clinical trial for ischemic stroke (NCT02222714). APC therapy holds potential to protect and restore cerebrovascular function and BBB breakdown in various other neurological disorders, as shown in different experimental models, and might have also applications in dementia and AD.
5.2.2. CypA and MMP-9 inhibitors
The detrimental effects of APOE4 on BBB and NVU function are mechanistically linked to the CypA-NFκB-MMP-9 proinflammatory pathway [29,35]. There are several new CypA inhibitors recently developed and investigated for use against hepatitis-C and HIV infections including CPI-431-32 [197], compound 25 [198], SCY-635 [199] and MM284 [200], which all may have a potential to stabilize the BBB in APOE4 carriers. In addition to CypA inhibitors, there are a number of identified MMP-9 inhibitors including minocycline [201], curcumin [202], SB-3CT(2) [203] and compound 18 [204]. For example, minocycline has been demonstrated to reduce hemorrhage frequency and increase endothelial tight junction and basement membrane proteins in aged 5xFAD/APOE4 transgenic mice [201]. Furthermore, recent studies showed that minocycline significantly reduced the infarct size, prevented tissue loss, improved perfusion, reduced BBB permeability, and increased tight junction protein levels in spontaneously hypertensive stroke-prone rats [205,206]. Future studies should determine the benefit of CypA and MMP-9 inhibitors on vascular dysfunction in AD and APOE transgenic mouse models.
5.2.3. Anti-hypertensive drugs
Many studies in experimental animal models and clinical studies show that pharmacological intervention by angiotensin receptor blocker, angiotensin converting enzyme inhibitor, or RAGE inhibitor is effective in treating hypertension related vascular injury [207–209], reducing AD related pathological changes in brain [209,210], and slowing cognitive decline [209,211]. Furthermore, developing future therapies capable of lowering expression of VCAM-1, ICAM-1 and/or blocking ET-1 receptor hold promise to further reduce hypertension-induced cerebrovascular dysfunction.
5.2.4. Fibrinogen depletion
Ancrod is a drug derived from the Malayan pit viper that contains a serine protease that cleaves fibrinopeptide A (FPA), essentially preventing conversion of fibrinogen to fibrin that results in fibrinogen depletion [212]. The potentially beneficial mechanism of Ancrod is its ability to lessen fibrinogen clot propagation and improve CBF via activation of endogenous fibrinolysis [212]. Studies in TgK21p55+/− mice, a MS model that exhibits clinical symptoms of paralysis and BBB breakdown, found that Ancrod treatment delayed the onset of inflammatory demyelination by diminishing fibrinogen deposition [213]. Additionally, clinical trials of Ancrod in ischemic stroke have yielded different results depending on time of treatment; for instance, Ancrod was clinically beneficial when administered within 3 hours post-stroke [214] but exhibited no benefit when administered within 6 hours post-stroke [215]. Ancrod’s mechanism was recently studied in vitro in cultured human endothelial cells grown in ischemic conditions and in stroke subjects. In both cases Ancrod decreased fibrinogen levels and increased FPA levels and reduced clot formation [212].
5.3. Lifestyle improvements for vascular health
5.3.1. Diet
Recent studies report that diet can mediate the vasculoplastic reserve of the hippocampus. For example, consuming high levels of cocoa flavanols increased capillary density and enhanced dentate gyrus-associated cognitive function in cognitively normal healthy subjects [216]. This suggests an interaction between vasculoplasticity and neuronal plasticity during normal aging and dementia, but how this relationship is affected by lifestyle and vascular risk factors is currently unclear and should be investigated in future studies [13].
Growing evidence supports the benefits of a Mediterranean diet in protecting against dementia and prolonging one’s cognitive reserve during aging. Age-related cognitive decline was attenuated in individuals consuming a Mediterranean diet, as found in a recent study of the Mediterranean-Dietary Approach to Systolic Hypertension (DASH) diet intervention for neurodegenerative delay (MIND) [217]. In comparing Mediterranean and Western diets, the primary difference is the source and proportion of dietary fats, with olive oil specifically being the main fat consumed in the Mediterranean diet and high levels of saturated fatty acids and simple carbohydrates being consumed in Western diets [218]. Microvascular dysfunction is evident in rodent models fed unhealthy diets, namely those fed diets of Western culture [219], high fat [220], and high cholesterol [221]. An intact BBB is needed for proper cholesterol metabolism [222]. In CSF, decreased cholesterol levels correlate with decreased Aβ42 and increased CSF APPα and APPβ (products of APP processing) levels, supporting an association between disrupted cholesterol metabolism and increased amyloidogenesis [223].
Resveratrol is a biologically active plant-derived phytoalexin. Resveratrol has been shown to cross BBB, and regulate expression of MMPs, reduce pericyte loss, maintain integrity of BBB, and promote Aβ clearance [224–226]. Treatment with resveratrol completely reversed diabetes-induced vascular dysfunction by reducing capillary leakage, pericyte degeneration, and VEGF protein expression in the murine retina [227]. An earlier study has shown that resveratrol inhibits RAGE expression in vascular cells [228], which is implicated in Aβ transport into the brain and accelerated Aβ pathology in a mouse model [91]. Furthermore, long-term consumption of resveratrol reduced oxidative stress and prevented behavioral deficits in a rat model with disrupted NVU [229].
Olive oil is high in essential omega-3 fatty acids, the major component of which is docosahexaenoic acid (DHA), and has long been reported to benefit cognition and overall brain health [230]. DHA cannot be synthesized by the body and thus must be consumed, and the primary transporter of DHA from blood-to-brain is the major facilitator superfamily domain containing 2A (MFSD2A) at the BBB [231]. Individuals with AD have lower CSF DHA lipid levels, and those with mild dementia have lower CSF α-liolenic acid levels [232]. Interestingly, reduced MFSD2A expression at the BBB can lead to a loss of its important functions, including maintenance of BBB integrity and omega-3 fatty acid transport into the brain [231,233,234]. Of note, transgenic APOE4 mice also exhibit reduced uptake of DHA into the brain compared with transgenic APOE2 mice [235], but whether this is related to reduced Mfsd2a expression is currently unknown. Additional studies are needed elucidate the underlying mechanisms of MFSD2A and fatty acids in relation to dementia and AD.
5.3.2. Exercise and environmental enrichment
Regular exercise and physical activity, particularly during midlife, are associated with improved cerebrovascular function and reduced rates of dementia and AD [236]. Individuals that exercised regularly for 28 days exhibited reduced plasma homocysteine levels and increased endothelial progenitor cells in peripheral blood, factors that protect against vascular damage and cognitive impairment [237]. Experimental studies in diabetic rats have shown that treadmill exercise maintains claudin-5 expression at the BBB compared to rats not receiving exercise [238]. Mechanistically, physical activity and cognitive stimulation in the form of enriched environment (e.g., tunnels, balls, ladders, and running wheel) accelerated Aβ enzymatic degradation and enhanced transvascular Aβ clearance, reducing Aβ accumulation in brains of AD transgenic mouse models [239,240]. Additionally, physical activity promoted Aβ clearance from brain to blood via upregulation of LRP1 [241,242] and downregulation of RAGE [91] at the BBB. Mice without access to a running wheel had decreased occludin tight junction levels and disrupted BBB integrity [243]. Although recent attention has been given to exercise, additional studies are needed to more completely understand the mechanism underlying its beneficial effects.
6. Conclusions and critical questions
Here, we have described the compelling evidence supporting the neurovascular link to cognitive impairment, dementia and AD. Specifically, how cerebrovascular functions in humans and animal models are affected and/or altered by genetic risk factors for AD and modifiable vascular risk factors, lifestyle and environment, which in turn can all influence development of dementia and AD.
The exact role of the vascular system in the pathophysiogenesis of dementia and AD and whether the vascular system is a viable therapeutic target for dementia and AD still remains, however, to be better understood and/or defined by future studies. Some critical questions that perhaps need to be addressed by studies in humans are, for example: i) Do cerebrovascular changes drive the initial pathogenic events in the living human brain leading to neuronal injury, disrupted structural and functional brain connectivity and early cognitive decline in sporadic AD and/or individuals with genetic risk for AD (i.e., APOE4) or ADAD (i.e., PSEN1) compared to those at a lower risk? ii) Do cerebrovascular changes lead to cognitive decline in individuals with vascular risk factors such as diabetes or hypertension? iii) Do reductions in the resting CBF and/or CBF dysregulation and BBB breakdown precede changes in structural and functional brain connectivity in the living human brain, and can these cerebrovascular abnormalities be detected early in the disease process in AD and ADAD patients and/or in individuals with vascular risk factors?
In parallel to addressing these translational questions, there is an emerging need for novel molecular and imaging biomarkers to predict cognitive decline, dementia and AD, including development of biomarkers for neurovascular dysfunction, and to determine whether these biomarkers can serve as reliable new diagnostic and/or prognostic tools for predicting cognitive impairment and the decline to dementia. From the experimental preclinical laboratory side, it would be extremely interesting to evaluate whether genetic, vascular and/or mechanical manipulation of the cerebrovascular system to advance vascular/BBB injury functions influence the course of neurological disorders and AD-like pathology in animal models of dementia and AD, as it does in some models of human rare monogenic diseases [83,244–246].
Finally, new experimental studies should reveal whether therapeutic targeting of cerebrovascular dysfunction, including BBB breakdown and dysregulated CBF, could influence the course of disease in experimental models of AD, and importantly, could these findings then be translated to humans affected by dementia and AD.
Highlights.
Cerebrovascular damage may initiate molecular events leading to neurodegeneration
Major genetic risk factors for AD are linked to brain vascular dysfunction
Vascular risk factors increase incidence of sporadic AD
Mid-life lifestyle changes may protect the neurovasculature and prevent AD
Vasculoprotective therapies may delay the onset and progression of AD
Acknowledgments
The work of B.V.Z. is supported by the National Institutes of Health grants R01AG023084, R01NS090904, R01NS034467, and R01AG039452 and the Cure for Alzheimer’s Fund. We apologize to those authors whose original work we were not able to cite due to limited length of this review.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Zlokovic BV. Neurovascular pathways to neurodegeneration in Alzheimer’s disease and other disorders. Nat Rev Neurosci. 2011;12:723–738. doi: 10.1038/nrn3114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Iadecola C. The pathobiology of vascular dementia. Neuron. 2013;80:844–866. doi: 10.1016/j.neuron.2013.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Moskowitz MA, Lo EH, Iadecola C. The science of stroke: mechanisms in search of treatments. Neuron. 2010;67:181–198. doi: 10.1016/j.neuron.2010.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zlokovic BV. The blood-brain barrier in health and chronic neurodegenerative disorders. Neuron. 2008;57:178–201. doi: 10.1016/j.neuron.2008.01.003. [DOI] [PubMed] [Google Scholar]
- 5.Zhao Z, Nelson AR, Betsholtz C, Zlokovic BV. Establishment and Dysfunction of the Blood-Brain Barrier. Cell. n.d;163:1064–1078. doi: 10.1016/j.cell.2015.10.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mann GE, Zlokovic BV, Yudilevich DL. Evidence for a lactate transport system in the sarcolemmal membrane of the perfused rabbit heart: kinetics of unidirectional influx, carrier specificity and effects of glucagon. Biochim Biophys Acta. 1985;819:241–248. doi: 10.1016/0005-2736(85)90179-8. [DOI] [PubMed] [Google Scholar]
- 7.Zlokovic BV, Begley DJ, Chain-Eliash DG. Blood-brain barrier permeability to leucine-enkephalin, D-alanine2-D-leucine5-enkephalin and their N-terminal amino acid (tyrosine) Brain Res. 1985;336:125–132. doi: 10.1016/0006-8993(85)90423-8. [DOI] [PubMed] [Google Scholar]
- 8.Zlokovid BV, Lipovac MN, Begley DJ, Davson H, Rakić L. Transport of leucine-enkephalin across the blood-brain barrier in the perfused guinea pig brain. J Neurochem. 1987;49:310–315. doi: 10.1111/j.1471-4159.1987.tb03431.x. [DOI] [PubMed] [Google Scholar]
- 9.Zlokovic BV. Cerebrovascular permeability to peptides: manipulations of transport systems at the blood-brain barrier. Pharm Res. 1995;12:1395–1406. doi: 10.1023/a:1016254514167. [DOI] [PubMed] [Google Scholar]
- 10.Begley DJ, Brightman MW. Structural and functional aspects of the blood-brain barrier. Prog Drug Res Fortschritte Arzneimittelforschung Prog Rech Pharm. 2003;61:39–78. doi: 10.1007/978-3-0348-8049-7_2. [DOI] [PubMed] [Google Scholar]
- 11.Ramanathan A, Nelson AR, Sagare AP, Zlokovic BV. Impaired vascular-mediated clearance of brain amyloid beta in Alzheimer’s disease: the role, regulation and restoration of LRP1. Front Aging Neurosci. 2015;7:136. doi: 10.3389/fnagi.2015.00136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Montagne A, Barnes SR, Sweeney MD, Halliday MR, Sagare AP, Zhao Z, Toga AW, Jacobs RE, Liu CY, Amezcua L, Harrington MG, Chui HC, Law M, Zlokovic BV. Blood-Brain Barrier Breakdown in the Aging Human Hippocampus. Neuron. 2015;85:296–302. doi: 10.1016/j.neuron.2014.12.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Montagne A, Pa J, Zlokovic BV. Vascular Plasticity and Cognition During Normal Aging and Dementia. JAMA Neurol. 2015 doi: 10.1001/jamaneurol.2014.4636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Montine TJ, Koroshetz WJ, Babcock D, Dickson DW, Galpern WR, Glymour MM, Greenberg SM, Hutton ML, Knopman DS, Kuzmichev AN, Manly JJ, Marder KS, Miller BL, Phelps CH, Seeley WW, Sieber BA, Silverberg NB, Sutherland M, Torborg CL, Waddy SP, et al. Recommendations of the Alzheimer’s disease-related dementias conference. Neurology. 2014;83:851–860. doi: 10.1212/WNL.0000000000000733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Snyder HM, Corriveau RA, Craft S, Faber JE, Greenberg SM, Knopman D, Lamb BT, Montine TJ, Nedergaard M, Schaffer CB, Schneider JA, Wellington C, Wilcock DM, Zipfel GJ, Zlokovic B, Bain LJ, Bosetti F, Galis ZS, Koroshetz W, Carrillo MC. Vascular contributions to cognitive impairment and dementia including Alzheimer’s disease. Alzheimers Dement J Alzheimers Assoc. 2015;11:710–717. doi: 10.1016/j.jalz.2014.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sweeney MD, Sagare AP, Zlokovic BV. Cerebrospinal fluid biomarkers of neurovascular dysfunction in mild dementia and Alzheimer’s disease. J Cereb Blood Flow Metab. 2015;35:1055–1068. doi: 10.1038/jcbfm.2015.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Winkler EA, Sengillo JD, Sullivan JS, Henkel JS, Appel SH, Zlokovic BV. Blood-spinal cord barrier breakdown and pericyte reductions in amyotrophic lateral sclerosis. Acta Neuropathol (Berl) 2013;125:111–120. doi: 10.1007/s00401-012-1039-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Korczyn AD. Vascular parkinsonism-characteristics, pathogenesis and treatment. Nat Rev Neurol. 2015;11:319–326. doi: 10.1038/nrneurol.2015.61. [DOI] [PubMed] [Google Scholar]
- 19.Drouin-Ouellet J, Sawiak SJ, Cisbani G, Lagacé M, Kuan WL, Saint-Pierre M, Dury RJ, Alata W, St-Amour I, Mason SL, Calon F, Lacroix S, Gowland PA, Francis ST, Barker RA, Cicchetti F. Cerebrovascular and blood-brain barrier impairments in Huntington’s disease: Potential implications for its pathophysiology. Ann Neurol. 2015;78:160–177. doi: 10.1002/ana.24406. [DOI] [PubMed] [Google Scholar]
- 20.Alzheimer’s Association. 2015 Alzheimer’s disease facts and figures. Alzheimers Dement J Alzheimers Assoc. 2015;11:332–384. doi: 10.1016/j.jalz.2015.02.003. [DOI] [PubMed] [Google Scholar]
- 21.Tanzi RE. The genetics of Alzheimer disease. Cold Spring Harb Perspect Med. 2012;2 doi: 10.1101/cshperspect.a006296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Winkler EA, Sagare AP, Zlokovic BV. The pericyte: a forgotten cell type with important implications for Alzheimer’s disease? Brain Pathol Zurich Switz. 2014;24:371–386. doi: 10.1111/bpa.12152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sagare AP, Bell RD, Zlokovic BV. Neurovascular defects and faulty amyloid-β vascular clearance in Alzheimer’s disease. J Alzheimers Dis JAD. 2013;33(Suppl 1):S87–100. doi: 10.3233/JAD-2012-129037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sagare AP, Bell RD, Zlokovic BV. Neurovascular dysfunction and faulty amyloid β-peptide clearance in Alzheimer disease. Cold Spring Harb Perspect Med. 2012;2 doi: 10.1101/cshperspect.a011452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zlokovic BV. Neurovascular mechanisms of Alzheimer’s neurodegeneration. Trends Neurosci. 2005;28:202–208. doi: 10.1016/j.tins.2005.02.001. [DOI] [PubMed] [Google Scholar]
- 26.Huang Y, Mahley RW. Apolipoprotein E: structure and function in lipid metabolism, neurobiology, and Alzheimer’s diseases. Neurobiol Dis. 2014;72(Pt A):3–12. doi: 10.1016/j.nbd.2014.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Corder EH, Saunders AM, Strittmatter WJ, Schmechel DE, Gaskell PC, Small GW, Roses AD, Haines JL, Pericak-Vance MA. Gene dose of apolipoprotein E type 4 allele and the risk of Alzheimer’s disease in late onset families. Science. 1993;261:921–923. doi: 10.1126/science.8346443. [DOI] [PubMed] [Google Scholar]
- 28.Verghese PB, Castellano JM, Holtzman DM. Apolipoprotein E in Alzheimer’s disease and other neurological disorders. Lancet Neurol. 2011;10:241–252. doi: 10.1016/S1474-4422(10)70325-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Halliday MR, Pomara N, Sagare AP, Mack WJ, Frangione B, Zlokovic BV. Relationship between cyclophilin a levels and matrix metalloproteinase 9 activity in cerebrospinal fluid of cognitively normal apolipoprotein e4 carriers and blood-brain barrier breakdown. JAMA Neurol. 2013;70:1198–1200. doi: 10.1001/jamaneurol.2013.3841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Halliday MR, Rege SV, Ma Q, Zhao Z, Miller CA, Winkler EA, Zlokovic BV. Accelerated pericyte degeneration and blood-brain barrier breakdown in apolipoprotein E4 carriers with Alzheimer’s disease. J Cereb Blood Flow Metab Off J Int Soc Cereb Blood Flow Metab. 2015 doi: 10.1038/jcbfm.2015.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hultman K, Strickland S, Norris EH. The APOEε4/ε4 genotype potentiates vascular fibrin(ogen) deposition in amyloid-laden vessels in the brains of Alzheimer’s disease patients. J Cereb Blood Flow Metab Off J Int Soc Cereb Blood Flow Metab. 2013;33:1251–1258. doi: 10.1038/jcbfm.2013.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zipser BD, Johanson CE, Gonzalez L, Berzin TM, Tavares R, Hulette CM, Vitek MP, Hovanesian V, Stopa EG. Microvascular injury and blood-brain barrier leakage in Alzheimer’s disease. Neurobiol Aging. 2007;28:977–986. doi: 10.1016/j.neurobiolaging.2006.05.016. [DOI] [PubMed] [Google Scholar]
- 33.Zonneveld HI, Goos JDC, Wattjes MP, Prins ND, Scheltens P, van der Flier WM, Kuijer JPA, Muller M, Barkhof F. Prevalence of cortical superficial siderosis in a memory clinic population. Neurology. 2014;82:698–704. doi: 10.1212/WNL.0000000000000150. [DOI] [PubMed] [Google Scholar]
- 34.Suri S, Mackay CE, Kelly ME, Germuska M, Tunbridge EM, Frisoni GB, Matthews PM, Ebmeier KP, Bulte DP, Filippini N. Reduced cerebrovascular reactivity in young adults carrying the APOE ε4 allele. Alzheimers Dement J Alzheimers Assoc. 2015;11:648–657.e1. doi: 10.1016/j.jalz.2014.05.1755. [DOI] [PubMed] [Google Scholar]
- 35.Bell RD, Winkler EA, Singh I, Sagare AP, Deane R, Wu Z, Holtzman DM, Betsholtz C, Armulik A, Sallstrom J, Berk BC, Zlokovic BV. Apolipoprotein E controls cerebrovascular integrity via cyclophilin A. Nature. 2012;485:512–516. doi: 10.1038/nature11087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Alata W, Ye Y, St-Amour I, Vandal M, Calon F. Human apolipoprotein E ε4 expression impairs cerebral vascularization and blood-brain barrier function in mice. J Cereb Blood Flow Metab Off J Int Soc Cereb Blood Flow Metab. 2015;35:86–94. doi: 10.1038/jcbfm.2014.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bell RD, Sagare AP, Friedman AE, Bedi GS, Holtzman DM, Deane R, Zlokovic BV. Transport pathways for clearance of human Alzheimer’s amyloid beta-peptide and apolipoproteins E and J in the mouse central nervous system. J Cereb Blood Flow Metab Off J Int Soc Cereb Blood Flow Metab. 2007;27:909–918. doi: 10.1038/sj.jcbfm.9600419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Deane R, Sagare A, Hamm K, Parisi M, Lane S, Finn MB, Holtzman DM, Zlokovic BV. apoE isoform-specific disruption of amyloid beta peptide clearance from mouse brain. J Clin Invest. 2008;118:4002–4013. doi: 10.1172/JCI36663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Miller SE, Sahlender DA, Graham SC, Höning S, Robinson MS, Peden AA, Owen DJ. The molecular basis for the endocytosis of small R-SNAREs by the clathrin adaptor CALM. Cell. 2011;147:1118–1131. doi: 10.1016/j.cell.2011.10.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Treusch S, Hamamichi S, Goodman JL, Matlack KES, Chung CY, Baru V, Shulman JM, Parrado A, Bevis BJ, Valastyan JS, Han H, Lindhagen-Persson M, Reiman EM, Evans DA, Bennett DA, Olofsson A, DeJager PL, Tanzi RE, Caldwell KA, Caldwell GA, et al. Functional links between Aβ toxicity, endocytic trafficking, and Alzheimer’s disease risk factors in yeast. Science. 2011;334:1241–1245. doi: 10.1126/science.1213210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhao Z, Sagare AP, Ma Q, Halliday MR, Kong P, Kisler K, Winkler EA, Ramanathan A, Kanekiyo T, Bu G, Owens NC, Rege SV, Si G, Ahuja A, Zhu D, Miller CA, Schneider JA, Maeda M, Maeda T, Sugawara T, et al. Central role for PICALM in amyloid-β blood-brain barrier transcytosis and clearance. Nat Neurosci. 2015;18:978–987. doi: 10.1038/nn.4025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Harold D, Abraham R, Hollingworth P, Sims R, Gerrish A, Hamshere ML, Pahwa JS, Moskvina V, Dowzell K, Williams A, Jones N, Thomas C, Stretton A, Morgan AR, Lovestone S, Powell J, Proitsi P, Lupton MK, Brayne C, Rubinsztein DC, et al. Genome-wide association study identifies variants at CLU and PICALM associated with Alzheimer’s disease. Nat Genet. 2009;41:1088–1093. doi: 10.1038/ng.440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lambert JC, Ibrahim-Verbaas CA, Harold D, Naj AC, Sims R, Bellenguez C, DeStafano AL, Bis JC, Beecham GW, Grenier-Boley B, Russo G, Thorton-Wells TA, Jones N, Smith AV, Chouraki V, Thomas C, Ikram MA, Zelenika D, Vardarajan BN, Kamatani Y, et al. Meta-analysis of 74,046 individuals identifies 11 new susceptibility loci for Alzheimer’s disease. Nat Genet. 2013;45:1452–1458. doi: 10.1038/ng.2802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lambert J-C, Heath S, Even G, Campion D, Sleegers K, Hiltunen M, Combarros O, Zelenika D, Bullido MJ, Tavernier B, Letenneur L, Bettens K, Berr C, Pasquier F, Fiévet N, Barberger-Gateau P, Engelborghs S, De Deyn P, Mateo I, Franck A, et al. Genome-wide association study identifies variants at CLU and CR1 associated with Alzheimer’s disease. Nat Genet. 2009;41:1094–1099. doi: 10.1038/ng.439. [DOI] [PubMed] [Google Scholar]
- 45.Lancaster TM, Brindley LM, Tansey KE, Sims RC, Mantripragada K, Owen MJ, Williams J, Linden DEJ. Alzheimer’s disease risk variant in CLU is associated with neural inefficiency in healthy individuals. Alzheimers Dement J Alzheimers Assoc. 2014 doi: 10.1016/j.jalz.2014.10.012. [DOI] [PubMed] [Google Scholar]
- 46.Zlokovic BV, Martel CL, Matsubara E, McComb JG, Zheng G, McCluskey RT, Frangione B, Ghiso J. Glycoprotein 330/megalin: probable role in receptor-mediated transport of apolipoprotein J alone and in a complex with Alzheimer disease amyloid beta at the blood-brain and blood-cerebrospinal fluid barriers. Proc Natl Acad Sci U S A. 1996;93:4229–4234. doi: 10.1073/pnas.93.9.4229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Armstrong RA. Spatial correlations between beta-amyloid (Abeta) deposits and blood vessels in familial Alzheimer’s disease. Folia Neuropathol Assoc Pol Neuropathol Med Res Cent Pol Acad Sci. 2008;46:241–248. [PubMed] [Google Scholar]
- 48.Niwa A, Matsuo K, Shindo A, Yata K, Shiraishi T, Tomimoto H. Clinical and neuropathological findings in a patient with familial Alzheimer disease showing a mutation in the PSEN1 gene: Familial Alzheimer disease with PSEN1. Neuropathology. 2013;33:199–203. doi: 10.1111/j.1440-1789.2012.01340.x. [DOI] [PubMed] [Google Scholar]
- 49.Gama Sosa MA, Gasperi RD, Rocher AB, Wang AC-J, Janssen WGM, Flores T, Perez GM, Schmeidler J, Dickstein DL, Hof PR, Elder GA. Age-related vascular pathology in transgenic mice expressing presenilin 1-associated familial Alzheimer’s disease mutations. Am J Pathol. 2010;176:353–368. doi: 10.2353/ajpath.2010.090482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wen PH, De Gasperi R, Sosa MAG, Rocher AB, Friedrich VL, Hof PR, Elder GA. Selective expression of presenilin 1 in neural progenitor cells rescues the cerebral hemorrhages and cortical lamination defects in presenilin 1-null mutant mice. Dev Camb Engl. 2005;132:3873–3883. doi: 10.1242/dev.01946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gorski DH, Walsh K. Control of vascular cell differentiation by homeobox transcription factors. Trends Cardiovasc Med. 2003;13:213–220. doi: 10.1016/s1050-1738(03)00081-1. [DOI] [PubMed] [Google Scholar]
- 52.Rovelet-Lecrux A, Legallic S, Wallon D, Flaman J-M, Martinaud O, Bombois S, Rollin-Sillaire A, Michon A, Le Ber I, Pariente J, Puel M, Paquet C, Croisile B, Thomas-Antérion C, Vercelletto M, Lévy R, Frébourg T, Hannequin D, Campion D Investigators of the GMAJ project. A genome-wide study reveals rare CNVs exclusive to extreme phenotypes of Alzheimer disease. Eur J Hum Genet EJHG. 2012;20:613–617. doi: 10.1038/ejhg.2011.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wu Z, Guo H, Chow N, Sallstrom J, Bell RD, Deane R, Brooks AI, Kanagala S, Rubio A, Sagare A, Liu D, Li F, Armstrong D, Gasiewicz T, Zidovetzki R, Song X, Hofman F, Zlokovic BV. Role of the MEOX2 homeobox gene in neurovascular dysfunction in Alzheimer disease. Nat Med. 2005;11:959–965. doi: 10.1038/nm1287. [DOI] [PubMed] [Google Scholar]
- 54.Iadecola C, Davisson RL. Hypertension and cerebrovascular dysfunction. Cell Metab. 2008;7:476–484. doi: 10.1016/j.cmet.2008.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Toledo JB, Arnold SE, Raible K, Brettschneider J, Xie SX, Grossman M, Monsell SE, Kukull WA, Trojanowski JQ. Contribution of cerebrovascular disease in autopsy confirmed neurodegenerative disease cases in the National Alzheimer’s Coordinating Centre. Brain J Neurol. 2013;136:2697–2706. doi: 10.1093/brain/awt188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wang L, Kittaka M, Sun N, Schreiber SS, Zlokovic BV. Chronic nicotine treatment enhances focal ischemic brain injury and depletes free pool of brain microvascular tissue plasminogen activator in rats. J Cereb Blood Flow Metab Off J Int Soc Cereb Blood Flow Metab. 1997;17:136–146. doi: 10.1097/00004647-199702000-00002. [DOI] [PubMed] [Google Scholar]
- 57.Ruitenberg A, den Heijer T, Bakker SLM, van Swieten JC, Koudstaal PJ, Hofman A, Breteler MMB. Cerebral hypoperfusion and clinical onset of dementia: the Rotterdam Study. Ann Neurol. 2005;57:789–794. doi: 10.1002/ana.20493. [DOI] [PubMed] [Google Scholar]
- 58.Roher AE, Debbins JP, Malek-Ahmadi M, Chen K, Pipe JG, Maze S, Belden C, Maarouf CL, Thiyyagura P, Mo H, Hunter JM, Kokjohn TA, Walker DG, Kruchowsky JC, Belohlavek M, Sabbagh MN, Beach TG. Cerebral blood flow in Alzheimer’s disease. Vasc Health Risk Manag. 2012;8:599–611. doi: 10.2147/VHRM.S34874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Paris D, Patel N, DelleDonne A, Quadros A, Smeed R, Mullan M. Impaired angiogenesis in a transgenic mouse model of cerebral amyloidosis. Neurosci Lett. 2004;366:80–85. doi: 10.1016/j.neulet.2004.05.017. [DOI] [PubMed] [Google Scholar]
- 60.Bell RD, Winkler EA, Sagare AP, Singh I, LaRue B, Deane R, Zlokovic BV. Pericytes control key neurovascular functions and neuronal phenotype in the adult brain and during brain aging. Neuron. 2010;68:409–427. doi: 10.1016/j.neuron.2010.09.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Chow N, Bell RD, Deane R, Streb JW, Chen J, Brooks A, Van Nostrand W, Miano JM, Zlokovic BV. Serum response factor and myocardin mediate arterial hypercontractility and cerebral blood flow dysregulation in Alzheimer’s phenotype. Proc Natl Acad Sci U S A. 2007;104:823–828. doi: 10.1073/pnas.0608251104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bell RD, Deane R, Chow N, Long X, Sagare A, Singh I, Streb JW, Guo H, Rubio A, Van Nostrand W, Miano JM, Zlokovic BV. SRF and myocardin regulate LRP-mediated amyloid-beta clearance in brain vascular cells. Nat Cell Biol. 2009;11:143–153. doi: 10.1038/ncb1819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hill RA, Tong L, Yuan P, Murikinati S, Gupta S, Grutzendler J. Regional Blood Flow in the Normal and Ischemic Brain Is Controlled by Arteriolar Smooth Muscle Cell Contractility and Not by Capillary Pericytes. Neuron. 2015;87:95–110. doi: 10.1016/j.neuron.2015.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hall CN, Reynell C, Gesslein B, Hamilton NB, Mishra A, Sutherland BA, O’Farrell FM, Buchan AM, Lauritzen M, Attwell D. Capillary pericytes regulate cerebral blood flow in health and disease. Nature. 2014;508:55–60. doi: 10.1038/nature13165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Iadecola C. Neurovascular regulation in the normal brain and in Alzheimer’s disease. Nat Rev Neurosci. 2004;5:347–360. doi: 10.1038/nrn1387. [DOI] [PubMed] [Google Scholar]
- 66.Sagare AP, Sweeney MD, Makshanoff J, Zlokovic BV. Shedding of soluble platelet-derived growth factor receptor-β from human brain pericytes. Neurosci Lett. 2015 doi: 10.1016/j.neulet.2015.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Bowman GL, Kaye JA, Quinn JF. Dyslipidemia and blood-brain barrier integrity in Alzheimer’s disease. Curr Gerontol Geriatr Res. 2012;2012:184042. doi: 10.1155/2012/184042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ryu JK, McLarnon JG. A leaky blood-brain barrier, fibrinogen infiltration and microglial reactivity in inflamed Alzheimer’s disease brain. J Cell Mol Med. 2009;13:2911–2925. doi: 10.1111/j.1582-4934.2008.00434.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Sengillo JD, Winkler EA, Walker CT, Sullivan JS, Johnson M, Zlokovic BV. Deficiency in mural vascular cells coincides with blood-brain barrier disruption in Alzheimer’s disease. Brain Pathol Zurich Switz. 2013;23:303–310. doi: 10.1111/bpa.12004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Akinyemi RO, Mukaetova-Ladinska EB, Attems J, Ihara M, Kalaria RN. Vascular risk factors and neurodegeneration in ageing related dementias: Alzheimer’s disease and vascular dementia. Curr Alzheimer Res. 2013;10:642–653. doi: 10.2174/15672050113109990037. [DOI] [PubMed] [Google Scholar]
- 71.Baloyannis SJ, Baloyannis IS. The vascular factor in Alzheimer’s disease: a study in Golgi technique and electron microscopy. J Neurol Sci. 2012;322:117–121. doi: 10.1016/j.jns.2012.07.010. [DOI] [PubMed] [Google Scholar]
- 72.Farkas E, Luiten PG. Cerebral microvascular pathology in aging and Alzheimer’s disease. Prog Neurobiol. 2001;64:575–611. doi: 10.1016/s0301-0082(00)00068-x. [DOI] [PubMed] [Google Scholar]
- 73.Cortes-Canteli M, Mattei L, Richards AT, Norris EH, Strickland S. Fibrin deposited in the Alzheimer’s disease brain promotes neuronal degeneration. Neurobiol Aging. 2015;36:608–617. doi: 10.1016/j.neurobiolaging.2014.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Yates PA, Desmond PM, Phal PM, Steward C, Szoeke C, Salvado O, Ellis KA, Martins RN, Masters CL, Ames D, Villemagne VL, Rowe CC AIBL Research Group. Incidence of cerebral microbleeds in preclinical Alzheimer disease. Neurology. 2014;82:1266–1273. doi: 10.1212/WNL.0000000000000285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Olazarán J, Ramos A, Boyano I, Alfayate E, Valentí M, Rábano A, Alvarez-Linera J. Pattern of and risk factors for brain microbleeds in neurodegenerative dementia. Am J Alzheimers Dis Other Demen. 2014;29:263–269. doi: 10.1177/1533317513517043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Shams S, Martola J, Granberg T, Li X, Shams M, Fereshtehnejad SM, Cavallin L, Aspelin P, Kristoffersen-Wiberg M, Wahlund LO. Cerebral microbleeds: different prevalence, topography, and risk factors depending on dementia diagnosis—the Karolinska Imaging Dementia Study. AJNR Am J Neuroradiol. 2015;36:661–666. doi: 10.3174/ajnr.A4176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Raven EP, Lu PH, Tishler TA, Heydari P, Bartzokis G. Increased iron levels and decreased tissue integrity in hippocampus of Alzheimer’s disease detected in vivo with magnetic resonance imaging. J Alzheimers Dis JAD. 2013;37:127–136. doi: 10.3233/JAD-130209. [DOI] [PubMed] [Google Scholar]
- 78.Horwood N, Davies DC. Immunolabelling of hippocampal microvessel glucose transporter protein is reduced in Alzheimer’s disease. Virchows Arch Int J Pathol. 1994;425:69–72. doi: 10.1007/BF00193951. [DOI] [PubMed] [Google Scholar]
- 79.Winkler EA, Nishida Y, Sagare AP, Rege SV, Bell RD, Perlmutter D, Sengillo JD, Hillman S, Kong P, Nelson AR, Sullivan JS, Zhao Z, Meiselman HJ, Wenby RB, Soto J, Abel ED, Makshanoff J, Zuniga E, De Vivo DC, Zlokovic BV. GLUT1 reductions exacerbate Alzheimer’s disease vasculo-neuronal dysfunction and degeneration. Nat Neurosci. 2015;18:521–530. doi: 10.1038/nn.3966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Protas HD, Chen K, Langbaum JBS, Fleisher AS, Alexander GE, Lee W, Bandy D, de Leon MJ, Mosconi L, Buckley S, Truran-Sacrey D, Schuff N, Weiner MW, Caselli RJ, Reiman EM. Posterior cingulate glucose metabolism, hippocampal glucose metabolism, and hippocampal volume in cognitively normal, late-middle-aged persons at 3 levels of genetic risk for Alzheimer disease. JAMA Neurol. 2013;70:320–325. doi: 10.1001/2013.jamaneurol.286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Mosconi L, De Santi S, Li J, Tsui WH, Li Y, Boppana M, Laska E, Rusinek H, de Leon MJ. Hippocampal hypometabolism predicts cognitive decline from normal aging. Neurobiol Aging. 2008;29:676–692. doi: 10.1016/j.neurobiolaging.2006.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Zeller K, Rahner-Welsch S, Kuschinsky W. Distribution of Glut1 glucose transporters in different brain structures compared to glucose utilization and capillary density of adult rat brains. J Cereb Blood Flow Metab Off J Int Soc Cereb Blood Flow Metab. 1997;17:204–209. doi: 10.1097/00004647-199702000-00010. [DOI] [PubMed] [Google Scholar]
- 83.Wang D, Kranz-Eble P, De Vivo DC. Mutational analysis of GLUT1 (SLC2A1) in Glut-1 deficiency syndrome. Hum Mutat. 2000;16:224–231. doi: 10.1002/1098-1004(200009)16:3<224::AID-HUMU5>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 84.Mawuenyega KG, Sigurdson W, Ovod V, Munsell L, Kasten T, Morris JC, Yarasheski KE, Bateman RJ. Decreased clearance of CNS beta-amyloid in Alzheimer’s disease. Science. 2010;330:1774. doi: 10.1126/science.1197623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Deane R, Wu Z, Sagare A, Davis J, Du Yan S, Hamm K, Xu F, Parisi M, LaRue B, Hu HW, Spijkers P, Guo H, Song X, Lenting PJ, Van Nostrand WE, Zlokovic BV. LRP/amyloid beta-peptide interaction mediates differential brain efflux of Abeta isoforms. Neuron. 2004;43:333–344. doi: 10.1016/j.neuron.2004.07.017. [DOI] [PubMed] [Google Scholar]
- 86.Tarasoff-Conway JM, Carare RO, Osorio RS, Glodzik L, Butler T, Fieremans E, Axel L, Rusinek H, Nicholson C, Zlokovic BV, Frangione B, Blennow K, Ménard J, Zetterberg H, Wisniewski T, de Leon MJ. Clearance systems in the brain-implications for Alzheimer disease. Nat Rev Neurol. 2015 doi: 10.1038/nrneurol.2015.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Zlokovic BV, Deane R, Sagare AP, Bell RD, Winkler EA. Low-density lipoprotein receptor-related protein-1: a serial clearance homeostatic mechanism controlling Alzheimer’s amyloid β-peptide elimination from the brain. J Neurochem. 2010;115:1077–1089. doi: 10.1111/j.1471-4159.2010.07002.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Donahue JE, Flaherty SL, Johanson CE, Duncan JA, Silverberg GD, Miller MC, Tavares R, Yang W, Wu Q, Sabo E, Hovanesian V, Stopa EG. RAGE, LRP-1, and amyloid-beta protein in Alzheimer’s disease. Acta Neuropathol (Berl) 2006;112:405–415. doi: 10.1007/s00401-006-0115-3. [DOI] [PubMed] [Google Scholar]
- 89.Sagare AP, Deane R, Zetterberg H, Wallin A, Blennow K, Zlokovic BV. Impaired lipoprotein receptor-mediated peripheral binding of plasma amyloid-β is an early biomarker for mild cognitive impairment preceding Alzheimer’s disease. J Alzheimers Dis JAD. 2011;24:25–34. doi: 10.3233/JAD-2010-101248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Drake J, Link CD, Butterfield DA. Oxidative stress precedes fibrillar deposition of Alzheimer’s disease amyloid beta-peptide (1–42) in a transgenic Caenorhabditis elegans model. Neurobiol Aging. 2003;24:415–420. doi: 10.1016/s0197-4580(02)00225-7. [DOI] [PubMed] [Google Scholar]
- 91.Deane R, Singh I, Sagare AP, Bell RD, Ross NT, LaRue B, Love R, Perry S, Paquette N, Deane RJ, Thiyagarajan M, Zarcone T, Fritz G, Friedman AE, Miller BL, Zlokovic BV. A multimodal RAGE-specific inhibitor reduces amyloid β-mediated brain disorder in a mouse model of Alzheimer disease. J Clin Invest. 2012;122:1377–1392. doi: 10.1172/JCI58642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Silverberg GD, Miller MC, Messier AA, Majmudar S, Machan JT, Donahue JE, Stopa EG, Johanson CE. Amyloid deposition and influx transporter expression at the blood-brain barrier increase in normal aging. J Neuropathol Exp Neurol. 2010;69:98–108. doi: 10.1097/NEN.0b013e3181c8ad2f. [DOI] [PubMed] [Google Scholar]
- 93.Yan SD, Chen X, Fu J, Chen M, Zhu H, Roher A, Slattery T, Zhao L, Nagashima M, Morser J, Migheli A, Nawroth P, Stern D, Schmidt AM. RAGE and amyloid-beta peptide neurotoxicity in Alzheimer’s disease. Nature. 1996;382:685–691. doi: 10.1038/382685a0. [DOI] [PubMed] [Google Scholar]
- 94.Smith EE, Schneider JA, Wardlaw JM, Greenberg SM. Cerebral microinfarcts: the invisible lesions. Lancet Neurol. 2012;11:272–282. doi: 10.1016/S1474-4422(11)70307-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Toledo JB, Arnold SE, Raible K, Brettschneider J, Xie SX, Grossman M, Monsell SE, Kukull WA, Trojanowski JQ. Contribution of cerebrovascular disease in autopsy confirmed neurodegenerative disease cases in the National Alzheimer’s Coordinating Centre. Brain J Neurol. 2013;136:2697–2706. doi: 10.1093/brain/awt188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Weller RO, Djuanda E, Yow H-Y, Carare RO. Lymphatic drainage of the brain and the pathophysiology of neurological disease. Acta Neuropathol (Berl) 2009;117:1–14. doi: 10.1007/s00401-008-0457-0. [DOI] [PubMed] [Google Scholar]
- 97.Xu W, Xu F, Anderson ME, Kotarba AE, Davis J, Robinson JK, Van Nostrand WE. Cerebral microvascular rather than parenchymal amyloid-β protein pathology promotes early cognitive impairment in transgenic mice. J Alzheimers Dis JAD. 2014;38:621–632. doi: 10.3233/JAD-130758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Morris AWJ, Carare RO, Schreiber S, Hawkes CA. The Cerebrovascular Basement Membrane: Role in the Clearance of β-amyloid and Cerebral Amyloid Angiopathy. Front Aging Neurosci. 2014;6:251. doi: 10.3389/fnagi.2014.00251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Brown WR. A review of string vessels or collapsed, empty basement membrane tubes. J Alzheimers Dis JAD. 2010;21:725–739. doi: 10.3233/JAD-2010-100219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Hunter JM, Kwan J, Malek-Ahmadi M, Maarouf CL, Kokjohn TA, Belden C, Sabbagh MN, Beach TG, Roher AE. Morphological and pathological evolution of the brain microcirculation in aging and Alzheimer’s disease. PloS One. 2012;7:e36893. doi: 10.1371/journal.pone.0036893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Richard E, van Gool WA, Hoozemans JJM, van Haastert ES, Eikelenboom P, Rozemuller AJM, van de Berg WDJ. Morphometric changes in the cortical microvascular network in Alzheimer’s disease. J Alzheimers Dis JAD. 2010;22:811–818. doi: 10.3233/JAD-2010-100849. [DOI] [PubMed] [Google Scholar]
- 102.Xu W, Tan L, Wang H-F, Jiang T, Tan M-S, Tan L, Zhao Q-F, Li J-Q, Wang J, Yu J-T. Meta-analysis of modifiable risk factors for Alzheimer’s disease. J Neurol Neurosurg Psychiatry. 2015 doi: 10.1136/jnnp-2015-310548. [DOI] [PubMed] [Google Scholar]
- 103.Freitag MH, Peila R, Masaki K, Petrovitch H, Ross GW, White LR, Launer LJ. Midlife pulse pressure and incidence of dementia: the Honolulu-Asia Aging Study. Stroke J Cereb Circ. 2006;37:33–37. doi: 10.1161/01.STR.0000196941.58869.2d. [DOI] [PubMed] [Google Scholar]
- 104.Shah NS, Vidal J-S, Masaki K, Petrovitch H, Ross GW, Tilley C, DeMattos RB, Tracy RP, White LR, Launer LJ. Midlife blood pressure, plasma β-amyloid, and the risk for Alzheimer disease: the Honolulu Asia Aging Study. Hypertension. 2012;59:780–786. doi: 10.1161/HYPERTENSIONAHA.111.178962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Sharp SI, Aarsland D, Day S, Sønnesyn H, Ballard C Alzheimer’s Society Vascular Dementia Systematic Review Group. Hypertension is a potential risk factor for vascular dementia: systematic review. Int J Geriatr Psychiatry. 2011;26:661–669. doi: 10.1002/gps.2572. [DOI] [PubMed] [Google Scholar]
- 106.Scuteri A, Nilsson PM, Tzourio C, Redon J, Laurent S. Microvascular brain damage with aging and hypertension: pathophysiological consideration and clinical implications. J Hypertens. 2011;29:1469–1477. doi: 10.1097/HJH.0b013e328347cc17. [DOI] [PubMed] [Google Scholar]
- 107.Uiterwijk R, Huijts M, Staals J, Rouhl RPW, De Leeuw PW, Kroon AA, Van Oostenbrugge RJ. Endothelial Activation Is Associated With Cognitive Performance in Patients With Hypertension. Am J Hypertens. 2015 doi: 10.1093/ajh/hpv122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Droste DW, Ritter MA, Dittrich R, Heidenreich S, Wichter T, Freund M, Ringelstein EB. Arterial hypertension and ischaemic stroke. Acta Neurol Scand. 2003;107:241–251. doi: 10.1034/j.1600-0404.2003.00098.x. [DOI] [PubMed] [Google Scholar]
- 109.Ueno M, Sakamoto H, Tomimoto H, Akiguchi I, Onodera M, Huang C-L, Kanenishi K. Blood-brain barrier is impaired in the hippocampus of young adult spontaneously hypertensive rats. Acta Neuropathol (Berl) 2004;107:532–538. doi: 10.1007/s00401-004-0845-z. [DOI] [PubMed] [Google Scholar]
- 110.Carnevale D, Mascio G, D’Andrea I, Fardella V, Bell RD, Branchi I, Pallante F, Zlokovic B, Yan SS, Lembo G. Hypertension induces brain β-amyloid accumulation, cognitive impairment, and memory deterioration through activation of receptor for advanced glycation end products in brain vasculature. Hypertension. 2012;60:188–197. doi: 10.1161/HYPERTENSIONAHA.112.195511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Faraco G, Park L, Zhou P, Luo W, Paul SM, Anrather J, Iadecola C. Hypertension enhances Aβ-induced neurovascular dysfunction, promotes β-secretase activity, and leads to amyloidogenic processing of APP. J Cereb Blood Flow Metab Off J Int Soc Cereb Blood Flow Metab. 2015 doi: 10.1038/jcbfm.2015.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Kruyer A, Soplop N, Strickland S, Norris EH. Chronic Hypertension Leads to Neurodegeneration in the TgSwDI Mouse Model of Alzheimer’s Disease. Hypertension. 2015;66:175–182. doi: 10.1161/HYPERTENSIONAHA.115.05524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Rosei EA, Rizzoni D, Muiesan ML, Sleiman I, Salvetti M, Monteduro C, Porteri E CENTRO (CandEsartaN on aTherosclerotic Risk factors) study investigators. Effects of candesartan cilexetil and enalapril on inflammatory markers of atherosclerosis in hypertensive patients with non-insulin-dependent diabetes mellitus. J Hypertens. 2005;23:435–444. doi: 10.1097/00004872-200502000-00027. [DOI] [PubMed] [Google Scholar]
- 114.Akter S, Jesmin S, Iwashima Y, Hideaki S, Rahman MA, Islam MM, Moroi M, Shimojo N, Yamaguchi N, Miyauchi T, Kawano S, Mizutani T, Kawano Y. Higher circulatory level of endothelin-1 in hypertensive subjects screened through a cross-sectional study of rural Bangladeshi women. Hypertens Res Off J Jpn Soc Hypertens. 2015;38:208–212. doi: 10.1038/hr.2014.160. [DOI] [PubMed] [Google Scholar]
- 115.Nagai M, Hoshide S, Kario K. Hypertension and dementia. Am J Hypertens. 2010;23:116–124. doi: 10.1038/ajh.2009.212. [DOI] [PubMed] [Google Scholar]
- 116.Larson EB, Yaffe K, Langa KM. New insights into the dementia epidemic. N Engl J Med. 2013;369:2275–2277. doi: 10.1056/NEJMp1311405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Profenno LA, Porsteinsson AP, Faraone SV. Meta-analysis of Alzheimer’s disease risk with obesity, diabetes, and related disorders. Biol Psychiatry. 2010;67:505–512. doi: 10.1016/j.biopsych.2009.02.013. [DOI] [PubMed] [Google Scholar]
- 118.Cheng G, Huang C, Deng H, Wang H. Diabetes as a risk factor for dementia and mild cognitive impairment: a meta-analysis of longitudinal studies. Intern Med J. 2012;42:484–491. doi: 10.1111/j.1445-5994.2012.02758.x. [DOI] [PubMed] [Google Scholar]
- 119.Barbagallo M, Dominguez LJ. Type 2 diabetes mellitus and Alzheimer’s disease. World J Diabetes. 2014;5:889–893. doi: 10.4239/wjd.v5.i6.889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Starr JM, Wardlaw J, Ferguson K, MacLullich A, Deary IJ, Marshall I. Increased blood-brain barrier permeability in type II diabetes demonstrated by gadolinium magnetic resonance imaging. J Neurol Neurosurg Psychiatry. 2003;74:70–76. doi: 10.1136/jnnp.74.1.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Mogi M, Horiuchi M. Neurovascular coupling in cognitive impairment associated with diabetes mellitus. Circ J Off J Jpn Circ Soc. 2011;75:1042–1048. doi: 10.1253/circj.cj-11-0121. [DOI] [PubMed] [Google Scholar]
- 122.Huber JD, VanGilder RL, Houser KA. Streptozotocin-induced diabetes progressively increases blood-brain barrier permeability in specific brain regions in rats. Am J Physiol Heart Circ Physiol. 2006;291:H2660–2668. doi: 10.1152/ajpheart.00489.2006. [DOI] [PubMed] [Google Scholar]
- 123.Hawkins BT, Lundeen TF, Norwood KM, Brooks HL, Egleton RD. Increased blood-brain barrier permeability and altered tight junctions in experimental diabetes in the rat: contribution of hyperglycaemia and matrix metalloproteinases. Diabetologia. 2007;50:202–211. doi: 10.1007/s00125-006-0485-z. [DOI] [PubMed] [Google Scholar]
- 124.Takeda S, Sato N, Uchio-Yamada K, Sawada K, Kunieda T, Takeuchi D, Kurinami H, Shinohara M, Rakugi H, Morishita R. Diabetes-accelerated memory dysfunction via cerebrovascular inflammation and Abeta deposition in an Alzheimer mouse model with diabetes. Proc Natl Acad Sci U S A. 2010;107:7036–7041. doi: 10.1073/pnas.1000645107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Ramos-Rodriguez JJ, Infante-Garcia C, Galindo-Gonzalez L, Garcia-Molina Y, Lechuga-Sancho A, Garcia-Alloza M. Increased Spontaneous Central Bleeding and Cognition Impairment in APP/PS1 Mice with Poorly Controlled Diabetes Mellitus. Mol Neurobiol. 2015 doi: 10.1007/s12035-015-9311-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Jayaraman A, Pike CJ. Alzheimer’s disease and type 2 diabetes: multiple mechanisms contribute to interactions. Curr Diab Rep. 2014;14:476. doi: 10.1007/s11892-014-0476-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.De Felice FG, Lourenco MV. Brain metabolic stress and neuroinflammation at the basis of cognitive impairment in Alzheimer’s disease. Front Aging Neurosci. 2015;7:94. doi: 10.3389/fnagi.2015.00094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Gupta A, Iadecola C. Impaired Aβ clearance: a potential link between atherosclerosis and Alzheimer’s disease. Front Aging Neurosci. 2015;7:115. doi: 10.3389/fnagi.2015.00115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Yarchoan M, Xie SX, Kling MA, Toledo JB, Wolk DA, Lee EB, Van Deerlin V, Lee VM-Y, Trojanowski JQ, Arnold SE. Cerebrovascular atherosclerosis correlates with Alzheimer pathology in neurodegenerative dementias. Brain J Neurol. 2012;135:3749–3756. doi: 10.1093/brain/aws271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Frazier DT, Seider T, Bettcher BM, Mack WJ, Jastrzab L, Chao L, Weiner MW, DeCarli C, Reed BR, Mungas D, Chui HC, Kramer JH. The role of carotid intima-media thickness in predicting longitudinal cognitive function in an older adult cohort. Cerebrovasc Dis Basel Switz. 2014;38:441–447. doi: 10.1159/000366469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Arntzen KA, Schirmer H, Johnsen SH, Wilsgaard T, Mathiesen EB. Carotid atherosclerosis predicts lower cognitive test results: a 7-year follow-up study of 4,371 stroke-free subjects - the Tromsø study. Cerebrovasc Dis Basel Switz. 2012;33:159–165. doi: 10.1159/000334182. [DOI] [PubMed] [Google Scholar]
- 132.Zhong W, Cruickshanks KJ, Schubert CR, Acher CW, Carlsson CM, Klein BEK, Klein R, Chappell RJ. Carotid atherosclerosis and 10-year changes in cognitive function. Atherosclerosis. 2012;224:506–510. doi: 10.1016/j.atherosclerosis.2012.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Bots ML, Launer LJ, Lindemans J, Hofman A, Grobbee DE. Homocysteine, atherosclerosis and prevalent cardiovascular disease in the elderly: The Rotterdam Study. J Intern Med. 1997;242:339–347. doi: 10.1046/j.1365-2796.1997.00239.x. [DOI] [PubMed] [Google Scholar]
- 134.McIlroy SP, Dynan KB, Lawson JT, Patterson CC, Passmore AP. Moderately elevated plasma homocysteine, methylenetetrahydrofolate reductase genotype, and risk for stroke, vascular dementia, and Alzheimer disease in Northern Ireland. Stroke J Cereb Circ. 2002;33:2351–2356. doi: 10.1161/01.str.0000032550.90046.38. [DOI] [PubMed] [Google Scholar]
- 135.Hassan A, Hunt BJ, O’Sullivan M, Bell R, D’Souza R, Jeffery S, Bamford JM, Markus HS. Homocysteine is a risk factor for cerebral small vessel disease, acting via endothelial dysfunction. Brain J Neurol. 2004;127:212–219. doi: 10.1093/brain/awh023. [DOI] [PubMed] [Google Scholar]
- 136.Miwa K, Tanaka M, Okazaki S, Yagita Y, Sakaguchi M, Mochizuki H, Kitagawa K. Increased Total Homocysteine Levels Predict the Risk of Incident Dementia Independent of Cerebral Small-Vessel Diseases and Vascular Risk Factors. J Alzheimers Dis JAD. 2015 doi: 10.3233/JAD-150458. [DOI] [PubMed] [Google Scholar]
- 137.Kamat PK, Kyles P, Kalani A, Tyagi N. Hydrogen Sulfide Ameliorates Homocysteine-Induced Alzheimer’s Disease-Like Pathology, Blood-Brain Barrier Disruption, and Synaptic Disorder. Mol Neurobiol. 2015 doi: 10.1007/s12035-015-9212-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Zhuo J-M, Wang H, Praticò D. Is hyperhomocysteinemia an Alzheimer’s disease (AD) risk factor, an AD marker, or neither? Trends Pharmacol Sci. 2011;32:562–571. doi: 10.1016/j.tips.2011.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Sudduth TL, Powell DK, Smith CD, Greenstein A, Wilcock DM. Induction of hyperhomocysteinemia models vascular dementia by induction of cerebral microhemorrhages and neuroinflammation. J Cereb Blood Flow Metab Off J Int Soc Cereb Blood Flow Metab. 2013;33:708–715. doi: 10.1038/jcbfm.2013.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Sudduth TL, Weekman EM, Brothers HM, Braun K, Wilcock DM. β-amyloid deposition is shifted to the vasculature and memory impairment is exacerbated when hyperhomocysteinemia is induced in APP/PS1 transgenic mice. Alzheimers Res Ther. 2014;6:32. doi: 10.1186/alzrt262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Franzblau M, Gonzales-Portillo C, Gonzales-Portillo GS, Diamandis T, Borlongan MC, Tajiri N, Borlongan CV. Vascular damage: a persisting pathology common to Alzheimer’s disease and traumatic brain injury. Med Hypotheses. 2013;81:842–845. doi: 10.1016/j.mehy.2013.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Glushakova OY, Johnson D, Hayes RL. Delayed increases in microvascular pathology after experimental traumatic brain injury are associated with prolonged inflammation, blood-brain barrier disruption, and progressive white matter damage. J Neurotrauma. 2014;31:1180–1193. doi: 10.1089/neu.2013.3080. [DOI] [PubMed] [Google Scholar]
- 143.Ju Y-ES, Lucey BP, Holtzman DM. Sleep and Alzheimer disease pathology--a bidirectional relationship. Nat Rev Neurol. 2014;10:115–119. doi: 10.1038/nrneurol.2013.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Peter-Derex L, Yammine P, Bastuji H, Croisile B. Sleep and Alzheimer’s disease. Sleep Med Rev. 2015;19:29–38. doi: 10.1016/j.smrv.2014.03.007. [DOI] [PubMed] [Google Scholar]
- 145.Xie L, Kang H, Xu Q, Chen MJ, Liao Y, Thiyagarajan M, O’Donnell J, Christensen DJ, Nicholson C, Iliff JJ, Takano T, Deane R, Nedergaard M. Sleep drives metabolite clearance from the adult brain. Science. 2013;342:373–377. doi: 10.1126/science.1241224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Pan W, Kastin AJ. Can sleep apnea cause Alzheimer’s disease? Neurosci Biobehav Rev. 2014;47:656–669. doi: 10.1016/j.neubiorev.2014.10.019. [DOI] [PubMed] [Google Scholar]
- 147.He J, Hsuchou H, He Y, Kastin AJ, Wang Y, Pan W. Sleep restriction impairs blood-brain barrier function. J Neurosci Off J Soc Neurosci. 2014;34:14697–14706. doi: 10.1523/JNEUROSCI.2111-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Sharma A, Muresanu DF, Lafuente JV, Patnaik R, Tian ZR, Buzoianu AD, Sharma HS. Sleep Deprivation-Induced Blood-Brain Barrier Breakdown and Brain Dysfunction are Exacerbated by Size-Related Exposure to Ag and Cu Nanoparticles Neuroprotective Effects of a 5-HT3 Receptor Antagonist Ondansetron. Mol Neurobiol. 2015 doi: 10.1007/s12035-015-9236-9. [DOI] [PubMed] [Google Scholar]
- 149.Calderón-Garcidueñas L, Franco-Lira M, Mora-Tiscareño A, Medina-Cortina H, Torres-Jardón R, Kavanaugh M. Early Alzheimer’s and Parkinson’s disease pathology in urban children: Friend versus Foe responses--it is time to face the evidence. BioMed Res Int. 2013;2013:161687. doi: 10.1155/2013/161687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Jung C-R, Lin Y-T, Hwang B-F. Ozone, particulate matter, and newly diagnosed Alzheimer’s disease: a population-based cohort study in Taiwan. J Alzheimers Dis JAD. 2015;44:573–584. doi: 10.3233/JAD-140855. [DOI] [PubMed] [Google Scholar]
- 151.Calderón-Garcidueñas L, Kavanaugh M, Block M, D’Angiulli A, Delgado-Chávez R, Torres-Jardón R, González-Maciel A, Reynoso-Robles R, Osnaya N, Villarreal-Calderon R, Guo R, Hua Z, Zhu H, Perry G, Diaz P. Neuroinflammation, hyperphosphorylated tau, diffuse amyloid plaques, and down-regulation of the cellular prion protein in air pollution exposed children and young adults. J Alzheimers Dis JAD. 2012;28:93–107. doi: 10.3233/JAD-2011-110722. [DOI] [PubMed] [Google Scholar]
- 152.Calderón-Garcidueñas L, Mora-Tiscareño A, Franco-Lira M, Zhu H, Lu Z, Solorio E, Torres-Jardón R, D’Angiulli A. Decreases in Short Term Memory, IQ, and Altered Brain Metabolic Ratios in Urban Apolipoprotein ε4 Children Exposed to Air Pollution. J Alzheimers Dis JAD. 2015;45:757–770. doi: 10.3233/JAD-142685. [DOI] [PubMed] [Google Scholar]
- 153.Calderón-Garcidueñas L, Vojdani A, Blaurock-Busch E, Busch Y, Friedle A, Franco-Lira M, Sarathi-Mukherjee P, Martínez-Aguirre X, Park S-B, Torres-Jardón R, D’Angiulli A. Air pollution and children: neural and tight junction antibodies and combustion metals, the role of barrier breakdown and brain immunity in neurodegeneration. J Alzheimers Dis JAD. 2015;43:1039–1058. doi: 10.3233/JAD-141365. [DOI] [PubMed] [Google Scholar]
- 154.Kim SH, Knight EM, Saunders EL, Cuevas AK, Popovech M, Chen L-C, Gandy S. Rapid doubling of Alzheimer’s amyloid-β40 and 42 levels in brains of mice exposed to a nickel nanoparticle model of air pollution. F1000Research. 2012;1:70. doi: 10.12688/f1000research.1-70.v1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Oppenheim HA, Lucero J, Guyot A-C, Herbert LM, McDonald JD, Mabondzo A, Lund AK. Exposure to vehicle emissions results in altered blood brain barrier permeability and expression of matrix metalloproteinases and tight junction proteins in mice. Part Fibre Toxicol. 2013;10:62. doi: 10.1186/1743-8977-10-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Yang Z, Liu ZW, Allaker RP, Reip P, Oxford J, Ahmad Z, Ren G. A review of nanoparticle functionality and toxicity on the central nervous system. J R Soc Interface. 2010;7:S411–S422. doi: 10.1098/rsif.2010.0158.focus. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Sharma HS, Hussain S, Schlager J, Ali SF, Sharma A. Influence of nanoparticles on blood-brain barrier permeability and brain edema formation in rats. Acta Neurochir Suppl. 2010;106:359–364. doi: 10.1007/978-3-211-98811-4_65. [DOI] [PubMed] [Google Scholar]
- 158.Sharma HS, Patnaik R, Sharma A. Diabetes aggravates nanoparticles induced breakdown of the blood-brain barrier permeability, brain edema formation, alterations in cerebral blood flow and neuronal injury An experimental study using physiological and morphological investigations in the rat. J Nanosci Nanotechnol. 2010;10:7931–7945. doi: 10.1166/jnn.2010.3616. [DOI] [PubMed] [Google Scholar]
- 159.Tang J, Xiong L, Wang S, Wang J, Liu L, Li J, Yuan F, Xi T. Distribution, translocation and accumulation of silver nanoparticles in rats. J Nanosci Nanotechnol. 2009;9:4924–4932. doi: 10.1166/jnn.2009.1269. [DOI] [PubMed] [Google Scholar]
- 160.Li C-H, Jhan C, Cheng Y-W, Tsai C-H, Liu C-W, Lee C-C, Chen R-M, Shyu M-K, Kang J-J. Gold nanoparticles increase endothelial paracellular permeability by altering components of endothelial tight junctions, and increase blood-brain barrier permeability in mice. Toxicol Sci Off J Soc Toxicol. 2015 doi: 10.1093/toxsci/kfv176. [DOI] [PubMed] [Google Scholar]
- 161.Takeda S, Sato N, Morishita R. Systemic inflammation, blood-brain barrier vulnerability and cognitive/non-cognitive symptoms in Alzheimer disease: relevance to pathogenesis and therapy. Front Aging Neurosci. 2014;6:171. doi: 10.3389/fnagi.2014.00171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Dunn N, Mullee M, Perry VH, Holmes C. Association between dementia and infectious disease: evidence from a case-control study. Alzheimer Dis Assoc Disord. 2005;19:91–94. doi: 10.1097/01.wad.0000165511.52746.1f. [DOI] [PubMed] [Google Scholar]
- 163.Nicolson Garth L, Haier Jorg. Role of Chronic Bacterial and Viral Infections in Neurodegenerative. Neurobehavioral, Psychiatric, Autoimmune and Fatiguing Illnesses: Part 1. 2009;2:20–28. [Google Scholar]
- 164.Lin WR, Shang D, Itzhaki RF. Neurotropic viruses and Alzheimer disease Interaction of herpes simplex type 1 virus and apolipoprotein E in the etiology of the disease. Mol Chem Neuropathol Spons Int Soc Neurochem World Fed Neurol Res Groups Neurochem Cerebrospinal Fluid. 1996;28:135–141. doi: 10.1007/BF02815215. [DOI] [PubMed] [Google Scholar]
- 165.Foley NC, Affoo RH, Martin RE. A systematic review and meta-analysis examining pneumonia-associated mortality in dementia. Dement Geriatr Cogn Disord. 2015;39:52–67. doi: 10.1159/000367783. [DOI] [PubMed] [Google Scholar]
- 166.Bircan HA, Çakir M, Yilmazer Kapulu I, Sütcü R, Kaya S, Öztürk O. Elevated serum matrix metalloproteinase-2 and -9 and their correlations with severity of disease in patients with community-acquired pneumonia. Turk J Med Sci. 2015;45:593–599. doi: 10.3906/sag-1402-51. [DOI] [PubMed] [Google Scholar]
- 167.Singhrao SK, Harding A, Simmons T, Robinson S, Kesavalu L, Crean S. Oral inflammation, tooth loss, risk factors, and association with progression of Alzheimer’s disease. J Alzheimers Dis JAD. 2014;42:723–737. doi: 10.3233/JAD-140387. [DOI] [PubMed] [Google Scholar]
- 168.Pisa D, Alonso R, Juarranz A, Rábano A, Carrasco L. Direct visualization of fungal infection in brains from patients with Alzheimer’s disease. J Alzheimers Dis JAD. 2015;43:613–624. doi: 10.3233/JAD-141386. [DOI] [PubMed] [Google Scholar]
- 169.Pisa D, Alonso R, Rábano A, Rodal I, Carrasco L. Different Brain Regions are Infected with Fungi in Alzheimer’s Disease. Sci Rep. 2015;5:15015. doi: 10.1038/srep15015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Haorah J, Schall K, Ramirez SH, Persidsky Y. Activation of protein tyrosine kinases and matrix metalloproteinases causes blood-brain barrier injury: Novel mechanism for neurodegeneration associated with alcohol abuse. Glia. 2008;56:78–88. doi: 10.1002/glia.20596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Haorah J, Knipe B, Leibhart J, Ghorpade A, Persidsky Y. Alcohol-induced oxidative stress in brain endothelial cells causes blood-brain barrier dysfunction. J Leukoc Biol. 2005;78:1223–1232. doi: 10.1189/jlb.0605340. [DOI] [PubMed] [Google Scholar]
- 172.Kousik SM, Napier TC, Carvey PM. The Effects of Psychostimulant Drugs on Blood Brain Barrier Function and Neuroinflammation. Front Pharmacol. 2012;3 doi: 10.3389/fphar.2012.00121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Northrop NA, Yamamoto BK. Methamphetamine effects on blood-brain barrier structure and function. Front Neurosci. 2015;9:69. doi: 10.3389/fnins.2015.00069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.McConnell SEA, O’Banion MK, Cory-Slechta DA, Olschowka JA, Opanashuk LA. Characterization of binge-dosed methamphetamine-induced neurotoxicity and neuroinflammation. Neurotoxicology. 2015 doi: 10.1016/j.neuro.2015.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Polesskaya O, Silva J, Sanfilippo C, Desrosiers T, Sun A, Shen J, Feng C, Polesskiy A, Deane R, Zlokovic B, Kasischke K, Dewhurst S. Methamphetamine causes sustained depression in cerebral blood flow. Brain Res. 2011;1373:91–100. doi: 10.1016/j.brainres.2010.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Yin R, Lu C, Chen Q, Fan J, Lu J. Microvascular damage is involved in the pathogenesis of heroin induced spongiform leukoencephalopathy. Int J Med Sci. 2013;10:299–306. doi: 10.7150/ijms.4830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Wollman SC, Alhassoon OM, Stern MJ, Hall MG, Rompogren J, Kimmel CL, Perez-Figueroa AM. White matter abnormalities in long-term heroin users: a preliminary neuroimaging meta-analysis. Am J Drug Alcohol Abuse. 2015;41:133–138. doi: 10.3109/00952990.2014.985829. [DOI] [PubMed] [Google Scholar]
- 178.Chen YH, Chang M, Davidson BL. Molecular signatures of disease brain endothelia provide new sites for CNS-directed enzyme therapy. Nat Med. 2009;15:1215–1218. doi: 10.1038/nm.2025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Geoghegan JC, Keiser NW, Okulist A, Martins I, Wilson MS, Davidson BL. Chondroitin Sulfate is the Primary Receptor for a Peptide-Modified AAV That Targets Brain Vascular Endothelium In Vivo. Mol Ther Nucleic Acids. 2014;3:e202. doi: 10.1038/mtna.2014.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Sagare A, Deane R, Bell RD, Johnson B, Hamm K, Pendu R, Marky A, Lenting PJ, Wu Z, Zarcone T, Goate A, Mayo K, Perlmutter D, Coma M, Zhong Z, Zlokovic BV. Clearance of amyloid-beta by circulating lipoprotein receptors. Nat Med. 2007;13:1029–1031. doi: 10.1038/nm1635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Sagare AP, Bell RD, Srivastava A, Sengillo JD, Singh I, Nishida Y, Chow N, Zlokovic BV. A lipoprotein receptor cluster IV mutant preferentially binds amyloid-β and regulates its clearance from the mouse brain. J Biol Chem. 2013;288:15154–15166. doi: 10.1074/jbc.M112.439570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Choeiri C, Staines W, Miki T, Seino S, Messier C. Glucose transporter plasticity during memory processing. Neuroscience. 2005;130:591–600. doi: 10.1016/j.neuroscience.2004.09.011. [DOI] [PubMed] [Google Scholar]
- 183.Kalaria RN, Harik SI. Reduced glucose transporter at the blood-brain barrier and in cerebral cortex in Alzheimer disease. J Neurochem. 1989;53:1083–1088. doi: 10.1111/j.1471-4159.1989.tb07399.x. [DOI] [PubMed] [Google Scholar]
- 184.Shang W, Guo C, Zhao J, Yu X, Zhang H. Ginsenoside Rb1 upregulates expressions of GLUTs to promote glucose consumption in adiopcytes. China J Chin Mater Medica. 2014;39:4448–4452. [PubMed] [Google Scholar]
- 185.Gschanes A, Boado R, Sametz W, Windisch M. The drug cerebrolysin and its peptide fraction E021 increase the abundance of the blood-brain barrier GLUT1 glucose transporter in brains of young and old rats. Histochem J. 2000;32:71–77. doi: 10.1023/a:1004003008683. [DOI] [PubMed] [Google Scholar]
- 186.Galasko D, Bell J, Mancuso JY, Kupiec JW, Sabbagh MN, van Dyck C, Thomas RG, Aisen PS the A.D.C. Study. Clinical trial of an inhibitor of RAGE-Aβ interactions in Alzheimer disease. Neurology. 2014;82:1536–1542. doi: 10.1212/WNL.0000000000000364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Burstein AH, Grimes I, Galasko DR, Aisen PS, Sabbagh M, Mjalli AMM. Effect of TTP488 in patients with mild to moderate Alzheimer’s disease. BMC Neurol. 2014;14:12. doi: 10.1186/1471-2377-14-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Zlokovic BV, Griffin JH. Cytoprotective protein C pathways and implications for stroke and neurological disorders. Trends Neurosci. 2011;34:198–209. doi: 10.1016/j.tins.2011.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Griffin JH, Zlokovic BV, Mosnier LO. Activated protein C: biased for translation. Blood. 2015;125:2898–2907. doi: 10.1182/blood-2015-02-355974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Thiyagarajan M, Fernández JA, Lane SM, Griffin JH, Zlokovic BV. Activated protein C promotes neovascularization and neurogenesis in postischemic brain via protease-activated receptor 1. J Neurosci Off J Soc Neurosci. 2008;28:12788–12797. doi: 10.1523/JNEUROSCI.3485-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Guo H, Zhao Z, Yang Q, Wang M, Bell RD, Wang S, Chow N, Davis TP, Griffin JH, Goldman SA, Zlokovic BV. An activated protein C analog stimulates neuronal production by human neural progenitor cells via a PAR1-PAR3-S1PR1-Akt pathway. J Neurosci Off J Soc Neurosci. 2013;33:6181–6190. doi: 10.1523/JNEUROSCI.4491-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Cheng T, Liu D, Griffin JH, Fernández JA, Castellino F, Rosen ED, Fukudome K, Zlokovic BV. Activated protein C blocks p53-mediated apoptosis in ischemic human brain endothelium and is neuroprotective. Nat Med. 2003;9:338–342. doi: 10.1038/nm826. [DOI] [PubMed] [Google Scholar]
- 193.Cheng T, Petraglia AL, Li Z, Thiyagarajan M, Zhong Z, Wu Z, Liu D, Maggirwar SB, Deane R, Fernández JA, LaRue B, Griffin JH, Chopp M, Zlokovic BV. Activated protein C inhibits tissue plasminogen activator-induced brain hemorrhage. Nat Med. 2006;12:1278–1285. doi: 10.1038/nm1498. [DOI] [PubMed] [Google Scholar]
- 194.Winkler EA, Sengillo JD, Sagare AP, Zhao Z, Ma Q, Zuniga E, Wang Y, Zhong Z, Sullivan JS, Griffin JH, Cleveland DW, Zlokovic BV. Blood-spinal cord barrier disruption contributes to early motor-neuron degeneration in ALS-model mice. Proc Natl Acad Sci U S A. 2014;111:E1035–1042. doi: 10.1073/pnas.1401595111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Williams PD, Zlokovic BV, Griffin JH, Pryor KE, Davis TP. Preclinical safety and pharmacokinetic profile of 3K3A-APC, a novel, modified activated protein C for ischemic stroke. Curr Pharm Des. 2012;18:4215–4222. doi: 10.2174/138161212802430413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Lyden P, Levy H, Weymer S, Pryor K, Kramer W, Griffin JH, Davis TP, Zlokovic B. Phase 1 safety, tolerability and pharmacokinetics of 3K3A-APC in healthy adult volunteers. Curr Pharm Des. 2013;19:7479–7485. doi: 10.2174/1381612819666131230131454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Gallay PA, Bobardt MD, Chatterji U, Trepanier DJ, Ure D, Ordonez C, Foster R. The Novel Cyclophilin Inhibitor CPI-431-32 Concurrently Blocks HCV and HIV-1 Infections via a Similar Mechanism of Action. PloS One. 2015;10:e0134707. doi: 10.1371/journal.pone.0134707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Yan W, Qing J, Mei H, Mao F, Huang J, Zhu J, Jiang H, Liu L, Zhang L, Li J. Discovery of Novel Small Molecule Anti-HCV Agents via the CypA Inhibitory Mechanism Using O-Acylation-Directed Lead Optimization. Mol Basel Switz. 2015;20:10342–10359. doi: 10.3390/molecules200610342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Hopkins S, Bobardt M, Chatterji U, Garcia-Rivera JA, Lim P, Gallay PA. The cyclophilin inhibitor SCY-635 disrupts hepatitis C virus NS5A-cyclophilin A complexes. Antimicrob Agents Chemother. 2012;56:3888–3897. doi: 10.1128/AAC.00693-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Heinzmann D, Bangert A, Müller A-M, von Ungern-Sternberg SNI, Emschermann F, Schönberger T, Chatterjee M, Mack AF, Klingel K, Kandolf R, Malesevic M, Borst O, Gawaz M, Langer HF, Katus H, Fischer G, May AE, Kaya Z, Seizer P. The Novel Extracellular Cyclophilin A (CyPA) - Inhibitor MM284 Reduces Myocardial Inflammation and Remodeling in a Mouse Model of Troponin I -Induced Myocarditis. PloS One. 2015;10:e0124606. doi: 10.1371/journal.pone.0124606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Yan P, Zhu A, Liao F, Xiao Q, Kraft AW, Gonzales E, Perez R, Greenberg SM, Holtzman DM, Lee J-M. Minocycline reduces spontaneous hemorrhage in mouse models of cerebral amyloid angiopathy. Stroke J Cereb Circ. 2015;46:1633–1640. doi: 10.1161/STROKEAHA.115.008582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Kundu P, De R, Pal I, Mukhopadhyay AK, Saha DR, Swarnakar S. Curcumin alleviates matrix metalloproteinase-3 and -9 activities during eradication of Helicobacter pylori infection in cultured cells and mice. PloS One. 2011;6:e16306. doi: 10.1371/journal.pone.0016306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Lee M, Chen Z, Tomlinson BN, Gooyit M, Hesek D, Juárez MR, Nizam R, Boggess B, Lastochkin E, Schroeder VA, Wolter WR, Suckow MA, Cui J, Mobashery S, Gu Z, Chang M. Water-Soluble MMP-9 Inhibitor Reduces Lesion Volume after Severe Traumatic Brain Injury. ACS Chem Neurosci. 2015 doi: 10.1021/acschemneuro.5b00140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Kawatani M, Fukushima Y, Kondoh Y, Honda K, Sekine T, Yamaguchi Y, Taniguchi N, Osada H. Identification of matrix metalloproteinase inhibitors by chemical arrays. Biosci Biotechnol Biochem. 2015;79:1597–1602. doi: 10.1080/09168451.2015.1045829. [DOI] [PubMed] [Google Scholar]
- 205.Yang Y, Salayandia VM, Thompson JF, Yang LY, Estrada EY, Yang Y. Attenuation of acute stroke injury in rat brain by minocycline promotes blood-brain barrier remodeling and alternative microglia/macrophage activation during recovery. J Neuroinflammation. 2015;12:26. doi: 10.1186/s12974-015-0245-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Yang F, Zhou L, Wang D, Wang Z, Huang Q-Y. Minocycline ameliorates hypoxia-induced blood-brain barrier damage by inhibition of HIF-1α through SIRT-3/PHD-2 degradation pathway. Neuroscience. 2015;304:250–259. doi: 10.1016/j.neuroscience.2015.07.051. [DOI] [PubMed] [Google Scholar]
- 207.Ando H, Zhou J, Macova M, Imboden H, Saavedra JM. Angiotensin II AT1 receptor blockade reverses pathological hypertrophy and inflammation in brain microvessels of spontaneously hypertensive rats. Stroke J Cereb Circ. 2004;35:1726–1731. doi: 10.1161/01.STR.0000129788.26346.18. [DOI] [PubMed] [Google Scholar]
- 208.Liu W, Yamashita T, Kurata T, Kono S, Hishikawa N, Deguchi K, Zhai Y, Abe K. Protective effect of telmisartan on neurovascular unit and inflammasome in stroke-resistant spontaneously hypertensive rats. Neurol Res. 2015;37:491–501. doi: 10.1179/1743132815Y.0000000002. [DOI] [PubMed] [Google Scholar]
- 209.Wang J, Ho L, Chen L, Zhao Z, Zhao W, Qian X, Humala N, Seror I, Bartholomew S, Rosendorff C, Pasinetti GM. Valsartan lowers brain beta-amyloid protein levels and improves spatial learning in a mouse model of Alzheimer disease. J Clin Invest. 2007;117:3393–3402. doi: 10.1172/JCI31547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Hajjar I, Brown L, Mack WJ, Chui H. Impact of Angiotensin receptor blockers on Alzheimer disease neuropathology in a large brain autopsy series. Arch Neurol. 2012;69:1632–1638. doi: 10.1001/archneurol.2012.1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Schneider P, Buerger K, Teipel S, Uspenskaya O, Hartmann O, Hansson O, Minthon L, Rujescu D, Moeller H-J, Zetterberg H, Blennow K, Ernst A, Bergmann A, Hampel H. Antihypertensive therapy is associated with reduced rate of conversion to Alzheimer’s disease in midregional proatrial natriuretic peptide stratified subjects with mild cognitive impairment. Biol Psychiatry. 2011;70:145–151. doi: 10.1016/j.biopsych.2011.01.036. [DOI] [PubMed] [Google Scholar]
- 212.Liu S, Marder VJ, Levy DE, Wang S-J, Yang F, Paganini-Hill A, Fisher MJ. Ancrod and fibrin formation: perspectives on mechanisms of action. Stroke J Cereb Circ. 2011;42:3277–3280. doi: 10.1161/STROKEAHA.111.622753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Akassoglou K, Adams RA, Bauer J, Mercado P, Tseveleki V, Lassmann H, Probert L, Strickland S. Fibrin depletion decreases inflammation and delays the onset of demyelination in a tumor necrosis factor transgenic mouse model for multiple sclerosis. Proc Natl Acad Sci U S A. 2004;101:6698–6703. doi: 10.1073/pnas.0303859101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Sherman DG, Atkinson RP, Chippendale T, Levin KA, Ng K, Futrell N, Hsu CY, Levy DE. Intravenous ancrod for treatment of acute ischemic stroke: the STAT study: a randomized controlled trial Stroke Treatment with Ancrod Trial. JAMA. 2000;283:2395–2403. doi: 10.1001/jama.283.18.2395. [DOI] [PubMed] [Google Scholar]
- 215.Levy DE, del Zoppo GJ, Demaerschalk BM, Demchuk AM, Diener H-C, Howard G, Kaste M, Pancioli AM, Ringelstein EB, Spatareanu C, Wasiewski WW. Ancrod in acute ischemic stroke: results of 500 subjects beginning treatment within 6 hours of stroke onset in the ancrod stroke program. Stroke J Cereb Circ. 2009;40:3796–3803. doi: 10.1161/STROKEAHA.109.565119. [DOI] [PubMed] [Google Scholar]
- 216.Brickman AM, Khan UA, Provenzano FA, Yeung L-K, Suzuki W, Schroeter H, Wall M, Sloan RP, Small SA. Enhancing dentate gyrus function with dietary flavanols improves cognition in older adults. Nat Neurosci. 2014;17:1798–1803. doi: 10.1038/nn.3850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Morris MC, Tangney CC, Wang Y, Sacks FM, Barnes LL, Bennett DA, Aggarwal NT. MIND diet slows cognitive decline with aging. Alzheimers Dement J Alzheimers Assoc. 2015 doi: 10.1016/j.jalz.2015.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Hsu TM, Kanoski SE. Blood-brain barrier disruption: mechanistic links between Western diet consumption and dementia. Front Aging Neurosci. 2014;6:88. doi: 10.3389/fnagi.2014.00088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Kanoski SE, Zhang Y, Zheng W, Davidson TL. The effects of a high-energy diet on hippocampal function and blood-brain barrier integrity in the rat. J Alzheimers Dis JAD. 2010;21:207–219. doi: 10.3233/JAD-2010-091414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Takechi R, Pallebage-Gamarallage MM, Lam V, Giles C, Mamo JC. Aging-related changes in blood-brain barrier integrity and the effect of dietary fat. Neurodegener Dis. 2013;12:125–135. doi: 10.1159/000343211. [DOI] [PubMed] [Google Scholar]
- 221.Takechi R, Galloway S, Pallebage-Gamarallage MM, Lam V, Dhaliwal SS, Mamo JC. Probucol prevents blood-brain barrier dysfunction in wild-type mice induced by saturated fat or cholesterol feeding. Clin Exp Pharmacol Physiol. 2013;40:45–52. doi: 10.1111/1440-1681.12032. [DOI] [PubMed] [Google Scholar]
- 222.Saeed AA, Genové G, Li T, Lütjohann D, Olin M, Mast N, Pikuleva IA, Crick P, Wang Y, Griffiths W, Betsholtz C, Björkhem I. Effects of a disrupted blood-brain barrier on cholesterol homeostasis in the brain. J Biol Chem. 2014;289:23712–23722. doi: 10.1074/jbc.M114.556159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Popp J, Lewczuk P, Kölsch H, Meichsner S, Maier W, Kornhuber J, Jessen F, Lütjohann D. Cholesterol metabolism is associated with soluble amyloid precursor protein production in Alzheimer’s disease. J Neurochem. 2012;123:310–316. doi: 10.1111/j.1471-4159.2012.07893.x. [DOI] [PubMed] [Google Scholar]
- 224.Marambaud P, Zhao H, Davies P. Resveratrol promotes clearance of Alzheimer’s disease amyloid-beta peptides. J Biol Chem. 2005;280:37377–37382. doi: 10.1074/jbc.M508246200. [DOI] [PubMed] [Google Scholar]
- 225.Shin JA, Oh S, Ahn J-H, Park E-M. Estrogen receptor-mediated resveratrol actions on blood-brain barrier of ovariectomized mice. Neurobiol Aging. 2015;36:993–1006. doi: 10.1016/j.neurobiolaging.2014.09.024. [DOI] [PubMed] [Google Scholar]
- 226.Wei H, Wang S, Zhen L, Yang Q, Wu Z, Lei X, Lv J, Xiong L, Xue R. Resveratrol attenuates the blood-brain barrier dysfunction by regulation of the MMP-9/TIMP-1 balance after cerebral ischemia reperfusion in rats. J Mol Neurosci MN. 2015;55:872–879. doi: 10.1007/s12031-014-0441-1. [DOI] [PubMed] [Google Scholar]
- 227.Kim YH, Kim YS, Roh GS, Choi WS, Cho GJ. Resveratrol blocks diabetes-induced early vascular lesions and vascular endothelial growth factor induction in mouse retinas. Acta Ophthalmol (Copenh) 2012;90:e31–37. doi: 10.1111/j.1755-3768.2011.02243.x. [DOI] [PubMed] [Google Scholar]
- 228.Jing Y-H, Chen K-H, Yang S-H, Kuo P-C, Chen J-K. Resveratrol ameliorates vasculopathy in STZ-induced diabetic rats: role of AGE-RAGE signalling. Diabetes Metab Res Rev. 2010;26:212–222. doi: 10.1002/dmrr.1076. [DOI] [PubMed] [Google Scholar]
- 229.Zhao H, Niu Q, Li X, Liu T, Xu Y, Han H, Wang W, Fan N, Tian Q, Zhang H, Wang Z. Long-term resveratrol consumption protects ovariectomized rats chronically treated with D-galactose from developing memory decline without effects on the uterus. Brain Res. 2012;1467:67–80. doi: 10.1016/j.brainres.2012.05.040. [DOI] [PubMed] [Google Scholar]
- 230.Stonehouse W, Conlon CA, Podd J, Hill SR, Minihane AM, Haskell C, Kennedy D. DHA supplementation improved both memory and reaction time in healthy young adults: a randomized controlled trial. Am J Clin Nutr. 2013;97:1134–1143. doi: 10.3945/ajcn.112.053371. [DOI] [PubMed] [Google Scholar]
- 231.Nguyen LN, Ma D, Shui G, Wong P, Cazenave-Gassiot A, Zhang X, Wenk MR, Goh ELK, Silver DL. Mfsd2a is a transporter for the essential omega-3 fatty acid docosahexaenoic acid. Nature. 2014;509:503–506. doi: 10.1038/nature13241. [DOI] [PubMed] [Google Scholar]
- 232.Fonteh AN, Cipolla M, Chiang J, Arakaki X, Harrington MG. Human cerebrospinal fluid fatty acid levels differ between supernatant fluid and brain-derived nanoparticle fractions, and are altered in Alzheimer’s disease. PloS One. 2014;9:e100519. doi: 10.1371/journal.pone.0100519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Ben-Zvi A, Lacoste B, Kur E, Andreone BJ, Mayshar Y, Yan H, Gu C. Mfsd2a is critical for the formation and function of the blood-brain barrier. Nature. 2014;509:507–511. doi: 10.1038/nature13324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Zhao Z, Zlokovic BV. Blood-brain barrier: a dual life of MFSD2A? Neuron. 2014;82:728–730. doi: 10.1016/j.neuron.2014.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Vandal M, Alata W, Tremblay C, Rioux-Perreault C, Salem N, Calon F, Plourde M. Reduction in DHA transport to the brain of mice expressing human APOE4 compared to APOE2. J Neurochem. 2014;129:516–526. doi: 10.1111/jnc.12640. [DOI] [PubMed] [Google Scholar]
- 236.Defina LF, Willis BL, Radford NB, Gao A, Leonard D, Haskell WL, Weiner MF, Berry JD. The association between midlife cardiorespiratory fitness levels and later-life dementia: a cohort study. Ann Intern Med. 2013;158:162–168. doi: 10.7326/0003-4819-158-3-201302050-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Choi JK, Moon KM, Jung SY, Kim JY, Choi SH, Kim DY, Kang S, Chu CW, Kwon SM. Regular exercise training increases the number of endothelial progenitor cells and decreases homocysteine levels in healthy peripheral blood. Korean J Physiol Pharmacol Off J Korean Physiol Soc Korean Soc Pharmacol. 2014;18:163–168. doi: 10.4196/kjpp.2014.18.2.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.de Senna PN, Xavier LL, Bagatini PB, Saur L, Galland F, Zanotto C, Bernardi C, Nardin P, Gonçalves CA, Achaval M. Physical training improves non-spatial memory, locomotor skills and the blood brain barrier in diabetic rats. Brain Res. 2015;1618:75–82. doi: 10.1016/j.brainres.2015.05.026. [DOI] [PubMed] [Google Scholar]
- 239.Lazarov O, Robinson J, Tang Y-P, Hairston IS, Korade-Mirnics Z, Lee VM-Y, Hersh LB, Sapolsky RM, Mirnics K, Sisodia SS. Environmental enrichment reduces Abeta levels and amyloid deposition in transgenic mice. Cell. 2005;120:701–713. doi: 10.1016/j.cell.2005.01.015. [DOI] [PubMed] [Google Scholar]
- 240.Ambrée O, Leimer U, Herring A, Görtz N, Sachser N, Heneka MT, Paulus W, Keyvani K. Reduction of amyloid angiopathy and Abeta plaque burden after enriched housing in TgCRND8 mice: involvement of multiple pathways. Am J Pathol. 2006;169:544–552. doi: 10.2353/ajpath.2006.051107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Prince M, Albanese E, Guerchet M, Prina M. World Alzheimer Report 2014: dementia and risk reduction: an analysis of protective and modifiable factors. Alzheimers Dis Int. 2014:1–99. [Google Scholar]
- 242.Herring A, Yasin H, Ambrée O, Sachser N, Paulus W, Keyvani K. Environmental enrichment counteracts Alzheimer’s neurovascular dysfunction in TgCRND8 mice. Brain Pathol Zurich Switz. 2008;18:32–39. doi: 10.1111/j.1750-3639.2007.00094.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Wolff G, Davidson SJ, Wrobel JK, Toborek M. Exercise maintains blood-brain barrier integrity during early stages of brain metastasis formation. Biochem Biophys Res Commun. 2015;463:811–817. doi: 10.1016/j.bbrc.2015.04.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Alakbarzade V, Hameed A, Quek DQY, Chioza BA, Baple EL, Cazenave-Gassiot A, Nguyen LN, Wenk MR, Ahmad AQ, Sreekantan-Nair A, Weedon MN, Rich P, Patton MA, Warner TT, Silver DL, Crosby AH. A partially inactivating mutation in the sodium-dependent lysophosphatidylcholine transporter MFSD2A causes a non-lethal microcephaly syndrome. Nat Genet. 2015;47:814–817. doi: 10.1038/ng.3313. [DOI] [PubMed] [Google Scholar]
- 245.Guemez-Gamboa A, Nguyen LN, Yang H, Zaki MS, Kara M, Ben-Omran T, Akizu N, Rosti RO, Rosti B, Scott E, Schroth J, Copeland B, Vaux KK, Cazenave-Gassiot A, Quek DQY, Wong BH, Tan BC, Wenk MR, Gunel M, Gabriel S, et al. Inactivating mutations in MFSD2A, required for omega-3 fatty acid transport in brain, cause a lethal microcephaly syndrome. Nat Genet. 2015;47:809–813. doi: 10.1038/ng.3311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Keller A, Westenberger A, Sobrido MJ, García-Murias M, Domingo A, Sears RL, Lemos RR, Ordoñez-Ugalde A, Nicolas G, da Cunha JEG, Rushing EJ, Hugelshofer M, Wurnig MC, Kaech A, Reimann R, Lohmann K, Dobričić V, Carracedo A, Petrović I, Miyasaki JM, et al. Mutations in the gene encoding PDGF-B cause brain calcifications in humans and mice. Nat Genet. 2013;45:1077–1082. doi: 10.1038/ng.2723. [DOI] [PubMed] [Google Scholar]