Abstract
Oxytocin has been shown to decrease cocaine taking and seeking in male rats, suggesting potential treatment efficacy for drug addiction. In the present study, we extended these findings to the assessment of cocaine seeking and taking in female rats. Further, we made direct comparisons of oxytocin’s impact on cocaine induced locomotor activity in both males and females. In females, systemic oxytocin (0.3, 1.0, 3.0 mg/kg) attenuated lever pressing for cocaine during self-administration and oxytocin (1.0 mg/kg) attenuated cue-induced cocaine seeking following extinction. Cocaine increased baseline locomotor activity to a greater degree in females relative to males. Oxytocin (0.1, 0.3, 1.0, and 3.0 mg/kg) reduced cocaine-induced locomotor activity in females, but not significantly in males. These data illustrate sex similarities in oxytocin’s attenuation of cocaine seeking, but sex differences in cocaine-induced locomotor effects. While reductions in cocaine seeking cannot be attributed to a reduction in locomotor activity in males, attenuation of locomotor function cannot be entirely ruled out as an explanation for a decrease in cocaine seeking in females suggesting that oxytocin’s effect on cocaine seeking may be mediated by different mechanisms in male and females.
Keywords: Self-Administration, Reinstatement, Oxytocin, Cocaine, Sex Differences
Oxytocin is an important hormone in a variety of social and stress-related behaviors including social interaction, maternal behavior, and anxiety reduction (Keverne and Curley, 2004; Lim and Young, 2006; Neumann, 2007). Early oxytocin research was largely limited to studying its role in female reproduction (Pederson and Prange, 1979). However, researchers subsequently discovered that oxytocin played a significant role in prosocial behaviors such as attachment (Carter, 1998) and social recognition (Ferguson et al., 2000). Recent studies have found that both central and peripheral administration of oxytocin produces anxiolytic and anti-stress effects in animal models (Ring et al., 2006) through modulation by oxytocin receptor function of the HPA axis (Landgraf and Neumann, 2004) and oxytocin innervation of forebrain regions (Windle et al., 2003).
Oxytocin potently modulates natural and drug reward processes via interactions with the brain dopamine system. Following synthesis in the magnocellular neurons of the supraoptic and paraventricular nuclei of the hypothalamus, oxytocin is secreted by axon terminals in the posterior pituitary into systemic circulation (Choy and Watkins 1977). Oxytocin produced in the parvocellular neurons of the paraventricular nuclei project to various brain regions, such as the nucleus accumbens (Knobloch et al., 2012). Growing evidence has suggested an important role for oxytocin in drug addiction (Sarnyai and Kovács, 2014). Some researchers have hypothesized that recreational drugs stimulate the oxytocin system (Dumont et al., 2009) and that social bonding and drug addiction might share underlying neural substrates (Liu et al., 2011; Young et al., 2011). This idea is further supported by findings that drug and alcohol dependent individuals typically display antisocial traits and poor socially conscious decision making (Dawe et al., 2004).
In regards to addiction, systemic and site-specific oxytocin administration decreased morphine tolerance and withdrawal (Kovacs et al., 1985; Sarnyai and Kovacs, 1994). Central oxytocin infusions impaired methamphetamine-induced conditioned place preference and stress-induced reinstatement (Qi et al., 2009). Moreover, oxytocin decreased both meth- and cocaine-seeking following self-administration (Carson et al., 2010a; Cox et al., 2013; Zhou et al., 2014) and attenuated cocaine-induced hyperlocomotion and stereotyped behavior (Sarnyai and Kovacs, 1994). Combined, this research suggests that oxytocin has potential as a therapeutic target for drug dependency and addiction (McGregor and Bowen, 2012).
The peripheral and central oxytocin systems are sexually dimorphic. Male and female rats differ in their distribution of oxytocin receptors within the reward circuitry (Dumais et al., 2013). Females display lower receptor expression in regions such as the nucleus accumbens, caudate putamen, and medial amygdala. Therefore, oxytocin may have different therapeutic effects in males and females. Sex differences have also been well characterized in cocaine addiction and dependency. Studies have found that while males are more likely to develop an addiction to cocaine (Brady and Randall, 1999), females are more likely to begin use at an earlier age (Weiss et al., 1997) and progress more rapidly from casual use to dependence and addiction (Westermeyer and Boedicker, 2000; O’Brien and Anthony, 2005). These findings are emulated in animal models. Female rats display greater motivation to seek cocaine than males across different stages of addiction (Lynch et al., 2002; for review see Carroll and Anker, 2010) and exhibit greater maintenance of responding for cocaine (Fuchs et al., 2005). While female rats reinstate less than or equally to males in a cue-induced reinstatement paradigm (Fuchs et al., 2005; Feltenstein, Henderson, and See, 2011), females display increased reinstatement following cocaine-prime or stress (Anker and Carroll, 2010; Buffalari et al., 2012; Feltenstein et al., 2011).
Previously, Zhou and colleagues (2014) reported that oxytocin decreased active lever presses, cocaine intake during self-administration, and conditioned cue induced cocaine seeking following extinction in males. Here, we expanded this question to females by assessing oxytocin’s effect on cocaine intake during self-administration and cue-induced reinstatement. Further, female rats display enhanced cocaine-induced locomotor activity relative to males (van Haaren and Meyer, 1991; Walker et al., 2001) and oxytocin decreased motor activity to a greater extent in females (Zhou et al., 2015). However, as the extent to which oxytocin impacts cocaine-induced locomotor activity in males and females has not been tested, we determined whether oxytocin administration before cocaine would impact locomotor activity in males and females. Given the sexual dimorphisms in the oxytocin system, we predicted that oxytocin would have a greater impact in females.
Methods and Procedures
Subjects
Adult male (weighing 275–300 g) and female (weighing 205–225 g) Sprague Dawley rats (Harlan, N=145) were single-housed on a reversed 12:12 light-dark cycle (lights off at 6:00 a.m.) in a temperature- and humidity-controlled vivarium. All experimental procedures were conducted during the dark cycle. Rats received water ad libitum and were kept on a stable intake diet 15–30 g of standard rat chow (Harlan, Indianapolis, IN, USA) daily throughout the study. Procedures were conducted in accordance with the “Guide for the Care and Use of Laboratory Rats” (Institute of Laboratory Animal Resources on Life Sciences, National Research Council, 2011) and approved by the IACUC of the Medical University of South Carolina.
Surgery
Anesthesia consisted of IP injections of ketamine (66 mg/kg, IP, Vedco Inc, St Joseph, MO, USA) mixed with xylazine (1.3 mg/kg, IP, Lloyd Laboratories, Shenandoah, IA, USA), and an additional injection of equithesin (0.5 ml/kg: sodium pentobarbital 9.72 mg/kg, chloral hydrate 42.5 mg/kg, and 21.3 mg/kg magnesium sulfate heptahydrate dissolved in 44% propylene glycol, 10 % ethanol solution). Ketorolac (2.0 mg/kg, IP, Sigma, St. Louis, MO, USA) was given before surgery as an analgesic. Surgical procedures were conducted using aseptic techniques. One end of a silastic catheter was implanted into the external right jugular. The other end ran subcutaneously, exited from a small incision on the back, and attached to an infusion cannula (PlasticsOne Inc. Roanoke, VA, USA). Cephazolin (10 mg/0.1 ml) was given post-surgery (0.1 ml IV) and during recovery along with 0.05 ml of TCS catheter locking solution. Cocaine self-administration began following at least 5 days of recovery from surgery.
Cocaine self-administration, extinction, and reinstatement
Rats were trained in self-administration chambers (30×20×20 cm, Med Associates) containing two retractable levers, two stimulus lights, a speaker, and a house light. All self-administration chambers were housed inside sound-attenuating cubicles. Each chamber contained tubing that extended through a spring leash attached to a swivel and a balanced metal arm. A 10 ml syringe was mounted on a pump outside the cubicle, which supplied drug infusions. Cocaine hydrochloride (provided by the National Institute on Drug Abuse, Research Triangle Park, NC, USA) was dissolved in 0.9% sterile saline and administered at 0.2 mg (males) or 0.15 mg (females) cocaine per 50 ul bolus. A computerized program (MED-PC, Med Associates, St Albans, VT) controlled the collection of data. Cocaine self-administration occurred during daily 2-hour sessions on an FR1 schedule of reinforcement. During the sessions, a response on the active lever resulted in a 2 s infusion and 5 s presentation of a light and tone stimulus complex, followed by a 20 s time out. Responses occurring during the time out and on the inactive lever were recorded without scheduled consequences. Before each session, catheters were flushed with 0.1 ml of saline. To verify catheter patency, rats occasionally received a 0.10–0.12 ml infusion of methohexital sodium (Eli Lilly, Indianapolis, IN, USA), a short-acting barbiturate that produces a rapid loss of muscle tone when administered intravenously. After each self-administration session, rats’ catheters were flushed wisth 0.1 ml Cefazolin and 0.05 ml TCS.
Rats underwent extinction following completion of cocaine self-administration. Extinction consisted of daily 2-hr sessions for at least 7 days and responding on either lever had no scheduled consequences. Extinction criterion was ≤20 active lever presses for two consecutive days. Upon meeting of extinction criterion, rats were tested on conditioned cued reinstatement tests (detailed below). During the conditioned cued test, responding on the active lever resulted in the presentation of light and tone stimulus complex.
Experiment 1a: Oxytocin’s effect on cocaine intake in females
In this experiment, females underwent self-administration as described above. First, we tested the effects of oxytocin on established cocaine maintained responding. Females (n=15) first learned to lever press for cocaine for at least 7 days (with > 10 infusions/session). Once cocaine intake stabilized (within 20% difference in number of infusions received between the last two days), each rat was tested with a unique order of vehicle, 0.1, 0.3, 1 and 3 mg/kg oxytocin at the volume of 1 ml/kg (IP, Cell Sciences, Canton, MA). Each solution was administered 30 min before daily self-administration sessions. To reach criteria between tests, rats were again required to have two consecutive days in which the numbers of infusions earned were within 20% of each other.
Experiment 1b: Oxytocin’s effect on cued-induced reinstatement in females
Females (n=18) underwent self-administration and extinction as previously described. Rats were trained to lever press for IV cocaine for 10 days, followed by at least 7 sessions of extinction. When extinction criterion was met, all rats underwent cue-induced reinstatement. In this reinstatement test, rats are returned their self-administration chambers and responding on the active lever resulted in the presentation of light and tone stimulus complex (identical to self-administration) but not an infusion of cocaine. Before each discrete reinstatement trial, with a minimum of 2 extinction sessions between reinstatement tests, rats received an injection of oxytocin (0.1, 0.3, or 1 mg/kg) or vehicle in a counterbalanced order for a total of four reinstatement tests. Previous work from our laboratory has consistently shown that responding remains stable over multiple reinstatement tests (Reichel and See, 2010; Cox et al., 2014).
Experiment 2: Oxytocin’s effect on cocaine-induced locomotor activity in males and females
To explore the effect of oxytocin on cocaine-induced locomotor activity in both males and females, rats underwent a single locomotor test assessed in clear acrylic chambers (approximately 40×40×30 cm) equipped with Digiscan monitors (AccuScan Instruments Inc., Columbus, OH, USA). Each chamber contained a 16×16 photobeam array for the x and y axes and 16 photobeams for the z axis. Photobeam breaks were detected by a Digiscan analyzer and horizontal activity was recorded by DigiPro software (Version 1.4). Males (n=10–12, per group) and females (n=10–12, per group) were injected IP with vehicle or one single dose of oxytocin (0.1, 0.3, 1, or 3 mg/kg) and placed in the locomotor chamber for 30 min to obtain an initial locomotor effect of oxytocin. After 30 min, rats were injected with 10 mg/kg cocaine and returned to the locomotor chamber for an additional 120 min to determine effect of oxytocin on cocaine-induced hyperlocomotion.
Estrous cycle monitoring
Females were habituated to vaginal cytology procedures during early extinction. Vaginal lumen samples were obtained by flushing 30 µl ddH2O with a sterile pipette tip and extracting the sample using a micro pipette immediately prior and following extinction sessions. Vaginal lumen samples were smeared onto a glass slide and stained with Quik-Dip Hematology Stain (Mercedes Medical, FL). Cycle phase (estrus, proestrus, diestrus) was classified based on previously published criteria (Marcondes et al., 2002; Feltenstein et al., 2011) with a light microscope set at 10× magnification. Rats were tested upon meeting of extinction criterion (< 25 lever presses for 2 consecutive days) and vaginal lumen samples were obtained prior to reinstatement test.
Data analysis
One-way repeated measures analysis of variance (ANOVA) was used to evaluate the effects of oxytocin on cocaine intake and active lever presses, while two-way ANOVA evaluated the effect of estrous cycle on reinstatement. Two-way ANOVA was used to analyze differences in cocaine-induced locomotor activity between males and females following oxytocin administration. Pairwise comparisons were conducted using Dunnett’s multiple comparisons test. All data are presented as the mean ±S.E.M., and α was set at p < 0.05.
Results
Experiment 1a: The effects of oxytocin on maintenance of cocaine self-administration in females
A one-way repeated measures ANOVA revealed that oxytocin decreased active lever presses for cocaine during the self-administration session [Figure 1C, F(4,74) = 9.63, p < 0.0001]. Specifically, oxytocin at doses of 0.3, 1.0, and 3.0 mg/kg resulted in significantly less active lever presses than rats administered vehicle (Dunnett p < 0.05). Likewise, cocaine intake (mg/kg body weight) was reduced during the session [Figure 1D, F(4,74) = 17.9, p < 0.0001] following administration of 0.1, 0.3, 1.0, and 3.0 mg/kg oxytocin doses relative to vehicle (Dunnett p < 0.05). To determine whether active lever responding decreased over time with multiple oxytocin injections, average active lever presses for two days prior to each test day were compared. Females did not display a decrease over time but instead a one-way repeated measures ANOVA revealed that active lever responding increased between the first and last oxytocin injection [F(4, 70) = 3.34, p < 0.05] indicating a training effect (Table 1).
Figure 1.
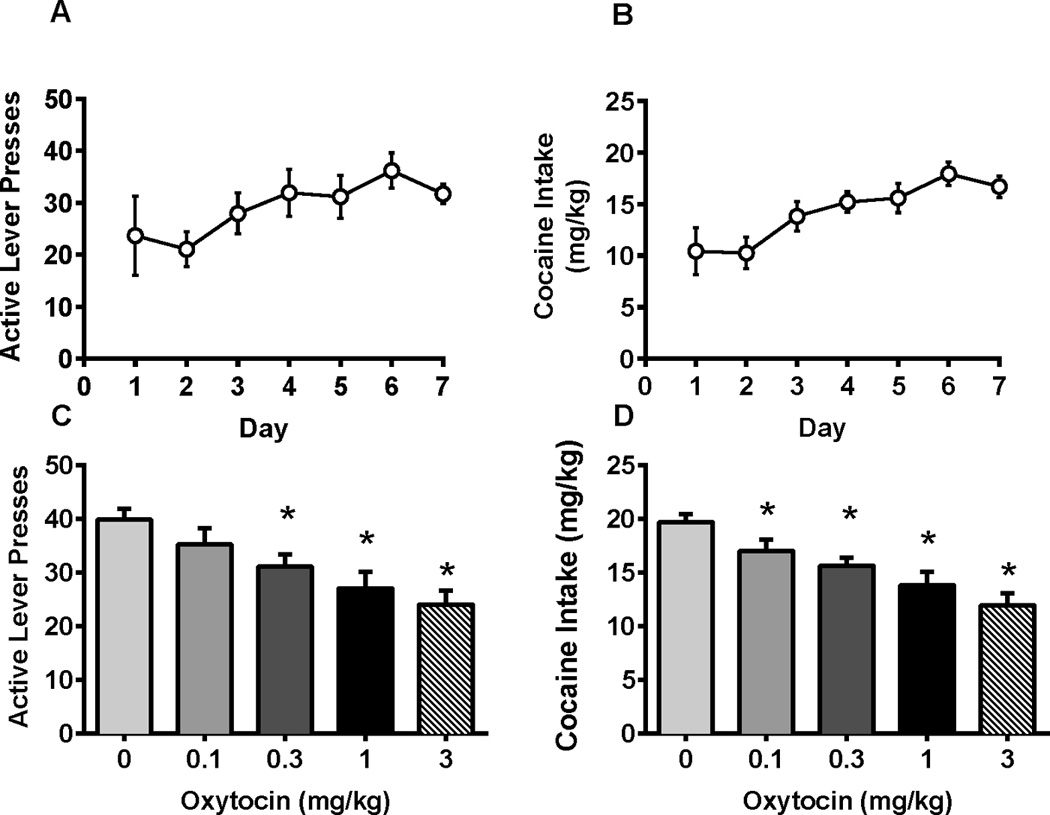
Active lever presses (A) and cocaine intake (B) during acquisition of cocaine responding and following oxytocin treatment in female rats. (C) Active lever presses. Oxytocin (0.3, 1.0, 3.0 mg/kg) decreased active lever presses for cocaine. (D) Adjusted cocaine intake by body weight. Oxytocin reduced cocaine intake across all doses. *significant difference from vehicle, p < 0.05
Table 1.
Lever presses and cocaine intake (mg/kg) between reinstatement test days.
| Lever Presses (Mean±SE) | Intake (Mean±SE) | |
|---|---|---|
| Test 1 | 31.90 ± 1.42 | 18.23±0.87 |
| Test 2 | 31.33 ± 1.53 | 19.05±1.10 |
| Test 3 | 33.67 ± 1.23 | 19.65±0.87 |
| Test 4 | 34.73 ± 1.02 | 19.83±0.60 |
| Test 5 | 37.47 ± 1.36* | 20.35±0.85 |
Notes.
denotes significant difference from Test 1, p<0.05
Experiment 1b: The effects of oxytocin on cue-induced reinstatement of cocaine seeking in females
A one-way repeated measures ANOVA revealed that oxytocin decreased active lever presses during the cue-induced reinstatement test [Figure 2C, F(3,71) = 3.96, p < 0.05]. Specifically, 1.0 mg/kg oxytocin decreased lever responding in the cue-induced reinstatement test relative to vehicle (Dunnett p < 0.05).
Figure 2.
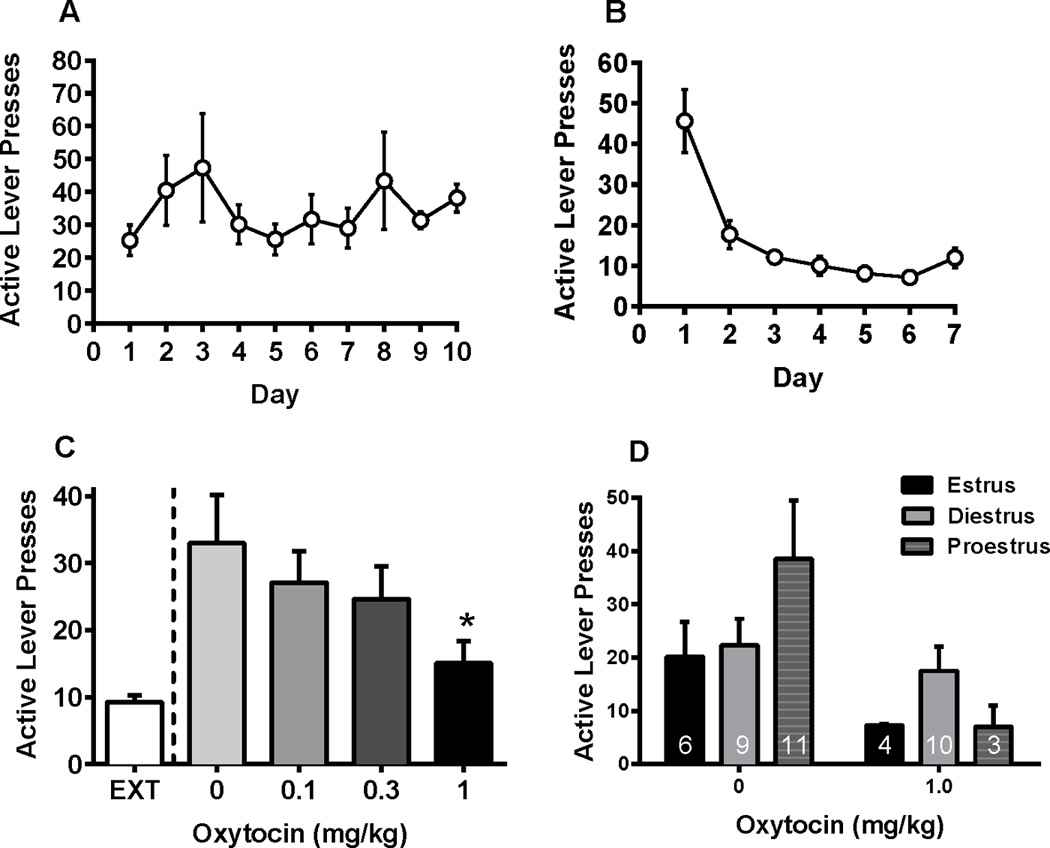
Cue-induced reinstatement to cocaine seeking in female rats following oxytocin pretreatment. (A) Active lever responding during acquisition of self-administration. (B) Active lever responding during extinction. (C) Active lever responding during reinstatement. Oxytocin (1 mg/kg) significantly decreased active lever responding as compared to vehicle (p < 0.05). (D) Active lever responding during reinstatement by estrous cycle and effective dose of oxytocin. No effect of cycle phase was seen during cue-induced reinstatement. *significant difference from vehicle, p < 0.05
Given that only one dose of oxytocin (1 mg/kg) decreased lever responding in the cue-induced reinstatement test, estrous cycle data was analyzed comparing the effective dose of oxytocin (1 mg/kg) against vehicle. Figure 2D depicts cue-induced reinstatement of cocaine seeking at the effective dose of oxytocin (1.0 mg/kg) during three phases of the estrous cycle (estrus, diestrus, proestrus). A two-way ANOVA revealed no significant interaction between oxytocin and cycle, and no significant main effect of cycle. Consistently, there was a main effect of oxytocin dose [F(1,37) = 4.66, p < 0.05]. To determine whether cocaine intake decreased over time with multiple oxytocin injections, average cocaine intake for two days prior to each test day were compared. Table 1 revealed that females displayed stable cocaine intake over multiple injections of oxytocin.
Experiment 2: Oxytocin reduced cocaine-induced locomotor activity in females, but not in males
Prior to administration of cocaine, oxytocin was administered to rats i.p. before placing them into locomotor chambers (Figure 3). Figure 3A depicts locomotor activity in males and females during the 30 min period following oxytocin injection without cocaine. In general, females had greater locomotor activity counts than males [sex main effect, F(4,102) = 4.07, p < 0.05] and oxytocin decreased responding in both sexes [oxytocin dose main effect, F(4,102) = 10.65, p < 0.001]. A follow up comparison showed that 0.3, 1.0, and 3.0 mg/kg and 0.1, 0.3, 1.0, and 3.0 mg/kg were effective in females and males, respectively.
Figure 3.
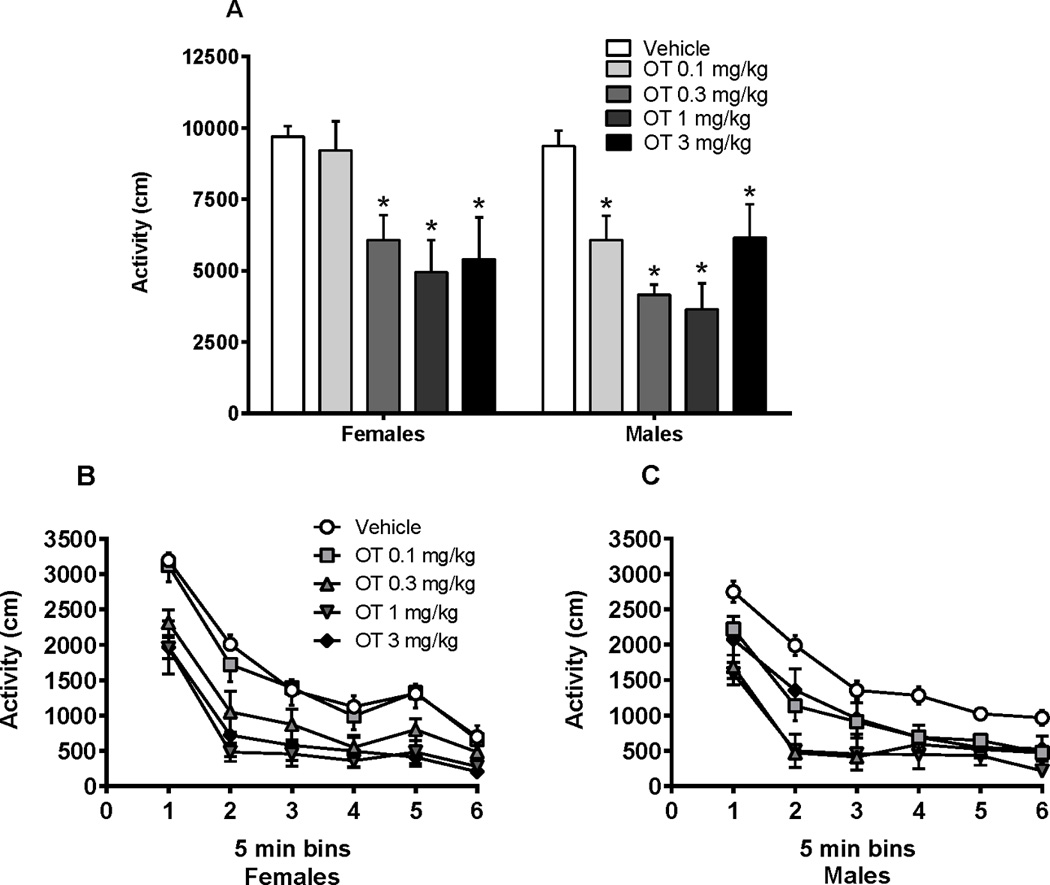
Locomotor activity in male and female rats following oxytocin injection (30 min prior to cocaine injection). (A) Locomotor activity over a 30 min session. Oxytocin attenuated activity in both males and females. (B) Female and (C) male locomotor activity scores represented in 5 min time bins throughout the 30 min period. Oxytocin attenuated distance traveled in females and males. *significant difference from same sex vehicle, p < 0.05
Consistently, the data revealed a similar pattern when analyzed through the time course of locomotor activity (5 min time bins over the 30 min period). In females, oxytocin significantly reduced locomotor activity [Figure 3B, F(4, 49) = 9.73, p < 0.001], with multiple doses of oxytocin (0.3, 1.0, and 3.0 mg/kg) reducing locomotion. In males, oxytocin impacted locomotor activity as well [Figure 3C, F (4, 51) = 8.46, p < 0.01), with all doses of oxytocin (0.1, 0.3, 1, and 3 mg/kg) decreasing distance traveled across the session.
Figure 4A depicts locomotor activity following cocaine injection with concurrent oxytocin. Specifically, Figure 4A shows sex differences in oxytocin’s ability to influence cocaine-induced locomotor activity (i.e., horizontal activity) over a 120 min period. A two-way ANOVA revealed a significant main effect of sex [F(1, 98) = 13.71, p < 0.001] and oxytocin dose [F(4, 98) = 4.85, p < 0.01). Furthermore, there was a sex × oxytocin dose interaction [F(4, 98) = 3.37, p < 0.05], suggesting that females were more prone to reduced cocaine-induced locomotor activity than males following oxytocin administration. In females, all four doses of oxytocin (0.1, 0.3, 1, and 3 mg/kg) decreased the distance traveled relative to vehicle (Dunnett, p < 0.05). In contrast, oxytocin had no effect on cocaine-induced locomotor activity in males. A similar pattern of results was obtained when evaluating distance traveled in 5 min time bins over the course of the session. Following cocaine injection, females had a significant dose × time interaction [F(92, 1081) = 4.41, p < 0.001], with all doses of oxytocin (0.1, 0.3, 1, and 3 mg/kg) decreasing the distance traveled across the session in the subsequent 120 min (Figure 4B). Following cocaine injection, males displayed no effect of oxytocin dose but did display a significant dose × time interaction [F(92, 1173) = 1.63, p < 0.01], driven by an early effect of oxytocin (0.1, 0.3, and 1.0 mg/kg) only in the first 10 min (Dunnett’s multiple comparisons, p < 0.05) (Figure 4C). However, no other effects of oxytocin dose on cocaine-induced locomotor activity were evident across the entire session. To account for variability on cocaine-induced locomotion, data was analyzed as a conversion to percent of control responding. Vehicle group locomotor activity was set at 100 percent responding and locomotor activity in groups of rats receiving oxytocin were normalized and compared to vehicle. This analysis yielded similar patterns of behavior. Females displayed a significant dose × time interaction [F(92, 1081) = 1.33, p < 0.05], with oxytocin (0.3 and 3.0 mg/kg) decreasing distance traveled across the session (Figure 4D). Males continued to display no effect of oxytocin dose indicating that oxytocin administration did not impact cocaine-induced locomotion (Figure 4E)
Figure 4.
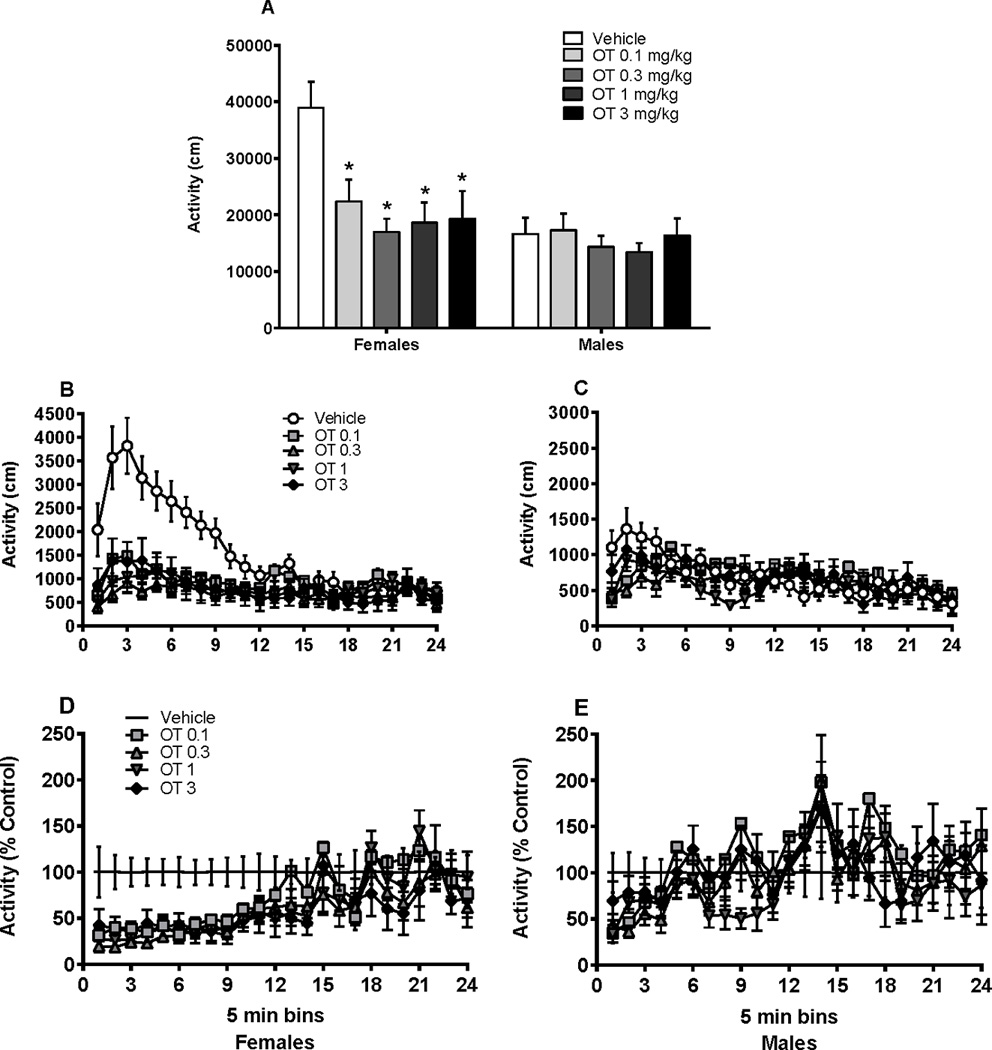
Cocaine-induced locomotor activity in male and female rats following oxytocin pretreatment (30 min oxytocin injection prior to cocaine injection). (A) Locomotor activity over 120 min session. Oxytocin at all doses attenuated activity relative to same-sex vehicle in females but not males. (B) Female and (C) male distance traveled scores represented in 5 min time bins throughout the 120 min session. Oxytocin attenuated activity across the entire session in females but not in males. (D) Female and (E) male distance traveled normalized to vehicle control group. Oxytocin attenuated activity across the session in females but not males. *significant difference from same sex vehicle, p < 0.05
Discussion
In the present set of experiments, an acute peripheral injection of oxytocin reduced active lever presses and cocaine intake during self-administration in female rats. Additionally, an acute injection of oxytocin reduced reinstatement of cocaine seeking as evidenced by decreased active lever presses during the conditioned cue-induced reinstatement test. Furthermore, acute administration of oxytocin had a significant impact on cocaine-induced locomotor activity in females.
The present findings are consistent with the attenuating effect of intra-cerebral (Morales-Rivera et al., 2014) and systemic (Zhou et al., 2014) oxytocin on cocaine self-administration and reinstatement in male rats. Both females (current report) and males (Zhou et al., 2014) used parallel doses and route of oxytocin injection in males and reported similar findings. Along the same vein, oxytocin decreased active lever responding for sucrose and sucrose intake in both males and females, although females were more sensitive to lower doses of oxytocin, with males only responding to higher doses (Zhou et al., 2015). Oxytocin’s attenuating effect on cocaine-seeking in male and female rats contributes to growing literature on oxytocin as a potential therapeutic target for psychostimulant addiction (Carson et al., 2010a; Cox et al., 2014). Additionally, repeated oxytocin (1.0 mg/kg, i.p.) during adolescence (PND 28–37) inhibited motivation, assessed on a progressive ratio (PR) schedule of reinforcement, to self-administer methamphetamine during adulthood (PND 62) in female rats (Hicks et al., 2015). Our present study shows that repeated intermittent administration of oxytocin did not decrease maintenance of active lever presses or cocaine intake in female rats (Table 1). This discrepancy may be attributed to differences in reinforcement schedule (FR vs. PR), drug reward (cocaine vs. methamphetamine), or age of oxytocin exposure (adulthood vs. adolescence). Oxytocin similarly attenuated cocaine-seeking in females and males (Zhou et al., 2014), which contrasts our reported sex differences in sucrose intake (i.e., a non-drug reinforcer) following oxytocin (Zhou et al., 2015). In these studies females were more sensitive to lower oxytocin doses when responding for sucrose-conditioned cues (Zhou et al., 2015). Taken together, these findings suggest oxytocin may mediate drug and natural reward processing via different mechanisms in male and female rats. For example, oxytocin had a greater impact in natural reward processing in females, while exerting a similar effect on drug reward processing. The differing circuitry underlying the drug reward and natural reward shows that dopaminergic activity within nucleus accumbens is important in drug seeking, while the lateral hypothalamus is a key structure in mediating food seeking (for review see DiLeone, Taylor, and Picciotto, 2012).
The ability of 1 mg/kg oxytocin to decrease cued reinstatement was not attributed to fluctuations in estrous cycle. Overall, circulating ovarian hormones influenced neither cue-induced reinstatement nor the oxytocin attenuation of behavior. These results echo previous research suggesting that female estrous cycle is not a primary factor influencing cue-induced reinstatement of cocaine seeking (Fuchs et al., 2005). Similarly, female estrous cycle did not influence meth-primed reinstatement or oxytocin’s ability to attenuate meth-primed reinstatement (Cox et al., 2013). Rather than cycle phase, oxytocin may impact cocaine-seeking through dopaminergic interactions. Oxytocin decreased dopamine release and receptor binding in mesolimbic brain structures (Sarnyai and Kovacs, 1994), and antagonized cocaine enhanced dopamine receptor binding and downstream signaling in the nucleus accumbens (Kovacs et al., 1990). Oxytocin also inhibited striatal dopamine transmission while simultaneously attenuating methamphetamine-induced hyperactivity (Qi et al., 2008). Combined, these findings suggest that oxytocin may directly counteract cocaine’s rewarding properties through attenuation of cocaine-induced dopaminergic signaling. Alternatively, oxytocin may be inherently rewarding, thus counter-acting the rewarding properties of both drug (e.g., cocaine) and non-drug (e.g., sucrose) reinforcers. Our enthusiasm for this line of reasoning is dampened because oxytocin does not condition a place preference (Qi et al., 2009; Baracz et al., 2012) unless at high doses (8 mg/kg; Liberzon et al., 1997). To date, it is unknown whether oxytocin acts as an instrumental reinforcer in a self-administration paradigm. Importantly, all of these aforementioned studies have only used male rats and one cannot assume simple extrapolation of these findings to females.
An important question in regards to oxytocin’s suppressive effects on locomotion is whether or not this suppression can account for the decrease in reinstated responding. Previously, we reported that female rats were more sensitive to locomotor attenuation following systemic injections of oxytocin (Zhou et al., 2015). In that report, only the highest dose of oxytocin attenuated locomotor activity in males. However in the current report, oxytocin equally decreased motor activity in males and females before cocaine administration (see Figure 3). These contrasting finding may be explained by methodological considerations. In the former study, rats received oxytocin and returned to the home cage for 30 min prior to placement in the locomotor chamber. In the later study, rats received oxytocin and then where immediately placed in the chamber. Thus, behavior was recorded during the time of oxytocin’s highest locomotor suppressing effects. In regards to cocaine induced locomotor activity, we found a greater reduction in cocaine-induced activity following oxytocin in females. Therefore, the present study suggests that the attenuation of cocaine seeking in male rats may likely not be attributed to a reduction in locomotor activity. However, locomotor activity cannot be ruled out as a potential explanation for decreased cocaine seeking in females.
Consistent with others (Festa et al., 2004; Walker et al., 2001), we found cocaine induced greater activity in female rats relative to male rats, and oxytocin brought activity down to a level consistent with a male baseline. These pattern of results were observed across the entire 120 min session as well as in the first ten 5 min bins (50 min) (data not shown), suggesting that this effect was not merely diminished cocaine action. In order to account for the variability in baseline locomotor activity, we further analyzed oxytocin’s effect on cocaine-induced locomotion by normalizing locomotor behavior to vehicle controls in males and females. This yielded a similar pattern of results, with oxytocin impacting cocaine-induced locomotion in females but not in males. These findings are interesting because oxytocin (1 mg/kg) attenuated meth-induced activity (Carson et al., 2010a). Albeit, there are sexual dimorphisms in central and peripheral oxytocin receptor distribution and function (Adan et al., 1995; Dumais et al., 2013; Fuchs et al., 1982; Vane & Williams, 1973), there are also inherent sex differences in cocaine-induced locomotor activity (Festa et al., 2004; Walker et al., 2001). Given the cocaine-induced sex difference in locomotor activity we report (Figure 4), the ability of oxytocin to inhibit hyperactivity in males is compromised because there is a reduced margin of baseline hyperactivity.
Numerous studies have determined that systemically administered oxytocin influences behavior through a central site of action. For example, systemic oxytocin reduced cocaine-induced sniffing behavior, an effect blocked by intracerebroventricular infusion of an oxytocin antagonist (Sarnyai et al., 1991). Similarly, peripherally administered oxytocin increased fos expression in hypothalamic nuclei (Carson et al., 2010b). However, these studies have been conducted in male rats and it remains unknown whether systemic oxytocin can influence behavior through a similar centrally mediated site of action in females. Few studies have examined the effect of peripherally administered oxytocin on central mechanisms in females. However, one study found that oxytocin influences sexual behavior differently depending on central or peripheral administration in female prairie voles (Witt, Carter, and Walton, 1990). Our data suggest that systemically administered oxytocin, while producing similar effects on cocaine seeking, potentially influences locomotion differently in female and male rats.
Overall, our findings reveal that systemically administered oxytocin reduced lever pressing for cocaine and cocaine intake during self-administration. Similarly, oxytocin attenuated cue-induced reinstatement of cocaine seeking following a period of extinction. These results parallel findings in male rats (Zhou et al., 2014). Furthermore, our results also display a potential sex-difference in oxytocin’s effect on cocaine-induced locomotor activity. Here, we showed that peripheral administration of oxytocin reduces cocaine-induced locomotor activity in females, but not in males, although possible caveats must be taken into consideration. Determining differences in peripherally vs. centrally driven oxytocin effects in males and females will be important as oxytocin continues to gain popularity as a therapeutic target for drug addiction.
References
- Adan RA, Van Leeuwen FW, Sonnemans MA, Brouns M, Hoffman G, Verbalis JG, Burbach JP. Rat oxytocin receptor in brain, pituitary, mammary gland, and uterus: partial sequence and immunocytochemical localization. Endocrinology. 1995;136(9):4022–4028. doi: 10.1210/endo.136.9.7649111. [DOI] [PubMed] [Google Scholar]
- Baracz SJ, Rourke PI, Pardey MC, Hunt GE, McGregor IS, Cornish JL. Oxytocin directly administered into the nucleus accumbens core or subthalamic nucleus attenuates methamphetamine-induced conditioned place preference. Behavioural brain research. 2012;228(1):185–193. doi: 10.1016/j.bbr.2011.11.038. [DOI] [PubMed] [Google Scholar]
- Brady KT, Randall CL. Gender differences in substance use disorders. Psychiatr Clin North Am. 1999;22:241–252. doi: 10.1016/s0193-953x(05)70074-5. [DOI] [PubMed] [Google Scholar]
- Buffalari DM, Baldwin CK, Feltenstein MW, See RE. Corticotrophin releasing factor (CRF) induced reinstatement of cocaine seeking in male and female rats. Physiology & behavior. 2012;105(2):209–214. doi: 10.1016/j.physbeh.2011.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell BM, Merchant KM. Serotonin 2C receptors within the basolateral amygdala induce acute fear-like responses in an open-field environment. Brain research. 2003;993(1):1–9. doi: 10.1016/s0006-8993(03)03384-5. [DOI] [PubMed] [Google Scholar]
- Carroll ME, Anker JJ. Sex differences and ovarian hormones in animal models of drug dependence. Hormones and behavior. 2010;58(1):44–56. doi: 10.1016/j.yhbeh.2009.10.001. [DOI] [PubMed] [Google Scholar]
- Carson DS, Cornish JL, Guastella AJ, Hunt GE, McGregor IS. Oxytocin decreases methamphetamine self-administration, methamphetamine hyperactivity, and relapse to methamphetamine-seeking behaviour in rats. Neuropharmacology. 2010a;58(1):38–43. doi: 10.1016/j.neuropharm.2009.06.018. [DOI] [PubMed] [Google Scholar]
- Carson DS, Hunt GE, Guastella AJ, Barber L, Cornish JL, Arnold JC, McGregor IS. Systemically administered oxytocin decreases methamphetamine activation of the subthalamic nucleus and accumbens core and stimulates oxytocinergic neurons in the hypothalamus. Addiction biology. 2010b;15(4):448–463. doi: 10.1111/j.1369-1600.2010.00247.x. [DOI] [PubMed] [Google Scholar]
- Carter CS. Neuroendocrine perspectives on social attachment and love. Psychoneuroendocrinology. 1998;23:779–818. doi: 10.1016/s0306-4530(98)00055-9. [DOI] [PubMed] [Google Scholar]
- Choy VJ, Watkins WB. Maturation of the hypothalamo-neurohypophysial system. Cell and tissue research. 1979;197(2):325–336. doi: 10.1007/BF00233923. [DOI] [PubMed] [Google Scholar]
- Cox BM, Young AB, See RE, Reichel CM. Sex differences in methamphetamine seeking in rats: Impact of oxytocin. Psychoneuroendocrinology. 2013;38(10):2343–2353. doi: 10.1016/j.psyneuen.2013.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis M. The role of the amygdala in fear and anxiety. Annual review of neuroscience. 1992;15(1):353–375. doi: 10.1146/annurev.ne.15.030192.002033. [DOI] [PubMed] [Google Scholar]
- Dawe S, Gullo MJ, Loxton NJ. Reward drive and rash impulsiveness as dimensions of impulsivity: implications for substance misuse. Addictive behaviors. 2004;29(7):1389–1405. doi: 10.1016/j.addbeh.2004.06.004. [DOI] [PubMed] [Google Scholar]
- DiLeone RJ, Taylor JR, Picciotto MR. The drive to eat: comparisons and distinctions between mechanisms of food reward and drug addiction. Nature neuroscience. 2012;15(10):1330–1335. doi: 10.1038/nn.3202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumais KM, Bredewold R, Mayer TE, Veenema AH. Sex differences in oxytocin receptor binding in forebrain regions: correlations with social interest in brain region-and sex-specific ways. Hormones and behavior. 2013;64(4):693–701. doi: 10.1016/j.yhbeh.2013.08.012. [DOI] [PubMed] [Google Scholar]
- Dumont GJH, Sweep FCGJ, Van der Steen R, Hermsen R, Donders ART, Touw DJ, Verkes RJ. Increased oxytocin concentrations and prosocial feelings in humans after ecstasy (3, 4-methylenedioxymethamphetamine) administration. Social neuroscience. 2009;4(4):359–366. doi: 10.1080/17470910802649470. [DOI] [PubMed] [Google Scholar]
- Feltenstein MW, Henderson AR, See RE. Enhancement of cue-induced reinstatement of cocaine-seeking in rats by yohimbine: sex differences and the role of the estrous cycle. Psychopharmacology. 2011;216(1):53–62. doi: 10.1007/s00213-011-2187-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson JN, Young LJ, Hearn EF, Matzuk MM, Insel TR, Winslow JT. Social amnesia in mice lacking the oxytocin gene. Nature genetics. 2000;25:284–288. doi: 10.1038/77040. [DOI] [PubMed] [Google Scholar]
- Festa ED, Russo SJ, Gazi FM, Niyomchai T, Kemen LM, Lin SN, Quinones-Jenab V. Sex differences in cocaine-induced behavioral responses, pharmacokinetics, and monoamine levels. Neuropharmacology. 2004;46(5):672–687. doi: 10.1016/j.neuropharm.2003.11.017. [DOI] [PubMed] [Google Scholar]
- Fuchs RA, Evans KA, Mehta RH, Case JM, See RE. Influence of sex and estrous cyclicity on conditioned cue-induced reinstatement of cocaine-seeking behavior in rats. Psychopharmacology. 2005;179(3):662–672. doi: 10.1007/s00213-004-2080-7. [DOI] [PubMed] [Google Scholar]
- Hicks C, Cornish JL, Baracz SJ, Suraev A, McGregor IS. Adolescent pre-treatment with oxytocin protects against adult methamphetamine-seeking behavior in female rats. Addiction biology. 2014 doi: 10.1111/adb.12197. [DOI] [PubMed] [Google Scholar]
- Kovács GL, Horváth Z, Sarnyai Z, Faludi M, Telegdy G. Oxytocin and a C-terminal derivative (Z-prolyl-D-leucine) attenuate tolerance to and dependence on morphine and interact with dopaminergic neurotransmission in the mouse brain. Neuropharmacology. 1985;24(5):413–419. doi: 10.1016/0028-3908(85)90026-7. [DOI] [PubMed] [Google Scholar]
- Kovacs GL, Sarnyai Z, Babarczi E, Szabo G, Telegdy G. The role of oxytocin-dopamine interactions in cocaine-induced locomotor hyperactivity. Neuropharmacology. 1990;29(4):365–368. doi: 10.1016/0028-3908(90)90095-9. [DOI] [PubMed] [Google Scholar]
- Keverne EB, Curley JP. Vasopressin, oxytocin and social behaviour. Current opinion in neurobiology. 2004;14:777–783. doi: 10.1016/j.conb.2004.10.006. [DOI] [PubMed] [Google Scholar]
- Knobloch HS, Charlet A, Hoffmann LC, Eliava M, Khrulev S, Cetin AH, Grinevich V. Evoked axonal oxytocin release in the central amygdala attenuates fear response. Neuron. 2012;73(3):553–566. doi: 10.1016/j.neuron.2011.11.030. [DOI] [PubMed] [Google Scholar]
- Landgraf R, Neumann ID. Vasopressin and oxytocin release within the brain: a dynamic concept of multiple and variable modes of neuropeptide communication. Frontiers in neuroendocrinology. 2004;25:150–176. doi: 10.1016/j.yfrne.2004.05.001. [DOI] [PubMed] [Google Scholar]
- Liberzon I, Trujillo KA, Akil H, Young EA. Motivational properties of oxytocin in the conditioned place preference paradigm. Neuropsychopharmacology. 1997;17(6):353–359. doi: 10.1016/S0893-133X(97)00070-5. [DOI] [PubMed] [Google Scholar]
- Liu Y, Young KA, Curtis JT, Aragona BJ, Wang Z. Social bonding decreases the rewarding properties of amphetamine through a dopamine D1 receptor-mediated mechanism. The Journal of Neuroscience. 2011;31(22):7960–7966. doi: 10.1523/JNEUROSCI.1006-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim MM, Young LJ. Neuropeptidergic regulation of affiliative behavior and social bonding in animals. Hormones and behavior. 2006;50:506–517. doi: 10.1016/j.yhbeh.2006.06.028. [DOI] [PubMed] [Google Scholar]
- Lynch WJ, Roth ME, Carroll ME. Biological basis of sex differences in drug abuse: preclinical and clinical studies. Psychopharmacology. 2002;164(2):121–137. doi: 10.1007/s00213-002-1183-2. [DOI] [PubMed] [Google Scholar]
- Marcondes FK, Bianchi FJ, Tanno AP. Determination of the estrous cycle phases of rats: Some helpful considerations. Brazilian Journal of Biology. 2002;62:609–614. doi: 10.1590/s1519-69842002000400008. [DOI] [PubMed] [Google Scholar]
- McGregor IS, Bowen MT. Breaking the loop: oxytocin as a potential treatment for drug addiction. Hormones and behavior. 2012;61(3):331–339. doi: 10.1016/j.yhbeh.2011.12.001. [DOI] [PubMed] [Google Scholar]
- Morales-Rivera A, Hernández-Burgos MM, Martínez-Rivera A, Pérez-Colón J, Rivera R, Montalvo J, Maldonado-Vlaar CS. Anxiolytic effects of oxytocin in cue-induced cocaine seeking behavior in rats. Psychopharmacology. 2014;231(21):4145–4155. doi: 10.1007/s00213-014-3553-y. [DOI] [PubMed] [Google Scholar]
- Neumann ID. Stimuli and consequences of dendritic release of oxytocin within the brain. Biochemical Society Transactions. 2007;35:1252–1257. doi: 10.1042/BST0351252. [DOI] [PubMed] [Google Scholar]
- O'Brien MS, Anthony JC. Risk of becoming cocaine dependent: epidemiological estimates for the United States, 2000–2001. Neuropsychopharmacology. 2005;30(5):1006–1018. doi: 10.1038/sj.npp.1300681. [DOI] [PubMed] [Google Scholar]
- Pedersen CA, Prange AJ. Induction of maternal behavior in virgin rats after intracerebroventricular administration of oxytocin. Proceedings of the National Academy of Sciences. 1979;76:6661–6665. doi: 10.1073/pnas.76.12.6661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi J, Yang JY, Song M, Li Y, Wang F, Wu CF. Inhibition by oxytocin of methamphetamine-induced hyperactivity related to dopamine turnover in the mesolimbic region in mice. Naunyn-Schmiedeberg's archives of pharmacology. 2008;376(6):441–448. doi: 10.1007/s00210-007-0245-8. [DOI] [PubMed] [Google Scholar]
- Qi J, Yang JY, Wang F, Zhao YN, Song M, Wu CF. Effects of oxytocin on methamphetamine-induced conditioned place preference and the possible role of glutamatergic neurotransmission in the medial prefrontal cortex of mice in reinstatement. Neuropharmacology. 2009;56(5):856–865. doi: 10.1016/j.neuropharm.2009.01.010. [DOI] [PubMed] [Google Scholar]
- Ren Z, Sun WL, Jiao H, Zhang D, Kong H, Wang X, Xu M. Dopamine D1 and N-methyl-D-aspartate receptors and extracellular signal-regulated kinase mediate neuronal morphological changes induced by repeated cocaine administration. Neuroscience. 2010;168(1):48–60. doi: 10.1016/j.neuroscience.2010.03.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reichel CM, See RE. Modafinil effects on reinstatement of methamphetamine seeking in a rat model of relapse. Psychopharmacology. 2010;210(3):337–346. doi: 10.1007/s00213-010-1828-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ring RH, Malberg JE, Potestio L, Ping J, Boikess S, Luo B, Rosenzweig-Lipson S. Anxiolytic-like activity of oxytocin in male mice: behavioral and autonomic evidence, therapeutic implications. Psychopharmacology. 2006;185:218–225. doi: 10.1007/s00213-005-0293-z. [DOI] [PubMed] [Google Scholar]
- Sarnyai Z, Babarczy E, Krivan M, Szabo G, Kovacs GL, Barth T, Telegdy G. Selective attenuation of cocaine-induced stereotyped behaviour by oxytocin: putative role of basal forebrain target sites. Neuropeptides. 1991;19(1):51–56. doi: 10.1016/0143-4179(91)90073-r. [DOI] [PubMed] [Google Scholar]
- Sarnyai Z, Kovács GL. Oxytocin in learning and addiction: from early discoveries to the present. Pharmacology Biochemistry and Behavior. 2014;119:3–9. doi: 10.1016/j.pbb.2013.11.019. [DOI] [PubMed] [Google Scholar]
- Sarnyai Z, Kovács GL. Role of oxytocin in the neuroadaptation to drugs of abuse. Psychoneuroendocrinology. 1994;19(1):85–117. doi: 10.1016/0306-4530(94)90062-0. [DOI] [PubMed] [Google Scholar]
- Sarnyai Z, Szabó G, Kovács GL, Telegdy G. Oxytocin attenuates the cocaine-induced exploratory hyperactivity in mice. Neuroreport. 1990;1(3):200–202. doi: 10.1097/00001756-199011000-00006. [DOI] [PubMed] [Google Scholar]
- Van Haaren F, Meyer ME. Sex differences in locomotor activity after acute and chronic cocaine administration. Pharmacology Biochemistry and Behavior. 1991;39(4):923–927. doi: 10.1016/0091-3057(91)90054-6. [DOI] [PubMed] [Google Scholar]
- Vane JR, Williams KI. The contribution of prostaglandin production to contractions of the isolated uterus of the rat. British journal of pharmacology. 1973;48(4):629–639. doi: 10.1111/j.1476-5381.1973.tb08250.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker QD, Cabassa J, Kaplan KA, Li ST, Haroon J, Spohr HA, Kuhn CM. Sex differences in cocaine-stimulated motor behavior: disparate effects of gonadectomy. Neuropsychopharmacology. 2001;25(1):118–130. doi: 10.1016/S0893-133X(00)00248-7. [DOI] [PubMed] [Google Scholar]
- Weiss RD, Martinez-Raga J, Griffin ML, Greenfield SF, Hufford C. Gender differences in cocaine dependent patients: a 6 month follow-up study. Drug and Alcohol Dependence. 1997;44(1):35–40. doi: 10.1016/s0376-8716(96)01319-1. [DOI] [PubMed] [Google Scholar]
- Westermeyer J, Boedicker AE. Course, severity, and treatment of substance abuse among women versus men. The American journal of drug and alcohol abuse. 2000;26(4):523–535. doi: 10.1081/ada-100101893. [DOI] [PubMed] [Google Scholar]
- Windle RJ, Kershaw YM, Shanks N, Wood SA, Lightman SL, Ingram CD. Oxytocin attenuates stress-induced c-fos mRNA expression in specific forebrain regions associated with modulation of hypothalamo–pituitary–adrenal activity. The Journal of neuroscience. 2004;24(12):2974–2982. doi: 10.1523/JNEUROSCI.3432-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witt DM, Carter CS, Walton DM. Central and peripheral effects of oxytocin administration in prairie voles (Microtus ochrogaster) Pharmacology Biochemistry and Behavior. 1990;37(1):63–69. doi: 10.1016/0091-3057(90)90042-g. [DOI] [PubMed] [Google Scholar]
- Young KA, Gobrogge KL, Wang Z. The role of mesocorticolimbic dopamine in regulating interactions between drugs of abuse and social behavior. Neuroscience & Biobehavioral Reviews. 2011;35(3):498–515. doi: 10.1016/j.neubiorev.2010.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou L, Ghee SM, See RE, Reichel CM. Oxytocin differentially affects sucrose taking and seeking in male and female rats. Behavioural brain research. 2015;283:184–190. doi: 10.1016/j.bbr.2015.01.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou L, Sun WL, Young AB, Lee K, McGinty JF, See RE. Oxytocin reduces cocaine seeking and reverses chronic cocaine-induced changes in glutamate receptor function. International Journal of Neuropsychopharmacology. 2014 doi: 10.1093/ijnp/pyu009. pyu009. [DOI] [PMC free article] [PubMed] [Google Scholar]


