Abstract
Introduction
Maps are powerful tools for visualization of differences in health indicators by geographical region, but multi-country maps of HIV indicators do not exist, perhaps due to lack of consistent data across countries. Our objective was to create maps of four HIV indicators in North, Central, and South American countries.
Methods
Using data from the North American AIDS Cohort Collaboration on Research and Design (NA-ACCORD) and the Caribbean, Central, and South America network for HIV epidemiology (CCASAnet), we mapped median CD4 at presentation for HIV clinical care, proportion retained in HIV primary care, proportion prescribed antiretroviral therapy (ART), and the proportion with suppressed plasma HIV viral load (VL) from 2010 to 2012 for North, Central, and South America. The 15 Canadian and US clinical cohorts and 7 clinical cohorts in Argentina, Brazil, Chile, Haiti, Honduras, Mexico, and Peru represented approximately 2–7% of persons known to be living with HIV in these countries.
Results
Study populations were selected for each indicator: median CD4 at presentation for care was estimated among 14,811 adults; retention was estimated among 87,979 adults; ART use was estimated among 84,757 adults; and suppressed VL was estimated among 51,118 adults. Only three US states and the District of Columbia had a median CD4 at presentation >350 cells/mm3. Haiti, Mexico, and several states had >85% retention in care; lower (50–74%) retention in care was observed in the US West, South, and Mid-Atlantic, and in Argentina, Brazil, and Peru. ART use was highest (90%) in Mexico. The percentages of patients with suppressed VL in the US South and Northeast were lower than in most of Central and South America.
Conclusions
These maps provide visualization of gaps in the quality of HIV care and allow for comparison between and within countries as well as monitoring policy and programme goals within geographical boundaries.
Keywords: Map, HIV indicators, CD4 T-lymphocyte count, retention in care, antiretroviral therapy, HIV RNA suppression, North America, Central America, South America, implementation science
Introduction
Geographical displays of medical data allow quick visualization of geographical associations and, because of this, can be more informative than other types of figures [1–3]. Maps enable visualization of gaps in the quality of care and serve as an important data source for monitoring geographically specific policies and programmes [4].
Many organizations currently produce maps of HIV indicators. Local maps of indicators for HIV testing and linkage of HIV-positive adults into care [5], state-level maps of the prevalence and incidence of HIV and other sexually transmitted infections [6], and country-level maps of demographic characteristics and other HIV indicators among HIV-positive adults are readily available [7–10]. These maps are accessible instruments for presenting important data to a broad audience of scientists, policy-makers, and the general public.
Monitoring trends in HIV clinical care indicators is essential to not only assessing the quality of HIV care, but also understanding the impact of suppressed HIV viral loads (VLs) on HIV transmission in a population [11,12]. In the context of changing health care policies and programmes (such as the Affordable Care Act in the United States [US] and the new global era of universal access to HIV treatment) capturing regional differences in indicators of HIV clinical care can inform our understanding of the impact of cost control and care improvement strategies.
To our knowledge, there are no publicly available, multi-country maps that focus on monitoring indicators of HIV clinical care among adults in care, perhaps due to the lack of consistent HIV population-based data across countries. Through the International Epidemiologic Databases to Evaluate AIDS (IeDEA) international research consortium, longitudinal clinical cohorts of adults in HIV care have harmonized their data across countries in seven geographical regions. Although these data are clinical care-driven and not population-based, they reflect adults receiving HIV care. Our objective was to create maps of 1) median CD4 cell count (CD4) at presentation for HIV care, 2) retention, 3) ART use and 4) HIV VL suppression indicators among adults in care for HIV infection, using data from the North American AIDS Cohort Collaboration on Research and Design (NA-ACCORD) and the Caribbean, Central and the South America network for HIV epidemiology (CCASAnet), both of which are regional collaborations of the IeDEA consortium.
Methods
NA-ACCORD and CCASAnet are multisite collaborations of cohort studies of HIV-positive adults. Details on the NA-ACCORD and CCASAnet collaborations have been published previously [13,14]. Briefly, cohorts contribute data on patient demographic characteristics, prescribed antiretroviral therapy (ART), dates of primary HIV clinical visits, clinical diagnoses, laboratory test results (including CD4 count and HIV-1 RNA VL), and vital status. All data are transferred securely to the collaborations’ centralized data management cores, where they undergo quality control per a standardized protocol [15]. Participants were consented locally and the activities of the NA-ACCORD and CCASAnet have been reviewed and approved by the local institutional review boards for each site and at Johns Hopkins School of Medicine and Vanderbilt University School of Medicine, respectively.
We present a cross-sectional analysis using data contributed by clinical cohorts from 2010 to 2012. The 15 Canadian and US clinical cohorts included in this analysis had contributing clinical sites in four Canadian provinces and 48 US states (although participants resided in all 50 states), Washington DC, the Virgin Islands, and Puerto Rico, representing approximately 7.1% of persons known to be living with HIV in the US and Canada in 2012. The seven Caribbean, Central American, and South American clinical cohorts included had sites in the following countries: Argentina, Brazil, Chile, Haiti, Honduras, Mexico, and Peru, representing approximately 1.6% of persons known to be living with HIV in these countries. Geographical residence of participants was used to map the indicators; if this information was not available, the clinic location was used.
We estimated median CD4 at presentation for HIV clinical care, defined as a CD4 measured within six months of entry into care at one of the participating clinical sites, among those who successfully linked into care (defined as ≥2 HIV primary care visits within 12 months, >90 days apart) and did not have a suppressed VL or report previous ART use [16,17]. Additionally, we estimated the last three steps in the HIV Care Continuum (HIVCC): a) retention in care, defined among patients with ≥1 HIV primary care visits from 2010 to 2012 (excluding the year of death for patients who died) as the percentage of patients with ≥2 HIV primary care visits >90 days apart during the last calendar year that the patient contributed data [18]; b) ART use, defined as the percentage of patients with ≥1 visit who were prescribed ART for ≥6 months in the last year the patient contributed data from 2010 to 2012 [19]; c) VL suppression, defined as the percentage of patients with ≥1 visit who had a plasma HIV-1 RNA ≤200 copies/mL at their last measurement contributed in a calendar year from 2010 to 2012 [19]. Laboratory measures, namely CD4 count and HIV VL, were surrogate measures when HIV primary care visits were not available, which may result in a slight underestimation of retention in care [20]. ART was defined as a regimen of ≥3 antiretroviral agents from at least two classes or a triple nucleoside/nucleotide reverse transcriptase inhibitor regimen containing abacavir or tenofovir. To estimate the indicators with fidelity to their established definitions, a study population (defined by the denominator of the indicator) was selected for each indicator.
The indicators were summarized at the state- or territory-level in the United States, the province-level in Canada, and the country-level in the Caribbean, Central and South America. These summary data were then used to generate choropleth maps. Indicators for the location of the clinical sites were included on the maps. Data were summarized using SAS 9.3 (SAS Institute, Cary, NC), and maps were created using ArcGIS version 10.1 (Esri, Redlands, CA).
Results
The study populations for each indicator were as follows: N=14,811 for median CD4 at presentation for care; N=87,979 for retention in care; N=84,757 for ART use; and N=51,118 for VL suppression (Table 1). The majority of participants (66–86%) in each of these populations were from the United States. The estimated indicators for Brazil, Chile, Haiti, Mexico, and Peru originated from one clinical cohort within those countries; many of these cohorts were multisite. Using the retention in care study population, the proportion of participants >50 years old was highest in the United States (51%), followed by Canada, Argentina, Brazil and Haiti (10–27%) and then Honduras, Mexico and Peru (8–9%). Median ages were 42–43 years in all countries except the United States (50 years), Canada (47 years), Mexico (39 years) and Peru (38 years). The country with the greatest proportion of women was Haiti (57%) followed by Honduras (47%). The United States had the greatest proportion of injection drug users (IDU) (20%). More than 40% of participants in Canada (47%), Chile (74%) and Mexico (67%) were men who have sex with men (MSM), but heterosexual contact was the primary transmission risk in Argentina (38%), Brazil (49%), Honduras (63%) and Peru (66%).
Table 1.
Number of adults contributing to the specified HIV indicators, by country, NA-ACCORD and CCASAnet, 2010–2012
| CD4 at presentation for HIV carea | Retention in careb | ART usec | HIV-1 RNA suppressiond | |||||
|---|---|---|---|---|---|---|---|---|
| Argentina | 399 | 3% | 2630 | 3% | 2667 | 3% | 2563 | 5% |
| Male | 291 | 73% | 1814 | 69% | 1858 | 70% | 1774 | 69% |
| ≥ 50 yo | 56 | 14% | 720 | 27% | 725 | 27% | 706 | 28% |
| Brazil | 400 | 3% | 2735 | 3% | 2809 | 3% | 2768 | 5% |
| Male | 274 | 69% | 1734 | 63% | 1791 | 64% | 1759 | 64% |
| ≥ 50 yo | 42 | 11% | 267 | 10% | 736 | 26% | 728 | 26% |
| Canada | 976 | 7% | 8104 | 9% | 687 | 1% | 1421 | 3% |
| Male | 780 | 80% | 6312 | 78% | 557 | 81% | 960 | 68% |
| ≥ 50 yo | 205 | 21% | 1006 | 12% | 327 | 48% | 363 | 26% |
| Chile | 527 | 4% | 1705 | 2% | 1969 | 2% | 1797 | 4% |
| Male | 476 | 90% | 1509 | 89% | 1744 | 89% | 1591 | 89% |
| ≥ 50 yo | 62 | 12% | 159 | 9% | 466 | 24% | 416 | 23% |
| Haiti | 1493 | 10% | 5271 | 6% | Not available | Not available | ||
| Male | 630 | 42% | 2249 | 43% | ||||
| ≥ 50 yo | 238 | 16% | 794 | 15% | ||||
| Honduras | 129 | 1% | 652 | 1% | 449 | 1% | 394 | 1% |
| Male | 89 | 69% | 344 | 53% | 204 | 45% | 171 | 43% |
| ≥ 50 yo | 20 | 16% | 61 | 9% | 67 | 15% | 62 | 16% |
| Mexico | 188 | 1% | 739 | 1% | 793 | 1% | 785 | 2% |
| Male | 166 | 88% | 653 | 88% | 702 | 89% | 695 | 89% |
| ≥ 50 yo | 16 | 9% | 57 | 8% | 130 | 16% | 127 | 16% |
| Peru | 1014 | 7% | 2070 | 2% | 2576 | 3% | 2555 | 5% |
| Male | 766 | 76% | 1420 | 69% | 1821 | 71% | 1796 | 70% |
| ≥ 50 yo | 119 | 12% | 180 | 9% | 401 | 16% | 394 | 15% |
| USA | 9685 | 65% | 64,073 | 73% | 72,807 | 86% | 38,835 | 76% |
| Male | 8239 | 85% | 53,825 | 84% | 61,312 | 84% | 31,884 | 82% |
| ≥ 50 yo | 2886 | 30% | 32,844 | 51% | 36,865 | 51% | 16,330 | 42% |
| Total | 14,811 | 100% | 87,979 | 100% | 84,757 | 100% | 51,118 | 100% |
yo=years old.
The percentages listed for a country are the proportion of the total study population contributed by that country's cohorts. The percentages listed for “Male” and “≥50 yo” are the proportion of the study population within countries that are male and ≥50 yo.
Presentation was defined as enrolment in a participating cohort between 2010 and 2012 and ≥1 CD4 within six months of enrolment.
Retention in care was measured using data from clinical HIV primary care visits, with the exception of Argentina, Peru, and the Canadian province of British Columbia (Canada), for which retention was measured using CD4 or VL measures as proxies for visits; the denominator was defined as those who had ≥1 visit in the study period.
The denominator was defined as those who had ≥1 visit in the study period.
The denominator was defined as those who had ≥1 visit and ≥1 HIV-1 RNA measurement in the study period.
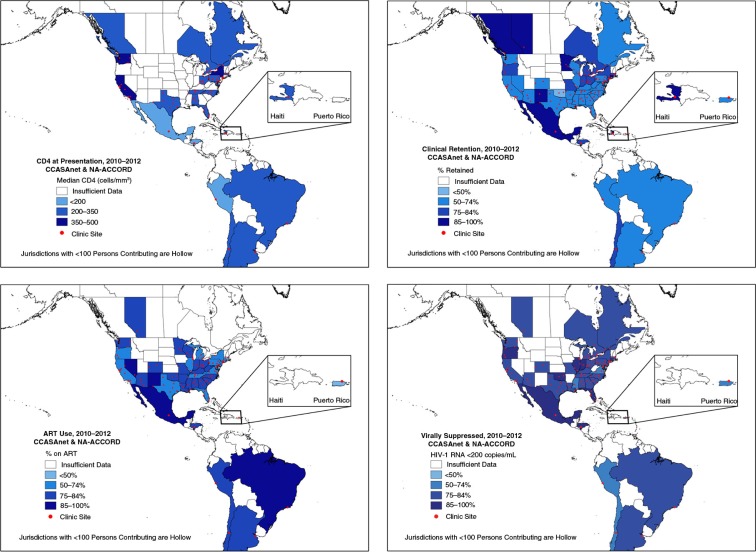
Mexico (127 cells/mm3), Honduras (163 cells/mm3) and Peru (175 cells/mm3) were the only countries with a median CD4 at HIV care presentation <200 cells/mm3; all other states, provinces and countries had a median CD4 ≥200 cells/mm3 at presentation for care (Figure 1a). Only three US states and the District of Columbia had a median CD4 at presentation >350 cells/mm3. Retention in care varied from 55 to 94%, with lower (50–74%) retention in the US West, South and Mid-Atlantic, as well as in Argentina, Brazil and Peru (Figure 1b). Haiti, Mexico and several states had >85% retention in care. ART use was highest (90%) in Mexico (Figure 1c). The percentages of patients with suppressed plasma HIV-1 RNA in the southern and north-eastern United States were lower than in most of Central and South America (Figure 1d). Estimates for each indicator at the state, province and country level can be found in the Supplementary file.
Figure 1.
(a–d) Maps of four indicators of HIV care in North, Central, and South American Countries, NA-ACCORD and CCASAnet, 2010–2012. (a) Median CD4 count at presentation for HIV clinical care, defined as CD4 cell count within six months of entry into care at one of the participating clinical sites, among those who did not have a suppressed viral load or report previous ART use. (b) Percentage retained in HIV clinical care, defined as the percentage of patients with ≥1 encounter from 2010 to 2012 who had ≥2 HIV primary care visits >90 days apart (measured in the last year an individual contributes data from 2010 to 2012). (c) Percentage of HIV-positive patients prescribed antiretroviral therapy, defined as a the percentage with ≥1 HIV clinical encounter who were prescribed ≥3 antiretroviral agents from at least two classes or a triple nucleoside/nucleotide reverse transcriptase inhibitor regimen containing abacavir or tenofovir for ≥6 months in the last year they contributed from 2010 to 2012. (d) Percentage of HIV-positive patients with suppressed plasma HIV viral load, defined as the percentage of patients with ≥1 HIV clinical encounter and ≥1 HIV-1 plasma RNA measurement who had an HIV-1 RNA ≤200 copies/mL at their last measurement contributed in a calendar year from 2010 to 2012.
Animated and static maps with options for stratification that display these indicators from 2000 to 2012 are available at www.naaccord.org.
Discussion
Mapping HIV indicators in North, Central and South America provides value to HIV researchers, epidemiologists, and programme and policymakers by 1) supporting research through the visualization of gaps in the quality of HIV care at the national level and by allowing for comparison between countries [4]; 2) allowing for monitoring of country-level policy and programme goals; and 3) providing estimates for quantitative models of HIV care. Maps of HIV indicators measured longitudinally among adults who have successfully linked to HIV care complement current efforts to geographically visualize other HIV indicators from population-based surveillance data and surveys [5–10].
Although the definitions of the HIV clinical care indicators we have presented were formalized in the United States, we show their utility to monitor progress in the HIVCC across the Western Hemisphere. Using these specific indicators to monitor HIV care in any given region is contingent upon whether these indicators reflect clinical care expectations in the region. Three of the four indicators mapped in this report have been endorsed for use to monitor progress towards US National HIV Strategy goals [21]. The retention in care indicator employed was endorsed by the US Institute of Medicine [18]. We have previously shown that this measure may slightly overestimate (by approximately 5%) the proportion of patients retained in care defined using the US Department of Health and Human Services (DHHS) definition and is better suited to measure trends over time [22]. The indicators for the proportion of patients prescribed ART and the proportion with suppressed HIV VL are endorsed by DHHS [19], but laboratory measures were surrogate measures of clinical visits when these data were not available. The median CD4 cell count at presentation indicator is a useful measure of immunologic status at entry into care and has been previously monitored in the NA-ACCORD [16,17].
Audiences for these maps may include government agencies, non-government and non-profit organizations, academic researchers, public health workers and officials, and industry. Easy-to-access geographical displays of HIV indicators can aid organizations with programme decision-making and policy. The consistent measurement of the specified indicators allows for direct comparison across states, provinces, regions and countries (assuming the estimates are generalizable within each region). Indicators can be employed over time to monitor progress towards HIV policy or programme goals endorsed by countries, or by international organizations, assuming the data required to measure the indicators are available cross-nationally. Additionally, estimates of HIV indicators are needed by researchers to understand the burden and the changing aspects of the HIV epidemics within geographical borders. Such estimates are particularly valuable for inputs informing models of cost-effectiveness of HIV interventions [23].
Alternatively, in communicating the changing nature of health care access and quality to the general public through the lens of policy and programme changes (e.g. altered trajectory of US National HIV Strategy goal indicators after expansion of publically funded health insurance programmes within the US), policy makers and scientists may employ many data visualization techniques, among which maps may be particularly engaging. Whether demonstrating need through mapping underperforming regions, such as increased HIV-related mortality throughout the American South [24], or demonstrating success through mapping the impact of successful regional interventions, maps are easily understood and convey a large amount of information. Dissemination of high quality analyses and evidence through the maps may impact the lives of persons living with HIV infection and alter the epidemiology of the epidemic by providing convincing visual evidence to motivate improvement of HIV health care services and the political will to implement needed changes.
The maps created here are possible because of the collaboration of the NA-ACCORD and CCASAnet. A standard data protocol is employed to merge data, even though the data originate from diverse clinical settings with varying levels of resources [15]. A limitation of these data, however, is the extrapolation of HIV clinical care indicator estimates from patients in the contributing clinical cohorts to HIV-positive adults in HIV care within a region, particularly in regions in which the data are from one single-site clinical cohort. Because these data are from HIV clinics and not population-based, comparisons of people in HIV clinical care in a given region with participants in our contributing HIV clinical cohorts are needed to determine generalizability of the indicators to all HIV-positive persons in HIV care in a given region. Although the NA-ACCORD population has been shown to have similar demographic characteristics as compared to persons living with HIV in the United States, comparable data do not exist for other IeDEA regions [25]. An additional important limitation is that the denominators of the retention in care, ART use and viral suppression indicators do not necessarily represent all HIV-positive persons in care, but rather include those in “active care” defined as those having ≥1 HIV primary care visit. For example, if a person was seen in the clinic in 2009, but not in 2010, that individual is not included in the retention in care indicator. The CD4 at presentation for care indicator is among those who successfully link into care. Potential underestimation of the proportion retained in care could result from our inability to determine if a participant transferred care, as opposed to falling out of care. Finally, a few of the individual participating HIV clinical cohorts have previously estimated some of these indicators, but there were differences in the selection criteria and indicator definitions used compared to this presented work.
In order for maps to be useful, the data collected must be representative of the region and analyzed in a timely fashion to have the biggest impact. Given the enormous effort needed to collect, clean and standardize the data, a delay of 2–4 years is expected. To improve the speed at which these data become available, annual updates of the maps (with interactive features that allow for stratification by demographic characteristics) will be posted at www.naaccord.org.
By mapping harmonized data and standardized definitions of HIV indicators across North, Central and South America, geographical associations of these HIV indicators are readily apparent. Although these mapped estimates are not population-based, they are useful tools for implementation science, monitoring progress towards geographically specified policy and programme goals, and serving as inputs for cost-effectiveness models.
Supplementary Material
Acknowledgements
NA-ACCORD Collaborating Cohorts and Representatives:
AIDS Link to the IntraVenous Experience: Gregory D. Kirk
Adult AIDS Clinical Trials Group Longitudinal Linked Randomized Trials: Constance A. Benson and Ronald J. Bosch
Fenway Health HIV Cohort: Stephen Boswell, Kenneth H. Mayer and Chris Grasso
HAART Observational Medical Evaluation and Research: Robert S. Hogg, P. Richard Harrigan, Julio SG Montaner, Angela Cescon and Hasina Samji
HIV Outpatient Study: John T. Brooks and Kate Buchacz
HIV Research Network: Kelly A. Gebo and Richard D. Moore
Johns Hopkins HIV Clinical Cohort: Richard D. Moore
John T. Carey Special Immunology Unit Patient Care and Research Database, Case Western Reserve University: Benigno Rodriguez
Kaiser Permanente Mid-Atlantic States: Michael A. Horberg
Kaiser Permanente Northern California: Michael J. Silverberg
Longitudinal Study of Ocular Complications of AIDS: Jennifer E. Thorne
Multicenter Hemophilia Cohort Study–II: James J. Goedert
Multicenter AIDS Cohort Study: Lisa P. Jacobson and
Gypsyamber D'Souza
Montreal Chest Institute Immunodeficiency Service Cohort: Marina B. Klein
Ontario HIV Treatment Network Cohort Study: Sean B. Rourke, Ann N. Burchell and Anita R. Rachlis
Retrovirus Research Center, Bayamon Puerto Rico: Robert F. Hunter-Mellado and Angel M. Mayor
Southern Alberta Clinic Cohort: M. John Gill
Studies of the Consequences of the Protease Inhibitor Era: Steven G. Deeks and Jeffrey N. Martin
Study to Understand the Natural History of HIV/AIDS in the Era of Effective Therapy: Pragna Patel, John T. Brooks
University of Alabama at Birmingham 1917 Clinic Cohort: Michael S. Saag, Michael J. Mugavero, and James Willig
University of North Carolina at Chapel Hill HIV Clinic Cohort: Joseph J. Eron and Sonia Napravnik
University of Washington HIV Cohort: Mari M. Kitahata, Heidi M. Crane and Daniel R. Drozd
Vanderbilt Comprehensive Care Clinic HIV Cohort: Timothy R. Sterling, David Haas, Sally Bebawy, and Megan Turner
Veterans Aging Cohort Study: Amy C. Justice, Robert Dubrow, and David Fiellin
Women's Interagency HIV Study: Stephen J. Gange and Kathryn Anastos
NA-ACCORD Study Administration:
Executive Committee: Richard D. Moore, Michael S. Saag, Stephen J. Gange, Mari M. Kitahata, Keri N. Althoff, Rosemary G. McKaig, Amy C. Justice and Aimee M. Freeman
Administrative Core: Richard D. Moore, Aimee M. Freeman and Carol Lent
Data Management Core: Mari M. Kitahata, Stephen E. Van Rompaey, Heidi M. Crane, Daniel R. Drozd, Liz Morton, Justin McReynolds, and William B. Lober
Epidemiology and Biostatistics Core: Stephen J. Gange, Keri N. Althoff, Alison G. Abraham, Bryan Lau, Jinbing Zhang, Jerry Jing, Elizabeth Golub, Shari Modur, Cherise Wong, Brenna Hogan, Weiqun Tong and Bin Lin
CCASAnet was supported by the National Institute of Allergy and Infectious Diseases (NIAID) and the National Cancer Institute (NCI) as part of the International Epidemiologic Databases to Evaluate AIDS (IeDEA): U01 AI069923.
CCASAnet Collaborating Cohorts and Representatives:
Fundación Huésped, Buenos Aires, Argentina: Pedro Cahn
Instituto National de Infectologia Evandro Chagas-Fundação Oswaldo Cruz, Rio de Janeiro, Brazil: Beatriz Grinsztejn
Universidad de Chile, Santiago, Chile: Marcelo Wolff Reyes
Le Groupe Haïtien d'Etude du Sarcome de Kaposi et des Infections Opportunistes (GHESKIO), Port-au-Prince, Haiti: Jean W. Pape, M.D.
Instituto Hondureño de Seguridad Social and Hospital Escuela, Tegucigalpa, Honduras: Denis Padgett
Instituto Nacional de Ciencias Médicas y Nutrición Salvador Zubiran, Mexico City, Mexico: Juan Sierra Madero
Instituto de Medicina Tropical Alexander von Humboldt, Lima, Peru: Eduardo Gotuzzo
Vanderbilt University, Nashville, TN, USA: Catherine McGowan
CCASAnet Study Administration:
Principal Investigators: Pedro Cahn and Catherine McGowan
Competing interests
Keri N Althoff reports service on a Medical Advisory Board for Gilead Sciences, Inc.
M John Gill has served as on HIV Advisory boards to Gilead Sciences, Inc, Merck, and ViiV.
Anita Rachlis reports institutional support from Merck, ViiV, and Gilead Sciences, Inc.
Jennifer E Thorne has served as a consultant for Gilead Sciences, Inc.
Pedro Cahn reports service on advisory boards for Abbive, BMS, Merck and ViiV. His institution has received grants on from Abbive, Merck, and ViiV.
Peter F Rebeiro, David B Hanna, Denis Padgett, Michael A Horberg, Beatriz Grinsztejn, Alison G Abraham, Robert Hogg, Marcelo J Wolff, Angel Mayor, Carolyn Williams, Timothy R Sterling, Mari M Kitahata, Kate Buchacz, Carina Cesar, Fernando Mejía Cordero, Sean B Rourke, Juan Sierra-Madero, Jean W Pape, and Catherine McGowan have nothing to report.
Authors' contributions
KNA, PFR, DBH, and CMG made substantial contributions to conception and design. DP, MAH, BG, AA, RH, MJG, MJW, AM, AR, CW, TRS, MK, KB, JET, CC, FMC, SBR, JSM, JWP, and PC contributed to acquisition of data. KNA, PFR, DBH, and CMG analysed and interpreted the data. All others were involved in drafting the manuscript or revising it critically for important intellectual content. All authors have read and approved the final version to be published.
Funding
The NA-ACCORD and collaborating cohorts were supported by grants U01-AI069918, U01-AA013566, U01-AA020790, U01-AI31834, U01-AI34989, U01-AI34993, U01-AI34994, U01-AI35004, U01-AI35039, U01-AI35040, U01-AI35041, U01-AI35042, UM1-AI35043, U01-AI37613, U01-AI37984, U01-AI38855, U01-AI38858, U01-AI42590, U01-AI68634, U01-AI68636, U01-AI69432, U01-AI69434, U01-DA036935, U01-HD32632, U10-EY08052, U10-EY08057, U10-EY08067, U24-AA020794, U54-MD007587, UL1-RR024131, UL1-TR000083, F31-DA037788, F31-DA030254, G12- MD007583, K01-AI071754, K01-AI093197, K23-EY013707, K24-DA00432, K24-AI065298, KL2-TR000421, MO1-RR-00052, N02-CP55504, P30-AI027763, P30-AI094189, P30-AI27757, P30-AI27767, P30-AI036219, P30-AI50410, P30-AI54999, P30-MH62246, R01-AA16893, R01-CA165937, R01-DA04334, R01-DA11602, R01-DA12568, R24-AI067039, R56-AI102622, Z01-CP010214, and Z01-CP010176 from the National Institutes of Health, USA; contract CDC200-2006-18797 from the Center's for Disease Control and Prevention, USA; contract 90047713 from the Agency for Healthcare Research and Quality, USA; contract 90051652 from the Health Resources and Services Administration, USA; grants TGF-96118, HCP-97105, CBR-86906, CBR-94036 from the Canadian Institutes of Health Research, Canada; Canadian Institutes of Health Research (CIHR) New Investigator award (A. Burchell); Ontario Ministry of Health and Long Term Care; and the Government of Alberta, Canada. Additional support was provided by the Intramural Research Program of the National Cancer Institute, and National Institutes of Health.
References
- 1.Richards TB, Croner C, Rushton G, Brown CK, Fowler L. Geographic information systems and public health: mapping the future. Public Health Rep. 1999;114:359–73. doi: 10.1093/phr/114.4.359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Koch T, Denike K. Medical mapping: the revolution in teaching and using maps for the analysis of medical issues. J Geogr. 2004;103:76–85. [Google Scholar]
- 3.Gao S, Mioc D, Anton F, Yi X, Coleman DJ. Online GIS services for mapping and sharing disease information. Int J Health Geogr. 2008;7:8. doi: 10.1186/1476-072X-7-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Proctor EK, Powell BJ, Baumann AA, Hamilton AM, Santens RL. Writing implementation research grant proposals: ten key ingredients. Implement Sci. 2012;7:96. doi: 10.1186/1748-5908-7-96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.AIDSVu. [cited 2015 June 10]. Available from: http://www.aidsvu.org.
- 6.Centers for Disease Control and Prevention. National Center for HIV/AIDS, Viral Hepatitis, STD, and TB Prevention Atlas. [cited 2015 June 10]. Available from: http://www.cdc.gov/nchhstp/atlas.
- 7.The Henry J Kaiser Family Foundation. The current state of the global HIV/AIDS epidemic tutorial. [cited 2015 June 10]. Available from: http://kff.org/interactive/the-current-state-of-the-global-hivaids-epidemic-tutorial.
- 8.UNAIDS. AIDSInfo. [cited 2015 June 10]. Available from: http://www.unaids.org/en/dataanalysis/datatools/aidsinfo.
- 9.World Health Organization. Global Health Observatory Data: HIV/AIDS. Adult HIV prevalence (15–29 years), 2013, by WHO region. [cited 2015 June 10]. Available from: http://www.who.int/gho/hiv/en.
- 10.The World Bank. Prevalence of HIV, total (% of population ages 15–49) [cited 2015 June 10]. Available from: http://data.worldbank.org/indicator/SH.DYN.AIDS.ZS/countries?display=map.
- 11.Gardner EM, McLees MP, Steiner JF, Del Rio C, Burman WJ. The spectrum of engagement in HIV care and its relevance to test-and-treat strategies for prevention of HIV infection. Clin Infect Dis. 2011;52:793–800. doi: 10.1093/cid/ciq243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cohen MS, Chen YQ, McCauley M, Gamble T, Hosseinipour MC, Kumarasamy N, et al. Prevention of HIV-1 infection with early antiretroviral therapy. N Engl J Med. 2011;365:493–505. doi: 10.1056/NEJMoa1105243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gange SJ, Kitahata MM, Saag MS, Bangsberg DR, Bosch RJ, Brooks JT, et al. Cohort profile: the North American AIDS Cohort Collaboration on Research and Design (NA-ACCORD) Int J Epidemiol. 2007;36:294–301. doi: 10.1093/ije/dyl286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McGowan CC, Cahn P, Gotuzzo E, Padgett D, Pape JW, Wolff M, et al. Cohort profile: Caribbean, Central, and South America Network for HIV Research (CCASAnet) collaboration within the International Epidemiologic Databases to Evaluate AIDS (IeDEA) programme. Int J Epidemiol. 2077;36:969–76. doi: 10.1093/ije/dym073. [DOI] [PubMed] [Google Scholar]
- 15.EuroCoord. HIV cohorts data exchange protocol. [cited 2015 June 10]. Available from: http://www.hicdep.org/
- 16.Althoff KN, Gange S, Klein M, Brooks JT, Hogg RS, Bosch RJ, et al. Late presentation for human immunodeficiency virus care in the United States and Canada. Clin Infect Dis. 2010;50:1512–20. doi: 10.1086/652650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Althoff KN, Gebo KA, Gange SJ, Klein MB, Brooks JT, Hogg RS, et al. CD4 count at presentation for HIV care in the United States and Canada: are those over 50 years more likely to have delayed presentation? AIDS Res Ther. 2010;7:45. doi: 10.1186/1742-6405-7-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Institute of Medicine. Monitoring HIV care in the United States: indicators and data systems. Washington, DC: The National Academies Press; 2012. [PubMed] [Google Scholar]
- 19.Valdiserri RO, Forsyth AD, Yakochenko V, Koh HK. Measuring what matters: development of standard HIV core indicators across the U.S. Department of Health and Human Services. Public Health Rep. 2013;128:354–9. doi: 10.1177/003335491312800504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rebeiro PF, Althoff KN, Lau B, Gill J, Abraham AG, Horberg MA, et al. Laboratory measures as proxies for primary care encounters: implications for quantifying clinical retention among HIV-infected adults in North America. Am J Epidemiol. 2015;182:952–60. doi: 10.1093/aje/kwv181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.White House Office of AIDS Policy. National HIV/AIDS strategy for the United States: update to 2020, July 2015. [cited 2015 Sep 18]. Available from: http://www.whitehouse.gov/sites/default/files/uploads/NHAS.pdf.
- 22.Rebeiro PF, Horberg MA, Gange SJ, Gebo KA, Yehia BR, Brooks JT, et al. Strong agreement of nationally recommended retention measures from the Institute of Medicine and Department of Health and Human Services. PLoS One. 2014;9:e111772. doi: 10.1371/journal.pone.0111772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Walensky RP. Cost effectiveness of HIV interventions: from cohort studies and clinical trials to policy. Top HIV Med. 2009;17:130–4. [PubMed] [Google Scholar]
- 24.Hanna DB, Selik RM, Tang T, Gange SJ. Disparities among US states in HIV-related mortality in persons with HIV infection, 2001–2007. AIDS. 2012;26:95–103. doi: 10.1097/QAD.0b013e32834dcf87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Althoff KN, Buchacz K, Hall HI, Zhang J, Hanna DB, Rebeiro P, et al. US trends in antiretroviral therapy use, HIV RNA plasma viral loads, and CD4 T-lymphocyte cell counts among HIV-infected persons, 2000 to 2008. Ann Intern Med. 2012;157:325–35. doi: 10.7326/0003-4819-157-5-201209040-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.