Abstract
The Suzuki–Miyaura cross-coupling is one of the most often utilized reactions in the synthesis of pharmaceutical compounds and conjugated materials. In its most common form, the reaction joins two sp2-functionalized carbon atoms to make a biaryl or diene/polyene unit. These substructures are widely found in natural products and small molecules and thus the Suzuki–Miyaura cross-coupling has been proposed as the key reaction for the automated assembly of such molecules, using protecting group chemistry to affect iterative coupling. We present herein, a significant advance in this approach, in which multiply functionalized cross-coupling partners can be employed in iterative coupling without the use of protecting groups. To accomplish this, the orthogonal reactivity of different boron substituents towards the boron-to-palladium transmetalation reaction is exploited. The approach is illustrated in the preparation of chiral enantioenriched compounds, which are known to be privileged structures in active pharmaceutical compounds.
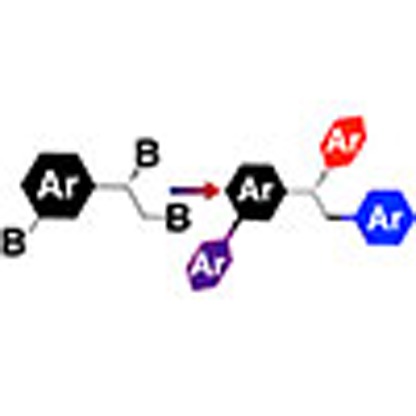 The Suzuki-Miyaura cross coupling is widely used in industrial and academic settings for the formation of carbon-carbon bonds. Here, the authors report a procedure whereby a molecule with multiple reactive carbon-boron bonds can undergo sequential, selective Suzuki-Miyaura reactions without the need for protecting groups.
The Suzuki-Miyaura cross coupling is widely used in industrial and academic settings for the formation of carbon-carbon bonds. Here, the authors report a procedure whereby a molecule with multiple reactive carbon-boron bonds can undergo sequential, selective Suzuki-Miyaura reactions without the need for protecting groups.
The Suzuki–Miyaura cross-coupling, in which organoboranes are coupled with organic halides or their equivalents (Fig. 1a), has changed the way organic molecules are assembled1,2. This reaction is the method of choice for the preparation of biaryl or polyene units in pharmaceutical3 and materials industries4. Because of the ease with which biaryl molecules are prepared using the Suzuki–Miyaura reaction, these substructures are now widely found in pharmaceutical products and precursors, perhaps even to the detriment of structural diversity. Indeed, evidence is emerging that ‘flat' molecules lacking stereochemistry are suboptimal drug candidates when compared with chiral molecules, which have a more complex and tunable three-dimensional shape and improved pharmacokinetic properties5,6. Molecules with chiral centres are found with significantly greater frequency (up to 30%) in final drugs than in discovery compounds, a fact attributed to improved drug performance compared with flat structures composed of sp2 centres5. Despite these compelling facts, the creation of C–C bonds with stereochemistry using the Suzuki–Miyaura reaction has only been demonstrated in the last few years, enabling the preparation of molecules with considerable diversity (Fig. 1b)7,8,9,10,11,12,13,14,15,16,17,18,19,20,21,22,23.
Figure 1. Advances in the Suzuki–Miyaura cross-coupling reaction.
(a) Classical Suzuki–Miyaura reaction for the formation of C–C bonds between aryl or alkenyl electrophiles and aryl or alkenyl organoboron nucleophiles. (b) Selected examples of molecules prepared by enantiospecific Suzuki–Miyaura cross-coupling reactions. (c) Iterative coupling concept employing the Suzuki–Miyaura reaction as the key structure-building component. (d) This work: chemoselective, protecting group-free cross-coupling of multiply borylated organic compounds, including the coupling of chiral, enantioenriched B–C bonds.
The development of methods to include the construction of chiral centres with control of stereochemistry is clearly critical to access more complex and valuable structures, and doing this in an iterative manner is a key for efficient, potentially automated applications. In fact, Burke has proposed the use of the Suzuki–Miyaura reaction as the key reaction with which the majority of non-peptidic organic molecules can be assembled in an automated manner (Fig. 1c)24,25. One critical component in any iterative synthesis involving organoboranes is the ability to modulate the reactivity of B–C bonds between ‘off' states, where no reaction takes place; and ‘on' states, from which coupling can occur. At present, this can only be accomplished through the use of blocking ligands on boron that deactivate the substrate towards coupling, which is followed by chemical removal of these ligands to generate an active-coupling partner26,27.
In a significant divergence from existing approaches, we describe herein the first example the iterative coupling of up to three B–C bonds within the same molecule without employing protecting group chemistry. This is accomplished by taking advantage of inherent differences in the transmetalation efficiency of closely related C–Bpin bonds (Fig. 1d). The orthogonal reactivity of B–C bonds in different positions in a single molecule permits the chemoselective, sequential coupling of aromatic, aliphatic and stereochemistry-bearing B–C bonds. This method permits the rapid generation of multiply arylated, chiral organic molecules with control of stereochemistry, without the need for protection/deprotection sequences. The method is simple, straightforward and holds considerable promise for the facile synthesis of new classes of pharmaceutical structures with improved properties. The Molander group has reported examples of protecting group-free iterative couplings accomplished employing the differential ability of benzylic RBF3K salts to undergo radical-based transmetallation19,28,29. Although this has not been reported on molecules containing more than one identical boron substituent, such reactivity can be envisioned.
Results
Orthogonal coupling of benzylic and non-benzylic C–B bonds
In 2009, we reported the first example of the cross-coupling of chiral benzylic boronic esters (1) that occurred with retention of stereochemistry (Fig. 2)9. This method has been extended to include the cross-coupling of allylic11,30, propargylic31 and doubly benzylic boronic esters14. Using different substrates, Suginome10, Hall13, Molander16 and Biscoe15 have reported groundbreaking invertive couplings under alternative conditions.
Figure 2. Orthogonal reactivity of branched benzylic and linear aliphatic boronic esters.
Observation of complete inversion of reactivity such that branched benzylic boronic esters (1) react in the presence of Ag2O, but not carbonate bases, while linear aliphatic isomers (3) are recovered unchanged when treated with Ag2O.
In our 2009 report, two key observations were critical for the current work. First, we noted that when bases other than silver oxide were employed with benzylic boronic ester 1, the starting boronic ester was recovered untouched, indicating that silver oxide was required for transmetalation. Second, under optimized conditions for the coupling of 1, namely Pd(0)/PPh3 and Ag2O, its linear achiral isomer 3 was completely unreactive, such that 3 could be recovered from the reaction mixture with the B–C bond intact (Fig. 2a)9. Thus, the mild silver oxide-promoted conditions employed for branched substrate 1 were clearly insufficient to promote transmetalation of isomer 3. This orthogonal reactivity offered the exciting opportunity to control the arylation of multiborylated organic compounds based on inherent differences in transmetalation efficiencies of different types of B–C bonds.
The critical question, then, was whether the relative reactivity described in Fig. 2 would translate to distinct B–C bonds in diborylated molecules. We first chose to examine the reactivity of 1,2 diboronates (5), which contain both a linear and a branched benzylic boron substituent. These compounds can be easily prepared with high enantioselectivity by diboration of the corresponding styrene derivative 4 (refs 32, 33, 34). Pinacol substituents were chosen on boron, as these are among the most common and stable ancillary groups for boron8,35. The coupling of one B–C(OR)2 unit in diborylated compounds followed by oxidation or, less commonly, amination or homologation of a remaining B–C bond has been amply demonstrated by Fernández36, Morken37,38,39 and Suginome40. However, with the exception of one example of a hydroxyl-promoted coupling39, there have been no reports describing the use of unprotected multiborylated compounds in iterative cross-couplings.
Initial attempts at reacting the benzylic, secondary B–C bond selectively in the presence of the adjacent linear B–C bond were unsuccessful, leading to a mixture of products, including those resulting from protodeboronation. However, the linear B–C bond in 5 could be enticed to undergo transmetalation and cross-coupling with aryl bromides in the presence of Pd(OAc)2 with RuPhos (2-dicyclohexylphosphino-2′,6′-diisopropoxybiphenyl) or SPhos (2-dicyclo-hexylphosphino-2′,6′-dimethoxybiphenyl) as ligand, and K2CO3 as base, leaving the branched B–C bond intact (Fig. 3a). Although counterintuitive, substrates showing sensitivity to deboronation benefited from the use of a significantly higher proportion of water (as seen in substrates 6aC, 6bC, 6gA, 6gE and 6hA, and later in the linear B–C(sp3) coupling of trisborylated substrate 11a (Fig. 4b)). On the basis of the work of the Lloyd-Jones group41, this could be due to a decrease in the effective pH of the reaction medium as the proportion of water is increased. In some cases, lower yields were obtained due to losses upon chromatography, as illustrated with compound 6hA (Fig. 3a). Under these relatively simple coupling conditions, a variety of coupling partners for the linear position were tolerated, including electron-rich, electron-poor and π-extended substrates. Heteroaromatic aryl bromides were less successful in this position, with 2-thienyl, 3-bromopyridyl and 3-bromoquinoline giving suboptimal yields, although 4-pyridylbromide could be effectively coupled as shown in Fig. 4c. In all cases studied, this reaction occurred without compromising the stereochemistry at the benzylic B–C bond.
Figure 3. Chemoselective Suzuki–Miyaura cross-coupling of chiral 1,2-diboronic esters.
(a) Demonstration of selective coupling of linear boronic ester, while the branched B–C bond remains intact. (b) Stereoretentive coupling of remaining secondary benzylic B–C bond in enantioenriched boronic esters, leading to enantiomerically enriched unsymmetrical 1,1′,2-triarylethanes with up to 92% enantiospecificity.
Figure 4. Chemoselective cross-couplings of bis- and tris-borylated chiral aromatics.
(a) Initial selective coupling of aryl B–C bonds using Pd/PtBu3 as the catalyst and carbonate bases, followed by second, stereospecific coupling of chiral secondary benzylic B–C bond leading to biphenyl-substituted 1,1–diaryl alkanes. (b) The introduction of three unique aryl groups sequentially by coupling at (1) the aryl B–C bond, (2) the linear achiral aliphatic B–C bond, and (3), the chiral benzylic B–C bond in a single pot for the first two steps, and the use of filtration through a short silica plug before the final step. (c) Illustration of the orthogonal coupling concept in the synthesis of phosphodiesterase inhibitor CDP 840 (14).
For the next step in the iterative sequence, the branched B–C bond was coupled using our previously described silver oxide-promoted conditions (Fig. 3b)9. Again, electron-rich, electron-poor, π-extended and heteroaromatic aryl iodides were well tolerated as coupling partners under the conditions shown in Fig. 3b. Enantiospecificities of this reaction are on the order of 90%, which is slightly lower than those observed in couplings of simpler 1-phenethyl boronic esters9,42, possibly indicating a slightly increased susceptibility to stereochemical erosion, because of the conjugated nature of any presumed stilbene intermediate resulting from β-hydride elimination; however, no regioisomeric products were observed, and thus the possibility of loss of stereochemistry during transmetalation must also be considered (enantiospecificity, e.s.=(e.e. product/e.e. starting material) × 100%). The electronics of the aryl iodide did not appear to play any effect.
Orthogonal coupling of benzylic sp3 versus sp2 C–B bonds
Having demonstrated that two adjacent aliphatic B–C bonds can be sequentially coupled with high chemoselectivity, we next probed whether the same approach could be used in molecules containing other types of B–C bonds with different propensities for transmetalation. Thus, we chose to pit B–C(sp2) bonds against secondary B–C(sp3) bonds, as in substrate 8, which was prepared by hydroboration of 4-pinacol boronato styrene. This reaction took place with 95:5 e.r. (ref. 43). In this case, the B–C(sp2) bond was targeted for initial coupling since it should clearly undergo transmetalation, the most readily based on >30 years of precedent. Although a number of conditions were effective for cross-coupling of this B–C bond and indeed left the secondary benzylic B–C bond untouched, small impurities derived from the incorporation of phenyl rather than aryl substituents were observed consistently. These side products originated from the use of triphenylphosphine as the ligand via well-documented P–Ph activation44. After several unsuccessful attempts to circumvent this unwanted side reaction, the use of PtBu3 proved successful45, giving the desired arylated products 9aX in high isolated yields, with the benzylic B–C bond intact for the next coupling (Fig. 4a). The reaction was readily scalable and could be routinely run on several hundred milligrams scale. Comparison of the enantiopurity of products 9aX, after coupling at the B–C(sp2) bond indicated, as expected, that there was no detectable loss of enantiopurity during this coupling.
With these products in hand, we next examined the scope of the Ag2O-promoted benzylic cross-coupling reaction (Fig. 4a). The enantiospecific cross-coupling took place as expected, except that π-extended biphenyl boronic esters 9aX displayed higher sensitivity to the loss of enantiomeric purity during coupling than previously observed for simple phenylated boronic esters9. In particular, we noted that electron-withdrawing substituents on the biphenyl derivative as in 9aJ led to a greater erosion in enantiospecificity (for example, 76% e.s. for coupling with 4-iodotrifluoromethyltoluene yielding 10aJk and 54% e.s. for coupling of the same boronic ester with 3-iodopyridine yielding 10aJi).
To confirm that this effect was due to the starting boronic ester, 3-iodopyridine and 4-iodotrifluoromethyltoluene were reacted with coupling partner 9aF, bearing a methoxyphenyl instead of phenacyl substituent. As shown in Fig. 4a, these reactions occurred with very high enantiospecificities (96% and 89%, respectively), confirming that it is the electron-withdrawing substituent on the biphenyl that leads to higher loss of enantiopurity in the coupling. Bulky aryl iodides such as 1-napthyliodide were effective, but also resulted in decreased enantiospecificity (that is, 10aDm). Comparing simple 1-phenethyl pinacol boronate with the π-extended systems illustrates the sensitivity of the latter systems: π-extended 9aD reacts with 1-naphthyliodide with 76% e.s., while PhCH(BPin)CH3 reacts with 85% e.s. when coupled with the same aryl iodide46. These results will provide important information in ongoing mechanistic studies of this reaction.
Protecting group-free iterative coupling of trisborylated species
To fully illustrate how the orthogonal reactivity of the various B–C bonds can be applied in iterative couplings, we prepared compound 11a containing three different types of B–C bonds: a B–C(sp2) bond, a primary B–C(sp3) bond and a secondary B–C(sp3) bond, and we attempted all three couplings in the same pot. For the first B–C(sp2) coupling, Fu-type conditions45, previously shown to be optimal for the coupling of diborylated substrate 8, were also effective with trisborylated substrate 11a, giving the desired product without loss of either aliphatic pinacol boronate. The yield of this first arylation was determined to be 92–95% by NMR analysis. Optimized conditions for the second-coupling reaction involved the use of solvent with a high proportion of water (1:2 ratio of organic solvent:water) as described above. In test studies, these conditions led to NMR yields on the order of 70%, boding well for a fully iterative coupling of 11. Indeed, we found the reaction proceeded with good yield, and importantly, leaving the benzylic B–C bond intact. Although the final benzylic cross-coupling worked well with isolated starting materials, when run in sequence, the one-pot procedure required filtration of the reaction mixture through a small silica plug, likely to deal with excess halides remaining from previous coupling reactions (Fig. 4b). In this manner, triarylated product 12aBaJ could be obtained in 32% overall yield, representing an average yield per step of just over 70%.
Application to compounds of medicinal importance
Finally, to illustrate the effectiveness of our orthogonal coupling methodology, we carried out the synthesis of a triarylated compound of pharmaceutical importance. Thus, compound 16 was prepared by diborylation of styrene 13 as shown in Fig. 4c, and, in only two steps, converted into biologically active derivative 14, otherwise known as CDP 840. This compound is a prototype orally active anti-inflammatory phosphodiesterase with potential inhibitory effects against phosphodiesterase-4 (ref. 47). The key coupling step occurred with 93% e.s. giving the final product in 95.5:4.5 e.r. Although outside the scope of this paper, we envisage that our iterative coupling approach will be highly effective for the rapid preparation of derivatives of active compounds by the introduction of a series of different aryl groups at various borylated positions of a central core molecule.
In conclusion, we have shown that the resistance of certain types of B–C bonds to transmetalation, one of the key steps in the Suzuki–Miyaura reaction48, can be capitalized upon to develop a protecting group-free sequential cross-coupling of multiply borylated organic compounds. This approach is complementary to other approaches that involve the manipulation of the substituents on boron to control reactivity of the B–C bond. In all cases, we employed pinacol esters since these are the most versatile and readily employed type of organoboron substituent8,35. Chiral substrates are compatible with the method, leading to products with significant complexity, which are likely to provide interesting leads for pharmaceutical and medicinal applications.
Methods
Coupling of linear B–C(sp3) bond in 1,2-diborylated compounds 5x
In a nitrogen-filled glovebox, diboronate 5x (1 equiv.), bromoarene (1.2 equiv.), Pd(OAc)2 (0.1 equiv.), RuPhos (0.25 equiv.) and K2CO3 (1.9 equiv.) were weighed into a vial and tetrahydrofuran was added. The reaction was sealed with a septum and removed from the glovebox, and placed under a flow of argon. Degassed water was added (20:1, organic:water) and the septum was replaced with a teflon cap. The reaction mixture was sonicated for about 2 min before being stirred at 80 °C for 15 h. The reaction mixture was cooled and filtered through a plug of silica, washed through with EtOAc and concentrated in vacuo. Purification by column chromatography was affected as the final purification.
Coupling of branched B–C(sp3) bond in 1-boryl,1,2-diaryl species 6xX
In a nitrogen-filled glovebox, boronic ester 6xX (1 equiv.), iodoarene (1.5 equiv.), Pd(dba)2 (0.08 equiv.), PPh3 (0.64 equiv.) and Ag2O (1.5 equiv.) were weighed into a 1 dram vial and dimethoxyethane (DME) was added. The reaction vessel was sealed, removed from the glovebox and heated at 70 °C for 16 h. The reaction mixture was cooled and filtered through a plug of silica, eluted with EtOAc and concentrated in vacuo. Purification by column chromatography was affected as the final purification.
Coupling of aromatic B–C(sp2) bond in diboryl species 8x
An oven-dried pressure tube with a stir bar in a glovebox was charged with diboronate 8x (1 equiv.), Pd2(dba)3 (0.05 equiv.), [(tBu)3PH]BF4 (0.2 equiv.), K2CO3 (3 equiv.), bromoarene (1.2 equiv.) and toluene (3.4 equiv.). The pressure tube was sealed with a rubber septum, removed from the glovebox and placed under argon. Water degassed (34:1 organic to water) was added and the rubber septum was replaced with a lid. The reaction was heated to 60 °C for 24 h. After cooling to room temperature, the reaction mixture was filtered through a plug of silica gel (ca. 2 mL) and a PROMAX 0.22 μm polytetrafluoroethylene (PTFE) syringe filter using copious ethyl acetate. The resulting product was purified by chromatography.
For NMR and super critical fluid chromatography (SFC) analysis of the compounds in this article, see Supplementary Figs 1–99.
Additional information
How to cite this article: Crudden, C. M. et al. Iterative protecting group-free cross-coupling leading to chiral multiply arylated structures. Nat. Commun. 7:11065 doi: 10.1038/ncomms11065 (2016).
Supplementary Material
Supplementary Figures 1-99, Supplementary Methods and Supplementary References
Acknowledgments
C.M.C. acknowledges the Natural Sciences and Engineering Research Council of Canada (NSERC) for funding in terms of Discovery, Accelerator and RTI grants, and the Canada Foundation for Innovation (CFI) for infrastructure funding. M.N. and C.M.C. acknowledge the Japan Society for the Promotion of Science and the Institute for Transformative Bio-Molecules/Nagoya University for funding. J.R. acknowledges NSERC for a postgraduate scholarship. C.Z., S.V., J.R., M.H. and V.L. acknowledge Queen's department of Chemistry for Queen's Graduate Awards.
Footnotes
Author contributions C.M.C. and D.I. conceived the overall concepts; C.Z., J.P.G.R, K.G., P.J.U., N.M., S.V., M.H., V.S.L. and Y.M. performed the experiments, compound characterization and data analysis. All authors contributed to the overall experiment design, discussions and manuscript preparation.
References
- Miyaura N., Yamada K. & Suzuki A. New stereospecific cross-coupling by the palladium-catalyzed reaction of 1-alkenylboranes with 1-alkenyl or 1-alkynyl halides. Tetrahedron Lett. 20, 3437–3440 (1979) . [Google Scholar]
- Suzuki A. Cross-coupling reactions of organoboranes: an easy way to construct C-C bonds (nobel lecture). Angew. Chem. Int. Ed. 50, 6722–6737 (2011) . [DOI] [PubMed] [Google Scholar]
- Carey J. S., Laffan D., Thomson C. & Williams M. T. Analysis of the reactions used for the preparation of drug candidate molecules. Org. Biomol. Chem. 4, 2337–2347 (2006) . [DOI] [PubMed] [Google Scholar]
- Schlüter A. D. The tenth anniversary of Suzuki polycondensation (spc). J. Polym. Sci. 39, 1533–1556 (2001) . [Google Scholar]
- Lovering F., Bikker J. & Humblet C. Escape from flatland: Increasing saturation as an approach to improving clinical success. J. Med. Chem. 52, 6752–6756 (2009) . [DOI] [PubMed] [Google Scholar]
- Over B. et al. Natural-product-derived fragments for fragment-based ligand discovery. Nature Chem 5, 21–28 (2013) . [DOI] [PubMed] [Google Scholar]
- Ding J., Rybak T. & Hall D. G. Synthesis of chiral heterocycles by ligand- controlled regiodivergent and enantiospecific suzuki miyaura cross-coupling. Nature Commun. 5, 5474 (2015) . [DOI] [PubMed] [Google Scholar]
- Leonori D. & Aggarwal V. K. Stereospecific couplings of secondary and tertiary boronic esters. Angew. Chem. Int. Ed. 54, 1082–1096 (2015) . [DOI] [PubMed] [Google Scholar]
- Imao D., Glasspoole B. W., Laberge V. S. & Crudden C. M. Cross coupling reactions of chiral secondary organoboronic esters with retention of configuration. J. Am. Chem. Soc. 131, 5024–5025 (2009) . [DOI] [PubMed] [Google Scholar]
- Ohmura T., Awano T. & Suginome M. Stereospecific suzuki-miyaura coupling of chiral alpha-(acylamino)benzylboronic esters with inversion of configuration. J. Am. Chem. Soc. 132, 13191–13193 (2010) . [DOI] [PubMed] [Google Scholar]
- Chausset-Boissarie L. et al. Enantiospecific, regioselective cross-coupling reactions of secondary allylic boronic esters. Chem. Eur. J. 19, 17698–17701 (2013) . [DOI] [PubMed] [Google Scholar]
- Molander G. A. & Wisniewski S. R. Stereospecific cross-coupling of secondary organotrifluoroborates: potassium 1-(benzyloxy)alkyltrifluoroborates. J. Am. Chem. Soc. 134, 16856–16868 (2012) . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J. C. H., MacDonald R. & Hall D. G. Enantioselective preparation and chemoselective cross-coupling of 1,1-diboron compounds. Nature Chem. 3, 894–899 (2011) . [DOI] [PubMed] [Google Scholar]
- Matthew S. C., Glasspoole B. W., Eisenberger P. & Crudden C. M. Synthesis of enantiomerically enriched triarylmethanes by enantiospecific suzuki-miyaura cross coupling reactions. J. Am. Chem. Soc. 136, 5828–5831 (2014) . [DOI] [PubMed] [Google Scholar]
- Li L., Zhao S., Joshi-Pangu A., Diane M. & Biscoe M. R. Stereospecific Pd-catalyzed cross-coupling reactions of secondary alkylboron nucleophiles and aryl chlorides. J. Am. Chem. Soc. 136, 14027–14030 (2014) . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandrock D. L., Jean-Gerard L., Chen C.-Y., Dreher S. D. & Molander G. A. Stereospecific cross-coupling of secondary alkyl beta-trifluoroboratoamides. J. Am. Chem. Soc. 132, 17108–17110 (2010) . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maity P., Shacklady-McAtee D. M., Yap G. P. A., Sirianni E. R. & Watson M. P. Nickel-catalyzed cross couplings of benzylic ammonium salts and boronic acids: Stereospecific formation of diarylethanes via C-N bond activation. J. Am. Chem. Soc. 135, 280–285 (2013) . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilsily A., Tramutola F., Owston N. A. & Fu G. C. New directing groups for metal-catalyzed asymmetric carbon-carbon bond-forming processes: Stereoconvergent alkyl-alkyl Suzuki cross-couplings of unactivated electrophiles. J. Am. Chem. Soc. 134, 5794–5797 (2012) . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tellis J. C., Primer D. N. & Molander G. A. Single-electron transmetalation in organoboron cross-coupling by photoredox/nickel dual catalysis. Science 345, 433–436 (2014) . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyamura S., Araki M., Suzuki T., Yamaguchi J. & Itami K. Stereodivergent synthesis of arylcyclopropylamines by sequential C-H borylation and Suzuki-Miyaura coupling. Angew. Chem. Int. Ed. 54, 846–851 (2015) . [DOI] [PubMed] [Google Scholar]
- Harris M. R., Hanna L. E., Greene M. A., Moore C. E. & Jarvo E. R. Retention or inversion in stereospecific nickel-catalyzed cross- coupling of benzylic carbamates with arylboronic esters: control of absolute stereochemistry with an achiral catalyst. J. Am. Chem. Soc. 135, 3303–3306 (2013) . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Awano T., Ohmura T. & Suginome M. Inversion or retention? Effects of acidic additives on the stereochemical course in enantiospecific suzuki-miyaura coupling of alpha-(acetylamino)benzylboronic esters. J. Am. Chem. Soc. 133, 20738–20741 (2011) . [DOI] [PubMed] [Google Scholar]
- Woerly E. M., Cherney A. H., Davis E. K. & Burke M. D. Stereoretentive suzuki-miyaura coupling of haloallenes enables fully stereocontrolled access to (-)-peridinin. J. Am. Chem. Soc. 132, 6941–6943 (2010) . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woerly E. M., Roy J. & Burke M. D. Synthesis of most polyene natural product motifs using just twelve building blocks and one coupling reaction. Nature Chem. 6, 484–491 (2014) . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J. et al. Synthesis of many different types of organic small molecules using one automated process. Science 34, 1221–1226 (2015) . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noguchi H., Hojo K. & Suginome M. Boron-masking strategy for the selective synthesis of oligoarenes via iterative Suzuki-Miyaura coupling. J. Am. Chem. Soc. 129, 758–759 (2007) . [DOI] [PubMed] [Google Scholar]
- Gillis E. P. & Burke M. D. A simple and modular strategy for small molecule synthesis: Iterative Suzuki-Miyaura coupling of B-protected haloboronic acid building blocks. J. Am. Chem. Soc. 129, 6716–6717 (2007) . [DOI] [PubMed] [Google Scholar]
- Primer D. N., Karakaya I., Tellis J. C. & Molander G. A. Single-electron transmetalation: an enabling technology for secondary alkylboron cross-coupling. J. Am. Chem. Soc. 137, 2195–2198 (2015) . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamashita Y., Tellis J. C. & Molander G. A. Protecting group-free, selective cross-coupling of alkyltrifluoroborates with borylated aryl bromides via photoredox/nickel dual catalysis. Proc. Natl Acad. Sci. USA 112, 12026–12029 (2015) . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glasspoole B. W., Ghozati K., Moir J. & Crudden C. M. Suzuki-Miyaura cross couplings of secondary allylic boronic esters. Chem. Commun. 48, 1230–1232 (2012) . [DOI] [PubMed] [Google Scholar]
- Partridge B. M., Chausset-Boissarie L., Burns M., Pulis A. P. & Aggarwal V. K. Enantioselective synthesis and cross-coupling of tertiary propargylic boronic esters using lithiation-borylation of propargylic carbamates. Angew. Chem. Int. Ed. 51, 11795–11799 (2012) . [DOI] [PubMed] [Google Scholar]
- Kliman L. T., Mlynarski S. N. & Morken J. P. Pt-catalyzed enantioselective diboration of terminal alkenes with B2(pin)2. J. Am. Chem. Soc. 131, 13210–13211 (2009) . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonet A., Pubill-Ulldemolins C., Bo C., Gulyas H. & Fernandez E. Transition-metal-free diboration reaction by activation of diboron compounds with simple Lewis bases. Angew. Chem. Int. Ed. 50, 7158–7161 (2011) . [DOI] [PubMed] [Google Scholar]
- Toribatake K. & Nishiyama H. Asymmetric diboration of terminal alkenes with a rhodium catalyst and subsequent oxidation: Enantioselective synthesis of optically active 1,2-diols. Angew. Chem. Int. Ed. 52, 11011–11015 (2013) . [DOI] [PubMed] [Google Scholar]
- Crudden C. M., Hleba Y. B. & Chen A. C. Regio and enantiocontrol in the room temperature hydroboration of vinyl arenes. J. Am. Chem. Soc. 125, 9200–9201 (2004) . [DOI] [PubMed] [Google Scholar]
- Penno D. et al. Multifaceted palladium catalysts towards the tandem diboration-arylation reactions of alkenes. Chem. Eur. J. 14, 10648–10655 (2008) . [DOI] [PubMed] [Google Scholar]
- Miller S. P., Morgan J. B., Nepveux F. J. & Morken J. P. Catalytic asymmetric carbohydroxylation of alkenes by a tandem diboration/Suzuki cross-coupling/oxidation reaction. Org. Lett. 6, 131–133 (2004) . [DOI] [PubMed] [Google Scholar]
- Mlynarski S. N., Schuster C. H. & Morken J. P. Asymmetric synthesis from terminal alkenes by cascades of diboration and cross-coupling. Nature 505, 386–390 (2014) . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaisdell T. P. & Morken J. P. Hydroxyl-directed cross-coupling: a scalable synthesis of debromohamigeran E and other targets of interest. J. Am. Chem. Soc. 137, 8712–8715 (2015) . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daini M. & Suginome M. Palladium-catalyzed, stereoselective, cyclizative alkenylboration of carbon-carbon double bonds through activation of a boron-chlorine bond. J. Am. Chem. Soc. 133, 4758–4761 (2011) . [DOI] [PubMed] [Google Scholar]
- Lennox A. J. J. & Lloyd-Jones G. C. Organotrifluoroborate hydrolysis: boronic acid release mechanism and an acid−base paradox in cross-coupling. J. Am. Chem. Soc. 134, 7431–7441 (2012) . [DOI] [PubMed] [Google Scholar]
- Li J. & Burke M. D. Pinene-derived iminodiacetic acid (pida): a powerful ligand for stereoselective synthesis and iterative cross-coupling of c(sp3) boronate building blocks. J. Am. Chem. Soc. 133, 13774–13777 (2011) . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi T., Matsumoto Y. & Ito Y. Catalytic asymmetric hydroboration of styrenes. J. Am. Chem. Soc. 111, 3426–3428 (1989) . [Google Scholar]
- Grushin V. V. Thermal stability, decomposition paths, and ph/ph exchange reactions of [(Ph3P)2Pd(Ph)X] (X=I, Br, Cl, F, and HF2). Organometallics 19, 1888–1900 (2000) . [Google Scholar]
- Littke A. F. & Fu G. C. A convenient and general method for Pd-catalyzed Suzuki cross-couplings of aryl chlorides and arylboronic acids. Angew. Chem. Int. Ed. 37, 3387–3388 (1998) . [DOI] [PubMed] [Google Scholar]
- Glasspoole B. W. The Chemoselective, Enantiospecific Cross-Coupling of Secondary Boronic Esters and the Stability of Mesoporous Silica Supports for Pd Catalysis (PhD thesis, Queen's Univ., 2011) . [Google Scholar]
- Alexander R. P. et al. CDP840. A prototype of a novel class of orally active anti-inflammatory phosphodiesterase 4 inhibitors. Bioorg. Med. Chem. Lett. 12, 1451–1456 (2002) . [DOI] [PubMed] [Google Scholar]
- Lennox A. J. J. & Lloyd-Jones G. C. Transmetalation in the Suzuki-Miyaura coupling: the fork in the trail. Angew. Chem. Int. Ed. 52, 7362–7370 (2013) . [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figures 1-99, Supplementary Methods and Supplementary References






