Abstract
Individuals vary widely in their tendency to seek stimulation and act impulsively, early developing traits with genetic origins. Failures to regulate these behaviors increase risk for maladaptive outcomes including substance abuse. Here, we explored the neuroanatomical correlates of sensation seeking and impulsivity in healthy young adults. Our analyses revealed links between sensation seeking and reduced cortical thickness that were preferentially localized to regions implicated in cognitive control, including anterior cingulate and middle frontal gyrus (n = 1015). These associations generalized to self-reported motor impulsivity, replicated in an independent group (n = 219), and correlated with heightened alcohol, tobacco, and caffeine use. Critically, the relations between sensation seeking and brain structure were evident in participants without a history of alcohol or tobacco use, suggesting that observed associations with anatomy are not solely a consequence of substance use. These results demonstrate that individual differences in the tendency to seek stimulation, act on impulse, and engage in substance use are correlated with the anatomical structure of cognitive control circuitry. Our findings suggest that, in healthy populations, covariation across these complex multidimensional behaviors may in part originate from a common underlying biology.
SIGNIFICANCE STATEMENT Impaired cognitive control may result in a tendency to seek stimulation impulsively and an increased risk for maladaptive outcomes, including substance abuse. Here, we examined the structural correlates of sensation seeking and impulsivity in a large cohort of healthy young adults. Our analyses revealed links between sensation seeking and reduced cortical thickness that were preferentially localized to regions implicated in cognitive control, including anterior cingulate and middle frontal gyrus. The observed associations generalized to motor impulsivity, replicated in an independent group, and predicted heightened alcohol, tobacco, and caffeine use. These data indicate that normal variability in cognitive control system anatomy predicts sensation seeking and motor impulsivity in the healthy populations, potentially increasing risk for substance use disorders.
Keywords: brain anatomy, cognitive control, impulsivity, individual differences, sensation seeking, substance use
Introduction
The tendency to seek stimulation and act impulsively, and the necessity to inhibit these drives, reflect core facets of human behavior. The consequences of even subtle shifts in the adaptive control of these processes are readily apparent. Lapses in self-regulation contribute to individual and societal problems including substance abuse and dependence (Horvath and Zuckerman, 1993; Baumeister and Heatherton, 1996). Suggesting the potential for a common brain basis, shared genetic factors contribute to the expression of sensation seeking, impulsivity, and substance use (Hur and Bouchard, 1997; Iacono et al., 1999; Verdejo-García et al., 2008). Convergent evidence in patient populations indicates that impairments in cognitive control system function encompassing aspects of middle frontal gyrus and anterior cingulate cortex (ACC) contribute to extreme forms of these seemingly discrete behaviors (e.g., attention-deficit/hyperactivity disorder (ADHD) and substance use; Bush et al., 1999; Willcutt et al., 2005; Seidman et al., 2006; Crockett et al., 2013; Volkow et al., 2013). Sensation seeking and impulsivity occur along continua. Normal variation in these traits contributes to both minor behaviors such as binge drinking, excessive caffeine consumption, and cigarette smoking; Waldeck and Miller, 1997; Ham and Hope, 2003; Jones and Lejuez, 2005; Gurpegui et al., 2007) and severe behaviors such as substance abuse and dependence. However, despite the important implications of individual variability in sensation seeking and impulsivity, we know relatively little about their neurobiological underpinnings in the general population.
The capacity to override or enhance habitual responses could influence the expression of sensation seeking and impulsivity in healthy populations. Consistent with this possibility, reduced middle frontal gyrus cortical thickness in regions implicated in cognitive control predicts impulsivity in adolescence (Schilling et al., 2013) and illness severity in patients with ADHD (Almeida et al., 2010). The neuroanatomical bases of sensation seeking and impulsivity in healthy adult samples remains equivocal (Gardini et al., 2009; Schilling et al., 2012), possibly due to limited sample sizes. The extent to which anatomical variability in the brain regions that underlie cognitive control contributes to sensation seeking and impulsivity in healthy adult populations is an open question, consequently the associated impact on health behaviors has remained largely unexplored.
Sensation seeking, impulsivity, and substance use may be regulated in part through the function of common brain systems. However, drug use can affect brain anatomy (Harper, 1998) and it is not yet clear to what degree abnormalities observed in individuals with substance use disorders might precede drug taking and reflect trait vulnerabilities for onset. The shift from occasional, controlled drug use to habitual consumption and chronic relapse is hypothesized to reflect a substance-mediated transition from cortical to striatal control of drug seeking/taking behaviors (Everitt and Robbins, 2005; Volkow et al., 2013) and decreased gray matter in prefrontal regions associated with self-regulation (Fein et al., 2002; Sullivan and Pfefferbaum, 2005; Ersche et al., 2013). An alternate, but not mutually exclusive, possibility is that impaired cognitive control and/or heightened striatal reactivity might reflect trait vulnerabilities, biasing healthy individuals toward sensation seeking, impulsivity, and increased substance use (Verdejo-García et al., 2008; Robbins et al., 2012). Supporting the possibility of predisposed brain circuitry, temperamental vulnerabilities in rodents increase susceptibility to drug use and subsequent addiction (Dalley et al., 2007; Diergaarde et al., 2008; Belin et al., 2011). Pathological substance use runs in families (Merikangas et al., 1998). Impulsivity in childhood predicts adult substance use disorders (Lee et al., 2011) and stimulant-dependent individuals and their nondependent siblings present with similar abnormalities in brain structure (Ersche et al., 2012). Collectively, these results suggest overlap in the underlying neurobiological mechanisms.
The goal of the present study was to explore the extent to which normal variability in brain structure can account for sensation seeking, impulsivity, and substance use in a large cohort of healthy young adults. Given evidence of shared associations with cognitive control processes, we hypothesized that common cortical territories would underlie their presentation.
Materials and Methods
Participants.
Between October 2008 and March 2013 native English speaking young adults (ages 18–35) with normal or corrected-to-normal vision were recruited from Harvard University, Massachusetts General Hospital, and the surrounding Boston communities (N = 1234) through an ongoing large-scale study of brain imaging and genetics (Holmes et al., 2015). History of psychiatric illness and medication usage was assessed through a structured phone screen. On the day of MRI data collection, participants were supervised during the completion of additional questionnaires concerning their physical health, past and present history of psychiatric illness, medication usage, and alcohol/tobacco/caffeine usage. Participants were excluded if their self-reported health information indicated a history of head trauma, current/past Axis I pathology or neurological disorder, current/past psychotropic medication usage, acute physical illness, and/or loss of consciousness. After the MRI session, participants completed an online self-report battery (see below) and were excluded if they failed to answer more than eight questions, admitted to seeking outside assistance during the completion of the battery, or if they did not complete the online assessment. Participants provided written informed consent in accordance with guidelines set by the Partners Health Care Institutional Review Board and the Harvard University Committee on the Use of Human Subjects in Research.
To address possible spurious effects resulting from cultural biases in self-reporting (Markus and Kitayama, 1991), initial analyses of the relations between sensation seeking, impulsivity, and brain structure were restricted to white, non-Hispanic participants of European ancestry. Data for the primary analyses consisted of 1015 participants (age: 21.38 ± 3.13; female: 52.90%; right handed: 91.50%; years of education: 14.62 ± 1.94; estimated IQ: 112.90 ± 8.97). The reliability and generalizability of observed effects were assessed through follow-up analyses of an independent group of white Hispanic and African-American participants (n = 219; age: 21.21 ± 3.27; female: 60.70%; right handed: 94.10%; years of education: 14.42 ± 1.86; estimated IQ: 110.40 ± 10.15).
MRI data acquisition.
Imaging data were collected on matched 3T Tim Trio scanners (Siemens) at Harvard University and Massachusetts General Hospital using the vendor-supplied 12-channel phased-array head coil. Structural data included a high-resolution multiecho T1-weighted magnetization-prepared gradient-echo image using the following parameters: TR = 2200 ms, TI = 1100 ms, TE = 1.54 ms for image 1–7.01 ms for image 4, FA = 7°, 1.2 × 1.2 × 1.2 mm and FOV = 230. Software upgrades (VB13, VB15, VB17) occurred during data collection. Reported results are after partialing out variance associated with scanner and software upgrade.
Online self-report battery.
After MRI data collection, the participants were provided a card with a random de-identified code and a web address to conduct online personality and cognitive measures. The battery was hosted on secure internal server and presented through the LimeSurvey user interface (http://www.limesurvey.org/).
Sensation seeking is characterized by a need for varied, novel, intense, and stimulating experiences and a willingness to take risks for the sake of such experiences (Zuckerman, 1979). The present analyses incorporated three self-reported measures of sensation seeking. These measures included the novelty seeking scale from the Temperament and Character Inventory (TCI; Cloninger, 1987), which assesses a tendency toward intense exhilaration or excitement in response to novel stimuli, cues for potential rewards, or potential relief of punishment; the behavioral activation fun seeking scale of the Behavioral Inhibition/Behavioral Activation Scale (BAS), which indexes a desire for new rewards and a willingness to approach potentially rewarding events on impulse (Carver and White, 1994); and the degree of risk taking from the Domain-specific Risk-attitude Scale, which assesses the likelihood of an individual to engage in risky activities/behaviors (Weber et al., 2002). In addition to sharing considerable conceptual overlap, the scales displayed moderate cross-measure correlations (Pearson r > 0.35; p < 0.001). The sensation-seeking composite score was calculated as the average of the z-scores for each individual scale.
Impulsivity consists of a lack of reflectiveness and planning, a tendency toward rapid decision making and action, a loss of inhibitory control, and carelessness (Evenden, 1999). Impulsivity was assessed through the completion of the Barratt Impulsiveness Scale, which measures facets of trait impulsivity including attention (focusing on the task at hand), motor (acting on the spur of the moment), self-control (planning and thinking carefully), cognitive complexity (enjoyment of challenging mental tasks), perseverance (a consistent life style; e.g., maintaining jobs/residences), and cognitive instability (thought insertions and racing thoughts; Patton et al., 1995).
The frequency and extent of current alcohol, tobacco, and caffeine use was assessed through self-report (n = 1112). Participants reported how often they drink alcohol (never; monthly or less; 2–4 times a month; 2–3 times a week; 4 or more times a week), how much alcohol they typically drink per occasion (1 or 2; 3 or 4; 5 or 6; 7, 8, or 9; 10 or more drinks), as well as the occurrence of binge drinking (> 4 drinks on any occasion) within the past 3 months (yes or no). Participants also reported their daily consumption of cigarettes (none; less than 2 cigarettes; about 1/2 pack; about 1 pack; more than 1 pack) and caffeine [none; 1 cup (12 oz soda, 6 oz coffee, 6 oz tea); 2–3 cups; 4–5 cups; 6 or more cups]. Approximately 9% of the sample (n = 110) reported never smoking or having a drink containing alcohol.
Although there is evidence suggesting temporally dissociable environmental and genetic correlates of the use of specific substances (Kendler et al., 2008), prior work linking impulsivity, sensation seeking, and a range of substance use behaviors indicate the potential for the influence of common brain systems (Waldeck and Miller, 1997; Jones and Lejuez, 2005; e.g., caffeine). Accordingly, a frequency of alcohol/tobacco/caffeine use composite score was calculated as the average of the z-scores for the self-reported frequency of use of each substance. To establish that any one aspect of the composite score did not drive the observed relations between anatomical variability and substance use, follow-up analyses were conducted on each of the included items.
Structural MRI data preprocessing.
Data were analyzed using FreeSurfer version 5.3.0 software (http://surfer.nmr.mgh.harvard.edu). FreeSurfer provides automated algorithms for the volumetric segmentation of subcortical structures and estimation of cortical thickness (Fischl and Dale, 2000; Fischl et al., 2002). Using the strategy detailed in Buckner et al. (2004), a study-optimized reference template was created from 700 subjects available through the existing dataset. Cortical thickness is calculated as the closest distance from the gray/white boundary to the gray/CSF boundary at each vertex on the tessellated surface (Fischl and Dale, 2000). After surface-based registration and before the analysis of cortical thickness, a 22 mm FWHM smoothing kernel was applied to each participant's data. For the purpose of visualization, resulting maps were displayed on the inflated Population-Average, Landmark-, and Surface-based (PALS) cortical surface using Caret software (Van Essen, 2005).
Statistical analyses.
Primary analyses began with block linear regressions examining the relations between cortical thicknesses and the sensation-seeking composite score after partialing out the variance associated with site, console software version, estimated IQ, age, and sex. Surface effects are plotted with the threshold p ≤ 5 × 10−4 corrected for multiple comparisons (Gaussian random field-corrected p ≤ 0.05; Friston et al., 1994). Additional block linear regressions were conducted separately for each subcortical structure. These analyses partialed out the variance associated with site, console software version, estimated IQ, age, sex, and estimated total intracranial volume and then examined the relation between volume and the sensation-seeking composite score. Follow-up analyses were conducted to assess the specificity of any observed effects across individual scales. Assessment of the anatomical correlates of impulsivity focused on the average cortical thickness of regions emerging from initial analyses of sensation seeking in the left and right hemisphere with the threshold p ≤ 5 × 10−3 (Bonferroni corrected p ≤ 0.05). To determine the reliability of the observed effects, we conducted independent follow-up analyses in a replication sample.
Results
Structural phenotypes are reliable
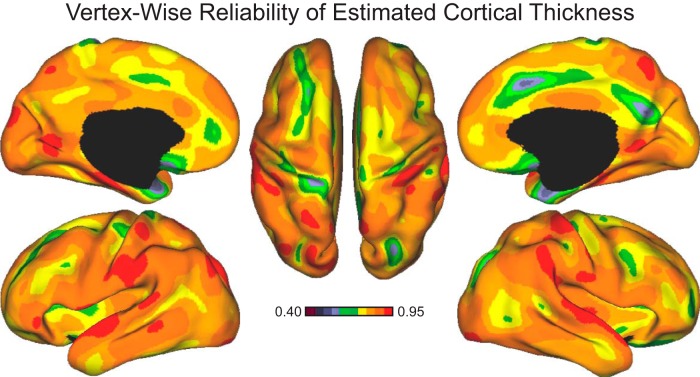
Efforts to identify meaningful biological markers of behavioral variability are limited by our understanding of the reliability of current methods. For the present study, we assessed the reliability of the vertex-level estimates of cortical thickness directly. To accomplish this, an additional dataset (n = 99; age: 21.52 ± 3.25; female: 47.50%; right handed: 91.90%; years of education: 14.48 ± 1.96; estimated IQ: 112.67 ± 11.22) was acquired over the course of the primary collection effort. Data were collected on 2 independent days (mean = 63 d apart; min = 2; max = 211). Each session was processed through the automated FreeSurfer pipeline separately. These data contain 43 participants whose initial runs are also included in the primary analyses. Pearson correlations were used to compare the two visits. Analyses revealed high test–retest reliability across the cortical surface (Fig. 1).
Figure 1.
Cortical thickness estimates are reliable. Vertex-wise estimated cortical thickness across two separate scanning sessions in 99 test–retest participants. Color bars reflect Pearson correlations of values between Visit 1 and Visit 2. Morphometric estimates were derived using the procedures of Fischl and Dale (2000) and then displayed on the lateral, medial, and dorsal surfaces of the left and right hemispheres (Van Essen, 2005).
Sensation seeking associates with decreased cortical thickness
Evidence suggests that impaired cognitive control marks patient populations characterized by extremes in impulsive behavior and sensation seeking. Our initial analyses focused on the associations between brain anatomy and sensation seeking in healthy young adults. The cortical thickness correlates of sensation seeking were quantified after correction for nuisance variance and multiple comparisons (Fig. 2). Analyses revealed relations linking increased sensation seeking with decreased cortical thickness in ACC (307.67 mm2; peak vertex MNI coordinates x = −10.2, y = 9.6, z = 38.6), caudal aspects of middle frontal gyrus (418.15 mm2; x = −23.3, y = −8.4, z = 48.6), and pericalcarine cortex (484.35 mm2; x = −9.8, y = −83.3, z = 13.2) in the left hemisphere, as well as caudal and rostral aspects of the middle frontal gyrus (1418.16 mm2; x = 35.0, y = 29.4, z = 34.1), ACC (255.40 mm2; x = 10.9, y = 10.1, z = 41.2), and supramarginal gyrus (337.22 mm2; x = 56.7, y = −30.3, z = 41.6) in the right hemisphere. None of the subcortical structures displayed a relation with sensation seeking that survived correction for multiple comparisons (p ≤ 5 × 10−4; Table 1).
Figure 2.
Sensation seeking associates with decreased cortical thickness. Surface-based renderings reflect the strength of the correlation between each vertex and sensation seeking (n = 1015). Reported correlations are after partialing out the variance associated with scanner, software version, estimated IQ, age, and sex. Display threshold for surface maps were set at p ≤ 5 × 10−4 (Gaussian random field-corrected p < 0.05). Color bars reflect Pearson correlations. Pcal, Pericalcarine cortex; MFG, middle frontal gyrus; SmG, supramarginal gyrus.
Table 1.
Sensation seeking, motor impulsivity, and subcortical volumes
| Sensation seeking |
Motor impulsivity |
|||||
|---|---|---|---|---|---|---|
| F(1,1007) | r2 | p-value | F(1,1007) | r2 | p-value | |
| Amygdala | ||||||
| Left | 0.85 | 0.001 | 0.36 | 2.87 | 0.002 | 0.09 |
| Right | 0.42 | < 0.001 | 0.52 | 0.25 | < 0.001 | 0.62 |
| Hippocampus | ||||||
| Left | 4.57 | 0.003 | 0.03 | 4.94 | 0.003 | 0.03 |
| Right | 2.15 | 0.001 | 0.14 | 4.92 | 0.003 | 0.03 |
| Thalamus | ||||||
| Left | 1.34 | 0.001 | 0.25 | 3.98 | 0.002 | 0.05 |
| Right | 7.63 | 0.003 | 0.006 | 8.44 | 0.004 | 0.004 |
| Caudate | ||||||
| Left | 3.05 | 0.002 | 0.08 | 0.14 | < 0.001 | 0.71 |
| Right | 4.00 | 0.002 | 0.05 | 0.53 | < 0.001 | 0.45 |
| Putamen | ||||||
| Left | 2.74 | 0.002 | 0.10 | 1.08 | 0.001 | 0.30 |
| Right | 1.74 | 0.001 | 0.19 | 1.82 | 0.001 | 0.18 |
| Globus pallidus | ||||||
| Left | 0.14 | < 0.001 | 0.71 | 1.95 | 0.001 | 0.16 |
| Right | < 0.01 | < 0.001 | 0.95 | < 0.01 | < 0.001 | 0.58 |
| Nucleus accumbens | ||||||
| Left | 0.37 | < 0.001 | 0.55 | 3.21 | 0.003 | 0.07 |
| Right | 0.37 | < 0.001 | 0.54 | < 0.01 | < 0.001 | 0.99 |
Reported p-values are uncorrected for multiple comparisons.
To establish that the observed relations between brain anatomy and sensation seeking were not driven by a subset of the included scales, follow-up analyses were conducted on the individual measures. Increased scores on each scale associated with reduced average thickness in the regions emerging from the sensation-seeking analyses in both the left (TCI Novelty Seeking: F(1,1008) = 36.02, p < 0.001, r2 = 0.033; Dospert Risk Taking: F(1,1008) = 11.72, p < 0.001, r2 = 0.011; BAS Fun Seeking: F(1,1008) = 17.33, p < 0.001, r2 = 0.016) and right hemispheres (TCI Novelty Seeking: F(1,1008) = 36.42, p < 0.001, r2 = 0.033; Dospert Risk Taking: F(1,1008) = 11.51, p < 0.001, r2 = 0.011; BAS Fun Seeking: F(1,1008) = 20.89, p < 0.001, r2 = 0.019; see Table 2 for region-specific analyses).
Table 2.
Associations between the cortical correlates of sensation seeking and individual scales
| Variable | Novelty seeking |
Fun seeking |
Risk taking |
Motor impulsivity |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| F(1,1008) | r2 | p-value | F(1,1008) | r2 | p-value | F(1,1008) | r2 | p-value | F(1,1008) | r2 | p-value | |
| Left middle frontal gyrus | 19.06 | 0.018 | 0.001 | 8.40 | 0.008 | 0.005 | 6.08 | 0.006 | 0.01 | 7.42 | 0.007 | 0.01 |
| Left anterior cingulate cortex | 24.25 | 0.022 | 0.001 | 9.62 | 0.009 | 0.005 | 4.94 | 0.005 | 0.03 | 15.03 | 0.014 | 0.001 |
| Left pericalcarine cortex | 13.07 | 0.013 | 0.001 | 11.40 | 0.011 | 0.001 | 9.83 | 0.010 | 0.005 | 5.80 | 0.006 | 0.05 |
| Right middle frontal gyrus | 30.51 | 0.028 | 0.001 | 16.91 | 0.016 | 0.001 | 6.08 | 0.006 | 0.01 | 17.49 | 0.016 | 0.001 |
| Right anterior cingulate cortex | 15.17 | 0.014 | 0.001 | 6.49 | 0.006 | 0.01 | 12.13 | 0.011 | 0.001 | 12.58 | 0.012 | 0.001 |
| Right supramarginal gyrus | 14.78 | 0.014 | 0.001 | 11.92 | 0.011 | 0.001 | 8.79 | 0.008 | 0.005 | 6.24 | 0.006 | 0.05 |
Males engage in more risk-taking behavior than females (Byrnes et al., 1999). Male participants in our sample reported increased sensation seeking relative to females (0.18 ± 0.77 vs 0.03 ± 0.75; t1013 = 3.37; p < 0.001). The observed increase was driven by heightened risk taking in the males (157.91 ± 26.81 vs 143.77 ± 23.91; t1013 = 8.88; p < 0.001). Males and females did not significantly differ in their self-reported novelty seeking (59.76 ± 9.10 vs 59.20 ± 10.02) or fun seeking (12.18 ± 2.19 vs 12.36 ± 2.08; ts < 1.33; p's > 0.18). Despite population differences in risk taking, each sex displayed relations with reduced thickness in the regions emerging from the initial sensation seeking analyses in both the left (males: F(1,472) = 13.01, p < 0.001, r2 = 0.026; females: F(1,531) = 19.76, p < 0.001, r2 = 0.034) and right hemispheres (males: F(1,472) = 17.65, p < 0.001, r2 = 0.034; females: F(1,531) = 16.95, p < 0.001, r2 = 0.029).
Impulsivity associates with decreased cortical thickness
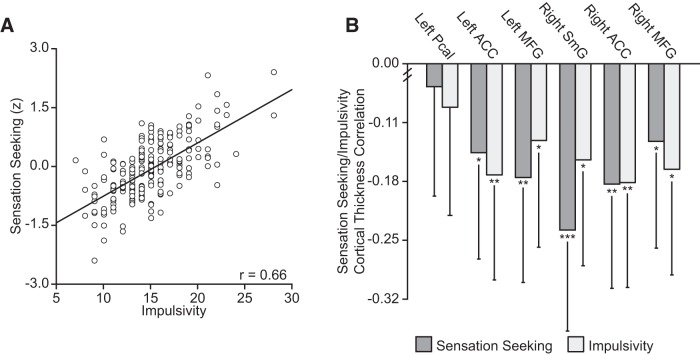
Heightened sensation seeking predicted increases in the motor (r = 0.62; Fig. 3A), attentional (r = 0.25), self-control (r = 0.44), cognitive complexity (r = 0.18), perseverance (r = 0.26), and cognitive instability (r = 0.27; p's < 0.001) facets of impulsivity. Consistent with a shared underlying circuitry, increased motor impulsivity associated with reduced cortical thickness in the regions emerging from the initial analyses of sensation seeking in the both the left (F(1,1008) = 20.75; p < 0.001; r2 = 0.019) and right (F(1,1008) = 20.89; p < 0.001; r2 = 0.019) hemispheres (see Fig. 3B, Table 2 for region-specific analyses). Despite the fact that these regions were, by definition, preselected to display strong associations with sensation seeking, sensation seeking did not predict anatomical variability to a greater extent than motor impulsivity (z's < 1.28; p's > 0.20; Fig. 3B). None of the subcortical structures displayed associations with motor impulsivity that survived correction for multiple comparisons (p ≤ 5 × 10−4; Table 1). When considering other facets of impulsivity (attention, self-control, cognitive complexity, perseverance, and cognitive instability), no associations with the cortical correlates of sensation seeking passed correction for multiple comparisons (F's < 6.19; p's > 0.01).
Figure 3.
Sensation seeking and motor impulsivity associate with decreased thickness in overlapping cortical territories. A, Scatter plot displaying the correlation between sensation seeking and motor impulsivity. B, Relations between motor impulsivity and the mean cortical thickness in regions emerging from the analyses of sensation seeking. The strength of the associations among sensation seeking, motor impulsivity, and cortical thickness do not significantly differ across these regions (Z < 1.60; p > 0.10). Error bars indicate 95% confidence intervals. *p < 0.05, **p < 0.01, ***p < 0.001. Pcal, Pericalcarine cortex; MFG, middle frontal gyrus; SmG, supramarginal gyrus. C, Surface-based renderings reflect the strength of the associations between each vertex and motor impulsivity. Display threshold for surface maps set at p ≤ 5 × 10−4 (Gaussian random field-corrected p < 0.05). Color bars reflect Pearson correlations.
The analyses with impulsivity detailed above were conducted within regions defined by their relations with sensation seeking. To examine the spatial specificity of the associations between impulsivity and brain anatomy, follow-up analyses considered the entire cortical surface. Consistent with a common cortical architecture supporting the regulation of both sensation seeking and impulsive behaviors, associations were observed linking reduced prefrontal cortical thickness and motor impulsivity (Fig. 3C). These relations spanned aspects of ACC (242.12 mm2) in the left hemisphere and rostral (536.66 mm2) and caudal (326.83 mm2) middle frontal gyrus and ACC (259.45 mm2) in the right hemisphere. When considering whole-brain analyses of attention, self-control, cognitive complexity, perseverance, or cognitive instability, no region survived correction for multiple comparisons.
When partialing out sensation seeking, the relation between motor impulsivity and the cortical thickness within the regions of interest no longer reached significance in either the left or right hemisphere (F's < 2.38; p's > 0.12). When accounting for motor impulsivity, the associations between prefrontal thickness and sensation seeking remained significant (left pericalcarine cortex: F(1,1007) = 12.06; p < 0.001; r2 = 0.012; left middle frontal gyrus: F(1,1007) = 9.31; p < 0.005; r2 = 0.009; left ACC: F(1,1007) = 5.98; p < 0.05; r2 = 0.005; right middle frontal gyrus: F(1,1007) = 10.18; p < 0.001; r2 = 0.009; right ACC: F(1,1007) = 6.15; p < 0.05; r2 = 0.006; right supramarginal gyrus: F(1,1007) = 12.14; p < 0.001; r2 = 0.011).
There are sex differences in the behavioral expression of impulsivity (Zuckerman, 1979) and in the risk for associated psychiatric illnesses (Kessler et al., 2005). Consistent with this evidence, male participants reported increased motor impulsivity relative to females (15.33 ± 3.46 vs 19.91 ± 2.66; t1013 = 2.01; p < 0.05). Despite these population differences, each sex displayed relations linking impulsivity and reduced thickness in the regions emerging from the initial sensation-seeking analyses in the left (males: F(1,472) = 8.12, p < 0.005, r2 = 0.016; females: F(1,531) = 7.35, p < 0.01, r2 = 0.013) and right (males: F(1,472) = 7.50, p < 0.01, r2 = 0.014; females: F(1,531) = 6.96, p < 0.01, r2 = 0.012) hemispheres. These analyses indicate a critical role for ACC, middle frontal, and supramarginal gyri in the guidance of sensation seeking and impulsive behaviors independent of sex.
Relations among sensation seeking, motor impulsivity, and brain structure replicate in an independent sample
Replicating our initial observations of covariation across sensation seeking and motor impulsivity in the discovery cohort (Fig. 3A), these correlations were present again in an independent group of participants (n = 219; r = 0.66; Fig. 4A). We next examined the relations between behavior and brain anatomy. Average anatomical variation within the cortical territories defined in our initial analyses of sensation seeking accounted for ∼3–4% of the behavioral variation in this independent sample (left hemisphere: F(1,212) = 9.25; p < 0.005; r2 = 0.038; right hemisphere: F(1,212) = 8.21; p < 0.005; r2 = 0.034). When examining each region independently, with the exception of the initial unexpected association between sensation seeking and pericalcarine cortical thickness (F(1,212) = 0.97; p = 0.33; r2 = 0.005), the cortical correlates of sensation seeking replicated in the independent group (Fig. 4B; left middle frontal gyrus: F(1,212) = 6.76; p < 0.01; r2 = 0.030; left ACC: F(1,212) = 4.63; p < 0.05; r2 = 0.020; right middle frontal gyrus: F(1,212) = 3.80; p < 0.05; r2 = 0.017; right ACC: F(1,212) = 7.40; p < 0.01; r2 = 0.031; right supramarginal gyrus: F(1,212) = 12.78; p < 0.001; r2 = 0.054).
Figure 4.
Cortical correlates of sensation seeking and motor impulsivity replicate in an independent sample. A, Scatter plot displaying the correlation between sensation seeking and motor impulsivity (n = 219). B, Relations between sensation seeking and the mean cortical thickness in regions emerging from initial analyses (n = 1015) in a replication sample (n = 219). Note that all cortical regions are replicated except Pcal. Pcal, Pericalcarine cortex; MFG, middle frontal gyrus; SmG, supramarginal gyrus. Error bars indicate 95% confidence intervals. *p < 0.05, **p < 0.01, ***p < 0.001.
Consistent with the replication analyses of sensation seeking, motor impulsivity associated with reduced average thickness within the cortical territories defined in our initial analyses (left: F(1,212) = 8.85; p < 0.005; r2 = 0.037; right: F(1,212) = 7.32; p < 0.01; r2 = 0.030). As above, no association was observed between self-reported motor impulsivity and pericalcarine cortical thickness (F(1,212) = 1.81; p = 0.18; r2 = 0.008). The remaining cortical correlates of sensation seeking each displayed relations with motor impulsivity (left middle frontal gyrus: F(1,212) = 3.74; p < 0.05; r2 = 0.017; left ACC: F(1,212) = 6.52; p < 0.01; r2 = 0.028; right middle frontal gyrus: F(1,212) = 6.03; p < 0.05; r2 = 0.027; right ACC: F(1,212) = 7.26; p < 0.01; r2 = 0.031; right supramarginal gyrus: F(1,212) = 5.20; p < 0.05; r2 = 0.024). No relations emerged when considering subcortical volumetric estimates and sensation seeking or motor impulsivity (F's < 2.10; p's > 0.14).
To further highlight the generalizability of the observed relations linking brain anatomy and behavior, the replication sample was subdivided into groups of white Hispanic and African-American participants. Within each group, heightened sensation seeking associated with reduced average cortical thickness in the left (white Hispanic: F(1,85) = 5.00, p < 0.05, r2 = 0.050; African-American: F(1,120) = 4.22, p < 0.05, r2 = 0.030) and right hemispheres (white Hispanic: F(1,85) = 3.98, p < 0.05, r2 = 0.042; African-American: F(1,120) = 4.73, p < 0.05, r2 = 0.034). This anatomical profile was also evident when considering impulsivity in both left (white Hispanic: F(1,85) = 3.78, p < 0.05, r2 = 0.027; African-American: F(1,120) = 6.69, p < 0.05, r2 = 0.064) and right hemispheres (white Hispanic: F(1,85) = 4.11, p < 0.05, r2 = 0.040; African-American: F(1,120) = 4.88, p < 0.05, r2 = 0.035).
Considering all of the results collectively, the majority of detected effects were found to generalize across samples drawn from three distinct racial/ethnic populations (white non-Hispanic, white Hispanic, and African-American).
Anatomy of sensation seeking and motor impulsivity localize preferentially in cognitive control regions
Extreme forms of sensation seeking and impulsivity are hypothesized to reflect impaired cognitive control system functioning (Bush et al., 1999; Willcutt et al., 2005; Seidman et al., 2006; e.g., ADHD). To search for indirect evidence of overlap in the cortical territories supporting these behavioral constructs in healthy young adults, a series of meta-analyses of terms relevant to cognitive control were conducted on a publicly available database of automatically extracted activation coordinates (Yarkoni et al., 2011). Analyses were performed for “cognitive control,” “conflict,” “error,” and “response inhibition.” Surface maps reflect z-scores corresponding to the likelihood that a region will activate if a study uses a particular term [i.e., P(Activation|Term); FDR corrected p < 0.01]. As reflected in Figure 5, the physical structure of aspects of cortex supporting a diverse set of cognitive control functions links to both sensation seeking and motor impulsivity in healthy young adults.
Figure 5.
Anatomical correlates of sensation seeking are preferentially localized to regions implicated in cognitive control. A, Surface-based rendering of the qualitative relation between each vertex on the medial surface of the right hemisphere and sensation seeking (Fig. 2). Meta-analyses were performed in Neurosynth (Yarkoni et al., 2011) for cognitive control (B), conflict (C), error (D), and response inhibition (E) and displayed on the medial surface of the right hemisphere (Van Essen, 2005). Surface maps reflect z-scores corresponding to the likelihood that a region will activate if a study uses a particular term [i.e., P(Activation|Term); FDR corrected p < 0.01]. A black border denotes the ACC region emerging from initial analyses of sensation seeking.
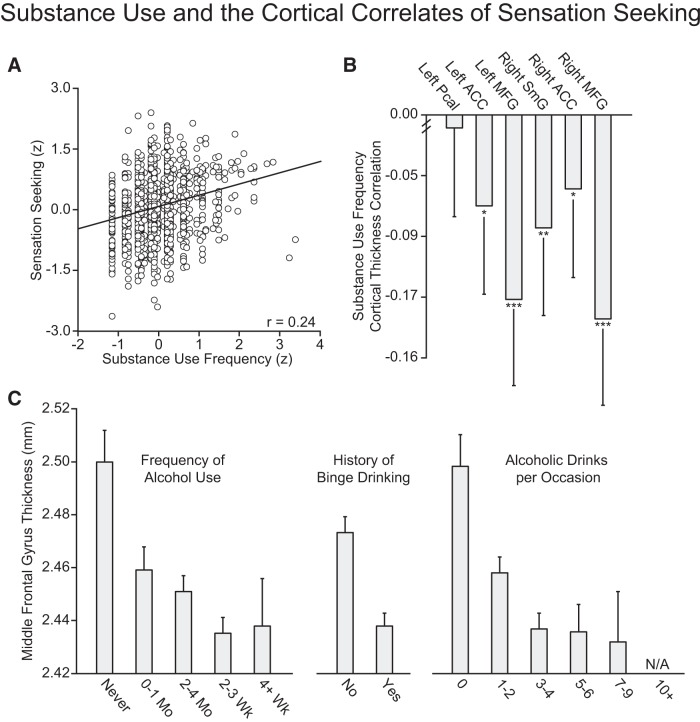
Sensation seeking and motor impulsivity correlate with increased alcohol, tobacco, and caffeine use
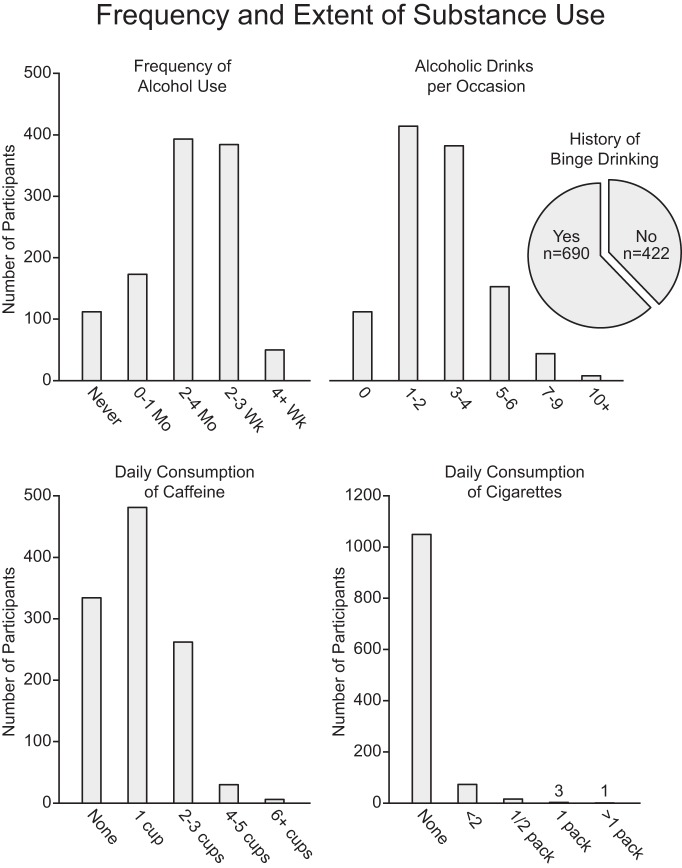
The frequency and extent of self-reported substance use are presented in Figure 6. In the present sample of healthy young adults, sensation seeking (r = 0.24; Fig. 7A) and motor impulsivity (r = 0.17) correlated with frequency of alcohol, tobacco, and caffeine use (p's < 0.001). These relations held when partialing out variance associated with estimated IQ, age, and sex (sensation seeking: F(1,1105) = 72.73, p < 0.001, r2 = 0.059; motor impulsivity: F(1,1105) = 15.73, p < 0.001, r2 = 0.013). When examining individual substance use/health behaviors, heightened sensation seeking and impulsivity associated with more frequent alcohol use (sensation seeking: F(1,1105) = 88.36, p < 0.001, r2 = 0.070; motor impulsivity: F(1,1105) = 21.48, p < 0.001, r2 = 0.018), an increase in the amount of alcohol consumed per occasion (sensation seeking: F(1,1105) = 82.27, p < 0.001, r2 = 0.063; motor impulsivity: F(1,1105) = 17.45, p < 0.001, r2 = 0.014), and an increased likelihood of having engaged in binge drinking within the past 3 months (sensation seeking: F(1,1104) = 64.07, p < 0.001, μ2 = 0.055; motor impulsivity: F(1,1104) = 13.43, p < 0.001, μ2 = 0.012). Suggesting broad relations across a range of health behaviors, heightened sensation seeking covaried with an increased frequency of cigarette smoking (sensation seeking: F(1,1105) = 18.34, p < 0.001, r2 = 0.016) and caffeine use (F(1,1105) = 7.90, p < 0.005, r2 = 0.007). These relations were not significant when considering motor impulsivity (cigarette smoking: F(1,1105) = 3.03, p = 0.082, r2 = 0.030; caffeine: F(1,1105) = 1.84, p = 0.18, r2 = 0.002).
Figure 6.
Wide variability exists in the frequency and extent of current alcohol, tobacco, and caffeine use. Graphs reflect available data for those participants who completed the alcohol, cigarettes, and caffeine survey (n = 1112).
Figure 7.
Sensation seeking and substance use are associated with decreased prefrontal cortical thickness. A, Scatter plot displaying the correlation between sensation seeking and substance use frequency. B, Relations between frequency of substance use and the average cortical thickness in regions emerging from the analyses of sensation seeking. Pcal, pericalcarine cortex; MFG, middle frontal gyrus; SmG, supramarginal gyrus. Error bars indicate 95% confidence intervals. *p < 0.05, **p < 0.01, ***p < 0.001. C, Association between middle frontal gyrus cortical thickness and frequency of alcohol use, presence of a binge drinking episode within the past 3 months, and amount of alcohol consumed per occasion. N/A, Not applicable.
Decreased prefrontal cortical thickness correlate with frequency of alcohol, tobacco, and caffeine use
Chronic substance use is believed to result in a loss of prefrontal functioning associated disruptions of self-control and the habitualization of maladaptive drug intake (Bechara, 2005; Volkow et al., 2013). It is not yet clear whether relations exist between shifts in brain structure and age-typical patterns of substance use in healthy adult populations, potentially predisposing individuals to the development of substance use disorders. Suggesting the presence of such associations, relations were observed between frequency of alcohol, tobacco, and caffeine usage and reduced average cortical thickness in the regions emerging from the initial analyses of sensation seeking in the both the left (F(1,1103) = 17.13, p < 0.001, r2 = 0.015) and right (F(1,1103) = 22.70, p < 0.001, r2 = 0.019) hemispheres. When considering individual regions, the association between frequency of substance use and decreased cortical thickness extended across the left (F(1,1103) = 19.39; p < 0.001; r2 = 0.017; Fig. 7B) and right middle frontal gyrus (F(1,1103) = 23.42; p < 0.001; r2 = 0.020), as well as the right supramarginal gyrus (F(1,1103) = 7.97; p < 0.005; r2 = 0.007). Marginally significant relations were observed in aspects of the left (F(1,1103) = 5.38; p < 0.05; r2 = 0.004) and right (F(1,1103) = 3.82; p < 0.05; r2 = 0.003) ACC. No association with substance use frequency was evident when considering pericalcarine cortical thickness (F(1,1103) = 1.61; p = 0.21; r2 = 0.001).
When partialing out sensation seeking, the bilateral relations between frequency of alcohol, tobacco, and caffeine use and middle frontal thickness remained (left: F(1,1102) = 10.63; p < 0.001; r2 = 0.009; right: F(1,1102) = 12.52; p < 0.001; r2 = 0.011). The remaining regions of interest no longer reached significance in either the left or right hemisphere (F's < 2.06; p's > 0.15).
The relation between middle frontal thickness and substance use was further explored with ANOVAs examining associations between hemisphere (left, right) and individual substance use behaviors after correction for nuisance variance. No association emerged when considering daily consumption of caffeine (F(1,1100) = 1.75, p = 0.138, μ2 = 0.006). Analyses revealed associations between frequency of alcohol use (F(1,1100) = 6.15, p < 0.001, μ2 = 0.022; Fig. 7C), the occurrence of binge drinking within the past 3 months (F(1,1100) = 20.32, p < 0.001, μ2 = 0.018), and the amount of alcohol consumed per occasion (F(1,1093) = 6.41, p < 0.001, μ2 = 0.023). Consistent with prior work suggesting prefrontal cortical thinning associates with nicotine addiction and relapse in smokers (Li et al., 2015), bilateral middle frontal thickness also displayed relations with self-reported daily consumption of cigarettes (F(1,1100) = 3.72, p < 0.005, μ2 = 0.013).
Male participants reported an increased frequency of alcohol, tobacco, and caffeine use relative to females (0.06 ± 0.67 vs −0.05 ± 0.64; t1108 = 2.79; p < 0.005). Although population differences in substance usage were evident, each sex displayed relations with reduced thickness in the regions emerging from the initial sensation seeking analyses in both the left (males: F(1,503) = 5.94, p < 0.05, r2 = 0.011; females: F(1,595) = 11.13, p < 0.001, r2 = 0.018) and right (males: F(1,503) = 4.57, p < 0.05, r2 = 0.009; females: F(1,595) = 20.45, p < 0.001, r2 = 0.031) hemispheres.
Associations between sensation seeking and decreased cortical thickness hold in the absence of substance use
Substance use can impact brain anatomy, making it difficult to separate the determinants and consequences of drug taking. Follow-up analyses searched for indirect evidence that the observed associations between sensation seeking and brain anatomy track the underlying trait itself and are not solely a consequence of substance use. When accounting for frequency of alcohol, tobacco, and caffeine usage, the associations between prefrontal thickness and sensation seeking (left pericalcarine cortex: F(1,1102) = 19.63; p < 0.001; r2 = 0.017; left middle frontal gyrus: F(1,1102) = 17.39; p < 0.001; r2 = 0.015; left ACC: F(1,1102) = 20.90; p < 0.001; r2 = 0.017; right middle frontal gyrus: F(1,1102) = 23.16; p < 0.001; r2 = 0.020; right ACC: F(1,1102) = 20.63; p < 0.001; r2 = 0.017; right supramarginal gyrus: F(1,1102) = 28.77; p < 0.001; r2 = 0.024) and motor impulsivity remained significant (left pericalcarine cortex: F(1,1102) = 7.84; p < 0.005; r2 = 0.007; left middle frontal gyrus: F(1,1102) = 7.48; p < 0.01; r2 = 0.006; left ACC: F(1,1102) = 19.94; p < 0.001; r2 = 0.016; right middle frontal gyrus: F(1,1102) = 18.95; p < 0.001; r2 = 0.016; right ACC: F(1,1102) = 16.17; p < 0.001; r2 = 0.014; right supramarginal gyrus: F(1,1102) = 10.21; p < 0.001; r2 = 0.009).
Critically, relations between increased sensation seeking and reduced cortical thickness were also evident when solely considering the subset of participants who presented without any history of tobacco or alcohol use (n = 110; left: F(1,103) = 6.75; p < 0.05; r2 = 0.058; right: F(1,103) = 4.35; p < 0.05; r2 = 0.038). Although limited by the cross-sectional design and reliance on self-reporting, the present analyses demonstrate that, in the absence of substance use, anatomical variability within aspects of the cognitive control system correlate with sensation seeking in healthy adults.
Discussion
Impairments in cognitive control underlie extreme forms of sensation seeking, impulsivity, and substance use in patient populations (Bush et al., 1999; Willcutt et al., 2005; Seidman et al., 2006; Crockett et al., 2013; Volkow et al., 2013). Subtle variations in these traits influence health behaviors, increasing risk for the development of abuse and dependence within the general population (Horvath and Zuckerman, 1993; Baumeister and Heatherton, 1996). Here, we explored whether cortical anatomy associates with sensation seeking and impulsivity in healthy young adults. In two independent groups, our analyses demonstrated links between sensation seeking, motor impulsivity, and reduced cortical thickness, preferentially localized to regions implicated in cognitive control, including the ACC and middle frontal gyrus. The significantly detected relations with brain anatomy were specific to motor impulsivity; no associations emerged when considering other facets of impulsivity. Notably, reduced gray matter thickness within these cortical territories also correlated with individual variation in self-reported substance use. The observed anatomical profile suggests that sensation seeking and motor impulsivity may arise in conjunction with shifts in ACC and middle frontal gyrus-dependent cognitive processes that broadly relate to goal-directed action and the inhibition of impulsive decisions (Hare et al., 2009; Figner et al., 2010; Essex et al., 2012).
The present analyses also suggest that construct overlap between sensation seeking and impulsivity arise, at least in part, through common anatomical variability (but see Jupp and Dalley, 2014). However, it is important to note that, although these behavioral constructs may rely on a shared cortical architecture, they are not interchangeable. Sensation seeking and impulsivity have distinct developmental trajectories (Steinberg et al., 2008) and dissociable heritability (Dalley et al., 2011). In adolescence, these behaviors may be best characterized by discrete factors individually associated with impulsivity and sensation seeking that are layered beneath a broad, externalizing phenotype (Castellanos-Ryan et al., 2014). In rodents, reactivity to novelty and impulsivity contribute to distinct phases of cocaine self-administration (initiation and persistence; Dalley et al., 2011). Rather than implying the existence of a fixed unitary factor, our analyses indicate a general role for ACC, middle frontal, and supramarginal gyri in the regulation of cognitive processes that broadly support sensation seeking and motor impulsivity. Critically, relations between sensation seeking and brain structure were evident in non-substance-using participants. This suggests that, within the context of currently assessed lifestyle factors, observed associations with anatomy track the underlying trait itself and are not solely a consequence of substance use.
Deficient cognitive control is hypothesized to increase vulnerability for the initiation of subsequent drug use (Hyman, 2007; Verdejo-García et al., 2008). However, despite much work in this area, results are equivocal and there is still considerable controversy regarding the predictive utility of sensation seeking and impulsivity (Lee et al., 2011). This may arise, to some extent, from efforts to relate questionnaire-based trait assessments with laboratory-based behavioral indices (Dick et al., 2010). For instance, a recent large-scale study revealed that seemingly common deficits in behavioral estimates of motor impulsivity observed in adolescent ADHD and substance abuse might arise through dissociable neurobiological pathways (Whelan et al., 2012). The present analyses suggest that shared cortical anatomy underlies aspects of self-reported impulsivity, sensation seeking, and substance use. Relatively few studies have employed both personality and behavioral measures of these broad constructs. Those that do find limited correlations between self-report and behavior (Reynolds et al., 2006). Accordingly, relations linking the present work, and questionnaire-based research more broadly, with behavioral evidence suggesting dissociable functional networks related to substance use and symptoms of ADHD remain to be explored.
Recreational drug use is common, yet a minority of people develop abuse or dependence (Chen and Kandel, 1995; Kessler et al., 2005). Impaired cognitive control and decreased prefrontal responsivity have been identified as putative risk markers for onset and relapse in substance use disorders (Everitt and Robbins, 2005; Verdejo-García et al., 2008; Koob and Volkow, 2010). In rodents, accelerated habit formation may underlie the transition of impulsive individuals from voluntary to compulsive substance use (Dalley et al., 2007; Diergaarde et al., 2008), whereas enhanced novelty seeking associates with the compulsive self-administration of stimulants (Belin et al., 2011). Striatal responses may be more likely to drive behavior in individuals with insufficient cognitive control, leading to the prioritization and overvaluation of immediate goals (e.g., impulsive use; Bechara, 2005). This is consistent with evidence suggesting effortful craving regulation in substance users associates with increased response in prefrontal regions supporting cognitive control and decreased activity in regions that process the predictive (nucleus accumbens) and motivational value (orbitofrontal cortex) of drug cues (Kober et al., 2010; Volkow et al., 2010). Reduced cortical thickness and heightened impulsivity precede significant substance use in adolescence (Schilling et al., 2013), whereas the rate of cortical thinning predicts both the severity of hyperactive and impulsive symptoms (Shaw et al., 2011) and drinking initiation (Luciana et al., 2013). Our analyses illustrate that, in healthy young adults, substance use covaries with sensation seeking, motor impulsivity, and decreased cortical thickness within brain regions theorized to support inhibitory control and goal-directed action. Although arising from cross-sectional data, these results suggest that normal variation in critical brain circuitry, and an accompanying increase in impulsive sensation seeking, could bias individuals toward substance use and the associated risk of developing abuse and dependence. Future longitudinal data will be needed to test this possibility, such as is being acquired as part of the upcoming Adolescent Brain Cognitive Development (ABCD) study.
The relations between compulsive drug use and the function of corticostriatal circuits are well established (Volkow et al., 2013). However, no associations emerged in our analyses of subcortical anatomy. While these data are in line with inconsistencies in the primary substance use literature (Ersche et al., 2013), our null subcortical findings should be interpreted with caution. Surface-based estimates of cortical thickness may be more precise than broad, segmentation-based measures of certain volumes, including subdivisions of the striatum. In addition, whereas sensation seeking and impulsivity are useful heuristics, these complex phenotypes can fractionate into distinct forms that are differentially expressed across time and individual (Olson et al., 1999; Zuckerman and Kuhlman, 2000; Dalley et al., 2011). Morphometric analyses may be insensitive to transient shifts in brain function. Subsequent work will be necessary to establish whether the observed variation in cortical anatomy occurs in isolation from, occurs in conjunction with, or serves to disrupt the mechanisms that regulate striatal function.
The present analyses indicate that anatomical variability in cognitive control circuitry tracks motor impulsivity and sensation seeking in healthy young adults. When considered in the context of our recent observation in the same sample that amygdala–medial prefrontal cortex circuit anatomy predicts negative affect (Holmes et al., 2012), the collective findings demonstrate that multiple behavioral traits selectively associate with anatomical variability in distinct brain systems. Although these discoveries hold potential for the identification of the discrete biological mechanisms contributing to normal variation in personality and temperament, open questions surround the extent to which complex brain–behavior associations can be characterized through static anatomical estimates. It is unclear why the anatomy of regions that underlie multiple complementary functions in task-evoked studies, for example, the prefrontal circuits that support the regulation of both affect and impulsivity (Ochsner and Gross, 2005; Dalley et al., 2011), would display such dissociable behavioral correlates. The associations between anatomy and behavior are subtle. One speculative possibility is that, even in large samples, our present methods are only powered to detect the most salient relations.
Several limitations warrant consideration when interpreting the present findings. First, the study participants were healthy, high functioning, young adults. Additional research should establish the generalizability of the current findings, particularly in the context of populations with increased vulnerability for psychiatric and substance use disorders (Ersche et al., 2012). Second, history of substance use was assessed through self-report. We did not collect information on illicit drug use. The lack of objective measures of consumption such as urine drug screenings is a potential limitation of this study. Finally, consistent with strong cross-scale correlations, overlap exists for some items in the self-reported measures of sensation seeking and impulsivity (e.g., motor impulsivity: “I do things without thinking,” Patton et al., 1995; fun seeking: “I often act on the spur of the moment,” Carver and White, 1994; novelty seeking: “I like to make quick decisions so I can get on with what has to be done,” Cloninger, 1987). Subsequent work coupling self-report with behavioral estimates of sensation seeking and impulsivity (e.g., impulsive motor errors, delay-discounting, risk taking) will be necessary to explore the relations linking these broad phenotypic categories with more precision (Reynolds et al., 2006).
The brain systems supporting cognitive control functions are dysregulated in populations marked by extremes of sensation seeking, impulsivity, and substance use. Prior research on patient populations has been important for understanding the relationship between biological processes and psychiatric illness. The present results establish that subtle shifts in the anatomy of cognitive control circuitry link to individual variability in sensation seeking and motor impulsivity, indicating that covariation across these complex multidimensional traits can be understood to originate in part from a common underlying biology. Critically, the observed associations are neurally instantiated in healthy young adults and correlate with substance use. These results reinforce the need to consider the neurobiological underpinnings of sensation seeking and impulsivity within healthy adult and early adolescent populations.
Footnotes
This work was supported by the Simons Foundation (R.L.B.), the Howard Hughes Medical Institute (R.L.B.), and the National Institute of Mental Health (Grants R01-MH079799 and K24MH094614 to J.W.S. and Grant K01MH099232 to A.J.H.). This research was made possible by the resources provided through Shared Instrumentation Grants 1S10RR023043 and 1S10RR023401. Data were provided in part by the Brain Genomics Superstruct Project of Harvard University and Massachusetts General Hospital (Principal Investigators: R.L.B., J.L.R., and J.W.S.) with support from the Center for Brain Science Neuroinformatics Research Group, the Athinoula A. Martinos Center for Biomedical Imaging, and the Center for Human Genetics Research. JWS is a Tepper Family MGH Research Scholar. We thank Justin Baker, Arielle Baskin-Sommers, Matt Hutchison, Marcia Johnson, and Lauren Patrick for their feedback on early versions of this manuscript; Timothy O'Keefe, Victor Petrov, and Gabriele Fariello for neuroinformatics support; and the Harvard FAS Research Computing for high-performance computing support.
The authors declare no competing financial interests.
References
- Almeida LG, Ricardo-Garcell J, Prado H, Barajas L, Fernández-Bouzas A, Avila D, Martínez RB. Reduced right frontal cortical thickness in children, adolescents and adults with ADHD and its correlation to clinical variables: a cross-sectional study. J Psychiatr Res. 2010;44:1214–1223. doi: 10.1016/j.jpsychires.2010.04.026. [DOI] [PubMed] [Google Scholar]
- Baumeister RF, Heatherton TF. Self-regulation failure: an overview. Psychological Inquiry. 1996;7:1–15. doi: 10.1207/s15327965pli0701_1. [DOI] [Google Scholar]
- Bechara A. Decision making, impulse control and loss of willpower to resist drugs a neurocognitive perspective. Nat Neurosci. 2005;8:1458–1463. doi: 10.1038/nn1584. [DOI] [PubMed] [Google Scholar]
- Belin D, Berson N, Balado E, Piazza PV, Deroche-Gamonet V. High-novelty-preference rats are predisposed to compulsive cocaine self-administration. Neuropsychopharmacology. 2011;36:569–579. doi: 10.1038/npp.2010.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckner RL, Head D, Parker J, Fotenos AF, Marcus D, Morris JC, Snyder AZ. A unified approach for morphometric and functional data analysis in young, old, and demented adults using automated atlas-based head size normalization: reliability and validation against manual measurement of total intracranial volume. Neuroimage. 2004;23:724–738. doi: 10.1016/j.neuroimage.2004.06.018. [DOI] [PubMed] [Google Scholar]
- Bush G, Frazier JA, Rauch SL, Seidman LJ, Whalen PJ, Jenike MA, Rosen BR, Biederman J. Anterior cingulate cortex dysfunction in attention-deficit/hyperactivity disorder revealed by fMRI and the counting stroop. Biol Psychiatry. 1999;45:1542–1552. doi: 10.1016/S0006-3223(99)00083-9. [DOI] [PubMed] [Google Scholar]
- Byrnes JP, Miller DC, Schafer WD. Gender differences in risk taking: a meta-analysis. Psychol Bull. 1999;125:367–383. doi: 10.1037/0033-2909.125.3.367. [DOI] [Google Scholar]
- Carver CS, White T. Behavioral inhibition, behavioral activation, and affective responses to impending reward and punishment: the BIS/BAS scales. J Pers Soc Psychol. 1994;67:319–333. doi: 10.1037/0022-3514.67.2.319. [DOI] [Google Scholar]
- Castellanos-Ryan N, Struve M, Whelan R, Banaschewski T, Barker GJ, Bokde AL, Bromberg U, Büchel C, Flor H, Fauth-Bühler M, Frouin V, Gallinat J, Gowland P, Heinz A, Lawrence C, Martinot JL, Nees F, Paus T, Pausova Z, Rietschel M, et al. IMAGEN Consortium. Neural and cognitive correlates of the common and specific variance across externalizing problems in young adolescence. Am J Psychiatry. 2014;171:1310–1319. doi: 10.1176/appi.ajp.2014.13111499. [DOI] [PubMed] [Google Scholar]
- Chen K, Kandel DB. The natural history of drug use from adolescence to the mid-thirties in a general population sample. Am J Public Health. 1995;85:41–47. doi: 10.2105/AJPH.85.1.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cloninger CR. A systematic method for clinical description and classification of personality variants: a proposal. Arch Gen Psychiatry. 1987;44:573–588. doi: 10.1001/archpsyc.1987.01800180093014. [DOI] [PubMed] [Google Scholar]
- Crockett MJ, Braams BR, Clark L, Tobler PN, Robbins TW, Kalenscher T. Restricting temptations: neural mechanisms of precommitment. Neuron. 2013;79:391–401. doi: 10.1016/j.neuron.2013.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalley JW, Fryer TD, Brichard L, Robinson ES, Theobald DE, Lääne K, Peña Y, Murphy ER, Shah Y, Probst K, Abakumova I, Aigbirhio FI, Richards HK, Hong Y, Baron JC, Everitt BJ, Robbins TW. Nucleus accumbens D2/3 receptors predict trait impulsivity and cocaine reinforcement. Science. 2007;315:1267–1270. doi: 10.1126/science.1137073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalley JW, Everitt BJ, Robbins TW. Impulsivity, compulsivity, and top-down cognitive control. Neuron. 2011;69:680–694. doi: 10.1016/j.neuron.2011.01.020. [DOI] [PubMed] [Google Scholar]
- Dick DM, Smith G, Olausson P, Mitchell SH, Leeman RF, O'Malley SS, Sher K. Understanding the construct of impulsivity and its relationship to alcohol use disorders. Addict Biol. 2010;15:217–226. doi: 10.1111/j.1369-1600.2009.00190.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diergaarde L, Pattij T, Poortvliet I, Hogenboom F, de Vries W, Schoffelmeer AN, De Vries TJ. Impulsive choice and impulsive action predict vulnerability to distinct stages of nicotine seeking in rats. Biol Psychiatry. 2008;63:301–308. doi: 10.1016/j.biopsych.2007.07.011. [DOI] [PubMed] [Google Scholar]
- Ersche KD, Jones PS, Williams GB, Turton AJ, Robbins TW, Bullmore ET. Abnormal brain structure implicated in stimulant drug addiction. Science. 2012;335:601–604. doi: 10.1126/science.1214463. [DOI] [PubMed] [Google Scholar]
- Ersche KD, Williams GB, Robbins TW, Bullmore ET. Meta-analysis of structural brain abnormalities associated with stimulant drug dependence and neuroimaging of addiction vulnerability and resilience. Curr Opin Neurobiol. 2013;23:615–624. doi: 10.1016/j.conb.2013.02.017. [DOI] [PubMed] [Google Scholar]
- Essex BG, Clinton SA, Wonderley LR, Zald DH. The impact of the posterior parietal and dorsolateral prefrontal cortices on the optimization of long-term versus immediate value. J Neurosci. 2012;32:15403–15413. doi: 10.1523/JNEUROSCI.6106-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evenden JL. Varieties of impulsivity. Psychopharmacology. 1999;146:348–361. doi: 10.1007/PL00005481. [DOI] [PubMed] [Google Scholar]
- Everitt BJ, Robbins TW. Neural systems of reinforcement for drug addiction: from actions to habits to compulsion. Nat Neurosci. 2005;8:1481–1489. doi: 10.1038/nn1579. [DOI] [PubMed] [Google Scholar]
- Fein G, Di Sclafani V, Cardenas VA, Goldmann H, Tolou-Shams M, Meyerhoff DJ. Cortical gray matter loss in treatment-naïve alcohol dependent individuals. Alcohol Clin Exp Res. 2002;26:558–564. [PMC free article] [PubMed] [Google Scholar]
- Figner B, Knoch D, Johnson EJ, Krosch AR, Lisanby SH, Fehr E, Weber EU. Lateral prefrontal cortex and self-control in intertemporal choice. Nat Neurosci. 2010;13:538–539. doi: 10.1038/nn.2516. [DOI] [PubMed] [Google Scholar]
- Fischl B, Dale AM. Measuring the thickness of the human cerebral cortex from magnetic resonance images. Proc Natl Acad Sci U S A. 2000;97:11050–11055. doi: 10.1073/pnas.200033797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischl B, Salat DH, Busa E, Albert M, Dieterich M, Haselgrove C, van der Kouwe A, Killiany R, Kennedy D, Klaveness S, Montillo A, Makris N, Rosen B, Dale AM. Whole brain segmentation: automated labeling of neuroanatomical structures in the human brain. Neuron. 2002;33:341–355. doi: 10.1016/S0896-6273(02)00569-X. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Worsley KJ, Frackowiak RS, Mazziotta JC, Evans AC. Assessing the significance of focal activations using their spatial extent. Hum Brain Mapp. 1994;1:210–220. doi: 10.1002/hbm.460010306. [DOI] [PubMed] [Google Scholar]
- Gardini S, Cloninger CR, Venneri A. Individual differences in personality traits reflect structural variance in specific brain regions. Brain Res Bull. 2009;79:265–270. doi: 10.1016/j.brainresbull.2009.03.005. [DOI] [PubMed] [Google Scholar]
- Gurpegui M, Jurado D, Luna JD, Fernández-Molina C, Moreno-Abril O, Gálvez R. Personality traits associated with caffeine intake and smoking. Prog Neuropsychopharmacol Biol Psychiatry. 2007;31:997–1005. doi: 10.1016/j.pnpbp.2007.02.006. [DOI] [PubMed] [Google Scholar]
- Ham LS, Hope DA. College students and problematic drinking: a review of the literature. Clin Psychol Rev. 2003;23:719–759. doi: 10.1016/S0272-7358(03)00071-0. [DOI] [PubMed] [Google Scholar]
- Hare TA, Camerer CF, Rangel A. Self-Control in decision-making involves modulation of the vmPFC valuation system. Science. 2009;324:646–648. doi: 10.1126/science.1168450. [DOI] [PubMed] [Google Scholar]
- Harper C. The neuropathology of alcohol-specific brain damage, or does alcohol damage the brain? J Neuropath Exp Neur. 1998;57:101–110. doi: 10.1097/00005072-199802000-00001. [DOI] [PubMed] [Google Scholar]
- Holmes AJ, Lee PH, Hollinshead MO, Bakst L, Roffman JL, Smoller JW, Buckner RL. Individual differences in amygdala-medial prefrontal anatomy link negative affect, impaired social functioning, and polygenic depression risk. J Neurosci. 2012;32:18087–18100. doi: 10.1523/JNEUROSCI.2531-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes AJ, Hollinshead MO, O'Keefe TM, Petrov VI, Fariello GR, Wald LL, Fischl B, Rosen BR, Mair RW, Roffman JL, Smoller JW, Buckner RL. Brain Genomics Superstruct Project initial data release with structural, functional, and behavioral measures. Sci Data. 2015;2:150031. doi: 10.1038/sdata.2015.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horvath P, Zuckerman M. Sensation seeking, risk appraisal, and risky behavior. Pers Indiv Differ. 1993;14:41–52. doi: 10.1016/0191-8869(93)90173-Z. [DOI] [Google Scholar]
- Hur YM, Bouchard TJ., Jr The genetic correlation between impulsivity and sensation seeking traits. Behav Genet. 1997;27:455–463. doi: 10.1023/A:1025674417078. [DOI] [PubMed] [Google Scholar]
- Hyman SE. The neurobiology of addiction: implications for voluntary control of behavior. Am J Bioeth. 2007;7:8–11. doi: 10.1080/15265160601063969. [DOI] [PubMed] [Google Scholar]
- Iacono WG, Carlson SR, Taylor J, Elkins IJ, McGue M. Behavioral disinhibition and the development of substance-use disorders: findings from the Minnesota Twin Family Study. Dev Psychopathol. 1999;11:869–900. doi: 10.1017/S0954579499002369. [DOI] [PubMed] [Google Scholar]
- Jones HA, Lejuez CW. Personality correlates of caffeine dependence: the role of sensation seeking, impulsivity, and risk taking. Exp Clin Psychopharmacol. 2005;13:259–266. doi: 10.1037/1064-1297.13.3.259. [DOI] [PubMed] [Google Scholar]
- Jupp B, Dalley JW. Behavioral endophenotypes of drug addiction: etiological insights from neuroimaging studies. Neuropharmacology. 2014;76:487–497. doi: 10.1016/j.neuropharm.2013.05.041. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Schmitt E, Aggen SH, Prescott CA. Genetic and environmental influences on alcohol, caffeine, cannabis, and nicotine use from early adolescence to middle adulthood. Arch Gen Psychiatry. 2008;65:674–682. doi: 10.1001/archpsyc.65.6.674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler RC, Berglund P, Demler O, Jin R, Merikangas KR, Walters EE. Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry. 2005;62:593–602. doi: 10.1001/archpsyc.62.6.593. [DOI] [PubMed] [Google Scholar]
- Kober H, Mende-Siedlecki P, Kross EF, Weber J, Mischel W, Hart CL, Ochsner KN. Prefrontal-striatal pathway underlies cognitive regulation of craving. Proc Natl Acad Sci U S A. 2010;107:14811–14816. doi: 10.1073/pnas.1007779107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF, Volkow ND. Neurocircuitry of addiction. Neuropsychopharmacology. 2010;35:217–238. doi: 10.1038/npp.2009.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SS, Humphreys KL, Flory K, Liu R, Glass K. Prospective association of childhood attention-deficit/hyperactivity disorder (ADHD) and substance use and abuse/dependence: a meta-analytic review. Clin Psychol Rev. 2011;31:328–341. doi: 10.1016/j.cpr.2011.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Yuan K, Cai C, Feng D, Yin J, Bi Y, Shi S, Yu D, Jin C, von Deneen KM, Qin W, Tian J. Reduced frontal cortical thickness and increased caudate volume within fronto-striatal circuits in young adult smokers. Drug Alcohol Depend. 2015;151:211–219. doi: 10.1016/j.drugalcdep.2015.03.023. [DOI] [PubMed] [Google Scholar]
- Luciana M, Collins PF, Muetzel RL, Lim KO. Effects of alcohol use initiation on brain structure in typically developing adolescents. Am J Drug Alcohol Abuse. 2013;39:345–355. doi: 10.3109/00952990.2013.837057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markus HR, Kitayama S. Culture and the self: implications for cognition, emotion, and motivation. Psychol Rev. 1991;98:224–253. doi: 10.1037/0033-295X.98.2.224. [DOI] [Google Scholar]
- Merikangas KR, Stolar M, Stevens DE, Goulet J, Preisig MA, Fenton B, Zhang H, O'Malley SS, Rounsaville BJ. Familial transmission of substance use disorders. Arch Gen Psychiatry. 1998;55:973–979. doi: 10.1001/archpsyc.55.11.973. [DOI] [PubMed] [Google Scholar]
- Ochsner KN, Gross JJ. The cognitive control of emotion. Trends Cogn Sci. 2005;9:242–249. doi: 10.1016/j.tics.2005.03.010. [DOI] [PubMed] [Google Scholar]
- Olson SL, Schilling EM, Bates JE. Measurement of impulsivity: construct coherence, longitudinal stability, and relationship with externalizing problems in middle childhood and adolescence. J Abnorm Child Psychol. 1999;27:151–165. doi: 10.1023/A:1021915615677. [DOI] [PubMed] [Google Scholar]
- Patton JH, Stanford MS, Barratt ES. Factor structure of the Barratt Impulsiveness Scale. J Clin Psychol. 1995;51:768–774. doi: 10.1002/1097-4679(199511)51:6<768::AID-JCLP2270510607>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- Reynolds B, Ortengren A, Richards JB, de Wit H. Dimensions of impulsive behavior: personality and behavioral measures. Pers Indiv Differ. 2006;40:305–315. doi: 10.1016/j.paid.2005.03.024. [DOI] [Google Scholar]
- Robbins TW, Gillan CM, Smith DG, de Wit S, Ersche KD. Neurocognitive endophenotypes of impulsivity and compulsivity: towards dimensional psychiatry. Trends Cogn Sci. 2012;16:81–91. doi: 10.1016/j.tics.2011.11.009. [DOI] [PubMed] [Google Scholar]
- Schilling C, Kühn S, Paus T, Romanowski A, Banaschewski T, Barbot A, Barker GJ, Brühl R, Büchel C, Conrod PJ, Dalley JW, Flor H, Ittermann B, Ivanov N, Mann K, Martinot JL, Nees F, Rietschel M, Robbins TW, Smolka MN, et al. Cortical thickness of superior frontal cortex predicts impulsiveness and perceptual reasoning in adolescence. Mol Psychiatry. 2013;18:624–630. doi: 10.1038/mp.2012.56. IMAGEN consortium ( www.imagen-europe.com) [DOI] [PubMed] [Google Scholar]
- Schilling C, Kühn S, Romanowski A, Schubert F, Kathmann N, Gallinat J. Cortical thickness correlates with impulsiveness in healthy adults. Neuroimage. 2012;59:824–830. doi: 10.1016/j.neuroimage.2011.07.058. [DOI] [PubMed] [Google Scholar]
- Seidman LJ, Valera EM, Makris N, Monuteaux MC, Boriel DL, Kelkar K, Kennedy DN, Caviness VS, Bush G, Aleardi M, Faraone SV, Biederman J. Dorsolateral prefrontal and anterior cingulate cortex volumetric abnormalities in adults with attention-deficit/hyperactivity disorder identified by magnetic resonance imaging. Biol Psychiatry. 2006;60:1071–1080. doi: 10.1016/j.biopsych.2006.04.031. [DOI] [PubMed] [Google Scholar]
- Shaw P, Gilliam M, Liverpool M, Weddle C, Malek M, Sharp W, Greenstein D, Evans A, Rapoport J, Giedd J. Cortical development in typically developing children with symptoms of hyperactivity and impulsivity: support for a dimensional view of attention deficit hyperactivity disorder. Am J Psychiatry. 2011;168:143–151. doi: 10.1176/appi.ajp.2010.10030385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinberg L, Albert D, Cauffman E, Banich M, Graham S, Woolard J. Age differences in sensation seeking and impulsivity as indexed by behavior and self-report: evidence for a dual systems model. Dev Psychol. 2008;44:1764–1778. doi: 10.1037/a0012955. [DOI] [PubMed] [Google Scholar]
- Sullivan EV, Pfefferbaum A. Neurocircuitry in alcoholism: a substrate of disruption and repair. Psychopharmacology. 2005;180:583–594. doi: 10.1007/s00213-005-2267-6. [DOI] [PubMed] [Google Scholar]
- Van Essen DC. A population-average, landmark-and surface-based (PALS) atlas of human cerebral cortex. Neuroimage. 2005;28:635–662. doi: 10.1016/j.neuroimage.2005.06.058. [DOI] [PubMed] [Google Scholar]
- Verdejo-García A, Lawrence AJ, Clark L. Impulsivity as a vulnerability marker for substance-use disorders: review of findings from high-risk research, problem gamblers and genetic association studies. Neurosci Biobehav Rev. 2008;32:777–810. doi: 10.1016/j.neubiorev.2007.11.003. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Fowler JS, Wang GJ, Telang F, Logan J, Jayne M, Ma Y, Pradhan K, Wong C, Swanson JM. Cognitive control of drug craving inhibits brain reward regions in cocaine abusers. Neuroimage. 2010;49:2536–2543. doi: 10.1016/j.neuroimage.2009.10.088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Tomasi D, Baler RD. Unbalanced neuronal circuits in addiction. Curr Opin Neurobiol. 2013;23:639–648. doi: 10.1016/j.conb.2013.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waldeck TL, Miller LS. Gender and impulsivity differences in licit substance use. J Subst Abuse. 1997;9:269–275. doi: 10.1016/S0899-3289(97)90021-3. [DOI] [PubMed] [Google Scholar]
- Weber EU, Blais ARE, Betz NE. A domain-specific risk-attitude scale: measuring risk perceptions and risk behaviors. J Behav Decis Making. 2002;15:263–290. doi: 10.1002/bdm.414. [DOI] [Google Scholar]
- Whelan R, Conrod PJ, Poline JB, Lourdusamy A, Banaschewski T, Barker GJ, Bellgrove MA, Büchel C, Byrne M, Cummins TD, Fauth-Bühler M, Flor H, Gallinat J, Heinz A, Ittermann B, Mann K, Martinot JL, Lalor EC, Lathrop M, Loth E, et al. IMAGEN Consortium. Adolescent impulsivity phenotypes characterized by distinct brain networks. Nat Neurosci. 2012;15:920–925. doi: 10.1038/nn.3092. [DOI] [PubMed] [Google Scholar]
- Willcutt EG, Doyle AE, Nigg JT, Faraone SV, Pennington BF. Validity of the executive function theory of attention-deficit/hyperactivity disorder: a meta-analytic review. Biol Psychiatry. 2005;57:1336–1346. doi: 10.1016/j.biopsych.2005.02.006. [DOI] [PubMed] [Google Scholar]
- Yarkoni T, Poldrack RA, Nichols TE, Van Essen DC, Wager TD. Large-scale automated synthesis of human functional neuroimaging data. Nat Methods. 2011;8:665–670. doi: 10.1038/nmeth.1635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuckerman M. Sensation seeking: beyond the optimal level of arousal. Hillsdale, NJ: Lawrence Erlbaum Associates; 1979. [Google Scholar]
- Zuckerman M, Kuhlman DM. Personality and risk-taking: common biosocial factors. J Pers. 2000;68:999–1029. doi: 10.1111/1467-6494.00124. [DOI] [PubMed] [Google Scholar]