Abstract
Eosinophilic esophagitis (EoE) is a chronic inflammatory condition characterized by symptoms of esophageal dysfunction and eosinophilic infiltration of the esophageal mucosa. The diagnosis requires esophageal biopsies demonstrating at least 15 eosinophils per high-powered field following a course of high-dose proton pump inhibitors. Management of EoE consists of the three Ds: drugs, dietary therapy, and esophageal dilation. In this review, we discuss the epidemiology, pathogenesis, diagnosis, and treatment of EoE to include the role of emerging therapies.
INTRODUCTION AND EPIDEMIOLOGY
Eosinophilic esophagitis (EoE) is a chronic inflammatory condition characterized by symptoms of esophageal dysfunction and eosinophil infiltration of the esophageal epithelium.1 EoE is three times more common in males than females,2, 3 more common in Caucasians compared with other races, and is strongly associated with other atopic disorders.3, 4, 5, 6, 7 It has a prevalence of 57/100,000 persons in the United States,8 and is present in 12–22% of patients undergoing upper endoscopy for the evaluation of dysphagia.9, 10, 11 EoE is also present in as high as 10% of those presenting with dysphagia with endoscopically normal mucosa.12
Although clinicians are becoming more familiar with EoE, the increase in prevalence observed cannot be simply attributed to heightened recognition alone.13, 14, 15, 16 There are several theories to explain this increase in prevalence. The hygiene hypothesis asserts that, as rates of infectious diseases decrease worldwide, rates of allergic and immunologic diseases increase. This may be because of changes in immune reliance from T helper type 1 (Th1) cells to T helper type 2 (Th2) cells, or because of decreases in antigen competition.17 Increased use of proton-pump inhibitors (PPIs) have been implicated in the increase in prevalence of EoE,18 given that PPIs increase esophageal mucosal permeability19 and may increase immunoglobulin E (IgE) formation against dietary antigens.20 The geographic distribution of EoE spans the entire United States, but is concentrated in rural areas in the bottom quartile of population density21 and in colder climate zones,22 implicating environmental triggers as a cause for the increase in prevalence. EoE is also more likely to be diagnosed during elevated exposures to allergens.4, 23, 24
PATHOGENESIS AND HISTOPATHOLOGY
The mucosa of the esophagus consists of nonkeratinized stratified squamous epithelium in three layers: the stratum corneum, stratum spinosum, and stratum germinativum.25 The stratum germinativum, or basal layer, is not more than 3 cells thick in a normal esophageal epithelium. In EoE, the total epithelium is thickened, particularly in the basal zone, to more than 3 cells. Other findings include dilated intercellular spaces (spongiosis), fibrosis of the lamina propria, microabscesses, and dense eosinophilic infiltration.26 In addition to an increase in eosinophil count, biopsies in EoE patients demonstrate an increase in tissue mast cells, T cells, increased expression of tumor necrosis factor-α, interleukin (IL)-5, and Th2-related cytokines.27
Many cytokines are implicated in the EoE inflammatory cascade. Eotaxin-3 (CCL26) is a highly upregulated chemokine in EoE that promotes eosinophil migration from the blood stream into mucosal tissue and correlates well with eosinophilia and mastocytosis.28, 29 IL-13 activates local inflammation in Th2-related diseases, and is increased in the mucosa of EoE patients.30 IL-13 also downregulates desmoglein-1 (DSG1), an intercellular adhesion molecule markedly decreased in esophageal biopsies of EoE patients.31 This decrease leads to impaired barrier function seen in EoE. Downregulation of DSG1 also overlaps with upregulation of periostin, an extracellular matrix molecule that facilitates eosinophil adhesion to fibronectin. EoE is also associated with increases in expression of thymic stromal lymphopoietin, a cytokine that increases allergic inflammation.32 In biopsies of EoE patients, there is increased thymic stromal lymphopoietin and basophils, suggesting a component of T cell-independent inflammation. However, serum levels of these biomarkers do not correlate well with EoE activity, and therefore the utility of measuring these proteins remains limited.33
DIAGNOSIS
The diagnosis of EoE requires symptoms of esophageal dysfunction, mucosal biopsies that demonstrate at least 15 eosinophils per high-powered field, and persistence of esophageal eosinophilia after a trial of high-dose PPI (see Figures 1 and 2).1 Secondary causes of esophageal eosinophilia should be ruled out, such as eosinophilic gastrointestinal diseases, celiac disease, Crohn's disease, hypereosinophilic syndrome, achalasia, and graft-vs.-host disease.1
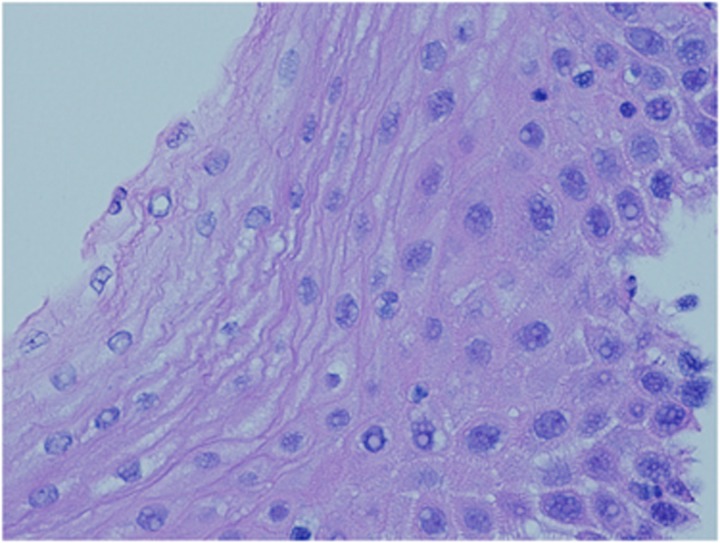
Figure 1.
Normal esophageal squamous mucosa, with a normal basal layer, no intraepithelial inflammatory cells, and no elongation of papillae from the lamina propia.
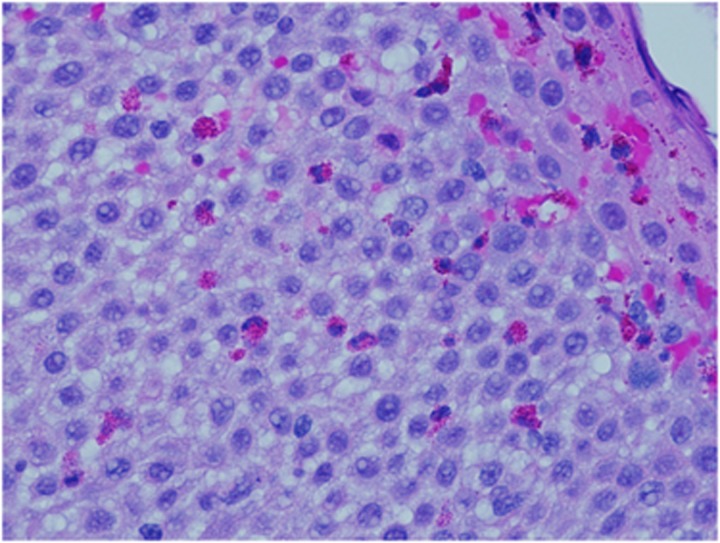
Figure 2.
Esophageal eosinophilia. Section shows abundant intraepithelial eosinophils and reactive epithelial changes including spongiosis and basal cell hyperplasia.
The most common clinical symptom of EoE is dysphagia that may be intermittent.9, 12 There can be significant delay in diagnosis, with one study reporting the presence of symptoms for 6 years before diagnosis.34 In children, EoE presents with emesis, abdominal pain, feeding refusal, weight loss, and failure to thrive. Adults, however, classically present with dysphagia and food impactions.1, 35 Race may influence clinical presentation. African Americans with EoE are more likely to experience heartburn, whereas Caucasians are more likely to present with dysphagia.3, 5 EoE patients are likely to have other atopic diseases, most commonly allergic rhinitis, and also asthma, dermatitis, and food allergies.36 In one study, 50% of patients with EoE had a positive skin test to at least one food allergen, and 93% had a positive skin test to at least one inhalant.37 The most common food allergens identified were peanuts, egg whites, wheat, soybeans, cow's milk, and tree nuts.37
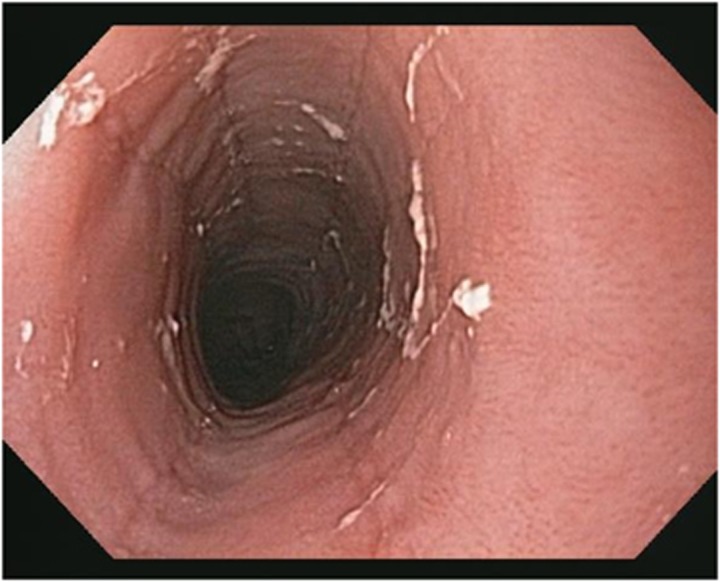
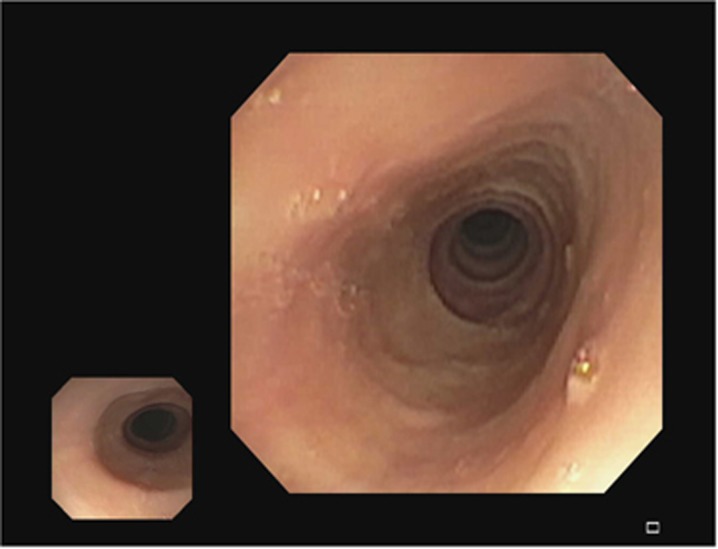
Patients with EoE can have several endoscopic findings. These include concentric rings (fixed or transient) (see Figure 3), longitudinal furrows (see Figure 4), white plaques (see Figure 5), reduced mucosal vascularity, fragile or crepe-like mucosa, and stricture (dominant or diffusely narrow esophagus) (see Figure 6).1, 38 Rings and furrows are the most common endoscopic findings, seen in 44% and 48% of patients respectively.39 The sensitivity of these endoscopic features for EoE is low (48% rings, 40% furrows, 15% strictures, and 27% white plaques), but the specificity is high (91% rings, 95% linear furrows, 95% strictures, and 94% white plaques).39 Up to 10% of EoE patients have no endoscopic findings, emphasizing that biopsies should be taken from all patients undergoing endoscopic evaluation for dysphagia.12 EREFS (Eosinophilic Esophagitis Endoscopic Reference Score), a classification system developed to standardize grading of endoscopic findings, has demonstrated good interobserver variability.38 This classification system assesses and scores each of the major endoscopic findings: rings (grades 0–3), exudates (grades 0–2), furrows (grades 0–1), mucosal edema (grades 0–1), and strictures (grades 0–1).38 Radiographic examination is of limited value in the diagnosis of EoE, although in certain circumstances, barium esophagrams can help detect subtle strictures or diffuse luminal narrowing.40
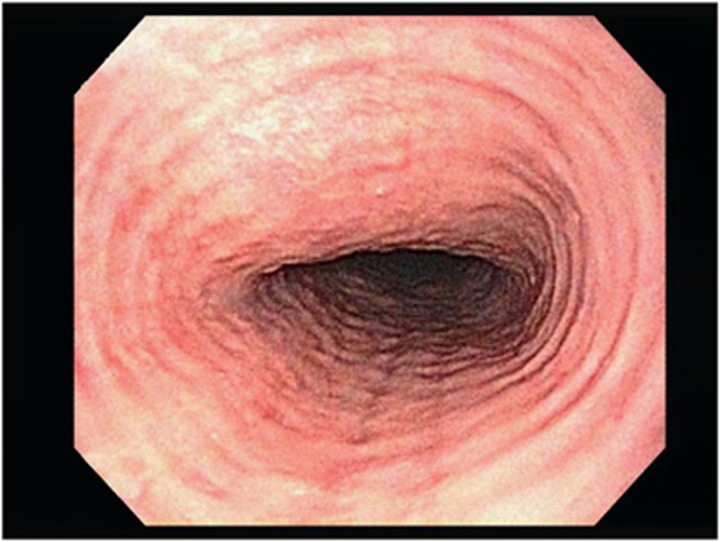
Figure 3.
Esophageal rings.
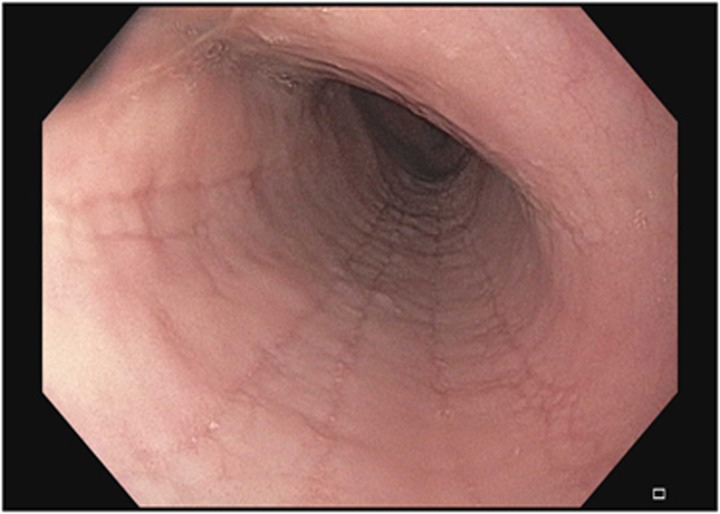
Figure 4.
Esophageal furrows.
Figure 5.
Esophageal white plaques.
Figure 6.
Esophageal stricture.
Biopsies are necessary to establish the diagnosis of EoE. Based on current clinical guidelines, the diagnosis requires 15 eosinophils per high-powered field or more in at least one esophageal site.1 EoE can be patchy and a single esophageal biopsy has low sensitivity (55%) for diagnosis.41, 42 Increasing to a minimum of six biopsies increases sensitivity to 99%,43 with biopsies in the mid and distal esophagus to help distinguish EoE from eosinophilia associated with reflux esophagitis. Targeting biopsies in furrows and exudates can also improve sensitivity.44 The mucosa may feel fibrotic while taking biopsies, requiring more effort to sample the mucosa. This is known as the “tug” sign45 or “pull” sign that has a sensitivity of 76% and specificity of 98% for EoE.46
The diagnosis of EoE requires mucosal eosinophilic infiltration that persists despite therapy with a PPI. Up to 50% of patients with an EoE phenotype respond clinically and histologically to PPI therapy.47, 48, 49 This entity, called PPI-responsive esophageal eosinophilia (PPI-REE), is indistinguishable clinically, endoscopically, and histologically from EoE.50, 51 There are two prevailing theories for the pathogenesis of PPI-REE. The first is that PPIs heal a defective barrier where acid increases mucosal permeability allowing the influx of allergens that activate a Th2-mediated inflammatory response.52 The second is that PPI therapy can reduce levels of key mediators of EoE such as eotaxin-3, IL-4, IL-5, and IL-13 independent of their antisecretory effects.47, 53 Even though biomarker staining can distinguish EoE from gastroesophageal reflux disease, this technique has not been useful in differentiating EoE from PPI-REE.50, 54 Recent data support that EoE and PPI-REE share a similar molecular basis.55 Treatment with PPIs, similar to treatment with steroids, reverses the molecular signature of EoE.56
As esophageal biopsies often necessitate upper endoscopy with sedation, new diagnostic methods are being investigated. Brush cytology with the Cytosponge is 69% sensitive and 67% specific for esophageal eosinophilia in the proximal esophagus, 72% sensitive and 75% specific for the distal esophagus,57 and correlates well with samples obtained during endoscopy.58 The Enterotest (HDC, Milpitas, CA) is a noninvasive capsule filled with 90 cm of string designed to sample luminal contents; in a pediatric population, the Enterotest accurately identified eosinophil-derived proteins in patients with EoE, with good sensitivity and specificity for esophageal eosinophilia.59 Further studies are needed before these technologies are implemented into clinical practice. Serum studies of proteins associated with EoE (such as IL-13 and eotaxin-3) have not yielded useful biomarkers for diagnosis or measuring response to therapy to date.33
There are two distinct phenotypes described in EoE. Patients with endoscopic findings limited to transient rings, furrows, and white plaques have the inflammatory phenotype, and those with fixed rings or strictures have the fibrostenotic phenotype. Although fibrostenosis is uncommon in children, adult EoE patients present with both phenotypes.34, 60 A study of the Swiss EoE database demonstrated that the duration of symptoms before diagnosis was the only factor that predicted stricture formation, suggesting that untreated inflammation is a major determinant of symptom development.34 These results were validated in a US institution where a significant difference in delayed diagnosis was observed in patients with an esophageal stricture vs. those with a patent esophageal diameter.61 Another retrospective study analyzing the differences between EoE phenotypes demonstrated that the likelihood of fibrostenotic disease doubled for every 10-year increase in age.60 These studies collectively suggest that the natural course of EoE includes the development of strictures over time that may be delayed or halted with treatment. Although there are no guidelines that delineate intervals for endoscopic surveillance of EoE patients, we conduct endoscopy annually in patients who have not shown resolution of esophageal eosinophilia.
The EndoFLIP system (Crospon Medical Devices, Galway, Ireland) is a device and program that uses impedance planimetry of a cylindrical bag to assess intraluminal pressure, and can give a measure of esophageal distensibility. In a study comparing EndoFLIP assessments in 35 EoE patients to 15 control patients, EoE patients had significantly less distensibility that did not correlate to eosinophilic density.62 This implicates esophageal dysmotility in symptom generation, and may explain why symptom resolution may not parallel resolution of eosinophilia.
MANAGEMENT
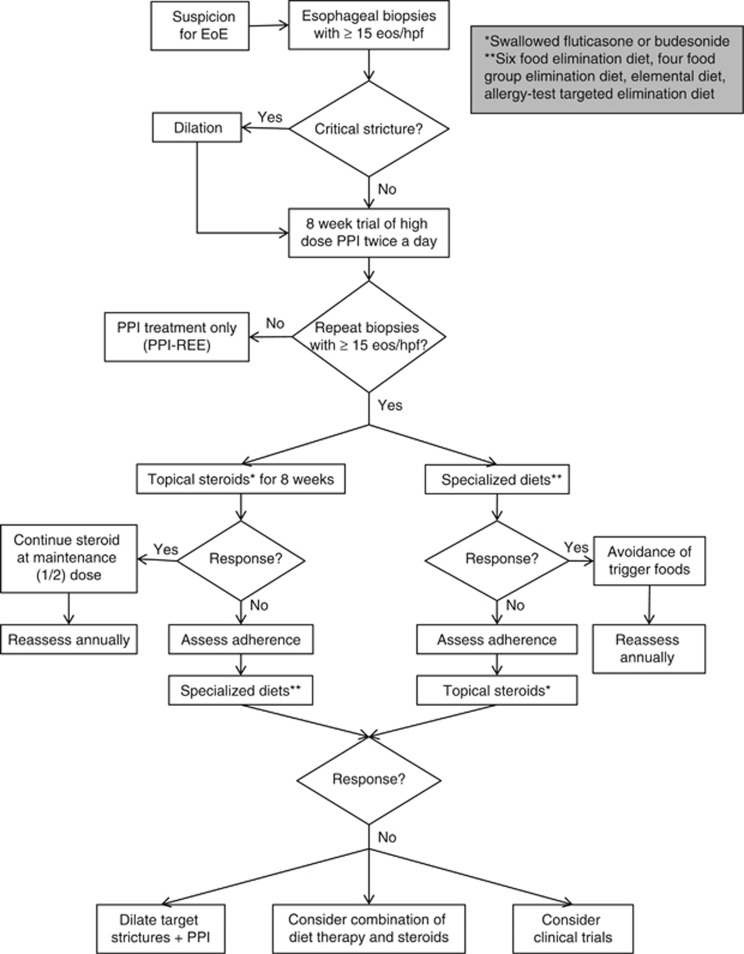
The management of EoE consists of the three Ds: drugs, diet, and dilation (see Figure 7). A major challenge in the management of EoE is the lack of well-defined therapeutic end points. From a patient's perspective, the goal is to improve symptoms and reduce the recurrence of food impaction. However, clinical end points are difficult to assess as patients develop adaptive techniques by chewing more carefully, eating slowly, and eliminating problem foods. The development of EEsAI (Eosinophilic Esophagitis Activity Index), a validated EoE symptom questionnaire that uses patient-reported outcomes, may help standardize clinical outcomes in EoE patients.63 Histologic outcomes are also difficult to assess. It is unclear which threshold for eosinophilia defines response to therapy, and symptoms do not accurately reflect degree of histologic inflammation.64, 65 Finally, endoscopic findings may not correspond to either symptoms or histology. It may be that future studies may use distensibility and submucosal fibrosis (as measured by systems like EndoFLIP) as treatment outcomes.
Figure 7.
Topical corticosteroids
Topical steroids remain the mainstay of EoE treatment. Steroids inhibit proinflammatory cytokines in the mucosa, thereby reducing eosinophil mucosal migration.1 Both swallowed aerosolized fluticasone and oral viscous budesonide induce a histologic and clinical response in randomized controlled trials.56, 66, 67, 68, 69, 70 In a pediatric study, swallowed fluticasone at 440 μg twice daily led to histologic improvement (>90% reduction in eosinophilia) after 3 months of treatment in half of subjects.66 In adults, histologic response was seen in 62% of subjects after treatment with 880 μg twice daily for 8 weeks.67 Recently, 65% of adult and pediatric patients treated with high-dose fluticasone (1,760 μg) for 3 months experienced histologic response.56 Extending therapy beyond 3 months in steroid-resistant patients did not help induce remission. It is noteworthy that in all three controlled trials, there was no significant difference in clinical symptoms between fluticasone and placebo.56, 66, 67 Although symptoms commonly recur in responsive patients after discontinuation of fluticasone,71 one recent study concluded that treatment with swallowed corticosteroids had a significant reduction in the frequent of food impactions over a 5-year follow-up period.72
Several controlled trials have used oral budesonide as a treatment option in EoE. In one pediatric study, oral viscous budesonide improved symptoms, endoscopic findings, and histologic eosinophilia in 87% of patients compared with a 0% placebo response.68 Another study in EoE children demonstrated that administration of medium-dose (cumulative dose >1.4 mg/day) and high-dose (cumulative dose > 2.8 mg/day) budesonide led to significant decreases in histologic eosinophilia and EoE symptoms scores.69 In adults, 1 mg budesonide administered in nebulized form for 15 days reduced symptoms and improved histology.70 After 50 weeks of therapy, patients maintained on low-dose budesonide (0.5 mg daily) had a better response compared with placebo, yet experienced an increase in eosinophilia without a significant difference in symptom response.73 In an open-label trial, viscous budesonide had more mucosal contact time and was more effective at reducing esophageal eosinophilia than swallowed nebulized budesonide.74 As budesonide respules are not intended for esophageal delivery, a slurry mixture (e.g., with a sugar substitute or honey) can be prepared by patients or their families. Otherwise, budesonide can be administered by continuously swallowing a nebulizer. A recent study showed equal efficacy of an effervescent budesonide formulation compared with an oral viscous formulation, with 80% of patients preferring the effervescent formulation and only 17% preferring the viscous formulation.75 There are no trials comparing fluticasone with budesonide. Adverse events with topical steroids are minimal, with esophageal candiasis being the most common (5–26%),67, 68, 70, 76, 77 that appears to be dose dependent.
Dietary therapy
Specialized diets are an effective treatment strategy in most EoE patients. There are three types of dietary treatment: elemental, targeted elimination, and empiric elimination of the most common allergen culprits. Studies in children have demonstrated that an elemental diet, which consists of an amino-acid free formula that eliminates all food triggers, is highly effective for both inducing histologic response and improving clinical symptoms.78, 79, 80 Elemental diet was 96% effective in inducing response in 10 children with EoE.80 Upon food challenge, patients redeveloped clinical symptoms and esophageal inflammation. In the only controlled study in adults, elemental diet was 72% (13/18) effective in inducing histologic response, defined as achieving ⩽10 eosinophils/high-powered field. However, 38% failed to adhere to the diet and all patients experienced esophageal inflammation when food was introduced.79 Although elemental diet is an effective treatment option, it has several drawbacks. It is expensive, may require a feeding tube for administration in children, can limit quality of life, has poor tolerability particularly in adults, and is not sustainable in the long term.
Given the challenges in maintaining this treatment, a diet tailored to remove specific allergens implicated in inflammation is more desirable. In a retrospective study of 22 adult EoE patients undergoing targeted food elimination based on results of allergy testing, 68% experienced clinical improvement, and 53% experienced endoscopic improvement with a significant improvement in posttreatment eosinophil count.81 In a retrospective cohort study in EoE children, 75% experienced clinical and histologic improvement after eliminating foods that tested positive on skin prick testing and atopic patch testing.82 Despite these studies demonstrating favorable outcomes when using allergy testing to eliminate foods, other controlled studies have not had similar outcomes. Gonsalves et al.83 reported that the predictive value of skin prick testing was only 13% in patients undergoing empiric elimination diets. Another effective strategy in treatment is the six-food elimination diet (SFED). This diet eliminates the six most common alimentary allergens: wheat, milk, eggs, nuts, soy, and seafood. Patients are monitored clinically and histologically as each food is slowly introduced to allow for identification of allergens. Some patients have more than one food trigger. In children, SFED led to 74% histologic remission. Eggs, soy, milk, and wheat were the most common allergens identified.84 In adults, a similar approach achieved 64% histologic remission with wheat and milk being the most common allergens identified.81 A prospective controlled study from Spain showed similar results with a 73% histologic response to empiric diet elimination.85 Of note, legumes were also eliminated in this study and found to be a cause in 23% of patients. Elimination diets, with culprits identified upon reintroduction, appear more effective than identification and elimination of allergens via skin prick testing.83 The treatment was durable with histologic remission seen after 3 years of therapy in patients who continue to avoid trigger foods.85 A recent study showed that a four-food group elimination diet (FFGED) eliminating dairy, eggs, legumes, and wheat yielded a 54% remission rate. In nonresponders, SFED led to an additional 18% remission.86 The number of endoscopies required to assess for histologic response is not practical in nonresearch settings and is a potential limitation of empiric diets. In a motivated patient who can adhere to specialized diets, FFGED or SFED are reasonable first-line approaches in lieu of topical steroids.
Dilation
EoE patients with features of fibrostenosis (fixed rings or strictures) benefit clinically from dilation, even though dilation does not influence esophageal inflammation or degree of eosinophilia. For this reason, clinicians should use dilation in conjunction with medical or dietary therapy. A study followed adult EoE patients for several years and demonstrated that dilation was effective in improving symptoms of 10/11 patients for a mean of 8 months.87 In a study of steroid-naive EoE patients treated only with PPI, dilation every other year was effective in maintaining clinical remission.88 Options for dilation include bougie, wire-guided dilator, or through-the-scope balloon dilator. No studies to date have compared the efficacy of these methods of dilation. In several retrospective studies, dilation improved dysphagia in 67–83% of patients, but improvement appears to be transient (∼1–2 years).89, 90, 91 Although there has historically been a concern about increased perforation rates when dilating patients with EoE,92, 93 recent studies have shown a perforation rate of 0.3%, similar to that of dilation of other esophageal conditions.90, 94, 95 Multiple dilations, especially in patients presenting with a critical esophageal stricture, appears to be a safe treatment.88 One study assessing esophageal distensibility has suggested that a target diameter of 17 mm may correlate to a lower risk of food impactions.96 Patients should be counseled about chest pain that commonly occurs after dilation in EoE.89 Once a mucosal tear develops after passage of the dilator or with the balloon, no further dilation is recommended during that session. Our practice is to target an esophageal diameter of at least 15 mm for patients to experience a sustained improvement in swallowing. Although there are no trials comparing dilation methods, we generally rely on bougie dilators that may result in dilation of strictures that were not otherwise detected during endoscopy. Empiric dilation (i.e., without an identified fixed ring or stricture) in EoE patients is controversial and data are lacking for its effectiveness. In such patients, dysphagia may be secondary other causes, such as reduced esophageal compliance.97
Emerging therapies
Given that at least one-third of EoE patients are unresponsive to topical steroids or specialized diets, researchers continue to explore options for antieosinophilic medications. Leukotriene inhibitors, such as montelukast, have not shown efficacy in maintaining steroid-free remission in EoE patients.98, 99 Therapies include drugs targeting key cytokines involved in the pathogenesis of EoE, such as IL-4, IL-5, and IL-13. There have been three well-designed studies targeting IL-5 cytokine.100, 101, 102 The largest was conducted in 226 children with EoE. All subjects received 2–4 infusions of anti-IL-5 treatment and achieved a significant eosinophil reduction, but symptom improvement was not significantly different from placebo.102
In the only anti-IL-13 study performed to date, 23 adult EoE patients were randomized in a 2:1 ratio to receive 3 doses of anti-IL-13 every 4 weeks.103 The primary end point (75% decrease in eosinophilia) was achieved in 40% of drug patients receiving anti-IL-13 treatment vs. 13% of placebo patients. Again, a significant difference was noted in histologic response, but there was no significant difference in clinical response.103 A larger international multicenter study evaluating the role of anti-IL-13 treatment for EoE is underway.
A recent randomized controlled trial compared Omalizumab, an anti-IgE medication, with placebo in EoE patients.104 Neither the primary end point (histologic improvement) nor the secondary end point (clinical response) was met in this study. This study concluded that EoE is not primarily an IgE-mediated allergy.104
Chemoattractant receptor-homologous molecule (CRTH2) is expressed in Th2 cells and mediates recruitment and activation of eosinophils.105 One randomized double-blinded placebo-controlled trial examined the role of CRTH2 antagonist (OC000459) in patients with severe EoE refractory to topical steroids.106 Although a significant improvement in eosinophil count was demonstrated in the 14 EoE patients treated with 100 mg of OC000459 twice daily for 8 weeks, patients did not achieve complete histologic response.106
In conclusion, EoE is a chronic inflammatory condition that is one of the most common causes of dysphagia. Its diagnosis requires endoscopic biopsies with demonstration of dense eosinophilia that persist after a course of PPI therapy to rule out PPI-REE. Treatment options available for patients include topical steroids, specialized diets, and dilation for critical strictures. Given the complexity in management of EoE, we recommend a multimodal approach with close follow-up and monitoring of symptoms to guide management. A multidisciplinary approach is useful, particularly in those patients with significant coexisting atopic conditions. Emerging therapies, although effective in reducing esophageal eosinophilia, have not yet demonstrated complete histologic response or improvements in clinical response.
Guarantor of the article: Manish B. Singla, MD.
Specific author contributions: Manish B. Singla and Fouad J. Moawad: literature search, manuscript drafting and revisions.
Financial support: No funding sources for this review article.
Potential competing interests: None.
References
- Dellon ES, Gonsalves N, Hirano I et al. ACG clinical guideline: evidenced based approach to the diagnosis and management of esophageal eosinophilia and eosinophilic esophagitis (EoE). Am J Gastroenterol 2013; 108: 679–692; quiz 693. [DOI] [PubMed] [Google Scholar]
- Kapel RC, Miller JK, Torres C et al. Eosinophilic esophagitis: a prevalent disease in the United States that affects all age groups. Gastroenterology 2008; 134: 1316–1321. [DOI] [PubMed] [Google Scholar]
- Sperry SL, Woosley JT, Shaheen NJ et al. Influence of race and gender on the presentation of eosinophilic esophagitis. Am J Gastroenterol 2012; 107: 215–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moawad FJ, Veerappan GR, Lake JM et al. Correlation between eosinophilic oesophagitis and aeroallergens. Aliment Pharmacol Ther 2010; 31: 509–515. [DOI] [PubMed] [Google Scholar]
- Moawad FJ, Veerappan GR, Dias JA et al. Race may play a role in the clinical presentation of eosinophilic esophagitis. Am J Gastroenterol 2012; 107: 1263; author reply 1263-1264. [DOI] [PubMed] [Google Scholar]
- Spergel JM, Brown-Whitehorn TF, Beausoleil JL et al. 14 years of eosinophilic esophagitis: clinical features and prognosis. J Pediatr Gastroenterol Nutr 2009; 48: 30–36. [DOI] [PubMed] [Google Scholar]
- Dellon ES. Epidemiology of eosinophilic esophagitis. Gastroenterol Clin North Am 2014; 43: 201–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dellon ES, Jensen ET, Martin CF et al. Prevalence of eosinophilic esophagitis in the United States. Clin Gastroenterol Hepatol 2014; 12: 589–596.e581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackenzie SH, Go M, Chadwick B et al. Eosinophilic oesophagitis in patients presenting with dysphagia--a prospective analysis. Aliment Pharmacol Ther 2008; 28: 1140–1146. [DOI] [PubMed] [Google Scholar]
- Ricker J, McNear S, Cassidy T et al. Routine screening for eosinophilic esophagitis in patients presenting with dysphagia. Therap Adv Gastroenterol 2011; 4: 27–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veerappan GR, Perry JL, Duncan TJ et al. Prevalence of eosinophilic esophagitis in an adult population undergoing upper endoscopy: a prospective study. Clin Gastroenterol Hepatol 2009; 7: 420–426, 426.e421-422. [DOI] [PubMed] [Google Scholar]
- Prasad GA, Talley NJ, Romero Y et al. Prevalence and predictive factors of eosinophilic esophagitis in patients presenting with dysphagia: a prospective study. Am J Gastroenterol 2007; 102: 2627–2632. [DOI] [PubMed] [Google Scholar]
- Hruz P, Straumann A, Bussmann C et al. Escalating incidence of eosinophilic esophagitis: a 20-year prospective, population-based study in Olten County, Switzerland. J Allergy Clin Immunol 2011; 128: 1349–1350.e1345. [DOI] [PubMed] [Google Scholar]
- van Rhijn BD, Verheij J, Smout AJ et al. Rapidly increasing incidence of eosinophilic esophagitis in a large cohort. Neurogastroenterol Motil 2013; 25: 47–52.e45. [DOI] [PubMed] [Google Scholar]
- Cherian S, Smith NM, Forbes DA. Rapidly increasing prevalence of eosinophilic oesophagitis in Western Australia. Arch Dis Child 2006; 91: 1000–1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Straumann A, Simon HU. Eosinophilic esophagitis: escalating epidemiology? J Allergy Clin Immunol 2005; 115: 418–419. [DOI] [PubMed] [Google Scholar]
- Okada H, Kuhn C, Feillet H et al. The 'hygiene hypothesis' for autoimmune and allergic diseases: an update. Clin Exp Immunol 2010; 160: 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merwat SN, Spechler SJ. Might the use of acid-suppressive medications predispose to the development of eosinophilic esophagitis? Am J Gastroenterol 2009; 104: 1897–1902. [DOI] [PubMed] [Google Scholar]
- Mullin JM, Valenzano MC, Whitby M et al. Esomeprazole induces upper gastrointestinal tract transmucosal permeability increase. Aliment Pharmacol Ther 2008; 28: 1317–1325. [DOI] [PubMed] [Google Scholar]
- Untersmayr E, Bakos N, Schöll I et al. Anti-ulcer drugs promote IgE formation toward dietary antigens in adult patients. FASEB J 2005; 19: 656–658. [DOI] [PubMed] [Google Scholar]
- Jensen ET, Hoffman K, Shaheen NJ et al. Esophageal eosinophilia is increased in rural areas with low population density: results from a national pathology database. Am J Gastroenterol 2014; 109: 668–675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurrell JM, Genta RM, Dellon ES. Prevalence of esophageal eosinophilia varies by climate zone in the United States. Am J Gastroenterol 2012; 107: 698–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf WA, Jerath MR, Dellon ES. De-novo onset of eosinophilic esophagitis after large volume allergen exposures. J Gastrointestin Liver Dis 2013; 22: 205–208. [PMC free article] [PubMed] [Google Scholar]
- Almansa C, Krishna M, Buchner AM et al. Seasonal distribution in newly diagnosed cases of eosinophilic esophagitis in adults. Am J Gastroenterol 2009; 104: 828–833. [DOI] [PubMed] [Google Scholar]
- Sleisenger MH, Feldman M, Friedman LS et al. Sleisenger and Fordtran's Gastrointestinal and Liver Disease : Pathophysiology, Diagnosis, Management, 9th edn. Saunders/Elsevier: Philadelphia, PA, 2010. [Google Scholar]
- Collins MH. Histopathology of eosinophilic esophagitis. Dig Dis 2014; 32: 68–73. [DOI] [PubMed] [Google Scholar]
- Straumann A, Bauer M, Fischer B et al. Idiopathic eosinophilic esophagitis is associated with a T(H)2-type allergic inflammatory response. J Allergy Clin Immunol 2001; 108: 954–961. [DOI] [PubMed] [Google Scholar]
- Blanchard C, Wang N, Stringer KF et al. Eotaxin-3 and a uniquely conserved gene-expression profile in eosinophilic esophagitis. J Clin Invest 2006; 116: 536–547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitaura M, Suzuki N, Imai T et al. Molecular cloning of a novel human CC chemokine (Eotaxin-3) that is a functional ligand of CC chemokine receptor 3. J Biol Chem 1999; 274: 27975–27980. [DOI] [PubMed] [Google Scholar]
- Mulder DJ, Justinich CJ. Understanding eosinophilic esophagitis: the cellular and molecular mechanisms of an emerging disease. Mucosal Immunol 2011; 4: 139–147. [DOI] [PubMed] [Google Scholar]
- Sherrill JD, Kc K, Wu D et al. Desmoglein-1 regulates esophageal epithelial barrier function and immune responses in eosinophilic esophagitis. Mucosal Immunol 2014; 7: 718–729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noti M, Wojno ED, Kim BS et al. Thymic stromal lymphopoietin-elicited basophil responses promote eosinophilic esophagitis. Nat Med 2013; 19: 1005–1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dellon ES, Rusin S, Gebhart JH et al. Utility of a noninvasive serum biomarker panel for diagnosis and monitoring of eosinophilic esophagitis: a prospective study. Am J Gastroenterol 2015; 110: 821–827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoepfer AM, Safroneeva E, Bussmann C et al. Delay in diagnosis of eosinophilic esophagitis increases risk for stricture formation in a time-dependent manner. Gastroenterology 2013; 145: 1230–1236.e1-2. [DOI] [PubMed] [Google Scholar]
- Kerlin P, Jones D, Remedios M et al. Prevalence of eosinophilic esophagitis in adults with food bolus obstruction of the esophagus. J Clin Gastroenterol 2007; 41: 356–361. [DOI] [PubMed] [Google Scholar]
- Roy-Ghanta S, Larosa DF, Katzka DA. Atopic characteristics of adult patients with eosinophilic esophagitis. Clin Gastroenterol Hepatol 2008; 6: 531–535. [DOI] [PubMed] [Google Scholar]
- Penfield JD, Lang DM, Goldblum JR et al. The role of allergy evaluation in adults with eosinophilic esophagitis. J Clin Gastroenterol 2010; 44: 22–27. [DOI] [PubMed] [Google Scholar]
- Hirano I, Moy N, Heckman MG et al. Endoscopic assessment of the oesophageal features of eosinophilic oesophagitis: validation of a novel classification and grading system. Gut 2013; 62: 489–495. [DOI] [PubMed] [Google Scholar]
- Kim HP, Vance RB, Shaheen NJ et al. The prevalence and diagnostic utility of endoscopic features of eosinophilic esophagitis: a meta-analysis. Clin Gastroenterol Hepatol 2012; 10: 988–996.e985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen BC, Baker ME, Falk GW. Role of barium esophagography in evaluating dysphagia. Cleve Clin J Med 2009; 76: 105–111. [DOI] [PubMed] [Google Scholar]
- Saffari H, Peterson KA, Fang JC et al. Patchy eosinophil distributions in an esophagectomy specimen from a patient with eosinophilic esophagitis: implications for endoscopic biopsy. J Allergy Clin Immunol 2012; 130: 798–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins MH. Histopathologic features of eosinophilic esophagitis. Gastrointest Endosc Clin N Am 2008; 18: 59–71 viii-ix. [DOI] [PubMed] [Google Scholar]
- Nielsen JA, Lager DJ, Lewin M et al. The optimal number of biopsy fragments to establish a morphologic diagnosis of eosinophilic esophagitis. Am J Gastroenterol 2014; 109: 515–520. [DOI] [PubMed] [Google Scholar]
- Salek J, Clayton F, Vinson L et al. Endoscopic appearance and location dictate diagnostic yield of biopsies in eosinophilic oesophagitis. Aliment Pharmacol Ther 2015; 41: 1288–1295. [DOI] [PubMed] [Google Scholar]
- Moawad FJ, Robinson CL, Veerappan GR et al. The tug sign: an endoscopic feature of eosinophilic esophagitis. Am J Gastroenterol 2013; 108: 1938–1939. [DOI] [PubMed] [Google Scholar]
- Dellon ES, Gebhart JH, Higgins LL et al. The esophageal biopsy "pull" sign: a highly specific and treatment-responsive endoscopic finding in eosinophilic esophagitis (with video). Gastrointest Endosc 2015; 83: 92–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molina-Infante J, Gisbert JP. Letter: PPI-responsive oesophageal eosinophilia--from initial scepticism to consistent prospective data. Aliment Pharmacol Ther 2014; 39: 229–230. [DOI] [PubMed] [Google Scholar]
- Molina-Infante J, Ferrando-Lamana L, Ripoll C et al. Esophageal eosinophilic infiltration responds to proton pump inhibition in most adults. Clin Gastroenterol Hepatol 2011; 9: 110–117. [DOI] [PubMed] [Google Scholar]
- Moawad FJ, Veerappan GR, Dias JA et al. Randomized controlled trial comparing aerosolized swallowed fluticasone to esomeprazole for esophageal eosinophilia. Am J Gastroenterol 2013; 108: 366–372. [DOI] [PubMed] [Google Scholar]
- Moawad FJ, Wells JM, Johnson RL et al. Comparison of eotaxin-3 biomarker in patients with eosinophilic oesophagitis, proton pump inhibitor-responsive oesophageal eosinophilia and gastro-oesophageal reflux disease. Aliment Pharmacol Ther 2015; 42: 231–238. [DOI] [PubMed] [Google Scholar]
- Dellon ES, Speck O, Woodward K et al. Clinical and endoscopic characteristics do not reliably differentiate PPI-responsive esophageal eosinophilia and eosinophilic esophagitis in patients undergoing upper endoscopy: a prospective cohort study. Am J Gastroenterol 2013; 108: 1854–1860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Rhijn BD, Weijenborg PW, Verheij J et al. Proton pump inhibitors partially restore mucosal integrity in patients with proton pump inhibitor-responsive esophageal eosinophilia but not eosinophilic esophagitis. Clin Gastroenterol Hepatol 2014; 12: 1815–1823.e1812. [DOI] [PubMed] [Google Scholar]
- Cheng E, Zhang X, Huo X et al. Omeprazole blocks eotaxin-3 expression by oesophageal squamous cells from patients with eosinophilic oesophagitis and GORD. Gut 2013; 62: 824–832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dellon ES, Speck O, Woodward K et al. Markers of eosinophilic inflammation for diagnosis of eosinophilic esophagitis and proton pump inhibitor-responsive esophageal eosinophilia: a prospective study. Clin Gastroenterol Hepatol 2014; 12: 2015–2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen T, Dellon ES, Moawad FJ et al. Transcriptome analysis of proton pump inhibitor-responsive esophageal eosinophilia reveals proton pump inhibitor-reversible allergic inflammation. J Allergy Clin Immunol 2015; 135: 187–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butz BK, Wen T, Gleich GJ et al. Efficacy, dose reduction, and resistance to high-dose fluticasone in patients with eosinophilic esophagitis. Gastroenterology 2014; 147: 324–333.e325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kern E, Lin D, Larson A et al. Prospective assessment of the diagnostic utility of esophageal brushings in adults with eosinophilic esophagitis. Dis Esophagus 2014; 29: 48–53. [DOI] [PubMed] [Google Scholar]
- Katzka DA, Geno DM, Ravi A et al. Accuracy, safety, and tolerability of tissue collection by Cytosponge vs endoscopy for evaluation of eosinophilic esophagitis. Clin Gastroenterol Hepatol 2015; 13: 77–83.e72. [DOI] [PubMed] [Google Scholar]
- Furuta GT, Kagalwalla AF, Lee JJ et al. The oesophageal string test: a novel, minimally invasive method measures mucosal inflammation in eosinophilic oesophagitis. Gut 2013; 62: 1395–1405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dellon ES, Kim HP, Sperry SL et al. A phenotypic analysis shows that eosinophilic esophagitis is a progressive fibrostenotic disease. Gastrointest Endosc 2014; 79: 577–585.e574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipka S, Kumar A, Richter JE. Impact of diagnostic delay and other risk factors on eosinophilic esophagitis phenotype and esophageal diameter. J Clin Gastroenterol 2015; 50: 134–140. [DOI] [PubMed] [Google Scholar]
- Kwiatek MA, Hirano I, Kahrilas PJ et al. Mechanical properties of the esophagus in eosinophilic esophagitis. Gastroenterology 2011; 140: 82–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoepfer AM, Straumann A, Panczak R et al. Development and validation of a symptom-based activity index for adults with eosinophilic esophagitis. Gastroenterology 2014; 147: 1255–1266.e1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sperry SL, Shaheen NJ, Dellon ES. Toward uniformity in the diagnosis of eosinophilic esophagitis (EoE): the effect of guidelines on variability of diagnostic criteria for EoE. Am J Gastroenterol 2011; 106: 824–832; quiz 833. [DOI] [PubMed] [Google Scholar]
- Pentiuk S, Putnam PE, Collins MH et al. Dissociation between symptoms and histological severity in pediatric eosinophilic esophagitis. J Pediatr Gastroenterol Nutr 2009; 48: 152–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konikoff MR, Noel RJ, Blanchard C et al. A randomized, double-blind, placebo-controlled trial of fluticasone propionate for pediatric eosinophilic esophagitis. Gastroenterology 2006; 131: 1381–1391. [DOI] [PubMed] [Google Scholar]
- Alexander JA, Jung KW, Arora AS et al. Swallowed fluticasone improves histologic but not symptomatic response of adults with eosinophilic esophagitis. Clin Gastroenterol Hepatol 2012; 10: 742–749.e741. [DOI] [PubMed] [Google Scholar]
- Dohil R, Newbury R, Fox L et al. Oral viscous budesonide is effective in children with eosinophilic esophagitis in a randomized, placebo-controlled trial. Gastroenterology 2010; 139: 418–429. [DOI] [PubMed] [Google Scholar]
- Gupta SK, Vitanza JM, Collins MH. Efficacy and safety of oral budesonide suspension in pediatric patients with eosinophilic esophagitis. Clin Gastroenterol Hepatol 2015; 13: 66–76.e63. [DOI] [PubMed] [Google Scholar]
- Straumann A, Conus S, Degen L et al. Budesonide is effective in adolescent and adult patients with active eosinophilic esophagitis. Gastroenterology 2010; 139: 1526–1537, 1537.e1521. [DOI] [PubMed] [Google Scholar]
- Helou EF, Simonson J, Arora AS. 3-yr-follow-up of topical corticosteroid treatment for eosinophilic esophagitis in adults. Am J Gastroenterol 2008; 103: 2194–2199. [DOI] [PubMed] [Google Scholar]
- Kuchen T, Straumann A, Safroneeva E et al. Swallowed topical corticosteroids reduce the risk for long-lasting bolus impactions in eosinophilic esophagitis. Allergy 2014; 69: 1248–1254. [DOI] [PubMed] [Google Scholar]
- Straumann A, Conus S, Degen L et al. Long-term budesonide maintenance treatment is partially effective for patients with eosinophilic esophagitis. Clin Gastroenterol Hepatol 2011; 9: 400–409.e401. [DOI] [PubMed] [Google Scholar]
- Dellon ES, Sheikh A, Speck O et al. Viscous topical is more effective than nebulized steroid therapy for patients with eosinophilic esophagitis. Gastroenterology 2012; 143: 321–324.e321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miehlke S, Hruz P, Vieth M et al. A randomised, double-blind trial comparing budesonide formulations and dosages for short-term treatment of eosinophilic oesophagitis. Gut 2015; 65: 390–399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaefer ET, Fitzgerald JF, Molleston JP et al. Comparison of oral prednisone and topical fluticasone in the treatment of eosinophilic esophagitis: a randomized trial in children. Clin Gastroenterol Hepatol 2008; 6: 165–173. [DOI] [PubMed] [Google Scholar]
- Aceves SS, Bastian JF, Newbury RO et al. Oral viscous budesonide: a potential new therapy for eosinophilic esophagitis in children. Am J Gastroenterol 2007; 102: 2271–2279; quiz 2280. [DOI] [PubMed] [Google Scholar]
- Markowitz JE, Spergel JM, Ruchelli E et al. Elemental diet is an effective treatment for eosinophilic esophagitis in children and adolescents. Am J Gastroenterol 2003; 98: 777–782. [DOI] [PubMed] [Google Scholar]
- Peterson KA, Byrne KR, Vinson LA et al. Elemental diet induces histologic response in adult eosinophilic esophagitis. Am J Gastroenterol 2013; 108: 759–766. [DOI] [PubMed] [Google Scholar]
- Kelly KJ, Lazenby AJ, Rowe PC et al. Eosinophilic esophagitis attributed to gastroesophageal reflux: improvement with an amino acid-based formula. Gastroenterology 1995; 109: 1503–1512. [DOI] [PubMed] [Google Scholar]
- Wolf WA, Jerath MR, Sperry SL et al. Dietary elimination therapy is an effective option for adults with eosinophilic esophagitis. Clin Gastroenterol Hepatol 2014; 12: 1272–1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spergel JM, Andrews T, Brown-Whitehorn TF et al. Treatment of eosinophilic esophagitis with specific food elimination diet directed by a combination of skin prick and patch tests. Ann Allergy Asthma Immunol 2005; 95: 336–343. [DOI] [PubMed] [Google Scholar]
- Gonsalves N, Yang GY, Doerfler B et al. Elimination diet effectively treats eosinophilic esophagitis in adults; food reintroduction identifies causative factors. Gastroenterology 2012; 142: 1451–1459.e1451; quiz e1414-1455. [DOI] [PubMed] [Google Scholar]
- Kagalwalla AF, Sentongo TA, Ritz S et al. Effect of six-food elimination diet on clinical and histologic outcomes in eosinophilic esophagitis. Clin Gastroenterol Hepatol 2006; 4: 1097–1102. [DOI] [PubMed] [Google Scholar]
- Lucendo AJ, Arias Á, González-Cervera J et al. Empiric 6-food elimination diet induced and maintained prolonged remission in patients with adult eosinophilic esophagitis: a prospective study on the food cause of the disease. J Allergy Clin Immunol 2013; 131: 797–804. [DOI] [PubMed] [Google Scholar]
- Molina-Infante J, Arias A, Barrio J et al. Four-food group elimination diet for adult eosinophilic esophagitis: a prospective multicenter study. J Allergy Clin Immunol 2014; 134: 1093–1099.e1091. [DOI] [PubMed] [Google Scholar]
- Straumann A, Spichtin HP, Grize L et al. Natural history of primary eosinophilic esophagitis: a follow-up of 30 adult patients for up to 11.5 years. Gastroenterology 2003; 125: 1660–1669. [DOI] [PubMed] [Google Scholar]
- Lipka S, Keshishian J, Boyce HW et al. The natural history of steroid-naïve eosinophilic esophagitis in adults treated with endoscopic dilation and proton pump inhibitor therapy over a mean duration of nearly 14 years. Gastrointest Endosc 2014; 80: 592–598. [DOI] [PubMed] [Google Scholar]
- Schoepfer AM, Gonsalves N, Bussmann C et al. Esophageal dilation in eosinophilic esophagitis: effectiveness, safety, and impact on the underlying inflammation. Am J Gastroenterol 2010; 105: 1062–1070. [DOI] [PubMed] [Google Scholar]
- Moawad FJ, Cheatham JG, DeZee KJ. Meta-analysis: the safety and efficacy of dilation in eosinophilic oesophagitis. Aliment Pharmacol Ther 2013; 38: 713–720. [DOI] [PubMed] [Google Scholar]
- Dellon ES, Gibbs WB, Rubinas TC et al. Esophageal dilation in eosinophilic esophagitis: safety and predictors of clinical response and complications. Gastrointest Endosc 2010; 71: 706–712. [DOI] [PubMed] [Google Scholar]
- Kaplan M, Mutlu EA, Jakate S et al. Endoscopy in eosinophilic esophagitis: "feline" esophagus and perforation risk. Clin Gastroenterol Hepatol 2003; 1: 433–437. [DOI] [PubMed] [Google Scholar]
- Eisenbach C, Merle U, Schirmacher P et al. Perforation of the esophagus after dilation treatment for dysphagia in a patient with eosinophilic esophagitis. Endoscopy 2006; 38 ((Suppl 2)): E43–E44. [DOI] [PubMed] [Google Scholar]
- Jacobs JW, Spechler SJ. A systematic review of the risk of perforation during esophageal dilation for patients with eosinophilic esophagitis. Dig Dis Sci 2010; 55: 1512–1515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohm ME, Richter JE. Review article: oesophageal dilation in adults with eosinophilic oesophagitis. Aliment Pharmacol Ther 2011; 33: 748–757. [DOI] [PubMed] [Google Scholar]
- Nicodème F, Hirano I, Chen J et al. Esophageal distensibility as a measure of disease severity in patients with eosinophilic esophagitis. Clin Gastroenterol Hepatol 2013; 11: 1101–1107.e1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roman S, Hirano I, Kwiatek MA et al. Manometric features of eosinophilic esophagitis in esophageal pressure topography. Neurogastroenterol Motil 2011; 23: 208–214, e111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucendo AJ, De Rezende LC, Jiménez-Contreras S et al. Montelukast was inefficient in maintaining steroid-induced remission in adult eosinophilic esophagitis. Dig Dis Sci 2011; 56: 3551–3558. [DOI] [PubMed] [Google Scholar]
- Stumphy J, Al-Zubeidi D, Guerin L et al. Observations on use of montelukast in pediatric eosinophilic esophagitis: insights for the future. Dis Esophagus 2011; 24: 229–234. [DOI] [PubMed] [Google Scholar]
- Assa'ad AH, Gupta SK, Collins MH et al. An antibody against IL-5 reduces numbers of esophageal intraepithelial eosinophils in children with eosinophilic esophagitis. Gastroenterology 2011; 141: 1593–1604. [DOI] [PubMed] [Google Scholar]
- Straumann A, Conus S, Grzonka P et al. Anti-interleukin-5 antibody treatment (mepolizumab) in active eosinophilic oesophagitis: a randomised, placebo-controlled, double-blind trial. Gut 2010; 59: 21–30. [DOI] [PubMed] [Google Scholar]
- Spergel JM, Rothenberg ME, Collins MH et al. Reslizumab in children and adolescents with eosinophilic esophagitis: results of a double-blind, randomized, placebo-controlled trial. J Allergy Clin Immunol 2012; 129: 456–463, 463.e451-453. [DOI] [PubMed] [Google Scholar]
- Rothenberg ME, Wen T, Greenberg A et al. Intravenous anti-IL-13 mAb QAX576 for the treatment of eosinophilic esophagitis. J Allergy Clin Immunol 2015; 135: 500–507. [DOI] [PubMed] [Google Scholar]
- Clayton F, Fang JC, Gleich GJ et al. Eosinophilic esophagitis in adults is associated with IgG4 and not mediated by IgE. Gastroenterology 2014; 147: 602–609. [DOI] [PubMed] [Google Scholar]
- Pettipher R, Vinall SL, Xue L et al. Pharmacologic profile of OC000459, a potent, selective, and orally active D prostanoid receptor 2 antagonist that inhibits mast cell-dependent activation of T helper 2 lymphocytes and eosinophils. J Pharmacol Exp Ther 2012; 340: 473–482. [DOI] [PubMed] [Google Scholar]
- Straumann A, Hoesli S, Bussmann C et al. Anti-eosinophil activity and clinical efficacy of the CRTH2 antagonist OC000459 in eosinophilic esophagitis. Allergy 2013; 68: 375–385. [DOI] [PubMed] [Google Scholar]