Abstract
The purpose of this study was to determine the relationship between total antioxidant capacity (TAC) from diet and supplements and prostate cancer aggressiveness among 855 African Americans (AA) and 945 European Americans (EA) in the North Carolina-Louisiana Prostate Cancer Project (PCaP). Cases were classified as either high aggressive, low aggressive, or intermediate aggressive. TAC was calculated from the vitamin C equivalent antioxidant capacity of 42 antioxidants measured via food frequency questionnaire. EA reported greater dietary TAC from diet and supplements combined (P < 0.0001). In both minimally and fully adjusted logistic regression models, TAC from diet and supplements combined was associated with a reduced odds of high aggressive prostate cancer in all men, AA and EA: ORs for highest vs. lowest level (> 1500 vs. < 500 mg VCE/d): 0.31 (95% CI: 0.15, 0.67; P-trend < 0.01), 0.28 (95% CI: 0.08, 0.96; P-trend < 0.001), and 0.36 (95% CI: 0.15, 0.86; P-trend = 0.58), respectively. These associations did not appear to differ between AA and EA. These data suggest that greater intake of antioxidants is associated with less aggressive prostate cancer. Additional research is needed to confirm these results and determine the underlying mechanisms.
Introduction
Prostate cancer is the most common non-cutaneous cancer and second most common cause of cancer related mortality in men in the US, with an estimated 238,590 new cases and 29,720 deaths in 2013 (1). The specific causes of prostate cancer have not yet been determined, but several risk factors have been identified for the disease, namely family history, age, and race (2, 3). There is also evidence that components of the diet, such as antioxidants found in the diet and supplements, also may be associated with prostate cancer risk. Men with prostate cancer have lower blood levels of vitamins E and C compared with controls (4), lower glutathione peroxidase and superoxide dismutase activity, and greater levels of malondialdehyde in erythrocytes (5, 6), all of which may reflect greater oxidative stress. Levels of antioxidants in the blood may interact with genetic variants for enzymes involved in inflammation (7) and DNA damage repair (8, 9). Based on this, as well as evidence from numerous human studies (10, 11), it is plausible that antioxidants from diet and supplements, such as vitamin E or carotenoids, may influence prostate cancer development and progression. Despite a large body of evidence for race and a growing body of evidence for diet as risk factors for prostate cancer relatively few studies have studied dietary antioxidants and prostate cancer in both African Americans (AA) and European Americans (EA) (12–14), two groups that exhibit a striking disparity in prostate cancer incidence and mortality (15).
However, studying the antioxidants present in diet and supplements is complicated by the sheer variety and magnitude of compounds that might affect prostate cancer. To examine the association between each individual antioxidant and prostate cancer risk would be impractical and likely to produce potentially spurious findings. One method for measuring dietary antioxidants that may circumvent such issues of multiplicity is the total antioxidant capacity (TAC) of the diet (16). TAC is a cumulative index of antioxidant intake. Greater dietary TAC has been associated with lower plasma concentrations of high-sensitivity C-reactive protein (17) and a reduced risk of all-cause mortality (18). There has been one study of dietary TAC and prostate cancer to date (19), but there have been no reported studies of the association between dietary antioxidants and prostate cancer aggressiveness separately in European and African Americans. And while advanced or lethal prostate cancer is relatively rare, these forms of the disease have a greater risk of mortality and are arguably more clinically relevant than indolent forms of the disease (20). This demonstrates a need to determine whether antioxidant intake might influence risk of and racial disparities in aggressive forms of the disease, an important step in identifying modifiable lifestyle factors in an effort to reduce prostate cancer severity and mortality.
Thus, the purpose of the current study was to determine the association between dietary TAC and prostate cancer aggressiveness using data from a population-based study of prostate cancer aggressiveness among similar numbers of AA and EA. We hypothesized that dietary TAC would be inversely associated with prostate cancer aggressiveness.
Methods
Data were analyzed from the North Carolina-Louisiana Prostate Cancer Project (PCaP), a population-based study of incident prostate cancer in AA and EA. Details of the study methods have been published (21). Research subjects were recruited from September 1, 2004 to April 31, 2009, from 42 counties in North Carolina and 21 parishes in Louisiana. Men between 40 and 79 years of age with a first diagnosis of histologically confirmed adenocarcinoma of the prostate on or after July 1, 2004 were eligible to participate in PCaP. Men were not eligible to participate in the study if they were unable to complete the interview in English, living in an institution or nursing home, cognitively impaired, under the influence of alcohol, severely medicated, or apparently psychotic. Research subjects also must have self-identified as at least part AA or Black, or Caucasian or White (EA) when they responded to the open-ended question “What is your race?” The project was approved by the institutional review boards at the University of North Carolina, Louisiana State University Health Sciences Center, and the Department of Defense Prostate Cancer Research Program. The current study was approved by the Institutional Review Board at the University of Connecticut. Informed consent was obtained from all individual participants included in the study.
Data were collected by a series of structured questionnaires administered by PCaP nurses. Dietary data for the year prior to diagnosis were collected using the National Cancer Institute Diet History Questionnaire (NCI DHQ) (22), which was modified to include several Southern foods (e.g. grits, watermelon, and okra). Values for the flavonoid and proanthocyanidin content of foods were added to the DHQ database using Nutrition Data System for Research, version 2011 (Nutrition Coordinating Center, University of Minnesota, Minneapolis, MN). Antioxidants from supplements (both multivitamin and individual) that were considered in analyses included β-carotene, lycopene, lutein, α-tocopherol, and vitamin C; since dietary data was only available on lutein and zeaxanthin combined, lutein from supplements was added to dietary lutein and zeaxanthin to determine the combined amount of dietary and supplemental lutein and zeaxanthin. Total antioxidant capacity (TAC) was calculated from the vitamin C equivalent antioxidant capacity of 42 antioxidants (carotenoids, vitamins C and E, flavonoids, isoflavones, and proanthocyanidins) (Equation 1). Vitamin C equivalent (VCE) antioxidant capacities of individual antioxidants were previously measured using the ABTS (2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) radical anion scavenging activity assay using a vitamin C standard (23). Using this method, daily intake of these 42 antioxidants was converted to vitamin C equivalents and summed to yield an estimate of average TAC per day.
| Equation 1 |
Calculation of theoretical TAC from diet and supplements, where D and S represent individual antioxidants from diet and supplements, respectively, in milligrams and C represents the vitamin C equivalent (VCE) antioxidant capacity per milligram of antioxidant.
Clinical stage, Gleason score, and prostate-specific antigen (PSA) at diagnosis were obtained from medical record abstraction. Research subjects were classified as either high aggressive (Gleason sum ≥8, or PSA >20 ng/ml, or Gleason sum = 7 and clinical stage T3–T4), low aggressive (Gleason sum <7 and clinical stage T1–T2 and PSA <10 ng/ml), or intermediate aggressive (all other cases). Results from PCaP using this categorization scheme have been published (24, 25), and are based on the risk strata described by the American Urological Association (26). A total of 2,258 men participated in PCaP. After excluding research subjects who did not respond to 5 or more questions on the DHQ (n=18), reported extreme or unlikely dietary TAC (defined as greater than 1.5 times the interquartile range of TAC from diet and supplements) (n=144), or were missing aggressiveness classification or data on covariates (n=292), 1,800 research subjects were included in all analyses.
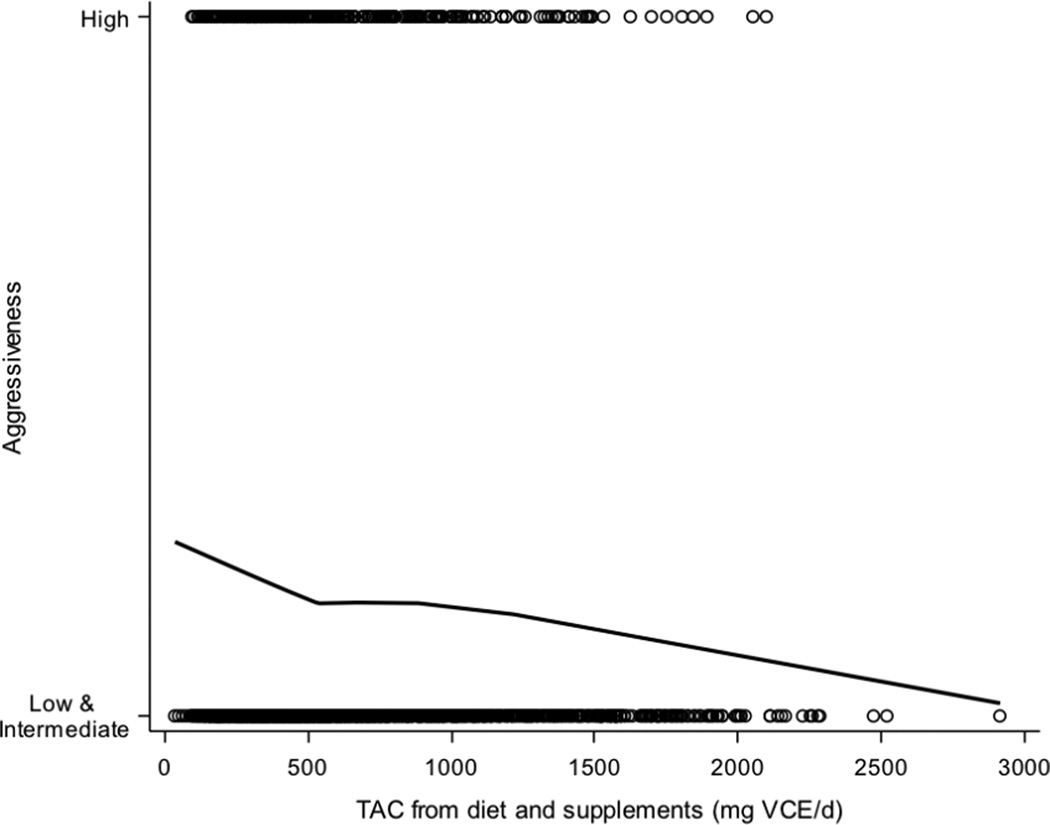
Dietary TAC was adjusted for total energy intake using the residual method (27) and energy adjusted values for TAC were used in all analyses. Research subjects were divided into four categories based on TAC both from diet and from diet and supplements combined: < 500, 500 ≤ 1000, 1000 ≤ 1500, and > 1500 mg VCE/d. These categories were determined based on visual assessment of a locally weighted scatter plot smoothing of TAC predicting aggressiveness (Figure 1). The relationship between TAC and prostate cancer aggressiveness was also determined by including TAC as a continuous predictor with the odds ratio reflecting a 500-unit increase in TAC. All statistical analyses were conducted using SAS software, version 9.3 (SAS Institute Inc., Cary, NC, USA). Chi-squared tests and Mann-Whitney-Wilcoxon tests were used to test for significant differences between AA and EA.
Figure 1.
LOESS plot of average daily TAC from diet and supplements predicting prostate cancer aggressiveness. The solid line is the smoothed LOESS line of TAC predicting aggressiveness; empty circles represent individual participants. The smoothing parameter was chosen based on minimization of the corrected Akaike information criterion (AICC). Abbreviations: TAC, total antioxidant capacity; VCE, vitamin C equivalents.
Logistic regression was used to compare high to low and intermediate aggressive prostate cancer cases by categories of TAC from both diet alone and from diet and supplements, with a TAC of < 500 mg VCE/d as the referent category. Since the relationship between antioxidant intake and high aggressive prostate cancer was of interest, low and intermediate aggressive cases were collapsed into a single category. Interactions between aggressiveness, TAC, other covariates, and race were determined based on the significance of the interaction term and assessment using 3-way tables; since a significant interaction was found between smoking status, race, and aggressiveness, an interaction term between smoking and race was included in logistic regression models. Logistic regression model 1 was adjusted for age, race, smoking status, and race-smoking interaction. Model 2 consisted of model 1, additionally adjusted for poverty index, marriage, previous digital rectal examination (DRE) and prostate specific antigen (PSA) screening, and body mass index. These control variables were chosen since they are likely to be associated with both antioxidant intake and prostate cancer. An interaction term between race and TAC was included in all models to allow for the calculation of odds ratios for all men, AA, and EA from each model. Linear contrasts were used to test for the significance of a linear trend in the odds ratios for each group. All P-values reported are two sided (α = 0.05). To visualize the relationship between TAC and prostate cancer aggressiveness for all men, and AA and EA separately, the odds ratios and 95% confidence intervals were plotted for TAC predicting prostate cancer aggressiveness in fully adjusted models as a linear predictor and as a restricted cubic spline with knots located at the 10th, 50th, and 90th percentiles (Figure 2 and 3).
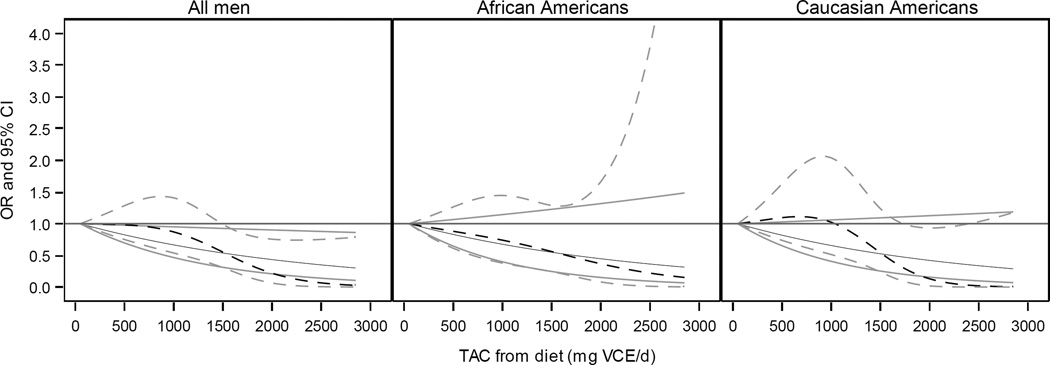
Figure 2.
Plot of odds ratio (black) and 95% confidence interval (grey) for total antioxidant capacity (TAC) of diet predicting odds of prostate cancer aggressiveness for all men, African Americans, and Caucasian Americans. Solid lines represent linear model of TAC on a continuous scale; broken lines represent model of TAC using a restricted cubic spline with knots located at the 10th, 50th, and 90th percentiles. Models adjusted for average energy intake, age, smoking, race, race-smoking interaction, poverty index, marriage, body mass index, and DRE and PSA screening. Results by race determined using an interaction term between TAC and race.
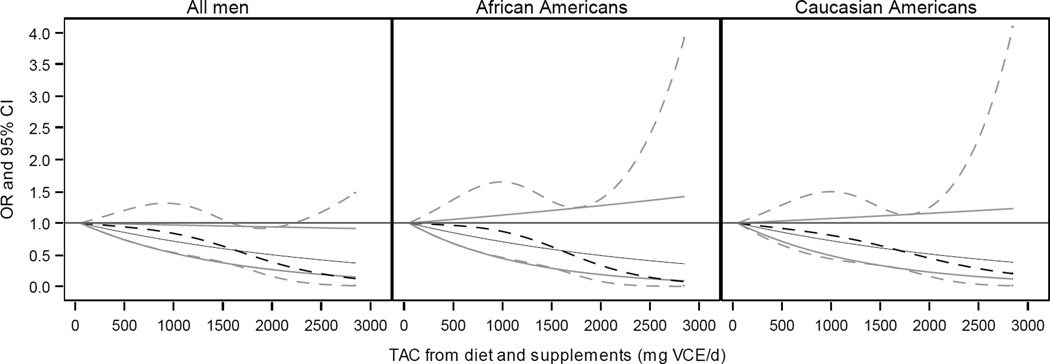
Figure 3.
Plot of odds ratio (black) and 95% confidence interval (grey) for total antioxidant capacity (TAC) of diet and supplements predicting odds of prostate cancer aggressiveness for all men, African Americans, and Caucasian Americans. Solid lines represent linear model of TAC on a continuous scale; broken lines represent model of TAC using a restricted cubic spline with knots located at the 10th, 50th, and 90th percentiles. Models adjusted for average energy intake, age, smoking, race, race-smoking interaction, poverty index, marriage, body mass index, and DRE and PSA screening. Results by race determined using an interaction term between TAC and race.
Results
Prostate cancer cases included 459 AA and 498 EA from Louisiana and 396 AA and 447 EA from North Carolina (Table 1). Since site was not a significant predictor (P = 0.99) it was excluded from all regression models. There were no significant differences between subjects included and excluded from analyses for variables in Table 1 (data not shown). Compared to EA, more AA had high aggressive prostate cancer, no previous PSA or DRE test, no health insurance, and were classified as borderline or below poverty thresholds by the US Census Bureau (P < 0.05). A greater proportion of EA were classified as overweight or obese, married, nonsmokers, and reported attaining college or professional education (P < 0.05). Compared with AA, EA consumed more antioxidants from supplements and from diet and supplements combined (Table 2).
Table 1.
Clinical, sociodemographic, and lifestyle characteristics of African and European Americans
| African Americans | European Americans | ||||
|---|---|---|---|---|---|
| n = 855 | n = 945 | ||||
| median | IQR | median | IQR | P-value1 | |
| Age (years) | 62 | (56, 67) | 64 | (59, 70) | < 0.0001 |
| n | % | n | % | P-value2 | |
| Site | |||||
| North Carolina | 396 | 47.0 | 447 | 53.0 | 0.68 |
| Louisiana | 459 | 48.0 | 498 | 52.0 | |
| Aggressiveness3 | |||||
| Low | 403 | 43.4 | 525 | 56.6 | < 0.01 |
| Intermediate | 285 | 50.5 | 279 | 49.5 | |
| High | 167 | 54.2 | 141 | 45.8 | |
| Previous PSA test | |||||
| No | 282 | 68.5 | 130 | 31.6 | < 0.0001 |
| Yes | 573 | 41.3 | 815 | 58.7 | |
| Previous DRE | |||||
| No | 174 | 63.7 | 99 | 36.3 | < 0.0001 |
| Yes | 681 | 44.6 | 846 | 55.4 | |
| BMI (kg/m2) | |||||
| < 18.5 | 11 | 57.9 | 8 | 42.1 | < 0.05 |
| 18.5 – 24.9 | 180 | 54.6 | 150 | 45.5 | |
| 25.0 – 29.9 | 336 | 43.9 | 430 | 56.1 | |
| 30 – 39.9 | 289 | 47.1 | 325 | 52.9 | |
| ≥ 40.0 | 39 | 54.9 | 32 | 45.1 | |
| Relative diagnosed <60 years old | |||||
| No | 664 | 45.5 | 796 | 54.5 | < 0.0001 |
| Yes | 119 | 60.7 | 77 | 39.3 | |
| Health insurance before diagnosis | |||||
| No | 124 | 73.4 | 45 | 26.6 | < 0.0001 |
| Yes | 725 | 44.7 | 896 | 55.3 | |
| Poverty Index4 | |||||
| Below | 50 | 75.8 | 16 | 24.2 | < 0.0001 |
| Borderline | 216 | 73.7 | 77 | 26.3 | |
| Above | 589 | 40.9 | 852 | 59.1 | |
| Education | |||||
| < High School | 246 | 72.8 | 92 | 27.2 | < 0.0001 |
| High School | 296 | 53.1 | 261 | 46.9 | |
| College | 254 | 39.2 | 394 | 60.8 | |
| Professional | 58 | 22.7 | 198 | 77.3 | |
| Married | |||||
| No | 269 | 63.4 | 155 | 36.6 | < 0.0001 |
| Yes | 586 | 42.6 | 790 | 57.4 | |
| Smoker | |||||
| Never | 263 | 43.6 | 340 | 56.4 | < 0.0001 |
| Current | 179 | 67.8 | 85 | 32.2 | |
| Former | 413 | 44.3 | 520 | 55.7 | |
Abbreviations: IQR, interquartile range; PSA, prostate specific antigen; DRE, digital rectal examination; BMI, body mass index
Due to rounding, percentages may not add to 100
Mann-Whitney-Wilcoxon test
Chi-squared test
Cases classified as low aggressive (Gleason score <7, stage ≤T2, PSA <10ng/ml), high aggressive (Gleason score ≥8; PSA ≥20 ng/ml, or Gleason score=7 if stage ≥ T3), or intermediate aggressive (all others)
Based on 2004 poverty thresholds by the US Census Bureau
Table 2.
Comparison of TAC from diet and supplements between African and European Americans
| African Americans | European Americans | ||||
|---|---|---|---|---|---|
| TAC, VCE/d | Median | IQR | Median | IQR | P value1 |
| Diet | 460 | (284, 718) | 448 | (295, 684) | 0.82 |
| Supplements | 0 | (0, 66) | 66 | (0, 135) | < 0.0001 |
| Diet and supplements | 504 | (317, 824) | 582 | (358, 956) | < 0.0001 |
Abbreviations: TAC, total antioxidant capacity; VCE/d, vitamin C equivalents per day; IQR, interquartile range
Mann-Whitney-Wilcoxon test
The results of logistic regression models of the association between TAC and odds of high aggressive prostate cancer are reported in Table 3. There were no statistically significant interactions between TAC and race (data not shown), which indicates that there is no evidence that the association between TAC and prostate cancer aggressiveness differed between AA and EA. In Model 1, there was evidence of a reduced odds of high aggressive prostate cancer in all men (P-trend < 0.05) with greater dietary TAC. This trend remained in Model 2 after adjusting for additional covariates (P-trend < 0.01). When stratified by race, a decreasing trend in the odds of high aggressive cancer was more evident among AA (Model 2, P-trend < 0.01). Among EA, greater dietary TAC was associated with a reduced odds of high aggressive prostate cancer, and most apparent among men reporting the greatest dietary TAC, OR 0.22 (95% CI: 0.05, 0.97). These results were consistent with those of dietary TAC modeled as a continuous variable (Figure 2), with a 500-unit increase in dietary TAC corresponding to odds ratios of 0.81 (95% CI: 0.67, 0.97) for all men, 0.81 (95% CI: 0.62, 1.07) for AA, and 0.80 (95% CI: 0.62, 1.03) for EA in analyses adjusted for all covariates (Table 3).
Table 3.
Odds of high aggressive prostate cancer1 by dietary TAC for all men, African Americans, and European Americans
| TAC, VCE/d (diet) | |||||||||||||||||
| < 500 mg VCE/d | 500 to 1000 mg VCE/d | 1000 to 1500 mg/VCE/d | > 1500 mg VCE/d | Continuous2 | |||||||||||||
| All Men | Cases3 | OR | 95% CI | Cases3 | OR | 95% CI | Cases3 | OR | 95% CI | Cases3 | OR | 95% CI | P-trend | Cases3 | OR | 95% CI | P-trend |
| Model 1 | 194/836 | 1.00 | Ref | 93/489 | 0.86 | (0.65, 1.13) | 17/106 | 0.68 | (0.38, 1.23) | 4/61 | 0.28 | (0.10, 0.78) | <0.05 | 308/1492 | 0.78 | (0.66, 0.93) | < 0.01 |
| Model 2 | 0.89 | (0.67, 1.18) | 0.70 | (0.38, 1.27) | 0.28 | (0.10, 0.80) | <0.01 | 0.81 | (0.67, 0.97) | < 0.05 | |||||||
| African American | |||||||||||||||||
| Model 1 | 111/407 | 1.00 | Ref | 49/221 | 0.88 | (0.60, 1.29) | 5/40 | 0.50 | (0.19, 1.31) | 2/20 | 0.32 | (0.07, 1.40) | <0.05 | 167/688 | 0.77 | (0.61, 0.98) | < 0.05 |
| Model 2 | 0.87 | (0.59, 1.30) | 0.54 | (0.20, 1.43) | 0.35 | (0.08, 1.60) | <0.01 | 0.81 | (0.62, 1.07) | 0.14 | |||||||
| European American | |||||||||||||||||
| Model 1 | 83/429 | 1.00 | Ref | 44/268 | 0.83 | (0.56, 1.24) | 12/66 | 0.94 | (0.48, 1.82) | 2/41 | 0.24 | (0.06, 1.02) | 0.49 | 141/804 | 0.80 | (0.63, 1.02) | 0.07 |
| Model 2 | 0.90 | (0.60, 1.35) | 0.91 | (0.46, 1.81) | 0.22 | (0.05, 0.97) | 0.71 | 0.80 | (0.62, 1.03) | 0.08 | |||||||
| TAC, VCE/d (diet and supplements) | |||||||||||||||||
| < 500 mg VCE/d | 500 to 1000 mg VCE/d | 1000 to 1500 mg/VCE/d | > 1500 mg VCE/d | Continuous2 | |||||||||||||
| All Men | Cases3 | OR | 95% CI | Cases3 | OR | 95% CI | Cases3 | OR | 95% CI | Cases3 | OR | 95% CI | P-trend | Cases3 | OR | 95% CI | P-trend |
| Model 1 | 167/668 | 1.00 | Ref | 95/514 | 0.77 | (0.58, 1.03) | 37/194 | 0.74 | (0.47, 1.16) | 9/116 | 0.29 | (0.14, 0.62) | < 0.001 | 308/1492 | 0.80 | (0.69, 0.93) | < 0.01 |
| Model 2 | 0.82 | (0.61, 1.09) | 0.81 | (0.51, 1.28) | 0.31 | (0.15, 0.67) | < 0.01 | 0.84 | (0.72, 0.98) | < 0.05 | |||||||
| African American | |||||||||||||||||
| Model 1 | 102/349 | 1.00 | Ref | 53/242 | 0.83 | (0.57, 1.21) | 9/60 | 0.59 | (0.28, 1.25) | 3/73 | 0.25 | (0.07, 0.84) | < 0.01 | 167/688 | 0.77 | (0.62, 0.96) | < 0.05 |
| Model 2 | 0.85 | (0.57, 1.25) | 0.65 | (0.30, 1.39) | 0.28 | (0.08, 0.96) | < 0.001 | 0.83 | (0.65, 1.06) | 0.14 | |||||||
| European American | |||||||||||||||||
| Model 1 | 65/319 | 1.00 | Ref | 42/272 | 0.73 | (0.48, 1.11) | 28/134 | 0.94 | (0.57, 1.53) | 6/79 | 0.34 | (0.14, 0.82) | 0.51 | 141/804 | 0.83 | (0.68, 1.01)) | 0.07 |
| Model 2 | 0.79 | (0.51, 1.21) | 1.02 | (0.61, 1.68) | 0.36 | (0.15, 0.86) | 0.58 | 0.84 | (0.68, 1.04) | 0.11 | |||||||
Abbreviations: OR, odds ratio; CI, confidence interval; TAC, total antioxidant capacity; VCE/d, vitamin C equivalents per day; Ref, referent
Model 1 adjusted for average energy intake, age, smoking, and race-smoking interaction
Model 2: Model 1, further adjusted for poverty index, marriage, body mass index, and DRE and PSA screening
Odds of high versus low and intermediate aggressive prostate cancer
Odds ratios and confidence intervals for a 500-unit increase in TAC
Number of high versus low and intermediate aggressive cases of prostate cancer
Greater TAC from both diet and supplements was associated with a significant reduction in odds of high aggressive prostate cancer among all men and AA in the fully adjusted Model, with greatest vs. lowest TAC ORs 0.31 (95% CI: 0.15, 0.67; P-trend < 0.01) and 0.28 (95% CI: 0.08, 0.96; P-trend < 0.001), respectively. For EA, men reporting the greatest TAC from diet and supplements had a significantly reduced odds of high aggressive prostate cancer, OR 0.36 (95% CI: 0.15, 0.86). These results are similar to those when TAC of diet and supplements was modeled as a continuous variable (Figure 3), though a dose response in the OR was more apparent for EA than for categorical analyses. In analyses adjusted for all covariates, a 500-unit increase in TAC from diet and supplements corresponded to an odds ratio of 0.84 (95% CI: 0.72, 0.98) for all men, 0.83 (95% CI: 0.65, 1.06) for AA, and 0.84 (95% CI: 0.68, 1.04) for EA (Table 3).
Discussion
Men with prostate cancer have been found to have low blood levels of dietary antioxidants (4), decreased activity of endogenous antioxidant enzymes, and increased levels of lipid peroxidation (5, 6). These findings could indicate either greater oxidative stress resulting in depletion of antioxidants or lower levels of antioxidants resulting in increased oxidative stress and lipid peroxidation. Furthermore, levels of antioxidants in the blood may interact with genetic variants for cyclooxygenase-2 and interleukin-8, which are involved in inflammatory processes (7), and human oxoguanine glycosylase 1 and X-ray repair cross-complementing group 1, which are involved in repairing DNA damage (8, 9). Thus, it is plausible that dietary antioxidants may influence prostate cancer development and progression by compensating for imbalances in oxidative stress.
In this study, dietary TAC was associated with a reduced odds of high aggressive prostate cancer. In logistic regression models, TAC from both diet alone and diet and supplements was associated with reduced odds of high aggressive prostate cancer among all men. We found no evidence that this association differed by race, with TAC from all sources associated with reduced odds of high aggressive prostate cancer among all men (Table 3 and Figures 2 and 3).
While there is evidence that antioxidants in the diet may influence risk of prostate cancer, previous studies have yielded conflicting results. Several antioxidants from both diet and supplements have been associated with prostate cancer risk (10, 28). For example, increased intake of vitamin E as supplemental α-tocopherol has been associated with a reduced risk of prostate cancer in the α-tocopherol, β-carotene Cancer Prevention Study (ATBC) (29), but also has been associated with an increased risk of prostate cancer in SELECT, the Selenium and Vitamin E Cancer Prevention Trial (30). Several other studies have found no effect of vitamin E on prostate cancer risk (31–33). However, Wright et al (34) found a significant association between increased dietary γ-tocopherol and decreased risk of prostate cancer. A similar finding was observed in the ATBC study (35). γ-Tocopherol has been shown to promote apoptosis (36) and inhibit growth (37) in prostate cancer cell lines. Similarly with vitamin E, studies on β-carotene have reported inverse (38–40) or positive (29) associations, and null associations with β-carotene or other carotenoids (41–46). And β-cryptoxanthin has been shown to be positively (47, 48), negatively (49), or not associated (50) with risk of prostate cancer. Previous studies on vitamin C and prostate cancer have observed no effect (31, 32, 41, 43, 47, 51, 52). There is limited evidence regarding dietary flavonoids, but in an Italian case-control study flavonoids were not associated with risk of prostate cancer (53). While an index of overall antioxidant intake, such as TAC, may serve as an alternative to measuring individual dietary antioxidants, the evidence to date suggests that the biological role of antioxidants in prostate cancer is unclear, with the benefits and harms of some antioxidants more apparent than others.
The results from studies using blood biomarkers of dietary antioxidants further demonstrates this variability. In a nested case-control study from SELECT, plasma α-tocopherol was positively associated with prostate cancer incidence, but no association was evident for γ-tocopherol (54). A nested case-control study from the Prostate, Lung, Colorectal and Ovarian (PLCO) Cancer Screening Trial found evidence of a possible association between serum γ-tocopherol and increased risk of prostate cancer (55). In another nested case-control-study of the PLCO cancer screening trial, serum β-carotene was positively associated with risk of aggressive prostate cancer, and there appears to be little evidence serum concentrations of other carotenoids (56). One exception is lycopene, where most of the evidence for a protective effect in some subgroups of men comes from the Health Professionals’ Follow-up Study (57). However, in general the data on associations between prostate cancer risk and blood biomarkers of antioxidant intake is unclear (56). This may be partly attributable to confounding by genetic variation affecting blood levels of carotenoids (58) and tocopherols (59), in which case future research should shed more light on the subtleties in the biological mechanisms of dietary components and their influence on disease risk.
While men with prostate cancer have been found to have significantly lower serum TAC (60), there is relatively little evidence on the association between dietary antioxidant capacity and its association with prostate cancer. To the author’s knowledge this is the first study of prostate cancer aggressiveness and TAC in both AA and EA. A recent publication by Russnes et al. (61) found no association between prostate cancer incidence and TAC from diet and supplements combined, although when considered separately there was a weak negative association with TAC from diet and prostate cancer incidence and a weak positive association with TAC from supplements for lethal and advanced prostate cancer. In the current study a reduced odds of high aggressive prostate cancer was observed with greater TAC from diet and supplements. One reason for this difference may be that Russnes et al. examined the association between TAC on prostate cancer incidence, whereas in the present, cross-sectional study the temporality of associations cannot be certain. Another reason may be the method used to determine TAC; the present study used the ABTS assay, which measures the radical scavenging potential of antioxidants, whereas Russnes et al. used the ferric-reducing antioxidant power (FRAP) assay, which measures the reducing power of antioxidants; thus, these two measures may not be directly comparable (62) and it has been argued that FRAP does not actually reflect total antioxidant capacity (63).
Furthermore, in this study TAC was estimated indirectly using the vitamin C equivalent antioxidant capacity of individual antioxidants, measured using the ABTS assay. Unlike most studies of TAC, in which TAC has been calculated based on oxygen radical absorbance capacity (ORAC) or FRAP databases, our research group developed a new method to assess dietary TAC by summing the VCE antioxidant capacities of major individual antioxidants using the ABTS assay (64). This approach has been validated and applied in several studies (23, 65, 66), as it agrees well with the phenolic and flavonoid content and experimentally determined TAC of foods (23). While this method does not measure the antioxidant capacity of foods directly, it has several advantages. It can be applied to any food composition data for which information on individual antioxidants is available, and the units, vitamin C equivalents, are more easily understood and comparable with Trolox equivalents used in other measures of TAC (67). However, a limitation of measuring TAC indirectly is that there are likely many antioxidants present in foods that are not accounted for in current food composition tables, and thus it is possible that this method may not precisely reflect the actual TAC of foods. This is especially the case for proanthocyanidins and flavonoids, data for which are sparse or lacking in most food composition databases. Russnes et al. measured the FRAP of individual foods directly, and thus it is plausible that measurements from this method in part reflect food components that have limited or no bioavailability. Thus, each method of measuring TAC is based on certain assumptions and has different strengths and limitations that should be considered.
This study had several strengths. Rapid case ascertainment used in PCaP limited the time between diagnosis and enrollment, thus reducing the likelihood of bias from time or treatment. PCaP was designed to accurately capture information on prostate cancer aggressiveness at diagnosis among an ethnically and geographically diverse population, while most studies have examined risk of developing prostate cancer. Furthermore, the use of less aggressive cases as controls allowed for the identification of factors associated with aggressive prostate cancer at diagnosis rather than risk of developing the disease.
Limitations of this study include a case-only design. All data were collected after diagnosis, thus treatment or disease may have biased the responses of research subjects. The time and reason for an initial diagnosis of prostate cancer also may have introduced bias. The measure of prostate cancer aggressiveness used may not reliably indicate future disease progression, particularly since participants were recruited during the PSA screening era. Dietary data were collected by a modified NCI DHQ that included Southern foods and this modified food frequency questionnaire has not been validated. However, the unmodified DHQ to which these changes were made has been previously validated (68) and while it is possible that these changes affected the validity of the DHQ, it seems unlikely that these changes would have introduced differential bias. Furthermore, antioxidant intake from supplements may be underestimated, as data on antioxidants in supplements were limited to vitamin C, α-tocopherol, β-carotene, lycopene, and lutein (e.g. the effects of selenium were not examined). Since the number of men in the upper categories of intake is very small these results should be interpreted cautiously. Since this study was cross-sectional, whether dietary antioxidants have an effect on the pathological progression of prostate cancer cannot be determined; however, these results warrant further research.
In conclusion, dietary and supplemental TAC was found to be inversely associated with odds of high aggressive prostate cancer in AA and EA. This study does not provide evidence that dietary antioxidants are associated with prostate cancer aggressiveness differently in AA and EA. However, further studies in other populations are required to confirm these results and determine whether dietary components effect the development and progression of prostate cancer.
Acknowledgments
The authors thank the North Carolina Central Cancer Registry, the Louisiana Tumor Registry, and the PCaP staff, advisory committees and participants for their important contributions.
Supported by the NIH Cancer Epidemiology Small Grant #1R03CA159421-01A1 and Department of Defense contract DAMD 17-03-2-0052.
Footnotes
The authors bear no conflict of interest regarding this manuscript.
Terrence M. Vance conducted data analyses and prepared the manuscript. Ock K. Chun designed the study and directed its implementation, including quality assurance and control. Joseph Su, Elizabeth T. H. Fontham, Jeannette T. Bensen, James L. Mohler, Susan E. Steck, and Lenore Arab helped supervise the field activities, designed the study’s analytic strategy, and contributed to the manuscript. Ying Wang helped conduct data analyses and prepare the Materials and Methods and the Discussion sections of the manuscript. Ming-Hui Chen provided statistical consultation on data analyses and interpretation of the results.
References
- 1.Siegel R, Ma J, Zou Z, Jemal A. Cancer statistics, 2014. CA Cancer J Clin. 2014;64:9–29. doi: 10.3322/caac.21208. [DOI] [PubMed] [Google Scholar]
- 2.Brawley OW. Prostate cancer epidemiology in the United States. World J Urol. 2012;30:195–200. doi: 10.1007/s00345-012-0824-2. [DOI] [PubMed] [Google Scholar]
- 3.Patel AR, Klein EA. Risk factors for prostate cancer. Nat Clin Pract Urol. 2009;6:87–95. doi: 10.1038/ncpuro1290. [DOI] [PubMed] [Google Scholar]
- 4.Akinloye O, Adaramoye O, Kareem O. Changes in antioxidant status and lipid peroxidation in Nigerian patients with prostate carcinoma. Pol Arch Med Wewn. 2009;119:526–532. [PubMed] [Google Scholar]
- 5.Arsova-Sarafinovska Z, Eken A, Matevska N, Erdem O, Sayal A, et al. Increased oxidative/nitrosative stress and decreased antioxidant enzyme activities in prostate cancer. Clin Biochem. 2009;42:1228–1235. doi: 10.1016/j.clinbiochem.2009.05.009. [DOI] [PubMed] [Google Scholar]
- 6.Aydin A, Arsova-Sarafinovska Z, Sayal A, Eken A, Erdem O, et al. Oxidative stress and antioxidant status in non-metastatic prostate cancer and benign prostatic hyperplasia. Clin Biochem. 2006;39:176–179. doi: 10.1016/j.clinbiochem.2005.11.018. [DOI] [PubMed] [Google Scholar]
- 7.Zhang J, Dhakal IB, Lang NP, Kadlubar FF. Polymorphisms in inflammatory genes, plasma antioxidants, and prostate cancer risk. Cancer Causes Control. 2010;21:1437–1444. doi: 10.1007/s10552-010-9571-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang J, Dhakal IB, Greene G, Lang NP, Kadlubar FF. Polymorphisms in hOGG1 and XRCC1 and risk of prostate cancer: effects modified by plasma antioxidants. Urology. 2010;75:779–785. doi: 10.1016/j.urology.2009.08.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Goodman M, Bostick RM, Ward KC, Terry PD, van Gils CH, et al. Lycopene intake and prostate cancer risk: effect modification by plasma antioxidants and the XRCC1 genotype. Nutr Cancer. 2006;55:13–20. doi: 10.1207/s15327914nc5501_2. [DOI] [PubMed] [Google Scholar]
- 10.Vance TM, Su J, Fontham ET, Koo SI, Chun OK. Dietary antioxidants and prostate cancer: a review. Nutr Cancer. 2013;65:793–801. doi: 10.1080/01635581.2013.806672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vance TM, Azabdaftari G, Pop EA, Lee SG, Su LJ, et al. Thioredoxin 1 in Prostate Tissue Is Associated with Gleason Score, Erythrocyte Antioxidant Enzyme Activity, and Dietary Antioxidants. Prostate Cancer. 2015;2015:728046. doi: 10.1155/2015/728046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hayes RB, Ziegler RG, Gridley G, Swanson C, Greenberg RS, et al. Dietary factors and risks for prostate cancer among blacks and whites in the United States. Cancer Epidemiol Biomarkers Prev. 1999;8:25–34. [PubMed] [Google Scholar]
- 13.Whittemore AS, Kolonel LN, Wu AH, John EM, Gallagher RP, et al. Prostate cancer in relation to diet, physical activity, and body size in blacks, whites, and Asians in the United States and Canada. J Natl Cancer Inst. 1995;87:652–661. doi: 10.1093/jnci/87.9.652. [DOI] [PubMed] [Google Scholar]
- 14.Lewis JE, Soler-Vila H, Clark PE, Kresty LA, Allen GO, et al. Intake of plant foods and associated nutrients in prostate cancer risk. Nutr Cancer. 2009;61:216–224. doi: 10.1080/01635580802419756. [DOI] [PubMed] [Google Scholar]
- 15.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013;63:11–30. doi: 10.3322/caac.21166. [DOI] [PubMed] [Google Scholar]
- 16.Serafini M, Del Rio D. Understanding the association between dietary antioxidants, redox status and disease: is the Total Antioxidant Capacity the right tool? Redox Rep. 2004;9:145–152. doi: 10.1179/135100004225004814. [DOI] [PubMed] [Google Scholar]
- 17.Brighenti F, Valtuena S, Pellegrini N, Ardigo D, Del Rio D, et al. Total antioxidant capacity of the diet is inversely and independently related to plasma concentration of high-sensitivity C-reactive protein in adult Italian subjects. Br J Nutr. 2005;93:619–625. doi: 10.1079/bjn20051400. [DOI] [PubMed] [Google Scholar]
- 18.Agudo A, Cabrera L, Amiano P, Ardanaz E, Barricarte A, et al. Fruit and vegetable intakes, dietary antioxidant nutrients, and total mortality in Spanish adults: findings from the Spanish cohort of the European Prospective Investigation into Cancer and Nutrition (EPIC-Spain) Am J Clin Nutr. 2007;85:1634–1642. doi: 10.1093/ajcn/85.6.1634. [DOI] [PubMed] [Google Scholar]
- 19.Russnes KM, Wilson KM, Epstein MM, Kasperzyk JL, Stampfer MJ, et al. Total antioxidant intake in relation to prostate cancer incidence in the health professionals follow up study. Int J Cancer. 2013 doi: 10.1002/ijc.28438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Albertsen PC, Hanley JA, Fine J. 20-year outcomes following conservative management of clinically localized prostate cancer. Jama. 2005;293:2095–2101. doi: 10.1001/jama.293.17.2095. [DOI] [PubMed] [Google Scholar]
- 21.Schroeder JC, Bensen JT, Su LJ, Mishel M, Ivanova A, et al. The North Carolina-Louisiana Prostate Cancer Project (PCaP): methods and design of a multidisciplinary population-based cohort study of racial differences in prostate cancer outcomes. Prostate. 2006;66:1162–1176. doi: 10.1002/pros.20449. [DOI] [PubMed] [Google Scholar]
- 22.National Institutes of Health, Applied Research Program, National Cancer Institute. Diet History Questionnaire, Version 1.0. 2007 DOI: http://appliedresearch.cancer.gov/DHQ/about/
- 23.Floegel A, Kim DO, Chung SJ, Song WO, Fernandez ML, et al. Development and validation of an algorithm to establish a total antioxidant capacity database of the US diet. Int J Food Sci Nutr. 2010;61:600–623. doi: 10.3109/09637481003670816. [DOI] [PubMed] [Google Scholar]
- 24.Arab L, Su LJ, Steck SE, Ang A, Fontham ET, et al. Coffee consumption and prostate cancer aggressiveness among African and Caucasian Americans in a population-based study. Nutr Cancer. 2012;64:637–642. doi: 10.1080/01635581.2012.676144. [DOI] [PubMed] [Google Scholar]
- 25.Su LJ, Arab L, Steck SE, Fontham ET, Schroeder JC, et al. Obesity and prostate cancer aggressiveness among African and Caucasian Americans in a population-based study. Cancer Epidemiol Biomarkers Prev. 2011;20:844–853. doi: 10.1158/1055-9965.EPI-10-0684. [DOI] [PubMed] [Google Scholar]
- 26.American Urological Association. Guideline for the Management of Clinically Localized Prostate Cancer: 2007 Update. 2007 [Google Scholar]
- 27.Willet W. Nutritional Epidemiology. USA: Oxford University Press; 2012. [Google Scholar]
- 28.Willis MS, Wians FH. The role of nutrition in preventing prostate cancer: a review of the proposed mechanism of action of various dietary substances. Clin Chim Acta. 2003;330:57–83. doi: 10.1016/s0009-8981(03)00048-2. [DOI] [PubMed] [Google Scholar]
- 29.Heinonen OP, Albanes D, Virtamo J, Taylor PR, Huttunen JK, et al. Prostate cancer and supplementation with alpha-tocopherol and beta-carotene: incidence and mortality in a controlled trial. J Natl Cancer Inst. 1998;90:440–446. doi: 10.1093/jnci/90.6.440. [DOI] [PubMed] [Google Scholar]
- 30.Klein EA, Thompson IM, Jr, Tangen CM, Crowley JJ, Lucia MS, et al. Vitamin E and the risk of prostate cancer: the Selenium and Vitamin E Cancer Prevention Trial (SELECT) Jama. 2011;306:1549–1556. doi: 10.1001/jama.2011.1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Heart Protection Study Collaborative Group. MRC/BHF Heart Protection Study of antioxidant vitamin supplementation in 20,536 high-risk individuals: a randomised placebo-controlled trial. Lancet. 2002;360:23–33. [Google Scholar]
- 32.Gaziano JM, Glynn RJ, Christen WG, Kurth T, Belanger C, et al. Vitamins E and C in the prevention of prostate and total cancer in men: the Physicians' Health Study II randomized controlled trial. Jama. 2009;301:52–62. doi: 10.1001/jama.2008.862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lonn E, Bosch J, Yusuf S, Sheridan P, Pogue J, et al. Effects of long-term vitamin E supplementation on cardiovascular events and cancer: a randomized controlled trial. Jama. 2005;293:1338–1347. doi: 10.1001/jama.293.11.1338. [DOI] [PubMed] [Google Scholar]
- 34.Wright ME, Weinstein SJ, Lawson KA, Albanes D, Subar AF, et al. Supplemental and dietary vitamin E intakes and risk of prostate cancer in a large prospective study. Cancer Epidemiol Biomarkers Prev. 2007;16:1128–1135. doi: 10.1158/1055-9965.EPI-06-1071. [DOI] [PubMed] [Google Scholar]
- 35.Hartman TJ, Albanes D, Pietinen P, Hartman AM, Rautalahti M, et al. The association between baseline vitamin E, selenium, and prostate cancer in the alpha-tocopherol, beta-carotene cancer prevention study. Cancer Epidemiol Biomarkers Prev. 1998;7:335–340. [PubMed] [Google Scholar]
- 36.Jiang Q, Rao X, Kim CY, Freiser H, Zhang Q, et al. Gamma-tocotrienol induces apoptosis and autophagy in prostate cancer cells by increasing intracellular dihydrosphingosine and dihydroceramide. Int J Cancer. 2012;130:685–693. doi: 10.1002/ijc.26054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Torricelli P, Caraglia M, Abbruzzese A, Beninati S. gamma-Tocopherol inhibits human prostate cancer cell proliferation by up-regulation of transglutaminase 2 and down-regulation of cyclins. Amino Acids. 2013;44:45–51. doi: 10.1007/s00726-012-1278-y. [DOI] [PubMed] [Google Scholar]
- 38.Ohno Y, Yoshida O, Oishi K, Okada K, Yamabe H, et al. Dietary beta-carotene and cancer of the prostate: a case-control study in Kyoto, Japan. Cancer Res. 1988;48:1331–1336. [PubMed] [Google Scholar]
- 39.Mettlin C, Selenskas S, Natarajan N, Huben R. Beta-carotene and animal fats and their relationship to prostate cancer risk. A case-control study. Cancer. 1989;64:605–612. doi: 10.1002/1097-0142(19890801)64:3<605::aid-cncr2820640307>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 40.McCann SE, Ambrosone CB, Moysich KB, Brasure J, Marshall JR, et al. Intakes of selected nutrients, foods, and phytochemicals and prostate cancer risk in western New York. Nutr Cancer. 2005;53:33–41. doi: 10.1207/s15327914nc5301_4. [DOI] [PubMed] [Google Scholar]
- 41.Hodge AM, English DR, McCredie MR, Severi G, Boyle P, et al. Foods, nutrients and prostate cancer. Cancer Causes Control. 2004;15:11–20. doi: 10.1023/B:CACO.0000016568.25127.10. [DOI] [PubMed] [Google Scholar]
- 42.Deneo-Pellegrini H, De Stefani E, Ronco A, Mendilaharsu M. Foods, nutrients and prostate cancer: a case-control study in Uruguay. Br J Cancer. 1999;80:591–597. doi: 10.1038/sj.bjc.6690396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.West DW, Slattery ML, Robison LM, French TK, Mahoney AW. Adult dietary intake and prostate cancer risk in Utah: a case-control study with special emphasis on aggressive tumors. Cancer Causes Control. 1991;2:85–94. doi: 10.1007/BF00053126. [DOI] [PubMed] [Google Scholar]
- 44.Ghadirian P, Lacroix A, Maisonneuve P, Perret C, Drouin G, et al. Nutritional factors and prostate cancer: a case-control study of French Canadians in Montreal, Canada. Cancer Causes Control. 1996;7:428–436. doi: 10.1007/BF00052669. [DOI] [PubMed] [Google Scholar]
- 45.Norrish AE, Jackson RT, Sharpe SJ, Skeaff CM. Prostate cancer and dietary carotenoids. Am J Epidemiol. 2000;151:119–123. doi: 10.1093/oxfordjournals.aje.a010176. [DOI] [PubMed] [Google Scholar]
- 46.Hsing AW, McLaughlin JK, Schuman LM, Bjelke E, Gridley G, et al. Diet, tobacco use, and fatal prostate cancer: results from the Lutheran Brotherhood Cohort Study. Cancer Res. 1990;50:6836–6840. [PubMed] [Google Scholar]
- 47.Schuurman AG, Goldbohm RA, Brants HA, van den Brandt PA. A prospective cohort study on intake of retinol, vitamins C and E, and carotenoids and prostate cancer risk (Netherlands) Cancer Causes Control. 2002;13:573–582. doi: 10.1023/a:1016332208339. [DOI] [PubMed] [Google Scholar]
- 48.Jain MG, Hislop GT, Howe GR, Ghadirian P. Plant foods, antioxidants, and prostate cancer risk: findings from case-control studies in Canada. Nutr Cancer. 1999;34:173–184. doi: 10.1207/S15327914NC3402_8. [DOI] [PubMed] [Google Scholar]
- 49.Jian L, Du CJ, Lee AH, Binns CW. Do dietary lycopene and other carotenoids protect against prostate cancer? Int J Cancer. 2005;113:1010–1014. doi: 10.1002/ijc.20667. [DOI] [PubMed] [Google Scholar]
- 50.Giovannucci E, Ascherio A, Rimm EB, Stampfer MJ, Colditz GA, et al. Intake of carotenoids and retinol in relation to risk of prostate cancer. J Natl Cancer Inst. 1995;87:1767–1776. doi: 10.1093/jnci/87.23.1767. [DOI] [PubMed] [Google Scholar]
- 51.Kirsh VA, Hayes RB, Mayne ST, Chatterjee N, Subar AF, et al. Supplemental and dietary vitamin E, beta-carotene, and vitamin C intakes and prostate cancer risk. J Natl Cancer Inst. 2006;98:245–254. doi: 10.1093/jnci/djj050. [DOI] [PubMed] [Google Scholar]
- 52.Bidoli E, Talamini R, Zucchetto A, Bosetti C, Negri E, et al. Dietary vitamins E and C and prostate cancer risk. Acta Oncol. 2009;48:890–894. doi: 10.1080/02841860902946546. [DOI] [PubMed] [Google Scholar]
- 53.Bosetti C, Bravi F, Talamini R, Parpinel M, Gnagnarella P, et al. Flavonoids and prostate cancer risk: a study in Italy. Nutr Cancer. 2006;56:123–127. doi: 10.1207/s15327914nc5602_1. [DOI] [PubMed] [Google Scholar]
- 54.Albanes D, Till C, Klein EA, Goodman PJ, Mondul AM, et al. Plasma tocopherols and risk of prostate cancer in the Selenium and Vitamin E Cancer Prevention Trial (SELECT) Cancer Prev Res (Phila) 2014;7:886–895. doi: 10.1158/1940-6207.CAPR-14-0058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Weinstein SJ, Peters U, Ahn J, Friesen MD, Riboli E, et al. Serum alpha-tocopherol and gamma-tocopherol concentrations and prostate cancer risk in the PLCO Screening Trial: a nested case-control study. PLoS One. 2012;7:e40204. doi: 10.1371/journal.pone.0040204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Peters U, Leitzmann MF, Chatterjee N, Wang Y, Albanes D, et al. Serum lycopene, other carotenoids, and prostate cancer risk: a nested case-control study in the prostate, lung, colorectal, and ovarian cancer screening trial. Cancer Epidemiol Biomarkers Prev. 2007;16:962–968. doi: 10.1158/1055-9965.EPI-06-0861. [DOI] [PubMed] [Google Scholar]
- 57.Wu K, Erdman JW, Jr, Schwartz SJ, Platz EA, Leitzmann M, et al. Plasma and dietary carotenoids, and the risk of prostate cancer: a nested case-control study. Cancer Epidemiol Biomarkers Prev. 2004;13:260–269. doi: 10.1158/1055-9965.epi-03-0012. [DOI] [PubMed] [Google Scholar]
- 58.Zubair N, Kooperberg C, Liu J, Di C, Peters U, et al. Genetic variation predicts serum lycopene concentrations in a multiethnic population of postmenopausal women. J Nutr. 2015;145:187–192. doi: 10.3945/jn.114.202150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Major JM, Yu K, Weinstein SJ, Berndt SI, Hyland PL, et al. Genetic variants reflecting higher vitamin e status in men are associated with reduced risk of prostate cancer. J Nutr. 2014;144:729–733. doi: 10.3945/jn.113.189928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Pace G, Di Massimo C, De Amicis D, Corbacelli C, Di Renzo L, et al. Oxidative stress in benign prostatic hyperplasia and prostate cancer. Urol Int. 2010;85:328–333. doi: 10.1159/000315064. [DOI] [PubMed] [Google Scholar]
- 61.Russnes KM, Wilson KM, Epstein MM, Kasperzyk JL, Stampfer MJ, et al. Total antioxidant intake in relation to prostate cancer incidence in the Health Professionals Follow-Up Study. Int J Cancer. 2014;134:1156–1165. doi: 10.1002/ijc.28438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Carocho M, Ferreira IC. A review on antioxidants, prooxidants and related controversy: natural and synthetic compounds, screening and analysis methodologies and future perspectives. Food Chem Toxicol. 2013;51:15–25. doi: 10.1016/j.fct.2012.09.021. [DOI] [PubMed] [Google Scholar]
- 63.Ou B, Huang D, Hampsch-Woodill M, Flanagan JA, Deemer EK. Analysis of antioxidant activities of common vegetables employing oxygen radical absorbance capacity (ORAC) and ferric reducing antioxidant power (FRAP) assays: a comparative study. J Agric Food Chem. 2002;50:3122–3128. doi: 10.1021/jf0116606. [DOI] [PubMed] [Google Scholar]
- 64.Kim D-O, Lee CY. Comprehensive study on vitamin C equivalent antioxidant capacity (VCEAC) of various polyphenolics in scavenging a free radical and its structural relationship. Crit Rev Food Sci Nutr. 2004;44:253–273. doi: 10.1080/10408690490464960. [DOI] [PubMed] [Google Scholar]
- 65.Wang Y, Yang M, Lee SG, Davis CG, Kenny A, et al. Plasma total antioxidant capacity is associated with dietary intake and plasma level of antioxidants in postmenopausal women. J Nutr Biochem. 2012;23:1725–1731. doi: 10.1016/j.jnutbio.2011.12.004. [DOI] [PubMed] [Google Scholar]
- 66.Wang Y, Chun OK, Song WO. Plasma and dietary antioxidant status as cardiovascular disease risk factors: a review of human studies. Nutrients. 2013;5:2969–3004. doi: 10.3390/nu5082969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kim DO, Lee KW, Lee HJ, Lee CY. Vitamin C equivalent antioxidant capacity (VCEAC) of phenolic phytochemicals. J Agric Food Chem. 2002;50:3713–3717. doi: 10.1021/jf020071c. [DOI] [PubMed] [Google Scholar]
- 68.Subar AF, Thompson FE, Kipnis V, Midthune D, Hurwitz P, et al. Comparative validation of the Block, Willett, and National Cancer Institute food frequency questionnaires : the Eating at America's Table Study. Am J Epidemiol. 2001;154:1089–1099. doi: 10.1093/aje/154.12.1089. [DOI] [PubMed] [Google Scholar]