Summary
In most species, females time reproduction to coincide with fertility. Thus, identifying factors that signal fertility to the brain can provide access to neural circuits that control sexual behaviors. In vertebrates, levels of key signaling molecules rise at the time of fertility to prime the brain for reproductive behavior [1–11], but how and where they regulate neural circuits is not known [12, 13]. Specifically, 17α,20β-dihydroxyprogesterone (DHP) and prostaglandin F2α (PGF2α) levels rise in teleost fish around the time of ovulation [10, 14, 15]. In an African cichlid fish, Astatotilapia burtoni, fertile females select a mate and perform a stereotyped spawning routine, offering quantifiable behavioral outputs of neural circuits. We show that within minutes, PGF2α injection activates a naturalistic pattern of sexual behavior in female A. burtoni. We also identify cells in the brain that transduce a prostaglandin signal to mate, and show that the gonadal steroid DHP modulates mRNA levels of the putative receptor for PGF2α (Ptgfr). We use CRISPR/Cas9 to generate the first targeted gene mutation in A. burtoni, and show that Ptgfr is necessary for the initiation of sexual behavior, uncoupling sexual behavior from reproductive status. Our findings are consistent with a model in which PGF2α communicates fertility status via Ptgfr to circuits in the brain that drive female sexual behavior. Our targeted genome modification in a cichlid fish shows that dissection of gene function can reveal basic control mechanisms for behaviors in this large family of species with diverse and fascinating social systems [16, 17].
Results
We sought to understand the control of the complex spawning behavioral routine in a cichlid fish, A. burtoni. In this species, the male dramatically displays his body coloration to a female while quivering vigorously, then attempts to lead her back to his territory (Figure 1A, Movie S1). If the female is ready to spawn, she follows him into his spawning site and pecks at egg-like spots on the anal fin of the male as he quivers in front of her. She then lays eggs and immediately collects them from the substrate into her mouth. As she searches for more eggs, she pecks again at egg spots near the site of sperm release from the male, fertilizing the eggs. The male and female circle around one another several times, repeating these behaviors in sequence. The female then carries the embryos in her mouth for ~2 weeks as they develop.
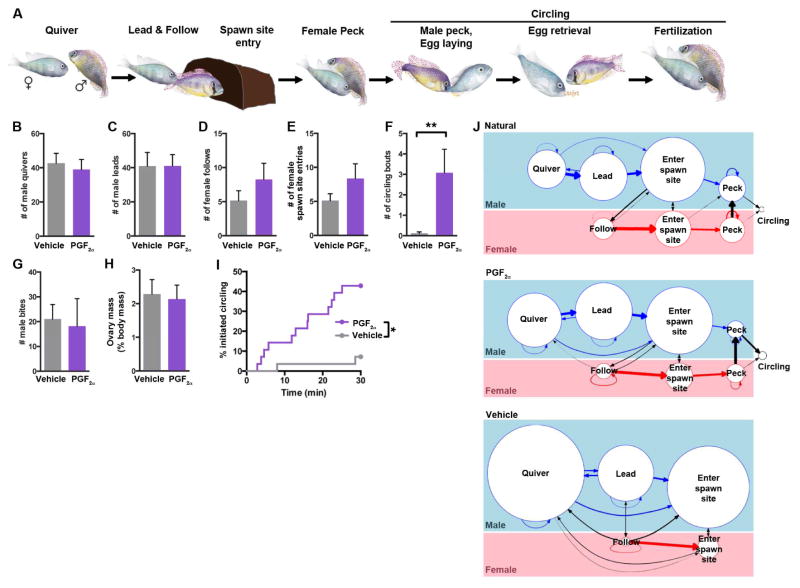
Figure 1. PGF2α activates female reproductive behavior in A. burtoni.
a, Natural progression of spawning behavior. After ovulation, females lay eggs during circling. B–I, Analysis of all reproductive assays. B, C, G, Males quiver, lead, and attack similarly with vehicle and PGF2α-injected females. D–F, I, Vehicle- and PGF2α-injected females show similar following and pot entry (D–E), but females show circling behavior more rapidly and frequently after PGF2α injection (F, I). H, Body weight-normalized ovary mass does not differ between groups. *, P = 0.0018, Mantel-Cox test; **, P = 0.0010, Mann-Whitney U-test. Mean ± SEM. n = 28 females per treatment. J, Ethograms reveal that naturally spawning and a reproductive subset of PGF2α-injected females exhibit quantitatively similar transitions between reproductive behaviors. Vehicle-treated females were matched for normalized ovary mass, but rarely circle. Diameters of circles are proportional to count of each behavior; weights of arrows are proportional to fraction followed by second behavior. Transitional probabilities do not differ between naturally spawning and PGF2α-injected females; all transitions P > 0.05, Mann-Whitney U-test. n = 5 assays per group. See also Figure S1; Movies S1, S2.
We first asked whether this spawning routine could be elicited by PGF2α, a factor whose titers rise in the fertile female fish [18]. Within 30 minutes of intraperitoneal PGF2α injection into females visually identified as non-fertile, they exhibited behavior quite similar to natural spawning behavior, whereas vehicle-injected controls rarely showed the full sequelae of reproductive behaviors (PGF2α-injected, 43% circled; vehicle-treated, 7% circled; n = 28/group; P = 0.0043, Fisher’s Exact Test) (Figure S1A, Movie S2). Females performed all behavioral sequences typical of reproductive behavior except egg laying, since we selected only fish that had not ovulated. Differences between PGF2α- and vehicle-injected fish cannot be ascribed to differences in male behavior or reproductive stage (assessed by ovary mass), as these parameters did not differ between assays with PGF2α- and vehicle-injected females (Figures 1B, 1C, 1G, 1H). Female following and spawning site entry was not significantly increased (Figures 1D, 1E), implying that PGF2α promotes the final stage of spawning behavior, circling (Figure 1F). We compared the behavior of naturally spawning females to a subset of PGF2α-injected females that exhibited comparable levels of reproductive behaviors by testing the frequency of transitions between behaviors. We found that PGF2α-injected females were similar to naturally spawning females in frequency and ordering of reproductive behaviors (Figure 1J). Thus, the behavioral sequelae elicited by PGF2α are very similar to those of naturally spawning females, but are performed outside the time of fertility.
This rapid generation of a complex behavior that we and others [8, 11] observe in cichlids led us to seek the mechanism of action for PGF2α at the genetic and neural levels. We identified a family of 11 putative prostaglandin G protein-coupled receptors in the A. burtoni genome and show that only a single receptor forms a monophyletic clade with PGF2α receptors (Ptgfr) from other vertebrate genomes (Figure S2A). This A. burtoni G protein-coupled receptor has conserved residues for PGF2α signaling, and elements of synteny are maintained from cichlid to human (Figures S2B, S2C), suggesting it is the sole Ptgfr ortholog, and has maintained PGF2α signaling capability.
A prior study in goldfish found that PGF2α injection directly into the brain is more potent than systemic injection for eliciting reproductive behavior, and that ovariectomized females spawn in response to PGF2α [19]. Together these results indicate that PGF2α acts on target(s) in the brain. Therefore, we localized cells expressing Ptgfr in the brain using in situ hybridization (ISH) and found expression in only four regions (Figures 2A–2B, S3A–S3D): the preoptic area (POA), a region implicated in sexual behavior across vertebrates [20]; the lateral tubular nucleus (NLT), a suggested homolog of the mammalian arcuate nucleus [21]; the vagal lobe (VL), a region that communicates with the internal viscera and controls mouth movements [22] but has not previously been implicated in female reproduction; and the dorsal compartment of the ventral telencephalon (Vd), a subpallial structure with no known function [23].
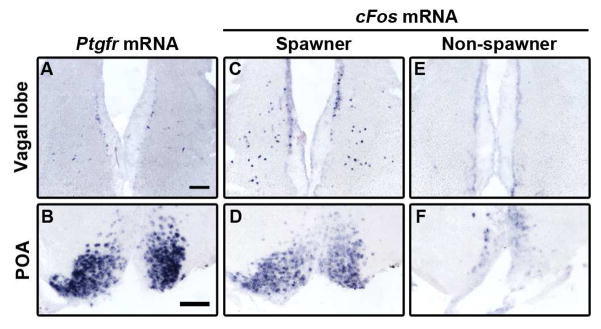
Figure 2. Ptgfr is expressed in regions active during spawning, and rises at the time of spawning.
A, B, Ptgfr mRNA is expressed in the POA and in scattered cells of the VL. C–H, POA and VL express cFos mRNA after being allowed to spawn naturally when exposed to a male (C, D). Females exposed to a courting male that did not spawn did not show cFos expression (E, F). Scale bars, 100 μm; n = 6–11 per group. See also Figures S2, S3; Table S1.
Since individual neurons activated during female spawning behavior have not been previously identified, we asked whether these brain regions were active during spawning by using cFos mRNA expression, a proxy for recent neural activity. We allowed uninjected females to interact with a courting male and compared cFos expression in females that spawned to those that did not. Cells in the POA and VL exhibited greater cFos expression in females that had spawned naturally when exposed to males compared to females that did not spawn (POA, 2.4-fold increase in spawning females, P = 0.012, Mann-Whitney test; VL, only had cFos expression in spawners; n ≥ 7/group) (Figure 2). NLT was cFos+ in females exposed to males regardless of spawning behavior (Figures S3I–S3L), but we did not detect a robust cFos induction in Vd in any fish (data not shown). Taken together, these results indicate that Ptgfr+ regions POA and VL play a role in reproductive behavior, while Ptgfr in Vd and NLT likely are involved in non-spawning behaviors.
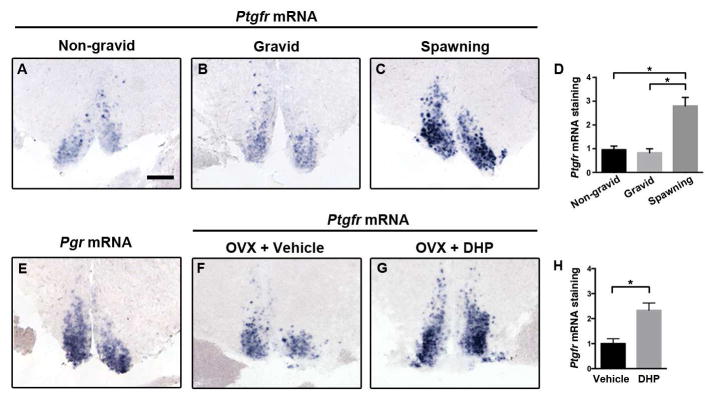
Ptgfr expression varied systematically across the reproductive cycle, with Ptgfr mRNA staining increased ~3-fold in the POA of females that had just spawned naturally, relative to females with small or large ovaries earlier in the reproductive cycle (Figures 3A–3D), indicating that its expression is enhanced only briefly around the time of spawning. The teleost progestin, 17α,20β-dihydroxyprogesterone (DHP), promotes final maturation and ovulation of oocytes prior to spawning in most teleosts including cichlids [15, 24, 25]. We found that the progesterone receptor (Pgr) is present in the female POA (Figure 3E). To test the role of this signaling pathway in Ptgfr expression, we treated ovariectomized females with either DHP or vehicle, and measured Ptgfr mRNA in the POA after 3 hours. DHP treatment caused an increase in Ptgfr mRNA in the POA similar to that observed in naturally spawning females (Figures 3F–3H), suggesting that Pgr signaling at ovulation increases sensitivity to PGF2α, promoting spawning behavior.
Figure 3. Progestin signaling upregulates Ptgfr mRNA expression in POA.
A–D, Ptgfr mRNA levels rise in the POA around the time of spawning. Females with small (A) or large (B) ovary mass but that did not spawn had less Ptgfr mRNA expression than females that spawned 30 minutes prior (C). *, P = 0.0009 by Kruskal-Wallis test and p < 0.05 by Dunn’s post-hoc test; n = 6–11 females per group. E, Progesterone receptor is expressed in the Ptgfr+ compartment of the POA. F–H, Treatment of ovariectomized (OVX) females with 17α,20β-dihydroxyprogesterone (DHP) results in a rise in Ptgfr mRNA levels. *, P = 0.0286 by Mann-Whitney test; n = 4 females/group. Mean ± SEM; scale bar, 100 μm.
To directly test the role of Ptgfr in spawning behavior, we generated A. burtoni carrying mutant Ptgfr using the CRISPR/Cas9 system [26]. We injected single-cell embryos with Cas9 mRNA [27, 28] and a single guide RNA targeting the second transmembrane domain of Ptgfr (Figures 4A–4B). We raised injected embryos to adulthood, separated them by sex, and housed them with WT fish of the opposite sex. We found that none of the injected female G0 fish produced offspring (n = 9 females). When we sequenced finclip genomic DNA from injected females, we found that CRISPR/Cas9 is efficient in A. burtoni: sequencing of genomic DNA from 20/22 (91%) injected fish exhibited extensive modification of the Ptgfr locus (Figure S4). Thus, CRISPR/Cas9 can be used to cause indels in A. burtoni, enabling the rapid detection phenotyping of mutant cichlids.
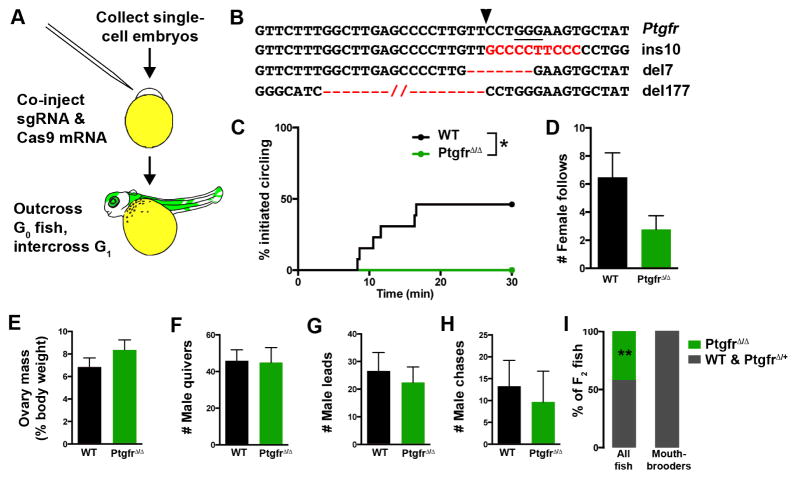
Figure 4. CRISPR/Cas9-mediated mutation of Ptgfr results in failure to spawn.
A, Schematic for generation of biallelic Ptgfr mutants. sgRNA, single guide RNA. B, Three Ptgfr alleles encoding a large deletion or frameshift mutations were analyzed in F1 females. The protospacer-adjacent motif is underlined, and Cas9 cut site indicated by arrowhead. C–D, PtgfrΔ/Δ females did not initiate circling behavior in response to PGF2α injection *, P = 0.031, Mantel-Cox test; n = 8–13 females/genotype. e, Ovary size was not different from WT males. F–H, males did not show different levels of courtship (F, G) or aggression (H) toward PtgfrΔ/Δ females. Mean ± SEM. I, Group-housed PtgfrΔ/Δ females were not found to carry offspring despite comprising 42% of the population. **, P < 0.0001, Fisher’s exact test, n = 93 group housed fish, n = 31 mouthbrooders. See also Figure S4, Table S2.
CRISPR/Cas9 has been suggested to cause low rates of off-target mutations [28], so we outcrossed G0 males to WT females to isolate the effect of targeted mutations. Ptgfr mutant males produced numerous broods with WT females. This contrast with Ptgfr mutant females suggested a sex-specific role for Ptgfr in spawning. Half of G1 offspring (49%) carried Ptgfr indel alleles, indicating numerous mutant cells in the germline (n = 92 fish). These G1 offspring carried a variety of indel alleles, so we selectively propagated those predicted to result in a loss of function (i.e. two frameshift and a 177 bp deletion). We intercrossed non-sibling G1 fish to obtain biallelic Ptgfr mutants (PtgfrΔ/Δ). These crosses transmitted modified Ptgfr alleles at expected frequencies, and PtgfrΔ/Δ fish are viable (Table S1). To assess whether PtgfrΔ/Δ females would reproduce naturally, we collected fin clips from females observed carrying broods. PCR amplification and sequencing revealed that no mouthbrooding females were PtgfrΔ/Δ (Figure 4I).
Although these results indicate that Ptgfr is critical for reproduction, we asked whether Ptgfr mutant females would perform complete spawning behavior routines. We injected these females and their WT siblings with PGF2α and paired each with a WT singly-housed male. PtgfrΔ/Δ females never exhibited the circling behavior typical of their WT siblings (P = 0.04, Fisher’s Exact test; n = 8–13/genotype), though they did perform the initial components of the routine (Figure 4D). These phenotypes were not a result of differential courtship by males or female size, and ovary mass indicated females had high GSI (Figures 4E–4H). Thus, PGF2α signaling through Ptgfr is necessary for the final stages of reproductive behavior, and there appears to be no redundancy in signaling pathways that activate spawning.
Discussion
Our results show that PGF2α signaling is necessary and sufficient to induce the final stages of reproductive behavior in cichlid fish, and we have identified regions in the brain likely important for generating this behavior. Although the neural circuit for spawning requires Ptgfr, other factors must modulate its action. For example, we found that PGF2α robustly activated spawning behavior in 8/10 WT females with larger ovaries (gonadosomatic index > 1.4), but 0/7 females with smaller ovaries (Figure S1B), suggesting that some factor(s) other than DHP inhibits sexual behavior after recent egg laying. PGF2α-insensitive periods have been observed later in the reproductive cycle in the paradise fish [10], though PGF2α appears uniformly effective in other species [11, 29]. Additionally, Ptgfr mRNA levels in the POA rise in A. burtoni during a separate short time window around spawning, suggesting that Ptgfr+ neurons become more sensitive to PGF2α when females are fertile.
We propose that individual Ptgfr+ regions control discrete aspects of female reproduction. The POA has been implicated in sexual behavior across vertebrates [20, 30, 31], and the expression of Ptgfr mRNA in this region is consistent with a role in spawning behavior. Given the known roles of the VL, Ptgfr mRNA expression here may identify neurons that are important for the initiation of mouthbrooding, or for receiving signals from viscera including the reproductive tract. Accordingly, females that exhibit circling behavior subsequent to PGF2α injection do not show cFos mRNA in the VL (data not shown), implying that egg laying and/or mouthbrooding is controlled by these neurons. Are these regions activated simultaneously to drive reproductive behavior, or might one region be the site at which the PGF2α signal from the periphery acts? Cells in the POA could be accessible to circulating prostaglandins, similar to mammals [32]. In an alternative model, fertility signals from the reproductive tract could be communicated to the brain via another signal, triggering the neural synthesis of PGF2α. One candidate for such a mediator is the vagus nerve, which innervates the viscera and communicates with the VL. A comparison of Ptgfr expression patterns across brain regions in species with divergent behavioral patterns may highlight the presence or absence of Ptgfr+ neuronal subsets, allowing inferences about specific functions for individual populations. The development of CRISPR/Cas9 in cichlids will allow tests of such hypotheses using cell-type specific gene modifications.
In mammals, PGF2α promotes both the onset of labor and maternal behavior [2, 6]. Our data, taken together with results from other vertebrates including ovoviviparous fish [33], suggests that PGF2α signaling has an ancestral function linking the release of offspring or eggs from the reproductive tract with the appropriate behavior. In mammals, for which sexual behavior is temporally dissociated from parturition, either progesterone or prostaglandin E2 (PGE2) is sufficient to drive female sexual behavior after priming by estradiol [1, 4, 34, 35]. PGE2 signaling therefore may act in a signaling pathway that anticipates ovulation, in order to time sexual behavior with fertility [29]. This function could result from a gain of expression of a PGE2 receptor in an ancestral mating circuit, or from co-opting of PGE2-sensitive cells into a mating circuit. Future experiments may reveal a conserved pattern of gene expression in cells that regulate mating in mammals [36, 37] and fish.
Given the remarkable diversity in reproductive behaviors among the ~1500 known cichlid species [17], genetically modified cichlids [38] have the potential to test the function of specific genes, neurons, and hormones that control social behaviors and to reveal their evolutionary trajectories. Furthermore, since the prolific speciation of East African cichlids is postulated to result from sexual selection [39], Ptgfr+ cells are a crucial component of a circuit through which females select a partner and initiate mating. Thus, mapping the inputs and outputs of these cells will permit an understanding of how females select a mate and ultimately shape evolution.
Experimental Procedures
Fish were bred and used at Stanford University from a colony derived from Lake Tanganyika [16] in accordance with AAALAC standards.
Non-gravid females were identified by absence of abdominal distension due to ovary size and injected intraperitoneally with PGF2α (~1.5 μg per g body; Cayman) or vehicle. Females were introduced into a male’s tank immediately after injection. Behaviors were video recorded for 30 minutes. Naturally spawning females in Figures 2 and S3 were collected by allowing a male access to uninjected females. Brains were dissected 30 minutes after observing spawning, and simultaneously from a control female from the same tank that did not spawn. Behavior assays were coded by an observer blind to treatment using custom MATLAB software [40].
Adult female A. burtoni were allowed to recover for 1–2 weeks after OVX, then were injected intraperitoneally with DHP (125 ng/g body weight; Sigma-Aldrich) or DMSO/saline vehicle. Brains were collected at 3 hours post-injection.
Raster plots and transitional probabilities were generated using a custom software package in R (http://fernaldlab.stanford.edu/resources). We used Mann-Whitney U-tests for two-group comparisons of continuous data and Fisher’s Exact test for categorical data. Transitional probabilities were calculated by dividing the total number of each behavior by the number of instances in which the subsequent behavior occurred. Arrow weights in Figure 1J are only shown for transitions with probability ≥4%. We selected 5 PGF2α-injected females with a similar number of circling bouts to compare with 5 naturally spawning females, and matched 5 vehicle-injected females by their comparable GSI. We used Mann-Whitney U-tests to compare transition probabilities across groups, with a Bonferroni-corrected cutoff of α=0.0027 to correct for 19 transitions we observed in Figure 1J.
For ISH, we subcloned Ptgfr and cFos (NM_001286320), into pCR-TOPO4 (Life Technologies). Ptgfr forward, 5′-AACCAAAGACTGGCTGGATG-3′; Ptgfr reverse, 5′-AAATTTCGAGCCACAACAGC-3′; cFos forward, 5′-AATTGGATCCAAGCCCAGATCTTCAGTGG-3′; cFos reverse, 5′-AATTGAATTCATAGCCCTGTGATCGGCAC-3′.
Mutations of Ptgfr were induced by injection of a single guide RNA (sgRNA) targeting the second transmembrane domain. We annealed oligonucleotides gPtgfrF, 5′ – TAGGCTTGAGCCCCTTGTTCCT – 3′, and gPtgfrR, 5′ – AAACAGGAACAAGGGGCTCAAG – 3′, and ligated the product into pT7-gRNA [27, 28]. We waited for 30 min of fertilization, then injected single-cell embryos. We delivered ~1 nL of 12 ng/μL Ptgfr sgRNA, 60 ng/μL nls-zCas9-nls mRNA, and 0.3% Texas Red-conjugated dextran (3000 MW, Life Technologies). In ~5 week embryos, we PCR amplified a 554 bp amplicon spanning the sgRNA binding site with the primers PtgfrFlankF, 5′ – CTTCTCCAACAGCCTTGCTC – 3′ and PtgfrFlankR, 5′ – CACAGCCTGTTAGCGTGTTG – 3′, and Sanger sequenced the product with PtgfrFlankF (ElimBio). We saved fish showing evidence of mutant Ptgfr, and crossed these fish to wild-types. G1 fish carrying an indel predicted to result in a null mutation were intercrossed to generate F1 fish. Additional information can be found in Supplemental Experimental Procedures.
Supplementary Material
Highlights.
Prostaglandin F2α injection rapidly leads to naturalistic female spawning behavior
A single receptor for prostaglandin F2α, Ptgfr, is expressed in four brain regions
Deletion of Ptgfr with CRISPR yields females that do not exhibit sexual behavior
Acknowledgments
L Benoit, N Gurtler, K Ceron, E Beaulieu for help in coding behavioral assays; Bénédict Rossi for artwork; N Shah for behavior analysis software; L Becker for help with fish maintenance; N Shah, K Maruska, B Grone, and C Yang for critical reading of the manuscript, and the Fernald Lab for useful discussions. Work was supported by NIH F32HD071755 to SAJ; NIH NS034950, MH101373, and NSF IOS-0923588 to RDF; NIH DK090065 and MH099647 to PM.
Footnotes
Author Contributions
Conceptualization, S.A.J. and R.D.F.; Methodology, S.A.J. and A.T.H.; Investigation, S.A.J., A.T.H., K.R.K., A.K., A.N., M.A.J., and P.M.; CRISPR/Cas9 genome editing – S.A.J. and P.M.; Writing – Original Draft, S.A.J.; Writing – Review & Editing, S.A.J., A.T.H., P.M. and R.D.F.; Funding Acquisition, S.A.J., P.M. and R.D.F.; Resources, J.L.L.; Supervision, S.A.J. and R.D.F.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Beach FA. Sexual attractivity, proceptivity, and receptivity in female mammals. Horm Behav. 1976;7:105–138. doi: 10.1016/0018-506x(76)90008-8. [DOI] [PubMed] [Google Scholar]
- 2.McCarthy MM, Bare JE, vom Saal FS. Infanticide and parental behavior in wild female house mice: effects of ovariectomy, adrenalectomy and administration of oxytocin and prostaglandin F2 alpha. Physiol Behav. 1986;36:17–23. doi: 10.1016/0031-9384(86)90066-1. [DOI] [PubMed] [Google Scholar]
- 3.Pedersen CA, Ascher JA, Monroe YL, Prange AJ., Jr Oxytocin induces maternal behavior in virgin female rats. Science. 1982;216:648–650. doi: 10.1126/science.7071605. [DOI] [PubMed] [Google Scholar]
- 4.Rodriguez-Sierra JF, Komisaruk BR. Effects of prostaglandin E2 and indomethacin on sexual behavior in the female rat. Horm Behav. 1977;9:281–289. doi: 10.1016/0018-506x(77)90063-0. [DOI] [PubMed] [Google Scholar]
- 5.Rodriguez-Sierra JF, Rosenblatt JS. Pregnancy termination by prostaglandin F2 alpha stimulates maternal behavior in the rat. Horm Behav. 1982;16:343–351. doi: 10.1016/0018-506x(82)90032-0. [DOI] [PubMed] [Google Scholar]
- 6.Widowski TM, Curtis SE. Behavioral responses of periparturient sows and juvenile pigs to prostaglandin F2 alpha. Journal of animal science. 1989;67:3266–3276. doi: 10.2527/jas1989.67123266x. [DOI] [PubMed] [Google Scholar]
- 7.Stacey NE. Effects of indomethacin and prostaglandins on the spawning behaviour of female goldfish. Prostaglandins. 1976;12:113–126. doi: 10.1016/s0090-6980(76)80010-x. [DOI] [PubMed] [Google Scholar]
- 8.Kidd MR, Dijkstra PD, Alcott C, Lavee D, Ma J, O’Connell LA, Hofmann HA. Prostaglandin F2 alpha facilitates female mating behavior based on male performance. Behav Ecol Sociobiol. 2013;67:1307–1315. [Google Scholar]
- 9.Liley NR, Tan ESP. The Induction of Spawning Behavior in Puntius-Gonionotus (Bleeker) by Treatment with Prostaglandin-Pgf2a. J Fish Biol. 1985;26:491–502. [Google Scholar]
- 10.Villars TA, Hale N, Chapnick D. Prostaglandin-F2 alpha stimulates reproductive behavior of female paradise fish (Macropodus opercularis) Horm Behav. 1985;19:21–35. doi: 10.1016/0018-506x(85)90003-0. [DOI] [PubMed] [Google Scholar]
- 11.Cole KS, Stacey NE. Prostaglandin induction of spawning behavior in Cichlasoma bimaculatum (Pisces cichlidae) Horm Behav. 1984;18:235–248. doi: 10.1016/0018-506x(84)90013-8. [DOI] [PubMed] [Google Scholar]
- 12.Forlano PM, Bass AH. Neural and hormonal mechanisms of reproductive-related arousal in fishes. Horm Behav. 2011;59:616–629. doi: 10.1016/j.yhbeh.2010.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yang CF, Shah NM. Representing Sex in the Brain, One Module at a Time. Neuron. 2014;82:261–278. doi: 10.1016/j.neuron.2014.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Goetz FW, Cetta F. Ovarian and plasma PGE and PGF levels in naturally ovulating brook trout (Salvelinus fontinalis) and the effects of indomethacin on prostaglandin levels. Prostaglandins. 1983;26:387–395. doi: 10.1016/0090-6980(83)90174-0. [DOI] [PubMed] [Google Scholar]
- 15.Nagahama Y, Yamashita M. Regulation of oocyte maturation in fish. Dev Growth Differ. 2008;50(Suppl 1):S195–219. doi: 10.1111/j.1440-169X.2008.01019.x. [DOI] [PubMed] [Google Scholar]
- 16.Fernald RD, Hirata NR. Field-Study of Haplochromis-Burtoni - Quantitative Behavioral Observations. Anim Behav. 1977;25:964–975. [Google Scholar]
- 17.Kocher TD. Adaptive evolution and explosive speciation: the cichlid fish model. Nat Rev Genet. 2004;5:288–298. doi: 10.1038/nrg1316. [DOI] [PubMed] [Google Scholar]
- 18.Stacey N. Hormones, pheromones and reproductive behavior. Fish Physiol Biochem. 2003;28:229–235. [Google Scholar]
- 19.Stacey NE, Peter RE. Central action of prostaglandins in spawning behaviour of female goldfish. Physiol Behav. 1979;22:1191–1196. doi: 10.1016/0031-9384(79)90275-0. [DOI] [PubMed] [Google Scholar]
- 20.Meisel RL, Sachs BD. The physiology of male sexual behavior. In: Knobil E, Neill JD, editors. The Physiology of Reproduction. 2. New York: Raven Press; 1994. pp. 3–105. [Google Scholar]
- 21.Lin X, Volkoff H, Narnaware Y, Bernier NJ, Peyon P, Peter RE. Brain regulation of feeding behavior and food intake in fish. Comparative biochemistry and physiology Part A, Molecular & integrative physiology. 2000;126:415–434. doi: 10.1016/s1095-6433(00)00230-0. [DOI] [PubMed] [Google Scholar]
- 22.Finger TE. Sorting food from stones: the vagal taste system in Goldfish, Carassius auratus. Journal of comparative physiology A, Neuroethology, sensory, neural, and behavioral physiology. 2008;194:135–143. doi: 10.1007/s00359-007-0276-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rink E, Wullimann MF. Connections of the ventral telencephalon and tyrosine hydroxylase distribution in the zebrafish brain (Danio rerio) lead to identification of an ascending dopaminergic system in a teleost. Brain research bulletin. 2002;57:385–387. doi: 10.1016/s0361-9230(01)00696-7. [DOI] [PubMed] [Google Scholar]
- 24.Senthilkumaran B, Sudhakumari CC, Chang XT, Kobayashi T, Oba Y, Guan GJ, Yoshiura Y, Yoshikuni M, Nagahama Y. Ovarian carbonyl reductase-like 20 beta-hydroxysteroid dehydrogenase shows distinct surge in messenger RNA expression during natural and gonadotropin-induced meiotic maturation in Nile tilapia. Biol Reprod. 2002;67:1080–1086. doi: 10.1095/biolreprod67.4.1080. [DOI] [PubMed] [Google Scholar]
- 25.Tacon P, Baroiller JF, Le Bail PY, Prunet P, Jalabert B. Effect of egg deprivation on sex steroids, gonadotropin, prolactin, and growth hormone profiles during the reproductive cycle of the mouthbrooding cichlid fish Oreochromis niloticus. Gen Comp Endocrinol. 2000;117:54–65. doi: 10.1006/gcen.1999.7388. [DOI] [PubMed] [Google Scholar]
- 26.Doudna JA, Charpentier E. Genome editing. The new frontier of genome engineering with CRISPR-Cas9. Science. 2014;346:1258096. doi: 10.1126/science.1258096. [DOI] [PubMed] [Google Scholar]
- 27.Varshney GK, Pei W, LaFave MC, Idol J, Xu L, Gallardo V, Carrington B, Bishop K, Jones M, Li M, et al. High-throughput gene targeting and phenotyping in zebrafish using CRISPR/Cas9. Genome Res. 2015;25:1030–1042. doi: 10.1101/gr.186379.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jao LE, Wente SR, Chen WB. Efficient multiplex biallelic zebrafish genome editing using a CRISPR nuclease system. Proc Natl Acad Sci U S A. 2013;110:13904–13909. doi: 10.1073/pnas.1308335110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stacey NE. Hormonal regulation of female reproductive behavior in fish. Am Zool. 1981;21:305–316. [Google Scholar]
- 30.Wong RY, Ramsey ME, Cummings ME. Localizing brain regions associated with female mate preference behavior in a swordtail. PLoS One. 2012;7:e50355. doi: 10.1371/journal.pone.0050355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Goodson JL. The vertebrate social behavior network: evolutionary themes and variations. Horm Behav. 2005;48:11–22. doi: 10.1016/j.yhbeh.2005.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Saper CB, Romanovsky AA, Scammell TE. Neural circuitry engaged by prostaglandins during the sickness syndrome. Nature neuroscience. 2012;15:1088–1095. doi: 10.1038/nn.3159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Venkatesh B, Tan CH, Lam TJ. Prostaglandins and teleost neurohypophyseal hormones induce premature parturition in the guppy, Poecilia reticulata. Gen Comp Endocrinol. 1992;87:28–32. doi: 10.1016/0016-6480(92)90146-b. [DOI] [PubMed] [Google Scholar]
- 34.Ring JR. The estrogen-progesterone induction of sexual receptivity in the spayed female mouse. Endocrinology. 1944;34:269–275. [Google Scholar]
- 35.Hall NR, Luttge WG, Berry RB. Intracerebral prostaglandin E2: effects upon sexual behavior, open field activity and body temperature in ovariectomized female rats. Prostaglandins. 1975;10:877–888. doi: 10.1016/0090-6980(75)90015-5. [DOI] [PubMed] [Google Scholar]
- 36.Musatov S, Chen W, Pfaff DW, Kaplitt MG, Ogawa S. RNAi-mediated silencing of estrogen receptor {alpha} in the ventromedial nucleus of hypothalamus abolishes female sexual behaviors. Proc Natl Acad Sci U S A. 2006;103:10456–10460. doi: 10.1073/pnas.0603045103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yang CF, Chiang MC, Gray DC, Prabhakaran M, Alvarado M, Juntti SA, Unger EK, Wells JA, Shah NM. Sexually dimorphic neurons in the ventromedial hypothalamus govern mating in both sexes and aggression in males. Cell. 2013;153:896–909. doi: 10.1016/j.cell.2013.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ma Y, Juntti SA, Hu CK, Huguenard JR, Fernald RD. Electrical synapses connect a network of gonadotropin releasing hormone neurons in a cichlid fish. Proc Natl Acad Sci U S A. 2015;112:3805–3810. doi: 10.1073/pnas.1421851112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wagner CE, Harmon LJ, Seehausen O. Ecological opportunity and sexual selection together predict adaptive radiation. Nature. 2012;487:366–369. doi: 10.1038/nature11144. [DOI] [PubMed] [Google Scholar]
- 40.Wu MV, Manoli DS, Fraser EJ, Coats JK, Tollkuhn J, Honda SI, Harada N, Shah NM. Estrogen Masculinizes Neural Pathways and Sex-Specific Behaviors. Cell. 2009;139:61–72. doi: 10.1016/j.cell.2009.07.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.