Abstract
Background
Phthalates are hypothesized to cause obesity, but few studies have assessed whether prenatal phthalate exposures are related to childhood body mass index (BMI).
Methods
We included 707 children from three prospective cohort studies enrolled in the United States between 1998 and 2006 who had maternal urinary phthalate metabolite concentrations measured during pregnancy, and measures of weight and height at ages 4 to 7 years. We calculated age- and sex-standardized BMI z-scores and classified children with BMI percentiles ≥85 as overweight/obese. We used mixed effects regression models to estimate associations between a 1-standard deviation increase in natural log phthalate metabolite concentrations and BMI z-scores and overweight/obesity. We estimated associations in multiple metabolite models adjusted for confounders, and evaluated heterogeneity of associations by child’s sex, race/ethnicity, and cohort.
Results
Mono-3-carboxypropyl phthalate (MCPP) concentrations were positively associated with overweight/obese status in children (odds ratio [95% credible interval] = 2.1 [1.2, 4.0]) but not with BMI z-scores (beta = −0.02 [−0.15, 0.11]). We did not observe evidence of obesogenic effects for other metabolites. However, monoethyl phthalate (MEP) and summed di-(2-ethylhexyl) phthalate metabolites (∑DEHP) concentrations were inversely associated with BMI z-scores among girls (MEP beta = −0.14 [−0.28, 0.00]; ∑DEHP beta = −0.12 [−0.27, 0.02]).
Conclusions
Maternal urinary MCPP, a non-specific metabolite of several phthalates, was positively associated with childhood overweight/obesity. Metabolites of diethyl phthalate and DEHP were associated with lower BMI in girls but not boys, suggesting prenatal exposures may have sexually dimorphic effects on physical development.
Introduction
One out of three children aged 2 to 19 years in the United States is overweight or obese.1 Obese children have poorer physical and psychosocial health compared to their normal-weight peers and exhibit early physiologic changes associated with chronic health conditions.2 While energy balance is a key determinant of weight change, the “environmental obesogen” hypothesis posits that prenatal exposure to endocrine disrupting chemicals, including phthalates, may also increase obesity risk by altering adipogenesis and lipid homeostasis.3
Phthalates are industrial chemicals with endocrine disrupting properties and widespread human exposure.4 Low molecular weight phthalates are used as solvents in products such as cosmetics, fragrances, and medications.5 High molecular weight phthalates enhance flexibility and durability of plastics and are found in building materials and food packaging.5 Urine is the optimal matrix for measurement of phthalates, which are rapidly transformed to polar metabolites that are eliminated by urinary excretion.6
Detection of phthalate metabolites in amniotic fluid and breast-milk demonstrate the potential for early life exposures4 and prenatal exposures to certain phthalates have been associated with reproductive and developmental outcomes in children.7 Some phthalates exhibit anti-androgenic activity in animals4 and human studies have reported sex differences in associations of phthalate exposures with child health.7 Furthermore, early life phthalate exposures may alter metabolic and homeostatic mechanisms related to the development of obesity.3 Toxicologic studies have demonstrated that certain phthalates affect steroid hormone levels and interfere with peroxisome proliferator-activated receptors, which regulate lipid metabolism and adipogenesis.8
Valvi et al.9 reported associations of maternal urinary summed di-(2-ethylhexyl) phthalate (∑DEHP) metabolites with higher body mass index (BMI) in girls but lower BMI in boys in a Spanish birth cohort. In the Mount Sinai Children’s Environmental Health and Disease Prevention Research Center (MSSM), Buckley et al.10 reported associations of maternal urinary ∑DEHP metabolite concentrations with lower percent fat mass in children with no differences in associations between girls and boys, though the study was underpowered to identify sex differences. Because animal studies suggest that certain phthalates exhibit sexually dimorphic effects,4 we pooled the MSSM cohort with two additional birth cohorts in the United States to examine associations of prenatal urinary phthalate metabolite concentrations and BMI assessed in children between ages 4 and 7 years and evaluated differences by child’s sex.
Methods
Children’s Environmental Health Center cohorts
The MSSM study enrolled 479 primiparous women with singleton pregnancies from the Mount Sinai prenatal clinic and two adjacent private practices in New York City between 1998 and 2002. Women delivered at the Mount Sinai Medical Center. Seventy-five women were subsequently excluded for reasons described elsewhere.11 The final cohort consists of 404 mother-infant pairs for whom birth data were available.
The Columbia Center for Children’s Environmental Health (CCCEH) enrolled 727 pregnant women between 1998 and 2006. The cohort was restricted to non-smoking women 18–35 years old who self-identified as either African American or Dominican and who had resided in Northern Manhattan or the South Bronx in New York City for >1 year prior to pregnancy. Additional details of the study population have been previously reported.12
The Health Outcomes and Measures of the Environment (HOME) Study, a prospective birth cohort located in Cincinnati, Ohio, enrolled 468 women between 2003 and 2006. Because the HOME Study contains a nested, randomized trial of in-home lead and injury hazard controls women had to be living in housing built before 1978. Additional eligibility criteria and study population characteristics have been described elsewhere.13 A total of 389 women delivered live-born, singleton infants without birth defects.
Questionnaires were administered to each mother at study enrollment to ascertain maternal characteristics including age at delivery, race/ethnicity, education, work status during pregnancy, parity, height, and pre-pregnancy BMI. We calculated gestational weight gain as last pregnancy weight minus self-reported pre-pregnancy weight. Women provided a spot urine sample at mean ± standard deviation (SD) gestational ages of 31.6 ± 5.1 (MSSM), 34.4 ± 3.0 (CCCEH), and 27.1 ± 2.2 (HOME) weeks. Child’s sex was ascertained from birth records and breastfeeding status of the index children was assessed by questionnaire.
For MSSM, we determined maternal smoking during pregnancy based on self-report. For the HOME Study, we classified women as active smokers during pregnancy if the average of three maternal cotinine serum concentrations (measured twice during pregnancy and at birth) exceeded 3 ng/mL.14 CCCEH excluded women with evidence of active smoking.12
Human subjects
Women provided informed consent prior to participation and children aged ≥ 7 years provided assent. The MSSM, CCCEH, and HOME studies received approval from the Institutional Review Boards (IRBs) of the Mount Sinai School of Medicine (MSSM), Columbia University (CCCEH), and the University of Cincinnati College of Medicine (HOME), respectively. The Centers for Disease Control and Prevention (CDC) IRB relied on the determinations made by the other IRBs for the CCCEH and HOME studies. For MSSM, the involvement of the CDC laboratory was determined not to constitute engagement in human subjects research. The current analysis was approved by the IRB of the University of North Carolina at Chapel Hill.
Phthalates exposure assessment
All spot urine samples were analyzed by CDC staff for monoethyl phthalate (MEP), mono-n-butyl phthalate (MnBP), mono-isobutyl phthalate (MiBP), mono(3-carboxypropyl) phthalate (MCPP), monobenzyl phthalate (MBzP), mono(2-ethylhexyl) phthalate (MEHP), mono(2-ethyl-5-hydroxyhexyl) phthalate (MEHHP), mono(2-ethyl-5-oxohexyl) phthalate (MEOHP), and mono(2-ethyl-5-carboxypentyl) phthalate (MECPP). Analytic methods and quality control procedures have been described.15,16 For each metabolite, we replaced values below the limit of detection (LOD) by the LOD/√2. We examined DEHP metabolites (MECPP, MEHHP, MEHP, MEOHP) as a micromolar sum (ΣDEHP, micromoles per liter).11 To facilitate effect size comparison, we standardized the natural log concentration of each phthalate metabolite or sum to its mean and SD in the pooled sample.
Outcome assessment
Weight and height were measured at follow-up visits scheduled for approximately ages 4–5.5, 6, and 7–9 years (MSSM), 5 and 7 years (CCCEH), and 4, 5, and 7–9 years (HOME). We measured children in bare or stocking feet while wearing light clothing (i.e., pediatric gown, underwear, or shorts and a t-shirt). We assessed weight using a digital scale (all HOME visits and CCCEH 5 year visit) or a pediatric Tanita scale (all MSSM visits and CCCEH 7 year visit) (models TBF-300 and BC-418, Tanita Corporation of America, Arlington Heights, Illinois) and determined height using wall-mounted stadiometers. We calculated BMI [weight (kg) / height (m)2], which is a moderately sensitive and highly specific indicator of adiposity, as a measure of excess weight for height.17 We computed age- and sex-standardized z-scores and percentiles for BMI using a CDC macro.18 We classified children as overweight/obese at each follow-up visit if their age- and sex-standardized BMI percentile was ≥85, which corresponds to a BMI z-score of approximately 1.
Statistical analysis
The current analysis includes infants of singleton pregnancies who were born at ≥32 weeks gestation and ≥1,500 grams and had measured prenatal maternal urinary phthalate metabolite concentrations. After excluding very dilute urine specimens due to the potential for inaccurate biomarker measurements (<10 mg/dL creatinine, n = 9),11 1,143 children met our baseline inclusion criteria. To examine BMI prior to puberty and ensure overlap in age assessments among cohorts, we further restricted the sample to children with weight and height data collected at one or more follow-up visits occurring between 4 and 7 years of age (N=707; 1,416 follow-up visits).
We assessed associations of prenatal urinary phthalate metabolite concentrations with body size using a Bayesian modeling framework to (1) account for missing at random covariate data, (2) stabilize estimates of correlated metabolites, and (3) conduct sensitivity analyses for potentially nonignorable (i.e., missing not at random) loss to follow-up.
MSSM and HOME measured creatinine to account for urinary dilution. CCCEH measured specific gravity in all urine samples (n = 339), with creatinine additionally measured in a subset (n = 202). In the subset with both measures, the Pearson correlation coefficient between creatinine and specific gravity was 0.78. To obtain a common measure of urine dilution, we predicted missing natural log creatinine concentrations in CCCEH at each iteration of the Markov Chain Monte Carlo (MCMC) algorithm in our Bayesian models using a linear regression model. We also used our Bayesian framework for multiple imputation of breastfeeding status (n = 6), maternal pre-pregnancy BMI (n = 58), and gestational weight gain (n = 107) assuming values were missing at random (see eAppendix).
To account for potential confounding among correlated phthalate metabolites, we estimated associations in multiple metabolite Bayesian hierarchical models.19 Following standard practice, the beta coefficient for each standardized phthalate metabolite was given an independent, normally distributed prior distribution with a mean of zero and variance of 1/τ2. We set τ = 1, which represents a prior belief that 95% of the effects of a SD difference in natural log phthalate metabolite concentration are within an odds ratio (OR) of 0.14 to 7.1 for overweight/obese status or approximately ± two SDs of the mean BMI z-score distribution in the study sample. Similarly, we specified independent, null-centered priors with τ = 1 for beta coefficients of covariates included in outcome and imputation models.
We estimated associations per SD increase in natural log phthalate metabolite concentrations in logistic and linear mixed effects models with random intercepts to account for multiple observations per child. Within our Bayesian framework, point and interval estimates were estimated as posterior mean ORs and beta coefficients and their associated 95% credible intervals (CI). CIs are more easily interpreted than confidence intervals from a traditional analysis: given the data and the model, there is a 95% chance that the true value is within this interval.
A priori, we chose variables to include in our final models that were either identified as potential confounders using directed acyclic graphs or were expected to be strong predictors of the outcome but not on the causal pathway. While we included a large number of covariates, our Bayesian framework stabilized estimates and protected against variance inflation using weakly informative prior information. Potential confounders included cohort, maternal race/ethnicity, maternal age at delivery, maternal education, maternal work status during pregnancy, maternal pre-pregnancy BMI, gestational weight gain, maternal smoking during pregnancy, calendar date of urine collection, and parity. Predictors of body size in children included maternal height, child’s sex, breastfeeding of the index child, and months of age at follow-up. Due to uncertainty regarding how best to account for urine dilution,20,21 we compared results with and without creatinine adjustment in preliminary models and found no important differences. Therefore, we accounted for urine dilution by including natural log creatinine as a covariate.20 To improve fit of BMI z-score models, we adjusted for an interaction between child’s sex and age at follow-up because outcome distributions by age differed between girls and boys. Continuous covariates were standardized to twice the population SD22 and we included a quadratic term for maternal pre-pregnancy BMI and cubic terms for maternal age and gestational weight gain based on their non-linear associations with outcomes.
We examined effect measure modification by cohort, child’s sex, and race/ethnicity by including interaction terms between the modifier and each metabolite concentration. In models assessing modification by both cohort and either sex or race/ethnicity, we included all two- and three-way interaction terms between metabolites, cohort, and the covariate. When assessing modification by race/ethnicity, we excluded children of “other race” in all cohorts and Hispanic children in HOME due to small numbers. We considered there to be meaningful effect modification if the 80% CI for the interaction term did not cross the null value (similar to an alpha of 0.2 in a frequentist framework). Finally, we explored dose-response relationships using restricted quadratic splines.23
In sensitivity analyses, we compared associations estimated in multiple metabolite models to those estimated in models including only one metabolite at a time, a common approach that may result in confounding by correlated metabolites. Single metabolite models specified the same prior distributions for phthalate beta coefficients as the main analyses, i.e. β ~ N(0,1).
Additionally, we conducted a sensitivity analysis for loss to follow-up due to concern that the probability of follow-up visit attendance may depend on a child’s outcome value at the time. Our primary models included children with at least one follow-up visit (N=707) under the assumption that outcome data are missing at random. We compared these results to a selection model approach24 that included all children with measured prenatal phthalate metabolite concentrations (N=1,143) under a potentially nonignorable (missing not at random) missing data mechanism. For this analysis, we constructed a binary missing outcome indicator variable for attendance at each follow-up visit based on the cohort-specific visit schedules (0 if observed, 1 if missing). Within our MCMC algorithm, we jointly fit the outcome model with cohort-specific models for the missing outcome indicator to allow predictors of missingness to vary by cohort. We specified the missing outcome indicator models as logistic mixed effects regression models with random intercepts, dependent on the outcome (potentially unobserved), maternal age, race/ethnicity, maternal education, maternal pre-pregnancy BMI, birthweight, child’s sex, calendar date of urine collection, and age (months) at follow-up. The CCCEH missing outcome indicator model additionally included four variables that were unmeasured in the other cohorts but previously reported to predict subject retention in CCCEH (receipt of public assistance during pregnancy, maternal satisfaction with living conditions, neighborhood poverty rate, and Spanish language linguistic isolation).25 Finally, because selection models are sensitive to unverifiable assumptions underlying the specification of the missing data model, we varied the parameterization of the missing outcome indicator models by using different functional forms for continuous variables, fitting a single missing indicator model for all three cohorts, and including interaction terms between age at follow-up and child’s sex and race/ethnicity.
We conducted descriptive analyses in SAS version 9.3 (SAS Institute, Cary, NC). We ran Bayesian models in WinBUGS version 1.4.3 (MRC Biostatistics Unit, Cambridge, UK) with a 10,000 iteration burn-in followed by 50,000 additional iterations. We ran ten chains for inference from the MCMC procedure for our sensitivity analyses for nonignorable missing data. We assessed model convergence using standard diagnostic measures.22
Results
The distributions of participant characteristics by cohort illustrate differences in the inclusion criteria and source populations of each study (Table 1). For example, HOME enrolled primarily white, non-Hispanic mothers who were older and had more years of education than women in the New York City cohorts. Mean (SD) maternal heights (cm) were 64 (2.9), 64 (2.8), 65 (2.8), and 64 (2.9) in MSSM, CCCEH, HOME, and the pooled sample, respectively.
Table 1.
Characteristics of the study sampleat baseline by cohort and pooled,n (%)
| Characteristic | MSSM | CCCEH | HOME | Pooled |
|---|---|---|---|---|
| Total (N) | 151 | 339 | 217 | 707 |
| Maternal age at delivery (years) | ||||
| < 20 | 42 (28) | 47 (14) | 13 (6) | 102 (14) |
| 20–24 | 53 (35) | 121 (36) | 37 (17) | 211 (30) |
| 25–29 | 21 (14) | 100 (30) | 62 (29) | 183 (26) |
| ≥ 30 | 35 (23) | 71 (21) | 105 (48) | 211 (30) |
| Race/ethnicity | ||||
| Non-Hispanic white | 31 (21) | 0 (0) | 135 (62) | 166 (23) |
| Non-Hispanic black | 41 (27) | 120 (35) | 69 (32) | 230 (33) |
| Hispanic | 76 (50) | 219 (65) | 4 (2) | 299 (42) |
| Other | 3 (2) | 0 (0) | 9 (4) | 12 (2) |
| Maternal education | ||||
| <High school | 34 (23) | 124 (37) | 20 (9) | 178 (25) |
| High school or GED | 34 (23) | 126 (37) | 27 (12) | 187 (26) |
| Some college | 45 (30) | 74 (22) | 64 (29) | 183 (26) |
| ≥ College degree | 38 (25) | 15 (4) | 106 (49) | 159 (22) |
| Mother worked during pregnancy (yes) | 92 (61) | 199 (59) | 181 (83) | 472 (67) |
| Parity (multiparous) | 0 (0) | 188 (55) | 117 (54) | 305 (43) |
| Maternal smoking during pregnancy (yes) | 26 (17) | 0 (0) | 22 (10) | 48 (7) |
| Pre-pregnancy body mass index (kg/m2) | ||||
| < 18.5 | 9 (6) | 16 (5) | 7 (4) | 32 (5) |
| 18.5–24.9 | 90 (60) | 170 (51) | 72 (43) | 332 (51) |
| 25–29.9 | 37 (25) | 75 (23) | 48 (29) | 160 (25) |
| ≥ 30 | 15 (10) | 70 (21) | 40 (24) | 125 (19) |
| Missing | 0 | 8 | 50 | 58 |
| Gestational weight gain (lb) | ||||
| < 25 | 21 (16) | 77 (25) | 75 (45) | 173 (29) |
| 25–34.9 | 38 (29) | 79 (26) | 38 (23) | 155 (26) |
| 35–44.9 | 26 (20) | 73 (24) | 35 (21) | 134 (22) |
| ≥ 45 | 45 (35) | 74 (24) | 19 (11) | 138 (23) |
| Missing | 21 | 36 | 50 | 107 |
| Year of urine collection | ||||
| 1998–2000 | 133 (88) | 94 (28) | 0 (0) | 227 (32) |
| 2001–2003 | 18 (12) | 125 (37) | 26 (12) | 169 (24) |
| 2004–2006 | 0 (0) | 120 (35) | 191 (88) | 311 (44) |
| Child's sex (male) | 80 (53) | 161 (47) | 94 (43) | 335 (47) |
| Breastfed (ever) | 94 (63) | 256 (76) | 176 (81) | 526 (75) |
| Missing | 1 | 4 | 1 | 6 |
Columbia Center for Children’s Environmental Health (CCCEH), Health Outcomes and Measures of the Environment Study (HOME), Mount Sinai School of Medicine Center for Children’s Environmental Health (MSSM)
Except for MEHP, the hydrolytic metabolite of DEHP, urinary phthalate metabolite concentrations were detectable in ≥95% of urine samples (see eTable 1). Although geometric mean urinary phthalate metabolite concentrations were lower in HOME than among MSSM and CCCEH participants, concentration distributions exhibited substantial overlap (see eTable 1). Spearman rank correlations among concentrations of phthalate metabolites in the study sample ranged from 0.37 for MEP and ∑DEHP to 0.78 for MnBP and MiBP. The percent of children classified as overweight or obese at all ages was lower in HOME than in the other two cohorts (Table 2). Similarly, mean age- and sex- standardized BMI z-scores were highest among CCCEH children and lowest in HOME (see eTable 2).
Table 2.
Percentof children in the study sample classified as overweight/obese by age at follow-up (N=707; 1,416 follow-up visits)
| Group/category | Age (years) | |||
|---|---|---|---|---|
| 4 (n = 420) | 5 (n = 361) | 6 (n = 258) | 7 (n = 377) | |
| Overall | 28 | 26 | 38 | 36 |
| Cohort | ||||
| MSSM | 35 | 30 | 28 | 36 |
| CCCEH | 36 | 35 | 47 | 44 |
| HOME | 17 | 19 | 0a | 21 |
| Child’s sex | ||||
| Girls | 28 | 27 | 39 | 35 |
| Boys | 27 | 25 | 37 | 37 |
| Race/ethnicity | ||||
| Non-Hispanic white | 17 | 16 | 13 | 15 |
| Non-Hispanic black | 22 | 30 | 37 | 32 |
| Hispanic | 43 | 34 | 44 | 48 |
| Other | 13 | 11 | 33 | 20 |
Columbia Center for Children’s Environmental Health (CCCEH), Health Outcomes and Measures of the Environment Study (HOME), Mount Sinai School of Medicine Center for Children’s Environmental Health (MSSM)
Fewer than 5 children in this stratum.
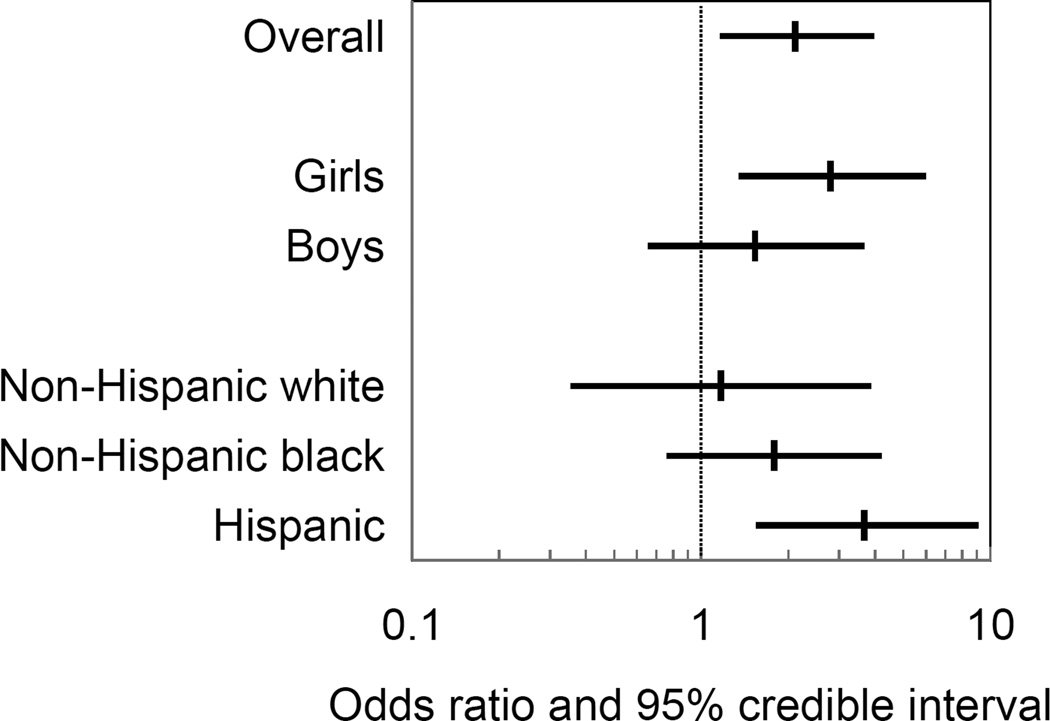
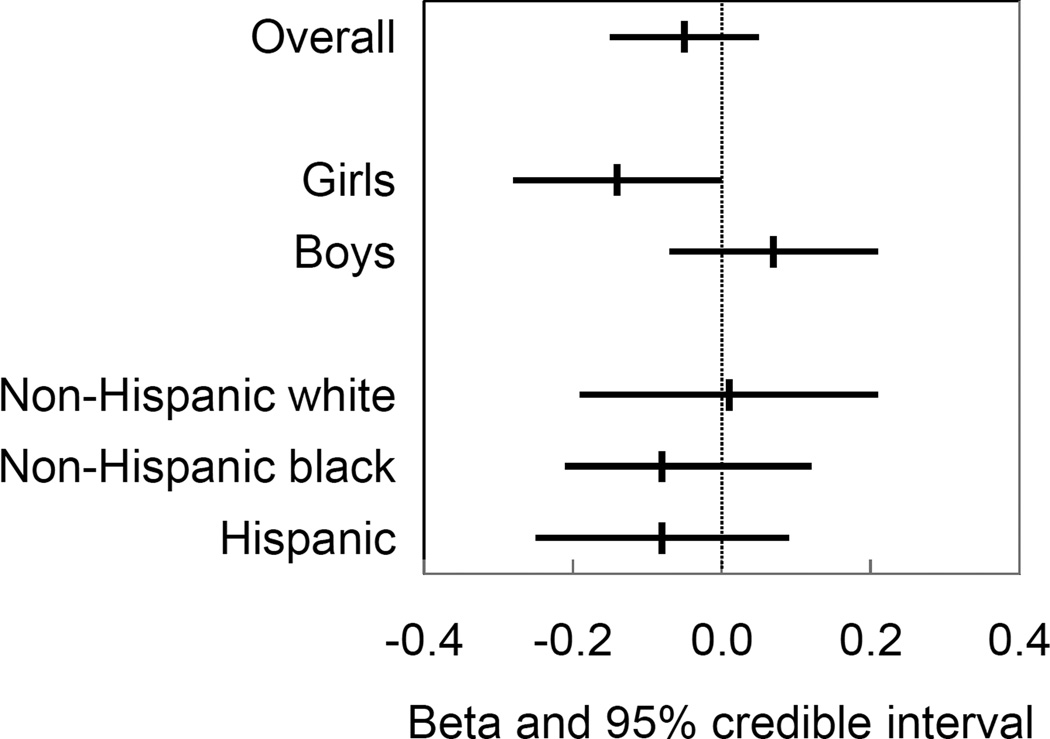
Adjusted associations of prenatal urinary phthalate metabolite concentrations with overweight/obese status and age- and sex-standardized BMI z-scores are reported in Table 3 (overall and by child’s sex) and Table 4 (by race/ethnicity). MCPP was associated with increased odds of overweight/obese status (OR [95% CI] = 2.1 [1.2, 4.0]). The association was stronger among Hispanic children compared to non-Hispanic black or white children (Figure 1). In contrast, MCPP was not associated with age- and sex- standardized BMI z-scores (beta = −0.02 [−0.15, 0.11]). We observed modification of the associations of MEP, MnBP, and ∑DEHP with BMI z-scores by child’s sex. MEP was associated with lower BMI z-scores among girls (beta = −0.14 [−0.28, 0.00]) but not boys (Figure 2). ∑DEHP was also associated with lower BMI z-scores among girls (beta = −0.12 [−0.27, 0.02]) whereas MnBP was associated with higher BMI z-scores among girls (beta = 0.16 [−0.06, 0.39]), though 95% CIs for sex-specific associations crossed the null value (Table 3).
Table 3.
Adjusted associations between prenatal urinary phthalate metabolite concentrations and overweight/obese status and body mass index (BMI) z-scores among children aged 4 to 7 years, overall and by child’s sex
| Metabolite / groupa | Overweight/obese OR (95% CI)b |
BMI z-score β (95% CI)c |
|---|---|---|
| MEP | ||
| Overall | 0.68 (0.42, 1.1) | −0.05 (−0.15, 0.05) |
| Girls | 0.60 (0.32, 1.1) | −0.14 (−0.28, 0.00) |
| Boys | 0.85 (0.44, 1.7) | 0.07 (−0.07, 0.21)d |
| MnBP | ||
| Overall | 1.0 (0.51, 2.0) | 0.03 (−0.12, 0.18) |
| Girls | 1.3(0.55, 3.0) | 0.16 (−0.06, 0.39) |
| Boys | 0.76 (0.30, 1.9) | −0.08 (−0.28, 0.13)d |
| MiBP | ||
| Overall | 0.84 (0.44, 1.6) | 0.01 (−0.14, 0.15) |
| Girls | 0.92 (0.41, 2.0) | −0.07 (−0.26, 0.13) |
| Boys | 0.67 (0.27, 1.6) | 0.02 (−0.18, 0.22) |
| MCPP | ||
| Overall | 2.1 (1.2, 4.0) | −0.02 (−0.15, 0.11) |
| Girls | 2.8 (1.4, 6.0) | 0.06 (−0.11, 0.23) |
| Boys | 1.5 (0.66, 3.7) | −0.09 (−0.28, 0.09) |
| MBzP | ||
| Overall | 0.63 (0.34, 1.2) | −0.07 (−0.20, 0.06) |
| Girls | 0.66 (0.31, 1.4) | −0.09 (−0.27, 0.09) |
| Boys | 0.69 (0.29, 1.6) | −0.04 (−0.22, 0.15) |
| ∑DEHP | ||
| Overall | 0.87 (0.53, 1.4) | −0.04 (−0.15, 0.06) |
| Girls | 0.68 (0.36, 1.3) | −0.12 (−0.27, 0.02) |
| Boys | 1.2(0.59, 2.4) | 0.04 (−0.11, 0.19)d |
Associations per standard deviation increase in natural log phthalate metabolite concentrations estimated in multiple metabolite logistic (overweight/obese) or linear (BMI z-score) mixed effects regression models, adjusted for cohort, maternal race/ethnicity, maternal age at delivery, maternal education, maternal work status during pregnancy, maternal pre-pregnancy BMI, maternal height, gestational weight gain, maternal smoking during pregnancy, natural log creatinine, calendar date of urine collection, parity, breast feeding, months of age at follow-up, and for overall models, child’s sex.
Number of children/number of follow-up visits for each group: 707/1,416 (overall), 372/756 (girls), and 335/660 (boys).
Posterior mean of odds ratios (95% credible intervals).
Posterior mean of beta coefficients (95% credible intervals).
Met criteria for heterogeneity compared to girls.
Table 4.
Adjusted associations between prenatal urinary phthalate metabolite concentrations and overweight/obese status and body mass index (BMI) z-scores among children aged 4 to 7 yearsby race/ethnicity
| Metabolite / groupa | Overweight/obese OR (95% CI)b |
BMI z-score β (95% CI)c |
|---|---|---|
| MEP | ||
| Non-Hispanic white | 0.74 (0.27, 2.0) | 0.01 (−0.19, 0.21) |
| Non-Hispanic black | 0.69 (0.34, 1.4) | −0.05 (−0.21, 0.12) |
| Hispanic | 0.62 (0.28, 1.3) | −0.08 (−0.25, 0.09) |
| MnBP | ||
| Non-Hispanic white | 1.1 (0.32, 3.9) | 0.04 (−0.23, 0.32) |
| Non-Hispanic black | 1.1(0.43, 2.6) | 0.00 (−0.27, 0.26) |
| Hispanic | 0.85 (0.30, 2.5) | 0.04 (−0.21, 0.29) |
| MiBP | ||
| Non-Hispanic white | 1.0 (0.32, 3.1) | −0.02 (−0.26, 0.22) |
| Non-Hispanic black | 0.55 (0.22, 1.4) | −0.04 (−0.32, 0.25) |
| Hispanic | 0.72 (0.27, 1.9) | 0.01 (−0.22, 0.25) |
| MCPP | ||
| Non-Hispanic white | 1.2(0.35, 3.9) | −0.13 (−0.39, 0.13) |
| Non-Hispanic black | 1.8(0.76, 4.2) | 0.00 (−0.25, 0.24) |
| Hispanic | 3.7(1.6, 9.1)d | 0.08 (−0.11, 0.27) |
| MBzP | ||
| Non-Hispanic white | 0.74 (0.22, 2.5) | −0.16 (−0.42, 0.10) |
| Non-Hispanic black | 0.58 (0.25, 1.4) | −0.08 (−0.32, 0.16) |
| Hispanic | 0.63 (0.26, 1.5) | −0.01 (−0.21, 0.18) |
| ∑DEHP | ||
| Non-Hispanic white | 1.4 (0.58, 3.6) | 0.10 (−0.09, 0.29) |
| Non-Hispanic black | 1.0 (0.46, 2.1) | −0.04 (−0.25, 0.16) |
| Hispanic | 0.59 (0.27, 1.3) | −0.14 (−0.31, 0.03) |
Associations per standard deviation increase in natural log phthalate metabolite concentrations estimated in multiple metabolite logistic (overweight/obese) or linear (BMI z-score) mixed effects regression models,adjusted for cohort, maternal age at delivery, maternal education, maternal work status during pregnancy, maternal pre-pregnancy BMI, maternal height, gestational weight gain, maternal smoking during pregnancy, natural log creatinine, calendar date of urine collection, parity, child’s sex, breast feeding, and months of age at follow-up.
Number of children/number of follow-up visits for each group: 166/358 (non-Hispanic white), 230/463 (non-Hispanic black), and 299/570 (Hispanic).
Posterior mean of odds ratios (95% credible intervals).
Posterior mean of beta coefficients (95% credible intervals).
Met criteria for heterogeneity compared to non-Hispanic blacks.
Figure 1.
Posterior mean of odds ratios and 95% credible intervals for the association of a one standard deviation increase in natural log urinary MCPP concentrations with overweight/obese status among children aged 4 to 7 years (N=707; 1,416 follow-up visits), overall and by child’s sex and race/ethnicity. Associations estimated in multiple metabolite logistic mixed effects regression models adjusted for cohort, maternal race/ethnicity, maternal age at delivery, maternal education, maternal work status during pregnancy, maternal pre-pregnancy BMI, maternal height, gestational weight gain, maternal smoking during pregnancy, natural log creatinine, calendar date of urine collection, parity, child’s sex, breastfeeding, and months of age at follow-up.
Figure 2.
Posterior mean of beta coefficients and 95% credible intervals for the association of a one standard deviation increase in natural log urinary MEP concentrations with BMI z-scores among children aged 4 to 7 years (N=707; 1,416 follow-up visits), overall and by child’s sex and race/ethnicity. Associations estimated in multiple metabolite linear mixed effects regression models adjusted for cohort, maternal race/ethnicity, maternal age at delivery, maternal education, maternal work status during pregnancy, maternal pre-pregnancy BMI, maternal height, gestational weight gain, maternal smoking during pregnancy, natural log creatinine, calendar date of urine collection, parity, child’s sex, breastfeeding, and months of age at follow-up.
Spline models assessing the shape of relationships between prenatal urinary phthalate metabolite concentrations and outcomes were consistent with null associations or a linear dose-response trend for all metabolites and outcomes (not shown). For example, the odds of overweight/obese status increased linearly with increasing MCPP concentration whereas the association of MCPP with BMI z-scores was null (see eFigure1).
Cohort-specific associations are reported in eTable 3 and eTable 4. Associations with overweight/obese status were not modified by cohort, though CIs for individual cohorts were wide. For example, ORs (95% CIs) for the association of MCPP with overweight/obese status were 2.2 (0.88, 5.3) in MSSM, 2.6 (1.1, 6.4) in CCCEH, and 1.3 (0.41, 4.5) in HOME. Cohort modified associations of two metabolites, MnBP and MEP, with BMI z-scores such that beta coefficients were on the opposite sides of the null in MSSM compared to CCCEH and HOME. However, MSSM was the smallest cohort and CIs were imprecise.
Point and interval estimates of most associations were similar whether estimated in single or multiple metabolite models (see eTable 5). However, the association between urinary MCPP concentrations and overweight/obese status was attenuated toward the null in the single (OR = 1.4 [0.88, 2.3]) compared to the multiple (OR = 2.1 [1.2, 4.0]) metabolite model. Our sensitivity analysis for loss to follow-up indicated that children with higher BMI z-scores were more likely to be followed-up. However, associations between prenatal phthalate concentrations and overweight/obese status and BMI z-scores were similar whether or not we accounted for potentially nonignorable missing outcomes (see eTable 5). Findings were comparable in models with alternative specifications of the missing outcome indicator model (not shown).
Discussion
In this pooled analysis of three birth cohorts, each SD increase in prenatal urinary concentrations of MCPP was associated with greater than twice the odds of overweight or obesity among 4 to 7 year old children. MCPP is a non-specific metabolite of several high molecular weight phthalates (e.g., di-isooctyl phthalate, di-n-octyl phthalate (DnOP), di-isononyl phthalate, di-isodecyl phthalate) and a minor metabolite of dibutyl phthalate (DBP).26 We also observed inverse associations of prenatal concentrations of MEP, the main metabolite of diethyl phthalate (DEP), and ∑DEHP with BMI z-scores among girls.
Three prospective studies have assessed early life phthalate exposures and childhood adiposity.9,10,27 In a small Dutch study utilizing infant cord blood measures of oxidative DEHP metabolites, high cord blood concentrations of one metabolite, MEOHP, were inversely associated with BMI among infant boys.27 However, phthalate concentrations measured in cord blood are susceptible to contamination6 and may arise from hospital-based exposures to DEHP at delivery.28 Valvi et al.9 assessed associations between the average of first and third trimester maternal urinary summed low (∑LMWP: MEP, MnBP, MiBP) and high (∑HMWP: MBzP and 4 DEHP metabolites) molecular weight phthalate metabolites concentrations and childhood anthropometry in a Spanish birth cohort study. ∑HMWP metabolites concentrations were associated with weight gain z-scores in the first 6 months and BMI z-scores at ages 1, 4, and 7 years in a sex-specific manner (lower among boys, higher among girls), and supplemental analyses indicated stronger associations for ∑DEHP than MBzP. ∑LMWP metabolite concentrations were not associated with outcomes and MCPP was not assessed. In a previous analysis of the MSSM cohort,10 children in the highest tertile of third trimester urinary ∑DEHP concentrations had lower percent fat mass than children in the first tertile. Other metabolites were not associated with percent fat mass and there was no evidence of sex-specific effects, though the study had limited sample size.
We evaluated heterogeneity of associations by child’s sex because development of body fat differs between girls and boys, and certain phthalates, including DEHP and DBP, exhibit anti-androgenic activity.4 In the current study, ∑DEHP was inversely associated with BMI z-scores among girls but not boys. The previous Spanish study, where prenatal urinary concentrations of DEHP metabolites were higher, reported inverse associations of ∑DEHP with body size among boys but positive associations among girls.9 Studies of high dose postnatal DEHP exposure in rodents have reported reductions in body weight mediated by peroxisome-proliferator activated receptor alpha.29,30 However, animal studies of early life DEHP exposures report effects on body weight that depend on timing, dose, and species31–35 with only one study reporting notable sex differences.33
We also observed sexual dimorphism for metabolites of DBP (MnBP) and DEP (MEP), which have not been assessed as potential obesogens in animal studies. As DEP does not produce anti-androgen effects4 it has been asserted that associations of its metabolite MEP with outcomes related to androgen insufficiency in human studies may be due to its correlation with other phthalates.4 Our associations were co-adjusted but could have been confounded by phthalates not measured in this study or other endocrine disrupting chemicals contained in personal care products. Alternatively, DEP may be related to sex differences in other pathways related to development, such as effects on thyroid hormones.
Ashley-Martin et al.36 assessed associations of first trimester urinary phthalate metabolite concentrations with cord blood levels of two adipocyte-produced hormones. They reported associations of MCPP, but not other phthalate metabolites, with leptin and adiponectin levels. Female infants in the highest quartile of MCPP concentrations had higher odds of high adiponectin (OR = 2.9, 95% CI = 1.0, 7.8) while male infants in all quartiles of MCPP concentrations had higher odds of high leptin compared to the lowest quartile. Because early childhood adiposity has been positively associated with cord blood adiponectin concentrations and negatively associated with cord blood leptin,37 these sexually-dimorphic associations are consistent with our finding of a stronger association of MCPP concentrations with overweight/obesity among girls than boys.
We observed an association of MCPP with overweight/obese status but not with differences in continuous BMI z-scores. BMI does not distinguish between lean and fat mass, whereas classifying individuals as overweight or obese identifies those at the high end of the BMI distribution who are more likely to have excess body fat.17 In children, use of BMI cut points to identify those with excess body fat is moderately sensitive and highly specific.17 Consequently, the association of MCPP with overweight/obese status, but not BMI z-scores, may reflect that BMI is a less sensitive marker of adiposity or that MCPP exposures are associated only with changes in the upper end of the BMI z-score distribution. Future analyses could examine associations of MCPP with the full distribution of BMI rather than assessing changes in the population mean (for example, using quantile regression).
Like DEHP, DnOP and other high molecular weight phthalates are used in plastics such as food packaging.38 The strongest predictor of childhood overweight is maternal overweight,39 and we adjusted for maternal pre-pregnancy BMI and gestational weight gain to control for potential confounding through shared maternal and child diet. We lacked information on diet and cannot rule out confounding by dietary sources of a woman’s phthalate exposures that may also be related to obesity in her child. Urinary concentrations of MCPP and DEHP metabolites have been associated with consumption of similar foods (meat, diary, and other fatty foods),38 suggesting that any confounding of the MCPP and ∑DEHP associations with body fatness would be in the same direction. Therefore, the inverse association of ∑DEHP concentrations with overweight/obese status provides indirect evidence that MCPP associations are not strongly confounded by dietary sources of high molecular weight phthalates exposures.
Associations by race/ethnicity must be interpreted with caution. Because over 80% of the non-Hispanic white children in the study sample were HOME Study participants, differences in associations among white compared to black or Hispanic children may reflect differences in unmeasured characteristics of the HOME population. HOME Study mothers were older and more educated and their children were leaner compared to the other two cohorts, leading to potential differences by cohort in the magnitude of residual or unmeasured confounding (e.g., environmental tobacco smoke) as well as susceptibility to obesogenic effects. In addition, the higher prevalence of overweight/obesity among Hispanic children may have led to higher odds ratios in this group since the odds ratio overestimates the risk ratio when outcomes are common. Hispanic participants in MSSM were primarily of Puerto Rican origin whereas CCCEH Hispanics were Dominican, which may explain differences in estimates of association among Hispanic children in MSSM compared to CCCEH. Cohort-specific estimates among non-Hispanic black children, who were included in all three studies, were more comparable.
We measured phthalate metabolite concentrations in a single spot urine sample, which may not represent exposures throughout pregnancy because phthalates are quickly eliminated from the body40 and exposure to phthalates sources is likely episodic. A study of the variability of urinary phthalate metabolite concentrations in four spot urine samples collected during pregnancy (range: 5–38 weeks gestation) suggested that sources of exposure to some phthalates (e.g., DBPs) may be more consistent during pregnancy than others (e.g., DEHP).41 Given the potential for exposure misclassification, our associations may be biased. However, under the assumptions of monotonicity and independent, non-differential measurement error of the exposures and outcomes, we expect that effect estimates are in the same directions as the true associations.42 In addition, we measured phthalate metabolite concentrations during late pregnancy, a potential sensitive period for exposure to obesogens due to rapid fetal growth and adipocyte replication.43
We restricted our analysis to early childhood because phthalate exposures may affect puberty,44,45 a developmental stage that is also strongly tied to BMI. Evaluating associations among older children is warranted to determine whether associations persist with age and to evaluate the timing of potential obesogenic effects with respect to puberty.
Despite limitations noted above, this prospective study has several important strengths. We assessed exposures during fetal development, which is thought to be a susceptible window for the origins of obesity.3 Pooling data from three independent cohorts with notable variation in population characteristics strengthened the robustness of our findings. Pooling also provided a large sample size to assess heterogeneity of associations by hypothesized modifying factors. Although we tested a relatively large number of associations, we employed a conservative Bayesian modeling approach that is robust to multiple testing bias. We minimized bias by multiply imputing missing covariate data and controlling for confounding among correlated phthalate metabolites. Finally, we conducted sensitivity analyses to explore potential bias from loss to follow-up.
In conclusion, we observed a positive association of prenatal urinary concentrations of MCPP, a non-specific metabolite of several phthalates, with overweight/obese status in children aged 4 to 7 years. Our findings do not suggest that prenatal urinary concentrations of other phthalate metabolites are associated with overweight/obesity. Indeed, we observed inverse associations of MEP, the main metabolite of DEP, and ∑DEHP with BMI z-scores among girls, indicating that exposure to DEP or DEHP may alter physical development. However, we cannot rule out confounding (e.g., from other environmental obesogens) as an alternative explanation to our findings.
Supplementary Material
Acknowledgments
Source of Funding:
Research reported in this publication was supported by the National Institute of Environmental Health Sciences of the National Institutes of Health under Award Number R21ES021700. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. JPB was supported by training grants from NIEHS (T32 ES007018) and the Eunice Kennedy Shriver National Institute of Child Health and Human Development (T32-HD052468-05). AHH was supported by a grant from NIEHS (ES020619).
The Mount Sinai Children’s Environmental Health Study was supported by grants from NIEHS (ES009584), US EPA (R827039 and RD831711), ATSDR, and The New York Community Trust.
The Columbia Children’s Environmental Health Center was supported by grants from NIEHS (P50 ES09600, RO1 ES013543, RO1 ES08977, RO1 ES11158), US EPA (R827027, R82860901), Irving General Clinical Research Center (grant RR00645), Bauman Family Foundation, Gladys and Roland Harriman Foundation, Hansen Foundation, W. Alton Jones Foundation, New York Community Trust, Educational Foundation of America, New York Times Company Foundation, Rockefeller Financial Services, Horace W. Smith Foundation, Beldon Fund, John Merck Fund, New York Community Trust, and V. Kann Rasmussen Foundation.
The Cincinnati HOME Study was supported by NIEHS (P30ES10126) and NIEHS/EPA (PO1 ES11261).
We thank Manori Silva, Ella Samandar and Jim Preau for the measurement of the phthalate metabolites. The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
Footnotes
Conflicts of Interest:
The authors declare no conflicts of interest.
References
- 1.Ogden CL, Carroll MD, Kit BK, Flegal KM. Prevalence of childhood and adult obesity in the United States, 2011–2012. JAMA. 2014;311(8):806–814. doi: 10.1001/jama.2014.732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Daniels SR, Arnett DK, Eckel RH, Gidding SS, Hayman LL, Kumanyika S, Robinson TN, Scott BJ, St Jeor S, Williams CL. Overweight in children and adolescents: pathophysiology, consequences, prevention, and treatment. Circulation. 2005;111(15):1999–2012. doi: 10.1161/01.CIR.0000161369.71722.10. [DOI] [PubMed] [Google Scholar]
- 3.Grun F, Blumberg B. Environmental obesogens: organotins and endocrine disruption via nuclear receptor signaling. Endocrinology. 2006;147(6 Suppl):S50–S55. doi: 10.1210/en.2005-1129. [DOI] [PubMed] [Google Scholar]
- 4.National Science Foundation. Phthalates and cumulative risk assessment: the tasks ahead. Washington, DC: National Academies Press; 2008. [PubMed] [Google Scholar]
- 5.Schettler T. Human exposure to phthalates via consumer products. Int J Androl. 2006;29(1):134–139. doi: 10.1111/j.1365-2605.2005.00567.x. [DOI] [PubMed] [Google Scholar]
- 6.Calafat AM, Longnecker MP, Koch HM, Swan SH, Hauser R, Goldman LR, Lanphear BP, Rudel RA, Engel SM, Teitelbaum SL, Whyatt RM, Wolff MS. Optimal Exposure Biomarkers for Nonpersistent Chemicals in Environmental Epidemiology. Environ Health Perspect. 2015;123(7):A166–A168. doi: 10.1289/ehp.1510041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Braun JM, Sathyanarayana S, Hauser R. Phthalate exposure and children's health. Curr Opin Pediatr. 2013;25(2):247–254. doi: 10.1097/MOP.0b013e32835e1eb6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Grun F, Blumberg B. Endocrine disrupters as obesogens. Mol Cell Endocrinol. 2009;304(1–2):19–29. doi: 10.1016/j.mce.2009.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Valvi D, Casas M, Romaguera D, Monfort N, Ventura R, Martinez D, Sunyer J, Vrijheid M. Prenatal Phthalate Exposure and Childhood Growth and Blood Pressure: Evidence from the Spanish INMA-Sabadell Birth Cohort Study. Environ Health Perspect. 2015;123(10):1022–1029. doi: 10.1289/ehp.1408887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Buckley JP, Engel SM, Mendez MA, Richardson DB, Daniels JL, Calafat AM, Wolff MS, Herring AH. Prenatal phthalate exposures and childhood fat mass in a New York City cohort. Environ Health Perspect. doi: 10.1289/ehp.1509788. http://dx.doi.org/10.1289/ehp.1509788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wolff MS, Engel SM, Berkowitz GS, Ye X, Silva MJ, Zhu C, Wetmur J, Calafat AM. Prenatal phenol and phthalate exposures and birth outcomes. Environ Health Perspect. 2008;116(8):1092–1097. doi: 10.1289/ehp.11007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Whyatt RM, Barr DB, Camann DE, Kinney PL, Barr JR, Andrews HF, Hoepner LA, Garfinkel R, Hazi Y, Reyes A, Ramirez J, Cosme Y, Perera FP. Contemporary-use pesticides in personal air samples during pregnancy and blood samples at delivery among urban minority mothers and newborns. Environ Health Perspect. 2003;111(5):749–756. doi: 10.1289/ehp.5768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Braun JM, Yolton K, Dietrich KN, Hornung R, Ye X, Calafat AM, Lanphear BP. Prenatal bisphenol A exposure and early childhood behavior. Environ Health Perspect. 2009;117(12):1945–1952. doi: 10.1289/ehp.0900979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Benowitz NL, Bernert JT, Caraballo RS, Holiday DB, Wang J. Optimal serum cotinine levels for distinguishing cigarette smokers and nonsmokers within different racial/ethnic groups in the United States between 1999 and 2004. Am J Epidemiol. 2009;169(2):236–248. doi: 10.1093/aje/kwn301. [DOI] [PubMed] [Google Scholar]
- 15.Kato K, Silva MJ, Needham LL, Calafat AM. Determination of 16 phthalate metabolites in urine using automated sample preparation and on-line preconcentration/high-performance liquid chromatography/tandem mass spectrometry. Anal Chem. 2005;77(9):2985–2991. doi: 10.1021/ac0481248. [DOI] [PubMed] [Google Scholar]
- 16.Silva MJ, Samandar E, Preau JL, Jr, Reidy JA, Needham LL, Calafat AM. Quantification of 22 phthalate metabolites in human urine. J Chromatogr B Analyt Technol Biomed Life Sci. 2007;860(1):106–112. doi: 10.1016/j.jchromb.2007.10.023. [DOI] [PubMed] [Google Scholar]
- 17.Freedman DS, Sherry B. The validity of BMI as an indicator of body fatness and risk among children. Pediatrics. 2009;124(Suppl 1):S23–S34. doi: 10.1542/peds.2008-3586E. [DOI] [PubMed] [Google Scholar]
- 18.Centers for Disease Control and Prevention. A SAS Program for the CDC Growth Charts. Atlanta, GA: Centers for Disease Control and Prevention; 2004. [Google Scholar]
- 19.MacLehose RF, Dunson DB, Herring AH, Hoppin JA. Bayesian methods for highly correlated exposure data. Epidemiology. 2007;18(2):199–207. doi: 10.1097/01.ede.0000256320.30737.c0. [DOI] [PubMed] [Google Scholar]
- 20.Barr DB, Wilder LC, Caudill SP, Gonzalez AJ, Needham LL, Pirkle JL. Urinary creatinine concentrations in the U.S. population: implications for urinary biologic monitoring measurements. Environ Health Perspect. 2005;113(2):192–200. doi: 10.1289/ehp.7337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yeh HC, Lin YS, Kuo CC, Weidemann D, Weaver V, Fadrowski J, Neu A, Navas-Acien A. Urine osmolality in the US population: implications for environmental biomonitoring. Environ Res. 2015;136:482–490. doi: 10.1016/j.envres.2014.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gelman A, Carlin JB, Stern HS, Dunson DB, Vehtari A, Rubin DB. Bayesian data analysis. CRC press; 2013. [Google Scholar]
- 23.Howe CJ, Cole SR, Westreich DJ, Greenland S, Napravnik S, Eron JJ., Jr Splines for trend analysis and continuous confounder control. Epidemiology. 2011;22(6):874–875. doi: 10.1097/EDE.0b013e31823029dd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Little RJA, Rubin DB. Statistical Analysis with Missing Data. 2nd. New York: John Wiley; 2002. [Google Scholar]
- 25.Rundle A, Hoepner L, Hassoun A, Oberfield S, Freyer G, Holmes D, Reyes M, Quinn J, Camann D, Perera F, Whyatt R. Association of childhood obesity with maternal exposure to ambient air polycyclic aromatic hydrocarbons during pregnancy. Am J Epidemiol. 2012;175(11):1163–1172. doi: 10.1093/aje/kwr455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Calafat AM, Silva MJ, Reidy JA, Earl Gray L, Samandar E, Preau JL, Herbert AR, Needham LL. Mono-(3-carboxypropyl) phthalate, a metabolite of di-n-octyl phthalate. J Toxicol Environ Health A. 2006;69(3–4):215–227. doi: 10.1080/15287390500227381. [DOI] [PubMed] [Google Scholar]
- 27.de Cock M, de Boer MR, Lamoree M, Legler J, van de Bor M. First Year Growth in Relation to Prenatal Exposure to Endocrine Disruptors - A Dutch Prospective Cohort Study. Int J Environ Res Public Health. 2014;11(7):7001–7021. doi: 10.3390/ijerph110707001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yan X, Calafat A, Lashley S, Smulian J, Ananth C, Barr D, Silva M, Ledoux T, Hore P, Robson MG. Phthalates Biomarker Identification and Exposure Estimates in a Population of Pregnant Women. Hum Ecol Risk Assess. 2009;15(3):565–578. doi: 10.1080/10807030902892554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Itsuki-Yoneda A, Kimoto M, Tsuji H, Hiemori M, Yamashita H. Effect of a hypolipidemic drug, Di (2-ethylhexyl) phthalate, on mRNA-expression associated fatty acid and acetate metabolism in rat tissues. Biosci Biotechnol Biochem. 2007;71(2):414–420. doi: 10.1271/bbb.60478. [DOI] [PubMed] [Google Scholar]
- 30.Xie Y, Yang Q, Nelson BD, DePierre JW. Characterization of the adipose tissue atrophy induced by peroxisome proliferators in mice. Lipids. 2002;37(2):139–146. doi: 10.1007/s11745-002-0873-7. [DOI] [PubMed] [Google Scholar]
- 31.Campioli E, Martinez-Arguelles DB, Papadopoulos V. In utero exposure to the endocrine disruptor di-(2-ethylhexyl) phthalate promotes local adipose and systemic inflammation in adult male offspring. Nutr Diabetes. 2014;4:e115. doi: 10.1038/nutd.2014.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Feige JN, Gerber A, Casals-Casas C, Yang Q, Winkler C, Bedu E, Bueno M, Gelman L, Auwerx J, Gonzalez FJ, Desvergne B. The pollutant diethylhexyl phthalate regulates hepatic energy metabolism via species-specific PPARalpha-dependent mechanisms. Environ Health Perspect. 2010;118(2):234–241. doi: 10.1289/ehp.0901217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hao C, Cheng X, Xia H, Ma X. The endocrine disruptor mono-(2-ethylhexyl) phthalate promotes adipocyte differentiation and induces obesity in mice. Biosci Rep. 2012;32(6):619–629. doi: 10.1042/BSR20120042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kobayashi K, Miyagawa M, Wang RS, Suda M, Sekiguchi S, Honma T. Effects of in utero and lactational exposure to di(2-ethylhexyl)phthalate on somatic and physical development in rat offspring. Ind Health. 2006;44(4):652–660. doi: 10.2486/indhealth.44.652. [DOI] [PubMed] [Google Scholar]
- 35.Schmidt JS, Schaedlich K, Fiandanese N, Pocar P, Fischer B. Effects of di(2-ethylhexyl) phthalate (DEHP) on female fertility and adipogenesis in C3H/N mice. Environ Health Perspect. 2012;120(8):1123–1129. doi: 10.1289/ehp.1104016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ashley-Martin J, Dodds L, Arbuckle TE, Ettinger AS, Shapiro G, Fisher M, Morisset AS, Taback S, Bouchard MF, Monnier P, Dallaire R, Fraser WD. A birth cohort study to investigate the association between prenatal phthalate and bisphenol A exposures and fetal markers of metabolic dysfunction. Environ Health. 2014;13(1):84. doi: 10.1186/1476-069X-13-84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mantzoros CS, Rifas-Shiman SL, Williams CJ, Fargnoli JL, Kelesidis T, Gillman MW. Cord blood leptin and adiponectin as predictors of adiposity in children at 3 years of age: a prospective cohort study. Pediatrics. 2009;123(2):682–689. doi: 10.1542/peds.2008-0343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Serrano SE, Braun J, Trasande L, Dills R, Sathyanarayana S. Phthalates and diet: a review of the food monitoring and epidemiology data. Environ Health. 2014;13(1):43. doi: 10.1186/1476-069X-13-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Salsberry PJ, Reagan PB. Dynamics of early childhood overweight. Pediatrics. 2005;116(6):1329–1338. doi: 10.1542/peds.2004-2583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Koch HM, Bolt HM, Preuss R, Angerer J. New metabolites of di(2-ethylhexyl)phthalate (DEHP) in human urine and serum after single oral doses of deuterium-labelled DEHP. Arch Toxicol. 2005;79(7):367–376. doi: 10.1007/s00204-004-0642-4. [DOI] [PubMed] [Google Scholar]
- 41.Ferguson KK, McElrath TF, Ko YA, Mukherjee B, Meeker JD. Variability in urinary phthalate metabolite levels across pregnancy and sensitive windows of exposure for the risk of preterm birth. Environ Int. 2014;70:118–124. doi: 10.1016/j.envint.2014.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.VanderWeele TJ, Hernan MA. Results on differential and dependent measurement error of the exposure and the outcome using signed directed acyclic graphs. Am J Epidemiol. 2012;175(12):1303–1310. doi: 10.1093/aje/kwr458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dietz WH. Critical periods in childhood for the development of obesity. Am J Clin Nutr. 1994;59(5):955–959. doi: 10.1093/ajcn/59.5.955. [DOI] [PubMed] [Google Scholar]
- 44.Ferguson KK, Peterson KE, Lee JM, Mercado-Garcia A, Blank-Goldenberg C, Tellez-Rojo MM, Meeker JD. Prenatal and peripubertal phthalates and bisphenol A in relation to sex hormones and puberty in boys. Reprod Toxicol. 2014;47:70–76. doi: 10.1016/j.reprotox.2014.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wolff MS, Teitelbaum SL, McGovern K, Windham GC, Pinney SM, Galvez M, Calafat AM, Kushi LH, Biro FM, Breast C, Environment Research P. Phthalate exposure and pubertal development in a longitudinal study of US girls. Hum Reprod. 2014;29(7):1558–1566. doi: 10.1093/humrep/deu081. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.