Abstract
Background
Malignant cerebral edema (CED) complicates at least 20 % of large hemispheric infarcts (LHI) and may result in neurological deterioration or death. Midline shift (MLS) is a standard but crude measure of edema severity. We propose that volumetric analysis of shifts in cerebrospinal fluid (CSF) over time provides a reliable means of quantifying the spectrum of edema severity after LHI.
Methods
We identified 38 patients from 2008 to 2014 with NIHSS ≥8, baseline CT <6 h after stroke onset, at least 1 follow-up (FU) CT, and no parenchymal hematoma. The volumes of CSF (sulci, ventricles, and cisterns) ipsilateral (IL) and contralateral (CL) to infarct on baseline and FU CTs were quantified by manually assisted outlining with MIPAV image analysis software, as was infarct volume and MLS on FU CTs. Percentage change in CSF volumes (ΔCSF) from baseline to FU scans was correlated with MLS and compared in those with vs. without malignant edema (defined as hemicraniectomy, osmotic therapy, or death/neurological deterioration with MLS ≥5 mm).
Results
11 of 38 subjects (29 %) developed malignant edema. Neither baseline NIHSS nor CSF volume differed between those with and without edema (median NIHSS 18 vs. 13, p = 0.12, CSF volume 102 vs. 124 ml, p = 0.16). Inter-rater reliability for CSF measurements was excellent (intraclass correlation coefficient 0.97). ΔCSF correlated strongly with MLS at peak edema (r = −0.75), even adjusting for infarct volume (p = 0.009). ΔCSF was also greater in those with malignant edema [−55 % (IQR −49 to −62) vs. −36 % (−27 to −45), p = 0.004]. ΔCSF was the greatest within IL sulci [−97 % (−86 to −99) vs. −71 % (−41 to −79), p = 0.002] but also significantly greater within CL sulci in those with malignant edema [−50 % (−29 to −65) vs. −25 % (0 to −31), p = 0.014]. More than half this CSF volume reduction occurred by the time of first FU CT around 24 h after stroke, while MLS rose later.
Conclusions
Volumetric CSF analysis reliably quantifies CED and distinguishes those with malignant edema and MLS from those with a more benign course after LHI. ΔCSF may provide an earlier and more sensitive indicator of edema severity across a broader dynamic range than MLS.
Keywords: Stroke, Brain edema, Cerebrospinal fluid, Neuroimaging
Introduction
Cerebral edema (CED) accounts for a large proportion of neurologic deterioration within the first week after large hemispheric infarction (LHI) and is the leading cause of early death [1]. Malignant edema develops in approximately 10–20 % of patients and can result in a rapid rise in compartmental pressure, subfalcine and uncal herniation, and clinical deterioration, often leading to death [2]. Mortality may be as high as 80 % despite medical management, unless surgical decompression is performed before herniation occurs [3]. Despite the importance of studying CED after LHI, little attention has been paid to understanding the kinetics of this process [4, 5]. No simple validated metric exists to easily and accurately quantify edema across the broad range of severities at several time points after stroke. Such a methodology would be important to characterize and thence understand the variability in trajectory of edema across patients while potentially providing a biomarker for studying and predicting outcomes after LHI, including early need for decompressive hemicraniectomy (DHC) [6].
Midline shift (MLS) and volume of infarct-related hypodensity are two commonly used surrogates of edema [7]. MLS is a crude estimate of subfalcine herniation which only becomes apparent later in the course and in severe cases, when edema has progressed beyond the point where reductions in intracranial blood and cerebrospinal (CSF) volume can compensate. It does not capture the full spectrum of edema severity, as those with milder degrees of edema may have little to no MLS, while others with temporal lobe infarction may develop uncal herniation despite minimal MLS. Hypodensity size is a variable combination of infarct and edema and so only approximates the actual edema-related increase in intracranial volume; it is also difficult to quantitate early when infarcts are subtle on computed tomography (CT) scans [8, 9]. In contrast, CSF is progressively displaced from the sulci and ventricles of the cerebral hemispheres as edema develops, and measures of CSF shifts may better capture the dynamic range and temporal course of CED after stroke.
We propose a CT-based volumetric analysis of shifts in CSF compartments as a novel method for more accurately quantifying CED after LHI. This leverages the widespread utilization of CT in acute stroke and the ease of CSF segmentation [10]. In this study, we aim to demonstrate the feasibility and reliability of CSF volumetrics in patients with LHI, and validate this novel metric against standard measures such as MLS and hypodensity volume. We will also evaluate whether changes in or ratios of specific CSF compartments (such as ipsilateral (IL) or contralateral (CL) sulci or ventricles) better correlate with MLS and discriminate those who develop malignant edema compared to estimates of stroke severity or infarct-related hypodensity volume.
Methods
Patient Selection and Clinical Endpoint
We retrospectively identified patients with hemispheric infarction from acute ischemic stroke patients who were enrolled in a prospective stroke genetics study at our institution between 2008 and 2014. Inclusion criteria were (1) initial National Institutes of Health Stroke Scale (NIHSS) ≥8; (2) baseline head CT within 6 h of stroke symptom onset; and (3) at least 1 additional follow-up (FU) CT within 5 days of stroke (and at least 4 h from baseline CT) confirming hemispheric infarction [i.e., hypodensity in middle cerebral artery (MCA) ± PCA/ACA territories] as well as some degree of edema, defined as the presence of ventricular or sulcal effacement, MLS, and/or transtentorial herniation. FU CT scans were categorized based on timing as either “early edema” (within 48 h of stroke onset) or “peak edema” (2–5 days from onset, but prior to DHC). In those with several FU CTs, the CT closest to 24 h from onset was used as early edema CT and the FU CT with the most MLS was used as the peak edema CT. Subjects were excluded if FU CT demonstrated parenchymal hematoma (i.e., homogenous hyperdensity occupying <30 % [PH1] or >30 % of infarct zone [PH2], latter with significant mass effect or located beyond borders of the infarct) [11].
Clinical and demographic information was extracted from the prospective study database and supplemented with review of medical records. This included age, sex, race, time of stroke onset, tPA use, baseline and 24-h NIHSS, and GCS at baseline and at the time of each FU CT. Radiographic data extracted from baseline CT included the presence of hyperdense MCA (hd-MCA) sign and Alberta Stroke Program Early CT Score (ASPECTS) [12]. The primary clinical outcome of “malignant edema” was defined by the development of edema requiring hemicraniectomy or osmotic drug therapy, or CT exhibiting MLS C5 mm associated with death or decline in GCS (of two or more points) from baseline. In our single-center institutional practice, osmotic therapy is only initiated at the time of neurologic worsening associated with edema on CT scan and is not used prophylactically. Similarly, DHC is performed in appropriate patients only after the development of edema and worsening mental status, but prior to signs of irreversible herniation.
Image Processing and Volumetric Analysis
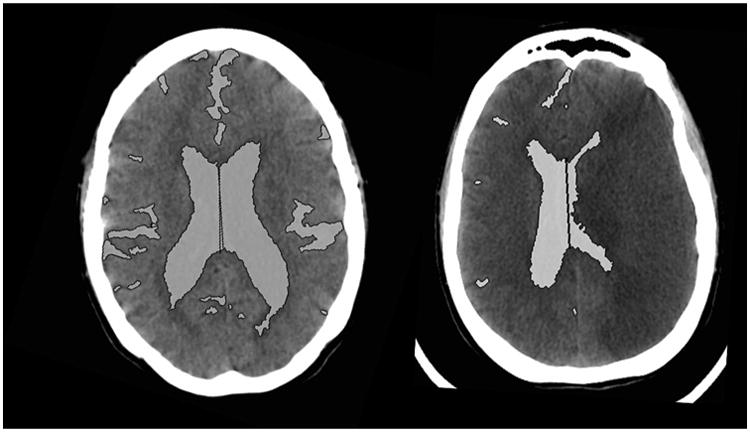
Each CT scan was analyzed using the NIH Medical Imaging Processing, Analysis, and Visualization (MIPAV) software. Supratentorial CSF spaces [sulci and ventricles ipsilateral (IL) and contralateral (CL) to stroke, third ventricle] and basal cisterns were outlined on each slice using the semi-automated level-set tool (with manual outlining, if required, see Fig. 1). The volumes of each compartment and the total volume of CSF were then quantified. Total hemispheric volume (perimeter of brain from vertex down to the level of the anterior clinoid process) was also quantified. Hemispheric and sulcal symmetry was calculated as the ratio of IL vs. CL CSF volumes. The proportion of CSF in the cerebral hemispheres was calculated as the ratio of total CSF volume to hemispheric volume. All FU scans also had measurement of MLS (displacement of the septum pellucidum) and infarct volume (by manual outlining of the visible hypodensity on each slice), and CED grading (grade 1 = focal brain swelling ≤1/3 of hemisphere, grade 2 = > 1/3 of hemisphere, grade 3 = edema with MLS) [13]. The absolute and percentage changes in volumes of CSF (total and each compartment) from baseline to early and peak edema FU CTs were calculated. ΔCSF was defined as the percentage change in total CSF volume from baseline to FU CT with peak edema in each subject. Continuous variables were reported as medians (with interquartile range, IQR) unless normality was confirmed by Shapiro-Wilk testing. Normally distributed variables were compared using t-tests, while non-parametric tests were employed for other comparisons of continuous variables.
Fig. 1. CSF spaces outlined on CT scan slice at baseline and peak edema in sample patient.
Reliability of Measurements
The CSF regions of interest were independently outlined by two different raters (K.Y. and R.D.) in a subset of 13 CTs from five subjects and volumes of each obtained. Inter-rater reliability was calculated using the intraclass correlation coefficient (ICC) derived from a two-way mixed model of absolute agreement of these volumes between raters. This testing was repeated for the measurement of MLS on FU CT scans.
Validation of ΔCSF
Baseline and FU CSF volumes were compared using paired t-tests. Hemispheric and sulcal symmetry was tested by comparing the observed ratios to one (i.e., perfect symmetry) using t-tests at each time point. Validation of ΔCSF as a metric for quantifying CED after LHI was accomplished by (1) correlating ΔCSF with MLS and hypodensity volume at peak edema (using Pearson correlation coefficients). Partial correlations were then obtained for ΔCSF and MLS, adjusting for baseline NIHSS and peak hypodensity volume. (2) The association between ΔCSF and the endpoint of malignant edema was analyzed using the Mann–Whitney U test. If an increased risk of edema was associated with ΔCSF, then this association was further tested in binomial logistic regression, adding baseline clinical and radiographic covariates including age, baseline NIHSS, hd-MCA sign, ASPECTS, tPA use, and baseline CSF volume. ΔCSF was compared between CED grades using one-way ANOVA. Receiver operating characteristic (ROC) analysis was performed to identify optimal ΔCSF thresholds for malignant edema. To further evaluate the internal face validity of CSF measurements, we correlated baseline CSF volume (as a proportion of hemispheric volume) against patient age to determine if this accurately reflected brain atrophy.
Results
We identified 38 subjects with LHI from the prospectively enrolled cohort. Median baseline NIHSS was 15.5 (IQR 10–20) (Table 1). Baseline CT scan was performed at 74 min from stroke onset (IQR 36–166), and median ASPECTS score was 9 (IQR 8–10); 13 (34 %) had an hd-MCA sign and 82 % were treated with tPA. All had at least one FU CT for CSF volumetric analysis; 33 (87 %) had an early FU scan performed at a median of 18 h (IQR 14–30), while 20 (53 %) had a scan at peak edema at a median of 67 h (52–88); 15 had both early and peak edema scans for serial analyses.
Table 1. Clinical and radiographic characteristics in all subjects, categorized by development of malignant cerebral edema.
| Variable | All subjects | Malignant edema (n = 11) | No malignant edema (n = 27) | p value |
|---|---|---|---|---|
| Age (years) | 62 ± 16 | 57 ± 17 | 64 ± 16 | 0.21 |
| Gender, female | 19 (50 %) | 7 (64 %) | 12 (44 %) | 0.48 |
| Race, AA (%) | 8 (21 %) | 2 (18 %) | 6 (22 %) | 1.00 |
| Baseline NIHSS | 15.5 (10–20) | 18 (14–21) | 13 (10–20) | 0.12 |
| ASPECTS | 9 (8–10) | 9 (8–10) | 10 (9–10) | 0.18 |
| Hd-MCA sign | 13 (34 %) | 6 (55 %) | 7 (26 %) | 0.09 |
| tPA treatment | 31 (82 %) | 9 (82 %) | 22 (82 %) | 1.00 |
| 24-h NIHSS | 17 (13–22) | 20 (17–25) | 16 (10–21) | 0.04 |
| Hypodensity volume | 201 (96–280) | 282 (227–329) | 135 (70–203) | 0.001 |
| Peak MLS | 4.9 ± 3.7 | 8.5 ± 2.6 | 2.5 ± 2.0 | <0.001 |
| Mortality | 5 (13 %) | 1 (9 %) | 4 (15 %) | 1.00 |
Values are reported as mean ± standard deviation, median (interquartile range), or proportions
AA African-American, Hd-MCA hyperdense MCA, MLS midline shift
Total baseline CSF volume was 120 ml (IQR 80–176 ml), of which just over two-thirds resided in the hemispheric sulci. The ratio of IL to CL hemispheric CSF volumes (i.e., hemispheric symmetry) was 0.96 ± 0.15 at baseline (p = 0.1 for comparison to 1). The ratio of IL:CL sulcal volumes (i.e., sulcal symmetry) was 0.94 ±0.16 (p = 0.048 for comparison to 1). CSF volume represented 11 ± 5 % of hemispheric volume at baseline. The correlation between % CSF volume (as an estimate of brain atrophy) and subject's age was 0.77 (p < 0.001, supplementary Fig.1). We did not find a correlation between ASPECTS score and baseline NIHSS or CSF volume/symmetry at baseline. However, hemispheric symmetry weakly correlated to baseline NIHSS (r = −0.36, p = 0.028). ICC for inter-rater measurement of CSF volumes was 0.92 for sulci and 0.99 for ventricles (both p < 0.001). Overall agreement for total CSF volumes was 0.97 (p < 0.001, supplementary Fig. 2). For comparison, ICC for the measurement of MLS was only 0.85.
Kinetics of Cerebral Edema
CSF volume fell 32 % from baseline to early FU scan (39 ± 24 ml, p < 0.001). The majority of this reduction occurred in IL sulci (22 ml or 56 %), but there was also a loss in CL sulci (12 ml/30 %) and IL ventricle (4 ml/ 28 %). Hemispheric symmetry fell to 0.63 ± 0.24 and sulcal symmetry to 0.57 ± 0.29 (both p < 0.001 compared to baseline). There was a further loss of CSF to scan with peak edema in those with both scans available: total CSF volume fell 55 ml compared to baseline (ΔCSF of 47 %) with IL sulci falling by 82 % (29 ml) and CL sulci by 35 % (14 ml). IL ventricular volume fell by 64 % (10 ml, all p < 0.001); there was no change in CL ventricular volume. Hemispheric symmetry fell to 0.31 ± 0.23 and sulcal symmetry to 0.21 ± 0.2 (both p < 0.001 in comparison to baseline and early CT). While over half the loss of CSF occurred by 24 h, the increase in MLS was seen predominantly on the peak edema scan (median of 5.3 mm, IQR 2.8–9.4) with little seen by 24 h after stroke (median 2 mm, IQR 0–2.7 mm).
Validation of CSF Volumetrics with MLS and Malignant Edema
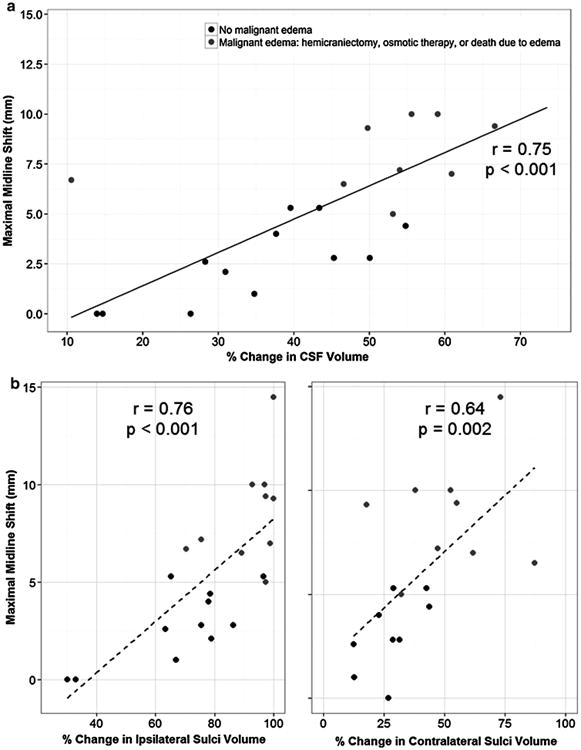
Malignant edema occurred in 11 subjects (29 %), with one additional subject having 5 mm or greater MLS without clinical deterioration. There was only a trend to higher baseline NIHSS in those with malignant edema, and hyperdense MCA sign was non-significantly more likely in those with edema (Table 1). There was no correlation between NIHSS and degree of MLS at peak edema (r = 0.13, p = 0.57). In contrast, we found a strong correlation between ΔCSF and MLS (r = −0.75, p < 0.001, Fig. 2). The volume of infarct-related hypodensity also correlated with MLS (r = 0.66, p = 0.001) and with ΔCSF (r = 0.54, p = 0.01), but the association of ΔCSF and MLS remained even after adjusting for baseline NIHSS and peak infarct-related hypodensity volume (partial correlation of −0.63, p = 0.005). MLS also inversely correlated with hemispheric (r = −0.64) and sulcal symmetry (r = −0.73, both p < 0.001). The CSF compartment most strongly correlated with MLS was % reduction in the volume of IL sulci (r = −0.76, p < 0.001).
Fig. 2. Correlation of peak midline shift with total ΔCSF (top) and in ipsilateral and contralateral sulci (bottom, left, and right).
There was a greater ΔCSF in those with malignant edema (55 vs. 35 % vs. baseline, p = 0.004). Absolute volume reduction was not statistically different (53 vs. 39 ml, p = 0.2). Adjusting the association of ΔCSF with malignant edema for baseline covariates (age, NIHSS, hyperdense MCA, ASPECTS, baseline CSF volume) did not temper its association (aOR 1.14, 95 % CI 1.02–1.28, p = 0.027). Reduction in IL and CL sulci volumes was also significantly greater in those with malignant edema (IL −92 vs. −65 %, p = 0.002; CL −43 vs. −19 %, p = 0.014). There was significantly larger ΔCSF in those with CED grades 3 (49 %) vs. 1 (14 %) or 2 (34 %, p = 0.005). ROC analysis revealed that ΔCSF had an area under curve of 0.85 (95 % CI 0.66–1.00, p = 0.006) with an optimal cutoff of 45 % providing 90 % sensitivity and 83 % specificity for malignant edema. For comparison, measurement of peak hypodensity volume provided 90 % sensitivity for volumes above 150 ml but with only 58 % specificity.
Discussion
In this validation study, we have demonstrated that severity of CED after LHI can be quantified using a CT-based volumetric measure of CSF shifts over time. This novel measure utilizes serial brain imaging with CT scans as routinely performed in patients with LHI at risk for malignant edema. Outlining CSF compartments on baseline and FU scans provided a reliable and quantitative metric (with extremely high inter-rater agreement) that correlated strongly with infarct volume and MLS and was associated with the development of malignant edema independent of baseline markers of stroke severity and even infarct volume itself. In fact, we found that baseline NIHSS was only weakly associated with more severe edema in this cohort of stroke patients with hemispheric infarcts, and there was absolutely no correlation of baseline NIHSS with maximal severity of midline shift. This highlights the need for better early predictors of malignant edema.
CSF volumetric measures also exhibited good temporal resolution, demonstrating easily measurable differences at two distinct FU time points after LHI compared to baseline imaging. The reduction in CSF volume (ΔCSF) that we were able to quantify also paralleled the known time course of cerebral edema, with over half the shifts occurring by 24 h, providing a potential early radiographic predictor of malignant edema at a time point when accurate decisions about need for surgical interventions are critical. MRI measurement of infarct volume within 24 h has been demonstrated to predict malignant edema, but we believe that a CT-based approach would offer more generalizable advantages if it was able to aid in early decision making [4].
Using CSF volumetrics to quantify CED also possesses intrinsic biologic merit as it reflects the direct physiologic compensation that the brain undergoes in response to edema, as outlined over two centuries ago by anatomist Alexander Monro but expanded upon by Burrows and later Cushing who noted the importance of CSF in intracranial dynamics [14–16]. Not only did we observe that the majority of hemispheric CSF resides in the bilateral sulci, but that the majority of CSF lost (i.e., compensation as edema develops, per the Monro-Kellie doctrine) comes from IL sulci. Measurement of % volume loss of IL sulci correlated most strongly with MLS and differentiated those with malignant edema from those less severely affected. Further exploring the compartmental dynamics of CSF might provide greater understanding of pressure shifts as edema develops. MLS is likely a later marker of edema when CSF compensation has been exhausted. Tracking early changes in CSF may allow elucidation of early kinetics not reflected simply by the measurement of MLS.
Few studies have attempted to identify biomarkers of edema. One recent attempt utilized radiographic volume change in a roughly analogous way, but with MR imaging (at a time point of 62 h that was quite similar to our peak edema CT scans) and increase of brain volume rather than reduction in CSF volume as their target measurement [17]. However, this study included only 12 subjects, of whom only three developed neurologic deterioration from edema. Nonetheless, it is interesting that they found that brain volume increased by 72 ml, a value not dissimilar to the 55-ml loss of CSF we measured. They found a trend to greater increase in volume in those with edema but no correlation of their measure with MLS. We believe that CSF volumetric measurements may provide a more sensitive marker of edema that detects subtle changes in sulcal effacement and compression of ventricles. Furthermore, performing MR scans in patients with large strokes who are often ventilated or unstable may be challenging, while CT scans are rapid and easier to obtain in this population; they are also more likely to be performed serially, which may facilitate the study of edema kinetics.
We found a 14-ml greater loss in CSF volume in those with malignant edema. While this may appear a minor difference, it is clear that small changes in intracranial volume can be quite clinically significant. A volumetric MR-based study of the effect of mannitol found an 8-ml reduction in brain volume after a bolus dose in LHI patients with MLS [18]. Despite this modest effect, it is apparent that osmotic agents like mannitol and hypertonic saline can reverse transtentorial herniation by reducing brain volume [19].
We also found a significant correlation between baseline CSF volume and age, further supporting the face validity of our measurements. While greater atrophy was not associated with lower risk of malignant edema in this study, having more CSF and room to compensate for edema may be significant and should be explored in future larger studies. One prior study did analyze total CSF volume at baseline (but not change over time) in relation to malignant edema after proximal occlusion [20]. They found that this intracranial volume reserve was lower in those developing malignant edema, while NIHSS was no different. In that study, only total (and not compartmental) CSF volume was measured and even then it was only estimated using a simple thresholding approach. While this method seemed feasible for baseline imaging, it cannot accurately delineate CSF in the presence of infarct-related hypodensity (with overlapping low density adjacent to sulci and ventricles) on FU CT scans. We applied a semi-automated CSF outlining approach with human input that is likely much more accurate in that setting. The next step in expanding the applicability of our approach to a broad spectrum of stroke patients is developing a fully automated algorithm that can outline CSF on serial CT scans. Not only could this be useful for understanding edema kinetics and prediction of malignant trajectories at early time points, but also be applied to large-scale genomic evaluations of edema after stroke, an endeavor that could uncover biologic targets to prevent edema formation [21–23].
There are several limitations to note in this preliminary analysis. While stroke patients were prospectively enrolled in this cohort, selection and image analysis was retrospectively performed and with some knowledge of radiographic outcomes (i.e., MLS) and clinical endpoints (i.e., need for hemicraniectomy), which could introduce a degree of bias. We also utilized a convenience sample of available scans that were generally performed at times of neurologic deterioration and so hopefully represent a close approximation of ‘peak edema,’ but were not standardized in timing. Larger prospective studies should validate the generalizable accuracy of CSF volumetrics to predict edema (adjusting for stroke severity and other important covariates) while employing automated techniques to negate any such biases. We also plan to further evaluate the utility of CSF changes at 24 h as a standardized early biomarker of edema severity.
Conclusions
CSF volumetrics offers a reliable and quantifiable measure of CED after LHI that better captures the full range of edema severity than MLS and does not simply recapitulate measuring infarct-hypodensity volume. We confirmed that those with more MLS and malignant edema manifest larger ΔCSF, validating our novel metric. We propose that this CT-based quantitative metric of edema will allow widespread study of early changes to predict malignant edema and further analyses of the early versus delayed kinetics of edema after LHI.
Supplementary Material
Acknowledgments
Funding: NIH KL2 (5KL2TR000450–08 and UL1 TR000448) to Rajat Dhar NINDS R01 (NS086419) to Jin-Moo Lee.
Footnotes
Electronic Supplementary Material: The online version of this article (doi:10.1007/s12028-015-0204-z) contains supplementary material, which is available to authorized users.
Compliance with Ethical Standards: Disclosures The authors have no relevant financial disclosures (outside of funding support, as listed) to report.
References
- 1.Silver FL, Norris JW, Lewis AJ, Hachinski VC. Early mortality following stroke: a prospective review. Stroke. 1984;15:492–6. doi: 10.1161/01.str.15.3.492. [DOI] [PubMed] [Google Scholar]
- 2.Hacke W, Schwab S, Horn M, Spranger M, De Georgia M, von Kummer R. “Malignant” middle cerebral artery territory infarction: clinical course and prognostic signs. Arch Neurol. 1996;53:309–15. doi: 10.1001/archneur.1996.00550040037012. [DOI] [PubMed] [Google Scholar]
- 3.Vahedi K, Hofmeijer J, Juettler E, Vicaut E, George B, Algra A, et al. Early decompressive surgery in malignant infarction of the middle cerebral artery: a pooled analysis of three randomised controlled trials. Lancet Neurol. 2007;6:215–22. doi: 10.1016/S1474-4422(07)70036-4. [DOI] [PubMed] [Google Scholar]
- 4.Thomalla G, Hartmann F, Juettler E, Singer OC, Lehnhardt FG, Köhrmann M, et al. Prediction of malignant middle cerebral artery infarction by magnetic resonance imaging within 6 hours of symptom onset: a prospective multicenter observational study. Ann Neurol. 2010;68:435–45. doi: 10.1002/ana.22125. [DOI] [PubMed] [Google Scholar]
- 5.Wijdicks EF, Diringer MN. Middle cerebral artery territory infarction and early brain swelling: progression and effect of age on outcome. Mayo Clin Proc. 1998;73:829–36. doi: 10.4065/73.9.829. [DOI] [PubMed] [Google Scholar]
- 6.Dohmen C, Bosche B, Graf R, Staub F, Kracht L, Sobesky J, et al. Prediction of malignant course in MCA infarction by PET and microdialysis. Stroke. 2003;34:2152–8. doi: 10.1161/01.STR.0000083624.74929.32. [DOI] [PubMed] [Google Scholar]
- 7.Manno EM, Adams RE, Derdeyn CP, Powers WJ, Diringer MN. The effects of mannitol on cerebral edema after large hemispheric cerebral infarct. Neurology. 1999;52:583–7. doi: 10.1212/wnl.52.3.583. [DOI] [PubMed] [Google Scholar]
- 8.Kasner SE, Demchuk A, Berrouschot J, Schmutzhard E, Harms L, Verro P, et al. Predictors of fatal brain edema in massive hemispheric ischemic stroke. Stroke. 2001;32:2117–23. doi: 10.1161/hs0901.095719. [DOI] [PubMed] [Google Scholar]
- 9.Krieger DW, Demchuk AM, Kasner SE, Jauss M, Hantson L. Early clinical and radiological predictors of fatal brain swelling in ischemic stroke. Stroke. 1999;30:287–92. doi: 10.1161/01.str.30.2.287. [DOI] [PubMed] [Google Scholar]
- 10.Nguyen BT, Mullins ME. Computed tomography follow-up imaging of stroke. Semin Ultrasound CT MR. 2006;27:168–76. doi: 10.1053/j.sult.2006.02.002. [DOI] [PubMed] [Google Scholar]
- 11.Fiorelli M, Bastianello S, von Kummer R, del Zoppo GJ, Larrue V, Lesaffre E, et al. Hemorrhagic transformation within 36 hours of a cerebral infarct: relationships with early clinical deterioration and 3-month outcome in the European Cooperative Acute Stroke Study I (ECASS I) cohort. Stroke. 1999;30:2280–4. doi: 10.1161/01.str.30.11.2280. [DOI] [PubMed] [Google Scholar]
- 12.Pexman JH, Barber PA, Hill MD, Sevick RJ, Demchuk AM, Hudon ME, et al. Use of the Alberta Stroke Program Early CT Score (ASPECTS) for assessing CT scans in patients with acute stroke. AJNR Am J Neuroradiol. 2001;22:1534–42. [PMC free article] [PubMed] [Google Scholar]
- 13.Strbian D, Meretoja A, Putaala J, Kaste M, Tatlisumak T. Cerebral edema in acute ischemic stroke patients treated with intravenous thrombolysis. Int J Stroke. 2013;8:529–34. doi: 10.1111/j.1747-4949.2012.00781.x. [DOI] [PubMed] [Google Scholar]
- 14.Monro A. Observations on the structure and functions of the nervous system. Edinburgh: Creech and Johnson; 1783. [Google Scholar]
- 15.Burrows G. On disorders of the cerebral circulation and on the connection between affections of the brain and diseases of the heart. Philadelphia: Lea & Blanchard; 1848. [Google Scholar]
- 16.Cushing H. The third circulation in studies in intracranial physiology and surgery. London: Oxford University Press; 1926. pp. 1–51. [Google Scholar]
- 17.Yoo AJ, Sheth KN, Kimberly WT, Chaudhry ZA, Elm JJ, Jacobson S, et al. Validating imaging biomarkers of cerebral edema in patients with severe ischemic stroke. J Stroke Cere-brovasc Dis. 2013;22:742–9. doi: 10.1016/j.jstrokecerebrovasdis.2012.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Videen TO, Zazulia a R, Manno EM, Derdeyn CP, Adams RE, Diringer MN, et al. Mannitol bolus preferentially shrinks non-infarcted brain in patients with ischemic stroke. Neurology. 2001;57:2120–2. doi: 10.1212/wnl.57.11.2120. [DOI] [PubMed] [Google Scholar]
- 19.Koenig MA, Bryan M, Lewin JL, Mirski MA, Geocadin RG, Stevens RD. Reversal of transtentorial herniation with hypertonic saline. Neurology. 2008;70:1023–9. doi: 10.1212/01.wnl.0000304042.05557.60. [DOI] [PubMed] [Google Scholar]
- 20.Minnerup J, Wersching H, Ringelstein EB, Heindel W, Niederstadt T, Schilling M, et al. Prediction of malignant middle cerebral artery infarction using computed tomography-based intracranial volume reserve measurements. Stroke. 2011;42:3403–9. doi: 10.1161/STROKEAHA.111.619734. [DOI] [PubMed] [Google Scholar]
- 21.Simard JM, Chen M, Tarasov KV, Bhatta S, Ivanova S, Melnitchenko L, et al. Newly expressed SUR1-regulated NC(Ca-ATP) channel mediates cerebral edema after ischemic stroke. Nat Med. 2006;12:433–40. doi: 10.1038/nm1390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kimberly WT, Battey TWK, Pham L, Wu O, Yoo AJ, Furie KL, et al. Glyburide is associated with attenuated vasogenic edema in stroke patients. Neurocrit Care. 2014;20:193–201. doi: 10.1007/s12028-013-9917-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dhar R, Murphy-Human T. A bolus of conivaptan lowers intracranial pressure in a patient with hyponatremia after traumatic brain injury. Neurocrit Care. 2011;14:97–102. doi: 10.1007/s12028-010-9366-x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


