Abstract
Given the volume and importance of research focusing on menstrual phase, a review of the strategies being used to identify menstrual phase and recommendations that will promote methodological uniformity in the field is needed.
We conducted a literature review via Ovid Medline and PsycINFO. Our goal was to review methods used to identify menstrual phase and subphases in biobehavioral research studies with women who had physiologically natural menstrual cycles. Therefore, we excluded articles that focused on any of the following: use of exogenous hormones, the postpartum period, menstrual-related problems (e.g. polycystic ovarian syndrome, endometriosis), and infertility/anovulation. We also excluded articles on either younger (<18 years old) or older (>45 years old) study samples.
We initially identified a total of 1,809 articles. After our exclusionary criteria were applied, 146 articles remained, within which our review identified six different methods used to identify menstrual phase and subphases. The most common method used was self-report of onset of menses (145/146 articles) followed by urine luteinizing hormone testing (50/146 articles) and measurement of hormones (estradiol and/or progesterone) in blood samples (49/146 articles).
Overall, we found a lack of consistency in the methodology used to determine menstrual phase and subphases. We provide several options to improve accuracy of phase identification, as well as to minimize costs and burden. Adoption of these recommendations will decrease misclassification within individual studies, facilitate cross-study comparisons and enhance the reproducibility of results.
Keywords: Women, Menstrual Phase, Sex Hormones, Methods, Recommendations, Review
Introduction
In addition to their role in reproductive functions, the sex hormones progesterone and estradiol play a significant role in a wide range of both biological and behavioral responses, such as stress response, neurotransmission, mood, and drug metabolism (Becker et al., 2008). Because these hormones vary dramatically across the menstrual cycle (Yen, Jaffee, & Barbieri, 1999), menstrual phase can be used as a natural proxy for endogenous progesterone and estradiol levels. Thus, menstrual phase is an important factor in biobehavioral research. In fact, according to PubMed, in the past 40 years, published literature that included either “menstrual phase” or “menstrual cycle” as a keyword has increased more than 10-fold, from 115 published articles in 1974 to a peak of 600 articles in 2009. Currently, there are around 500 articles published annually. This research has indicated that menstrual phase may be related to a variety of behavioral outcomes, from the perception of attention, memory, and pain (Hoeger Bement et al., 2009; Kowalczyk et al., 2010; Nielsen, Ahmed, & Cahill, 2014; Pletzer, Petasis, & Cahill, 2014) to calorie intake and drug use (Brennan et al., 2009; Carpenter, Upadhyaya, LaRowe, Saladin, & Brady, 2006; Holdstock & de Wit, 2000; Reed, Levin, & Evans, 2010; Reed, Evans, Bedi, Rubin, & Foltin, 2011; Reed, Levin, & Evans, 2008). Unfortunately, despite the rapid growth in this area, research methodology for determination of menstrual phase lacks consistency and precision. Furthermore, depending on the methodology employed, a high probability of misclassification (e.g. inaccurate identification of menstrual phase) may occur. It is likely that this lack of precision, as well as other potential errors in menstrual phase determination, contribute to inconsistent findings in the literature and hamper efforts to compare findings across studies.
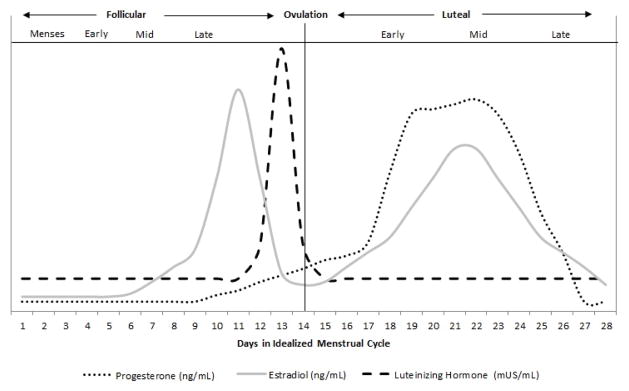
The menstrual cycle is commonly characterized with respect to specific milestones and events denoting various phases. The first day of the ovarian cycle is defined by the onset of blood flow (menses), which also marks the beginning of the follicular phase (Yen et al., 1999). Ovulation occurs approximately halfway through the menstrual cycle, denoting the onset of the luteal phase. These two phases are often further subdivided into early-, mid-, and late-follicular, as well as early-, mid-, and late-luteal (Figure 1; Table 1) as characterized by levels of estradiol and progesterone. Specifically, progesterone is very low throughout the follicular phase, whereas estradiol is very low during the early follicular phase and is followed by a rise, as the follicle develops, that peaks sharply in the late follicular phase. The end of the follicular phase is concomitant with a surge in luteinizing hormone (LH), which is caused by the increase in estradiol. The LH surge heralds ovulation and the formation of the corpus luteum, which secretes progesterone, marking the start of the luteal phase. Approximately half-way through the luteal phase (mid-luteal), progesterone peaks and estradiol has a secondary peak.
Figure 1.
Relative Change in Hormones across Menstrual Cycle
Table 1.
Characterization of Menstrual Phases
| Follicular | Ovulation | Luteal | ||||||
|---|---|---|---|---|---|---|---|---|
| Menses | Early | Mid | Late | Early | Mid | Late | ||
| Days based on 28-day cycle | 1–4 | 4–5 | 5–7 | 8–12 | 13–15 | 16–20 | 21–23 | 24–28 |
| Progesterone | ||||||||
| Absolute Values (ng/mL) | < 2 | < 2 | < 2 | < 2 | 2–20 | 2–20 | 2–30 | 2–20 |
| Relative Change | Stable | Stable | Stable | Mild Increase | Increase | Large Increase | Peak | Large Decline |
| Estradiol | ||||||||
| Absolute Values (ng/mL) | 20–60 | 20–100 | 100–200 | >200 | >200 | 100–200 | 100–200 | 20–60 |
| Relative Change | Stable | Mild Increase | Large Increase | Primary Peak | Large Decline | Mild Increase | Secondary Peak | Moderate Decline |
| Luteinizing Hormone | ||||||||
| Absolute Values (mUS/mL) | 5–25 | 5–25 | 5–25 | 5–25 | 25–100 | 5–25 | 5–25 | 5–25 |
| Relative Change | Stable | Stable | Stable | Stable | Peak | Stable | Stable | Stable |
Conventionally, a “normal” menstrual cycle is considered to be 28 days in length, with a standard 14-day luteal phase (Yen et al., 1999). However, recent research indicates considerable variability in menstrual-cycle length, as well as the length of each phase (Cole, Ladner, & Byrn, 2009; Jukic, Weinberg, Baird, & Wilcox, 2007). In fact, at a population level, the mean cycle length is 27 to 29 days (Becker et al., 2008; Cole et al., 2009). Cole and colleagues recommend that a menstrual cycle may be considered “normal” as long as a menses occurs every 23–32 days, with the follicular and luteal phases lasting anywhere from 10–20 days and 9–17 days, respectively (Cole et al., 2009). Variation in menstrual-cycle length depends on when the follicle begins to develop (producing predominantly estradiol) and the viability of the corpus luteum (which produces the peak of progesterone). This cycle variation is subject to a variety of both genetic and environmental factors (Jukic et al., 2007; Liu, Gold, Lasley, & Johnson, 2004) and therefore reliance on the conventional 28-day determination methodology (e.g., follicular- versus luteal-phase identification based on number of days since onset of menses only) can contribute to misclassification within the expanding biobehavioral literature. Although other methods (e.g., urine LH testing, measurement of progesterone and/or estradiol) are often used in the literature to determine menstrual phase, how consistently these methods are used to define phase in biobehavioral research has never been examined. Further, despite recommendations for standardization (Reed & Evans, 2009), research methodology for determination of menstrual phase lacks consistency and precision. Therefore, the goal of this review is to provide information and recommendations to researchers on how best to identify a menstrual phase and/or subphase of interest within the context of biobehavioral research. Specifically, we aimed to review the current methods being used in the literature, and provide methodological recommendations, with consideration to the cost and burden, for phase and subphase determination to improve the accuracy of their identification, as well as to facilitate comparison of results across studies. Following these recommendations will aid in the reproducibility of biomedical research publications, which has been recently prioritized by the National Institutes of Health as an area needing improvement (Collins & Tabak, 2014).
Methods
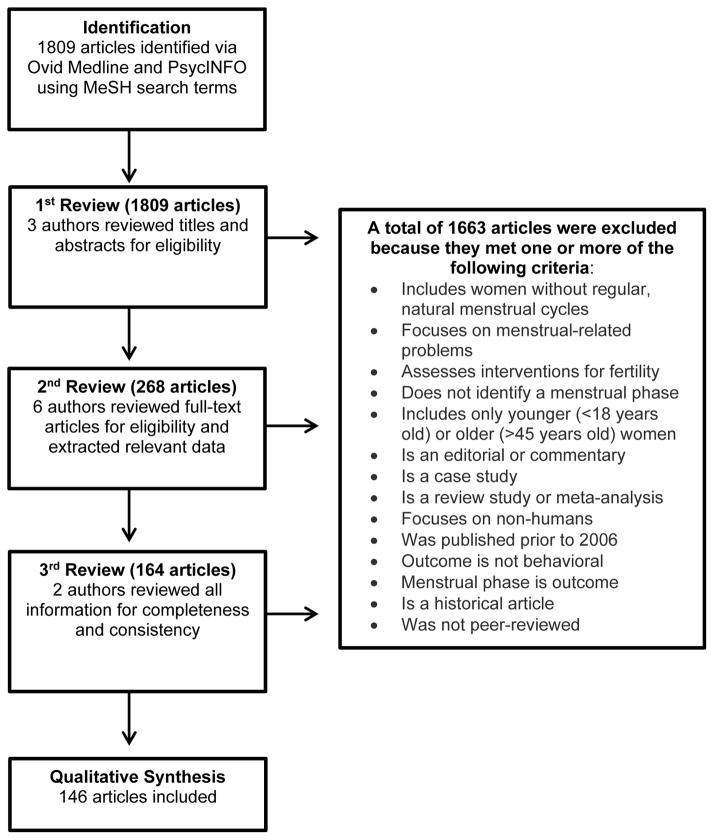
Given our goal of reviewing the methodologies used to identify menstrual phases and subphases, we limited our search results to articles that used menstrual phase as an independent variable. Further, although publications on menstrual phase are available from as early as 1974, we reviewed those published between January 2006 and November 2014 to focus on current methodologies. We further restricted the articles to include those published in English and describing studies conducted with humans. We applied these criteria to a literature search conducted via Ovid Medline and PsycINFO for the following Medical Subject Headings (MeSH) search terms: menstrual cycle, fertile period, follicular phase, luteal phase, and menstruation. Additional keywords searched included female cycle, follicular, luteal, and phase. The keyword search also included a multiple character wildcard search for the following ovula*, pre ovula*, preovula*, post ovula*, and postovula*. A multiple character wildcard search allows for a search of the truncated word, thus words like ovulation and ovulating would be identified by the term “ovula*”. Using this search strategy, shown in Table 2, we identified a total of 1,809 articles. Next, given that our goal was to examine methodology used to identify menstrual phases in the natural, non-manipulated menstrual cycle in biobehavioral research, inclusion criteria included articles that focused on behavioral outcomes. Further, we excluded any articles primarily focused on the following topics: use of exogenous hormones, the postpartum period, menstrual-related problems (e.g., polycystic ovarian syndrome, endometriosis) and infertility/anovulation. We also excluded articles that did not identify menstrual phases (e.g., those that only studied a specific day in the menstrual cycle such as two days after onset of menses without reference to what menstrual phase was being investigated), and articles that exclusively enrolled either younger (<18 years old) or older (>45 years old) women, as well as case studies, review articles, historical articles, dissertations, editorials and commentaries Figure 2).
Table 2.
Search Strategies for Publications on Menstrual Phase Methodology
| Search Number | Search Term |
|---|---|
| 1 | exp Menstrual Cycle/ |
| 2 | (menses or menstruation or menstrual).mp. |
| 3 | 1 or 2 |
| 4 | female cycle.mp. |
| 5 | follicular.mp. |
| 6 | luteal.mp. |
| 7 | ovula*.mp. |
| 8 | (pre ovula* or preovula*).mp. |
| 9 | (post ovula* or postovula*).mp. |
| 10 | 4 or 5 or 6 or 7 or 8 or 9 |
| 11 | 3 and 10 |
| 12 | phase.ti,ab. |
| 13 | 11 and 12 |
| 14 | limit 13 to (English language and humans and yr=“2006 -Current”) |
| 15 | remove duplicates from 14 |
- exp <term> This command explodes the term entered onto the command line. In this case, exp Menstrual Cycle retrieves records that contain the term “menstrual cycle” and any of the narrower, more specific terms including the following: fertile period, follicular phase, luteal phase and menstruation.
- <term>.ab Indicates a search for a certain term in the abstract.
- <term>.mp Signifies a “multi-purpose” search in which a search of multiple fields (e.g., title, abstract, subject heading, registry words) will be done.
- <term>.ti This command is a search command that searches for specified term in the title.
- <term>* Indicates a search for a truncated work. For example, ovula* would search for ovulation and ovulate.
Figure 2.
Flow Diagram of Article Selection
Using those article eligibility criteria, we applied a three-level review (Figure 2). First, three authors (A. Allen, A. McRae-Clark, S. Carlson) reviewed 1,809 articles based on information provided in the title and/or abstract. This first level of review resulted in the exclusion of 1,541 articles. Second, six authors (A. McRae-Clark, M. Saladin, K. Gray, C.L. Wetherington, S. McKee, S. Allen) reviewed full articles to determine eligibility and perform data extraction. Finally, the data from these articles was reviewed by two authors (A. Allen, S. Carlson) for completeness and consistency. Any disagreements were reviewed and resolved by the full coauthor group. The second and third level of review resulted in exclusion of 122 additional articles. Therefore, our final review included 146 articles (see supplementary information for additional details). Within those articles six different methodologies for menstrual phase determination were identified: self-report of onset of menses, urinary LH testing, sex hormone measurement via saliva sample, sex hormone measurement via blood sample, basal-body temperature, and sonography. To be included in the review a method had to appear in two or more articles, either alone or in combination with another method.
Results
Self-Report of Onset of Menses
A total of 145 out of 146 articles (99%) included self-report of onset of menses to determine menstrual phase. The majority of the articles (60%; 83/146 articles) used forward counting, which determines menstrual phase by counting the number of days from the onset of menses prospectively. Fewer articles (16%; 23/146 articles) used backward counting, which retrospectively counted backwards from the onset of menses to determine phase. The remaining 50 articles (34%) did not specify how self-report was used to determine menstrual phase. A major strength of this method is that it has no cost and a very low participant burden. With this method, menstrual phases are often identified by classifying the first 14 days following the onset of menses as the follicular phase and all other days as the luteal phase. As noted above, this method is based on the often incorrect assumption that ovulation occurs consistently 14 days after the onset of menses. Thus, misclassification of the mid-follicular and late follicular subphases and the early luteal, mid-luteal, and late luteal subphases is quite prevalent using this method. Further, research indicates that the accuracy of self-reported data is prone to error and is influenced by many factors including cycle length and time since menses (Bean, Leeper, Wallace, Sherman, & Jagger, 1979; Creinin, Keverline, & Meyn, 2004; Jukic et al., 2007; Small, Manatunga, & Marcus, 2007; Wegienka & Baird, 2005). In fact, estimates indicate as much as 50% of menstrual phase determination based on self-report may be wrong (Shirtcliff, Granger, Schwartz, & Curran, 2001). Therefore, when used alone, this method may reliably identify only menses and the early follicular subphase.
Urinary LH Testing
A total of 50 of the 146 articles (34%) used urinary LH testing to determine phase. All of them used urine LH testing in combination with self-report of onset of menses, and over half (58%; 29/50 articles) also used retrospective confirmation via blood or saliva sex hormone measurement. With this method, participants are instructed to test for the presence of LH in the urine via urinating on a test kit stick during the first morning void. This is typically done for 10 consecutive days starting on the 18th day prior to the end of the woman’s typical cycle. For example, if a woman reports a 26-day cycle, she would start LH testing on day 8 (i.e., 26 – 18 = 8). If a woman reports a range of cycle length (e.g., 27–33), erring on the side of caution is done by initiating urine LH testing based on the shorter cycle length (e.g., cycle day 9), with the caveat that additional days of testing may be necessary. Thus, a limitation of this method is that its accuracy relies on a woman knowing the length of her cycle, which, as noted above, may not be reliable information (Bean et al., 1979; Creinin et al., 2004; Jukic et al., 2007; Small et al., 2007; Wegienka & Baird, 2005). A positive LH test (typically indicated by a dark test line) indicates a LH surge, which predicts ovulation to occur within 28–36 hours after the beginning of the LH rise (Kerin, 1982). Therefore, in addition to the moderately low cost and participant burden, a strength of this method is that it can prospectively identify ovulation. Limitations of this method include reliance on self-reported data from participants, as well as test results that sometimes are inconclusive (e.g. a test line that appears light rather than dark may indicate the LH surge for some women). Combining this method with self-report of onset of menses can yield accurate prospective identification of certain follicular and luteal subphases (e.g., menses, early follicular, ovulation, and early luteal). However, when the self-report of onset of menses, urinary LH testing and measurement of sex hormone levels via blood or saliva samples (as described below) are used together, all subphases (i.e., menses, early follicular, mid-follicular, late follicular, ovulation, early luteal, mid-luteal, and late luteal) can be identified.
Sex Hormone Measurement via Saliva Sample
Of the 146 articles, 26 (18%) included measurement of the sex hormones estradiol and progesterone via saliva samples, with all of these articles also including self-report of onset of menses and 29% (7/29) also including LH testing. The majority of these articles (62%; 18/29 articles) did not specify hormone values used to determine phase. Approximately 21% (6/29 articles) provided a relative definition such as progesterone is higher in the luteal phase than the follicular phase. The remaining five articles (17%) reported specific values for each hormone. For example, Schoofs and colleagues (2009) reported using a definition for luteal phase as a progesterone level in the range of 127-446 pg/ml (Schoofs & Wolf, 2009). It was unclear whether definitions were made a priori in all cases. A major strength of measuring sex hormones via daily saliva samples is that it provides physiological measures that allow for the precise identification of each subphase. An additional strength is that the saliva sample can be collected outside the clinic in a noninvasive manner and easily stored prior to delivery to the analyzing laboratory. In addition, the hormone concentrations measured in saliva, as opposed to blood, more accurately represent the biologically “available” fraction (i.e., it excludes the fraction bound to sex hormone-binding globulin that would hence be biologically unavailable) (Riad-Fahmy, Read, Walker, Walker, & Griffiths, 1987). Whereas early work suggested that salivary progesterone was a poor indicator of ovulation (Metcalf, Evans, & Mackenzie, 1984), more recent work has shown salivary estradiol and progesterone measurement to be a feasible and appropriate method for menstrual subphase determination (Celec et al., 2009; Gandara, Leresche, & Mancl, 2007). Limitations of using saliva samples for hormone measurement include assay cost and delay in receiving results from laboratories, making it very difficult to use this method to prospectively determine menstrual phase without easy access to an analyzing laboratory that can provide analysis immediately prior to the experimental protocol. Further, the high variability of hormone levels from woman to woman has impeded the establishment of standard reference ranges for defining menstrual phase. Finally, onsite laboratory availability for salivary assays may be limited. Thus, this method is generally used as a retrospective confirmation of menstrual phase or to relate behavioral outcomes to hormonal levels.
Sex Hormone Measurement via Blood Sample
Of the 146 studies reviewed, 49 (34%) included assessment of sex hormones via blood samples. Most (88%; 43/49 articles) studies assessed both progesterone and estradiol. We found no consistency among the studies in the specific criteria used to determine phase. Articles included either specific progesterone or estradiol criteria to determine menstrual phase (e.g.,a progesterone level of 0.15–1.5 ng/mL was defined as follicular phase in Choi et al., 2006), subjective and/or relative definitions (e.g., a higher estrogen/progesterone ratio indicated follicular phase in Allen, Bade, Center, Finstad, & Hatsukami, 2008) or no definition at all (e.g., Gordon & Girdler, 2014). It was unclear whether definitions were made a priori in all cases.
Blood-hormone assays have the virtue of being fairly sensitive measures. For instance, the sensitivity of estradiol and progesterone in two of the studies reviewed was reported at 10 pg/mL and 0.08–0.20 ng/mL, respectively (Allen, Hatsukami, Christianson, & Nelson, 1999; Derntl et al., 2008). However, they also possess a number of limitations. First, like saliva samples, standard references ranges cannot be established due to a high level of variability from woman to woman. Second, as with sex hormone measurement in saliva samples, prospective confirmation of menstrual phase is difficult. Third, participants must attend the clinic to provide a blood sample, which may add substantial burden for the participant and limit the frequency of sample collection. Fourth, the collection of blood samples is also more invasive than the previously described methods. Fifth, the cost for collection, processing, and analyzing blood samples is substantially higher than either self-report of onset of menses or urine LH testing. Sixth, hormone levels change daily depending on the physiology of the developing follicle, egg release, and formation and maintenance of the corpus luteum (Yen et al., 1999); thus, frequent measurement may be necessary but impractical. Lastly, as noted above, hormone values must be used for retrospective confirmation given the time it takes for laboratories to analyze the lab samples.
Basal-Body Temperature
Out of the 146 articles identified, 11 (8%) reported use of the basal-body temperature (BBT) method in combination with self-report of onset of menses (100%; 11/11 articles), urinary luteinizing hormone testing (45%; 5/11 articles), and/or blood hormones (36%; 4/11 articles). No articles used the BBT method alone to identify menstrual phase. Compared with a mean value computed from BBT values obtained during the menses or early follicular phases, BBT values slightly increase (2–3° F) up to a few days after ovulation (Becker et al., 2008; Yen et al., 1999). Thus, this method requires participants to measure the basal sublingual body temperature daily beginning during menses at a standard time each day to avoid diurnal variability. Participants then self-report the temperatures to researchers who compute an average value prior to ovulation. Because BBT increase may occur a few days after ovulation (Becker et al., 2008; Yen et al., 1999), prospective identification of ovulation is difficult; however, BBT does allow for prospective identification of the early luteal phase. While the cost of obtaining daily temperature measurement is minimal, it does involve a moderate level of participant burden due to required daily measurements at standard times and reliance on accurate participant reporting. Because many factors (e.g., illness, alcohol consumption, sleep patterns) may influence BBT, identification of ovulation using BBT alone fails in an estimated 20% of ovulatory cycles (Moghissi, 1976). However, the combination of this method with self-report of onset of menses may yield more accurate determination of menses, early follicular, ovulation, and early luteal.
Sonography
Three studies (2%) included the use of sonography, along with self-report of onset of menses and blood hormone levels, as a method to identify menstrual phase. Although this method can accurately identify all menstrual phase and subphases via various anatomical markers, its utility is substantially limited by its high invasiveness, participant burden, and cost.
Discussion
The results of this qualitative review indicate that a variety of methodologies have been used to identify menstrual phases and subphases in biobehavioral research. Unfortunately, these methods have many inconsistencies. The lack of consistency in ascertainment of menstrual phase presents several problems for biobehavioral researchers as a whole. First, without rigorous definitions and recommendations in place, the likelihood of misclassification occurring within a study is high and when misclassification occurs, study results are compromised. Second, comparison between studies that use different methodologies is difficult, if not impossible. The inability to compare studies poses a major challenge for scientific advancement within the field. Therefore, the remainder of this review provides suggested guidance for biobehavioral researchers who study menstrual phase effects; we also offer recommendations for menstrual phase and subphase identification.
Recommendations for Selecting Subphase of Interest
The first step in conducting research on menstrual phase effects is to identify a menstrual phase or subphase(s) of interest. Given the constant changes in hormonal levels across the menstrual cycle and within its subphases, we recommend that biobehavioral researchers select a subphase(s) of interest, rather than a phase (e.g., follicular versus luteal) of interest. Selecting a narrower subphase(s) of interest confers greater experimental precision than merely choosing follicular versus luteal phases without regard to subphases. For example, a frequent observation in drug-abuse behavioral literature is that, in preclinical studies, estradiol facilitates drug-seeking behaviors, whereas progesterone protects against these behaviors (Anker & Carroll, 2010; Lynch & Sofuoglu, 2010). Therefore, a biobehavioral researcher in this area may be interested in comparing the late follicular subphase (low progesterone, high estradiol) with the mid-luteal subphase (high progesterone, medium estradiol). The selection of these two subphases would allow for a comparison when the progesterone to estradiol (P/E) ratios are most divergent. A second example is a researcher wishing to compare an outcome in men and women during a subphase in which the hormone levels are most similar in men and women. The researcher would select the early follicular phase during which E2 and P levels in women are lowest and most similar to those of men (Yen et al., 1999). Alternatively, if the researcher also wanted to compare men and women during a subphase when the hormones are most divergent, then selection of the mid-luteal phase would be appropriate given that P is at its peak and E has a secondary peak at this time. Identifying a subphase of interest in older women (e.g. > 35 years of age) should be done with additional caution given that cycle to cycle variability within an individual woman increases dramatically as the menopausal transition approaches (Cramer, Barbieri, Fraer, & Harlow, 2002).
Recommendations for Eligibility Criteria and Measurement of Confounders
Upon selection of subphase(s) of interest, we recommend consideration of eligibility criteria and measurement of potential confounders of biobehavioral research. Several medical conditions and medications have been reported to influence sex hormone levels and the menstrual cycle (Table 3). We recommend that all of these medical conditions and medications be considered as exclusionary items. At the very least, we recommend excluding for pregnancy, breastfeeding, amenorrhea, ovarian hypofunction, and use of exogenous hormones such as hormonal contraceptives or hormonal treatments, unless of course their effects are assessed. Further, age, physical activity, stress, time of day, depression, and changes in body weight, can also play a role in the regularity of the menstrual cycle (Becker et al., 2008; Harlow, Wise, Otto, Soares, & Cohen, 2003). Therefore, these confounding variables should be carefully tracked during data collection and considered during statistical analyses.
Table 3.
Examples of Medical Conditions, Medications, and Other Items that Can Influence Sex Hormone Levels and the Menstrual Cycle
| Medical Conditions | Medications and Other Items |
|---|---|
| Pregnancy | Contraceptives |
| Breastfeeding | Hormone treatments |
| Amenorrhea | St. John’s wort |
| Ovarian hypofunction | Phenobarbital |
| Rapid weight loss or low body fat | Carbamazepine |
| Klinefelter syndrome, Turner syndrome | Rifampicin |
| Adrenal disorders | Erythromycin, clarithromycin |
| Alcoholism | Ketoconazole, itraconazole |
| Hemochromatosis | Ritonavir |
| Sickle cell disease | Grapefruit juice |
| Depression | Clomiphene |
| Naloxone | |
| Digoxin |
Recommendations for Selecting Saliva or Blood for Sex Hormone Assessment
If sex hormone levels are to be used to determine menstrual phase, one decision that needs to be considered is the use of saliva versus blood samples. First, as noted above, sex hormones measured via blood samples measure both the bound and unbound hormones, whereas sex hormones measured via saliva samples measure only the unbound hormones. In other words, hormones measured in saliva samples are the biologically available value. Depending on the research question at hand, this may be an important distinction to consider. Second, onsite laboratory availability to conduct hormonal measurement via saliva samples may be more limited. Third, for both saliva and blood samples, one needs to consider the frequency of collection and how this may impact cost and participant burden. For instance, if the researcher hypothesizes that change in a hormone level (versus absolute hormone level) may be related to the outcome of interest, then more frequent sampling may be necessary. In this scenario, at-home saliva-sample collection may be more favorable. Alternatively, if the researcher is examining whether an absolute level of a hormone is related to an outcome of interest, then a single time point blood sample may be adequate. Fourth, some research suggests that hormone levels assessed via saliva samples may have more diurnal variability (Wood, 2009). If this is the case, multiple samples or standardized times for sample collection may be necessary. Finally, one must consider the type of materials that will be used to collect specimens. For instance, the use of cotton versus polyester salivettes can impact reliability of saliva measurement and may also vary in price (Gröschl & Rauh, 2006; Shirtcliff et al., 2001).
Recommendations for Menstrual Subphase Identification
To identify menstrual cycle subphases, we recommend use of one of the following six methodological options. These options (summarized in Tables 4 and 5) are offered in order from most to least preferred with respect to their ability to accurately and prospectively identify all subphases. However, the selection of an option will depend upon the experimental question, whether some versus all subphases are of interest, and the need for prospective or retrospective identification of subphase. The cost and invasiveness of each option should also be weighed against the accuracy and necessity of accurate identification per the study goals.
Table 4.
Relative Cost, Burden, Precision and Accuracy by Method of Menstrual-Phase Determination
| Method | Relative Cost | Participant Burden | Precision of Measurement | Estimated Overall Accuracy of Subphase Identification |
|---|---|---|---|---|
| Self-Report of Onset of Menses | $ | Very Low | Low | Low |
| Basal-Body Temperature | $$ | Low | Low | Medium |
| Urine LH Testing | $$ | Low | Low | Medium |
| Saliva Sex Hormone Measurement | $$$ | Low | Medium | High |
| Blood Sex Hormone Measurement | $$$ | Medium | Medium | High |
| Sonography | $$$$$ | High | High | Very High |
Table 5.
Prospective and Retrospective Identification of Menstrual Subphases by Different Recommended Methodologies
| Methodology | Identification of Subphase | |||||||
|---|---|---|---|---|---|---|---|---|
| Menses | Early Follicular | Mid-Follicular | Late Follicular | Ovulation | Early Luteal | Mid-Luteal | Late Luteal | |
| Option #1: Sonography | P | P | P | P | P | P | P | P |
| Option #2: Self-Report of Onset of Menses, Urinary LH Testing, and Sex Hormone Measurement | P | P | R | R | P | P | R | R |
| Option #3: Self-Report of Onset of Menses and Sex Hormone Measurement | P | P | R | R | R | R | R | R |
| Option #4: Self-Report of Onset of Menses and Urinary LH Testing | P | P | ? | ? | P | P | ? | ? |
| Option #5: Self-Report of Onset of Menses and Basal-Body Temperature | P | P | ? | ? | R | P | ? | ? |
| Option #6: Self-Report of Onset of Menses | P | P | - | - | - | - | - | - |
Key: P = Accurate Prospective identification, R = Accurate Retrospective identification, ? = Questionable identification, - = Identification not possible
Option #1 – Sonography
This method will identify all menstrual phases and subphases accurately and prospectively. However, as discussed above, this method is limited by the high cost and burden involved. Therefore, it may prove infeasible in most biobehavioral research projects.
Option #2 - Self-Report of Onset of Menses + Urinary LH Testing + Sex Hormone Measurement
The combination of these three methods permits reliable prospective identification of menses and the early follicular phase (via self-report of onset of menses), as well as ovulation and the early luteal phase (via urinary LH testing). Further, retrospective identification of other subphases (e.g., mid-follicular, late follicular, mid-luteal, and late luteal) can be estimated and then later confirmed via sex hormone values. For instance, using the information provided in Table 1, a progesterone value >2 ng/mL would signify ovulation and, thus, the luteal phase. Further, if a series of three progesterone values were collected over the course of a week and the second was the highest value, this day may correspond to the mid-luteal phase. The strength of combining these three methodologies is the increased accuracy of prospective identification of menses, early follicular, ovulation, and early luteal subphases, while being less costly and burdensome than sonography. However, this option’s limitation is its reliance on retrospective confirmation of the remaining subphases (i.e., mid-follicular, late follicular, mid-luteal, and late luteal). This option also burdens the participant with frequent use of measurement kits (LH surge kit and saliva collection kit).
Option #3 - Self-Report of Onset of Menses + Sex Hormone Measurement
Prospectively, the use of these two methods can only identify menses and the early follicular phase. However, retrospective identification of follicular subphases could be accomplished with estradiol measurements (via saliva or blood), and luteal subphases could be identified with progesterone measurements (via saliva or blood). Given the inability of this method to prospectively and reliably identify any luteal subphases, we recommend prospective use of this method only when menses or early follicular subphases are of interest.
Option #4 - Self-Report of Onset of Menses + Urinary LH Testing
This combination of methods can be used prospectively to identify four subphases: menses, early follicular, ovulation, and early luteal. In the absence of hormonal confirmation, identification of the remaining subphases (mid-follicular, late follicular, mid-luteal, and late luteal) would be questionable.
Option #5 - Self-Report of Onset of Menses + Basal-Body Temperature
With this option the prospective identification of menses, early-follicular and early-luteal are possible, with retrospective identification of ovulation. The remaining phases (mid-follicular, late follicular, mid-luteal, and late luteal) would not be accurately identified.
Option #6 - Self-Report of Onset of Menses Alone
We recommend the use of this final option for menstrual-subphase identification only when budget, logistics or other constraints allow for no other methods. However, in population-based research, this method is often the only feasible option. With self-report of onset of menses, accurate prospective identification of menses and early follicular subphases is possible. However, the prospective identification of the remaining menstrual subphases will likely contain substantial error (Bean et al., 1979; Creinin et al., 2004; Jukic et al., 2007; Shirtcliff et al., 2001; Small et al., 2007; Wegienka & Baird, 2005). Given daily hormonal fluctuations, along with the high variability among women in length of menstrual phases, we do not recommend use of this method to identify any luteal menstrual phase. Identification of mid- and late-follicular phases is questionable also as is the ability to make comparisons of outcomes between subphases. At the very least, we strongly caution researchers regarding the high likelihood of misclassification with this option.
Recommendations for Describing Methodology Used
Finally, for presentation and publication of results of biobehavioral research in which menstrual phase has been examined, we offer the following recommendations to facilitate cross-study comparisons. First, presentation of study findings should include an explicit description of which methodology was used to identify menstrual subphase, including how the subphase was defined, if identification of phase was made prospectively or retrospectively, and if operational definitions were made a priori. Further, researchers should include a frank discussion of the strengths and limitations of the methodology used as it applies to their observations. Second, when sex hormone values are available, mean values for each subphase should be provided. We recommend that sex hormone levels be presented as absolute values for each hormone (e.g., mean value of progesterone and estradiol), a ratio of the hormones (progesterone/estradiol), and the change(s) in hormone levels between two or more time points. See Table 6 for a selection of published articles that utilized exemplar approaches.
Table 6.
Articles with Exemplar Approaches
| Allen SS, Bade T, Center B, Finstad D, Hatsukami D. (2008). Menstrual phase effects on smoking relapse. Addiction, 103, 809–821. |
| Childs E, Dlugos A, de Wit H. (2010). Cardiovascular, hormonal, and emotional responses to the TSST in relation to sex and menstrual cycle phase. Psychophysiology, 47, 550–559. |
| Evans SM, Levin FR. (2011). Response to alcohol in women: Role of the menstrual cycle and a family history of alcoholism. Drug and Alcohol Dependence, 114, 18–30. |
| Gordon JL, Girdler SS. (2014). Mechanisms underlying hemodynamic and neuroendocrine stress reactivity at different phases of the menstrual cycle. Psychophysiology, 51, 309–318. |
| Gray KM, DeSantis SM, Carpenter MJ, Saladin ME, LaRowe DS, Upadhyaya HP. (2010). Menstrual cycle and cue reactivity in women smokers. Nicotine and Tobacco Research, 12, 174–178. |
| Nillni YI, Rohan KJ, Zvolensky MJ. (2012). The role of menstrual cycle phase and anxiety sensitivity in catastrophic misinterpretation of physical symptoms during a CO2 challenge. Archives of Women’s Mental Health, 15, 413–422. |
| Ossewaarde L, Hermans EJ, van Wigen GA, Kooijman SC, Johansson IM, Backstrom T, Fernandez G. (2010). Neural mechanisms underlying changes in stress-sensitivity across the menstrual cycle. Psychoneuroendocrinology, 35, 47–55. |
| Veldhuijzen DS, Keaser ML, Traub DS, Zhuo J, Gullapalli RP, Greenspan JD. (2013). The role of circulating sex hormones in menstrual cycle-dependent modulation of pain-related brain activation. Pain, 154, 548,559. |
Research focused on quantifying and improving accurate identification of menstrual phase is needed. Specifically, future research should quantify the validity and reliability of our recommended methods for identifying menstrual phase. Additional research on alternative methods for identifying menstrual phase is ongoing. For instance, a new methodology may soon become available that would provide an alternative to urine LH testing. Recently, a series of papers has been published on determining ovulation by monitoring ovarian activity via hormone metabolites (glucuronide and pregnanediol glucuronide; metabolites of estradiol and progesterone, respectively) found in the urine (Blackwell et al., 2003, 2012; Blackwell, Vigil, Cooke, d’Arcangues, & Brown, 2013). This type of testing was used by one of the articles in our review (Fischer et al., 2011). With this method, participants tested their urine daily for 10 days around expected ovulation using Ovarian Monitor tests. Greater than 90% agreement was observed between lay users and lab tests in identification of ovulation within one day (Blackwell et al., 2012). This shows slightly improved reliability over the identification of ovulation via urine LH home tests (89% agreement within one day) (Martinez, Bernardus, Vermeiden, & Schoemaker, 1992). However, the cost and availability of these new home tests may initially present more of a challenge than those associated with the current widely used urine LH home tests. A second new methodology is dried blood spots (DBS). DBS are drops of whole blood that have been collected on filter paper from a finger prick (McDade, Williams, & Snodgrass, 2007). Collection of DBS is becoming more common in both clinical and population-based research given its low-cost and low participant burden (McDade et al., 2007). Many different biomarkers and hormones can be measured via DBS (McDade et al., 2007), including estradiol, luteinizing hormone, and progesterone(Shirtcliff et al., 2001; Worthman & Stallings, 1994). Therefore, this low-cost, low-burden method could be used to identify menstrual phase (Shirtcliff et al., 2001). No articles in our review used DBS to assess menstrual phase. These alternative methodologies, though promising, have yet to be fully evaluated in biobehavioral research.
This review is not without limitations. First, our eligibility criteria for article inclusion have resulted in the exclusion of some seminal publications. Specifically, there are additional studies that were not included in this review even though they operationally defined menstrual phases using similar techniques (e.g., self-report of onset of menses, assessment of hormone values in blood samples, urine luteinizing hormone testing) and used it as an independent variable in biobehavioral research. These studies were excluded for a variety of reasons such as the inclusion of a group of women on hormonal contraceptives (e.g., Pletzer, Kronbichler, Nuerk, & Kerschbaum, 2014, Kowalczyk et al., 2010; Kowalczyk, Evans, Bisaga, Sullivan, & Comer, 2006), the inclusion of a group of women with menstrual-related problems such as premenstrual dysphoric disorder (e.g., (Gingnell, Bannbers, Wikström, Fredrikson, & Sundström-Poromaa, 2013; Reed et al., 2008) or delivery of other exogenous hormones during the study (e.g., (Craig et al., 2008; Evans, Blank, Sams, Weaver, & Eissenberg, 2006; Reed et al., 2010). Therefore, this review does not comprehensively contain all articles with relevant methodology. However, our selected articles met specific criterion for this review and provides an accurate reflection of the current state of methodologies contained in the literature at large. A second limitation is that our recommendations have not yet been directly compared to one another. Thus, our rank ordering has been determined based on expert opinion rather than scientific data. Future work should compare the validity of these recommended strategies. Despite these limitations, our recommendations have the potential to significantly increase the consistency of methods used in the field.
Conclusions
In sum, substantial inconsistencies currently exist in the methodologies used to identify menstrual phase in biobehavioral research. Enhancing the reproducibility of biomedical research publications has become a priority for the National Institutes of Health (Collins & Tabak, 2014). Therefore, we have provided biobehavioral researchers with six recommended methodological options for accurately identifying menstrual phases and subphases. The first methodology (sonography) will usually be prohibited by cost and burden, and the last methodology, self-report of onset of menses, has limited application given the between- and within-woman variation of the length of menstrual phases and, thus, does not provide capability for phase or subphase comparisons across studies. In lieu of sonography, the most favorable approaches include direct measurement of hormones combined with additional techniques (e.g. self-report, urinary LH testing). In choosing among the remaining three methodologies, in addition to cost and participant burden considerations, the major deciding factor should be which subphases are of interest given the research question and which methodology can most accurately identify those phases. Finally, special consideration should also be given to eligibility criteria and measurement of potential confounders. Publication of study results should include explicit description of methodologies used, along with mean hormonal values observed for each subphase (when available). Ultimately, the adoption of these recommendations will reduce misclassification and related bias within studies, improve cross-study comparisons and allow for reproducibility of study results, which will lead to an overall improvement in the state of the literature.
Supplementary Material
Contributor Information
Alicia M. Allen, Email: alle0299@umn.edu, Department of Family Medicine & Community Health, Medical School, University of Minnesota; Mailing Address: 717 Delaware Street SE, Room 422, Minneapolis, Minnesota, USA 55414
Aimee L. McRae-Clark, Email: mcraeal@musc.edu, Department of Psychiatry and Behavioral Sciences, Medical University of South Carolina; Mailing Address: 67 President Street, Charleston, SC, USA 29425
Samantha Carlson, Email: carl4713@umn.edu, Department of Family Medicine & Community Health, Medical School, University of Minnesota; Mailing Address: 717 Delaware Street SE, Room 400, Minneapolis, Minnesota, USA 55414.
Michael E. Saladin, Email: saladinm@musc.edu, Department of Health Sciences and Research, College of Health Professions, Medical University of South Carolina; Mailing Address: 77 President St., Charleston, SC, USA 29425
Kevin M. Gray, Email: graykm@musc.edu, Department of Psychiatry and Behavioral Sciences, Medical University of South Carolina; Mailing Address: 125 Doughty Street, Suite 190, MSC861, Charleston SC, USA 29425
Cora Lee Wetherington, Email: cwetheri@nida.nih.gov, National Institute on Drug Abuse, National Institutes of Health; Mailing Address: National Institute on Drug Abuse, 6001 Executive Blvd, Suite 3155, Bethesda, MD, USA 20892-9593
Sherry A. McKee, Email: sherry.mckeey@yale.edu, Department of Psychiatry, Yale University School of Medicine; Mailing Address: 2 Church St. South, #109, New Haven, CT, USA 06519
Sharon S. Allen, Email: allen001@umn.edu, Department of Family Medicine & Community Health, Medical School, University of Minnesota; Mailing Address: 420 Delaware Street SE, MMC 381 Mayo, Minneapolis, Minnesota, USA 55455
References
- Allen SS, Bade T, Center B, Finstad D, Hatsukami D. Menstrual phase effects on smoking relapse. Addiction (Abingdon, England) 2008;103(5):809–21. doi: 10.1111/j.1360-0443.2008.02146.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen SS, Hatsukami DK, Christianson D, Nelson D. Withdrawal and pre-menstrual symptomatology during the menstrual cycle in short-term smoking abstinence: effects of menstrual cycle on smoking abstinence. Nicotine & Tobacco Research: Official Journal of the Society for Research on Nicotine and Tobacco. 1999;1(2):129–42. doi: 10.1080/14622299050011241. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/11072394. [DOI] [PubMed] [Google Scholar]
- Anker JJ, Carroll ME. Sex differences in the effects of allopregnanolone on yohimbine-induced reinstatement of cocaine seeking in rats. Drug and Alcohol Dependence. 2010;107(2–3):264–7. doi: 10.1016/j.drugalcdep.2009.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bean JA, Leeper JD, Wallace RB, Sherman BM, Jagger H. Variations in the reporting of menstrual histories. American Journal of Epidemiology. 1979;109(2):181–5. doi: 10.1093/oxfordjournals.aje.a112673. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/425957. [DOI] [PubMed] [Google Scholar]
- Becker JB, Berkley KJ, Geary N, Hampson E, Herman JP, Young EA, editors. Sex Differences in the Brain: From Genes to Behavior. New York, NY: Oxford University Press, Inc; 2008. [Google Scholar]
- Blackwell LF, Brown JB, Vigil P, Gross B, Sufi S, d’Arcangues C. Hormonal monitoring of ovarian activity using the Ovarian Monitor, part I. Validation of home and laboratory results obtained during ovulatory cycles by comparison with radioimmunoassay. Steroids. 2003;68(5):465–76. doi: 10.1016/s0039-128x(03)00049-7. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/12798498. [DOI] [PubMed] [Google Scholar]
- Blackwell LF, Vigil P, Cooke DG, d’Arcangues C, Brown JB. Monitoring of ovarian activity by daily measurement of urinary excretion rates of oestrone glucuronide and pregnanediol glucuronide using the Ovarian Monitor, Part III: Variability of normal menstrual cycle profiles. Human Reproduction (Oxford, England) 2013;28(12):3306–15. doi: 10.1093/humrep/det389. [DOI] [PubMed] [Google Scholar]
- Blackwell LF, Vigil P, Gross B, d’Arcangues C, Cooke DG, Brown JB. Monitoring of ovarian activity by measurement of urinary excretion rates of estrone glucuronide and pregnanediol glucuronide using the Ovarian Monitor, Part II: reliability of home testing. Human Reproduction (Oxford, England) 2012;27(2):550–7. doi: 10.1093/humrep/der409. [DOI] [PubMed] [Google Scholar]
- Brennan IM, Feltrin KL, Nair NS, Hausken T, Little TJ, Gentilcore D, Tj L. Effects of the phases of the menstrual cycle on gastric emptying, glycemia, plasma GLP-1 and insulin, and energy intake in healthy lean women. American Journal of Gastrointestional Liver Physiology. 2009;297:G602–G610. doi: 10.1152/ajpgi.00051.2009. [DOI] [PubMed] [Google Scholar]
- Carpenter MJ, Upadhyaya HP, LaRowe SD, Saladin ME, Brady KT. Menstrual cycle phase effects on nicotine withdrawal and cigarette craving: a review. Nicotine & Tobacco Research: Official Journal of the Society for Research on Nicotine and Tobacco. 2006;8(5):627–38. doi: 10.1080/14622200600910793. [DOI] [PubMed] [Google Scholar]
- Celec P, Ostaniková D, Skoknová M, Hodosy J, Putz Z, Kúdela M. Salivary sex hormones during the menstrual cycle. Endocrine Journal. 2009;56(3):521–3. doi: 10.1507/endocrj.k09e-020. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/19194049. [DOI] [PubMed] [Google Scholar]
- Childs E, Dlugos A, de Wit H. Cardiovascular, hormonal, and emotional responses to the TSST in relation to sex and menstrual cycle phase. Psychophysiology. 2010;47:550–559. doi: 10.1111/j.1469-8986.2009.00961.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi JC, Park SK, Kim YH, Shin YW, Kwon JS, Kim JS, Lee MS. Different brain activation patterns to pain and pain-related unpleasantness during the menstrual cycle. Anesthesiology. 2006;105(1):120–7. doi: 10.1097/00000542-200607000-00021. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/16810003. [DOI] [PubMed] [Google Scholar]
- Cole La, Ladner DG, Byrn FW. The normal variabilities of the menstrual cycle. Fertility and Sterility. 2009;91(2):522–7. doi: 10.1016/j.fertnstert.2007.11.073. [DOI] [PubMed] [Google Scholar]
- Collins FS, Tabak LA. Policy: NIH plans to enhance reproducibility. Nature. 2014;505(7485):612–3. doi: 10.1038/505612a. Retrieved from http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=4058759&tool=pmcentrez&rendertype=abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig MC, Fletcher PC, Daly EM, Picchioni MM, Brammer M, Giampietro V, Murphy DGM. A study of visuospatial working memory pre- and post-Gonadotropin Hormone Releasing Hormone agonists (GnRHa) in young women. Hormones and Behavior. 2008;54(1):47–59. doi: 10.1016/j.yhbeh.2008.01.012. [DOI] [PubMed] [Google Scholar]
- Cramer DW, Barbieri RL, Fraer AR, Harlow BL. Determinants of early follicular phase gonadotrophin and estradiol concentrations in women of late reproductive age. Human Reproduction (Oxford, England) 2002;17(1):221–7. doi: 10.1093/humrep/17.1.221. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/11756392. [DOI] [PubMed] [Google Scholar]
- Creinin MD, Keverline S, Meyn LA. How regular is regular? An analysis of menstrual cycle regularity. Contraception. 2004;70(4):289–92. doi: 10.1016/j.contraception.2004.04.012. [DOI] [PubMed] [Google Scholar]
- Derntl B, Windischberger C, Robinson S, Lamplmayr E, Kryspin-Exner I, Gur RC, Habel U. Facial emotion recognition and amygdala activation are associated with menstrual cycle phase. Psychoneuroendocrinology. 2008;33(8):1031–40. doi: 10.1016/j.psyneuen.2008.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans SE, Blank M, Sams C, Weaver MF, Eissenberg T. Transdermal nicotine-induced tobacco abstinence symptom suppression: nicotine dose and smokers’ gender. Experimental and Clinical Psychopharmacology. 2006;14(2):121–35. doi: 10.1037/1064-1297.14.2.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans SM, Levin FR. Response to alcohol in women: Role of the menstrual cycle and a family history of alcoholism. Drug and Alcohol Dependence. 2011;114:18–30. doi: 10.1016/j.drugalcdep.2010.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer J, Semple S, Fickenscher G, Jürgens R, Kruse E, Heistermann M, Amir O. Do women’s voices provide cues of the likelihood of ovulation? The importance of sampling regime. PloS One. 2011;6(9):e24490. doi: 10.1371/journal.pone.0024490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gandara BK, Leresche L, Mancl L. Patterns of salivary estradiol and progesterone across the menstrual cycle. Annals of the New York Academy of Sciences. 2007;1098:446–50. doi: 10.1196/annals.1384.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gingnell M, Bannbers E, Wikström J, Fredrikson M, Sundström-Poromaa I. Premenstrual dysphoric disorder and prefrontal reactivity during anticipation of emotional stimuli. European Neuropsychopharmacology: The Journal of the European College of Neuropsychopharmacology. 2013;23(11):1474–83. doi: 10.1016/j.euroneuro.2013.08.002. [DOI] [PubMed] [Google Scholar]
- Gordon JL, Girdler SS. Mechanisms underlying hemodynamic and neuroendocrine stress reactivity at different phases of the menstrual cycle. Psychophysiology. 2014;51(4):309–18. doi: 10.1111/psyp.12177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray KM, DeSantis SM, Carpenter MJ, Saladin ME, LaRowe DS, Upadhyaya HP. Menstrual cycle and cue reactivity in women smokers. Nicotine and Tobacco Research. 2010;12:174–178. doi: 10.1093/ntr/ntp179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gröschl M, Rauh M. Influence of commercial collection devices for saliva on the reliability of salivary steroids analysis. Steroids. 2006;71(13–14):1097–100. doi: 10.1016/j.steroids.2006.09.007. [DOI] [PubMed] [Google Scholar]
- Harlow BL, Wise LA, Otto MW, Soares CN, Cohen LS. Depression and its influence on reproductive endocrine and menstrual cycle markers associated with perimenopause: the Harvard Study of Moods and Cycles. Archives of General Psychiatry. 2003;60(1):29–36. doi: 10.1001/archpsyc.60.1.29. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/12511170. [DOI] [PubMed] [Google Scholar]
- Hoeger Bement MK, Rasiarmos RL, DiCapo JM, Lewis A, Keller ML, Harkins AL, Hunter SK. The role of the menstrual cycle phase in pain perception before and after an isometric fatiguing contraction. European Journal of Applied Physiology. 2009;106(1):105–12. doi: 10.1007/s00421-009-0995-8. [DOI] [PubMed] [Google Scholar]
- Holdstock L, de Wit H. Effects of ethanol at four phases of the menstrual cycle. Psychopharmacology. 2000;150(4):374–82. doi: 10.1007/s002130000461. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/10958078. [DOI] [PubMed] [Google Scholar]
- Jukic AMZ, Weinberg CR, Baird DD, Wilcox AJ. Lifestyle and reproductive factors associated with follicular phase length. Journal of Women’s Health (2002) 2007;16(9):1340–7. doi: 10.1089/jwh.2007.0354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerin J. Ovulation detection in the human. Clinical Reproduction and Fertility. 1982;1(1):27–54. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/6821195. [PubMed] [Google Scholar]
- Kowalczyk WJ, Evans SM, Bisaga AM, Sullivan MA, Comer SD. Sex differences and hormonal influences on response to cold pressor pain in humans. The Journal of Pain: Official Journal of the American Pain Society. 2006;7(3):151–60. doi: 10.1016/j.jpain.2005.10.004. [DOI] [PubMed] [Google Scholar]
- Kowalczyk WJ, Sullivan MA, Evans SM, Bisaga AM, Vosburg SK, Comer SD. Sex differences and hormonal influences on response to mechanical pressure pain in humans. The Journal of Pain: Official Journal of the American Pain Society. 2010;11(4):330–42. doi: 10.1016/j.jpain.2009.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Gold EB, Lasley BL, Johnson WO. Factors affecting menstrual cycle characteristics. American Journal of Epidemiology. 2004;160(2):131–40. doi: 10.1093/aje/kwh188. [DOI] [PubMed] [Google Scholar]
- Lynch WJ, Sofuoglu M. Role of progesterone in nicotine addiction: evidence from initiation to relapse. Experimental and Clinical Psychopharmacology. 2010;18(6):451–61. doi: 10.1037/a0021265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez AR, Bernardus RE, Vermeiden JP, Schoemaker J. Reliability of home urinary LH tests for timing of insemination: a consumer’s study. Human Reproduction (Oxford, England) 1992;7(6):751–3. doi: 10.1093/oxfordjournals.humrep.a137731. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/1500469. [DOI] [PubMed] [Google Scholar]
- McDade TW, Williams S, Snodgrass JJ. What a drop can do: dried blood spots as a minimally invasive method for integrating biomarkers into population-based research. Demography. 2007;44(4):899–925. doi: 10.1353/dem.2007.0038. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/18232218. [DOI] [PubMed] [Google Scholar]
- Metcalf MG, Evans JJ, Mackenzie JA. Indices of ovulation: comparison of plasma and salivary levels of progesterone with urinary pregnanediol. The Journal of Endocrinology. 1984;100(1):75–80. doi: 10.1677/joe.0.1000075. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/6690646. [DOI] [PubMed] [Google Scholar]
- Moghissi KS. Accuracy of basal body temperature for ovulation detection. Fertility and Sterility. 1976;27(12):1415–21. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/1001528. [PubMed] [Google Scholar]
- Nielsen SE, Ahmed I, Cahill L. Postlearning stress differentially affects memory for emotional gist and detail in naturally cycling women and women on hormonal contraceptives. Behavioral Neuroscience. 2014;128(4):482–93. doi: 10.1037/a0036687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nillni YI, Rohan KJ, Zvolensky MJ. The role of menstrual cycle phase and anxiety sensitivity in catastrophic misinterpretation of physical symptoms during a CO2 challenge. Archives of Women’s Mental Health. 2012;15:413–422. doi: 10.1007/s00737-012-0302-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ossewaarde L, Hermans EJ, van Wigen GA, Kooijman SC, Johansson IM, Backstrom T, Fernandez G. Neural mechanisms underlying changes in stress-sensitivity across the menstrual cycle. Psychoneuroendocrinology. 2010;35:47–55. doi: 10.1016/j.psyneuen.2009.08.011. [DOI] [PubMed] [Google Scholar]
- Pletzer B, Kronbichler M, Nuerk HC, Kerschbaum H. Hormonal contraceptives masculinize brain activation patterns in the absence of behavioral changes in two numerical tasks. Brain Research. 2014;1543:128–42. doi: 10.1016/j.brainres.2013.11.007. [DOI] [PubMed] [Google Scholar]
- Pletzer B, Petasis O, Cahill L. Switching between forest and trees: opposite relationship of progesterone and testosterone to global-local processing. Hormones and Behavior. 2014;66(2):257–66. doi: 10.1016/j.yhbeh.2014.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed SC, Evans SM. In: Women and Addiction: A Comprehensive Handbook. Brady KT, Back SE, Greenfield SF, editors. New York, NY: The Guildford Press; 2009. pp. 14–31. [Google Scholar]
- Reed SC, Evans SM, Bedi G, Rubin E, Foltin RW. The effects of oral micronized progesterone on smoked cocaine self-administration in women. Hormones and Behavior. 2011;59(2):227–35. doi: 10.1016/j.yhbeh.2010.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed SC, Levin FR, Evans SM. Changes in mood, cognitive performance and appetite in the late luteal and follicular phases of the menstrual cycle in women with and without PMDD (premenstrual dysphoric disorder) Hormones and Behavior. 2008;54(1):185–93. doi: 10.1016/j.yhbeh.2008.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed SC, Levin FR, Evans SM. The effects of progesterone pretreatment on the response to oral d-amphetamine in Women. Hormones and Behavior. 2010;58(3):533–43. doi: 10.1016/j.yhbeh.2010.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riad-Fahmy D, Read GF, Walker RF, Walker SM, Griffiths K. Determination of ovarian steroid hormone levels in saliva. An overview. The Journal of Reproductive Medicine. 1987;32(4):254–72. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/3585869. [PubMed] [Google Scholar]
- Schoofs D, Wolf OT. Stress and memory retrieval in women: no strong impairing effect during the luteal phase. Behavioral Neuroscience. 2009;123(3):547–54. doi: 10.1037/a0015625. [DOI] [PubMed] [Google Scholar]
- Shirtcliff EA, Granger DA, Schwartz E, Curran MJ. Use of salivary biomarkers in biobehavioral research: cotton-based sample collection methods can interfere with salivary immunoassay results. Psychoneuroendocrinology. 2001;26(2):165–73. doi: 10.1016/s0306-4530(00)00042-1. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/11087962. [DOI] [PubMed] [Google Scholar]
- Small CM, Manatunga AK, Marcus M. Validity of self-reported menstrual cycle length. Annals of Epidemiology. 2007;17(3):163–70. doi: 10.1016/j.annepidem.2006.05.005. [DOI] [PubMed] [Google Scholar]
- Veldhuijzen DS, Keaser ML, Traub DS, Zhuo J, Gullapalli RP, Greenspan JD. The role of circulating sex hormones in menstrual cycle-dependent modulation of pain-related brain activation. Pain. 2013;154:548, 559. doi: 10.1016/j.pain.2012.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wegienka G, Baird DD. A comparison of recalled date of last menstrual period with prospectively recorded dates. Journal of Women’s Health (2002) 2005;14(3):248–52. doi: 10.1089/jwh.2005.14.248. [DOI] [PubMed] [Google Scholar]
- Wood P. Salivary steroid assays - research or routine? Annals of Clinical Biochemistry. 2009;46(Pt 3):183–96. doi: 10.1258/acb.2008.008208. [DOI] [PubMed] [Google Scholar]
- Worthman CM, Stallings JF. Measurement of gonadotropins in dried blood spots. Clinical Chemistry. 1994;40(3):448–53. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/8131281. [PubMed] [Google Scholar]
- Yen SSC, Jaffee RB, Barbieri RL. Reproductive Endocrinology: Physiolog, Pathophysiology and Clinical Management. Philadelphia: W.B. Saunders Company; 1999. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.