Abstract
Silicone wristbands can be used as passive sampling tools for measuring personal environmental exposure to organic compounds. Due to the lightweight and simple design, the wristband may be a useful technique for measuring children’s exposure. In this study, we tested the stability of flame retardant compounds in silicone wristbands and developed an analytical approach for measuring 41 flame retardants in the silicone wristband in order to evaluate exposure to these compounds in preschool-aged children. To evaluate the robustness of using wristbands to measure flame retardants, we evaluated the stability of 3 polybrominated diphenyl ethers (BDEs), and 2 organophosphate flame retardants (OPFRs) in wristbands over 84 days and did not find any evidence of significant loss over time at either 4 or −20°C (p > 0.16). We recruited a cohort of 92 preschool aged children in Oregon to wear the wristband for 7 days in order to characterize children’s acceptance of the technology, and to characterize their exposure to flame retardants. Seventy-seven parents returned the wristbands for analysis of 35 BDEs, 4 OPFRs, and 2 other brominated flame retardants although 5 were excluded from the exposure assessment due to protocol deviations (n=72). A total of 20 compounds were detected above the limit of quantitation, and 11 compounds including 4 OPFRs and 7 BDEs were detected in over 60% of the samples. Children’s gender, age, race, recruitment site, and family context were not significantly associated with returning wristbands or compliance with protocols. Comparisons between flame retardant data and socio-demographic information revealed significant differences in total exposures to both ΣBDEs and ΣOPFRs based on age of house, vacuuming frequency, and family context. These results demonstrate that preschool children in Oregon are exposed to BDEs that are no longer being produced in the United States and to OPFRs that have been used as an alternative to polybrominated compounds. Silicone wristbands were well tolerated by young children and were useful for characterizing personal exposure to flame retardants that were not bound to particulate matter.
Keywords: personal monitoring, exposure assessment, BDEs, OPFRs, triphenyl phosphate
1. INTRODUCTION
Polybrominated diphenyl ethers (BDEs), organophosphate flame retardants (OPFRs), and other brominated flame retardants (BFRs) such as bis(2-ethylhexyl)-2,3,4,5-tetrabromophthalate, and 2-ethylhexyl 2,3,4,5-tetrabromobenzoate are incorporated into building materials, polyurethane foam, and electronic products to meet flammability standards (Stapleton et al., 2011; Stapleton et al., 2009). As these compounds are released from these products, they enter the indoor environment where they may accumulate in dust and human tissues (Ryan and Rawn, 2014; Sjodin et al., 2008a; Sjodin et al, 2008b; Sundkvist et al., 2010; Thomsen et al., 2010). Due to widespread occurrence, the ability to bioaccumulate, and concerns about toxicity, the European Union banned the marketing and use of pentaBDE (BDEs: 17, 28, 47, 66, 85, 99, 100, 138, 153, 154) and octaBDE (BDEs: 153, 183, 196, 197, 203, 206, 207 and 209) effective in 2004 (The European Parliament, 2003), and phased them out of electronic equipment by 2006 (La Guardia et al., 2006; The European Parliament, 2003). DecaBDE has been phased out in electronics under the European Court of Justice for the European Union (2008), but risk assessments are still on-going to characterize direct and indirect risks of this technical mixture (Alcock et al., 2011). During this same time period, manufacturers in the United States voluntarily ceased production of pentaBDE and many states adopted legislation to ban the manufacture and distribution of commercial products containing pentaBDE and octaBDE(van der Veen and de Boer, 2012). Additionally, the United Nations Environmental Programme has included tetraBDE, pentaBDE, hexaBDE, and heptaBDE to the inventory of chemicals monitored through the Stockholm Convention (UNEP, 2009).
Since these restrictions have gone into effect, data indicates that BDEs are still detected frequently in environmental and biomonitoring studies, but the concentration of the restricted compounds may be stabilizing or even decreasing in some populations (Fang et al., 2015; Law et al., 2014).. Restrictions on BDEs, has led to an increased use of OPFRs including triphenyl phosphate (TPHP), tris(1,3-dichloroisopropyl) phosphate (TDCIPP), tris(2-chloroethyl) phosphate (TCEP), and tris(1-chloro-2-propyl) phosphate (TCPP) (van der Veen and de Boer, 2012). In addition to their use as flame retardants, these OPFRs are commonly added to varnishes and floor polishes as a plasticizer (van der Veen and de Boer, 2012). OPFRs have been detected in water, sediment, and indoor air (van der Veen and de Boer, 2012), as well as human breast milk (Sundkvist et al., 2010). This indicates that OPFRs are pervasive in the environment and human exposure is occurring.
Currently, there are several methods for measuring exposure to BDEs and OPFRs including active and passive air sampling (Jaward et al., 2004; Bergh et al., 2011; van der Veen and de Boer, 2012), dust and hand wipe sampling (Bergh et al., 2011; Stapleton et al., 2014), and biological monitoring (Ryan and Rawn, 2014; Sundkvist et al., 2010; Cooper et al., 2011; Sjodin et al., 2008b). Current exposure assessment methods typically focus on measuring flame retardants associated with particles such as house dust or in human serum. Conversely, passive samplers rely on diffusion of volatile and semi-volatile chemicals into the polymer (e.g. silicone) or receiving phase. Compounds are not simply adsorbed to the surface of the membrane, but absorbed into and permeate throughout the polymer over time. As chemicals diffuse and accumulate in passive samplers, they represent the unbound fraction of contaminants (or the freely dissolved fraction in water, and vapor phase in air). These fractions are a surrogate for exposure that is biologically relevant (Anderson and Hillwalker, 2008). A recent advance in personal passive sampling devices (PSDs) using commercially-available silicone wristbands offers many benefits for measuring a wide range of organic compounds. Specific benefits include their ease of use and familiarity to the general public through their adoption in health awareness campaigns such as LIVESTRONG© wristbands for supporting cancer research. To date, silicone wristbands have been used to quantify exposure to polycyclic aromatic hydrocarbons (PAHs) worn in an occupational setting, but it was not possible to quantitatively measure flame retardants at that time (O'Connell et al., 2014). Since accurate measurement of chemical exposure is a critical component for estimating health effects, a quantitative methodology that can measure volatile and semi-volatile flame retardants in silicone wristband passive samplers would complement current particulate-based and biological monitoring methods. Furthermore, the simplicity of these samplers to be worn as bracelets or anklets potentially makes them beneficial to monitoring children’s chemical exposures.
The objectives for this study were three-fold: i) demonstrate that volatile and semi-volatile flame retardants (e.g. BDEs and OPFRs) are captured, stable, and recovered from wristband passive samplers; ii) compare and characterize flame retardants detected in silicone wristbands worn by preschool children; and iii) determine if concentrations found in the children’s wristbands are correlated with selected socio-demographic and household characteristics.
2. MATERIALS AND METHODS
2.1 Reagents
Analytical grade standards were obtained from Accustandard (New Haven, CT) as either single 50µg/mL analyte solutions, or as a composite at 10µg/mL. In total, 36 BDEs, 4 OPFRs, and 2 BFRs were obtained for analyses. For a complete list of individual compounds, see Supplemental Information Table-S1. Acronyms for flame retardants were taken from a recent publication addressing the uncertainty in some abbreviations (Bergman et al., 2012). Perylene-d12, 2 fluorinated polybrominated biphenyl ethers (FBDEs), and 1 brominated biphenyl (2-BBP) were used as reference standards and were obtained from either ChemService (for perylene-d12, West Chester, PA) or Accustandard (FBDEs and 2-BBP). All solvents were Optima-grade or equivalent (Fisher Scientific, Pittsburgh, PA), and all laboratory water used for infusions or post-deployment cleaning was from a Barnstead purifier (Dubuque, IA).
2.2 Wristband cleaning, deployment, and extraction
Prior to deployment, wristbands (width: 1.3 cm; inner diameter: 5.8 cm, 24 hour wristbands.com, Houston, TX) were soaked with ethyl acetate, hexane and methanol at 30°C to remove analytical interferences as previously described (O'Connell et al., 2014). Wristbands had an average weight of 4.64g ± 0.03g, and were stored at −20°C after treatment. Each batch of solvent-cleaned wristbands was evaluated with criteria for quality control QC purposes, and is described further in the supporting information. Wristbands were transported to and from the field in clean, air-tight polytetrafluoroethylene (PTFE) bags as previously described.(O'Connell et al., 2014) Wristbands and PTFE bags were given to the parents, along with instructions on use and a pre-paid, self-addressed business envelope in which to return the PSD to the laboratory. Parents were instructed to remove excess air in the PTFE bag prior to transport by squeezing the bag prior to sealing in order to minimizing headspace. Trip blanks were mailed in the same manner as samples. After deployment, PSDs were rinsed with water twice to remove any surface debris, and quickly rinsed with isopropanol to remove any additional surface material along with residual water as reported previously (O'Connell et al., 2014) and stored in amber jars at −20°C until extraction. Visual inspection showed no particles on any wristband after rinsing.
A recovery surrogate, either FBDE-118 or FBDE-126 was added at 500 ng prior to all sample extractions, with either FBDE performing similarly (average recovery of FBDE-118 was 73 ± 32 %, and FBDE-126 was 93 ± 29 %). Compounds were removed from the silicone wristband with two rounds of 100 mL of ethyl acetate as previously described (O'Connell et al., 2014). The ethyl acetate was combined and reduced to nominally 300 µL with nitrogen evaporators (Turbo-Vap L, Biotage, Charlotte, NC and N-EVAP 111, Organomation Associates Inc., Berlin, MA). Three mL of acetonitrile was added to each sample, and extracts were loaded onto 500mg C18 SPE (solid phase extraction) cartridges (Supelco, Bellefonte, PA). The cartridges were pre-rinsed with 6 mL of acetonitrile at 3mL/min. Samples were loaded at 1.8mL/min and finally eluted with 9 mL of acetonitrile at 3mL/min using a Rapid Trace, automated SPE workstation (Biotage, Uppsala, Sweden). The 9 mL extracts were reduced as above with Turbo-Vaps to 0.5mL, and solvent-exchanged to hexane to be more amenable to GC analysis. Rationale for this additional cleanup derived from early results identified in earlier wristband analyses that revealed high fatty acids and/or personal care products in some samples that might interfere with analyses (O'Connell et al., 2014). Additionally, other work with high fat biological material showed advantages to using acetonitrile, resulting in less GC-MS background of carbohydrates, lipids, and proteins than other solvents (Forsberg et al., 2011; Lehotay et al., 2001). Since the SPE steps involved additional manipulation of the samples than previously reported, quality control samples were assessed, and consisted of mixtures of all 41 compounds added to several blank wristband extracts prior to the SPE steps at two concentrations, 10 ng/mL and 100 ng/mL. The resulting QC extracts were used to evaluate recovery and any matrix interferences with the additional SPE cleanup.
2.3 Wristband flame retardant capture, transport, and storage stability
To simulate capture and stability of flame retardants within the samplers, 52 wristbands were infused with TPHP, TCDPP, BDE-47, BDE-99 and BDE-154. These compounds represent both current and banned flame retardants, and span a wide range in physical chemical properties, with log Koa ranging from 8.5 to 13.3, and log Kow from 3.7 to 8.6, covering much of the chemical diversity of our study compounds. In a method described previously (Booij et al., 2002), 1 liter amber jars were each filled with 400 mL MeOH and flame retardant spiking standards. Next, 8–10 wristbands were added one at a time to each jar. Water was added next to make a 1:1 mixture of MeOH:H2O, and the solution and wristbands were left mixing at 60 rpm for 3 days. Immediately after the 3 day infusion, 12 random wristbands were extracted from the infusion set, which illustrated that the wristbands can absorb flame retardants, and represented starting concentrations (t=0). All other wristbands were stored in clean, air-tight polytetrafluoroethylene (PTFE) bags under various temperatures and time points described below. Replication in the storage stability experiments consisted of four wristbands extracted for each time and temperature scenario. Wristbands were compared to starting concentrations after 7 and 14 days following storage at both 4°C and 30°C simulating transportation of the wristbands from participants to the laboratory. The 30°C temperature and 14 day samplers represent a “worse-case” scenario for transport, while the 4°C and 7 day sets test transport under more ideal conditions.
The stability of flame retardants in wristbands was evaluated at −20°C, and included 7, 14, 28, and 84 days where the frozen temperature and lack of UV light represents ideal storage conditions. Longer-term accelerated storage stability studies are generally performed at least 10°C higher than the actual storage temperature (ICH, 2003). The degradation time is nominally double based on typical reaction rates that increases at a factor of 2 for every 10°C increase. Our ideal storage, −20°C, was used as the basis of our stability studies, and the accelerated storage stability conditions were performed at 4°C, an increase of 24°C for 28 and 84 days. Therefore, the assumption is that the stability at 4°C for 84 days is the equivalent to about 500 days (84 × 22.4) at −20°C.
2.4 Instrumental analysis
Aliquots were fortified with perylene-d12 at 500 ng as the internal standard prior to analysis. Samples were analyzed by GC-MS with an Agilent 7890A gas chromatograph (Santa Clara, CA) interfaced with an Agilent 5975C mass spectrometer equipped with a triple axis detector. The GC-MS was operated in electron impact mode, operating at 70eV, with the MS source and quadruple temperature set to 250°C and 150°C respectively. The MS detector transfer line was set at 300°C.
The 41 flame retardant GC-MS oven profile was set to have an initial temperature of 90°C for 1.25 minutes, followed by a 10°C/min ramp to 240°C. The temperature ramp was then increased to 20°C/min to 310°C where it held for 10 minutes. One µl was loaded onto a DB5-MS column through a dimpled liner (Agilent, 2mm i.d.) with pulsed splitless injection at 290 °C. The pulse pressure was set to 30psi for 0.5 minutes with a 3mL/min septum purge and a 35mL/min purge to the split vent after one minute. An example of the resulting chromatography can be observed in the supporting information (SI-Figure 1). Limits of detection (LODs) were determined using an EPA method (EPA, 1995), and described further in the supporting information. Limits of quantitation LOQs were set as 5-fold higher than the LODs.
2.5 Population sample and data collection
From October 2012 to January 2013, 92 children aged 3–5 years old were recruited through preschools in two counties in the state of Oregon. Overall all, 36% of this population was female, and 64% was male. Children’s ages ranged from 3.12 – 5.75 years. All research activities were approved by Oregon State University’s Institutional Review Board. All parents gave informed written consent and children gave verbal assent before partaking in any research activity. Researchers visited participants’ homes where they asked parents to complete a series of structured questionnaires to capture socio-demographic information (e.g. household income, parental education levels, race, etc.) and household characteristics (e.g. housing stock characteristics, housekeeping behaviors, etc.). Each child was given a wristband to wear around their wrist or ankle. Parents were requested to have their child wear the wristband continuously for 7 days, although it could be taken off at night and placed next to the child’s bed on a table if preferred. After 7 days, parents were instructed to seal the wristband in the PTFE bag, note the number of days the child wore the wristband on the chain of custody label, and place it in the mail. Analytical results were reported back to the participants.
2.6 Quality control samples
To ensure data quality, over 30% of the total samples analyzed were QC samples. QC included two trip blanks: non-deployed wristbands that were placed in sealed PTFE bags during wristband deployment and mailed via the United States Postal Service from both field locations to Oregon State University. Other QC analyzed during the study included continuing calibration verification standards for instrument performance (CCVs), instrument blanks (IBs), extraction blanks, post-deployment cleaning blanks, and SPE blanks.
All CCVs were within 15% of the true value for all compounds on 12 separate days indicating that instrument performance remained consistent throughout the analyses. All analytes measured in one trip blank were below their respective LOQs. The second trip blank had 40 analytes below limits of quantitation, and 1 analyte above the LOQ, TPHP. However, while TPHP was above the LOQ in this single QC sample, it was 6 fold lower than the lowest TPHP concentration found in children’s wristband, and therefore no correction was made to the sample set.
2.7 Statistical Analysis
In the stability studies, samples were analyzed with two-way analysis of variance (ANOVA) if the data passed assumptions of equal variances and normal distributions with Bartlett’s and Shapiro-Wilk tests, respectively. If assumptions were not met, non-parametric Kruskal-Wallis tests were used (i.e. for time and for temperature). Outliers, defined as a studentized residual beyond ± 2.5, were removed prior to statistical analysis and parametric assumptions. Only 6 data points in the experimental data were identified as outliers out of the 156 measurements used to evaluate the capture, retention and characterization of 41 flame retardants under varying laboratory conditions. Data were determined to be parametric for TPHP, and data were non-parametric for TDCIPP, BDE-47, BDE-99 and BDE-154.
The concentrations of each flame retardant collected from preschool children, was normalized by the mass of the average wristband (4.64g) and by the number of days the parent reported that the child wore the wristband since not all children wore the PSD for the requested duration. This resulted in units of ng flame retardant per gram silicone per day (ng/g/day). This information was used to calculate descriptive statistics including mean, standard deviation, and range of each flame retardant. Compliance with returning the wristband and wearing it for 7 days was evaluated with regards to selected sociodemographic variables using chi-square tests and linear regression. Due to the small sample size of some of the selected socio-demographic characteristics, we created an aggregated variable, “family context”. This family context score was created using parents self-reported total years of education (e.g. 12 = completed high school; 16 = 4-yr college degree, etc.), self-reported parental employment status (e,g, part- or full-time were coded as “1”; not employed were coded as “0”), annual self-reported household income (e.g. scale from 1 = <$22,000 to 8 = ≥$70,001), and the home learning environment was measured with 14 items from the Parenting Questionnaire related to literacy and numeracy activities in the household (Morrison and Cooney, 2002). Then, values for all six variables were standardized and averaged to create the family context variable (Cronbach’s alpha = 0.74). As such, the family context score is a surrogate for the household’s socio-economic status. A higher score would be indicative of a higher level of maternal education, greater household income, and a more enriched home learning environment.
For the 11 compounds that were detected at or above the LOD in at least 60% of the wristbands worn by children, further statistical tests were conducted. Compounds below the LOD were assigned a value equal to the LOD divided by the square root of 2. Since the concentrations were highly skewed, data was transformed to their natural log to approximate a more normal distribution. Spearman correlation coefficients were computed to evaluate the relationship between individual compounds (SI-Table 2). Since congeners in the same class were more closely correlated with each other (SI-Table 2), we created a sum score for the different flame retardant classes when assessing the association between exposures to selected socio-demographic characteristics to minimize the number of comparisons [lnΣ(BDEs) and lnΣ(OPFRs)]. Then, general linear regression models were used to evaluate the association between these summed scores and selected sociodemographic data and household characteristics to identify factors that could be associated with these exposures (SI-Table 3).
3. RESULTS AND DISCUSSION
3.1 Wristband Capture and Stability of flame retardants
An efficient flame retardant analytical method was optimized for analyzing all 41 flame retardants used in this study. Method performance was good, and the LOQ in wristbands ranged from 0.77 to 26.5 ng/g wristband depending on the analyte. Overall, our LODs were lower by a factor of 2–10 depending on the flame retardant compared with recently reported limits of detection in a similar method (Ryan and Rawn, 2014). Additional details about method performance including the additional SPE cleanup steps can be found in the supporting information.
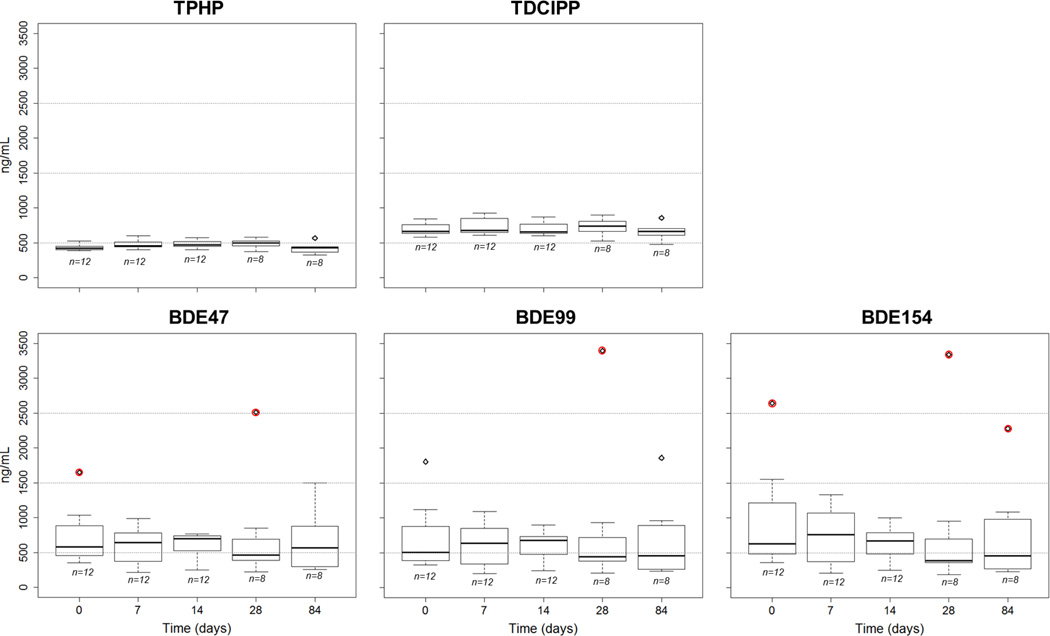
Using data collected in laboratory experiments, we examined wristband transport and storage stability to determine if temperature or duration influenced the recovery of compounds from the wristband passive samplers. Compound stability and potential degradation during transport and storage of flame retardants are commonly cited problems in complex environmental matrices (La Guardia et al., 2006). For instance, epidemiological studies requiring collection of biological samples in the field require special transport considerations, including freezing samples immediately upon collection, which can further complicate a study design and reduce compliance.(Cariou et al., 2005) Furthermore, storage stability data is not always preformed or reported which can contribute to misinterpretation of data. In our stability investigation, the recovered concentrations of the flame retardants did not statistically differ by time in storage (p>0.16 for all compounds), or between temperature treatments (p>0.30 for all compounds). Figure 1 shows all replicates for each time point. Standard deviations were higher than expected for BDEs throughout the stability study, and was traced to the setup used for the initial infusion process highlighted further in the supporting information. However, only 6 outliers were identified (and excluded) out of 156 measurements (Figure 1). The experimental results illustrate that no significant loss of flame retardants occurred after the wristband samplers were placed in the air-tight containers over the time period studied, including the 4°C temperature out to 84 days. If the degradation process proceeds as according to accelerated storage assumptions, then the stability of the flame retardants at −20°C extends well beyond 84 days, and possibly up to 500. Thus, the compounds extracted in the laboratory can reasonably be interpreted as the concentrations that were in the sampler at the end of the preschool study deployment. This is because all wristbands worn by the participants in the preschool demonstration were transported over conditions less severe than those measured in the transport and storage experiments. The lack of degradation of flame retardants at elevated temperatures (e.g. 30°C) or over longer periods of time (e.g. 84 days) provides many practical advantages with regards to shipping samples and time to analysis.
Figure 1.
Boxplots illustrating transport and storage stability for five flame retardants at five time points (0, 7, 14, 28 and 84 days). The number of wristbands for each time point is listed under each boxplot. Outliers circled in red were removed prior to statistical tests by having studentized residuals beyond ±2.5. Data from each time point represents the aggregate of multiple temperatures (−20, 4, 30°C), and are shown here because no statistical differences between any temperature treatment were found across the dataset for any compound (p >0.3). Boxplots show the median (black line), and the edges of each box (bottom and top) are drawn from the first and third quartiles illustrating the interquartile range (IRQ), and whiskers are drawn to the data ranging between 1.5 X IRQ below the first and above the third quartile.
3.2 Flame retardant in wristbands worn by pre-school aged children
Of the 92 wristbands distributed to children, 84% (n=77) were returned by parents to Oregon State University through the mail. Compliance with returning the wristband or wearing it for all 7 days did not differ between county (p=0.43), ethnicity (p=0.32), child age (p=0.41), or family context (p=0.29, Table 1). Parents reported that the silicone wristbands were easily worn by their preschool aged children. There were very few issues noted by parents who returned wristbands: one parent reported that the child did not want to wear it at night, and two parents reported their child did not want to wear it all day and would take it on and off. Some children seemed to like the wristbands and called them “their own personal science bracelet.” Sixty-four percent (N=50) of the children wore the wristband for all 7 days, indicating reasonable compliance for all phases of the protocol. No socio-demographic factors (e.g. county, ethnicity, child age, or family context) differed between those families that returned the wristband and those that did not. Nor did these factors differ between children who wore the wristband for all seven days compared to those who only wore it for less than 7 days.
Table 1.
Children’s compliance data from caretaker questionnaires. We compared selected characteristics of the 92 preschool children recruited in Oregon in comparison to the 77 children who returned the PSD (2012–2013). The frequency of the selected characteristics of the participants who returned the PSD were consistent with the larger sample.
| Overall | Returned PSD | p-value | |||
|---|---|---|---|---|---|
| (n=92) | (n=77) | ||||
| Selected Characteristics | N | Frequency (%) |
N | Frequency (%) |
|
| County | |||||
| Benton | 29 | 32 | 26 | 34 | 0.29* |
| Deschutes | 63 | 69 | 51 | 66 | |
| Sex | 0.96* | ||||
| Boys | 59 | 65 | 50 | 50 | |
| Girls | 32 | 35 | 27 | 77 | |
| Missing/Refused | 1 | ||||
| Ethnicity | |||||
| White | 74 | 80 | 64 | 83 | 0.14* |
| Other | 18 | 20 | 13 | 17 | |
| Maternal Education | |||||
| No degree | 4 | 5 | 3 | 4 | 0.06^ |
| High school | 17 | 20 | 12 | 16 | |
| College degree (2 or 4 yrs.) | 8 | 9 | 8 | 11 | |
| Graduate degree | 49 | 57 | 46 | 61 | |
| Other | 8 | 9 | 6 | 8 | |
| Missing/Refused | 6 | 2 | |||
| Paternal Education | |||||
| No degree | 3 | 5 | 3 | 5 | 0.96^ |
| High school | 11 | 18 | 10 | 18 | |
| College degree (2 or 4 yrs.) | 7 | 11 | 6 | 11 | |
| Graduate degree | 40 | 65 | 36 | 64 | |
| Other | 1 | 2 | 1 | 2 | |
| Missing/Refused | 30 | 21 | |||
| Income | |||||
| <$22,000 | 20 | 23 | 16 | 21 | 0.53^ |
| $22,001 – $70,000 | 35 | 41 | 31 | 41 | |
| >$70,000 | 31 | 36 | 28 | 37 | |
| Missing/Refused | 6 |
Mean (SD) |
2 |
Mean (SD) |
|
| Family Context Score | 89 | −0.03 (0.68) |
77 | 0.03 (0.64) |
0.08ǂ |
Chi-square test
Fisher exact test
Linear regression
For the subsequent statistical analysis and all 77 wristbands returned to the laboratory, six were returned without data regarding the number of days the wristband was worn by the child, and were assumed to be worn for 7 days for the following statistical analysis. By assuming the maximum time frame, this decision provides the most conservative estimate of exposure. Additionally, five wristbands were excluded from the following statistical analysis due to parent reports of substantial deviance from the protocol (e.g. never worn by child, lost at school for several weeks, laundered, etc.) resulting in a final sample size of 72 for the demonstration. While compliance was not 100%, we found that relying on parents to return the PSD via the mail greatly simplified logistics by eliminating the need to re-contact participants or collect the wristbands in person.
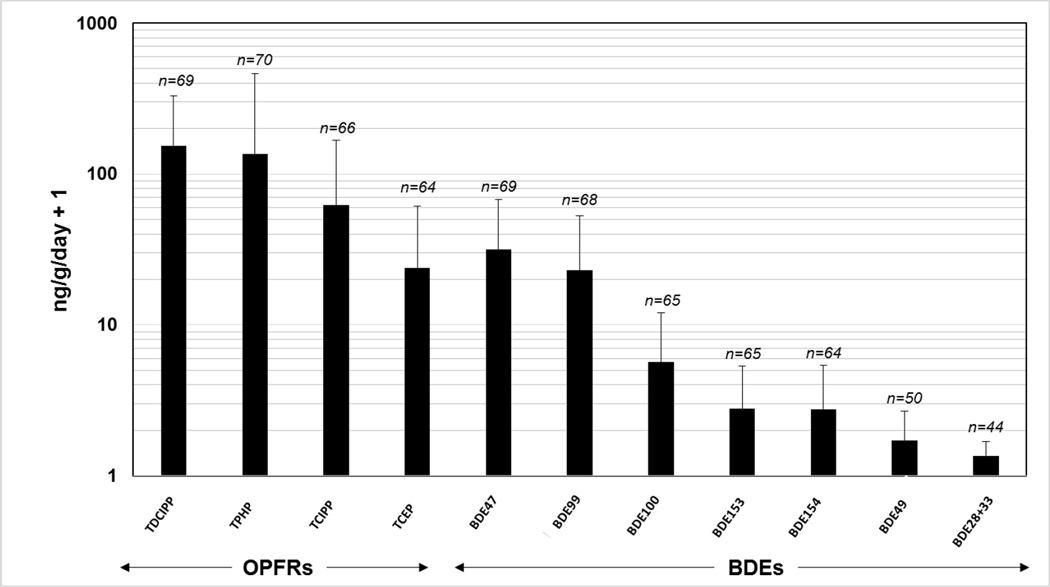
All wristbands were able to be extracted adequately, and many samples had concentrations of flame retardants high enough to require dilution before analysis. More information about extraction performance can be found in the supporting information. Of the 41 compounds included in the analytical method, 20 were detected above the LOQ in at least one of the 72 wristband worn by children (SI-Table 4). Overall, the OPFRs were detected with high frequency (≥ 89%, see SI-Table 4) and at relatively high concentrations (averaging 22.8–154 ng/g/day, Figure 2). BDEs that have been phased out of use in the United States were also detected at relatively high frequencies (with 5 BDEs over 88%, SI-Table 4), but at relatively lower concentrations (0.05–30.4 ng/g/day, Figure 2). Focusing on the 11 compounds that were detected in 60% or more of the samples, OPFRs were correlated with each other, but weakly correlated with most BDE compounds (SI-Table 2). Conversely, BDEs congeners were strongly associated with one another (SI-Table 2). The significant correlations between some compounds within the same class could suggest a common origin for each flame retardant group (e.g. OPFRs or PentaBDE). This, coupled with the high frequency of the OPFRs studied may indicate a shift in the distribution of flame retardants in the indoor environment away from the BDEs that have been restricted for nearly a decade in the United States and is worth further investigation.
Figure 2.
An illustration of the most abundant flame retardants (60% or more detections) measured in the 72 wristbands worn by children in Oregon. The number of samples exceeding reporting limits is given above each flame retardant. Data is reported in ng/g/day plus one for illustrative purposes since the y-axis is in log scale. Averages include values below reporting limits as equal to the LOD divided by the square root of 2 as stated in the text. Error bars illustrate the variation of the population studied, and are one standard deviation from the mean.
3.3 Sociodemographic correlations with flame retardants
We explored whether socio-demographic characteristics or household behaviors were associated with the lnΣ(BDEs) and lnΣ(OPFRs) measured in the silicone wristbands worn by preschool children. Using generalized linear regression models, the year the home was built and the frequency of vacuuming was significantly related to the lnΣOPFRs and lnΣBDEs (SI-Table 3, p=0.02). In a multiple regression model, family context inversely associated with lnΣOPFRs where households with a greater family context score had lower levels of these compounds detected in the child’s wristband but the strength of the association was not as strong for lnΣBDEs (Table 2). Since OPFRs and BDEs are semivolatile compounds, physical agitation and heated air generated from vacuuming may further increase the volatility of these compounds from house dust. Also, the type and disposal method of accumulated material may also play a role in exposure. However, differentiating the impact of household characteristics on children’s exposures to these two classes of flame retardants is hindered by the lack of time activity patterns. Future exposure assessment studies should incorporate time spent in each microenvironment to overcome this limitation, and also target recruitment based on household characteristics in order to have a larger sample size for a more thorough assessment of housing features and cleaning behaviors on flame retardant exposure.
Table 2.
Effect estimates of wristband data using multivariate regressions with potential predictors of sociodemographic information. Reference groups are children who live in homes where vacuum frequency is <6 times per month, with homes constructed before 2005, and with an average family context score.
| LnΣ OPFR (ng/g/d) | Estimate (β) | SE | p-value |
|---|---|---|---|
| Intercept | 4.67 | 0.36 | <0.01 |
| Vacuum frequency (≥ 6 times/month) | −0.10 | 0.23 | 0.68 |
| Home age (≥ 2005) | 0.74 | 0.27 | 0.01 |
| Family context | −0.36 | 0.18 | 0.05 |
| LnΣBDE (ng/g/d) | |||
| Intercept | 4.53 | 0.46 | <0.01 |
| Vacuum frequency (≥ 6 times/month) | 0.48 | 0.29 | 0.10 |
| Home age (≥ 2005) | −0.65 | 0.35 | 0.06 |
| Family context | −0.28 | 0.23 | 0.21 |
In fully saturated models, gender, age of home, recruitment site, vacuuming frequency, and family context only explained approximately one-eighth of the observed variability in lnΣOPFRs (R2=0.13) and approximately one-sixth of the observed variability in lnΣBDEs (R2=0.16) detected in the wristbands worn these preschool children. Previous exposure assessment studies have shown an increase in OPFRs in indoor dust samples collected in the U.S. after the 2004 phase out of PentaBDE and OctaBDE (Dodson et al., 2012). These studies have identified several factors that are associated with flame retardant exposure. For instance, the presence of napping equipment made out of foam in in early childhood education environments is associated with increased concentrations of PentaBDE, TCEP, and TDCIPP in dust samples but not indoor air samples (Bradman et al., 2014). Data also shows that sofas purchased in the United States prior to 2005 had higher levels of BDEs whereas those purchased after 2005 had higher concentrations of OPFRs (Stapleton et al., 2012). In the United Kingdom, the presence of foam chairs in classrooms is associated with higher OPFR concentrations in floor dust (Brommer and Harrad, 2015). The presence of upholstered furniture with crumbling or exposed foam in Northern Californian households was related to higher concentrations of PentaBDE congeners in house dust (Whitehead et al., 2013), and individuals from lower income households had higher BDE body burdens (Zota et al., 2008). While further research is needed to identify sources of flame retardant exposures that could be incorporated into prevention activities, the data seems to indicate trends in higher OPFRs in the indoor environment since the restrictions against BDEs have been enacted, which is similar to the results observed in our population.
3.4 Study limitations
This is the first study using silicone wristbands as personal monitors for children and to quantify their exposure to a mixture of 41 volatile/semi-volatile flame retardants. However, there are several limitations in our study that are worth noting. First, our study population relied upon a convenience sample of preschool children in two communities in Oregon and may not be representative of a wider population. The small sample size and lack of time activity data limited our ability to determine all factors that could influence flame retardant concentrations in the environment inhabited by preschool aged children. Second, the amount of flame retardants detected in the silicone wristbands provide information about volatile and semi-volatile compounds in the air and may represent direct dermal contact as well. Subsequently, it is not easy to isolate a single route of exposure using this approach. It would also likely underestimate total exposure to flame retardants since it would not capture exposure related to housedust. Additionally, we did not infuse performance reference compounds into the wristbands and therefore cannot estimate air concentrations in the environment in this study. Performance reference compounds are used as in situ calibration standards to estimate environmental concentrations (Booij et al., 2002). Because air concentrations were not estimated, and the affinity for silicone and flame retardants differ between compounds, we cannot infer directly that the magnitudes of flame retardants found in wristbands would reflect the same relative occurrence in the environment (see the supporting information about silicone sampler absorption). For this reason, we kept all findings in this study in the context of what was found in the wristband itself (i.e. ng/g wristband) and in relationship to this population, but future studies could include performance reference compounds and overcome this limitation. Finally, we relied on parental report for compliance and the wristbands may not have been worn by the children at all times.
4. CONCLUSIONS
Ultimately, personal silicone wristband samplers offer an innovative and non-invasive approach towards measuring personal exposure to multiple volatile and semi-volatile organic chemical mixtures such as flame retardants and were well tolerated by children. The sampled population show that young children are exposed to a mixture of flame retardant chemicals, and that inhalation and dermal routes of exposure deserves further study. The data collected in this Oregon population indicated that the OPFRs, which are not subject to the same regulatory policies as BDEs, are present in children’s environment and are abundant flame retardants detected by the wristbands.
Supplementary Material
HIGHLIGHTS.
Silicone wristbands are a non-invasive approach for personal sampling of chemical mixtures
Flame retardants were stable in a simulation of transport and storage stability in wristband samplers
A total of 20 flame retardants were detected in silicone wristbands worn by children
Some flame retardants measured in wristbands were associated with house age, vacuum frequency, and family context.
Acknowledgments
This research was supported by a pilot grant awarded by the NIEHS-funded Environmental Health Sciences Center at Oregon State University (P30 ES000210). The authors thank Ted Haigh and Glenn Wilson of the OSU Food Safety and Environmental Stewardship Program, and Kenneth C. Willard for help with analysis. The authors also acknowledge the Hallie Ford Center. The authors thank Pete Hoffman, Josh Willmarth, and Kevin Hobbie for proof-reading this manuscript.
FUNDING SOURCES
This research was supported by a pilot grant awarded by the NIEHS-funded Environmental Health Sciences Center at Oregon State University (P30 ES000210).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
RESEARCH WITH HUMAN SUBJECTS
All research activities were approved by Oregon State University’s Institutional Review Board and written informed consent was given prior to participation in the study.
CONFLICT OF INTEREST DISCLOSURE
Kim Anderson and Steven O’Connell, authors of this research, disclose a financial interest in MyExposome, Inc. which is marketing products related to the research being reported. The terms of this arrangement have been reviewed and approved by Oregon State University in accordance with its policy on research conflicts of interest. The authors have no other disclosures.
AUTHOR CONTRIBUTIONS
The manuscript was written through contributions of all authors. All authors have given approval to the final version of the manuscript.
REFERENCES
- Alcock RE, MacGilivray BH, Busby JS. Understanding the mismatch between the demands of risk assessment and practice of scientists — The case of Deca-BDE. Environ Int. 2011;37(1):216–225. doi: 10.1016/j.envint.2010.06.002. [DOI] [PubMed] [Google Scholar]
- Anderson KA, Hillwalker WE. Bioavailability. Encyclopedia of Ecology. 2008;1:348–357. [Google Scholar]
- Bergh C, Torgrip R, Emenius G, Östman C. Organophosphate and phthalate esters in air and settled dust- a multi-locatoin indoor study. Indoor Air. 2011;2:67–76. doi: 10.1111/j.1600-0668.2010.00684.x. [DOI] [PubMed] [Google Scholar]
- Bergman A, Ryden A, Law RJ, de Boer J, Covaci A, Alaee M, Birnbaum L, Petreas M, Rose M, Sakai S, Van den Eede N, van der Veen I. A novel abbreviation standard for organobromine, organochlorine and organophosphorus flame retardants and some characteristics of the chemicals. Environ Int. 2012;49:57–82. doi: 10.1016/j.envint.2012.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Booij K, Smedes F, van Weerlee EM. Spiking of performance reference compounds in low density polyethylene and silicone passive water samplers. Chemosphere. 2002;46(8):1157–1161. doi: 10.1016/s0045-6535(01)00200-4. [DOI] [PubMed] [Google Scholar]
- Bradman A, Castorina R, Gaspar F, Nishioka M, Weathers W, Egeghy PP, Maddalena R, Williams J, Jenkins PL, McKone TE. Flame retardant exposures in California early childhood education environments. Chemosphere. 2014;116:61–66. doi: 10.1016/j.chemosphere.2014.02.072. [DOI] [PubMed] [Google Scholar]
- Brommer S, Harrad S. Sources and human exposure implications of concentrations of organophosphate flame retardants in dust from UK cars, classrooms, living rooms, and offices. Environ Int. 2015;83:202–207. doi: 10.1016/j.envint.2015.07.002. [DOI] [PubMed] [Google Scholar]
- Cariou R, Antignac JP, Marchand P, Berrebi A, Zalko D, Andre F, Le Bizec B. New multiresidue analytical method dedicated to trace level measurement of brominated flame retardants in human biological matrices. J Chromatogr A. 2005;1100:144–152. doi: 10.1016/j.chroma.2005.09.040. [DOI] [PubMed] [Google Scholar]
- Cooper E, Covaci A, van Nuijus A, Webster T, Stapleton H. Analysis of the flame retardant metabolites bis(1,3-dichloro-2-propyl) phosphate (BDCPP) and diphenyl phosphate (DPP) in urine using liquid chromatography-tandem mass spectrometry. Anal. Bioanal. Chem. 2011;401(7):2123–2132. doi: 10.1007/s00216-011-5294-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodson RE, Perovich LJ, Covaci A, Van den Eede N, Ionas AC, Dirtu AC, Brody JG, Rudel RA. After the PBDE Phase-Out: A Broad Suite of Flame Retardants in Repeat House Dust Samples from California. Environ Sci Technol. 2012;46(24):13056–13066. doi: 10.1021/es303879n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EPA (Environmental Protection Agency) Definition and procedure for determination of the Method Detection Limit. 40 CFR Part 136, Appendix B, Revision 1.11. 1995:343–345. [Google Scholar]
- Fang J, Nyberg E, Winnberg U, Bignert A, Bergman A. Spatial and temporal trends of the Stockholm Convention POPs in mother’s milk- a global review. Environmental Science and Pollution Research. 2015;22(12):8989–9041. doi: 10.1007/s11356-015-4080-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forsberg ND, Wilson GR, Anderson KA. Determination of Parent and Substituted Polycyclic Aromatic Hydrocarbons in High-Fat Salmon Using a Modified QuEChERS Extraction, Dispersive SPE and GC-MS. J Agric Food Chem. 2011;59(5):8108–8116. doi: 10.1021/jf201745a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ICH. International Conference on Harmonization, Vol. Q1A (R2) Geneva: 2003. Stability testing for new drug substances and products. [Google Scholar]
- Jaward FM, Farrar NJ, Harner T, Sweetman AJ, Jones KC. Passive air sampling of PCBs, PBDEs, and organochlorine pesticides across Europe. Environmental Science & Technology. 2004;38(1):34–41. doi: 10.1021/es034705n. [DOI] [PubMed] [Google Scholar]
- La Guardia MJ, Hale RC, Harvey E. Detailed polybrominated diphenyl ether (PBDE) congener composition of the widely used penta-, octa-, and deca-PBDE technical flame-retardant mixtures. Environmental Science & Technology. 2006;40(20):6247–6254. doi: 10.1021/es060630m. [DOI] [PubMed] [Google Scholar]
- Law RJ, Covaci A, Harrad S, Herzke D, Abdallah MAE, Fermie K, Toms LML, Takigami H. Levels and trends of PBDEs and HBCDs in the global environment: Status at the end of 2012. Environment International. 2014;65:147–158. doi: 10.1016/j.envint.2014.01.006. [DOI] [PubMed] [Google Scholar]
- Lehotay SJ, Lightfield AR, Harman-Fetco JA, Donoghue DJ. Analysis of pesticide residues in eggs by direct sample introduction/gas chromatography/tandem mass spectrometry. J Agric Food Chem. 2001;49(10):4589–4596. doi: 10.1021/jf0104836. [DOI] [PubMed] [Google Scholar]
- Morrison FJ, Cooney RR. Parenting and academic achievement: Multiple paths to early literacy. In: Borkowski JG, et al., editors. Parenting and the child's world: Influences on academic, intellectual, and social-emotional development. Mahwah, NJ, US: Lawrence Erlbaum Associates Publishers; 2002. pp. 141–160. [Google Scholar]
- O'Connell SG, Kincl LD, Anderson KA. Silicone Wristbands as Personal Passive Samplers. Environ Sci Technol. 2014;48(6):3327–3335. doi: 10.1021/es405022f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Official Journal of the European Union, European Parliament (C-14/06), Kingdom of Denmark (C-295/06) v Commission of the European Communities (Directive 2002/95/EC — Electrical and electronic equipment— Restriction of use of certain hazardous substances —Decabromodiphenyl ether (‘DecaBDE’) — Commission Decision 2005/717/EC — Exemption of DecaBDE from the prohibition on use — Actions for annulment — Commission's implementing powers — Infringement of the enabling provision). Court of Justice of the European Communities, V. 2008/C 116/04. 2008 [Google Scholar]
- Ryan JJ, Rawn DFK. The brominated flame retardants, PBDEs and HBCD, in Canadian human milk samples collected from 1992 to 2005; concentrations and trends. Environ Int. 2014;70:1–8. doi: 10.1016/j.envint.2014.04.020. [DOI] [PubMed] [Google Scholar]
- Sjodin A, Papke O, McGahee E, Focant JF, Jones RS, Pless-Mulloli T, Toms LML, Hermann T, Muller J, Needham LL, Patterson DG. Concentration of polybrominated diphenyl ethers (PBDEs) in household dust from various countries. Chemosphere. 2008a;73(1):S131–S136. doi: 10.1016/j.chemosphere.2007.08.075. [DOI] [PubMed] [Google Scholar]
- Sjodin A, Wong LY, Jones RS, Park A, Zhang Y, Hodge C, Dipietro E, McClure C, Turner W, Needham LL, Patterson DG. Serum concentrations of polybrominated diphenyl ethers (PBDEs) and polyhrominated biphenyl (PBB) in the United States population: 2003–2004. Environmental Science & Technology. 2008b;42(4):1377–1384. doi: 10.1021/es702451p. [DOI] [PubMed] [Google Scholar]
- Stapleton HM, Klosterhaus S, Eagler S, Fuh J, Meeder JD, Blum A, Webster TF. Detection of Organophosphate Flame Retardants in Furniture Foam and U.S. House Dust. Environmental Science & Technology. 2009;43(19):7490–7495. doi: 10.1021/es9014019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stapleton HM, Klosterhaus S, Keller A, Ferguson PL, van Bergen S, Cooper E, Webster TF, Blum A. Identification of Flame Retardants in Polyurethane Foam Collected from Baby Products. Environmental Science & Technology. 2011;45(12):5323–5331. doi: 10.1021/es2007462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stapleton HM, Misenheimer J, Hoffman K, Webster TF. Flame retardant associations between children's handwipes and house dust. Chemosphere. 2014;116:54–60. doi: 10.1016/j.chemosphere.2013.12.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stapleton HM, Sharma S, Getzinger G, Ferguson PL, Gabriel M, Webster TF, Blum A. Novel and High Volume Use Flame Retardants in US Couches Reflective of the 2005 PentaBDE Phase Out. Environ Sci Technol. 2012;46(24):13432–13439. doi: 10.1021/es303471d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundkvist AM, Olofsson U, Haglund P. Organophosphorus flame retardants and plasticizers in marine and fresh water biota and in human milk. Journal of Environmental Monitoring. 2010;12(4):943–951. doi: 10.1039/b921910b. [DOI] [PubMed] [Google Scholar]
- The Euopean Parliament, Directive 2003/11/EC of the European Parliament and of the Council amending for the 24th time Council Directive 76/769/EEC relating to restrictions on the marketing and use of certain dangerous substances and preparations (pentabromodiphenyl ether, octabromodiphenyl ether) 2003 [Google Scholar]
- Thomsen C, Stigum H, Froshaug M, Broadwell SL, Becher G, Eggesbo M. Determinants of brominated flame retardants in breast milk from a large scale Norwegian study. Environ Int. 2010;36(1):68–74. doi: 10.1016/j.envint.2009.10.002. [DOI] [PubMed] [Google Scholar]
- UNEP. The new POPs under the Stockholm Convention. Vol. 2015. Stockholm Convention. 2009 [Google Scholar]
- van der Veen I, de Boer J. Phosphorus flame retardants: Properties, production, environmental occurrence, toxicity and analysis. Chemosphere. 2012;88(10):1119–1153. doi: 10.1016/j.chemosphere.2012.03.067. [DOI] [PubMed] [Google Scholar]
- Whitehead TP, Brown FR, Metaver C, Park JS, Does M, Petreas MX, Buffler PA, Rappaport SM. Polybrominated diphenyl ethers in residential dust: sources of variability. Environ Int. 2013;57–58:11–24. doi: 10.1016/j.envint.2013.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zota AR, Rudel RA, Morello-Frosch RA, Brody JG. Elevated House Dust and Serum Concentrations of PBDEs in California: Unintended Consequences of Furniture Flammability Standards? Environ Sci Technol. 2008;42(21):8158–8164. doi: 10.1021/es801792z. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.