Abstract
Mitochondria are important for providing cellular energy ATP through the oxidative phosphorylation pathway. They are also critical in regulating many cellular functions including the fatty acid oxidation, the metabolism of glutamate and urea, the anti-oxidant defense, and the apoptosis pathway. Mitochondria are an important source of reactive oxygen species leaked from the electron transport chain while they are susceptible to oxidative damage, leading to mitochondrial dysfunction and tissue injury. In fact, impaired mitochondrial function is commonly observed in many types of neurodegenerative diseases, including Alzheimer’s disease, Parkinson’s disease, Huntington’s disease, alcoholic dementia, brain ischemia-reperfusion related injury, and others, although many of these neurological disorders have unique etiological factors. Mitochondrial dysfunction under many pathological conditions is likely to be promoted by increased nitroxidative stress, which can stimulate post-translational modifications (PTMs) of mitochondrial proteins and/or oxidative damage to mitochondrial DNA and lipids. Furthermore, recent studies have demonstrated that various antioxidants, including naturally occurring flavonoids and polyphenols as well as synthetic compounds, can block the formation of reactive oxygen and/or nitrogen species, and thus ultimately prevent the PTMs of many proteins with improved disease conditions. Therefore, the present review is aimed to describe the recent research developments in the molecular mechanisms for mitochondrial dysfunction and tissue injury in neurodegenerative diseases and discuss translational research opportunities.
Keywords: oxidative stress, mitochondrial dysfunction, neuronal cell death, neurodegenerative diseases, post-translational protein modifications, antioxidants, translational research
1. Introduction
The mitochondria are present in most eukaryotic cells. Their sizes range from 0.5 to 1.0 micrometers in diameter, but recent data suggest that their shape and size can vary by a process of mitochondrial fusion and fission following exposure to toxic agents or under pathophysiological conditions (Chaturvedi and Beal, 2013; Duboff et el., 2013). Alternatively, the damaged mitochondria can be removed by mitochondrial autophagy (mitophagy) (Ashrafi and Schwarz, 2013; Chaturvedi and Beal, 2013). Mitochondria are considered as ‘power plants’ of the cell since they generate most of the cellular energy ATP needed for numerous reactions. They are also involved in the intermediary metabolism, such as conversion of pyruvate and other metabolites through the citric acid cycle, urea metabolism, steroid synthesis pathway, etc. In addition, they are involved in heat production, storage of calcium ion, regulation of the membrane potential and transports, and programmed cell death signaling (i.e. apoptosis) (Chaturvedi and Beal, 2013; Dashdorj et al., 2013; Mailloux and Harper, 2011).
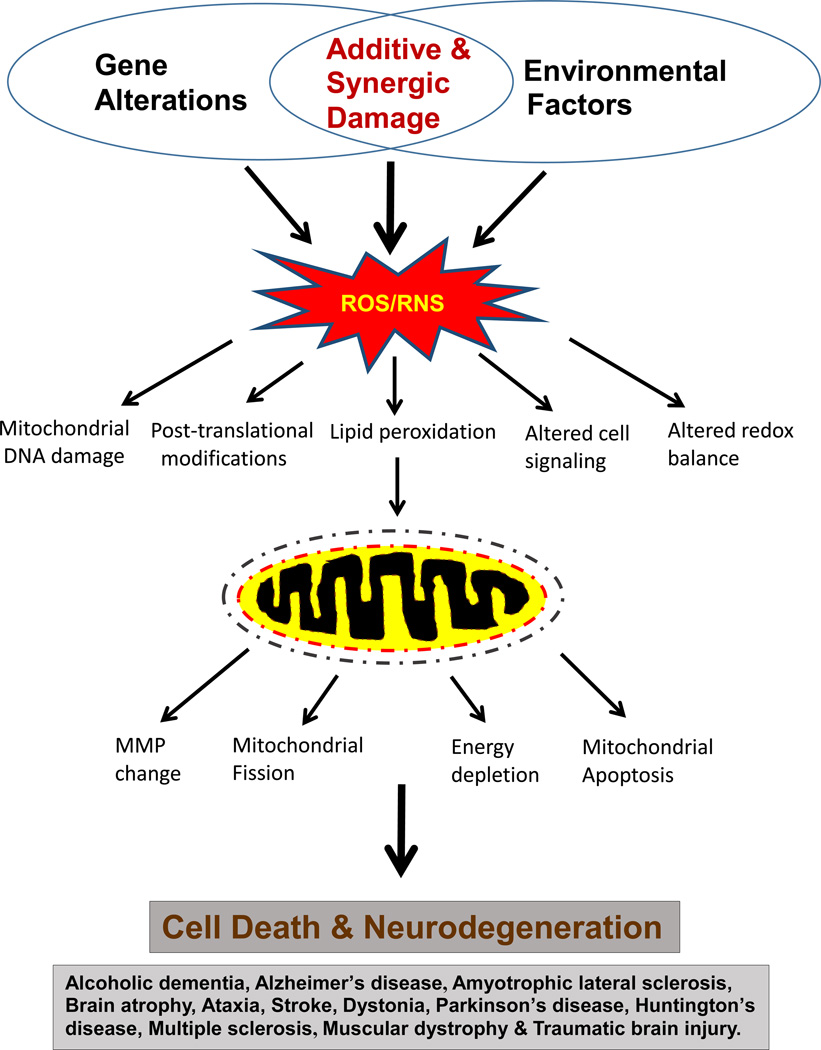
Under normal conditions, less than 2% of oxygen is leaked from the mitochondrial electron transport chain (ETC) (Boveris et al., 1972). However, it is well-established that greater amounts of reactive oxygen species (ROS) can be produced out of the damaged mitochondria under various pathological conditions (Chaturvedi and Beal, 2013). In addition, mitochondria are known to produce reactive nitrogen species (RNS) including nitric oxide (NO) through nitric oxide synthase (NOS) expressed in mitochondria (Navarro and Boveris, 2008). Combination of ROS and RNS produces a much more potent oxidant peroxynitrite (ONOO-). For instance, peroxynitrite can nitrate Tyr or Trp and S-nitrosylate Cys residues of many mitochondrial proteins, contributing to their inactivation and mitochondrial dysfunction, followed by cell death and organ damage (Abdelmegeed and Song, 2014; Franco et al., 2013; Song et al., 2013, 2014). In fact, both nitroxidative stress and mitochondrial dysfunction are considered as the major contributing factors for the development and progression of many pathological conditions, including neurodegenerative diseases such as Alzheimer’s disease (AD), Parkinson’s disease (PD), Huntington’s disease (HD), alcoholic dementia, brain ischemia-reperfusion injury and traumatic brain injury (TBI) (Johri and Beal, 2012; Navarro and Boveris, 2008) (Figure 1). Many of these neurological diseases may have different etiological factors, but may share common factors of increased nitroxidative stress and mitochondrial dysfunction, likely resulting from the protein post-translational modifications (PTMs) including oxidation, nitration, hyperphosphorylation, acetylation, glycosylation and formation of various protein adducts (Abdelmegeed and Song, 2014; Byun et al., 2012, 2014; Franco et al., 2013; Liu et al., 2015; Jin et al., 2014; Narayan et al., 2015; Song et al., 2014; Sultana et al., 2013; Tepper et al., 2014; Zhang et al., 2013).
Figure 1.
Schematic diagram showing the major causes and consequences of ROS/RNS-mediated mitochondrial dysfunction and cell death in many neurodegenerative diseases.
Over-production of ROS/RNS usually lead to an increased nitroxidative stress inside the cells. However, cells have evolved to contain many defensive enzymes and cellular anti-oxidants in order to protect themselves against ROS/RNS-mediated cell or organ damage (Weinberg and Chandel, 2009). In addition, various small molecule antioxidants can delay or prevent the oxidative modification of the ‘oxidizable’ substrates. Furthermore, recent studies have demonstrated that several antioxidants prevent the formation of the ROS/RNS to the manageable levels (Correia et al., 2010). A variety of edible plants contain many different kinds of antioxidants such as polyphenols, flavonoids and other types. Some polyphenols are effective as metal chelators and in scavenging ROS/RNS due to the presence of multiple hydroxyl groups (Amit and Priyadarsini, 2011). It is reasonable to consider that the most rational approach for preventing or treating neurodegenerative disorders is to understand the molecular mechanisms of the adverse consequences of these ROS/RNS and other free radicals (Chaturvedi and Beal, 2013; Tajuddin et al., 2013). Therefore the present article is aimed to briefly review the consequences of increased nitroxidative stress and mechanisms of mitochondrial dysfunctions in some selected neurodegenerative diseases. Based on the mechanistic understanding of neurodegenerative disorders, we also describe potential translational opportunities and challenges.
2. Consequences of Increased Nitroxidative Stress
2.1. Changes in Mitochondrial Shapes and Functions
Mitochondria are dynamic organelles, often transported by cytoskeletal proteins. In normal conditions, they merge (e.g., fusion mediated by OPA1, Mfn-1 and Mfn-2 proteins) and divide [e.g., fission by dynamin-related protein (Drp1) and other proteins] while this fusion-fission cycle is regulated by GTPases in the dynamin family. Mitochondrial fusion protects cells from excessive degradation of mitochondria, prevents mitophagy and rescues damaged mitochondria. Mitochondrial fission is mediated by cytosolic Drp1, which is translocated to mitochondria to initiate fission and thus plays an important role in mitochondrial quality control and bioenergetics (Dickey and Strack, 2011). After the fission, the two daughter mitochondria are either subjected to mito-fusion or elimination by mitophagy. The mitochondria with higher membrane potential would undergo fusion while the other depolarized daughter mitochondria with dysfunctional aggregated proteins are eliminated by mitophagy (Twig et al., 2008; Zhang and Ney, 2010). However, in certain disease conditions, the imbalance between fusion and fission leads to elongation and excessive mitochondrial fragmentation, which suppresses normal mitochondrial function (Johri and Beal, 2012). For instance, mitochondrial fusion by Mfn-2 is required for proper development and maintenance of cerebellar granular cells. In contrast, a mutation in Mfn-2 gene results in the development of axonal disorder, Charcot-Marie-Tooth type 2A disease (CMT), a hereditary peripheral neuropathy that affects both motor neurons and sensory neurons (Chen et al., 2007; Kijima et al., 2005). In addition, under increased oxidative stress after exposure to neurotoxic glutamate, mitochondrial fusion protein OPA1, normally present in the mitochondrial inner membrane, is discharged to cytosol with concomitant release of cytochrome c. These events are accompanied by mitochondrial fragmentation and apoptosis of HT22 cells while an antioxidant tocopherol significantly prevented these events (Sanderson et al., 2015a). Similar incidences of release of mitochondrial OPA1 and cytochrome c followed by apoptosis were observed in primary rat neuronal cells in a simulated model of ischemia-reperfusion hypoxic injury (Sanderson et al., 2015b). These results from at least two different models of neuronal injury suggest that increased oxidative stress is involved in regulating the mitochondrial fusion and fission process and cell death, although the detailed mechanism by which increased oxidative stress stimulates OPA1 release from mitochondria needs to be further studied.
Mitochondria can actively pass through the cytosol on the dynein and kinesin tracks and this mitochondrial transport also regulates fission. Many reports demonstrated that the altered mitochondrial trafficking and fusion/fission dynamics are observed in various neurodegenerative diseases including CMT (Chen et al., 2007). Similarly, mitochondrial dysfunction is implicated in the aging process due to the accumulation of damaged or mutated mitochondrial DNA (mtDNA) by increased ROS production, resulting in a change in mitochondrial mass (Chaturvedi and Beal, 2013). Axonal degeneration, as observed in CMT, is another example where axonal mitochondria cannot carry out bioenergy metabolism with abnormal Ca2+ homeostasis and protease activation. The removal of damaged mitochondria can be processed through mitophagy. Thus, mitochondrial numbers are regulated by mitophagy, which selectively surrounds the damaged and depolarized mitochondria in autophagic vacuoles for subsequent elimination in lysosomes (Tolkovsky, 2009). Recent reports suggest that the PINK1/Parkin and autophagy receptors play an important role in mitophagy. The accumulation of PINK1 results in recruitment of E3 ubiquitin ligase, Parkin. Upon recruitment of Parkin, ubiquitination of various proteins such as hexokinase 1, voltage dependent anion channel 1 (VDAC1), mitochondrial rho family GTPase (Miro) and Mfn1/2 takes place (Geisler et al., 2010; Okatsu et al., 2012; Tanaka et al., 2010; Wang et al., 2011). The anchoring of the damaged mitochondria to the cytoskeleton is mediated by Miro (possibly with VDAC1 and hexokinase 1) and subsequent degradation is carried out by the PINK1/Parkin pathway. Another pathway for the elimination of damaged, aggregated and dysfunctional organelles is accomplished through mitochondrial autophagy receptors.
The proteins and lipids, located on the outer mitochondrial membrane, sometimes work as mitophagy receptors. Cardiolipin, FUNDC1, Nix/BNIP3L, and BNIP3 (which are only present on the outer mitochondrial membrane) can bind to LC3 on the autophagosome and thus contribute to apoptosis (Hanna et al., 2012; Novak et al., 2010). Thus the Nix/BNIP3L is important for the maintenance of the healthy mitochondrial pool to keep the equilibrium between mitophagy and cellular homeostasis. Most of the mitochondrial proteins, involved in mitochondrial fission and fusion, are present to maintain normal cellular functions under healthy conditions. In contrast, impaired mitochondrial function is frequently observed in many disease states, including several neurodegenerative disorders. Therefore, normalization of mitochondrial function can become a potential target for pharmacological interventions to prevent or treat many metabolic and neurodegenerative diseases.
2.2. Post-Translational Modifications of Mitochondrial Proteins
Under elevated nitroxidative stress, many mitochondrial proteins can undergo different types of PTM, such as oxidation, nitration, S-nitrosylation, phosphorylation, acetylation, glycosylation, carbonylation, palmitoylation, myristoylation and other modifications including protein adduct formation (Song et al., 2014). Consequently, modified mitochondrial proteins likely become inactivated and thus contribute to impaired mitochondrial function, ATP depletion and necroapoptosis (Dexter and Jenner, 2013; Song et al., 2014; Sultana and Butterfield, 2013). In this review, we will briefly discuss a few examples of PTM of mitochondrial proteins, such as nitration, S-nitrosylation, phosphorylation, and acetylation, as they are often identified in the experimental models and human specimens of neurodegenerative disorders. However, many other types of PTM are also known to be associated with various neurodegenerative conditions. These PTMs, not discussed in this review, include: adduct formation with DNA, lipids and proteins (de la Monte and Tong, 2014; Sultana et al., 2013), carbonylation (Ergin et al., 2013; Oikawa et al., 2014; Sultana et al., 2013), glycation (e.g., advanced glycation end products) (Byun et al., 2012; Choi et al., 2014), methylation (Pattaroni and Jacob, 2013; Peña-Altamira et al., 2013; Tradewell et al., 2012), N-linked glycosylation with Asn (Gonçalves et al., 2015; Mkhikian et al., 2011; Schedin-Weiss et al., 2014), O-linked glycosylation with Ser or Thr (Karababa et al., 2014; Lozano et al., 2014; Tan et al., 2014; Zhu et al., 2014), sumoylation (Gebriel et al., 2014; ; Nistico et al., 2014; Shahpasandzadeh et al., 2014), ubiquitination (Bishop et al., 2014; Gebriel et al., 2014; Karababa et al., 2014; Nistico et al., 2014; Schmid et al., 2013), etc.
The brain utilizes about 20% three fourths of the total oxygen supply of the body despite its relative low weight (approximately 2% of the total body weight) (Bunevicius et al., 2013). Under pathological conditions and after exposure to neurotoxic agents, increased amounts of superoxide anion (O2-.) and NO can be produced, resulting in nitroxidative stress in the brain. Co-existence of NO and superoxide anion can generate more cytotoxic agents, peroxynitrite (ONOO-) and peroxynitrous acid (ONOOH), both of which have been implicated in neuronal cell death (Brown, 2010; Chung and David, 2010; Franco et al., 2013; Gould et al., 2013; Maggio et al., 2012; Malinski, 2007; Schildknecht et al., 2013). Since the brain contains relatively low amounts of glutathione (GSH) and other energy substrates compared to other peripheral tissues, including the liver (Aoyama and Nakaki, 2013; Wali et al., 2011), it is relatively sensitive to severe nitroxidative damage.
NO produced in the brain plays both physiological and pathological roles (Brown, 2010; Navarro and Boveris, 2008). Cellular NO can be synthesized from L-arginine by NOS isozymes, which require oxygen and NADPH for their activities. The three isoforms of NOS exist: neuronal (cNOS or nNOS), endothelial (eNOS), and the inducible NO synthase (iNOS). The nNOS and eNOS are known to be constitutively expressed and Ca-calmodulin-dependent isozymes (Knowles and Moncada, 1994). In contrast, the inducible isozyme iNOS, Ca-independent and virtually not expressed in normal tissues, can be rapidly activated by inflammation and oxidative stress in many cell types, including astrocytes, microglia, macrophages, neurons, myocytes, platelets, leukocytes, endothelial cells and others. Small amounts of NO in the brain and various neurons can act as a neurotransmitter (Brown, 2010; Dai et al., 2013). However, NO, produced at greater amounts (0.1 – 1 µM) usually by iNOS and nNOS or in inflammatory disease states, can act as a neurotoxic agent and is believed to nitrate many proteins in the neuron, causing cell death and severe tissue injury (Brown, 2010; Dai et al., 2013; Franco et al., 2013). For instance, large amounts of NO seems to be important in causing ischemic brain injury (Garcia-Bonilla et al., 2014) and many other neuronal diseases (Butterfield et al., 2007, 2014). Direct evidence for the involvement of iNOS in neuronal diseases was provided by the experimental results of using iNOS-knockout mice, a specific inhibitor of iNOS 1400W (Kumar et al., 2014), or an anti-sense oligonucleotide specific to iNOS (Maggio et al., 2012). For instance, iNOS-knockout mice were resistant to behavioral and synaptic deficits or cerebral plaque formation and premature mortality in animal models of AD (Medeiros et al., 2007; Nathan et al., 2005). These iNOS-knockout mice were also protected from dopaminergic neurodegeneration in mouse models of PD (Kumar et al., 2015; Liberatore et al., 1999) and ischemic brain injury (Garcia-Bonilla et al., 2014), as similar to their resistance to inflammatory diseases and peripheral tissue injury caused by alcohol and non-alcoholic substances (Nobunaga et al., 2014; Spruss et al., 2011). In addition, nNOS seems to be responsible for DNA damage in a mouse model of PD (Hoang et al., 2009). Markedly increased NO levels can stimulate nitration of many proteins, which tend to aggregate, as reported in the neuronal tissues of patients with neurodegenerative diseases including AD, PD, HD and amyotrophic lateral sclerosis (ALS) (Brown, 2010; Butterfield et al., 2007, 2014; Chung and David, 2010; Dexter and Jenner, 2013; Maggio et al., 2012; Malinski, 2007; Schildknecht et al., 2013).
In addition to lipid peroxidation and DNA damage, increased nitroxidative stress can promote oxidative modifications of cysteine (Cys), methionine (Met), and other amino acids, in most proteins, as recently reviewed (Butterfield et al., 2014; Song et al., 2014; Sultana and Butterfield, 2013). For instance, Cys residue(s) can be oxidatively-modified in many forms [sulfenic acid, disulfide, sulfinic/sulfonic acids, NO- or peroxynitrite-dependent S-nitrosylation, NO-independent ADP-ribosylation, mixed disulfide formation between Cys residues and glutathione (glutathionylation), cysteine (cystathionylation), succinic acid, myristic acid (Bastidas et al., 2013), palmitic acid (Cys-palmitoylation)] (Song et al., 2014) for membrane attachment or cellular trafficking, and adduct formation with reactive metabolites of drugs or lipid peroxides. S-nitrosylation is a reversible modification of Cys residues in proteins to produce the corresponding nitrosothiols that affect cell survival through changes in gene transcription, vesicular trafficking, receptor mediated signal transduction and apoptosis, depending on the sources of ROS/RNS, their cellular levels, spatiotemporal production, and nitrosylated proteins (Gould et al., 2013; Malinski, 2007; Sha and Marshall, 2012; Shahani and Sawa, 2012; Song et al., 2010). More importantly, some of the neuroprotective proteins such as parkin and XIAP have been found to be modulated by S-nitrosylation, contributing to their suppression and neural cell death. These results suggests that nitroxidative stress is an important contributing factor of neurodegeneration (Chung et al., 2004).
If one of these Cys residues serves as the active site or is located near the active site of certain enzymes, it is highly likely that oxidative modifications of these Cys residues can result in their inactivation, although a few exceptions exist for the activation of S-nitrosylated proteins, as recently reviewed (Song et al., 2014). Glyceraldehyde-3-phosphate dehydrogenase (GAPDH), an enzyme involved in the glycolysis, is S-nitrosylated on Cys-150, before its translocation from cytosol to nucleus and then interacts with Siah1, an E3-ubiquitin ligase, to promote the degradation of nuclear target proteins (Hara et al., 2006). This NO-dependent nuclear translocation of GAPDH followed by its interaction with Siah1 has been shown in neuronal damage in an animal model of PD induced by MPTP exposure. In contrast, Deprenyl, a known MAO-B inhibitor, disrupts the GAPDH-Siah1 complex-induced neuronal cell death, resulting in cell survival. Recent reports also showed that many cellular proteins are S-nitrosylated in several neurodegenerative diseases such as AD, PD, ALS, and stroke. These S-nitrosylated proteins include: Parkin, protein disulfide isomerase (PDI), peroxiredoxin 2 (Prx2), XIAP, Drp1, heat shock protein 90 (HSP90), matrix metalloproteinase-9 (MMP-9), phosphatase and tensin homolog (PTEN), as described (Butterfield et al., 2010; Cho et al., 2009; Fang et al., 2007; Kwak et al., 2010; Walker et al., 2010).
Phosphorylation is another form of PTM by which many cellular proteins are regulated by reversible cycles of phosphorylation and dephosphorylation by protein kinases and phosphatases, respectively. In general, the activities of these protein kinases and phosphatases can be changed following exposure to exogenous agents including neurotoxic agents. The kinase proteins include cAMP-dependent protein kinases (PKA), phosphatidylinositol triphosphate-dependent kinase (PI-3K), protein kinase B (AKT or PKB), protein kinase C isoforms (PKC), calcium-calmodulin-dependent protein kinases (CAMK), glycogen synthase kinase-3β (GSK3β), stress activated mitogen-activated protein kinases (MAPKs), including c-Jun N-terminal protein kinase (JNK) and p38 kinase (p38K), and receptor-mediated tyrosine kinases. In addition, the activities of these kinases are generally altered in pathological conditions, including neurodegenerative diseases (Ferraris et al., 2013; Ron and Messing, 2013; Yang et al., 2013; Yin et al., 2013). These kinases can work independently or together synergistically or antagonize each other’s activities. In fact, crosstalk between different classes of protein kinases becomes complicated, contributing to different outcomes, depending on their induction, tissue distribution, subcellular localization or translocation to another compartment, cellular environment, treatment agent, and activity status of phosphoprotein phosphatases, as recently reported (Kamata et al., 2005; Son et al., 2013).
Under increased nitroxidative stress, MAPKs can be activated through oxidative modifications and inactivation of the redox-sensitive critical Cys residues of the corresponding MAPK phosphatases, which dephosphorylate the phosphorylated (activated) upstream kinase(s) of each MAPK (Son et al., 2011). For instance, oxidative stress induced by neurotoxic glutamate can also activate the MAPK pathways that are involved in many cellular processes, such as cell growth, differentiation, inflammation, and cell death. However, a recent review indicated that the extracellular signal-regulated kinase (ERK) pathway can be also involved in oxidative neuronal injury (Subramaniam and Unsicker, 2010), instead of its usual roles in cell growth and proliferation. In contrast, JNK and/or the p38K pathways, activated by stress stimuli such as pro-inflammatory cytokines, ultraviolet irradiation, heat shock, osmotic shock, hydrogen peroxide, alcohol, drugs, anti-cancer agents, hypoxia and neurotoxic agents, are involved in cell differentiation and apoptosis (Byun et al., 2012, 2014; Nijboer et al., 2013; Pan et al., 2009; Park et al., 2013; Shah et al., 2015). The activated p-JNK and p-p38K in neuronal tissues can phosphorylate many target proteins, including Tau protein. In fact, it was shown that activated p-JNK, p-p38K and other kinases can phosphorylate many of 80 serine and threonine residues and 5 tyrosine residues of the microtubule-associated Tau proteins, contributing to tauopathies in humans and experimental animal models (Chen et al., 2004; Wang et al., 2013). In the normal brain, there are 2–3 mols of phosphate per mole of Tau, whereas Tau is hyper-phosphorylated at least three-fold greater in AD brain than normal brains, supporting the notion that Tau hyper-phosphorylation is a critical step in Aβ aggregation (Wang et al., 2013). Consequently, inhibiting the oxidative stress with activated p-JNK and/or p-p38K caused by glutamate, dopamine, or other neurotoxic agents or in neuropathological conditions, including cerebral ischemia (Nijboer et al., 2013), may be a promising strategy for the development of new drugs to prevent and/or treat neurodegenerative diseases.
Another protein kinase, which plays an important role in causing mitochondrial dysfunction via phosphorylation of VDAC1 in AD patients, is GSK3β. Hyper-activation of GSK3β has been linked to Aβ production, Aβ deposits, hyper-phosphorylated Tau, and neurofibrillary tangle formation in AD (Wang et al., 2013) as well as insulin resistance in diabetes (Jope et al., 2007). GSK3β can phosphorylate VDAC1 on threonine 51, resulting in the detachment of hexokinase from VDAC1. Increasing evidence also suggests that in AD pathogenesis, elevated GSK3β activity is a key event in abnormal amyloid precursor protein (APP) processing, increased Aβ production, and hyper-phosphorylation of Tau (Forlenza et al., 2011; Jimenez et al., 2011; Jo et al., 2011).
Another important PTM in various neurodegenerative diseases is reversible acetylation and deacetylation of neuronal proteins, as recently reviewed (Bahari-Javan et al., 2014; Fischer, 2014; Lazo-Gomez et al., 2013). For instance, the acetylated status of certain proteins, such as nuclear histone and several mitochondrial proteins (Wagner et al., 2012), is very important in epigenetic changes and impaired energy supply in many different neurodegenerative diseases. The acetylation-deacetylation cycle depends on the activities of acetylases and deacetylases, growth factors, proper nutrient signaling and mitochondria-dependent bioenergetics, most of which are regulated through FOXO and Sirtuin (SIRT) proteins (Guedes-Dias and Oliverira, 2013). Mammalian Sirtuins include the mitochondrial deacetylase SIRT3 and recently mitochondrial lysine acetylation (AcLys) was found to initiate mitochondrial degradation by autophagy. This mitophagy process is closely regulated by PINK1 and Parkin (Ashrafi et al., 2014; Auberger et al., 2014), two interacting proteins that relocalize to mitochondria to stimulate deficient proton gradients. Deacetylation of mitochondrial proteins and altered SIRT3 levels occur in rodent models of PD before the onset of the toxic aggregate formation. The development of site-specific AcLys-antibodies, characterizations of acetylated proteins in patients, and chemical modulators of SIRT proteins may have clinical applications (Han, 2009).
Several PTMs of many key mitochondrial proteins can simultaneously take place under increased nitroxidative stress in neuropathological conditions. Therefore, it is very difficult to determine the functional implication of each PTM or demonstrate which PTM plays a critical role in causing mitochondrial dysfunction and injury to neuronal cells. In addition, it is challenging to identify the modified amino acid(s) and their roles in activity changes, especially those expressed in low amounts in a particular cell in the brain, following exposure to a neurotoxic agent or in neurological disease specimens. Therefore, we may need to use an in vitro model system to clearly demonstrate the pattern of PTMs for a specific protein(s) of interest to evaluate their functional role. Once we obtain the useful information from the in vitro model systems, the identities of the proteins modified by different PTMs and their roles in mitochondrial dysfunction and apoptosis need to be verified in a specific model of neurodegenerative diseases and/or human pathological specimens. Molecular elucidation of different PTMs of target proteins is a critical step in helping future development of preventive and/or therapeutic agents against neurodegenerative diseases. For this reason, we have briefly described the mechanisms of mitochondrial dysfunction in four major neurodegenerative diseases.
2.3. Multiple Mechanisms of Neuronal Cell Death by Increased Nitroxidative Stress
In the previous section, we have described the role of increased nitroxidative stress in promoting oxidative modifications of cellular macromolecules DNA, lipid and proteins. We also mentioned a few examples of various PTMs of many cellular proteins, contributing to mitochondrial dysfunction and neuronal cell death, which ultimately leads to neurodegeneration (Fig. 1). For instance, we described modifications (e.g., phosphorylation) of some critical proteins involved in the cell signaling pathways, resulting in different outcomes. Several reports on the redox-related activation of the JNK and/or p38K or their upstream kinases such as mitogen activated protein kinase kinase (M2K) and mitogen activated protein kinase kinase kinase (M3K), as reviewed (Son et al., 2011). Previous results with hepatoma cells showed that pro-apoptotic Bax in the cytosol can be phosphorylated (activated) by p-JNK and/or p-p38K, resulting in its conformational change with exposure of the C-terminal membrane domain. These events were followed by translocation of the activated Bax to mitochondria, leading to changes in mitochondrial permeability transition (MPT) and apoptosis of cultured hepatoma cells (Kim et al., 2006). The importance of phosphorylation was further confirmed by using site-directed mutagenesis of potential amino acids for phosphorylation followed by functional analysis of cell death rates. Site-directed mutagenesis results revealed that Thr167 of Bax was phosphorylated before it was translocated to mitochondria to stimulate mitochondria-dependent apoptosis (Kim et al., 2006). In addition, activated p-JNK is known to translocate to mitochondria to initiate MPT change and damage following exposure to other hepatotoxic agents including acetaminophen (Hanawa et al., 2008; Win et al., 2011; Xie et al., 2014). Since JNK does not contain the canonical mitochondrial leader sequence (Ferramosa & Zara, 2013), it would be interesting to identify the mechanism(s) by which activated p-JNK is translocated to mitochondria and then phosphorylates mitochondrial matrix proteins such as mitochondrial aldehyde dehydrogenase 2 isoform (ALDH2) in carbon tetrachloride-exposed rats (Moon et al., 2010). One of the potential mechanisms of JNK translocation to mitochondria could be through its interaction with a scaffold protein Sab (Win et al., 2011; Win et al., 2014) or Bcl-XL (Kharbanda et al., 2000) on the mitochondrial outer membrane before p-JNK was transported to mitochondria. In fact, an in vitro study using bioenergetically competent mitochondria revealed that the recombinant JNK can phosphorylate many mitochondrial proteins such as ATP synthase β subunit, α-ketoglutarate dehydrogenase (α-KGDH), pyruvate dehydrogenase (PDH) E1α and β subunits (Schroeter et al., 2003). A recent report with mass-spectral analysis of the purified phosphoproteins showed that more than 106 mitochondrial proteins can be phosphorylated at 2 h by activated p-JNK in carbon tetrachloride-exposed mouse livers (Jang et al., 2015). The JNK-target proteins include ALDH2, ATP synthase α subunit, α-KGDH, PDH E1α and β subunits, and many other mitochondrial proteins or enzymes. By carefully studying the time-dependent changes in enzyme activities, mitochondrial permeability and histological features in the absence or presence of specific JNK inhibitors, it was concluded that translocation of activated p-JNK to mitochondria and subsequent phosphorylation of many mitochondrial proteins are critically important in promoting mitochondrial dysfunction, mitochondrial permeability change and necrotic tissue injury usually observed at later time points (Jang et al., 2015). Similarly, phosphorylation of many proteins such as Tau (Wang et al., 2013) and Bax in neuronal tissues can produce neurofibrillary tangles (NFTs) and apoptosis of neuronal cells, respectively, contributing to neurodegeneration (Byun et al., 2012; Byun et al., 2014; Yarza et al., 2016). However, the number and identity of the neuronal proteins, that can be phosphorylated by activated p-JNK, p-p38K, and/or GSK3β in brains (Forlenza et al., 2011; Jimenez et al., 2011; Jo et al., 2011), need to be investigated to further delineate the causal role of protein phosphorylation in promoting neurodegenerative diseases.
Many laboratories reported that PTMs of some mitochondrial proteins such as SOD2, Gpx and ATP synthase (complex V) can lead to their inactivation (Abdelmegeed et. al, 2013; Ribas et al., 2014). In addition, mitochondrial complexes I (NADH-dependent ubiquinone oxidoreductase) and IV (cytochrome c oxidase) can be oxidatively-modified and inactivated under increased oxidative stress (Opii et al., 2007; Song et al., 2013). Suppression of these mitochondrial complex proteins leads to impairment of the mitochondrial ETC, resulting in insufficient electron transfer and greater ROS leakage from the respiratory chain. Cytosolic caspases can be also activated by S-nitrosylated proliferating nuclear cell antigen (PCNA)and thus activated (Yin et al., 2015), thus resulting in elevated apoptosis of neuronal cells. Consequently, we expect to see markedly increased oxidative stress, lipid peroxidation and decreased energy production following oxidative modifications of many proteins, as reported in neurodegenerative diseases. In addition, nitration of chaperon protein HSP90 can cause apoptosis of neuronal cells (Franco et al., 2013), contributing to neurodegeneration. In fact, increased oxidative modifications including protein nitration of cellular proteins and DNA in neuronal tissues have been reported by many different laboratories (Dias et al., 2013; Grimm et al., 2011; Malkus et al., 2009). Furthermore, increased nitroxidative stress can activate cell-death associated MAPKs such as JNK and p38K (Song et al., 2014), contributing to neuronal cell death (Zhu et al, 2015) and neurodegeneration, as reported (Antonio et al., 2011; Byun et al., 2012; Byun et al., 2014; Klein et al., 2003; Upreti et al., 2010). Moreover, increased levels of peroxynitrite, produced from increased nitroxidative stress, can activate poly-ADP-ribose polymerase and mitochondrial permeability change, contributing to neuronal damage. Extensive activation of poly(ADP-ribose) polymerase-1 (PARP-1) by DNA damage induces caspase-independent cell death via ischemia and inflammation. Thus subsequent NAD+ depletion and mitochondrial permeability transition (MPT) are sequential and necessary steps in PARP-1-mediated cell death (Alano et al., 2004; Alano et al., 2010; Lu et al., 2014). Furthermore, many other laboratories reported the importance of S-nitrosylation and/or nitrated proteins such as parkin, GAPDH, XIAP, Prx2, PDI and caspase 3 in neurological diseases (Butterfield et al., 2010, 2014; Cho et al., 2009; Fang et al., 2007; Kwak et al., 2010; Walker et al., 2010; Yin et al., 2015). However, it is likely that additional proteins in the brain could have been nitrated and/or S-nitrosylated under increased nitroxidative stress after exposure to neurotoxic agents or in neurological conditions. Therefore, additional proteins, that can be S-nitrosylated and/or nitrated, need to be identified and characterized to further evaluate the role of protein nitrative protein modifications in causing mitochondrial dysfunction, apoptosis and neurodegeneration.
The small amounts of ROS/RNS, which can be used as cell signaling molecules (Rhee et al., 2000), can be properly handled by various cellular antioxidant defense systems including the compensatory activation of certain antioxidant genes under normal physiological conditions. However, chronic and/or over-production of ROS/RNS under pathological conditions or after exposure to neurotoxic agents, can elevate nitroxidative stress, which subsequently promotes oxidative damage to mitochondrial DNA, proteins (with multiple PTMs), and lipids accompanied with altered cellular signaling and redox balance (Fig. 1). All these changes can cause ER stress with elevated levels of unfolded aggregated proteins, as seen in neurofibrillary tangles in Alzheimer’s disease (Barbero-Camps et al., 2014; Mota et al., 2015; Plácido et al., 2014). In addition, these changes can cause mitochondrial dysfunction (Chaturvedi and Beal, 2013; Dashdorj et al., 2013; Johri and Beal, 2012; Mailloux and Harper, 2011) with increased mitochondrial permeability changes, altered mitochondrial fission/fusion process (with smaller mitochondrial mass), energy depletion and apoptosis/necrosis of neuronal cells. Repeated apoptosis/necrosis with chronic inflammation in the brain can contribute to neurodegeneration, as summarized (Fig. 1).
3. Mitochondrial Dysfunctions in Selected Neurodegenerative Diseases
3.1. Mitochondrial Dysfunction in Alzheimer’s Disease
AD is a progressive neurodegenerative disease that directly affects memory loss and disturbs other important brain functions with decreased quality of life. It represents the most common form of age-related dementia that results in loss of social and intellectual skills. Mostly, these changes are severe enough to interfere with daily activities of affected individuals. The AD is an epidemic that is incurable, irreversible, and progressive particularly in old ages. At the onset of AD, neuronal cells start degenerating, and whole brain size shrinks eventually. Consequently, the brain tissue would have progressively fewer nerve cells with reduced mitochondrial function and lost synaptic connections between each other (Garcia-Escudero et al., 2013; Mondragon-Rodriguez et al., 2013).
Recent studies have shown that a common cause of death in the developed world is AD through the plaque formation outside the nerve cells and neurofibrillary tangles accumulated inside the nerve cells. Aging is the biggest risk factor for acquiring AD in addition to the genetic and environmental factors. Ten percent of the population is expected to acquire AD at the age of 65 years while approximately one third of the people over 85 years old have this disease (Alzheimer’s Association, 2014; Ortman et al., 2014). Other risk factors can be coronary artery disease, high blood pressure (hypertension), stroke, serum cholesterol contents (Proitsi et al, 2014), etc. Recent studies suggest that metabolic syndromes, such as diabetes and obesity with insulin resistance, also play an important role in causing sporadic AD (de la Monte and Tong, 2009, 2014; Nuzzo et al., 2015). Diets containing high fat with or without high fructose/sucrose and sedentary life style may represent another risk factor that contributes to the cognitive decline in animal models (Agrawal et al., 2015; Chang et al., 2014; Liu et al., 2014b; McNeilly et al., 2012; Morrison et al., 2010). The environmental lead (Pb) has been also reported to play an important role in amyloidogenesis, resulting from the combined interactions between mutated or damaged genes, such as APP gene, and a variety of environmental factors (Rajan et al., 2014; Wirth et al., 2014). Exposure to Pb has been shown to produce methylation of the APP gene in early development (Adwan et al., 2014; Bihaqi and Zawia, 2013). The gene, most frequently associated with the late-onset AD (i.e., after age 65), is APO-lipoprotein E4 (APOE4), a variant of an APOE gene (Rajan et al., 2014; Wirth et al., 2014). The generation of 8-hydroxyguanine by Pb results in the formation of one or more CpG (Cytosine Guanine) groups in the APOE gene and is responsible to produce amyloid from APP. The toxic effects of Pb on the brain are due to the oxidative stress that increases the production of amyloid from the APP gene, which is eventually accumulated as amyloid plaques in AD brains.
Another important metal, also implicated in neurodegenerative diseases, is copper. Aβ peptide binds to copper with a very high affinity (1010 M−1 range) (Faller et al., 2013), reducing Cu(II) to Cu(I) with a catalytic generation of H2O2 and Aβ aggregate. This high affinity of peptide Aβ(1–42) for copper is due to its content of β-sheet conformation which favors high-affinity copper binding. In fact, high levels of copper were found in senile plaques and in the cerebrospinal fluids of AD patients. Sub-stoichiometric Cu2+ binding to Aβ results in rapid Aβ fibril formation, where Cu2+ bound Aβ can produce fibrils twice as fast as apo-Aβ. Once generated, these fibrils seed monomeric Aβ to stimulate further fibril formation. After Aβ aggregates are initially formed, metallothionein is unable to facilitate disaggregation, resulting in further Aβ aggregation followed by neuronal cell death. Consequently synaptic loss begins to occur in the brain, eventually resulting in widespread memory loss and decline of brain function frequently associated with severe AD pathology. Some studies have shown that gender difference may also play an important role in AD pathogenesis (Vina and Lloret, 2010). According to this report, females are more likely to develop AD possibly through loss of the protective effect of estrogen against mitochondrial damage observed in older women. Moreover, individuals with a genetic variation in certain genes [e.g., APOE4] are almost four times more susceptible to the development of AD in comparison to people with a normal gene (Sadigh-Eteghad et al., 2012).
Regardless of different risk factors of AD, mitochondrial dysfunction is considered one of the most prominent features observed in vulnerable neurons of the AD patients’ brain (Garcia-Escudero et al., 2013; Mondragon-Rodriguez et al., 2013). Generally, there are two different types of AD, including sporadic (SAD) and familial (FAD). In FAD, the disease is caused by the presence of specific mutations in at least one out of three genes of APP (Garcia-Escudero et al., 2013). Although there is little information regarding the onset of SAD, the main risk for AD is known to be age-related oxidative damage. This oxidative damage may accelerate the expression of beta-secretase-1 (BACE1), an enzyme involved in Aβ generation. Furthermore, increased Aβ may increase the ROS/RNS levels and nitroxidative stress, resulting in mitochondrial dysfunction, possibly through various PTMs of proteins and mitochondrial DNA damage. In addition, mitochondrial ROS may cause modified Aβ formation, while the increased Aβ can lead to oxidative mitochondrial dysfunctions, which may in turn increase the ROS levels (LaFerla et al., 2007). This vicious feed-forward cycle can be repeated and the ROS/RNS generation would be greatly elevated. Consequently, the increased nitroxidative stress can activate stress-activated MAPKs including the JNK and p38K, which can phosphorylate Tau protein at various amino acid residues as observed in the Alzheimer’s patients (Wang et al., 2013). Furthermore, Tau accumulation can cause significant deficits in the mitochondrial distribution in AD patients (Kopeikina et al., 2011). The combination of increased mitochondrial fission and decreased fusion promotes Aβ interaction with the mitochondrial fission protein Drp1, resulting in mitochondrial fragmentation with impaired the axonal transport of mitochondria and synaptic degeneration in neurons. Drp1 interactions with Aβ and phosphorylated Tau are likely to cause excessive mitochondrial fragmentation accompanied with mitochondrial and synaptic deficiencies, leading to neuronal damage and cognitive decline. The GTPase activity, which is important for the mitochondrial fragmentation, was significantly increased in postmortem brain tissues from AD patients and brain tissues from APP, APP/PS1 and 3X Tg.AD mice (Manczak and Reddy, 2012). AD can actively progress, when the accumulated amounts of Aβ increase and disturb the normal functions, leading to neuronal cell death and memory loss. Mostly, main characteristic pathological features of AD include: extensive neurodegeneration in different regions of the brain, including the parietal lobe, regions of frontal cortex, the median temporal lobe and the cingulate gyrus (Rohrer, 2012). Despite all these findings, the molecular mechanisms by which elevated Tau and Aβ promote mitochondrial dysfunction and neuronal cell death are still elusive and thus need to be further studied. However, it is reasonable to assume that various PTMs of many mitochondrial proteins (Butterfields et al., 2014; Sultana et al., 2013) are important in promoting mitochondrial dysfunction and apoptosis, as shown in the liver disease caused by different toxic agents or ischemia reperfusion (Abdelmegeed et al., 2013; Moon et al., 2006, 2008, 2010).
Oxidative stress is also known to play an important role in the onset and progression of AD by promoting multiple PTMs of proteins, oxidative DNA damage and lipid peroxidation in patients with mild cognitive impairment. In a double-transgenic mouse model of AD (APP/DAL), over-expression of a mutant form of human amyloid precursor protein (APP) and a dominant-negative mutant of mitochondrial aldehyde dehydrogenase 2 (ALDH2) was shown to increase oxidative stress. Furthermore, these transgenic APP/DAL mice showed impaired performance on Y-maze and object recognition tests at 3 months of age, suggesting that oxidative stress accelerates cognitive dysfunction and pathological insults in the brain (Ohsawa et al., 2008; Kanamaru et al., 2015). Although the underlying causes for cognitive deficits are uncharacterized, it is likely that mitochondrial function of these animals would be significantly suppressed due to increased oxidative stress, compared to those of the corresponding wild-type mice. Recently obesity was shown as another risk factor for AD in mice fed a high fat diet (HFD). In the brains of HFD-fed mice, the levels of APP and Aβ40/Aβ42 were significantly elevated in both hippocampal and cortical regions (Nuzzo et al., 2015). In addition, the decreased number of insulin receptors and the inhibition of Akt-Foxo3 signaling were observed. These results suggest that HFD induces oxidative stress and mitochondrial dysfunction, leading to cell death and eventually neurodegeneration (Nuzzo et al., 2015).
3.2. Mitochondrial dysfunction in Parkinson’s disease
PD, which is also known as paralysis agitans or hypokinetic rigid syndrome (HRS), usually affect people over the age of 50 years old. PD mainly results from the death of dopamine-generating neurons in the substantia nigra (SN) (Schildknecht et al., 2013). The most difficult part of this disease is that the tremor and shaking interferes with regular daily activities of affected people, as the disease progresses (Ciccone et al., 2013). Typically, this disease with abnormally aggregated proteins results from Parkinson’s gene mutations with altered levels of a protein Parkin, E3 ubiquitin ligase (Sekigawa et al., 2013). The progression of PD is tightly linked to the histopathological features, which are characterized by the formation of both Lewy Neurites (LNs) and Lewy Bodies (LBs). The intracellular inclusions, principally constituted of a small protein α-Synuclein (α-Syn), expressed in SN, neocortex, hippocampus and cerebellum. Accumulation of α-Syn is known to cause neural degeneration via a specific mechanism in the mitochondria (Ciccone et al., 2013; Schildknecht et al., 2013; Sekigawa et al., 2013). Recent reports suggest that the aggregation of α-Syn occurs due to the interaction between α-Syn and cytochrome C oxidase (COX, mitochondrial complex IV), resulting in mitochondrial dysfunction and neuronal degeneration (Al-Mansoori et al., 2013; Ciccone et al., 2013). Furthermore, α-Syn can be nitrated and/or oxidatively-modified, resulting in its accumulation with decreased function and ultimately synucleinopathies (Ciccone et al., 2013; Puschmann, 2013; Sekigawa et al., 2013). Several studies, performed on autopsied individual specimens with PD, showed that there is an enrichment of the autophagosome-like structure. In addition, the decreased concentration of GSH and increased levels of oxidized proteins, lipids and DNA were likely to promote the PD progression (Puschmann, 2013). However, it is still poorly understood what proteins are oxidatively-modified and how these oxidized proteins promote mitochondrial dysfunction and neuronal death. These areas need further investigation.
The anti-oxidative defense enzymes such as aldehyde dehydrogenases (ALDH) generally remove reactive aldehydes including lipid peroxides in the brain and other tissues. Reduced ALDH1 expression in surviving midbrain dopamine neurons has been reported in brains of patients who died with PD (Galter et al., 2003; Liu et al., 2014a). In addition, impaired mitochondrial complex I activity, which is well-documented in PD, reduces the availability of the NAD+ co-factor required for the activities of various ALDH isoforms in removing reactive aldehydes. The decreased functions of ALDH isozymes following exposure to environmental toxins and/or reduced ALDH expression (Song et al., 2011) are likely to play an important role in the pathophysiology of PD (Liu et al., 2015). The knockout mice deficient of Aldh1a1 and Aldh2 (Aldh1a1(−/−) ×Aldh2(−/−) genes, exhibited age-dependent deficits in motor performance on rotarod and gait analysis. On the other hand, intraperitoneal administration of L-DOPA plus benserazide alleviated the deficits in motor performance (Wey et al., 2012). A mitochondrial complex I inhibitor rotenone, which is frequently used to produce an animal model of PD, increases intracellular levels of the toxic dopamine metabolite 3, 4-dihydroxyphenyl-acetaldehyde (DOPAL) through decreased DOPAL metabolism by suppressed ALDH and reduced vesicular sequestration of cytoplasmic dopamine by the vesicular monoamine transporter (VMAT). The results provide a novel mechanism for rotenone-induced toxicity in dopaminergic neurons. According to the ‘catechol aldehyde hypothesis’, buildup of the autotoxic dopamine metabolite DOPAL contributes to PD pathogenesis (Goldstein et al., 2015). Moreover, pesticide exposure has also been shown to associate with PD occurrence, since pesticides can interfere with several cellular processes potentially relevant to PD pathogenesis. For instance, Benomyl, via its thiocarbamate sulfoxide metabolite, inhibits ALDH, leading to accumulation DOPAL and preferential degeneration of dopaminergic neurons for the development of PD (Fitzmaurice et al., 2013).
It has been suggested that the Parkin and PINK1 kinase (PTEN induced putative kinase 1) mutations are implicated in the quality control of the mitochondria while mutations of these genes can lead to the development of autosomal recessive PD (Cookson, 2012; Haskin et al., 2013). PINK1 is expressed in various regions of the brain, including the hippocampus, SN, and Purkinje cells of the cerebellum. This protein normally contains a mitochondrial signal motif both in the C-terminal auto-regulatory region and the N-terminal domain (de Vries and Przedborski, 2013). PINK1 has been involved in the mitochondria dynamics, metabolism, oxidative stress, and ubiquitin-mediated protein degradation (Pickrell and Youle, 2015). The PINK1 and Parkin signaling is a key pathway through which mitophagy can occur, since knocking down PINK1 or Parkin gene decreased mitophagy, resulting in accumulation of damaged mitochondrial fragments intracellularly (Wu et al., 2015). The main role of PINK1 in regulating PD can be related to the inhibition of LBs formation, whereas the lack of PINK1 is likely to result in the accumulation of LBs and mitochondrial impairment of dopaminergic neurons. Consequently, PINK1 mutation has been considered as one of the major causes for the onset of autosomal recessive early-onset PD, as reported (de Vries and Przedborski, 2013; McCoy and Cookson, 2012).
Parkin, E3 ubiquitin-dependent protein ligase, is important in controlling protein degradation and aggregation. Mutation and over-expression of Parkin have been linked to the increased ROS generation accompanied with mitochondrial and lysosomal dysfunction (Narendra and Youle, 2011; Navarro & Boveris, 2009; Winklhofer, 2014). Since Parkin is associated with mitochondrial DNA, this may explain for its ability to protect against the oxidative stress (Mailloux and Harper, 2011; Narendra and Youle, 2011). Another study demonstrated that increased oxidative stress is mostly related to the dopamine metabolism which generates ROS (i.e., H2O2) that can react with Fe2+ and form the highly reactive hydroxyl ion (OH−). This in turn alters the oxidative defense system and increases the levels of oxidized glutathione disulfide (GSSG) with decreased GSH concentration. A decreased GSH concentration is an early sign of oxidative stress that can lead to the degradation of dopamine neurons in PD (Ciccone et al., 2013). Furthermore, the important role of mtDNA-related cell death in PD has been shown with transgenic mice carrying a mitochondria-targeted restriction enzyme (mito-PstI). Since mito-PstI promotes double-strand DNA break in the mtDNA, it is likely that mito-PstI reduces the rate of oxidative phosphorylation, thus contributing to cell death (Pickrell et al., 2011). In fact, PD patients were reported to have a mild deficiency in the NADH-dependent ubiquinone reductase (mitochondrial complex I) activity and impaired mitochondria function with decreased membrane potential. A number of proteins such as PINK1, DJ-1, α-Syn, leucine-rich repeat kinase 2, and Parkin, all of which are genetically linked to familial PD, are either mitochondrial proteins or associated with mitochondria. Failure of mitophagy has been shown to promote the onset of PD pathogenesis (McCoy and Cookson, 2012; Narendra and Youle, 2011; Navarro and Boveris, 2009; Winklhofer, 2014).
3.3. Mitochondrial Dysfunction in Ischemic Stroke
Cerebral ischemia is a condition of inadequate blood supply or flow to the brain. There are two types of the ischemia conditions, namely global and focal ischemia. The global ischemia encompasses wide areas of the brain tissues, whereas focal ischemia is confined to a specific region in the brain. Ischemic process, accompanied with increased accumulation of metabolic wastes, can lead to poor supply of nutrients and oxygen, called as ‘cerebral hypoxia’ that can induce cell death in brain tissues. Consequently, an ischemic condition in brain severely suppresses metabolic processes and generates energy crisis with decreased visual, cognitive and neuromuscular functions. It is likely that the suppressed neuromuscular and cognitive functions can be attributed to mitochondrial dysfunctions either through hypoxia-mediated decreased levels of mitochondrial fusion (Sanderson et al., 2015b) and/or increased oxidative stress (Sun et al., 2014), which can impair mitochondrial function by promoting various PTMs of mitochondrial proteins and oxidative mitochondrial DNA damage. By using a specific peptide inhibitor of JNK, Nijboer and colleagues also demonstrated an important role of mitochondrial JNK in causing neuroinflammation and neuronal damage under ischemic hypoxia (Nijboer et al., 2013), as similar to an acute liver injury by exposure to a hepatotoxin carbon tetrachloride (Moon et al., 2010; Jang et al., 2015) or following hepatic ischemia reperfusion (Moon et al., 2008). However, the JNK-mediated phosphorylated mitochondrial proteins and underlying mechanisms of cell death in the ischemic brain tissues are still unknown and need to be further studied to determine the role of each target protein in altering mitochondrial integrity and neuronal injury.
The brain, in particular, is unable to switch to anaerobic metabolism since the brain does not contain sufficient amounts of ATP as a long term energy reservoir. In addition, brain does not have a system (i.e., gluconeogenesis) to produce glucose for ATP synthesis from other non-glucose molecules or other energy substrates, compared to the liver. Thus, normal blood circulation and proper oxygenation are essential for healthy brain tissues. Following ischemia, however, the ATP level rapidly drops below a threshold level and it takes long time to restore ATP to the original level, even after reperfusion for extended time periods (Borutaite et al., 2013; Bunevicius et al., 2013). In the absence of or lower cellular ATP, the neuronal cells start to lose their ability to maintain the normal electrochemical gradients, resulting in induction of membrane permeability with a huge influx of the calcium into the cytosol with massive accumulation of toxic glutamate and urea possibly due to impaired mitochondrial function, as reported (Borutaite et al., 2013; Li et al., 2012; Xu et al., 2008). It is also likely that greater secondary oxidative damage including peroxynitrite-mediated neuronal damage (Eliasson et al., 1999) can take place during the reperfusion period following ischemia (Rodrigo et al., 2013), as shown in the hepatic ischemia-reperfusion injury (Moon et al., 2008). In fact, the activities of mitochondrial complexes I (NADH-dependent ubiquinone oxidoreductase) and IV (cytochrome c oxidase) were suppressed possibly through protein nitrosylation and/or nitration during reperfusion following ischemia, suggesting mitochondrial dysfunction with depressed function of the respiratory chain. In addition, mitochondrial cytochrome c was released to cytosol to stimulate caspases to further augment cell death. Lysosomal proteases including calpain could also be activated after ischemic insult, as shown in C. elegans (Syntichaki et al., 2002). Accumulation of metabolic wastes further slows down or interrupts blood flow to the brain. All these changes occurring during the ischemia-reperfusion period can lead to immediate loss of consciousness and permanent neuronal damage. For instance, if this bad situation continues for more than 20 minutes, the brain loses the electrical activity, the condition also called as ‘Penumbra’. Under ischemic conditions, the amounts of mitochondrial ROS were significantly greater in comparison to that with the ischemic post-conditioning (Liang et al., 2013). Furthermore, recent results also showed that heavy alcohol intake can aggravate the ischemic brain injury with increased infarct size and neuronal deficit through inactivating ALDH2 (Wang et al., 2015), which metabolizes various reactive aldehydes including lipid peroxides including 4-HNE and MDA (Song et al., 2011, 2015). In this study, activation of ALDH2 by a chemical activator Alda-1 or overexpression of ALDH2 gene abolished neuronal cell death, infarct size and neurological deficit caused by heavy alcohol in ischemic rats.
Manganese superoxide dismutase (MnSOD2) is a mitochondrial enzyme responsible for clearing superoxide radicals. The activities of MnSOD2 and other defensive enzymes including the cytosolic SOD1 have been reported to be suppressed in response to ischemia, possibly via various PTMs including nitration (Abdelmegeed et al., 2010, 2013). This would possibly exacerbate the glutamate toxicity with increased levels of mitochondrial ROS following brain ischemia, contributing to apoptosis of the neuronal cells. Indeed, MnSOD-mimetics can prevent neural apoptosis and reduce the consequences of brain ischemia by properly maintaining the mitochondrial redox status. Therefore, the MnSOD2 plays an important role in maintaining the mitochondrial function by decreasing the levels of mitochondrial ROS produced after brain ischemia (Huang et al., 2012).
Furthermore, recent studies have shown that the mitochondrial damage exhibited a severe collapse in both outer and inner membranes at 24 hours after the reperfusion following 2 hours of ischemia (Kilinc et al., 2010; Li et al., 2012; Liang et al., 2013; Lipton, 2013), as similar to the hepatic ischemia-reperfusion injury (Moon et al., 2008). Additionally the irregular shape, fragmented cristae, electron-lucent matrix, and extreme dilation of the intercristal space of damaged mitochondria were also observed following cerebral ischemia. The swelling of the mitochondria has been related to the opening of the mitochondrial permeability transition pore with Ca2+ concentration which is proportionally increased to the extent of open pores. The Ca2+ overload is considered pathological characteristics of brain ischemia while ischemia post-condition has a limited overload of Ca2+ through modulating the NCX3 isoform of the Na+/Ca2+ exchange. Moreover, the lowering of Ca2+ concentration and the inhibition of the opening of mitochondrial permeability transition pore can reduce mitochondrial swelling (Scorziello et al., 2013).
3.4. Mitochondrial Dysfunction in Traumatic Brain Injury
TBI is an acquired injury from an external blow to the brain with temporarily or permanently impaired cognitive function. In some cases, secondary injury arises that may or may not be detectable (Ilvesmaki et al., 2014). One of the predominant concerns after TBI is the secondary injury accompanied with swelling and release of various pro-inflammatory chemicals, contributing to inflammation and cell injury or death (Hsieh and Yang, 2013). The organelle that is susceptible during and after this traumatic insult is mitochondria. Experimental models of TBI have shown that mitochondria sustain considerable structural and functional damage and that brain survival is closely linked to mitochondrial homeostasis (Gilmer et al., 2009). Mitochondrial swelling has been observed in the early stages of TBI in both experimental models and human brain specimens explanted from TBI patients (Bullock et al., 1991). One of the major causes of the secondary injury from a TBI is an overproduction of ROS (Abdul-Muneer et al., 2015). TBI is also associated with an induction of iNOS with increased production of NO and subsequent peroxynitrite, a very powerful nitrosating/nitrating agent that causes significant impairment of the mitochondrial respiration by inhibiting complexes I (NADH-dependent ubiquinone oxidoreductase), III (cytochrome c reductase) and V (ATP synthase). Elevated NO may also lower cellular ATP via direct suppression of ATP synthase through nitration (Abdelmegeed et al., 2013; Moon et al., 2008) and/or the poly-ADP-ribose polymerase (PARP)-1 route in neurons (Zhang et al., 1994).
Secondary injuries resulting from TBI-related nitroxidative stress are associated with DNA strand breaks that activate PARP-1 and produce another level of DNA damage (Besson, 2009). In response to the severe DNA damage after TBI, PARP-1 becomes over-activated and depletes the cells’ energy sources, resulting in neuronal death (Barr and Conley, 2007). In vitro studies also support the importance of PARP-1-dependent NAD+/NADH depletion as a mechanism of cytotoxic hypoxia caused by neuroinflammatory mediators. Pyruvate is the end-product of glycolysis and is either reduced to lactate or enters the mitochondrial Krebs cycle to be oxidized to CO2 and H2O. Mitochondrial PDH catalyzes the reaction of converting pyruvate to acetyl-coenzyme A. This enzyme is tightly regulated by both end-product inhibition and reversible phosphorylation. PDH, a critical gateway enzyme between the cytosolic glycolysis and the mitochondrial Krebs cycle, can be modified by various forms of PTM, resulting in its inactivation under increased nitroxidative stress (Folmes et al., 2010; Martin et al., 2005; Schroeter et al., 2003). Inactivation of PDH, even in the presence of sufficient oxygen supply, limits the flux of the substrate through the Krebs cycle and causes pyruvate to accumulate, leading to an increased production of lactate and decreased ATP synthesis. In fact, significantly decreased respiratory rates and ATP production have been observed in mitochondria isolated from both experimental models and TBI patients (Verweij et al., 1997). Increased levels of the cerebral extracellular lactate concentrations and in the lactate/pyruvate ratio have been observed in severe TBI, even when both cerebral blood flow (CBF) and brain tissue oxygen tension (PbtO2) are within the normal range. This suggests that even in the absence of brain ischemia, the brain might experience a “metabolic crisis”, a term coined by Vespa and colleagues (2005). These authors identified mitochondrial dysfunction as the probable cause of this crisis (Vespa et al., 2003, 2005). Experimental evidence has confirmed that mitochondria play a pivotal role in metabolic dysfunction after TBI and this impairment consequently contributes to post-traumatic neuronal death (Soustiel and Sviri, 2007).
Thus the aforementioned evidences clearly demonstrate that mitochondrial dysfunction is an important etiological mechanism for the altered brain energy metabolism and the apoptotic cell death occurring after both ischemic stroke and TBI. Nevertheless, the causes and consequences of these abnormalities remain poorly understood and intervention to prevent secondary injury is the prime area for future translational studies.
4. Translational Research Opportunities and Challenges
4.1. Natural Antioxidants against Mitochondrial Dysfunction
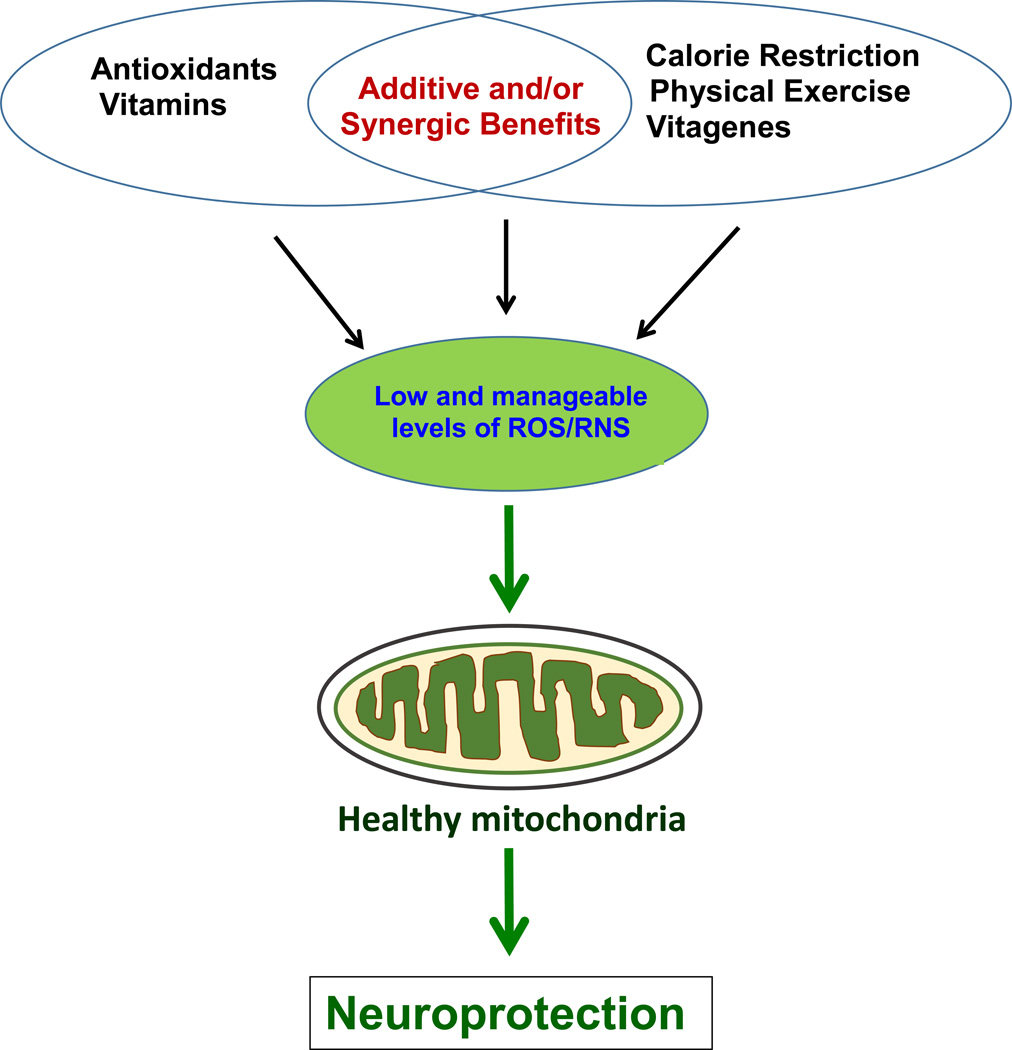
Recent studies have demonstrated that antioxidants, such as polyphenols at low concentrations, typically contained in the diet, are working inside the cell by activating one or more adaptive cellular stress responsive pathways. Antioxidants such as resveratrol, caffeic acid, epicatechin ECGC can activate hormetic pathways in neurons and mediate cytoprotection by activating anti-apoptotic proteins (Chen et al., 2005; Kurauchi et al., 2012; Mandel et al., 2008; Ren et al., 2011; Schroeter et al., 2007). For instance, a redox-sensitive transcription factor NF-E2-related factor-2 (Nrf2) is released from the cytosolic repressor protein Keap1 (Kelch ECH associating protein) under oxidative stress, translocates to the nucleus and binds to the antioxidant-response element (ARE), thus activating antioxidant defense enzymes, including glutathione S-transferase, glutathione peroxidase (GPx) and heme oxygenase-1 (HO-1) (Lee and Johnson, 2004). The antioxidant systems are generally classified into two groups either enzymatic or non-enzymatic antioxidants. The first group consists of a few enzymes such as SOD, Gpx and catalase, all of which act as the first line defense inside the cell against ROS by converting them to inert or less reactive molecules. The second group represents small antioxidant molecules, including GSH, ascorbate (vitamin C), α-tocopherol (vitamin E), and bilirubin, as shown in Figure 2. All these small molecule antioxidants can scavenge the ROS/RNS directly and indirectly by preventing their production (Winterbourn, 2008).
Figure 2.
Potential prevention and protection against mitochondrial dysfunction and many neurodegenerative diseases by natural or synthetic antioxidants, physical exercise, and others.
A variety of edible fruits, plants, and tree nuts, such as apples, grapes, oranges, pomegranates and walnuts, contain the antioxidant molecules. The antioxidant properties of these prophylactic substances of the edible plants have been attributed to the polyphenols, flavonoids, anthocyanins, and other antioxidants. Polyphenols with more than 800 structural variants are considered as secondary metabolites of the plants with aromatic rings containing one or many hydroxyl moieties. Phenolic acids are mainly found in foods and are divided into two classes either derivatives of benzoic or cinnamic acid. The hydroxybenzoic acid derivatives are present in very low amounts in edible plants, although some vegetables and fruits such as onions and black radish are known to contain more than 10 ppm on fresh weight basis. The hydroxycinnamic acids are more common in comparison to hydroxybenzoic acids, while they consist of ferulic, sinapic acids, p-coumaric, caffeic acid, etc (Vauzour, 2012). Examples of polyphenolic molecules and natural antioxidants derived from plant sources include: flavonoids, curcumin, tannins, cinnamic acid derivatives, caffeine, gallic acid derivatives, catechins, salicylic acid derivatives, resveratrol, folate, hesperidin, lutein, lycopene, silimarin (silibinin), chlorogenic acid, ellagic acid and anthocyanins. Moreover, other non-phenolic compounds also derived from plants with antioxidant properties include: α-linolenic acid (ALA), eicosapentaenoic acid, docosahexaenoic acid (DHA), ascopyrones, carotenoids, retinal, thiols, melatonin, jasmonic acid and allicin. Recently, docosahexaenoic acid (DHA) and phosphatidylcholine (PC) have been shown to prevent AD and vascular dementia in Aβ25–35-induced cognitive deficient rats. DHA+PC combination was shown to improve learning and memory in a dose dependent manner by increasing the GPx and SOD activities and decreasing Tau phosphorylation in both the hippocampal and cortical regions of the brain (Qu et al., 2016).
All these compounds show excellent antioxidant activities and beneficial effects in preventing oxidative stress, mitochondrial dysfunction and inflammation in the experimental models of neurodegenerative disorders (Calabrese et al., 2012; Lee et al., 2015; Li et al., 2014; Nataraj et al., 2015; Rege et al., 2013; Singhal et al., 2013; Subash et al., 2014a, 2014b, 2015; Tapias et al., 2014; Vauzour, 2012; Vepsäläinen et al., 2013; Wang et al., 2014]. One of the well-known polyphenols is resveratrol (3, 5, 4’-trihydroxy-trans-stilbene) contained in red grapes and it has been shown to protect the brain from oxidative damage (Gacar et al., 2011). In addition, the green tea polyphenols have been shown to inhibit amyloid fibril formation, protects neurons from oxidative damage caused by Aβ in AD, decrease hyper-phosphorylated Tau, and ameliorate primary hippocampal neuronal damage induced by okadaic acid (Li et al., 2014). Similarly, polyphenols from pomegranate juice have been shown to inhibit rotenone-induced inflammation and neurodegeneration in AD and PD animal models (Tapias et al., 2014). Oral administration of pomegranate juice prevented dopaminergic neuron loss and the inflammatory responses in subtantia nigra in an experimental model of PD. Vepsäläinen et al. (2013) have demonstrated the protective role of anthocyanins from bilberry and blackcurrant extracts in significantly inhibiting the levels of soluble Aβ40 and Aβ42 in a transgenic APdE9 AD mouse model. Both berry-containing diets improved the spatial working memory deficit of aged APdE9 AD mice compared to the corresponding mice fed the control diet, suggesting that anthocyanin-rich bilberry and blackcurrant supplements favorably modulate APP processing and improve behavioral abnormalities in the AD mouse model (Tapias et al., 2014). Long-term oral administration of pomegranate extracts significantly prevented the elevated production of pro-inflammatory cytokines in the serum and brain tissues as well as neuronal contents of Aβ1–40 and Aβ1–42 in a transgenic mouse model of AD (Essa et al., 2015).
In addition to polyphenols, anthocyanins and other small molecule antioxidants, the long-term maintenance of the health conditions can be achieved by another method of activating the complex network of longevity assurance genes termed as vitagenes. Vitagenes represent a group of genes involved in preserving cellular homeostasis during stressful conditions by encoding heat shock proteins (Hsp) Hsp32, Hsp70, the thioredoxin and the sirtuin protein systems. As described, the dietary antioxidants such as polyphenols and L-carnitine/acetyl-L-carnitine activate the hormetic pathways, including vitagenes, and thus stimulate neuroprotective responses in neurodegenerative conditions (Vepsäläinen et al., 2013). The endogenous cellular defense pathways, including sirtuins, Nrfs and related pathways are vital to mediate adaptive stress responses for the prevention of oxidative stress, mitochondrial dysfunction and neuronal cell death prior to severe neurodegenerative diseases. The aforementioned studies provided strong evidence for the beneficial effects of natural compounds found in edible plants in the prevention of neurodegenerative diseases. This should provide ample research opportunities to characterize the extent and mechanism(s) of protection, particularly for mitochondrial homeostasis. It would also be of interest to evaluate whether this protection may be completely or partially effective against the pre-existing disease states. Further, the synergistic or additive effects of a combination of different natural compounds need to be studied on the development and/or progression of neurodegenerative diseases in experimental animal models simulating human disease conditions. The need for various animal models to evaluate the effects and mechanisms of action by these compounds are of extreme importance. In addition, translational studies on human of both genders should be performed to ultimately determine the beneficial effects of these compounds. Collectively, the use of natural compounds to study the mechanism of prevention and /or treatment of neurodegenerative diseases might shed light to better understanding of the mechanism of the disease itself and eventually to the development of better therapeutic strategies. Based on the scientific evidence, people with increased risks regardless of genetic backgrounds and ages should consider taking dietary antioxidants along with proper hormetic approaches for their health benefit.
4.2 Synthetic Agents against Mitochondrial Dysfunction
Despite the use of antioxidants from natural resources such as plants, fruits and vegetables, the list of synthetic drugs and antioxidants for preventing and/or treating the neurological diseases is generally increasing. They constitute many synthetic drugs that act as either agonists or antagonists. Among them is huperzine A (Calabrese et al., 2012), Ladostigil (Zhang, 2012), Rofecoxib and Nimesulide (Weinreb et al., 2012), Zonisamide (Kalonia et al., 2010), and Centrophenoxine (Costa et al., 2010). In addition, there are several inhibitors of various metabolic enzymes. These inhibitors were shown to be beneficial against neurodegenerative diseases by preventing mitochondrial dysfunction. Further, a peptide inhibitor P110 inhibits Drp-1related enzyme activity, blocks Drp1/Fis1 interaction in vitro and protects neurons by preventing mitochondrial fragmentation and ROS generation in primary dopaminergic neurons in a cell culture model for PD. In addition, P110, improved mitochondrial membrane potential and its integrity, while it reduced apoptosis and neurite loss (Verma and Nehru, 2009). Similarly, the inhibitors of type B monoamine oxidase, such as Selegiline and Rasagiline, protect neuronal cells by inducing pro-survival anti-apoptotic Bcl-2 protein family and neurotrophic factors. Selegiline and Rasagiline have been reported to increase the various neurotrophic factors, neurotrophins such as nerve growth factor (NGF), brain-derived neurotrophic factor (BDNF), neurotrophin 3 (NT-3) and ligands of glial cell line-derived neurotrophic factor (GDNF), respectively (Qi et al., 2013). The activators of sirtuin family members (SIRT1–7) include Splitomycin and its derivatives, Sirtinol, AGK2, Tenovin, Suramin, Cambinol, Salermide and Thiobarbiturates. These Sirtuin activators are gaining importance as potential inhibitors of neurological disorders (Naoi and Maruyama, 2010).
The potent acetyl cholinesterase inhibitors (AChEI), PMS777, a tetrahydrofuran derivative, and galantamine, another AChEI, decreased ROS generation while they activated mitochondrial activity in neuronal SK-N-SH cells when these cells were exposed to LPS+IL-1β (Ezoulin et al., 2005). Specific inhibitors of activated p-JNK3, p-p38K, and GSK3β and other enzymes related to the cell death pathways can be evaluated as potential drug candidates for the treatment of AD and other neuropathological conditions, based on their increased levels, as recently reported (Forlenza et al., 2011; Pan et al, 2009). For instance, CEP-1347/KT-7515, a potent inhibitor of the SAPK/JNK pathway by regulating the transcriptional activity of c-jun, blocked Aβ-induction of Cyclin D1 and the Bcl-2 family members DP5 and Bim genes, and promoted cell survival (Harris et al., 2002). CEP-1347/KY-7515 has been also shown to inhibit JNK activation in PC12 cells and in primary sympathetic neurons, thus showing protection from neuronal cell death (Bozyczko-Coyne et al., 2001). Roscovitine, a Cdk5 inhibitor, prevented neuronal cell death by inhibiting c-Jun activation and JNK inhibiting phosphorylation in primary cortical neurons (Sun et al, 2009). Similarly, SP600125 was reported to inhibit JNK activity and reduce the levels of c-Jun phosphorylation, while it protected dopaminergic neurons from apoptosis, and partly restored the levels of dopamine in MPTP-induced PD model in C57BL/6N mice (Wang et al., 2004). Inhibition of JNK by CEP-11004 in mice reduced the levels of phosphorylated c-Jun and cyclooxygenase-2, which are responsible for neuronal apoptosis (Hidding et al., 2002).
In addition, many chelators of metal ions, such as zinc, copper and iron, have been reported to become potential therapeutic candidates in treating various neurological disorders. For instance, a few metal chelators such as Desferal (DFO), VK28, clioquinol, and M10 have also been shown to possess neuroprotective activities in cell cultures and animal models. An eight-amino-acid peptide, NAPVSIPQ (NAP), has been identified as the smallest active element of activity-dependent neuroprotective protein (ADNP), a glial cell mediator of vasoactive Intestinal Peptide (VIP)-induced neuroprotection (Quintana et al., 2006). NAP analogs M98 and M99 display potent protection, similar to their parent peptide NAP or DFO, against 6-hydroxy-dopamine (6-OH-DA) toxicity in cultured SH-SY5Y cells at 1 mM. In cultured PC12 cells, M98 and M99 afford protection against 6-OH-DA toxicity at the range of 0.001–1 mM, and 0.001 or 1 mM, respectively, suggesting that M98 and M99 should be further investigated as potential drug candidates for neuroprotection (Gozes et al., 2003, 2004).
Furthermore, the protection with mitochondria-targeted anti-oxidants, such as ubiquinone (Mito-Q) and carboxy-propyl (Mito-CP), against neurodegenerative diseases appears promising, although these synthetic compounds need further evaluations. These mitochondria-targeted antioxidants were effective in preventing mitochondrial dysfunction and nitroxidative tissue injury in various disease models including AD (Ng et al., 2014), ALS (Miquel et al., 2014), colitis (Dashdorj et al., 2013) and Friedreich Ataxia fibroblasts (Jauslin et al., 2003). In the AD model in C. elegans, these mitochondria-targeted compounds such as Mito-Q restored the suppressed activities of mitochondrial complexes I and IV. Mito-Q improved the suppressed mitochondrial function and extended lifespan of C. elegans. However, it did not affect the status of oxidative damage of mitochondrial DNA, suggesting its specific action on the mitochondrial membrane (Ng et al., 2014). These results indicate important implications of PTMs of mitochondrial ETC proteins over oxidative DNA damage. These promising results from different laboratories suggest that mitochondria-targeted antioxidants are more effective against elevated nitroxidative stress than the untargeted natural antioxidants. Because of the recent development of these antioxidant agents and clinical testing (Smith and Murphy, 2010), we expect increased approval of some of these beneficial agents in treating mitochondrial dysfunction-related organ damage and thus preventing some of the neurodegenerative conditions.
5. Conclusion
In the present review, we have briefly described an overview of the latest research developments on the role of mitochondrial dysfunction in a few selected neurodegenerative diseases. Since the mitochondrial dysfunction was first reported in the 1960s, the critical role of mitochondrial function in both health and disease has been widely documented. By using various advanced techniques, several mitochondrial proteins, that are post-translationally modified, have been identified. Despite these identifications, the actual number of post-translationally modified mitochondrial proteins in neurodegenerative diseases could be markedly increased by using the advanced instruments with greater detection sensitivity and sophisticated techniques. Any damage or impairment in the mitochondria is now understood to play a significant role in a wide range of disease states, including several neurodegenerative diseases such as Alzheimer’s disease, Parkinson’s disease, Huntington’s disease, alcoholic dementia, stroke-related dementia, brain ischemia-reperfusion injury, TBI, etc (Chaturvedi and Beal, 2013; Dashdorj et al., 2013). The most rational approach to minimize the socioeconomic impacts of any disease is to understand the underlying molecular mechanisms of mitochondrial dysfunction and cell death under increased nitroxidative stress and to counteract the deleterious consequences with nutritional supplements and/or specific medications. For instance, prevention of mitochondrial dysfunction and cell death can also be achieved by ingesting healthy diets. Taken together, based on the results from our laboratories and other scientists, regular consumption of diets composed of fruits, vegetables, nuts that contain adequate amounts of antioxidants, and in combination with physical exercise and social interactions (Figure. 2), may prevent metabolic and neurological disorders (McCormack et al., 2013). In particular, people who are at risk to develop metabolic, cardiovascular and neurological disorders, including AD and PD, should consider consuming higher levels of antioxidants (Slavin and Llyod, 2012), omega-3 fatty acids (Calon and Cole 2007), and do some physical exercise to achieve or maintain healthy living style. Since mitochondrial dysfunction plays an important role in the initiation/progression of these chronic disorders, a better understanding of the mitochondrial dynamics and the role of nitroxidative stress is likely to lead to the development of new therapeutic agents to prevent and treat mitochondria-related neurodegenerative diseases.
Acknowledgments
The work is supported by the Intramural Program Fund of the National Institute on Alcohol Abuse and Alcoholism. The authors (MME and GD) also highly appreciate the research supports from the Research Council, Oman (RC/AGR/FOOD/11/01 to MME) and the internal grant (IG/AGR/FOOD/14/01) from Sultan Qaboos University, Oman. Youngshim Choi is supported by the KRIBB Research Initiative Program (Korean Biomedical Scientist Fellowship Program), Korea Research Institute of Bioscience and Biotechnology, Republic of Korea.
Abbreviations
- Aβ
Amyloid-β
- α-Syn
α-Synuclein
- AChEI
Acetyl cholinesterase inhibitors
- AcLys
Acetylated Lys
- AD
Alzheimer’s disease
- ALS
Amyotrophic lateral sclerosis
- APOE
APO-lipoprotein E
- APP
Amyloid precursor protein
- BACE1
Beta-secretase
- CBP
CREB-binding protein
- CMT
Charcot-Marie-Tooth type 2A disease
- COX
Cytochrome C oxidase
- Drp1
Dynamin-related protein-1
- ER
Endoplasmic reticulum
- FAD
Familial Alzheimer’s disease
- GAPDH
Glyceraldehyde-3-phosphate dehydrogenase
- GSH
Glutathione
- GSK3β
Glycogen synthase kinase-3β
- Hsp
Heat shock protein
- iNOS
inducible nitric oxide synthase
- LBs
Lewy bodies
- MAPK
Mitogen activated protein kinases
- Miro
Mitochondrial rho family GTPase
- Mitophagy
Mitochondrial autophagy
- mtDNA
Mitochondrial DNA
- MnSOD
Mitochondrial manganese superoxide dismutase
- NO
Nitric oxide
- NOS
Nitric oxide synthase
- PARP
Poly-ADP-ribose polymerase
- PD
Parkinson’s disease
- PINK1
PTEN induced putative kinase 1
- PTM
post-translational protein modification
- ROS
Reactive oxygen species
- RNS
Reactive nitrogen species
- SAD
Sporadic Alzheimer’s disease
- SAPK/JNK
Stress activated/c-Jun N-terminal protein kinase
- SIRT
Sirtuin
- SN
Substantia nigra
- SOD
Superoxide dismutase
- TBI
Traumatic brain injury
- VDAC1
Voltage dependent anion channel-1
Footnotes
Conflict of Interests
The authors declare that there is no conflict of interests regarding the publication of this paper.
References
- Abdelmegeed MA, Jang S, Banerjee A, Hardwick JP, Song BJ. Robust protein nitration contributes to acetaminophen-induced mitochondrial dysfunction and acute liver injury. Free Radic. Biol. Med. 2013;60:211–222. doi: 10.1016/j.freeradbiomed.2013.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abdelmegeed MA, Moon KH, Chen C, Gonzalez FJ, Song BJ. Role of cytochrome P450 2E1 in protein nitration and ubiquitin-mediated degradation during acetaminophen toxicity. Biochem. Pharmacol. 2010;79:57–66. doi: 10.1016/j.bcp.2009.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abdelmegeed MA, Song BJ. Functional role of protein nitration in acute and chronic liver diseases. Oxid. Med. Cell. Longev. 2014;2014:149627. doi: 10.1155/2014/149627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abdul-Muneer PM, Chandra N, Haorah J. Interactions of oxidative stress and neurovascular inflammation in the pathogenesis of traumatic brain injury. Mol. Neurobiol. 2015;51:966–979. doi: 10.1007/s12035-014-8752-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adwan L, Subaiea GM, Zawia NH. Tolfenamic acid downregulates BACE1 and protects against lead-induced upregulation of Alzheimer’s disease related biomarkers. Neuropharmacology. 2014;79:596–602. doi: 10.1016/j.neuropharm.2014.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agrawal R, Noble E, Vergnes L, Ying Z, Reue K, Gomez-Pinilla F. Dietary fructose aggravates the pathobiology of traumatic brain injury by influencing energy homeostasis and plasticity. [2015 Oct 1];J. Cereb. Blood Flow Metab. 2015 doi: 10.1177/0271678X15606719. pii: 0271678x15606719. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akiyama H, Arai T, Kondo H, Tanno E, Haga C, Ikeda K. Cell mediators of inflammation in the Alzheimer disease brain. Alzheimer Dis. Assoc. Disord. 2000;14(Suppl 1):S47–S53. doi: 10.1097/00002093-200000001-00008. [DOI] [PubMed] [Google Scholar]
- Al-Mansoori KM, Hasan MY, Al-Hayani A, El-Agnaf OM. The role of α-synuclein in neurodegenerative diseases: from molecular pathways in disease to therapeutic approaches. Curr. Alzheimer Res. 2013;10:559–568. doi: 10.2174/1567205011310060002. [DOI] [PubMed] [Google Scholar]
- Alano CC, Ying W, Swanson RA. Poly(ADP-ribose) polymerase-1-mediated cell death in astrocytes requires NAD+ depletion and mitochondrial permeability transition. J. Biol. Chem. 2004;279(18):18895–18902. doi: 10.1074/jbc.M313329200. [DOI] [PubMed] [Google Scholar]
- Alano CC, Garnier P, Ying W, Higashi Y, Kauppinen TM, Swanson RA. NAD+ depletion is necessary and sufficient for poly(ADP-ribose) polymerase-1-mediated neuronal death. J. Neurosci. 2010;30(8):2967–2978. doi: 10.1523/JNEUROSCI.5552-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alzheimer’s Association. Alzheimer’s Disease Facts and Figures, Alzheimer’s & Dementia. 2014;10(2) doi: 10.1016/j.jalz.2014.02.001. [DOI] [PubMed] [Google Scholar]
- Amit K, Priyadarsini I. Free radicals, oxidative stress and importance of antioxidants in human health. J. Med. Allied Sci. 2011;1:53–60. [Google Scholar]
- Antoniou X, Falconi M, Di Marino D, Borsello T. JNK3 as a therapeutic target for neurodegenerative diseases. J. Alzheimers Dis. 2011;24(4):633–642. doi: 10.3233/JAD-2011-091567. [DOI] [PubMed] [Google Scholar]
- Aoyama K, Nakaki T. Impaired glutathione synthesis in neurodegeneration. Int. J. Mol. Sci. 2013;14:21021–21044. doi: 10.3390/ijms141021021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashrafi G, Schlehe JS, LaVoie MJ, Schwarz TL. Mitophagy of damaged mitochondria occurs locally in distal neuronal axons and requires PINK1 and Parkin. J. Cell Biol. 2014;206:655–670. doi: 10.1083/jcb.201401070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashrafi G, Schwarz TL. The pathways of mitophagy for quality control and clearance of mitochondria. Cell Death Differ. 2013;20:31–42. doi: 10.1038/cdd.2012.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auburger G, Gispert S, Jendrach M. Mitochondrial acetylation and genetic models of Parkinson’s disease. Prog. Mol. Biol. Transl. Sci. 2014;127:155–182. doi: 10.1016/B978-0-12-394625-6.00006-4. [DOI] [PubMed] [Google Scholar]
- Bahari-Javan S, Sananbenesi F, Fischer A. Histone-acetylation: a link between Alzheimer’s disease and post-traumatic stress disorder? Front. Neurosci. 2014;8:1–7. doi: 10.3389/fnins.2014.00160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbero-Camps E, Fernández A, Baulies A, Martinez L, Fernández-Checa JC, Colell A. Endoplasmic reticulum stress mediates amyloid β neurotoxicity via mitochondrial cholesterol trafficking. Am. J. Pathol. 2014;184(7):2066–2081. doi: 10.1016/j.ajpath.2014.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barr TL, Conley YP. Poly (ADP-ribose) polymerase-1 and its clinical applications in brain injury. J. Neurosci. Nurs. 2007;39:278–284. doi: 10.1097/01376517-200710000-00004. [DOI] [PubMed] [Google Scholar]
- Bastidas AC, Pierce LC, Walker RC, Johnson DA, Taylor SS. Influence of N-myristylation and ligand binding on the flexibility of the catalytic subunit of protein kinase A. Biochemistry. 2013;52:6368–6379. doi: 10.1021/bi400575k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Besson VC. Drug targets for traumatic brain injury from poly(ADP-ribose) polymerase pathway modulation. Br. J. Pharmacol. 2009;157:695–704. doi: 10.1111/j.1476-5381.2009.00229.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bihaqi SW, Zawia N. Enhanced tauopathy and AD-like pathology in aged primate brains decades after infantile exposure to lead (Pb) Neurotoxicology. 2013;39:95–101. doi: 10.1016/j.neuro.2013.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop P, Rubin P, Thomson AR, Rocca D, Henley JM. The ubiquitin C-terminal hydrolase L1 (UCH-L1) C terminus plays a key role in protein stability, but Its farnesylation is not required for membrane association in primary neurons. J. Biol. Chem. 2014;289:36140–36149. doi: 10.1074/jbc.M114.557124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borutaite V, Toleikis A, Brown GC. In the eye of the storm: mitochondrial damage during heart and brain ischaemia. FEBS J. 2013;280:4999–5014. doi: 10.1111/febs.12353. [DOI] [PubMed] [Google Scholar]
- Boveris A, Oshino N, Chance B. The cellular production of hydrogen peroxide. Biochem. J. 1972;128:617–630. doi: 10.1042/bj1280617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bozyczko-Coyne D, O’Kane TM, Wu ZL, Dobrzanski P, Murthy S, Vaught JL, Scott RW. CEP-1347/KT-7515, an inhibitor of SAPK/JNK pathway activation, promotes survival and blocks multiple events associated with Abeta-induced cortical neuron apoptosis. J. Neurochem. 2001;77:849–863. doi: 10.1046/j.1471-4159.2001.00294.x. [DOI] [PubMed] [Google Scholar]
- Brown GC. Nitric oxide and neuronal death. Nitric Oxide. 2010;23:153–165. doi: 10.1016/j.niox.2010.06.001. [DOI] [PubMed] [Google Scholar]
- Bullock R, Maxwell WL, Graham DI, Teasdale GM, Adams JH. Glial swelling following human cerebral contusion: an ultrastructural study. J. Neurol. Neurosurg. Psychiatry. 1991;54:427–434. doi: 10.1136/jnnp.54.5.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunevicius A, Yuan H, Lin W. The potential roles of 18F-FDG-PET in management of acute stroke patients. Biomed Res. Int. 2013;2013:634598. doi: 10.1155/2013/634598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butterfield DA, Di Domenico F, Swomley AM, Head E, Perluigi M. Redox proteomics analysis to decipher the neurobiology of Alzheimer-like neurodegeneration: overlaps in Down’s syndrome and Alzheimer’s disease brain. Biochem. J. 2014;463:177–189. doi: 10.1042/BJ20140772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butterfield DA, Hardas SS, Lange ML. Oxidatively modified glyceraldehyde-3-phosphate dehydrogenase (GAPDH) and Alzheimer’s disease: many pathways to neurodegeneration. J. Alzheimers Dis. 2010;20:369–393. doi: 10.3233/JAD-2010-1375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butterfield DA, Reed TT, Perluigi M, De Marco C, Coccia R, Keller JN, Markesbery WR, Sultana R. Elevated levels of 3-nitrotyrosine in brain from subjects with amnestic mild cognitive impairment: implications for the role of nitration in the progression of Alzheimer’s disease. Brain Res. 2007;1148:243–248. doi: 10.1016/j.brainres.2007.02.084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byun K, Bayarsaikhan D, Bayarsaikhan E, Son M, Oh S, Lee J, Son HI, Won MH, Kim SU, Song BJ, Lee B. Microglial AGE-albumin is critical in promoting alcohol-induced neurodegeneration in rats and humans. PLoS One. 2014;9:e104699. doi: 10.1371/journal.pone.0104699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byun K, Bayarsaikhan E, Kim D, Kim CY, Mook-Jung I, Paek SH, Kim SU, Yamamoto T, Won MH, Song BJ, Park YM, Lee B. Induction of neuronal death by microglial AGE-albumin: implications for Alzheimer’s disease. PLoS One. 2012;7:e37917. doi: 10.1371/journal.pone.0037917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calabrese V, Cornelius C, Dinkova-Kostova AT, Iavicoli I, Di Paola R, Koverech A, Cuzzocrea S, Rizzarelli E, Calabrese EJ. Cellular stress responses, hermetic phytochemicals and vitagenes in aging and longevity. Biochim. Biophys. Acta. 2012;1822:753–783. doi: 10.1016/j.bbadis.2011.11.002. [DOI] [PubMed] [Google Scholar]
- Calon F, Cole G. Neuroprotective action of omega-3 polyunsaturated fatty acids against neurodegenerative diseases: evidence from animal studies. Prostaglandins Leukot. Essent. Fatty Acids. 2007;77(5–6):287–293. doi: 10.1016/j.plefa.2007.10.019. [DOI] [PubMed] [Google Scholar]
- Chaturvedi RK, Beal MF. Mitochondrial diseases of the brain. Free Radic. Biol. Med. 2013;63:1–29. doi: 10.1016/j.freeradbiomed.2013.03.018. [DOI] [PubMed] [Google Scholar]
- Chang HC, Tai YT, Cherng YG, Lin JW, Liu SH, Chen TL, Chen RM. Resveratrol attenuates high-fat diet-induced disruption of the blood-brain barrier and protects brain neurons from apoptotic insults. J. Agric. Food Chem. 2014;62(15):3466–3475. doi: 10.1021/jf403286w. [DOI] [PubMed] [Google Scholar]
- Chen CY, Jang JH, Li MH, Surh YJ. Resveratrol upregulates heme oxygenase-1 expression via activation of NF-E2-related factor 2 in PC12 cells. Biochem. Biophys. Res. Commun. 2005;331:993–1000. doi: 10.1016/j.bbrc.2005.03.237. [DOI] [PubMed] [Google Scholar]
- Chen F, Dravid D, Ferrari A, Gotz J. Postranslational modifications of tau role in human taupathies and modeling in transgenic animals. Curr. Drug Targets. 2004;5:503–515. doi: 10.2174/1389450043345236. [DOI] [PubMed] [Google Scholar]
- Chen H, McCaffery JF, Chan DC. Mitochondrial fusion protects against neurodegeneration in the cerebellum. Cell. 2007;130:548–562. doi: 10.1016/j.cell.2007.06.026. [DOI] [PubMed] [Google Scholar]
- Cho DH, Nakamura T, Fang J, Cieplak P, Godzik A, Gu Z, Lipton SA. S-nitrosylation of Drp1 mediates beta-amyloid-related mitochondrial fission and neuronal injury. Science. 2009;324:102–105. doi: 10.1126/science.1171091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi BR, Cho WH, Kim J, Lee HJ, Chung C, Jeon WK, Han JS. Increased expression of the receptor for advanced glycation end products in neurons and astrocytes in a triple transgenic mouse model of Alzheimer’s disease. Exp. Mol. Med. 2014;46:e75. doi: 10.1038/emm.2013.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung KK, David KK. Emerging roles of nitric oxide in neurodegeneration. Nitric Oxide. 2010;22:290–295. doi: 10.1016/j.niox.2010.02.002. [DOI] [PubMed] [Google Scholar]
- Chung KK, Thomas B, Li X, Pletnikova O, Troncoso JC, Marsh L, Dawson VL, Dawson TM. S-nitrosylation of parkin regulates ubiquitination and compromises parkin’s protective function. Science. 2004;304:1328–1331. doi: 10.1126/science.1093891. [DOI] [PubMed] [Google Scholar]
- Ciccone S, Maiani E, Bellusci G, Diederich M, Gonfloni S. Parkinson’s disease: a complex interplay of mitochondrial DNA alterations and oxidative Stress. Int. J. Mol. Sci. 2013;14:2388–2409. doi: 10.3390/ijms14022388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cookson MR. Parkinsonism due to mutations in Pink1, Parkin and DJ-1 and oxidative stress and mitochondrial pathways. Cold Spring Harb. Perspect. Med. 2012;2:a009415. doi: 10.1101/cshperspect.a009415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Correia SC, Carvalho C, Cardoso S, Santos RX, Santos MS, Oliveira CR, Perry G, Zhu X, Smith MA, Moreira PI. Mitochondrial preconditioning: a potential neuroprotective strategy. Front. Aging Neurosci. 2010;2:1–13. doi: 10.3389/fnagi.2010.00138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa C, Tozzi A, Luchetti E, Siliquini S, Belcastro V, Tantucci M, Picconi B, Ientile R, Calabresi P, Pisani F. Electrophysiological actions of zonisamide on striatal neurons: Selective neuroprotection against complex I mitochondrial dysfunction. Exp. Neurol. 2010;221:217–224. doi: 10.1016/j.expneurol.2009.11.002. [DOI] [PubMed] [Google Scholar]
- Dai Z, Wu Z, Yang Y, Wang J, Satterfield MC, Meininger CJ, Bazer FW, Wu G. Nitric oxide and energy metabolism in mammals. Biofactors. 2013;39:383–391. doi: 10.1002/biof.1099. [DOI] [PubMed] [Google Scholar]
- Dashdorj A, Jyothi KR, Sangbin L. Mitochondria-targeted antioxidant MitoQ ameliorates experimental mouse colitis by suppressing NLRP3 inflammasome-mediated inflammatory cytokines. BMC Med. 2013;11:178. doi: 10.1186/1741-7015-11-178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de la Monte S, Tong M. Mechanisms of nitrosamine-mediated neurodegeneration: potential relevance to sporadic Alzheimer’s disease. J. Alzheimers Dis. 2009;17:817–825. doi: 10.3233/JAD-2009-1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de la Monte S, Tong M. Brain metabolic dysfunction at the core of Alzheimer’s disease. Biochem. Pharmacol. 2014;88:548–559. doi: 10.1016/j.bcp.2013.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Vries RL, Przedborski S. Mitophagy and Parkinson’s disease: be eaten to stay healthy. Mol. Cell. Neurosci. 2013;55:37–43. doi: 10.1016/j.mcn.2012.07.008. [DOI] [PubMed] [Google Scholar]
- Dexter DT, Jenner P. Parkinson disease: from pathology to molecular disease mechanisms. Free Radic. Biol. Med. 2013;62:132–144. doi: 10.1016/j.freeradbiomed.2013.01.018. [DOI] [PubMed] [Google Scholar]
- Dias V, Junn E, Mouradian MM. The role of oxidative stress in Parkinson’s disease. J. Parkinsons Dis. 2013;3(4):461–491. doi: 10.3233/JPD-130230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickey AS, Strack S. PKA/AKAP1 and PP2A/Bβ2 regulate neuronal morphogenesis via Drp1 phosphorylation and mitochondrial bioenergetics. J. Neurosci. 2011;31:15716–15726. doi: 10.1523/JNEUROSCI.3159-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DuBoff B, Feany M, Götz J. Why size matters - balancing mitochondrial dynamics in Alzheimer’s disease. Trends Neurosci. 2013;36:325–335. doi: 10.1016/j.tins.2013.03.002. [DOI] [PubMed] [Google Scholar]
- Eliasson MJ, Huang Z, Ferrante RJ, Sasamata M, Molliver ME, Snyder SH, Moskowitz MA. Neuronal nitric oxide synthase activation and peroxynitrite formation in ischemic stroke linked to neural damage. J. Neurosci. 1999;19(14):5910–5918. doi: 10.1523/JNEUROSCI.19-14-05910.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ergin V, Hariry RE, Karasu C. Carbonyl stress in aging process: role of Vitamins and phytochemicals as redox regulators. Aging Dis. 2013;4:276–294. doi: 10.14336/AD.2013.0400276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Essa MM, Subash S, Akbar M, Al-Adawi S, Guillemin GJ. Long-term dietary supplementation of pomegranates, figs and dates alleviate neuroinflammation in a transgenic mouse model of Alzheimer’s disease. PLoS One. 2015;10:e0120964. doi: 10.1371/journal.pone.0120964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ezoulin MJ, Li J, Wu G, Dong CZ, Ombetta JE, Chen HZ, Massicot F, Heymans F. Differential effect of PMS777, a new type of acetylcholinesterase inhibitor, and galanthamine on oxidative injury induced in human neuroblastoma SK-N-SH cells. Neurosci. Lett. 2005;389:61–65. doi: 10.1016/j.neulet.2005.07.026. [DOI] [PubMed] [Google Scholar]
- Faller PC, Hureau C, O. Berthoumieu O. Role of metal ions in the self-assembly of the Alzheimer’s amyloid-β peptide. Inorg. Chem. 2013;52:12193–12206. doi: 10.1021/ic4003059. [DOI] [PubMed] [Google Scholar]
- Fang J, Nakamura T, Cho DH, Gu Z, Lipton SA. S-nitrosylation of peroxiredoxin 2 promotes oxidative stress-induced neuronal cell death in Parkinson’s disease. Proc. Nat. Acad. Sci. USA. 2007;104:18742–18747. doi: 10.1073/pnas.0705904104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferraris D, Yang Z, Welsbie D. Dual leucine zipper kinase as a therapeutic target for neurodegenerative conditions. Future Med. Chem. 2013;5:1923–1934. doi: 10.4155/fmc.13.150. [DOI] [PubMed] [Google Scholar]
- Ferramosca A, Zara V. Biogenesis of mitochondrial carrier proteins: molecular mechanisms of import into mitochondria. Biochim. Biophysic. Acta. 2013;1833(3):494–502. doi: 10.1016/j.bbamcr.2012.11.014. [DOI] [PubMed] [Google Scholar]
- Fischer A. Targeting histone-modifications in Alzheimer’s disease. What is the evidence that this is a promising therapeutic avenue? Neuropharmacology. 2014;80:95–102. doi: 10.1016/j.neuropharm.2014.01.038. [DOI] [PubMed] [Google Scholar]
- Fitzmaurice AG, Rhodes SL, Lulla A, Murphy NP, Lam HA, O’Donnell KC, Barnhill L, Casida JE, Cockburn M, Sagasti A, Stahl MC, Maidment NT, Ritz B, Bronstein JM. Aldehyde dehydrogenase inhibition as a pathogenic mechanism in Parkinson disease. Proc. Natl. Acad. Sci. USA. 2013;110:636–641. doi: 10.1073/pnas.1220399110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folmes CD, Sawicki G, Cadete VJ, Masson G, Barr AJ, Lopaschuk GD. Novel O-palmitolylated beta-E1 subunit of pyruvate dehydrogenase is phosphorylated during ischemia/reperfusion injury. Proteom. Sci. 2010;8:38. doi: 10.1186/1477-5956-8-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forlenza OV, Torres CA, Talib LL, de Paula VJ, Joaquim HP, Diniz BS, Gattaz WF. Increased platelet GSK3B activity in patients with mild cognitive impairment and Alzheimer’s disease. J. Psychiat. Res. 2011;45:220–224. doi: 10.1016/j.jpsychires.2010.06.002. [DOI] [PubMed] [Google Scholar]
- Franco MC, Ye Y, Refakis CA, Feldman JL, Stokes AL, Basso M, Melero Fernández de Mera RM, Sparrow NA, Calingasan NY, Kiaei M, Rhoads TW, Ma TC, Grumet M, Barnes S, Beal MF, Beckman JS, Mehl R, Estévez AG. Nitration of Hsp90 induces cell death. Proc. Natl. Acad. Sci. U S A. 2013;110:E1102–E1111. doi: 10.1073/pnas.1215177110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gacar N, Mutlu O, Utkan T, Komsuoglu Celikyurt I, Gocmez SS, Ulak G. Beneficial effects of resveratrol on scopolamine but not mecamylamine induced memory impairment in the passive avoidance and Morris water maze tests in rats. Pharmacol. Biochem. Behav. 2011;99:316–323. doi: 10.1016/j.pbb.2011.05.017. [DOI] [PubMed] [Google Scholar]
- Galter D, Buervenich S, Carmine A, Anvret M, Olson L. ALDH1 mRNA: presence in human dopamine neurons and decreases in substantia nigra in Parkinson’s disease and in the ventral tegmental area in schizophrenia. Neurobiol. Dis. 2003;14:637–647. doi: 10.1016/j.nbd.2003.09.001. [DOI] [PubMed] [Google Scholar]
- Garcia-Bonilla L, Moore JM, Racchumi G, Zhou P, Butler JM, Iadecola C, Anrather J. Inducible nitric oxide synthase in neutrophils and endothelium contributes to ischemic brain injury in mice. J. Immunol. 2014;193:2531–2537. doi: 10.4049/jimmunol.1400918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- García-Escudero V, Martín-Maestro P, Perry G, Avila J. Deconstructing mitochondrial dysfunction in Alzheimer disease. Oxid. Med. Cell. Longev. 2013;2013:162152. doi: 10.1155/2013/162152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gebriel M, Prabhudesai S, Uleberg KE, Larssen E, Piston D, Bjørnstad AH, Møller SG. Zebrafish brain proteomics reveals central proteins involved in neurodegeneration. J. Neurosci. Res. 2014;92:104–115. doi: 10.1002/jnr.23297. [DOI] [PubMed] [Google Scholar]
- Geisler S, Holmström KM, Skujat D, Fiesel FC, Rothfuss OC, Kahle PJ, Springer W. PINK1/Parkin-mediated mitophagy is dependent on VDAC1 and p62/SQSTM1. Nature Cell Biol. 2010;12:119–131. doi: 10.1038/ncb2012. [DOI] [PubMed] [Google Scholar]
- Gilmer LK, Roberts KN, Joy K, Sullivan PG, Scheff SW. Early mitochondrial dysfunction after cortical contusion injury. J. Neurotrauma. 2009;26:1271–1280. doi: 10.1089/neu.2008.0857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein DS, Sullivan P, Cooney A, Jinsmaa Y, Kopin IJ, Sharabi Y. Rotenone decreases intracellular aldehyde dehydrogenase activity: implications for the pathogenesis of Parkinson’s disease. J. Neurochem. 2015;133:14–25. doi: 10.1111/jnc.13042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonçalves M, Tillack L, de Carvalho M, Pinto S, Conradt HS, Costa J. Phosphoneurofilament heavy chain and N-glycomics from the cerebrospinal fluid in amyotrophic lateral sclerosis. Clin. Chim. Acta. 2015;438:342–349. doi: 10.1016/j.cca.2014.09.011. [DOI] [PubMed] [Google Scholar]
- Gould N, Doulias PT, Tenopoulou M, Raju K, Ischiropoulos H. Regulation of protein function and signaling by reversible cysteine S-nitrosylation. J. Biol. Chem. 2013;288:26473–26479. doi: 10.1074/jbc.R113.460261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gozes I, Divinsky I, Pilzer I, Fridkin M, Brenneman DE, Spier AD. From vasoactive intestinal peptide (VIP) through activity-dependent neuroprotective protein (ADNP) to NAP: A view of neuroprotection and cell division. J. Mol. Neurosci. 2003;20:315–322. doi: 10.1385/JMN:20:3:315. [DOI] [PubMed] [Google Scholar]
- Gozes I, Steingart RA, Spier AD. NAP mechanisms of neuroprotection. J. Mol. Neurosci. 2004;24:67–72. doi: 10.1385/JMN:24:1:067. [DOI] [PubMed] [Google Scholar]
- Grimm S, Hoehn A, Davies KJ, Grune T. Protein oxidative modifications in the ageing brain: consequence for the onset of neurodegenerative disease. Free Radic. Res. 2011;45(1):73–88. doi: 10.3109/10715762.2010.512040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guedes-Dias P, Oliverira JMA. Lysine deacetylase and mitochondrial dynamics in neurodegeneration. Biochim. Biophys. Acta. 2013;1832:1345–1359. doi: 10.1016/j.bbadis.2013.04.005. [DOI] [PubMed] [Google Scholar]
- Han SH. Potential role of sirtuin as a therapeutic target for neurodegenerative diseases. J. Clin. Neurol. 2009;5:120–125. doi: 10.3988/jcn.2009.5.3.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanawa N, Shinohara M, Saberi B, Gaarde WA, Han D, Kaplowitz N. Role of JNK translocation to mitochondria leading to inhibition of mitochondria bioenergetics in acetaminophen-induced liver injury. J. Biol. Chem. 2008;283(20):13565–13577. doi: 10.1074/jbc.M708916200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanna RA, Quinsay MN, Orogo AM, Giang K, Rikka S, Gustafsson AB. Microtubule-associated protein 1 light chain 3 (LC3) interacts with Bnip3 protein to selectively remove endoplasmic reticulum and mitochondria via autophagy. J. Biol. Chem. 2012;287:19094–19104. doi: 10.1074/jbc.M111.322933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hara MR, Thomas B, Cascio MB, Bae BI, Hester LD, Dawson VL, Dawson TM, Sawa A, Snyder SH. Neuroprotection by pharmacologic blockade of the GAPDH death cascade. Proc. Natl. Acad. Sci. USA. 2006;103:3887–3889. doi: 10.1073/pnas.0511321103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris C, Maroney AC, Johnson EM., Jr Identification of JNK-dependent and -independent components of cerebellar granule neuron apoptosis. J. Neurochem. 2002;83:992–1001. doi: 10.1046/j.1471-4159.2002.01219.x. [DOI] [PubMed] [Google Scholar]
- Haskin J, Szargel R, Shani V, Mekies LN, Rott G, Lim G, Lim KL, Bandopadhyay R, Wolosker H, Engelender S. AF-6 is a positive modulator of the PINK1/parkin pathway and is deficient in Parkinson’s disease. Human Mol. Genet. 2013;22:2083–2096. doi: 10.1093/hmg/ddt058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hidding U, Mielke K, Waetzig V, Brecht S, Hanisch U, Behrens A, Wagner E, Herdegen T. The c-Jun N-terminal kinases in cerebral microglia: immunological functions in the brain. Biochem. Pharmacol. 2002;64:781–788. doi: 10.1016/s0006-2952(02)01139-5. [DOI] [PubMed] [Google Scholar]
- Hoang T, Choi DK, Naga i M, Wu DC, Nagata T, Prou D, Wilson GL, Vila M, Jackson-Lewis V, Dawson VL, Dawson TM, Chesselet MF, Przedborski S. Neuronal NOS and cyclooxygenase-2 contribute to DNA damage in a mouse model of Parkinson’s disease. Free Radic. Biol. Med. 2009;47:1049–1056. doi: 10.1016/j.freeradbiomed.2009.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsieh HL, Yang CM. Role of redox signaling in neuroinflammation and neurodegenerative diseases. Biomed. Res. Int. 2013;2013:484613. doi: 10.1155/2013/484613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang H, Guo F, Cao Y. Neuroprotection by manganese superoxide dismutase (MnSOD) mimics: antioxidant effect and oxidative stress regulation in acute experimental Stroke. CNS Neurosci. 2012;18:811–818. doi: 10.1111/j.1755-5949.2012.00380.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ilvesmaki T, Luoto TM, Hakulinen U, Brander A, Ryymin P, Eskola H, Iverson GL, Ohman J. Acute mild traumatic brain injury is not associated with white matter change on diffusion tensor imaging. Brain. 2014;137:1876–1882. doi: 10.1093/brain/awu095. [DOI] [PubMed] [Google Scholar]
- Jang SH, Yu LR, Abdelmegeed MA, Gao Y, Banerjee A, Song BJ. Critical role of c-Jun N-terminal protein kinase in promoting mitochondrial dysfunction and acute liver injury. Redox Biol. 2015;6:552–564. doi: 10.1016/j.redox.2015.09.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jauslin ML, Meier T, Smith RA, Murphy MP. Mitochondria-targeted antioxidants protect Friedreich Ataxia fibroblasts from endogenous oxidative stress more effectively than untargeted antioxidants. FASEB J. 2003;17:1972–1974. doi: 10.1096/fj.03-0240fje. [DOI] [PubMed] [Google Scholar]
- Jimenez S, Torres M, Vizuette M, Sanchez-Varo R, Sanchez-Meijis E, Truijillo-Estrada L, Carmona-Cuenca I, Caballero C, Ruano D, Guitierrez A, Victoria J. Age-dependant accumulation of soluble amyloid Beta (Ab) oligomers reverses the neuroprotective effect of soluble amyloid precursor protein-alpha (sAPPα) by modulating phosphatidylinositol 3-kinase/ Akt-GSK-3β pathway in Alzheimer’s mouse model. J. Biol. Chem. 2011;286:18414–18425. doi: 10.1074/jbc.M110.209718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin H, Kanthasamy A, Harischandra DS, Kondru N, Ghosh A, Panicker N, Anantharam V, Rana A, Kanthasamy AG. Histone hyperacetylation up-regulates protein kinase Cδ in dopaminergic neurons to induce cell death: relevance to epigenetic mechanisms of neurodegeneration in Parkinson disease. J. Biol. Chem. 2014;289:34743–34767. doi: 10.1074/jbc.M114.576702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jo J, Whitcomb DJ, Olsen KM, Kerrigan TL, Lo SC, Bru-mercier G, Dickinson B, Scullion S, Sheng M, Collingridge G, Cho K. Ab (1-42) inhibition of LTP is mediated by a signaling pathway involving caspase-3, Akt1 and GSK-3β. Nat. Neurosci. 2011;14:545–547. doi: 10.1038/nn.2785. [DOI] [PubMed] [Google Scholar]
- Johri A, Beal MF. Mitochondrial dysfunction in neurodegenerative diseases. J. Pharmacol. Exp. Ther. 2012;342:619–630. doi: 10.1124/jpet.112.192138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jope RS, Yuskaitis CJ, Beurel E. Glycogen synthase kinase-3 (GSK3): inflammation, diseases, and therapeutics. Neurochem. Res. 2007;32:577–595. doi: 10.1007/s11064-006-9128-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalonia H, Kumar P, Kumar A, Nehru B. Protective effect of rofecoxib and nimesulide against intra-striatal quinolinic acid-induced behavioral, oxidative stress and mitochondrial dysfunctions in rats. Neurotoxicology. 2010;31:195–203. doi: 10.1016/j.neuro.2009.12.008. [DOI] [PubMed] [Google Scholar]
- Kamata H, Honda S, Maeda M, Chang L, Hirata H, Karin M. Reactive oxygen species promote TNFalpha-induced death and sustained JNK activation by inhibiting MAP kinase phosphatases. Cell. 2005;120:649–661. doi: 10.1016/j.cell.2004.12.041. [DOI] [PubMed] [Google Scholar]
- Kanamaru T, Kamimura N, Yokota T, Iuchi K, Nishimaki K, Takami S, Akashiba H, Shitaka Y, Katsura K, Kimura K, Ohta S. Oxidative stress accelerates amyloid deposition and memory impairment in a double-transgenic mouse model of Alzheimer’s disease. Neurosci. Lett. 2015;587:126–131. doi: 10.1016/j.neulet.2014.12.033. [DOI] [PubMed] [Google Scholar]
- Karababa A, Görg B, Schliess F, Häussinger D. O-GlcNAcylation as a novel ammonia-induced posttranslational protein modification in cultured rat astrocytes. Metab. Brain Dis. 2014;29:975–982. doi: 10.1007/s11011-013-9454-7. [DOI] [PubMed] [Google Scholar]
- Kharbanda S, Saxena S, Yoshida K, Pandey P, Kaneki M, Wang Q, Cheng K, Chen YN, Campbell A, Sudha T, Yuan ZM, Narula J, Weichselbaum R, Nalin C, Kufe D. Translocation of SAPK/JNK to mitochondria and interaction with Bcl-xL in response to DNA damage. J. Biol. Chem. 2000;275:322–327. doi: 10.1074/jbc.275.1.322. [DOI] [PubMed] [Google Scholar]
- Kijima K, Numakura C, Izumino H, Umetsu K, Nezu A, Shiiki T, Ogawa M, Ishizaki Y, Kitamura T, Shozawa Y, Hayasaka K. Mitochondrial GTPase mitofusin 2 mutation in Charcot-Marie-Tooth neuropathy type 2A. Hum. Genet. 2005;116:23–27. doi: 10.1007/s00439-004-1199-2. [DOI] [PubMed] [Google Scholar]
- Kilinc M, Gürsoy-Ozdemir Y, Gürer G, Erdener SE, Erdemli E, Can A, Dalkara T. Lysosomal rupture, necroapoptotic interactions and potential crosstalk between cysteine proteases in neurons shortly after focal ischemia. Neurobiol. Dis. 2010;40:293–302. doi: 10.1016/j.nbd.2010.06.003. [DOI] [PubMed] [Google Scholar]
- Kim BJ, Ryu SW, Song BJ. JNK- and p38 kinase-mediated phosphorylation of Bax leads to its activation and mitochondrial translocation and to apoptosis of human hepatoma HepG2 cells. J. Biol. Chem. 2006;281(30):21256–21265. doi: 10.1074/jbc.M510644200. [DOI] [PubMed] [Google Scholar]
- Klein JA, Ackerman SL. Oxidative stress, cell cycle, and neurodegeneration. J. Clin. Invest. 2003;111(6):785–793. doi: 10.1172/JCI18182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knowles RG, Moncada S. Nitric oxide synthases in mammals. Biochem. J. 1994;298:24–258. doi: 10.1042/bj2980249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopeikina KJ, Carlson GA, Pitstick R, et al. Tau accumulation causes mitochondrial distribution deficits in neurons in a mouse model of tauopathy and in human Alzheimer’s disease brain. Am. J. Pathol. 2011;179:2071–2082. doi: 10.1016/j.ajpath.2011.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar A, Chen SH, Kadiiska MB, Hong JS, Zielonka J, Kalyanaraman B, Mason RP. Inducible nitric oxide synthase is key to peroxynitrite-mediated, LPS-induced protein radical formation in murine microglial BV2 cells. Free Radic. Biol. Med. 2014;73:51–59. doi: 10.1016/j.freeradbiomed.2014.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar A, Leinisch F, Kadiiska MB, Corbett J, Mason RP. Formation and Implications of alpha-synuclein radical in Maneb- and Paraquat-induced models of Parkinson’s disease. Mol. Neurobiol. 2015 May 8; doi: 10.1007/s12035-015-9179-1. Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurauchi K, Hisatsune A, Isohama Y, Mishima S, Katsuki H. Caffeic acid phenethyl ester protects nigral dopaminergic neurons via dual mechanisms involving heme oxygenase-1 and brain-derived neurotrophic factor. Br. J. Pharmacol. 2012;166:1151–1168. doi: 10.1111/j.1476-5381.2012.01833.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwak YD, Ma T, Diao S, Zhang X, Chen Y, Hsu J, Lipton SA, Masliah E, Xu H, Liao FF. NO signaling and S-nitrosylation regulate PTEN inhibition in neurodegeneration. Mol. Neurodegen. 2010;5:49. doi: 10.1186/1750-1326-5-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaFerla FM, Green KN, Oddo S. Intracellular amyloid-β in Alzheimer’s Disease. Nat. Rev. Neurosci. 2007;8:499–509. doi: 10.1038/nrn2168. [DOI] [PubMed] [Google Scholar]
- Lazo-Gómez R, Ramírez-Jarquín UN, Tovar-Y-Romo LB, Tapia R. Histone deacetylases and their role in motor neuron degeneration. Front. Cell. Neurosci. 2013;7:1–7. doi: 10.3389/fncel.2013.00243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JM, Johnson JA. An important role of Nrf2-ARE pathway in the cellular defense mechanism. J. Biochem. Mol. Biol. 2004;37:139–143. doi: 10.5483/bmbrep.2004.37.2.139. [DOI] [PubMed] [Google Scholar]
- Lee Y, Park HR, Chun HJ, Lee J. Silibinin prevents dopaminergic neuronal loss in a mouse model of Parkinson’s disease via mitochondrial stabilization. J. Neurosci. Res. 2015;93:755–765. doi: 10.1002/jnr.23544. [DOI] [PubMed] [Google Scholar]
- Li H, Wu X, Wu Q, Gong D, Shi M, Guan L, Zhang J, Liu J, Yuan B, Han G, Zou Y. Green tea polyphenols protect against okadaic acid-induced acute learning and memory impairments in rats. Nutrition. 2014;30:337–342. doi: 10.1016/j.nut.2013.08.021. [DOI] [PubMed] [Google Scholar]
- Li J, Ma X, Yu W, Lou Z, Mu D. Reperfusion promotes mitochondrial dysfunction following focal cerebral ischemia in rats. PLoS One. 2012;7:e46498. doi: 10.1371/journal.pone.0046498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang JM, Xu HY, Zhang XJ, Li X, Zhang HB, Ge PF. Role of mitochondrial function in the protective effects of ischemic post conditioning on ischemia/reperfusion cerebral damage. J. Int. Med. Res. 2013;41:618–627. doi: 10.1177/0300060513476587. [DOI] [PubMed] [Google Scholar]
- Liberatore GT, Jackson-Lewis V, Vukosavic S, Mandir AS, Vila M, McAuliffe WG, Dawson VL, Dawson TM, Przedborski S. Inducible nitric oxide synthase stimulates Dopaminergic neurodegeneration in the MPTP model of Parkinson disease. Nat. Med. 1999;5:1403–1409. doi: 10.1038/70978. [DOI] [PubMed] [Google Scholar]
- Lipton P. Lysosomal membrane permeabilization as a key player in brain ischemic cell death: a “lysosomocentric” hypothesis for ischemic brain damage. Translat. Stroke Res. 2013;4:672–684. doi: 10.1007/s12975-013-0301-2. [DOI] [PubMed] [Google Scholar]
- Liu G, Yu J, Ding J, Xie C, Sun L, Rudenko I, Zheng W, Sastry N, Luo J, Rudow G, Troncoso JC, Cai H. Aldehyde dehydrogenase 1 defines and protects a nigrostriatal dopaminergic neuron subpopulation. J. Clin. Invest. 2014a;124:3032–3046. doi: 10.1172/JCI72176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu XY, Li HL, Su JB, Ding FH, Zhao JJ, Chai F, Li YX, Cui SC, Sun FY, Wu ZY, Xu P, Chen XH. Regulation of RAGE splicing by hnRNP A1 and Tra2β-1 and its potential role in AD pathogenesis. J. Neurochem. 2015;133:187–198. doi: 10.1111/jnc.13069. [DOI] [PubMed] [Google Scholar]
- Liu Y, Fu X, Lan N, Li S, Zhang J, Wang S, Li C, Shang Y, Huang T, Zhang L. Luteolin protects against high fat diet-induced cognitive deficits in obesity mice. Behav. Brain Res. 2014b;267:178–188. doi: 10.1016/j.bbr.2014.02.040. [DOI] [PubMed] [Google Scholar]
- Lozano L, Lara-Lemus R, Zenteno E, Alvarado-Vásquez N. The mitochondrial O-linked N-acetylglucosamine transferase (mOGT) in the diabetic patient could be the initial trigger to develop Alzheimer disease. Exp. Gerontol. 2014;58:198–202. doi: 10.1016/j.exger.2014.08.008. [DOI] [PubMed] [Google Scholar]
- Lu P, Kamboj A, Gibson SB, Anderson CM. Poly(ADP-ribose) polymerase-1 causes mitochondrial damage and neuron death mediated by Bnip3. J. Neurosci. 2014;34(48):15975–15987. doi: 10.1523/JNEUROSCI.2499-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maggio DM, Chatzipanteli K, Masters N, Patel SP, Dietrich WD, Pearse DD. Acute molecular perturbation of inducible nitric oxide synthase with an antisense approach enhances neuronal preservation and functional recovery after contusive spinal cord injury. J. Neurotrauma. 2012;29:2244–2249. doi: 10.1089/neu.2012.2371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mailloux RJ, Harper ME. Uncoupling proteins and the control of mitochondrial reactive oxygen species production. Free Radic. Biol. Med. 2011;51:1106–1115. doi: 10.1016/j.freeradbiomed.2011.06.022. [DOI] [PubMed] [Google Scholar]
- Malinski T. Nitric oxide and nitroxidative stress in Alzheimer’s disease. J. Alzheimers Dis. 2007;11:207–218. doi: 10.3233/jad-2007-11208. [DOI] [PubMed] [Google Scholar]
- Malkus KA, Tsika E, Ischiropouloss H. Oxidative modifications, mitochondrial dysfunction, and impaired protein degradation in Parkinson’s disease: how neurons are lost in the Bermuda triangle. Mol. Neurodegener. 2009;4:24. doi: 10.1186/1750-1326-4-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manczak M, Reddy PH. Abnormal interaction between the mitochondrial fission protein Drp1 and hyperphosphorylated tau in Alzheimer’s disease neurons: implications for mitochondrial dysfunction and neuronal damage. Human Mol. Genet. 2012;21:2538–2547. doi: 10.1093/hmg/dds072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandel SA, Amit T, Kalfon L, Reznichenko L, Weinreb O, Youdim MBH. Cell signaling pathways and iron chelation in the neurorestorative activity of green tea polyphenols: special reference to epigallocatechin gallate (EGCG) J. Alzheimers Dis. 2008;15:211–222. doi: 10.3233/jad-2008-15207. [DOI] [PubMed] [Google Scholar]
- Martin E, Rosenthal RE, Fiskum G. Pyruvate dehydrogenase complex: metabolic link to ischemic brain injury and target of oxidative stress. J. Neurosci. Res. 2005;79:240–247. doi: 10.1002/jnr.20293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCoy MK, Cookson MR. Mitochondrial quality control and dynamics in Parkinson’s disease. Antioxid. Redox Signal. 2012;16:869–882. doi: 10.1089/ars.2011.4019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormack D, McFadden D. A Review of pterostilbene antioxidant activity and disease modification. Oxid. Med. Cell. Longev. 2014;2013:575482. doi: 10.1155/2013/575482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNeilly AD, Williamson R, Balfour DJ, Stewart CA, Sutherland C. A high-fat-diet-induced cognitive deficit in rats that is not prevented by improving insulin sensitivity with metformin. Diabetologia. 2012;55(11):3061–3070. doi: 10.1007/s00125-012-2686-y. [DOI] [PubMed] [Google Scholar]
- Medeiros R, Prediger R D, Passos GF, Pandolfo P, Duarte FS, Franco JL, Dafre AL, Di Giunta G, Figueiredo CP, Takahashi RN, Campos MM, Calixto JB. Connecting TNF-alpha signaling pathways to iNOS expression in a mouse model of Alzheimer’s disease: relevance for the behavioral and synaptic deficits induced by amyloid beta protein. J. Neurosci. 2007;27:5394–5404. doi: 10.1523/JNEUROSCI.5047-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miquel E, Cassina A, Martínez-Palma L, Souza JM, Bolatto C, Rodríguez-Bottero S, Logan A, Smith RA, Murphy MP, Barbeito L, Radi R, Cassina P. Neuroprotective effects of the mitochondria-targeted antioxidant MitoQ in a model of inherited amyotrophic lateral sclerosis. Free Radic. Biol. Med. 2014;70:204–213. doi: 10.1016/j.freeradbiomed.2014.02.019. [DOI] [PubMed] [Google Scholar]
- Mkhikian H, Grigorian A, Li CF, Chen HL, Newton B, Zhou RW, Beeton C, Torossian S, Tatarian GG, Lee SU, Lau K, Walker E, Siminovitch KA, Chandy KG, Yu Z, Dennis JW, Demetriou M. Genetics and the environment converge to dysregulate N-glycosylation in multiple sclerosis. Nat. Commun. 2011;2:334. doi: 10.1038/ncomms1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mondragón-Rodríguez S, Perry G, Zhu X, Moreira PI, Acevedo-Aquino MC, Williams S. Phosphorylation of tau protein as the link between oxidative stress, mitochondrial dysfunction, and connectivity failure: implications for Alzheimer’s disease. Oxid. Med. Cell. Longev. 2013;2013:940603. doi: 10.1155/2013/940603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moon KH, Hood BL, Kim BJ, Hardwick JP, Conrads TP, Veenstra TD, Song BJ. Inactivation of oxidized and S-nitrosylated mitochondrial proteins in alcoholic fatty liver of rats. Hepatology. 2006;44:1218–1230. doi: 10.1002/hep.21372. [DOI] [PubMed] [Google Scholar]
- Moon KH, Hood BL, Mukhopadhyay P, Rajesh M, Abdelmegeed MA, Kwon YI, Conrads TP, Veenstra TD, Song BJ, Pacher P. Oxidative inactivation of key mitochondrial proteins leads to dysfunction and injury in hepatic ischemia reperfusion. Gastroenterology. 2008;135:1344–1357. doi: 10.1053/j.gastro.2008.06.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moon KH, Lee YM, Song BJ. Inhibition of hepatic mitochondrial aldehyde dehydrogenase by carbon tetrachloride through JNK-mediated phosphorylation. Free Radic. Biol. Med. 2010;48:391–398. doi: 10.1016/j.freeradbiomed.2009.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison CD, Pistell PJ, Ingram DK, Johnson WD, Liu Y, Fernandez-Kim SO, White CL, Purpera MN, Uranga RM, Bruce-Keller AJ, Keller JN. High fat diet increases hippocampal oxidative stress and cognitive impairment in aged mice: implications for decreased Nrf2 signaling. J. Neurochem. 2010;114(6):1581–1589. doi: 10.1111/j.1471-4159.2010.06865.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mota SI, Costa RO, Ferreira IL, Santana I, Caldeira GL, Padovano C, Fonseca AC, Baldeiras I, Cunha C, Letra L, Oliveira CR, Pereira CM, Rego AC. Oxidative stress involving changes in Nrf2 and ER stress in early stages of Alzheimer’s disease. Biochim. Biophys. Acta. 2015;1852(7):1428–1441. doi: 10.1016/j.bbadis.2015.03.015. [DOI] [PubMed] [Google Scholar]
- Naoi M, Maruyama W. Monoamine oxidase inhibitors as neuroprotective agents in age-dependent neurodegenerative disorders. Curr. Pharmaceut. Design. 2010;16:2799–2817. doi: 10.2174/138161210793176527. [DOI] [PubMed] [Google Scholar]
- Narayan PJ, Lill C, Faull R, Curtis MA, Dragunow M. Increased acetyl and total histone levels in post-mortem Alzheimer’s disease brain. Neurobiol. Dis. 2015;74:281–294. doi: 10.1016/j.nbd.2014.11.023. [DOI] [PubMed] [Google Scholar]
- Narendra DP, Youle RJ. Targeting mitochondrial dysfunction: role for PINK1 and Parkin in mitochondrial quality control. Antioxid. Redox Signal. 2011;14:1929–1938. doi: 10.1089/ars.2010.3799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nataraj J, Manivasagam T, Thenmozhi AJ, Essa MM. Lutein protects dopaminergic neurons against MPTP-induced apoptotic death and motor dysfunction by ameliorating mitochondrial disruption and oxidative stress. Nutr. Neurosci. 2015 doi: 10.1179/1476830515Y.0000000010. 2015 Mar 2. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- Nathan C, Calingasan N, Nezezon J, Ding A, Lucia MS, La Perle K, Fuortes M, Lin M, Ehrt S, Kwon NS, Chen J, Vodovotz Y, Kipiani K, Beal MF. Protection from Alzheimer’s-like disease in the mouse by genetic ablation of inducible nitric oxide synthase. J Exp. Med. 2005;202:1163–1169. doi: 10.1084/jem.20051529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navarro A, Boveris A. Mitochondrial nitric oxide synthase, mitochondrial brain dysfunction in aging, and mitochondria-targeted antioxidants. Adv. Drug Deliv. Rev. 2008;60:1534–1544. doi: 10.1016/j.addr.2008.05.002. [DOI] [PubMed] [Google Scholar]
- Navarro A, Boveris A. Brain mitochondrial dysfunction and oxidative damage in Parkinson’s disease. J. Bioenerg. Biomemb. 2009;41:517–521. doi: 10.1007/s10863-009-9250-6. [DOI] [PubMed] [Google Scholar]
- Ng LF, Gruber J, Cheah IK, Goo CK, Cheong WF, Shui G, Sit KP, Wenk MR, Halliwell B. The mitochondria-targeted antioxidant MitoQ extends lifespan and improves healthspan of a transgenic Caenorhabditis elegans model of Alzheimer disease. Free Radic. Biol. Med. 2014;71:390–401. doi: 10.1016/j.freeradbiomed.2014.03.003. [DOI] [PubMed] [Google Scholar]
- Nijboer CH, Bonestroo HJ, Zijlstra J, Kavelaars A, Heijnen CJ. Mitochondrial JNK phosphorylation as a novel therapeutic target to inhibit neuroinflammation and apoptosis after neonatal ischemic brain damage. Neurobiol. Dis. 2013;54:432–444. doi: 10.1016/j.nbd.2013.01.017. [DOI] [PubMed] [Google Scholar]
- Nisticò R, Ferraina C, Marconi V, Blandini F, Negri L, Egebjerg J, Feligioni M. Age-related changes of protein SUMOylation balance in the AβPP Tg2576 mouse model of Alzheimer’s disease. Front. Pharmacol. 2014;5:1–9. doi: 10.3389/fphar.2014.00063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nobunaga M, Obukuro K, Kurauchi Y, Hisatsune A, Seki T, Tsutsui M, Katsuki H. High fat diet induces specific pathological changes in hypothalamic orexin neurons in mice. Neurochem. Int. 2014;78:61–66. doi: 10.1016/j.neuint.2014.09.002. [DOI] [PubMed] [Google Scholar]
- Novak I, Kirkin V, McEwan DG, Zhang J, Wild P, Rozenknop A, Rogov V, Löhr F, Popovic D, Occhipinti A, Reichert AS, Terzic J, Dötsch V, Ney PA, Dikic I. Nix is a selective autophagy receptor for mitochondrial clearance. EMBO Reports. 2010;11:45–51. doi: 10.1038/embor.2009.256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nuzzo D, Picone P, Baldassano S, Caruana L, Messina E, Marino-Gammazza A, Cappello F, Mulè F, Carlo MD. Insulin resistance as common molecular denominator linking obesity to Alzheimer’s disease. Curr Alzheimers Res. 2015;12:723–735. doi: 10.2174/1567205012666150710115506. [DOI] [PubMed] [Google Scholar]
- Oikawa S, Kobayashi H, Kitamura Y, Zhu H, Obata K, Minabe Y, Dazortsava M, Ohashi K, Tada-Oikawa S, Takahashi H, Yata K, Murata M, Yamashima T. Proteomic analysis of carbonylated proteins in the monkey substantia nigra after ischemia-reperfusion. Free Radic. Res. 2014;48:694–705. doi: 10.3109/10715762.2014.901509. [DOI] [PubMed] [Google Scholar]
- Okatsu K, Iemura S, Koyano F, Go E, Kimura M, Natsume T, Tanaka K, Matsuda N. Mitochondrial hexokinase HKI is a novel substrate of the Parkin ubiquitin ligase. Biochem. Biophys. Res. Commun. 2012;428:197–202. doi: 10.1016/j.bbrc.2012.10.041. [DOI] [PubMed] [Google Scholar]
- Ohsawa I, Nishimaki K, Murakami Y, Suzuki Y, Ishikawa M, Ohta S. Age-dependent neurodegeneration accompanying memory loss in transgenic mice defective in mitochondrial aldehyde dehydrogenase 2 activity. J. Neurosci. 2008;28:6239–6249. doi: 10.1523/JNEUROSCI.4956-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Opii WO, Nukala VN, Sultana R, Pandya JD, Day KM, Merchant ML, Klein JB, Sullivan PG, Butterfield DA. Proteomic identification of oxidized mitochondrial proteins following experimental traumatic brain injury. J. Neurotrauma. 2007;24(5):772–789. doi: 10.1089/neu.2006.0229. [DOI] [PubMed] [Google Scholar]
- Ortman JM, Velkoff VA, Hogan H. An aging nation: The older population in the United States. U.S. Census Bureau May. 2014 [Google Scholar]
- Pan J, Xiao Q, Sheng CY, Hong Z, Yang HQ, Wang G, Ding JQ, Chen SD. Blockade of the translocation and activation of c-Jun N-terminal kinase 3 (JNK3) attenuates dopaminergic neuronal damage in mouse models of Parkinson’s disease. Neurochem. Int. 2009;54:418–425. doi: 10.1016/j.neuint.2009.01.013. [DOI] [PubMed] [Google Scholar]
- Park SY, Kim J-H, Lee SJ, Kim Y. Surfactin exhibits neuroprotective effects By inhibiting amyloid β-mediated microglial activation. Neurotoxicology. 2013;38:115–123. doi: 10.1016/j.neuro.2013.07.004. [DOI] [PubMed] [Google Scholar]
- Pattaroni C, Jacob C. Histone methylation in the nervous system: functions and dysfunctions. Mol. Neurobiol. 2013;47:740–756. doi: 10.1007/s12035-012-8376-4. [DOI] [PubMed] [Google Scholar]
- Peña-Altamira LE, Polazzi E, Monti B. Histone post-translational modifications in Huntington’s and Parkinson’s diseases. Curr. Pharmac. Design. 2013;19:5085–5092. doi: 10.2174/13816128113199990355. [DOI] [PubMed] [Google Scholar]
- Pickrell AM, Fukui H, Wang X, Pinto M, Moraes CT. The striatum is highly susceptible to mitochondrial oxidative phosphorylation dysfunctions. J. Neurosci. 2011;31:9895–9904. doi: 10.1523/JNEUROSCI.6223-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pickrell AM, Youle RJ. The roles of PINK1, parkin, and mitochondrial fidelity in Parkinson’s disease. Neuron. 2015;85:257–273. doi: 10.1016/j.neuron.2014.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plácido AI, Pereira CM, Duarte AI, Candeias E, Correia SC, Santos RX, Carvalho C, Cardoso S, Oliveira CR, Moreira PI. The role of endoplasmic reticulum in amyloid precursor protein processing and trafficking: implications for Alzheimer’s disease. Biochim. Biophys. Acta. 2014;1842(9):1444–1453. doi: 10.1016/j.bbadis.2014.05.003. [DOI] [PubMed] [Google Scholar]
- Proitsi P, Lupton MK, Velayudhan L, Newhouse S, Fogh I, Tsolaki M, Daniilidou M, Pritchard M, Kloszewska I, Soininen H, Mecocci P, Vellas B, Alzheimer’s Disease Neuroimaging Initiative. Williams J, GERAD1 Consortium. Stewart R, Sham P, Lovestone S, Powell JF. Genetic predisposition to increased blood cholesterol and triglyceride lipid levels and risk of Alzheimer disease: a Mendelian randomization analysis. PLoS Med. 2014;11(9):e1001713. doi: 10.1371/journal.pmed.1001713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puschmann A. Monogenic Parkinson’s disease and parkinsonism: clinical phenotypes and frequencies of known mutations. Parkinson. Related Disorders. 2013;19:407–415. doi: 10.1016/j.parkreldis.2013.01.020. [DOI] [PubMed] [Google Scholar]
- Qi X, Qvit N, Su YS, Mochly-Rosen D. A novel Drp1 inhibitor diminishes aberrant mitochondrial fission and neurotoxicity. J. Cell Sci. 2013;126:789–802. doi: 10.1242/jcs.114439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qu MH, Yang X, Wang Y, Tang Q, Han H, Wang J, Wang GD, Xue C, Gao Z. Docosahexaenoic acid-phosphatidylcholine improves cognitive deficits in an Aβ23-35-induced Alzheimer’s disease rat model. Curr. Top. Med. Chem. 2016;16:558–564. doi: 10.2174/1568026615666150813144437. [DOI] [PubMed] [Google Scholar]
- Quintana FJ, Zaltzman R, Fernandez-Montesinos R, Herrera JL, Gozes I, Cohen IR, Pozo D. NAP, a peptide derived from the activity-dependent neuroprotective protein, modulates macrophage function. Ann. N. Y. Acad. Sci. 2006;1070:500–506. doi: 10.1196/annals.1317.069. [DOI] [PubMed] [Google Scholar]
- Rajan KB, Skarupski KA, Rasmussen HE, Evans DA. Gene-environment Interaction of body mass index and apolipoprotein E ε4 allele on cognitive decline. Alzheimer Dis. Assoc. Dis. 2014;28:134–140. doi: 10.1097/WAD.0000000000000013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rege SD, Kumar S, Wilson DN, Tamura L, Geetha T, Mathews ST, Huggins KW, Broderick TL, J. R. Babu JR. Resveratrol protects the brain of obese mice from oxidative damage. Oxid. Med. Cell. Longev. 2013;2013:419092. doi: 10.1155/2013/419092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren J, Fan C, Chen N, Huang J, Yang Q. Resveratrol pretreatment attenuates cerebral ischemic injury by upregulating expression of transcription factor Nrf2 and HO-1 in rats. Neurochem. Res. 2011;36:2352–2362. doi: 10.1007/s11064-011-0561-8. [DOI] [PubMed] [Google Scholar]
- Rhee SG, Bae YS, Lee SR, Kwon J. Hydrogen peroxide: a key messenger that modulates protein phosphorylation through cysteine oxidation. Sci STKE. 2000;10(53):e1. doi: 10.1126/stke.2000.53.pe1. 2000. [DOI] [PubMed] [Google Scholar]
- Ribas V, García-Ruiz C, Fernández-Checa JC. Glutathione and mitochondria. Front. Pharmacol. 2014;5:151. doi: 10.3389/fphar.2014.00151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodrigo R, Fernández-Gajardo R, Gutiérrez R, Matamala JM, Carrasco R, Miranda-Merchak A, Feuerhake W. Oxidative stress and pathophysiology of ischemic stroke: novel therapeutic opportunities. CNS Neurol. Disord. Drug Targets. 2013;12:698–714. doi: 10.2174/1871527311312050015. [DOI] [PubMed] [Google Scholar]
- Rohrer JD. Structural brain imaging in frontotemporal dementia. Biochim. Biophys. Acta Mol. Basis Dis. 2012;1822:325–332. doi: 10.1016/j.bbadis.2011.07.014. [DOI] [PubMed] [Google Scholar]
- Ron D, Messing RO. Signaling pathways mediating alcohol effects. Curr. Top. Behav. Neurosci. 2013;13:87–126. doi: 10.1007/7854_2011_161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadigh-Eteghad S, Talebi M, Farhoudi M. Association of apolipoprotein E epsilon 4 allele with sporadic late onset Alzheimer`s disease, a meta-analysis. Neurosciences. 2012;17:321–326. [PubMed] [Google Scholar]
- Sanderson TH, Raghunayakula S, Kumar R. Release of mitochondrial Opa1 following oxidative stress in HT22 cells. Mol. Cell Neurosci. 2015a;64:116–122. doi: 10.1016/j.mcn.2014.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanderson TH, Raghunayakula S, Kumar R. Neuronal hypoxia disrupts mitochondrial fusion. Neuroscience. 2015b;301:71–78. doi: 10.1016/j.neuroscience.2015.05.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schedin-Weiss S, Winblad B, Tjernberg LO. The role of protein glycosylation in Alzheimer disease. FEBS J. 2014;281:46–62. doi: 10.1111/febs.12590. [DOI] [PubMed] [Google Scholar]
- Schildknecht S, Gerding HH, Karreman C, Drescher M, Lashuel HA, Outeiro TF, di Monte DA, Leist M. Oxidative and nitrative alpha-synuclein modifications and proteostatic stress: implications for disease mechanisms and interventions in synucleinopathies. J. Neurochem. 2013;125:491–511. doi: 10.1111/jnc.12226. [DOI] [PubMed] [Google Scholar]
- Schmid AW, Fauvet B, Moniatte M, Lashuel HA. Alpha-synuclein post-translational modifications as potential biomarkers for Parkinson disease and other synucleinopathies. Mol. Cell. Proteom. 2013;12:3543–3558. doi: 10.1074/mcp.R113.032730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroeter H, Bahia P, Spencer JPE, et al. (-)Epicatechin stimulates ERK-dependent cyclic AMP response element activity and up-regulates GluR2 in cortical neurons. J. Neurochem. 2007;101:1596–1606. doi: 10.1111/j.1471-4159.2006.04434.x. [DOI] [PubMed] [Google Scholar]
- Schroeter H, Boyd CS, Ahmed R, Spencer JP, Duncan RF, Rice-Evans C, Cadenas E. c-Jun N-terminal kinase (JNK)-mediated modulation of brain mitochondria function: new target proteins for JNK signalling in mitochondrion-dependent apoptosis. Biochem. J. 2003;372:359–369. doi: 10.1042/BJ20030201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scorziello A, Savoia C, Sisalli MJ, Adornetto A, Secondo A, Boscia F, Esposito A, Polishchuk EV, Polishchuk RS, Molinaro P, Carlucci A, Lignitto L, Di Renzo G, Feliciello A, Annunziato L. NCX3 regulates mitochondrial Ca(2+) handling through the AKAP121-anchored signaling complex and prevents hypoxia-induced neuronal death. J. Cell Sci. 2013;126:5566–5577. doi: 10.1242/jcs.129668. [DOI] [PubMed] [Google Scholar]
- Sekigawa A, Takamatsu Y, Sekiyama K, Takenouchi T, Sugama S, Waragai M, Fujita M, Hashimoto H. Diversity of mitochondrial pathology in a mouse model of axonal degeneration in synucleinopathies. Oxid. Med. Cell. Longev. 2013;2013:817807. doi: 10.1155/2013/817807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sha Y, Marshall HE. S-nitrosylation in the regulation of gene transcription. Biochim. Biophys. Acta. 2012;1820:701–711. doi: 10.1016/j.bbagen.2011.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah SA, Yoon GH, Kim MO. Protection of the developing brain with Anthocyanins against ethanol-induced oxidative stress and neurodegeneration. Mol. Neurobiol. 2015;51:1278–1291. doi: 10.1007/s12035-014-8805-7. [DOI] [PubMed] [Google Scholar]
- Shahani N, Sawa A. Protein S-nitrosylation: role for nitric oxide signaling in neuronal death. Biochim. Biophys. Acta. 2012;1820:736–742. doi: 10.1016/j.bbagen.2011.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shahpasandzadeh H, Popova B, Kleinknecht A, Fraser PE, Outeiro TF, Braus GH. Interplay between sumoylation and phosphorylation for protection against α-synuclein inclusions. J. Biol. Chem. 2014;289:31224–31240. doi: 10.1074/jbc.M114.559237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singhal NK, Chauhan AK, Jain SK, Shanker R, Singh C, Singh MP. Silymarin-and melatonin-mediated changes in the expression of selected genes in pesticides-induced Parkinsonism. Mol. Cell Biochem. 2013;384:47–58. doi: 10.1007/s11010-013-1780-x. [DOI] [PubMed] [Google Scholar]
- Slavin JL, Lloyd B. Health benefits of fruits and vegetables. Adv. Nutr. 2012;3(4):506–516. doi: 10.3945/an.112.002154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith RA, Murphy MP. Animal and human studies with the mitochondria-targeted antioxidant MitoQ. Ann. New York Acad. Sci. 2010;1201:96–103. doi: 10.1111/j.1749-6632.2010.05627.x. [DOI] [PubMed] [Google Scholar]
- Son Y, Kim S, Chung HT, Pae HO. Reactive oxygen species in the activation of MAP kinases. Methods Enzymol. 2013;528:27–48. doi: 10.1016/B978-0-12-405881-1.00002-1. [DOI] [PubMed] [Google Scholar]
- Son Y, Cheong YK, Kim NH, Chung HT, Kang DG, Pae HO. Mitogen-activated protein kinases and reactive oxygen species: How can ROS activate MAPK pathways? J. Signal Transd. 2011;2011:792639. doi: 10.1155/2011/792639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song BJ, Abdelmegeed MA, Henderson LE, Yoo SH, Wan J, Purohit V, Hardwick JP, Moon KH. Increased nitroxidative stress promotes mitochondrial dysfunction in alcoholic and nonalcoholic fatty liver disease. Oxid. Med. Cell. Longev. 2013;2013:781050. doi: 10.1155/2013/781050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song BJ, Abdelmegeed MA, Yoo SH, Kim BJ, Jo SA, Jo I, Moon KH. Post-translational modifications of mitochondrial aldehyde dehydrogenase and biomedical implications. J Proteomics. 2011;74:2691–2702. doi: 10.1016/j.jprot.2011.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song BJ, Akbar M, Abdelmegeed MA, Byun K, Lee B, S. K. Yoon SK, Hardwick HP. Mitochondrial dysfunction and tissue injury by alcohol, high fat, nonalcoholic substances and pathological conditions through post-translational protein modifications. Redox Biol. 2014;3:109–123. doi: 10.1016/j.redox.2014.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song BJ, Akbar M, Jo I, Hardwick JP, Abdelmegeed MA. Translational Implications of the alcohol-metabolizing enzymes, including cytochrome P450-2E1, in alcoholic and nonalcoholic liver disease. Adv. Pharmacol. 2015;74:303–372. doi: 10.1016/bs.apha.2015.04.002. [DOI] [PubMed] [Google Scholar]
- Song BJ, Suh SK, Moon KH. A simple method to systematically study oxidatively modified proteins in biological samples and its applications. Methods Enzymol. 2010;473:251–264. doi: 10.1016/S0076-6879(10)73013-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soustiel JF, Sviri GE. Monitoring of cerebral metabolism: non-ischemic impairment of oxidative metabolism following severe traumatic brain injury. Neurol. Res. 2007;29:654–660. doi: 10.1179/016164107X240017. [DOI] [PubMed] [Google Scholar]
- Spruss A, Kanuri G, Uebel K, Bischoff SC, Bergheim I. Role of the inducible nitric oxide synthase in the onset of fructose-induced steatosis in mice. Antioxid. Redox Signal. 2011;14:2121–2135. doi: 10.1089/ars.2010.3263. [DOI] [PubMed] [Google Scholar]
- Subash S, Braidy N, Essa MM, Zayana AB, Ragini V, Al-Adawi S, Al-Asmi A, Guillemin GJ. Long-term (15 mo) dietary supplementation with pomegranates from Oman attenuates cognitive and behavioral deficits in a transgenic mice model of Alzheimer’s disease. Nutrition. 2015;31:223–229. doi: 10.1016/j.nut.2014.06.004. [DOI] [PubMed] [Google Scholar]
- Subash S, Essa MM, Al-Adawi S, Memon MA, Manivasagam T, Akbar M. Neuroprotective effects of berry fruits on neurodegenerative diseases. Neural Regen. Res. 2014a;9:1557–1566. doi: 10.4103/1673-5374.139483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subash S, Essa MM, Al-Asmi A, Al-Adawi S, Vaishnav R. Chronic dietary supplementation of 4% figs on the modification of oxidative stress in Alzheimer’s disease transgenic mouse model. Biomed. Res. Int. 2014b;2014:546357. doi: 10.1155/2014/546357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subramaniam S, Unsicker K. ERK and cell death: ERK1/2 in neuronal death. FEBS J. 2010;277:22–29. doi: 10.1111/j.1742-4658.2009.07367.x. [DOI] [PubMed] [Google Scholar]
- Sultana R, Perluigi M, Butterfield DA. Lipid peroxidation triggers neurodegeneration: a redox proteomics view into the Alzheimer disease brain. Free Radic. Biol. Med. 2013;62:157–169. doi: 10.1016/j.freeradbiomed.2012.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sultana R, Butterfield DA. Oxidative modification of brain proteins in lzheimer’s disease: perspective on future studies based on results of redox proteomics studies. J Alzheimers Dis. 2013;(33 Suppl 1):S243–S251. doi: 10.3233/JAD-2012-129018. [DOI] [PubMed] [Google Scholar]
- Sun KH, Lee HG, Smith MA, Shah K. Direct and indirect roles of cyclin-dependent kinase 5 as an upstream regulator in the c-Jun NH2-terminal kinase cascade: relevance to neurotoxic insults in Alzheimer’s disease. Mol. Biol Cell. 2009;20:4611–4619. doi: 10.1091/mbc.E09-05-0433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun J, Li YZ, Ding YH, Wang J, Geng J, Yang H, Ren J, Tang JY, Gao J. Neuroprotective effects of gallic acid against hypoxia/reoxygenation-induced mitochondrial dysfunctions in vitro and cerebral ischemia/reperfusion injury in vivo. Brain Res. 2014;1589:126–139. doi: 10.1016/j.brainres.2014.09.039. [DOI] [PubMed] [Google Scholar]
- Syntichaki P, Xu K, Driscoll M, Tavernaraki N. Specific aspartyl and calpain proteases are required for neurodegeneration in C. elegans. Nature. 2002;419(6910):939–944. doi: 10.1038/nature01108. [DOI] [PubMed] [Google Scholar]
- Tajuddin NF, Przybycien-Szymanska MM, Pak TR, Neafsey EJ, Collins MA. Effect of repetitive daily ethanol intoxication on adult rat brain: significant changes in phospholipase A2 enzyme levels in association with increased PARP-1 indicate neuroinflammatory pathway activation. Alcohol. 2013;47:39–45. doi: 10.1016/j.alcohol.2012.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan EP, Villar MT, Lezi E, Lu J, Selfridge JE, Artigues A, Swerdlow RH, Slawson C. Altering O-linked β-N-acetylglucosamine cycling disrupts mitochondrial function. J. Biol. Chem. 2014;289:14719–14730. doi: 10.1074/jbc.M113.525790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka A, Cleland MM, Xu S, Narendra DP, Suen DF, Karbowski M, Youle RJ. Proteasome and p97 mediate mitophagy and degradation of mitofusins induced by Parkin. J. Cell Biol. 2010;191:1367–1380. doi: 10.1083/jcb.201007013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tapias V, Cannon JR, Greenamyre JT. Pomegranate juice exacerbates oxidative stress and nigrostriatal degeneration in Parkinson’s disease. Neurobiol. Aging. 2014;5:1162–1176. doi: 10.1016/j.neurobiolaging.2013.10.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tepper K, Biernat J, Kumar S, Wegmann S, Timm T, Hübschmann S, Redecke L, Mandelkow EM, Müller DJ, Mandelkow E. Oligomer formation of tau protein hyperphosphorylated in cells. J. Biol. Chem. 2014;289:34389–34407. doi: 10.1074/jbc.M114.611368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tolkovsky AM. Mitophagy. Biochim. Biophys. Acta. 2009;1793:1508–1515. doi: 10.1016/j.bbamcr.2009.03.002. [DOI] [PubMed] [Google Scholar]
- Tradewell ML, Yu Z, Tibshirani M, Boulanger MC, Durham HD, Richard SS. Arginine methylation by PRMT1 regulates nuclear-cytoplasmic localization and toxicity of FUS/TLS harbouring ALS-linked mutations. Human Mol. Genet. 2012;21:136–149. doi: 10.1093/hmg/ddr448. [DOI] [PubMed] [Google Scholar]
- Twig G, Elorza E, Molina AJ, Mohamed H, Wikstrom JD, Walzer G, Stiles L, Haigh SE, Katz S, Las G, Alroy J, Wu M, Py BF, Yuan J, Deeney JT, Corkey BE, Shirihai OS. Fission and selective fusion govern mitochondrial segregation and elimination by autophagy. EMBO. J. 2008;27:433–446. doi: 10.1038/sj.emboj.7601963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Upreti VV, Moon KH, Yu LR, Lee IJ, Eddington ND, Ye X, Veenstra TD, Song BJ. Increased oxidative-modifications of cytosolic proteins in 3,4 methylenedioxymethamphetamine (MDMA, ecstasy)-exposed rat liver. Proteomics. 2011;11(2):202–211. doi: 10.1002/pmic.201000203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vauzour D. Dietary polyphenols and modulation of brain functions: Biological actions and molecular mechanisms underpinning their beneficial effects. Oxid. Med. Cell. Longev. 2012;2012:914273. doi: 10.1155/2012/914273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vepsäläinen S, Koivisto H, Pekkarinen E, Mäkinen P, Dobson G, McDougall GJ, Stewart D, Haapasalo A, Karjalainen RO, Tanila H, Hiltunen M. Anthocyanin-enriched bilberry and blackcurrant extracts modulate amyloid precursor protein processing and alleviate behavioral abnormalities in the APP/PS1 mouse model of Alzheimer’s disease. J. Nutr. Biochem. 2013;24:360–370. doi: 10.1016/j.jnutbio.2012.07.006. [DOI] [PubMed] [Google Scholar]
- Verma R, Nehru B. Effect of centrophenoxine against rotenone-induced oxidative stress in an animal model of Parkinson’s disease. Neurochem. Int. 2009;55:369–375. doi: 10.1016/j.neuint.2009.04.001. [DOI] [PubMed] [Google Scholar]
- Verweij BH, Muizelaar JP, Vinas FC, Peterson PL, Xiong Y, Lee CP. Mitochondrial dysfunction after experimental and human brain injury and its possible reversal with a selective N-type calcium channel antagonist (SNX-111) Neurol. Res. 1997;19:334–339. doi: 10.1080/01616412.1997.11740821. [DOI] [PubMed] [Google Scholar]
- Vespa P, Bergsneider M, Hattori N, Wu HM, Huang SC, Martin NA, Glenn TC, McArthur DL, Hovda DA. Metabolic crisis without brain ischemia is common after traumatic brain injury: a combined microdialysis and positron emission tomography study. J. Cereb. Blood Flow Metab. 2005;25:763–774. doi: 10.1038/sj.jcbfm.9600073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vespa PM, McArthur D, O’Phelan K, Glenn T, Etchepare M, Kelly D, Bergsneider M, Martin NA, Hovda DA. Persistently low extracellular glucose correlates with poor outcome 6 months after human traumatic brain injury despite a lack of increased lactate: a microdialysis study. J. Cereb. Blood Flow Metab. 2003;23:865–877. doi: 10.1097/01.WCB.0000076701.45782.EF. [DOI] [PubMed] [Google Scholar]
- Viña J, Lloret A. Why women have more Alzheimer’s disease than men: gender and mitochondrial toxicity of amyloid-beta peptide. J. Alzheimers Dis. 2010;20(Suppl. 2):S527–S533. doi: 10.3233/JAD-2010-100501. [DOI] [PubMed] [Google Scholar]
- Wagner GR, Pride PM, Babbey CM, Payne RM. Friedreich’s ataxia reveals a mechanism for coordinate regulation of oxidative metabolism via feedback inhibition of the SIRT3 deacetylase. Human Mol. Genet. 2012;21:2688–2697. doi: 10.1093/hmg/dds095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wali M, Kharbanda KK, Deth RC. Ethanol lowers glutathione in rat liver and brain and inhibits methionine synthase in a cobalamin-dependent manner. Alcoholism: Clin. Exp. Res. 2011;35:277–283. doi: 10.1111/j.1530-0277.2010.01343.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker AK, Farg MA, Bye CR, McLean CA, Horne MK, Atkin JD. Protein disulphide isomerase protects against protein aggregation and is S-nitrosylated in amyotrophic lateral sclerosis. Brain. 2010;133:105–116. doi: 10.1093/brain/awp267. [DOI] [PubMed] [Google Scholar]
- Wang D, Liu L, Zhu X, Wu W, Wang Y. Hesperidin alleviates cognitive impairment, mitochondrial dysfunction and oxidative stress in a mouse model of Alzheimer’s disease. Cell. Mol. Neurobiol. 2014;34:1209–1221. doi: 10.1007/s10571-014-0098-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang JZ, Xia YY, Grundke-Iqbal I, Iqbal K. Abnormal hyperphosphorylation of tau: sites, regulation, and molecular mechanism of neurofibrillary degeneration. J. Alzheimers Dis. 2013;33:S123–S139. doi: 10.3233/JAD-2012-129031. [DOI] [PubMed] [Google Scholar]
- Wang W, Shi l, Xie Y, Ma C, Li W, Su X, Huang S, Chen R, Zhu Z, Mao Z, Han Y, Li M. SP600125, a new inhibitor, protects dopaminergic neurons in the MPTP model of Parkinson’s disease. Neurosci. Res. 2004;48:195–202. doi: 10.1016/j.neures.2003.10.012. [DOI] [PubMed] [Google Scholar]
- Wang W, Lin LL, Guo JM, Cheng YQ, Qian J, Mehta JL, Su DF, Luan P, Liu AJ. Heavy ethanol consumption aggravates the ischemic cerebral injury by inhibiting ALDH2. Int. J. Stroke. 2015 Jul 14; doi: 10.1111/ijs.12560. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- Wang W, Winter D, Ashrafi G, Schlehe J, Wong YL, Selkoe D, Rice S, Steen J, LaVoie MJ, Schwarz TL. PINK1 and Parkin target Miro for phosphorylation and degradation to arrest mitochondrial motility. Cell. 2011;147:893–906. doi: 10.1016/j.cell.2011.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberg F, Chandel NS. Mitochondrial metabolism and cancer. Ann. New York Acad. Sci. 2009;1177:66–73. doi: 10.1111/j.1749-6632.2009.05039.x. [DOI] [PubMed] [Google Scholar]
- Weinreb O, Amit T, Bar-Am O, Youdim MB. Ladostigil: a novel multimodal neuroprotective drug with cholinesterase and brain-selective monoamine oxidase inhibitory activities for Alzheimer’s disease treatment. Curr. Drug Targets. 2012;13:483–494. doi: 10.2174/138945012799499794. [DOI] [PubMed] [Google Scholar]
- Wey MC, Fernandez E, Martinez PA, Sullivan P, Goldstein DS, Strong R. Neurodegeneration and motor dysfunction in mice lacking cytosolic and mitochondrial aldehyde dehydrogenases: implications for Parkinson’s disease. PLoS One. 2012;7:e31522. doi: 10.1371/journal.pone.0031522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Win S, Than TA, Fernandez-Checa JC, Kaplowitz N. JNK interaction with Sab mediates ER stress induced inhibition of mitochondrial respiration and cell death. Cell Death Dis. 2014;5:e989. doi: 10.1038/cddis.2013.522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Win S, Than TA, Han D, Petrovic LM, Kaplowitz N. c-Jun N-terminal Kinase (JNK)-dependent acute liver injury from acetaminophen or tumor necrosis factor (TNF) requires mitochondrial Sab protein expression in mice. J. Biol. Chem. 2011;286:35071–35078. doi: 10.1074/jbc.M111.276089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winklhofer KF. Parkin and mitochondrial quality control: toward assembling the puzzle. Trends Cell Biol. 2014;24:332–341. doi: 10.1016/j.tcb.2014.01.001. [DOI] [PubMed] [Google Scholar]
- Winterbourn C. Reconciling the chemistry and biology of reactive oxygen species. Nat. Chem. Biol. 2008;4:278–286. doi: 10.1038/nchembio.85. [DOI] [PubMed] [Google Scholar]
- Wirth M, Villeneuve S, La Joie R, Marks SM, Jagust WJ. Gene-environment interactions: lifetime cognitive activity, APOE genotype, and β-amyloid burden. J. Neurosci. 2014;34:8612–8617. doi: 10.1523/JNEUROSCI.4612-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu W, Xu H, Wang Z, Mao Y, Yuan L, Luo W, Cui Z, Cui T, Wang XL, Shen YH. PINK1-Parkin-mediated mitophagy protects mitochondrial integrity and prevents metabolic stress-induced endothelial injury. PLoS One. 2015;10:e0132499. doi: 10.1371/journal.pone.0132499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie Y, McGill MR, Dorko K, Kumer SC, Schmitt TM, Forster J, Jaeschke H. Mechanisms of acetaminophen-induced cell death in primary human hepatocytes. Toxicol. Appl. Pharmacol. 2014;279(3):266–274. doi: 10.1016/j.taap.2014.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu K, Puchowicz MA, Sun X. Mitochondrial dysfunction in aging rat brain following transient global ischemia. Adv. Exp. Med. Biol. 2008;614:379–386. doi: 10.1007/978-0-387-74911-2_42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang S, Zhou G, Liu H, Zhang B, Li J, Cui R, Du Y. Protective effects of p38 MAPK inhibitor SB202190 against hippocampal apoptosis and spatial learning and memory deficits in a rat model of vascular dementia. Biomed. Res. Int. 2013;2013:215798. doi: 10.1155/2013/215798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yarza R, Vela S, Solas M, Ramirez MJ. c-Jun N-terminal Kinase (JNK) Signaling as a Therapeutic Target for Alzheimer’s Disease. Front. Pharmacol. 2016;6:321. doi: 10.3389/fphar.2015.00321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin F, Jiang T, Cadenas E. Metabolic triad in brain aging: mitochondria, insulin/IGF-1 signalling and JNK signaling. Biochem. Soc. Transact. 2013;41:101–105. doi: 10.1042/BST20120260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin L, Xie Y, Yin S, Lv X, Zhang J, Gu Z, Sun H, Liu S. The S-nitrosylation status of PCNA localized in cytosol impacts the apoptotic pathway in a Parkinson’s disease paradigm. PLoS One. 2015;10(2):e0117546. doi: 10.1371/journal.pone.0117546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang F, Chen L, Liu C, Qiu P, Wang A, Li L, Wang H. Up-regulation of protein tyrosine nitration in methamphetamine-induced neurotoxicity through DDAH/ADMA/NOS pathway. Neurochem. Int. 2013;62:1055–1064. doi: 10.1016/j.neuint.2013.03.016. [DOI] [PubMed] [Google Scholar]
- Zhang HY. New insights into huperzine A for the treatment of Alzheimer’s disease. Acta Pharmacol. Sinica. 2012;33:1170–1175. doi: 10.1038/aps.2012.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Dawson VL, Dawson TM, Snyder SH. Nitric oxide activation of poly(ADPribose) synthetase in neurotoxicity. Science. 1994;263:687–689. doi: 10.1126/science.8080500. [DOI] [PubMed] [Google Scholar]
- Zhang J, Ney PA. Reticulocyte mitophagy: monitoring mitochondrial clearance in a mammalian model. Autophagy. 2010;6:405–408. doi: 10.4161/auto.6.3.11245. [DOI] [PubMed] [Google Scholar]
- Zhu Y, Shan X, Yuzwa SA, Vocadlo DJ. The Emerging Link between O-GlcNAc and Alzheimer Disease. J. Biol. Chem. 2014;289:34472–34481. doi: 10.1074/jbc.R114.601351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Z, Li R, Stricker R, Reiser G. Extracellular α-crystallin protects astrocytes from cell death through activation of MAPK, PI3K/Akt signaling pathway and blockade of ROS release from mitochondria. Brain Res. 2015;1620:17–28. doi: 10.1016/j.brainres.2015.05.011. [DOI] [PubMed] [Google Scholar]