Abstract
B-cell dysregulation in aging is thought to mostly occur in conventional B2 cells without affecting innate B1 cells. Elderly humans and mice also accumulate 4-1BBL+ MHC class-IHi CD86Hi B cells of unknown origin. Here we report that these cells, termed 4BL cells, are activated murine and possibly human B1a cells. The activation is mediated by aging human monocytes and murine peritoneal macrophages. The 4BL cells induce expression of 4-1BBL and IFNγR1 on B1a cells resulting in subsequent up regulation of membrane TNFα (mTNFα) and CD86. As a result, B1a cells induce expression of granzyme B in CD8+T cells by targeting TNFR2 via mTNFα while providing co-stimulation with CD86. Thus, for the first time, these results indicate that aging affects the function of B1a cells. Upon aging, these cells lose their tumor-supporting activity and become inducers of potentially antitumor and autoimmune CD8+T cells.
Keywords: Aging, macrophage, antitumor CD8+T cells, 4BL cells
Introduction
The immune system becomes dysregulated upon aging in mammals and this dysregulation affects both myeloid and lymphoid immune cell compartments (see reviews (1, 2)). Aging-associated intrinsic and extrinsic factors skew hematopoiesis towards myelopoiesis (3, 4), resulting in reduced B-cell-committed progenitors in bone marrow (BM) (5-7) and changes in the prevalence and function of myeloid cells and lymphocytes in circulation and in tissues. For example, the elderly accumulate monocytes and macrophages expressing pro-inflammatory cytokines such as TNFα and IFNγ (8, 9) and, in mouse peritoneum, M1 macrophages expressing reactive oxygen (10). Aging also impairs phagocytosis of human PB CD14+ monocytes, murine neutrophils, and PC macrophages (8, 11, 12). Compounded with a life-long antigenic exposure, changes in T cells, and increased half-life of mature B cells (13, 14), B cells subsequently become enriched for antigen-experienced memory and mature conventional B2 cells at the expense of naive B cells (15-18). In contrast, very little is known as to whether aging affects innate B1a cells, the key producers of natural antibodies to pathogens and endogenous antigens. Although the capacity to generate B1 cells is reduced in BM after birth (19), aging does not appear to affect their generation in BM of mice (20) and even increases their frequency in peritoneum (PC) and spleen of old mice (11, 21). B1 cells differ from B2 cells by a distinct cellular origin, such as the generation from specific fetal progenitors and a limited self-renewing capacity, and by their preferential localization in the coelomic cavity, Peyer’s patches, and tonsils (22-25). They also represent less than 5% of B cells in circulation and in the peripheral lymphoid tissues of young adult mice (22, 25). Murine B1 cells consist of CD5+(B1a) and CD5−(B1b) CD11b+B cells (25), while their human counterparts remain poorly characterized (26-28) but have been shown to be a subset of memory CD27+CD43+CD69−CD20+ B cells (27). Aging mice also accumulate CD23− CD21/CD35−B cells (so-called aging-associated B cells, ABCs) refractory to BCR and CD40 engagement (29). Recently, we reported that aging humans, macaques, and mice also accumulate another type of CD19+ B cells of unknown origin. These cells, designated 4BL cells, express high levels of HLA-I, the co-stimulatory molecule CD86, and a TNF superfamily type 2 transmembrane protein 4-1BBL/CD137L (30). Although macrophages and dendritic cells (DC) express 4-1BBL (31), it can be transiently expressed on B cells upon a combined stimulation of BCR and CD40 (32). This cell surface molecule receives and transmits signals upon engagement with its receptor 4-1BB/CD137 expressed on activated NK and T cells and splenic DCs (31, 32), resulting in activation of cytolytic CD8+T cells and NK cells (33-36). In synergy with B7 and ICAM, 4-1BBL can also induce proliferation of naïve CD4+T cells when antigen is limiting (37). We have reported that, by utilizing the 4-1BBL/4-1BB and MHC class-I/TCR signaling pathways, 4BL cells also induce the generation of antigen-specific perforin+ GrB+ CD8+ T cells (30). In fact, this feature of 4BL cells explains the enrichment of highly differentiated GrB+CD45RA+CD8+T cells in aging humans (15) and accounts for a paradoxical retarded growth of poorly immunogenic tumors in the elderly (30).
Here, we report that murine B1a cells, and possibly human B1 cells, become converted into 4BL cells upon aging. Surprisingly, the conversion is not due to intrinsic changes in aging B cells, but instead is induced by myeloid cells of aging subjects, such as human peripheral blood monocytes and murine peritoneal macrophages. First, they induce BCR and CD40 signaling that leads to expression of 4-1BBL, IFNγR1, and membrane (m) TNFα in B1a cells. Then, upon engagement of 4-1BBL and IFNγR1 with their respective stimuli (4-1BB and IFNγ also provided by aging myeloid cells), B1a cells further up-regulate expression of mTNFα and CD86. The resulting 4BL cells then use mTNFα in the induction of GrB expression in CD8+T cells by targeting TNFR2 while providing co-stimulation with CD86. Overall, our results reveal, for the first time, a unique functional consequence of the crosstalk between dysregulated myeloid and lymphoid cell compartments upon aging. This process converts innate B1a cells from presumably being immune suppressive cells into the inducers of cytolytic GrB+CD8+T cells.
METHODS
PBMC were collected from peripheral blood (PB) of elderly (n= 19, years ± SD: 79 ± 6.45) and young (n=11, years ± SD: 41 ± 7.2) healthy humans from the Baltimore Longitudinal Study of Aging (BLSA, NIA) under the Human Subject Protocol # 2003054 and Tissue Procurement Protocol # 2003-076. Young (5-8 weeks) female C57BL/6j and BALB/c, congenic JHT mice with B-cell deficiency (B6.129P2-Igh-Jtm1Cgn/J) and GFR+ mice [C57BL/6-Tg(UBC-GFP)30Scha/J] were from the Jackson Laboratory (Bar Harbor, ME); and 4-1BBL and 4-1BB deficient (KO) mice (4-1BBL KO and 4-1BB KO) were reported elsewhere (33, 38). Old mice (18-22 months old) were from Aged Rodent Colony, NIA. ARE-Del mice (39) and TNFα, TNFR1, TNFR2, and IFNγR KO mice were from NCI-Frederick, MD.
Flow cytometry
All Ab were from BioLegend (San Diego, CA) except otherwise specified. Human cells Fc receptors were blocked using Human TruStain FcX™ 5 min prior to staining with antibodies (Ab), such as anti-human Ab: TNFα-APC (BD Pharmingen, Piscataway, NJ) or –FITC (clone MAb11), IFNγ-FITC (BD Pharmingen) or -Pacific Blue (clone 4S.B3), CD19-PerCP/Cy5.5 or -Alexa Fluor® 780 (clone HIB19), 4-1BBL-PE or -APC (5F4), CD86-PE (BD Pharmingen, clone FUN-1), CD27-PerCP/Cy5.5 (clone M-T271), CD119 (IFNγR α chain)-PE or –Biotin (clone GIR-208), Streptavidin-Pacific blue or –APC, IL10-PerCP/Cy5.5 (JES3-9D7), CD14-Pacific Blue (clone M5E2) or –FITC (clone HCD14), CD3-PE (BD Pharmingen, clone SP34), CD8-APC or -PE (clone HIT8a), GrB-FITC (clone GB11), CD11b-FITC or –Alexa Fluor® 700 (clone M1/70), CD138-PECy7 (eBioscience, clone DL101), CD43-APC (eBioscience, clone eBio 84-3C1), CD69-APCCy7 (BD Pharmingen, clone FN50), and CD120b (TNFR2)-PE (clone 3G7A02). For murine cells phenotype analysis, cells were preincubated with Tru Stain FcX™ before immunostaining with different combinations of the following anti-mouse Ab: TNFα-FITC (clone MP6-XT22), 4-1BBL- PerCP-eFluor®710 (eBioscience) or -PE (clone TKS-1), CD5-PerCP/Cy5.5 (eBioscience), -FITC or -APC (clone 53-7.3), CD119 (IFNγR α chain)-Biotin (clone 2E2), Streptavidin-Pacific blue or –APC, CD19-APC-eFluor® 780 (eBioscience, clone 1D3) or -PerCP/Cy5.5 or –APC (clone 6D5), CD86-PerCP/Cy5.5 (clone GL-1), CD23-FITC (BD Pharmingen, clone B3B4), CD21/CD35-APC (clone 7G6), CD93-PeCy7 or –FITC (clone AA4.1), CD1d-PE or –Pacific Blue (clone 1B1), CD120b (TNFR2)-PE (clone TR75-89), CD8-APC (clone 53-6.7). For intracellular cytokine staining, freshly isolated human PBMC or murine cells were activated with Phorbol 12-Myristate 13-Acetate (PMA, 5 ng/ml; R&D, Minneapolis, MN) + Ionomycin (500 ng/ml; R&D), and brefeldin A (1/1000, eBioscience) for 5 h at 37 °C. The cells were stained using the Intracellular Fixation and Permeabilization Buffers Kit (eBioscience) following the manufacturer’s protocol. Appropriate isotype controls were used throughout all experiments.
In vitro assays
B cells from human peripheral blood (PB) and murine spleens were negatively isolated using B-cell Isolation Kit II (≥98% purity, Miltenyi Biotec, Auburn, CA) and the EasySepTM Mouse B cell Isolation Kit (≥95% purity, StemCell Technologies, Vancouver, Canada), respectively. 4-1BBL expressing B cells (named as 4BL cells) were magnetically sorted using anti-4-1BBL-PE (human: clone 5F4; mouse: clone TKS-1; both from BioLegend) + anti-PE MicroBeads (Miltenyi Biotec). CD8+ T cells were isolated negatively using the Human CD8+ T Cell Isolation Kit or Mouse CD8a+ T Cells Isolation Kit (≥97% purity Miltenyi Biotec). The B-cell-mediated activation of CD8+ T cells was performed as previously reported (30). Briefly, young subject CD8+ T cells were labeled with eFluor® 450 cell proliferation dye (10 min at 37 °C, 5 μM, eBioscience) and mixed with respective syngeneic (murine) or allogeneic (human) B cells from young and aging subjects at 1:1 ration in presence of 1.5 μg/ml of anti-CD3 Ab (UCHT1 and HIT3a clones, respectively, for human and murine cells; BD Pharmingen) for 4-5 days in complete RPMI (cRPMI) at 37 °C in humidified atmosphere with 5% CO2. To test the function of TNFα/TNFR2 axis in induction of GrB+CD8+ T-cells, we used B cells and CD8+ T cells from TNFα and TNFR2 deficient congenic mice, respectively. Alternatively, WT cells were incubated with 5 μg/ml TNFα blocking Ab with murine (clone MP6-XT22, Rat IgG1κ; eBioscience) or human cells (clone MAb-1, Mouse IgG1κ; eBioscience); or human CD8+ T cells were pretreated 10 min at RT with 10 μg/ml of anti-human TNFR2 blocking Ab (clone hTNFR-M1, Rat IgG2b; BD Pharmingen) prior to mixing with B cells. To test the importance of cell contact, we performed a transwell assay in a 24-well tissue plate (3-μm pore size, Corning Life Science, Acton, MA). To test the role of CD86 function, murine 4BL cells were pretreated with 10 μg/ml of anti-CD86 Ab (clone PO3.1; eBioscience) or isotype control (Rat IgG2b, κ; eBioscience) at RT prior mixing at 1:1 ratio with eFluor® 450-labeled CD8+ T cells and 1.5 μg/ml soluble anti-mouse CD3.
Induction of TNFR2 expression on human CD8+ T cells was evaluated after 24 h activation with beads coupled with anti-CD3/CD28 or anti-CD3/CD28/CD137 Abs at 1:30 bead to cell ratio (Invitrogen), or with soluble anti-CD3 (1.5 μg/ml, clone HIT3a; BD Pharmingen) and anti-CD28 (1.5 μg/ml, clone 37.51; BD Pharmingen) and 4-1BB agonistic 3H3 Ab as described elsewhere (40). Alternatively, 4-1BBL+B cells were cultured overnight with eFluor® 450-labeled CD8+ T cells from young mice at a 1:1 ratio in the presence of 1.5 μg/ml soluble anti-CD3 Ab. 4-1BBL and IFNγR1 up-regulation in murine splenic B cells (2x106/ml) was induced with 10 μg/ml IgM (AffiniPure F(ab’)2 Fragment Goat anti-mouse IgM; Jackson Laboratories) and 5 μg/ml of anti-mouse CD40 agonistic Ab (FGK45, ENZO Lifesciences) by treatment for 18 h. CD86 was induced by treating B cells with 100 U/ml IFNγ (R&D) for 18 h. To evaluate the effect of 4-1BBL ligation on TNFα induction, cells were stimulated with LPS (1 μg/ml), or plate-bound 4-1BB:Fc fusion protein (R&D), or control antibody (human IgG1; R&D) in 96-flat bottomed plates for 24 h at 37 °C.
4BL cell induction by myeloid cells
PB monocytes (isolated using the Human Monocyte Isolation Kit II, Miltenyi Biotec) or murine PC myeloid (depleted of B, T, and NK cells treated with mixture of anti-mouse CD19-PE, CD3-PE and DX5-PE Abs and anti-PE MicroBeads; Miltenyi Biotec) were mixed with eFluor® 450-labelled (5 μM; eBioscience) B cells from respective young subjects at a 1:1 ratio. To block BCR signaling, B cells were pre-treated with Btk (20 nM, PC1-32765; Selleckchem, Boston, MA) and SYK (4 μM, R406; Selleckchem) inhibitors for 30 min at 37 °C prior to mixing with myeloid cells. CD40 signaling was blocked with anti-mouse CD40L blocking Ab (10 μg/ml, clone MR1; eBioscience).
Antigen-specific induction of CD8+ T cells by 4BL cells
To test whether naïve 4BL cells can process and present self-antigen, young (10 weeks old) and old (21 months old) C57BL/6 mice were either s.c. challenged with B16 melanoma cells as described elsewhere (41) or i.p. injected with 3 mg/ml of albumin from chicken egg white (Sigma-Aldrich, St. Louis, MO) in 200 μl PBS. After 4 days, the mice were euthanized and splenic B cells were isolated with the EasySepTM Mouse B Cell Isolation Kit (≥95% purity; StemCell Technologies, Vancouver, Canada). Cells were further separated into CD5+ and CD5− B cells using anti-mouse CD5-PE (clone 53-7.3) and anti-PE MicroBeads. Alternatively, peritoneal cavity B cells were depleted of CD23+ cells using anti-mouse CD23-PE (clone B3B4, BioLegend) and anti-PE MicroBeads. CD19+ B cells were then positively selected using anti-mouse CD19-PE (clone 6D5) and anti-PE MicroBeads (>98% purity). B cells were enriched with 70-90% B1a cells according to CD11b and CD5 staining. To prepare target CD8+T cells, 8-11 weeks old female pmel and OT-1 mice with gp10025-33 or OVA257-264 –specific TCR-transgenic CD8+ T cells, respectively, were s.c. immunized with 10 μg human gp10025-33 peptide or 5 μg OVA257-264 peptide (SIINFEKL, ANASpec) in incomplete Freud’s adjuvant. After 4-5 days, splenic CD8+ T cells from immunized mice were isolated using the EasyStep™Mouse CD8+ T Cell Enrichment Kit (StemCell Technologies) and labeled with eFluor450 cell proliferation dye (eBioscience). Then, to test for B-cell-induced activation of CD8+ T cells, the eFluor450+ CD8+ T cells from pmel and OT-1 mice were cultured with B cells from young or old WT mice challenged with B16 melanoma or ovalbumin protein, respectively, at 1:1 ratio for 5 days in cRPMI without any stimulation.
In vivo manipulations
Animals were housed in a pathogen-free environment at the National Institute on Aging Animal Facility, Baltimore, MD, under the Guide for the Care and Use of Laboratory Animals (NIH Publication No. 86-23, 1985). For adoptive transfer experiments, 5x106 eFluor® 450-labeled B cells from spleens and PC of young WT or GFP-Tg mice were injected (i.v. or i.p.) into congenic old and young mice to evaluate PC B cells after 5-6 days. PC macrophages were depleted in old mice by 2 i.p. injections of 150 μl of clodronate liposomes (Chlophosome™) 2 days prior to B-cell transfer. The generation of Old-restored mice, i.e., induction of new B-cell lymphopoiesis in old mice treated with anti-CD20 Ab) was described elsewhere (30). B1a cells and FOB cells were magnetically or FACS sorted from C57BL/6 mice and i.v. injected (2x106 cells) into JHT mice one day after s.c. challenge with B16-F10 melanoma cells (105 in 100 μL PBS, American Type Culture Collection).
Statistical Analysis
The results are presented as the mean ± SEM, and significance was assessed by Mann-Whitney and non-parametric test (Prism 6; Graph Pad Software, Inc., San Diego, CA). A p-value less than 0.05 was considered statistically significant.
RESULTS
Aging milieu activates innate B1a cells
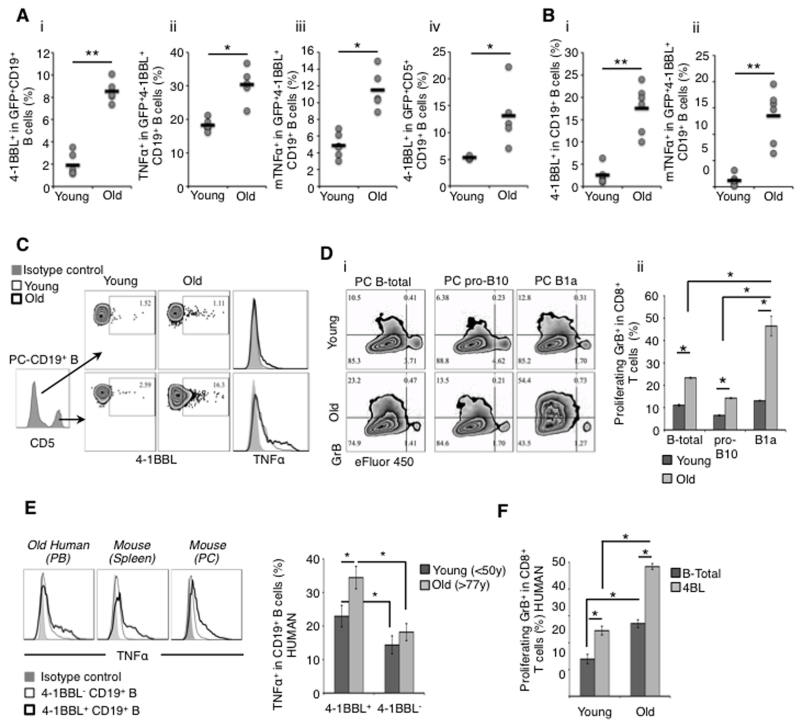
We previously observed that the reappearance of 4BL cells after transient B-cell depletion in old mice (Old-restored) is delayed for one month compared to the rest of B cells (personal communication, M.B. & A.B. and (30)) and that cancer patients also accumulate 4BL cells upon autologous hematopoietic stem cell transfer (30). These data suggest that the generation of 4BL cells could be induced by extrinsic factors. To test this possibility, we injected splenic B cells of young GFP-expressing mice into the peritoneal cavity (PC) of 18-month and 8-10 weeks old congenic mice (old and young, respectively, n=6/group; SFig.1Ai). After 6 days, mice were euthanized to evaluate PC B cells. The injected GFP+B cells markedly induced expression of 4-1BBL in old, but not young, mice (p<0.01; Fig. 1Ai), indicating that aging milieu induces 4BL cells. The 4-1BBL+GFP+B cells also up-regulated TNFα (both intracellular and membrane (m) forms; Fig.1Aii, iii) and surprisingly expressed CD5 (p<0.05; Fig.1Aiv). Since CD5 defines murine B1a cells (23-25), they could be the source of 4BL cells upon aging. To test this possibility, we repeated the experiment by intra-peritoneally (i.p.) injecting a separate group of young and old mice with GFP+B cells isolated from PC (instead of spleen) of young mice. Compared with splenic B cells, PC GFP+B cells induced markedly higher levels of 4-1BBL and mTNFα in aging mice (SFig.1Aii-iv). We also detected 4-1BBL+TNFα+CD5+GFP+B cells in PC and spleens of old mice injected via tail vein (SFig.1B). Importantly, host PC B cells (GFP−) were markedly enriched for 4-1BBL+TNFα+ CD5High B cells in old mice (p<0.005 as compared to young mice; Fig.1B). To further implicate B1a cells in the conversion, we analyzed PC B-cell subsets and found that CD5+ pro-B10 cells (which generate regulatory B10 cells (42)) and CD5−B cells were only marginally positive (<2%) for 4-1BBL and TNFα regardless of the age of mice (Fig.1C and SFig.1C). We also tested whether the increase of 4-1BBL+TNFα+ B cells is due to proliferation of preexisting 4BL cells. Upon adoptive transfer of eFluor450-labelled B cells into PC of old and young mice, we did not detect dilution of eFluor450 even 6 days following injection (SFig.1D). Thus, the aging milieu mostly activates B1a cells without expanding their preexisting 4BL-cell subsets.
Figure 1. The aging milieu activates B1a cells.
(a and b) Young and old C57BL/6 WT mice were i.p. injected with 5x106 splenic B cells from congenic GFP+ young mice. Shown is the frequency (dots) and the mean value (lines) of 4-1BBL+ in GFP+ B cells (a-i, iv) and host GFP−B cells (b-i) co-expressing TNFα (a-ii), membrane TNFα (mTNFα, a-iii and b-ii), and CD5 (iv) in PC of each mouse after 6 days, as indicated in the Y-axis. The results are from 3-4 mice/group and experiments were reproduced three times. (c) PC CD5+ B cells of old mice mostly express 4-1BBL and TNFα. Arrows indicate the B-cell population tested (center panel). (d) Aging PC B1a cells mostly induce GrB+CD8+ T cells. Unsorted B cells (B-total) and FACS-sorted pro-B10 (pro-B10) and B1a B (B1a) cells from PC of young and old mice were cultured for 4-5 days with eFluor® 450-labeled CD8+ T cells stimulated with 1.5 μg/ml anti-mouse CD3 Ab. Shown is a representative dot plot (i) of a summary graph (mean ± SEM, ii) of 2 independent experiments performed in triplicate. (e) Elderly human 4-1BBL+B cells express high levels of TNFα. Shown is the frequency mean ± SEM of TNFα+ in 4-1BBL− and 4-1BBL+B cells in young and elderly people PB after PMA/Ionomycin/Brefeldin A activation. (f) 4-1BBL+B cells (4BL cells) from elderly people induce higher levels of GrB+CD8+ T cells. Shown is the frequency mean ± SEM of a representative result reproduced with 5 old and 5 young human cells. From hereon, *p<0.05, **p<0.01, ***p<0.005, and NS = not statistically significant.
Next, to further link the activated B1a cells with 4BL cells, we tested whether they can induce the generation of cytolytic CD8+ T cells expressing GrB (the key function of 4BL cells (30)). Sort-purified B cells (B-total), pro-B10, and B1a cells from PC of old and young mice were in vitro co-cultured with young mouse eFluor 450-labeled CD8+T cells stimulated with anti-CD3 Ab for 5 days. Although anti-CD3 Ab stimulation induced proliferation of CD8+T cells, i.e., the cells comparably diluted eFluor 450 regardless of the B cells or age of the mice (SFig.1E), old mouse B cells per se induced greater GrB expression in CD8+T cells compared to young mouse B cells (B-total: 22.8 ± 0.42% vs 10.5 ± 0.42%, and pro-B10: 14 ± 0.27% vs 6.2 ± 0.29%, respectively old vs young, p≤0.05; Fig.1D). However, compared to young or old mouse B-total cells or even pro-B10 cells, B1a cells of old mice induced significantly higher levels of GrB+CD8+T cells (45 ± 4.4% vs 12.8 ± 0.23%, respectively, old vs young, p<0.05; Fig.1D). Hence, B1a cells can indeed become 4BL cells in aging mice. To test if there is a similar conversion in humans, we compared PB of young (<50 years of age, n=11) and old (>75 years old, n=19) healthy donors. The frequency of 4-1BBL+TNFα+ memory (CD27+CD43−) and B1 cells (CD27+CD43+CD69−CD20+ (27)) was increased in the elderly (SFig.1F. Note: the definition of human B1a cells remains unclear and thus we only evaluated B1 cells usually found within memory B-cells in PB). As in mice, the 4BL cells of elderly people expressed higher levels of mTNFα than 4-1BBL−B cells (p<0.01) and 4BL cells of young donors (p<0.05; Fig.1E). In the mixed lymphocyte reaction (MLR) assay with allogeneic CD8+T cells of young people, 4BL cells within memory CD27+B cells of the elderly induced higher levels of GrB expression in CD8+T cells as compared to unsorted B cells (B-total) or 4BL cells of young donors (p≤0.05; Fig.1F and SFig.1G).
Aging converts splenic B1a cells into 4BL cells and disables their tumor-supporting activity
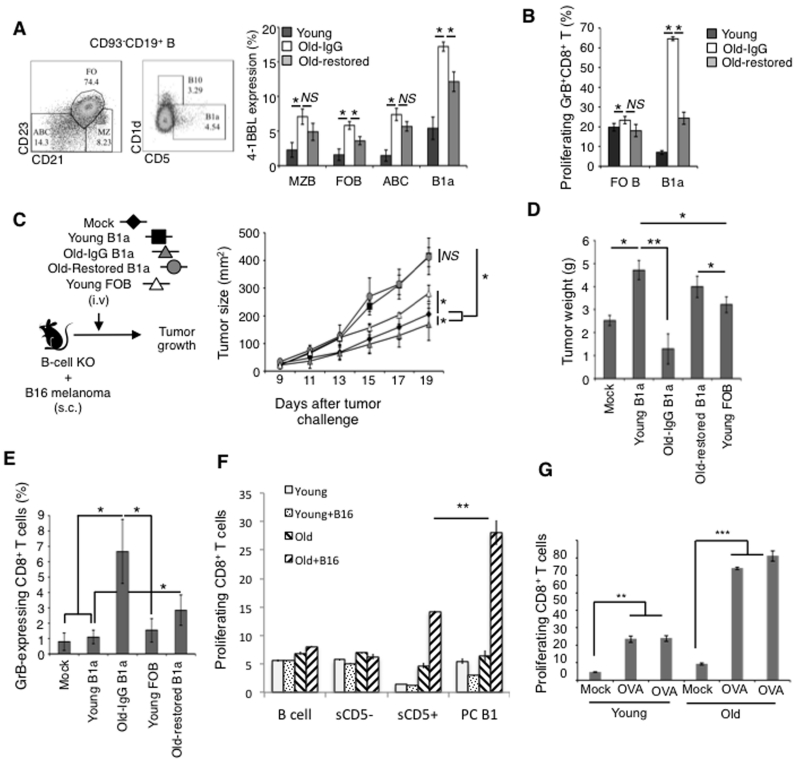
Although B1a cells represent less than 5% of B cells in spleens of young adult mice (22, 25), their conversion into 4BL cells probably explains the induction of GrB+CD8+T cells that we previously linked with the splenic B cells of aging mice (30). To test this possibility, we analyzed expression of 4-1BBL in various subsets of splenic B cells. 4-1BBL was increased in aging marginal zone B (MZB), follicular B cells (FOB), and ABCs, but the increase only reached the level of sB1a cells of young mice (Fig. 2A). In contrast, aging markedly up-regulated 4-1BBL in sB1a cells (17.23% ± 0.67 vs 5.39% ± 1.62, respectively, old vs young, p<0.001; Fig. 2A), suggesting that they become 4BL cells. To further confirm this possibility, we tested the function of sB1a cells and FOB cells of young and old mice in a 5-day GrB+CD8+T cell induction assay (as used above). Note that ABCs were not tested here, as they do not function as 4BL cells (30). Aging mouse sB1a cells strongly induced expression of GrB in CD8+ T cells, while young mouse sB1a cells or FOB cells of young or old mice failed to do so (Fig. 2B). To confirm this finding, we also tested sB1a cells from Old-restored mice, the old mice whose 4BL cells were reduced despite the restoration of the B-cell compartment after depletion of B cells with anti-CD20 Ab (30). Compared to control old mice treated with an irrelevant IgG (Old-IgG), B cells of Old-restored mice contained less 4-1BBL+sB1a cells (Fig. 2A) and could not efficiently induce GrB+CD8+ T cells (p<0.05; Fig. 2B).
Figure 2. Aging splenic B1a cells express 4-1BBL (a), induce GrB+CD8+ T cells (b), and reduce tumor growth (c).
Shown is the 4-1BBL+ subset (left panel) within splenic mature B-cell subsets (CD93−CD19+) and their ability to induce GrB+CD8+ T cells (b) as in Fig. 1. Y-axis, frequency mean ± SEM of 3 independent experiments performed in triplicate with B cells sort-purified from young (dark bars), old (Old-IgG, open bars), and old mice with a restored B-cell compartment (Old-restored, light grey bars). Sort-purified FOB and sB1a cells of young, Old-restored mice (Old-restored), or old mice pretreated with control IgG (Old-IgG) were transferred into B-cell deficient JHT mice with B16 melanoma to test their ability to affect growth (c) and weight (d) of tumor and generate GrB+CD8+T cells at day 20 post tumor challenge (e). The experiment was reproduced twice with 4-5 mice/group. (f and g) Aging B1a cells induce antigen-specific GrB+CD8+T cells by presenting endogenous antigens. Sort purified CD5− and CD5+ splenic B cells or CD23- depleted PC B cells (B1) from old and young C57BL/6 mice either with B16 melanoma or i.p. injected OVA protein were cultured for 5 days with TCR transgenic CD8+T cells from young pmel (f) or OT-1 mice (g), respectively. Note: In (f and g) no exogenous antigen or anti-CD3 Ab stimulation was used during B cell and T cell co-culture; the TCR transgenic T cells were pre-activated in pmel and OT-1 mice for 5 days by s.c. injecting 5 μg of a respective peptide in IFA. Shown is the mean (%) ± SEM of 3 mice/per group experiment (f) and individual mice (g).
A consequence of 4BL cell enrichment in mice is that it retards growth of poorly immunogenic B16 melanoma cells (30). Thus, to test the in vivo function of sB1a cells, we adoptively transferred B-cell deficient JHT mice (JHT mice) with sB1a or FOB cells sort-purified from congenic naïve young or old mice. Then the mice were subcutaneously challenged with a lethal dose of B16 melanoma cells (Fig. 2C). Transfer of FOB cells and, particularly sB1a cells of young mice, significantly increased growth of the B16 melanoma (p<0.05 Fig. 2C,D), confirming their tumor-supporting functions (43, 44). In contrast, the transfer of sB1a cells of old mice failed to do so and instead retarded tumor growth (p<0.05 as compared with mock-treatment; Fig. 2C,D). This response was lost if sB1a cells were from Old-restored mice, as the tumor grew as fast as after the transfer of young sB1a cells (Fig. 2C,D). The JHT mice replenished only with aging sB1a cells contained high levels of GrB+CD8+T cells in the circulation (Fig. 2E) and in the secondary lymphoid organs (SFig. 1H), while transfer of sB1a cells of Old-restored or young mice failed to increase those cells (Fig. 2E and SFig. 1H). This result corroborates our previous finding that 4BL cells retard B16 melanoma growth by inducing antitumor GrB+CD8+ T cells (30), suggesting that aging B1a cells became potent inducers of antigen-specific GrB+CD8+T cells. We tested this possibility using TCR-transgenic CD8+T cells from pmel and OT-1 mice, which recognize gp10025-33 peptide (of a pigment synthesis antigen expressed by B16 melanoma cells (45)) and OVA257-264 peptide, respectively. First, we in vivo primed B cells with an antigen by challenging young and old WT C57BL/6 mice with B16 melanoma or i.p. injecting 200 μl ovalbumin (intact protein, 3 mg/ml). Then, after 4-5 days, B cells were isolated and co-cultured with respective TCR-transgenic CD8+T cells for 5-day without any cognate antigen or anti-CD3 Ab stimulation. Control B cells from naïve or mock-treated mice did not activate CD8+T cells, verifying that their activation requires a cognate antigen presentation (Fig. 2F,G). In contrast, splenic CD5+ B1 cells and, particularly, PC B1 cells of old mice with B16 melanoma, but not splenic CD5− B cells of old mice or B cells and B1a cells of young mice, markedly induced proliferation of pmel CD8+T cells (Fig. 2F). Similarly, B1 cells of OVA-injected WT mice also activated OT-1 CD8+T cells, which was more pronounced for aging B1 cells (Fig. 2G). Thus, aging B1a cells can induce antigen-specific CD8+T cell responses.
Myeloid cells induce the generation of 4BL cells
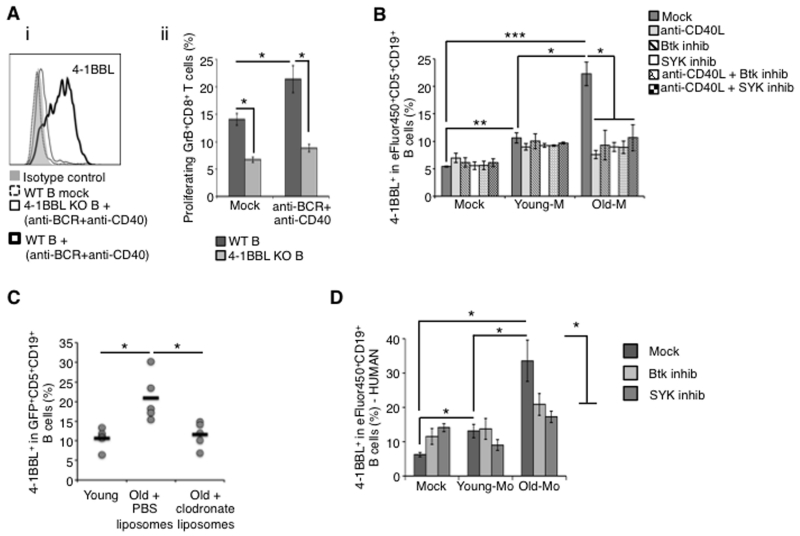
To understand the mechanism of the 4BL cell induction, we treated B cells of young mice with PC lavage of old mice. It did not induce 4BL cells (not depicted). Thus, given that BCR/CD40 stimulation up-regulates (albeit transiently) expression of 4-1BBL on B cells (32), the induction is probably mediated by PC cells. To test this possibility, we first confirmed the up-regulation of 4-BBL on young mouse B cells upon stimulation with anti-IgM/CD40 Ab (Fig. 3Ai). The stimulated B cells also induced higher levels of GrB+CD8+ T cells in our in vitro induction assay (p<0.05; Fig. 3Aii). However, this induction was lost if B cells were from 4-1BBL deficient (KO) mice (Fig. 3Aii), underscoring the importance of 4-1BBL (30). Since the BCR/CD40 signaling can be triggered by macrophages (46, 47) dysregulated upon aging (8), we also evaluated CD11b+ myeloid cells and macrophages in PC of old and young mice. These cells expressed higher levels of CD40L (needed for CD40 signaling) and IFNγ in PC of aging mice compared to young mice (SFig. 2A-F). To test their function, we in vitro co-cultured CD11b+ cells purified from PC of young or old mice (respectively, Young-M and Old-M, >70% pure after depletion of T, NK, and B cells; SFig. 3A) with eFluor450-labeled PC CD5+B cells of young mice. Upon overnight co-culture, the Old-M, but not Young-M, cells strongly up-regulated expression of 4-1BBL in B1a cells (p<0.005; Fig. 3B). Since the CD5+B cells failed to dilute eFluor450 even after 5-6 days of co-culture (SFig. 3B), Old-M cells induced conversion of 4BL cells without expansion of preexisting 4-1BBL+B1a cells. Next, to link the fact that induction requires BCR/CD40 signaling, we repeated the experiment by co-culturing Old-M cells with B1a cells in the presence of anti-CD40L blocking Ab or with B1a cells pre-treated with spleen tyrosine kinase (Syk) or Bruton’s tyrosine kinase (Btk) inhibitors, which inactivate BCR signaling (48). Both treatments completely disabled the induction of 4-1BBL+B1a cells (p<0.05; Fig. 3B), indicating the importance of the BCR/CD40 signaling axis. To confirm the importance of aging myeloid cells in vivo, we modified the experiment shown in Fig. 1A by transferring young mouse GFP+B cells into congenic old mice treated with chlodronate liposomes to deplete macrophages. While control macrophage-sufficient old mice significantly increased the frequency of 4-1BBL-expressing CD5+GFP+B cells (p=0.02; Fig. 3C, see also Fig. 1A), the macrophage-depleted old mice failed to do so just as observed in young mice (Fig. 3C). Thus, the conversion of B1a cells into 4BL cells is at least in part mediated by macrophages of aging mice. To test if a similar induction occurs in human 4BL cells, we co-cultured overnight peripheral blood (PB) B cells of young humans with PB monocytes of young or elderly humans. As in mice, only elderly human monocytes strongly induced expression of 4-1BBL in B cells (p<0.05; Fig. 3D), and this induction was lost if human B cells were pre-treated with Btk and Syk inhibitors (p<0.05, Btk or Syk vs Mock; Fig. 3D).
Figure 3. PC myeloid cells induce the generation of 4BL cells.
Resting B cells of young mice up-regulate 4-1BBL upon overnight stimulation with anti-mouse IgM (Fab, 10 μg/ml) and CD40 agonistic Ab (5 μg/ml; a) or with myeloid cells from PC of old, but not young, WT mice (b). The up-regulation was associated with the ability to induce GrB+CD8+T cells if B cells were from WT, but not 4-1BBL KO mice (a-ii). Myeloid cells cannot induce 4-1BBL+B cells if B cells were pretreated with Btk (20 nM) or SYK (4 μM) inhibitors, or stimulated in the presence of CD40 blocking Ab (b). Clodronate liposome-induced depletion of phagocytic cells in PC of old mice abrogates the induction of 4-BBL expression in i.p. injected congenic young mouse GFP+ B cells. Shown is the individual mouse results (dots) and the mean value (lines) of 5-6 representative mice/group. This experiment was reproduced twice. (d) Unlike young (Young-Mo), elderly human PB monocytes (Old-Mo) also convert young human B cells into 4BL cells. The induction was lost if B cells were pretreated with Btk or SYK inhibitors. Shown is the mean ± SEM of 4-1BBL+ in eFluor® 450-labeled B cells treated with myeloid cells from 3 young and 5 elderly people.
4BL cells up-regulate mTNFα via signaling with 4-1BBL
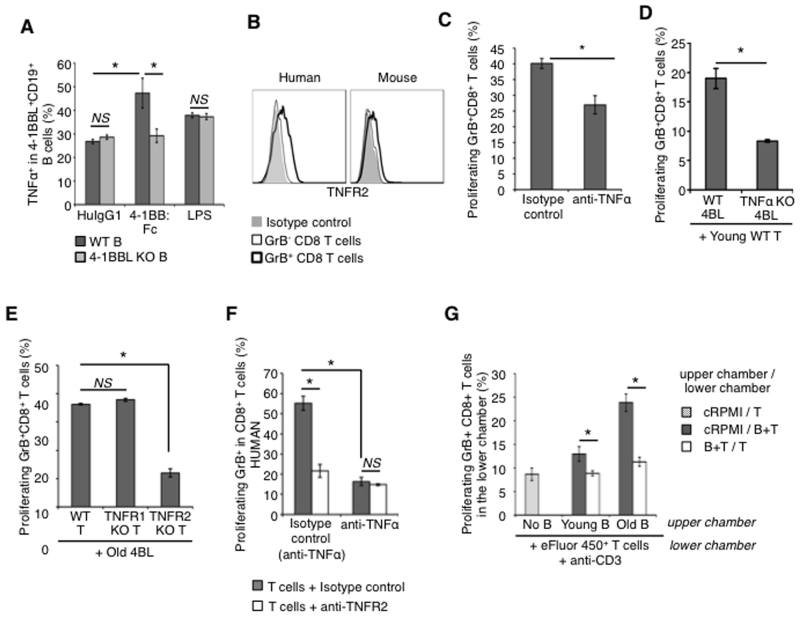
Since the conversion of 4BL cells leads to up-regulation of mTNFα (Fig. 1A,B), we next wanted to understand the mechanism of this induction. Macrophages require 4-1BBL signaling to sustain expression of TNFα (49), suggesting that a similar mechanism could also be used in 4BL cells. To test this possibility, we stimulated B cells from WT and 4-1BBL KO mice with anti-BCR/CD40 Ab alone or together with plate-bound 4-1BB-Fc protein (4-1BBL agonist (49)) or with a non-specific B-cell mitogen, LPS (50). While LPS or anti-IgM/CD40 Ab induced comparable expression of TNFα in B cells regardless of the presence or absence of 4-1BBL (i.e., WT and 4-1BBL KO; Fig. 4A), a combined treatment with BCR/CD40 Ab and 4-1BB-Fc l strongly up-regulated TNFα in WT B cells, but not in 4-1BBL KO B cells (Fig. 4A). Hence, BCR/CD40 signaling initiates expression of 4-1BBL and TNFα (as also discussed for Fig. 3B,C), and then 4-1BBL is needed to further up-regulate TNFα in 4BL cells upon engagement with 4-1BB.
Figure 4. 4BL cells induce GrB+CD8+ T cells using TNFα.
(a) The 4-1BBL expression in B cells induced with anti-mouse IgM/anti-mouse CD40 Ab is further up-regulated upon overnight stimulation with plate-bound 4-1BB:Fc protein, but not control human IgG1 (HuIgG1, 10 μg/ml). The up-regulation is lost in 4-BBL KO B cells. Control cells were treated with LPS (1 μg/ml) as a non-specific stimulator. (b) A representative histogram that shows that TNFR2 is mostly expressed on GrB+CD8+ T cells. Thick and thin lines are for GrB+ and GrB−CD8+ T cells, respectively. Murine (c,d,e,g) and human (f) 4BL cells lose the ability to induce GrB+CD8+ T cells in the presence of TNFα neutralizing Ab (5 μg/ml; c and f), anti-TNFR2 Ab (10 μg/ml; f), or if B cells are TNFα KO (d) or if T cells are from TNFR2 KO, but not TNFR1 KO, mice (e). No GrB+CD8+T cells were induced if B cells and T cells were physically separated in a trans-well assay (g). Shown is the frequency mean ± SEM of GrB+CD8+ T cells of a representative experiment repeated at least 3 times with 2-4 mice and human cells per group.
Since TNFα can be involved in expression of GrB (51), we evaluated expression of its receptors on CD8+T cells. Unlike TNFR1, TNFR2 was up-regulated on GrB+CD8+ cells of aging mice and humans (Fig. 4B). Thus, considering high levels of expression of its ligand mTNFα on 4BL cells (Fig. 1A,B), we next tested whether the mTNFα/TNFR2 axis is used to induce expression of GrB in CD8+T cells. First, we included TNFα neutralizing Ab or control IgG1 in our in vitro GrB+CD8+T cell-induction assay with 4BL cells and we observed that the depletion of TNFα significantly retarded the induction of GrB expression in CD8+T cells (Fig. 4C). The induction of GrB+CD8+T cells was also markedly retarded if we used 4BL cells from TNFα KO mice (Fig. 4D). Moreover, WT 4BL cells failed to efficiently induce expression of GrB in CD8+T cells from TNFR2 KO mice (Fig. 4E), while the induction was unimpaired in TNFR1 KO CD8+ T cells (Fig. 4E). Similarly for human cells, TNFR2 or TNFα neutralizing Abs also abrogated the induction of GrB+CD8+ T cells in an MLR assay utilizing elderly human 4BL cells (Fig. 4F). Since the mTNFα/TNFR2 axis probably requires a cell contact, we also cultured 4BL cells with CD8+T cells either in the same well or separated with a porous membrane in trans-well assay. The separation of the two cells completely abrogated the induction of GrB+CD8+ T cells (Fig. 4G). Together, these data demonstrate that BCR/CD40 signaling induces expression of 4-1BBL and mTNFα on 4BL cells. Furthermore, upon activation of 4-1BBL, mTNFα is further up-regulated to induce GrB+CD8+T cells by targeting TNFR2.
4BL cells up-regulate TNFR2 in CD8+T cells by targeting 4-1BB
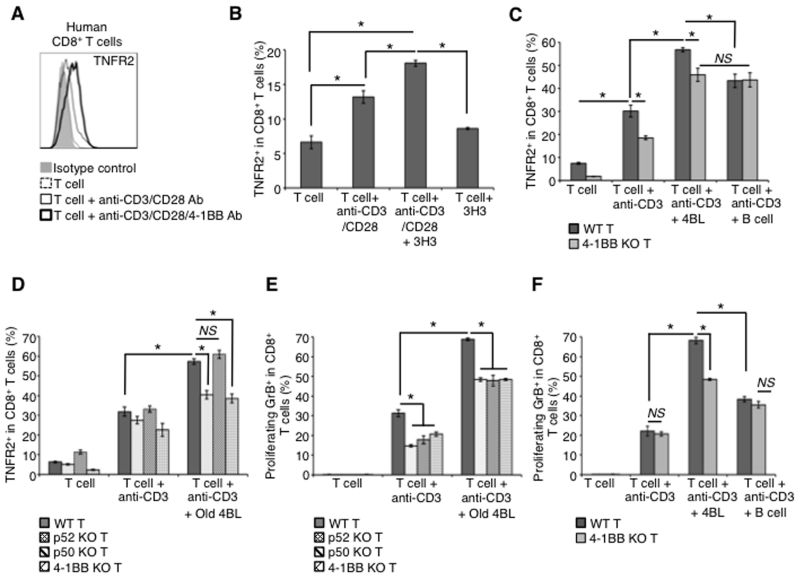
Next, we wanted to understand how CD8+ T cells up-regulate TNFR2. Since TCR stimulation induces expression of 4-1BB (32, 33, 36), we tested whether it can also up-regulate TNFR2. Indeed, CD8+T cells stimulated with anti-CD3 or anti-CD3/CD28 Ab not only induced expression of 4-1BB (SFig. 3C) but also TNFR2 (Fig. 5A-D). However, expression of TNFR2 was further and considerably up-regulated if the stimulation also contained agonistic 4-1BB Ab (3H3; Fig. 5A,B). Since treatment with 3H3 alone did not induce expression of TNFR2 (Fig. 5B) (presumably due to low levels of 4-1BB on resting T cells (SFig. 3C)) (33), these results suggest that activated T cells first need to induce expression of 4-1BB, which upon receiving signaling from 4-BBL expressed on 4BL cells can further up-regulate expression of TNFR2. In support of this model, the anti-CD3/CD28 Ab-induced expression of TNFR2 in CD8+T cells was markedly augmented upon co-culture with sort-purified 4-1BBL+, but not 4-1BB−, B cells of old mice (4BL and B cells, respectively; Fig. 5C). However, if CD8+T cells were from 4-1BB KO mice, the 4BL cell-induced up-regulation of TNFR2 was lost, as its expression failed to increase beyond the levels induced by anti-CD3/CD28 Ab stimulation alone (Fig. 5C). Thus, the induction of TNFR2 expression in CD8+T cells is bimodal as its expression induced via TCR signaling is further up-regulated upon 4-1BB signaling induced by 4BL cells. Since 4-1BB can signal via both the canonical and non-canonical NF-κB pathway (52), we also compared the induction of TNFR2 in CD8+T cells from mice deficient in classical (p50) and non-canonical (p52) NF-κB. While 4BL cells in the presence of anti-CD3 Ab up-regulated TNFR2 in p50 KO CD8+ T cells as efficiently as in WT T cells (Fig. 5D), they failed to do so in p52 KO T cells (Fig. 5D) as if the T cells were 4-1BB KO (Fig. 5C,D). Hence, 4BL cells use 4-1BBL to up-regulate expression of TNFR2 in CD8+T cells via inducing non-canonical NFκB signaling from 4-1BB. As discussed above, the TNFR2 is then targeted to induce expression of GrB in CD8+ T cells. Indeed, the TNFR2 up-regulation mirrored the induction of GrB+CD8+ T cells, i.e., the induction of GrB expression in p52 KO (Fig. 5E) and 4-1BB KO (Fig. 5F) CD8+T cells was reduced to the levels induced by anti-CD3 Ab stimulation alone despite the presence of old mouse 4BL cells. Of note, GrB induction was also reduced in p50 KO CD8+ T cells (Fig. 5E), suggesting that, unlike TNFR2, its expression requires canonical NF-κB (53).
Figure 5. 4BL cells up-regulate TNFR2 in CD8+ T cells via the 4-1BB/4-1BBL axis.
While TNFR2 is induced on resting human (a) and murine (b) CD8+ T cells after stimulation with anti-CD3/CD28 Ab (thin line) for 24 h, it is further up-regulated upon 4-1BB engagement (anti-CD3/CD28/4-1BBL Ab beads, thick line in a, and anti-CD3/CD28 Ab with 3H3 Ab, 5 μg/ml, b). Dotted line is for non-stimulated T cells. Unlike 4-1BBL−B cells (B cell; c), aging 4BL cells induce TNFR2 in CD8+ T cells (c and d). The up-regulation is lost in T cells from 4-1BB KO (c and d) and p52, but not p50, NFκB KO (d) mice. Before mixing with B cells, T cells were pretreated with anti-CD3 Ab (1.5 μg/ml) for 24 h to up-regulate 4-1BB. CD8+ cells shown in c and d also express GrB after 5 days culture (e and f). Shown is the mean ± SEM of a representative experiment repeated at least twice with 3-4 mice/group.
Myeloid cells enhance co-stimulatory function of 4BL cells via IFNγ
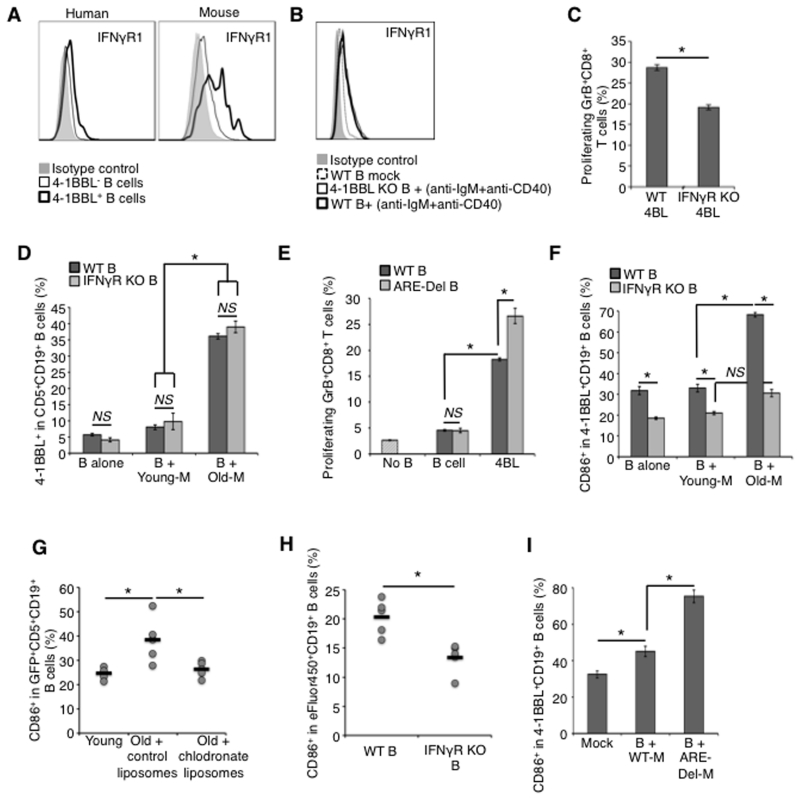
Interestingly, besides being high expressers of 4-1BBL, MHC class-I and CD86 (30), as well as mTNFα, 4BL cells also expressed IFNγR1 (Fig. 6A). Its expression appears to also depend on BCR/CD40 signaling, as resting murine and human B cells only markedly up-regulated IFNγR1 upon anti-IgM/CD40 Ab stimulation (Fig. 6B). Since IFNγ is a potential inducer of CD86 (54), we hypothesized that 4BL cells probably enhance their T-cell costimulatory functions by receiving IFNγ expressed in aging CD11b+myeloid cells (SFig. 2E,F). In concordance, 4BL cells from IFNγR1 KO mice failed to efficiently induce the generation of GrB+CD8+T cells in vitro (Fig. 6C). Conversely, the presence of blocking CD86 Ab (but not control Ab) was sufficient to abrogate the induction of GrB+CD8+T cells with WT 4BL cells (SFig. 4A). Although IFNγ alone up-regulated CD86 on resting B cells (SFig. 4B), it was not sufficient to convert B cells into 4BL cells. IFNγ and IFNγR (IFNγ KO and IFNγR1 KO) did not affect the low frequency of 4BL cells present in young mice (SFig. 4C-E, and B-alone in Fig. 6D), which is usually around 5% of B cells (30). Similarly, the proportion of 4BL cells was not increased in ARE-Del mice (SFig. 4F), which constitutively express IFNγ (39). Although B cells of ARE-Del mice demonstrate highly up regulated CD86 compared to WT mice (SFig. 4G), their sort-purified 4-1BBL−B cells failed to induce GrB+CD8+T cells stimulated with anti-CD3 Ab (Fig. 6E) despite their chronic exposure to IFNγ. In contrast, ARE-Del 4-1BBL+ (4BL cells) markedly induced GrB+CD8+T cells with a significantly higher potency than WT 4BL cells (p<0.05; Fig. 6E). Hence, these results suggest that 4BL cells use IFNγR1 to up-regulate CD86 and thereby contribute to the generation of GrB+CD8+ T cells.
Figure 6. Myeloid cells also enhance the co-stimulatory function of 4BL cells via IFNγ.
(a) Although human and murine 4BL cells mostly express IFNγR1 (thick line, a), both 4-BBL KO and WT B cells up-regulate its expression upon anti-IgM/CD40 stimulation (red and black thick lines, b). IFNγR1 KO 4BL cells less efficiently induce GrB+CD8+ T cells in vitro (c). Unlike 4-BBL that is induced on both IFNγR KO and WT CD5+B cells stimulated with myeloid cells of old mice (Old-M) but not young mice (Young-M); CD86 requires IFNγ signaling, as it was not induced on IFNγR1 KO B (f). Compared to WT 4BL cells, 4BL cells from ARE-Del mice induce a higher frequency of GrB+CD8+ T cells (d). Shown is the mean ± SEM of a representative experiment repeated at least twice in triplicate experiments with 3-4 mice/group. PC macrophages of old mice in vivo induce CD86 (g). B1a cells from young GFP+ mice (g) were i.p. injected into congenic young and old mice pre-treated with control liposome or clodronate liposomes (g), as in Fig. 3c. In (h), eFluor® 450-labeled B cells from WT or IFNγR1 KO mice were i.p. injected into old WT mice. Shown is the frequency of CD86+ in GFP+ B1a (g) or eFlur450+B1a (h) from individual mice (dots) and the mean value (lines) of a representative 5-6 mice/group experiment repeated twice. Ex vivo, myeloid cells of young ARE-Del mice (B+ ARE-Del-M) also strongly up-regulate CD86 on B cells of young WT mice compared to WT myeloid cells (B+WT-M, i).
To test the role of myeloid cells in this process, we co-cultured purified CD5+B cells (B-alone) from young WT and IFNγR1 KO mice with congenic PC Young-M and Old-M cells. While Old-M up-regulated 4-1BBL on both WT and IFNγR1 KO B cells (Fig. 3 and Fig. 6D), they failed to induce CD86 in IFNγR1 KO B cells (Fig. 6F and SFig. 4E). Young mouse B cells also significantly up-regulated CD86 upon injection into PC of congenic and macrophage-sufficient old mice (Fig. 6G), but failed to do so in old mice depleted of macrophages (Fig. 6G) or if B cells were from IFNγR1 KO mice (Fig. 6H). Control B cells both in vitro and in vivo exposed to Young-M cells did not induce expression of CD86 and 4-1BBL (see B-alone or mock with B + Young-M, Fig. 6D,F and SFig. 4E, and Young in Fig. 6G). Furthermore, CD11b+ myeloid cells from PC of young ARE-Del mice (which constitutively express IFNγ (39)) also strongly up-regulated CD86 upon their in vitro co-culture, while WT CD11b+ cells of young mice failed to do so (Fig. 6I), probably accounting for the enhanced ability of 4-1BBL+B cells from ARE-Del mice to induce GrB+CD8+ T cells (Fig. 6E). Together, these results indicate that aging myeloid cells, such as macrophages, use the IFNγ/IFNγR1 signaling axis to enhance the co-stimulatory function of 4BL cells by up-regulating CD86.
DISCUSSION
Aging significantly dysregulates the function and distribution of B cells in the circulation (15-18), resulting in impaired humoral responses to new antigens and vaccines in the elderly (55) (see reviews 1, 2). We recently reported that elderly humans, macaques, and mice accumulate 4-1BBL+B cells (4BL cells) of unknown origin (30). They phenotypically and functionally differ from other B cells enriched in aging, such as mature and antigen-experienced memory B2 cells of mammals (15-18) and exhausted mature B2 cells (ABCs) in mice (29). Here, we demonstrate that at least in mice 4BL cells are B1a cells converted upon aging, while in human PB they appear to be memory CD43−CD27+ B cells. The failure to link 4BL cells with human memory CD43+CD27 + B cells that contain B1 cells (27) could be due to reduced CD43 expression in aging or the species difference. Although aging is not known to affect the function of innate B1 cells (11, 20, 21), it increases the numbers of both B1a cells (as well as B2 cells) in PC of mice (11), presumably due to their enhanced longevity (13), self-renewal capabilities (21), and homing in response to elevated production of CXCL13 (56-58). Aging B1a cells increase 3-fold in mice (21, 59) and, being CXCR5High, infiltrate into spleen and PC with a higher efficiency than B2 cells (56-58). Functionally, aging B1 cells are implicated in autoimmune diseases, such as Sjogren’s syndrome in humans (60) and lupus erythematosus in mice (61), and are primarily considered the producers of natural antibodies, mediators of tissue homeostasis, and involved in the clearance of apoptotic cells (62). In contrast, our data indicate that aging dramatically affects the function of B1a cells, as they lose tumor-supporting (43) and possibly immune suppressive activities but gain a new property—the induction of GrB+CD8+T cells. For example, while young B1a cells strongly enhance the growth of B16 melanoma in congenic B-cell-deficient mice, aging B1a cells fail to do so. Instead they retard tumor growth by inducing antitumor GrB+CD8+T cells, the function of which we previously reported for 4BL cells in old mice with poorly immunogenic tumors and in breast cancer patients after autologous hematopoietic stem transplantation (auto-HSCT) (30). Thus, aging renders B1a cells/4BL cells into APCs that can uptake endogenous antigens in vivo, (e.g., B16 melanoma or i.p. injected OVA protein) and induce antigen-specific CD8+T cell responses such as TCR-transgenic CD8+T cells from pmel and OT-1 mice, respectively. This is in line with the fact that 4BL cells highly express an array of surface molecules needed for the induction of CD8+ T cells, including MHC class-I, CD86, CD40, and 4-1BBL (30).
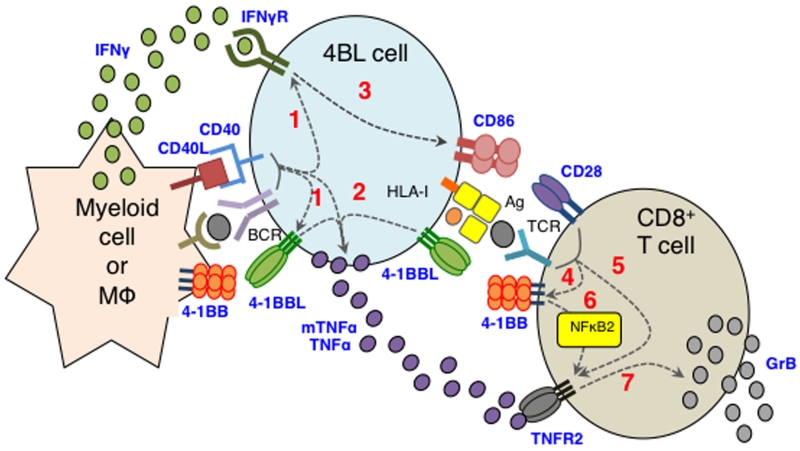
In our previous report we hypothesized that the generation of 4BL cells is induced by extrinsic factors, as these cells also accumulate in cancer patients after auto-HSCT and their reappearance after B-cell depletion in Old-restored mice was significantly delayed compared to the rest of the B cells (30). Here, we experimentally confirm this hypothesis by demonstrating the importance of aging myeloid cells, such as human monocytes and murine PC macrophages, in the conversion of 4BL cells. This is in line with a plethora of changes reported for monocytes and macrophages upon aging in humans and rodents. For example, old rats enrich for CD11b+ monocytes expressing IFNγ (63), while elderly human PB accumulate so-called intermediate and non-classical monocytes producing high levels of TNFα (8). Aging also impairs phagocytosis of myeloid cells, such as human neutrophils and CD14+ monocytes and murine PC macrophages (8, 11), and the responses of monocytes and macrophages to IFNγ and TLR stimulations (64-66). We also found that old mice show an increase in CD11b+ myeloid cells and macrophages expressing IFNγ and CD40L. Importantly, our in vitro and in vivo experiments indicate that aging mouse PC CD11b+ myeloid cells can directly convert young mouse B1 cells into 4BL cells. Since control myeloid cells from young mice or old mice depleted of phagocytic cells such as macrophages failed to do so, we conclude that the conversion is primarily mediated by aging macrophages. In humans, PB monocytes of the elderly appear to be the inducers of 4BL cells. Thus, by corroborating the importance of crosstalk between myeloid cells/macrophages and B cells (67-69), our data suggest that the dysregulation of macrophages in the elderly can initiate a chain of events including the conversion of B1a cells into 4BL cells and concurrent induction of cytolytic CD8+T cells, as summarized in Fig. 7. First, they trigger BCR and CD40 signaling and thereby induce expression of 4-1BBL, mTNFα, and IFNγR1 in B1a cells, converting them into 4BL cells (step 1, Fig. 7). This initial step can be abrogated by blocking BCR or CD40 signaling, indicating the importance of these pathways in the conversion of 4BL cells as well as in the development and function of B1a cells (25, 48). Since human and murine monocytes and macrophages express CD40L (70) and CD40L+CD11b+ myeloid cells that are enriched in PC of aging mice, they could provide CD40L to activate CD40 on B1a cells. In contrast, we do not know the mechanism of the BCR signaling. Since macrophages can stimulate BCR during exchange of antigens (46, 47) via FcγRIIB-captured and recycled immune complexes (71), we can only speculate that myeloid cells of aging subjects activate B1a cells by utilizing autoreactive antibody that is increased upon aging (72). As a result, 4BL cells express 4-1BBL to further up-regulate expression of mTNFα and IFNγR1 upon engagement with 4-1BB (step 2, Fig. 7). This is consistent with the fact that signaling of 4-1BBL is required for sustained production of TNFα in macrophages (49, 73) and that its receptor is also expressed on myeloid cells such as splenic DCs (32). By expressing IFNγR1, 4BL cells also up-regulate CD86 in response to IFNγ (step 3, Fig. 7). Using neutralizing antibody or cells with impaired expressions of IFNγ or IFNγR1, we demonstrate that aging CD11b+ myeloid cells and macrophages provide IFNγ, although in vivo it can probably be supplied by IFNγ-producing cells such as Th1 T cells and NK cells. In addition, considering its importance in MHC class-I expression and antigen presentation (74), IFNγ is probably responsible for the enhanced expression of MHC class-I on 4BL cells (30). Thus, aging myeloid cells induce high levels of expression of 4-BBL, mTNFα, CD86, and MHC-I on B1a cells. As a result, the activated B1a cells/4BL cells become APCs that induce antigens-specific GrB+CD8+T cell responses requiring the 4-1BBL/4-1BB signaling axis (30). First, aging B1a cells induce expression of 4-1BB (step 4) and TNFR2 (step 5, Fig. 7) in CD8+T cells. Then, they target 4-1BB via 4-1BBL to further up-regulate expression of TNFR2 in CD8+T cells (step 6, Fig. 7). Confirming the use of the non-canonical NF-κB signaling by 4-1BB (52), 4BL cells failed to up-regulate TNFR2 in CD8+T cells deficient in the non-canonical, but not canonical, NF-kB signaling pathway. Then, using mTNFα, 4BL cells target TNFR2 and induce expression of GrB in CD8+T cells (step 7, Fig. 7), while providing co-stimulation with CD86. In summary, our data suggest that aging converts B1a cells into potentially pathogenic and autoimmune 4BL cells via dysregulation of the myeloid cell compartment.
Figure 7. Summary schema.
Aging CD11b+ myeloid cells initiate a chain of events that convert B1a cells into 4BL cells and concurrently induce GrB+CD8+ T cells. First, they convert B1a cells into 4BL cells by inducing expression of 4-1BBL, mTNFα, and IFNγR [1]. Then, 4BL cells further up-regulate mTNFα and CD86 via activating 4-1BBL [2] and IFNγR1 [3] signaling from 4-1BB and IFNγ provided by aging myeloid cells, respectively. 4BL cells activate CD8+T cells to express 4-1BB [4] and TNFR2 [5]. This activation enables 4BL cells to trigger non-canonical NFκB signaling from 4-1BB and further increase expression of TNFR2 on CD8+T cells [6]. The TNFR2 is targeted with mTNFα to induce expression of GrB in CD8+T cells, while providing co-stimulation with CD86.
Supplementary Material
ACKNOWLEDGMENTS
We are grateful to Linda Zukley (NIA/NIH) for help with human blood samples; Drs. Robert Wiltrout and Jonathan Weiss (NCI/NIH) for IFNγR mice; Mary Kaileh (NIA) for p50 and p52 KO mice; Nan-Ping Weng for OT-1 mice; and Dr. Joost Oppenheim (NCI/NIH) for helpful discussions and comments. The authors are also grateful to Cindy Clark (NIH Library) for proofreading of the manuscript.
Financial Support This research was supported by the Intramural Research Program of the National Institute on Aging, NIH.
Footnotes
AUTHOR CONTRIBUTIONS
C.L-C. and M.B. designed and performed the research and collected and analyzed data; K.M., X.C., R.W., R.S., H.A.Y., M.C., and L.F. contributed to vital new reagents and provided critical interpretation; C.L-C. and A.B. wrote the manuscript; and A.B. conceived, designed, and supervised the study.
Disclosures
The authors have no financial conflicts of interest.
Supplementary Materials include S.Fig.1-4.
References
- 1.Le Saux S, Weyand CM, Goronzy JJ. Mechanisms of immunosenescence: lessons from models of accelerated immune aging. Ann. N. Y. Acad. Sci. 2012;1247:69–82. doi: 10.1111/j.1749-6632.2011.06297.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rodier F, Campisi J. Four faces of cellular senescence. J. Cell Biol. 2011;192:547–556. doi: 10.1083/jcb.201009094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sudo K, Ema H, Morita Y, Nakauchi H. Age-associated characteristics of murine hematopoietic stem cells. J. Exp. Med. 2000;192:1273–1280. doi: 10.1084/jem.192.9.1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Morrison SJ, Wandycz AM, Akashi K, Globerson A, Weissman IL. The aging of hematopoietic stem cells. Nat Med. 1996;2:1011–1016. doi: 10.1038/nm0996-1011. [DOI] [PubMed] [Google Scholar]
- 5.Roeder I, Horn K, Sieburg HB, Cho R, Muller-Sieburg C, Loeffler M. Characterization and quantification of clonal heterogeneity among hematopoietic stem cells: a model-based approach. Blood. 2008;112:4874–4883. doi: 10.1182/blood-2008-05-155374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cho RH, Sieburg HB, Muller-Sieburg CE. A new mechanism for the aging of hematopoietic stem cells: aging changes the clonal composition of the stem cell compartment but not individual stem cells. Blood. 2008;111:5553–5561. doi: 10.1182/blood-2007-11-123547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kuranda K, Vargaftig J, de la Rochere P, Dosquet C, Charron D, Bardin F, Tonnelle C, Bonnet D, Goodhardt M. Age-related changes in human hematopoietic stem/progenitor cells. Aging Cell. 2011;10:542–546. doi: 10.1111/j.1474-9726.2011.00675.x. [DOI] [PubMed] [Google Scholar]
- 8.Hearps AC, Martin GE, Angelovich TA, Cheng WJ, Maisa A, Landay AL, Jaworowski A, Crowe SM. Aging is associated with chronic innate immune activation and dysregulation of monocyte phenotype and function. Aging Cell. 2012;11:867–875. doi: 10.1111/j.1474-9726.2012.00851.x. [DOI] [PubMed] [Google Scholar]
- 9.Bouchlaka MN, Sckisel GD, Chen M, Mirsoian A, Zamora AE, Maverakis E, Wilkins DE, Alderson KL, Hsiao HH, Weiss JM, Monjazeb AM, Hesdorffer C, Ferrucci L, Longo DL, Blazar BR, Wiltrout RH, Redelman D, Taub DD, Murphy WJ. Aging predisposes to acute inflammatory induced pathology after tumor immunotherapy. J Exp Med. 2013;210:2223–2237. doi: 10.1084/jem.20131219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Smallwood HS, Lopez-Ferrer D, Squier TC. Aging enhances the production of reactive oxygen species and bactericidal activity in peritoneal macrophages by upregulating classical activation pathways. Biochemistry. 2011;50:9911–9922. doi: 10.1021/bi2011866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Linehan E, Dombrowski Y, Snoddy R, Fallon PG, Kissenpfennig A, Fitzgerald DC. Aging impairs peritoneal but not bone marrow-derived macrophage phagocytosis. Aging Cell. 2014;13:699–708. doi: 10.1111/acel.12223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mancuso P, McNish RW, Petersw-Golden M, Brock TG. Evaluation of phagocytosis and arachidonate metabolism by alveolar macrophages and recruited neutrophils from F344xBN rats of different ages. Mech Ageing Dev. 2001;122:1899–1913. doi: 10.1016/s0047-6374(01)00322-0. [DOI] [PubMed] [Google Scholar]
- 13.Kline GH, Hayden TA, Klinman NR. B cell maintenance in aged mice reflects both increased B cell longevity and decreased B cell generation. J Immunol. 1999;162:3342–3349. [PubMed] [Google Scholar]
- 14.Johnson KM, Owen K, Witte PL. Aging and developmental transitions in the B cell lineage. Int Immunol. 2002;14:1313–1323. doi: 10.1093/intimm/dxf092. [DOI] [PubMed] [Google Scholar]
- 15.Koch S, Larbi A, Derhovanessian E, Ozcelik D, Naumova E, Pawelec G. Multiparameter flow cytometric analysis of CD4 and CD8 T cell subsets in young and old people. Immun Ageing. 2008;5:6. doi: 10.1186/1742-4933-5-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lerner A, Yamada T, Miller RA. Pgp-1hi T lymphocytes accumulate with age in mice and respond poorly to concanavalin A. European journal of immunology. 1989;19:977–982. doi: 10.1002/eji.1830190604. [DOI] [PubMed] [Google Scholar]
- 17.Guerrettaz LM, Johnson SA, Cambier JC. Acquired hematopoietic stem cell defects determine B-cell repertoire changes associated with aging. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:11898–11902. doi: 10.1073/pnas.0805498105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cancro MP, Allman DM. Connecting the dots: revealing the interactions of lymphocyte development and homeostasis in the immunobiology of aging. Semin Immunol. 2005;17:319–320. doi: 10.1016/j.smim.2005.05.017. [DOI] [PubMed] [Google Scholar]
- 19.Barber CL, Montecino-Rodriguez E, Dorshkind K. Reduced production of B-1-specified common lymphoid progenitors results in diminished potential of adult marrow to generate B-1 cells. Proc Natl Acad Sci U S A. 2011;108:13700–13704. doi: 10.1073/pnas.1107172108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Alter-Wolf S, Blomberg BB, Riley RL. Old mice retain bone marrow B1 progenitors, but lose B2 precursors, and exhibit altered immature B cell phenotype and light chain usage. Mech Ageing Dev. 2009;130:401–408. doi: 10.1016/j.mad.2009.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stall AM, Farinas MC, Tarlinton DM, Lalor PA, Herzenberg LA, Strober S, Herzenberg LA. Ly-1 B-cell clones similar to human chronic lymphocytic leukemias routinely develop in older normal mice and young autoimmune (New Zealand Black-related) animals. Proc Natl Acad Sci U S A. 1988;85:7312–7316. doi: 10.1073/pnas.85.19.7312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Herzenberg LA, Baumgarth N, Wilshire JA. B-1 cell origins and VH repertoire determination. Curr Top Microbiol Immunol. 2000;252:3–13. doi: 10.1007/978-3-642-57284-5_1. [DOI] [PubMed] [Google Scholar]
- 23.Hayakawa K, Hardy RR, Parks DR, Herzenberg LA. The “Ly-1 B” cell subpopulation in normal immunodefective, and autoimmune mice. The Journal of experimental medicine. 1983;157:202–218. doi: 10.1084/jem.157.1.202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Caligaris-Cappio F, Gobbi M, Bofill M, Janossy G. Infrequent normal B lymphocytes express features of B-chronic lymphocytic leukemia. The Journal of experimental medicine. 1982;155:623–628. doi: 10.1084/jem.155.2.623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hardy RR, Hayakawa K. B cell development pathways. Annu Rev Immunol. 2001;19:595–621. doi: 10.1146/annurev.immunol.19.1.595. [DOI] [PubMed] [Google Scholar]
- 26.Perez-Andres M, Grosserichter-Wagener C, Teodosio C, van Dongen JJ, Orfao A, van Zelm MC. The nature of circulating CD27+CD43+ B cells. J Exp Med. 2011;208:2565–2566. doi: 10.1084/jem.20112203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Griffin DO, Holodick NE, Rothstein TL. Human B1 cells in umbilical cord and adult peripheral blood express the novel phenotype CD20+ CD27+ CD43+ CD70. The Journal of experimental medicine. 2011;208:67–80. doi: 10.1084/jem.20101499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Descatoire M, Weill JC, Reynaud CA, Weller S. A human equivalent of mouse B-1 cells? J Exp Med. 2011;208:2563–2564. doi: 10.1084/jem.20112232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hao Y, O’Neill P, Naradikian MS, Scholz JL, Cancro MP. A B-cell subset uniquely responsive to innate stimuli accumulates in aged mice. Blood. 2011;118:1294–1304. doi: 10.1182/blood-2011-01-330530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lee-Chang C, Bodogai M, Moritoh K, Olkhanud PB, Chan AC, Croft M, Mattison JA, Holst PJ, Gress RE, Ferrucci L, Hakim F, Biragyn A. Accumulation of 4-1BBL+ B cells in the elderly induces the generation of granzyme-B+ CD8+ T cells with potential antitumor activity. Blood. 2014 doi: 10.1182/blood-2014-03-563940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang C, Lin GH, McPherson AJ, Watts TH. Immune regulation by 4-1BB and 4-1BBL: complexities and challenges. Immunol Rev. 2009;229:192–215. doi: 10.1111/j.1600-065X.2009.00765.x. [DOI] [PubMed] [Google Scholar]
- 32.Futagawa T, Akiba H, Kodama T, Takeda K, Hosoda Y, Yagita H, Okumura K. Expression and function of 4-1BB and 4-1BB ligand on murine dendritic cells. Int Immunol. 2002;14:275–286. doi: 10.1093/intimm/14.3.275. [DOI] [PubMed] [Google Scholar]
- 33.Cooper D, Bansal-Pakala P, Croft M. 4-1BB (CD137) controls the clonal expansion and survival of CD8 T cells in vivo but does not contribute to the development of cytotoxicity. European journal of immunology. 2002;32:521–529. doi: 10.1002/1521-4141(200202)32:2<521::AID-IMMU521>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 34.Watts TH, DeBenedette MA. T cell co-stimulatory molecules other than CD28. Curr Opin Immunol. 1999;11:286–293. doi: 10.1016/s0952-7915(99)80046-6. [DOI] [PubMed] [Google Scholar]
- 35.Shuford WW, Klussman K, Tritchler DD, Loo DT, Chalupny J, Siadak AW, Brown TJ, Emswiler J, Raecho H, Larsen CP, Pearson TC, Ledbetter JA, Aruffo A, Mittler RS. 4-1BB costimulatory signals preferentially induce CD8+ T cell proliferation and lead to the amplification in vivo of cytotoxic T cell responses. J Exp Med. 1997;186:47–55. doi: 10.1084/jem.186.1.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kim HJ, Lee JS, Kim JD, Cha HJ, Kim A, Lee SK, Lee SC, Kwon BS, Mittler RS, Cho HR, Kwon B. Reverse signaling through the costimulatory ligand CD137L in epithelial cells is essential for natural killer cell-mediated acute tissue inflammation. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:E13–22. doi: 10.1073/pnas.1112256109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gramaglia I, Cooper D, Miner KT, Kwon BS, Croft M. Co-stimulation of antigen-specific CD4 T cells by 4-1BB ligand. Eur J Immunol. 2000;30:392–402. doi: 10.1002/1521-4141(200002)30:2<392::AID-IMMU392>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 38.Zhao Y, Croft M. Dispensable role for 4-1BB and 4-1BBL in development of vaccinia virus-specific CD8 T cells. Immunology letters. 2012;141:220–226. doi: 10.1016/j.imlet.2011.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hodge DL, Berthet C, Coppola V, Kastenmuller W, Buschman MD, Schaughency PM, Shirota H, Scarzello AJ, Subleski JJ, Anver MR, Ortaldo JR, Lin F, Reynolds DA, Sanford ME, Kaldis P, Tessarollo L, Klinman DM, Young HA. IFN-gamma AU-rich element removal promotes chronic IFN-gamma expression and autoimmunity in mice. J Autoimmun. 2014;53:33–45. doi: 10.1016/j.jaut.2014.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhao Y, Tahiliani V, Salek-Ardakani S, Croft M. Targeting 4-1BB (CD137) to enhance CD8 T cell responses with poxviruses and viral antigens. Front Immunol. 2012;3:332. doi: 10.3389/fimmu.2012.00332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schiavo R, Baatar D, Olkhanud P, Indig FE, Restifo N, Taub D, Biragyn A. Chemokine receptor targeting efficiently directs antigens to MHC class I pathways and elicits antigen-specific CD8+ T-cell responses. Blood. 2006;107:4597–4605. doi: 10.1182/blood-2005-08-3207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yanaba K, Bouaziz JD, Haas KM, Poe JC, Fujimoto M, Tedder TF. A regulatory B cell subset with a unique CD1dhiCD5+ phenotype controls T cell-dependent inflammatory responses. Immunity. 2008;28:639–650. doi: 10.1016/j.immuni.2008.03.017. [DOI] [PubMed] [Google Scholar]
- 43.Perez EC, Machado J, Jr., Aliperti F, Freymuller E, Mariano M, Lopes JD. B-1 lymphocytes increase metastatic behavior of melanoma cells through the extracellular signal-regulated kinase pathway. Cancer science. 2008;99:920–928. doi: 10.1111/j.1349-7006.2008.00776.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bodogai M, Lee Chang C, Wejksza K, Lai J, Merino M, Wersto RP, Gress RE, Chan AC, Hesdorffer C, Biragyn A. Anti-CD20 antibody promotes cancer escape via enrichment of tumor-evoked regulatory B cells expressing low levels of CD20 and CD137L. Cancer research. 2013;73:2127–2138. doi: 10.1158/0008-5472.CAN-12-4184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Overwijk WW, Theoret MR, Finkelstein SE, Surman DR, de Jong LA, Vyth-Dreese FA, Dellemijn TA, Antony PA, Spiess PJ, Palmer DC, Heimann DM, Klebanoff CA, Yu Z, Hwang LN, Feigenbaum L, Kruisbeek AM, Rosenberg SA, Restifo NP. Tumor regression and autoimmunity after reversal of a functionally tolerant state of self-reactive CD8+ T cells. J.Exp.Med. 2003;198:569–580. doi: 10.1084/jem.20030590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Carrasco YR, Batista FD. B cells acquire particulate antigen in a macrophage-rich area at the boundary between the follicle and the subcapsular sinus of the lymph node. Immunity. 2007;27:160–171. doi: 10.1016/j.immuni.2007.06.007. [DOI] [PubMed] [Google Scholar]
- 47.Harvey BP, Quan TE, Rudenga BJ, Roman RM, Craft J, Mamula MJ. Editing antigen presentation: antigen transfer between human B lymphocytes and macrophages mediated by class A scavenger receptors. J Immunol. 2008;181:4043–4051. doi: 10.4049/jimmunol.181.6.4043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Seda V, Mraz M. B-cell receptor signalling and its crosstalk with other pathways in normal and malignant cells. Eur J Haematol. 2015;94:193–205. doi: 10.1111/ejh.12427. [DOI] [PubMed] [Google Scholar]
- 49.Langstein J, Michel J, Fritsche J, Kreutz M, Andreesen R, Schwarz H. CD137 (ILA/4-1BB), a member of the TNF receptor family, induces monocyte activation via bidirectional signaling. J Immunol. 1998;160:2488–2494. [PubMed] [Google Scholar]
- 50.Quintans J, Lefkovits I. Clonal expansion of lipopolysaccharide-stimulated B lymphocytes. J Immunol. 1974;113:1373–1376. [PubMed] [Google Scholar]
- 51.Guilloton F, de Thonel A, Jean C, Demur C, Mansat-De Mas V, Laurent G, Quillet-Mary A. TNFalpha stimulates NKG2D-mediated lytic activity of acute myeloid leukemic cells. Leukemia. 2005;19:2206–2214. doi: 10.1038/sj.leu.2403952. [DOI] [PubMed] [Google Scholar]
- 52.McPherson AJ, Snell LM, Mak TW, Watts TH. Opposing roles for TRAF1 in the alternative versus classical NF-kappaB pathway in T cells. J Biol Chem. 2012;287:23010–23019. doi: 10.1074/jbc.M112.350538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Huang C, Bi E, Hu Y, Deng W, Tian Z, Dong C, Hu Y, Sun B. A novel NF-kappaB binding site controls human granzyme B gene transcription. J Immunol. 2006;176:4173–4181. doi: 10.4049/jimmunol.176.7.4173. [DOI] [PubMed] [Google Scholar]
- 54.Curiel RE, Garcia CS, Rottschafer S, Bosco MC, Espinoza-Delgado I. Enhanced B7-2 gene expression by interferon-gamma in human monocytic cells is controlled through transcriptional and posttranscriptional mechanisms. Blood. 1999;94:1782–1789. [PubMed] [Google Scholar]
- 55.Sasaki S, Sullivan M, Narvaez CF, Holmes TH, Furman D, Zheng NY, Nishtala M, Wrammert J, Smith K, James JA, Dekker CL, Davis MM, Wilson PC, Greenberg HB, He XS. Limited efficacy of inactivated influenza vaccine in elderly individuals is associated with decreased production of vaccine-specific antibodies. The Journal of clinical investigation. 2011;121:3109–3119. doi: 10.1172/JCI57834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wols HA, Johnson KM, Ippolito JA, Birjandi SZ, Su Y, Le PT, Witte PL. Migration of immature and mature B cells in the aged microenvironment. Immunology. 2010;129:278–290. doi: 10.1111/j.1365-2567.2009.03182.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Berberich S, Forster R, Pabst O. The peritoneal micromilieu commits B cells to home to body cavities and the small intestine. Blood. 2007;109:4627–4634. doi: 10.1182/blood-2006-12-064345. [DOI] [PubMed] [Google Scholar]
- 58.Ishikawa S, Sato T, Abe M, Nagai S, Onai N, Yoneyama H, Zhang Y, Suzuki T, Hashimoto S, Shirai T, Lipp M, Matsushima K. Aberrant high expression of B lymphocyte chemokine (BLC/CXCL13) by C11b+CD11c+ dendritic cells in murine lupus and preferential chemotaxis of B1 cells towards BLC. J Exp Med. 2001;193:1393–1402. doi: 10.1084/jem.193.12.1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Weksler ME. Changes in the B-cell repertoire with age. Vaccine. 2000;18:1624–1628. doi: 10.1016/s0264-410x(99)00497-1. [DOI] [PubMed] [Google Scholar]
- 60.Dauphinee M, Tovar Z, Talal N. B cells expressing CD5 are increased in Sjogren’s syndrome. Arthritis Rheum. 1988;31:642–647. doi: 10.1002/art.1780310509. [DOI] [PubMed] [Google Scholar]
- 61.Ito T, Ishikawa S, Sato T, Akadegawa K, Yurino H, Kitabatake M, Hontsu S, Ezaki T, Kimura H, Matsushima K. Defective B1 cell homing to the peritoneal cavity and preferential recruitment of B1 cells in the target organs in a murine model for systemic lupus erythematosus. J.Immunol. 2004;172:3628–3634. doi: 10.4049/jimmunol.172.6.3628. [DOI] [PubMed] [Google Scholar]
- 62.Chen Y, Park Y-B, Patel E, Silverman GJ. IgM antibodies to apoptosis-associated determinants recruit C1q and enhance dendritic cell phagocytosis of apoptotic cells. J Immunol. 2009;182:6031–6043. doi: 10.4049/jimmunol.0804191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Martinez L, Gomez C, Vazquez-Padron RI. Age-related changes in monocytes exacerbate neointimal hyperplasia after vascular injury. Oncotarget. 2015;6:17054–17064. doi: 10.18632/oncotarget.3881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Yoon P, Keylock KT, Hartman ME, Freund GG, Woods JA. Macrophage hypo-responsiveness to interferon-gamma in aged mice is associated with impaired signaling through Jak-STAT. Mech Ageing Dev. 2004;125:137–143. doi: 10.1016/j.mad.2003.11.010. [DOI] [PubMed] [Google Scholar]
- 65.Herrero C, Marques L, Lloberas J, Celada A. IFN-gamma-dependent transcription of MHC class II IA is impaired in macrophages from aged mice. J Clin Invest. 2001;107:485–493. doi: 10.1172/JCI11696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Renshaw M, Rockwell J, Engleman C, Gewirtz A, Katz J, Sambhara S. Cutting edge: impaired Toll-like receptor expression and function in aging. J Immunol. 2002;169:4697–4701. doi: 10.4049/jimmunol.169.9.4697. [DOI] [PubMed] [Google Scholar]
- 67.Kilmon MA, Wagner NJ, Garland AL, Lin L, Aviszus K, Wysocki LJ, Vilen BJ. Macrophages prevent the differentiation of autoreactive B cells by secreting CD40 ligand and interleukin-6. Blood. 2007;110:1595–1602. doi: 10.1182/blood-2006-12-061648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Szakal AK, Aydar Y, Balogh P, Tew JG. Molecular interactions of FDCs with B cells in aging. Semin Immunol. 2002;14:267–274. doi: 10.1016/s1044-5323(02)00059-3. [DOI] [PubMed] [Google Scholar]
- 69.Wong SC, Puaux AL, Chittezhath M, Shalova I, Kajiji TS, Wang X, Abastado JP, Lam KP, Biswas SK. Macrophage polarization to a unique phenotype driven by B cells. Eur J Immunol. 2010;40:2296–2307. doi: 10.1002/eji.200940288. [DOI] [PubMed] [Google Scholar]
- 70.Grewal IS, Flavell RA. CD40 and CD154 in cell-mediated immunity. Annu Rev Immunol. 1998;16:111–135. doi: 10.1146/annurev.immunol.16.1.111. [DOI] [PubMed] [Google Scholar]
- 71.Bergtold A, Desai DD, Gavhane A, Clynes R. Cell surface recycling of internalized antigen permits dendritic cell priming of B cells. Immunity. 2005;23:503–514. doi: 10.1016/j.immuni.2005.09.013. [DOI] [PubMed] [Google Scholar]
- 72.Howard WA, Gibson KL, Dunn-Walters DK. Antibody quality in old age. Rejuvenation research. 2006;9:117–125. doi: 10.1089/rej.2006.9.117. [DOI] [PubMed] [Google Scholar]
- 73.Kang YJ, Kim SO, Shimada S, Otsuka M, Seit-Nebi A, Kwon BS, Watts TH, Han J. Cell surface 4-1BBL mediates sequential signaling pathways ‘downstream’ of TLR and is required for sustained TNF production in macrophages. Nat Immunol. 2007;8:601–609. doi: 10.1038/ni1471. [DOI] [PubMed] [Google Scholar]
- 74.Zhou F. Molecular mechanisms of IFN-gamma to up-regulate MHC class I antigen processing and presentation. Int Rev Immunol. 2009;28:239–260. doi: 10.1080/08830180902978120. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.