Abstract
A few decades ago it was discovered that two regions of the adult brain retain the ability to generate new neurons. These regions include the subgranular zone of the hippocampal dentate gyrus and the ventricular-subventricular zone (V-SVZ) located at the border of the lateral ventricle. In the V-SVZ, it was discovered that neural progenitor cells share many features of mature astrocytes and are often referred as V-SVZ astrocytes. We will first describe the markers, the morphology, and the neurophysiological characteristics of V-SVZ astrocytes. We will then discuss the fact that V-SVZ astrocytes constitute a mixed population with respect to their neurogenic properties, e.g., quiescent versus activated state, neurogenic fate, and transcription factors expression. Finally, we will describe two functions of V-SVZ astrocytes, their metabolic coupling to blood vessels and their neurogenic supportive role consisting of providing guidance and survival cues to migrating newborn neurons.
Keywords: Subventricular zone, Adult neurogenesis, Neural stem cells, Astrocyte
Introduction
Until recently, it was thought that the generation of neurons in mammals occurred only during the embryonic period. By now it has been clearly demonstrated that new neurons continue to be generated in two regions of the adult brain, the subgranular zone (SGZ) of the dentate gyrus of the hippocampus and the ventricular-subventricular zone (V-SVZ) lining the lateral wall of the lateral ventricle. The neural progenitor cells in the V-SVZ give rise to transit amplifying precursors that themselves give birth to neuroblasts (Doetsch et al. 1999). These neuroblasts migrate tangentially to the olfactory bulb to become interneurons (Luskin 1993, Lois and Alvarez-Buylla 1994). The neural progenitor cells of the V-SVZ arise during embryonic development and persist into adulhood. In the embryonic brain, a very particular cell type, called radial glia was initially described with the classical Golgi silver impregnation method at the end of nineteenth century (Magini, 1888; Ramon y Cajal, 1890; Retzius, 1893; von Lenhossék, 1895), and found to provide a scaffolding for the migration and placement of newborn neurons (Rakic 1971). Radial glia were found to possess another critical function, first identified in songbirds, which is their ability to proliferate in the ventricular zone coinciding with sites of neurogenesis (Alvarez-Buylla et al. 1990). This finding was later confirmed and expanded in the embryonic mammalian brain and it is now well-accepted that radial glia act as neural progenitor cells (NPCs) and generate the majority of neurons in the embryonic brain (Miyata et al. 2001, Noctor et al. 2001 , Malatesta et al. 2003). After birth, most radial glia transform into parenchymal astrocytes throughout the central nervous system (Schmechel and Rakic 1979 , Voigt 1989 , Alves et al. 2002 , Merkle et al. 2004), except in the two postnatal neurogenic regions, where radial glia act as NPCs and generate the three main neural cell types, including neurons, oligodendrocytes, and astrocytes (Kriegstein and Alvarez-Buylla 2009). These NPCs persist throughout adult life in these two regions, in all mammalian species examined including humans (Bonfanti and Peretto 2011). The exact fate of NPCs is being more carefully examined using novel labeling methods and lines of transgenic mice based on the concept that not all NPCs are equal in terms of their fate. NPCs in the dorsal V-SVZ were shown to generate oligodendrocyte precursors (OPCs) that migrated radially into the white matter (Marshall and Goldman 2002, Marshall et al. 2003, Menn et al. 2006). Nevertheless, the generation of neurons predominates over that of oligodendrocytes (Menn et al. 2006) although OPC production is significantly increased following injury, in particular demyelination (Picard-Riera et al. 2002, Aguirre et al. 2007, El Waly et al. 2014)). Importantly, it was elegantly shown that a single NPC exclusively generates OPCs or immature neurons, but not both (Ortega et al. 2013). Here, we do not distinguish between OPC or neuroblast-fated NPCs with respect to their properties.
In the V-SVZ, It was found that NPCs share many features of mature astrocytes including morphological and biophysical characteristics as well as antigens such as glial fibrillary acidic protein (GFAP) (Doetsch et al. 1997, Doetsch et al. 1999, Liu et al. 2006). Here, we will focus on describing specific properties of the V-SVZ NPCs, referred to as V-SVZ astrocytes. We will first describe the unique set of markers, the morphology, and the neurophysiological characteristics of V-SVZ astrocytes. We will discuss the fact that although the population of V-SVZ astrocytes seems homogenous with respect to their neurophysiological properties (electrophysiological, coupling, neurotransmitter receptors, and transporters expression) they differ with respect to their neurogenic properties, e.g., stages of the cell cycle, quiescence versus activation state, neurogenic fate, and transcription factor expression. Finally, we will describe two functions of V-SVZ astrocytes, their coupling to blood vessels and their neurogenic supportive role consisting of providing guidance and survival cues to migrating newborn neurons.
1. Morphological and antigenic properties defining V-SVZ astrocytes
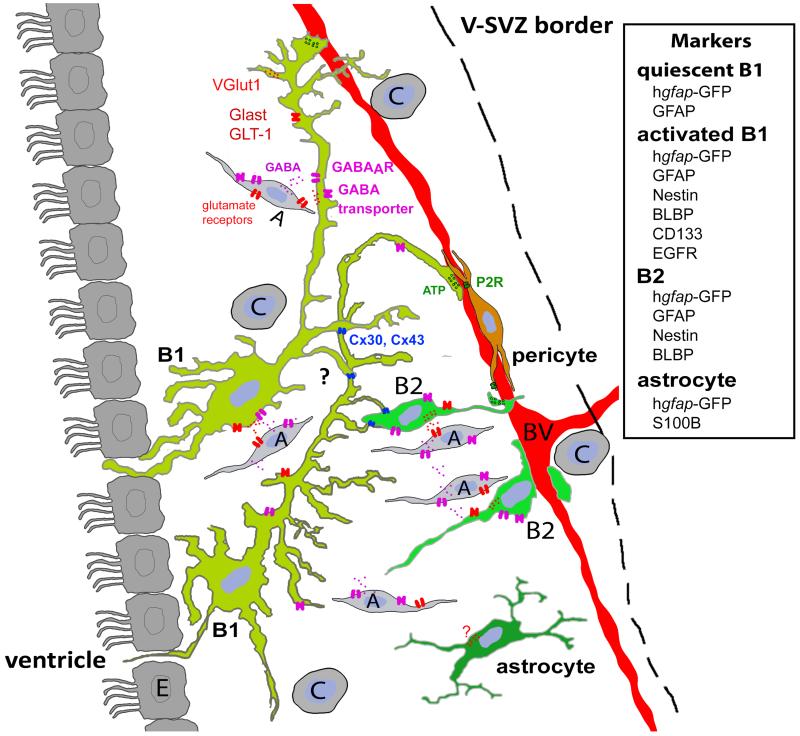
Two main populations of GFAP-positive cells and a third more discret population were observed in the V-SVZ (Jankovski and Sotelo 1996, Doetsch et al. 1997)(Fig. 1). The two main populations were called type B1 and B2 cells (Doetsch et al. 1997), both of them exhibiting characteristics of astrocytes. B1 cells were initially described morphologically with a simple fusiform cell body, only 2 to 3 processes with few branches (Jankovski and Sotelo 1996). These cells were identified as the NPCs of the V-SVZ (Doetsch et al. 1999). Type B1 slowly divide to give birth to highly proliferative transit amplifying precursors (Type C cells) that themselves give birth to neuroblasts (Type A cells), which migrate to the olfactory bulb to become interneurons (Doetsch et al. 1999). Both type B cells directly contact neuroblasts and form a glial sheath around chains of newborn neurons as they migrate toward the olfactory bulb (Lois et al. 1996 , Doetsch et al. 1997). Through improvement in labeling technique, the morphology of type B1 cells has been found to be more specialized and complex than initially described. In particular, the cell bodies of B1 cells are located beneath the ependymal layer and their apical process is frequently intercalated between ependymal cells (Doetsch et al. 1999, Mirzadeh et al. 2008) while their basal process projects across the V-SVZ thickness and terminates on blood vessels, in particular capillaries (Mirzadeh et al. 2008 , Shen et al. 2008, Tavazoie et al. 2008 , Lacar et al. 2011). The basal processes are of variable lengths depending on the proximity of the vessels to the ependyma and confer a radial glia-like morphology to NPCs (Mirzadeh et al. 2008 , Lacar et al. 2011)(Fig. 1). B1 cells have fewer processes than typical stellate astrocytes. Nevertheless by examining the very fine morphology of individually labeled B1 cells, it became apparent that the complexity of their morphology has been underestimated. They possess a high density of short and thin projections extending from their apical and basal processes (Fig. 1) (Mirzadeh et al. 2008 , Lacar et al. 2011, Lacar et al. 2012). The function, the molecular composition, and the presence of receptors on these thin processes are unknown, but they may allow B1 cells to better sense cues in their microenvironment.
Figure 1.
The different populations of astrocyte like cells and their markers in the adult V-SVZ drawn from cells expressing GFP after electroporation.
Ependymal cells (E) are multiciliated located at the border of the lateral ventricle. Activated and quiescent type B1 cells extend an apical process intercalated between ependymal cells while their basal process contact the blood vessels. Type B2 cells, also called niche astrocytes, display an intermediate fusiform/stellate appearance and are located mostly at the periphery of the V-SVZ. They never contact the ventricle but interact with blood vessels. A third very discreet population of astrocyte was observed in the V-SVZ and is S100B positive. These cells present a stellate appearance (Platel et al. 2009) but their arborisation is not well developped. It is unknown if they connect to blood vessels. Type A cell (neuroblasts) have a migratory morphology and are located throughout the V-SVZ. Type C cells (transit amplifying progenitors) are located throughout the V-SVZ and apposed to blood vessels. Pericytes are located on capillaries and present several processes that run on the blood vessel.
The second population of astrocytes, the type B2 cells, also called niche astrocytes, displays a stellate appearance resembling that of parenchymal astrocytes (Mirzadeh et al. 2008, Lacar et al. 2011 )(Fig. 1). They are mostly located at the periphery of the V-SVZ creating a physical boundary between the SVZ and the striatum (Liu et al., 2005) and are probably non-neurogenic. They do not contact the ventricle but interact with blood vessels (Mirzadeh et al. 2008, Lacar et al. 2011 ). A third very small population of astrocytes was observed in the V-SVZ of 4 weeks old animals and represent only very few cells (Platel et al. 2009). These astrocytes are S100B positive (Platel et al. 2009), but surprisingly have not been observed in s100b-GFP mice (Raponi et al. 2007). In human (h) Gfap-GFP mice, few S100B-positive cells are GFP-positive while the others are GFP-negative (Platel et al. 2009). Approximately half of these S100+ cells are also NG2 positive. These cells present a relatively immature stellate appearance and don’t have a specific location along the V-SVZ, but are never in contact with the ventricle (Platel et al. 2009). This population may represent astrocytes or NG2 cells born in the V-SVZ. Their function and potential migration pattern remain unknown.
Type B cells have been reported to express several markers associated with astrocytes or radial glia, like GFAP (Jankovski and Sotelo 1996, Doetsch et al. 1997), nestin, vimentin (Doetsch et al. 1997), the glutamate transporter GLAST (Braun et al. 2003 , Bolteus and Bordey 2004 ), and brain lipid binding protein (BLBP)(Platel et al. 2009, Giachino et al. 2014) (Table 1). Therefore, type B cells are also frequently referred to as V-SVZ astrocytes. Additional markers expressed in other cell types outside of the neurogenic region have been identified in V-SVZ astrocytes and further divide this population of cells, including CD133 (prominin-1), EGFR, and Sox2 (Komitova and Eriksson 2004, Codega et al. 2014). The different combination of markers is thought to reflect different activation states of V-SVZ astrocytes from quiescent to activated stages. CD133 has been shown to label V-SVZ cells with stem cell characteristics (Fischer et al. 2011) while EGFR+ cells label activated (i.e., proliferative) V-SVZ astrocytes as well as transit amplifying cells (Pastrana et al. 2009). It is thus important to use a combination of markers to identify the quiescent and activated V-SVZ NPCs with for example CD133+/EGFR-(quiescent) and CD133+/EGFR+ (proliferative, activated). It was also recently shown that BLBP is more restricted that initially thought and that it is expressed in mitotically activated and not in quiescent V-SVZ astrocytes (Giachino et al. 2014).
Table 1.
Anatomical, antigenic, and neurophysiological properties of V-SVZ cells.
| Type | Subtype | Morphology | Location | Markers | Glutamate related marker |
GABA related marker |
|
|---|---|---|---|---|---|---|---|
| V-SVZ astrocytes |
Type B1 | Overall Type B1 |
fusiform | Attached to the ventricle and blood vessels Cell body in V-SVZ |
GFAP + CD133 +/− Nestin +/− C×43 ; C×30 hgfap-GFP BLBP +/− |
GLAST GLT-1 VGlut1 Glutamate mGluR1-5 |
GAT4 GABAAR |
| quiescent | " | " | GFAP +; CD133 +/− |
? | ? | ||
| activated | " | " | GFAP +; Nestin + EGFR +; CD133 + hgfap-GFP BLBP |
? | ? | ||
| Type B2 | Intermediate fusiform /stellate |
Periphery of the V-SVZ, not in contact with the ependymal layer |
GFAP +; BLBP + Nestin +; C×43 +; Cx30? hgfap-GFP |
GLAST; GLT-1 VGlut1 ? Glutamate mGluR1-5 ? |
GAT4 GABAAR | ||
| Other astrocytes | Stellate | no specific location | hgfap-GFP +/− S100B +; GFAP? C×43 ? ; C×30? |
? | ? | ||
| Type C | Larger, more spherical than type B |
Core SVZ | Mash1 +; EGFR + Lex +; Dl×2 + BLBP+/− |
? | ? | ||
| Type A | bipolar elongated | Core SVZ | DCX +; Tuj 1 + PSA-NCAM + |
NMDA-R Kainate-R AMPA-R mGluR |
GAT1 VGAT GABAAR GABA |
||
| Ependymal cells | cubic | border of the ventricle | S100B +; CD24 + Nestin +; C×43+ CD133 (cilia) + |
- | - | ||
+/− indicates that the marker is not expressed in all the population
2. Neurophysiological characteristics of V-SVZ astrocytes
Studies during the last decade have characterized the neurophysiological properties of SVZ astrocytes. Because the V-SVZ is very densely populated by several cell types, including V-SVZ astrocytes, transit amplifying cells, neuroblasts, ependymal cells, and microglial cells, it is complicated to identify V-SVZ astrocytes from the other cell types in acute brain slices without a counter-staining. Thus, most neurophysiological studies took advantage of transgenic mice, such as the hgfap-GFP mice originally generated to study mature astrocytes (Liu et al. 2006) and hgfap-DsRed mouse line (Young et al. 2010). In these lines, V-SVZ astrocytes display GFP or DsRed expression that matches the expression of GFAP detected by immunostaining. However, the number of GFAP+ cells expressing DsRed is lower than in the GFP mouse line and DsRed persists in neuroblasts due to its long half-life although fainter than in V-SVZ astrocytes (Young et al. 2010). Therefore, in most studies using brain slices from these lines, B1 and B2 V-SVZ astrocytes were not distinguished. Electrophysiological recordings of GFP-positive cells have shown that V-SVZ astrocytes display K+ conductances at rest like mature astrocytes, but have a low level of expression of barium-sensitive inwardly rectifying K+-mediated current (Wang et al. 2003, Liu et al. 2006), which is a hallmark of astrocytic differentiation and cell cycle exit (Bordey and Sontheimer, 1997; Macfarlane and Sontheimer, 2000). In addition, V-SVZ astrocytes express several other functional ion channels, neurotransmitter receptors, and transporters detailed below.
GABA signaling has been the most studied neurotransmitter signaling in the V-SVZ. Functional GABAA receptors have been identified on V-SVZ astrocytes (Stewart et al. 2002, Nguyen et al. 2003, Wang et al. 2003, Gascon et al. 2006). The exact subunit composition remains to be examined. The expression of GABAB and GABAC receptors has not been explored. V-SVZ astrocytes also express the high affinity GABA transporter, GAT4 (Bolteus and Bordey 2004) while GAT1 is expressed by neuroblasts. It was demonstrated that GABA controls the proliferation of V-SVZ astrocytes through GABAA receptor activation (Nguyen et al. 2003, Liu et al. 2005). Neuroblasts are a paracrine source of GABA. They synthesize and release GABA in a calcium-dependent but non-vesicular manner (Liu et al. 2005 ). Such a paracrine release of GABA increases V-SVZ astrocyte proliferation and was proposed to act as a negative proliferative cue from neuroblasts to V-SVZ astrocytes (Liu et al. 2005). However, GABA can also come from an external source. It was shown that striatal GABAergic neurons located at the border of the V-SVZ could regulate intracellular Ca2+ dynamics in V-SVZ astrocytes through GABAA receptors activation and depolarization of L- and T-type voltage-gated calcium channels (Young et al. 2010, Young et al. 2012).
Glutamate signaling is also present in the V-SVZ. V-SVZ astrocytes do not display any NMDA, kainate, or AMPA-induced currents or calcium increases in acute slices (Liu et al., 2006; Platel et al., 2010). In vitro, metabotropic glutamate receptor subtypes 3 and 5 are expressed by GFAP-positive cells isolated from the V-SVZ (Di Giorgi-Gerevini et al. 2005). Nevertheless, it is not clear whether these receptors are expressed in V-SVZ astrocytes in vivo especially since mGluR5-induced calcium currents were detected in neuroblasts but not in surrounding cells (Platel et al. 2008). As mentioned above, V-SVZ astrocytes express the glutamate transporters GLAST (EAAT1) and GLT-1 (EAAT2) (Braun et al. 2003 , Bolteus and Bordey 2004 , Liu et al. 2006). Although it seems that V-SVZ astrocytes do not express any glutamate receptors, we will further detail in part 5 their role as master regulators of glutamate signaling (Platel et al. 2008, Platel et al. 2010).
It was recently shown that V-SVZ astrocytes express α3 and α4 nicotinic and muscarinic receptors. Local subependymal choline acetyl transferase positive (ChAT+) neurons can release acetylcholine into the V-SVZ niche in an activity-dependent manner (Paez-Gonzalez et al. 2014). Indeed, optogenetic stimulation of these subependymal ChAT+ neurons revealed that their activity could trigger release of acetylcholine that activates V-SVZ astrocytes and increase SVZ cell number (Paez-Gonzalez et al. 2014). It is nevertheless possible that this effect on proliferation is indirect through the release of other factors such as GABA from striatal neurons.
V-SVZ astrocytes express connexin 30 and 43, components of gap junctions in mature astrocytes that allow intercellular communication (Liu et al. 2006, Nomura et al. 2010, Lacar et al. 2011). Connexin can also form hemi-channels, which unlike gap junctional coupling allow direct communication between the intracellular and extracellular milieu (Bennett et al. 2003). Hemi-channels have been implicated in the initiation and propagation of calcium waves between radial glia during corticogenesis (Weissman et al. 2004). Similarly, it was reported that V-SVZ astrocytes display functional coupling involving 50–60 cells as well as intercellular calcium waves (Lacar et al. 2011, Lacar et al. 2012). These waves travelled bidirectionally between type B1 and B2 cells and propagated onto blood vessels. They were absent in the presence of a gap junction blocker, but persisted with purinergic receptor blockers (Lacar et al. 2011). Such functional coupling among V-SVZ astrocytes and between V-SVZ astrocytes and blood vessels are another evidence that V-SVZ astrocytes do not merely have a structural role, but may play an active role in coordinating intercellular communication and cell behavior in this neurogenic region.
3. Do all V-SVZ astrocytes possess the same neurophysiological properties?
The neurophysiological characteristics of V-SVZ astrocytes have been measured in hgfap-GFP-positive V-SVZ astrocytes without distinguishing the different subpopulations mentioned earlier leading to the conculsions that neurophysiological properties described in the previous chapter are homogenous among these cells. For example, all V-SVZ astrocytes were reported to express the neurotransmitter transporters GLAST, GLT-1, and GAT4 as well as connexin 43 resulting in functional coupling. All V-SVZ astrocytes were also reported to respond to focal applications of GABA, which induced inward currents (Liu et al. 2005).
These results are surprising since the major subpopulations of V-SVZ astrocytes, type B1 and B2 cells, are different with respect to their antigenic markers, morphology, and location in the V-SVZ. In addition, different subtypes of V-SVZ astrocytes were identified based on their location along the ventricle, their transcription factor expression, and the fate of their neuronal progeny (Kohwi et al. 2007, Merkle et al. 2007, Fiorelli et al. 2015). . For example, dorsal V-SVZ astrocytes express the transcription factor Emx1 and Pax6 and mainly generate periglomerular dopaminergic neurons and superficial granule cells. The ventral V-SVZ astrocytes are mainly Gli1-positive and produce deep granule cells and calbindin-positive periglomerular cells whereas the lateral V-SVZ is Gsx2- and Dlx5/6-positive and produce all periglomerular neurons subtypes (Kohwi et al. 2007).
These data lead to the following intriguing questions: are the neurophysiological characteristics of the different populations of V-SVZ astrocytes similar? Do these characteristics (e.g., expression of receptors) change depending on the phase of the cell cycle? Are these neurophysiological characteristics similar in different compartments of the V-SVZ, i.e. dorsal, lateral, ventral and medial?
It is possible that some of the neurophysiological characteristics of V-SVZ astrocytes described above represent only basic functions that are also present in parenchymal astrocytes (Wang and Bordey 2008). Nevertheless, it has been shown that several specific signaling are different between compartments. For example, the Wnt family of soluble ligands regulates the self-renewal of a small population of V-SVZ astrocytes, which are located in the dorsal compartment and generate glutamatergic olfactory bulb neurons (Azim et al. 2014). Another example is the sonic hedgehog (Shh) signaling. Shh is selectively produced by a small group of ventral forebrain neurons and its receptor gli1 is only expressed in the ventral V-SVZ astrocytes. This signaling regulates the formation of calbindin periglomerular neurons and deep granule cells (Ihrie et al. 2011).
Collectively, we hypothetize that some mechanisms are common in all the V-SVZ astrocytes while some differences and specificity appear in the different populations or during different phases of the cell cycle. New neurophysiological studies will need to take into account the different V-SVZ populations showing distinct expression of molecular determinants along the ventricle axes.
In the next two sections, we specifcally focus on two specific functions of V-SVZ astrocytes, the metabolic coupling and their supportive role during neuroblast migration.
4. Metabolic coupling in the V-SVZ niche
In the brain, neuronal activity dictates transfer of oxygen and nutrients from the blood stream into active neuronal assemblies through a local “neurovascular coupling” in part carried out by astrocytes (Giaume et al. 2010). Although SVZ cells do not generate action potentials, the V-SVZ contains cells undergoing proliferation, which is a metabolically demanding process (Bolanos et al. 2010). It is thus not completely surprising that the V-SVZ contains a large network of blood vessels and in particular capillaries (Mercier et al. 2002, Shen et al. 2008, Tavazoie et al. 2008, Snapyan et al. 2009, Lacar et al. 2012). A pioneering study reported that V-SVZ astrocytes closely ensheath blood vessels around the V-SVZ (Mercier et al. 2002). Additional studies showed that dividing V-SVZ astrocytes and type C cells are tightly apposed to V-SVZ blood vessels (Shen et al. 2008, Tavazoie et al. 2008). They frequently contact the vasculature at specific sites that lack astrocyte endfeet and pericyte coverage, and display a more permeable blood brain barrier allowing small diffusible molecules to enter the neurogenic zone (Tavazoie et al. 2008 ). As such, type B and C cells are uniquely poised to receive signals from the vasculature, like hormones and growth factors. In addition, both hormones and growth factors can control cell proliferation in the V-SVZ under normal condition and following injury (for review (Silva-Vargas et al. 2013)).
Reciprocally, it was demonstrated that V-SVZ cells have the ability to modify blood flow, which is expected to alter metabolite supply into this region. It has been shown that a 30 minutes injection of epidermal and basic fibroblast growth factor (EGF and bFGF) into the lateral ventricular significantly increased the number of cells entering S-phase of the cell cycle (i.e., analyzed with BrdU uptake) in the V-SVZ. In addition, EGF + bFGF injection led to a sustained rise in blood flow in the V-SVZ. Because a similar injection in the cortex, where there is no proliferation, did not lead to an increase in blood flow, the authors suggested that the increase in the number of cycling cells was the biological process leading to an increase in blood flow.
Based on the anatomical arrangement of astrocytes-capillaries in the V-SVZ, these same authors examined whether V-SVZ astrocytes could regulate capillary diameter in acute slices and blood flow in vivo. A series of elegant studies have been performed on mature, parenchymal astrocytes suggesting that calcium signaling in astrocytes leads to the release of vasoconstricting or dilating factors. Similarly, in the V-SVZ, astrocytes display spontaneous calcium waves and stimulation-induced (electrical and GABA application) calcium increases (Lacar et al. 2011 , Lacar et al. 2012). To selectively increase calcium in V-SVZ astrocytes and not in surrounding V-SVZ cells, Lacar et al. used transgenic mice, in which GFAP+ cells express a Gq-protein-coupled receptor (called Mas-related gene A1, MrgA1) that is not expressed in the brain and has no endogenous ligands (Fiacco et al. 2007). These mice were initially generated to study astrocytic functions at synapses (Fiacco et al. 2007). Application of the MrgA1-selective peptide agonist FLRFα resulted in calcium increases in V-SVZ astrocytes. Using these mice, they then showed that intracellular calcium increases in V-SVZ astrocytes induces ATP release followed by purinergic (P2Y2/4 receptor activation on pericytes and dilation (Lacar et al. 2012). Perhaps the most elegant and challenging experiment was to selectively express MrgA1 receptors in V-SVZ astrocytes in vivo using neonatal electroporation and show that ventricular injection of the ligand led to increase in blood flow in the V-SVZ monitored using laser Doppler flowmetry. Using neonatal electroporation allows to express a plasmid of interest in V-SVZ cells which are radial glila cells in neonates. Over time, the fast cycling cells dilute the plasmid and the neuroblasts migrate away resulting in plasmid expression selectively in ependymal cells and quiescent or slow-cycling NPCs (Lacar et al. 2010).
This coupling underscores the intimate reciprocal interaction of V-SVZ astrocytes and niche cells. Considering that V-SVZ astrocytes receive signals from other V-SVZ cells such as GABA-induced depolarizing signal leading to calcium increases, these findings further suggest that V-SVZ astrocytes may act as transducers of neurometabolic demand and neural-vascular coupling in the V-SVZ.
5. V-SVZ astrocytes support neuroblast survival during migration by releasing glutamate
Type B1 cells generate neuroblasts that migrate a long distance to the olfactory bulb through the rostral migratory stream (RMS). While V-SVZ astrocytes don’t express any ionotropic glutamate receptors, neuroblasts express functional AMPA (Platel et al. 2007), kainate (Platel et al. 2008), NMDA receptors (Platel et al. 2010), and mGluR5 (Di Giorgi Gerevini et al. 2004, Platel et al. 2008) shown using neurophysiological recordings in acute slices and immunohistochmistry in fixed sections. Each of these receptor subtypes has a different function in neuroblasts. Kainate receptors control migration (Platel et al. 2008), mGluR5 regulate proliferation (Di Giorgi Gerevini et al. 2004) while NMDARs are important for neuroblast survival (Platel et al. 2010). Electrophysiological recordings showed that these receptors were activated by endogenous levels of glutamate (Platel et al. 2008, Platel et al. 2010) suggesting a source of glutamate onto neuroblasts. Projection of glutamergic terminals had not been observed in the V-SVZ, but given the role of astrocytes in glutamate homeostasis during synaptic transmission, it was speculated that V-SVZ astrocytes may be a local source of glutamate (Bordey 2006). Although there are no synaptic contacts between V-SVZ astrocytes and neuroblasts, V-SVZ astrocytes closely ensheath them (Doetsch et al. 1997). In addition, V-SVZ astrocytes contain high level of glutamate (Platel et al. 2007) and express glutamate transporters GLAST and GLT-1 conferring them the ability to regulate ambient glutamate levels (Bolteus and Bordey 2004, Liu et al. 2006). In addition, vesicular glutamate transporter 1 (VGLUT1) was found in V-SVZ astrocytes, located mainly in the rostral V-SVZ (Platel et al. 2007) using immunostaining as well as post-embedding immunogold labeling (Platel et al. 2010). In order to demonstrate a potential calcium-dependent glutamate release from V-SVZ astrocytes, transgenic mice, in which V-SVZ astrocytes express MrgA1 receptors were used. The MrgA1-selective peptide agonist, FLRFα, which increased calcium in V-SVZ astrocytes, led to an increase in the frequency of NMDA receptor-mediated channel activity in neuroblasts recorded with the patch clamp technique in acute slices (Platel et al. 2010). It was concluded that V-SVZ astrocytes release glutamate in a calcium-dependent manner onto neuroblasts. It is noticeable that in acute hippocampal slices from MrgA1 mice, FLRFa application did not affect synaptic transmission (Fiacco et al. 2007). This emphasizes the importance of the glutamatergic signal from V-SVZ astrocytes to neuroblasts in the neurogenic zone. These findings raise additional questions; it was shown that V-SVZ astrocytes tonically release glutamate in a calcium-dependent manner. However, the signal(s) leading to intracellular calcium increases in V-SVZ astrocytes that could trigger the release of glutamate remain unclear. One possibility is the neurotransmitter GABA released from neuroblasts (Liu et al., 2005).
Conclusion
The subventricular zone is a more complex region than previously appreciated in terms of its cellular diversity among a similar group of cells, like NPCs. The neurogenesis community is progressively finding new markers and generating new genetic tools such as new lines of transgenic mice to better characterize the morphological and neurophysiological properties of NSCs. In addition, additional work is needed to better identify the similarities and the differences between the neurogenic domains in the V-SVZ. This is particularly important given the regional specification of NPCs in terms of their fate, neuronal vs oligodendroglial or the different types of neurons generated. Moreover, while we have gained insights into how V-SVZ astrocytes can control blood flow and perhaps address the metabolic demand of proliferating and migrating cells in the niche, it is important to determine how this metabolic coupling is modulated by physiological states and how this will impact the neurogenic niche. It is also important to better understand the interactions between neurotransmitters and intracellular rresponses, and how this impact NPC and neuroblast behavior.
In conclusion, one of the most exciting findings in neurogenesis and the astrocyte field is the discovery that a specialized type of astrocytes acts as NPCs in the adult neurogenic zone. Future studies will uncover the mechanisms that allow these specialized neurogenic region and cells to retain their proliferative capacity in the adult brain. As a correlate of this discovery is a question related to parenchymal astrocytes and in particular addressing whether parenchymal astrocytes could regain their ability to generate neurons given the right environment and set of neurogenic factors. Ultimately, understanding the biology of the V-SVZ astrocytes has important therapeutic potential in treating brain injuries and neurological disorders.
Neurogenesis persist in the brain in the ventricular-subventricular zone (V-SVZ)
Neural progenitor cells in the V-SVZ have characteristics of astrocytes
Do all V-SVZ astrocytes possess the same features?
V-SVZ astrocytes act as transducers of neurometabolic coupling in the SVZ
V-SVZ astrocytes support neuroblast survival during migration
Acknowledgements
Platel Jean-Claude is supported by the Institute National de la Santé et de la Recherche Médicale.
Abbreviations
- RMS
rostral migratory stream
- GFAP
glial fibrillary acidic protein
- V-SVZ
ventricular-subventricular zone
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Bibliography
- Aguirre A, et al. A functional role for EGFR signaling in myelination and remyelination. Nat Neurosci. 2007;10(8):990–1002. doi: 10.1038/nn1938. [DOI] [PubMed] [Google Scholar]
- Alvarez-Buylla A, et al. Proliferation “hot spots” in adult avian ventricular zone reveal radial cell division. Neuron. 1990;5(1):101–109. doi: 10.1016/0896-6273(90)90038-h. [DOI] [PubMed] [Google Scholar]
- Alves JA, et al. Initial stages of radial glia astrocytic transformation in the early postnatal anterior subventricular zone. J Neurobiol. 2002;52(3):251–265. doi: 10.1002/neu.10087. [DOI] [PubMed] [Google Scholar]
- Azim K, et al. Persistent Wnt/beta-catenin signaling determines dorsalization of the postnatal subventricular zone and neural stem cell specification into oligodendrocytes and glutamatergic neurons. Stem Cells. 2014;32(5):1301–1312. doi: 10.1002/stem.1639. [DOI] [PubMed] [Google Scholar]
- Bennett MV, et al. New roles for astrocytes: gap junction hemichannels have something to communicate. Trends Neurosci. 2003;26(11):610–617. doi: 10.1016/j.tins.2003.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolanos JP, et al. Glycolysis: a bioenergetic or a survival pathway? Trends Biochem Sci. 2010;35(3):145–149. doi: 10.1016/j.tibs.2009.10.006. [DOI] [PubMed] [Google Scholar]
- Bolteus AJ, et al. GABA release and uptake regulate neuronal precursor migration in the postnatal subventricular zone. J Neurosci. 2004;24(35):7623–7631. doi: 10.1523/JNEUROSCI.1999-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonfanti L, et al. Adult neurogenesis in mammals--a theme with many variations. Eur J Neurosci. 2011;34(6):930–950. doi: 10.1111/j.1460-9568.2011.07832.x. [DOI] [PubMed] [Google Scholar]
- Bordey A. Adult neurogenesis: basic concepts of signaling. Cell Cycle. 2006;5(7):722–728. doi: 10.4161/cc.5.7.2614. [DOI] [PubMed] [Google Scholar]
- Braun N, et al. Expression of the ecto-ATPase NTPDase2 in the germinal zones of the developing and adult rat brain. Eur J Neurosci. 2003;17(7):1355–1364. doi: 10.1046/j.1460-9568.2003.02567.x. [DOI] [PubMed] [Google Scholar]
- Codega P, et al. Prospective identification and purification of quiescent adult neural stem cells from their in vivo niche. Neuron. 2014;82(3):545–559. doi: 10.1016/j.neuron.2014.02.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Giorgi Gerevini VD, et al. The mGlu5 metabotropic glutamate receptor is expressed in zones of active neurogenesis of the embryonic and postnatal brain. Brain Res Dev Brain Res. 2004;150(1):17–22. doi: 10.1016/j.devbrainres.2004.02.003. [DOI] [PubMed] [Google Scholar]
- Di Giorgi-Gerevini V, et al. Endogenous activation of metabotropic glutamate receptors supports the proliferation and survival of neural progenitor cells. Cell Death Differ. 2005;12(8):1124–1133. doi: 10.1038/sj.cdd.4401639. [DOI] [PubMed] [Google Scholar]
- Doetsch F, et al. Subventricular zone astrocytes are neural stem cells in the adult mammalian brain. Cell. 1999;97(6):703–716. doi: 10.1016/s0092-8674(00)80783-7. [DOI] [PubMed] [Google Scholar]
- Doetsch F, et al. Cellular composition and three-dimensional organization of the subventricular germinal zone in the adult mammalian brain. J Neurosci. 1997;17(13):5046–5061. doi: 10.1523/JNEUROSCI.17-13-05046.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doetsch F, et al. Regeneration of a germinal layer in the adult mammalian brain. Proc Natl Acad Sci U S A. 1999;96(20):11619–11624. doi: 10.1073/pnas.96.20.11619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El Waly B, et al. Oligodendrogenesis in the normal and pathological central nervous system. Front Neurosci. 2014;8:145. doi: 10.3389/fnins.2014.00145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiacco TA, et al. Selective stimulation of astrocyte calcium in situ does not affect neuronal excitatory synaptic activity. Neuron. 2007;54(4):611–626. doi: 10.1016/j.neuron.2007.04.032. [DOI] [PubMed] [Google Scholar]
- Fiorelli R, et al. Adding a spatial dimension to postnatal ventricular-subventricular zone neurogenesis. Development. 2015;142(12):2109–2120. doi: 10.1242/dev.119966. [DOI] [PubMed] [Google Scholar]
- Fischer J, et al. Prospective isolation of adult neural stem cells from the mouse subependymal zone. Nat Protoc. 2011;6(12):1981–1989. doi: 10.1038/nprot.2011.412. [DOI] [PubMed] [Google Scholar]
- Gascon E, et al. GABA regulates dendritic growth by stabilizing lamellipodia in newly generated interneurons of the olfactory bulb. J Neurosci. 2006;26(50):12956–12966. doi: 10.1523/JNEUROSCI.4508-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giachino C, et al. Molecular diversity subdivides the adult forebrain neural stem cell population. Stem Cells. 2014;32(1):70–84. doi: 10.1002/stem.1520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giaume C, et al. Astroglial networks: a step further in neuroglial and gliovascular interactions. Nat Rev Neurosci. 2010;11(2):87–99. doi: 10.1038/nrn2757. [DOI] [PubMed] [Google Scholar]
- Ihrie RA, et al. Persistent sonic hedgehog signaling in adult brain determines neural stem cell positional identity. Neuron. 2011;71(2):250–262. doi: 10.1016/j.neuron.2011.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jankovski A, et al. Subventricular zone-olfactory bulb migratory pathway in the adult mouse: cellular composition and specificity as determined by heterochronic and heterotopic transplantation. J Comp Neurol. 1996;371(3):376–396. doi: 10.1002/(SICI)1096-9861(19960729)371:3<376::AID-CNE3>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- Kohwi M, et al. A subpopulation of olfactory bulb GABAergic interneurons is derived from Emx1- and Dlx5/6-expressing progenitors. J Neurosci. 2007;27(26):6878–6891. doi: 10.1523/JNEUROSCI.0254-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komitova M, et al. Sox-2 is expressed by neural progenitors and astroglia in the adult rat brain. Neurosci Lett. 2004;369(1):24–27. doi: 10.1016/j.neulet.2004.07.035. [DOI] [PubMed] [Google Scholar]
- Kriegstein A, et al. The glial nature of embryonic and adult neural stem cells. Annu Rev Neurosci. 2009;32:149–184. doi: 10.1146/annurev.neuro.051508.135600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacar B, et al. Neural progenitor cells regulate capillary blood flow in the postnatal subventricular zone. J Neurosci. 2012;32(46):16435–16448. doi: 10.1523/JNEUROSCI.1457-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacar B, et al. Imaging and recording subventricular zone progenitor cells in live tissue of postnatal mice. Front Neurosci. 2010;4 doi: 10.3389/fnins.2010.00043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacar B, et al. Gap junction-mediated calcium waves define communication networks among murine postnatal neural progenitor cells. Eur J Neurosci. 2011;34(12):1895–1905. doi: 10.1111/j.1460-9568.2011.07901.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacar B, et al. Gap junction-mediated calcium waves define communication networks among murine postnatal neural progenitor cells. Eur J Neurosci. 2011 doi: 10.1111/j.1460-9568.2011.07901.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, et al. GFAP-expressing cells in the postnatal subventricular zone display a unique glial phenotype intermediate between radial glia and astrocytes. Glia. 2006;54(5):394–410. doi: 10.1002/glia.20392. [DOI] [PubMed] [Google Scholar]
- Liu X, et al. Nonsynaptic GABA signaling in postnatal subventricular zone controls proliferation of GFAP-expressing progenitors. Nat Neurosci. 2005;8(9):1179–1187. doi: 10.1038/nn1522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lois C, et al. Long-distance neuronal migration in the adult mammalian brain. Science. 1994;264(5162):1145–1148. doi: 10.1126/science.8178174. [DOI] [PubMed] [Google Scholar]
- Lois C, et al. Chain migration of neuronal precursors. Science. 1996;271(5251):978–981. doi: 10.1126/science.271.5251.978. [DOI] [PubMed] [Google Scholar]
- Luskin MB. Restricted proliferation and migration of postnatally generated neurons derived from the forebrain subventricular zone. Neuron. 1993;11(1):173–189. doi: 10.1016/0896-6273(93)90281-u. [DOI] [PubMed] [Google Scholar]
- Malatesta P, et al. Neuronal or glial progeny: regional differences in radial glia fate. Neuron. 2003;37(5):751–764. doi: 10.1016/s0896-6273(03)00116-8. [DOI] [PubMed] [Google Scholar]
- Marshall CA, et al. Subpallial dlx2-expressing cells give rise to astrocytes and oligodendrocytes in the cerebral cortex and white matter. J Neurosci. 2002;22(22):9821–9830. doi: 10.1523/JNEUROSCI.22-22-09821.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall CA, et al. Gliogenic and neurogenic progenitors of the subventricular zone: who are they, where did they come from, and where are they going? Glia. 2003;43(1):52–61. doi: 10.1002/glia.10213. [DOI] [PubMed] [Google Scholar]
- Menn B, et al. Origin of oligodendrocytes in the subventricular zone of the adult brain. J Neurosci. 2006;26(30):7907–7918. doi: 10.1523/JNEUROSCI.1299-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mercier F, et al. Anatomy of the brain neurogenic zones revisited: fractones and the fibroblast/macrophage network. J Comp Neurol. 2002;451(2):170–188. doi: 10.1002/cne.10342. [DOI] [PubMed] [Google Scholar]
- Merkle FT, et al. Mosaic organization of neural stem cells in the adult brain. Science. 2007;317(5836):381–384. doi: 10.1126/science.1144914. [DOI] [PubMed] [Google Scholar]
- Merkle FT, et al. Radial glia give rise to adult neural stem cells in the subventricular zone. Proc Natl Acad Sci U S A. 2004;101(50):17528–17532. doi: 10.1073/pnas.0407893101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirzadeh Z, et al. Neural stem cells confer unique pinwheel architecture to the ventricular surface in neurogenic regions of the adult brain. Cell Stem Cell. 2008;3(3):265–278. doi: 10.1016/j.stem.2008.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyata T, et al. Asymmetric inheritance of radial glial fibers by cortical neurons. Neuron. 2001;31(5):727–741. doi: 10.1016/s0896-6273(01)00420-2. [DOI] [PubMed] [Google Scholar]
- Nguyen L, et al. Autocrine/paracrine activation of the GABA(A) receptor inhibits the proliferation of neurogenic polysialylated neural cell adhesion molecule-positive (PSA-NCAM+) precursor cells from postnatal striatum. J Neurosci. 2003;23(8):3278–3294. doi: 10.1523/JNEUROSCI.23-08-03278.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noctor SC, et al. Neurons derived from radial glial cells establish radial units in neocortex. Nature. 2001;409(6821):714–720. doi: 10.1038/35055553. [DOI] [PubMed] [Google Scholar]
- Nomura T, et al. EphB signaling controls lineage plasticity of adult neural stem cell niche cells. Cell Stem Cell. 2010;7(6):730–743. doi: 10.1016/j.stem.2010.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortega F, et al. Oligodendrogliogenic and neurogenic adult subependymal zone neural stem cells constitute distinct lineages and exhibit differential responsiveness to Wnt signalling. Nat Cell Biol. 2013;15(6):602–613. doi: 10.1038/ncb2736. [DOI] [PubMed] [Google Scholar]
- Paez-Gonzalez P, et al. Identification of distinct ChAT(+) neurons and activity-dependent control of postnatal SVZ neurogenesis. Nat Neurosci. 2014;17(7):934–942. doi: 10.1038/nn.3734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pastrana E, et al. Simultaneous prospective purification of adult subventricular zone neural stem cells and their progeny. Proc Natl Acad Sci U S A. 2009;106(15):6387–6392. doi: 10.1073/pnas.0810407106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picard-Riera N, et al. Experimental autoimmune encephalomyelitis mobilizes neural progenitors from the subventricular zone to undergo oligodendrogenesis in adult mice. Proc Natl Acad Sci U S A. 2002;99(20):13211–13216. doi: 10.1073/pnas.192314199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Platel JC, et al. Control of neuroblast production and migration by converging GABA and glutamate signals in the postnatal forebrain. J Physiol. 2008;586(16):3739–3743. doi: 10.1113/jphysiol.2008.155325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Platel JC, et al. NMDA receptors activated by subventricular zone astrocytic glutamate are critical for neuroblast survival prior to entering a synaptic network. Neuron. 2010;65(6):859–872. doi: 10.1016/j.neuron.2010.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Platel JC, et al. GFAP-GFP neural progenitors are antigenically homogeneous and anchored in their enclosed mosaic niche. Glia. 2009;57(1):66–78. doi: 10.1002/glia.20735. [DOI] [PubMed] [Google Scholar]
- Platel JC, et al. Tonic activation of GLUK5 kainate receptors decreases neuroblast migration in whole-mounts of the subventricular zone. J Physiol. 2008;586(16):3783–3793. doi: 10.1113/jphysiol.2008.155879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Platel JC, et al. GABA and glutamate signaling: homeostatic control of adult forebrain neurogenesis. J Mol Histol. 2007;38(6):602–610. doi: 10.1007/s10735-007-9153-y. [DOI] [PubMed] [Google Scholar]
- Rakic P. Guidance of neurons migrating to the fetal monkey neocortex. Brain Res. 1971;33(2):471–476. doi: 10.1016/0006-8993(71)90119-3. [DOI] [PubMed] [Google Scholar]
- Raponi E, et al. S100B expression defines a state in which GFAP-expressing cells lose their neural stem cell potential and acquire a more mature developmental stage. Glia. 2007;55(2):165–177. doi: 10.1002/glia.20445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmechel DE, et al. A Golgi study of radial glial cells in developing monkey telencephalon: morphogenesis and transformation into astrocytes. Anat Embryol (Berl) 1979;156(2):115–152. doi: 10.1007/BF00300010. [DOI] [PubMed] [Google Scholar]
- Shen Q, et al. Adult SVZ stem cells lie in a vascular niche: a quantitative analysis of niche cell-cell interactions. Cell Stem Cell. 2008;3(3):289–300. doi: 10.1016/j.stem.2008.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva-Vargas V, et al. Adult neural stem cells and their niche: a dynamic duo during homeostasis, regeneration, and aging. Curr Opin Neurobiol. 2013;23(6):935–942. doi: 10.1016/j.conb.2013.09.004. [DOI] [PubMed] [Google Scholar]
- Snapyan M, et al. Vasculature guides migrating neuronal precursors in the adult mammalian forebrain via brain-derived neurotrophic factor signaling. J Neurosci. 2009;29(13):4172–4188. doi: 10.1523/JNEUROSCI.4956-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart RR, et al. Neural progenitor cells of the neonatal rat anterior subventricular zone express functional GABA(A) receptors. J Neurobiol. 2002;50(4):305–322. doi: 10.1002/neu.10038. [DOI] [PubMed] [Google Scholar]
- Tavazoie M, et al. A specialized vascular niche for adult neural stem cells. Cell Stem Cell. 2008;3(3):279–288. doi: 10.1016/j.stem.2008.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voigt T. Development of glial cells in the cerebral wall of ferrets: direct tracing of their transformation from radial glia into astrocytes. J Comp Neurol. 1989;289(1):74–88. doi: 10.1002/cne.902890106. [DOI] [PubMed] [Google Scholar]
- Wang DD, et al. The astrocyte odyssey. Prog Neurobiol. 2008;86(4):342–367. doi: 10.1016/j.pneurobio.2008.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang DD, et al. Biophysical properties and ionic signature of neuronal progenitors of the postnatal subventricular zone in situ. J Neurophysiol. 2003;90(4):2291–2302. doi: 10.1152/jn.01116.2002. [DOI] [PubMed] [Google Scholar]
- Wang DD, et al. GABA depolarizes neuronal progenitors of the postnatal subventricular zone via GABAA receptor activation. J Physiol. 2003;550(Pt 3):785–800. doi: 10.1113/jphysiol.2003.042572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weissman TA, et al. Calcium waves propagate through radial glial cells and modulate proliferation in the developing neocortex. Neuron. 2004;43(5):647–661. doi: 10.1016/j.neuron.2004.08.015. [DOI] [PubMed] [Google Scholar]
- Young SZ, et al. GABA(A) Increases Calcium in Subventricular Zone Astrocyte-Like Cells Through L- and T-Type Voltage-Gated Calcium Channels. Front Cell Neurosci. 2010;4:8. doi: 10.3389/fncel.2010.00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young SZ, et al. NKCC1 knockdown decreases neuron production through GABA(A)-regulated neural progenitor proliferation and delays dendrite development. J Neurosci. 2012;32(39):13630–13638. doi: 10.1523/JNEUROSCI.2864-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]