Abstract
Background/Objective
Puberty is a period defined by large changes in adipose tissue accumulation and distribution, however longitudinal patterns of ectopic fat development have not been shown. We have previously shown significant declines in beta-cell function (BCF) across puberty and hypothesize that accumulation of ectopic fat deposition, particularly hepatic fat, will predict this fall.
Subject/Methods
We conducted a longitudinal study and examined 2-year change in abdominal fat distribution and type 2 diabetes risk markers in 76 Hispanic children and young adults (16.1 ±0.5 years, 66% obese, 52% male, 51% post-pubertal). Subcutaneous abdominal adipose tissue (SAAT), visceral adipose tissue (VAT), hepatic fat fraction (HFF) and pancreatic fat fraction (PFF) were measured by 3-Tesla MRI, and markers of type 2 diabetes risk were collected at fasting and during an oral glucose tolerance test (OGTT).
Results
Baseline pubertal status significantly moderated 2-year change in ectopic fat deposition, such that VAT, HFF and PFF increased in individuals during late and post-pubertal growth whereas children earlier in their pubertal development decreased ectopic accumulation and had less VAT accumulation (VAT: pTanner*time =0.044, 0.31±0.08L vs. 0.03±0.10L; HFF: pTanner*time=0.007, 1.34±0.87% vs. −2.61±1.11%; PFF: pTanner*time<0.001, 1.61±0.39% vs. −0.96±0.50%). Independent of pubertal status, two-year increase in HFF and VAT significantly associated with a decline in BCF (β=−1.04, p=0.038; β=−1.81, p=0.020) and metabolic function, while accumulation of SAAT significantly associated with BCF (β=1.36, p=0.012) and metabolic improvement. HFF accumulation was the only depot to significantly predict clinical markers of type 2 diabetes risk, fasting glucose and HbA1c, and circulating free fatty acid levels (β=1.00, p=0.034; β=1.00, p=0.015; β=01.01, p=0.024).
Conclusions
The accumulation of SAAT defends against type 2 diabetes risk and potentially ectopic fat accumulation. Intra-abdominal VAT and HFF accumulation both associate with metabolic decline and BCF, while HFF predicts an even greater number of metabolic risk features.
Introduction
Adipose tissue expansion is a normal part of childhood development, and involves a period of accelerated growth during puberty 1,2. This pubertal growth is defined by changes in regional body fat distribution and body composition, with changes in subcutaneous fat depots well characterized 3–5. However there is limited data on the development of the specific intra-abdominal depots and liver fat that are most closely tied with type 2 diabetes and metabolic dysfunction 6–12. The impact of these pubertal changes in body fat distribution are important for understanding the pathophysiology leading to development of type 2 diabetes during puberty.
The development of type 2 diabetes arises from the progression of insulin resistance and the subsequent inability of beta-cells to adequately compensate through increased insulin secretion 13. We have previously shown that beta-cell compensation declines across puberty 10,14. Although the exact factors contributing to this decline are unknown, we hypothesize that pubertal increases in intra-abdominal fat and/or liver fat might contribute to these findings. In this regard, it has also been hypothesized that increases in visceral and ectopic fat stem from an inability of subcutaneous fat to increase in size and thus contributes to spillover of triglycerides to other undesirable sites for storage, such as the visceral depot, or accumulation in non-adipose tissue sites such as the liver or pancreas 15,16. However, there is a lack of human data to support this concept. We have previously shown that intra-abdominal fat associates with BCF decline 11, but have not been able to investigate longitudinal effects of liver or pancreatic accumulation before this analysis. Furthermore, there is mixed evidence on which intra-abdominal depot is central to metabolic dysfunction, with recent cross-sectional studies supporting hepatic over visceral deposition 17–21. Longitudinal studies examining simultaneous changes in multiple fat depots and risk for diabetes are needed in order to confirm that hepatic deposition is primary in metabolic decline.
Therefore, the primary purpose of this paper is to investigate the patterns of 2-year change in abdominal fat accumulation in a cohort of Hispanic children and young adults, and to test how change in specific depots predicts change in metabolic outcomes. First, we hypothesized that there would be “saturation” of subcutaneous adipose tissue storage capacity during pubertal growth, driving deposition of fat into ectopic depots, and that this will be moderated by baseline pubertal or obesity status. Second, we hypothesized that change in ectopic fat, primarily hepatic, would predict 2-year change in metabolic parameters, confirming the primary role of liver fat deposition in the pathophysiology of obesity.
Materials and Methods
Participants
Data were collected from the longitudinal cohort project, Study of Latino Adolescents at Risk (SOLAR) conducted at the University of Southern California (USC) and some data from this study has been previously reported 11,22,23. Participants for the current analysis included a subset of 76 patients who had complete measures of subcutaneous abdominal adipose tissue (SAAT), visceral adipose tissue (VAT), hepatic fat fraction (HFF) and pancreatic fat fraction (PFF) for 2 consecutive visits that were on average 2 years apart (range 1.40–2.54yrs, median 1.97yrs). The 76 participants were all of Hispanic descent, (defined by parental/self-report of Hispanic descent for self, parents, and grandparents), and included normal weight, overweight and obese (11/15/50, respective frequency- categorized by body mass index) male and female participants, ranging in age from 10–23 years. This is the first analysis for this study to examine 2-year change in abdominal fat depots SAAT, VAT, HFF and PFF and their predictive effect on metabolic outcomes. Prior to testing, informed written consent/assent was obtained from the participant and parents, and the USC Institutional Review Board approved this study.
Adiposity and Metabolic Measures
At each of the two study visits, the following measures were collected. Height and weight were collected in duplicate and obesity status using by body mass index (BMI) was determined based on the age of the participant. Participants 8–18 years of age were categorized based on BMI percentile for age and sex according to the Centers for Disease Control and Prevention (CDC) guidelines. Categories included normal weight (≥5–85th percentile), overweight (≥85–95th percentile) and obese (≥95th percentile). For participants >18 years of age, adult BMI categories were used. Total body fat mass (TFM) was assessed by dual-energy X-ray absorptiometry (Hologic QDR 4500W; Hologic, Bedord, MA). All participants underwent multibreath-hold, 3-D whole-abdomen magnetic resonance imaging (MRI) scanning on a General Electric 3-Tesla magnet (Excite HD; GE Healthcare, Waukesha, Wisconsin) equipped with an 8-channel abdominal coil array to measure abdominal fat distribution and ectopic fat accumulation as previously described12. The MRI pulse sequence consisted of a chemical-shift technique known as IDEAL (GE Healthcare), and generated separate water and fat datasets of the anatomy on a voxel-wise basis. After MRI data acquisition, SAAT and VAT quantity (measured in liters), and HFF and PFF percentage (quantified as the percentage of fat found within the organ tissue) were measured by a single analyst using the commercial semiautomated software tool SliceOmatic (TomoVision, Inc., Montreal, Québec, Canada). This approach has been described in detail in literature and has been validated against other modalities 24–27.
A full medical history and physical exam where pubertal stage was determined 28 was conducted at the Clinical Trials Unit (CTU) at the USC University Hospital as previously reported 22. Blood was collected at fasting and 15, 30, 45, 60, and 120 minutes during an oral glucose tolerance test (OGTT) (1.75 g oral glucose solution/kg body weight, maximum 75 g). Glucose was assayed using a Yellow Springs Instruments analyzer (YSI Inc., Yellow Springs, Ohio) using the glucose oxidase method. Insulin was assayed using an ELISA immunoassay (EMD Millipore, Billercia, MA: intra-assay coefficient of variation 4.6–7.6% and inter-assay coefficient of variation 9.1–11.4%). An in vitro enzymatic colormetric method assay was used for the quantitative determination of FFA (Wako NEFA-HR (2) series Wako Diagnostics, USA: minimum detectable level ~0.0014 mmol/L). Glucose, insulin, and FFA values from the 2-hour OGTT were used to calculate glucose, insulin, and FFA area under the curve (AUC), in addition to the Insulin Sensitivity Index (ISI, aka the Matsuda Index), the Insulinogenic Index (IGI) (indicator of insulin secretion), and an indicator of beta-cell function (BCF; product of the ISI*IGI), with formulas adjusted for 15, 30, 45, 60 and 120 minute OGTT collection 29,30.
Statistical Analysis
Examination of changes in abdominal fat depots over 2-year period and interaction with baseline obesity status and Tanner stage
Linear mixed effects regression models, using repeated measures, were used to analyze the changes in abdominal fat depots across a 2-year period. The repeated measurements of abdominal depots, SAAT, VAT, HFF and PFF, were tested for change over time and if that level of change over time was influenced by baseline pubertal stage and/or obesity status by examination of interaction terms (Tanner*time, obesity*time). Baseline pubertal stage was categorized at baseline as either early (Tanner Stages 2–3) or advanced sexual maturity (Tanners 4–5). This split was based on our previous findings that beta-cell compensation changed after Tanner stage 3 14. Baseline weight status was categorized as normal, overweight or obese at baseline using BMI categories. In addition, the role of sex was examined as a covariate and if sex influenced how the depots changed over time (time*sex).
Changes in abdominal depots influence on metabolic parameters
Linear mixed effects regression models, using repeated measures, were also used to analyze how the baseline and changes in abdominal fat depots associated with metabolic parameters. In all models, the repeated measurements of metabolic parameters (fasting glucose, insulin and FFA; hemoglobin A1c (HbA1c); 2-hr OGTT glucose, insulin and FFA AUC; 2-hr OGTT glucose; ISI, IGI and BCF) were the dependent variable and the abdominal fat depots were the primary predictors. A priori covariates included baseline pubertal stage, age, sex, TFM and change in TFM. TFM, SAAT, VAT, HFF, and PFF were treated as time-varying covariates and were modeled as baseline (TFMBaseline, SAATBaseline, VATBaseline, HFFBaseline, PFFBaseline,) and change-from-baseline (ΔTFM, ΔSAAT, ΔVAT, ΔHFF, ΔPFF). All abdominal depots were tested in a single model to examine which best predicted change in the metabolic parameters. The baseline estimate represents a standard cross-sectional estimate of the association between x and y at baseline. Change-from-baseline is a longitudinal estimate that is not confounded by genetic make-up or other unmeasured time-invariant covariates, and is interpreted as the association between a change in x from baseline and the concomitant change in y over the same period 31. Metabolic parameters were natural log transformed to meet model assumptions and back-transformed βs are reported. Since all abdominal fat depot and FFA variables were examined as continuous variables, when significant change-from-baseline relationships were found, post-hoc comparisons across time at high (+1SD) levels of fat depot or FFA change were performed in order to display the effect on metabolic parameters (% relative change).
All models were run using proc mixed, repeated measures in SAS 9.3. Since there were only 2 repeated measures (2 time points of MRI and metabolic measurement), the models were linear and a compound symmetry covariance structure was used. Statistical significance was set to P<0.05, and descriptive data are reported as mean ± standard error of the mean (SEM).
Results
The baseline characteristics of the 76 participants are reported in Table 1. Sixty-six percent of the participants were obese and 52% were male. The average age at baseline was 16.1 ±0.5 years and 51% of participants were post-pubertal (Tanner stage 5). On average the SAAT depot made up approximately 20% of the fat mass in our population ([6.1L(SAAT)*0.905kg/L (density of adipose tissue32)]/27.7kg(TFM)).
Table 1.
Baseline Characteristics (n=76)
| Variable | Frequency |
|---|---|
| Obesity status (normal/overweight/obese) | (11/15/50) |
| Sex (M/F) | (40/36) |
| Tanner stage (2/3/4/5) | (34/5/7/40) |
|
| |
| Mean ± SEM | |
|
| |
| Age (yrs) | 16.1 ± 0.5 |
| Height (cm) | 160.3 ± 1.5 |
| Weight (kg) | 79.6 ± 2.8 |
| BMI (kg/m2) | 30.5 ± 0.8 |
| BMI%ile* | 93.6 ± 1.2 |
| Total fat mass (kg) | 27.7 ± 1.3 |
| SAT (L) | 6.1 ± 0.4 |
| VAT (L) | 1.6 ± 0.1 |
| HFF (%) | 6.5 ± 0.7 |
| PFF (%) | 4.6 ± 0.3 |
| Fasting glucose (mg/dL) | 90.2 ± 0.8 |
| 2-h glucose (mg/dL) | 121.2 ± 2.3 |
| Glucose AUCOGTT (mg/dL x min) | 260.3 ± 3.5 |
| HbA1c (%) | 5.5 ± 0.0 |
| Fasting Insulin (μU/mL) | 8.3 ± 0.6 |
| Insulin AUCOGTT (μU/mL x min) | 234.0 ± 17.8 |
| Mastuda Index (ISI) | 4.1 ± 0.3 |
| Insulinogenic Index (IGI) | 3.1 ± 0.3 |
| Disposition Index (ISI* IGI) | 10.6 ± 1.0 |
| Fasting FFA (mmol/L) | 0.62 ± 0.02 |
| FFA AUCOGTT (mmol/L x min) | 0.66 ± 0.02 |
For participants ≤18 yrs.
BMI= body mass index, SAT= subcutaneous abdominal adipose tissue, VAT= visceral adipose tissue, HFF= hepatic fat fraction, PFF= pancreatic fat fraction, AUC= area under the curve, OGTT= oral glucose tolerance test, ISI= insulin sensitivity index, IGI= insulinogenic index, FFA= free fatty acid
Patterns of two-year change in abdominal fat depots
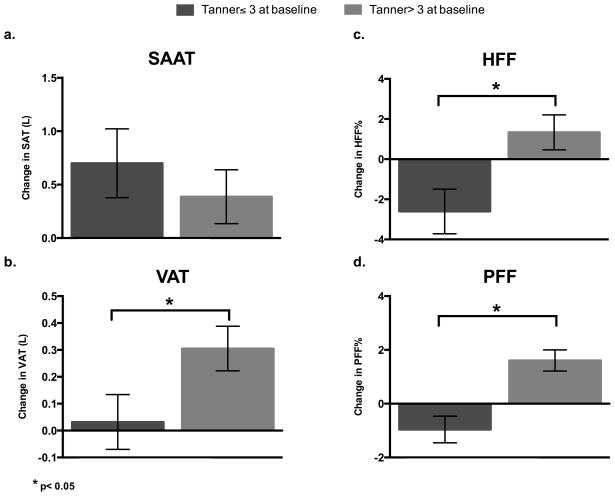
For the group as a whole, SAAT (+0.60±0.35 L) and VAT (+0.20±0.11 L) significantly increased over the 2-year period (p=0.004, p=0.010, respectively). However, as shown in Figure 1, there was a significantly greater increase in VAT in those with a baseline Tanner stage >3 (pTanner*time = 0.044, 0.31±0.08L vs. 0.03±0.10L). There were no significant time-related changes in HFF or PFF, yet, there was a pattern of change that significantly differed by baseline pubertal status and time (pTanner*time = 0.007 for HFF, pTanner*time < 0.001 for PFF; Figure 1). Specifically, there was a decrease in HFF (−2.61±1.11%) and PFF (−0.96±0.50%) in those with baseline Tanner stage ≤3, relative to an increase in HFF (1.34±0.87%) and PFF (1.61±0.39%) in those with more advanced pubertal stage (baseline Tanner stage>3). Adjusting for time between scans and sex did not alter these findings (data not shown), however these findings were not independent of age. Although controlling for sex did not change any of these findings, there was an interaction with time in the prediction of SAAT. Specifically, in girls there was a significantly greater increase in SAAT over the 2-year period relative to boys (psex*time =0.049; 0.98±0.29L vs. 0.17±0.28L;).
Figure 1. Two-year change in abdominal fat depots by Tanner stage.
a. SAAT accumulation does not significantly differ by Tanner stage b. There is a significant difference in VAT change by baseline Tanner stage, with a greater increase in participants with Tanner>3 (ptanner*time = 0.044) c. There is a significant difference in HFF change by baseline Tanner stage, with a decrease in HFF in participants with Tanner ≤3 and an increase in participants with Tanner>3 (ptanner*time = 0.007) d. There is a significant difference in PFF change by baseline Tanner stage, with a decrease in PFF in participants with Tanner≤3 and an increase in participants with Tanner>3 (ptanner*time < 0.001). SAAT= subcutaneous abdominal adipose tissue, VAT= visceral adipose tissue, HFF= hepatic fat fraction, PFF= pancreatic fat fraction
Associations between abdominal fat depots at baseline and type 2 diabetes risk factors
Glucose, Insulin and FFA
VAT and HFF at baseline were the only fat depots to significantly associate with any glucose measure. VATBaseline and HFFBaseline were both significantly associated with higher glucose AUC (β= 1.09, p=0.022; β= 1.01, p=0.004); however only VATBaseline was positively associated with fasting glucose (β= 1.05, p=0.019) and only HFFBaseline was positively associated with HbA1c (β= 1.00, p=0.045). Furthermore, HFFBaseline and PFFBaseline positively associated with FFA AUC (β= 1.01, p=0.036; β= 1.03, p=0.034). SAAT was the only depot at baseline to significantly associate with any insulin measure, such that SAATBaseline was significantly associated with higher insulin AUC (β= 1.18, p=0.001). However PFFBaseline was associated with a trend of higher fasting insulin (β= 1.06, p=0.093). No baseline depot significantly associated with 2-hr OGTT glucose or fasting FFA.
Insulin sensitivity, secretion, and beta-cell function
Higher SAATBaseline was significantly associated with lower insulin sensitivity (ISI- β= −1.11, p=0.027), but was positively associated with insulin secretion (IGI- β= 1.19, p=0.003). HFFBaseline was the only baseline value to significantly associate with BCF, such that higher baseline HFF associated with lower BCF (β= −1.04, p=0.043), and HFFBaseline was associated with a trend for lower insulin sensitivity (β= −1.03, p=0.054).
Associations between two-year changes in abdominal fat depots and type 2 diabetes risk factors (Table 2 and Figure 2)
Table 2.
Significant associations between changes in each fat depot and risk factors for type 2 diabetes
| Δ SAAT | Δ VAT | Δ HFF | Δ PFF | |||||
|---|---|---|---|---|---|---|---|---|
| beta | p-value | beta | p-value | beta | p-value | beta | p-value | |
| Fasting glucose | −1.00 | NS | 1.05 | 0.082 | 1.00 | 0.034 | 1.00 | NS |
| Glucose AUC | −1.09 | 0.002 | 1.15 | 0.015 | 1.01 | 0.001 | 1.00 | NS |
| HbA1c | −1.01 | 0.068 | 1.01 | NS | 1.00 | 0.015 | 1.00 | NS |
| Fasting insulin | 1.18 | 0.087 | −1.11 | NS | 1.00 | NS | 1.06 | 0.078 |
| Insulin AUC | 1.03 | NS | 1.00 | NS | 1.02 | NS | 1.03 | NS |
| FFA AUC | −1.06 | NS | 1.08 | NS | 1.01 | 0.024 | 1.02 | 0.078 |
| IGI | 1.37 | 0.002 | −1.74 | 0.007 | −1.02 | NS | −1.02 | NS |
| ISI | −1.01 | NS | −1.07 | NS | −1.02 | NS | −1.04 | NS |
| BCF | 1.35 | 0.012 | −1.81 | 0.020 | −1.04 | 0.038 | 1.02 | NS |
Bold indicates p<0.05; Each model is adjusted for baseline SAAT, VAT, HFF, PFF, tanner, age, sex, total fat and change in total fat; No change in fat depot significantly predicted 2hr glucose, insulin AUC, insulin sensitivity, or fasting FFA; SAAT= subcutaneous abdominal adipose tissue, VAT= visceral adipose tissue, HFF= hepatic fat fraction, PFF= pancreatic fat fraction, AUC= area under the curve, IGI= insulinogenic index (insulin secretion), ISI=insulin sensitivity index (matsuda), BCF=beta-cell function, FFA= free fatty acid.
Figure 2. Change in fat depot predicting 2-year change in risk for type 2 diabetes.
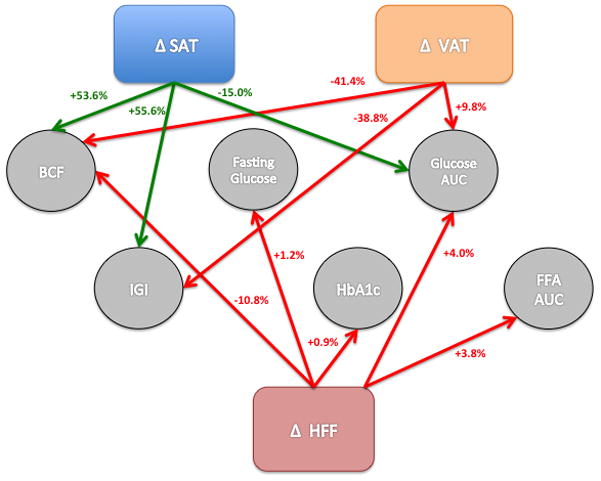
These lines represent how a 1-SD increase in each fat depot predicts a % change in risk for type 2 diabetes. Relationships that are beneficial to risk for type 2 diabetes have green arrows, while relationships that are detrimental to risk for type 2 diabetes have red arrows. All displayed relationships are significant (p<0.05) and are adjusted for baseline SAAT, VAT, HFF, PFF, tanner, age, sex, total fat and change in total fat, and change in other respective fat depots (SAAT, VAT, HFF, PFF). SAAT= subcutaneous abdominal adipose tissue, VAT= visceral adipose tissue, HFF= hepatic fat fraction, PFF= pancreatic fat fraction, BCF= beta-cell function, IGI= insulinogenic index (insulin secretion), AUC= area under the curve, FFA= free fatty acid
Glucose, Insulin and FFA
As shown in Table 2, of the four abdominal depots measured, only ΔHFF significantly associated with the 2-year change in fasting glucose and HbA1c. Specifically, a 1-SD increase in HFF was associated with a 1.2% increase in fasting glucose (β= 1.00, p=0.034) and a 0.9% increase in HbA1c (β= 1.00, p=0.015) (Figure 2). ΔSAAT, ΔVAT and ΔHFF were significantly associated with 2-year change in glucose AUC, following opposite patterns for subcutaneous versus intra-abdominal deposition. A 1-SD increase in SAAT was significantly associated with a 15.0% decrease in glucose AUC (β= −1.09, p=0.002) and a 1-SD increase in VAT and HFF was significantly associated with a 9.8% and 4.0% increase in glucose AUC (β= 1.15, p=0.015 and β= 1.01, p=0.001). ΔPFF showed no significant association with any glucose measure, and no depot change significantly associated with 2-hr OGTT glucose.
HFF was the only depot to associate with a FFA measure, such that ΔHFF significantly associated with a 2-year increase in FFA AUC (β= 01.01, p=0.024). Accordingly, a 1-SD increase in HFF was significantly associated with a 3.8% increase in FFA AUC (β= 01.01, p=0.024). No depot change significantly related to insulin measures; however an increase in ΔPFF and ΔSAAT was associated with a trend in 2-year increase in fasting insulin (β= 1.06, p=0.078; β= 1.18, p=0.087),
Insulin sensitivity, secretion, and beta-cell function
Changes in abdominal fat depots were not significantly associated with 2-year changes in insulin sensitivity (ISI); however ΔSAAT and ΔVAT were significantly associated with 2-year change in insulin secretion (IGI) in opposite directions. Namely, a 1-SD increase in SAAT was significantly associated with a 55.6% increase in IGI (β= 1.37, p=0.002), and a 1-SD increase in VAT was significantly associated with a 38.8% decrease in IGI (β= −1.74, p=0.007) (Table 2 and Figure 2). ΔSAAT, ΔVAT, and ΔHFF were significantly associated with 2-year change in BCF (product of insulin sensitivity and insulin secretion). Specifically, a 1-SD increase in SAAT was significantly associated with a 44.5% increase in BCF (β= 1.36, p=0.012) whereas an increase in VAT and HFF was significantly associated with a 35.6% and 10.5% respective decrease in BCF (β= −1.81, p=0.020; β= −1.04, p=0.038). The overall summary of the significant relationships between change in fat depots and 2-year change in metabolic risk measures are displayed in Figure 2.
Discussion
In the present longitudinal study, we found that over a 2-year period children earlier in their pubertal development defended against VAT, HFF and PFF accumulation, whereas youth with a more advanced pubertal stage at baseline had significantly greater accumulation of ectopic fat. Demonstrating the harmful effects of ectopic fat accumulation on risk for type 2 diabetes, we found that increases in hepatic and visceral fat predicted metabolic decline, while the accumulation of subcutaneous fat predicted metabolic improvement/protection over 2 years. Finally, supporting previous work by our group 17, this is the first longitudinal study to show that HFF, more than VAT, predicted a greater number of markers for type 2 diabetes risk; however VAT and SAAT had stronger effects on BCF and were the only significant predictors of insulin secretion.
This is the first study to show a pubertal divergence in longitudinal ectopic fat accumulation. Over the 2 years, there was no overall increase in HFF and PFF in our population of Hispanic children and young adults; however when separated by baseline pubertal development, there was greater accumulation of VAT, HFF and PFF in participants with advanced pubertal stage at baseline, versus a decrease in HFF and PFF in those with earlier pubertal stage at baseline. These findings support our hypothesis that a “saturation” of adipose tissue expansion occurs with later stages of pubertal development, thereby leading to fat deposition into ectopic depots. It is unknown exactly what causes the shift of accumulation from SAAT to ectopic depots. It is unlikely that the relatively small amount of adipose tissue that accumulates in ectopic regions could not be accommodated by the large SAAT depot that makes up approximately 20% of the fat mass in our population, and rather other signals may be dictating site specific deposition. The pattern of abdominal fat changes over time was not affected by baseline obesity status as expected, supporting the idea that signals other than total adiposity may dictate fat deposition patterning. Rosenbaum et al. have shown that SAAT in pre-pubertal children is less responsive to basal lipolysis and more responsive to anti-lipolytic signals compared to adults, potentially allowing for the increased SAAT expansion and lack of HFF and PFF development seen in our study 33. Additionally, studies in genetically modified mice have shown that extracellular matrix collagen VI development is the signal that restricts adipocyte hypertrophy, which leads to failed adipose expansion and metabolic dysfunction 34. The lipolytic capacity and extracellular matrix development of adipose tissue may be developmentally programmed and these observations potentially explain the hypothesis that following puberty, children are less able to expand their SAAT depot and defend against ectopic deposition. Adipose tissue extra-cellular matrix development and expandability should be studied across the pubertal development, as natural signals to prevent or reverse failed adipose expansion could be discovered.
We next examined whether changes in these fat depots were associated with 2-year change in type 2 diabetes risk and as hypothesized, found significant associations between increased hepatic fat and increases in metabolic risk for type 2 diabetes. The strength of this data lies in the repeated measurement, and the ability to simultaneously test whether changes in multiple hypothesized fat depots were independently associated with decline in metabolic health. Baseline levels of SAAT, VAT, HFF and PFF are highly correlated and while previous cross-sectional analysis have attempted to account for this using matching strategies 17–19, the effect of each depot type can more accurately be assessed using longitudinal change which is less biased by collinearity. This longitudinal study confirms the cross-sectional work suggesting that hepatic fat accumulation is central to metabolic decline. As hypothesized, the accumulation of hepatic fat over a two-year period predicted BCF and was the only fat depot to significantly predict clinical markers of type 2 diabetes, fasting glucose and HbA1c, and to predict FFA in response to an oral glucose load (FFA AUC). The liver is primarily responsible for endogenous glucose production and also plays a role in fatty acid disposal 35,36. Our data indicate that the paracrine activity of increasing hepatic fat impairs this process, supporting that hepatic fat has a greater effect on glycemic control than visceral fat or other adipose depots 37, and plays a role in circulating FFA levels, thought to be a key mechanism linking obesity to insulin resistance, beta-cell failure and transition to type 2 diabetes 36.
While our data suggests that hepatic fat accumulation is important, changes in visceral and subcutaneous fat also associated with key metabolic outcomes and with even greater strength. Change in HFF, SAAT and VAT all significantly and independently predicted BCF, arguably the most important risk factor for transition to type 2 diabetes 38. However, our analyses showed that change in VAT and SAAT depot had 4 and 5 times the effect on BCF compared to HFF (−41.4% and +53.6% vs. −10.8%), and had 2 and 4 times the effect on glucose AUC compared to HFF (+9.8% and −15.6% vs +4%). Furthermore, 2-year change in VAT and SAT were the only depots significantly associated with insulin secretion in response to a glucose challenge (−38.8% and +55.6%). While the causal benefit of SAAT expandability have been described 15,34,39, the causal role of VAT accumulation in metabolic decline is in question40. It has been proposed that VAT has a greater impact on metabolic health through the release of FFA acids and more directly impacts liver functioning through the portal vein, however our data showed no significant relationship between change in VAT and any of the FFA measures. It has been shown that the surgical removal of the omentum (a component of VAT) does not improve metabolic health 40, therefore accumulation of VAT is part of the picture of transition to type 2 diabetes, but in of itself seems insufficient to cause disease. Our data demonstrates strong associations due to change in VAT and SAAT, and therefore longitudinal accumulation of VAT rather than SAAT may be an overall indication of adipose organ dysfunction that has independent effects on BCF and metabolic health other than leading to ectopic spillover. Future studies should examine if adipose features, such as levels of adipose inflammation or adipokine release, could predict such patterns of longitudinal change. Additionally, it has previously been questioned whether hepatic and visceral fat accumulation are symptoms of insulin resistance and thus simply co-associate with metabolic dysfunction, or whether they play an independent causal role in insulin resistance and other features of metabolic decline (glycemic control, beta-cell function). We included baseline and change in insulin sensitivity in all of our models to test this hypothesis and all of the reported relationships were unchanged (data not reported). This novel data separates the detrimental effect of hepatic and visceral accumulation from simply symptoms of insulin resistance and furthers the case of a causal role in metabolic decline.
The longitudinal nature of this study provides strong evidence for fat depot accumulation leading to changes in metabolic health, however causality still cannot be assumed. The analysis of change variables provides associations free of confound from baseline characteristics, such as gender or genetic make-up, but other unmeasured time-varying factors may be important. This is the first study to assess longitudinal change in ectopic depot (HFF and PFF) accumulation patterns in youth and the effect on metabolic function. While there were significant relationships with clinical markers, 1-SD change in HFF lead to limited relative change in metabolic outcomes. The effect may be greater if the observation time was greater than the 2-years of this study, or if other features of hepatic function were assessed beyond simple fat accumulation, such as recently identified hepatokines, particularly fetuin-A 41,42. Hepatokine signaling in fatty liver may be central to metabolic dysfunction, and future studies should assess hepatokine signaling in addition to other features of hepatic function over time in parallel with changes in fat accumulation to better understand these relationships. In addition, we did not find any significant associations between changes in fat depots and insulin sensitivity as expected, despite associations with baseline SAAT and HFF. This could indicate an unmeasured baseline variable as the underlying driver of the association between fat deposition and insulin resistance, such as sex hormone signaling, which would be relevant in our primarily adolescent population43.
In conclusion, this is the first longitudinal study to show that early pubertal development is associated with accumulation of SAAT and stability of HFF and PFF, whereas in later pubertal stage (after Tanner 3), there is significant accumulation of VAT, HFF and PFF. Independent of pubertal development, 2-year changes in HFF, VAT and SAAT associated with increased risk for type 2 diabetes, and importantly beta-cell dysfunction. While intra-abdominal fat accumulation predicted metabolic decline, the expansion of subcutaneous adipose tissue was metabolically protective.
Acknowledgments
The project described was supported by Grant Number R01DK059211 from the National Institute of Diabetes And Digestive And Kidney Diseases. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Diabetes And Digestive And Kidney Diseases or the National Institutes of Health. We would like to thank all the Childhood Obesity Research Core (CORC) research team, as well as the nursing staff at the CTU. In addition, we are grateful for our study participants and their families for their involvement.
Footnotes
Conflict of Interest: The authors declare no conflict of interest.
References
- 1.Knittle JL, Timmers K, Ginsberg-Fellner F, Brown RE, Katz DP. The growth of adipose tissue in children and adolescents. Cross-sectional and longitudinal studies of adipose cell number and size. Journal of Clinical Investigation. 1979;63:239–246. doi: 10.1172/JCI109295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Landgraf K, Rockstroh D, Wagner IV, Weise S, Tauscher R, Schwartze JT, et al. Evidence of early alterations in adipose tissue biology and function and its association with obesity-related inflammation and insulin resistance in children. Diabetes. 2015;64:1249–1261. doi: 10.2337/db14-0744. [DOI] [PubMed] [Google Scholar]
- 3.Taylor RW, Grant AM, Williams SM, Goulding A. Sex differences in regional body fat distribution from pre- to postpuberty. Obesity (Silver Spring) 2010;18:1410–1416. doi: 10.1038/oby.2009.399. [DOI] [PubMed] [Google Scholar]
- 4.Goulding A, Taylor RW, Gold E, Lewis-Barned NJ. Regional body fat distribution in relation to pubertal stage: a dual-energy X-ray absorptiometry study of New Zealand girls and young women. Am J Clin Nutr. 1996;64:546–551. doi: 10.1093/ajcn/64.4.546. [DOI] [PubMed] [Google Scholar]
- 5.Roemmich JN, Rogol AD. Hormonal changes during puberty and their relationship to fat distribution. Am J Hum Biol. 1999;11:209–224. doi: 10.1002/(SICI)1520-6300(1999)11:2<209::AID-AJHB9>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 6.Huang TT, Johnson MS, Figueroa-Colon R, Dwyer JH, Goran MI. Growth of visceral fat, subcutaneous abdominal fat, and total body fat in children. Obes Res. 2001;9:283–289. doi: 10.1038/oby.2001.35. [DOI] [PubMed] [Google Scholar]
- 7.Johnson MS, Figueroa-Colon R, Huang TT, Dwyer JH, Goran MI. Longitudinal changes in body fat in African American and Caucasian children: influence of fasting insulin and insulin sensitivity. J Clin Endocrinol Metab. 2001;86:3182–3187. doi: 10.1210/jcem.86.7.7665. [DOI] [PubMed] [Google Scholar]
- 8.Huang TT-K, Johnson MS, Gower BA, Goran MI. Effect of changes in fat distribution on the rates of change of insulin response in children. Obes Res. 2002;10:978–984. doi: 10.1038/oby.2002.133. [DOI] [PubMed] [Google Scholar]
- 9.Ball GDC, Huang TT-K, Gower BA, Cruz ML, Shaibi GQ, Weigensberg MJ, et al. Longitudinal changes in insulin sensitivity, insulin secretion, and beta-cell function during puberty. The Journal of Pediatrics. 2006;148:16–22. doi: 10.1016/j.jpeds.2005.08.059. [DOI] [PubMed] [Google Scholar]
- 10.Goran MI, Shaibi GQ, Weigensberg MJ, Davis JN, Cruz ML. Deterioration of insulin sensitivity and beta-cell function in overweight Hispanic children during pubertal transition: a longitudinal assessment. Int J Pediatr Obes. 2006;1:139–145. doi: 10.1080/17477160600780423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Goran MI, Lane C, Toledo-Corral C, Weigensberg MJ. Persistence of pre-diabetes in overweight and obese Hispanic children: association with progressive insulin resistance, poor beta-cell function, and increasing visceral fat. Diabetes. 2008;57:3007–3012. doi: 10.2337/db08-0445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Toledo-Corral CM, Alderete TL, Hu HH, Nayak K, Esplana S, Liu T, et al. Ectopic fat deposition in prediabetic overweight and obese minority adolescents. J Clin Endocrinol Metab. 2013;98:1115–1121. doi: 10.1210/jc.2012-3806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pratley RE, Weyer C. The role of impaired early insulin secretion in the pathogenesis of Type II diabetes mellitus. Diabetologia. 2001;44:929–945. doi: 10.1007/s001250100580. [DOI] [PubMed] [Google Scholar]
- 14.Kelly LA, Lane CJ, Weigensberg MJ, Toledo-Corral CM, Goran MI. Pubertal changes of insulin sensitivity, acute insulin response, and β-cell function in overweight Latino youth. The Journal of Pediatrics. 2011;158:442–446. doi: 10.1016/j.jpeds.2010.08.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ravussin E, Smith SR. Increased fat intake, impaired fat oxidation, and failure of fat cell proliferation result in ectopic fat storage, insulin resistance, and type 2 diabetes mellitus. Ann N Y Acad Sci. 2002;967:363–378. doi: 10.1111/j.1749-6632.2002.tb04292.x. [DOI] [PubMed] [Google Scholar]
- 16.McQuaid SE, Hodson L, Neville MJ, Dennis AL, Cheeseman J, Humphreys SM, et al. Downregulation of adipose tissue fatty acid trafficking in obesity: a driver for ectopic fat deposition? Diabetes. 2011;60:47–55. doi: 10.2337/db10-0867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Alderete TL, Toledo-Corral CM, Desai P, Weigensberg MJ, Goran MI. Liver fat has a stronger association with risk factors for type 2 diabetes in African-American compared with Hispanic adolescents. J Clin Endocrinol Metab. 2013;98:3748–3754. doi: 10.1210/jc.2013-1138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fabbrini E, Magkos F, Mohammed BS, Pietka T, Abumrad NA, Patterson BW, et al. Intrahepatic fat, not visceral fat, is linked with metabolic complications of obesity. 2009:15430–15435. doi: 10.1073/pnas.0904944106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.D’Adamo E, Cali AMG, Weiss R, Santoro N, Pierpont B, Northrup V, et al. Central role of fatty liver in the pathogenesis of insulin resistance in obese adolescents. Dia Care. 2010;33:1817–1822. doi: 10.2337/dc10-0284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kim JS, Lê K-A, Mahurkar S, Davis JN, Goran MI. Influence of elevated liver fat on circulating adipocytokines and insulin resistance in obese Hispanic adolescents. Pediatr Obes. 2012;7:158–164. doi: 10.1111/j.2047-6310.2011.00014.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wicklow BA, Wittmeier KDM, MacIntosh AC, Sellers EAC, Ryner L, Serrai H, et al. Metabolic consequences of hepatic steatosis in overweight and obese adolescents. Dia Care. 2012;35:905–910. doi: 10.2337/dc11-1754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Toledo-Corral CM, Vargas LG, Goran MI, Weigensberg MJ. Hemoglobin A1c above Threshold Level is Associated with Decreased β-Cell Function in Overweight Latino Youth. The Journal of Pediatrics. 2012;160:751–756. doi: 10.1016/j.jpeds.2011.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Toledo-Corral CM, Alderete TL, Richey J, Sequeira P, Goran MI, Weigensberg MJ. Fasting, post-OGTT challenge, and nocturnal free fatty acids in prediabetic versus normal glucose tolerant overweight and obese Latino adolescents. Acta Diabetol. 2015;52:277–284. doi: 10.1007/s00592-014-0634-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hu HH, Nayak KS, Goran MI. Assessment of abdominal adipose tissue and organ fat content by magnetic resonance imaging. Obes Rev. 2011;12:e504–15. doi: 10.1111/j.1467-789X.2010.00824.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hu HH, Kim H-W, Nayak KS, Goran MI. Comparison of fat-water MRI and single-voxel MRS in the assessment of hepatic and pancreatic fat fractions in humans. Obesity (Silver Spring) 2010;18:841–847. doi: 10.1038/oby.2009.352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Alabousi A, Al-Attar S, Joy TR, Hegele RA, McKenzie CA. Evaluation of adipose tissue volume quantification with IDEAL fat-water separation. J Magn Reson Imaging. 2011;34:474–479. doi: 10.1002/jmri.22603. [DOI] [PubMed] [Google Scholar]
- 27.Reeder SB, Cruite I, Hamilton G, Sirlin CB. Quantitative assessment of liver fat with magnetic resonance imaging and spectroscopy. J Magn Reson Imaging. 2011;34:729–749. doi: 10.1002/jmri.22580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Marshal WA, Tanner JM. Variations in the Pattern of Pubertal Changes in Boys. Archives of Disease in Childhood. 1970;45:13–23. doi: 10.1136/adc.45.239.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Matsuda M, DeFronzo RA. Insulin sensitivity indices obtained from oral glucose tolerance testing: comparison with the euglycemic insulin clamp. Dia Care. 1999;22:1462–1470. doi: 10.2337/diacare.22.9.1462. [DOI] [PubMed] [Google Scholar]
- 30.DeFronzo RA, Matsuda M. Reduced time points to calculate the composite index. Dia Care. 2010;33:e93–e93. doi: 10.2337/dc10-0646. [DOI] [PubMed] [Google Scholar]
- 31.Diggle P, Heagerty P, Liang K-Y, Zeger S. Analysis of Longitudinal Data. Oxford University Press; 2002. [Google Scholar]
- 32.Martin AD, Daniel MZ, Drinkwater DT, Clarys JP. Adipose tissue density, estimated adipose lipid fraction and whole body adiposity in male cadavers. Int J Obes Relat Metab Disord. 1994;18:79–83. [PubMed] [Google Scholar]
- 33.Rosenbaum M, Presta E, Hirsch J, Leibel RL. Regional differences in adrenoreceptor status of adipose tissue in adults and prepubertal children. J Clin Endocrinol Metab. 1991;73:341–347. doi: 10.1210/jcem-73-2-341. [DOI] [PubMed] [Google Scholar]
- 34.Khan T, Muise ES, Iyengar P, Wang ZV, Chandalia M, Abate N, et al. Metabolic dysregulation and adipose tissue fibrosis: role of collagen VI. Mol Cell Biol. 2009;29:1575–1591. doi: 10.1128/MCB.01300-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Perseghin G. Viewpoints on the way to a consensus session: where does insulin resistance start? The liver Dia Care. 2009;32 (Suppl 2):S164–7. doi: 10.2337/dc09-S303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Boden G, Shulman GI. Free fatty acids in obesity and type 2 diabetes: defining their role in the development of insulin resistance and beta-cell dysfunction. Eur J Clin Invest. 2002;32 (Suppl 3):14–23. doi: 10.1046/j.1365-2362.32.s3.3.x. [DOI] [PubMed] [Google Scholar]
- 37.Kantartzis K, Machann J, Schick F, Fritsche A, Häring H-U, Stefan N. The impact of liver fat vs visceral fat in determining categories of prediabetes. Diabetologia. 2010;53:882–889. doi: 10.1007/s00125-010-1663-6. [DOI] [PubMed] [Google Scholar]
- 38.Kahn SE. The Importance of β-Cell Failure in the Development and Progression of Type 2 Diabetes. 2013;86:4047–4058. doi: 10.1210/jcem.86.9.7713. http://dxdoiorg/101210/jcem8697713. [DOI] [PubMed] [Google Scholar]
- 39.Virtue S, Vidal-Puig A. Adipose tissue expandability, lipotoxicity and the Metabolic Syndrome--an allostatic perspective. Biochim Biophys Acta. 2010;1801:338–349. doi: 10.1016/j.bbalip.2009.12.006. [DOI] [PubMed] [Google Scholar]
- 40.Fabbrini E, Tamboli RA, Magkos F, Marks-Shulman PA, Eckhauser AW, Richards WO, et al. Surgical removal of omental fat does not improve insulin sensitivity and cardiovascular risk factors in obese adults. Gastroenterology. 2010;139:448–455. doi: 10.1053/j.gastro.2010.04.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Stefan N, Häring HU. The role of hepatokines in metabolism. Nat Rev Endocrinol. 2013;9:144–152. doi: 10.1038/nrendo.2012.258. [DOI] [PubMed] [Google Scholar]
- 42.Stefan N, Sun Q, Fritsche A, Machann J, Schick F, Gerst F, et al. Impact of the adipokine adiponectin and the hepatokine fetuin-A on the development of type 2 diabetes: prospective cohort- and cross-sectional phenotyping studies. PLoS ONE. 2014;9:e92238. doi: 10.1371/journal.pone.0092238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Linder K, Springer F, Machann J, Schick F, Fritsche A, Häring HU, et al. Relationships of body composition and liver fat content with insulin resistance in obesity-matched adolescents and adults. Obesity (Silver Spring) 2014;22:1325–1331. doi: 10.1002/oby.20685. [DOI] [PubMed] [Google Scholar]