Abstract
OBJECTIVES:
Literature describing the risk factors predisposing inflammatory bowel disease (IBD) patients to anal squamous neoplasia is very scarce. Case reports and small case series have implicated perianal Crohn's disease (CD), long-standing IBD, human papillomavirus (HPV) infection, and immunosuppressive treatment. In this study, we retrospectively examined the association between HPV infection and anal squamous neoplastic lesions among IBD patients from our center.
METHODS:
We reviewed the pathology records and slides of IBD patients diagnosed with anal squamous cell carcinomas (SCCs), high-grade squamous intraepithelial lesions (HSILs), and low-grade squamous intraepithelial lesions (LSILs) who presented at our center between 1 March 1994 and 9 September 2014. The HPV status of the neoplasms was assessed histologically, by immunohistochemical staining for p16 overexpression, and by global and type-specific HPV PCR.
RESULTS:
SCCs, HSILs, LSILs, and small cell carcinoma were identified, respectively, in six, nine, two, and one IBD patients. All six patients with SCC had CD with perianal involvement. HPV-related neoplasia was identified in 3/6 cases of SCC (all HPV-16), 1/1 small cell carcinoma (HPV-18), and 9/9 HSIL (7 HPV-16, 2 not typed); 2/2 LSILs were negative for high-risk HPV.
CONCLUSIONS:
In our experience, anal squamous neoplastic lesions in IBD are associated with HPV infection and SCC seem to be associated with perianal CD. Prospective studies are needed to confirm these results.
INTRODUCTION
Anal neoplasia is rare, with an annual incidence of 2 cases per 100,000 and accounts for only 1–4% of colorectal malignancies.1, 2, 3, 4, 5 Anal canal cancers include: squamous cell carcinoma (SCC), adenocarcinoma, small cell carcinoma, and undifferentiated carcinoma. SCC arising from the anal transitional zone or squamous mucosa accounts for the majority of cases.
Multiple risk factors for developing anal squamous carcinoma have been studied, including history of receptive anal intercourse, history of sexually transmitted diseases, >10 sexual partners, men who have sex with men, history of other anogenital cancer (cervical, vulvar, or vaginal), and immunosuppression associated with solid-organ transplantation or HIV. As with cervical cancer, infection with human papillomavirus (HPV), especially types 16 and 18, has a strong association with perianal and anal canal cancer.6, 7
Recently, a high prevalence of anal HPV infection was reported in patients with inflammatory bowel disease (IBD).8 However, the prevalence of anal HPV infection in IBD patients with anal neoplastic lesions is currently unknown. A recent study published by Shah et al.9 reported a positive HPV in 5.3% of IBD subjects with atypical squamous cells of undetermined significance. In this cohort, there were no cases of low-grade squamous intraepithelial lesions (LSILs), high-grade squamous intraepithelial lesions (HSILs), or anal cancer.9 The literature describing the phenotype of IBD patients who develop anal neoplasia is scarce and consists mainly of case reports and limited case series with conflicting data.10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26 The aim of our study was to determine the prevalence of HPV infection in IBD patients with anal squamous neoplastic lesions who have been treated at our institution during the past 20 years, utilizing retrospective HPV testing on paraffin-embedded tissue blocks.
We also assessed predisposing risk factors reported in the literature such as disease duration, perianal involvement, and immunosupressive treatment in our cohort of patients.
METHODS
We retrospectively reviewed the records of IBD patients diagnosed with anal squamous neoplastic lesions presenting at Mount Sinai Hospital in New York, between 1 March 1994 and 9 September 2014. This retrospective research was approved by Mount Sinai Medical Center's research ethics committee.
Case identification and histological evaluation
The Mount Sinai Hospital Pathology Department and Gastrointestinal Pathology Division databases were searched retrospectively for biopsies, and surgical resections of anal squamous lesions in patients with IBD examined between 1 March 1994 and 9 September 2014 (Figures 1 and 2). The databases were queried for entries corresponding to ulcerative colitis (UC), Crohn's disease (CD), or indeterminate colitis combined with the terms “squamous dysplasia,” “anal intraepithelial”, “AIN”, “HPV”, or “squamous cell carcinoma”. Patients diagnosed with anal neoplastic lesions prior to diagnosis of IBD were excluded.
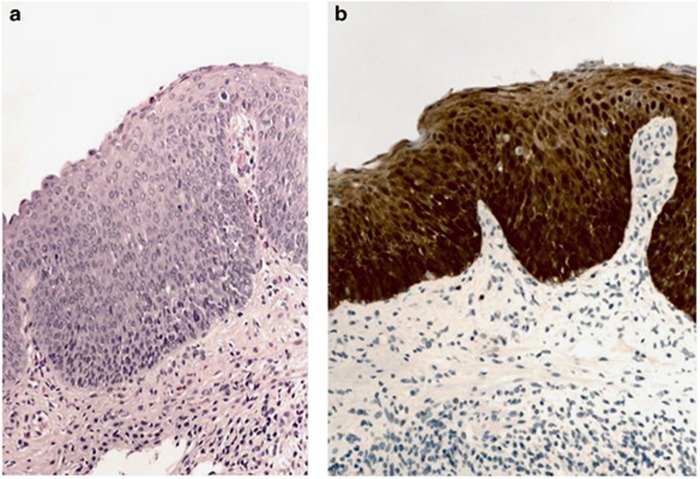
Figure 1.
Photomicrographs of anal squamous intraepithelial neoplasia, high grade. (a) Anal mucosa with dysplasia extending beyond the basal 1/3 of the epithelium, hematoxylin and eosin, × 200; (b) p16 “block positive” staining pattern supports the diagnosis.
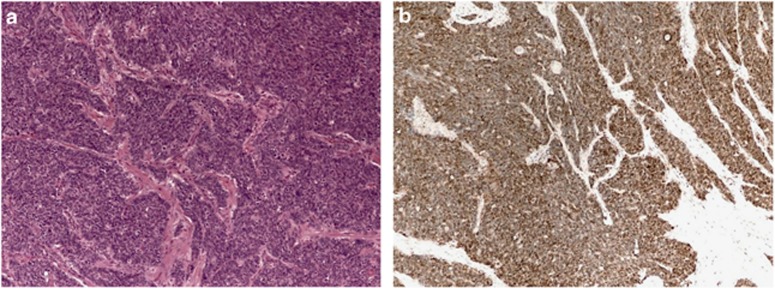
Figure 2.
Photomicrographs of anal squamous cell carcinoma. (a) Hematoxylin and eosin stain, × 100; (b) immunostain for p16 shows overexpression pattern consistent with transcriptionally active human papillomavirus.
Corresponding hematoxylin and eosin–stained biopsy slides were reviewed by a gastrointestinal pathologist (N.H.) and a gynecological pathologist (N.P.) and graded as LSIL, HSIL, SCC, or small cell carcinoma.
The presence of HPV was assessed using immunohistochemical staining for p16 overexpression in combination with global and type-specific molecular PCR for HPV-16 and -18. It is important to note that p16 immunostain is a surrogate marker for dysregulation of the cell cycle as a consequence of active HPV oncoprotein E7, commonly, but not exclusively HPV-16.
Deparaffinized sections were stained immunohistochemically for p16 with p16INK4 antibody (clone E6H4; prediluted; Ventana, Tucson, AZ). The stained slides were evaluated by the authors (H.M.K., N.P., N.H.) and the pathological diagnoses were made by consensus. The grade of anal squamous intraepithelial lesions was diagnosed using p16 staining and morphology as per the CAP-ASCCP LAST project guidelines.27
Cases with p16 block–positive staining but negative HPV by PCR (see methods below) underwent additional immunostaining for p53 (clone DO-7; prediluted; Ventana).
Molecular analysis
Genomic DNA was extracted from formalin-fixed, paraffin-embedded tissue sections using a Maxwell 16 MDx FFPE Tissue LEV DNA Purification Kit on the Maxwell 16 MDx Instrument (Promega, Madison, WI) according to the manufacturer's instructions. The L1 region of the HPV genome was amplified by real-time PCR using consensus primers GP5+ and GP6+, to allow the amplification of global HPV genotypes (>27 genotypes, including HPV-6, -11, -16, -18, -31, -33, and -35) in a single reaction,28 and a LightCycler 480 High Resolution Melting Master Kit (Roche, Indianapolis, IN). The PCR cycle consisted of (1) denature at 95 °C for 10 min; (2) 45 cycles: denature at 95 °C for 10 s, then annealing at 55 °C for 1 min, and then extension at 72 °C for 20 s; (3) additional extension at 72 °C for 5 min. A Cp value >40 was considered as negative. If HPV DNA was amplified in the sample, a High Resolution Melting Curve Analysis was performed on the LightCycler 480 instrument to confirm HPV genotype. If the sample was either HPV-16 or -18 based on the melting curve analysis, a HPV-16/18-specific probe based on real-time PCR was performed to further confirm the HPV-16 or -18 genotypes using above PCR cycle parameters. In addition, β-actin (forward primer 5′-CACTGTGCCCATCTACG-3′ reverse primer 5′-TTAATGTCACGCACGATTTCC-3′) was amplified in a separate tube as an internal control to confirm the quality of the DNA by PCR.
Chart review
Abstracted data from chart analysis included gender, IBD type (CD, UC, or indeterminate colitis), ileal pouch anastomosis, colectomy, condyloma, HIV, colorectal cancer, disease duration, perianal disease, smoking status, and immunosuppressive therapy at diagnosis.
RESULTS
Patient's characteristics
We identified 18 IBD patients who were diagnosed with anal squamous neoplastic lesions presenting at our medical center between 1 March 1994 and 9 September 2014. Lesions were found during perianal exams, colonoscopies, or incidentally on colectomy specimen. Eight patients were male. Ten patients (56%) carried a clinical diagnosis of UC, 7 (39%) CD, and 1 (6%) indeterminate colitis. Eight of the 18 patients had perianal disease involvement, including one isolated case of UC with a unique perianal fistula. Invasive SCCs, small cell carcinoma, HSILs, and LSILs were identified respectively in six, one, nine, and two patients (Table 1). The small cell carcinoma occurred in a patient with a 10-year history of UC who had undergone proctocolectomy with ileo-anal pouch anastomosis 11 years earlier. Biopsies showed histologically that the carcinoma arose directly from the overlying HSILs.
Table 1. Patient characteristics according to anal neoplastic lesion type (n=18).
| Total (n=18) | SCC (n=6) | HSIL (n=9) | LSIL (n=2) | Small cell carcinoma (n=1) | |
|---|---|---|---|---|---|
| Gender, M/F (n) | 8/10 | 2/4 | 3/6 | 2/0 | 1/0 |
| IBD type, UC/CD/IC (n) | 10/7/1 | 0/6/0 | 7/1/1 | 2/0/0 | 1/0/0 |
| Ileo-anal pouch anastomosis +/− (n) | 3/15 | 1/5 | 1/8 | 0/2 | 1/0 |
| Colectomy +/− (n) | 6/12 | 2/4 | 3/6 | 1/1 | 1/0 |
| HIV status +/− (n) | 1/17 | 0/6 | 1/8 | 0/2 | 0/1 |
| Condyloma +/− (n) | 1/17 | 0/6 | 1/8 | 0/2 | 0/1 |
| Perianal disease +/− (n) | 8/10 | 6/0 | 1/8 | 1/1 | 0/1 |
| Disease duration in years >10/5 to 10/<5/unknown (n) | 8/1/2/7 | 2/0/1/3 | 5/1/1/2 | 1/0/0/1 | 1/0/0/0 |
| Smoking status +/−/unknown (n) | 2/8/8 | 0/3/3 | 2/3/4 | 0/1/1 | 0/1/0 |
| Colorectal cancer +/− (n) | 1/17 | 0/6 | 0/9 | 1/1 | 0/1 |
CD, Crohn's disease; F, female; HSIL, high-grade squamous intraepithelial lesion; IBD, inflammatory bowel disease; IC, indeterminate colitis; LSIL, low-grade squamous intraepithelial lesion; M, male; SCC, squamous cell carcinoma; UC, ulcerative colitis.
In the SCC group, all 6 patients had CD with perianal involvement compared with 1/9 and 1/2 in the HSIL and LSIL groups, respectively. Information regarding medical therapy at the time of anal cancer diagnosis was available in 10 patients, of which 4, 2, and 2 were on thiopurine, steroids, and biological therapy, respectively (Table 2).
Table 2. Immunosuppressive therapy at the time of anal cancer diagnosis.
| Immunosuppressive therapy (n) | Total (n=18) | SCC (n=6) | HSIL (n=9) | LSIL (n=2) | Small cell carcinoma (n=1) |
|---|---|---|---|---|---|
| Immunomodulators (azathioprine/6-MP/methotrexate) | 4 | 1 | 2 | 1 | 0 |
| Biological therapy | 2 | 0 | 2 | 0 | 0 |
| Immunomodulators and anti-TNF (combination) | 1 | 0 | 1 | 0 | 0 |
| Steroids | 2 | 0 | 2 | 0 | 0 |
| Cyclosporine/tacrolimus | 2 | 0 | 1 | 1 | 0 |
| Mycophenolate | 1 | 0 | 0 | 1 | 0 |
| None | 5 | 2 | 3 | 0 | 0 |
| Unknown | 8 | 3 | 3 | 1 | 1 |
HSIL, high-grade squamous intraepithelial lesion; LSIL, low-grade squamous intraepithelial lesion; SCC, squamous cell carcinoma; TNF, tumor necrosis factor.
Tumor immunohistochemical and molecular analysis
Fifteen of the 18 lesions were HPV-related based on histology, p16 immunostaining, and PCR (Table 3). Of the six SCC cases, three were HPV-driven based on positive HPV-16 by PCR and concordant p16 overexpression. One additional case of SCC was positive for HPV-16 but did not overexpress p16, suggesting that the HPV was not biologically tumorigenic.29 The remaining two cases of SCC were negative for HPV by global HPV PCR analysis. Incidentally, one of those cases overexpressed p16 and p53 by immunostaining, suggesting a p53-mutated SCC.
Table 3. HPV testing results.
| Total (n=18) | SCC (n=6) | HSIL (n=9) | LSIL (n=2) | Small cell carcinoma (n=1) | |
|---|---|---|---|---|---|
| HPV-related lesion | 15 | 3 | 9 | 2 | 1 |
| Non-HPV-related lesion | 3 | 3 | 0 | 0 | 0 |
HPV, human papillomavirus; HSIL, high-grade squamous intraepithelial lesion; LSIL, low-grade squamous intraepithelial lesion; SCC, squamous cell carcinoma.
All nine cases of HSILs were HPV-related based on morphology (HPV viral cytopathic changes, including koilocytes and basal zone proliferation) and p16 block-positive overexpression. Of these, seven were positive for HPV-16 by PCR, whereas 2 were negative for HPV, owing to loss of lesional tissue in deep sections of the tissue block.
The two LSIL lesions were morphologically consistent with HPV but were negative for HPV by PCR and for p16 overexpression as commonly occurs in low-risk HPV subtypes.
The single case of small cell carcinoma arising from HSILs was the only lesion that was positive for HPV-18.
Interestingly, three of the female patients had cervical cytology available. All three had negative testing results, either at the same time as their anal lesion or afterward. The same three patients all had HPV-16-positive anal lesions (two high-grade lesions and one SCC).
DISCUSSION
Albeit limited by its retrospective design, this study to our knowledge is the largest combined clinicopathological and molecular study of IBD patients diagnosed with anal squamous neoplastic lesions. Keeping in mind that this study did not include a control group of IBD-free controls with anal neoplastic lesions, chronic perianal CD, prolonged IBD, and evidence of HPV exposure were implicated as potential risk factors for squamous neoplasia. Perianal disease was present in 44% of the patients overall, including all those diagnosed with SCC. Disease duration prior to the diagnosis of squamous neoplasia exceeded 10 years in 44% of the patients and in 33% of those with SCC. Molecular, immunohistochemical, and/or morphological evidence of HPV was identified in 15 of the 18 cases overall, including 3 of the 6 cases of SCC, 1 case of small cell carcinoma, and all 11 cases of LSILs and HSILs. Besides careful perianal examination, screening of IBD patients for anal HPV could be considered but it seems too early to provide clear recommendations for screening a specific subset of patients. A prospective study is needed to confirm these findings.
Information regarding medical therapy at the time of diagnosis of anal squamous neoplasia was available in 10 patients only, of which 4, 2, and 2 were on thiopurine, steroids, and biological therapy, respectively. Unfortunately, exposure to immunosuppressants before their diagnosis is unknown.
HPV is a small, non-enveloped, double-stranded DNA virus. More than 200 subtypes have been identified based on the genetic sequence of the outer capsid protein L1. Approximately 40 subtypes infect the mucosal epithelium; they represent the most common sexually transmitted group of pathogens in humans. These mucosotropic HPV subtypes are further classified into non-oncogenic or low-risk types, including HPV-6 and -11, and potentially oncogenic or high-risk types, including HPV-16 and -18. The transformation zone at the junction between distal columnar epithelium of the rectum and proximal squamous epithelium of the anal canal is particularly susceptible to HPV infection. Although persistent infection of the basal and parabasal cells of the squamous mucosa with high-risk, oncogenic HPV types is implicated in HSILs (the anal cancer precursor) and invasive SCC, low-grade non-oncogenic HPV types are rarely associated with malignancy but are causally linked to venereal warts (condyloma) and respiratory papillomatosis.30, 31, 32
In the general population, infection with HPV, especially types 16 and 18, has a strong association with perianal and anal canal cancer.33 Frisch et al.,34 in a population-based study, found that 88% of anal squamous cancer specimens were positive for HPV infection, with 73% of specimens positive for HPV type 16 infection. Anal HPV infection occurred in 50% of a cohort of sexually active women >1 year, with more than half spontaneously clearing the infection within 1 year.35 The prevalence and clearance of anal HPV infection are similar in heterosexual men. However, men who have sex with men have more persistent infection and a higher proportion of oncogenic types.36 Additional risk factors include a history of receptive anal intercourse, men who have sex with men, history of sexually transmitted diseases, multiple sexual partners, history of anogenital cancer (cervical, vulvar, or vaginal), and immunosuppression after solid-organ transplantation.37, 38
In patients with IBD, the prevalence of anal HPV infection was recently reported to be high.8 In this study of 26 IBD patients, despite the fact that <50% were on immunomodulators, 81% of patients had anal HPV detected (80% had ⩾1 high-risk HPV types) and 42% of patients had an abnormal anal cytology. All patients taking a thiopurine had ⩾1 anal high-risk HPV detected. No patient with undetectable anal HPV was taking an immunosuppressant. Among patients who had biopsies, 38% had LSILs (anal intraepithelial neoplasia (AIN) grade 1) and 15% HSILs (AIN grade 2). Forty-three percent of patients with anal dysplasia were taking immunosuppressants.
The effect of immunosuppression on the rates of anal HPV infection in IBD patients or on the prevalence of high-grade neoplastic lesions and its progression to anal cancer in the IBD population is largely unknown. A prospective study of 230 IBD patients demonstrated a significant increase in viral warts in the group receiving AZA/6-MP compared with those who were not on immunosuppression (17.2% vs. 3.3%, P=0.004).39 Similarly, an increase in abnormal pap smears in women with IBD compared with controls (42% vs. 7%, P<0.001) has been reported and also in women with a history of exposure to immunosuppression,40 supporting the recommendation for HPV vaccination and regular gynecological examinations in female IBD patients on immunosuppressive therapy.41
The prevalence of HSILs or even SCCs in patients with IBD treated with immunosuppressive medications is unknown. Past studies have shown that oncogenic HPV strains are the causative infectious agent leading to HSILs, which is considered the precursor lesion for invasive anal squamous cell cancer. HSIL has been shown to be present more commonly in immunocompromised patients, including patients with HIV and those who underwent solid-organ transplantation, and therefore IBD patients on immunosuppressive drugs or with chronic perianal lesions could be considered to be an at-risk group. However, it must be acknowledged that the CESAME cohort did not demonstrate an association between thiopurine use and anal cancer (whether adenocarcinoma or SCC) in IBD patients although the crude risk in the subset of patients with perianal CD exposed to thiopurines was 0.42 per 1000 patient-years.42 In addition, the TREAT and the ENCORE registries have not demonstrated an excess of anal cancers in patients treated with infliximab.43, 44
In conclusion, our study suggests that anal squamous neoplasia in IBD is associated with HPV infection and that SCC seem to be associated with perianal CD. Besides careful perianal examination, screening of IBD patients for anal HPV could be considered but it seems too early to provide clear recommendations for screening a specific subset of patients. A prospective study is needed to confirm these findings. Although based on low-level evidence from uncontrolled studies, annual perianal examination with or without anal cytology (microscopic evaluation of anal cells collected by sampling the anal canal with a brush) could be considered in IBD patients with longstanding perianal fistulizing disease/anal stricture or known HPV infection. In addition, IBD patients with other risk factors for HPV infection such as history of receptive anal intercourse, men who have sex with men, history of sexually transmitted diseases including HIV, multiple sexual partners, and history of anogenital cancer (cervical, vulvar, or vaginal) should also be screened in an effort to prevent anal cancer.
Study Highlights
Guarantor of the article: Jean-Frédéric Colombel, MD, PhD.
Specific author contributions: Planning and conducting the study: Jean-Frédéric Colombel, Noam Harpaz, and Joannie Ruel. Collecting data: Joannie Ruel, Huaibin Mabel Ko, Giulia Roda, Ninad Patil, David Zhang, and Bindia Jharap. Interpreting data: all authors. Drafting the manuscript: Joannie Ruel, Huaibin Mabel Ko, Giulia Roda, and Bindia Jharap. All authors have approved the final draft submitted.
Financial support: None.
Potential competing interests: None.
References
- Johnson LG, Madeleine MM, Newcorner LM et al. Anal cancer incidence and survival: the surveillance, epidemiology, and end results experience, 1973-2000. Cancer 2004; 101: 281–288. [DOI] [PubMed] [Google Scholar]
- Schneider TC, Schulte WT. Management of carcinoma of anal canal. Surgery 1981; 90: 729–733. [PubMed] [Google Scholar]
- Beahrs OH, Wilson SM. Carcinoma of the anus. Ann Surg 1976; 184: 422–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corman ML, Haggitt RC. Carcinoma of the anal canal. Surg Gynecol Obstet 1977; 145: 674–676. [PubMed] [Google Scholar]
- Newlin HE, Zlotecki RA, Morris CG et al. Squamous cell carcinoma of the anal margin. J Surg Oncol 2004; 86: 55–62. [DOI] [PubMed] [Google Scholar]
- Hoots BE, Palefsky JM, Pimenta JM et al. Human papillomavirus type distribution in anal cancer and anal intraepithelial lesions. Int J Cancer 2009; 124: 2375–2383. [DOI] [PubMed] [Google Scholar]
- Machalek DA, Poynten M, Jin F et al. Anal human papillomavirus infection and associated neoplastic lesions in men who have sex with men: a systematic review and meta-analysis. Lancet Oncol 2012; 13: 487–500. [DOI] [PubMed] [Google Scholar]
- Cranston R, Baker J, Mowrey C et al. Inflammatory Bowel Disease Patients Have a High Prevalence of Anal Human Papillomavirus and Anal Dysplasia. Abstract Su1143. DDW 2013.
- Shah SB, Pickham D, Araya H et al. Prevalence of anal dysplasia in patients with inflammatory bowel disease. Clin Gastro Hepatol 2015; 13: 1955–1961. [DOI] [PubMed] [Google Scholar]
- Frisch M, Johansen C. Anal carcinoma in inflammatory bowel disease. Br J Cancer 2000; 83: 89–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zagoni T, Peter Z, Sipos F et al. Carcinoma arising in enterocutaneous fistulae of Crohn's disease patients: description of two cases. Int J Colorectal Dis 2006; 21: 461–464. [DOI] [PubMed] [Google Scholar]
- Daly JJ, Madrazo A. Anal Crohn's disease with carcinoma in situ. Dig Dis Sci 1980; 25: 464–466. [DOI] [PubMed] [Google Scholar]
- Ball CS, Wujanto R, Haboubi NY et al. Carcinoma in anal Crohn's disease: discussion paper. J R Soc Med 1988; 81: 217–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lumley JW, Stitz RW. Crohn's disease and anal carcinoma: an association? A case report and review of the literature. Aust NZ J Surg 1991; 61: 76–77. [DOI] [PubMed] [Google Scholar]
- Devon KM, Brown CJ, Burnstein M et al. Cancer of the anus complicating perianal Crohn's disease. Dis Colon Rectum 2009; 52: 211–216. [DOI] [PubMed] [Google Scholar]
- Kang J, Min BS, Lee KY et al. Squamous cell carcinoma of the anus in a patient with perianal Crohn's disease. Int J Colorectal Dis. 2010; 25: 411–413. [DOI] [PubMed] [Google Scholar]
- Iesalnieks I, Gaertner WB, Glass H et al. Fistula-associated anal adenocarcinoma in Crohn's disease. Inflamm Bowel Dis 2010; 16: 1643–1648. [DOI] [PubMed] [Google Scholar]
- Egea-Valenzuela J, Belchí-Segura E, Essouri N et al. Adenocarcinoma of the rectum and anus in a patient with Crohn's disease treated with infliximab. Rev Esp Enferm Dig 2010; 102: 501–504. [DOI] [PubMed] [Google Scholar]
- Chandramohan K, Mathew AP, Muralee M et al. Squamous cell carcinoma arising from long-standing perianal fistula. Int Wound J 2010; 7: 515–518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baars JE, Kuipers EJ, Dijkstra G et al. Malignant transformation of perianal and enterocutaneous fistulas is rare: results of 17 years of follow up from the Netherlands. Scand J Gastroenterol 2011; 46: 319–325. [DOI] [PubMed] [Google Scholar]
- Connell WR, Sheffield JP, Kamm MA et al. Lower gastrointestinal malignancy in Crohn's disease. Gut 1994; 35: 347–352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ky A, Sohn N, Weinstein MA et al. Carcinoma arising in anorectal fistulas of Crohn's disease. Dis Colon Rectum 1998; 41: 992–996. [DOI] [PubMed] [Google Scholar]
- Slater G, Greenstein A, Aufses AH Jr. Anal carcinoma in patients with Crohn's disease. Ann Surg 1984; 199: 348–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith R, Hicks D, Tomljanovich PI et al. Adenocarcinoma arising from chronic perianal Crohn's disease: case report and review of the literature. Am Surg 2008; 74: 59–61. [PubMed] [Google Scholar]
- Thomas M, Bienkowski R, Vandermeer TJ et al. Malignant transformation in perianal istulas of Crohn's disease: a systematic review of literature. J Gastrointest Surg 2010; 14: 66–73. [DOI] [PubMed] [Google Scholar]
- Bahadursingh AM, Longo WE. Malignant transformation of chronic perianal Crohn's fistula. Am J Surg 2005; 189: 61–62. [DOI] [PubMed] [Google Scholar]
- Darragh TM, Colgan TJ, Cox JT et al. The lower anogenital squamous terminology standardization project for HPV-associated lesions: background and consensus recommendations from the College of American Pathologists and the American Society for Colposcopy and Cervical Pathology. Arch Pathol Lab Med 2012; 136: 1266–1297. [DOI] [PubMed] [Google Scholar]
- de Roda Husman AM, Walboomers JM, van den Brule AJ et al. The use of general primers GP5 and GP6 elongated at their 3' ends with adjacent highly conserved sequences improves human papillomavirus detection by PCR. J Gen Virol 1995; 76: 1057–1062. [DOI] [PubMed] [Google Scholar]
- Lewis JS Jr, Thorstad WL, Chernock RD et al. p16 positive oropharyngeal squamous cell carcinoma: an entity with a favorable prognosis regardless of tumor HPV status. Am J Surg Pathol 2010; 34: 1088–1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- zur Hausen H. Papillomaviruses and cancer: from basic studies to clinical application. Nat Rev Cancer 2002; 2: 342–359. [DOI] [PubMed] [Google Scholar]
- Chow LT, Broker TR, Steinberg BM. The natural history of human papillomavirus infections of the mucosal epithelia. APMIS 118: 422–449. [DOI] [PubMed] [Google Scholar]
- de Villiers EM, Fauquet C, Broker TR et al. Classification of papillomaviruses. Virology 2004;324:17–27. [DOI] [PubMed] [Google Scholar]
- Bjorge T, Engeland A, Luostarinen T et al. Human papillomavirus infection as a risk factor for anal and perianal skin cancer in a prospective study. Br J Cancer 2002; 87: 61–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frisch M, Glimelius B, van den Brule AJC et al. Sexually transmitted infection as a cause of anal cancer. N Engl J Med 1997; 337: 1350–1358. [DOI] [PubMed] [Google Scholar]
- Frisch M, Glimelius B, van den Brule AJ et al. Benign anal lesions, inflammatory bowel disease and risk for high-risk human papillomavirus-positive and -negative anal carcinoma. Br J Cancer 1998; 78: 1534–1538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shvetsov YB, Hernandez BY, McDuffie K et al. Duration and clearance of anal human papillomavirus (HPV) infection among women: the Hawaii HPV cohort study. Clin Infect Dis 2009; 48: 536–546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gervasoni JE Jr, Wanebo HJ. Cancers of the anal canal and anal margin. Cancer Invest 2003; 21: 452–464. [DOI] [PubMed] [Google Scholar]
- Sunesen KG, Norgaard M, Thorlacius-Ussing O et al. Immunosuppressive disorders and risk of anal squamous cell carcinoma: a nationwide cohort study in Denmark, 1978-2005. Int J Cancer 2010; 127: 675–684. [DOI] [PubMed] [Google Scholar]
- Seksik P, Cosnes J, Sokol H et al. Incidence of benign upper respiratory tract infections, HSV and HPV cutaneous infections in inflammatory bowel disease patients treated with azathioprine. Aliment Pharmacol Ther 2009; 29: 1106–1113. [DOI] [PubMed] [Google Scholar]
- Kane S, Khatibi B, Reddy D. Higher incidence of abnormal Pap smears in women with inflammatory bowel disease. Am J Gastroenterol 2008; 103: 631–636. [DOI] [PubMed] [Google Scholar]
- Singh H, Demers AA, Nugent Z et al. Risk of cervical abnormalities in women with inflammatory bowel disease: a population-based nested case-control study. Gastroenterology 2009; 136: 451–458. [DOI] [PubMed] [Google Scholar]
- Beaugerie L. Inflammatory bowel disease therapies and cancer risk: where are we and where are we going? Gut 2012; 61: 476–483. [DOI] [PubMed] [Google Scholar]
- Lichtenstein GR, Feagan BG, Cohen RD et al. Serious infections and mortality in association with therapies for Crohn's disease: TREAT registry. Clin Gastroenterol Hepatol 2006; 4: 621–630. [DOI] [PubMed] [Google Scholar]
- Colombel J, Prantera C, Rutgeerts PJ. No new safety signals identified in Crohn's disease patients treated with infliximab in an interim review of the ENCORE registry. Gastroenterology 2008; 134: A-472. [Google Scholar]